Recent trend in visible-light photoelectrocatalytic systems for degradation of organic contaminants in water/wastewater
Moses G.
Peleyeju
ab and
Omotayo A.
Arotiba
 *abc
*abc
aDepartment of Applied Chemistry, University of Johannesburg, Doornfontein, South Africa. E-mail: oarotiba@uj.ac.za
bDST/Mintek Nanotechnology Innovation Centre, University of Johannesburg, South Africa
cCentre for Nanomaterials Science Research, University of Johannesburg, South Africa
First published on 13th July 2018
Abstract
Electrochemical advanced oxidation process and heterogeneous photocatalysis have received great attention in the last few years as alternative/complementary water treatment technologies. Recent reports have demonstrated that coupling these two techniques to form a photoelectrocatalytic system does not only significantly minimise their drawbacks, but also offer synergistic benefits in the treatment process. The possibility of using sunlight as light energy source further lends photoelectrocatalysis to sustainability. Herein, recent works on photoelectrocatalysis are discussed with focus on the methods of synthesis, solar receptive tuning and water treatment applications of selected visible-light sensitive materials such as TiO2, ZnO, WO3, BiVO4 and their composites towards organic pollutant degradation.
Water impactThe increasing load and chemical complexity of pollutants in wastewater have led to inefficient treatment by conventional methods. Photoelectrochemical oxidation (PEO), a subset of advanced oxidation process, has emerged as a promising complement or alternative because it combines the strengths of photocatalysis and electrochemistry. This method can become more sustainable if research is focused at solar (not UV) responsive semiconductors. |
1.0 Introduction
In the quest to purify water contaminated by a myriad of toxic substances, researchers have been developing methods/processes that are not only suitable for the specific application but are also efficient and economically viable. The scarcity of quality and copious amount of water to meet the consumption needs of humans has driven this search, giving rise to a substantial number of scientific reports on wastewater treatment approaches. In particular, advanced oxidation processes (AOPs) have been extensively explored for the removal of harmful organic compounds from water. AOPs are a group of purification methods characterised by in situ production of hydroxyl radicals.1 This oxidant specie has high oxidising potential and can react unselectively with a broad range of organic compounds until their mineralisation.2 This attribute makes the hydroxyl radical an excellent candidate for the oxidative degradation of diverse organic substances often found in wastewater. A few examples of AOPs are Fenton reaction, heterogeneous photocatalysis, and electrochemical advanced oxidation processes (EAOP). AOPs have emerged as effective water treatment technologies because of their ability to degrade refractory organic pollutants which are resistant to conventional treatment technologies. There are a vast number of investigations dealing with the effectiveness of AOPs in destroying organic substances including dyes, pharmaceuticals, chlorinated organics, phenolic compounds, herbicides, disinfection by-products, etc. The suitability of AOPs in remediating water contaminated by recalcitrant organics has been well demonstrated.3–9Heterogeneous photocatalysis is one of the most researched water treatment technologies in recent years. Since the water splitting experiment of Fujishima et al. in 1972,10 thousands of scientific articles have been published on the application of TiO2 and other semiconductors for water treatment. Earlier studies focused on TiO2 because of its excellent photocatalytic characteristic under UV irradiation, chemical stability, large surface area, non-toxicity and low cost amongst others.11,12 The major drawbacks associated with this catalyst are its large band gap, its fast recombination of photogenerated charges and the recovery of the photocatalyst from water after use.13–15 The typical bad gap of TiO2 is 3.2 eV, this wide gap requires high energy for the excitation of electron from the valence band of TiO2. Generally, a photocatalyst must absorb photon with energy equal or higher than its band-gap energy for electron to be promoted from the valence band to the conduction band, leaving behind holes in the valence band. This energy requirement limits the application of TiO2 with visible light as the energy obtainable within the visible region is inadequate. Fast recombination of holes and electrons is also a notable disadvantage with TiO2 photocatalysis, adversely affecting the efficiency of the process.
To mitigate these challenges, efforts have been made to incorporate metal and non-metal species into the lattice of the semiconductor.16–18 Similarly, heterostructured materials have also been explored to tackle this challenge.19–22 It is of note that many other semiconducting materials have been investigated for photocatalytic applications, and some of these challenges are still observed with them. Without doubt, tangible advances have been achieved in photocatalytic water treatment technology. However, much is still being done to develop photocatalytic treatment systems in which the drawbacks highlighted previously are overcome. Such treatment systems are expected to offer improved performance and show promise for large-scale applications. In this regard, coupling electrochemical system and photocatalysis represents a plausible approach to efficient and effective water treatment system. In electrochemical oxidation process, hydroxyl radicals are generated by the oxidation of water molecules on the surface of the anode.23–25 The formed radicals then react with the organic substrates in the solution until they are destroyed. Direct electron transfer reactions in which electron leaves the contaminant for the anode are also possible. This leads to the degradative oxidation of the contaminants. Several studies have depicted anodic oxidation process as a promising technology for water treatment and anodes such as boron-doped diamond (BDD), SnO2, PbO2, IrO2etc. are well documented.23,24 However, the success of this technique depends largely on the nature of the anodic material. BDD is still the choice anode for electrochemical oxidation of organic pollutants. Hydroxyl radicals are produced in sufficient amount at the surface of BDD and the interaction between the radicals and the electrode surface is weak. These features have been indicated to be advantageous for the mineralisation of the target pollutants.26 Despite the exciting results reported with the electrochemical oxidation method, the cost implications of the process in terms of electrical energy and electrode materials require more attention. Development of improved electrochemical water treatment systems with reduced energy utilisation and overall lower cost is worthwhile. One of the ways of realising this is by combining treatment methods.
The combination of electrochemical oxidation and photocatalysis is referred to as photoelectrocatalysis. In this hybrid process, the inherent challenges of heterogeneous photocatalysis are largely resolved. Also, the anodic potential needed in a typical photoelectrocatalytic cell is lower compared to what is required for electrochemical degradation of organics. A few review articles have been published on photoelectrocatalysis for water treatment.27–32 This review is an addition to the existing body of knowledge with a particular focus on photoelectrocatalytic systems in which visible light source has been utilised (especially in the last six years). In this review, attention is given to the electrode materials used and their methods of preparation. We considered various approaches that have been employed to enhance solar efficiency of the anodic materials. Furthermore, a summary of the experimental conditions employed are also presented.
2.0 Photoelectrocatalysis: an overview
Photoelectrocatalysis (PEC) is an advanced oxidation process which couples both photocatalytic oxidation process and electrochemical oxidation process. Simply, a photoactive semiconducting material is employed as anodic material and both light of suitable wavelength and electrical energy are applied to achieve the degradation of the target organic compound. In this process, recombination of photogenerated electrons and holes which occurs in photocatalytic process is retarded by the applied bias potential. This leads to longer lifespan of the charges and consequently improvement of the photocatalytic degradation process. In the same vein, production of hydroxyl radicals via water oxidation at the anode surface and the possible occurrence of direct electron transfer reactions are bound to increase the oxidation rate of the pollutant. On the other hand, the challenge of catalyst recovery after treatment in a photocatalytic setup is absent as the catalyst is localised on a conducting substrate in PEC.When light photons (hν) of sufficient energy are incident on a semiconductor (S), the process in eqn (1) occurs,
| S + hν → e−CB + h+VB | (1) |
The photogenerated charge pairs are transient, with estimated lifetime in the order of nanoseconds.33 These charges are capable of interacting with species in the heterogeneous system. For instance, the holes are powerful oxidant which can react with organics and water molecules which are adsorbed onto the surface of the catalyst. The oxidative degradation of organic compounds by valence-bound holes has been documented.34 Furthermore, the trapped holes have been indicated to undergo one-electron oxidation step with water molecules to generate the highly oxidising hydroxyl radicals. Formation of hydroxylated intermediates during the photocatalytic degradation of organic molecules has been given as evidence for the involvement of hydroxyl radicals.33 These intermediates were said to be consistent with those obtained when similar molecules were reacted with a known source of hydroxyl radicals. In addition, electron spin resonance studies have been conducted in support of the existence of hydroxyl radicals upon light irradiation of TiO2.35,36 It should also be noted that some reactive species such as coumarin, p-chlorobenzoic, p-benzoquinone and terephthalic acid have been employed as fluorescent probes to establish the formation of hydroxyl radicals in aqueous media in advanced oxidation processes.37–40 At present, photoluminescence study of the interaction between terephthalic acid and hydroxyl radicals in solution is often provided as evidence for the formation of hydroxyl radicals in photocatalytic processes.38,41–43eqn (2) and (3) depict the interaction of photogenerated holes with organics and water molecule. In addition, it is assumed that the positive holes can also oxidise hydroxide ions to produce hydroxyl radicals44 as shown in eqn (4).
| R + h+ → R˙ | (2) |
| H2O + h+ → OH˙ + H+ | (3) |
| OH− + h+ → OH˙ | (4) |
The electrons at the conduction band can interact with adsorbed or dissolved oxygen in the reaction system to form superoxide ion as shown in eqn (5). The superoxide ions are also known to possess considerable oxidising power (eqn (6)). Further reactions can take place between the various species in the mixture as depicted by eqn (7) and (8).
| e− + O2 → O2˙− | (5) |
| O2˙− + H+ → OOH˙ | (6) |
| OOH˙ + H+ + e− → H2O2 | (7) |
| H2O2 + hν → 2OH˙ | (8) |
In a typical electrolytic cell, water oxidation occurs at the anode surface upon application of electrical energy according to eqn (9).
| H2O → OH˙ + H+ + e− | (9) |
In photoelectrocatalytic system, therefore, oxidant species can be generated via photocatalytic process and electrolytic process. The applied bias potential minimises electron–hole recombination phenomenon and the photogenerated electrons are channeled away via the counter electrode. Direct electron transfer reactions, in which there is a transfer of electrons from the target pollutant to the anode are also a possibility. In essence, the oxidation routes of organic contaminants in a photoelectrocatalytic setup are multiple. Fig. 1 is an example of a photoelectrocatalytic cell.
 | ||
| Fig. 1 A typical photoelectrocatalytic cell for degradation of organic pollutants.45 | ||
3.0 Anodic materials for photoelectrocatalytic oxidation
Similar to what obtains in anodic oxidation process, the nature of the electrode material plays an important role in the photoelectrocatalytic process. Expectedly, any semiconductor that would be used as a photoanode should have a suitable band gap and exhibit photochemical stability in aqueous media. Such material should offer high quantum yield and considerable adsorptive capability for the organic contaminant. It is also desirable that such photoanode material is capable of harvesting substantial portion of the visible spectrum, as this is important to address the question of sustainability and overall cost implications of the process.3.1 TiO2 photoanode
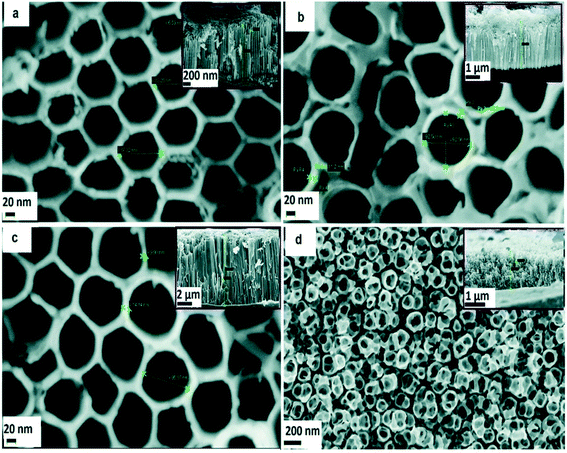
TiO2 is undoubtedly the most investigated metal oxide semiconductor for water treatment applications. It has been extensively explored as anodic material in photoelectrocatalytic oxidation of organic contaminants in water. As stated earlier, TiO2 is an n-type semiconductor which exhibits excellent properties including high photocatalytic activity, photostability and non-toxicity. Its wide utilisation as photoanode can also be related to its very good photoelectrochemical performance. Various approaches have been employed to obtain TiO2 anodes, including anodisation, sol–gel and coating, magnetron sputtering etc.Several other reports exist on the synthesis of TiO2 NTAs by anodisation method.52–63 This method has been shown to enable the production of TiO2 photoanodes with controllable nanoscale features and considerably high surface area. Stability of the NTAs is also a key factor that is given due consideration when optimising anodisation conditions. It is needless to say that TiO2 NTAs for photocatalytic applications must demonstrate high photo-to-current conversion property. Some electron images of ordered TiO2 films obtained via anodisation can be seen in Fig. 2.
Pablos et al.64 prepared TiO2 films on the surfaces of Ti and ITO glass by dipping the supports in a suspension of P25–TiO2. The photoelectrocatalytic performance of the TiO2/Ti and TiO2/ITO electrodes were evaluated against that of a TiO2 electrode prepared by annealing of Ti. Although the thermally prepared electrode was found to display superior properties for charge carrier separation upon application of bias potential, the particulate TiO2 electrodes appeared to be more effective for charge carrier transfer on TiO2 and thus favour the formation of hydroxyl radicals. TiO2/Ti was reported to be most suitable for the degradation of the selected organic compound. The performance of this electrode (TiO2/Ti) was attributed firstly to the good charge carrier properties resulting from its particulate nature and then to the good interaction between the catalyst layer and the Ti substrate, which promotes charge carrier separation when an electric bias potential is applied. Islam and Basu65 prepared a TiO2/Ti electrode by dip-coating technique. Prior to coating, diethylene glycol was added to the TiO2 sol and the mixture was thoroughly mixed. Citing Kajitvichyanukul and Amornchat,66 the authors submitted that the presence of the additive in the sol enhances the adhesion of the thin film to the surface of the substrate, thus improving the stability of the electrode. Cervantes et al.67 also prepared a solution of P25–TiO2 and coated it onto a Ti plates by painter method. The TiO2 electrode obtained after oven drying and calcination was employed for the degradation of a mixture of synthetic dyes. In the same vein, Liu et al.68 prepared a film of P25–TiO2 on a Ti mesh substrate. The powder photocatalyst was mixed with isopropanol solution of zinc nitrate and electrophoretically deposited onto the clean Ti support. Factors such as electrophoretic deposition time and potential were found to have influence on the morphology of the films, which in turn has direct correlation with the stability of the photoanode. In the work of Zhou et al.,69 doped TiO2 film was prepared on a Ni support by sol–gel and dip-coating methods. To ensure complete coverage of the substrate by the catalyst, the dipping of the substrate into the sol of the catalyst was done five times. Mancilla et al.70 also prepared a film of TiO2/Ti by sol–gel and dip-coating methods for photoelectrocatalytic treatment of aqueous organics. The authors optimised the coating process and reported that dipping the Ti substrate into the sol of the organic precursor one time produced a better film with fewer cracks. While the electrode obtained by dipping the substrate into the precursor three times presented a warped and cracked surface. The more cracks observed in the latter were attributed to the increase in capillary pressure during solvent evaporation while the electrode was being calcined. Furthermore, the one-layer thick film was found to exhibit a higher photocurrent compared to the multiple-layer films. Wang et al.71 prepared TiO2 films electrodes by depositing the catalyst paste onto FTO glass support by doctor-blade technique using transparent adhesive tapes as spacers. Similarly, Oliveira et al.72 prepared porous films of TiO2 and its composite by spreading aliquots from the aqueous suspensions of the catalysts onto FTO substrates with a glass rod, using adhesive tape as spacer. Preparation of TiO2 films by sol gel and dip-coating methods is simple and does not require the use of any special equipment but the long-term stability and consequently reusability of the thin film electrodes are a concern.
