One-pot synthesis of β-cyclodextrin modified Au nanoclusters with near-infrared emission†
Wenjing
Li
,
Xi
Wang
,
Tao
Jiang
,
Xiang
Ma
 * and
He
Tian
* and
He
Tian
Key Laboratory for Advanced Materials and Feringa Nobel Prize Scientist Joint Research Center, Institute of Fine Chemicals, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: maxiang@ecust.edu.cn
First published on 3rd April 2020
Abstract
β-Cyclodextrin modified gold nanoclusters (AuNCs@β-CD) with near-infrared (NIR) emission were facilely and successfully prepared based on a one-pot and green synthesis route, which provides an interesting and efficient strategy to realize NIR emissive nanoclusters via supramolecular macrocycle modification.
Metal nanoclusters have attracted great attention and a series of important advances have been made in the fields of synthesis and application.1–4 In the past few years, the emerging application of luminescent metal (e.g., gold and silver) nanoclusters has gained increased attention for bioimaging, drug delivery and photodynamic therapy.5–9 Au nanoclusters (AuNCs), with diameters below 3 nm, have molecular-like properties with their size between that of a single atom and nanoparticles. The discrete energy levels due to the small size result in corresponding optical transition absorption and emission and give unique optical properties to AuNCs.10–12 In addition, the large specific surface area, large Stokes shift and high photostability have enabled AuNCs to achieve significant development in sensing, imaging and catalysis.13,14 Many studies have found that near-infrared (NIR) emission, with wavelengths longer than 780 nm, compared with conventional visible-infrared imaging, can further reduce background noise in biological tissues, which has become a popular research direction.15 Chemical reduction, photoreduction and chemical etching are all applied to the process of Au3+ synthesis of AuNCs in the presence of protecting groups and reducing agents.16 For the in-depth study of the synthesis process of AuNCs, some proteins and polymers have been used as protective agents and reducing agents, which enable a more convenient, low-toxicity and environment-friendly synthesis process.17–21
Meanwhile, supramolecular macrocycles (such as cyclodextrin, cucurbituril and calixarenes) are also widely used in various fields due to their unique host–guest interactions.22 Naturally occurring cyclodextrins (CDs), α-, β- and γ-CD, with six, seven and eight glucose units in cyclic oligomers, have been extensively studied for their high biocompatibility, low toxicity and good water-solubility. Cyclodextrin molecules have a hollow cylindrical structure with a wide top and a narrow bottom, open at both ends. Hydrophobic regions are formed inside the cavity due to the effect of C–H bonds, while hydroxyl groups outside the molecule endow CDs with good hydrophilicity.23 Among various macrocycles, β-CD is most widely used. Due to advantages such as non-toxicity, water-solubility, environment-friendliness, biocompatibility, and host–guest recognition ability of β-CD, the design of β-CD functionalized nanomaterials has great development prospects.24 According to the literature, the CD-modified AuNCs usually require complex surface modifications or the aid of reducing agents, which is quite complicated and involves many environment-unfriendly agents.25–27 In addition, most of the CD-modified AuNCs have green emission.28,29 Thus, the development of a simple and convenient method for preparing luminescent AuNCs with a wide colour range is still attracting much attention from researchers.
Herein, AuNCs with near-infrared emission were synthesized using sulfhydryl modified β-CD (β-SH-CD) as a reductant and protector. And the effects of different preparation conditions, mainly the acid–base properties of the reaction solution, on the NIR emission of CD-modified AuNCs have been explored. To the best of our knowledge, this is the first successful attempt to use supramolecular macrocyclic compounds to modify AuNCs with NIR emission.
