Nanofabrication within unimolecular nanoreactors
Youfu
Wang
 * and
Xinyuan
Zhu
* and
Xinyuan
Zhu

School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, People's Republic of China. E-mail: wyfown@sjtu.edu.cn
First published on 18th May 2020
Abstract
Nanoparticles (NPs) have been a research focus over the last three decades owing to their unique properties and extensive applications. It is crucial to precisely control the features of NPs including topology, architecture, composition, size, surface and assembly because these features will affect their properties and then applications. Ingenious nanofabrication strategies have been developed to precisely control these features of NPs, especially for templated nanofabrication within predesigned nanoreactors. Compared with conventional nanoreactors (hard templates and supramolecular nanoreactors), unimolecular nanoreactors exhibit (1) covalently stable nanostructures uninfluenced by environmental variations, (2) extensively regulated features of the structure including topology, composition, size, surface and valence due to the rapid development of polymer chemistry, and (3) effective encapsulation of abundant guests with or without strong interaction to achieve the function of loading, delivery and conversion of guests. Thus, unimolecular nanoreactors have shown fascinating prospects as templates for nanofabrication. Various NPs with expected topologies (sphere, rod, tube, branch, and ring), architectures (compact, hollow, core–shell, and necklace-like), compositions (metal, metal oxide, semiconductor, doping, alloy, silica, and composite), sizes (generally 1–100 nm), surface properties (hydrophilic, hydrophobic, reactivity, valence and responsivity) and assemblies (oligomer, chain, and aggregate) can be fabricated easily within reasonably designed unimolecular nanoreactors in a programmable way. In this review, we provide a brief introduction of the properties and types of unimolecular nanoreactors, a condensed summary of representative methodologies of nanofabrication within various unimolecular nanoreactors and a predicted outlook of the potential further developments of this charming nanofabrication approach.
1. Introduction
Nanomaterials have attracted widespread attention owing to their unique physical, chemical and biological properties distinguished from their bulk counterparts. Nanomaterials have been used in broad applications and have exerted deep influence on our daily life.1–4 NPs with three dimensions at the nanoscale are one of the most famous classes of nanomaterials. Their nanoscale effects lead to various properties that can satisfy a variety of applications, such as catalysis, photonics, electronics and biology. The basic features of NPs, such as topology (sphere, rod, tube, branch, ring, polyhedron, and plate),5–9 architecture (compact, hollow, core–shell, yolk–shell, and oligomer),10–14 composition (metal, metal oxide, semiconductor, doping, alloy, silica, and composite),15–17 size,18–20 surface (hydrophilic, hydrophobic, reactivity, valence and responsivity)21–24 and assembly (oligomer, chain, aggregate, and superlattice),25,26 profoundly affect their properties and then corresponding applications (Fig. 1). Therefore, it is important to develop delicate nanofabrication strategies to obtain high-quality NPs with good control over these basic features. Generally, there are two strategies for the fabrication of NPs, namely top-down and bottom-up strategies. For the top-down strategy, NPs are broken off from bulk materials through strong external forces in high price, making this method incapable of precisely tailoring the topology, architecture, size and surface of the resulting NPs.27 For the bottom-up strategy, NPs build up via nucleation and growth of precursors with good control over the above-mentioned features of NPs. The bottom-up strategies such as the hydrothermal and solvothermal method,28 interface-mediated synthesis,29 sol–gel processes,30 microwave-assisted synthesis,31 sonochemical synthesis32 and template synthesis33 are all wet chemical processes which can fabricate NPs in a much easier, better controllable and more frugal way compared to the top-down strategies.The template synthesis method is a representative bottom up strategy for the precisely controlled fabrication of NPs. With this method, a pre-existing hard or soft template with desired nanostructures was used to direct the production of NPs with expected and novel features which are inherited from the templates and difficult to obtain with other methods. Using predesigned nano-structural carbon or silica as hard templates, novel nanomaterials with unique nanostructures, various compositions and highly thermal stabilities have been fabricated in a controlled way.34 However, these finely tailored hard templates are usually fabricated from soft templates.35 Soft templates such as surfactants and polymers are very powerful tools for nanofabrication.36,37 Compared with supramolecular nanoreactors assembled from multiple molecules or multiple macromolecules with dynamic stability affected by environmental variations, unimolecular nanoreactors with covalently stable and predesigned nanostructures are one of the most attractive types of templates to fabricate NPs in a programmable way.38 Due to the rapid development of polymer chemistry, various types of unimolecular nanoreactors with diverse compositions and nanostructures have been prepared and nanofabrication of NPs with expected features within these unimolecular nanoreactors has shown charming prospects.
2. Properties and types of unimolecular nanoreactors
Unimolecular nanoreactors have drawn considerable attention in recent years because of their unique properties. Unimolecular nanoreactors are composed of covalent bonds within one nanoscale macromolecule exhibiting low viscosity and high solubility due to their rigid architectures avoiding chain entanglements. The advantages of unimolecular nanoreactors for the fabrication of NPs are prominent: (1) the structural stability of unimolecular nanoreactors is remarkable compared to that of supramolecular nanoreactors, which are the assemblies of multiple molecules (such as surfactants, amphiphilic polymers and so on) via weak interactions. The structural stability of supramolecular nanoreactors could be influenced seriously by environmental variations such as solvent, concentration, pH, ionic strength, temperature and so on. However, unimolecular nanoreactors, by contrast, can maintain their original architectures regardless of the outside changes (Fig. 2A).39 The soft but steady structures of unimolecular nanoreactors can interact with metal precursors at the molecular level and direct the formation of NPs in a robust way. (2) The structural features of unimolecular nanoreactors could be extensively regulated including topology, architecture, composition, size, surface and valence due to the rapid development of polymer chemistry and vast types of available monomers.40 All these delicately predesigned structural features of unimolecular nanoreactors will be inherited to the fabricated NPs with diverse topologies, various architectures, multifarious compositions, aplenty but uniform sizes, abundant guest interactions, and ample surface chemistry. (3) What's more, there are a variety of guests that can be effectively encapsulated into the cavities of unimolecular nanoreactors with or without strong interaction to achieve the function of loading, delivery and conversion of the guests. After metal precursors enrichment through strong interaction and subsequent reduction or pyrolysis, then NPs will form within unimolecular nanoreactors. The outstanding features of unimolecular nanoreactors are summarized in Fig. 2B. | ||
| Fig. 2 (A) Different behaviors of unimolecular nanoreactors and supramolecular nanoreactors under dilution. Reproduced with permission from Elsevier,39 copyright 2015. (B) The structural advantages of unimolecular nanoreactors. (C) The primary types of unimolecular nanoreactors: dendrimers (including dendrons), hyperbranched polymers (HBPs), single-chain nanoparticles (SCNPs), star polymers, bottlebrush polymers and cyclic brush polymers. | ||
The primary types of unimolecular nanoreactors are dendrimers (including dendrons), hyperbranched polymers (HBPs), single-chain nanoparticles (SCNPs), star polymers, bottlebrush polymers and cyclic brush polymers as shown in Fig. 2C. The unique properties and various types of unimolecular nanoreactors with finely structural regulations make them powerful nanoreactors for templated nanofabrication of NPs.41 The synthesis of NPs templated from various unimolecular nanoreactors will be presented in the next section.
