Pathways toward high-performance inorganic perovskite solar cells: challenges and strategies
Bo
Li
 ,
Lin
Fu
,
Lin
Fu
 ,
Shuang
Li
,
Hui
Li
,
Lu
Pan
,
Lian
Wang
,
Bohong
Chang
and
Longwei
Yin
,
Shuang
Li
,
Hui
Li
,
Lu
Pan
,
Lian
Wang
,
Bohong
Chang
and
Longwei
Yin
 *
*
Key Laboratory for Liquid-Solid Structural Evolution and Processing of Materials, Ministry of Education, School of Materials Science and Engineering, Shandong University, Jinan 250061, P. R. China. E-mail: yinlw@sdu.edu.cn; Fax: +86 531 88396970; Tel: +86 531 88396970
First published on 2nd September 2019
Abstract
High-efficiency and low-cost perovskite solar cells (PSCs) are desirable candidates for addressing the scalability challenge of renewable solar energy. The dynamically evolving research field of cesium-based inorganic PSCs has achieved immense progress in the power conversion efficiency (PCE) of over 17% within just a few years. Compared with thermally vulnerable organic–inorganic perovskites, inorganic perovskites exhibit greater stability advantages for commercial applications. However, there are still a large number of issues and challenges for inorganic perovskites such as unstable phase structures, serious defect traps and limited absorption range, obstructing further development of inorganic PSCs. In this review, we present a unique outlook on the current progress of inorganic PSCs along with their structure–property–synthesis–performance traits. Importantly, we analyse comprehensively the challenges on the paths towards inorganic PSC commercialization, followed by highlighting the state-of-the-art materials engineering strategies, including phase-structure and composition regulation, surface-interfacial modification, ion-doping engineering and lead-free perovskite material design. Combined with the in-depth analyses of advanced materials characterization technologies and theoretical calculations, we present detailed exciton/charge-carrier dynamics and the physical mechanism of defects in inorganic PSCs. Finally, potential research directions to further improve the photovoltaic performance of inorganic PSCs are proposed, with an aim to gain insight into inorganic perovskites and their future research prospects.
1. Introduction
Organic–inorganic hybrid halide perovskites have been one of the most-studied photovoltaic materials in recent years.1–10 Since the initial discovery of their potential photovoltaic applications, the power conversion efficiency of organic–inorganic hybrid halide PSCs has reached over 24%, which is comparable with that of the state-of-the-art copper indium gallium diselenide (CIGS) solar cells and close to that of commercial crystalline silicon solar cells.11–20 This demonstrable rapid development of perovskite photovoltaic technology is largely due to the simple and reproducible solution/vapor-chemistry fabrication techniques and excellent optoelectronic properties such as suitable direct bandgap, extra-long carrier diffusion length and high absorption coefficient of perovskites.21–26Despite the high efficiencies and excellent properties, the poor stability of organic–inorganic hybrid halide perovskite materials is still a critical challenge preventing this photovoltaic technology from being commercialized.27–35 To be marketable for commercial applications, perovskite photovoltaic devices must be able to work continuously for over 20 years under outdoor conditions.36 Thus extensive attention has recently been focused on the long-term stability of perovskite materials with regard to prolonged exposure to heat, moisture, oxygen and light.37–43 Inorganic materials normally exhibit better stability compared to organic materials, especially in terms of high temperature. One of the feasible ways forward, therefore, is substituting the organic cations with cesium cations (Cs+) due to their inherent inorganic stability.44–48
Cesium-based inorganic perovskites were first applied as the light absorber in perovskite solar cells with around 6% PCE by Hodes's group in 2015.49 In this pioneering report, inorganic CsPbBr3 was demonstrated to work equally as well as the organic–inorganic one, especially generating a high open circuit voltage (Voc) which is a prominent feature of these solar cells. Furthermore, the inorganic halide perovskite CsPbBr3 was found to show better stability compared with the organic–inorganic hybrid halide perovskite MAPbBr3.50 During an illumination period of 5 h, the unencapsulated MAPbBr3 device exhibited a stronger and faster photocurrent density decay of 55% compared to the maximum value, in contrast to the CsPbBr3 one with only 13% decay. Aging tests were conducted in ambient relative humidity of 60–70% over 2 weeks. The MAPbBr3-based solar cell suffered a heavy efficiency loss of 85%, while the CsPbBr3-based one showed no significant decay. However, the inorganic PSCs did not attract much attention because of their low efficiency levels. In 2016, Snaith's group reported bandgap-tunable cesium lead halide PSCs by adjusting the halide (iodide and bromide) stoichiometric ratio, achieving close to 10% power conversion efficiency.51 Using an all-vacuum-deposition strategy, the mixed halide inorganic perovskite solar cells exhibited a stabilized efficiency exceeding 11%.52 In the meantime, cubic phase CsPbI3 quantum dot PSCs achieved close to 11% efficiency and worked out the issues of cubic phase instability of inorganic perovskites.53 Since then, inorganic halide perovskites have attracted enormous attention from researchers previously working on organic–inorganic perovskites or other optoelectronic materials, and these research efforts have promoted inorganic perovskite material development and understanding of device principles (Table 1).
| Formula | Bandgap (eV) | Preparation method | Device structure | PCE (%) | Year | Ref. |
|---|---|---|---|---|---|---|
| CsPbI3 | 1.73 | Spin-coating | Au/spiro-OMeTAD/perov/c-TiO2/FTO | 2.9 | 2015 | 62 |
| CsPb0.96Bi0.02I3 | 1.56 | Spin-coating | Au/CuI/perov/c-TiO2/FTO | 13.2 | 2017 | 106 |
| CsPb0.95Ca0.05I3 | 1.72 | Spin-coating | Au/P3HT/perov/m-TiO2/c-TiO2/FTO | 13.5 | 2018 | 178 |
| CsPbI3 | 1.73 | Colloidal solution | Al/MoOx/spiro-OMeTAD/perov/c-TiO2/FTO | 10.8 | 2016 | 53 |
| Vacuum evaporation | Ag/SWCNT/perov/SnO2/FTO | 8.8 | 2017 | 96 | ||
| Vacuum evaporation | Au/P3HT/perov/c-TiO2/ITO | 10.5 | 2016 | 105 | ||
| CsPb0.96Sb0.02I3 | 1.84 | Spin-coating | Carbon/perov/m-Al2O3/m-TiO2/c-TiO2/FTO | 5.2 | 2018 | 179 |
| CsSn0.6Pb0.4I3 | 1.63 | Colloidal solution | Au/spiro-OMeTAD/perovQD/m-TiO2/c-TiO2/FTO | 0.1 | 2017 | 180 |
| CsPb0.88Mn0.12I3 | 1.70 | Spin-coating | Ag/spiro-OMeTAD/perov/c-TiO2/FTO | 2017 | 181 | |
| CsPbBr3 | 2.30 | Spin-coating | Au/spiro-OMeTAD/perov/m-TiO2/FTO | 6.0 | 2015 | 49 |
| Spin-coating | Carbon/perov/m-TiO2/c-TiO2/FTO | 6.7 | 2016 | 182 | ||
| Spin-coating | Carbon/GQDs/perov/m-TiO2/c-TiO2/FTO | 9.7 | 2018 | 183 | ||
| Colloidal solution | Au/spiro-OMeTAD/perov/c-TiO2/FTO | 5.5 | 2017 | 184 | ||
| Spin-coating | Carbon/PQDs/perov/GQDs/FTO | 4.1 | 2018 | 185 | ||
| CsPb0.97Sm0.03Br3 | 2.30 | Spin-coating | Carbon/perovs/m-TiO2/c-TiO2/FTO | 10.1 | 2018 | 186 |
| CsPb0.97Tb0.03Br3 | 2.30 | Spin-coating | Carbon/NiOx//SnS:ZnS/perovs/m-TiO2/c-TiO2/FTO | 10.3 | 2018 | 187 |
| CsPb0.97Sr0.03Br3 | 2.24 | Spin-coating | Carbon/perovs/m-TiO2/c-TiO2/FTO | 9.2 | 2019 | 188 |
| Cs0.91Rb0.09PbBr3 | 2.24 | Spin-coating | Carbon/perovs/m-TiO2/c-TiO2/FTO | 9.9 | 2018 | 120 |
| CsPbI2Br | 1.92 | Spin-coating | Ag/spiro-OMeTAD/perov/c-TiO2/FTO | 9.8 | 2016 | 51 |
| Al/BCP/PCBM/perov/PEDOT:PSS/ITO | 6.7 | 2016 | 63 | |||
| Au/spiro-OMeTAD/perov/c-TiO2/FTO | 10.7 | 2017 | 189 | |||
| Ag/ZnO-C60/perov/NiOx/FTO | 13.3 | 2018 | 190 | |||
| Cs0.925K0.075PbI2Br | 1.92 | Au/spiro-OMeTAD/perov/c-TiO2/FTO | 10.0 | 2017 | 119 | |
| CsPb0.98Sr0.02I2Br | 1.87 | Au/P3HT/perov/m-TiO2/c-TiO2/FTO | 11.2 | 2017 | 126 | |
| CsPb0.98Mn0.02I2Br | 1.92 | Carbon/perov/m-TiO2/c-TiO2/FTO | 13.5 | 2018 | 125 | |
| CsPbI2Br | Colloidal solution | Au/PTAA/perov/c-TiO2/FTO | 12.4 | 2018 | 191 | |
| Vacuum evaporation | Ag/TAPC/MoO3/perov/C60/Ca/ITO | 11.7 | 2017 | 53 | ||
| Spin-coating | Au/P3HT/perov/c-TiO2/FTO | 7.7 | 2017 | 192 | ||
| CsPb0.9Sn0.1IBr2 | 1.79 | Carbon/perov/m-TiO2/FTO | 11.3 | 2017 | 121 | |
| CsPb0.95Eu0.05I2Br | 1.98 | Au/spiro-OMeTAD/perov/TiO2/FTO | 13.7 | 2019 | 193 | |
| CsPb0.995Mn0.005I1.01Br1.99 | 1.85 | Carbon/perov/m-TiO2/c-TiO2/FTO | 7.36 | 2018 | 124 | |
| CsPbIBr2 | 2.05 | Spray-coating | Au/spiro-OMeTAD/perov/m-TiO2/c-TiO2/FTO | 5.4 | 2016 | 194 |
| CsPbIBr2 | Thermal evaporation | Au/perov/c-TiO2/FTO | 4.7 | 2016 | 95 | |
| Spin-coating | Au/MoOx/perov/NiOx/FTO | 5.5 | 2017 | 195 | ||
| CsSnBr3 | 1.75 | Spin-coating | Au/spiro-OMeTAD/perov/m-TiO2/FTO | 2.17 | 2016 | 141 |
| Au/PTAA/perov/m-TiO2/FTO | 3.04 | 2017 | 142 | |||
| Vapor deposition | Ag/BCP/C60/perov/MoO3/ITO | 0.38 | 2016 | 196 | ||
| CsSnI2.9Br0.1 | 1.27 | Spin-coating | Au/spiro-OMeTAD/perov/m-TiO2/FTO | 1.76 | 2015 | 140 |
| CsSnIBr2 | 1.65 | Carbon/perov/m-TiO2/FTO | 3.2 | 2016 | 143 | |
| CsGeI3 | 1.63 | Au/spiro-OMeTAD/CsGeI3/m-TiO2/FTO | 0.11 | 2015 | 197 | |
| Cs2AgBiBr6 | 2.02 | Vacuum sublimation | Au/P3HT/perov/c-TiO2/FTO | 1.37 | 2018 | 198 |
| Cs2NaBiI6 | 1.66 | Spin-coating | Au/spiro-OMeTAD/perov/m-TiO2/c-TiO2/FTO | 0.44 | 2018 | 199 |
| Cs2TiBr6 | 1.82 | Two-step vapor deposition method | Au/P3HT/perov/C60/c-TiO2/FTO | 3.3 | 2018 | 144 |
| Cs2SnI6 | 1.48 | Two-step vapor deposition and solid state reaction | Ag/P3HT/perov/c-TiO2/FTO | 1.0 | 2017 | 145 |
In this review, we focus on the progress of state-of-the-art inorganic perovskite materials and photovoltaic devices, and offer a unique outlook on the path towards the commercialization of inorganic perovskites from the perspective of existing challenges and coping strategies. First, we review the structure–property-synthesis characteristics and inherent material-level issues and challenges of inorganic perovskites. Theoretical investigations and experimental results of the structures and electronic properties along with the underlying issues of inorganic halide perovskites are beneficial for comprehensive understanding of inorganic materials. Second, on the basis of the issues in the inorganic perovskite research field, we discuss the current state-of-the-art engineering strategies from the aspect of phase-structure and composition regulation, surface-interfacial modification, ion-doping engineering and lead-free perovskite materials design as shown in Fig. 1. These represent the key research areas contributing to the rapid development of inorganic halide PSCs within this short number of years. In addition, the current physical understanding of inorganic halide perovskite materials and photovoltaic devices is summarized systematically. Finally, we propose the prospective research directions to further improve the photovoltaic performance of inorganic PSCs which may help to gain insight into inorganic perovskites and future research prospects.