Basically, AuNCs@β-CD with NIR emission were synthesized in a facile, one-pot route. Chloroauric acid and β-SH-CD aqueous solution were mixed under alkaline conditions, and the mixture was gently stirred at 80 °C for 24 hours to obtain an aqueous AuNC solution with NIR emission (Scheme 1). The AuNCs@β-CD show NIR emission at 795 nm (ΦPL = 0.02) under an excitation wavelength of 450 nm (Fig. 1A and Table S1, ESI†). NIR emission longer than 780 nm can further reduce the background noise of biological tissue compared with traditional visible-infrared imaging, thereby providing better application potential in the biological field. As shown in Fig. 1B, the AuNCs@β-CD have a long luminescence lifetime containing two components, 3.56 μs (99.9%) and 17.26 μs (0.1%). The average lifetime is 3.63 μs, which is longer than most analogous near-infrared emitting AuNCs.
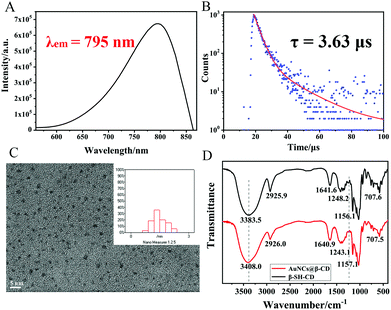 | ||
| Fig. 1 (A) Photoluminescence spectrum of AuNCs@β-CD (λex = 450 nm); (B) lifetime spectra of AuNCs@β-CD; (C) TEM image of AuNCs@ β-CD and (D) IR spectra of β-SH-CD and AuNCs@β-CD. | ||
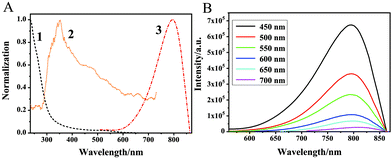
Fig. 1C shows AuNCs@β-CD with an average particle size of 1.49 nm. In addition, Raman scattering spectra also prove that β-SH-CD has been modified on the surface of AuNCs. From the Raman spectrum (Fig. S1, ESI†), it can be seen that the peaks at 117 cm−1 and 300 cm−1 represent the signal peaks of Au–S–C in-plane bending vibration and Au–S bond stretching vibration, respectively. Since the excitation wavelength of the Raman spectrometer exciter is 785 nm, AuNCs@β-CD with emission at 795 nm will have a certain effect on the Raman signal, which weakens the generated signal. But from the spectrum, it can still be proved that β-SH-CD has been modified on the surface of AuNCs. In addition, the valence and composition of Au elements in AuNCs@β-CD can be determined by X-ray photoelectron spectroscopy. As shown in Fig. S2 (ESI†), the binding energy shows two peaks at 84.4 eV and 88.1 eV, which can be attributed to the Au(I) and Au(0) characteristic peaks of the 4f7/2 and 4f5/2 orbitals.30 The data also show that AuNCs@β-CD with a portion of Au(I) and S–Au(I) on the surface can effectively improve the stability and quantum yield of the resulting AuNCs.31,32 In order to further prove the modification of β-SH-CD on the surface of AuNCs, infrared spectra of β-CD and AuNCs@β-CD are shown in Fig. 1D. It can be seen that the two spectral lines coincide with each other, and the peaks are basically the same. The peak at 3300–3400 cm−1 corresponds to the –OH stretching vibration peak, which results in a certain red shift and widening after the formation of AuNCs@β-CD; the peak at 1243 cm−1 corresponds to the –CH2–S out-of-plane bending vibration peak; and the peak at 1157 cm−1 corresponds to the C–O stretching vibration peak in the cyclodextrin cavity. Based on the above data, it is also confirmed that β-SH-CD has been modified on the surface of AuNCs. Hence, a new type of AuNCs, with NIR emission, modified with supramolecular macrocyclic compounds, was successfully synthesized using a simple and facile method. Further analysis of the absorption spectrum (Fig. 2A, curve 1), excitation spectrum (Fig. 2A, curve 2) and emission spectrum (Fig. 2A, curve 3) of AuNCs@β-CD shows that the peak of the excitation spectrum is at 360 nm, and a small absorption peak appears at the wavelength greater than 400 nm. In order to eliminate the interference of the frequency doubling peaks, a longer wavelength was used as the excitation wavelength. It can be seen that different excitation wavelengths do not change the maximum emission wavelength of AuNCs@β-CD, only changing the emission intensity (Fig. 2B), and the wavelength is consistent with the trend of curve 2 in Fig. 2A.
