Insights on the interaction of calcein with calcium carbonate and its implications in biomineralization studies†
Giulia
Magnabosco
 *a,
Iryna
Polishchuk
b,
Jonathan
Erez
c,
Simona
Fermani
a,
Boaz
Pokroy
*a,
Iryna
Polishchuk
b,
Jonathan
Erez
c,
Simona
Fermani
a,
Boaz
Pokroy
 b and
Giuseppe
Falini
b and
Giuseppe
Falini
 *a
*a
aDipartimento di chimica “Giacomo Ciamician”, Alma Mater Studiorum-Università di Bologna, Via F. Selmi 2, 40126 Bologna, Italy. E-mail: giulia.magnabosco3@unibo.it; giuseppe.falini@unibo.it
bDepartment of Material Sciences and Engineering and the Russel Berrie Nanotechnology Institute Technion-Israel Institute of Technology, 32000 Haifa, Israel
cInstitute of Earth Sciences, The Hebrew University of Jerusalem, Edmond Safra Campus, Jerusalem 91904, Israel
First published on 25th June 2018
Abstract
The effects of calcein, a fluorescent marker commonly used to assess mineral growth in calcifying organisms, on calcite and aragonite structure have been investigated. Calcein is entrapped within calcite and aragonite and modifies the shape and morphology of both polymorphs. Moreover, in the presence of Mg2+, it inhibits aragonite formation in favor of magnesium calcite.
For studying and understanding of the biomineralization processes, various methods to mark the growth of the inorganic components in the organisms have been employed, allowing researchers to correlate a particular region of the skeleton to the instant this region was deposited. A common method is to use fluorescent molecules to label a particular stage of the deposition process.1 Calcein, a strongly fluorescent organic dye synthesized via modification of fluorescein with ethylenediaminetetraacetic acid analogues (Fig. SI1†),2 has been widely used to study the growth of calcified skeletons because it coordinates calcium ions and is entrapped within the growing shells of various organisms.3–5 Calcein has been successfully applied to mark bone growth6 as well as shell and coral skeleton formation.1,7 However, even if the transport of this dye inside living organisms was deeply characterized,3,8,9 no information on the effect of calcein on the morphology and polymorphism of calcium carbonate crystals is readily available.
The effect of soluble additives, both synthetic and biogenic, on calcium carbonate crystals has been extensively studied to understand biomineralization10–15 and to fabricate novel advanced materials.16–22 Based on these studies, models on the mechanisms of interaction between various additives and the growing mineral have been proposed. Interaction and entrapment of calcein within the crystal structure of calcium carbonates are known to occur due its chemical structure.23,24
In this study, we examined the in vitro effect of calcein on the growth of calcite and aragonite crystals, the two main polymorphs of CaCO3 found in living organisms, using the calcein concentration in the range usually adopted for in vivo labelling.
For this goal, we used the vapor diffusion method, involving the diffusion of NH3(g) and CO2(g) obtained from the decomposition of (NH4)2CO3(s) into a Ca2+ solution containing the dye. This method is relevant for biomineralization processes due to the slow increase of the concentration of CO32− ions in the crystallization solution. It exploits carbonate speciation in water resembling the process suggested to occur in living systems, in which carbonic anhydrase supplies carbonate to the calcification site.25
In our experiment, calcite precipitated when solely Ca2+ was present in the crystallization solution, while aragonite was the main polymorph obtained when Mg2+ was co-present with a Mg2+/Ca2+ molar ratio equal to 4. Mg2+ favors the precipitation of aragonite by (i) adsorbing on calcite nuclei and preventing the integration of Ca2+ in the lattice, which leads to the growth of calcite and (ii) forming magnesium calcite, which is more soluble than calcite and can be as soluble as aragonite. In these conditions, supersaturation levels suitable for aragonite precipitation are generated.26,27
No detectable inhibition or promotion of precipitation due to the presence of calcein was observed, as evaluated by the measure of total Ca2+ deposited in each experiment (see Table SI1†). Moreover, no detectable amounts of ammonium ions or chloride ions were detected in the precipitated calcium carbonate crystals.
