Influence of nanomedicine mechanical properties on tumor targeting delivery
Zheng
Li†
a,
Chen
Xiao†
a,
Tuying
Yong†
a,
Zifu
Li
 *abc,
Lu
Gan
*abc and
Xiangliang
Yang
*abc
*abc,
Lu
Gan
*abc and
Xiangliang
Yang
*abc
aNational Engineering Research Center for Nanomedicine, College of Life Science and Technology, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, 430074, China. E-mail: zifuli@hust.edu.cn; lugan@hust.edu.cn; yangxl@hust.edu.cn
bKey Laboratory of Molecular Biophysics of Ministry of Education, College of Life Science and Technology, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, 430074, China
cHubei Key Laboratory of Bioinorganic Chemistry and Materia Medical, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, 430074, China
First published on 26th March 2020
Abstract
Modulating nanomedicine mechanical properties for enhanced drug delivery to tumors has attracted increasing attention in the past few decades. In this tutorial review, we analyze the impact of nanomedicine mechanical properties on in vivo transport processes and highlight the most recent advances in drug delivery efficiency and antitumor efficacy. Typical nanoparticles that have been explored for this purpose since 2000 are summarized while the methods to tune and the techniques to characterize nanomedicine mechanical properties are introduced. In the end, challenges and perspectives on tailoring nanomedicine mechanical properties for tumor targeting delivery are discussed.
Key learning points1. The significance of nanomedicine mechanical properties in delivery processes.2. Five critical in vivo transport processes of cancer nanomedicines. 3. Typical nano drug delivery systems with tailored mechanical properties. 4. Techniques to measure and approaches to adjust nanoparticle mechanical properties. 5. Current challenges and future directions in nanomedicine mechanical properties. |
1. Introduction
Treating cancer with nanomedicines has received great attention in the past few decades. Compared with conventional chemotherapeutics, loading free drugs within nanoparticles can change their pharmacokinetics and biodistribution, thereby contributing to tumor targeted drug delivery and reducing adverse effects to normal tissues.1 As a result, Doxil®, Abraxane®, and many other nanoparticle-based therapeutics have entered the market for cancer therapy. Nonetheless, these nanotherapeutics have achieved limited survival benefits compared with conventional chemotherapy drugs in clinical settings. This is because pathophysiological barriers prevent nanomedicines from realizing their therapeutic potential. Upon intravenous (i.v.) administration, nanomedicines encounter numerous barriers from the mononuclear phagocyte system (MPS), aberrant tumor vasculature, intratumoral pressure, and drug resistance.2 In this scenario, an ideal nanomedicine should possess the following characters, including long circulation, tumor accumulation, deep penetration, cellular internalization, and drug release, for effective cancer treatment. Extensive studies have shown that nanoparticle physicochemical properties, for instance surface charge and hydrophobicity, size, shape, composition, and so on, have a profound impact on their in vivo transport processes, and therefore dictate therapeutic efficacy and safety of nanomedicines.2 Accordingly, optimizing these physicochemical properties is a long-lasting research topic for cancer nanomedicines.1Tailoring nanomedicine mechanical properties for enhanced drug delivery to tumors is a rather new field. In fact, the interest in modulating nanomedicine mechanical properties largely originated from several exciting discoveries in biology fields. For example, tissue cells, including fibroblasts, myocytes, neurons, and other cell types, were revealed to develop their own mechanotransduction machinery to feel and respond to the stiffness of their substrates.3 As a result, environmental mechanical cues, for instance matrix elasticity, alone could direct mesenchymal stem cell lineage specification toward neurons in matrixes mimicking brain elasticity (0.1–1 kPa), myoblasts in matrixes similar to muscles (8–17 kPa), and osteoblasts in matrixes whose elasticity (25–40 kPa) is close to that of collagenous bones, respectively.4 In embryogenesis during development, it was further demonstrated that the mechanical properties of the cell, the cell softness, dictated stress-induced spreading and differentiation of embryonic stem cells (0.5 kPa in stiffness).5 The 120 day lifetime of erythrocytes, or red blood cells (RBCs), is largely ascribed to their extraordinary deformability (26 kPa), which is lost after many thousands of times of deformation during circulation, leading to ultimate splenic filtration and clearance. With atomic force microscopy (AFM), it was revealed that cancer cells (0.5 kPa), in particular the metastatic cancer cells, were softer than the cells in adjacent healthy tissues (1.13 kPa).6 The increased deformability has played a critical role in cancer cell extravasation from blood vessels and metastasis to distant organs. Target rigidity was found to play a decisive role in phagocytosis.7 Macrophages preferred rigid (30 kPa) hydrogel microparticles to soft (14 kPa) counterparts. It was also reported that soft (115 MPa) mature human immunodeficiency virus (HIV) particles could infect cells efficiently whereas stiff (930 MPa) immature particles could not.8 In this way, HIV particles tuned their mechanical properties for efficient infectivity, soft during entry but stiff during viral budding. All these reports suggested that mechanical properties played a critical role in key biological events, for instance blood circulation, extravasation, and cellular internalization. Inspired by the extraordinary long circulation of RBCs, DeSimone et al. studied the effect of microgel elastic modulus on blood circulation and correlated an 8-fold decrease (from 63.9 kPa to 7.8 kPa) in microgel modulus to a remarkable 30-fold increase in the elimination phase half-life of microgels.9 Since this pioneering work published in 2011, many research groups have followed up10 and the initial investigations have been summarized in two excellent reviews.11 Nonetheless, it was not until very recently that we demonstrated that the softness of nanomedicines regulated their delivery efficiency and, more importantly, antitumor efficacy.12 Our results revealed that the softness of tumor cell derived microparticles influenced every step of in vivo transport processes.
In this tutorial review, we briefly introduce the techniques to characterize nanoparticle mechanical properties. The chemical and biological approaches developed to tune nanoparticle mechanical properties will be highlighted. The influence of nanomedicine mechanical properties on in vivo transport processes, including long circulation, tumor accumulation, deep penetration, cellular internalization, and drug release, will be reviewed comprehensively, Fig. 1. The impact of mechanical properties on therapeutic efficacy and safety will be emphasized. Importantly, the performance of six representative nanoparticles will be compared in terms of five critical transport processes. Finally, current challenges and future opportunities will be provided.
2. Characterization and modulation of nanomedicine mechanical properties
2.1 Characterization of nanomedicine mechanical properties
Mechanical properties refer to the response of a material toward applied forces.11 Elasticity and stiffness are two most widely used terms in the literature for characterizing material mechanical properties. Elasticity reflects the ability of a material to withstand deformation caused by stress and to resume its original shape as the stress is removed. The elasticity of a material is conventionally presented as its elastic moduli, including Young's modulus (E), shear modulus (G) and bulk modulus (K). E is defined as the ratio between stress and strain in a material in the linear elasticity regime of a uniaxial deformation, G is the ratio of shear stress to the corresponding shear strain, and K is the ratio of the pressure increase to the resulting relative decrease of volume. According to these definitions, E describes the response of a material to linear stress, G reflects the response to shear stress, and K portrays the resistance to compressive stress. Experimentally, E is obtained by a stress–strain diagram with an axial extensometer. G is measured with a rheometer or dynamic mechanical analysis. K of an impermeable object can be determined by measuring the volume change at varied pressures.10 These three moduli are intrinsically correlated. Their relationship can be described in equations 2G(1 + ν) = E = 3K(1 − 2ν), where ν is Poisson's ratio. Therefore, measuring one modulus and knowing the Poisson's ratio of a material, other moduli can be deduced from these equations. Although bulk elasticity does not fully characterize nanoparticles’ mechanical properties, bulk elastic moduli are often measured for the ease of operation on macroscopic materials consisting of similar compositions as nanoparticles.10 Stiffness, or rigidity, measures the resistance of a material to elastic deformation or deflection and is defined as the ratio between applied force and the resultant length change. The inverse of stiffness is softness or deformability, which refers to the ability of an object to change shape under an applied stress and is widely used for biological samples, for instance RBCs, embryonic stem cells, and cancer cells. For instance, the elastic modulus of breast cancer MCF-7 cells has been measured and compared with optical stretching, parallel-plate rheology, cell monolayer rheology, AFM, particle tracking analysis, and magnetic twisting cytometry.13 As different techniques probed varied mechanical responses of cancer cells to applied forces, the measured elastic modulus had a broad range from 0.004 to 13.5 kPa.Four techniques have been leveraged to characterize nanoparticle mechanical properties.10 AFM has been the most reliable and widely used technique to probe nanoparticle mechanical properties.11 Based on a typical force–distance curve in nano-indentation, Young's modulus (E) of a nanoparticle can be calculated by applying the Hertz or Sneddon model. Conventional AFM sharp tips (∼20 nm) can differentiate mechanical heterogeneity within a single nanoparticle. However, such sharp tips have an additional adhesion problem. To address this issue, colloidal particles, with a diameter of several micrometers, have been attached to AFM sharp tips.13 Colloidal AFM probe thus measures the mechanical properties of the whole nanoparticle under a larger contact area. But this mode suffers from the limitation of insensitivity to mechanical heterogeneity within a single nanoparticle. As biological samples exhibit distinct variation in the dehydration process, caution should be exercised when AFM tests are performed in aqueous solution, especially for long time experimentation. Bulk modulus (K) of the whole nanoparticle can be extracted from measuring the volume changes under varied osmotic pressures. The size of the nanoparticle is usually measured with laser light scattering. Similar to other ensemble-average based light scattering techniques, however, this technique offers limited valuable information on a single nanoparticle, not to mention mechanical heterogeneity within a single nanoparticle. Since nanoparticles are always dispersed in aqueous solution, there is no dehydration issue. Shear modulus (G) of nanoparticles can be acquired from the damping of an oscillating crystal in a quartz crystal microbalance with dissipation monitoring (QCM-D).14 But this technique is only applicable to nanomaterials capable of adsorbing onto a surface.15 QCM-D cannot resolve mechanical heterogeneity within a single nanoparticle. Filtration tests, in which objects are pushed to pass through filters, have also been adopted for the characterization of nanoparticle mechanical properties. Normally, the filter pores are smaller than the diameter of the nanomedicine. But quantitative mechanical properties can be hardly obtained from filtration tests, not to mention mechanical heterogeneity within a single nanoparticle. Since varied techniques have different principles and probe distinctive mechanical responses, it is important and useful to characterize the same nanoparticle using various techniques. As an example, E of lysozyme–dextran nanogels was measured ranging from 48 to 71 kPa in AFM while the QCM-D measurement gave a close G value of ∼68 kPa.15
