Bacterial symbionts and natural products
Jason M.
Crawford
and
Jon
Clardy
*
Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, 240 Longwood Avenue, Boston, MA 02115, USA. E-mail: jon_clardy@hms.harvard.edu; Fax: +1 617 432-6424; Tel: +1 617 432-2845
First published on 19th May 2011
Abstract
The study of bacterial symbionts of eukaryotic hosts has become a powerful discovery engine for chemistry. This highlight looks at four case studies that exemplify the range of chemistry and biology involved in these symbioses: a bacterial symbiont of a fungus and a marine invertebrate that produce compounds with significant anticancer activity, and bacterial symbionts of insects and nematodes that produce compounds that regulate multilateral symbioses.
 Jason M. Crawford | Jason M. Crawford carried out his doctoral research in the Department of Chemistry at the Johns Hopkins University with Professor Craig A. Townsend. There, he studied the biosynthesis of bioactive small molecules, primarily aromatic polyketides produced by fungi. He is currently a National Institutes of Health Pathway to Independence postdoctoral fellow at Harvard Medical School with Professor Jon Clardy. His current research focuses on mutualistic and pathogenic interactions at the molecular level to understand the chemical signaling events between bacteria and their animal hosts and to apply these insights for the discovery of new molecules with biomedical potential. |
 Jon Clardy | Jon Clardy has forty years of experience with biologically active small molecules, mostly naturally occurring ones, and his laboratory currently focuses on bacterially produced small molecules and anti-malaria therapeutic agents. He holds the Hsien Wu and Daisy Yen Wu Chair at Harvard Medical School and is also a Senior Associate Member of the Broad Institute of Harvard and MIT, where he co-directs the Infectious Disease Initiative. He co-founded the Chemical Biology Graduate Program at Harvard University and teaches a popular undergraduate course with David Liu to nonscience students. |
In the last ten years, a series of shocking revelations—the molecular equivalents of a reality TV show's uncovering the true parents of a well known individual or a deeply hidden family secret—altered the study of genetically encoded small molecules, natural products for short. These revelations all involved natural products produced by bacterial symbionts, and while details differed, two main plot lines emerged: parentage, in which the real producers of well known natural products with medical potential were not the organisms from which they were originally discovered, and hidden relationships, in which bacterially produced small molecules turned out to be the unsuspected regulators of complex interactions. For chemists, these studies led to new molecules, new biosynthetic pathways, and an understanding of the biological functions these molecules fulfill.
Natural products, symbionts, and hosts
Natural products have repeatedly transformed our understanding of what small molecules can do in biological contexts.1 In the not so distant past, everyone “knew” that it would not be possible to find selective kinase inhibitors, especially inhibitors bound at the ATP-binding site, because these sites would be essentially identical in all kinases. Staurosporine (1) completely altered that bit of conventional wisdom, and while it was not selective enough to be therapeutically useful, the road to selective kinase inhibitors like Gleevec, Tarceva, and Iressa began with staurosporine (Fig. 1). In a similar vein, FK506 (2) and rapamycin (3) showed that a small molecule could simultaneously bind two different proteins to create a tripartite aggregate with biological properties different from any of the individual contributors, and enediyne antibiotics like dynemicin (4) showed how an implausible looking arrangement of functional groups could be triggered to rearrange and create potent DNA damaging agents. Intensive studies over the last half of the 20th-century on the sort of bacteria that produce staurosporine, FK506, rapamycin and the enediynes led to many useful discoveries and ultimately to a feeling that this particular discovery vein had been mined out. In the last decade, three lines of research have shown that bacterial small molecules are far from a played out resource. The first line began with systematic efforts to discover the small molecules made by the vast majority of bacteria that could not be, or at least had not been, cultured in the laboratory.2,3 The second line began with the sequencing of bacterial genomes, which led to the realization that these genomes contained easily recognizable biosynthetic gene clusters for many small molecules that had never been seen in the laboratory.4–6 The third line, which will be the focus of this brief essay, began with the discovery of bacterial symbionts where none had been expected and the important roles played by the small molecules they produced.7 | ||
| Fig. 1 Selected bacterial products that have changed conventional wisdom in both chemistry and biology. | ||
In biology, a symbiotic relation is the close and often long-term interaction between two or more different organisms. We will follow convention by calling the bacterial participant the symbiont and the larger (eukaryotic) partner the host. Symbiotic relationships, which span a continuum and can vary with time, are traditionally divided into three broad categories: mutualist in which both partners benefit, parasitic/pathogenic in which one partner benefits at the expense of the other, and commensal in which one partner benefits but the other partner does not pay much of a price. The most interesting associations for this essay are the mutualistic relations, as they comprise the most rapidly growing and least studied class. We will address only four illustrative cases—two in which studies of well-known and potentially useful natural products led to the discovery of their production by symbiotic bacteria and two in which the intentional study of bacterial symbionts led to the discovery of new chemistry. Our selections hit only a few highlights, and we apologize in advance to the many researchers whose research is omitted.