In essence, the choice of preparation technique for TiO2 thin film electrodes should be premised on factors such as film thickness control, electrode stability and service life, up-scalability, cost implications and simplicity amongst others.
3.2 Zinc oxide (ZnO) photoanodes
Zinc oxide is an n-type semiconductor which has a direct and wide band gap in the near-UV region and a large free-exciton binding energy.91 Heterogeneous photocatalysis with ZnO is similar to what obtains with TiO2 since the values of the band-gap energy of both semiconductors are about the same. ZnO, however, does offer some merits such as high absorption coefficient and high electron mobility,92 low cost and environment friendliness. In addition, the strong luminescent characteristic of ZnO allows for investigation of the phenomenon of photogenerated charges recombination,93 which impedes photocatalytic activity of the catalyst. The effect of any dopant which is incorporated to minimise this phenomenon can thus be evaluated and a highly active photocatalyst can be obtained. ZnO absorbs only a very small fraction of the solar spectrum in the UV region due to its large band-gap energy,94 and this tends to limit its practical application as a photocatalyst for water purification. Furthermore, it is susceptible to photocorrosion.95Several studies have reported the application of ZnO-based photocatalysts for oxidation of organic contaminants.96–99 In most of these studies, the optical properties of ZnO was tuned to improve its photocatalytic characteristic either by the incorporation of dopants such as metals and non-metal species into the lattice of the semiconductor or by coupling it to another photocatalyst.
Preparation of ZnO film electrodes can be achieved by various methods including those described for TiO2 films electrode. For instance, Huang et al.100 obtained ZnO film electrode by sol–gel and spin-coating methods using a glass as a support. Furthermore, Yi et al. prepared ZnO film on silicon substrate by magnetron sputtering technique using metal zinc disk as a target.98 Shetty and Nanda (2012) prepared a Zn film electrode by anodisation using slightly acidic deionised water as an electrolyte.101 In the same vein, Basu et al. employed a highly pure Zn metal and oxalic acid to produce Zn film electrode in the presence of UV light.102 Similarly, Lee et al.103 obtained ZnO/Zn photoanode by anodising Zn plate in a solution of oxalic acid. Also, Ramirez-Canon et al.104 synthesised a nanostructured film of ZnO by anodisation, utilising pure Zn foil and electrolytes such as ethanolic solutions of H3PO4, HNO3, HCl, H2C2O4 (oxalic acid), NaOH and aqueous solution of KHCO3. The morphology and dimensions of the films formed were reportedly dependent on the type and the nature of the electrolyte used. Spray pyrolysis technique has also been used to produce ZnO film on conductive substrate. In a study by Sapkal et al.,105 the precursor solution (alcoholic solution of zinc acetate) of ZnO was coated onto FTO glass by spray pyrolysis method. ZnO film has also been prepared by liquid phase deposition. In their experiment on photoelectrocatalytic degradation of p-nitrophenol, Fan et al.106 prepared the photoanode by immersing a previously treated Ti plate in an acidic solution of ZnO for a set period of time. Recently, Zhang and co-workers also reported the preparation by liquid phase deposition and photoelectrocatalytic application of doped ZnO or ZnO composite anodes for degradation of organic pollutants in water.107–109 Graphitic material such as exfoliated/expanded graphite has been used as support for the immobilisation of ZnO. Arotiba and group110 reported the fabrication of exfoliated graphite–ZnO nanocomposite into an electrode by mechanical mixing of the ZnO nanoparticles and exfoliated graphite and subsequent compression of the composite material into pellets. ZnO has been indicated to absorb a larger fraction of the solar spectrum and more light quanta than TiO2 (ref. 111) and as stated earlier, it has excellent charge carrier mobility. ZnO catalyst has been judged to present higher photocatalytic efficiency than TiO2. However, it has not been extensively explored for photoelectrocatalytic degradation of organic pollutants. Since the major drawback associated with ZnO photocatalysis is photocorrosion during light irradiation,112 this challenge should be taken into consideration when preparing ZnO films for photoelectrocatalytic applications. Research efforts can be directed at modifying the surface of this catalyst to effect possible changes in surface charge and functionality. This can lead to the production of ZnO films with enhanced stability. It is also necessary to employ immobilisation approach that ensures firm adherence of the films to the conductive substrate.
3.3 Tungsten trioxide (WO3) photoanode
Tungsten trioxide (WO3) is notable as a semiconductor for electrochromic applications owing to its good stability in acidic media, strong adherence to immobilisation supports and large optical modulation.113 It is an n-type semiconductor with a fairly narrow band gap (2.4–2.8 eV). Its relatively high conductivity, low susceptibility to photocorrosion and considerable oxidation power of the valence band114 holes have drawn attention to it as a potential anodic material for water purification. WO3, because of its narrow band gap, has visible light absorption capability close to about 500 nm of the solar spectrum.115,116 This makes it a promising candidate for visible-light driven catalytic processes. The photocatalytic activity of pristine WO3 is however low owing to the rapid recombination of electron–hole pairs and the somewhat positive conduction band edge.117 It has been stated that the bias potential which is usually applied in the photoelectrocatalytic process counters the recombination of charges at the semiconductor. WO3 therefore may represent an impressive photoanode for organic pollutants oxidation.Thin films of WO3 have been prepared by a number of methods. Kim et al.118 prepared a dispersion of WO3 by mixing measured amounts of the catalyst, ethylene glycol and triton. The dispersion was drop-cast onto a titanium support, dried and thermally treated. Mohite et al.119 also synthesised a film of WO3 on a conductive substrate by dissolving tungsten powder in hydrogen peroxide and mixing the resulting peroxotungstic acid with ethanol to form the precursor solution. The WO3 precursor was subsequently sprayed onto the preheated FTO glass substrate and annealed in air to produce the crystalline film. Longobucco et al.115 reported preparation of two WO3 films electrodes via colloidal solution and anodisation. The colloidal solution, which was obtained using sodium tungstate as starting material, was deposited onto cleaned FTO glass by spin-coating. The other electrode was prepared by accelerated potentiostatic anodisation of W sheet. The surface of the FTO glass coated with WO3 suspension showed the presence of homogeneous and spherical particles with small size distribution while the surface of the anodised W sheet displayed roughly spherical and strongly fused particles with larger size. It is noteworthy however that the photoelectrocatalytic activities of both electrodes are comparable. In another work by Zhu et al.,116 WO3/FTO electrode was prepared by doctor blade method. The WO3 paste was obtained by mixing the catalyst powder, previously prepared by hydrothermal process, with terpineol and ethyl cellulose. The cellulose played the role of a binder. Arotiba and co-workers also reported the fabrication and application of a photoanode from a composite of WO3 and exfoliated graphite. WO3 was synthesised by hydrothermal method and the photocatalyst particles were trapped within the pores of the carbonaceous material and the composite material was subsequently compressed under high pressure to produce pellets for the fabrication of the electrode. The highly compressible nature of exfoliated graphite was beneficial for trapping WO3 nanoparticles.120 Vidyarthi et al.121 prepared a polycrystalline WO3 films by reactive magnetron sputtering using a very pure metallic tungsten target and a Ti coated silicon wafers substrate. The schematic representation of this procedure is shown in Fig. 3. The micro-structural features of the thin films produced were found to be dependent upon the applied sputter pressures. And the photocurrent response of the films were influenced by their structures.
 | ||
| Fig. 3 Scheme of the fabrication of thickness-gradient WO3 thin film materials library by reactive sputter deposition. A typical photograph of WO3 thickness-gradient thin film materials library patterned using a micro-machined Si-mask is shown. The positions 1 and 9 correspond to the thinnest and the thickest films in the materials library, respectively. Reproduced from ref. 121, with permission from Elsevier. | ||
3.4 Bismuth vanadate (BiVO4) photoanode
There are not many reports on the use of BiVO4 as anodes for the photoelectrocatalytic oxidation of organic pollutants. However, BiVO4 has been gaining attention as a photocatalyst for harvesting sunlight energy in photoelectrochemical water oxidation process.122–124 This catalyst is suitable for visible-light driven processes because of its relatively small band-gap energy of 2.4 eV. In addition, BiVO4 is stable to photocorrosion, non-toxic and consists of earth-abundant elements. The major factor hampering its utilisation as photoanodes is its low photo-efficiency, resulting from the poor charge separation in the catalyst. Much efforts have been directed at ameliorating this challenge and exciting results have been reported in the literature, especially in the areas of water oxidation for hydrogen generation. However, reports on the use of BiVO4 as photoanodes for degradation of organic contaminants are still very sparse. Xia et al.125 reported on the photoelectrochemical performance of BiVO4 and α-Fe2O3/BiVO4. The photoanodes, fabricated by coating the precursor solutions of BiVO4 and Fe2O3 onto FTO glass in a successive manner, displayed impressive incident photon-to-current efficiency (ICPE). Photoelectrocatalytic degradation of phenol at these electrodes yielded very significant abatement in the chemical oxygen demand (COD) of the aqueous solution of the compound. Monfort et al.126 also synthesised pure and Nb doped BIVO4 by sol–gel method using Triton X-100 as structure-controlling agent. The films of the materials were deposited onto FTO glass by doctor blade method. Both materials did not only show promise for hydrogen generation but also for degradation of Rhodamine B and stearic acid. There exist other articles in which BiVO4 is combined with other well explored semiconductors for photoelectrocatalytic water treatment. These articles will be presented in section 5.0.4.0 Trends in the preparation of visible-light active semiconductors
For practical application and economic viability of photocatalytic processes in solving environmental and energy problems in this era, it is crucial for potential catalysts to be considerably active under visible light irradiation. As such, solar energy can be utilised to drive these processes and the cost and the danger associated with UV irradiation can be circumvented. Several efforts have been made to enhance the photoabsorption capability of well-known semiconductors with large band-gap energies in the lower energy region of the solar spectrum.4.1 Metal and non-metal dopants
A large number of articles have reported on the incorporation of metal and non-metal ions into the lattices of photocatalysts in order to improve their absorption in the visible-light region. For instance, tailoring the band gap of TiO2 by doping with metal ions has successfully shifted its optical absorption to the visible light region. It is believed that doping with metal ions leads to the narrowing of band gaps because the ions constitute impurities in the forbidden energy band of the photocatalysts.127,128 Modification of TiO2 with noble metals such as Au, Ag, Pt and Pd has been indicated to improve the sensitivity of the catalyst under solar irradiation. This improvement can be related to the visible light absorption of plasmonic nanoparticles via localised surface plasmon resonance (SPR).129–131 It has however been noted that doping with metal ions often gives localised d states deep in the band gap of the semiconductor and the states have been indicated to serve as recombination centres for photoexcited charges.132 In the same vein, doping with non-metal ions has been shown to lead to the extension of the optical absorption of photocatalysts to the visible light region. Substitutional doping with non-metals such as N, C, S etc. have been indicated to lead to band-gap narrowing of TiO2 by mixing with the oxygen 2p states.132,133 N doping of TiO2 produces a significant result in extending the sensitivity of TiO2 to the visible region. This is owing to the size of N which is comparable to that of oxygen. Co-doping with metal and non-metal ions to obtain the synergistic effect of the dopants has also been exploited. Tang et al.134 investigated the effect of Fe in the lattices of TiO2 on the band gap and photoresponse of the catalyst. The Fe-doped titania showed lower band gap energy and greatly improved sensitivity in the visible region. In a study by Chen et al.,135 TiO2 modified with Au nanoparticles displayed superior light absorption under simulated sunlight irradiation compared to the unmodified TiO2. The enhancement in the photoelectrocatalytic activity of the modified electrode was as a result of the SPR of Au nanoparticles arising from the interband transitions from 5d band to and within the 6sp band of the noble metal. Daghrir et al.74 observed a red shift in the optical absorption of TiO2 when it was doped with N and the N doped TiO2 electrode displayed a superior photoelectrocatalytic performance in the visible region compared with undoped TiO2. Zhou et al.69 also reported an improved photoelectrocatalytic activity of TiO2 under visible light irradiation when the catalyst was co-doped with La and N. In a study by Song et al.136 TiO2 modified with N and activated carbon was synthesised and fabricated into electrode for the photoelectrocatalytic oxidation of salicylic acid. The particle electrode was reported to display high absorption in the visible-light region. The high performance of the electrode in this energy range was attributed to the presence of nitrogen and carbon in the semiconductor. Also, enhancement in the photoelectrochemical properties of ZnO under visible light was observed by Salem et al.137 when modified with Fe ions. Optimising the Fe content of the catalyst, the authors obtained a more than 80% enhancement in photocurrent response with 0.5% atomic loading. The performance of the Fe doped ZnO under visible light was attributed to the modification in the band gap width of the semiconductor. Incorporation of Cu into the lattices of ZnO has also been reported to induce band gap narrowing and significant improvement in the sensitivity of the semiconductor under solar illumination.138 In the same vein, a report by Ahmad et al.139 indicated that doping ZnO with Ce extended its optical absorption into the visible light range and reduced recombination of holes and electrons recombination. Also, Khan et al.140 reported that ZnO doped with reduced graphene oxide (rGO) displayed a ten-fold performance over the pristine ZnO under visible light. The performance of the rGO modified ZnO was due to the decrease in band gap, efficient charge separation between the valence band and conduction band, and the excellent electronic property of graphene. It should be noted that carbon materials such as graphene, activated carbon, carbon nanodots etc. do not only improve light absorption properties of photocatalysts under solar irradiation but also enhance photocatalytic degradation of organic pollutants at these photocatalysts owing to their excellent adsorption characteristic.The sensitivity of photocatalysts such as WO3 and BiVO4 has also been shown to improve under visible light irradiation upon modifications with metal and non-metal. For instance, Mohite et al.141 reported that there was a slight shift towards the visible region in the absorption spectrum of Ga modified WO3. Similarly, Li et al.142 reported that WO3 doped with Al exhibited a better photoelectrochemical response than pristine WO3. The presence of Al in the lattices of the WO3 resulted in decrease in the band-gap energy of the metal oxide, and the authors ascribed this red shift to the +3 valence state of Al which serves as electron acceptor level. Interestingly, the photoresponse of the Al doped WO3 showed no substantial improvement in the visible light region and the impressive photoelectrochemical performance was thus attributable to efficient charge transfer in the doped catalyst. In another study by Liu et al.,143 it was reported that N doped WO3 displayed higher photocurrent density and better photoelectrocatalytic performance under visible light than the undoped WO3. The authors attributed the superior optical absorption of the doped catalyst in the visible light range to the modification of its band edge caused by the presence of the N dopant in the crystal lattice of WO3. In a report by Wang et al.,144 loading metallic Bi onto BiVO4 film was said to improve its visible-light response and positive photon excitations. The authors also suggested that the formation of metal/semiconductor junction favoured separation of carriers and consequently lead to enhanced photoelectrochemical performance. Similarly, BiVO4 has been modified with N and the obtained results showed that the band gap of the catalyst reduced with N doping. The absorption edges extended to higher wavelengths and the photocatalytic activity of the doped BiVO4 surpassed that of the pristine BiVO4. The presence of N was believed to induce a distortion and created oxygen vacancies in the crystal lattice of BiVO4, and there was increasing molar ratio of V4+/V5+ with N doping. The result is prolonged lifetime of photogenerated charge carriers since their recombination is inhibited by the phenomena stated above.145 Luo et al. also studied the effect of incorporating Mo into BiVO4 on its photocatalytic characteristic.146 It was reported that shallow level Mo doping resulted in significantly enhanced photocurrent response of the modified catalyst under visible light.