3. Nanoparticles templated from various unimolecular nanoreactors
As mentioned above, the metal precursors occupied the specific domain of the unimolecular nanoreactors via strong interactions, such as coordination and electrostatic force, and subsequent pyrolysis or reduction to form NPs inheriting the nanostructures from the unimolecular nanoreactors in a controlled way. If there is no strong interaction between metal precursors and unimolecular nanoreactors, NPs without expected nanostructures will form. Notably, the outer domains of the unimolecular nanoreactors intimately and permanently tethered on the obtained NP surface to provide stability, solubility, biocompatibility, responsivity, assembly, reactivity and valences of the resulting nanohybrids. The integrated organic–inorganic hybrid NPs exhibit varied properties and broad applications.3.1 Dendrimers and dendrons as nanoreactors
Dendrimers have highly regular structures, well-defined chemical formulae and near spherical architectures with three recognizable areas: (1) the core, (2) branched layers radiating from the core, and (3) terminal groups on the periphery. These three structural regions can be widely regulated to satisfy various functions and applications. Dendrimers with unique physical, photophysical, chemical and biological properties have been broadly applied in sensing, electronics, photonics, catalysis and nanomedicine.42 As a structurally defined unimolecular nanoreactor, dendrimers can be used as encapsulating, stabilizing and directing agents for the fabrication of NPs with controlled architecture, composition, size and surface.43,44 Metal precursors can occupy the cavity of dendrimers with or without strong interactions and subsequently be chemically reduced to yield NPs in a controlled or uncontrolled way with dimensions of less than 3 nm.45–47 The terminal groups of the dendrimer can be further modified before or after the nanofabrication to provide the dispersity, stability, biocompatibility and assembly of the obtained NPs.48 With dendrimers as nanoreactors, various NPs and clusters (metal, metal oxide, and semiconductor) with mono- and multi-metallic compositions, compact and core–shell architectures have been fabricated.49–51 This approach provides the ability to create new materials integrating different metals together at the sub-nanometer scale.Without a strong interaction, the metal precursor can be loaded into the cavity of the dendrimer in a statistical way. So, the obtained NPs are poorly controlled in topology, architecture, surface, size and location (Fig. 3A).53 With a strong interaction, such as in poly(amidoamine) (PAMAM) and phenylazomethine dendrimer (DPA), the metal precursor can accumulate in the cavity of the dendrimer in a precise coordination way. The dendrimer has a gradient in branch density from the core to the terminal groups, which cause an energy gradient that directs the transfer of energy and charge from the periphery to the core. Metal ions complex to the coordination sites within those dendrimers in a stepwise way, which make it possible for finely regulating the number and location of the occupied metal ions.54 With this method, subnanoscale TiO2 NPs in anatase and rutile forms52 and Pt NPs55 with very narrow size distributions have been fabricated within DPA nanoreactors in a controllable way (Fig. 3B). Multimetallic NPs containing 2–8 elements with compact56,57 and core–shell architectures58–60 were also prepared in a controllable way using PAMAM or DPA as the nanoreactor (Fig. 4). Structural rearrangement has been investigated in experimental and theoretical aspects for the ultrasmall core–shell NPs (<∼2 nm). These obtained NPs which were incapable of being prepared by other wet chemical methods exhibited remarkable catalytic properties benefiting from the ultrasmall size and multielemental composition.51
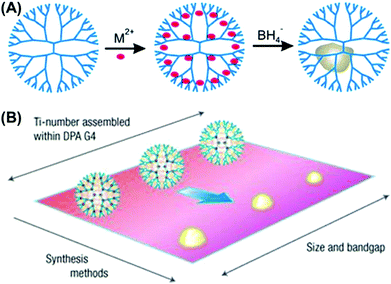 | ||
| Fig. 3 (A) Scheme of the NP fabrication within dendrimers without strong interaction. (B) Scheme of the formation of size-controlled TiO2 NPs within the G4 phenylazomethine dendrimer (DPA) with strong interaction. Reproduced with permission from Springer Nature,52 copyright 2008. | ||
 | ||
| Fig. 4 Dendrimer templated ultrasmall and multimetallic NPs with (A) compact and (B) core–shell architectures. Reproduced with permission from Springer Nature,57 copyright 2018 and ACS,58,59 copyright 2013. | ||
NPs templated from dendrimers are mostly applied in catalysis benefiting from the ultrasmall sizes and multi-metal features.45,47,50,51,56 Pt NPs with diameters of 2–5 nm are regarded as the ideal catalysts for the oxygen reduction reaction but are limited to preparation by conventional methods. Prof. Yamamoto fabricated ultrafine Pt clusters composed of defined numbers of atoms (12, 28, and 60) templated from DPA through stepwise complexation and reduction. As a result of exceptionally small particle sizes (0.9, 1.0, and 1.2 nm), the clusters exhibited very high catalytic activity for the four-electron reduction of oxygen molecules.55 The templated NPs within the dendrimer were also applied in bioimaging, drug delivery and sensing.44
As a branch of dendrimers, dendrons with highly structural regularity, well-defined chemical formula and 3D branched architecture radiating from one direction of the core can also be used as a nanoreactor to fabricate NPs in a controlled way. The functional groups in the core except those in the radiating direction of dendrons can function as valences of the resulting NPs. Interestingly, monofunctional Au NPs with a mean size of 1.3 nm were obtained within a benzylic thioether dendron equipped with a protected acetylene group in the core. After applying the deprotection and oxidative coupling protocol, the monofunctionalized Au NPs reacted with each other and exclusively dimerized to form Au NP dimers bridged through a diyne group.61 The monofunctional NPs with the acetylene group can also be covalently coupled to form oligomers (dimers, trimers and tetramers) via click chemistry in a wet chemical protocol (Fig. 5A).62 Different monofunctional groups in the core of the dendrons exerted important influence on the nucleation and growth during the NP formation, and also stabilized the obtained Au NPs in both experimental and theoretical studies.63 A nitro monofunctional polyester dendron and its assembly tethered on carbon nanotubes and cellulose were also utilized as nanoreactors for the fabrication of CdS quantum dots (QDs) and QD assemblies (Fig. 5B).64,65
 | ||
| Fig. 5 (A) Monofunctional Au NPs templated from monofunctional dendrons and then oligomerization to form dimers, trimers and tetramers via click chemistry. Reproduced with permission from ACS,61 copyright 2012 and Wiley-VCH,62 copyright 2014. (B) Dendron and its assembly tethered on carbon nanotubes as nanoreactors for the preparation of the CdS QD and QD assembly. Reproduced with permission from ACS,65 copyright 2006. | ||
Due to the definite composition and structure of dendrimers and dendrons, they can template the nanofabrication of NPs in a programmable way if there are strong interactions between metal precursors and internal regions of dendrimers and dendrons. Ultrasmall NPs with uniform size less than 3 nm, especially for well-defined clusters, alloys and monofunctional NPs, can be obtained within dendrimers or dendrons and are hard to prepare with other methods. However, the sizes of dendrimers and dendrons are limited because of the steric hindrance which also restricts the dimensions of the templated NPs. The huge cavities within dendrimers can also increase the difficulty for the NP formation during the reduction process for the low contact probability between metal precursors.
3.2 Hyperbranched polymers as nanoreactors
As analogues to dendrimers, hyperbranched polymers (HBPs) have nearly spherical architecture yet lower structural regularity compared to dendrimers. The globular and dendritic structures of HBPs exhibit unique properties such as plentiful internal and terminal groups, vast components, intramolecular cavities, high solubility and low viscosity. HBPs are generally prepared through simple one-pot polymerization of small molecular monomers or functional macromolecules.66,67 Due to the easy synthesis, tunable structures, and unique properties, HBPs have found their excellent performance in various fields such as supramolecular chemistry, nanotechnology, optoelectronics materials, biomaterials, adhesives, modifiers and coatings.68,69 The NPs fabricated within HBPs can not only obtain multifunctionality, uniform dispersibility, and splendid solubility but also can acquire extra properties. There are three strategies for the nanofabrication of NPs within HBPs, that is HBPs first, NPs first, and ligand exchange (Fig. 6).70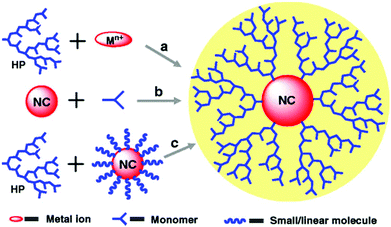 | ||
| Fig. 6 Three strategies for the nanofabrication of NPs within HBPs: (a) HBPs first, (b) NPs first, and (c) ligand exchange. Reproduced with permission from Springer,70 copyright 2011. | ||
Although being easy to synthesize, the controllability of HBP synthesis is poor resulting in indefinite molecular structure and large polymer dispersity index. Even with specifically strong interaction, HBPs cannot template the synthesis of NPs in a precisely controlled way, especially in topology, architecture and size. Prof. Xinyuan Zhu and colleagues have focused on hydrophilic hyperbranched PAMAM, HPAMAM, as unimolecular nanoreactors for nanofabrication. As analogues to PAMAM dendrimers, HPAMAM also exhibits strong coordination capacity with metal precursors and can effectively direct the templated formation of NPs. PEG grafted HPAMAM through pH responsive acylhydrazone bonds, HPAMAM-g-PEG, as a double-hydrophilic HBP was utilized as a unimolecular nanoreactor for the fabrication of CdS QDs in aqueous solution through metal precursor enrichment and reduction. When PEG arms withdrew from the HPAMAM core under pH < 5.5, the obtained CdS QDs within HPAMAM-g-PEG HBPs exhibited significantly enhanced fluorescence. Such a pH-responsive feature of these nanocomposites has been demonstrated in a novel biosensor in acidic lysosomes (Fig. 7).71 An amphiphilic HBP with HPAMAM core and aliphatic chain arms formed stable unimolecular nanoreactors in chloroform and was employed to accumulate Cd2+ ions from the aqueous phase. After the reaction with aqueous S2− ions, monodisperse CdS QDs stabilized by HBP were obtained. This amphiphilic HBP nanoreactor provided a new two-phase route to fabricate uniform CdS QDs.72 Polystyrene grafted hyperbranched polyglycerol, denoted as HPG-b-PS, was prepared and used as a unimolecular nanoreactor for fabricating colloidal Au NPs. The accumulation of Au precursors via coordination with the internal HPG core and following chemical reduction allowed the construction of well-dispersed and stable Au NPs with broad diameters of 10–30 nm.73 The length and density of the PS chain on HPG affect not only the solubility of HPG-b-PS during nanofabrication but also the dispersity and stability of the resulting Au NPs. Various NPs with metal, metal oxide and semiconductor compositions have also been synthesized and applied in catalysis and biology.74–77
 | ||
| Fig. 7 (A) Scheme for PEG grafted hyperbranched PAMAM (HPAMAM-g-PEG) through the pH sensitive acylhydrazone bond as the nanoreactor for fabricating CdS QDs. Reproduced with permission from ACS,71 copyright 2010. | ||
The HBPs with less structural regularity can fabricate NPs in a less controlled way especially for size and surface. However, for some practical applications, HBPs are still potential candidates for nanofabrication benefiting from easy synthesis and commercial availability in low cost.