2.2 Modulation of nanoparticle mechanical properties
Nine categories of nanoparticles have been reported so far for tumor targeted drug delivery, Fig. 2. The first three classes are polymer based nanocarriers, hydrogel particles, polymeric micelles and cylindrical polymer brushes, Fig. 2A–C. Templating methods have been used to prepare another three types of nanocarriers, silica nanocapsules, layer-by-layer capsules, and discoidal particles, Fig. 2D–F. The last three all are bioinspired nanocarriers, nanovesicles, hybrid nanoparticles, and tumor cell derived microparticles, Fig. 2G–I. The mechanical properties of the first eight nanoparticles are controlled by three chemical means, by adjusting the crosslinker ratio, solid content, and compositions.11 For tumor cell derived microparticles, three biological tools, including using cancer cells of varied softness, chemical drug treatment, and small RNA interference, have been developed to control their mechanical properties.12 Of these nine nanocarriers, AFM is the most widely used technique for quantifying mechanical properties while Young's modulus, E, is the main reported mechanical property. Oftentimes, nanoparticles’ Young's moduli are measured without loaded drugs. The key assumption here is that loading free drugs will not significantly change nanocarrier mechanical properties. This assumption is, however, still unsubstantiated experimentally.Hydrogel particles, including nanogels and microgels, are the most widely investigated nanocarriers, Fig. 2A. The first method to obtain hydrogel particles is emulsion polymerization and the mechanical properties are controlled by tuning the crosslinking densities. A typical crosslinker used for this purpose is N,N′-methylene-bis-acrylamide (BIS). As an example, Giasson et al. prepared four N,N-diethyl acrylamide and 2-hydroxyethyl methacrylate nanogels.16 The resultant nanogels had diameters of 160 nm and exhibited increased E from 18 to 211 kPa with increased BIS content from 1.7% to 15%. Lyon et al. prepared two ∼1 μm N-isopropylacrylamide (NIPAM) microgels with BIS content of 1% and 3%.17 But their mechanical properties were not determined. Gao et al. constructed four 2-hydroxyethyl methacrylate (HEMA) microgels with diameters of 800–1000 nm.18 The mechanical properties of microgels were deduced from compressing bulk hydrogels in a mechanical tester. As the BIS content increased from 3% to 15%, E increased from 16.7 to 155.7 kPa. Wang et al. prepared a 6 μm soft acrylamide microgel showing a bulk Young's modulus of 14 kPa with 0.05% BIS.7 When the BIS content was increased to 2%, bulk E increased to 30 kPa. Inverse micro-emulsion polymerization is the second method to prepare hydrogel particles. For instance, Mitragotri et al. fabricated a series of 200 nm nanogels of distinct stiffness by increasing crosslinker poly(ethylene glycol) diacrylate (PEGDA) volume fraction from 0.05 to 0.4.19 Rheology tests revealed that G of the bulk hydrogel increased from 0.255 to 3000 kPa. By changing the solid content, Eniola-Adefeso et al. obtained 2 μm hydrogel microparticles and 500 nm nanoparticles.20 AFM demonstrated that E increased from 23 to 508 kPa. Adopting a similar micro-emulsion polymerization method, Jiang et al. obtained 250 nm gold nanoparticle encapsulated poly(carboxybetaine) zwitterionic nanogels.21 Increasing the cross-linking density from 2% to 15% led to an increase in bulk elastic moduli from 0.26 to 1.35 MPa. Templating on mesoporous silica microparticles, Caruso et al. developed the third method to prepare hydrogel particles. Different amounts of succinimidyl carboxyl methyl ester functionalized 8-arm-PEG were used to crosslink amine functionalized 8-arm-PEG, producing 7–9 μm PEG microgels.22 Colloidal probe AFM revealed that E increased from 0.2 to 3.3 kPa with increased crosslinker from 0.5 to 4 mg mL−1. In another instance, Xu et al. modulated E from 0.63 to 0.87, 2.53 and 3.41 MPa while keeping both size and surface charge of 300 nm cationized gelatin nanoparticles constant by increasing the amount of gelatin together with glutaraldehyde during sample preparation.23 Given emulsion polymerization and crosslinker BIS are most frequently used for hydrogel particle preparation, special attention should be paid.24 First of all, the monomers need to be selected based on the polymerization rate. Monomers should possess a similar polymerization rate to BIS, otherwise, a heterogeneous crosslinked hydrogel particle will be produced. Second, the effect of residue surfactants cannot be neglected. Surfactants, such as sodium dodecyl sulphate, are normally added to assist emulsion polymerization and removed afterwards. But removing residue surfactants is not a trivial task. In particular, the mesh size of a three dimensional polymer network is smaller for stiff hydrogels than that of soft hydrogels. Thus, it takes longer for surfactant molecules to diffuse out in stiff hydrogels than that in soft hydrogels. Residue surfactants significantly affect hydrogel particle hydrophobicity.
The second type of nanoparticle is a polymeric micelle, Fig. 2B. Two distinct methods were developed to control polymeric micelle softness. Benny et al. self-assembled amphiphilic mono methoxy-poly(ethylene glycol)–poly(lactide) (PEG–PLA) copolymer into polymer micelles, which had a diameter of 68 nm and E of 165 kPa.25 Upon lyophilization and hydration processes, the original polymer micelle solidified to have an E of 260 kPa and a diameter of 86 nm. Qin et al. reported the second method to modulate E of 150 nm polymer micelles by varying the compositions in amphipathic poly(ethylene glycol)-b-(poly ε-caprolactone-g-poly butyl acrylate) (PEG–(PCL-g-PBA)) micelles.26 PCL was a semi-crystalline polymer while PBA was an amorphous polymer with a low glass transition temperature. Herein, the content of PBA was increased to augment softness. AFM revealed that E decreased from 71 GPa for PEG–PCL micelles to 28.5 GPa for (PEG–(PCL-g-PBA120)) micelles.
The third nanoparticle is a cylindrical polymer brush, Fig. 2C. Caruso et al. first prepared poly(HEMA) backbones, and subsequently obtained soft 200 nm or 1150 nm cylindrical polymer brushes by grafting side chains consisting of random polymers of poly[(ethylene glycol)methyl ether methacrylate] (PEGMA) and poly(glycidyl methacrylate) (PGMA).27 To increase the stiffness of cylindrical polymer brushes, a PCL homopolymer brush was synthesized as a core before polymerization of EGMA and GMA. Rigid core shell cylindrical polymer brushes had diameters of 260 nm and 1200 nm. Because of the rigid PCL core, the degree of coiling of the brushes decreased in AFM images, implying reduced softness. But quantitative mechanical properties of polymer brushes were not provided.
The fourth nanoparticle is a silica nanocapsule, Fig. 2D. Two different templating techniques have been developed to prepare silica nanocapsules of varied mechanical properties. As an example, Zhao et al. synthesized 150 nm silica nanocapsules based on a combination of nano-emulsion and biosilicification dual-templating technology.28 Two silica precursors, triethoxyvinylsilane (TEVS) and tetraethylorthosilicate (TEOS), were utilized to obtain soft and stiff silica nanocapsules, which had E values of 704 kPa and 9.7 GPa, respectively. Alternatively, Lu et al. synthesized 240–310 nm hollow periodic mesoporous organosilica nanocapsules by combining silica nanoparticle templating with preferential etching.29 Young's moduli of periodic mesoporous organosilica were controlled from 350.6 to 250.9 and 233.4 MPa with benzene-, ethane-, and thioether-linkage. Interestingly, upon preferential removal of structurally stable Si–(O)4 tetrahedra, AFM showed that soft deformable organosilica nanocapsules had corresponding E values of 91.3, 3.95, and 47.7 MPa.
The fifth nanoparticle is a hollow polymeric microcapsule, Fig. 2E. Typically, these hollow microparticles are obtained by combining layer-by-layer assembly of oppositely charged polyelectrolytes with subsequent removal of sacrificial templates. The mechanical properties were tuned by variation of the number of polymer layers or the polymers used. As an example, Parak et al. synthesized 4.1 and 4.7 μm polymer microcapsules of varied stiffness,30 ranging from 0.2 to 10 N m−1, by templating on sacrificial CaCO3 microparticles with both non-degradable and degradable polymers. Caruso et al. prepared ∼3 μm hyaluronic acid (HA) microcapsules via atom transfer radical polymerization-mediated continuous assembly of polymers on silica templates.31 Increasing the layers of HA leads to increased stiffness from 7.5 to 17.6 and 27.2 mN m−1. While the thickness can be as thin as several nanometers, the diameter of the final polymeric microcapsules is normally in the range of several micrometers.
Fig. 2F schematically illustrates the sixth type of nanoparticle, discoidal particles, which are prepared with particle replication in nonwetting templates, PRINT. DeSimone et al. invented this unique technology.9 Because of the swelling property of 2-hydroxyethyl acrylate and poly(ethylene glycol)diacrylate (PEGDA) hydrogel, a 2 or 3 μm mold was used to fabricate red blood cell mimics (RBCMs), which exhibited similar size, shape, and stiffness to RBCs. Decreasing the size of mold to 0.2 μm, 0.78 μm biconcave discoidal particles could also be harvested.32 The elastic moduli of these biconcave discoidal particles were deduced from measurements in bulk hydrogels. Single particle based mechanical analysis is urgently needed. Decuzzi et al. further leveraged this PRINT technique to construct 1000 × 400 nm discoidal polymeric particles (DPN) of varied E, 1.3 kPa and 15 kPa,33 and 1 or 2 μm circular, quadrangular, and elliptical discoidal particles with E ranging from 100 kPa to 10 MPa.34 The shape, size and thickness of resultant discoidal particles are facilely implemented with well-controlled molds while the stiffness is modulated via changing compositions in the polymer paste.
The seventh nanoparticle is a nanovesicle, Fig. 2G. Lipid nanovesicles, such as nanoliposomes, consisting of a lipid bilayer structure and encapsulating an aqueous interior, are widely used for drug delivery. The mechanical properties of nanovesicles can be facilely adjusted by controlling either the lipid chain lengths or the ratio of unsaturated lipids.35 As an example, Gan et al. prepared six 85 nm nanovesicles with E ranging from 5.8 to 19.9 and 42.8 MPa in aqueous solution.36 Intriguingly, the deformations of these nanovesicles were probed under applied forces from 100 pN to 1 nN.