4.2 Combination of two or more semiconductors
Another smart approach to obtaining appreciably efficient photoanodes with excellent light-harvesting property is by coupling two or more semiconductors. This approach is a significant advancement in semiconductor photocatalysis because recombination of carrier charges is inhibited and interfacial charge transfer is improved in coupled semiconductors.147 Combining appropriate semiconductors is thought to create heterojunctions that generate sufficient potential which is advantageous for separation electrons–holes pairs.148 When a p-type semiconductor (in which holes are the predominant charge carriers) is coupled to an n-type semiconductor (in which electrons are the main charge carriers), a p–n heterojunction results. In a p–n heterojunction, the migration of holes from the p-type semiconductor to the n-type semiconductor and electrons from the n-type to the p-type semiconductor result in charged regions. The existence of this charged regions can produce built-in potential which serves to retard the recombination of photogenerated charge carriers. Formation of n–n and p–p heterojunctions is also possible between like-type semiconductors. Generally, the alignment of the band-energy levels of two semiconductors can lead to migration of charges and thus creation of electric field which can prolong the lifetime of photogenerated holes and electrons. Heterojunctions formed by semiconductors have been categorised into three on the basis of the positions of the band energies. In the first case (type I), semiconductor A has a more negative conduction band and a more positive valence band than semiconductor B. In type II, semiconductor A has a more negative conduction band but a less positive valence band than semiconductor B. In type III, the valence band of semiconductor A is above the conduction band of semiconductor B. Heterojunction types I, II and III are otherwise termed straddling, staggered and broken respectively, based on their band diagrams. The staggered architecture has been indicated to be most advantageous for extending the lifespan of photogenerated charge carriers. This is because the electric field created therein allows for separation of the photo-excited holes and electrons in the two semiconductors, leading to improved photocatalytic properties. In the straddling type, the holes and electrons tend to migrate to the semiconductor with smaller band gap where they can easily recombine. In the broken architecture, it is practically impossible for the photo-excited electrons and holes to migrate from one semiconductor to the other. From the forgoing, it is clear that the nature and consequently the relevance in photoelectrocatalytic applications of heterojunctions formed between semiconductors depend on the type and the band edges of the coupled semiconductors.Furthermore, it should be noted that coupling a primarily UV active semiconductor such as TiO2 to a visible-light driven semiconductor such as BiVO4 can produce a bi-component photocatalyst with improved solar energy absorption efficiency. The synergic absorption of the two semiconductors can extend the light sensitivity to broad solar spectrum.149 Wang et al. investigated the photoelectrocatalytic activity of a photoanode consisting of N doped TiO2 and BiVO4. The photoresponse of the heterostructured electrode under visible light was superior to those of the pure TiO2 and BiVO4.150 Similarly, Guaraldo et al.151 reported that depositing WO3 on TiO2 film resulted in enhanced photocatalytic performance of the electrode in the visible light region. Liu et al.94 also showed that water oxidation and organic pollutants degradation were more enhanced at ZnO/BiOBr heterostructured anode than at the pure ZnO and BiOBr anodes. Li et al.152 evaluated the photoelectrocatalytic performance of a ternary electrode fabricated with ZnO, Cu2O and TiO2 under visible light. The activity of the ternary electrode was found to be greater than that of the binary electrode (Cu2O/TiO2). It was over 100% more active than the unitary electrode (TiO2 nanotubes arrays). Xia et al.153 also reported the activities of BiVO4/WO3 heterojunction photoelectrodes towards organic substance degradation. The absorption edges of the hybrid electrode shifted to higher wavelength and it exhibited stronger absorption intensity compared to WO3 electrode. Also, the photocurrent density obtained at the heterostructured anode was much higher than those obtained at the pristine BiVO4 and WO3 anodes. There are other reports on the construction of semiconductor heterojunctions for improved sensitivity and photocatalytic performance in the visible light region.154–161
4.3 Tuning morphology and size
It should be noted that tailoring the morphology, crystal structure and size of photocatalysts can also lead to significant improvement in their photoresponse in the visible region.162,163 The catalyst precursors, solvents and reaction conditions such as pH, concentration and temperature are some of the parameters that must be carefully selected to produce materials which offer the best activities. In addition, the method of preparation also has impact on the final product. For instance, in the work of Wang et al.,164 it was reported that quantum-sized TiO2 synthesised by microwave-assisted approach exhibited impressive activity with visible irradiation. This performance was ascribed to the small size and specific surface states of the catalyst which were posited to lead to reduction in migration path of charge carriers, promotion of valence band position and narrowing of band-gap energy, and minimisation of charges recombination. Zhao et al.,165 in their study of BiVO4, showed that engineering the morphology and crystalline structure of photocatalysts is important for efficient solar energy conversion. Varying the pH of the precursors yielded different morphologies and crystalline structures of BiVO4 (Fig. 4). The activity of the preferred BiVO4 was also related to the energy levels of the catalyst which was influenced by the crystal phase and morphology. | ||
| Fig. 4 SEM images of the BiVO4 samples prepared by hydrothermal method under different pH values. (a) pH = 0.25, (b) pH = 0.5, (c) pH = 0.75, (d) pH = 1.0, (e) pH = 1.5, (f) pH = 2.5, (g) pH = 3.0, (h) pH = 3.5, and (i) pH = 4.0. Reproduced from ref. 165, with permission from American Chemical Society. | ||
In a report on BiFeO3, Wang et al.166 observed that the photocatalytic efficiency of the catalyst was dependent on morphology. Of the three morphologies (spindles, cubes and plates) synthesised, the plates displayed the best activity under the same experimental conditions. In another study by Mazzarolo et al.,167 it was reported that varying the anodisation conditions produced TiO2 nanotubes with different tube top morphologies. They obtained four distinct top appearances, namely, ‘grassy’ tubes, tubes with initiation layers double anodised tubes and open tubes (Fig. 5). The tubes with open and defined open mouth reportedly showed outstanding photocatalytic and photoelectrochemical properties.
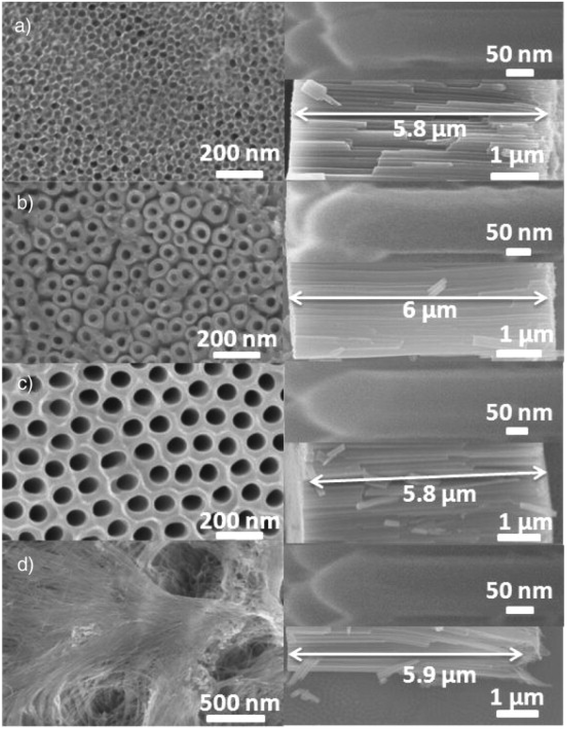 | ||
| Fig. 5 SEM micrographs showing top surfaces and cross sections for the “initiation layer” (a), “open tubes” (b), “double anodization” morphologies (c) and “grass” (d), with cross-sectional image and detail of the tube on the right panel. Reproduced from ref. 167, with permission from Elsevier. | ||
Li et al.168 also showed that both morphology and configuration have effects on the activities of nanostructured catalyst comprising CdS, ZnS and Zn1−xCdxS in the visible light region. By using different solvents in a one-pot hydrothermal synthesis, the authors produced different ternary heterostructures which displayed distinct activities. Meenakshi et al.169 also reported that ZnO nanorods exhibited significant activity under visible irradiation. The morphology was produced by optimising the concentrations of precursors, temperature and aging time. Similarly, Byzynski et al.170 reported that different morphologies of ZnO with different synthesis methods. And the activity of the ZnO catalyst under visible irradiation varied with morphologies. Also, Li et al.171 prepared different ZnO nanostructured networks having different morphologies (Fig. 6) by varying the concentration of one of the precursors (the precipitating agent). ZnO with the preferred structure (ultralong and ultrathin branching nanowires Fig. 6(e)) was indicated to be advantageous for better sunlight utilisation, improved facilitation of charge transport and showed reduced diffusion distance.
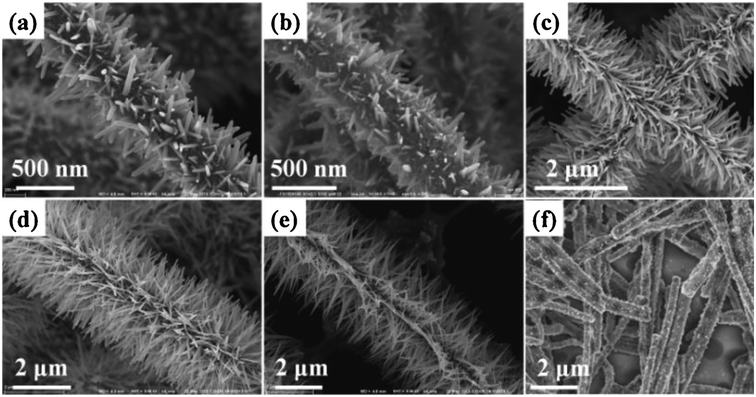 | ||
| Fig. 6 SEM images showing the structural evolution of the “caterpillar-like” ZnO nanostructures prepared hydrothermally at 95 °C for 5 h in solution with 0.025 M Zn(NO3)2·6H2O, 0.025 M HMTA, 0.0035 M PEI, and different NH4OH concentrations: (a) 0 M, (b) 0.05 M, (c) 0.10 M, (d) 0.15 M, (e) 0.20 M, and (f) 0.25 M. Reproduced from ref. 171, with permission from Elsevier. * HMTA – hexamethylenetetramine, PEI – polyethylene imine. | ||
Memar et al.172 also studied the effect of surfactant size on the physical properties of nanostructured crystalline WO3 films produced. It was observed that the size of the surfactant used influenced the size of the catalyst synthesised which in turn correlates with the photoelectrochemical performance of the material. Similarly, Zhou et al.173 reported that varying the amount of acid used in the synthesis of WO3 resulted in different nanostructures. The photoelectrochemical activity of the WO3 was morphology-dependent with plate-like and rod-like shapes displaying the best performance.