3.3 Single-chain nanoparticles as nanoreactors
Single-chain nanoparticles (SCNPs) prepared by intramolecular chain collapse of precisely defined linear polymers containing crosslinkable components.78 Due to the development of polymer chemistry, components, sizes and valences of SCNPs can be tailored systematically. Advances in this technology have been applied in catalysis, sensing, nanoreactors, nanomedicine, etc.79SCNPs can also be used as unimolecular nanoreactors for the synthesis of NPs especially if there is strong interaction between their scaffold and metal precursors.80,81 Poly(N,N-dimethylaminoethyl methacrylate) containing 7% or 13% monomer hanging coumarin units can undergo intramolecular chain collapse to obtain SCNPs with controllable cross-linking densities triggered by photo-induced dimerization of coumarin. AuNPs can be fabricated via strong coordination interaction between Au precursors and tertiary amine groups in the SCPNs and subsequent reduction. The formation speed of the colloidal AuNP was subjected to the cross-linking density of SCNPs, which was faster with higher cross-linking density. This phenomenon was due to the fact that more dense tertiary amine groups in SCNPs can coordinate more Au precursors effectively converting to the final AuNPs.82 Poly(acrylic acid) (PAA) based SCNPs with tunable sizes were collapsed from poly(benzyl acrylate) containing enediyne units triggered by Bergman cyclization followed by hydrogenolysis. Because of the extensive coordination capability of PAA with various metal ions, ZnS and CdS QDs can be prepared and encapsulated within these SCNPs in one-pot and show high solubility in aqueous solution.83 With similar nanofabrication processes mentioned above, NPs with metal, metal oxide and semiconductor compositions have been fabricated within SCNPs.82–84
Interestingly, monofunctional SCNPs were prepared through ring-opening metathesis polymerization of three norbornene dicarboximide monomers equipped with fluorescein diacetate, tri-O-allyl-Tris and protected diol, and then ring-closing metathesis induced intramolecular chain collapse. Before and after deprotection, the organic- and aqueous-soluble SCNPs with narrow molecular weight (MW) distributions, MW of about 50 kDa and size of about 15–20 nm were prepared in a controlled manner.86 These monofunctional SCNPs can also be used as nanoreactors for the fabrication of Au NPs via the strong occupation of metal precursors with hydroxyl groups in their scaffolds of deprotected SCNPs. After DNA grafting onto the monofunctional group, the DNA strand on the formed Au NPs functions as the valence (Fig. 8).85 With a similar protocol, NPs with different valences (mono-valence or two different multi-valences), different sizes (6–10 nm, 15–20 nm or 30–40 nm), and different compositions (organic, Au, Pt or Pd) were fabricated within this type of SCNP. These obtained NPs with controlled valences, sizes, and compositions have demonstrated their potential applications in accurate assembly, ratiometric sensing, and targeted biomedicine.87
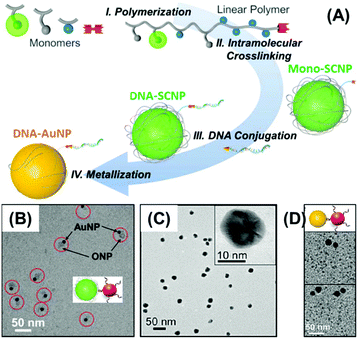 | ||
| Fig. 8 (A) Scheme of the fabrication of monofunctional Au NPs using monofunctional SCNPs as nanoreactors. (B) TEM image of heterodimers from monofunctional SCNPs and commercial 10 nm Au NPs. (C) TEM images of the obtained monofunctional Au NPs templated within SCNPs. (D) TEM images of the heterodimer from the mono-DNA Au NPs and commercial 10 nm AuNPs with complementary DNA strands. Reproduced with permission from ACS,85 copyright 2017. | ||
3.4 Star polymers as nanoreactors
Star polymers containing linear “arms” radiating from a “core” exhibit highly regular structures and unique properties owing to the spatially defined while compact architecture and controlled components and sizes.88 The synthetic approaches of star polymers could be roughly categorised as core-first, arm-first, and grafting-onto approaches. Generally, the core-first approach has better structural controllability for the star polymer construction.β-Cyclodextrin (β-CD) has been demonstrated to be a wonderful “core” molecule for star polymers due to low cost, accessibility and compact hydroxyl groups. The hydroxyl groups in β-CD can not only initiate ring-opening polymerization directly but also can be modified with bromine substitutes or RAFT (reversible addition–fragmentation chain transfer) agents, which can initiate ATRP (atom transfer radical polymerization) or RAFT polymerization to achieve block co-polymers from β-CD. These β-CD based star polymers have been validated to be powerful nanoreactors to fabricate NPs.
Plenty of NPs with diverse nanostructures for various applications templated from β-CD based star polymers have been studied thoroughly by Prof. Zhiqun Lin and colleagues.37 The β-CD based star polymers used for nanofabrication were prepared through the core-first approach via successive ATRP steps. β-CD equipped with 21 bromides, 21Br-β-CD, was synthesized and employed as an ATRP macroinitiator resulting in star polymers with well-defined and compact architectures, controlled molecular weights and distribution, and regulatable ratios of two or more radiating blocks.89 Finally, the obtained star polymers were utilized as nanoreactors to fabricate NPs resulting from the strong interaction between the specific polymer blocks and metal precursors. With this method, not only compact NPs, but core–shell and hollow NPs could also be fabricated (Fig. 9).90 Taking core–shell NPs as an example, β-CD based star polymers armed with triblock copolymers, P4VP-b-PtBA-b-PS, have been utilized as unimolecular nanoreactors. The core metal precursor can be occupied in the P4VP block via strong interaction (coordination or electrostatic interaction) and then be reduced to form the core. After hydrolysis of the PtBA block to the PAA block, the shell metal precursor can be further occupied in the PAA domain via strong interaction and then be reduced to create the shell. The sizes of the core and shell in resulting NPs could be tailored by adjusting polymerization time during the ATRP process. The surface tethered PS block which can be designed as other polymers could provide intimately and permanently surface features of the obtained NPs (Fig. 9C). Furthermore, the capped polymer tethered to the surface of the NPs eliminates the aggregation of NPs and ensures the solution stability and dispersity in polar or nonpolar solvents. Thus, polymer layer capped NPs are fabricated successfully in a controlled way. The star polymer as the unimolecular nanoreactor for nanofabrication is a general and robust approach for the controlled and effective preparation of nearly uniform NPs with regulated architectures, compositions, sizes and surfaces.