The eighth nanoparticle, a hybrid particle, has a typical core shell structure, Fig. 2H. Four different methods have been reported to control the stiffness. Jiang et al. prepared two types of poly(lactic-co-glycolic acid) (PLGA) core lipid shell hybrid nanoparticles in a two-stage microfluidic device. Firstly, by changing the injection order, these authors fabricated 40 nm PLGA–lipid and PLGA–water–lipid nanoparticles.37 AFM characterization revealed that the addition of interfacial water layer resulted in a reduction in E from 1.2 to 0.76 GPa. Adopting a similar approach, these authors tuned the shell structure with either a monolayer lipid or a bilayer lipid on 100 nm PLGA nanoparticles.38 The bilayer lipid covered nanoparticles exhibited a much higher deformation and energy dissipation than that of the monolayer lipid under AFM tips. But E values of these two hybrid nanoparticles were not reported. Gao et al. further leveraged this two-stage microfluidic platform to prepare the third type of hybrid nanoparticle with hydrodynamic diameters around 200 nm.39 The resultant hybrid nanoparticle had a PLGA core, interfacial water layer, and lipid shell, which was further functionalized with Pluronic F127. The mechanical properties were controlled by modulating the hydrodynamic diameter of the PLGA core. E increased from 5 MPa without the PLGA core to 50 MPa with a 70 nm PLGA core and 110 MPa with a 160 nm PLGA core. Similar to nanovesicles, the deformations of these hybrid nanoparticles were evaluated under well-controlled forces with AFM sharp tips. Moses et al. described the fourth type of hybrid nanoparticle with 160 nm nanolipogels, consisting of an alginate core and a lipid shell.40 The mechanical properties were also controlled by tuning the core. Without the alginate hydrogel core, nanoliposome had an E of 45 kPa. Introducing the alginate hydrogel core led to an increase in E to 1.6 MPa while adding crosslinker calcium ions further increased E to 5.3, 13.8, and 19 MPa.
Fig. 2I illustrates the ninth nanocarrier, tumor cell derived microparticles. Being one type of extracellular vesicle with a hydrodynamic diameter of 100–1000 nm, these microparticles are shed by tumor cells in response to external stimuli, such as ultraviolet radiation, and could be used as efficient nanocarriers for a variety of chemotherapeutic agents to eradicate ovarian cancer cells, hepatocellular carcinoma cells and cancer stem cells in lung cancer patients.41 Yang et al. developed three methods to control the softness of 500 nm tumor cell derived microparticles.12 Firstly, using soft tumor repopulating cells (TRCs) selected from 90 Pa three dimensional (3D) soft fibrin hydrogels as a parental cellular resource,42 these authors demonstrated that microparticles released by TRCs (3D-MPs) were softer than microparticles derived from tumor cells cultured in conventional two dimensional plastics (2D-MPs). AFM nano-indentation measurements in aqueous solution revealed that 3D-MPs had an E of 1 kPa whereas 2D-MPs exhibited an E of 3 kPa. The higher deformability of 3D-MPs than 2D-MPs had also been validated with high and low osmotic pressures in hypertonic and hypotonic solutions.12 But quantitative K values were not calculated. Secondly, the softness of the microparticles was regulated with chemical agents. These authors demonstrated that 3D-MPs could be stiffened after jasplakinolide (Jasp) treatment while 2D-MPs could be softened with latrunculin A (LatA). Thirdly, identifying that cytospin-A had been significantly decreased in 3D-MPs, these authors reduced E of 2D-MPs from 3 kPa to around 1 kPa by knocking down cytospin-A in 2D-MPs with cytospin-A small interference RNA (siRNA). Nonetheless, it is important to note that, besides cytospin-A, the expression of several cytoskeleton related proteins was also significantly changed in 3D-MPs.12 The influence of these proteins on microparticle physicochemical properties, for instance hydrophobicity, and in vivo drug delivery processes cannot be neglected.
In all the nanocarriers mentioned above, the measured elastic moduli span an extremely wide range of almost 9 orders of magnitude from 0.2 kPa in PEG microgels to 71 GPa in PEG–PCL polymer micelles. Table 1 summarizes the advantages and limitations of six different strategies used for tailoring nanomedicine mechanical properties. But how to precisely modulate nanoparticle mechanical properties is still an unresolved issue.11 Aside from mechanical properties, huge differences exist in other physicochemical properties, including size, shape, composition, surface charge, and hydrophobicity. Therefore, it is a vast challenge to make a direct comparison among different nanoparticles in terms of absolute elastic moduli solely. For this reason, the trends drawn with various nanomedicines are analyzed and compared in this tutorial review.
| Strategies | Advantages | Limitations | Ref. | |
|---|---|---|---|---|
| Chemical means | Crosslinker ratio | Low cost | Poor biodegradation | 7, 9, 16–19, 21, 22 and 32 |
| Massive production | ||||
| Solid content | Massive production | Limited range of elasticity (23 kPa–3.4 MPa) | 20, 23 and 31 | |
| Controllable surface properties | ||||
| Composition | Controllable surface properties | Difficulty in mass production | 25–30 and 33–40 | |
| Wide range in elasticity (1.3 kPa–71 GPa) | ||||
| Biological tools | Cancer cells with varied softness | Biodegradation | Potential Tumorigenicity | 12 |
| Unique multiple biological functions | Limited range of elasticity (1–3 kPa) | |||
| Chemical drug treatment | Controllable surface properties | Difficulty in mass production | 12 | |
| Precise modulation of mechanical properties | Limited range of elasticity (1–3 kPa) | |||
| Small RNA interference | Controllable surface properties | High cost | 12 | |
| Precise modulation of mechanical properties | Limited range of elasticity (1–3 kPa) | |||
3. Influence of nanomedicine mechanical properties on transport processes
3.1 Long circulation
Prolonged blood circulation is highly desirable for i.v. administered nanomedicines. By taking advantage of the unique characteristics of solid tumors, the leaky vasculature and the defective lymphatic vessels, nanomedicines passively but preferentially accumulate in tumor tissues via the enhanced permeability and retention (EPR) effect.1 Even for actively targeted nanomedicines, they need to arrive at tumor tissues before binding to the receptors overexpressed on the surface of cancer cells. Accordingly, the longer the nanomedicines circulate in the blood stream the more they can enrich the tumor tissues.2 To this end, nanomedicines need to overcome at least three biological barriers, namely, opsonisation, clearance by the mononuclear phagocytic system (MPS) or the reticuloendothelial system (RES), and physical filtrations in small blood capillaries, spleen, and lungs. In this section, we summarize the effect of nanomedicine mechanical properties on protein adsorption, phagocytosis, and physical filtrations, which altogether determine the blood circulation time of the nanomedicine, Fig. 3. | ||
| Fig. 3 The impact of nanomedicine mechanical properties on blood circulation. (A) The effect of nanomedicine mechanical properties on protein adsorption. Reprinted with permission from ref. 18. Copyright 2012. Royal Society of Chemistry. (B) and (C) Soft 3D tumor cell derived microparticles can withstand phagocytosis of RAW264.7 macrophages. Reprinted with permission from ref. 12. Copyright 2019 Nature Publishing Group. (D) A schematic illustration of a microfluidic device for the evaluation of RBCM softness. (E) An image sequence showing soft RBCMs squeezing to pass through a channel. (F) Stiff RBCMs stuck in the entrance of the channel. Scale bar, 30 μm. Figures (D–F) reveal that soft particles can squeeze and pass through narrow filtration. Reprinted with permission from ref. 9. Copyright 2011 National Academy of Sciences. (G) Softer nanogels have longer blood circulation time. Reprinted with permission from ref. 21. Copyright 2012 American Chemical Society. | ||
Upon systematic administration, the first biological barrier nanomedicines encountered is opsonisation, during which proteins and other biomolecules adsorb onto nanomedicines to form a protein corona. The newly adsorbed proteins bestow nanomedicines a new identity, which can be recognized and captured by the macrophages residing in the RES. Therefore, the adsorptive proteins affect the nanomedicine blood circulation time. The adsorption of proteins onto nanomedicines largely depends on nanoparticle physicochemical properties, including surface charge and hydrophobicity, size, and shape.43 A simple and effective solution to preventing protein adsorption is surface modification, such as PEGylation. The impact of nanoparticle mechanical properties on protein adsorption has also been studied. As an example, both stiff (30 kPa) and soft (14 kPa) polyacrylamide microbeads and sheets adsorbed a similar amount of proteins.7 Consistently, four types of hydrogel particles exhibited identical protein adsorption capability, around 40 mg proteins per gram hydrogel particles, Fig. 3A.18 Collectively, it seems that protein adsorption has no preference onto either stiff or soft nanoparticles in terms of the amount of adsorbed proteins. But it is unclear whether there is any difference between the types of adsorbed proteins. Furthermore, these limited studies were all performed in relatively simple in vitro static settings. It is unknown which kind of protein will adsorb onto nanomedicines when they are in blood flow, which has both opsonins, including complements and immunoglobulins, and dysopsonins, such as albumin and apolipoproteins.43 Therefore, there is still an urgent need to correlate nanomedicine mechanical properties with protein adsorption, the subsequent MPS clearance and blood circulation. In performing the above-mentioned investigations, the role of surface modification, for instance PEGylation, should be taken into consideration, as there might exist a coupled effect between surface modification and mechanical properties in terms of nanomedicine protein adsorption. The interplay between surface modification and mechanical properties is interesting but seldom explored.28
The effect of nanomedicine mechanical properties on phagocytosis has been widely studied utilizing various nanoparticles with different macrophages. Macrophages are a type of professional phagocytic cell and are the main components of MPS present in the liver and spleen. As an exogenous object, nanoparticles need to evade phagocytosis. The seminal work, performed by Wang et al., identified mechanical properties of a target as a novel key criterion to determine the efficiency of Fc-receptor mediated phagocytosis.7 Their results demonstrated that bone-marrow-derived macrophages from mice exhibited a 6-fold preference for stiff (30 kPa) over soft (14 kPa) opsonized microbeads. The conclusion that macrophages prefer rigid particles is supported by numerous follow-up studies. As an example, Mitragotri et al. demonstrated that J774 macrophages phagocytosed 200 nm hard (3000 kPa) nanogels 3.5-fold higher than soft (10 kPa) nanogels.19 Decuzzi et al. reported that 1.3 kPa DPNs were 2 to 3 times less internalized as compared to 15 kPa DPNs in J774.A1 macrophages. Interestingly, using intravital microscopy, they observed that stiff DPNs deposited 4 times more than soft DPNs in the liver of mice, especially in Kupffer cells.33 This study emphasizes that tissue resident macrophages also engulf more rigid objects over soft counterparts. With another cell line, RAW 264.7 macrophages, higher cellular uptake was consistently detected for all three types of stiff (9.7 GPa) silica nanocapsules, unmodified, PEG-modified, and FA–PEG-modified, over their soft (704 kPa) counterparts.28 In another example, it was found that RAW 264.7 macrophages internalized stiff 2D-MPs (3 kPa) more than soft 3D-MPs (1 kPa),12Fig. 3B. Importantly, the amount of phagocytosed stiff 2D MPs decreased when 2D MPs were treated with LatA to reduce E, Fig. 3C. Correspondingly, more 3D-MPs were internalized by macrophages when their stiffness was increased after Jasp treatment. Although tumor cell derived microparticles bear the endogenous nature, these well-controlled experiments corroborate that nanomedicine mechanical properties are a key determinant for phagocytosis. While a clear consensus, that macrophages prefer stiff over soft nanomedicines, has been arrived based on most of the studies, there are some exceptions. With a murine RAW 264.7 macrophage, Giasson et al. found that macrophage uptake pathways were dependent on nanogel mechanical properties.16 Soft hydrogel particles (18 kPa) were preferentially engulfed via micropinocytosis whereas stiff nanogels (211 kPa) were internalized with a clathrin-dependent pathway. Nanogels of intermediate stiffness (36 and 136 kPa) were taken via multiple pathways, which resulted in an enhanced uptake amount and rate.