5.0 Photoelectrocatalytic degradation of organic contaminants under visible irradiation
There are several reports on the application of nanostructured materials using UV excitation for photoelectrocatalytic destruction of organic pollutants. But recent years have witnessed a huge shift of attention to visible-light excitation as a viable option. Both natural sunlight and simulated sunlight have been utilised by researchers to induce reactions at the surface of semiconducting photocatalyts.174–176 Many reports have attested to the efficacy of visible light in catalysing reactions at the surface of photoanodes consisting of materials with appropriate properties. Some organic pollutants which are commonly found in water/wastewater including pharmaceuticals, dyes, phenolics etc. have been subjected to photoelectrocatalytic treatment with solar light. Interesting results have been recorded with emerging pharmaceutical contaminants such as diclofenac, sulfamethoxazole, norfloxacin, tetracycline etc. (please see Table 1). Many organic dyes such as methylene blue, Rhodamine B, methyl orange, methyl blue, reactive red, titan yellow etc. have also been degraded at different photoanodes under solar irradiation. Similarly, attempts have been made at destroying some recalcitrant phenolic compounds via photoelectrocatalytic process. To assess the extent of removal of these organic substances after photoelectrocatalytic treatment, techniques such as UV-visible spectrometry, gas/liquid chromatography often coupled with mass spectrometry, chemical oxygen demand (COD) measurement and total organic carbon (TOC) analysis can be used. UV-visible spectrometer is suitable for monitoring decolourisation of dyes, while concentration abatement of pharmaceuticals and phenols are better measured using appropriate chromatographic methods. It should also be of interest to researchers to study the various intermediate products formed during photoelectrocatalytic degradation of organic compounds owing to the fact that some intermediate compounds can be resistant to further degradation and may be more toxic than the parent compound. Analysis of intermediate products can be achieved by using chromatography-mass spectrometry setup. In addition, aliquots can be taken from the treatment reactor from time to time during the treatment process to assess the level of toxicity. Both COD and TOC analyses give important information on the organic load of the water being treated. But TOC analysis provides useful hint on the organic carbon content of the water, thus a good measure of the effectiveness of photoelectrocatalytic treatment. Importantly, TOC analysis gives the extent of mineralisation (conversion to H2O, CO2 and inorganic ions) of the targeted compound(s). As can be seen in Table 1, many works, particularly those dealing with dyes, only measured the extent of degradation of the original compound. It is known that dyes lose their colours once their chromophores are cleaved. It therefore follows that decolourisation of a dye solution is not an indication of the abatement of the organic load since many organic moieties can still be present in the solution. To truly assess the performance of a photoelectrocatalytic system towards the destruction of any organic contaminant, TOC (or at least COD) analysis should be carried out. To further advance photoelectrocatalysis for degradation of organic pollutants in water, it is necessary that simulated wastewater which has similarity to real wastewater in terms of components is used to conduct initial assessment of any proposed reactor. Parameters such as pH, supporting electrolytes and organic load should be chosen to mimic those of the targeted wastewater.| Anodes | Contaminants | Experimental conditions | Removal efficiency | Rate constant | Ref. |
|---|---|---|---|---|---|
| F–TiO2 | Methylene blue | 1.4 V, C0 = 10 mg L−1, 0.05 M Na2SO4, 4 h | UV-vis (92.9%) | 18.3 × 10−5 s−1 | 177 |
| F, Sn–TiO2 | Phenol | 1.5 V, C0 = 10 mg L−1, 0.01 M Na2SO4, 5 h | TOC (70.7%) | 178 | |
| Bi/TiO2 | Phenol | 0.5 V, C0 = 50 mg L−1, 8 h | UV-vis (40.3%) | 6.45 × 10−2 h−1 | 179 |
| Au/TiO2 | Methylene blue | 0.5 V, C0 = 5 mg L−1, 0.5 M Na2SO4, 6 h | UV-vis (66%) | — | 180 |
| ZnSe/Au/TiO2 | Methylene blue | 0.5 V, C0 = 5 mg L−1, 0.5 M Na2SO4, 180 min | UV-vis (87%) | — | 181 |
| AgInS2/TiO2 | Norfloxacin | 0.6 V, C0 = 20 mg L−1, 0.01 M Na2SO4, 150 min | UV-vis (92%) | 1.56 × 10−2 | 182 |
| Pd, C, N & S co-doped TiO2 | Acetylsalicylic acid | 2 V, C0 = 10 mg L−1, 0.1 M Na2SO4, 120 min | HPLC (89.1%) | 0.0182 min−1 | 183 |
| TiO2/CdS | Methyl blue (MB) and methyl orange (MO) | 1 V, 0.1 M K2SO4, 120 min | UV-vis (MB, 97.4%, MO, 81.3%) | — | 184 |
| CeO2/TiO2 | Methyl orange | 2 V, C0 = 5 mg L−1, 0. 1 M Na2SO4, 60 min | UV-vis (98.1%) | — | 185 |
| RGO-CeO2–TiO2 | Bisphenol A | 9 V, C0 = 10 mg L−1, 0.05 mol L−1, 120 min | HPLC (40%) | 0.0045 min−1 | 186 |
| RGO-CeO2–TiO2 | Tetrabromobisphenol A | 9 V, C0 = 10 mg L−1, 0.05 mol L−1, 100 min | HPLC (85%) | 0.0191 h−1 | 187 |
| Fe2O3/TiO2 | Rhodamine B | 2.0 V, C0 = 10 mg L−1, 0.1 M NaCl, 3 h | UV-vis (92.3%) | — | 188 |
| FeTiO3/TiO2 | Titan yellow | 0.5 V, C0 = 0.5 mg L−1, 0.1 M NaNO3, 60 min | UV-vis (43%) | — | 189 |
| ZnFe2O4/TiO2 | Methyl orange | 0.8 V, C0 = 10 mg L−1, 0.1 M Na2SO4, 120 min | UV-vis (100%) | 0.11 h−1 | 190 |
| Cu2O/TiO2 | 2,4,6-Trichlorophenol | 1 V, C0 = 5 mg L−1, 0.1 M Na2SO4, 120 min | HPLC (99.9%) | — | 191 |
| MoS2/TiO2 | Methylene blue (MB) and sulfadiazinmu (SD) | C 0 = 20 mg L−1 (MB), 10 mg L−1 (SD), 0.01 M Na2SO4, 240 min | MB, UV-vis (60%); SD, HPLC (64%) | MB (3.6 × 10−3 min−1), SD (3.9 × 10−3 min−1) | 192 |
| g-C3N4 QDs/TiO2 | Phenol | 0.6 V, C0 = 5 mg L−1, 0.1 M Na2SO4, 120 min | HPLC (98.6%) | 0.0361 min−1 | 193 |
| Expanded graphite–TiO2 | p-Nitrophenol | 5 mA cm−2, C0 = 0.4 mM, 0.1 M Na2SO4, 90 min | UV-vis (62%) | 10.4 × 10−3 min−1 | 88 |
| Nano-graphite/TiO2 | Phenol, Rhodamine B (RhB) | 2 V, C0 = 25 mg L−1 (phenol), 10 mg L−1 (RhB), 0.1 M Na2SO4, 90 min (phenol), 60 min (RhB) | UV-vis phenol (99.2%), RhB (99.9%) | — | 194 |
| TiO2-Exfoliated graphite | Sulfamethoxazole | 10 mA cm−2, C0 = 25 mg L−1, 0.1 M Na2SO4, 240 min | UV-vis (82%) | 5.74 × 10−3 min−1 | 45 |
| BiVO4/TiO2 (N2) | Methylene blue | 1 V, C0 = 10 mg L−1, 0.1 M Na2SO4, 80 min | UV-vis (91.8%) | — | 195 |
| WO3/TiO2 | Rhodamine B (RhB) and reactive red 152 (RR) | 1.5 V, C0 = 1 mM (500 mL) | UV-vis (RhB (98%, 30 min), RR (94%, 40 min)) | 3.71 × 10−4 s−1 (RhB), 5.62 × 10−5 (RR) | 196 |
| WO3 | Methyl orange | 1.5 V, C0 = 1 mM, 320 min | UV-vis (98%) | 5.34 × 10−6 s−1 | 197 |
| WO3 | Methyl orange | 1 V, C0 = 50 μM, 0.1 M H2SO4 | UV-vis (100%) | — | 198 |
| CdS/WO3 | Rhodamine B | 1 V, C0 = 6 mg L−1, 0.1 M Na2SO4, 2 h | UV-vis (69.3%) | 1.01 × 10−2 min−1 | 199 |
| WO3-Exfoliated graphite | 2-Nitrophenol | 10 mA cm−2, C0 = 20 mg L−1, 0.1 M Na2SO4, 180 min | UV-vis (82%) | 9.54 × 10−3 min−1 | 120 |
| WO3/BiVO4 | Methylene blue | 0.2 V (Vs Ag/AgCl), 0.1 M Na2SO4 supporting electrolyte (SE), pH = 7, C0 = 5 mg L−1, 120 min | UV-vis (80%) | — | 200 |
| WO3/Mo–BiVO4 | Tetracycline hydrochloride (TCH), phenol, congo red (CR) | 1 V (SCE), 0.1 M Na2SO4 SE, C0 = 10 mg L−1, 180 min | — | (TCH) 0.683 h−1, phenol (0.385 h−1), CR (1.05 h−1) | 201 |
| Fe2O3/BiVO4 | Phenol | 0.6 V (Vs Ag/AgCl), 0.1 M Na2SO4 SE, pH = 7, C0 = 50 mg L−1, 120 min | COD (68.89%) | — | 125 |
| g-C3N4/BiVO4 | Diclofenac sodium | 1 V, C0 = 10 mg L−1, 0.5 M KH2PO4, 2 h | HPLC (29.4%) | 3.13 × 10−3 min−1 | 202 |
| Ag3PO4/BiVO4 | Norfloxacin | 0.5 V, C0 = 5 mg L−1, 10 mM NaClO4, 90 min | HPLC (100%) | 2.63 × 10−3 min−1 | 203 |
| Co–ZnO | Ofloxacin | 0.8 V, C0 = 10 mg L−1, 50 mL, 0.1 M Na2SO4, 6 h | HPLC (86.7%) | — | 108 |
| α-Fe2O3/ZnO | 4-Chlorophenol (4-CP), diclofenac (DCF) | 1.0 V, C0 = 10 mg L−1, 50 mL, 0.1 M Na2SO4, 10 h | HPLC (4-CP, (72.3 ± 0.6)%, DCF, (84.6 ± 0.6)%) | 2.02 × 10−3 min−1 (4-CP), 2.86 × 10−3 min−1 (DCF) | 107 |
| g-C3N4@ZnO | Phenol | 1.5 V, C0 = 5 mg L−1, 0.1 M Na2SO4, 3 h | HPLC (97.3%) | 1.216 h−1 | 204 |
| WO3/ZnO | Phthalic acid | 1.5 V, C0 = 1 mM, 320 min | COD (63.63%) | 7.17 × 10−8 s−1 | 205 |
| BiVO4/ZnO | Tetracycline | 0.8 V, C0 = 10 mg L−1, 80 mL, 0.1 M Na2SO4, 2 h | HPLC (90%) | 0.00867 min−1 | 109 |
| BiVO4/ZnO | Tetracycline | 0.8 V, C0 = 20 mg L−1, 0.1 M Na2SO4, 2 h | HPLC (66.1%) | 0.00867 min−1 | 206 |
| γ-Bi2MoO6 | Diclofenac sodium | 1.5 V (SCE), C0 = 10 mg L−1, pH = 5.62, 120 min | HPLC (19.4%) | 0.1112 h−1 | 207 |
| BiOI@rGO | Rhodamine B (RhB), methyl orange (MO), phenol | 2 V, 0.5 M Na2SO4 SE, C0 = 5 mg L−1, 180 min | UV-vis (RhB-85%, MO-70.1%), HPLC (phenol-65.9%, 240 min) | — | 208 |
| CdS/ITO | Bisphenol A | 0.8 V (Ag/AgCl), 0.05 mol L−1 NaCl, C0 = 0.1 mmol L−1, 20 min | Differential pulse voltammetry (94.1%) | 209 | |
| Bi2WO6/CA | Nonylphenol | +0.6 V (SCE), C0 = 1 mg L−1 pH 13, 8 h | HPLC (99.3%) | — | 210 |
| In2O3/In2S3/CdS | 3-Fluoro-3-methylphenol | 0.6 V (Ag/AgCl), 0.1 mol L−1 Na2SO4, C0 = 50 mg L−1, 180 min | UV-vis (87.3%) | 0.279 h−1 | 211 |
| Au/CuI | Methylene blue | 0.2 V, C0 = 5 mg L−1, 0.1 M Na2SO4, 2 h | UV-vis (95%) | 1.48 h−1 | 212 |
| Cu2O/α-Fe2O3 | Oxytetracycline | 0.5 V, C0 = 10 mg L−1, 50 mL, 0.1 M H2SO4, 60 min | HPLC (73.3%) | 2.14 × 10−2 min−1 | 213 |
| Bi2O3–BiOI | Phenol | 2.5 V, C0 = 10 mg L−1, 0.2 M Na2SO4, 3 h | HPLC (80%) | 0.577 h−1 | 154 |
It is of note that many works on solar light photoelectrocatalysis for the removal of organic contaminants from water employed TiO2-based anodes (Table 1). This implies that there is an ever increasing drive towards improving the visible-light sensitivity of this unrivalled metal oxide photocatalyst. Notably, catalysts such as BiVO4, WO3, Fe2O3, CdS, ZnSe, MoS2, g-C3N4 and Cu2O which have relatively narrower band gaps, and are capable of better absorption of visible light, are being coupled to this photocatalyst to fabricate electrodes which retain the photo-efficiency and many other desirable properties of TiO2 outlined section 3.1. Even though visible-light active photocatalysts such as BiVO4, WO3etc. are receiving attention in many photocatalytic applications including photoelectrocatalysis for water splitting for hydrogen generation and destruction of organic pollutants in water, it is envisaged that research efforts will still be directed towards obtaining a TiO2-based photoelectrodes with impressive performance under visible irradiation for these applications for many years to come. The fate of ZnO as a candidate for fabricating photoanodes for degradation of organics in water will hinge on not only improving its activity under solar light but also coming up with practicable synthesis and modification approach(es) to enhance its photostability in water. It is also interesting to note that other much less utilised catalysts such as bismuth-based catalysts and those found towards the end of Table 1 (and in composites with the well-known ones) are also being advanced for photoelectrocatalysis. It may be necessary to carry out more fundamental investigations to gain insights into the properties of these upcoming photocatalysts.
6.0 Perspective and conclusion
Research in the past six years have shown that photoelectrocatalytic process holds huge potential for the removal of organic pollutants from water. This coupled process has been shown to possess advantages over the individual processes of heterogeneous photocatalysis and electrolysis. Many works have reported more efficient degradation of pollutants by photoelectrocatalysis compared to electrolysis and photocatalysis under similar experimental conditions. Since one of the most important component of the photoelectrocatalytic setup is the anodic material, a lot of efforts are being put into developing catalysts which can give optimal performance under mild conditions. In particular, photocatalytic materials which are capable of light absorption in the visible region of the solar spectrum are being advanced. In this regard, various approaches are being utilised to improve the light absorption properties of traditionally UV-active catalysts such as TiO2 and ZnO. Visible-light active materials such as BiVO4, WO3etc. are also gaining tremendous attention as suitable anodic materials for photoelectrocatalytic degradation of organic pollutants. Without doubt significant advances have been made in the exploration of this water treatment technique. However, for the eventual realisation of the potentials of this technique and practical application, serious attention needs to be given to the stability and lifespan of the photoelectrodes. Methods that ensure strong adherence of the photocatalytic materials to the conductive substrates should be adopted. Furthermore, since a typical wastewater contains a variety of substances, some of which are capable of ‘poisoning’ the catalysts, workable approaches for regenerating the catalyst surface to obtain a fairly durable electrode must be considered. For real applications, treatment reactors must be designed to harness direct sunlight energy, especially in countries where this is abundant. Furthermore, other advanced oxidation processes such as the Fenton process can be incorporated into photoelectrocatalysis to maximise degradation efficiencies of the organic contaminants. This will lead to a shorter treatment time and possibly a reduced energy consumption and therefore a lower treatment cost.It is noteworthy that heavy metal ions which are often present in wastewater alongside organic pollutants can be removed at the cathode of a reactor via photoelectrocatalytic reduction if the operational conditions are appropriately optimised. There are a few recent works in this area.214–218 A combination of photoelectrocatalytic cathode (for reduction of inorganics) and photoelectrochemical anode (for organics) can be a plausible route of investigation for a more robust approach to water treatment.
Photoelectrocatalytic process for water treatment is still evolving, there are already works which have considered the possibilities of developing a single photoelectrocatalytic reactor for the dual purposes of water treatment and energy generation using visible-light sensitive materials. This interesting pursuit, if well developed and perfected, will undoubtedly serve to meet the environmental and energy needs of man utilising sunlight.
Conflicts of interest
There are no conflicts of interest.Acknowledgements
Financial supports from the DST/Mintek Nanotechnology Innovation Centre, University of Johannesburg; the National Research Foundations, South Africa (Grant Number: 98887); the Faculty of Science, University of Johannesburg and the Centre for Nanomaterials Science Research, University of Johannesburg are gratefully acknowledged.References
- M. A. Oturan and J.-J. Aaron, Advanced oxidation processes in water/wastewater treatment: principles and applications. A review, Crit. Rev. Environ. Sci. Technol., 2014, 44, 2577–2641 CrossRef
.
-
S. Malato, P. Fernández-Ibáñez, M. Maldonado and I. Oller, Solar Photocatalytic Processes: Water Decontamination and Disinfection, New and Future Developments in Catalysis, Elsevier, Amsterdam, Netherlands, 2013, pp. 371–393 Search PubMed
.
- O. Rodriguez, J. M. Peralta-Hernandez, A. Goonetilleke and E. R. Bandala, Treatment Technologies for Emerging Contaminants in water: A review, Chem. Eng. J., 2017, 323, 361–380 CrossRef
.