 | ||
| Fig. 9 (A–C) Synthetic schemes of the fabrication of NPs with various architectures (compact, core–shell and hollow) utilizing star polymers as unimolecular nanoreactors. (D) TEM images and cartoons of polymer capped NPs with compact, core–shell and hollow architectures from left to right, respectively. Reproduced with permission from Wiley-VCH,37 copyright 2018 and Springer Nature,90 copyright 2013. | ||
With this method, NPs with various compositions such as noble metals, metal oxides, semiconductors, silica and composites can be obtained by choosing appropriate precursors and subsequent growth conditions. A β-CD based star polymer armed with triblock copolymer, PS-b-PtBA-b-PS, was developed to craft colloidal Au or Ag hollow NPs. The internal cavity diameter and intermediate shell thickness in the obtained hollow NPs could be precisely tailored through controlling the degrees of polymerization of internal PS and intermediate PtBA blocks, respectively, during the star polymer preparation. It is worth mentioning that the plasmonic properties of these hollow NPs could be easily tailored by altering the outer shell diameter or shell thickness of the hollow NPs, which verified well with simulations. Compared to those of the compact counterparts with the same outer diameter, the plasmonic peaks of the hollow NPs exhibited increasing red-shift with decreased shell thicknesses.91 High-quality perovskite NPs have witnessed rapid advances over the past few years, however, the stability of perovskite NPs is a critical factor that promotes further progress in optoelectronic fields. Using β-CD based star polymers armed with triblock copolymers, P4VP-b-PtBA-b-PEO or P4VP-b-PtBA-b-PS, as unimolecular nanoreactors, a methylammonium lead bromide (MAPbBr3) core was prepared within the P4VP block and the SiO2 shell was formed via hydrolysing the tetramethyl orthosilicate occupying the PAA block pyrolyzed from the PtBA block. The delicately designed star polymers as nanoreactors can template the controlled fabrication of core–shell NPs with tailored perovskite diameter, SiO2 thickness, and tethered polymers. The PS or PEO tethered perovskite@SiO2 core–shell nanocomposite exhibited improved stabilities (colloidal stability, water stability and photostability) and appealing solution processability (Fig. 10A). Uniform organo-silica hybrid NPs with compact and hollow architectures were also crafted within β-CD based star polymers armed with a Si-containing homopolymer and a diblock copolymer with a Si-containing block as the outer layer, respectively.92 β-CD based star polymers can also be used as nanoreactors for the organic NP fabrication, including conjugated93 or non-conjugated94 polymer NPs with compact or hollow architectures via the cross-linking process. This class of soft NPs might be developed as a new branch of nanocarriers and drug nano-vehicles.
 | ||
| Fig. 10 (A) Scheme and digital images of PS-tethered MAPbBr3@SiO2 core–shell NPs thin film immersed in water for 0, 15, and 30 min, respectively. (B) Scheme of the photo-crosslinking and photocleavage behaviours of coumarin-containing polymer tethered on the obtained Au NPs and their reversibly light-triggered self-assembly and disassembly. Reproduced with permission from AAAS,95 copyright 2019 and NAS,96 copyright 2018. | ||
Not only can the hydrophobic and hydrophilic features of templated NPs be adjusted as PS and PEO capped perovskite@SiO2 core–shell nanocomposites mentioned above (Fig. 10A), because of the intimate and permanent connection between the outer block and the obtained NPs, the stability, dispersity, reactivity and responsivity of the formed NPs can also be controlled by choosing an appropriate polymerization approach and component. The terminal bromines on β-CD based star polymers after ATRP can be converted to azide groups, which can be modified with acetylene terminated polymers (PEDOT, PVDF and so on) via the effective click reaction. Conjugated-polymer-capped Pb chalcogenide NPs with compact and hollow architectures were fabricated within β-CD based star polymers armed with PAA-b-PEDOT and PS-b-PAA-b-PEDOT copolymers. PEDOT capped PbTe NPs with long-term stability and dispersion were promising in thermoelectric materials after doping with polystyrene sulfonate, which could function as heat-to-electricity converters and provide an alternative route for power generation and refrigeration.97 PEDOT capped PbS and PbTe hollow NPs showed an absorption maximum blue-shift fitted with theoretical predictions.98 Notably, ferroelectric PVDF capped ferroelectric BaTiO3 fabricated within star polymers with uniform and stable nanocomposites99 were promising when used in electronics, catalysis, energy conversion and storage, and biotechnology. The tethered polymer on the obtained NPs can also exhibit reactivity or responsivity and further affect the assembly behaviour and properties. Au NPs can be fabricated within the β-CD based star polymer armed with PAA-b-poly(7-methylacryloyloxy-4-methylcoumarin), PAA-b-PMAMC. The coumarin unit in PMAMC tethered on the surface of Au NPs showed photo-crosslinking and photocleavage behaviours and then induced the reversible assembly and disassembly of the Au NPs triggered through light. Under irradiation with 365 nm light, the coumarin units underwent a photodimerization process resulting in gradually formed Au NP aggregates with irregular shape and size (Fig. 10B).96 Remarkably, using 254 nm light as a trigger, the aggregates gradually disassembled and finally went back to the isolated NP state with original shape and size. The plasmonic absorptions of the assembled and isolated Au NPs were also tuned reversibly under different light irradiation. Thermo-responsive poly(N-isopropylacrylamide) (PNIPAM) capped Au NPs can induce red-shift and increasing intensity of plasmonic peak through increasing temperature because of the collapse of PNIPAM on the surface of Au NPs restricting the assembly of Au NPs. Interestingly, when adding linear PNIPAM, the Au NPs assembled to form aggregates through increasing temperature and the plasmonic peak shows greater red-shift and decreased intensity. All above thermal triggered processes were reversible. Markedly, the absence and presence of free PNIPAM induced the switchable catalytic and optical performance of PNIPAM capped Au NPs.100
Interestingly, Prof. Zhiqun Lin even designed an intriguing 1D necklace-like nanostructure comprising α-CD nanodiscs modified with Br substitutes to initiate sequential ATRPs and then metal ion gathering and reduction. Different from the normal morphology of star polymer nanoreactors, the assembled uniform functional nanodiscs along the PEG flexible polymer chain assumed the rectangle shape due to the crush between these unimolecular nanoreactor units. Such 1D organic–inorganic nano-necklaces composed of uniform inorganic NPs, capped with hydrophobic polymer chains and connected with PEG chain offering good solubility of the nano-necklaces in common solvents (Fig. 11). Nano-necklaces with various compositions, such as semiconductor CdSe, magnetic Fe3O4 and ferroelectric BaTiO3 have been prepared. These novel structures of nano-necklaces may be developed in some new applications for optoelectronics, catalysis, ferroelectrics, plasmonics, magnetic devices and other areas.101
 | ||
| Fig. 11 (A) Scheme of the synthesis of a necklace-like star polymer and its templated fabrication of a necklace-like nanostructure. (B–D) TEM images of semiconductor CdSe, magnetic Fe3O4 and ferroelectric BaTiO3 nanonecklaces, respectively. Reproduced with permission from AAAS,101 copyright 2015. | ||
3.5 Bottlebrush polymers as nanoreactors
Similar to a star polymer, a bottlebrush polymer has radiating linear “arms” from a linear core instead of a compact core for a star polymer. Bottlebrush polymers show high density of side chains and robust 3D architectures because of one or more side chains radiating from every repeating unit in the linear core. The synthetic strategies of the bottlebrush polymer can be categorized as grafting-from, grafting-through and grafting-onto approaches.102,103 The bottlebrush polymer is also a powerful nanoreactor for nanofabrication similar to the star polymer. Due to the novel topology, the bottlebrush polymer can template 1D NP fabrication, such as nanorods and nanotubes, which have shown myriad applications, such as catalysis, electronics, photonics, sensing and fundamental research in crystallization kinetics of nanorods, etc.104–107Prof. Zhiqun Lin used cellulose as a rigid backbone with intensive hydroxyl groups that allow the grafting of dense block copolymer side chains through bromine modification and sequential ATRPs. Similar to the β-CD strategy, robust 1D nanorods with compact, core–shell and hollow architectures have been fabricated using a cylindrical cellulose-based bottlebrush polymer as the unimolecular nanoreactor (Fig. 12). Taking a compact nanorod as an example, the cellulose-g-(PAA-b-PS) bottlebrush can be synthesized and used as the nanoreactor, in which the inner PAA block directs nanorod growth through metal concentration and reduction and the outer PS block provides dispersity and stability (Fig. 12A).108 Various nanorods with precisely controlled architectures, compositions, sizes and surfaces could also be obtained in a similar method. The length of the obtained nanorod can be tuned from the nanoscale to the micron scale choosing cellulose with different molecular weights as linear backbones of bottlebrush polymers.