Bacteria in a fungal host: rhizoxin
Rhizoxin (5) emerged as a potentially useful anticancer candidate by a circuitous route. Fungi belonging to the Rhizopus genus caused a serious rice disease, seedling blight, and the critical pathogenicity factor, rhizoxin (5), was isolated from Rhizopus cultures in the early 1980s and shown to induce the root swelling characteristic of the disease.8 Rhizoxin's macrolactone ring binds to β-tubulin, which inhibits microtubule assembly and leads to eukaryotic cell cycle arrest. This antimitotic activity, which was characteristic of other anticancer agents,9 prompted the synthesis of rhizoxin analogs and extensive clinical trials to evaluate them as potential antitumor drugs.Hertweck and co-workers, initiated an effort to define rhizoxin's biosynthetic pathway—possibly as a prelude to a biosynthetic approach to additional rhizoxin analogs—and discovered that an unsuspected intracellular bacterium in the Burkholderia genus was the actual rhizoxin producer.10 They carried out an elegant set of studies to first identify candidate biosynthetic genes of bacterial but not fungal origin. The rhizoxin-positive phytopathogenic fungus Rhizopus microsporus was consistently associated with a bacterium now known as Burkholderia rhizoxinica.11 Using fluorescent dyes, the living bacterium was visualized as an intracellular symbiont of the host fungus.10 Introducing rhizoxin-producing bacteria grown in isolation into a non-producing symbiont-free fungal host conferred the ability to produce rhizoxin thereby unambiguously establishing a molecular basis for the symbiosis.10 In this association between fungus and bacteria, both partners benefit from rhizoxin's phytopathogenic activity, as the dying plant feeds both. The bacteria gain a safe haven inside fungal cells with stable conditions and plentiful cytosolic metabolites. The fungus also requires the bacteria to sporulate,12 while avoiding rhizoxin's toxicity via resistance-conferring mutations in its β-tubulin gene.13
This pioneering study also led to the genes responsible for the biosynthesis of rhizoxin, which contained insights for chemists. Rhizoxin's biosynthetic genes encode a hybrid modular (type I) polyketide synthase/nonribosomal peptide synthetase complex and occupy a continuous, ∼81 kB stretch, of the bacterial genome.14,15 The gene cluster is flanked by two peripherally located transposase genes, which indicate that the producing bacteria acquired the genes from a bacterial neighbor by a route called horizontal gene transfer. Rhizoxin's biosynthesis, unlike its laboratory synthesis, involves a long linear sequence of steps in which small fragments are added sequentially until the entire skeleton is complete. The growing rhizoxin molecule moves along this long linear pathway as a series of thioesters, and after the addition of each new building block, the thiol on the next module accepts the product from the thiol of the preceding module (Fig. 2A). At the end of the modular pathway, a thioesterase releases the product. These pathways can be analyzed by disabling the terminal thioesterase, which causes the intermediate thioesters to pile up until they are gradually hydrolyzed from their enzyme partner. These released intermediates can be characterized to provide a series of molecular snap shots that can be assembled into a movie of the molecule's biosynthesis.16–18
 | ||
| Fig. 2 Natural products produced via modular enzymatic assembly lines. (a) Hypothetical mixed polyketide synthase (blue) and nonribosomal peptide synthetase (red) complex. Polyketide synthases accept and condense short fatty acyl-Coenzyme A (CoA) substrates. Nonribosomal peptide synthetases accept and condense amino acid monomer units at the expense of ATP. Both modular enzymes carry the intermediates via labile thioester attachments. Individual enzyme domains are represented as “balls on a string”. For simplification, the domain functionalities are not shown or discussed. The chain termination event is typically catalyzed by a thioesterase domain. The reader is directed to general reviews on polyketide77 and nonribosomal peptide biosynthesis.78,79 (b) Structure of rhizoxin (5). One of the intermediates, 5a, was identified by inactivating the terminal thioesterase domain. Intermediate 5a represents a substrate for the β-branch module, which installs rhizoxin's δ-lactonevia a Michael addition. | ||