- M. Tokumura, A. Sugawara, M. Raknuzzaman, M. Habibullah-Al-Mamun and S. Masunaga, Comprehensive study on effects of water matrices on removal of pharmaceuticals by three different kinds of advanced oxidation processes, Chemosphere, 2016, 159, 317–325 CrossRef PubMed
.
- M. Sillanpää, M. C. Ncibi and A. Matilainen, Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review, J. Environ. Manage., 2018, 208, 56–76 CrossRef PubMed
.
- G. Boczkaj and A. Fernandes, Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review, Chem. Eng. J., 2017, 320, 608–633 CrossRef
.
- B. Bethi, S. H. Sonawane, B. A. Bhanvase and S. P. Gumfekar, Nanomaterials-based advanced oxidation processes for wastewater treatment: A review, Chem. Eng. Process.: Process Intesif., 2016, 109, 178–189 CrossRef
.
- S. Garcia-Segura and E. Brillas, Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters, J. Photochem. Photobiol., C, 2017, 31, 1–35 CrossRef
.
- F. C. Moreira, R. A. Boaventura, E. Brillas and V. J. Vilar, Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters, Appl. Catal., B, 2017, 202, 217–261 CrossRef
.
- A. Fujishima and K. Honda, Electrochemical photolysis of water at a semiconductor electrode, Nature, 1972, 238, 37–38 CrossRef PubMed
.
- R. Daghrir, P. Drogui and D. Robert, Modified TiO2 for environmental photocatalytic applications: a review, Ind. Eng. Chem. Res., 2013, 52, 3581–3599 CrossRef
.
-
M. G. Antoniou, C. Zhao, K. E. O'Shea, G. Zhang, D. D. Dionysiou, C. Zhao, C. Han, M. N. Nadagouda, H. Choi, T. Fotiou, T. M. Triantis and A. Hiskia, in Photocatalysis: Applications, The Royal Society of Chemistry, 2016, pp. 1–34 Search PubMed
.
- H. Liang, Z. Jia, H. Zhang, X. Wang and J. Wang, Photocatalysis oxidation activity regulation of Ag/TiO2 composites evaluated by the selective oxidation of Rhodamine B, Appl. Surf. Sci., 2017, 422, 1–10 CrossRef
.
- Y. Hu, W. Chen, J. Fu, M. Ba, F. Sun, P. Zhang and J. Zou, Hydrothermal synthesis of BiVO4/TiO2 composites and their application for degradation of gaseous benzene under visible light irradiation, Appl. Surf. Sci., 2018, 436, 319–326 CrossRef
.
- A. Di Paola, E. García-López, G. Marcì and L. Palmisano, A survey of photocatalytic materials for environmental remediation, J. Hazard. Mater., 2012, 211, 3–29 CrossRef PubMed
.
- L. G. Devi and R. Kavitha, Review on modified N–TiO 2 for green energy applications under UV/visible light: selected results and reaction mechanisms, RSC Adv., 2014, 4, 28265–28299 RSC
.
- P. K. Sharma, M. A. L. Cortes, J. W. Hamilton, Y. Han, J. A. Byrne and M. Nolan, Surface modification of TiO2 with copper clusters for band gap narrowing, Catal. Today, 2017 DOI:10.1016/j.cattod.2017.12.002
.
- N. Rahimi, R. A. Pax and E. M. Gray, Review of functional titanium oxides. I: TiO2 and its modifications, Prog. Solid State Chem., 2016, 44, 86–105 CrossRef
.
- B. Sun, G. Zhou, T. Gao, H. Zhang and H. Yu, NiO nanosheet/TiO2 nanorod-constructed p–n heterostructures for improved photocatalytic activity, Appl. Surf. Sci., 2016, 364, 322–331 CrossRef
.
- F. Petronella, A. Truppi, T. Sibillano, C. Giannini, M. Striccoli, R. Comparelli and M. L. Curri, Multifunctional TiO2/FexOy/Ag based nanocrystalline heterostructures for photocatalytic degradation of a recalcitrant pollutant, Catal. Today, 2017, 284, 100–106 CrossRef
.
- W. Zhou, Y. Leng, D. Hou, H. Li, L. Li, G. Li, H. Liu and S. Chen, Phase transformation and enhanced photocatalytic activity of S-doped Ag2O/TiO2 heterostructured nanobelts, Nanoscale, 2014, 6, 4698–4704 RSC
.
- X. Zhang, Y. Wang, B. Liu, Y. Sang and H. Liu, Heterostructures construction on TiO2 nanobelts: A powerful tool for building high-performance photocatalysts, Appl. Catal., B, 2017, 202, 620–641 CrossRef
.
- E. Brillas and C. A. Martínez-Huitle, Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review, Appl. Catal., B, 2015, 166, 603–643 CrossRef
.
- J. B. Rocha, M. S. Gomes, E. V. dos Santos, E. M. de Moura, D. R. da Silva, M. Quiroz and C. Martínez-Huitle, Electrochemical degradation of Novacron Yellow C-RG using boron-doped diamond and platinum anodes: Direct and Indirect oxidation, Electrochim. Acta, 2014, 140, 419–426 CrossRef
.
- B. P. Chaplin, Critical review of electrochemical advanced oxidation processes for water treatment applications, Environ. Sci.: Processes Impacts, 2014, 16, 1182–1203 RSC
.
- T. González, J. R. Domínguez, P. Palo, J. Sánchez-Martín and E. M. Cuerda-Correa, Development and optimization of the BDD-electrochemical oxidation of the antibiotic trimethoprim in aqueous solution, Desalination, 2011, 280, 197–202 CrossRef
.
- E. Zarei and R. Ojani, Fundamentals and some applications of photoelectrocatalysis and effective factors on its efficiency: a review, J. Solid State Electrochem., 2017, 21, 305–336 CrossRef
.
- Y. Zhang, X. Xiong, Y. Han, X. Zhang, F. Shen, S. Deng, H. Xiao, X. Yang, G. Yang and H. Peng, Photoelectrocatalytic degradation of recalcitrant organic pollutants using TiO2 film electrodes: an overview, Chemosphere, 2012, 88, 145–154 CrossRef PubMed
.
- S. Garcia-Segura and E. Brillas, Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters, J. Photochem. Photobiol., C, 2017, 31, 1–35 CrossRef
.
- G. G. Bessegato, T. T. Guaraldo, J. F. de Brito, M. F. Brugnera and M. V. B. Zanoni, Achievements and trends in photoelectrocatalysis: from environmental to energy applications, Electrocatalysis, 2015, 6, 415–441 CrossRef
.
- T. Ochiai and A. Fujishima, Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification, J. Photochem. Photobiol., C, 2012, 13, 247–262 CrossRef
.
- R. Daghrir, P. Drogui and D. Robert, Photoelectrocatalytic technologies for environmental applications, J. Photochem. Photobiol., A, 2012, 238, 41–52 CrossRef
.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Environmental applications of semiconductor photocatalysis, Chem. Rev., 1995, 95, 69–96 CrossRef
.
- E. R. Carraway, A. J. Hoffman and M. R. Hoffmann, Photocatalytic oxidation of organic acids on quantum-sized semiconductor colloids, Environ. Sci. Technol., 1994, 28, 786–793 CrossRef PubMed
.
- M. Grela, M. Coronel and A. Colussi, Quantitative spin-trapping studies of weakly illuminated titanium dioxide sols. Implications for the mechanism of photocatalysis, J. Phys. Chem., 1996, 100, 16940–16946 CrossRef
.
- C. D. Jaeger and A. J. Bard, Spin trapping and electron spin resonance detection of radical intermediates in the photodecomposition of water at titanium dioxide particulate systems, J. Phys. Chem., 1979, 83, 3146–3152 CrossRef
.
- H. Czili and A. Horváth, Applicability of coumarin for detecting and measuring hydroxyl radicals generated by photoexcitation of TiO2 nanoparticles, Appl. Catal., B, 2008, 81, 295–302 CrossRef
.
- Y. Jing and B. P. Chaplin, Mechanistic Study of the Validity of Using Hydroxyl Radical Probes To Characterize Electrochemical Advanced Oxidation Processes, Environ. Sci. Technol., 2017, 51, 2355–2365 CrossRef PubMed
.
- J. Farner Budarz, A. Turolla, A. F. Piasecki, J.-Y. Bottero, M. Antonelli and M. R. Wiesner, Influence of aqueous inorganic anions on the reactivity of nanoparticles in TiO2 photocatalysis, Langmuir, 2017, 33, 2770–2779 CrossRef PubMed
.
- S. Issarapanacheewin, K. Wetchakun, S. Phanichphant, W. Kangwansupamonkon and N. Wetchakun, Efficient photocatalytic degradation of Rhodamine B by a novel CeO2/Bi2WO6 composite film, Catal. Today, 2016, 278, 280–290 CrossRef
.
- K.-i. Ishibashi, A. Fujishima, T. Watanabe and K. Hashimoto, Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique, Electrochem. Commun., 2000, 2, 207–210 CrossRef
.
- T. Charbouillot, M. Brigante, G. Mailhot, P. R. Maddigapu, C. Minero and D. Vione, Performance and selectivity of the terephthalic acid probe for OH as a function of temperature, pH and composition of atmospherically relevant aqueous media, J. Photochem. Photobiol., A, 2011, 222, 70–76 CrossRef
.
- Q. Xiao and L. Ouyang, Photocatalytic activity and hydroxyl radical formation of carbon-doped TiO2 nanocrystalline: effect of calcination temperature, Chem. Eng. J., 2009, 148, 248–253 CrossRef
.
- D. Robert and S. Malato, Solar photocatalysis: a clean process for water detoxification, Sci. Total Environ., 2002, 291, 85–97 CrossRef PubMed
.
- M. G. Peleyeju, E. H. Umukoro, L. Tshwenya, R. Moutloali, J. O. Babalola and O. A. Arotiba, Photoelectrocatalytic water treatment systems: degradation, kinetics and intermediate products studies of sulfamethoxazole on a TiO2–exfoliated graphite electrode, RSC Adv., 2017, 7, 40571–40580 RSC
.
- J. Yajun, Growth mechanism and photocatalytic performance of double-walled and bamboo-type TiO2 nanotube arrays, RSC Adv., 2014, 4, 40474–40481 RSC
.
- C. Adán, J. Marugán, E. Sánchez, C. Pablos and R. van Grieken, Understanding the effect of morphology on the photocatalytic activity of TiO2 nanotube array electrodes, Electrochim. Acta, 2016, 191, 521–529 CrossRef
.
- J. Dong, J. Han, Y. Liu, A. Nakajima, S. Matsushita, S. Wei and W. Gao, Defective black TiO2 synthesized via anodization for visible-light photocatalysis, ACS Appl. Mater. Interfaces, 2014, 6, 1385–1388 CrossRef PubMed
.
- S. Joseph and P. Sagayaraj, A cost effective approach for developing substrate stable TiO2 nanotube arrays with tuned morphology: a comprehensive study on the role of H2O2 and anodization potential, New J. Chem., 2015, 39, 5402–5409 RSC
.
- H. Lee, T.-H. Park and D.-J. Jang, Preparation of anatase TiO2 nanotube arrays dominated by highly reactive facets via anodization for high photocatalytic performances, New J. Chem., 2016, 40, 8737–8744 RSC
.
- R. S. Hyam and D. Choi, Effects of titanium foil thickness on TiO2 nanostructures synthesized by anodization, RSC Adv., 2013, 3, 7057–7063 RSC
.
- D. Jia, Z. Qi, X. Li, L. Li, L.-H. Shao and H. Liu, 3D hierarchical macro/mesoporous TiO2 with nanoporous or nanotubular structures and their core/shell composites achieved by anodization, CrystEngComm, 2017, 19, 2509–2516 RSC
.
- A. Apolinario, C. Sousa, J. Ventura, J. Costa, D. Leitao, J. Moreira, J. Sousa, L. Andrade, A. Mendes and J. Araujo, The role of the Ti surface roughness in the self-ordering of TiO2 nanotubes: a detailed study of the growth mechanism, J. Mater. Chem. A, 2014, 2, 9067–9078 RSC
.
- S. Rani, S. C. Roy, M. Paulose, O. K. Varghese, G. K. Mor, S. Kim, S. Yoriya, T. J. LaTempa and C. Grimes, Synthesis and applications of electrochemically self-assembled titania nanotube arrays, Phys. Chem. Chem. Phys., 2010, 12, 2780–2800 RSC
.
- K. Gulati, A. Santos, D. Findlay and D. Losic, Optimizing anodization conditions for the growth of titania nanotubes on curved surfaces, J. Phys. Chem. C, 2015, 119, 16033–16045 CrossRef
.
- Y. Sun, Q. Zhao, G. Wang and K. Yan, Influence of water content on the formation of TiO 2 nanotubes and photoelectrochemical hydrogen generation, J. Alloys Compd., 2017, 711, 514–520 CrossRef
.
- H. Song, K. Cheng, H. Guo, F. Wang, J. Wang, N. Zhu, M. Bai and X. Wang, Effect of ethylene glycol concentration on the morphology and catalytic properties of TiO2 nanotubes, Catal. Commun., 2017, 97, 23–26 CrossRef
.
- N. Nyein, W. K. Tan, G. Kawamura, A. Matsuda and Z. Lockman, TiO2 nanotube arrays formation in fluoride/ethylene glycol electrolyte containing LiOH or KOH as photoanode for dye-sensitized solar cell, J. Photochem. Photobiol., A, 2017, 343, 33–39 CrossRef
.
- Y. Wong, M. Affendy, S. Lau, P. Teh, H. Lee, C. Tan and S. Ramesh, Effects of anodisation parameters on thin film properties: a review, Mater. Sci. Technol., 2017, 33, 699–711 CrossRef
.
- G. Ali, C. Chen, S. H. Yoo, J. M. Kum and S. O. Cho, Fabrication of complete titania nanoporous structures via electrochemical anodization of Ti, Nanoscale Res. Lett., 2011, 6, 332 CrossRef PubMed
.
- X. Nie, J. Chen, G. Li, H. Shi, H. Zhao, P. K. Wong and T. An, Synthesis and characterization of TiO2 nanotube photoanode and its application in photoelectrocatalytic degradation of model environmental pharmaceuticals, J. Chem. Technol. Biotechnol., 2013, 88, 1488–1497 CrossRef
.
- C. Ampelli, F. Tavella, S. Perathoner and G. Centi, Engineering of photoanodes based on ordered TiO2-nanotube arrays in solar photo-electrocatalytic (PECa) cells, Chem. Eng. J., 2017, 320, 352–362 CrossRef
.
- J. Wu, H. Xu and W. Yan, Photoelectrocatalytic degradation rhodamine B over highly ordered TiO2 nanotube arrays photoelectrode, Appl. Surf. Sci., 2016, 386, 1–13 CrossRef
.