 | ||
| Fig. 1 The typical strategies for inorganic halide perovskite material design and the challenges and issues in the inorganic halide perovskite solar cell research field including phase structure instability,59 internal and surface defects,127 limited absorption range,54 mechanism ambiguity96 and the development of lead-free perovskite materials.200 | ||
2. Fundamental background and challenges
Different from organic–inorganic hybrid perovskites, inorganic halide perovskites embrace unique crystal structures due to the incorporation of cesium ions. The unique crystal structure characteristics endow inorganic perovskites with distinctive optical properties and photovoltaic performance, meanwhile, causing new issues and challenges in the perovskite photovoltaic field. In particular, the phase transition temperatures vary dramatically in inorganic perovskites compared with the organic–inorganic ones, and are dependent on chemical composition, which poses serious issues of phase-stability. In this section, we briefly overview the current research progress in understanding the fundamental properties of inorganic perovskites in terms of the crystal structure, electronic properties, theoretical investigations and synthesis methods. On the basis of the specific characteristics of inorganic perovskite materials, we summarized comprehensively the issues and challenges for developing high-performance inorganic perovskite solar cells at the present stage.2.1. Crystal structure
Generally, perovskite materials possess a typical formula structure of ABX3, where cation A occupies the corner positions (0, 0, 0), cation B is located at the centre positions (1/2, 1/2, 1/2) and anion X is at the centre of the six planes (1/2, 1/2, 0), constituting an ideal cubic unit cell. To maintain a high-symmetry cubic ABX3 perovskite crystal structure, the tolerance factor should be considered to be within a range from 0.813 to 1.107, where RA, RB and RX are the ionic radii of the corresponding cations and anions.54,55 For inorganic halide perovskites, the cesium ion (Cs+, 1.81 Å) can match the requirement of t and has been identified as the preferred alternative inorganic cation substitute for the organic cation methylamine (MA+, 2.70 Å). The Goldschmidt tolerance factors of MAPbI3 and CsPbI3 are calculated to be 1.02 and 0.84 respectively, which are both in the range (0.81–1.11) for structurally stable perovskites.
should be considered to be within a range from 0.813 to 1.107, where RA, RB and RX are the ionic radii of the corresponding cations and anions.54,55 For inorganic halide perovskites, the cesium ion (Cs+, 1.81 Å) can match the requirement of t and has been identified as the preferred alternative inorganic cation substitute for the organic cation methylamine (MA+, 2.70 Å). The Goldschmidt tolerance factors of MAPbI3 and CsPbI3 are calculated to be 1.02 and 0.84 respectively, which are both in the range (0.81–1.11) for structurally stable perovskites.
The general crystal structures of inorganic halide perovskites can be divided into orthorhombic, tetragonal, and cubic phase crystal structures according to the environmental temperature as shown in Fig. 2a.56 The differences between crystal structures result in different optical and electronic properties.57 Here, taking a cesium lead halide perovskite (CsPbX3) as an example, the typical cubic phase of cesium lead halide perovskites generally appears at high temperature.58–60 As the temperature decreases, a phase transition with lower-symmetry is observed. The typical chlorine-based inorganic perovskite CsPbCl3 stabilizes in the cubic phase at a temperature over 50 °C, and shows phase transition from the cubic phase to tetragonal phase at around 46 °C, and to the orthorhombic phase around 42 °C. For bromide-based perovskite (CsPbBr3), the structural phase transition occurs at 130 °C and 88 °C, at which temperature the cubic perovskite structure turns to tetragonal and further to orthorhombic, respectively.61 However, for an iodide-based perovskite (CsPbI3), the cubic phase CsPbI3 can only remain stable at a high temperature of over 300 °C.61 Below 300 °C, the cubic phase of CsPbI3 would change to a more thermodynamically favourable orthorhombic phase, which is also known as the “yellow phase” and is unusable for photovoltaic applications due to its broad band gap of 2.82 eV.63 The unfavourable phase transition can be restrained by partial substitution of iodide with bromide (CsPbBrxI1−x), which can decrease the cubic phase transition temperature. Comparatively, the incorporation of bromide into CsPbI3 increases the band gap and decreases the visible light absorption range.64
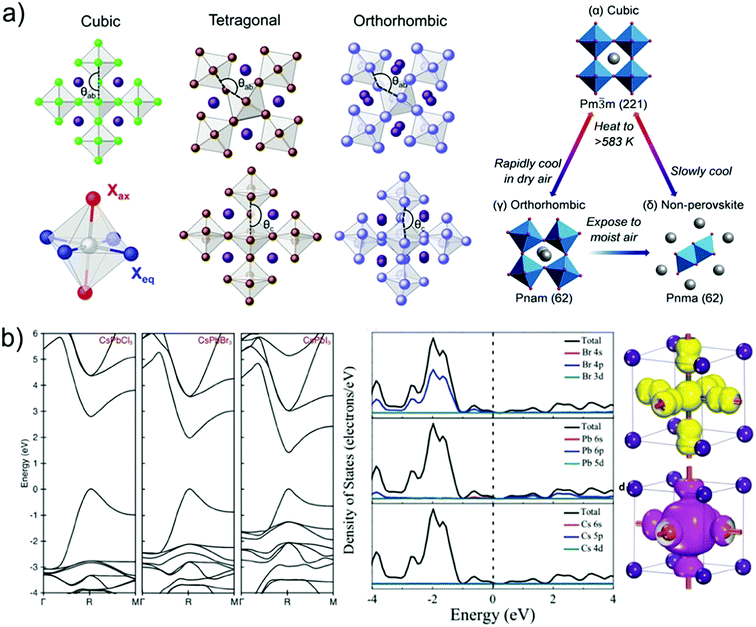 | ||
| Fig. 2 (a) Polyhedral models of the different crystal structures of inorganic halide perovskites (cubic, tetragonal, or orthorhombic) and their structural transitions under different conditions.59,110 (b) Calculated electronic band structures for cubic-phase CsPbCl3, CsPbBr3, and CsPbI3, and the density of states of cubic CsPbBr3 with elemental contributions to the energy band.72 | ||
2.2. Electronic properties
In perovskite semiconductors and photovoltaic devices, optical transitions (absorption and photoluminescence) and charge transfer (injection and extraction) greatly determine the optoelectronic properties and performances, and these parameters strongly rely on the nature of the electronic properties, such as the energy level of the valence band and conduction band.65 These electronic structures and properties of inorganic halide perovskites with different halide compositions are found to show analogical features.66–68 For typical 3D structured inorganic perovskites, the valence bands are constituted by the combination of 6s orbitals of lead and np orbitals of the halides (Cl: n = 3, Br: n = 4, and I: n = 5), while the conduction band is composed of the combination of lead (6p) orbitals and the halide np orbitals with primary contributions of the lead (6p) orbitals.69,70 The electronic states of inorganic perovskite are only slightly affected by the cesium cation, and thus making no direct electronic contribution to bandgap.71Fig. 2b depicts the calculated electronic structures of cubic phase inorganic halide perovskites CsPbCl3, CsPbBr3, and CsPbI3, respectively, according to density functional theory in consideration of spin–orbit interactions and relativistic corrections.72 It is clear that the energy band structures of inorganic halide perovskites are almost independent of the halogen compositions. Apart from the difference of bandgap values, all the inorganic halide perovskites exhibit direct bandgaps.69,70,72,73 Furthermore, comparable effective masses of electrons and holes can be predicted based on the electronic band dispersion around the band edges, contributing to the high carrier mobilities.74–76As the halide varies from iodide to bromide to chloride, the energy levels of the halide np6 orbitals decrease, shifting the valence band toward more positive potentials.77 The ratio of the valence band and conduction band of inorganic perovskites with compositions CsPbBrxI3−x, CsPbBr3 and CsPbBrxCl3−x is calculated to be 6.1/4.3 eV, 6.5/4.15 eV and 6.5/3.8 eV, respectively.78 Meanwhile, a consecutive variation of the halide composition from iodide to bromide to chloride results in systematic change of the optical bandgap. Based on the UV-vis absorption spectra, the optical bandgap values of CsPbX3 with compositions CsPbBrxI3−x, CsPbBr3 and CsPbBrxCl3−x are calculated to be 1.8 eV, 2.35 eV and 2.7 eV, respectively.79 In addition, for tin-based inorganic perovskites, the calculated bandgaps decrease from 2.8 to 1.3 eV as the composition of cesium tin halides of CsSnX3 perovskites varies from chloride to bromide to iodide.80 The optical spectra of tin-based perovskites show a red shift compared with those of analogous lead perovskites, which can be assigned to the higher electro-negativity of tin in the “B” site in the ABX3 perovskite lattice.81,82 The bandgaps of germanium (Ge) based analogues CsGeX3 are similar to those of the Pb counterparts (3.43, 2.38, and 1.51 eV for Cl, Br and I, respectively).83,84
2.3. Theoretical calculations
With the help of advanced experimental characterization methods, the physicochemical properties of inorganic perovskites are obtained to some extent. However, in order to gain deeper insight into the relationships between the crystal structures, electronic structures and power conversion performances of inorganic perovskites, theoretical studies are required urgently. Primarily, theoretical calculations point out that the replacement of organic cations with inorganic Cs+ makes no difference in the optical or electronic properties.85 Alkali metal lead halides (RbPbI3 and CsPbI3) with the cubic perovskite structure were examined using PbI2 as an initial structural model, suggesting that while the Pb2+ 6s lone-pairs were stereochemically inert, the presence of proximal instabilities could have an effect on the functional properties of these materials.86 By high-resolution in situ synchrotron XRD measurement, Marronnier et al.87 found that undercooled CsPbI3 can temporarily maintain a metastable perovskite polytype (black γ-phase) structure at room temperature, stabilizing the metastable perovskite polytype (black γ-phase) structure crucial for photovoltaic applications. The competition among low-temperature phases of CsPbI3 was also inspected through energy and vibrational entropy calculations, indicating that the key to avoiding the transition from the black phase to yellow phase was preventing the order–disorder entropy term arising from double-well instabilities.88In addition to helping in understanding the physicochemical properties, the theoretical calculations have also been made it easy to find new compositions and structures of efficient and stable kinds of perovskites. In addition to the typical 3D perovskite structure of ABX3, heterovalent elements also have been studied to explore new perovskite systems. With the B-site cation in the +4 oxidation state, Cs2PdBr6, with the formula A2BX6, was indicated to have dispersive electronic bands by density functional theory calculations.89 And this solution-processable Cs2PdBr6 was observed to be resistant to water, indicating excellent long-term stability for optoelectronic applications. Composed of a zero-dimensional dimer form and a two-dimensional layered form, it was proposed that Cs3Sb2I9 possessed a nearly direct bandgap with a similar high level of absorption to MAPbI3, giving guidance to experiments and assistantly demonstrating the experimental results.90 The basic physical properties of lead-free Ge-based inorganic perovskites AGeX3 (A = Cs or Rb; X = I, Br, or Cl), including dielectric constants, photoabsorption coefficients, effective masses of charge carriers, exciton binding energies, and electronic band structures, were predicted using density functional theory calculations, which were proposed to act as light absorbers for solar cells.91 Besides, chalcogenide perovskites ABX3 (X = S or Se; A, B = metals with a combined valence of 6) were computed as a new family for photovoltaic applications because of their more environmentally friendly compositions than lead halide perovskites. CaTiS3, BaZrS3, CaZrSe3 and CaHfSe3 were identified to be promising materials for solar cells since their calculated absorption properties were relatively suitable.92
2.4. Synthesis strategies
For fabricating high-performance inorganic halide perovskite solar cells, the morphologies and qualities of perovskite thin films are largely studied for photovoltaic performance. In terms of polycrystalline films, solution-chemistry routes and vapor deposition processes are predominant due to the facile and reproducible process in fabricating high-quality perovskite thin films. The solution-chemistry techniques for depositing perovskite thin films can be divided into two main strategies: one-step and two-step methods. Fig. 3a shows the typical one-step deposition method of inorganic perovskite films. In this method, the perovskite films are directly deposited from the precursor solution on planar or mesoporous substrates. Because the inorganic halide perovskite CsPbX3 can be synthesized by the interaction of CsX and PbX2, a typical two-step method constitutes the first step of PbX2 deposition, and then the conversion of CsPbX3 during the second step as shown in Fig. 3b.93 The qualities of the inorganic perovskite film deposited by the one-step method are primarily determined by the film shrinkage during the crystallization of perovskite due to the removal of the solvent.94 The main challenge for the two-step method is the volume expansion of the PbX2 precursor due to the intercalation of CsX. Thus, the key factor for solution-chemistry deposition of perovskite films is to control film shrinkage in the one-step method and film expansion in the two-step method.94 | ||
| Fig. 3 (a) Schematic of the fabrication process and the SEM image of CsPbI2Br perovskite films passivated using Pb(NO3)2 methyl acetate solution.130 (b) Schematic illustration and SEM image of the CsPbBr3 perovskite film fabricated using multistep solution-processed deposition technology.93 (c) Schematic illustration and SEM image of the CsPbI3 perovskite film fabricated by vapor deposition route.105 | ||
In addition to the solution-chemistry route, a dual-source vacuum vapour deposition technique is also introduced to fabricate highly uniform polycrystalline inorganic halide perovskite films. As shown in Fig. 3c, the PbX2 and CsX vapours obtained by heating elemental sources, are transported to the substrate and crystallize to form perovskites after annealing. The first uniform inorganic perovskite films were prepared by Ma et al., in which CsPbIBr2 was carefully deposited via a dual source thermal evaporation process.95 Large size perovskite crystal grains of 500–1000 nm were acquired by controlling the annealing and substrate temperature of 250 °C and 75 °C, respectively. It is also shown that precise control of precursor co-deposition is essential for the formation of desired perovskite films with an excellent crystal structure. Hutter et al. investigated the charge carrier dynamics of CsPbI3 thin films via the comparison of vapor and solution deposition strategies.96 The time-resolved microwave conductivity technique was applied to detect the charge carrier mobilities, which demonstrates that the mobilities of both vapour and solution deposited films were approximately 25 cm2 V−1 s−1. However, an extra-long lifetime of tens of microseconds was observed in the vapour-deposited CsPbI3 thin film, whereas the perovskite films fabricated by the solution deposited method exhibited a photo-excited decay lifetime of only 200 ns. The trap densities of the vapor deposited perovskite film based on time-resolved microwave conductivity curves were far lower than the value for solution-deposited films, which explained the excellent carrier lifetime of vapour deposited films due to less trapping and recombination.