To evaluate the quantity of calcein associated to the inorganic phase, the crystals were dissolved in an acidic buffer and the UV-vis absorption spectrum of calcein was measured. The high molar absorption coefficient of calcein allows an accurate quantification of the dye in solution even for very low concentrations, making this approach more accurate than other techniques (e.g., thermogravimetric analysis).28
Calcein loading was calculated with respect to the calcium content measured on pristine precipitates and bleached precipitates. Calcite crystals entrap significantly higher quantity of calcein as compared to aragonite crystals (p ≤ 0.05, Fig. 1). Interestingly, bleaching does not significantly change the content of calcein in calcite crystals (p > 0.05), while in aragonite crystals, its content significantly decreases after bleaching (p ≤ 0.05), implying that the quantity of surface adsorbed dye is significant only for aragonite. Indeed, it has been reported that aragonite has a surface area almost ten times higher than that of calcite, justifying a higher adsorption efficiency per unit mass of CaCO3.29
 | ||
| Fig. 1 Calcein content (wt%) measured in (a) calcite and (b) aragonite precipitated in the presence of the different dye concentration examined (Table SI1†). The data on bleached samples are reported in light gray and those on untreated ones are reported in dark gray. | ||
Experimental data indicate that the amount of dye entrapped within calcium carbonate increases with an increase in its solution concentration. Moreover, the percentage of dye removed from the solution during the crystallization process decreases with its concentration. This observation indicates that 40 μM, which is the concentration used in in vivo experiments, does not allow maximum loading into calcium carbonate in the experiments carried out in vitro. In fact, increasing the concentration to 400 μM results in the increase in the loading of almost ten times. Thus, if an organism accumulates calcein at the mineral growing sites, higher entrapment can be achieved with an enhancement of the fluorescence signal.
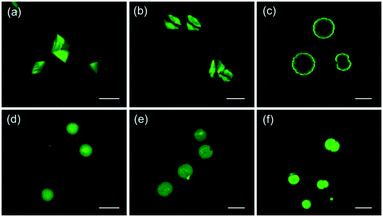
The spatial distribution of calcein entrapped within the crystals was investigated by confocal microscopy. This analysis was carried out on the samples chemically bleached prior to imaging to exclude any fluorescence contribution from the dye adsorbed onto crystal surfaces.
Confocal images of calcite crystals grown in the presence of calcein (Fig. 2a–c) show an inhomogeneous distribution of the dye within the crystals. The dye localizes preferentially on rhombohedral faces, which are more extended in the external layers in case of spherulitic calcite (Fig. 2c). In addition to this, it cannot be excluded that the signal from the center of the spherulitic crystals is not detected since the thickness of the sample and the geometry of the microscope do not allow the laser to reach the top layers (Fig. SI2†). Confocal images of aragonite crystals grown in the presence of calcein (Fig. 2d–f) show a homogeneous distribution of the dye inside the crystals. Since aragonite particles as well as those of magnesium calcite are composed of packed acicular crystals,30 it can be assumed that the dye is mainly entrapped among the crystalline domains, and only partly is embedded within the lattice. This observation is also confirmed by the results on dye loading, which is significantly reduced after bleaching.
Rietveld refinement applied to the high resolution X-ray powder diffraction data allowed quantifying the CaCO3 polymorphic distribution and the lattice distortions (see Table SI4†) induced by calcein interaction with calcium carbonate crystals.20,31 The crystalline phase analysis shows that calcein does not affect the precipitation of calcite, which always occurs regardless of its concentration. Interestingly, in the presence of calcein, pure calcite is obtained, while in the absence of the additive, traces of aragonite and vaterite are present (Fig. SI4†). The scenario is different in the precipitation conditions used for aragonite. The presence of calcein promotes precipitation of magnesium calcite at the expense of aragonite and when 400 μM calcein is present in the crystallization solution, only 4 wt% of aragonite co-precipitate is obtained (Fig. SI5†). This trend could be related to the higher complexation constant of calcein for Ca2+ than for Mg2+.32 Thus, increasing the concentration of free Mg2+ ions with respect to Ca2+ affects CaCO3 supersaturation of the precipitating solution and the thermodynamic stability of magnesium calcite and aragonite.33 However, it has been reported that the presence of calcein does not impact the incorporation of Mg2+ into biologically and inorganically precipitated calcium carbonate.34
The presence of calcein also induces a change in the lattice parameters of calcite (Table SI5†). This change is visualized in Fig. 3a, where the (104) diffraction peak of calcite shifts to a lower 2θ value when it is grown in the presence of 400 μM of calcein. This is mainly related to an expansion of the c-axis (Table SI5†) associable to the entrapping of the additive. No significant shift of aragonite peaks is observed (Fig. 3b), indicating that calcein is mainly localized among the crystalline units of aragonite, as suggested by the bleaching results and confocal microscopy experiments as well as the non-significant variation of the lattice parameters (Table SI6†). In the magnesium calcite crystals, the lattice distortions are due to a combined opposed effect of calcein and Mg2+ ions, with the former expanding the c-axis, as observed for calcite, and the latter decreasing the a-axis (Table SI6†).