- C. Pablos, J. Marugán, R. van Grieken, C. Adán, A. Riquelme and J. Palma, Correlation between photoelectrochemical behaviour and photoelectrocatalytic activity and scaling-up of P25-TiO2 electrodes, Electrochim. Acta, 2014, 130, 261–270 CrossRef
.
- M. M. Islam and S. Basu, Understanding photoelectrochemical degradation of methyl orange using TiO2/Ti mesh as photocathode under visible light, J. Environ. Chem. Eng., 2016, 4, 3554–3561 CrossRef
.
- P. Kajitvichyanukul and P. Amornchat, Effects of diethylene glycol on TiO2 thin film properties prepared by sol–gel process, Sci. Technol. Adv. Mater., 2005, 6, 344–347 CrossRef
.
- T. N. M. Cervantes, D. A. M. Zaia, G. J. Moore and H. de Santana, Photoelectrocatalysis Study of the Decolorization of Synthetic Azo Dye Mixtures on Ti/TiO2, Electrocatalysis, 2013, 4, 85–91 CrossRef
.
- C.-F. Liu, C. Huang, C.-C. Hu, Y. Juang and C. Huang, Photoelectrochemical degradation of dye wastewater on TiO2-coated titanium electrode prepared by electrophoretic deposition, Sep. Purif. Technol., 2016, 165, 145–153 CrossRef
.
- X. Zhou, X. Zhang, X. Feng, J. Zhou and S. Zhou, Preparation of a La/N co-doped TiO2 film electrode with visible light response and its photoelectrocatalytic activity on a Ni substrate, Dyes Pigm., 2016, 125, 375–383 CrossRef
.
- F. J. Mancilla, S. F. Rojas, A. F. Gualdrón-Reyes, M. I. Carreño-Lizcano, L. J. Duarte and M. Niño-Gómez, Improving the photoelectrocatalytic performance of boron-modified TiO2/Ti sol–gel-based electrodes for glycerol oxidation under visible illumination, RSC Adv., 2016, 6, 46668–46677 RSC
.
- D. Wang, X. Li, J. Chen and X. Tao, Enhanced visible-light photoelectrocatalytic degradation of organic contaminants at iodine-doped titanium dioxide film electrode, Ind. Eng. Chem. Res., 2011, 51, 218–224 CrossRef
.
- H. G. Oliveira, L. H. Ferreira, R. Bertazzoli and C. Longo, Remediation of 17-α-ethinylestradiol aqueous solution by photocatalysis and electrochemically-assisted photocatalysis using TiO2 and TiO2/WO3 electrodes irradiated by a solar simulator, Water Res., 2015, 72, 305–314 CrossRef PubMed
.
- C. Montero-Ocampo, A. Gago, G. Abadias, B. Gombert and N. Alonso-Vante, In situ photoelectrochemical/photocatalytic study of a dye discoloration in a microreactor system using TiO2 thin films, Environ. Sci. Pollut. Res., 2012, 19, 3751–3762 CrossRef PubMed
.
- R. Daghrir, P. Drogui, N. Delegan and M. A. El Khakani, Electrochemical degradation of chlortetracycline using N-doped Ti/TiO2 photoanode under sunlight irradiations, Water Res., 2013, 47, 6801–6810 CrossRef PubMed
.
- C. W. Yeh and K. R. Wu, Photoelectrocatalytic activity of Cu2O films prepared on different substrates in connection with TiO2 electrodes, Adv. Mater. Res., 2013, 774–776, 987–991 Search PubMed
.
- M. Qamar, Q. Drmosh, M. I. Ahmed, M. Qamaruddin and Z. H. Yamani, Enhanced photoelectrochemical and photocatalytic activity of WO3-surface modified TiO2 thin film, Nanoscale Res. Lett., 2015, 10, 54 CrossRef PubMed
.
- R. Daghrir, P. Drogui, N. Delegan and M. A. El Khakani, Removal of chlortetracycline from spiked municipal wastewater using a photoelectrocatalytic process operated under sunlight irradiations, Sci. Total Environ., 2014, 466, 300–305 CrossRef PubMed
.
- S. Han, X. Zhang, Q. Yu and L. Lei, Preparation of B-doped TiO2/ITO film electrode by MOCVD and its visible-lightdriven photoelectrocatalytic properties, J. Chem. Eng. Chin. Univ., 2013, 27, 488–493 Search PubMed
.
- C.-Y. Chang, Y.-H. Hsieh and Y.-Y. Chen, Photoelectrocatalytic degradation of sodium oxalate by TiO2/Ti thin film electrode, Int. J. Photoenergy, 2012, 2012, 576089 Search PubMed
.
- S. Han, X. Zhang, Q. Yu and L. Lei, Preparation of TiO2/ITO film electrode by AP-MOCVD for photoelectrocatalytic application, Sci. China: Chem., 2012, 1–9 CrossRef
.
- M. Zhang, W. Pu, S. Pan, O. K. Okoth, C. Yang and J. Zhang, Photoelectrocatalytic activity of liquid phase deposited α-Fe2O3 films under visible light illumination, J. Alloys Compd., 2015, 648, 719–725 CrossRef
.
- H. Maki, Y. Okumura, H. Ikuta and M. Mizuhata, Ionic equilibria for synthesis of TiO2 thin films by the liquid-phase deposition, J. Phys. Chem. C, 2014, 118, 11964–11974 CrossRef
.
- S. Deki, A. Nakata, Y. Sakakibara and M. Mizuhata, Deposition of Metal Oxide Films at Liquid− Liquid Interface by the Liquid Phase Deposition Method, J. Phys. Chem. C, 2008, 112, 13535–13539 CrossRef
.
- L. Dan, H.-x. Tong and L. Zhang, Preparation of TiO2/ITO film by liquid phase deposition and its photoelectrocatalytic activity for degradation of 4-aminoantipyrine, Trans. Nonferrous Met. Soc. China, 2013, 23, 3306–3311 CrossRef
.
- T.-F. Wu, W.-C. Lin, Y.-C. Hsiao, C.-H. Su, C.-C. Hu, C.-P. Huang and R. Muniyandi, Electrochemical Photocatalytic Degradation of Orange G Using TiO2 Films Prepared by Cathodic Deposition, J. Electrochem. Soc., 2014, 161, H762–H769 CrossRef
.
- L. Özcan, S. Yurdakal, V. Augugliaro, V. Loddo, S. Palmas, G. Palmisano and L. Palmisano, Photoelectrocatalytic selective oxidation of 4-methoxybenzyl alcohol in water by TiO2 supported on titanium anodes, Appl. Catal., B, 2013, 132, 535–542 CrossRef
.
- D. Li, X. Cheng, X. Yu and Z. Xing, Preparation and characterization of TiO2-based nanosheets for photocatalytic degradation of acetylsalicylic acid: influence of calcination temperature, Chem. Eng. J., 2015, 279, 994–1003 CrossRef
.
- B. Ntsendwana, S. Sampath, B. Mamba and O. Arotiba, Photoelectrochemical oxidation of p-nitrophenol on an expanded graphite–TiO2 electrode, Photochem. Photobiol. Sci., 2013, 12, 1091–1102 RSC
.
- G. Palmisano, V. Loddo, H. H. El Nazer, S. Yurdakal, V. Augugliaro, R. Ciriminna and M. Pagliaro, Graphite-supported TiO2 for 4-nitrophenol degradation in a photoelectrocatalytic reactor, Chem. Eng. J., 2009, 155, 339–346 CrossRef
.
- D. Li, J. Jia, Y. Zhang, N. Wang, X. Guo and X. Yu, Preparation and characterization of Nano-graphite/TiO 2 composite photoelectrode for photoelectrocatalytic degradation of hazardous pollutant, J. Hazard. Mater., 2016, 315, 1–10 CrossRef PubMed
.
- A. Janotti and C. G. Van de Walle, Fundamentals of zinc oxide as a semiconductor, Rep. Prog. Phys., 2009, 72, 126501 CrossRef
.
- Q. Cheng, M. K. Benipal, Q. Liu, X. Wang, P. A. Crozier, C. K. Chan and R. J. Nemanich, Al2O3 and SiO2 Atomic Layer Deposition Layers on ZnO Photoanodes and Degradation Mechanisms, ACS Appl. Mater. Interfaces, 2017, 9, 16138–16147 CrossRef PubMed
.
- R. Georgekutty, M. K. Seery and S. C. Pillai, A highly efficient Ag-ZnO photocatalyst: synthesis, properties, and mechanism, J. Phys. Chem. C, 2008, 112(35), 13563–13570 CrossRef
.
- Z.-Q. Liu, P.-Y. Kuang, R.-B. Wei, N. Li, Y.-B. Chen and Y.-Z. Su, BiOBr nanoplate-wrapped ZnO nanorod arrays for high performance photoelectrocatalytic application, RSC Adv., 2016, 6, 16122–16130 RSC
.
- H. Zhang, R. Zong and Y. Zhu, Photocorrosion inhibition and photoactivity enhancement for zinc oxide via hybridization with monolayer polyaniline, J. Phys. Chem. C, 2009, 113, 4605–4611 CrossRef
.
- Q. Huang and J. Li, Enhanced photocatalytic and SERS properties of ZnO/Ag hierarchical multipods-shaped nanocomposites, Mater. Lett., 2017, 204, 85–88 CrossRef
.
- N. Kumaresan, K. Ramamurthi, R. R. Babu, K. Sethuraman and S. M. Babu, Hydrothermally grown ZnO nanoparticles for effective photocatalytic activity, Appl. Surf. Sci., 2017, 418, 138–146 CrossRef
.
- X. Yi, C. Ma, F. Yuan, N. Wang, F. Qin, B. Hu and Q. Zhang, Structural, morphological, photoluminescence and photocatalytic properties of Gd-doped ZnO films, Thin Solid Films, 2017, 636, 339–345 CrossRef
.
- K. M. Lee, C. W. Lai, K. S. Ngai and J. C. Juan, Recent developments of zinc oxide based photocatalyst in water treatment technology: a review, Water Res., 2016, 88, 428–448 CrossRef PubMed
.
- M. C. Huang, J. C. Lin, S. H. Cheng and W. H. Weng, Influence of Ga dopant on photoelectrochemical characteristic of Ga-doped ZnO thin films deposited by sol–gel spin-coating technique, Surf. Interface Anal., 2017, 49, 434–440 CrossRef
.
- A. Shetty and K. K. Nanda, Synthesis of zinc oxide porous structures by anodization with water as an electrolyte, Appl. Phys. A: Mater. Sci. Process., 2012, 109, 151–157 CrossRef
.
- P. Basu, N. Saha, S. Maji, H. Saha and S. Basu, Nanoporous ZnO thin films deposited by electrochemical anodization: effect of UV light, J. Mater. Sci.: Mater. Electron., 2008, 19, 493–499 CrossRef
.
- S.-L. Lee, L.-N. Ho, S.-A. Ong, Y.-S. Wong, C.-H. Voon, W. F. Khalik, N. A. Yusoff and N. Nordin, Enhanced electricity generation and degradation of the azo dye Reactive Green 19 in a photocatalytic fuel cell using ZnO/Zn as the photoanode, J. Cleaner Prod., 2016, 127, 579–584 CrossRef
.
- A. Ramirez-Canon, D. O. Miles, P. J. Cameron and D. Mattia, Zinc oxide nanostructured films produced via anodization: a rational design approach, RSC Adv., 2013, 3, 25323–25330 RSC
.
- R. Sapkal, S. Shinde, M. Mahadik, V. Mohite, T. Waghmode, S. Govindwar, K. Rajpure and C. Bhosale, Photoelectrocatalytic decolorization and degradation of textile effluent using ZnO thin films, J. Photochem. Photobiol., B, 2012, 114, 102–107 CrossRef PubMed
.
- M. Fan, C. Yang, W. Pu and J. Zhang, Liquid phase deposition of ZnO film for photoelectrocatalytic degradation of p-nitrophenol, Mater. Sci. Semicond. Process., 2014, 17, 104–109 CrossRef
.
- L. Cheng, L. Liu, R. Li and J. Zhang, Liquid phase deposition of α-Fe2O3/ZnO heterojunction film with enhanced visible-light photoelectrocatalytic activity for pollutant removal, J. Electrochem. Soc., 2017, 164, H726–H733 CrossRef
.
- Y. Tian, L. Cheng and J. Zhang, Fabrication of Co-Doped ZnO Photoanode by Liquid Phase Deposition for Photoelectrocatalytic Degradation of Ofloxacin under Visible Light, J. Electrochem. Soc., 2018, 165, H284–H290 CrossRef
.
- J. Feng, L. Cheng, J. Zhang, O. K. Okoth and F. Chen, Preparation of BiVO4/ZnO composite film with enhanced visible-light photoelectrocatalytic activity, Ceram. Int., 2018, 44, 3672–3677 CrossRef
.
- B. Ntsendwana, S. Sampath, B. Mamba, O. Oluwafemi and O. Arotiba, Photoelectrochemical degradation of eosin yellowish dye on exfoliated graphite–ZnO nanocomposite electrode, J. Mater. Sci.: Mater. Electron., 2016, 27, 592–598 CrossRef
.
- H. Fu, T. Xu, S. Zhu and Y. Zhu, Photocorrosion inhibition and enhancement of photocatalytic activity for ZnO via hybridization with C60, Environ. Sci. Technol., 2008, 42, 8064–8069 CrossRef PubMed
.
- B. Subash, B. Krishnakumar, M. Swaminathan and M. Shanthi, Highly efficient, solar active, and reusable photocatalyst: Zr-loaded Ag–ZnO for reactive red 120 dye degradation with synergistic effect and dye-sensitized mechanism, Langmuir, 2013, 29, 939–949 CrossRef PubMed
.
- M. Nunes, C. Moura, A. R. Hillman and C. Freire, Novel hybrid based on a poly [Ni (salen)] film and WO3 nanoparticles with electrochromic properties, Electrochim. Acta, 2017, 238, 142–155 CrossRef
.
- B. Weng, J. Wu, N. Zhang and Y.-J. Xu, Observing the role of graphene in boosting the two-electron reduction of oxygen in graphene–WO3 nanorod photocatalysts, Langmuir, 2014, 30, 5574–5584 CrossRef PubMed
.
- G. Longobucco, L. Pasti, A. Molinari, N. Marchetti, S. Caramori, V. Cristino, R. Boaretto and C. A. Bignozzi, Photoelectrochemical mineralization of emerging contaminants at porous WO3 interfaces, Appl. Catal., B, 2017, 204, 273–282 CrossRef
.
- J. Zhu, W. Li, J. Li, Y. Li, H. Hu and Y. Yang, Photoelectrochemical activity of NiWO4/WO3 heterojunction photoanode under visible light irradiation, Electrochim. Acta, 2013, 112, 191–198 CrossRef
.
- P. Dong, B. Yang, C. Liu, F. Xu, X. Xi, G. Hou and R. Shao, Highly enhanced photocatalytic activity of WO3 thin films loaded with Pt–Ag bimetallic alloy nanoparticles, RSC Adv., 2017, 7, 947–956 RSC
.