2.5. Inorganic halide perovskites: issues and challenges
3. Engineering strategies for inorganic halide perovskites
On the basis of the issues and challenges discussed above, in this section, we present and summarize systemically the current state-of-the-art engineering strategies, including phase-structure engineering, composition regulation, surface-interfacial modification, lead-free perovskite materials design, and physical mechanism analyses. Specifically, focusing on the phase structure instability, the studies of cesium lead iodide (CsPbI3) which possesses the most desired bandgap and highly unstable phase structure are reviewed based on four different strategies. The limited absorption range can be regulated by composition engineering by A-site, B-site and halide-site ion substitution and doping. The issues of interfacial and surface defects can be inhibited by interfacial engineering and rational transport layer design. In addition, systematic investigations of lead-free perovskites based on homovalent elements and heterovalent elements are summarized to address lead toxicity. The in-depth analyses of the photophysical performance of several inorganic PSCs (such as transient-absorption/-photoluminescence) are reviewed.3.1. Phase-stable cesium lead iodide
Cubic-phase stabilization is extremely important for the application of cesium lead halide inorganic perovskite materials in the fabrication of perovskite solar cells. From a light absorption point of view, cesium lead iodide (CsPbI3) is the most desirable choice due to its bandgap of about 1.7 eV. However, CsPbI3 in the black cubic phase (α) generally formed at high temperature (over 300 °C) is inclined to convert into the yellow orthorhombic phase (δ) at room temperature and is sensitive to moisture as shown in Fig. 4a. This non-perovskite structure material is less photoactive with an optical bandgap of more than 2.8 eV.104 In this section, the strategies on how to stabilize the CsPbI3 inorganic perovskite cubic-phase have been discussed. By decreasing the perovskite grain size, the perovskite surface energy can be increased for obtaining a stable cubic-phase CsPbI3 inorganic perovskite. Meanwhile, substituting metal cations with different ions or doping metal ions in the CsPbI3 crystal lattice can also produce cubic-phase stable CsPbI3 inorganic perovskites. In addition, surface engineering via various organic additives has also been applied to stabilize cubic-phase CsPbI3 for photoelectric applications. | ||
| Fig. 4 (a) Diagrammatic structure of CsPbI3 with different phases.62 (b) Schematic and SEM cross-sectional view of the CsPbI3 nanocrystal solar cell.53 (c) Schematic of the film deposition process and AX salt post-treatment. (d) Comparison of the extracted mobility and terahertz lifetimes for each of the films.103 | ||
 | ||
| Fig. 5 (a and b) TRMC traces for (a) vapour-deposited CsPbI3 thin films with a thickness of 260 nm and (b) 350 nm CsPbI3 film spin-coated from a DMF/DMSO solution.96 (c) Schematic of the crystal structures of γ-CsPbI3 without surface ligands, OA-stabilized α-CsPbI3 and PEA-stabilized β-CsPbI3.107 (d) Mechanism of α-CsPbI3 stabilization by zwitterions. The zwitterion molecules are expelled toward the grain surface and grain boundaries during the growth of CsPbI3 grains, impeding the grains from growing larger.108 (e) Mechanism of PVP-induced α-CsPbI3. The unbound lone pairs of N/O atoms in the acylamino groups of PVP molecules offer excess electrons and interact with Cs ions in CsPbI3.109 | ||
3.2. Composition engineering
Similar to organic–inorganic hybrid perovskites, inorganic halide perovskites share the common optical properties of composition-tunable bandgap and emission spectrum.110 By changing the halide elements (Cl, Br, and I), the optical absorption and photoluminescence spectrum can be finely tuned throughout the whole visible light range from 400 to 700 nm (Fig. 6a and b).111 Theoretical studies have revealed that the electronic structures of inorganic halide perovskites are related to the p orbitals of halide and the p orbitals of lead, and thus, the optical properties and bandgaps can generally be tuned by rational composition engineering. By adjusting the proportion of precursors during synthesis, their optical absorption and emission colors can be easily tuned.112 In this way, their chemical compositions can be precisely tuned to yield various optical properties. | ||
| Fig. 6 (a) Images of the J–V curve of the CsPbI2Br-based solar cell. (b) Photoluminescence spectra of CsPb(IxBr1−x)3 films.51 (c) Photographs and (d) absorption spectra of CsPbI3−xBrx films.63 | ||
To balance these two crucial metrics, all kinds of ratios of iodine to bromine have been explored. Snaith and his co-workers first demonstrated continuous bandgap tuning in mixed halide inorganic perovskite CsPbI3−xBrx films via tuning the Br/I ratios, as shown in Fig. 6a and b.51 Beal et al. reported based on their studies on CsPbI3−xBrx that the variation of x ranging from 0 to 3 can bring about great changes to the optical properties.63Fig. 6c and d show the photos of a series of CsPbI3−xBrx perovskites and the corresponding continuous change of absorption or photoluminescence (PL) spectra. In addition, since the radius of Br− is smaller than that of I−, the substitution of Br with I improves the structural stability from the viewpoint of the tolerance factor. From this point of view, the smallest halide was also incorporated to tune the value of the tolerance factor in an attempt to obtain a better structural stability under moisture and low temperature.114 To avoid the problem of poor solvability of the precursor, the thermal evaporation method with a dual source was applied to deposit CsPbBr2I films by Ma et al.95 The grain size of CsPbIBr2 is not uniform, varying from 500 to 1000 nm, and a Au electrode was deposited directly on the CsPbIBr2 film without any hole transport material. These studies revealed the promising potential of bandgap-tunable inorganic halide perovskites applied in tandem solar cells. For instance, in CsPbI3−xBrx, the compounds with 0.6 < x < 1.2 are the most appropriate ones for solar cells. When x is less than 0.6, the undesired orthorhombic phase is thermodynamically preferred at room temperature. When x is more than 1.2, phase separation would occur under illumination. Thus, CsPbBrI2 with a bandgap of 1.9 eV is selected as a desired target composition as an inorganic halide perovskite absorber.64
In addition to the studies of iodide–bromide mixed CsPbI3−xBrx, the halide site can also be regulated by Cl ions and pseudo halogen molecules. Just as organic–inorganic hybrid perovskites that the Cl mixture is difficult to obtain with a high Cl content, only small content of Cl can be detected a in inorganic halide perovskites CsPbBr3 with Cl doping, and the crystal structures of CsPbBr3 transform from orthorhombic to cubic phase after Cl doping.115,116 Pseudo halogen molecules such as SCN have been incorporated into inorganic halide perovskites to engineer the optoelectronic properties. Interestingly, the photoluminescence of the CsPbBr3 perovskite successfully doped with SCN− exhibits an abnormal blue shift in the optical properties.117 The theoretical investigations and experimental results reveal that SCN− doped CsPbBr3 perovskite had disorder in the crystal structure, leading to crystal lattice expansion, thus resulting in the variation of electronic structures with band gap broadening.
 | ||
| Fig. 7 (a) Schematic of the solar cell structure of the planar architecture and zoom of the K+ cation site in the lattice.119 (b) Schematic of all-inorganic PSCs. Optical images and J–V curves of CsPbBr3, CsPbIBr2, and CsPb0.9Sn0.1IBr2 films and the corresponding solar cells, respectively. (c) Optical images of the CsPb1−xSnxIBr2 films.121 | ||
3.3. Interfacial engineering and transport layer design
It is well known that there are considerable coordination sites on the surface of perovskite films, causing negative effects like the hysteresis phenomenon and trap centers. For example, the inhomogeneous interface in the perovskite bulk layer may result in a long exciton diffusion length and substantial current leakage,127,128 while the structural defects, metallic lead atoms and halogen vacancies may reduce Voc by accelerating the nonradiative charge recombination.129 Therefore, interfacial functionalization and transport layer design are important strategies to boost the photovoltaic performance and durability against the environment like humidity, and sometimes to stabilize the cubic phase of Cs-based inorganic perovskites in the meantime. | ||
| Fig. 8 (a) TRPL decay of the Pb(Ac)2 passivated CsPbI2Br films on compact-TiO2; (b) the schematic of charge transport properties in PbO- and Pb(Ac)2-modified films;102 (c) mechanism of HI/PEAI-induced cubic phase CsPbI3 stability.132 (d) J–V characteristics of all-inorganic CsPbI2Br PSCs with a SnO2/ZnO bilayer ETL and SnO2 ETL; (e) the time-dependent stabilized power output of the PSCs for both SnO2/ZnO- and SnO2-based PSCs.135 | ||
In addition to the functionalization of the inorganic perovskite solar device interface, crystal functionalization is also investigated. Wang et al.131 reported a bifunctional strategy using a simple phenyltrimethylammonium bromide (PTABr) post-treatment, which achieved gradient Br doping and surface passivation. The gradient Br doping and PTA surface termination increase the crystal size and improve the phase stability, achieving a champion PCE of 17%. Wang et al.132 applied the synergistic effect of hydroiodic acid (HI) and phenylethylammonium lead iodide (HPI3+x) additives to stabilize the cubic phase CsPbI3 perovskite crystals. As shown in Fig. 8c, during the crystallization process, the additives can act as the blocking element to hinder the phase transition by functionalizing the crystal units. The optimized inorganic perovskite solar cell shows a record PCE of 15.07% with remarkable stability. Liao et al. designed a CsPbBr3/carbon interface for interfacial energy difference modulation.133 By setting intermediate energy levels with carbon quantum dots and red phosphorus quantum dots, the interfacial electron–hole recombination can be significantly suppressed. Arising from the suppressed interfacial charge recombination, the optimal device yields a PCE of up to 8.20% under one sun illumination, which is much higher than that of a QD-free device.
The ETLs in PSCs can be divided into two types: inorganic and organic ones. In general, inorganic ETLs are n-type metal oxides, such as TiO2, SnO2 and ZnO, which are applied in a planar or mesoporous structure because of the high annealing temperature of these oxides. Organic ETLs are usually fullerene (C60) and its derivatives, [6,6]-phenyl C61 butyric acid methyl ester (PCBM), for example. This kind of ETL has been employed in inverted PSCs because of the suitable energy level alignment, decent electron mobility, and low temperature solution process. The former inorganic one is widely studied in the field of inorganic PSCs. Aamir et al.134 fabricated CsPbBr2I inorganic perovskite solar cells using a single step spin-coating method based on a low-temperature processed ZnO layer on an ITO glass substrate. The results show that the ZnO substrate temperature during spin coating is a critical parameter to generate the required crystalline morphology of the inorganic perovskite thin films, while it does not affect the stoichiometry of the inorganic perovskite materials. Yan et al.135 fabricated a SnO2/ZnO bilayer electron transporting layer (ETL), aiming for low energy loss and larger Voc (Fig. 8d and e). The interfacial trap-assisted recombination can be suppressed via a desirable cascade energy level alignment between the inorganic perovskite and SnO2/ZnO bilayer ETL. A high Voc of 1.23 V and power conversion efficiency of 14.6% were obtained.