This thioesterase disabling approach provided fascinating insights into unusual features of rhizoxin's biosynthesis (Fig. 2B). For example, the α,β-unsaturated thioester intermediate 5a, characterized as its released free acid, was one of the intermediates identified.16 The next advanced intermediate in the sequence contained the δ-lactone, suggesting that an enzymatic Michael addition took place to generate an atypical β-branch modification. Genetic deletion of the putative β-branch module destroyed δ-lactone intermediates, leaving 5a as the most advanced intermediate detected. The enzymatic details of how the β-branch module constructs the Michael product remain unknown, but the module functionally incorporates one acetate. It is also not clear whether the δ-lactone forms via release of an enzyme-bound thioester intermediate as shown or through spontaneous cyclization of the open free acid form. In any case, conversion of the free carboxyl group into an ester leads to semi-synthetic derivatives that are three to four orders of magnitude more potent in antimitotic assays, ranking them among the top known antiproliferative agents.19 The identification of rhizoxin's biosynthetic genes allows other bacteria with the potential to make rhizoxin, or close relatives, to be identified, and the free-growing Pseudomonas fluorescensPf-5 isolate, for example, has a rhizoxin gene cluster.20,21
Cyanobacteria in sea squirts: cyanobactins
Whole animal extracts from the ascidian Lissoclinum patella contained cyclic peptide macrolactams—patellamide C (6), ulithiacyclamide (7), patellin 6 (8), or trunkamide A (9) are representative—with significant anticancer activity (Fig. 3).22–24 Their structures suggested bacterial origin, so the sea squirt's bacterial symbionts were collected, and the collective genome of all the symbionts, their metagenome, was sequenced. Bioinformatic analysis of the resulting sequences led to a candidate patellamide biosynthetic gene cluster and the producing organism, a Cyanobacterium called Prochloron didemni that photosynthesizes nutrients for the sea squirt. This candidate gene cluster, when introduced into a non-producing E. colihost, conferred the ability to biosynthesize the patellamides.25,26 This study highlights features that have become commonplace in this field of research: the power of large-scale sequencing to discover biosynthetic gene clusters, and the introduction of these clusters into alternative hosts (a technique called heterologous expression) to confirm the assignment.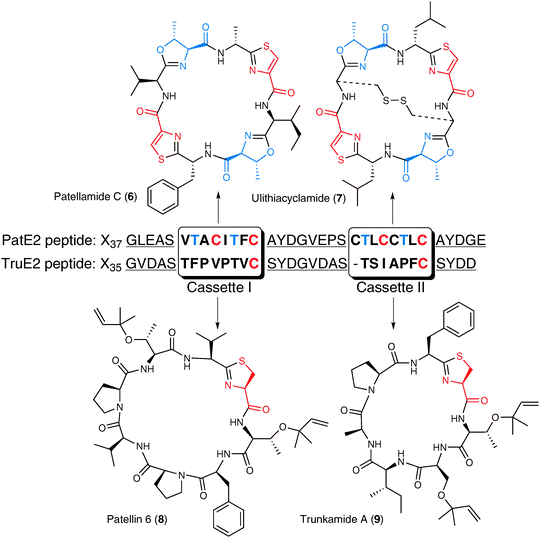 | ||
| Fig. 3 Ribosomally produced cyanobactins. Precursor peptides PatE2 and TruE2 are shown, which can be prenylated, as in the case of the trunkamides (bottom), and heterocyclized in both cases (top and bottom). The final product cassette sequences are boxed. Heterocyclized oxazolines are shown in blue and thiazolines/thiazoles are shown in red. The protease recognition sites surrounding the two cassettes in each precursor peptide are underlined. The specific leader sequences (Xn) are not shown. | ||
Of course the discovery of the initial cluster led to the discovery of many similar clusters and an expanding class of natural products now known as the cyanobactins.24,27 The cyanobactins are ribosomally synthesized peptides made by excising a smaller peptide from a highly post-translationally modified precursor peptide. For example, the residues for patellamide C and ulithiacyclamide, their structural cassettes, lie on the same precursor peptide (Fig. 3). Select serine, threonine, or cysteine residues in the precursor peptides can be prenylated, as in the case of the trunkamides,28 or heterocyclized to form oxazoline, methyloxazoline, or thiazoline moieties. Subsequent enzymatic oxidation of the heterocycles can lead to oxazole, methyloxazole, or thiazole residues.29
The structural cassettes are flanked by protease recognition sequences where two subtilisin-like proteases cleave the modified peptides.30,31 The first protease cleaves at the N-terminal recognition sites. The second protease cleaves at the C-terminal sites, but rather than releasing a linear peptide through hydrolysis, this enzyme catalyzes N–C macrocyclization and product release via a transamidation reaction. The cassette regions that encode the cyanobactin residues are naturally hypervariable (reported as low as 46%) while the surrounding genes maintain high sequence conservation (>99%).32 Understanding the mechanism for cassette hypermutation within the 7 or 8 product codons remains an interesting future challenge. Relaxed substrate specificity by the biosynthetic enzymes allows the efficient synthesis of alternative macrolactams, and this relaxed specificity and cassette hypervariability are almost certainly essential features of the bacterium's strategy for evolving new chemical defenses in an ever-changing world. By tracking the bacterial gene clusters responsible for the production of cyanobactins in ascidians through metagenomic approaches, new biosynthetic enzymes have been connected to the post-translational processing steps, new bioactive products have been discovered and engineered, and a bacterial natural product evolution strategy analogous to adaptive immunity has been proposed.32
Actinobacteria in ants and beetles: dentigerumycin and mycangimycin