- G. Kim, E. T. Igunnu and G. Z. Chen, A sunlight assisted dual purpose photoelectrochemical cell for low voltage removal of heavy metals and organic pollutants in wastewater, Chem. Eng. J., 2014, 244, 411–421 CrossRef
.
- S. Mohite, V. Ganbavle and K. Rajpure, Solar photoelectrocatalytic activities of rhodamine-B using sprayed WO3 photoelectrode, J. Alloys Compd., 2016, 655, 106–113 CrossRef
.
- E. H. Umukoro, M. G. Peleyeju, J. C. Ngila and O. A. Arotiba, Towards wastewater treatment: Photo-assisted electrochemical degradation of 2-nitrophenol and orange II dye at a tungsten trioxide-exfoliated graphite composite electrode, Chem. Eng. J., 2017, 317, 290–301 CrossRef
.
- V. S. Vidyarthi, M. Hofmann, A. Savan, K. Sliozberg, D. König, R. Beranek, W. Schuhmann and A. Ludwig, Enhanced photoelectrochemical properties of WO3 thin films fabricated by reactive magnetron sputtering, Int. J. Hydrogen Energy, 2011, 36, 4724–4731 CrossRef
.
- K. R. Tolod, S. Hernández and N. Russo, Recent advances in the BiVO4 photocatalyst for sun-driven water oxidation: Top-performing photoanodes and scale-up challenges, Catalysts, 2017, 7, 13 CrossRef
.
- C. M. Suarez, S. Hernández and N. Russo, BiVO4 as photocatalyst for solar fuels production through water splitting: A short review, Appl. Catal., A, 2015, 504, 158–170 CrossRef
.
- B. Anke, M. Rohloff, M. G. Willinger, W. Hetaba, A. Fischer and M. Lerch, Improved photoelectrochemical performance of bismuth vanadate by partial O/F-substitution, Solid State Sci., 2017, 63, 1–8 CrossRef
.
- L. Xia, J. Bai, J. Li, Q. Zeng, L. Li and B. Zhou, High-performance BiVO4 photoanodes cocatalyzed with an ultrathin α-Fe 2 O 3 layer for photoelectrochemical application, Appl. Catal., B, 2017, 204, 127–133 CrossRef
.
- O. Monfort, S. Sfaelou, L. Satrapinskyy, T. Plecenik, T. Roch, G. Plesch and P. Lianos, Comparative study between pristine and Nb-modified BiVO4 films employed for photoelectrocatalytic production of H2 by water splitting and for photocatalytic degradation of organic pollutants under simulated solar light, Catal. Today, 2017, 280, 51–57 CrossRef
.
- X. Qiu, M. Miyauchi, H. Yu, H. Irie and K. Hashimoto, Visible-Light-Driven Cu(II)−(Sr1−yNay)(Ti1−xMox)O3 Photocatalysts Based on Conduction Band Control and Surface Ion Modification, J. Am. Chem. Soc., 2010, 132, 15259–15267 CrossRef PubMed
.
- X. Yan, K. Yuan, N. Lu, H. Xu, S. Zhang, N. Takeuchi, H. Kobayashi and R. Li, The interplay of sulfur doping and surface hydroxyl in band gap engineering: Mesoporous sulfur-doped TiO2 coupled with magnetite as a recyclable, efficient, visible light active photocatalyst for water purification, Appl. Catal., B, 2017, 218, 20–31 CrossRef
.
- C. Peng, W. Wang, W. Zhang, Y. Liang and L. Zhuo, Surface plasmon-driven photoelectrochemical water splitting of TiO2 nanowires decorated with Ag nanoparticles under visible light illumination, Appl. Surf. Sci., 2017, 420, 286–295 CrossRef
.
- Z. Wei, L. Rosa, K. Wang, M. Endo, S. Juodkazis, B. Ohtani and E. Kowalska, Size-controlled gold nanoparticles on octahedral anatase particles as efficient plasmonic photocatalyst, Appl. Catal., B, 2017, 206, 393–405 CrossRef PubMed
.
- E. H. Umukoro, M. G. Peleyeju, J. C. Ngila and O. A. Arotiba, Photocatalytic degradation of acid blue 74 in water using Ag–Ag2O–ZnO nanostuctures anchored on graphene oxide, Solid State Sci., 2016, 51, 66–73 CrossRef
.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Visible-light photocatalysis in nitrogen-doped titanium oxides, Science, 2001, 293, 269–271 CrossRef PubMed
.
- Y. Ma, L. Han, H. Ma, J. Wang, J. Liu, L. Cheng, J. Yang and Q. Zhang, Improving the visible-light photocatalytic activity of interstitial carbon-doped TiO2 with electron-withdrawing bidentate
carboxylate ligands, Catal. Commun., 2017, 95, 1–5 CrossRef
.
- W. Tang, X. Chen, J. Xia, J. Gong and X. Zeng, Preparation of an Fe-doped visible-light-response TiO2 film electrode and its photoelectrocatalytic activity, Mater. Sci. Eng., B, 2014, 187, 39–45 CrossRef
.
- Q. Chen, H. Liu, Y. Xin and X. Cheng, Coupling immobilized TiO2 nanobelts and Au nanoparticles for enhanced photocatalytic and photoelectrocatalytic activity and mechanism insights, Chem. Eng. J., 2014, 241, 145–154 CrossRef
.
- J. Song, X. Wang, J. Huang, J. Ma, X. Wang, H. Wang, R. Ma, P. Xia and J. Zhao, High performance of N-doped TiO2-magnetic activated carbon composites under visible light illumination: Synthesis and application in three-dimensional photoelectrochemical process, Electrochim. Acta, 2016, 222, 1–11 CrossRef
.
- M. Salem, S. Akir, T. Ghrib, K. Daoudi and M. Gaidi, Fe-doping effect on the photoelectrochemical properties enhancement of ZnO films, J. Alloys Compd., 2016, 685, 107–113 CrossRef
.
- M. Salem, I. Massoudi, S. Akir, Y. Litaiem, M. Gaidi and K. Khirouni, Photoelectrochemical and opto-electronic properties tuning of ZnO films: Effect of Cu doping content, J. Alloys Compd., 2017, 722, 313–320 CrossRef
.
- M. Ahmad, E. Ahmed, F. Zafar, N. Khalid, N. Niaz, A. Hafeez, M. Ikram, M. A. Khan and H. Zhanglian, Enhanced photocatalytic activity of Ce-doped ZnO nanopowders synthesized by combustion method, J. Rare Earths, 2015, 33, 255–262 CrossRef
.
- I. Khan, A. A. Ibrahim, M. Sohail and A. Qurashi, Sonochemical assisted synthesis of RGO/ZnO nanowire arrays for photoelectrochemical water splitting, Ultrason. Sonochem., 2017, 37, 669–675 CrossRef PubMed
.
- S. Mohite, V. Ganbavle and K. Rajpure, Photoelectrochemical performance and photoelectrocatalytic degradation of organic compounds using Ga: WO3 thin films, J. Photochem. Photobiol., A, 2017, 344, 56–63 CrossRef
.
- W. Li, F. Zhan, J. Li, C. Liu, Y. Yang, Y. Li and Q. Chen, Enhancing photoelectrochemical water splitting by aluminum-doped plate-like WO3 electrodes, Electrochim. Acta, 2015, 160, 57–63 CrossRef
.
- Y. Liu, Y. Li, W. Li, S. Han and C. Liu, Photoelectrochemical properties and photocatalytic activity of nitrogen-doped nanoporous WO3 photoelectrodes under visible light, Appl. Surf. Sci., 2012, 258, 5038–5045 CrossRef
.
- Q. Wang, J. He, Y. Shi, S. Zhang, T. Niu, H. She and Y. Bi, Designing non-noble/semiconductor Bi/BiVO4 photoelectrode for the enhanced photoelectrochemical performance, Chem. Eng. J., 2017, 326, 411–418 CrossRef
.
- M. Wang, Q. Liu, Y. Che, L. Zhang and D. Zhang, Characterization and photocatalytic properties of N-doped BiVO4 synthesized via a sol–gel method, J. Alloys Compd., 2013, 548, 70–76 CrossRef
.
- W. Luo, Z. Li, T. Yu and Z. Zou, Effects of surface electrochemical pretreatment on the photoelectrochemical performance of Mo-doped BiVO4, J. Phys. Chem. C, 2012, 116, 5076–5081 CrossRef
.
- S. Balachandran, N. Prakash, K. Thirumalai, M. Muruganandham, M. Sillanpää and M. Swaminathan, Facile construction of heterostructured BiVO4–ZnO and its dual application of greater solar photocatalytic activity and self-cleaning property, Ind. Eng. Chem. Res., 2014, 53, 8346–8356 CrossRef
.
- Y. Wang, J. Sun, J. Li and X. Zhao, Electrospinning Preparation of Nanostructured g-C3N4/BiVO4 Composite Films with an Enhanced Photoelectrochemical Performance, Langmuir, 2017, 33, 4694–4701 CrossRef PubMed
.
- Y. Wang, Q. Wang, X. Zhan, F. Wang, M. Safdar and J. He, Visible light driven type II heterostructures and their enhanced photocatalysis properties: a review, Nanoscale, 2013, 5, 8326–8339 RSC
.
- R. Wang, J. Bai, Y. Li, Q. Zeng, J. Li and B. Zhou, BiVO4/TiO2(N2) Nanotubes Heterojunction Photoanode for Highly Efficient Photoelectrocatalytic Applications, Nano-Micro Lett., 2017, 9, 14 CrossRef
.
- T. Guaraldo, V. Goncales, B. Silva, S. de Torresi and M. Zanoni, Hydrogen production and simultaneous photoelectrocatalytic pollutant oxidation using a TiO2/WO3 nanostructured photoanode under visible light irradiation, J. Electroanal. Chem., 2016, 765, 188–196 CrossRef
.
- J. Li, S. Lv, Y. Liu, J. Bai, B. Zhou and X. Hu, Photoeletrocatalytic activity of an n-ZnO/p-Cu2O/n-TNA ternary heterojunction electrode for tetracycline degradation, J. Hazard. Mater., 2013, 262, 482–488 CrossRef PubMed
.
- L. Xia, J. Bai, J. Li, Q. Zeng, X. Li and B. Zhou, A highly efficient BiVO4/WO3/W heterojunction photoanode for visible-light responsive dual photoelectrode photocatalytic fuel cell, Appl. Catal., B, 2016, 183, 224–230 CrossRef
.
- Y. Cong, Y. Ji, Y. Ge, H. Jin, Y. Zhang and Q. Wang, Fabrication of 3D Bi2O3-BiOI heterojunction by a simple dipping method: Highly enhanced visible-light photoelectrocatalytic activity, Chem. Eng. J., 2017, 307, 572–582 CrossRef
.
- C. Gao, Z. Zhang, X. Li, L. Chen, Y. Wang, Y. He, F. Teng, J. Zhou, W. Han and E. Xie, Synergistic effects in three-dimensional SnO2/TiO2/CdS multi-heterojunction structure for highly efficient photoelectrochemical hydrogen production, Sol. Energy Mater. Sol. Cells, 2015, 141, 101–107 CrossRef
.
- F. Zhan, J. Li, W. Li, Y. Liu, R. Xie, Y. Yang, Y. Li and Q. Chen, In situ formation of CuWO4/WO3 heterojunction plates array films with enhanced photoelectrochemical properties, Int. J. Hydrogen Energy, 2015, 40, 6512–6520 CrossRef
.
- P. Guo, J. Jiang, S. Shen and L. Guo, ZnS/ZnO heterojunction as photoelectrode: Type II band alignment towards enhanced photoelectrochemical performance, Int. J. Hydrogen Energy, 2013, 38, 13097–13103 CrossRef
.
- F. Zhan, J. Li, W. Li, Y. Yang, W. Liu and Y. Li, In situ synthesis of CdS/CdWO4/WO3 heterojunction films with enhanced photoelectrochemical properties, J. Power Sources, 2016, 325, 591–597 CrossRef
.
- S. Juntrapirom, D. Tantraviwat, S. Suntalelat, O. Thongsook, S. Phanichphant and B. Inceesungvorn, Visible light photocatalytic performance and mechanism of highly efficient SnS/BiOI heterojunction, J. Colloid Interface Sci., 2017, 504, 711–720 CrossRef PubMed
.
- D. Sharma, V. R. Satsangi, R. Shrivastav, U. V. Waghmare and S. Dass, Understanding the photoelectrochemical properties of nanostructured CeO2/Cu2O heterojunction photoanode for efficient photoelectrochemical water splitting, Int. J. Hydrogen Energy, 2016, 41, 18339–18350 CrossRef
.
- S. Wang, J.-H. Yun, B. Luo, T. Butburee, P. Peerakiatkhajohn, S. Thaweesak, M. Xiao and L. Wang, Recent progress on visible light responsive heterojunctions for photocatalytic applications, J. Mater. Sci. Technol., 2017, 33, 1–22 CrossRef
.
- Q. Wu, R. Han, P. Chen, X. Qi and W. Yao, Novel synthesis and photocatalytic performance of BiVO4 with tunable morphologies and macroscopic structures, Mater. Sci. Semicond. Process., 2015, 38, 271–277 CrossRef
.
- H. Yaghoubi, Z. Li, Y. Chen, H. T. Ngo, V. R. Bhethanabotla, B. Joseph, S. Ma, R. Schlaf and A. Takshi, Toward a visible light-driven photocatalyst: the effect of midgap-states-induced energy gap of undoped TiO2 nanoparticles, ACS Catal., 2014, 5, 327–335 CrossRef
.
- S. Wang, S. Lin, D. Zhang, G. Li and M. K. Leung, Controlling charge transfer in quantum-size titania for photocatalytic applications, Appl. Catal., B, 2017, 215, 85–92 CrossRef
.
- Y. Zhao, R. Li, L. Mu and C. Li, The Significance of Crystal Morphology Controlling in Semiconductor-based Photocatalysis: A Case Study on BiVO4 Photocatalyst, Cryst. Growth Des., 2017, 17(6), 2923–2928 CrossRef
.
- X. Wang, W. Mao, Q. Zhang, Q. Wang, Y. Zhu, J. Zhang, T. Yang, J. Yang, X. A. Li and W. Huang, PVP assisted hydrothermal fabrication and morphology-controllable fabrication of BiFeO3 uniform nanostructures with enhanced photocatalytic activities, J. Alloys Compd., 2016, 677, 288–293 CrossRef
.
- A. Mazzarolo, K. Lee, A. Vicenzo and P. Schmuki, Anodic TiO2 nanotubes: influence of top morphology on their photocatalytic performance, Electrochem. Commun., 2012, 22, 162–165 CrossRef
.
- K. Li, R. Chen, S.-L. Li, S.-L. Xie, X.-L. Cao, L.-Z. Dong, J.-C. Bao and Y.-Q. Lan, Engineering the Morphology and Configuration of Ternary Heterostructures for Improving Their Photocatalytic Activity, ACS Appl. Mater. Interfaces, 2016, 8, 4516–4522 CrossRef PubMed
.