For the HTLs, spiro-OMeTAD is the most commonly used HTM in inorganic PSCs on account of its matched HOMO level of −5.22 eV with perovskites and high solubility in nonpolar solvents such as chlorobenzene. Nevertheless, high cost and poor long-term stability limit the application of spiro-OMeTAD. To address these two issues at the same time, Chen et al. exploited a carbon electrode serving as both the hole transport material and the metal electrode in CsPbBr3 all-inorganic PSCs. This carbon-based HTL-free device achieved significantly better thermal stability compared to the CH3NH3PbI3 one.136 A carbon electrode was also used in CsPbIBr2 PSCs fabricated by dual source thermal evaporation.95
3.4. Lead-free perovskites
Despite exciting progress of excellent thermal stability and photovoltaic performance for inorganic perovskites, the concern of lead toxicity is still the biggest obstacle for the commercialization of this emerging technology. In recent years, significant efforts have been made to develop light active lead-free halide perovskite materials, especially inorganic lead-free perovskites. In general, there are two categories of lead-free halide perovskite materials: homovalent element (Sn and Ge) based perovskites (such as CsSnI3 or CsGeI3) and heterovalent element (Bi and Sb) based perovskites (such as Cs2AgBiI6 or Cs2AgSbCl6). However, these inorganic lead-free perovskite solar cells show significantly inferior photovoltaic performances to the lead based perovskite counterparts. In this section, we provide reviews on the fundamental understanding of the material properties and photovoltaic performance of lead-free inorganic perovskite materials based on homovalent and heterovalent elements.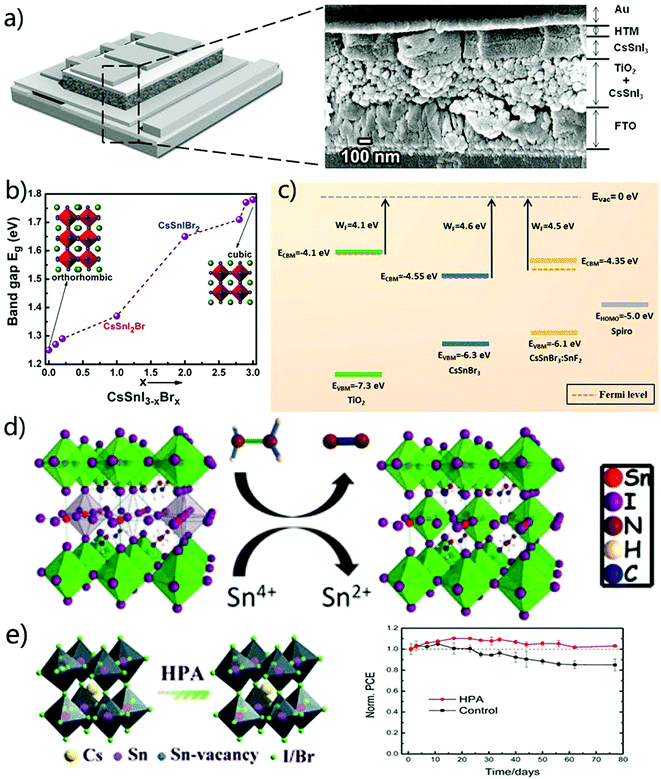 | ||
| Fig. 9 (a) Schematic and cross-sectional SEM image of the device consisting of a conducting FTO layer, TiO2 blocking layer, and TiO2 nanoparticle scaffold infiltrated with CsSnI3 and covered with an overlayer of 100 nm, hole transporting layer and Au.139 (b) The variation of bandgaps and crystal structures as a function of the Br concentration.140 (c) Band structures of dense TiO2, pristine CsSnBr3 and CsSnBr3 with 20 mol% SnF2.141 (d) Possible mechanism of the reaction of hydrazine vapor with Sn-based perovskites.142 (e) The effect of HPA on the CsSnIBr2 structure and normalized PCE of the encapsulated CsSnIBr2 device under ambient conditions for 77 days.143 | ||
On the other hand, a mixed halide Sn-based inorganic perovskite was also studied by Sabba et al.140 They systematically investigated CsSnI3−xBrx as a function of varying x from 0 to 3 to tune the band structure. As the Br ratio increased, the crystal structure changes from orthorhombic CsSnI3 to cubic CsSnBr3, accompanied with a blue shift of the absorption spectra (Fig. 9b). The current densities and voltage were significantly improved due to the decreased Sn vacancies. In addition, Gupta et al.141 found that the addition of SnF2 could decrease the work function of CsSnBr3, making its conduction band (CB) and valence band (VB) more close to the CB of TiO2 and the VB of spiro-OMeTAD (Fig. 9c). That is to say, the charge transfer at these two interfaces was improved. The final PCE of the fabricated solar cells was boosted from 0.01% of the pristine CsSnBr3 device to 2.1%. Additive engineering also employed vapour and acid.142,143 N2H4, as a reducing vapour atmosphere during the one-step spin coating, was supposed to react in the following way: 2SnI62− + N2H4 → 2SnI42− + N2 + 4HI (Fig. 9d), reducing Sn4+ and hence suppressing the carrier recombination in the prepared films. Hypo-phosphorous acid (HPA) was added to the precursor solution of CsSnIBr2, acting as a complex to promote the nucleation process while reducing the carrier mobility and charge carrier density in CsSnIBr2 films. Therefore, the Voc and FF of the HTM-free solar cells boosted the PCE up to 3.2% with highly stable performance for 77 days (Fig. 9e).
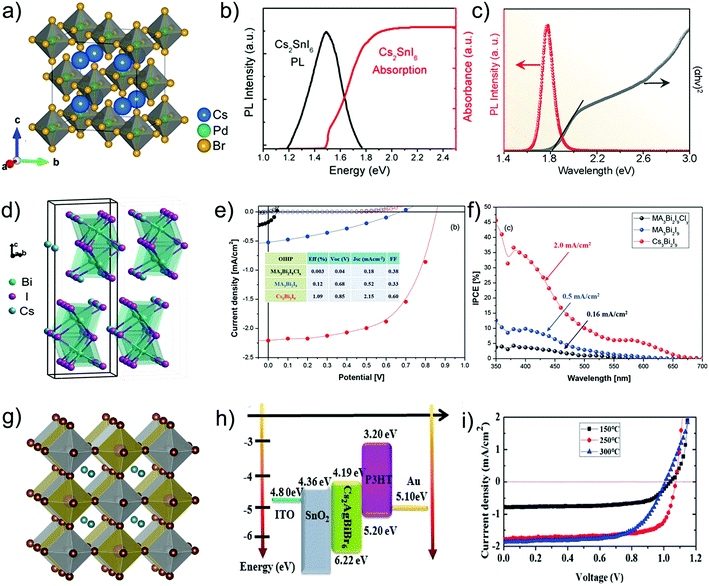 | ||
| Fig. 10 (a) Crystal structure diagram of the Cs2PdBr6 perovskite.121 (b) Absorption and PL spectra of the Cs2SnI6 film.43 (c) Tauc plot and PL spectrum of the Cs2TiBr6 thin film.144 (d) Schematic of the crystal structure of Cs3Bi2I9.148 (e) J–V curves and (f) IPCE curves for A3B2X9 perovskite solar cells using three different materials. (g) Crystal structure diagram of Cs2AgBiBr6.155 (h) Schematic of the energy alignment diagram of the Cs2AgBiBr6 perovskite solar cell. (i) J–V curves of Cs2AgBiBr6 perovskite solar cells at different annealing temperatures. | ||
Trivalent cations (i.e. Bi3+ and Sb3+) have also attracted much attention due to their potential photovoltaic properties for lead-free perovskite solar cell applications. For such trivalent cation substitution, the 3D perovskite structure transforms into a new chemical formula of A3B2X9 by removing 1/3 B-site cations from the original ABX3. Similar to the A2BX6 perovskite, A3B2X9 also exhibits a 0D nonperovskite structure as shown in Fig. 10d. A typical Cs3Bi2I9 perovskite was demonstrated to occupy a band gap of about 2.2 eV with instinct stability in an ambient atmosphere.148 However, these A3B2X9 perovskites show undesirable optoelectronic properties because of the low-dimensional structure induced electronic dimensionality. Although no hysteresis and high stability were observed in the FTO/m-TiO2/Cs3Bi2I9/spiro-OMeTAD/Ag device, only 1.09% PCE was exhibited because of the heavy loss in Jsc of merely 2.15 mA cm−2. Moreover, the inorganic Cs3Bi2I9 exhibits superior photovoltaic performance to the organic–inorganic hybrid MA3Bi2I9 as shown in Fig. 10e and f. Since then, many efforts have been devoted to improve the photovoltaic performance of Cs3B2X9-based perovskite solar cells.149–151 Kim et al.152 developed stable cubic-AgBi2I7 by dissolving BiI3 and AgI in n-butylamine. The corresponding solar cells achieved 1.22% photovoltaic conversion efficiency. Similarly, on account of the higher electron and hole mobility and better defect tolerance, Cs3Sb2I9 in the layered structure is a promising candidate for photovoltaic applications. Using the two-step thermal evaporation method, Saparov et al.90 avoided the presence of the dimer structure which is usually mixed with the layered structure when fabricated by the solution method. But the efficiency of the solar cells in the structure of FTO/c-TiO2/Cs3Sb2I9/PTAA/Au was not so satisfying, exhibiting a PCE lower than 1%, which was proposed to originate from the deep level defects in layered Cs3Sb2I9. Similar to traditional 3D lead perovskites, Rb was also applied to corporate with Cs or fully replace it in the layered ones.153 However, all of these research studies are under development and need a significant breakthrough in the conversion efficiency.
An inorganic halide double perovskite (Cs2B′B′′X6) formed through replacing two toxic Pb cations in the 3D perovskite crystal structure with a pair of nontoxic monovalent and trivalent metal cations is also a promising candidate to achieve high-efficiency and non-toxic inorganic perovskite photovoltaic devices. The Cs2B′B′′X6 structure provides the possibility of incorporating various organic and inorganic species into the A-site, various metal cations with different oxidation states into the B-site, and different halide anions into the X-site. The typical inorganic halide double perovskite crystal structure is shown in Fig. 10g. For example, in the Cs2AgBiBr6 crystal structure, both Ag+ and Bi3+ occupy the B-site of the crystal lattice with slightly varied metal halide bond lengths, which is attributed to the difference of the ionic radii of Ag+ (129 pm) and Bi3+ (117 pm) relative to that of Br− (183 pm). The changed ionic radii results in different atomic packing in the cubic unit cell. Recent reports revealed that the halide double perovskites Cs2B′B′′X6 with Ag+ in the B′-site and heavy halogens in the X-site yield smaller bandgaps, which are suitable for photovoltaic device application. Ning et al. first fabricated inorganic halide double perovskite Cs2AgBiBr6 solar cells and achieved a PCE of 1.22%.101 Although with unsatisfactory photovoltaic performance, this perovskite film still exhibited long carrier diffusion length of 110 nm and the photovoltaic device showed no hysteresis. Subsequently, by fine-tuning the film deposition parameters for growing high-quality Cs2AgBiBr6 films, Pantaler et al. also realized a hysteresis-free Cs2AgBiBr6 mesoporous double perovskite solar cell with increased Voc.154 However, it is worth noting that halide double perovskite materials such as Cs2AgBiBr6 exhibit low-energy-forming lattice defects, which improve the probability of defect emergence and ion migration in these materials. To overcome these issues, a number of film fabrication strategies have been designed to modulate the film morphology and improve the surface coverage for enhanced device performance. For instance, Wu et al. proposed a low-pressure-assisted annealing strategy to fabricate double perovskite planar heterojunction solar cells with a high quality Cs2AgBiBr6 film.155 Specifically, the perovskite solution was first spin-coated onto a glass/ITO substrate, followed by an annealing in a low-pressure chamber (20 Pa), achieving a uniform film with good crystallinity. The obtained device exhibited a PCE of up to 1.44%. In addition, the Cs2AgBiBr6 film morphology was also improved by an anti-solvent assisted annealing approach.156
3.5. Physical mechanism discussion
Inorganic perovskite materials exhibit a series of distinctive characteristics in their optoelectronic response which have a crucial influence on the photovoltaic performance, particularly for long-time response. In this section, a survey of recent advances concerning physical mechanisms both in inorganic perovskite materials and optoelectronic devices is provided, with the aim of comprehensively covering the generation, transport and recombination dynamics of excitons and charge carriers.The time-resolved photoluminescence of inorganic halide perovskites provide a wealth of information about the photophysical properties behind inorganic perovskite photovoltaic devices. Fig. 11a,b depict the PL dynamics of CsPbX3 with different halide compositions in nanocrystal forms.110 It is obvious that the PL decay rates of CsPbX3 decline generally with the variation of halides from Cl to Br to I, and the lifetimes of charge carriers change from several nanoseconds to several tens of nanoseconds. The experimental studies based on CsPbX3 perovskite films show a similar tendency of PL decays and lifetimes.115,119 As the ratio of I/Br increases, the PL decay rates decrease and the carrier lifetimes increase. Remarkably, the cubic phase CsPbI3 film exhibits an extra-long carrier lifetime of over 300 ns, which is much higher than that of organic–inorganic hybrid perovskites.109 In addition, as the bandgap broadens with the change of halides from I to Br to Cl, the radiative recombination becomes faster. However, the PL decay rate is much higher than the rate of bandgap variation with regard to the halide substitution. On the other hand, the transient PL decays of the CsPbX3 perovskites are not a single-exponential decay, demonstrating the presence of multichannel electron–hole recombination. Moreover, the multichannel electron–hole recombination detected in PL transient decays of CsPbX3 indicates that the radiative recombination originated from the multiple carrier states, demonstrating the processes of carrier-trapping in these inorganic halide perovskites.