The fact that soft particles outperform rigid counterparts in physical filtrations has been demonstrated in a variety of scenarios. For instance, BIS crosslinked PNIPAM microgels were capable of deforming and squeezing through cylindrical pores as small as 1/10th of the microgel diameter under renal filtration relevant pressure differentials (4.8–10.3 kPa).17 This study highlights the outstanding softness of such microgels. However, because the polymerization rate of BIS is much faster than that of NIPAM, these microgels adopt a dense core loose shell structure and are heterogeneous in terms of stiffness within a single microgel.24 Therefore, the effective size is much smaller than the measured diameter. Caruso et al. prepared PEG microgels with diameters similar to red blood cells but of varied softness and evaluated their passage through 5 μm microfluidic channels under blood capillary relevant pressure gradients (0.1–1 kPa).22 Similar to RBCs, soft PEG hydrogel particles (1 kPa) could deform to fit into and pass through the microchannels. In contrast, stiff PEG microgels (3.3 kPa) had trouble in passing through the microfluidic blood capillary, particularly at low pressure differentials. Remarkably, the most deformable PEG microgel, with an E of 0.2 kPa, exhibited superior performance compared with RBCs when the capillary pressure gradient was smaller than 1 kPa. Using the PRINT technique, DeSimone et al. fabricated 6 μm RBCMs.9 In a 3 μm tall, 3 μm wide and 50 μm long microfluidic channel, Fig. 3D, 7.8 kPa RBCM was stretched by flow and pushed through the microchannel, Fig. 3E. The stiff RBCM with an E of 63.9 kPa was stuck in the entrance of the 3 μm wide channel, Fig. 3F. As a result, the stiff RBCM was largely sequestrated in lung filtrations, leading to lung embolization and severe distress to mice. By contrast, the most deformable 7.8 kPa RBCM was well tolerated in mice and capable of avoiding retarding in the lungs and kidneys. Soft nanoparticles capable of navigating splenic filtrations were also illustrated with zwitterionic nanogels.21 However, more studies are still needed to understand intra-organ distribution and interactions between deformable nanoparticles and macrophages with advanced intravital microscopy.
Owing to the superior performance in terms of phagocytosis and physical infiltrations, soft particles exhibit prolonged circulation compared to stiff counterparts. With an intravital imaging method, it was found that the elimination half-life of RBCMs increased from 2.88 to 93.29 hours when bulk E decreased from 63.9 to 7.8 kPa.9 In another circumstance, by tracking radio-labelled nanogels, the elimination half-life of nanogels was found to elongate from 9.7 to 61.4 hours by reducing the bulk modulus from 3000 to 10 kPa.19 The collective in vivo circulation half-life of zwitterionic hybrid nanogels extended from 9.1 to 15.0 hours by decreasing the bulk modulus from 1.35 to 0.26 MPa, Fig. 3G. The rigidified cylindrical polymer brush exhibited a plasma residence time of 18.9 hours while its soft counterpart exhibited 20.4 hours.27 The circulation half-life of a radiolabelled soft discoidal particle (1.3 kPa) was estimated to be ∼24 hours.33 However, the performance of its stiff counterpart (15 kPa) was not evaluated.
In conclusion, all above examples demonstrate that soft nanomedicines, with identical surface properties, size, and shape, outperform their stiff counterparts in blood circulation. Therefore, soft nanomedicines would have a higher chance to accumulate at tumor tissues than their stiff counterparts.
3.2 Tumor accumulation
The success of cancer nanomedicines relies on tumor accumulation, the combination outcome of prolonged blood circulation and the EPR effect.1 Nonetheless, upon i.v. administration, the blood-borne nanomedicines inevitably interact with normal organs. These noncancerous tissues present as physiological barriers for tumor accumulation. For instance, macrophages in the liver and spleen are the major scavengers of nanomedicines. The anatomo-physiological fenestrations in the spleen and kidneys and constricted capillaries in the lungs physically trap nanomedicines. In this section, we summarize the influence of nanomedicine mechanical properties on biodistribution and tumor accumulation, Fig. 4. | ||
| Fig. 4 The influence of nanomedicine mechanical properties on biodistribution and tumor accumulation. DOX distribution in normal organs: (A) heart; (B) liver; (C) spleen; (D) lungs; (E) kidneys; and tumor (F) after treatment with various formulations. These figures corroborated that soft nanomedicines exhibited higher tumor accumulation and less accumulation in normal organs than their stiff counterparts. Reprinted with permission from ref. 12. Copyright 2019 Nature Publishing Group. | ||
The impact of nanoparticle mechanical properties on biodistribution has been studied with numerous nanomedicines. Micrometer-sized nanomedicines are easily sequestered in the lungs, which are the first downstream tissue with micro-capillaries of diameters between 2 and 13 μm. Around 40% of 6.0 μm RBCMs with bulk E larger than 40 kPa were stuck in the lungs two hours post dosing, and caused remarkable distress to mice.9 In contrast, only ∼10% of more deformable RBCMs, with bulk E smaller than 17 kPa, were detected in the lungs. The most deformable RBCMs, with E of 7.8 kPa, mainly amassed (∼70%) in the spleen two hours post injection. Intriguingly, these highly deformable RBCMs were only transiently and physically entrapped in the fenestrations of the spleen and released back into blood circulation later, a possible explanation for the extremely long elimination half-life of 93.29 hours. But the clearance mechanism of these RBCMs was not clarified. Stiff (15 kPa) discoidal particles were found to accumulate 4 times higher in the liver than soft counterparts (1.3 kPa) over a 2 hour observation period via intravital microscopy.33 Similarly, 48 hours post injection, splenic accumulation of nanogels increased monotonically with increased stiffness, which inversely correlated with blood circulation half-life.21 Collectively, all above examples corroborated that nanoparticle softness regulated their organ accumulation. More importantly, nanomedicine softness also modulated payloads’ biodistribution,12Fig. 4A–E. Encapsulated within soft 3D-MPs (1 kPa), less doxorubicin (DOX) was retained in normal organs, including the heart, liver, spleen, lungs, and kidneys, compared with that loaded in stiff 2D-MPs (3 kPa), free DOX and Doxil®. Nanomedicine softness mediated biodistribution is beneficial for not only reduced side effects but also enhanced tumor accumulation and potent antitumor efficacy of delivered payloads.
The effect of nanomedicine mechanical properties on tumor accumulation is less explored. Quantitative drug delivery efficiency has only been reported in DOX loaded tumor cell derived microparticles.12 Specifically, soft 3D-MPs achieved a delivery efficiency of ∼8% injected dose per gram of tumor tissue whereas stiff 2D-MPs achieved less than 3% and Doxil® around 1%, Fig. 4F. Of note, the delivery efficiencies of these tumor cell derived microparticles, both 3D and 2D, are much higher than 0.7%, the median delivery efficiency for the past 10 years.44 The enhanced tumor delivery efficiency was ascribed to the improved biodistribution, Fig. 4A–E. Furthermore, DOX delivery efficiency of 3D-MPs reduced markedly when the stiffness of 3D-MPs was increased with either chemical or biological approaches. Consistently, DOX delivery efficiency of 2D-MPs increased significantly when the stiffness of 2D-MPs was reduced. These results corroborate that microparticle softness regulates drug delivery efficiency and biodistribution. With confocal intravital microscopy imaging, it was revealed that soft (1.3 kPa) discoidal particles amassed 6 fold higher in tumor than their stiff counterparts (15 kPa).33 Based on ex vivo near infrared (NIR) fluorescent images, semi-quantitative tumor-to-liver and tumor-to-spleen ratios were obtained to study tumor uptake and clearance of both rigid (9.7 GPa) and soft (704 kPa) silica nanocapsules.28 Three conclusions could be drawn. First, both soft and rigid silica nanocapsules were mainly cleared by the spleen, as the tumor-to-liver ratios were around 10 times higher than the tumor-to-spleen ratios. Second, soft nanocapsules accumulated less in the spleen than rigid groups. Third, functionalization with an active targeting agent, folic acid (FA), improved rigid nanocapsule tumor uptake to some extent but exerted little influence on tumor accumulation of soft silica nanocapsules. But no statistical difference can be detected between soft and rigid nanocapsules in terms of tumor accumulation. Similarly, in vivo NIR fluorescent images indicated that tumor uptake of nanolipogels decreased monotonically as E increased from 45 kPa to 1.6 MPa, 13.8 MPa and 19 MPa both 6 hours and 48 hours post dosing.40Ex vivo NIR fluorescent images further confirmed that particles of smaller Young's moduli were more likely to accumulate at tumor tissues 48 hours post dosing. Though qualitative, these observations all argue that soft nanomedicines accumulate more in tumor tissues than their stiff counterparts. A lipid–PLGA hybrid nanoparticle is, however, an exception. Stiff monolayer lipid covered nanoparticles accumulated more than soft bilayer lipid decorated nanoparticles, as demonstrated in both in vivo and ex vivo NIR imaging.38
Overall, the above examples illustrate that soft nanomedicines outperform their stiff counterparts in biodistribution and tumor accumulation. The superior performance of soft nanomedicines over rigid counterparts is ascribed to prolonged blood circulation.