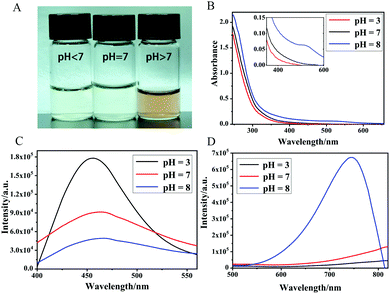
The effects of preparation conditions on the NIR emission were studied and the optimal preparation conditions were obtained. To determine the influence of the acid–base properties of the reaction solution on the emission of AuNCs@β-CD, AuNCs@β-CD were synthesized using 0.8 mM HCl acidic solution, 0.1 M NaHCO3 alkaline solution and water solution as the reaction solvents. As shown in Fig. 3A, we obtained the aqueous solutions of AuNCs@β-CD with pale yellow (pH < 7, pH = 7) and reddish brown (pH > 7) color under visible light. As shown in Fig. 3D, only AuNCs@β-CD prepared under alkaline conditions produced NIR emission. The AuNCs@β-CD obtained at different pH values all have emission at 455 nm, and with the increase of acidity, the emission at 455 nm gradually increased (Fig. 3C). The UV-visible absorption spectrum also shows that only the AuNCs@β-CD aqueous solution produced under the alkaline preparation conditions has a gentle peak at 470 nm, corresponding to the colour of the obtained solution (Fig. 3B). During the reaction, Au(III) reacts with sulfhydryl groups to form Au(I)–S, and then these Au(I)–S bonds are gradually reduced to form a Au(0) kernel under alkaline and heated conditions. As the reaction time increases, both the intensity and displacement of the absorption curve become larger (Fig. S4C, ESI†), which indicates the formation of the Au(0) kernel. And the emission at 795 nm gradually increased with the increase of reaction time (Fig. S4B, ESI†), indicating that AuNCs with a higher degree of reduction had NIR emission. Alkaline conditions can significantly improve the reducibility of thiol groups, which is the main reason for the easier preparation of AuNCs@β-CD with NIR emission under alkaline conditions. The pH value of AuNCs@β-CD solution prepared under alkaline conditions was regulated (back and forth between pH = 2 and pH = 9), and the solution showed a relatively stable fluorescence intensity (Fig. S3, ESI†), which proved that after the Au(0) kernel was formed, the effect of pH on its luminescence became relatively small. The data above show that the acidity and basicity of the reaction solution affect only the luminescence of AuNCs, and not the formation of AuNCs.
In addition, we also explored the effects of heating time, the molar ratio of Au/SH and reaction temperature on the preparation of AuNCs@β-CD. According to Fig. S4 (ESI†), it can be seen that the longer the heating time in the preparation of AuNCs, the greater their photoluminescence intensity. A possible reason is that the –SH group in β-SH-CD requires a longer reaction process due to the effect of steric hindrance. Increasing the reaction time can improve the yield of AuNCs with NIR emission.
The UV-visible absorption spectrum of AuNCs@β-CD, Fig. S5A (ESI†), shows the relationship between the cluster radius and the Au/SH molar ratio. When the ratio of Au/SH is 1, surface plasmon resonance (SPR) of the Au nanoparticles is observed. In essence, the appearance of SPR basically depends on the size of the particles, and large particles would cause an obvious red shift. This is due to insufficient free β-SH-CD ligands binding to the surface of Au nanoparticles to prevent the binding and aggregation of Au atoms. When the ratio of Au/SH is 1/2, the number of ligands is sufficient with a high etching rate, and the growth rate of the gold nucleus is low. The emission spectrum is attenuated to the visible light region in an exponential manner. The SPR peak disappears and smaller AuNCs are formed. When the ratio of Au/SH is 1/1.5, the etching rate of the ligand and the growth rate of the gold nucleus are suitable for forming AuNCs with near-infrared emission with a larger diameter. The ultraviolet absorption also decays exponentially, but the absorption intensity after 470 nm in the visible light region slightly increased, as shown in Fig. S5B (ESI†).