While humans justifiably regard the development of agriculture as a signature achievement and the basis of modern civilizations, insects had evolved efficient agricultural systems tens of millions of years earlier and used them to support large social colonies. Fungus-farming ants, for example, create fungal gardens deep underground in which they grow an obligate food fungus that they supply with organic matter they forage on the surface.33 These ant colonies can contain several million individuals performing specialized tasks. This ant-fungal mutualism has been so successful that it evolved from a single origin in the Amazon some 50–60 million years ago to become over 230 ant species that are the major herbivores in the New World tropics. Fungal gardens are subject to infection by a parasitic fungus, and the ants defend their food source through a symbiosis with antibiotic-producing Actinobacteria that are housed in anatomically specialized crypts on the ant's surface. The crypts appear to be supplied by exocrine glands from the ant's interior. Since Actinobacteria produce most of our current natural product-based drugs, insects appear to have beaten us to more than agriculture.Chemical examination of an Actinobacterium isolated from an ant crypt, a Pseudonocardia species,34 led to the discovery of a new antifungal, dentigerumycin (10, Fig. 4), which exhibits roughly ten-fold greater activity against the parasitic fungus (an Escovopsis species) than the farmed fungus.35 Dentigerumycin has a cyclic depsipeptide scaffold containing highly modified amino acids initiated by a polyketide-derived pyran unit. Like rhizoxin (5), dentigerumycin is most plausibly synthesized by a mixed polyketide synthase/nonribosomal peptide synthetase complex. Dentigerumycin's ability to kill fast-growing eukaryotes (parasitic fungus) in the presence of slow-growing eukaryotes (farmed fungus) has led to an ongoing systematic investigation of this and other small molecules associated with fungal symbionts as potential anticancer agents since cancer cells are typically fast-growing cells in a milieu of slow-growing cells.36,37
 | ||
| Fig. 4 Selected bacterial products from Actinobacteria-insect mutualisms. The antifungals dentigerumycin (10) and mycangimycin (11) both exhibit specificity against the parasitic fungus over the food fungus. | ||
Bark beetles like the Southern Pine beetles that are responsible for widespread destruction of trees in parts of the United States employ a similar strategy. They engage in a symbiosis with a food fungus under the bark of infested trees, and the fungus is transported in a specialized compartment, called the mycangium. A parasitic fungus threatens the beetles and their food fungus through an ability to overwhelm the food fungus. In response, the mycangium also houses symbiotic Actinobacteria, one of which was shown to produce a selective fungicide, mycangimycin (11, Fig. 4).38,39 The biosynthesis of mycangimycin's endoperoxide and the roles of other naturally occurring small molecules that can be identified in the bacterial symbiont's sequenced genome remain as subjects for future investigations.
Proteobacteria in nematodes: rhabduscin and stilbenes
Gammaproteobacteria belonging to the genera Photorhabdus and Xenorhabdus engage in a complex symbiosis consisting of the bacteria, their mutualistic nematode hosts, the insect larvae that they parasitize in the soil, and microbial competitors.40–46 Any given Photorhabdus or Xenorhabdus isolate harbors dozens of genes encoding proteins similar to well-studied biosynthetic systems: polyketide synthases, nonribosomal peptide synthetases, and β-lactam-producing enzymes among others.47 These bacteria encode a secondary metabolic repertoire comparable to that of the prolific antibiotic-producing Streptomyces genus. The bacteria form a close partnership with their nematode host—Photorhabdus with Heterorhabditis nematodes and Xenorhabdus with Steinernema nematodes—but the nematode-bacteria pairs are capable of infecting a wide variety of insect larvae in the environment, and this promiscuous lethality makes for an especially effective agricultural biocontrol agent. When a nematode successfully invades an insect, it regurgitates the bacteria, which then produce toxins, proteases and esterases that kill and liquefy their prey. They also produce signals that promote the infective juvenile nematodes that invaded to become reproducing adults, molecular counters to the insect's innate immune defenses, and antibiotics to ward off microbial competitors. These multiple biological functions require a suite of small molecule effectors as exemplified by two examples, stilbenes and an isocyanide product.Stilbenes are common phenylpropanoid plant metabolites that exhibit phytoalexin, nematicidal, herbivore deterrent, and insecticidal activities as part of the plant's natural defense mechanisms.48Photorhabdus species are the only known bacterial producers of stilbenes (see 12),49 and these small molecules play a variety of roles in the bacteria-worm-insect symbiosis. In addition to serving as defenses against microbial competitors trying to share the decomposing insect meal,50 stilbenes inhibit phenoloxidase activity,51 a key component of the insect's innate immune system, to provide a survival advantage to the bacterial symbionts. The stilbene biosynthetic pathway produces as yet unidentified small molecules critical for the development of the free-living infective juvenile into reproducing adults.52 While plants and bacteria evolved structurally similar stilbene-based systems, they utilize markedly different biosynthetic strategies. Plants use a type III polyketide synthase, stilbene synthase, to homologate malonyl-CoA units onto a phenylpropanoid starter unit and initiate a regiospecific cyclization, whereas Photorhabdus luminescens employs free-standing (type II) polyketide synthase and fatty acid synthase proteins involved in the construction and condensation of two β-keto-acyl-carrier protein intermediates (Fig. 5A).52