 | ||
| Fig. 11 (a) Typical optical absorption and PL spectra of CsPbX3 nanocrystals. (b) Time-resolved PL decays for CsPbX3.110 (c) Transient absorption (TA) spectra of CsPbBr3 nanocrystals and temporal evolution of ΔmOD after photoexcitation at the (a) first excitonic band and (b) higher energy state.162 | ||
Ultrafast transient absorption spectroscopy can provide complementary information of time-revolved PL experiments. It has been revealed that hot-carrier relaxation dynamics in perovskite films causes the long carrier diffusion lengths, which is beneficial to the enhancement of power conversion efficiencies of solar cell devices.158–161 In contrast, light emitting devices prefer more efficient hot-carrier dynamics because the hot-carrier intra-band relaxations would compete with the charge carrier trapping. Fig. 11c depicts the dynamics of intra-band hot-carrier relaxation with respect to anion compositions. All the inorganic halide perovskite samples exhibit ultra-short cooling times on the scale of sub-picoseconds. Moreover, a general decline trend of cooling rates can be observed with the increase of the Br/I ratio, which is attributed to the overlap of phonons and holes in the Br-rich inorganic halide perovskites. The sub-picosecond hot-carrier cooling dynamics strikes down the restriction of photons and benefits the construction of the lowest transition states.162
Time-resolved terahertz spectroscopy is also an effective method to investigate the transient photoconductivity within inorganic halide perovskites.163 From the experimental results reported by Yettapu et al., a considerably large diffusion length (over 9.2 μm) and high carrier mobility (about 4500 cm2 V−1 s−1) are detected in the inorganic halide perovskite CsPbBr3.164 The excellent carrier transmission properties are greatly due to the negligible influence of surface defects on trapping charge carriers. The carrier transmission values of inorganic halide perovskites are comparable with or better than those of single-crystalline semiconductors, which demonstrates that inorganic halide perovskites have great potential for the investigations and applications in optoelectronic and photovoltaic devices.
For the investigations of perovskite photovoltaic devices, the exciton binding energy is one of the important parameters, which play a decisive role in the electron–hole separation ability and free charge carrier output under irradiation of sunlight. In general, a semiconductor with a low exciton binding energy value easily generates more free electrons and holes under an equivalent irradiation. In particular, recent studies have revealed the coexistence of excitons and free carriers in perovskite materials.166 However, compared with low exciton binding energy values obtained in organic–inorganic perovskites (2–10 meV), inorganic halide perovskites show a higher exciton binding energy, representing a huge energy barrier for exciton separation as the intrinsic thermal energy can be obtained even at room temperature.22,160,167 The relatively high exciton binding energy values in inorganic halide perovskites may cause insufficient free charge carriers through exciton dissociation at room temperature.165 Therefore, in contrast to nearly full separation of excitons in organic–inorganic halide perovskites due to their extremely low binding energy, it is necessary to design the inorganic halide perovskites with lower exciton binding energy or construct a reasonable interfacial structure and compositions with increasing exciton dissociation rates in inorganic perovskite photovoltaic devices.
As the size of the inorganic halide perovskite crystal decreases to be smaller than the Bohr diameter of the exciton, the quantum confinement effect and dielectric effect would be the decisive influencing factors. As a result, the exciton binding energies of inorganic halide perovskite nanocrystals are higher than those of their bulk materials, contributing to the high photoluminescence quantum yield. Inorganic perovskite CsPbBr3 nanocrystals generally exhibit a higher quantum yield in the weak-confinement regime, where the sizes of the nanocrystals are comparable to or even larger than the Bohr excitonic diameter.110,168 The differences in optical behaviours between traditional chalcogen-based nanocrystals and inorganic halide perovskite nanocrystals might originate from the intrinsic electronic structures, whereas the similar effective mass of electrons and holes in CsPbBr3 nanocrystals result in a nearly identical confinement for both electrons and holes, which, in turn, increases the probability of radiative transition by the enhanced overlap effect between the electron–hole wave function.169,170 However, owing to the inhomogeneity in size and the existence of organic ligands on the surface, it is inaccurate to estimate the exciton binding energy of inorganic halide perovskite nanocrystals based on temperature-dependent emission or absorption measurements.
 | ||
| Fig. 12 (a) Excited-state distribution profiles of CsPbI2Br for Δt = 0 ps (red diamonds) and Δt = 800 ps (black circles). (b) Plot of Δβ2vs. Δt: the slope is proportional to the diffusion constant, and error bars correspond to 90% confidence intervals.174 (c) Histogram of diffusion constants measured for 12 individual domains. (d and e) Ionic conductivity of CsPbI2Br films under different light intensities. (f) Ionic conductivity of MAPbI3 films under different light intensities.177 | ||
In addition, the ion migration behaviour is also compared between inorganic perovskites and organic–inorganic hybrid perovskites.177 By calculating the ionic conductivity as a function of temperature and light intensity, the activation energy of ion migration can be obtained. The fitted parameters revealed that the ion migration energy barrier of MAPbI3 decreased from 0.62 to 0.07 eV with light illumination, and in contrast, the value for CsPbI2Br remained constant (around 0.45 eV) (Fig. 12d–f). This result demonstrated that light illumination had no effect on ion migration in inorganic halide perovskites. This difference was also demonstrated by PL measurements, showing suppressed photo-induced halide ion segregation in inorganic perovskites. The photovoltaic perovskite stability of the solar devices was examined under continuous illumination, exhibiting that the CsPbI2Br-based solar cell retained its initial efficiency for 1500 h, while the MAPbI3-based solar cell degraded after 50 h. Therefore, inorganic halide perovskites have potential as an alternative to organic–inorganic hybrid perovskites as photostable photovoltaic materials.
4. Conclusions and outlook
In summary, a survey of the literature reveals the merits and demerits of inorganic halide perovskite materials and photovoltaic devices. During the early stage of perovskite development, composition tuning was investigated to pursue the widest light absorption range of the cubic phase. With the development of various chemical strategies, stable cubic phase inorganic halide perovskites have been successfully produced. Furthermore, a variety of engineering strategies for the aspects of composition, structure, interface, and dimensions have been proposed to enrich inorganic perovskite material systems, optimize optoelectronic device configurations and improve the optoelectronic performance of inorganic PSCs. These studies have led to unprecedented progress in fabricating high-performance inorganic perovskite photovoltaics with over 17% efficiency by various research groups via different material and configuration modulation strategies.Very recently, all-inorganic PSCs using a carbon counter electrode or novel inorganic transport materials have been developed based on several studies, resulting in lower cost and longer lifetime. For the commercialization of these novel photovoltaic techniques, it is still of primary importance to fabricate high-stability and low-cost PSCs. In addition, despite the exciting progress in stability and efficiency, the presence of toxic lead in their composition is still regarded as one of the major limiting factors hindering their commercialization. Thus, addressing the toxicity issues in these compounds by developing lead-free inorganic perovskites represents a promising direction in the perovskite research field. Furthermore, inorganic halide perovskites with a bandgap of around 1.7 eV can be the most competitive top cells for tandem photovoltaic devices matched with silicon solar cells. For this purpose, cesium lead iodide (CsPbI3) perovskites have become a promising candidate due to their desirable bandgap and excellent thermal stability. Finally, in addition to the advances in inorganic perovskite device performance, further investigations including theoretical calculations and photophysical characterization toward bulk and polycrystalline materials will help to obtain a better understanding towards the fundamental properties of inorganic perovskites.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Bo Li and Lin Fu contributed equally to this work. We acknowledge support from a project supported by the State Key Program of National Natural Science of China (no. 51532005), the National Natural Science Foundation of China (no. 51872171, 51272137), and the Tai Shan Scholar Foundation of Shandong Province.References
- J. Burschka, N. Pellet, S. J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Gratzel, Nature, 2013, 499, 316 CrossRef CAS PubMed
.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395 CrossRef CAS PubMed
.
- H. S. Kim, C. R. Lee, J. H. Im, K. B. Lee, T. Moehl, A. Marchioro, S. J. Moon, R. Humphry-Baker, J. H. Yum, J. E. Moser, M. Gr_tzel and N. G. Park, Sci. Rep., 2012, 2, 591 CrossRef PubMed
.
- H. Zhou, Q. Chen, G. Li, S. Luo, T. B. Song, H. S. Duan, Z. Hong, J. You, Y. Liu and Y. Yang, Science, 2014, 345, 542 CrossRef CAS PubMed
.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. I. Seok, Nat. Mater., 2014, 13, 897 CrossRef CAS PubMed
.
- N. J. Jeon, J. H. Noh, W. S. Yang, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Nature, 2015, 517, 476 CrossRef CAS PubMed
.
- J. H. Heo, S. H. Im, J. H. Noh, T. N. Mandal, C. S. Lim, J. A. Chang, Y. H. Lee, H. J. Kim, A. Sarkar, M. K. Nazeeruddin, M. Gratzel and S. I. Seok, Nat. Photonics, 2013, 7, 486 CrossRef CAS
.
- D. Liu and T. L. Kelly, Nat. Photonics, 2014, 8, 133 CrossRef CAS
.
- Q. Chen, H. Zhou, Z. Hong, S. Luo, H. S. Duan, H. H. Wang, Y. Liu, G. Li and Y. Yang, J. Am. Chem. Soc., 2014, 136, 622 CrossRef CAS PubMed
.
- G. E. Eperon, V. M. Burlakov, P. Docampo, A. Goriely and H. J. Snaith, Adv. Funct. Mater., 2014, 24, 151 CrossRef CAS
.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050 CrossRef CAS PubMed
.
- F. Bella, G. Griffini, J.-P. Correa-Baena, G. Saracco, M. Gratzel, A. Hagfeldt, S. Turri and C. Gerbaldi, Science, 2016, 354, 203 CrossRef CAS PubMed
.
- W. Chen, Y. Wu, Y. Yue, J. Liu, W. Zhang, X. Yang, H. Chen, E. Bi, I. Ashraful, M. Graetzel and L. Han, Science, 2015, 350, 944 CrossRef CAS PubMed
.
- L. Dou, A. B. Wong, Y. Yu, M. Lai, N. Kornienko, S. W. Eaton, A. Fu, C. G. Bischak, J. Ma, T. Ding, N. S. Ginsberg, L.-W. Wang, A. P. Alivisatos and P. Yang, Science, 2015, 349, 1518 CrossRef CAS PubMed
.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643 CrossRef CAS PubMed
.
- X. Li, D. Bi, C. Yi, J.-D. Decoppet, J. Luo, S. M. Zakeeruddin, A. Hagfeldt and M. Gratzel, Science, 2016, 353, 58 CrossRef CAS PubMed
.
- D. P. McMeekin, G. Sadoughi, W. Rehman, G. E. Eperon, M. Saliba, M. T. Hoerantner, A. Haghighirad, N. Sakai, L. Korte, B. Rech, M. B. Johnston, L. M. Herz and H. J. Snaith, Science, 2016, 351, 151 CrossRef CAS PubMed
.
- A. Mei, X. Li, L. Liu, Z. Ku, T. Liu, Y. Rong, M. Xu, M. Hu, J. Chen, Y. Yang, M. Graetzel and H. Han, Science, 2014, 345, 295 CrossRef CAS PubMed
.
- H. Tan, A. Jain, O. Voznyy, X. Lan, F. P. G. de Arquer, J. Z. Fan, R. Quintero-Bermudez, M. Yuan, B. Zhang, Y. Zhao, F. Fan, P. Li, L. N. Quan, Y. Zhao, Z.-H. Lu, Z. Yang, S. Hoogland and E. H. Sargent, Science, 2017, 355, 722 CrossRef CAS PubMed
.