3.3 Deep penetration
Arriving at tumor tissues is not the end of cancer nanomedicines, on the contrary, it is just another beginning.2 Particularly for solid tumors, such as breast cancer, hepatocellular carcinoma, and pancreatic cancer, to name a few, what is waiting for nanomedicines are tortuous and irregular tumor blood vasculature, a dense extracellular matrix (ECM), high interstitial fluid pressure, and accumulated solid stress due to fast growing cancer cells. These altogether form an aberrant tumor microenvironment (TME) and serve as a pathological obstacle preventing nanomedicines from accessing cancer cells.2 What is worse, the nanoscale size of nanomedicines critically limits their extravasation from blood vessel and penetration into tumor tissues. Therefore, successful nanomedicines need to have the capability of efficient extravasation and deep penetration. In this section, we summarize the effect of nanomedicine mechanical properties on diffusion in hydrogels, mucus, and simulated extracellular matrix, penetration in a multicellular spheroid (MCS) model, and blood vessel extravasation and tumor penetration in zebrafish and mice, Fig. 5. | ||
| Fig. 5 The influence of nanomedicine mechanical properties on blood extravasation and deep penetration. Down-regulating cytospin-A softens 2D-MPs and promotes their extravasation and penetration into H22 tumor tissues. Scale bars, 100 μm. Reprinted with permission from ref. 12. Copyright 2019 Nature Publishing Group. | ||
The advantages of soft nanomedicines over stiff counterparts on blood extravasation and tumor penetration have been nicely demonstrated with tumor cell derived microparticles in both in vitro and in vivo settings.12 First of all, soft DOX@3D-MPs penetrated deeper than stiff DOX@2D-MPs, Doxil®, and free DOX in H22 tumor spheroids. Second, DOX from DOX@3D-MPs exhibited the lowest co-localization with blood vessels in ex vivo tumor slides compared to DOX@2D-MPs, Doxil®, and free DOX. Third, a dorsal window chamber was inserted into the skin of mice and H22 tumor tissues implanted within the chamber were directly observed with intravital confocal microscopy to record blood extravasation and tumor penetration. DOX@3D-MPs extravasated from blood vessels 10 minutes post injection and penetrated gradually through the tumor tissue whereas DOX@2D-MPs, Doxil®, and free DOX were mainly retained within blood vessels. Fourth, DOX@3D-MPs displayed the highest extravascular DOX fluorescence intensity compared to DOX@2D-MPs, Doxil®, and free DOX in transgenic zebrafish with green fluorescent vessels. Collectively, all these results corroborated that DOX@3D-MPs had the best blood extravasation and tumor penetration compared to DOX@2D-MPs, Doxil®, and free DOX.12 Furthermore, blood extravasation and tumor penetration of these microparticles were ascribed to their softness. Reducing or increasing the stiffness, with either chemical treatment or biological intervention, of stiff 2D-MPs and soft 3D-MPs can directly change their subsequent blood extravasation and tumor penetration. For instance, down-regulating cytospin-A with siRNA reduced 2D-MPs Young's modulus and endowed 2D-MPs with enhanced extravasation and tumor penetration, Fig. 5. Soft nanomedicines surpassing rigid counterparts have also been displayed in other systems. As an example, the penetration depths of PEG–PCL polymer micelles increased monotonically in collagen gels, 4T1 tumor spheroids, and 4T1 tumor slides and tissues as E decreased from 62.6 to 44.8, 32.8 and 28.5 GPa.26 But quantitative analysis has not been carried out. Soft (704 kPa) FA–PEG-modified silica nanocapsules also penetrated deeper than stiff nanocapsules (9.7 GPa) in MCS of human ovarian adenocarcinoma SKOV3 cells.28 Being deformable, soft nanomedicines can squeeze and pass through the narrow interstitials of endothelium cells and ECM to extravasate from blood vasculature and penetrate deep into tumor tissues.
By stark contrast, being more internalized by cancer cells, stiff PEG–PLA polymer micelles (260 kPa) exhibited better tumor penetration in human pancreatic adenocarcinoma BxPC3 tumor spheroids than their soft counterpart (165 kPa).25 However, these polymeric micelles were only studied in relatively simple monolayer cancer cells or MCS models.
Complicating things further, hybrid nanoparticles and nanovesicles of intermediate rigidity were demonstrated to have better tumor penetration than stiffer and/or softer counterparts. Specifically, 50 MPa PLGA core lipid shell hybrid nanoparticles outperformed 5 MPa soft and 110 MPa hard hybrid nanoparticles in E12 cell monolayers with mucus, BxPC-3 and human pancreatic stellate cell (HPSC) MCS, ex vivo rat mucus and rat intestines, and in vivo tumor tissues.39 Super-resolution high-speed confocal microscopy provided direct evidence that 50 MPa spherical hybrid nanoparticles deformed into ellipsoids, similar to that in AFM with an applied force of 50 nN. In contrast, 110 MPa hard hybrid nanoparticles distorted negligibly while 5 MPa soft hybrid nanoparticles deformed excessively and irregularly. The sphere-to-ellipsoid deformation facilitated rotation and penetration of 50 MPa hybrid nanoparticles was further rationalized with molecular dynamics (MD) simulations. As a result of superior mucus-penetrating capability, DOX loaded hybrid nanoparticles of intermediate rigidity achieved the highest plasma DOX concentration, which was 14.8-fold, 4.9-fold, and 2.8-fold higher than those obtained from free DOX, and DOX loaded softer and stiffer nanoparticles. Consistently, nanovesicles of intermediate rigidity (19.9 MPa) exhibited better tumor penetration in simulated ECM (collagen hydrogel), tumor spheroids, and tumor tissues than stiffer (42.8 MPa) and softer (5.8 MPa) counterparts.36 Nanovesicles of intermediate rigidity were also observed to transform into rod-like shape during transportation in the ECM. More importantly, this study elegantly differentiates the contribution of cellular internalization from tumor penetration by comparing the penetration between nanovesicles of intermediate rigidity and stiff nanovesicles in two well-designed MCS models. Similar to PEG–PLA polymer micelles,25 stiff nanovesicles exhibited higher cellular uptake and therefore deeper penetration in MCS of BxPC-3 cells than nanovesicles of intermediate rigidity. But BxPC-3 cell derived MCS lacks ECM, which is abundant in tumor tissues in vivo. In an MCS model consisting of both BxPC-3 and HPSCs cells, stiff nanovesicles underperformed nanovesicles of intermediate rigidity in terms of penetration. Consistently, nanovesicles of intermediate rigidity displayed better tumor penetration than stiff counterparts in tumor tissues with the ECM. These well-controlled experiments clarify that high diffusivity in the ECM is essential whereas cellular internalization is insufficient for tumor penetration.
No consistent conclusion can be drawn from the above-mentioned studies. 1 kPa tumor cell derived microparticles, 28.5 GPa PEG–PCL polymer micelles, 704 kPa silica nanocapsules, 260 kPa PEG–PLA polymer micelles, 50 MPa hybrid nanoparticles and 19.9 MPa nanovesicles all exhibit better tumor penetration than their controls respectively. Nonetheless, almost all other physicochemical properties are different among these nanomedicines.11 Besides, the cell lines and in vitro and in vivo animal models differ markedly. It is therefore not possible to compare the performance of nanomedicines in terms of absolute elastic moduli. To further understand the influence of nanomedicine mechanical properties on tumor penetration, nanomedicines, cell lines, and animal models should be strictly selected. In particular, it is of utmost significance to control mechanical properties as a single variable with all other physicochemical properties identical.
3.4 Cellular internalization
In essence, it is the drugs encapsulated within nanocarriers that exert a therapeutic effect and eradicate malignant cells. Therefore, after the long journey of blood circulation, tumor accumulation, and permeation through the ECM, nanomedicines need to be internalized efficiently by cancer cells.1,2 In this section, we summarize the effect of nanomedicine mechanical properties on cellular internalization, supplemented with theoretical modelling, Fig. 6. The combination effect of nanomedicine stiffness in tandem with targeting agents on internalization will be emphasized. While the effect of stiffness on cellular uptake is mostly investigated in in vitro settings, cellular uptake based on cells separated from tumor tissues will also be introduced.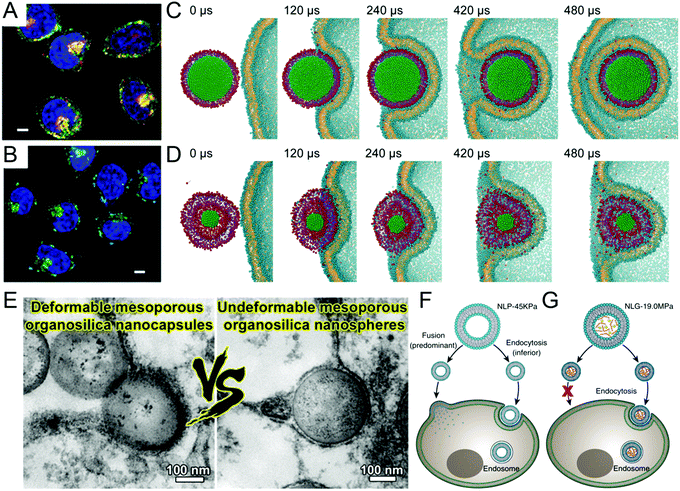 | ||
| Fig. 6 The influence of nanomedicine mechanical properties on cellular internalization. In vitro cellular uptake of stiff (A) and soft (B) nanoparticles. Scale bars, 5 μm. Molecular dynamics simulation of cellular uptake with stiff (C) and soft (D) nanoparticles. Figures (A–D) argue for stiff nanomedicine in terms of cellular internalization. Reprinted with permission from ref. 37. Copyright 2015 Wiley-VCH. (E) Soft silica nanocapsules deforming during cellular uptake. Scale bars, 100 nm. Reprinted with permission from ref. 29. Copyright 2018 American Chemical Society. (F) Soft nanoliposomes enter cells via two pathways, fusion (dominant) and endocytosis (inferior). (G) Stiff nanolipogels enter cells via only endocytosis. Reprinted with permission from ref. 40. Copyright 2018 Nature Publishing Group. Figures (E–G) support that soft nanomedicines have higher cellular uptake than stiff counterparts. | ||
The fact that cancer cells prefer stiff nanomedicines over soft counterparts has been substantiated with several nanomedicines in in vitro settings. For example, stiff (1.2 GPa) PLGA–lipid nanoparticles displayed higher cellular uptake, in HeLa cells, than soft (0.76 GPa) PLGA–water–lipid nanoparticles,37Fig. 6A and B. Both hybrid nanoparticles were internalized through energy dependent clathrin-mediated endocytosis. Adopting the Canham–Helfrich framework, MD simulations revealed that stiff nanoparticles can be engulfed smoothly by cancer cells, Fig. 6C, whereas soft nanoparticles deformed, which impeded their entry into cancer cells, Fig. 6D. Therefore, more energy was needed for cancer cells to internalize soft nanoparticles. For this reason, soft nanoparticles were less internalized and thus inferior to stiff nanoparticles in killing HeLa cancer cells after encapsulating two drugs, DOX and combretastatin A4, CA4. Similarly, stiff monolayer lipid covered PLGA nanoparticles illustrated higher cellular uptake than soft bilayer lipid decorated PLGA nanoparticles and therefore achieved higher cytotoxicity to both HeLa cancer cells and human umbilical vein endothelial cells (HUVECs).38 These two systems corroborated the theoretical analysis and molecular simulations on cellular internalization of deformable nanomedicines.45 Along the same line, cellular internalization, via clatherin-mediated endocytosis, increased monotonically with increased stiffness from 5.8 to 19.9 and 42.8 MPa, in both BxPC3 and HPSC.36 Accordingly, stiff nanovesicles (42.8 MPa) exhibited the lowest IC50 among all tested nanovesicles against both cell lines. In another instance, stiff (260 kPa) PEG–PLA micelles displayed superior uptake kinetics and amount compared with soft (165 kPa) counterparts in BxPC3 cells.25 Instead of utilizing the Canham–Helfrich Hamiltonian, a simple coarse-grained theory was developed to account for the observed rigidity-dependent cellular internalization. While cancer cells demonstrated intrinsic preference for stiff nanomedicines over soft counterparts in the above studies, their inclination towards stiff nanogels could be further amplified by conjugating with targeting agents, such as anti-ICAM antibodies.19 In another example, folate receptor positive SKOV3 cells internalized much more FA modified stiff (9.7 GPa) silica nanocapsules than soft (704 kPa) counterparts.28 Without FA modification, however, no difference could be detected between soft and stiff nanocapsules, suggesting a coupled effect between stiffness and surface modification.
