Molecular rotors as reporters for viscosity of solutions of collagen like peptides†
Christopher D.
McTiernan
 a,
Matias
Zuñiga-Bustos
b,
Roberto
Rosales-Rojas
bc,
Pablo
Barrias
a,
Matias
Zuñiga-Bustos
b,
Roberto
Rosales-Rojas
bc,
Pablo
Barrias
 d,
May
Griffith
d,
May
Griffith
 ef,
Horacio
Poblete
ef,
Horacio
Poblete
 bg,
Peter S.
Sherin
bg,
Peter S.
Sherin
 h,
Ismael
López-Duarte
h,
Ismael
López-Duarte
 h,
Marina K.
Kuimova
h,
Marina K.
Kuimova
 h and
Emilio I.
Alarcon
h and
Emilio I.
Alarcon
 *ai
*ai
aDivision of Cardiac Surgery, University of Ottawa Heart Institute, 40 Ruskin Street, Ottawa, Canada. E-mail: ealarcon@ottawaheart.ca
bDepartamento de Bioinformática, Centro de Bioinformática, Simulación y Modelado (CBSM), Facultad de Ingeniería, Universidad de Talca, Campus Talca, 1 Poniente No. 1141, Casilla 721, Talca, Chile
cDoctorado en ciencias Mención Modelado de Sistemas Químicos y Biológicos, Facultad de Ingeniería, Universidad de Talca, Campus Talca, 1 Poniente No. 1141, Casilla 721, Talca, Chile
dDepartamento de Ciencias del Ambiente, Facultad de Química y Biología, Universidad de Santiago de Chile, Casilla 40 Correo 33, Santiago, Chile
eCentre de Recherche Hôpital Maisonneuve-Rosemont, Montréal, QC, Canada
fDépartement d'ophtalmologie, Université de Montréal, Montréal, QC, Canada
gMillennium Nucleus of Ion Channels-Associated Diseases (MiNICAD), Universidad de Talca, Talca, Chile
hChemistry Department, Molecular Sciences Research Hub, Imperial College London, 82 Wood Lane, London W12 0BZ, UK
iDepartment of Biochemistry, Microbiology, and Immunology, Faculty of Medicine, University of Ottawa, Ottawa, Canada
First published on 21st October 2021
Abstract
We have studied the suitability of using a molecular rotor-based steady-state fluorometric assay for evaluating changes in both the conformation and the viscosity of collagen-like peptide solutions. Our results indicate that a positive charge incorporated on the hydrophobic tail of the BODIPY molecular rotor favours the dye specificity as a reporter for viscosity of these solutions.
Fluorescent molecules that have variable emission states dependent upon different molecular conformations have been used as biological sensors in applications such as lipid membrane status1–4 and monitoring local viscosities in vitro and in vivo.5–7 Molecular rotors (MR), in particular, have been used to evaluate viscosity in solution that is reliant on the fact that the fluorescent lifetime and quantum yield (QY) of the fluorescent MR change as a function of viscosity. Given that intramolecular twisting and rotation of such rotors typically lead to non-radiative decay of the excited state back to the ground state; it would seem apparent that a local increase in viscosity7–12 and/or crowding13–15 around these probes would affect this twisting and rotation. This leads to deactivation of the non-radiative decay pathway, which results in an increase in fluorescence QY and lifetime.8–10,16–18 It has been previously demonstrated that BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) based molecular rotors are capable of probing the micro-viscosity of solutions, cellular membranes, cellular compartments and tissues within in vitro, ex vivo, and in vivo settings.7,19,20 The exhibited changes in fluorescence are typically characterized as changes in fluorescence lifetime (τ) as measured via Fluorescence Lifetime Imaging Microscopy (FLIM) or other specialized equipment fitted with detectors capable of Time-Correlated Single Photon Counting (TCSPC).8,21–27 The BODIPY probes being tested here have shown drastic changes in fluorescence lifetime as a function of viscosity, ranging from 0.01 Pa s to 10 Pa s, with the highest sensitivity being observed in the viscosity range of 0.1 Pa s to approximately 4–5 Pa s, while viscosities of 0.001–0.05 Pa s result in little to no sensitivity.23
While this approach provides quantitative viscosity measurements, it may be advantageous to use intensity-based measurements that allow for use of more easily and readily accessible equipment.12,28 The advantage of use of fluorescence intensity is primarily due to the accessibility and cost of steady-state fluorimeters over their time-resolved FLIM counterparts. Using fluorescence intensities for determining conformation, and by extension viscosities in solutions of self-assembling structures, could serve as a reliable quality control tool for streamlining quality check of materials such as bioinks, often produced as aqueous solutions of short peptides.