- Y. Zhang, B. Li, L. Y. Zhang and L. Yin, Sol. Energy Mater. Sol. Cells, 2017, 170, 187 CrossRef CAS
.
- S. D. Stranks, G. E. Eperon, G. Grancini, C. Menelaou, M. J. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Science, 2013, 342, 341 CrossRef CAS PubMed
.
- Q. Lin, A. Armin, R. C. R. Nagiri, P. L. Burn and P. Meredith, Nat. Photonics, 2015, 9, 106 CrossRef CAS
.
- W. Nie, H. Tsai, R. Asadpour, J.-C. Blancon and A. J. Neukirch, Science, 2015, 347, 522 CrossRef CAS PubMed
.
- Q. Dong, Y. Fang, Y. Shao, P. Mulligan, J. Qiu, L. Cao and J. Huang, Science, 2015, 347, 967 CrossRef CAS PubMed
.
- D. W. deQuilettes, S. M. Vorpahl, S. D. Stranks, H. Nagaoka, G. E. Eperon, M. E. Ziffer, H. J. Snaith and D. S. Ginger, Science, 2015, 348, 683 CrossRef CAS PubMed
.
- B. Li, Y. Zhang, L. Zhang and L. Yin, Adv. Mater., 2017, 29, 1701221 CrossRef PubMed
.
- N. Tsvetkov, Q. Lu, L. Sun, E. J. Crumlin and B. Yildiz, Nat. Mater., 2016, 15, 1010 CrossRef CAS PubMed
.
- J. You, L. Meng, T.-B. Song, T.-F. Guo, Y. Yang, W.-H. Chang, Z. Hong, H. Chen, H. Zhou, Q. Chen, Y. Liu, N. De Marco and Y. Yang, Nat. Nanotechnol., 2016, 11, 75 CrossRef CAS PubMed
.
- N. Ahn, K. Kwak, M. S. Jang, H. Yoon, B. Y. Lee, J.-K. Lee, P. V. Pikhitsa, J. Byun and M. Choi, Nat. Commun., 2016, 7, 13422 CrossRef CAS PubMed
.
- Y. Bai, Q. Dong, Y. Shao, Y. Deng, Q. Wang, L. Shen, D. Wang, W. Wei and J. Huang, Nat. Commun., 2016, 7, 12806 CrossRef CAS PubMed
.
- Y. Li, J. K. Cooper, W. Liu, C. M. Sutter-Fella, M. Amani, J. W. Beeman, A. Javey, J. W. Ager, Y. Liu, F. M. Toma and I. D. Sharp, Nat. Commun., 2016, 7, 12446 CrossRef CAS PubMed
.
- Q. Tai, P. You, H. Sang, Z. Liu, C. Hu, H. L. W. Chan and F. Yan, Nat. Commun., 2016, 7, 11105 CrossRef CAS PubMed
.
- Y. Zhao, J. Wei, H. Li, Y. Yan, W. Zhou, D. Yu and Q. Zhao, Nat. Commun., 2016, 7, 10228 CrossRef CAS PubMed
.
- K. O. Brinkmann, J. Zhao, N. Pourdavoud, T. Becker, T. Hu, S. Olthof, K. Meerholz, L. Hoffmann, T. Gahlmann, R. Heiderhoff, M. F. Oszajca, N. A. Luechinger, D. Rogalla, Y. Chen, B. Cheng and T. Riedl, Nat. Commun., 2017, 8, 13938 CrossRef CAS PubMed
.
- Y. Yang and J. You, Nature, 2017, 544, 155 CrossRef CAS PubMed
.
- S. Chen, K. R. Choudhury, J. Subbiah, C. M. Amb, J. R. Reynolds and F. So, Adv. Energy Mater., 2011, 1, 963 CrossRef CAS
.
- P. Docampo and T. Bein, Acc. Chem. Res., 2016, 49, 339 CrossRef CAS PubMed
.
- Y. Rong, L. Liu, A. Mei, X. Li and H. Han, Adv. Energy Mater., 2015, 5, 1501066 CrossRef
.
- T. Leijtens, G. E. Eperon, N. K. Noel, S. N. Habisreutinger, A. Petrozza and H. J. Snaith, Adv. Energy Mater., 2015, 5, 1500963 CrossRef
.
- T. A. Berhe, W. Su, C. Chen, C. Pan, J. Cheng, H. Chen, M. Tsai, L. Chen, A. A. Dubale and B. Hwang, Energy Environ. Sci., 2016, 9, 323 RSC
.
- M. Ye, X. Hong, F. Zhang and X. Liu, J. Mater. Chem. A, 2016, 4, 6755 RSC
.
- H. T. Nguyen, Z. Ku and H. J. Fan, Adv. Energy Mater., 2016, 6, 1501420 CrossRef
.
- A. B. Djurisic, F. Liu, A. M. C. Ng, Q. Dong, M. K. Wong, A. Ng and C. Surya, Phys. Status Solidi RRL, 2016, 10, 281 CrossRef CAS
.
- Z. Li, M. Yang, J. S. Park, S. H. Wei, J. J. Berry and K. Zhu, Chem. Mater., 2016, 28, 284 CrossRef CAS
.
- X. Xia, W. Wu, H. Li, B. Zheng, Y. Xue, J. Xu, D. Zhang, C. Gaob and X. Liu, RSC Adv., 2016, 6, 14792 RSC
.
- T. Liu, Y. Zong, Y. Zhou, M. Yang, Z. Li, O. S. Game, K. Zhu, R. Zhu, Q. Gong and N. P. Padture, Chem. Mater., 2017, 29, 3246 CrossRef CAS
.
- W. Zhu, C. Bao, F. Li, T. Yu, H. Gao, Y. Yi, J. Yang, G. Fu, X. Zhou and Z. Zou, Nano Energy, 2016, 19, 17 CrossRef CAS
.
- X. Zhang, X. Ren, B. Liu, R. Munir, X. Zhu, D. Yang, J. Li, Y. Liu, D. M. Smilgies, R. Li, Z. Yang, T. Niu, X. Wang, A. Amassian, K. Zhao and S. F. Liu, Energy Environ. Sci., 2017, 10, 2095 RSC
.
- M. Kulbak, D. Cahen and G. Hodes, J. Phys. Chem. Lett., 2015, 6, 2452 CrossRef CAS PubMed
.
- M. Kulbak, S. Gupta, N. Kedem, I. Levine, T. Bendikov, G. Hodes and D. Cahen, J. Phys. Chem. Lett., 2016, 7, 167 CrossRef CAS PubMed
.
- R. J. Sutton, G. E. Eperon, L. Miranda, E. S. Parrott, B. A. Kamino, J. B. Patel, M. T. Hörantner, M. B. Johnston, A. A. Haghighirad, D. T. Moore and H. J. Snaith, Adv. Energy Mater., 2016, 6, 1502458 CrossRef
.
- C.-Y. Chen, H.-Y. Lin, K.-M. Chiang, W.-L. Tsai, Y.-C. Huang, C.-S. Tsao and H.-W. Lin, Adv. Mater., 2017, 29, 1605290 CrossRef PubMed
.
- A. Swarnkar, A. R. Marshall, E. M. Sanehira, B. D. Chernomordik, D. T. Moore, J. A. Christians, T. Chakrabarti and J. M. Luther, Science, 2016, 354, 92 CrossRef CAS PubMed
.
- G. E. Eperon, S. D. Stranks and C. Menelaou, Energy Environ. Sci., 2014, 7, 982–988 RSC
.
- G. Kieslich, S. Sun and A. K. Cheetham, Chem. Sci., 2014, 5, 4712 RSC
.
- C. C. Stoumpos, C. D. Malliakas, J. A. Peters, Z. Liu, M. Sebastian, J. Im, T. C. Chasapis, A. C. Wibowo, D. Y. Chung, A. J. Freeman, B. W. Wessels and M. G. Kanatzidis, Cryst. Growth Des., 2013, 13, 2722 CrossRef CAS
.
- U. Maitra, U. Gupta, M. De, R. Datta, A. Govindaraj and C. N. R. Rao, Angew. Chem., Int. Ed., 2013, 52, 13057 CrossRef CAS PubMed
.
- M. Sakata, T. Nishiwaki and J. Harada, J. Phy. Soc. Jpn, 1979, 47, 232 CrossRef CAS
.
- R. J. Sutton, M. R. Filip and A. A. Haghighirad, ACS Energy Lett., 2018, 3, 1787 CrossRef CAS
.
- F. Bertolotti, L. Protesescu, M. Kovalenko, S. Yakunin, A. Cervellino, S. Billinge, M. Terban, J. Pedersen, N. Masciocchi and A. Guagliardi, ACS Nano, 2017, 11, 3819 CrossRef CAS PubMed
.
- P. Cottingham and R. Brutchey, Chem. Commun., 2016, 52, 5246 RSC
.
- G. E. Eperon, G. M. Paterno, R. J. Sutton, A. Zampetti, A. A. Haghighirad, F. Caciallibc and H. J. Snaith, J. Mater. Chem. A, 2015, 3, 19688 RSC
.
- R. Beal, D. Slotcavage, T. Leijtens, A. Bowring, R. Belisle, W. Nguyen, G. Burkhard, E. Hoke and M. McGehee, J. Phys. Chem. Lett., 2016, 7, 746 CrossRef CAS PubMed
.
- W. Yin, Y. Yan and S. Wei, J. Phys. Chem. Lett., 2014, 5, 3625 CrossRef CAS PubMed
.
- A. Swarnkar, V. K. Ravi and A. Nag, ACS Energy Lett., 2017, 2, 1089 CrossRef
.
- C. C. Stoumpos and M. G. Kanatzidis, Adv.
Mater., 2016, 28, 5778 CrossRef CAS PubMed
.
- R. A. Jishi, O. B. Ta and A. A. Sharif, J. Phys. Chem. C, 2014, 118, 28344 CrossRef CAS
.
- M. Sebastian, J. A. Peters, C. C. Stoumpos, J. Im, S. S. Kostina, Z. Liu, M. G. Kanatzidis, A. J. Freeman and B. W. Wessels, Phys. Rev. B, 2015, 92, 235210 CrossRef
.
- X. Li, Y. Wu, S. Zhang, B. Cai, Y. Gu, J. Song and H. Zeng, Adv. Funct. Mater., 2016, 26, 2435 CrossRef CAS
.
- X. Li, F. Cao, D. Yu, J. Chen, Z. Sun, Y. Shen, Y. Zhu, L. Wang, Y. Wei, Y. Wu and H. Zeng, Small, 2017, 13, 1603996 CrossRef PubMed
.
- Q. Chen, N. De Marco, Y. Yang, T.-B. Song, C.-C. Chen, H. Zhao, Z. Hong, H. Zhou and Y. Yang, Nano Today, 2015, 10, 355 CrossRef CAS
.
- Y. Wang and H. Sun, Small Methods, 2018, 2, 1700252 CrossRef
.
- K. Heidrich, W. Schafer, M. Schreiber, J. Sochtig, G. Trendel, J. Treusch, T. Grandke and H. J. Stolz, Phys. Rev. B, 1981, 24, 5642 CrossRef CAS
.
- V. K. Ravi, G. B. Markad and A. Nag, ACS Energy Lett., 2016, 1, 665 CrossRef CAS
.
- M. Ahmad, G. Rehman, L. Ali, M. Shafiq, R. Iqbal, R. Ahmad, T. Khan, S. Jalali-Asadabadi, M. Maqbool and I. Ahmad, J. Alloys Compd., 2017, 705, 828 CrossRef CAS
.
- J. Even, J. Phys. Chem. Lett., 2015, 6, 2238 CrossRef CAS PubMed
.
- V. K. Ravi, G. B. Markad and A. Nag, ACS Energy Lett., 2016, 1, 665 CrossRef CAS
.
- Q. V. Le, M. Park, W. Sohn, H. W. Jang and S. Y. Kim, Adv. Electron. Mater., 2017, 1600388 Search PubMed
.
- J. Kang and L.-W. Wang, J. Phys. Chem. Lett., 2017, 8, 489 CrossRef CAS PubMed
.
- L. Peedikakkandy and P. Bhargava, RSC Adv., 2016, 6, 19857 RSC
.
- T. C. Jellicoe, J. M. Richter, H. F. J. Glass, M. Tabachnyk, R. Brady, S. E. Dutton, A. Rao, R. H. Friend, D. Credgington, N. C. Greenham and M. L. Bohm, J. Am. Chem. Soc., 2016, 138, 2941 CrossRef CAS PubMed
.
- L. C. Tang, C. S. Chang and J. Y. Huang, J. Phys.: Condens. Matter, 2000, 12, 9129 CrossRef CAS
.
- L. C. Tang, J. Y. Huang, C. S. Chang, M. H. Lee and L. Q. Liu, J. Phys.: Condens. Matter, 2005, 17, 7275 CrossRef CAS
.