 | ||
| Fig. 5 Bacterial stilbenes and vinyl isocyanide natural products. (a) The stilbene biosynthetic pathway in bacteria is initiated from two amino acid substrates. Phenylalanine is converted to cinnamic acid by a phenylalanine ammonia lyase (StlA), which is converted to its CoA thioester by a CoA ligase (StlB). Leucine is metabolized via the branched chain fatty acid pathway (BkdA/B) and elongated by BkdC. The two advanced substrates are cyclized in a head-to-head fashion by StlC to produce stilbene 12. (b) Vinyl isocyanide biosynthetic genes. IsnA excises the C2 of ribulose-5-phosphate to produce the carboxy indole isocyanide intermediate. IsnB accepts the diffusible IsnA product and catalyzes an oxidative decarboxylation to the final indole vinyl isocyanide product 15. (c) The insect pathogens X. nematophila and P. luminescens both produce the glycoside isocyanide rhabduscin (13, relative configuration). The plant pathogen E. carotovora and an Enterobacter species produce byelyankacin (14). | ||
The Clarke laboratory used a genetic strategy to identify a transcriptional repressor, HexA, in Photorhabdus temperata involved in the mutualist-pathogen transition.53 Deleting the hexAgene in Photorhabdus temperata53 and Photorhabdus luminescens led to the up-regulation (derepression) of stilbene production.54 Examination of the P. luminescens mutant led to an expansion of the stilbene family from three to at least nine members.54 It is possible that these new molecules might be the downstream signals involved in the development of the infective juvenile nematodes, but this has yet to be proven.
P. luminescens and X. nematophila share only a distant relationship, but both produce rhabduscin (13), a glycoside isocyanide.55 Since natural products rarely contain an isocyanide functional group, rhabduscin's co-occurrence in genetically distant bacteria occupying similar ecological niches suggested an important functional role. A clue to function came from finding a closely related molecule, byelyankacin (14), in an Enterobacter species56,57 and in the plant pathogen Erwinia carotovora.58 Researchers were led to 14 through a screen to identify inhibitors of tyrosinase, a tyrosine specific phenoloxidase.56,57 Phenoloxidase plays a key role in an insect's defense against microbial invaders, as the oxidation of various phenols creates a defensive melanin barrier that walls off the pathogen and exhibits bactericidal activity. The biosynthesis of the vinyl isocyanide is known in outline as the pathway for a similar molecule (15) was discovered in a functional antibiotic screen of a library of E. coli bacteria heterologously expressing DNA sequences isolated from soil.59,60 The pathway begins with an amino acid, tyrosine in the case of 13 and 14, tryptophan in the case of 15, and two genes, isnA and isnB, convert the amino acid into the vinyl isocyanide (Fig. 5B,C). The added carbon comes from C-2 of ribulose-5-phosphate,60 and the mechanism of its incorporation remains a future challenge.
Future prospects
The small molecule repertoires of bacterial symbionts represent an extraordinary discovery opportunity for both chemistry and biology. While the handful of examples in this highlight have focused on interesting features of some illustrative symbioses, there are more general arguments for such symbioses as an ever-expanding field of inquiry. Bacterial symbionts are everywhere. All plants, animals, and fungi originated and evolved on a planet awash in bacteria, and as a result have bacterial symbionts that have co-evolved with them. Many structurally diverse and pharmacologically relevant products have been identified in a number of these bacterial symbionts, and the list will undoubtedly continue to expand (See example reviews for host fungi,61–63 plants,64,65 invertebrate animals,66–68 and even humans69,70). As Nobel laureate Joshua Lederberg noted at the beginning of this century: “We should think of each host and its parasites as a superorganism with the respective genomes yoked into a chimera of sorts.”71Our own bodies, for example, have at least 1000 species of gut bacteria, and the metagenome of all of those bacterial symbionts contains 100 times more genes than our own DNA. Many of these bacteria are not parasites, as Lederberg called them, but mutualists that complement our own genome by breaking down dietary fiber or making vitamins. Indeed, certain bacteria on our skin produce products that inhibit pathogenic bacterial growth72 or modulate our immune system.73–76 Uncovering the many roles of the human microbiome will require the focused effort of many laboratories.