The effect of reaction temperature on the reaction was also studied. Since the temperature can further increase the reactivity of the –SH group and accelerate the reaction rate of –SH and Au3+, keeping the reaction time constant and increasing the reaction temperature will easily give small size AuNCs@β-CD with green emission (Fig. S6A and B, ESI†). As shown in Fig. S6C and D (ESI†), when the reaction solution is heated at 80 °C, the reaction time needs to be shortened to obtain better AuNCs@β-CD of NIR emission.
In conclusion, AuNCs@β-CD with NIR emission were prepared, using β-SH-CD as a reducing agent and a protective agent under alkaline conditions. In the process of preparing NIR emitting AuNCs, the acid–base properties of the reaction solution were found to have a large effect on their luminescence performance, and AuNCs@β-CD with NIR emission can be obtained only under alkaline conditions. At the same time, detailed characterization of AuNCs@β-CD was performed and the luminescence properties were investigated. Compared with analogous near-infrared emitting AuNCs, the system has a longer luminescence lifetime. In addition, by optimizing the synthesis method, we found that the optimal preparation conditions are a ratio of Au/SH of 1/1.5, heated at 80 °C for 24 hours, or heated at 70 °C for 72 hours, and the photophysical properties were determined, which provide an effective method for the synthesis of near-infrared AuNCs. The study is beneficial for expanding the design and application of in vivo imaging with higher contrast and resolution.
We gratefully acknowledge the financial support from the NSFC (21788102, 21722603 and 21871083), the Project supported by Shanghai Municipal Science and Technology Major Project (Grant No. 2018SHZDZX03), the ‘Shu Guang’ project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (19SG26), the Innovation Program of Shanghai Municipal Education Commission (2017-01-07-00-02-E00010), and the Fundamental Research Funds for the Central Universities.
Conflicts of interest
There are no conflicts to declare.Notes and references
- K. Kwak and D. Lee, Acc. Chem. Res., 2019, 52, 12–22 CrossRef CAS PubMed
.
- Y. Chen, M. L. Phipps, J. H. Werner, S. Chakraborty and J. S. Martinez, Acc. Chem. Res., 2018, 51, 2756–2763 CrossRef CAS PubMed
.
- X. Kang and M. Zhu, Coord. Chem. Rev., 2019, 394, 1–38 CrossRef CAS
.
- Z. Wang, Q.-P. Qu, H.-F. Su, P. Huang, R. K. Gupta, Q.-Y. Liu, C.-H. Tung, D. Sun and L.-S. Zheng, Sci. China: Chem., 2019, 63, 16–20 CrossRef
.
- J. Ge, C. Li, Y. Zhao, X. Yu and G. Jie, Chem. Commun., 2019, 55, 7350–7353 RSC
.
- M. Yu, J. Xu and J. Zheng, Angew. Chem., Int. Ed., 2019, 58, 4112–4128 CrossRef CAS PubMed
.
- G. Gao, R. Chen, M. He, J. Li, J. Li, L. Wang and T. Sun, Biomaterials, 2019, 194, 36–46 CrossRef CAS PubMed
.
- O. J. H. Chai, Z. Liu, T. Chen and J. Xie, Nanoscale, 2019, 11, 20437–20448 RSC
.
- C.-Y. Song, J.-Y. Zhang, Y. Qiu, H.-P. Jin, H.-M. Zhang, S. Liu, H. Liu, H.-B. Qiu and G.-G. Gao, Sci. China: Chem., 2019, 62, 347–354 CrossRef CAS
.
- T. Jiang, X. Wang, J. Wang, G. Hu and X. Ma, ACS Appl. Mater. Interfaces, 2019, 11, 14399–14407 CrossRef CAS PubMed
.
- W. Lan, Q. Tan, J. Qiao, G. Shen and L. Qi, Chin. Chem. Lett., 2019, 30, 1627–1630 CrossRef CAS
.
- L. Yan, Y. Yu and Z. Xia, Sci. China: Chem., 2018, 61, 619–626 CrossRef CAS
.
- A. Bonanno, I. Perez-Herraez, E. Zaballos-Garcia and J. Perez-Prieto, Chem. Commun., 2020, 56, 587–590 RSC
.
- X. Ran, Z. Wang, F. Pu, Z. Liu, J. Ren and X. Qu, Chem. Commun., 2019, 55, 15097–15100 RSC
.
- X. Wang, Y. Li, L. Huang, X. W. Jiang, L. Jiang, H. Dong, Z. Wei, J. Li and W. Hu, J. Am. Chem. Soc., 2017, 139, 14976–14982 CrossRef CAS PubMed
.
- X. R. Song, N. Goswami, H. H. Yang and J. Xie, Analyst, 2016, 141, 3126–3140 RSC
.
- S. Malola and H. Hakkinen, Chem. Commun., 2019, 55, 9460–9462 RSC
.
- D. Cuaran-Acosta, P. Londono-Larrea, E. Zaballos-Garcia and J. Perez-Prieto, Chem. Commun., 2019, 55, 1604–1606 RSC
.
- X. Wang, Y. Xu, X. Ma and H. Tian, Ind. Eng. Chem. Res., 2018, 57, 2866–2872 CrossRef CAS
.
- T. Yu, C. Xu, J. Qiao, R. Zhang and L. Qi, Chin. Chem. Lett., 2019, 3, 660–663 CrossRef
.
- Y. Chen, J. Qiao, Q. Liu and L. Qi, Chin. Chem. Lett., 2018, 29, 366–370 CrossRef CAS
.
- X. Ma and H. Tian, Acc. Chem. Res., 2014, 47, 1971–1981 CrossRef CAS PubMed
.
- D. Li, J. Wang and X. Ma, Adv. Opt. Mater., 2018, 6, 1800273 CrossRef
.
- X. Ma, J. Wang and H. Tian, Acc. Chem. Res., 2019, 52, 738–748 CrossRef CAS PubMed
.
- T. Das, D. K. Poria and P. Purkayastha, Nanomedicine, 2016, 12, 1105–1112 CrossRef CAS PubMed
.
- C. K. Lo, M. C. Paau, X. P. Yang and M. M. F. Choi, J. Phys. Chem. C, 2010, 114, 15995–16003 CrossRef
.
- A. Mathew, G. Nataranjan, L. Lehtovaara, H. Häkkinen, R. M. Kumar, V. Subramanian, A. Jaleel and T. Pradeep, ACS Nano, 2013, 8, 139–152 CrossRef PubMed
.
- Y. Li, Q.-L. Wen, A.-Y. Liu, Y. Long, P. Liu, J. Ling, Z.-T. Ding and Q.-E. Cao, Microchim. Acta, 2020, 187, 106 CrossRef CAS PubMed
.
- M. I. Halawa, F. Wu, T. H. Fereja, B. Lou and G. Xu, Sens. Actuators, B, 2018, 254, 1017–1024 CrossRef CAS
.
- Y. Chen, Y. Wang, C. Wang, W. Li, H. Zhou, H. Jiao, Q. Lin and C. Yu, J. Colloid Interface Sci., 2013, 396, 63–68 CrossRef CAS PubMed
.
- Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. T. Leong, J. Y. Lee and J. Xie, J. Am. Chem. Soc., 2012, 134, 16662–16670 CrossRef CAS PubMed
.
- Z. Luo, V. Nachammai, B. Zhang, N. Yan, D. T. Leong, D. E. Jiang and J. Xie, J. Am. Chem. Soc., 2014, 136, 10577–10580 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthesis, quantum yield data, Raman, XPS, photoluminescence spectra and so forth. See DOI: 10.1039/d0cc00713g |
| This journal is © The Royal Society of Chemistry 2020 |