- L. Kang, D. M. Ramo, Z. Lin, P. D. Bristowe, J. Qin and C. Chen, J. Mater. Chem. C, 2013, 1, 7363 RSC
.
- G. R. Berdiyorov, A. Kachmar, F. El-Mellouhi, M. A. Carignano and M. El-Amine Madjet, J. Phys. Chem. C, 2016, 120, 16259 CrossRef CAS
.
- J. Brgoch, A. J. Lehner, M. Chabinyc and R. Seshadri, J. Phys. Chem. C, 2014, 118(48), 27721 CrossRef CAS
.
- A. Marronnier, G. Roma, S. Boyer-Richard, L. Pedesseau, J.-M. Jancu, Y. Bonnassieux, C. Katan, C. C. Stoumpos, M. G. Kanatzidis and J. Even, ACS Nano, 2018, 12, 3477 CrossRef CAS PubMed
.
- G. Xiao, Y. Cao, G. Qi, L. Wang, C. Liu, Z. Ma, X. Yang, Y. Sui, W. Zheng and B. Zou, J. Am. Chem. Soc., 2017, 139, 10087 CrossRef CAS PubMed
.
- N. Sakai, A. A. Haghighirad, M. R. Filip, P. K. Nayak, S. Nayak, A. Ramadan, Z. Wang, F. Giustino and H. J. Snaith, J. Am. Chem. Soc., 2017, 139, 6030 CrossRef CAS PubMed
.
- B. Saparov, F. Hong, J. -P. Sun, H. -S. Duan, W. Meng, S. Cameron, I. G. Hill, Y. Yan and D. B. Mitzi, Chem. Mater., 2015, 27, 5622 CrossRef CAS
.
- U. G. Jong, C. J. Yu, Y. H. Kye, Y. G. Choe, W. Hao and S. Li, Inorg. Chem., 2019, 58, 4134 CrossRef CAS PubMed
.
- Y.-Y. Sun, M. L. Agiorgousis, P. Zhang and S. Zhang, Nano Lett., 2015, 15, 581 CrossRef CAS PubMed
.
- J. Duan, Y. Zhao, B. He and Q. Tang, Small, 2018, 14, 1704443 CrossRef PubMed
.
- Y. Zhao and K. Zhu, Chem. Soc. Rev., 2016, 45, 655 RSC
.
- Q. Ma, S. Huang, X. Wen, M. A. Green and A. W. Y. Ho-Baillie, Adv. Energy Mater., 2016, 6, 1502202 CrossRef
.
- E. M. Hutter, R. J. Sutton, S. Chandrashekar, M. Abdi-Jalebi, S. D. Stranks, H. J. Snaith and T. J. Savenije, ACS Energy Lett., 2017, 2, 1901 CrossRef CAS PubMed
.
- Z. Yu, M. Leilaeioun and Z. Holman, Nat. Energy, 2016, 1, 16137 CrossRef
.
- X. Qiu, Y. Jiang, H. Zhang, Z. Qiu, S. Yuan, P. Wang and B. Cao, Phys. Status Solidi RRL, 2016, 10, 587 CrossRef CAS
.
- N. Sakai, A. A. Haghighirad, M. R. Filip, P. K. Nayak, S. Nayak, A. Ramadan, Z. Wang, F. Giustino and H. J. Snaith, J. Am. Chem. Soc., 2017, 139, 6030 CrossRef CAS PubMed
.
- J. Xu, J. B. Liu, B. X. Liu and B. Huang, J. Phys. Chem. Lett., 2017, 8(18), 4391 CrossRef CAS PubMed
.
- W. Ning, F. Wang, B. Wu, J. Lu, Z. Yan, X. Liu, Y. Tao, J.-M. Liu, W. Huang, M. Fahlman, L. Hultman, T. C. Sum and F. Gao, Adv. Mater., 2018, 30, 1706246 CrossRef PubMed
.
- Z. Zeng, J. Zhang, X. Gan, H. Sun, M. Shang, D. Hou, C. Lu, R. Chen, Y. Zhu and L. Han, Adv. Energy Mater., 2018, 8, 1801050 CrossRef
.
- E. Sanehira, A. Marshall, J. Christians, S. Harvey, P. Ciesielski, L. Wheeler, P. Schulz, L. Lin, M. Beard and J. Luther, Sci. Adv., 2017, 3, 4204 CrossRef PubMed
.
- H. Choi, J. Jeong, H.-B. Kim, S. Kim, B. Walker, G.-H. Kim and J. Y. Kim, Nano Energy, 2014, 7, 80 CrossRef CAS
.
- L. A. Frolova, D. V. Anokhin, A. A. Piryazev, S. Y. Luchkin, N. N. Dremova, K. J. Stevenson and P. A. Troshin, J. Phys. Chem. Lett., 2017, 8, 67 CrossRef CAS PubMed
.
- Y. Hu, F. Bai, X. Liu, Q. Ji, X. Miao, T. Qiu and S. Zhang, ACS Energy Lett., 2017, 2, 2219 CrossRef CAS
.
- Y. Fu, M. T. Rea, J. Chen, D. J. Morrow, M. P. Hautzinger, Y. Zhao, D. Pan, L. H. Manger, J. C. Wright, R. H. Goldsmith and S. Jin, Chem. Mater., 2017, 29, 8385 CrossRef CAS
.
- Q. Wang, X. Zheng, Y. Deng, J. Zhao, Z. Chen and J. Huang, Joule, 2017, 1, 371 CrossRef CAS
.
- B. Li, Y. Zhang, L. Fu, T. Yu, S. Zhou, L. Zhang and L. Yin, Nat. Commun., 2018, 9, 1076 CrossRef PubMed
.
- L. Protesescu, S. Yakunin, M. Bodnarchuk, F. Kriegm, R. Caputo, C. Hendon, R. Yang, A. Walsh and M. Kovalenko, Nano Lett., 2015, 15, 3692 CrossRef CAS PubMed
.
- G. Nedelcu, L. Protesescu, S. Yakunin, M. I. Bodnarchuk, M. J. Grotevent and M. V. Kovalenko, Nano Lett., 2015, 15, 5635 CrossRef CAS PubMed
.
- Q. Akkerman, V. D'Innocenzo, S. Accornero, A. Scarpellini, A. Petrozza, M. Prato and L. Manna, J. Am. Chem. Soc., 2015, 137, 10276 CrossRef CAS PubMed
.
- G. Liao, J. Duan, Y. Zhao and Q. Tang, Sol. Energy, 2018, 171, 279 CrossRef CAS
.
- L. Fu, Y. Zhang, B. Chang, B. Li, S. Zhou, L. Zhang and L. Yin, J. Mater. Chem. A, 2018, 6, 13263 RSC
.
- B. Li, Y. Zhang, L. Zhang and L. Yin, J. Power Sources, 2017, 360, 11 CrossRef CAS
.
- Q. Van Le, M. Park, W. Sohn, H. W. Jang and S. Y. Kim, Adv. Electrode Mater., 2017, 3, 1600448 CrossRef
.
- Y. Lou, Y. Niu, D. Yang, Q. Xu, Y. Hu, Y. Shen, J. Ming, J. Chen, L. Zhang and Y. Zhao, Nano Res., 2018, 11, 2715 CrossRef CAS
.
- M. Saliba, T. Matsui, K. Domanski, J. -Y. Seo, A. Ummadisingu, S. M. Zakeeruddin, J. -P. Correa-Baena, W. R. Tress, A. Abate and A. Hagfeldt, Science, 2016, 354, 206 CrossRef CAS PubMed
.
- J. K. Nam, S. U. Chai, W. Cha, Y. J. Choi, W. Kim, M. S. Jung, J. Kwon, D. Kim and J. H. Park, Nano Lett., 2017, 17, 2028 CrossRef CAS PubMed
.
- Y. Li, J. Duan, H. Yuan, Y. Zhao, B. He and Q. Tang, Sol. RRL, 2018, 2, 1800164 CrossRef
.
- J. Liang, P. Zhao, C. Wang, Y. Wang, Y. Hu, G. Zhu, L. Ma, J. Liu and Z. Jin, J. Am. Chem. Soc., 2017, 139, 14009 CrossRef CAS PubMed
.
- N. Li, Z. Zhu, J. Li, A. K. Y. Jen and L. Wang, Adv. Energy Mater., 2018, 8, 1800525 CrossRef
.
- S. Zou, Y. Liu, J. Li, C. Liu, R. Feng, F. Jiang, Y. Li, J. Song, H. Zeng, M. Hong and X. Chens, J. Am. Chem. Soc., 2017, 139, 11443 CrossRef CAS PubMed
.
- J. Liang, Z. Liu, L. Qiu, Z. Hawash, L. Meng, Z. Wu, Y. Jiang, L. K. Ono and Y. Qi, Adv. Energy Mater., 2018, 499, 1800504 CrossRef
.
- D. Bai, J. Zhang, Z. Jin, H. Bian, K. Wang, H. Wang, L. Liang, Q. Wang and S. F. Liu, ACS Energy Lett., 2018, 3, 970 CrossRef CAS
.
- C. F. J. Lau, M. Zhang, X. Deng, J. Zheng, J. Bing, Q. Ma, J. Kim, L. Hu, M. A. Green, S. Huang and A. H. Baillie, ACS Energy Lett., 2017, 2, 2319 CrossRef CAS
.
- Q. Zeng, X. Zhang, X. Feng, S. Lu, Z. Chen, X. Yong and W. Zhang, Adv. Mater., 2018, 30, 1705393 CrossRef PubMed
.
- H. Cho, S. H. Jeong, M. H. Park, Y. H. Kim, C. Wolf, C. L. Lee, J. H. Heo, A. Sadhanala, N. Myoung, S. Yoo, S. H. Im, R. H. Friend and T. W. Lee, Science, 2015, 350, 1222 CrossRef CAS PubMed
.
- R. Lindblad, N. K. Jena, B. Philippe, J. Oscarsson, D. Bi, A. Lindblad, S. Mandal, B. Pal, D. D. Sarma, O. Karis, H. Siegbahn, E. M. J. Johansson, M. Odelius and H. Rensmo, J. Phys. Chem. C, 2015, 119, 1818 CrossRef CAS
.
- J. Yuan, L. Zhang, C. Bi, M. Wang and J. Tian, Sol. RRL, 2018, 2, 1800188 CrossRef
.
- Y. Wang, T. Zhang, M. Kan and Y. Zhao, J. Am. Chem. Soc., 2018, 140, 12345 CrossRef CAS PubMed
.
- K. Wang, Z. Jin, L. Liang, H. Bian, D. Bai and H. Wang, Nat. Commun., 2018, 9, 4544 CrossRef PubMed
.
- G. Liao, J. Duan, Y. Zhao and Q. Tang, Sol. Energy, 2018, 171, 279 CrossRef CAS
.
- M. Aamir, T. Adhikari, M. Sher, N. Revaprasadu, W. Khalid, J. Akhtar and J.-M. Nunzi, New J. Chem., 2018, 42, 14104 RSC
.
- L. Yan, Q. Xue, M. Liu, Z. Zhu, J. Tian, Z. Li, Z. Chen, H. Yan, H.-L. Yip and Y. Cao, Adv. Mater., 2018, 1802509 CrossRef PubMed
.
- X. Chang, W. Li, L. Zhu, H. Liu, H. Geng, S. Xiang, H. Chen, J. Liu and H. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 33649 CrossRef CAS PubMed
.
- I. Chung, B. Lee, J. He, R. P. H. Chang and M. G. Kanatzidis, Nature, 2012, 485, 486 CrossRef CAS PubMed
.
- Z. Chen, J. J. Wang, Y. Ren, C. Yu and K. Shum, Appl. Phys. Lett., 2012, 101, 093901 CrossRef
.
- M. H. Kumar, S. Dharani, W. L. Leong, P. P. Boix, R. R. Prabhakar, T. Baikie, C. Shi, H. Ding, R. Ramesh, M. Asta, M. Graetzel, S. G. Mhaisalkar and N. Mathews, Adv. Mater., 2014, 26, 7122 CrossRef CAS PubMed
.
- D. Sabba, H. K. Mulmudi, R. R. Prabhakar, T. Krishnamoorthy, T. Baikie, P. P. Boix, S. Mhaisalkar and N. Mathews, J. Phys. Chem. C, 2015, 119, 1763 CrossRef CAS
.
- S. Gupta, T. Bendikov, G. Hodes and D. Cahen, ACS Energy Lett., 2016, 1, 1028 CrossRef CAS
.
- T.-B. Song, T. Yokoyama, C. C. Stoumpos, J. Logsdon, D. H. Cao, M. R. Wasielewski, S. Aramaki and M. G. Kanatzidis, J. Am. Chem. Soc., 2017, 139, 836 CrossRef CAS PubMed
.