Here, we used fluorogenic molecular rotor-based reporter molecules to quantify the viscosity of short collagen like peptides (CLP) solutions with viscosities ranging between 0.25 to ca. 100 Pa s, considerably higher than those typically probed in the literature using BODIPY dyes. We hypothesised that our rotors would interact with the peptide structures and, therefore, will respond to the change in their supramolecular conformation, as reported previously for other environmentally sensitive molecules,15,18,29–31 thus indirectly reporting on viscosity. Two different BODIPY molecular rotors bearing a carbon chain (non-charged BDPY1 and charged BDPY2) were used in this study (see Scheme 1). To the best of our knowledge, the use of fluorescence intensities of molecular rotors to determine conformation and viscosities of collagen like peptide solutions had not yet been explored.
We confirmed that the emission lifetimes and quantum yields of BDPY1 and BDPY2 (Scheme 1) strongly vary with the solution viscosity (Fig. S1, ESI†). Then we investigated the interaction of BDPY1 and BDPY2 with solutions of CLP-PEG (collagen-like-peptide polyethylene glycol conjugate),32 at variable temperature (Fig. 1 and Fig. S2, ESI†). It is clear to see that the emission of BDPY2 changes significantly as a function of temperature of these solutions (Fig. 1A and B). However, spectral shape of BDPY1 may be indicative of the dye aggregation (see Fig. S2, ESI†).
It should be noted that the photophysical properties of BODIPY rotors were previously shown to be temperature-independent, i.e. their fluorescence depends only on viscosity,17 while the temperature affects their properties indirectly, through the effect of temperature on solution viscosity. While, both BDPY1 and BDPY2 have similar fluorogenic cores and viscosity sensitivity (Fig. S1, ESI†), BDPY2 with its charge bearing tail is considerably more hydrophilic. As such it might be less prone to aggregation, see Fig. S2 (ESI†), and more suited for the analysis of the aqueous based collagen like peptide solutions, similar to what was previously shown in biological tissue analysis.7 Isothermal titration calorimetry measurements for the association of BDPY2 to the CLP structure using a multi-site model for plotting, rendered an association constant ≈1 × 105 M−1 (see Fig. S3, ESI†). This association constant is within the range of that reported for other BODIPY dyes to other biopolymers.33,34
Fig. 1A and B display emission spectra obtained for BDPY2 in the presence and absence of 0.1% w/w CLP-PEG over the temperature range of 10–70 °C. In both cases there appears to be a consistent and drastic decrease in the fluorescence intensity of the rotor as a function of increasing temperature. The slight increase in initial BDPY2 fluorescence intensity observed in the sample containing 0.1% w/w CLP-PEG as compared to BDPY2 water only should be assigned to the increased solution viscosity due to the presence of CLP-PEG. The subsequent decrease in intensity as a function of temperature observed in both samples may be related to temperature induced changes in viscosity,17,35 dielectric constant of the solution,35 or extent of hydrogen bonding.36 To ensure that the incorporation of the BODIPY rotors did not disrupt the triple helical structure of CLP-PEG molecules, CD melting curves were recorded for 0.1% w/w CLP-PEG (Fig. 1C and D) in the presence and absence of 10 μM BDPY2. As can be seen in Fig. 1C and D, the addition of the charged rotor (BDPY2) did not influence the melting curve of the CLP-PEG or inhibit its reassembly upon cooling, nor did it affect the conformation as comparable CD spectra for CLP-PEG were obtained in its presence (Fig. 1E) and absence (Fig. 1F). Note that similar trends were also observed in the melting curves of the type I collagen solutions (Fig. S4, ESI†) however, as expected, there was no reassembly of the helical structure observed upon heating above the melting/denaturation temperature of the type I collagen solution in the presence or absence of the BODIPY rotors.