 | ||
| Fig. 12 (A–C) Synthetic steps for the fabrication of nanorods with different architectures (compact, core–shell and hollow) templated from bottlebrush polymers. (D) TEM images of nanorods with compact, core–shell and hollow architectures from left to right, respectively. Reproduced with permission from AAAS,108 copyright 2016. | ||
The bottlebrush polymer can also be used to fabricate nanocomposites. Using cellulose-g-PAA as the nanoreactor, after the concentration of SnO2 precursors and templated growth of polydopamine (PDA), the PDA-coated SnO2 nanocomposites were crafted and used as electrode materials for lithium-ion batteries (Fig. 13A). The PDA performed as a passivating solid electrolyte interphase layer.109 Compared with uncoated SnO2 electrode based batteries, excellent electrochemical performance and superior long-term cycling stability (over 300 cycles) have been achieved after coating the protective PDA to enhance the Sn/Li2O interface fraction of SnO2-based electrodes. The bottlebrush polymers can also be prepared using a linear polymer with a bromine-containing repeating unit as the backbone and subsequent ATRP processes. These bottlebrush polymers have lower brush density compared to cellulose based bottlebrush polymers. Generally, 1D soft nanotubes are usually obtained using these bottlebrush polymers as unimolecular nanoreactors. In this way, water soluble organo-silica hybrid nanotubes (Fig. 13B)110 and organo-soluble organic nanotubes111,112 were fabricated with good controllability in dimensions and compositions. Organic nanotubes with well-defined one or two open ends were also fabricated via crosslinking of specific bottlebrush blocks within a multiblock bottlebrush along the backbone.113,114
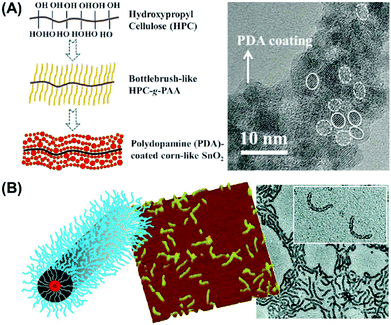 | ||
| Fig. 13 (A) Synthetic scheme and TEM image of bottlebrush polymer templated fabrication of PDA-coated SnO2 nanocrystals. (B) Scheme, AFM image and TEM images of organo-silica nanotubes templated from bottlebrush polymers. Reproduced with permission from Wiley-VCH,109 copyright 2017 and ACS,110 copyright 2010. | ||
Star like bottlebrush polymers, bottlebrush polymers with multi-arms, are also synthesized and used for nanofabrication.115 The three armed bottlebrush polymer, amphiphilic PHEMA-g-(PAA-b-cPS) was synthesized via ATRP followed by partial crosslinking of PS. After nanofabrication within this armed bottlebrush polymer, cPS-capped CsPbBr3 perovskite NPs were obtained with greatly improved stabilities over those obtained through conventional approaches due to the crosslinked hydrophobic PS on the surface. The internal PAA block directed the critical accumulation of metal ions and then formation of CsPbBr3 NPs. Concurrently, the outer cPS shell enhanced the passivation of NPs against external stimuli, consequently endowing the final perovskite NPs with excellent structural stability (UV light, heat, water, moisture, and oxygen) (Fig. 14).116 The obtained CsPbBr3 NPs were nearly spherical instead of the expected three-armed topology (branch) which may be due to the low brush density allowing the collapse of the PAA block during nanofabrication and the low length ratio of the PHEMA backbone to the PAA brush. In a similar way, a three-armed organic nanotube was fabricated through crosslinking the outer layer of the three armed bottlebrush polymer with a larger length ratio of the backbone to the brush.117
 | ||
| Fig. 14 Synthetic scheme and (a, b) TEM images of cPS-capped CsPbBr3 NPs templated from the three armed PHEMA-g-(PAA-b-cPS) bottlebrush polymer. Reproduced with permission from ACS,116 copyright 2019. | ||
3.6 Cyclic brush polymers as nanoreactors
Cyclic brush polymers have radiating linear arms from a cyclic core instead of from a compact core for the star polymer or a linear core for the bottlebrush polymer.118 Benefiting from the discovery of ring expansion metathesis polymerization (REMP),119,120 a cyclic brush polymer with uniform morphology can be prepared in a controlled way.121–124 Similar to the star polymer and bottlebrush polymer, the cyclic brush polymer can also be used as a unimolecular nanoreactor for cyclic NP fabrication.Cyclic NPs have attracted considerable attention because of the unique topology and the corresponding anisotropic properties.125 Several effective approaches have been developed for the fabrication of cyclic particles. However, most of the existing methods are high-cost, low yield and not robust. Although some research studies have been conducted on the cyclic particles, the dimension of the resulting particles is limited to the microscale.126 It is urgent to develop new methods to fabricate cyclic NPs in a robust way. Prof. Ke Zhang used the cyclic brush polymers as unimolecular nanoreactors for cyclic NP fabrication. The cyclic brush polymers were synthesized via the REMP and “grafting-onto” technique via the click reaction. With the organosilicon contained polymer or PGMA as the internal block in the cyclic brush polymer, cyclic silica or Au NPs can be obtained via hydrolysis of organosilicon or Au cluster coordination and reduction (Fig. 15).127
 | ||
| Fig. 15 (A) Scheme of the cyclic brush polymer and its templated nanofabrication of cyclic NPs. (B and C) TEM images of the obtained cyclic silica and Au NPs, respectively. Reproduced with permission from ACS,127 copyright 2016. | ||
4. Summary and outlook
In this review, we provided a brief summary of the fabrication of NPs within unimolecular nanoreactors. Rapid development in polymer chemistry has promoted remarkable progress in the controlled synthesis of unimolecular nanoreactors with diverse but uniform topologies, architectures, components and sizes. The unimolecular nanoreactors with well-defined and stable nanofeatures can direct the fabrication of various NPs with prospective nanostructures. The inside cavity concentrated plenty of precursors and the outside tethered polymer chains provided dispersion stability of the gained NPs. In this way, NPs with expected topologies (sphere, rod, tube, branch, and ring), architectures (compact, hollow, core–shell, and necklace-like), compositions (metal, metal oxide, semiconductor, doping, alloy, silica, and composite), sizes (generally 1–100 nm), surface properties (hydrophilic, hydrophobic, reactivity, valence and responsivity) and assemblies (oligomer, chain, and aggregate) can be fabricated easily within reasonably designed unimolecular nanoreactors. Diverse properties of the obtained NPs templated from unimolecular nanoreactors have led to a large number of potential applications. This kind of robust and unconventional strategy is promising for ingenious nanofabrication and has a positive influence on the fields of electronics, photonics, sensing, catalysis, biomedicine, etc.As an ingenious nanofabrication method, the further development of NPs templated from unimolecular nanoreactors may open new horizons in fabricating NPs with novel but unaccessible nanostructures (Fig. 16). Firstly, the topologies of the templated NPs can be expanded. Branched NPs can be fabricated within easily synthesized multi-armed bottlebrush polymers.115 Nanoplates can be fabricated within 2D brush polymers which can be potentially synthesized from the fast developing 2D polymers.128,129 3D nanopore nanomaterials can be fabricated from compacted oligomers of unimolecular nanoreactors if their outer layers have strong interaction with metal precursors.130 Secondly, the architectures of the templated NPs can be broadened. NP oligomers (homo- and hetero-oligomers)131,132 can be fabricated within unimolecular nanoreactors with or without interconnected spaces, such as star polymer oligomers,133 bottlebrush polymer oligomers,134 multi-block bottlebrush polymers,135 Janus HBPs136 or Janus SCNPs.137 Porous NPs can be fabricated with star-like bottlebrush polymers if their outer layers have strong interaction with metal precursors. Yolk–shell NPs can be fabricated within star polymers, bottlebrush polymers or cyclic brush polymers with four-block brushes. Thirdly, the compositions of the templated NPs can be enriched. Not only dendrimers but also all the unimolecular nanoreactors could template the fabrication of nanoalloys with or without phase separation.17,107 The nano-composites containing inorganic nonmetals can also be fabricated easily. Fourthly, the surface of the templated NPs can be designed intelligently. The intimately and permanently connected polymers on the surface of NPs can direct their assembly to superstructures or responsivity to external stimuli. Fifthly, the valences of the templated NPs can be controlled precisely.23,87 The unimolecular nanoreactors with precise functional groups (number, type and location)138–140 can be maintained after nanofabrication and function as the valences of the obtained NPs for further nano-reactions between NPs like small molecules to form a complex and designable NP assembly. The new horizons of this robust and unconventional nanofabrication strategy will show striking fascination in nanostructure crafting and application expansion.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The National Natural Science Foundation of China (21805130) and the Natural Science Foundation of Shanghai (17ZR1441300) are gratefully acknowledged for their funding support.Notes and references
- Q. Zhang, E. Uchaker, S. L. Candelaria and G. Cao, Chem. Soc. Rev., 2013, 42, 3127–3171 RSC
.
- J. A. Barreto, W. O'Malley, M. Kubeil, B. Graham, H. Stephan and L. Spiccia, Adv. Mater., 2011, 23, H18–H40 CrossRef CAS PubMed
.
- M. M. Khin, A. S. Nair, V. J. Babu, R. Murugan and S. Ramakrishna, Energy Environ. Sci., 2012, 5, 8075–8109 RSC
.
- H. Wei and E. Wang, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC
.
- L. Yang, Z. Zhou, J. Song and X. Chen, Chem. Soc. Rev., 2019, 48, 5140–5176 RSC
.
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60–103 CrossRef CAS PubMed
.
- S. Mostafa, F. Behafarid, J. R. Croy, L. K. Ono, L. Li, J. C. Yang, A. I. Frenkel and B. R. Cuenya, J. Am. Chem. Soc., 2010, 132, 15714–15719 CrossRef CAS PubMed
.
- Z. Zhu, H. Meng, W. Liu, X. Liu, J. Gong, X. Qiu, L. Jiang, D. Wang and Z. Tang, Angew. Chem., Int. Ed., 2011, 50, 1593–1596 CrossRef CAS PubMed
.
- Z. Wu, S. Yang and W. Wu, Nanoscale, 2016, 8, 1237–1259 RSC
.
- R. Ghosh Chaudhuri and S. Paria, Chem. Rev., 2012, 112, 2373–2433 CrossRef CAS
.
- X. Pang, C. Wan, M. Wang and Z. Lin, Angew. Chem., Int. Ed., 2014, 53, 5524–5538 CrossRef CAS PubMed
.
- A. Li, W. Zhu, C. Li, T. Wang and J. Gong, Chem. Soc. Rev., 2019, 48, 1874–1907 RSC
.
- W. Li, H. Palis, R. Mérindol, J. Majimel, S. Ravaine and E. Duguet, Chem. Soc. Rev., 2020, 49, 1955–1976 RSC
.
- X. Wang, J. Feng, Y. Bai, Q. Zhang and Y. Yin, Chem. Rev., 2016, 116, 10983–11060 CrossRef CAS PubMed
.
- M. B. Cortie and A. M. McDonagh, Chem. Rev., 2011, 111, 3713–3735 CrossRef CAS PubMed
.
- G. Chen, H. Qiu, P. N. Prasad and X. Chen, Chem. Rev., 2014, 114, 5161–5214 CrossRef CAS PubMed
.
- P.-C. Chen, X. Liu, J. L. Hedrick, Z. Xie, S. Wang, Q.-Y. Lin, M. C. Hersam, V. P. Dravid and C. A. Mirkin, Science, 2016, 352, 1565–1569 CrossRef CAS PubMed
.
- B. E. Hayden, Acc. Chem. Res., 2013, 46, 1858–1866 CrossRef CAS PubMed
.
- A. Albanese, P. S. Tang and W. C. W. Chan, Annu. Rev. Biomed. Eng., 2012, 14, 1–16 CrossRef CAS PubMed
.
- W. Li, R. Zamani, P. Rivera Gil, B. Pelaz, M. Ibáñez, D. Cadavid, A. Shavel, R. A. Alvarez-Puebla, W. J. Parak, J. Arbiol and A. Cabot, J. Am. Chem. Soc., 2013, 135, 7098–7101 CrossRef CAS PubMed
.
- S. Wang, K. Liu, X. Yao and L. Jiang, Chem. Rev., 2015, 115, 8230–8293 CrossRef CAS PubMed
.
- M. A. Boles, D. Ling, T. Hyeon and D. V. Talapin, Nat. Mater., 2016, 15, 141–153 CrossRef CAS PubMed
.
- L. Song and Z. Deng, ChemNanoMat, 2017, 3, 698–712 CrossRef CAS
.
- K. E. Sapsford, W. R. Algar, L. Berti, K. B. Gemmill, B. J. Casey, E. Oh, M. H. Stewart and I. L. Medintz, Chem. Rev., 2013, 113, 1904–2074 CrossRef CAS PubMed
.
- M. A. Boles, M. Engel and D. V. Talapin, Chem. Rev., 2016, 116, 11220–11289 CrossRef CAS PubMed
.
- C. R. Laramy, M. N. O'Brien and C. A. Mirkin, Nat. Rev. Mater., 2019, 4, 201–224 CrossRef CAS
.
- J.-K. Lung, J.-C. Huang, D.-C. Tien, C.-Y. Liao, K.-H. Tseng, T.-T. Tsung, W.-S. Kao, T.-H. Tsai, C.-S. Jwo, H.-M. Lin and L. Stobinski, J. Alloys Compd., 2007, 434–435, 655–658 CrossRef CAS
.
- D. R. Modeshia and R. I. Walton, Chem. Soc. Rev., 2010, 39, 4303–4325 RSC
.
- X. Wang, Q. Peng and Y. Li, Acc. Chem. Res., 2007, 40, 635–643 CrossRef CAS PubMed
.
- R. Sui and P. Charpentier, Chem. Rev., 2012, 112, 3057–3082 CrossRef CAS PubMed
.
- M. N. Nadagouda, T. F. Speth and R. S. Varma, Acc. Chem. Res., 2011, 44, 469–478 CrossRef CAS PubMed
.
- H. Xu, B. W. Zeiger and K. S. Suslick, Chem. Soc. Rev., 2013, 42, 2555–2567 RSC
.
- Y. Liu, J. Goebl and Y. Yin, Chem. Soc. Rev., 2013, 42, 2610–2653 RSC
.
- D. Gu and F. Schüth, Chem. Soc. Rev., 2014, 43, 313–344 RSC
.
- Y. Wan and D. Zhao, Chem. Rev., 2007, 107, 2821–2860 CrossRef CAS
.
- B. Li, N. You, Y. Liang, Q. Zhang, W. Zhang, M. Chen and X. Pang, Energy Environ. Mater., 2019, 2, 38–54 CrossRef CAS
.
- X. Li, J. Iocozzia, Y. Chen, S. Zhao, X. Cui, W. Wang, H. Yu, S. Lin and Z. Lin, Angew. Chem., Int. Ed., 2018, 57, 2046–2070 CrossRef CAS PubMed
.
- D. G. Shchukin and G. B. Sukhorukov, Adv. Mater., 2004, 16, 671–682 CrossRef CAS
.
- M. C. Lukowiak, B. N. S. Thota and R. Haag, Biotechnol. Adv., 2015, 33, 1327–1341 CrossRef CAS PubMed
.
- J.-F. Lutz, J.-M. Lehn, E. W. Meijer and K. Matyjaszewski, Nat. Rev. Mater., 2016, 1, 16024 CrossRef CAS
.
- X. Fan, Z. Li and X. J. Loh, Polym. Chem., 2016, 7, 5898–5919 RSC
.
- D. Astruc, E. Boisselier and C. Ornelas, Chem. Rev., 2010, 110, 1857–1959 CrossRef CAS PubMed
.
- L. M. Bronstein and Z. B. Shifrina, Chem. Rev., 2011, 111, 5301–5344 CrossRef CAS PubMed
.
- S. R. Barman, A. Nain, S. Jain, N. Punjabi, S. Mukherji and J. Satija, J. Mater. Chem. B, 2018, 6, 2368–2384 RSC
.
- R. Ye, A. V. Zhukhovitskiy, C. V. Deraedt, F. D. Toste and G. A. Somorjai, Acc. Chem. Res., 2017, 50, 1894–1901 CrossRef CAS PubMed
.
- K. Yamamoto and T. Imaoka, Acc. Chem. Res., 2014, 47, 1127–1136 CrossRef CAS PubMed
.
- R. M. Crooks, M. Zhao, L. Sun, V. Chechik and L. K. Yeung, Acc. Chem. Res., 2001, 34, 181–190 CrossRef CAS PubMed
.
- J. Yang, Q. Zhang, H. Chang and Y. Cheng, Chem. Rev., 2015, 115, 5274–5300 CrossRef CAS PubMed
.
- X. Peng, Q. Pan and G. L. Rempel, Chem. Soc. Rev., 2008, 37, 1619–1628 RSC
.
- V. S. Myers, M. G. Weir, E. V. Carino, D. F. Yancey, S. Pande and R. M. Crooks, Chem. Sci., 2011, 2, 1632–1646 RSC
.
- K. Yamamoto, T. Imaoka, M. Tanabe and T. Kambe, Chem. Rev., 2020, 120, 1397–1437 CrossRef PubMed
.
- N. Satoh, T. Nakashima, K. Kamikura and K. Yamamoto, Nat. Nanotechnol., 2008, 3, 106–111 CrossRef CAS PubMed
.
- X. Liu, D. Gregurec, J. Irigoyen, A. Martinez, S. Moya, R. Ciganda, P. Hermange, J. Ruiz and D. Astruc, Nat. Commun., 2016, 7, 13152 CrossRef CAS PubMed
.
- K. Yamamoto, M. Higuchi, S. Shiki, M. Tsuruta and H. Chiba, Nature, 2002, 415, 509–511 CrossRef CAS PubMed
.
- K. Yamamoto, T. Imaoka, W.-J. Chun, O. Enoki, H. Katoh, M. Takenaga and A. Sonoi, Nat. Chem., 2009, 1, 397–402 CrossRef CAS PubMed
.
- M. Takahashi, H. Koizumi, W.-J. Chun, M. Kori, T. Imaoka and K. Yamamoto, Sci. Adv., 2017, 3, e1700101 CrossRef PubMed
.
- T. Tsukamoto, T. Kambe, A. Nakao, T. Imaoka and K. Yamamoto, Nat. Commun., 2018, 9, 3873 CrossRef PubMed
.
- R. M. Anderson, L. Zhang, J. A. Loussaert, A. I. Frenkel, G. Henkelman and R. M. Crooks, ACS Nano, 2013, 7, 9345–9353 CrossRef CAS PubMed
.
- L. Zhang, R. Iyyamperumal, D. F. Yancey, R. M. Crooks and G. Henkelman, ACS Nano, 2013, 7, 9168–9172 CrossRef CAS PubMed
.
- M. R. Knecht, M. G. Weir, A. I. Frenkel and R. M. Crooks, Chem. Mater., 2008, 20, 1019–1028 CrossRef CAS
.
- J. P. Hermes, F. Sander, U. Fluch, T. Peterle, D. Thompson, R. Urbani, T. Pfohl and M. Mayor, J. Am. Chem. Soc., 2012, 134, 14674–14677 CrossRef CAS PubMed
.
- F. Sander, U. Fluch, J. P. Hermes and M. Mayor, Small, 2014, 10, 349–359 CrossRef CAS PubMed
.
- K. C.-F. Leung, X.-B. Li, X. Li, S.-F. Lee, J. C. Yu, P. M. Mendes, K. E. Hermann and M. A. Van Hove, Mater. Chem. Front., 2019, 3, 1555–1564 RSC
.
- S.-H. Hwang, C. N. Moorefield, P. Wang, K.-U. Jeong, S. Z. D. Cheng, K. K. Kotta and G. R. Newkome, Chem. Commun., 2006, 3495–3497 RSC
.
- S.-H. Hwang, C. N. Moorefield, P. Wang, K.-U. Jeong, S. Z. D. Cheng, K. K. Kotta and G. R. Newkome, J. Am. Chem. Soc., 2006, 128, 7505–7509 CrossRef CAS PubMed
.
- C. Gao and D. Yan, Prog. Polym. Sci., 2004, 29, 183–275 CrossRef CAS
.
- Y. Segawa, T. Higashihara and M. Ueda, Polym. Chem., 2013, 4, 1746–1759 RSC
.
- Y. Zheng, S. Li, Z. Weng and C. Gao, Chem. Soc. Rev., 2015, 44, 4091–4130 RSC
.
- W. Wu, R. Tang, Q. Li and Z. Li, Chem. Soc. Rev., 2015, 44, 3997–4022 RSC
.
- X. Hu, L. Zhou and C. Gao, Colloid Polym. Sci., 2011, 289, 1299–1320 CrossRef CAS
.
- L. Zhu, Y. Shi, C. Tu, R. Wang, Y. Pang, F. Qiu, X. Zhu, D. Yan, L. He, C. Jin and B. Zhu, Langmuir, 2010, 26, 8875–8881 CrossRef CAS PubMed
.
- Y. Shi, J. Liang, L. Liu, L. He, C. Tu, X. Guo, B. Zhu, C. Jin, D. Yan, T. Han and X. Zhu, J. Appl. Polym. Sci., 2011, 120, 991–997 CrossRef CAS
.
- J. Iocozzia and Z. Lin, Langmuir, 2016, 32, 7180–7188 CrossRef CAS PubMed
.
- S. Saliba, C. Valverde Serrano, J. Keilitz, M. L. Kahn, C. Mingotaud, R. Haag and J.-D. Marty, Chem. Mater., 2010, 22, 6301–6309 CrossRef CAS
.
- H. Li, J. K. Jo, L. D. Zhang, C.-S. Ha, H. Suh and I. Kim, Langmuir, 2010, 26, 18442–18453 CrossRef CAS PubMed
.
- C. Frangville, M. Gallois, Y. Li, H. H. Nguyen, N. Lauth-de Viguerie, D. R. Talham, C. Mingotaud and J.-D. Marty, Nanoscale, 2016, 8, 4252–4259 RSC
.
- Y. Shi, L. Liu, F. Zhang, M. Niu, Y. Zhao, Y. Fan, Y. Liang, M. Liu, Z. Zhang and J. Wang, Polymers, 2017, 9, 459 CrossRef PubMed
.
- S. Mavila, O. Eivgi, I. Berkovich and N. G. Lemcoff, Chem. Rev., 2016, 116, 878–961 CrossRef CAS PubMed
.
- C. K. Lyon, A. Prasher, A. M. Hanlon, B. T. Tuten, C. A. Tooley, P. G. Frank and E. B. Berda, Polym. Chem., 2015, 6, 181–197 RSC
.
- H. Rothfuss, N. D. Knöfel, P. W. Roesky and C. Barner-Kowollik, J. Am. Chem. Soc., 2018, 140, 5875–5881 CrossRef CAS PubMed
.
- N. D. Knöfel, H. Rothfuss, C. Barner-Kowollik and P. W. Roesky, Polym. Chem., 2019, 10, 86–93 RSC
.
- J. He, L. Tremblay, S. Lacelle and Y. Zhao, Soft Matter, 2011, 7, 2380–2386 RSC
.
- G. Qian, B. Zhu, Y. Wang, S. Deng and A. Hu, Macromol. Rapid Commun., 2012, 33, 1393–1398 CrossRef CAS PubMed
.
- A. Latorre-Sanchez and J. A. Pomposo, Chem. Commun., 2015, 51, 15736–15738 RSC
.
- H. Xing, Y. Bai, Y. Bai, L. H. Tan, J. Tao, B. Pedretti, G. A. Vincil, Y. Lu and S. C. Zimmerman, J. Am. Chem. Soc., 2017, 139, 3623–3626 CrossRef CAS PubMed
.
- Y. Bai, H. Xing, G. A. Vincil, J. Lee, E. J. Henderson, Y. Lu, N. G. Lemcoff and S. C. Zimmerman, Chem. Sci., 2014, 5, 2862–2868 RSC
.
- Y. Bai, H. Xing, Y. Bai, L. H. Tan, K. Hwang, J. Li, Y. Lu and S. C. Zimmerman, Chem. Sci., 2020, 11, 1564–1572 RSC
.
- J. M. Ren, T. G. McKenzie, Q. Fu, E. H. H. Wong, J. Xu, Z. An, S. Shanmugam, T. P. Davis, C. Boyer and G. G. Qiao, Chem. Rev., 2016, 116, 6743–6836 CrossRef CAS PubMed
.
- X. Pang, L. Zhao, M. Akinc, J. K. Kim and Z. Lin, Macromolecules, 2011, 44, 3746–3752 CrossRef CAS
.
- X. Pang, L. Zhao, W. Han, X. Xin and Z. Lin, Nat. Nanotechnol., 2013, 8, 426–431 CrossRef CAS PubMed
.
- Y. Chen, D. Yang, Y. J. Yoon, X. Pang, Z. Wang, J. Jung, Y. He, Y. W. Harn, M. He, S. Zhang, G. Zhang and Z. Lin, J. Am. Chem. Soc., 2017, 139, 12956–12967 CrossRef CAS PubMed
.
- C. Feng, X. Pang, Y. He, Y. Chen, G. Zhang and Z. Lin, Polym. Chem., 2015, 6, 5190–5197 RSC
.
- Y. Bian, C. Yang, Q. Gu, X. Zhu, Y. Wang and X. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 1550–1555 CrossRef CAS
.
- C. Feng, X. Pang, Y. He, B. Li and Z. Lin, Chem. Mater., 2014, 26, 6058–6067 CrossRef CAS
.
- Y. He, Y. J. Yoon, Y. W. Harn, G. V. Biesold-McGee, S. Liang, C. H. Lin, V. V. Tsukruk, N. Thadhani, Z. Kang and Z. Lin, Sci. Adv., 2019, 5, eaax4424 CrossRef
.
- Y. Chen, Z. Wang, Y. He, Y. J. Yoon, J. Jung, G. Zhang and Z. Lin, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E1391–E1400 CrossRef CAS PubMed
.
- H. Xu, X. Pang, Y. He, M. He, J. Jung, H. Xia and Z. Lin, Angew. Chem., Int. Ed., 2015, 54, 4636–4640 CrossRef CAS PubMed
.
- Y. He, X. Pang, B. Jiang, C. Feng, Y. W. Harn, Y. Chen, Y. J. Yoon, S. Pan, C. H. Lu, Y. Chang, M. Zebarjadi, Z. Kang, N. Thadhani, J. Peng and Z. Lin, Angew. Chem., Int. Ed., 2017, 56, 12946–12951 CrossRef CAS PubMed
.
- B. Jiang, X. Pang, B. Li and Z. Lin, J. Am. Chem. Soc., 2015, 137, 11760–11767 CrossRef CAS PubMed
.
- Y. Chen, Z. Wang, Y. W. Harn, S. Pan, Z. Li, S. Lin, J. Peng, G. Zhang and Z. Lin, Angew. Chem., Int. Ed., 2019, 58, 11910–11917 CrossRef CAS
.
- H. Xu, Y. Xu, X. Pang, Y. He, J. Jung, H. Xia and Z. Lin, Sci. Adv., 2015, 1, e1500025 CrossRef
.
- R. Verduzco, X. Li, S. L. Pesek and G. E. Stein, Chem. Soc. Rev., 2015, 44, 2405–2420 RSC
.
- T. Pelras, C. S. Mahon and M. Müllner, Angew. Chem., Int. Ed., 2018, 57, 6982–6994 CrossRef CAS
.
- T. Zhai, L. Li, Y. Ma, M. Liao, X. Wang, X. Fang, J. Yao, Y. Bando and D. Golberg, Chem. Soc. Rev., 2011, 40, 2986–3004 RSC
.
- R. K. Joshi and J. J. Schneider, Chem. Soc. Rev., 2012, 41, 5285–5312 RSC
.
- M. Serra, R. Arenal and R. Tenne, Nanoscale, 2019, 11, 8073–8090 RSC
.
- B. C. Steimle, J. L. Fenton and R. E. Schaak, Science, 2020, 367, 418–424 CrossRef CAS PubMed
.
- X. Pang, Y. He, J. Jung and Z. Lin, Science, 2016, 353, 1268–1272 CrossRef CAS PubMed
.
- B. Jiang, Y. He, B. Li, S. Zhao, S. Wang, Y. B. He and Z. Lin, Angew. Chem., Int. Ed., 2017, 56, 1869–1872 CrossRef CAS PubMed
.
- M. Müllner, J. Yuan, S. Weiss, A. Walther, M. Förtsch, M. Drechsler and A. H. E. Müller, J. Am. Chem. Soc., 2010, 132, 16587–16592 CrossRef
.
- K. Huang, D. P. Canterbury and J. Rzayev, Chem. Commun., 2010, 46, 6326–6328 RSC
.
- K. Huang and J. Rzayev, J. Am. Chem. Soc., 2011, 133, 16726–16729 CrossRef CAS PubMed
.
- K. Huang and J. Rzayev, J. Am. Chem. Soc., 2009, 131, 6880–6885 CrossRef CAS
.
- K. Huang, D. P. Canterbury and J. Rzayev, Macromolecules, 2010, 43, 6632–6638 CrossRef CAS
.
- Y. Deng, S. Zhang, G. Lu and X. Huang, Polym. Chem., 2013, 4, 1289–1299 RSC
.
- Y. Liu, Z. Wang, S. Liang, Z. Li, M. Zhang, H. Li and Z. Lin, Nano Lett., 2019, 19, 9019–9028 CrossRef CAS
.
- Z. He, A. Zhong, H. Zhang, L. Xiong, Y. Xu, T. Wang, M. Zhou and K. Huang, Macromol. Rapid Commun., 2016, 37, 1566–1572 CrossRef CAS
.
- S. Zhang, Y. Tezuka, Z. Zhang, N. Li, W. Zhang and X. Zhu, Polym. Chem., 2018, 9, 677–686 RSC
.
- C. W. Bielawski, D. Benitez and R. H. Grubbs, Science, 2002, 297, 2041–2044 CrossRef CAS PubMed
.
- T. Yamamoto and Y. Tezuka, Polym. Chem., 2011, 2, 1930–1941 RSC
.
- A. J. Boydston, T. W. Holcombe, D. A. Unruh, J. M. J. Fréchet and R. H. Grubbs, J. Am. Chem. Soc., 2009, 131, 5388–5389 CrossRef CAS PubMed
.
- Y. Xia, A. J. Boydston and R. H. Grubbs, Angew. Chem., Int. Ed., 2011, 50, 5882–5885 CrossRef CAS PubMed
.
- K. Zhang, M. A. Lackey, Y. Wu and G. N. Tew, J. Am. Chem. Soc., 2011, 133, 6906–6909 CrossRef CAS PubMed
.
- A. Narumi, M. Yamada, Y. Unno, J. Kumaki, W. H. Binder, K. Enomoto, M. Kikuchi and S. Kawaguchi, ACS Macro Lett., 2019, 8, 634–638 CrossRef
.
- A. R. Halpern and R. M. Corn, ACS Nano, 2013, 7, 1755–1762 CrossRef CAS
.
- B. Senyuk, Q. Liu, S. He, R. D. Kamien, R. B. Kusner, T. C. Lubensky and I. I. Smalyukh, Nature, 2013, 493, 200–205 CrossRef CAS PubMed
.
- L. Xiao, L. Qu, W. Zhu, Y. Wu, Z. Liu and K. Zhang, Macromolecules, 2017, 50, 6762–6770 CrossRef CAS
.
- J. Sakamoto, J. van Heijst, O. Lukin and A. D. Schlüter, Angew. Chem., Int. Ed., 2009, 48, 1030–1069 CrossRef CAS PubMed
.
- P. Payamyar, B. T. King, H. C. Öttinger and A. D. Schlüter, Chem. Commun., 2016, 52, 18–34 RSC
.
- T. Zhao, A. Elzatahry, X. Li and D. Zhao, Nat. Rev. Mater., 2019, 4, 775–791 CrossRef CAS
.
- M. R. Buck, J. F. Bondi and R. E. Schaak, Nat. Chem., 2012, 4, 37–44 CrossRef CAS PubMed
.
- M. R. Buck and R. E. Schaak, Angew. Chem., Int. Ed., 2013, 52, 6154–6178 CrossRef CAS PubMed
.
- M. Zhang, Q. Xiong, Y. Wang, Z. Zhang, W. Shen, L. Liu, Q. Wang and Q. Zhang, Polym. Chem., 2014, 5, 4670–4678 RSC
.
- A. Li, Z. Li, S. Zhang, G. Sun, D. M. Policarpio and K. L. Wooley, ACS Macro Lett., 2012, 1, 241–245 CrossRef CAS
.
- Y. Yamauchi, K. Yamada, N. N. Horimoto and Y. Ishida, Polymer, 2017, 120, 68–72 CrossRef CAS
.
- Y. Liu, C. Yu, H. Jin, B. Jiang, X. Zhu, Y. Zhou, Z. Lu and D. Yan, J. Am. Chem. Soc., 2013, 135, 4765–4770 CrossRef CAS PubMed
.
- L. Jiang, M. Xie, J. Dou, H. Li, X. Huang and D. Chen, ACS Macro Lett., 2018, 7, 1278–1282 CrossRef CAS
.
- M. X. Xie, L. Jiang, Z. P. Xu and D. Y. Chen, Chem. Commun., 2015, 51, 1842–1845 RSC
.
- B. Huang, M. Chen, S. Zhou and L. Wu, Polym. Chem., 2015, 6, 3913–3917 RSC
.
- Y. Wang, R. Li, Y. Bian, X. Zhang and X. Zhu, Polym. Chem., 2019, 10, 5168–5171 RSC
.
| This journal is © The Royal Society of Chemistry 2020 |