The chemistry of bacterial symbionts is, like most of natural products chemistry, highly idiosyncratic, and there is no way to predict what molecules, pathways and biological functions will have evolved. Understanding will require the sort of experimental analysis at which chemists excel and in which many of them delight. What might come from all of these efforts? A lot of surprises, and many of them will reveal new ways that small molecules function in biological contexts, the historically important role of natural products.
Acknowledgements
Our work on bacterial symbioses is supported by the US National Institutes of Health (Grant R01 GM086258 to J.C.) and the New England Regional Center of Excellence for Biodefense and Emerging Infectious Diseases (Grant U54 AI057159 to J.C.). During the course of the work, J.M.C. was supported by a Damon Runyon Cancer Research Foundation postdoctoral fellowship (DRG-2002-09) and a National Institutes of Health Pathway to Independence award (Grant 1K99 GM097096-01).References
- J. Clardy and C. Walsh, Nature, 2004, 432, 829–837 CrossRef CAS
.
- S. F. Brady, L. Simmons, J. H. Kim and E. W. Schmidt, Nat. Prod. Rep., 2009, 26, 1488–1503 RSC
.
- K. Lewis, S. Epstein, A. D'Onofrio and L. L. Ling, J. Antibiot., 2010, 63, 468–476 Search PubMed
.
- C. T. Walsh and M. A. Fischbach, J. Am. Chem. Soc., 2010, 132, 2469–2493 CrossRef CAS
.
- J. Davies, Curr. Opin. Chem. Biol., 2011, 15, 5–10 Search PubMed
.
- J. M. Winter, S. Behnken and C. Hertweck, Curr. Opin. Chem. Biol., 2011, 15, 22–31 Search PubMed
.
- E. W. Schmidt, Nat. Chem. Biol., 2008, 4, 466–473 CrossRef CAS
.
- S. Iwasaki, H. Kobayashi, J. Furukawa, M. Namikoshi, S. Okuda, Z. Sato, I. Matsuda and T. Noda, J. Antibiot., 1984, 37, 354–362 CAS
.
- D. G. Kingston, J. Nat. Prod., 2009, 72, 507–515 CrossRef CAS
.
- L. P. Partida-Martinez and C. Hertweck, Nature, 2005, 437, 884–888 CrossRef CAS
.
- L. P. Partida-Martinez, I. Groth, I. Schmitt, W. Richter, M. Roth and C. Hertweck, Int. J. Syst. Evol. Microbiol., 2007, 57, 2583–2590 Search PubMed
.
- L. P. Partida-Martinez, S. Monajembashi, K. O. Greulich and C. Hertweck, Curr. Biol., 2007, 17, 773–777 CrossRef CAS
.
- I. Schmitt, L. P. Partida-Martinez, R. Winkler, K. Voigt, E. Einax, F. Dolz, S. Telle, J. Wostemeyer and C. Hertweck, ISME J., 2008, 2, 632–641 CrossRef CAS
.
- L. P. Partida-Martinez and C. Hertweck, ChemBioChem, 2007, 8, 41–45 CrossRef CAS
.
- G. Lackner, N. Moebius, L. Partida-Martinez and C. Hertweck, J. Bacteriol., 2011, 193, 783–784 Search PubMed
.
- B. Kusebauch, B. Busch, K. Scherlach, M. Roth and C. Hertweck, Angew. Chem., Int. Ed., 2009, 48, 5001–5004 CrossRef CAS
.
- J. Moldenhauer, D. C. Gotz, C. R. Albert, S. K. Bischof, K. Schneider, R. D. Sussmuth, M. Engeser, H. Gross, G. Bringmann and J. Piel, Angew. Chem. Int. Ed., 2010, 49, 1465–1467 CAS
.
- B. Kusebauch, B. Busch, K. Scherlach, M. Roth and C. Hertweck, Angew. Chem. Int. Ed., 2010, 49, 1460–1464 CAS
.
- K. Scherlach, L. P. Partida-Martinez, H. M. Dahse and C. Hertweck, J. Am. Chem. Soc., 2006, 128, 11529–11536 CrossRef CAS
.
- N. Brendel, L. P. Partida-Martinez, K. Scherlach and C. Hertweck, Org. Biomol. Chem., 2007, 5, 2211–2213 RSC
.
- J. E. Loper, M. D. Henkels, B. T. Shaffer, F. A. Valeriote and H. Gross, Appl. Environ. Microbiol., 2008, 74, 3085–3093 CrossRef CAS
.
- C. M. Ireland, A. R. Durso, R. A. Newman and M. P. Hacker, J. Org. Chem., 1982, 47, 1807–1811 CrossRef CAS
.
- A. R. Carroll, J. C. Coll, D. J. Bourne, J. K. MacLeod, T. M. Zabriskie, C. M. Ireland and B. F. Bowden, Aust. J. Chem., 1996, 49, 659–667
.
- E. W. Schmidt and M. S. Donia, Curr. Opin. Biotechnol., 2010, 21, 827–833 Search PubMed
.