In contrast, soft HIV particles are more efficient in cellular uptake than stiff counterparts,8 as many nanomedicines. As an example, soft (47.7 MPa) silica nanocapsules were more internalized than stiff (233.4 MPa) counterparts by human breast cancer MCF-7 cells,29Fig. 6E. Along the same line, cellular uptake of nanolipogels reduced monotonically with strengthened stiffness from 45 kPa to 1.6 MPa, 13.8 MPa, and 19 MPa in MDA-MB-231, MCF-7 and MCF-10A cells.40 The enhanced cellular internalization of soft nanolipogels was ascribed to the predominant fusion endocytosis pathway, Fig. 6F. In stark contrast, stiff nanolipogels were mainly internalized via an energy consuming clathrin-mediated endocytosis pathway, Fig. 6G. However, it has to be mentioned that lipid layers possess similar components and structure to cell membrane, leading to the lower energy cost of fusion pathway. This fusion pathway might not fit other types of nanocarriers. In another instance, soft (16.7 kPa) HEMA nanogels showed superior internalization kinetics and amount compared with stiff (155 kPa) counterparts in human hepatocellular carcinoma HepG2 cells.18 Mechanistically, soft nanogels were mainly internalized via micropinocytosis pathways whereas stiff ones were largely taken up via caveolae- and clatherin-mediated endocytosis. Nonetheless, the correlation between cellular uptake and endocytosis pathways has yet to be established. Though the detailed uptake pathway is not clarified, soft HA capsule with a stiffness of 7.5 mN m−1 displayed higher cell surface binding, cellular association, and cellular uptake in the case of HeLa cells than stiff counterparts with a stiffness of 17.6–28.9 mN m−1.31 Moreover, soft 3D-MPs displayed superior cellular internalization compared with stiff 2D-MPs in both cancer cells, including murine hepatocarcinoma H22 and melanoma B16-F10 cells, and their stem cells selected from a soft fibrin gel.42 As a consequence, DOX loaded 3D-MPs had much lower IC50 values than DOX@2D-MPs against all cancer cells, including cancer stem cells. Remarkably, the highest DOX cellular uptake was also observed in DOX@3D-MPs among DOX@2D-MPs, free DOX and Doxil® on green fluorescent protein expressing H22 tumor cells separated from tumor tissues. More importantly, DOX cellular uptake in both cancer cells and side population cells, a kind of cancer stem cell, could be adjusted by tailoring the softness of 2D-MPs and 3D-MPs with either chemical drug treatment or siRNA interference. This study emphasizes that nanomedicine softness regulates cancer cells’ cellular uptake in both in vitro and in vivo settings.
In contrast to the above two scenarios, an optimum stiffness was revealed to be a key determinant for successful siRNA delivery to hard-to-transfect suspension cancer cells, human leukemia K562.23 For cationized gelatin nanoparticles, cellular uptake was enhanced with decreased stiffness from 3.41 to 2.53 MPa but reached a maximum value at 0.87 MPa, and then decreased again at 0.63 MPa. While the detailed cellular internalization pathways called for more investigations, 0.87 MPa nanoparticles down-regulated BCR-ABL oncogene in both gene and protein expression levels in in vitro settings.
In summary, the above twelve examples underpin that distinct cancer cells exhibit specific preference over nanomedicines of varied stiffness, ranging from 1 kPa to several GPa. While no clear consensus can be drawn from experimental investigations, theoretical analysis and molecular simulations corroborate that cells prefer stiff nanoparticles over soft counterparts, especially in the initial step, membrane wrapping. Nonetheless, cellular internalization of nanomedicines involves much more complex processes.45 Therefore, multiscale modeling frameworks are urgently needed to account for different length and time scales as well as multiple events during cellular internalization.
3.5 Drug release
Following cancer cell cellular internalization, nanomedicines are anticipated to go through a series of intracellular sorting and processing, which are predetermined by endocytosis pathways, while payloads loaded within nanomedicines will be ultimately released to work on cytoplasmic or nuclear targets.2 In this section, we summarize the influence of nanoparticle mechanical properties on drug release and intracellular processing, Fig. 7. | ||
| Fig. 7 The impact of nanomedicine mechanical properties on drug release and intracellular processing. (A) Sustained release of FITC-dextran from nanoliposome and nanolipogels. Reprinted with permission from ref. 40. Copyright 2018 Nature Publishing Group. (B) DOX release profiles from 3D-MPs and 2D-MPs at different pH values. Intracellular processing of DOX@3D-MPs (C) and DOX@2D-MPs (D) in B16-F10 cancer cells. Scale bars, 20 μm. Reprinted with permission from ref. 12. Copyright 2019 Nature Publishing Group. | ||
Nanomedicine mechanical properties are expected to affect drug release kinetics. Cargos encapsulated within stiff nanomedicines would encounter a higher resistance than those in soft counterparts. This expectation is confirmed in the release of fluorescein isothiocyanate (FITC) labelled dextran from nanolipogels,40Fig. 7A. Calcium ion crosslinking decreases the mesh size of the alginate hydrogel network in the nanolipogel core and therefore slows down the release of FITC-dextran. Without a hydrogel core, no difference could be detected in DOX release between stiff 2D-MPs and soft 3D-MPs at pH 4.5, 6.5 and 7.4,12Fig. 7B. In another instance, 6-coumarin was released at a similar rate from both stiff (260 kPa) and soft (165 kPa) PEG–PLA polymeric micelles.25 These results highlight that the methods used to tune nanomedicine mechanical properties affect both nanomedicine intrinsic structures and interactions between payloads and carriers.
The effect of nanomedicine mechanical properties on intracellular processing has been studied with several different types of nanoparticles. Upon binding to cancer cells, nanoparticles are internalized via endocytic pathways and are transferred from the extracellular medium to intracellular vesicles, and end up in the lysosomes.1 Nonetheless, loaded drugs need to escape from lysosomes and exert therapeutic effects on cytoplasmic or nuclear targets. In such a case, intracellular processing is a key step for nanomedicines in eradicating cancer cells. As an example, pH responsive polymeric microcapsules were fabricated to detect the pH difference between extracellular medium and intracellular vesicles, including endosomes and lysosomes, and more importantly to measure intracellular processing kinetics.30 Soft microcapsules were processed faster to lysosomes than stiff counterparts when the stiffness was smaller than 5 N m−1. However, in another polymeric microcapsule system, microcapsule intracellular deformation and transportation to lysosomes were independent of stiffness.31 Though a HeLa cell line was used for both studies, polymers used, size and stiffness of microcapsules were distinctively different. For DNA-targeting chemotherapeutics, including DOX and cisplatin, nuclear entry is a key step. Tumor cell derived microparticles had been revealed to facilitate DNA-targeting chemotherapeutics nuclear entry via microtubules.41Fig. 7C and D illustrate that more DOX are colocalized with nuclei in soft DOX@3D-MPs than stiff DOX@2D-MPs.12 Nonetheless, the higher nucleus entry of DOX@3D-MPs than DOX@2D-MPs could be ascribed to either higher cellular uptake or faster intracellular processing. Systematic studies are therefore needed to clarify this issue.
Though it is evident that nanomedicine stiffness affects drug release and intracellular processing inside cancer cells, no clear conclusion can be drawn from the limited available studies.
3.6 Antitumor efficacy
Since nanomedicine mechanical properties affect every aspects of in vivo delivery processes, they regulate antitumor efficacy and adverse effects towards normal organs. This assertion is well demonstrated in chemotherapeutics encapsulated tumor cell derived microparticles.12 As soft DOX@3D-MPs displayed higher accumulation in tumor tissues, enhanced blood vessel extravasation and penetration into tumor tissues, and preferential uptake by both cancer cells and cancer stem cells, they achieved better antitumor efficacy and prolonged survival in H22 tumor bearing mice than stiff DOX@2D-MPs, Fig. 8A and B. Strikingly, compared to state-of-the-art nanotherapeutics, Doxil®, DOX@3D-MPs exhibited better efficiency at a dosage 1/8th of Doxil® in terms of DOX, suggesting that DOX@3D-MPs is at least 8 times more potent in killing cancer cells than Doxil®. Because of reasonable biodistribution and promoted tumor accumulation, DOX@3D-MPs exerted negligible adverse effects towards normal organs. More importantly, reducing the stiffness of 2D-MPs with cytospin-A siRNA significantly boosted the antitumor efficacy of DOX@2D-MPs and extended mice survival, Fig. 8C and D. These results underscore a causal role of nanomedicine softness in drug delivery efficiency and antitumor efficacy. The nanomedicine softness regulated blood circulation, extravasation, and tumor accumulation and penetration is reminiscent of cancer cells.42 Soft cancer cells also outperform stiff counterparts in tumor metastasis,46 tumor dormancy47 and resistance of T cell recognition.48 In another scenario, decent antitumor efficacy was attained with hydroxycamptothecin loaded nanovesicles of intermediate stiffness (19.9 MPa), Lip3, in BxPC-3-HPSC tumor xenografts.36 Different treatment outcomes were mainly ascribed to tumor penetration. Lip3 deformed moderately and transformed from spherical to ellipsoidal shape, which facilitated efficient diffusion in the dense ECM. In stark contrast, neither softer (Lip2 ∼ 10 MPa, Lip5 ∼ 5 MPa) nor stiffer (Lip4 42.8 MPa) nanovesicles could permeate through the dense ECM surrounding tumor tissues. In the third example, dual drugs, DOX and CA4, encapsulated stiff monolayer lipid PLGA nanoparticles achieved better tumor inhibition in HeLa tumor bearing nude mice than soft bilayer lipid PLGA nanoparticle.38 The enhanced tumor suppression of stiff nanoparticles was attributed to enhanced cellular internalization and subsequent promoted tumor accumulation. Hemolysis assay, histopathological staining, and serological examination all suggested that both hybrid nanoparticles were safe in mice. Because of different cancer models and nanomedicines, no consistent conclusion can be drawn from the limited available studies. Whether stiff or soft nanomedicines are beneficial for potent antitumor efficacy and reduced adverse effects is still an open question. | ||
| Fig. 8 The impact of nanomedicine mechanical properties on antitumor efficacy and mice survival. Tumor growth curves (A) and Kaplan–Meier survival plot (B) of H22 tumor bearing mice after intravenous injection of various DOX formulations. Knocking down cytospin-A in stiff 2D-MPs retards H22 tumor growth (C) and prolongs mice survival (D). Soft 3D-MPs confer better tumor suppression and longer survival than stiff counterparts. Reprinted with permission from ref. 12. Copyright 2019 Nature Publishing Group. | ||
Table 2 summarizes the performance of six representative nanoparticles in terms of five critical delivery processes and antitumor efficacy. Four conclusions can be drawn. First of all, the mechanical properties of nanoparticles affect every aspect of in vivo delivery processes. Second, no single nanoparticle has been evaluated in all five critical processes. Tumor cell derived microparticles have been studied in most processes.12 Although soft 3D-MPs exhibited higher resistance to macrophage phagocytosis than stiff 2D-MPs, their performance in long circulation is not clear. Besides, no definite conclusion can be drawn in terms of intracellular processing. Third, taking all nanoparticles into the consideration of each single transport process, except long circulation and tumor accumulation, contradictory preferences are noted in cellular internalization and deep penetration. In particular for cellular internalization, three types of nanomedicines argue for soft whereas another four kinds support stiff. Fourth, while many nanoparticles have been investigated under complex in vivo conditions, limited systems have been carefully examined in drug release and intracellular processing.