SEM images show that the dye modifies calcite crystal shape and morphology (Fig. 4a–d). When grown in the presence of 4 or 40 μM calcein, the crystals show only {10.4} rhombohedral faces (Fig. 4b) or demonstrate additional crystalline {hk.l} faces almost parallel to the c-axis, respectively. The presence of 400 μM calcein in the crystallization solution leads to the formation of spherulitic aggregates exposing small {10.4} faces. A similar trend in the evolution of the shape and morphology with the increase in the concentration of an additive has been observed in the presence of block copolymer poly(ethylene glycol)-block-poly(methacrylic acid),35 which similarly to calcein contains carboxylate functional groups. Aragonite crystals precipitated in the absence of additives (Fig. 4e) present a dumbbell shape, composed of needles with a triangular section. Crystals grown in the presence of 4 μM or 40 μM calcein (Fig. 4f and g) appear elongated and show {hk0} faces, and the dimensions decrease with the increase in the concentration of the additive. The mass ratio of magnesium calcite increases at higher calcein concentration, and the crystals grown in the presence of 400 μM calcein (Fig. 4h) are composed of small particles aggregated in a dumbbell shape, in which the characteristic stepped {01.1} faces are observable.36
 | ||
| Fig. 4 SEM images of crystals grown in the presence of 10 mM Ca2+ (a) without additives and in the presence of (b) 4 μM, (c) 40 μM and (d) 400 μM calcein and crystals grown in the presence of 10 mM Ca2+ and 40 mM Mg2+ (e) without additives and in the presence of (f) 4 μM, (g) 40 μM and (h) 400 μM. Scalebar is 10 μm in the main picture and 1 μm in the inset. Images are representative of the whole crystal population (Fig. SI3†). | ||
Although it is evident that the morphological variations of the crystals are due to the stabilization of crystalline faces by calcein, the complexity of the molecule and the difficulty in identifying specific crystalline planes of interaction, several crystalline faces ({hk.l}) almost parallel to the c-axis can be involved, make it difficult for us to propose a structural model of interaction.
In conclusion, we studied the effect of calcein, a dye commonly used to mark the growth of calcifying organisms, on the growth of calcite and aragonite crystals in vitro. The obtained data reveal a strong influence of calcein on the precipitation of calcium carbonate, affecting both polymorphic distribution and crystal morphology. Calcein is loaded into the structure of both polymorphs, localizing within the lattice preferentially along (104) planes in calcite crystals and adsorbing among crystalline domains in aragonite. These in vitro observations do not find a documented correspondence in in vivo systems, for which no effect of calcein on the calcification process have been reported so far. Thus, it is evident that the organisms' capability to control the deposition of the mineral phases can overcome the potential interference caused by calcein.
Conflicts of interest
There are no conflicts to declare.References
- M. Holcomb, A. L. Cohen and D. C. McCorkle, J. Exp. Mar. Biol. Ecol., 2010, 440, 126–131 CrossRef
.
- H. Diehl and J. L. Ellingboe, Anal. Chem., 1956, 28, 882–884 CrossRef
.
- É. Tambutté, S. Tambutté, N. Segonds, D. Zoccola, A. Venn, J. Erez and D. Allemand, Proc. Biol. Sci., 2012, 279, 19–27 CrossRef PubMed
.
- A. L. Moran, Mar. Biol., 2000, 137, 893–898 CrossRef
.
- J. M. Bernhard, J. Foraminiferal Res., 2004, 34, 96–101 CrossRef
.
- S. J. Du, V. Frenkel, G. Kindschi and Y. Zohar, Dev. Biol., 2001, 238, 239–246 CrossRef PubMed
.
- J. Thébault, L. Chauvaud, J. Clavier, R. Fichez and E. Morize, Mar. Biol., 2005, 149, 257–267 CrossRef
.
- S. Bentov, C. Brownlee and J. Erez, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 21500–21504 CrossRef PubMed
.
- D. Evans, W. Müller and J. Erez, Geochim. Cosmochim. Acta, 2018 DOI:10.1016/j.gca.2018.02.048
.
- G. Falini, S. Albeck, S. Weiner and L. Addadi, Science, 1996, 271, 67–69 CrossRef
.
- M. Reggi, S. Fermani, V. Landi, F. Sparla, E. Caroselli, F. Gizzi, Z. Dubinsky, O. Levy, J.-P. Cuif, Y. Dauphin, S. Goffredo and G. Falini, Cryst. Growth Des., 2014, 14, 4310–4320 CrossRef
.
- M. Reggi, S. Fermani, C. Samorì, F. Gizzi, F. Prada, Z. Dubinsky, S. Goffredo and G. Falini, CrystEngComm, 2016, 18, 8829–8833 RSC
.
- A. Berman, L. Addadi and S. Weiner, Nature, 1988, 331, 546–548 CrossRef
.
- S. Albeck, J. Aizenberg, L. Addadi and S. Weiner, J. Am. Chem. Soc., 2002, 115, 11691–11697 CrossRef
.