- E. W. Schmidt, J. T. Nelson, D. A. Rasko, S. Sudek, J. A. Eisen, M. G. Haygood and J. Ravel, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 7315–7320 CrossRef CAS
.
- P. F. Long, W. C. Dunlap, C. N. Battershill and M. Jaspars, ChemBioChem, 2005, 6, 1760–1765 CrossRef CAS
.
- E. W. Schmidt and M. S. Donia, Methods Enzymol., 2009, 458, 575–596 CAS
.
- M. S. Donia, J. Ravel and E. W. Schmidt, Nat. Chem. Biol., 2008, 4, 341–343 CrossRef CAS
.
- J. A. McIntosh, M. S. Donia and E. W. Schmidt, J. Am. Chem. Soc., 2010, 132, 4089–4091 CrossRef
.
- J. Lee, J. McIntosh, B. J. Hathaway and E. W. Schmidt, J. Am. Chem. Soc., 2009, 131, 2122–2124 CrossRef CAS
.
- J. A. McIntosh, C. R. Robertson, V. Agarwal, S. K. Nair, G. W. Bulaj and E. W. Schmidt, J. Am. Chem. Soc., 2010, 132, 15499–15501 Search PubMed
.
- M. S. Donia, B. J. Hathaway, S. Sudek, M. G. Haygood, M. J. Rosovitz, J. Ravel and E. W. Schmidt, Nat. Chem. Biol., 2006, 2, 729–735 CrossRef CAS
.
- E. J. Caldera, M. Poulsen, G. Suen and C. R. Currie, Environ. Entomol., 2009, 38, 78–92 Search PubMed
.
- M. J. Cafaro, M. Poulsen, A. E. Little, S. L. Price, N. M. Gerardo, B. Wong, A. E. Stuart, B. Larget, P. Abbot and C. R. Currie, Proc. Biol. Sci., 2010 Search PubMed
, Nov. 24. PMID: 21106596.
- D. C. Oh, M. Poulsen, C. R. Currie and J. Clardy, Nat. Chem. Biol., 2009, 5, 391–393 CrossRef CAS
.
- J. Clardy, M. A. Fischbach and C. R. Currie, Curr. Biol., 2009, 19, R437–441 Search PubMed
.
- M. Poulsen, Drug News Perspect., 2010, 23, 203–210 Search PubMed
.
- J. J. Scott, D. C. Oh, M. C. Yuceer, K. D. Klepzig, J. Clardy and C. R. Currie, Science, 2008, 322, 63 CrossRef CAS
.
- D. C. Oh, J. J. Scott, C. R. Currie and J. Clardy, Org. Lett., 2009, 11, 633–636 CrossRef CAS
.
- S. A. Joyce, R. J. Watson and D. J. Clarke, Curr. Opin. Microbiol., 2006, 9, 127–132 CrossRef CAS
.
- H. Goodrich-Blair and D. J. Clarke, Mol. Microbiol., 2007, 64, 260–268 CrossRef CAS
.
- H. Goodrich-Blair, Curr. Opin. Microbiol., 2007, 10, 225–230 CrossRef CAS
.
- E. E. Herbert and H. Goodrich-Blair, Nat. Rev. Microbiol., 2007, 5, 634–646 Search PubMed
.
- D. J. Clarke, Cell. Microbiol., 2008, 10, 2159–2167 Search PubMed
.
- N. R. Waterfield, T. Ciche and D. Clarke, Annu. Rev. Microbiol., 2009, 63, 557–574 Search PubMed
.
- G. R. Richards and H. Goodrich-Blair, Cell. Microbiol., 2009 Search PubMed
.
- E. Duchaud, C. Rusniok, L. Frangeul, C. Buchrieser, A. Givaudan, S. Taourit, S. Bocs, C. Boursaux-Eude, M. Chandler, J. F. Charles, E. Dassa, R. Derose, S. Derzelle, G. Freyssinet, S. Gaudriault, C. Medigue, A. Lanois, K. Powell, P. Siguier, R. Vincent, V. Wingate, M. Zouine, P. Glaser, N. Boemare, A. Danchin and F. Kunst, Nat. Biotechnol., 2003, 21, 1307–1313 CrossRef CAS
.
- J. Chong, A. Poutaraud and P. Hugueney, Plant Sci., 2009, 177, 143–155 Search PubMed
.
- W. H. Richardson, T. M. Schmidt and K. H. Nealson, Appl. Environ. Microbiol., 1988, 54, 1602–1605 CAS
.
- K. Hu and J. M. Webster, FEMS Microbiol. Lett., 2000, 189, 219–223 CrossRef CAS
.
- I. Eleftherianos, S. Boundy, S. A. Joyce, S. Aslam, J. W. Marshall, R. J. Cox, T. J. Simpson, D. J. Clarke, R. H. Ffrench-Constant and S. E. Reynolds, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 2419–2424 CrossRef CAS
.