| Elastic moduli | Long circulation | Tumor accumulation | Deep penetration | Cell internalization | Drug release | Antitumor efficacy | Ref. | |
|---|---|---|---|---|---|---|---|---|
| N.D. stands for not determined and N.P. for no preference. | ||||||||
| Tumor cell microparticles | Soft, 1 kPa | N.D. | Soft✓ | Soft✓ | Soft✓ | N.P. | Soft✓ | 12 |
| Stiff, 3 kPa | ||||||||
| Nanovesicles | 5.8–42.8 MPa | N.D. | N.D. | Intermediate✓ | Stiff✓ | N.D. | Intermediate✓ | 36 |
| Hybrid nanoparticles | 45 kPa–19 MPa | N.D. | Soft✓ | N.D. | Soft✓ | Stiff✓ | N.D. | 40 |
| N.D. | N.D. | Stiff✓ | N.D. | Stiff✓ | N.D. | Stiff✓ | 38 | |
| Silica nanocapsules | Soft, 704 kPa | N.D. | Soft✓ | Soft✓ | Stiff✓ | N.D. | N.D. | 28 |
| Stiff, 9.7 GPa | ||||||||
| Soft, 47.7 MPa | N.D. | N.D. | N.D. | Soft✓ | N.D. | N.D. | 29 | |
| Stiff, 233.4 MPa | ||||||||
| Nanogels | Soft, 10 kPa | Soft✓ | N.D. | N.D. | Stiff✓ | N.D. | N.D. | 19 |
| Stiff, 3000 kPa | ||||||||
| 0.18–1.35 MPa | Soft✓ | N.D. | N.D. | N.D. | N.D. | N.D. | 21 | |
| Discoidal particles | Soft, 1.3 kPa | Soft✓ | Soft✓ | N.D. | N.D. | N.D. | N.D. | 33 |
| Stiff, 15 kPa | ||||||||
| 7.8–63.9 kPa | Soft✓ | N.D. | N.D. | N.D. | N.D. | N.D. | 9 | |
4. Concluding remarks
Nanomedicine mechanical properties have emerged as a novel key nano property. Although some progress has been made in nanomedicine mechanical properties in tumor targeting delivery, there are numerous fundamental challenges and opportunities ahead.First of all, how to guarantee mechanical properties as a single variable with all other physicochemical properties identical becomes an urgent problem in revealing the impact of nanomedicine mechanical properties on tumor targeting delivery. While size, shape, and surface charge are always reported in most of the studied nanomedicines, surface hydrophobicity is seldom determined. Surface properties dictate nanomedicine protein adsorption and the subsequent delivery processes.43 However, quantifying single nanoparticle hydrophobicity is not a trivial task. By means of combining freeze-fracture shadow-casting with cryo-scanning electron microscopy,49 nanoparticle hydrophobicity can be determined precisely at an oil–water interface in situ. Because the stiffness of nanovesicles is mediated by varying lipid components, their hydrophobicity might change as well. Complicating things further, hydrophobicity is intrinsically intertwined with mechanical properties in some nanoparticles. As such, polymeric nanogels should not be selected.24 By contrast, hybrid nanoparticles with a typical core shell, which can be further functionalized with various molecules, are ideal candidates.
Second, for the nine classes of nanoparticles discussed above, PEG–PLA micelles, nanoliposomes1 and tumor cell derived microparticles41 have already been applied to cancer patients in clinical settings. Recent studies from different groups clearly indicate that tailoring mechanical properties of these nanocarriers is beneficial for tumor targeted drug delivery. While nanoliposomes of intermediate stiffness and soft tumor cell derived microparticles have achieved better antitumor efficacy than their controls in vivo, stiff PEG–PLA micelles only demonstrate modest benefit compared with soft counterparts in in vitro settings. Therefore, it is essential to compare the antitumor efficacy of chemotherapeutics loaded stiff and soft PEG–PLA micelles in mice and large animals before clinical translation. To apply soft tumor cell derived microparticles in clinical settings, numerous grand challenges need to be addressed.12 Soft 3D-MPs are obtained from highly tumorigenic tumor repopulating cells and might contain tumor related biomolecules. Therefore, the safety issue is a big concern. In addition, large scale preparation of soft 3D-MPs at a reasonable cost is indispensable for even preclinical evaluations in large animals. From a translational perspective, nanoliposomes of intermediate stiffness have advantages over stiff PEG–PLA micelles and soft 3D-MPs in terms of scale-up production, quality control, and regulations.
Third, recent studies demonstrated that nanomedicines of intermediate stiffness exhibited superior performance over their softer and stiffer counterparts in tumor penetration36 and cellular internalization,23 respectively. These findings raise the interesting question of whether there exists an optimum stiffness for nanomedicines in each delivery process. To answer this question, the mechanical forces nanomedicines experienced in five critical transport processes need to be measured precisely. Specifically, the stresses, including shear, compressive and tensile stresses, deforming nanomedicines in blood flow, phagocytosis, extravasation, diffusion in the ECM, cancer cell cellular internalization and intracellular processing, need to be quantified with well-designed probes. As shown in hybrid nanoparticles39 and nanovesicles36 recently, different magnitudes of force are required to deform nanomedicines of varied stiffness. Revealing such forces would clarify the mechanical preference of nanomedicines in each delivery process, reconcile the contradictory conclusions drawn from different research groups in deep penetration, cellular internalization, and antitumor efficacy, and rationalize the design of mechanically adaptive nanomedicines for precise drug delivery to tumors.
Fourth, matching mechanical stiffness between cells and substrates is critical for both normal cell and stem cell functions.5 Achieving a delicate mechanical stiffness balance between nanomedicines and cancer cells might also be a prerequisite for efficient drug delivery. That is probably the reason why soft 3D-MPs,12 which have a Young's modulus close to cancer cells,13 are outstanding in drug delivery and tumor inhibition.
Fifth, different cancers have distinctive varied pathophysiological characteristics.46 It is not feasible for one type of nanomedicine to eradicate all cancers. The questions regarding which kind of malignant tumor and which in vivo delivery process will benefit from soft or hard nanomedicines remain largely uncharted. It is speculated that solid tumors with ample ECM, such as pancreatic cancers and hepatocellular carcinoma, benefit the most from mechanically optimized nanomedicines.
Finally, most of the current nanomedicines aim at eradicating tumor cells directly. Ma et al. have demonstrated that soft pliable Pickering emulsions are better adjuvants than stiff PLGA microparticles for targeted delivery antigens to dendritic cells and enhanced vaccination against tumors.14 Furthermore, recent studies published by various groups have clearly demonstrated that macrophage phagocytosis is significantly affected by nanomedicine mechanical properties.7,33,34 However, leveraging nanomedicine mechanical properties to specifically target at tumor associated macrophages remains largely unexplored.50 Whether cytotoxic T cells, neutrophils, natural killer cells, regulatory T cells, and myeloid-derived suppressor cells prefer stiff or soft nanoparticles is mainly present as an open question. Targeting these immune cells with mechanically optimized nanomedicines might induce robust cancer immunotherapy.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by grants from the National key research and development program of China (2018YFA0208900), the National Basic Research Program of China (2015CB931802), the National Science Foundation of China (31972927, 31700867, 81672937, 81974459, 81627901, 81803018 and 81773653), PCSIRT (IRT13016), the Scientific Research Foundation of Huazhong University of Science and Technology (3004170130), and the Program for HUST Academic Frontier Youth Team (2018QYTD01).References
- J. Shi, P. W. Kantoff, R. Wooster and O. C. Farokhzad, Nat. Rev. Cancer, 2017, 17, 20–37 CrossRef CAS PubMed
.
- H. Chen, Z. Gu, H. An, C. Chen, J. Chen, R. Cui, S. Chen, W. Chen, X. Chen, X. Chen, Z. Chen, B. Ding, Q. Dong, Q. Fan, T. Fu, D. Hou, Q. Jiang, H. Ke, X. Jiang, G. Liu, S. Li, T. Li, Z. Liu, G. Nie, M. Ovais, D. Pang, N. Qiu, Y. Shen, H. Tian, C. Wang, H. Wang, Z. Wang, H. Xu, J.-F. Xu, X. Yang, S. Zhu, X. Zheng, X. Zhang, Y. Zhao, W. Tan, X. Zhang and Y. Zhao, Sci. China: Chem., 2018, 61, 1503–1552 CrossRef CAS
.
- D. E. Discher, P. Janmey and Y. L. Wang, Science, 2005, 310, 1139–1143 CrossRef CAS PubMed
.
- A. J. Engler, S. Sen, H. L. Sweeney and D. E. Discher, Cell, 2006, 126, 677–689 CrossRef CAS PubMed
.
- F. Chowdhury, S. Na, D. Li, Y.-C. Poh, T. S. Tanaka, F. Wang and N. Wang, Nat. Mater., 2010, 9, 82–88 CrossRef CAS PubMed
.
- M. Plodinec, M. Loparic, C. A. Monnier, E. C. Obermann, R. Zanetti-Dallenbach, P. Oertle, J. T. Hyotyla, U. Aebi, M. Bentires-Alj, R. Y. H. Lim and C.-A. Schoenenberger, Nat. Nanotechnol., 2012, 7, 757–765 CrossRef CAS PubMed
.