- E. Weber, L. Bloch, C. Guth, A. N. Fitch, I. M. Weiss and B. Pokroy, Chem. Mater., 2014, 26, 4925–4932 CrossRef
.
- G. Magnabosco, M. D. Giosia, I. Polishchuk, E. Weber, S. Fermani, A. Bottoni, F. Zerbetto, P. G. Pelicci, B. Pokroy, S. Rapino, G. Falini and M. Calvaresi, Adv. Healthcare Mater., 2015, 4, 1510–1516 CrossRef PubMed
.
- D. C. Green, J. Ihli, P. D. Thornton, M. A. Holden, B. Marzec, Y.-Y. Kim, A. N. Kulak, M. A. Levenstein, C. Tang, C. Lynch, S. E. D. Webb, C. J. Tynan and F. C. Meldrum, Nat. Commun., 2016, 7, 13524 CrossRef PubMed
.
- Y.-Y. Kim, L. Ribeiro, F. Maillot, O. Ward, S. J. Eichhorn and F. C. Meldrum, Adv. Mater., 2010, 22, 2082–2086 CrossRef PubMed
.
- Y.-Y. Kim, J. D. Carloni, B. Demarchi, D. Sparks, D. G. Reid, M. E. Kunitake, C. C. Tang, M. J. Duer, C. L. Freeman, B. Pokroy, K. Penkman, J. H. Harding, L. A. Estroff, S. P. Baker and F. C. Meldrum, Nat. Mater., 2016, 15, 903–910 CrossRef PubMed
.
- E. Weber and B. Pokroy, CrystEngComm, 2015, 17, 5873–5883 RSC
.
- M. D. Giosia, I. Polishchuk, E. Weber, S. Fermani, L. Pasquini, N. M. Pugno, F. Zerbetto, M. Montalti, M. Calvaresi, G. Falini and B. Pokroy, Adv. Funct. Mater., 2016, 26, 5569–5575 CrossRef
.
- Y.-Y. Kim, K. Ganesan, P. Yang, A. N. Kulak, S. Borukhin, S. Pechook, L. Ribeiro, R. Kröger, S. J. Eichhorn, S. P. Armes, B. Pokroy and F. C. Meldrum, Nat. Mater., 2011, 10, 890–896 CrossRef PubMed
.
- L. Addadi, J. Moradian, E. Shay, N. G. Maroudas and S. Weiner, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 2732–2736 CrossRef
.
- L. Addadi and S. Weiner, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 4110–4114 CrossRef
.
- A. Moya, S. Tambutté, A. Bertucci, É. Tambutté, S. Lotto, D. Vullo, C. T. Supuran, D. Allemand and D. Zoccola, J. Biol. Chem., 2008, 283, 25475–25484 CrossRef PubMed
.
-
S. Mann, Biomineralization: principles and concepts in bioinorganic materials chemistry, Oxford University Press, 2001 Search PubMed
.
- G. Falini, S. Fermani, G. Tosi and E. Dinelli, Cryst. Growth Des., 2009, 9, 2065–2072 CrossRef
.
-
P. Gabbott, Principles and applications of thermal analysis, John Wiley & Sons, 2008 Search PubMed
.
- H. Rademaker and M. Launspach, Beilstein J. Nanotechnol., 2011, 2, 222–227 CrossRef PubMed
.
- S. Fermani, B. N. Džakula, M. Reggi, G. Falini and D. Kralj, CrystEngComm, 2017, 19, 2451–2455 RSC
.
- B. Pokroy, J. P. Quintana, E. N. Caspi, A. Berner and E. Zolotoyabko, Nat. Mater., 2004, 3, 900–902 CrossRef PubMed
.
- G. Arena, S. Musumeci, R. Purrello and S. Sammartano, Thermochim. Acta, 1983, 61, 129–138 CrossRef
.
- D. Wang, L. M. Hamm, A. J. Giuffre, T. Echigo, J. D. Rimstidt, J. J. De Yoreo, J. Grotzinger and P. M. Dove, Faraday Discuss., 2013, 159, 371–386 RSC
.
- D. Dissard, G. Nehrke, G. J. Reichart, J. Nouet and J. Bijma, Geochem., Geophys., Geosyst., 2009, 10 CrossRef
, 1–13.
- S.-H. Yu, H. Cölfen and M. Antonietti, J. Phys. Chem. B, 2003, 107, 7396–7405 CrossRef PubMed
.
- G. Falini, S. Fermani, M. Gazzano and A. Ripamonti, J. Mater. Chem., 1998, 8, 1061–1065 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure and additional characterization. See DOI: 10.1039/c8ce00853a |
| This journal is © The Royal Society of Chemistry 2018 |