- G. Meenakshi and A. Sivasamy, Synthesis and characterization of zinc oxide nanorods and its photocatalytic activities towards degradation of 2, 4-D, Ecotoxicol. Environ. Saf., 2017, 135, 243–251 CrossRef PubMed
.
- G. Byzynski, C. Melo, D. P. Volanti, M. M. Ferrer, A. F. Gouveia, C. Ribeiro, J. Andrés and E. Longo, The interplay between morphology and photocatalytic activity in ZnO and N-doped ZnO crystals, Mater. Des., 2017, 120, 363–375 CrossRef
.
- Q. Li, X. Sun, K. Lozano and Y. Mao, Dependence of Photoelectrochemical Properties on Geometry Factors of Interconnected “Caterpillar-like” ZnO Networks, Electrochim. Acta, 2016, 222, 232–245 CrossRef
.
- A. Memar, C. M. Phan and M. O. Tade, Controlling particle size and photoelectrochemical properties of nanostructured WO3 with surfactants, Appl. Surf. Sci., 2014, 305, 760–767 CrossRef
.
- J. Zhou, S. Lin, Y. Chen and A. Gaskov, Facile morphology control of WO3 nanostructure arrays with enhanced photoelectrochemical performance, Appl. Surf. Sci., 2017, 403, 274–281 CrossRef
.
- H. Wang, Y. Liang, L. Liu, J. Hu and W. Cui, Highly ordered TiO2 nanotube arrays wrapped with g-C3N4 nanoparticles for efficient charge separation and increased photoelectrocatalytic degradation of phenol, J. Hazard. Mater., 2018, 344, 369–380 CrossRef PubMed
.
- S. Garcia-Segura, S. Dosta, J. M. Guilemany and E. Brillas, Solar photoelectrocatalytic degradation of Acid Orange 7 azo dye using a highly stable TiO2 photoanode synthesized by atmospheric plasma spray, Appl. Catal., B, 2013, 132, 142–150 CrossRef
.
- R. Daghrir, P. Drogui, N. Delegan and M. A. El Khakani, Electrochemical degradation of chlortetracycline using N-doped Ti/TiO2 photoanode under sunlight irradiations, Water Res., 2013, 47, 6801–6810 CrossRef PubMed
.
- D. Liu, R. Tian, J. Wang, E. Nie, X. Piao, X. Li and Z. Sun, Photoelectrocatalytic degradation of methylene blue using F doped TiO2 photoelectrode under visible light irradiation, Chemosphere, 2017, 185, 574–581 CrossRef PubMed
.
- D. Liu, J. Zhou, J. Wang, R. Tian, X. Li, E. Nie, X. Piao and Z. Sun, Enhanced visible light photoelectrocatalytic degradation of organic contaminants by F and Sn co-doped TiO2 photoelectrode, Chem. Eng. J., 2018, 344, 332–341 CrossRef
.
- I. Ali, S.-R. Kim, S.-P. Kim and J.-O. Kim, Anodization of bismuth doped TiO2 nanotubes composite for photocatalytic degradation of phenol in visible light, Catal. Today, 2017, 282, 31–37 CrossRef
.
- Y. Yin, E. Liu, H. Li, J. Wan, J. Fan, X. Hu, J. Li, C. Tang and C. Pu, Fabrication of plasmonic Au/TiO2 nanotube arrays with enhanced photoelectrocatalytic activities, Ceram. Int., 2016, 42, 9387–9395 CrossRef
.
- Y. Yin, J. Li, Y. Wang, J. Wan, X. Du, X. Hu, E. Liu and J. Fan, Constructing ZnSe and Au co-sensitized TiO2 nanotube arrays for high-efficiency photoelectrocatalytic activities, Mater. Res. Bull., 2017, 88, 33–40 CrossRef
.
- M. Zhang, X. Li, Q. Zhao, S. Fan, Z. Jiang and G. Chen, AgInS2 nanoparticles modified TiO2 nanotube array electrodes: Ultrasonic-assisted SILAR preparation and mechanism of enhanced photoelectrocatalytic activity, Mol. Catal., 2017, 442, 97–106 CrossRef
.
- D. Li, J. Jia, T. Zheng, X. Cheng and X. Yu, Construction and characterization of visible light active Pd nano-crystallite decorated and CNS-co-doped TiO2 nanosheet array photoelectrode for enhanced photocatalytic degradation of acetylsalicylic acid, Appl. Catal., B, 2016, 188, 259–271 CrossRef
.
- Q. Wang, Q. Yuan, Z. Liu, R. Jin, Y. Cui and S. Gao, Ultrasound-assisted synthesis and solar-light-driven photoelectrocatalytic activity of CdS sensitized TiO2 nanotube array photocatalysts, Sep. Purif. Technol., 2018, 194, 216–221 CrossRef
.
- X. Deng, H. Zhang, R. Guo, Y. Cui, Q. Ma, X. Zhang, X. Cheng, B. Li, M. Xie and Q. Cheng, Effect of fabricating parameters on photoelectrocatalytic performance of CeO2/TiO2 nanotube arrays photoelectrode, Sep. Purif. Technol., 2018, 193, 264–273 CrossRef
.
- Q. Zhou, A. Xing, J. Li, D. Zhao, K. Zhao and M. Lei, Synergistic enhancement in photoelectrocatalytic degradation of bisphenol A by CeO2 and reduced graphene oxide co-modified TiO2 nanotube arrays in combination with Fenton oxidation, Electrochim. Acta, 2016, 209, 379–388 CrossRef
.
- Q. Zhou, A. Xing, D. Zhao and K. Zhao, Tetrabromobisphenol A photoelectrocatalytic degradation using reduced graphene oxide and cerium dioxide comodified TiO2 nanotube arrays as electrode under visible light, Chemosphere, 2016, 165, 268–276 CrossRef PubMed
.
- W. Tang, Y. Zhang, X. Chen and X. Zeng, Fe2O3/TiO2 film electrodes prepared by the forced hydrolysis method and their photoelectrocatalytic performance, Mater. Lett., 2018, 217, 109–112 CrossRef
.
- A. Watoni, D. Wibowo and M. Nurdin, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 267, 012005 Search PubMed
.
- S. Xie, K. Ouyang, Y. Lao, P. He and Q. Wang, Heterostructured ZnFe2O4/TiO2 nanotube arrays with remarkable visible-light photoelectrocatalytic performance and stability, J. Colloid Interface Sci., 2017, 493, 198–205 CrossRef PubMed
.
- Q. Ma, H. Zhang, Y. Cui, X. Deng, R. Guo, X. Cheng, M. Xie and Q. Cheng, Fabrication of Cu2O/TiO2 nano-tube arrays photoelectrode and its enhanced photoelectrocatalytic performance for degradation of 2, 4, 6-trichlorophenol, J. Ind. Eng. Chem., 2018, 57, 181–187 CrossRef
.
- W. Teng, Y. Wang, H. Huang, X. Li and Y. Tang, Enhanced photoelectrochemical performance of MoS2 nanobelts-loaded TiO2 nanotube arrays by photo-assisted electrodeposition, Appl. Surf. Sci., 2017, 425, 507–517 CrossRef
.
- B. Sun, N. Lu, Y. Su, H. Yu, X. Meng and Z. Gao, Decoration of TiO2 nanotube arrays by graphitic-C3N4 quantum dots with improved photoelectrocatalytic performance, Appl. Surf. Sci., 2017, 394, 479–487 CrossRef
.
- D. Li, J. Jia, Y. Zhang, N. Wang, X. Guo and X. Yu, Preparation and characterization of Nano-graphite/TiO2 composite photoelectrode for photoelectrocatalytic degradation of hazardous pollutant, J. Hazard. Mater., 2016, 315, 1–10 CrossRef PubMed
.
- R. Wang, J. Bai, Y. Li, Q. Zeng, J. Li and B. Zhou, BiVO4/TiO2(N2) nanotubes heterojunction photoanode for highly efficient photoelectrocatalytic applications, Nano-Micro Lett., 2017, 9, 14 CrossRef
.
- Y. Hunge, A. Yadav, M. Mahadik, R. Bulakhe, J. Shim, V. Mathe and C. Bhosale, Degradation of organic dyes using spray deposited nanocrystalline stratified WO3/TiO2 photoelectrodes under sunlight illumination, Opt. Mater., 2018, 76, 260–270 CrossRef
.
- Y. Hunge, M. Mahadik, S. Kumbhar, V. Mohite, K. Rajpure, N. Deshpande, A. Moholkar and C. Bhosale, Visible light catalysis of methyl orange using nanostructured WO3 thin films, Ceram. Int., 2016, 42, 789–798 CrossRef
.
- R. Fernández-Domene, R. Sánchez-Tovar, B. Lucas-Granados, C. García-Zamora and J. García-Antón, Customized WO3 nanoplatelets as visible-light photoelectrocatalyst for the degradation of a recalcitrant model organic compound (methyl orange), J. Photochem. Photobiol., A, 2018, 356, 46–56 CrossRef
.
- L. Cheng, L. Liu, K. Yan and J. Zhang, Visible light-driven photoelectrocatalysis coupling with electroenzymatic process for degradation of chloramphenicol, Chem. Eng. J., 2017, 330, 1380–1389 CrossRef
.
- P. Chatchai, A. Y. Nosaka and Y. Nosaka, Photoelectrocatalytic performance of WO3/BiVO4 toward the dye degradation, Electrochim. Acta, 2013, 94, 314–319 CrossRef
.
- Q. Zeng, J. Li, L. Li, J. Bai, L. Xia and B. Zhou, Synthesis of WO3/BiVO4 photoanode using a reaction of bismuth nitrate with peroxovanadate on WO3 film for efficient photoelectrocatalytic water splitting and organic pollutant degradation, Appl. Catal., B, 2017, 21–29 CrossRef
.
- J. Sun, Y. Guo, Y. Wang, D. Cao, S. Tian, K. Xiao, R. Mao and X. Zhao, H2O2 assisted photoelectrocatalytic degradation of diclofenac sodium at g-C3N4/BiVO4 photoanode under visible light irradiation, Chem. Eng. J., 2018, 332, 312–320 CrossRef
.
- D. Cao, Y. Wang, M. Qiao and X. Zhao, Enhanced photoelectrocatalytic degradation of norfloxacin by an Ag3PO4/BiVO4 electrode with low bias, J. Catal., 2018, 360, 240–249 CrossRef
.
- J. Wang, Z. Yang, X. Gao, W. Yao, W. Wei, X. Chen, R. Zong and Y. Zhu, Core-shell g-C3N4@ ZnO composites as photoanodes with double synergistic effects for enhanced visible-light photoelectrocatalytic activities, Appl. Catal., B, 2017, 217, 169–180 CrossRef
.
- Y. Hunge, M. Mahadik, A. Moholkar and C. Bhosale, Photoelectrocatalytic degradation of phthalic acid using spray deposited stratified WO3/ZnO thin films under sunlight illumination, Appl. Surf. Sci., 2017, 420, 764–772 CrossRef
.
- J. Feng, L. Cheng, J. Zhang, O. K. Okoth and F. Chen, Preparation of BiVO4/ZnO composite film with enhanced visible-light photoelectrocatalytic activity, Ceram. Int., 2018, 44, 3672–3677 CrossRef
.
- S. Liu, X. Zhao, H. Zeng, Y. Wang, M. Qiao and W. Guan, Enhancement of photoelectrocatalytic degradation of diclofenac with persulfate activated by Cu cathode, Chem. Eng. J., 2017, 320, 168–177 CrossRef
.
- H. Wang, Y. Liang, L. Liu, J. Hu, P. Wu and W. Cui, Enriched photoelectrocatalytic degradation and photoelectric performance of BiOI photoelectrode by coupling rGO, Appl. Catal., B, 2017, 208, 22–34 CrossRef
.
- J.-Y. Luo, L.-L. Chen, X.-H. Liang, Q.-W. Zhao and H. Li, Anodic deposition-assisted photoelectrocatalytic degradation of bisphenol A at a cadmium sulfide modified electrode based on visible light-driven fuel cells, Electrochim. Acta, 2015, 186, 420–426 CrossRef
.
- Z. Fan, H. Shi, H. Zhao, J. Cai and G. Zhao, Application of carbon aerogel electrosorption for enhanced Bi2WO6 photoelectrocatalysis and elimination of trace nonylphenol, Carbon, 2018, 126, 279–288 CrossRef
.
- Y. Xiao, G. Tian, Y. Chen, X. Zhang, H. Fu and H. Fu, Exceptional visible-light photoelectrocatalytic activity of In2O3/In2S3/CdS ternary stereoscopic porous heterostructure film for the degradation of persistent 4-fluoro-3-methylphenol, Appl. Catal., B, 2018, 225, 477–486 CrossRef
.
- M. Sun, C. Zhai, J. Hu, M. Zhu and J. Pan, Plasmon enhanced electrocatalytic oxidation of ethanol and organic contaminants on gold/copper iodide composites under visible light irradiation, J. Colloid Interface Sci., 2018, 511, 110–118 CrossRef PubMed
.
- L. Cheng, Y. Tian and J. Zhang, Construction of pn heterojunction film of Cu2O/α-Fe2O3 for efficiently photoelectrocatalytic degradation of oxytetracycline, J. Colloid Interface Sci., 2018, 526, 470–479 CrossRef PubMed
.
- Q. Wang, Q. Zheng, R. Jin, S. Gao, Q. Yuan, W. Rong and R. Wang, Photoelectrocatalytic removal of organic dyes and Cr (VI) ions using Ag3PO4 nanoparticles sensitized TiO2 nanotube arrays, Mater. Chem. Phys., 2017, 199, 209–215 CrossRef
.
- Y. Zhang, Q. Wang, J. Lu, Q. Wang and Y. Cong, Synergistic photoelectrochemical reduction of Cr (VI) and oxidation of organic pollutants by g-C3N4/TiO2-NTs electrodes, Chemosphere, 2016, 162, 55–63 CrossRef PubMed
.
- Q. Wang, R. Jin, C. Yin, M. Wang, J. Wang and S. Gao, Photoelectrocatalytic removal of dye and Cr (VI) pollutants with Ag2S and Bi2S3 co-sensitized TiO2 nanotube arrays under solar irradiation, Sep. Purif. Technol., 2017, 172, 303–309 CrossRef
.
- L. Liu, R. Li, Y. Liu and J. Zhang, Simultaneous degradation of ofloxacin and recovery of Cu(II) by photoelectrocatalysis with highly ordered TiO2 nanotubes, J. Hazard. Mater., 2016, 308, 264–275 CrossRef PubMed
.
- L. Yang, X. Zheng, M. Liu, S. Luo, Y. Luo and G. Li, Fast photoelectro-reduction of CrVI over MoS2@TiO2 nanotubes on Ti wire, J. Hazard. Mater., 2017, 329, 230–240 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2018 |