- W. Li, J. Li, J. Li, J. Fan, Y. Mai and L. Wang, J. Mater. Chem. A, 2016, 4, 17104 RSC
.
- M. Chen, M. G. Ju, A. D. Carl, Y. Zong, R. L. Grimm, J. Gu, X. C. Zeng, Y. Zhou and N. P. Padture, Joule, 2018, 2, 558 CrossRef CAS
.
- X. Qiu, B. Cao, S. Yuan, X. Chen, Z. Qiu, Y. Jiang, Q. Ye, H. Wang, H. Zeng, J. Liu and M. G. Kanatzidis, Solar Energy Mater, Sol. Cells, 2017, 159, 227 CrossRef CAS
.
- B. Lee, C. C. Stoumpos, N. Zhou, F. Hao, C. Malliakas, C.-Y. Yeh, T. J. Marks, M. G. Kanatzidis and R. P. H. Chang, J. Am. Chem. Soc., 2014, 136, 15379 CrossRef CAS PubMed
.
- B. Lee, A. Krenselewski, S. I. Baik, D. N. Seidman and R. P. H. Chang, Sustainable Energy Fuels, 2017, 1, 710 RSC
.
- B.-W. Park, B. Philippe, X. Zhang, H. Rensmo, G. Boschloo and E. M. J. Johansson, Adv. Mater., 2015, 27, 6806 CrossRef PubMed
.
- M. Abulikemu, S. Ould-Chikh, X. Miao, E. Alarousu, B. Murali, G. O. Ngongang Ndjawa, J. Barbé, A. El Labban, A. Amassian and S. Del Gobbo, J. Mater. Chem. A, 2016, 4, 12504 RSC
.
- X. Zhang, G. Wu, Z. Gu, B. Guo, W. Liu, S. Yang, T. Ye, C. Chen, W. Tu and H. Chen, Nano Res., 2016, 9, 2921 CrossRef CAS
.
- M. Lyu, J.-H. Yun, M. Cai, Y. Jiao, P. V. Bernhardt, M. Zhang, Q. Wang, A. Du, H. Wang, G. Liu and L. Wang, Nano Res., 2016, 9, 692 CrossRef CAS
.
- Y. Kim, Z. Yang, A. Jain, O. Voznyy, G.-H. Kim, M. Liu, L. N. Quan, F. P. García de Arquer, R. Comin, J. Z. Fan and E. H. Sargent, Angew. Chem., Int. Ed., 2016, 55, 9586 CrossRef CAS PubMed
.
- J. Pal, S. Manna, A. Mondal, S. Das, K. V. Adarsh and A. Nag, Angew. Chem., Int. Ed., 2017, 56, 14187 CrossRef CAS PubMed
.
- M. Pantaler, K. T. Cho, V. I. E. Queloz, I. G. Benito, C. Fettkenhauer, I. Anusca, M. K. Nazeeruddin, D. C. Lupascu and G. Grancini, ACS Energy Lett., 2018, 3, 1781 CrossRef CAS
.
- C. Wu, Q. Zhang, Y. Liu, W. Luo, X. Guo, Z. Huang, H. Ting, W. Sun, X. Zhong, S. Wei, S. Wang, Z. Chen and L. Xiao, Adv. Sci., 2018, 5, 1700759 CrossRef PubMed
.
- Y. Zhang, J. Yin, M. R. Parida, G. H. Ahmed, J. Pan, O. M. Bakr, J.-L. Brédas and O. F. Mohammed, J. Phys. Chem. Lett., 2017, 8, 3173 CrossRef CAS PubMed
.
- D. Wang, D. Wu, D. Dong, W. Chen, J. Hao, J. Qin, B. Xu, K. Wang and X. Sun, Nanoscale, 2016, 8, 11565 RSC
.
- Y.-H. Kim, C. Wolf, Y.-T. Kim, H. Cho, W. Kwon, S. Do, A. Sadhanala, C. G. Park, S.-W. Rhee, S. H. Im, R. H. Friend and T.-W. Lee, ACS Nano, 2017, 11, 6586 CrossRef CAS PubMed
.
- H.-K. Seo, H. Kim, J. Lee, M.-H. Park, S.-H. Jeong, Y.-H. Kim, S.-J. Kwon, T.-H. Han, S. Yoo and T.-W. Lee, Adv. Mater., 2017, 29, 1605587 CrossRef PubMed
.
- A. Miyata, A. Mitioglu, P. Plochocka, O. Portugall, J. T.-W. Wang, S. D. Stranks, H. J. Snaith and R. J. Nicholas, Nat. Phys., 2015, 11, 582 Search PubMed
.
- S. Zhou, R. Tang and L. Yin, Adv. Mater., 2017, 29, 1703682 CrossRef PubMed
.
- H. Chung, S. I. Jung, H. J. Kim, W. Cha, E. Sim, D. Kim, W.-K. Koh and J. Kim, Angew. Chem., Int. Ed., 2017, 56, 4160 CrossRef CAS PubMed
.
- N. Yarita, H. Tahara, T. Ihara, T. Kawawaki, R. Sato, M. Saruyama, T. Teranishi and Y. Kanemitsu, J. Phys. Chem. Lett., 2017, 8, 1413 CrossRef CAS PubMed
.
- G. R. Yettapu, D. Talukdar, S. Sarkar, A. Swarnkar, A. Nag, P. Ghosh and P. Mandal, Nano Lett., 2016, 16, 4838 CrossRef CAS PubMed
.
- C. Wu, Y. Zou, T. Wu, M. Ban, V. Pecunia, Y. Han, Q. Liu, T. Song, S. Duhm and B. Sun, Adv. Funct. Mater., 2017, 27, 1700338 CrossRef
.
- J. Shi, H. Zhang, Y. Li, J. J. Jasieniak, Y. Li, H. Wu, Y. Luo, D. Li and Q. Meng, Energy Environ. Sci., 2018, 11, 1460 RSC
.
- Y. Yang, D. P. Ostrowski, R. M. France, K. Zhu, J. Lagemaat, J. M. Luther and M. C. Beard, Nat. Photonics, 2016, 10, 53 CrossRef CAS
.
- A. Swarnkar, R. Chulliyil, V. K. Ravi, M. Irfanullah, A. Chowdhury and A. Nag, Angew. Chem., Int. Ed., 2015, 127, 15644 CrossRef
.
- X. Zhang, B. Xu, J. Zhang, Y. Gao, Y. Zheng, K. Wang and X. W. Sun, Adv. Funct. Mater., 2016, 26, 4595 CrossRef CAS
.
- H. Huang, L. Polavarapu, J. A. Sichert, A. S. Susha, A. S. Urban and A. L. Rogach, NPG Asia Mater., 2016, 8, 328 CrossRef
.
- J. M. Frost, K. T. Butler, F. Brivio, C. H. Hendon, M. van Schilfgaarde and A. Walsh, Nano Lett., 2014, 14, 2584 CrossRef CAS PubMed
.
- K. Miyata, T. L. Atallah and X.-Y. Zhu, Sci. Adv., 2017, 3, 1701469 CrossRef PubMed
.
- E. M. Hutter, J.-J. Hofman, M. L. Petrus, M. Moes, R. D. Abellon, P. Docampo and T. J. Savenije, Adv. Energy Mater., 2017, 7, 1602349 CrossRef
.
- C. L. Kennedy, A. H. Hill, E. S. Massaro and E. M. Grumstrup, ACS Energy Lett., 2017, 2, 1501 CrossRef CAS
.
- G. E. Eperon, E. Jedlicka and D. S. Ginger, J. Phys. Chem. Lett., 2017, 9, 104 CrossRef PubMed
.
- S. Dastidar, S. Li, S. Y. Smolin, J. B. Baxter and A. T. Fafarman, ACS Energy Lett., 2017, 2, 2239 CrossRef CAS
.
- W. Zhou, Y. Zhao, X. Zhou, R. Fu, Q. Li, Y. Zhao, K. Liu, D. Yu and Q. Zhao, J. Phys. Chem. Lett., 2017, 8, 4122 CrossRef CAS PubMed
.
- C. F. J. Lau, X. Deng, J. Zheng, J. Kim, Z. Zhang, M. Zhang, J. Bing, B. Wilkinson, L. Hu, R. Patterson, S. Huang and A. Ho-Baillie, J. Mater. Chem. A, 2018, 6, 5580 RSC
.
- S. Xiang, W. Li, Y. Wei, J. Liu, H. Liu, L. Zhu and H. Chen, Nanoscale, 2018, 10, 9996 RSC
.
- F. Liu, C. Ding, Y. Zhang, T. S. Ripolles, T. Kamisaka, T. Toyoda, S. Hayase, T. Minemoto, K. Yoshino and S. Dai,
et al.
, J. Am. Chem. Soc., 2017, 139, 16708 CrossRef CAS PubMed
.
- Q. A. Akkerman, D. Meggiolaro, Z. Dang, F. De Angelis and L. Manna, ACS Energy Lett., 2017, 2, 2183 CrossRef CAS PubMed
.
- J. Liang, C. Wang, Y. Wang, Z. Xu, Z. Lu, Y. Ma, H. Zhu, Y. Hu, C. Xiao, X. Yi, G. Zhu, H. Lv, L. Ma, T. Chen, Z. Tie, Z. Jin and J. Liu, J. Am. Chem. Soc., 2016, 138, 15829 CrossRef CAS PubMed
.
- J. Duan, Y. Zhao, B. He and Q. Tang, Angew. Chem., Int. Ed., 2018, 57, 3787 CrossRef CAS PubMed
.
- J. B. Hoffman, G. Zaiats, I. Wappes and P. V. Kamat, Chem. Mater., 2017, 29, 9767 CrossRef CAS
.
- J. Duan, Y. Zhao, B. He and Q. Tang, Small, 2018, 1704443 CrossRef PubMed
.
- J. Duan, Y. Zhao, X. Yang, Y. Wang, B. He and Q. Tang, Adv. Energy Mater., 2018, 8, 1802346 CrossRef
.
- H. Yuan, Y. Zhao, J. Duan, Y. Wang, X. Yang and Q. Tang, J. Mater. Chem. A, 2018, 6, 24324 RSC
.
- Y. Zhao, Y. Wang, J. Duan, X. Yang and Q. Tang, J. Mater. Chem. A, 2019, 7, 6877 RSC
.
- J. K. Nam, M. S. Jung, S. U. Chai, Y. J. Choi, D. Kim and J. H. Park, J. Phys. Chem. Lett., 2017, 8, 2936 CrossRef CAS PubMed
.
- C. Liu, W. Li, C. Zhang, Y. Ma, J. Fan and Y. Mai, J. Am. Chem. Soc., 2018, 140, 3825 CrossRef CAS PubMed
.
- J. Zhang, D. Bai, Z. Jin, H. Bian, K. Wang, J. Sun, Q. Wang and S. F. Liu, Adv. Energy Mater., 2018, 356, 1703246 CrossRef
.
- Q. Ma, S. Huang, S. Chen, M. Zhang, C. F. J. Lau, M. N. Lockery, H. K. Mulmudi, Y. Shan, J. Yao, J. Zheng, X. Deng, K. Catchpole, M. A. Green and A. W. Y. Ho-Baillie, J. Phys. Chem. C, 2017, 121, 19642 CrossRef CAS
.
- W. Xiang, Z. Wang, D. J. Kubicki, W. Tress, J. Luo, D. Prochowicz and M. Grätzel, Joule, 2019, 3, 205 CrossRef CAS
.
- C. F. J. Lau, X. Deng, Q. Ma, J. Zheng, J. S. Yun, M. A. Green, S. Huang and A. W. Y. Ho-Baillie, ACS Energy Lett., 2016, 1, 573 CrossRef CAS
.
- C. Liu, W. Li, J. Chen, J. Fan, Y. Mai and R. E. Schropp, Nano Energy, 2017, 41, 75 CrossRef CAS
.
- D. Moghe, L. Wang, C. J. Traverse, A. Redoute, M. Sponseller, P. R. Brown and R. R. Lunt, Nano Energy, 2016, 28, 469 CrossRef CAS
.
- T. Krishnamoorthy, H. Ding, C. Yan, W. L. Leong, T. Baikie, Z. Zhang and S. G. Mhaisalkar, J. Mater. Chem. A, 2015, 3, 23829 RSC
.
- M. Wang, P. Zeng, S. Bai, J. Gu, F. Li, Z. Yang and M. Liu, Sol. RRL, 2018, 2, 1800217 CrossRef
.
- C. Zhang, L. Gao, S. Teo, Z. Guo, Z. Xu, S. Zhao and T. Ma, Sustainable Energy Fuels, 2018, 2, 2419 RSC
.
- Z. Xiao, Z. Song and Y. Yan, Adv. Mater., 2019, 1803792 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2019 |