- S. A. Joyce, A. O. Brachmann, I. Glazer, L. Lango, G. Schwar, D. J. Clarke and H. B. Bode, Angew. Chem., Int. Ed., 2008, 47, 1942–1945 Search PubMed
.
- S. A. Joyce and D. J. Clarke, Mol. Microbiol., 2003, 47, 1445–1457 Search PubMed
.
- R. Kontnik, J. M. Crawford and J. Clardy, ACS Chem. Biol., 2010, 5, 659–665 Search PubMed
.
- J. M. Crawford, R. Kontnik and J. Clardy, Curr. Biol., 2010, 20, 69–74 CrossRef CAS
.
-
A. Nakagawa, Y. Fuchu-Shi, S. Takahashi, T. Miyazaki, Y. Osanai, K. Kosaka, K. Nagai, N. Arao and K. Tanaka, US Patent, 2006, US 7,115,721 B2 Search PubMed
.
- S. Takahashi, H. Iwai, K. Kosaka, T. Miyazaki, Y. Osanai, N. Arao, K. Tanaka, K. Nagai and A. Nakagawa, J. Antibiot., 2007, 60, 717–720 Search PubMed
.
- S. F. Brady, J. D. Bauer, M. F. Clarke-Pearson and R. Daniels, J. Am. Chem. Soc., 2007, 129, 12102–12103 CrossRef CAS
.
- S. F. Brady and J. Clardy, Angew. Chem., Int. Ed., 2005, 44, 7063–7065 CrossRef CAS
.
- S. F. Brady and J. Clardy, Angew. Chem., Int. Ed., 2005, 44, 7045–7048 CrossRef CAS
.
- G. Lackner, L. P. Partida-Martinez and C. Hertweck, Trends Microbiol., 2009, 17, 570–576 Search PubMed
.
- E. Stocker-Worgotter, Nat. Prod. Rep., 2008, 25, 188–200 RSC
.
- K. Molnar and E. Farkas, Zeitschrift fur Naturforschung, 2010, 65, 157–173 Search PubMed
.
- R. P. Ryan, K. Germaine, A. Franks, D. J. Ryan and D. N. Dowling, FEMS Microbiol. Lett., 2008, 278, 1–9 Search PubMed
.
- A. C. Newton, B. D. Fitt, S. D. Atkins, D. R. Walters and T. J. Daniell, Trends Microbiol., 2010, 18, 365–373 Search PubMed
.
- J. Piel, Curr. Med. Chem., 2006, 13, 39–50 CrossRef CAS
.
- T. R. Thomas, D. P. Kavlekar and P. A. LokaBharathi, Mar. Drugs, 2010, 8, 1417–1468 CrossRef
.
- H. B. Bode, Curr. Opin. Chem. Biol., 2009, 13, 224–230 CrossRef CAS
.
- A. L. Cogen, V. Nizet and R. L. Gallo, Br. J. Dermatol., 2008, 158, 442–455 Search PubMed
.
- E. A. Grice and J. A. Segre, Nat. Rev. Microbiol., 2011, 9, 244–253 Search PubMed
.
- J. Lederberg, Science, 2000, 288, 287–293 CrossRef CAS
.
- A. L. Cogen, K. Yamasaki, K. M. Sanchez, R. A. Dorschner, Y. Lai, D. T. MacLeod, J. W. Torpey, M. Otto, V. Nizet, J. E. Kim and R. L. Gallo, J. Invest. Dermatol., 2010, 130, 192–200 Search PubMed
.
- Y. Lai, A. Di Nardo, T. Nakatsuji, A. Leichtle, Y. Yang, A. L. Cogen, Z. R. Wu, L. V. Hooper, R. R. Schmidt, S. von Aulock, K. A. Radek, C. M. Huang, A. F. Ryan and R. L. Gallo, Nat. Med., 2009, 15, 1377–1382 CrossRef CAS
.
- Y. Lai, A. L. Cogen, K. A. Radek, H. J. Park, D. T. Macleod, A. Leichtle, A. F. Ryan, A. Di Nardo and R. L. Gallo, J. Invest. Dermatol., 2010, 130, 2211–2221 Search PubMed
.
- C. W. Lo, Y. K. Lai, Y. T. Liu, R. L. Gallo and C. M. Huang, J. Invest. Dermatol., 2011, 131, 401–409 Search PubMed
.
- Y. K. Lee and S. K. Mazmanian, Science, 2010, 330, 1768–1773 Search PubMed
.
- C. Hertweck, Angew. Chem., Int. Ed., 2009, 48, 4688–4716 CrossRef CAS
.
- A. Koglin and C. T. Walsh, Nat. Prod. Rep., 2009, 26, 987–1000 RSC
.
- M. Strieker, A. Tanovic and M. A. Marahiel, Curr. Opin. Struct. Biol., 2010, 20, 234–240 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2011 |