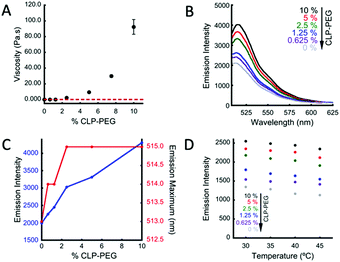
To further examine the interaction of BDPY2 with CLP-PEG, we prepared solutions with concentrations of CLP-PEG ranging from 0% to 10% w/w and 10 μM BDPY2. These solutions ranged in viscosity from below 0.25 Pa s (limit of detection of the employed system) to 92 Pa s, as measured by bulk rheometry (Fig. 2A). As seen in Fig. 2B and C, when we measured the fluorescence spectrum of BDPY2 in these solutions we find that as the concentration of CLP-PEG increases there is a concomitant increase in fluorescence intensity and a slight red shift in the emission maxima. Further, as shown in Fig. 2D, the fluorescence emission intensity of BDPY2 decreases as temperatures increase, irrespective of the starting CLP-PEG concentration. For BDPY1 we observed minor, non-significant, changes in the emission intensity as a function of the CLP-PEG content (Fig. S5, ESI†). Together with temperature dependent data presented in Fig. S2 (ESI†), this data suggests partial aggregation of BDPY1 and its lack of sensitivity to the presence of CPL, hence, the studies with this dye were discontinued.
The atomic interactions between BDPY2 and CLP were studied using classical molecular dynamics in an aqueous environment at different temperatures. Our results show that BDPY2 interacted closely with CLP throughout the course of the simulation (for greater than 60% of the simulation time). This data indicates that the BODIPY rotors bind to CLP instead of diffusing freely in solution.
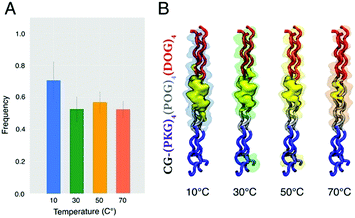
As shown in Fig. 3, the contact frequency of BDPY2 with CLP decreased as a function of increasing temperature, highlighting that the dye remains attached to CLP at lower temperatures and in viscous environments throughout the 500 ns simulation. The three-dimensional volumetric contact maps (Fig. 3B) of BDPY2 in the presence of CLP at 10 °C, 30 °C, 50 °C, and 70 °C illustrate that the contacts of the probe to the peptide are governed by the generation of electrostatic interactions between the polar head groups of the CLP and the charged tail of BDPY2 at lower temperatures, whereas at higher temperatures, lower viscosities, the dye tends to diffuse freely in the solvent. Additionally, to better highlight the nature of the contacts of BDPY1 and BDPY2 with the CLP at 10 °C, 30 °C, 50 °C, and 70 °C; Fig. S6 (ESI†) breaks down the simulated contact frequency with each amino acid residue of the CLP peptide. Overall, despite the neutral BDPY1 showing a similar contact behavior to BDPY2 (see Fig. S7, ESI†), experimentally BDPY1 showed lower hydrophilicity compared to BDPY2 and insufficient fluorescence response to CLP.