- K. A. Beningo and Y. L. Wang, J. Cell Sci., 2002, 115, 849–856 CAS
.
- N. Kol, Y. Shi, M. Tsvitov, D. Barlam, R. Z. Shneck, M. S. Kay and I. Rousso, Biophys. J., 2007, 92, 1777–1783 CrossRef CAS PubMed
.
- T. J. Merkel, S. W. Jones, K. P. Herlihy, F. R. Kersey, A. R. Shields, M. Napier, J. C. Luft, H. Wu, W. C. Zamboni, A. Z. Wang, J. E. Bear and J. M. DeSimone, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 586–591 CrossRef CAS PubMed
.
- A. C. Anselmo and S. Mitragotri, Adv. Drug Delivery Rev., 2017, 108, 51–67 CrossRef CAS PubMed
.
- Y. Hui, X. Yi, F. Hou, D. Wibowo, F. Zhang, D. Zhao, H. Gao and C.-X. Zhao, ACS Nano, 2019, 13, 7410–7424 CrossRef CAS PubMed
.
- Q. Liang, N. Bie, T. Yong, K. Tang, X. Shi, Z. Wei, H. Jia, X. Zhang, H. Zhao, W. Huang, L. Gan, B. Huang and X. Yang, Nat. Biomed. Eng., 2019, 3, 729–740 CrossRef CAS PubMed
.
- P.-H. Wu, D. R.-B. Aroush, A. Asnacios, W.-C. Chen, M. E. Dokukin, B. L. Doss, P. Durand-Smet, A. Ekpenyong, J. Guck, N. V. Guz, P. A. Janmey, J. S. H. Lee, N. M. Moore, A. Ott, Y.-C. Poh, R. Ros, M. Sander, I. Sokolov, J. R. Staunton, N. Wang, G. Whyte and D. Wirtz, Nat. Methods, 2018, 15, 491–498 CrossRef CAS PubMed
.
- Y. Xia, J. Wu, W. Wei, Y. Du, T. Wan, X. Ma, W. An, A. Guo, C. Miao, H. Yue, S. Li, X. Cao, Z. Su and G. Ma, Nat. Mater., 2018, 17, 187–194 CrossRef CAS PubMed
.
- J. W. Myerson, B. Braender, O. McPherson, P. M. Glassman, R. Y. Kiseleva, V. V. Shuvaev, O. Marcos-Contreras, M. E. Grady, H.-S. Lee, C. F. Greineder, R. V. Stan, R. J. Composto, D. M. Eckmann and V. R. Muzykantov, Adv. Mater., 2018, 30, 1802373 CrossRef PubMed
.
- X. Banquy, F. Suarez, A. Argaw, J.-M. Rabanel, P. Grutter, J.-F. Bouchard, P. Hildgen and S. Giasson, Soft Matter, 2009, 5, 3984–3991 RSC
.
- G. R. Hendrickson and L. A. Lyon, Angew. Chem., Int. Ed., 2010, 49, 2193–2197 CrossRef CAS PubMed
.
- W. Liu, X. Zhou, Z. Mao, D. Yu, B. Wang and C. Gao, Soft Matter, 2012, 8, 9235–9245 RSC
.
- A. C. Anselmo, M. Zhang, S. Kumar, D. R. Vogus, S. Menegatti, M. E. Helgeson and S. Mitragotri, ACS Nano, 2015, 9, 3169–3177 CrossRef CAS PubMed
.
- M. B. Fish, C. A. Fromen, G. Lopez-Cazares, A. W. Golinski, T. F. Scott, R. Adili, M. Holinstat and O. Eniola-Adefeso, Biomaterials, 2017, 124, 169–179 CrossRef CAS PubMed
.
- L. Zhang, Z. Cao, Y. Li, J.-R. Ella-Menye, T. Bai and S. Jiang, ACS Nano, 2012, 6, 6681–6686 CrossRef CAS PubMed
.
- J. Cui, M. Bjoernmalm, K. Liang, C. Xu, J. P. Best, X. Zhang and F. Caruso, Adv. Mater., 2014, 26, 7295–7299 CrossRef CAS PubMed
.
- W. Zhang, B. Han, X. Lai, C. Xiao, S. Xu, X. Meng, Z. Li, J. Meng, T. Wen, X. Yang, J. Liu and H. Xu, Chem. Commun., 2020, 56, 1255–1258 RSC
.
- F. A. Plamper and W. Richtering, Acc. Chem. Res., 2017, 50, 131–140 CrossRef CAS PubMed
.
- T. Stern, I. Kaner, N. L. Zer, H. Shoval, D. Dror, Z. Manevitch, L. Chai, Y. Brill-Karniely and O. Benny, J. Controlled Release, 2017, 257, 40–50 CrossRef CAS PubMed
.
- H. Deng, K. Song, J. Zhang, L. Deng, A. Dong and Z. Qin, Chem. Commun., 2018, 54, 3014–3017 RSC
.
- M. Muellner, S. J. Dodds, N. Tri-Hung, D. Senyschyn, C. J. H. Porter, B. J. Boyd and F. Caruso, ACS Nano, 2015, 9, 1294–1304 CrossRef PubMed
.
- Y. Hui, D. Wibowo, Y. Liu, R. Ran, H.-F. Wang, A. Seth, A. P. J. Middelberg and C.-X. Zhao, ACS Nano, 2018, 12, 2846–2857 CrossRef CAS PubMed
.
- Z. Teng, C. Wang, Y. Tang, W. Li, L. Bao, X. Zhang, X. Su, F. Zhang, J. Zhang, S. Wang, D. Zhao and G. Lu, J. Am. Chem. Soc., 2018, 140, 1385–1393 CrossRef CAS PubMed
.
- R. Hartmann, M. Weidenbach, M. Neubauer, A. Fery and W. J. Parak, Angew. Chem., Int. Ed., 2015, 54, 1365–1368 CrossRef CAS PubMed
.
- H. Sun, E. H. H. Wong, Y. Yan, J. Cui, Q. Dai, J. Guo, G. G. Qiao and F. Caruso, Chem. Sci., 2015, 6, 3505–3514 RSC
.
- T. J. Merkel, K. Chen, S. W. Jones, A. A. Pandya, S. Tian, M. E. Napier, W. E. Zamboni and J. M. DeSimone, J. Controlled Release, 2012, 162, 37–44 CrossRef CAS PubMed
.
- J. Key, A. L. Palange, F. Gentile, S. Aryal, C. Stigliano, D. Di Mascolo, E. De Rosa, M. Cho, Y. Lee, J. Singh and P. Decuzzi, ACS Nano, 2015, 9, 11628–11641 CrossRef CAS PubMed
.
- R. Palomba, A. L. Palange, I. F. Rizzuti, M. Ferreira, A. Cervadoro, M. G. Barbato, C. Canale and P. Decuzzi, ACS Nano, 2018, 12, 1433–1444 CrossRef CAS PubMed
.
- M. Yu, W. Song, F. Tian, Z. Dai, Q. Zhu, E. Ahmad, S. Guo, C. Zhu, H. Zhong, Y. Yuan, T. Zhang, X. Yi, X. Shi, Y. Gan and H. Gao, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 5362–5369 CrossRef CAS PubMed
.
- Z. Dai, M. Yu, X. Yi, Z. Wu, F. Tian, Y. Miao, W. Song, S. He, E. Ahmad, S. Guo, C. Zhu, X. Zhang, Y. Li, X. Shi, R. Wang and Y. Gan, ACS Nano, 2019, 13, 7676–7689 CrossRef CAS PubMed
.
- J. Sun, L. Zhang, J. Wang, Q. Feng, D. Liu, Q. Yin, D. Xu, Y. Wei, B. Ding, X. Shi and X. Jiang, Adv. Mater., 2015, 27, 1402–1407 CrossRef CAS PubMed
.
- L. Zhang, Q. Feng, J. Wang, S. Zhang, B. Ding, Y. Wei, M. Dong, J.-Y. Ryu, T.-Y. Yoon, X. Shi, J. Sun and X. Jiang, ACS Nano, 2015, 9, 9912–9921 CrossRef CAS PubMed
.
- M. Yu, L. Xu, F. Tian, Q. Su, N. Zheng, Y. Yang, J. Wang, A. Wang, C. Zhu, S. Guo, X. Zhang, Y. Gan, X. Shi and H. Gao, Nat. Commun., 2018, 9, 2607 CrossRef PubMed
.
- P. Guo, D. Liu, K. Subramanyam, B. Wang, J. Yang, J. Huang, D. T. Auguste and M. A. Moses, Nat. Commun., 2018, 9, 130 CrossRef PubMed
.
- J. Ma, Y. Zhang, K. Tang, H. Zhang, X. Yin, Y. Li, P. Xu, Y. Sun, R. Ma, T. Ji, J. Chen, S. Zhang, T. Zhang, S. Luo, Y. Jin, X. Luo, C. Li, H. Gong, Z. Long, J. Lu, Z. Hu, X. Cao, N. Wang, X. Yang and B. Huang, Cell Res., 2016, 26, 713–727 CrossRef CAS PubMed
.
- J. Liu, Y. H. Tan, H. F. Zhang, Y. Zhang, P. W. Xu, J. W. Chen, Y. C. Poh, K. Tang, N. Wang and B. Huang, Nat. Mater., 2012, 11, 734–741 CrossRef CAS PubMed
.
- R. Cai and C. Chen, Adv. Mater., 2019, 31, 1805740 CrossRef CAS PubMed
.
- S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet, H. F. Dvorak and W. C. W. Chan, Nat. Rev. Mater., 2016, 1, 16014 CrossRef CAS
.
- S. Zhang, H. Gao and G. Bao, ACS Nano, 2015, 9, 8655–8671 CrossRef CAS PubMed
.
- D. Wirtz, K. Konstantopoulos and P. C. Searson, Nat. Rev. Cancer, 2011, 11, 512–522 CrossRef CAS PubMed
.
- Y. Y. Liu, J. D. Lv, X. Y. Liang, X. N. Yin, L. Zhang, D. G. Chen, X. Jin, R. Fiskesund, K. Tang, J. W. Ma, H. F. Zhang, W. Q. Dong, S. Q. Mo, T. Z. Zhang, F. R. Cheng, Y. B. Zhou, J. Xie, N. Wang and B. Huang, Cancer Res., 2018, 78, 3926–3937 CrossRef CAS PubMed
.
- M. Huse, Nat. Rev. Immunol., 2017, 17, 679–690 CrossRef CAS PubMed
.
- L. Isa, F. Lucas, R. Wepf and E. Reimhult, Nat. Commun., 2011, 2, 438 CrossRef PubMed
.
- L. Cassetta and J. W. Pollard, Nat. Rev. Drug Discovery, 2018, 17, 887–904 CrossRef CAS PubMed
.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |






