Improving nanoparticle diffusion through tumor collagen matrix by photo-thermal gold nanorods†
Vahid
Raeesi
ab and
Warren C. W.
Chan
*abcd
aDepartment of Material Science and Engineering, University of Toronto, Toronto M5S 3E1, Canada. E-mail: warren.chan@utoronto.ca
bInstitute of Biomaterials and Biomedical Engineering, University of Toronto, Toronto, Ontario M5S 3G9, Canada
cDepartment of Chemical Engineering Toronto, University of Toronto, Toronto, Ontario M5S 3E5, Canada
dDepartment of Chemistry, University of Toronto, Toronto, Ontario M5S 3H6, Canada
First published on 7th January 2016
Abstract
Collagen (I) impairs the targeting of nanoparticles to tumor cells by obstructing their diffusion inside dense tumor interstitial matrix. This potentially makes large nanoparticles (>50 nm) reside near the tumor vessels and thereby compromises their functionality. Here we propose a strategy to locally improve nanoparticle transport inside collagen (I) component of the tumor tissue. We first used heat generating gold nanorods to alter collagen (I) matrix by local temperature elevation. We then explored this impact on the transport of 50 nm and 120 nm inorganic nanoparticles inside collagen (I). We demonstrated an increase in average diffusivity of 50 nm and 120 nm in the denatured collagen (I) by ∼14 and ∼21 fold, respectively, compared to intact untreated collagen (I) matrix. This study shows how nanoparticle-mediated hyperthermia inside tumor tissue can improve the transport of large nanoparticles through collagen (I) matrix. The ability to increase nanoparticles diffusion inside tumor stroma allows their targeting or other functionalities to take effect, thereby significantly improving cancer therapeutic or diagnostic outcome.
A major focus in cancer nanomedicine is to transport nanoparticles to cells within tumor milieu.1–3 Nanoparticles are designed as imaging probes4–6 and therapeutic agents7–10 to target tumor cells. Nanoparticles are surface modified with a ligand that is specifically recognized by tumor cells11,12 and are then injected into the bloodstream. These nanoparticles are next transported through blood circulation to the tumor vessels, where it has been proposed they escape the leaky tumor vessels via leaky vessels.13–15 Once nanoparticles cross the vessel wall, they need to be transported through the tumor interstitial matrix to reach the cells. Collagen (I), a major protein in the tumor interstitium,16 forms a dense 3D network of fibrilar structure in the interstitial space between the tumor cells and blood vessels, and acts as one of the dominant physiological barrier against diffusion inside the tumor.17–19 This poses a great challenge for many nanoparticle designs, especially for larger sizes (e.g., 100 nm) that get stuck within the collagen network.20 If the nanoparticles are unable to diffuse through the tumor interstitial matrix, they will reside near the vessel. There are two consequences to this: (1) targeted nanoparticles will not be able to interact with receptors on the cells, and (2) tumor retention may be shortened as they can easily diffuse out of the tumor because of high interstitial pressure (IFP).21 The inability of nanoparticles to transport through the tumor interstitial matrix may be a reason that a number of recent studies showed a lack of difference in total tumor accumulation between active and passive-design nanoparticles.22–25 Hence, there is a need to develop strategies to alter the tumor matrix to enable transport of nanoparticles through it. A number of strategies have been proposed: (a) the use and incorporation of proteolytic enzyme collagenase26 in the nanoparticle design27–29 and (b) design of larger nanoparticles to degrade and release smaller nanoparticles within the tumor matrix.30 However, these strategies have limitations. First, free collagenase can not be systematically administered as collagen is the structural protein in other organs31 or it may lose activity during nanoparticle formulation process.32 Second, conversion of large particle assemblies to small nanoparticles require intricate design chemistry to minimize degradation during circulation33 and rapid release34 within tumor matrix before being cleared by elevated IFP.35 Given the heterogeneity of tumors plus variable tumor retention rates based on nanoparticle physical-chemical properties, it may be difficult to design a unified nanosystem for this purpose.
Here we proposed a strategy to locally improve nanoparticle transport through collagen (I) component of the tumor tissue. In a collagen (I) μ-channel setup, we first introduced gold nanorods (GNRs) to alter the collagen (I) matrix under near-infrared (NIR) light stimulation. We showed local irreversible denaturation of collagen (I) fibrils by using GNRs to photo-thermally increase the local temperature to 45–55 °C. We then introduced two sizes of 50 nm and 120 nm nanoparticles with the same surface chemistry into both treated and untreated collagen (I) μ-channels. We demonstrated an increase in average diffusivity of 50 nm and 120 nm in the denatured collagen (I) by ∼14 and ∼21 fold, respectively, compared to intact untreated collagen (I) matrix. This study shows how nanoparticle-mediated hyperthermia inside tumor tissue can improve the transport of large nanoparticles (>50 nm) through collagen (I) matrix.
GNRs were selected for this study because the heat generated by these nanoparticles have a theoretical photo-thermal (PTT) conversion efficiency of >90% with NIR wavelength excitation between 700–850 nm. Also, this wavelength range has been shown to yield the largest tissue penetration depth.36 GNRs have been applied in cancer therapy as a hyperthermia agent37–40 or as part of a combinatorial strategy for synergistic killing41–43 of tumor cells. The experimental set-up to study the effect of GNRs generated heat on transport through a collagen (I) matrix is described in Fig. 1a and b. The glass μ-chip had two reservoirs, which were connected through a μ-channel. We filled the channel with bovine collagen (I) solution and neutralized it to form a rigid gel structure in 4 h. The collagen (I) matrix appeared turbid inside the μ-channel (Fig. 1c). Reflectance confocal imaging revealed a porous structure with randomly oriented collagen fibers at a concentration ranging from 2–7 mg mL−1 (Fig. S1†). A 7 mg ml−1 collagen concentration (Fig. 1d) was used for the rest of the experiments as it was in the range of the reported collagen (I) contents in tumors.44 The transport mode in our setup was diffusion (derived by concentration gradient) as there was no convective flow (Peclet number ∼0). GNRs were synthesized via a directional growth of gold seeds in cetyltrimethyl ammonium bromide (CTAB) surfactant and ascorbic acid41,45 (see Materials and methods in the ESI†). Next, GNRs were coated with polyethylene glycol (PEG) to stabilize them in a buffer medium. The GNRs had an average length to width ratio of 28 nm × 7 nm (Fig. 2a), longitudinal absorption peak at 740 nm and a transverse peak at 520 nm (Fig. 2b). 30 μl GNRs were added to one reservoir and the other reservoir was filled with equal volume of phosphate buffered saline (PBS). Then we used a continuous wavelength near-IR laser (785 nm) to excite the GNRs and the temperature was monitored using thermal imaging. We first correlated the relationship between GNR concentration and laser power to control temperature elevation (Fig. 2c and S2†). We utilized this characterization to study the effect of heat on collagen (I) gel structure in the μ-channels. We raster irradiated the channel horizontally with a 5 mm diameter laser beam in one side of the μ-channel. We optimized the GNR concentration (6 nM), laser power density (3 W cm−2) so that the channel exposed-area exhibit an average temperature between 45–55 °C, in the range of reported collagen (I) denaturation temperatures46,47 (see Fig. 2d). When the μ-channel was heated, the exposed area became clear. The heated medium converted from a dense hydrogel to a liquid-like medium. We detected no reflectance signal from collagen fibrils in the photo-thermally exposed area (Fig. 2e and f) with 88% loss of turbidity (as measured by absorbance measurements at 405 nm (ref. 48)) compared to non-exposed areas (Fig. 2g), suggesting the disappearance of collagen fibers. After cooling, the fiber structure did not re-form, indicating irreversible denaturation of collagen fibers. This is likely due to the transformation of the native triple helical structure into a random coiled structure as reported by previous studies.49 This change in conformation was further confirmed by the observation of agglomerates at the bottom of the channel wall (Fig. 2h).
The GNR's PTT experiments clearly showed that the fibrilar structure was altered in the collagen (I) matrix. We next evaluated whether this change in collagen (I) matrix would lead to an increase in transport of spherical nanoparticles. We selected gold nanoparticles of 50 and 120 nm core diameters as model particle systems. These spherical gold nanoparticles were synthesized using a hydroquinone-seed mediated growth method and surface coated with polyethylene glycol and AlexaFluor dye molecules using the previous developed methods from our laboratory (Fig. 3a, see Materials and methods†). This dye was selected because it can be excited at 647 nm and the emission is far from the plasmon band of the gold nanoparticles, so that minimal quenching of the dye by gold nanoparticle surface would occur. The 50 nm and 120 nm nanoparticles showed plasmon peaks at 535 nm and 595 nm, respectively (Fig. 3b). TEM images revealed their size and shape with narrow size distribution (Fig. S3†) and this was supported by dynamic light scattering measurements with a PDI < 0.06.
To demonstrate GNR's PTT effect on diffusion, we added GNRs (6 nM) in one reservoir of both μ-channels and then illuminated the channel with a laser for 6 min using our previously optimized conditions. We then added the fluorescently-tagged nanoparticles on top of the GNRs reservoir and then traced the motion of the nanoparticles as they were diffusing along the collagen (I) matrix channel length using confocal fluorescence microscopy (Materials and Methods). Images were recorded in 12 h at different distances along the μ-channel and processed using ImageJ and Matlab to develop spatio-temporal intensity profiles and calculate diffusivity values, respectively. The confocal fluorescent images in Fig. 3c illustrate the diffusion of the 50 nm (red) and 120 nm (green) nanoparticles along the collagen (I) matrix channel before and after PTT treatment. The red/green colors are meant to distinguish between 50 nm and 120 nm nanoparticles and not indicative of actual emission channel. The diffusion direction is from left (high concentration) to right (low concentration) at the same time point and the position for each particle size. Regardless of nanoparticle size, in untreated collagen (I) matrix, the average intensity along the channel length is low and most of the nanoparticles are populated at the left end of the channel whereas in a photo-thermally treated (PTT) collagen (I) matrix, we detected higher average fluorescence intensity through the entire length of the channel. Qualitatively, we can make two conclusions. (1) At a defined time t, the number of nanoparticles along the length of the channel is higher in PTT-treated channel for both nanoparticle sizes. (2) At the same nanoparticle concentration, the penetration depth through the collagen (I) matrix is increased when PTT is applied. This suggested that there was an increase in the diffusivity of nanoparticles through the collagen (I) matrix after PTT. We further scrutinized these results by developing intensity profile along the mid-channel for each particle size. Fig. 3d and e show temporal intensity profiles for the 50 nm nanoparticles in control and PTT-treated μ-channels, respectively. The PTT process increased the rate of intensity change compared to an intact collagen (I) matrix, regardless of the penetration depth. For a fixed penetration depth (same concentration gradient), the rate of increase in intensity can be a measure of how resistant the medium is against diffusion. For penetration depth of 0–500 μm, we calculated ∼9 fold increase in the intensity rate for PTT treated channel. This means in PTT denatured collagen (I) matrix, 50 nm nanoparticles reached their initial local maximum concentration 9 times faster than intact collagen (I) matrix (control). We then plotted the normalized intensity versus penetration depth at t = 4 h which was the required time for x = 0 (Reference point) to reach its maximum point (Fig. 3f). For penetration depth of 100 μm–1000 μm, we found 2.1–18.0 fold raise in the intensity. We inferred in a PTT treated channel, the local concentration of 50 nm nanoparticles at different penetration depth was increased. Also, an increase in the intensity from 0% to 54% at x = 1000 μm suggests that the penetration depth in PTT-treated channel was increased. The effect of the PTT process for 120 nm particles followed the same trend as 50 nm nanoparticles, shown in Fig. 3g and h. 120 nm nanoparticles reached saturation in PTT-treated channel in ∼3 h while in untreated channel the signal for the same spot started to saturate after 12 h. 120 nm nanoparticles showed fluorescent signals at 500 μm for PTT treat collagen (I) matrix as compared to untreated matrix. This suggested more than a 300 μm increase in penetration depth for 120 nm nanoparticles. We quantified the change in collagen (I) matrix diffusivity (D) by developing intensity profiles versus penetration distance at fixed time points and fitted the data to fick's law solution as described in equation 1:
 | (1) |
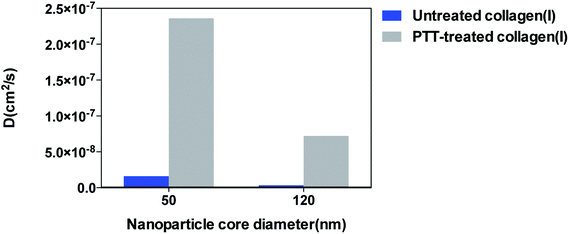 | ||
| Fig. 4 Effect of GNRs PTT on the diffusivity of 50 nm and 120 nm gold nanoparticles inside collagen (I). | ||
In conclusion, we used an in vitro model to show that a 2-step process can enhance the penetration of nanoparticles transport within a collagen matrix. The first step requires the denaturation of collagen structure by using gold nanorods heating, which effectively opens up the collagen (I) matrix for larger nanoparticles to transport through. We clearly showed a deeper penetration depth for 50 and 120 nm spherical gold nanoparticles within the collagen (I) matrix after gold nanorod priming. Further experiments will require the confirmation of improved transport of larger nanoparticles through the tumor matrix in animal models. The tumor extracellular matrix may prevent the transport of nanoparticles to the targeted tumor cells and the use of gold nanorods heating may prime the tumor for the “actual” nanoparticle therapeutic or diagnostic formulation to be successfully delivered to the tumor cells. This may be the case for both direction injection of “second” nanoparticles into tumors as well as via a systemic injection, where nanoparticles may have to diffuse through the matrix to reach the targeted cells. The gold nanorods may be introduced into the tumor via direct or systemic injection. In vivo, even if these gold nanorods do not penetrate deeply, the ability to heat and denature the localized tumor matrix may allow more of the subsequent or secondary nanoparticles to diffuse through. The incorporation of heat-generating components that denatures the tumor extracellular matrix may allow entire nanosystems to be transported through the tumor extracellular matrix and achieve specific tumor cell targeting.
Acknowledgements
WCWC would also like to acknowledge the Canadian Institute of Health Research (MOP-130143 and RMF-111623), Natural Sciences and Engineering Research Council (2015-06397), Prostate Cancer Canada (D2014-12) for supporting his research program.References
- R. K. Jain and T. Stylianopoulos, Nat. Rev. Clin. Oncol., 2010, 7, 653–664 CrossRef CAS PubMed
.
- E. Ruoslahti, S. N. Bhatia and M. J. Sailor, J. Cell Biol., 2010, 188, 759–768 CrossRef CAS PubMed
.
- W. Jiang, B. Y. Kim, J. T. Rutka and W. C. Chan, Expert Opin. Drug Delivery, 2007, 4, 621–633 CrossRef CAS PubMed
.
- J. V. Jokerst, T. Lobovkina, R. N. Zare and S. S. Gambhir, Nanomedicine, 2011, 6, 715–728 CrossRef CAS PubMed
.
- V. P. Zharov and D. O. Lapotko, IEEE J. Sel. Top. Quantum Electron., 2005, 11, 733–751 CrossRef CAS
.
- L. Y. T. Chou and W. C. W. Chan, Adv. Healthcare Mater., 2012, 1, 714–721 CrossRef CAS PubMed
.
- L. Zhang, F. Gu, J. Chan, A. Wang, R. Langer and O. Farokhzad, Clin. Pharmacol. Ther., 2007, 83, 761–769 CrossRef PubMed
.
- J. Hrkach, D. Von Hoff, M. M. Ali, E. Andrianova, J. Auer, T. Campbell, D. De Witt, M. Figa, M. Figueiredo, A. Horhota, S. Low, K. McDonnell, E. Peeke, B. Retnarajan, A. Sabnis, E. Schnipper, J. J. Song, Y. H. Song, J. Summa, D. Tompsett, G. Troiano, T. Van Geen Hoven, J. Wright, P. LoRusso, P. W. Kantoff, N. H. Bander, C. Sweeney, O. C. Farokhzad, R. Langer and S. Zale, Sci. Transl. Med., 2012, 4, 128ra39–128ra39 Search PubMed
.
- C. J. Langer, M. A. Socinski and K. J. O’Byrne, Proc. Am. Soc. Clin. Oncol., 2005, 23(suppl 16) CAS
, LBA7011.
- D. Yoo, H. Jeong, C. Preihs, J.-S. Choi, T.-H. Shin, J. L. Sessler and J. Cheon, Angew. Chem., Int. Ed., 2012, 51, 12482–12485 CrossRef CAS PubMed
.
- A. P. R. Johnston, M. M. J. Kamphuis, G. K. Such, A. M. Scott, E. C. Nice, J. K. Heath and F. Caruso, ACS Nano, 2012, 6, 6667–6674 CrossRef CAS PubMed
.
- T. A. ElBayoumi and V. P. Torchilin, Clin. Cancer Res., 2009, 15, 1973–1980 CrossRef CAS PubMed
.
- M. E. Davis, Z. G. Chen and D. M. Shin, Nat. Rev. Drug Discovery, 2008, 7, 771–782 CrossRef CAS PubMed
.
- C. J. Cheng, G. T. Tietjen, J. K. Saucier-Sawyer and W. M. Saltzman, Nat. Rev. Drug Discovery, 2015, 14, 239–247 CrossRef CAS PubMed
.
- S. D. Perrault, C. Walkey, T. Jennings, H. C. Fischer and W. C. W. Chan, Nano Lett., 2009, 9, 1909–1915 CrossRef CAS PubMed
.
- Y.-L. Yang, S. Motte and L. J. Kaufman, Biomaterials, 2010, 31, 5678–5688 CrossRef CAS PubMed
.
- P. A. Netti, D. A. Berk, M. A. Swartz, A. J. Grodzinsky and R. K. Jain, Cancer Res., 2000, 60, 2497–2503 CAS
.
- S. Ramanujan, A. Pluen, T. D. McKee, E. B. Brown, Y. Boucher and R. K. Jain, Biophys. J., 2002, 83, 1650–1660 CrossRef CAS PubMed
.
- O. Tredan, C. M. Galmarini, K. Patel and I. F. Tannock, JNCI, J. Natl. Cancer Inst., 2007, 99, 1441–1454 CrossRef CAS PubMed
.
- A. Pluen, Y. Boucher, S. Ramanujan, T. D. McKee, T. Gohongi, E. di Tomaso, E. B. Brown, Y. Izumi, R. B. Campbell, D. A. Berk and R. K. Jain, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 4628–4633 CrossRef CAS PubMed
.
- L. T. Baxter and R. K. Jain, Microvasc. Res., 1989, 37, 77–104 CrossRef CAS PubMed
.
- E. A. Sykes, J. Chen, G. Zheng and W. C. W. Chan, ACS Nano, 2014, 8, 5696–5706 CrossRef CAS PubMed
.
- E. Huynh and G. Zheng, WIREs Nanomed. Nanobiotechnol., 2013, 5, 250–265 CrossRef CAS PubMed
.
- S. Kunjachan, R. Pola, F. Gremse, B. Theek, J. Ehling, D. Moeckel, B. Hermanns-Sachweh, M. Pechar, K. Ulbrich, W. E. Hennink, G. Storm, W. Lederle, F. Kiessling and T. Lammers, Nano Lett., 2014, 14, 972–981 CrossRef CAS PubMed
.
- D. B. Kirpotin, Cancer Res., 2006, 66, 6732–6740 CrossRef CAS PubMed
.
- M. Magzoub, S. Jin and A. S. Verkman, FASEB J., 2007, 22, 276–284 CrossRef PubMed
.
- T. T. Goodman, P. L. Olive and S. H. Pun, Int. J. Nanomed., 2007, 2, 265–274 CrossRef CAS PubMed
.
- S. Murty, T. Gilliland, P. Qiao, T. Tabtieng, E. Higbee, A. A. Zaki, E. Puré and A. Tsourkas, Part. Part. Syst. Charact., 2014, 31, 1307–1312 CrossRef CAS PubMed
.
- S. J. Kuhn, S. K. Finch, D. E. Hallahan and T. D. Giorgio, Nano Lett., 2006, 6, 306–312 CrossRef CAS PubMed
.
- C. Wong, T. Stylianopoulos, J. Cui, J. Martin, V. P. Chauhan, W. Jiang, Z. Popovic, R. K. Jain, M. G. Bawendi and D. Fukumura, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 2426–2431 CrossRef CAS PubMed
.
- R. K. Jain, J. Clin. Oncol., 2013, 31, 2205–2218 CrossRef CAS PubMed
.
- M. R. Villegas, A. Baeza and M. Vallet Regí, ACS Appl. Mater. Interfaces, 2015, 151013114646000 Search PubMed
.
- F. Tewes, E. Munnier, B. Antoon, L. Ngaboni Okassa, S. Cohen-Jonathan, H. Marchais, L. Douziech-Eyrolles, M. Soucé, P. Dubois and I. Chourpa, Eur. J. Pharm. Biopharm., 2007, 66, 488–492 CrossRef CAS PubMed
.
- A. P. Esser-Kahn, S. A. Odom, N. R. Sottos, S. R. White and J. S. Moore, Macromolecules, 2011, 44, 5539–5553 CrossRef CAS
.
- R. K. J. S. R. Chary, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 5385–5385 CrossRef
.
- Z. Qin and J. C. Bischof, Chem. Soc. Rev., 2012, 41, 1191 RSC
.
- C. Ungureanu, R. Kroes, W. Petersen, T. A. M. Groothuis, F. Ungureanu, H. Janssen, F. W. B. van Leeuwen, R. P. H. Kooyman, S. Manohar and T. G. van Leeuwen, Nano Lett., 2011, 11, 1887–1894 CrossRef CAS PubMed
.
- T. B. Huff, M. N. Hansen, Y. Zhao, J.-X. Cheng and A. Wei, Langmuir, 2007, 23, 1596–1599 CrossRef CAS PubMed
.
- W.-S. Kuo, C.-N. Chang, Y.-T. Chang, M.-H. Yang, Y.-H. Chien, S.-J. Chen and C.-S. Yeh, Angew. Chem., Int. Ed., 2010, 49, 1–6 CrossRef PubMed
.
- T. B. Huff, L. Tong, Y. Zhao, M. N. Hansen, J.-X. Cheng and A. Wei, Nanomedicine, 2007, 2, 125–132 CrossRef CAS PubMed
.
- T. S. Hauck, T. L. Jennings, T. Yatsenko, J. C. Kumaradas and W. C. W. Chan, Adv. Mater., 2008, 20, 3832–3838 CrossRef CAS
.
- K. C. Hribar, M. H. Lee, D. Lee and J. A. Burdick, ACS Nano, 2011, 5, 2948–2956 CrossRef CAS PubMed
.
- D. Wang, Z. Xu, H. Yu, X. Chen, B. Feng, Z. Cui, B. Lin, Q. Yin, Z. Zhang, C. Chen, J. Wang, W. Zhang and Y. Li, Biomaterials, 2014, 35, 8374–8384 CrossRef CAS PubMed
.
- S. Ramanujan, A. Pluen, T. D. McKee, E. B. Brown, Y. Boucher and R. K. Jain, Biophys. J., 2002, 83, 1650–1660 CrossRef CAS PubMed
.
- N. R. Jana, Small, 2005, 1, 875–882 CrossRef CAS PubMed
.
- C. A. Miles and A. J. Bailey, Proc. – Indian Acad. Sci., Chem. Sci., 1999, 111, 71–80 CAS
.
- L. Bozec and M. Odlyha, Biophys. J., 2011, 101, 228–236 CrossRef CAS PubMed
.
- A. O. Brightman, B. P. Rajwa, J. E. Sturgis, M. E. McCallister, J. P. Robinson and S. L. Voytik-Harbin, Biopolymers, 2000, 54, 222–234 CrossRef CAS PubMed
.
- N. T. Wright and J. D. Humphrey, Annu. Rev. Biomed. Eng., 2002, 4, 109–128 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nr08463f |
| This journal is © The Royal Society of Chemistry 2016 |