To better understand the effect of viscosity, temperature, and conformation, we set out to further compare the characteristics of 10 μM BDPY2 in aqueous solutions consisting of 0% and 5% w/w CLP-PEG, an intermediate concentration. Fluorescence emission data for BDPY1 and 5% w/w CLP-PEG did not show any trend to link the dye emission with viscosity, Fig. S5 (ESI†). For BDPY2, as seen in Fig. 4A and B, the absorption and emission spectra of the dye are similar in both the 0% and 5% CLP-PEG solutions, with absorption maxima centered at 495 nm and emission maxima centered around 517 nm at 25 °C. In monitoring the steady-state fluorescence emission spectrum of both the solutions at temperatures ranging from 10–70 °C we recorded that the emission of BDPY2 decreases and becomes slightly red shifted with increasing temperature for both the 0% CLP-PEG (Fig. 4C and E) and 5% CLP-PEG (Fig. 4D and F) solutions. Further, there is a large jump in the emission intensity of BDPY2 in 5% w/w CLP-PEG between 10 °C and 20 °C, which corresponds to a significant change in viscosity of the CLP-PEG solution as measured in Fig. 4G.
Upon closer examination of the steady-state fluorescence anisotropy of BDPY2 in water and 5% w/w CLP-PEG solutions (Fig. 4H) we found that the fluorescence anisotropy of BDPY2 was higher in the viscous CLP-PEG solution. There was a decrease in anisotropy as the temperature of the solutions increased and the supramolecular CLP-PEG conformation is lost, which resulted in a reduced viscosity. This data provides further evidence that the viscosity of the solutions are correlated with the rotation/relaxation of BDPY2, probably due to the interactions of BDP2 with the helical structure of the assembled CLP-PEG.
Molecular analyses for the density of atomic contacts between BDPY2 and CLP show the “head-group” of the dye sitting closest to CLP structure at 10 °C (see Fig. 4A). This interaction weakens at higher temperatures (see Fig. 4B–D). Our results indicate the binding of BDPY2 to CLP favors a stable head planar conformation of BDPY2. The modelling data suggests that the conformation of the BDPY2 upon binding to CLP increases the energy barrier for the transition to a butterfly conformation (core-bended structure), which increases the viscosity sensitivity for the dye.37
In summary, we propose that the fluorescence intensity of BDPY2 rotor is strongly correlated to the viscosity of aqueous CLP solutions, in the range between 0.25 to ca. 100 Pa s, as measured by standard rheometry. Our results suggest that under well controlled conditions, one could use the emissive properties of the BDPY2 dye to develop a rapid quality control test whereby emission intensities could be directly correlated with viscosity. Such a simple quality control system will considerably simplify the standardization of peptide-based gels and bioinks.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
EIA thanks the Natural Sciences and Engineering Research Council (NSERC) RGPIN-2015-0632, the Canadian Institutes of Health Sciences (CIHR) and to the Government of Ontario for an Early Career Research Award. HP thanks to Fondecyt grant (#1211143), also, the Millennium Nucleus of Ion Channels-Associated Diseases (MiNICAD) is a Millennium Nucleus supported by the Iniciativa Científica Milenio of the Ministry of Economy, Development, and Tourism (Chile), and the Fondo de Equipamiento Científico y Tecnologico (FONDEQUIP, #160063). MZ-B thanks Fondecyt Postdoctoral fellowship 3200252. RRR thanks ANID Chile PhD fellowship (#21212080). MG acknowledges support from the Canada Research Chair program (#950-232398). PB thanks CONICYT PhD fellowship (#2116065). M. K. K. is thankful to the EPSRC for a Career Acceleration Fellowship (EP/I003983/1). P. S. S. is thankful to the European Commission for Marie Sklodowska-Curie Fellowship (#834809). EIA would like to thank Dr Jean-François Couture at University of Ottawa for access to the ITC system used in this study.References
- A. Colom, E. Derivery, S. Soleimanpour, C. Tomba, M. D. Molin, N. Sakai, M. González-Gaitán, S. Matile and A. Roux, Nat. Chem., 2018, 10, 1118–1125 CrossRef CAS PubMed
.
- D. Su, C. L. Teoh, L. Wang, X. Liu and Y.-T. Chang, Chem. Soc. Rev., 2017, 46, 4833–4844 RSC
.
- M. Dal Molin, Q. Verolet, A. Colom, R. Letrun, E. Derivery, M. Gonzalez-Gaitan, E. Vauthey, A. Roux, N. Sakai and S. Matile, J. Am. Chem. Soc., 2015, 137, 568–571 CrossRef CAS PubMed
.
- J. García-Calvo, J. Maillard, I. Fureraj, K. Strakova, A. Colom, V. Mercier, A. Roux, E. Vauthey, N. Sakai, A. Fürstenberg and S. Matile, J. Am. Chem. Soc., 2020, 142, 12034–12038 CrossRef PubMed
.
- D. Zhu, M. A. Haidekker, J.-S. Lee, Y.-Y. Won and J. C. M. Lee, Macromolecules, 2007, 40, 7730–7732 CrossRef CAS
.
- S. Toliautas, J. Dodonova, A. Žvirblis, I. Čiplys, A. Polita, A. Devižis, S. Tumkevičius, J. Šulskus and A. Vyšniauskas, Chem. – Eur. J., 2019, 25, 10342–10349 CrossRef CAS PubMed
.
- L. E. Shimolina, M. A. Izquierdo, I. López-Duarte, J. A. Bull, M. V. Shirmanova, L. G. Klapshina, E. V. Zagaynova and M. K. Kuimova, Sci. Rep., 2017, 7, 41097 CrossRef CAS PubMed
.
- M. K. Kuimova, S. W. Botchway, A. W. Parker, M. Balaz, H. A. Collins, H. L. Anderson, K. Suhling and P. R. Ogilby, Nat. Chem., 2009, 1, 69–73 CrossRef CAS PubMed
.
- M. K. Kuimova, G. Yahioglu, J. A. Levitt and K. Suhling, J. Am. Chem. Soc., 2008, 130, 6672–6673 CrossRef CAS PubMed
.
- M. K. Kuimova, Phys. Chem. Chem. Phys., 2012, 14, 12671–12686 RSC
.
- Z. Yang, J. Cao, Y. He, J. H. Yang, T. Kim, X. Peng and J. S. Kim, Chem. Soc. Rev., 2014, 43, 4563–4601 RSC
.
- M. A. Haidekker and E. A. Theodorakis, Org. Biomol. Chem., 2007, 5, 1669–1678 RSC
.
- A. J. Thompson, T. W. Herling, M. Kubánková, A. Vyšniauskas, T. P. Knowles and M. K. Kuimova, J. Phys. Chem. B, 2015, 119, 10170–10179 CrossRef CAS PubMed
.
- M. Kubánková, I. López-Duarte, J. A. Bull, D. M. Vadukul, L. C. Serpell, M. de Saint Victor, E. Stride and M. K. Kuimova, Biomaterials, 2017, 139, 195–201 CrossRef PubMed
.
- A. K. Mora, P. K. Singh, B. S. Patro and S. Nath, Chem. Commun., 2016, 52, 12163–12166 RSC
.
- S.-C. Lee, J. Heo, H. C. Woo, J.-A. Lee, Y. H. Seo, C.-L. Lee, S. Kim and O.-P. Kwon, Chem. – Eur. J., 2018, 24, 13706–13718 CrossRef CAS PubMed
.
- A. Vyšniauskas and M. K. Kuimova, Int. Rev. Phys. Chem., 2018, 37, 259–285 Search PubMed
.
- P. K. Singh, M. Kumbhakar, H. Pal and S. Nath, J. Phys. Chem. B, 2010, 114, 5920–5927 CrossRef CAS PubMed
.
- S. Raut, J. Kimball, R. Fudala, H. Doan, B. Maliwal, N. Sabnis, A. Lacko, I. Gryczynski, S. V. Dzyuba and Z. Gryczynski, Phys. Chem. Chem. Phys., 2014, 16, 27037–27042 RSC
.
- P. S. Sherin, I. Lopez-Duarte, M. R. Dent, M. Kubankova, A. Vysniauskas, J. A. Bull, E. S. Reshetnikova, A. S. Klymchenko, Y. P. Tsentalovich and M. K. Kuimova, Chem. Sci., 2017, 8, 3523–3528 RSC
.
- W. Miao, C. Yu, E. Hao and L. Jiao, Front. Chem., 2019, 7, 825 CrossRef CAS PubMed
.
- M. R. Dent, I. López-Duarte, C. J. Dickson, N. D. Geoghegan, J. M. Cooper, I. R. Gould, R. Krams, J. A. Bull, N. J. Brooks and M. K. Kuimova, Phys. Chem. Chem. Phys., 2015, 17, 18393–18402 RSC
.
- Y. Wu, M. Štefl, A. Olzyńska, M. Hof, G. Yahioglu, P. Yip, D. R. Casey, O. Ces, J. Humpolíčková and M. K. Kuimova, Phys. Chem. Chem. Phys., 2013, 15, 14986–14993 RSC
.
- X. Peng, Z. Yang, J. Wang, J. Fan, Y. He, F. Song, B. Wang, S. Sun, J. Qu, J. Qi and M. Yan, J. Am. Chem. Soc., 2011, 133, 6626–6635 CrossRef CAS PubMed
.
- E. Gatzogiannis, Z. Chen, L. Wei, R. Wombacher, Y.-T. Kao, G. Yefremov, V. W. Cornish and W. Min, Chem. Commun., 2012, 48, 8694–8696 RSC
.
- L. Wang, Y. Xiao, W. Tian and L. Deng, J. Am. Chem. Soc., 2013, 135, 2903–2906 CrossRef CAS PubMed
.
- Z. Yang, Y. He, J.-H. Lee, N. Park, M. Suh, W.-S. Chae, J. Cao, X. Peng, H. Jung, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2013, 135, 9181–9185 CrossRef CAS PubMed
.
- E. M. Woodcock, P. Girvan, J. Eckert, I. Lopez-Duarte, M. Kubánková, J. van Loon, N. J. Brooks and M. K. Kuimova, Biophys. J., 2019, 116, 1984–1993 CrossRef CAS PubMed
.
- G. Chakraborty, A. K. Ray, P. K. Singh and H. Pal, Chem. Commun., 2018, 54, 8383–8386 RSC
.
- P. K. Singh, A. K. Mora, S. Murudkar and S. Nath, RSC Adv., 2014, 4, 34992–35002 RSC
.
- P. K. Singh, J. Sujana, A. K. Mora and S. Nath, J. Photochem. Photobiol., A, 2012, 246, 16–22 CrossRef CAS
.
- M. M. Islam, R. Ravichandran, D. Olsen, M. K. Ljunggren, P. Fagerholm, C. J. Lee, M. Griffith and J. Phopase, RSC Adv., 2016, 6, 55745–55749 RSC
.
- N. Dorh, S. Zhu, K. B. Dhungana, R. Pati, F.-T. Luo, H. Liu and A. Tiwari, Sci. Rep., 2015, 5, 18337 CrossRef CAS PubMed
.
- L. P. Jameson, N. W. Smith, O. Annunziata and S. V. Dzyuba, Phys. Chem. Chem. Phys., 2016, 18, 14182–14185 RSC
.
- A. Vyšniauskas, M. Qurashi, N. Gallop, M. Balaz, H. L. Anderson and M. K. Kuimova, Chem. Sci., 2015, 6, 5773–5778 RSC
.
- A. Polita, S. Toliautas, R. Žvirblis and A. Vyšniauskas, Phys. Chem. Chem. Phys., 2020, 22, 8296–8303 RSC
.
- X. Liu, W. Chi, Q. Qiao, S. V. Kokate, E. P. Cabrera, Z. Xu, X. Liu and Y.-T. Chang, ACS Sens., 2020, 5, 731–739 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cp04398f |
| This journal is © the Owner Societies 2021 |